Abstract
Purpose
Central post-stroke pain (CPSP) is a neuropathic disorder resulting in pain and disability. An emerging treatment for CPSP is non-invasive brain stimulation including direct current stimulation [tDCS] and repetitive transcranial magnetic stimulation [rTMS]. This systematic review analyzes the efficacy and quality of non-invasive brain stimulation intervention studies for CPSP.
Methods
Studies were sought from three research databases published between 2007 and 2017. Studies were included if the sole intervention was non-invasive brain stimulation and the primary outcome either clinical or experimental pain intensity. Studies were qualitatively assessed for risk of bias.
Results
Of 1107 articles extracted, six met eligibility criteria. Five studies found a decrease in pain intensity (p<0.05) immediately and 3 weeks after rTMS or tDCS was delivered over the primary motor cortex. For experimental pain, one study found thermal pain thresholds improved for those receiving tDCS compared to sham (p<0.05), while another found normalization of the cold detection threshold only after rTMS (p<0.05). Qualitative assessment revealed only one study rated as “excellent/good” quality, while the other five were rated as “fair” or “poor”.
Conclusion
Non-invasive brain stimulation may have a therapeutic effect on pain level for individuals with CPSP, as evidenced by significant decreases in clinical and experimental pain scores. However, despite the impact of CPSP and the promise of non-invasive brain stimulation, few rigorous studies have been performed in this area. Future studies should aim to standardize treatment parameters, measure both clinical and experimental pain, and include long-term follow-up.
Keywords: stroke, pain management, transcranial direct current stimulation, transcranial magnetic stimulation
Plain Language Summary
A number of people who have suffered a stroke may also suffer from central post-stroke pain, which can increase overall pain levels that can negatively affect post-stroke recovery. Although pharmacological treatments exist, one non-pharmacological option is the use of transcranial brain stimulation, a safe and effective mode of altering or enhancing brain function. This systematic review aims to evaluate how effective this treatment is in reducing both clinical pain (such as on a 0–10 pain rating scale) and experimental pain (pain related to changes in temperature or pressure on skin) to gain a more holistic understanding of its effects. Six studies were included in the review, and of these, five reported significant decreases in overall clinical pain levels and two reported changes in experimental pain. However, nearly all of the studies differed in the parameters of the study and the characteristics of the participants, and only one of the studies had a quality rating of “excellent/good,” meaning that each study was conducted differently, and thus it is difficult to generalize these results. Future studies should aim to measure both clinical and experimental pain and aim for higher quality with more standardized study protocols and patient recruitment, in order to improve the likelihood that this treatment can be used clinically to treat central post-stroke pain.
Introduction
Central post-stroke pain (CPSP), also known as Dejerine–Roussy syndrome or thalamic pain syndrome, is a central neuropathic pain disorder that affects from 10 to 35 percent of the post-stroke population.1 Clinical characteristics of CPSP vary greatly but most commonly include allodynia (the production of pain by a normally painless stimulus) and dysesthesia (abnormal sensation).2,3 The nature of the pain can include burning or aching to freezing or numb, and the pain intensity has been reported to be nearly 8/10 on a visual analog scale (VAS).4 Equally variable is the onset and duration of symptoms, with some experiencing symptoms within 6 months of stroke, to others having symptoms 10 years later.2 Nevertheless, a commonality of CPSP is the impact it has on the individual’s quality of life and interference with rehabilitation.2,3
While the pathophysiology of CPSP is not entirely clear, research points to diverse involvement of the spinothalamic tracts, the somatosensory cortex, and multiple areas of the cerebral cortex.4,5 A promising non-pharmacological treatment to directly target such areas is brain stimulation. Typically, brain stimulation is separated by mode of delivery: 1) application of magnets or electrodes to the scalp surface or 2) surgical implantation of electrodes. The latter, including motor cortex stimulation (MCS) and deep brain stimulation (DBS), has been found to reduce short-term pain intensity.6–8 However, due to the invasiveness of both MCS and DBS, these interventions are proving more difficult to generalize to clinical care.
In contrast, surface-derived techniques like repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) may be faster to translate to clinical care. Such interventions do not require surgery and are thus non-invasive. Moreover, research thus far on rTMS and tDCS has shown a positive effect for pain in a multitude of conditions, including spinal cord injury,9 hyperalgesia,10 and motor recovery post-stroke.11 Emerging evidence also suggests that rTMS and tDCS may also have promising pain-reducing effects in persons with CPSP.12,13 Evidence is limited because a majority of the research outcomes in this area has been isolated in using clinical pain intensity. Since both rTMS and tDCS may target brain regions involved in pain processing, a key ancillary outcome measure to consider is laboratory correlates of pain processing, which can be assessed by administering an experimental pain measure such as quantitative sensory testing (QST). To our knowledge, systematic appraisal of both clinical and experimental pain outcome measures has not been performed.
Therefore, the purpose of the current study was to evaluate and summarize current literature studying the effect of rTMS and tDCS on central post-stroke pain, including both clinical and experimental pain measures. Other secondary outcomes were examined when reported. We anticipated the opportunity to provide recommendations for future research by examining the efficacy and safety of non-invasive brain stimulation among individuals with central-post stroke pain to improve outcomes and quality of life.
Methods
Protocol and Registration
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed during the search and reporting phase of the research process.14 The initial protocol and systematic review were prospectively registered on July 24, 2017 through the PROSPERO database for systematic reviews (ID: CRD42017070563).
Search Strategy
A comprehensive literature search was performed using the databases PubMed, Embase, and Web of Science between 2007 and 2018. The literature search plan was developed and performed in collaboration with a Research Librarian (LL) with expertise in systematic reviews and senior authors with expertise in stroke and pain science and rehabilitation (JF, CS). The search used key words and subject headings, appropriate for each database, related to neurostimulation, pain, and thalamic diseases.
Selection Criteria
Type of Population
Studies involving adult patients with stroke between the ages of 18 and 85 years with a primary condition of central post-stroke pain were included without any restrictions for sex, length of post-stroke CPSP onset, or anatomical side of stroke. The stroke diagnosis was required to meet criteria as defined by the American Heart Association.15 Further, the CPSP diagnosis had to meet the most up-to-date diagnosis definition.16 The type of stroke was restricted to ischemic or hemorrhagic stroke. Cerebellar and brainstem strokes were excluded due to symptom complexity. Transient ischemic attacks were also excluded.
Study Intervention
The study intervention was non-invasive brain stimulation including transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS). Papers with co-interventions in addition to brain stimulation were not included unless the other co-intervention was included as a separate comparison arm.
Study Outcome
Outcomes included participant-reported pain measures and quantitative sensory testing measures. We opted to query all studies assessing pain outcome, regardless of measure, although commonly, either the numeric pain rating scale or the visual analog scale17 are employed. The VAS is a validated self-report outcome measure of pain intensity that involves marking a 10-cm line between two descriptive anchors; the left VAS end point is commonly marked “no pain,” while the right VAS end point is commonly marked “intolerable pain.”14 QST is an umbrella term for employing experimental pain paradigms as a correlate for endogenous pain processing.18 QST is typically separated into either static (e.g. pain threshold, tolerance) or dynamic (e.g. temporal summation of second pain, conditioned pain modulation) measures.
Type of Study Design
Only randomized controlled trials and observational studies (cohort, case–control, and cross-sectional studies) were included in this study.
Publication Type
Only studies published in English journals among the three databases were considered for review. Grey literature (e.g. conference posters or abstracts) were not included.
Study Selection
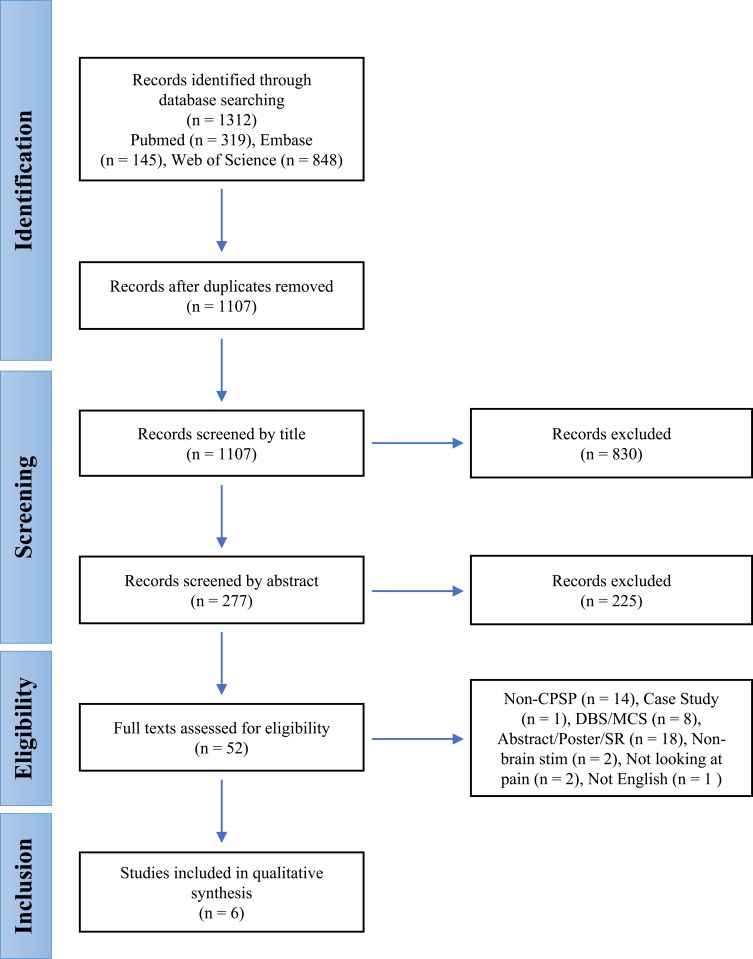
The databases were systematically searched in June 2017, and afterwards, the yield was imported into EndNote™ (Clarivate Analytics, Philadelphia, PA). A follow-up search was conducted in April 2018 to ensure that no studies published since the initial search were left out of the current review. This allowed for the consolidation of literature searches from multiple databases, elimination of duplicate articles, and precise tracking for the construction of a PRISMA flow sheet (Figure 1). After deleting duplicates, the titles were screened by four viewers (BR, KB, SD, DS). Abstracts from the remaining publications were screened by two reviewers (BR, DS). Finally, full-text articles of remaining articles were retrieved and examined for eligibility by two reviewers (KB, SD). A third, blinded reviewer (BR) served as the deciding vote in the event conflicts arose between reviewers. If in doubt, the two senior authors (JF, CS) were consulted.
Figure 1.
PRISMA flow chart for selection of studies.
Data Extraction
One reviewer (KB) performed data extraction independently and a second reviewer (BR) checked the extraction. For each article, the following information was extracted using a standard data extraction form: study design, number of participants, participant characteristics (e.g. age, sex, time since stroke, type of stroke), pain duration, stimulation intervention type, control group information, and outcome measures (e.g. pain intensity, QST). Within- and between-subject mean differences were extracted and/or calculated for each of the outcome measures. Data were then summarized qualitatively.
Critical Appraisal
Quality assessment was performed on all studies meeting inclusion criteria. Two reviewers (SD, DS) employed the Modified Downs and Black Checklist,19 along with a third senior author review (CS), and scores were mutually agreed upon. This tool allows raters to judge elements from five domains (reporting, external validity, internal validity bias and confounding factors, and power). Based on scores from each domain, a total score is calculated, and from this score, the study is rated as either “excellent/good”, “fair”, or “poor” quality.
Results
Search Results
A total of 1312 articles were identified through the comprehensive database search (Figure 1). After the removal of duplicates, 1107 titles and abstracts remained for screening. After screening the titles and abstracts, 1055 articles were excluded. Of the remaining 52 articles, 46 were excluded upon full-text review. Reasons for exclusion include studies not related to CPSP, studies using DBS or MCS as the primary intervention, studies that did not involve brain stimulation, studies where pain was not the primary outcome, case studies/abstracts/posters, and studies not written or translated to English. Six studies20–25 remained and were included in the qualitative synthesis.
Study Characteristics
Of the six studies included in the final synthesis, one was a randomized controlled trial (RCT), three were non-randomized intervention studies with either between-subjects or within-subjects design, while two others were purely descriptive designs (e.g., case study). Sample sizes ranged from 14 to 23, with a mean of 18.5 subjects (Table 1). Only de Oliveira et al21 reported subjects who did not complete the study (n=2), due to changes in medication within the duration of enrollment. All studies were conducted between 2012 and 2015 (Table 1).
Table 1.
Demographic Characteristics of the Included Studies
| Author, Year | Study Type | Subject Number Completing Study (Number Recruited) | Sex (M, F) | Age Range (Mean or Mean ± SD) | Type of Stroke | Time Post-Stroke (Months) | Pain Duration (Range in Months) | |
|---|---|---|---|---|---|---|---|---|
| Ischemic | Hemorrhagic | |||||||
| Bae et al, 201420 | RCT | 14 (14) | 7, 7 | 45–55 (51.7) | 8 | 6 | NR | 14.6 (Mean) |
| De Oliveira et al, 201421 | Prospective cohort | 21 (23) | 11, 10 | 37–73 (56.3) | 17 | 4 | NR | 12–144 |
| Hasan et al, 201422 | Case series | 14 (14) | 10, 4 | 57 (median) | 11 | 3 | NR | NR |
| Kobayashi et al, 201523 | Cross-over | 18 (18) | 12, 6 | 39–81 (63.0 ± 9.9) | 5 | 13 | 6–39 | 3–26 |
| Matsumura et al, 201324 | Cross-over | 20 (20) | 12, 8 | 54–85 (63.6 ± 8.1) | 7 | 13 | NR | 6–180 |
| Ohn et al, 201225 | Case series | 22 (22) | 13, 9 | (54.0 ± 9) | 15 | 7 | NR | 21.9 ± 17.2 (Mean ± SD) |
Participant Characteristics
The participants were recruited from local pain centers or else was not specified. The six studies included subjects with either ischemic or hemorrhagic stroke, greater than 6 months post-stroke, typically more males than females and ages 37–85 years (Table 1). All studies included subjects with CPSP with onset starting from as soon as 3 months to as long as 180 months and duration of pain ranging from 3 months to 15 years.
Brain Stimulation Parameters
Five studies utilized rTMS, while Bae et al20 used tDCS. Five studies stimulated the primary motor cortex, while one stimulated the pre-motor cortex and dorsolateral pre-frontal cortex.21 The intensity and flow of the stimulation varied across studies (Table 2). The frequency of stimulation ranged from once a day for 10 days to once a week for 12 weeks, and the length of intervention ranged from 1 day to 12 weeks.
Table 2.
Summary of Interventions from Included Studies
| Author, Year | Type of Stimulation | Stimulation Location | Intensity | Current Flow | Frequency of Intervention | Length of Intervention | Control Group |
|---|---|---|---|---|---|---|---|
| Bae et al, 201420 | tDCS | Primary motor cortex | 2 mA | 20 min | 3×/week | 3 weeks | Sham tDCS |
| De Oliveira et al, 201421 | rTMS | Primary motor cortex/dorsolateral prefrontal cortex | 120% RMT | 10 Hz − 25 × 5 sec | 1×/day | 10 days | Sham rTMS |
| Hasan et al, 201422 | rTMS | M1 predetermined ‘hotspot’ | 80–90% RMT | 10 Hz − 20 × 10 s | 1 session/3–5 days | 5 sessions | None |
| Kobayashi et al, 201523 | rTMS | Primary motor cortex | 90% RMT | 5 Hz −10 × 10 s | 1×/week | 12 weeks | Sham rTMS |
| Matsumura et al, 201324 | rTMS | Primary motor cortex | 100% RMT | 5 Hz −10 × 50 pulses | 1 session | 1 day | Sham rTMS |
| Ohn et al, 201225 | rTMS | Motor ‘hotspot’ of first dorsal interossei of affected hand | 90% RMT | 10 Hz − 50 × 5 s | 1/day | 5 days | None |
Abbreviation: RMT, Resting Motor Threshold.
Outcome Measures
Patient-Reported Pain Measures
Five of the six studies utilized the VAS for pain intensity as the primary outcome measure. Hasan et al22 collected their pain outcome via a pain diary as a secondary measure, which included self-reported Nominal Rating Scale (NRS) overall pain ratings, from 0 to 10 with 0 being “no pain” and 10 being “worst pain imaginable”, for overall pain, touch-related pain, and sensitivity to hot/cold.
QST
Two studies administered QST paradigms.20,22 In both cases, static pain threshold measures were used, but not dynamic. These measures included both thermal (i.e. warm and cold sensation and/or pain) and mechanical (i.e. tactile, pressure) thresholds. Hasan et al22 measured affected versus unaffected regions at baseline and found differences in thermal but not mechanical thresholds. Despite using different stimulation techniques (rTMS vs tDCS), both studies found a change in cold sensation threshold before and after stimulation (p<0.05). In addition, Bae et al20 found changes in warm sensation as well as cold pain thresholds, with some of these changes lasting up to 3 weeks post-stimulation.
Brain Stimulation Effect
Patient-Reported Pain Measures
Four of the studies reported significant decreases in VAS after the trial (p<0.05),20,23–25 with all four utilizing either rTMS or tDCS on the motor cortex, and one study revealing a significant time course effect over 12 weeks on VAS score. Table 3 reports the findings discussed hereafter. Bae et al20 found a decrease of 1.15 in pain intensity 3 weeks after a 3-week trial in the real rTMS group compared to a sham rTMS group, though no immediate or 1-week change. Matsumura et al24 reported a one-time stimulation reduced pain intensity by 17.4% for up to 300 mins post-stimulation versus only 3.4% for sham rTMS. Ohn et al25 found that the majority of subjects responded positively to rTMS, with a decrease of 0.9 on the VAS immediately after a 5-day trial of rTMS, and sustained benefits for up to 2 weeks post-stimulation. Kobayashi et al23 found a time course effect on pain intensity after 1 and 3 weeks of stimulation on VAS as far out as 12 weeks, with a plateau occurring around week 8. Of those who were considered “non-responders” to rTMS, the mean VAS scores were 5.1 at initial assessment and increased slightly after rTMS to 5.5, and then further to 6.0 at 2 weeks post-rTMS. One study21 utilized rTMS, but rather than stimulating the motor cortex, the pre-motor cortex (PMC) and dorsolateral pre-frontal cortex (DLPFC) were targeted. However, no significant changes in VAS scores were detected after 10 days with 10 sessions of stimulation compared to sham rTMS (effect size = 0.02; observed power = 0.03).
Table 3.
Intervention Results from Included Studies
| Author, Year | Measurement Time Point | VAS | |
|---|---|---|---|
| Within Subjects Mean Differences | |||
| Experimental Group | Control Group | ||
| Bae et al, 201420 | Baseline, immediate post | −0.15 | −0.28 |
| Baseline, 1 week post | −0.58 | −0.42 | |
| Baseline, 3 weeks post | −1.15* | −0.14 | |
| De Oliveira et al, 201421 | Baseline, day 10 post | −0.07 | −0.10 |
| Hasan et al, 201422 | Baseline, immediate post | −0.70* | |
| Kobayashi et al, 201523 | Baseline, immediate post | Time course effect on VAS score F(1, 10) = −22.273** | |
| Baseline, weekly for 12 weeks | Time course effect on VAS score F(12, 204) = −13.476** | ||
| Matsumura et al, 201324 | Baseline, multiple time points post (0, 60, 120, 180, 240, 300 mins, and 24 hrs) | −1.18* (significant time course effect up to 300 mins) | −0.21 |
| Ohn et al, 201225 | Baseline, immediately post | −0.90* | |
Notes: * p<0.05, ** p< 0.01.
Abbreviations: VAS, Visual Analog Scale.
One study22 did not report VAS scores but did report numeric rating scale (NRS) scores via pain diaries. Pain scores were averaged out over each week following rTMS. The authors found pain intensity decreased by 10% 1 week following the intervention that was not maintained in subsequent weeks.
Quantitative Sensory Testing
Bae et al17 found that the threshold for warm sensation (38.19°C to 35.93°C) decreased, while cold sensation (25.50°C to 26.26°C) and cold pain (12.24°C to 14.03°C) increased when compared to sham tDCS at 3 weeks post-treatment (p<0.05, Table 4). Hasan et al used QST as their primary outcome measure and discovered similar results to those found by Bae et al. Changes in cold sensation threshold (4.7±2.5°C) significantly increased in subjects receiving rTMS, while changes in warm threshold (−2.6±1.3°C) and pain cold threshold (−2.1±2.8°C) were notable but not statistically significant.22
Table 4.
Intervention Results from Included Studies
| Author, Year | Measurement Time Point | QST | ||
|---|---|---|---|---|
| Within Subjects Mean Differences | ||||
| Type | Experimental Group | Control Group | ||
| Bae et al, 201420 | Baseline, immediate post | CST | 0.39 | 0.32 |
| WST | −0.48 | −0.49 | ||
| CPT | 0.33 | 0.10 | ||
| WPT | −0.07 | −0.41 | ||
| Baseline, 1 week post | CST | 0.56* | 0.40 | |
| WST | −0.79 | −0.23 | ||
| CPT | 0.52* | 0.10 | ||
| WPT | −0.31 | −0.04 | ||
| Baseline, 3 weeks post | CST | 0.76* | 0.38 | |
| WST | −2.26** | −0.37 | ||
| CPT | 1.79*** | 0.23 | ||
| WPT | −0.37 | 0.15 | ||
| De Oliveira et al, 201421 | ||||
| Hasan et al, 201422 | Baseline, immediate post | CST | 4.70* | 1.10 |
| WST | −2.60 | −0.10 | ||
| CPT | −2.10 | −0.80 | ||
| WPT | 0.70 | 0.30 | ||
| TT | −0.01 | −0.01 | ||
| PT | –2.00 | –0.50 | ||
| Kobayashi et al, 201523 | ||||
| Matsumura et al, 201324 | ||||
| Ohn et al, 201225 | ||||
Notes: * p<0.05, ** p < 0.01, *** p< 0.001. A positive change represents an increase in temperature or pressure.
Abbreviations: QST, Quantitative Sensory Test; CST, cold sensation threshold; WST, warm sensation threshold; CPT, cold pain threshold; WPT, warm pain threshold; TT, tactile threshold; PT, pressure threshold.
Other Reported Outcomes
Beyond the primary pain outcomes, Kobayashi et al23 assessed the differences in response to brain stimulation based on the severity of reported pain at baseline. Those with severe dysesthesia (>70 VAS on 0 to 100 VAS scale) did not see significant changes in VAS scores after 12 weeks of once-weekly rTMS, whereas those without severe dysesthesia (<70 VAS) did see a significant reduction after 12 weeks (p<0.001). Matsumura et al24 evaluated the relationship between pharmacological evaluation and rTMS. VAS pain reduction via ketamine (r=0.503, p=0.012), morphine (r=0.527, p=0.009), and thiopental (r-0.609, p=0.002) were correlated with VAS score reductions via rTMS of the primary motor cortex. Importantly, some of the included studies reported the possibility of subjects taking pain medications during the study but did control for their potential impact on treatment effects.
Qualitative Appraisal
Results are summarized in Table 5. Overall, quality assessments revealed that the majority of included studies had a high susceptibility to bias, with only one study scoring as “good/excellent” quality.21 Only one study was a RCT,20 whereas three others were within-subject randomized cross-over studies.21,23,24 The extent to which study participants were representative of the population from which they were recruited could not be confirmed, in part because of the variability in timing from stroke and CPSP onset to enrollment, variability in stroke severity, and limited information provided on previous rehabilitation. Further, blinding both for participants and for study developers in studies involving randomization was deemed to be minimal.
Table 5.
Quality Assessment, Modified Downs and Black Checklist. Key for Total Score: Poor <14; Fair 14–18; Excellent/Good 19–28
| Author, Year | Reporting | External Validity | Internal Validity-Bias | Internal Validity-Confounding | Power | Total |
|---|---|---|---|---|---|---|
| Bae et al, 201420 | 7 | 0 | 5 | 1 | 0 | 13 |
| De Oliveira et al, 201421 | 10 | 0 | 7 | 4 | 1 | 22 |
| Hasan et al, 201422 | 6 | 0 | 4 | 0 | 0 | 10 |
| Kobayashi et al, 201423 Study 1 | 8 | 0 | 5 | 3 | 0 | 16 |
| Kobayashi et al, 201423 Study 2 | 8 | 0 | 5 | 3 | 0 | 16 |
| Matsumura et al, 201324 | 7 | 0 | 3 | 0 | 0 | 10 |
| Ohn et al, 201225 | 8 | 0 | 4 | 2 | 1 | 15 |
Discussion
The aim of this review was to systematically characterize the efficacy of non-invasive brain stimulation techniques on central post-stroke pain, as defined by changes in participant-reported pain intensity and laboratory correlates of pain processing (QST). Our findings suggest that non-invasive stimulation of the motor cortex, utilizing either tDCS or rTMS, has positive effects on CPSP. Given the variability in intervention follow-up time points, the duration of effects is not comparable across studies. However, brain stimulation was found to have both short-term (e.g., immediately after the session) and long-term (e.g., 3 weeks to 8 weeks post-stimulation) effects. Despite suggested benefits of non-invasive brain stimulation on CPSP, few studies have been performed in this area, and those that were of lower quality. To determine the clinical viability of brain stimulation for CPSP, additional higher rigor studies must be performed.
All but one study in our review utilized rTMS as the main intervention, and all studies focused their location of stimulation around the motor cortex. However, exact motor cortex stimulation targets could not be accurately determined without other methods (e.g., magnetic resonance imaging (MRI)). Stimulation current was either 5 Hz or 10 Hz for most studies, although the frequency and duration varied (range of 10 to 50 pulses for 5–10 s). Four of the six studies20,21,23,24 included a sham stimulation control group. While only one study23 reported time since stroke for study participants and all but one study reported time of pain onset which was greater than 6 months.
Despite similarities, there were actually more differences across studies, with the most striking being the differences in frequency and length of the intervention. Frequencies varied from a single session to daily sessions up to 3 times a week, but only two of the six studies had the same frequency. The length of the intervention was different among all six studies, ranging from one session to multiple sessions across 12 weeks. Intensity varied from 80% to 120% of resting motor threshold (RMT), with one study using the same intensity (2 mA) for every participant. With such variation in intervention frequency, duration, and intensity, it is difficult to pinpoint ideal parameters for effective non-invasive stimulation, which would be required to make clinical recommendations. Furthermore, participant variability likely played a role in outcomes across the six studies, including age, gender, type of stroke, time since pain onset, and severity of pain levels pre-intervention, which could all affect the outcome of a stimulation intervention. Only two studies23,25 reported on stroke location with both reporting no difference or association in results by stroke location. The heterogeneity in response to treatment may be a result of the heterogeneity of participants. Future studies must consider enrollment of more homogenous participant groups with clear delineation of the intervention protocol including stimulation parameters and dosage.
More concerning was the quality of the studies, as determined by the Modified Downs and Black scale, varied widely, with the majority found to have a high susceptibility to bias. The only study rated as “good/excellent” quality was discontinued midway through the trial due to a lack of positive findings. The poor external validity scores indicate a need for the utilization of better sampling techniques and making samples more representative of the population. A poor internal validity-confounding section also indicates that future research needs to incorporate random assignment, blinding, concealment, and/or a control for losses to follow-up to reduce the amount of error in results. Future studies should place more emphasis on controlling for bias in all areas of research to improve the quality of studies and the validity of their results.
Another concern is the lack of measures used to assess the multi-factorial nature of pain. The majority of studies in this used VAS as the primary outcome measure, which is undoubtedly a valuable and reliable assessor of pain intensity. However, because patients with CPSP are susceptible to both pain and disability, it will be important to measure pain interference and pain-related disability via questionnaires and performance measures. Performance measures of movement-evoked pain and disability would be preferred, as they are less dependent on recall capacity26 and are a more robust measure of pain evoked with activity.27 Moreover, previous work found that the majority of individuals with CPSP experienced pain that was movement-evoked.28 Spontaneous measures of pain intensity (e.g. VAS) would fail to capture pain with activity unless measured in real time (i.e. with performance measures).29
Another measure to consider is QST, as it provides insight into endogenous pain processing. However, despite the mechanistic value of QST as an ancillary measure to assess treatment effects for CPSP, we found only two studies that utilized such paradigms.20,22 Nevertheless, both studies found experimentally induced thermal sensitivity to change before and after stimulation. These findings are notable for two reasons: 1) QST changes were predominantly for cold sensation measures and 2) the studies utilized different stimulation techniques (rTMS for Bae et al, tDCS for Hasan et al). Despite a paucity of literature specific to QST differences among CPSP patients receiving brain stimulation, a number of earlier studies in this population assessed the propensity for cold pain. Interestingly, patients with CPSP demonstrated cold hypoalgesia rather than hyperalgesia when compared to the unaffected side.22 Nonetheless, many individuals with CPSP also experience cold allodynia,28 and a large contingent describe cold to be an aggravating factor.21,30 As such, it is possible that both rTMS and tDCS have differential specificity to cold pain. More studies would be required to confirm such specificity and would also need to account for limitations found in previous QST paradigms among the CPSP population.
Studies that utilize QST to assess brain stimulation effects for CPSP will also require a more comprehensive assessment. First, such studies should expand upon both experimental and clinical pain descriptors, as well as expand upon pain locations.28 Second, future studies will need to employ a more robust battery of QST paradigms that includes dynamic tests. Current literature is limited to static tests of threshold, which only indicate the moment at which participants feel a sensation. In contrast, dynamic QST tests such as temporal summation of second pain31 and conditioned pain modulation32 measure experimental pain perception over time and subsequently are believed to represent endogenous pain facilitation and inhibition capacity. Finally, QST study designs should include adequate training of participants before formal testing, which was not apparent in the studies we reviewed. QST training familiarizes participants to experimentally induced pain and subsequent reporting of pain. Such training is particularly important with dynamic QST tests since pain is reported at multiple time points and often in a short duration.
There are a number of limitations in our systematic review. While comprehensive, our review is limited to studies within the 3 databases we queried, and only English manuscripts were included, thus limiting the scope of the data collection and analysis. Furthermore, not every question on our qualitative assessment tool (Modified Downs and Black) is applicable to all study designs. Small sample size and variability in intervention techniques and parameters were also a limitation. That being said, the strengths of the current review include an in-depth analysis of the study parameters as well as analysis of experimental pain measures (e.g. QST).
Conclusion
In summary, given that CPSP is a complex diagnosis that can present in a variety of ways, it is pertinent to understand how this treatment can be individualized across the CPSP population. Being able to reduce symptoms of pain and temperature sensitivity may ultimately increase the quality of life of patients and allow for more effective therapy. This review has confirmed that non-invasive stimulation over the motor cortex may indeed be an effective treatment. Furthermore, experimental pain measures give promise to a better understanding of specific pain mechanisms in CPSP, which may lead to improved clinical treatment. Although stimulating the dLPFC was not found to be effective, it may still be worth investigating other pain pathways and what role they play in the symptoms manifested in CPSP. It has not yet been determined who exactly are responders versus non-responders to brain stimulation in this population, but compared to other invasive techniques such as deep brain stimulation or motor cortex stimulation, non-invasive brain stimulation appears to have potential as a clinical treatment for CPSP.
Author Contributions
All authors contributed to conceptualization, data collection, formal analysis, investigation, writing – original draft, writing – review and editing, visualization, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. In addition, BR, CS, and JF contributed to project administration.
Disclosure
The authors report no conflicts of interest or sources of financial support for this work.
References
- 1.Flaster M, Meresh E, Rao M, Biller J. Central poststroke pain: current diagnosis and treatment. Top Stroke Rehabil. 2013;20(2):116–123. doi: 10.1310/tsr2002-116 [DOI] [PubMed] [Google Scholar]
- 2.Hansson P. Post-stroke pain case study: clinical characteristics, therapeutic options and long-term follow-up. Eur J Neurol. 2004;11:22–30. doi: 10.1111/j.1471-0552.2004.00793.x [DOI] [PubMed] [Google Scholar]
- 3.Kumar G, Soni CR. Central post-stroke pain: current evidence. J Neurol Sci. 2009;284(1–2):10–17. doi: 10.1016/j.jns.2009.04.030 [DOI] [PubMed] [Google Scholar]
- 4.Oh H, Seo W. A comprehensive review of central post-stroke pain. Pain Manag Nurs. 2015;16(5):804–818. doi: 10.1016/j.pmn.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 5.Boivie J. Central post-stroke pain. Pain. 2006;81:715–730. [DOI] [PubMed] [Google Scholar]
- 6.Tanei T, Kajita Y, Noda H, et al. Efficacy of motor cortex stimulation for intractable central neuropathic pain: comparison of stimulation parameters between post-stroke pain and other central pain. Neurol Med Chir (Tokyo). 2011;51(1):8–14. doi: 10.2176/nmc.51.8 [DOI] [PubMed] [Google Scholar]
- 7.Nandi D, Smith H, Owen S, Joint C, Stein J, Aziz T. Peri-ventricular grey stimulation versus motor cortex stimulation for post stroke neuropathic pain. J Clin Neurosci. 2002;9(5):557–561. doi: 10.1054/jocn.2001.1042 [DOI] [PubMed] [Google Scholar]
- 8.Fagundes-Pereyra WJ, Teixeira MJ, Reyns N, et al. Motor cortex electric stimulation for the treatment of neuropathic pain. Arq Neuropsiquiatr. 2010;68(6):923–929. doi: 10.1590/S0004-282X2010000600018 [DOI] [PubMed] [Google Scholar]
- 9.Fregni F, Boggio PS, Lima MC, et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain. 2006;122(1–2):197–209. doi: 10.1016/j.pain.2006.02.023 [DOI] [PubMed] [Google Scholar]
- 10.Sacco P, Prior M, Poole H, Nurmikko T. Repetitive transcranial magnetic stimulation over primary motor vs non-motor cortical targets; effects on experimental hyperalgesia in healthy subjects. BMC Neurol. 2014;14:166. doi: 10.1186/s12883-014-0166-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoyer EH, Celnik PA. Understanding and enhancing motor recovery after stroke using transcranial magnetic stimulation. Restor Neurol Neurosci. 2011;29(6):395–409. doi: 10.3233/RNN-2011-0611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothwell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005;76(6):833–838. doi: 10.1136/jnnp.2004.055806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefaucheur JP, Drouot X, Menard-Lefaucheur I, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry. 2004;75(4):612–616. doi: 10.1136/jnnp.2003.022236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 15.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century. Stroke. 2013;44(7):2064–2089. doi: 10.1161/STR.0b013e318296aeca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henry JL, Lalloo C, Yashpal K. Central poststroke pain: an abstruse outcome. Pain Res Manag. 2008;13(1):41–49. doi: 10.1155/2008/754260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain. 2011;152(10):2399–2404. doi: 10.1016/j.pain.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 18.Felix ER, Widerstrom-Noga EG. Reliability and validity of quantitative sensory testing in persons with spinal cord injury and neuropathic pain. J Rehabil Res Dev. 2009;46(1):69–83. doi: 10.1682/JRRD.2008.04.0058 [DOI] [PubMed] [Google Scholar]
- 19.Hootman Jennifer M, Driban Jeffrey B, Sitler Michael R, Harris Kyle P, Cattano Nicole M. Reliability and validity of three quality rating instruments for systematic reviews of observational studies. Res Synth Methods. 2011;2(2):110–118. doi: 10.1002/jrsm.v2.2 [DOI] [PubMed] [Google Scholar]
- 20.Bae SH, Kim GD, Kim KY. Analgesic effect of transcranial direct current stimulation on central post-stroke pain. Tohoku J Exp Med. 2014;234(3):189–195. doi: 10.1620/tjem.234.189 [DOI] [PubMed] [Google Scholar]
- 21.de Oliveira RA, de Andrade DC, Mendonca M, et al. Repetitive transcranial magnetic stimulation of the left premotor/dorsolateral prefrontal cortex does not have analgesic effect on central poststroke pain. J Pain. 2014;15(12):1271–1281. doi: 10.1016/j.jpain.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 22.Hasan M, Whiteley J, Bresnahan R, et al. Somatosensory change and pain relief induced by repetitive transcranial magnetic stimulation in patients with central poststroke pain. Neuromodulation. 2014;17(8):731–736. doi: 10.1111/ner.2014.17.issue-8 [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi M, Fujimaki T, Mihara B, Ohira T. Repetitive transcranial magnetic stimulation once a week induces sustainable long-term relief of central poststroke pain. Neuromodulation. 2015;18(4):249–254. doi: 10.1111/ner.2015.18.issue-4 [DOI] [PubMed] [Google Scholar]
- 24.Matsumura Y, Hirayama T, Yamamoto T. Comparison between pharmacologic evaluation and repetitive transcranial magnetic stimulation-induced analgesia in poststroke pain patients. Neuromodulation. 2013;16(4):349–354; discussion 354. doi: 10.1111/ner.12019 [DOI] [PubMed] [Google Scholar]
- 25.Ohn SH, Chang WH, Park CH, et al. Neural correlates of the antinociceptive effects of repetitive transcranial magnetic stimulation on central pain after stroke. Neurorehabil Neural Repair. 2012;26(4):344–352. doi: 10.1177/1545968311423110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stull D, Leidy N, Parasiraman B, Chassany O. Optimal recall periods for patient-reported outcomes: challenges and potential solutions. Curr Med Res Opin. 2009;25(4):929–942. doi: 10.1185/03007990902774765 [DOI] [PubMed] [Google Scholar]
- 27.Wideman TH, Finan PH, Edwards RR, et al. Increased sensitivity to physical activity among individuals with knee osteoarthritis: relation to pain outcomes, psychological factors, and responses to quantitative sensory testing. Pain. 2014;155(4):703–711. doi: 10.1016/j.pain.2013.12.028 [DOI] [PubMed] [Google Scholar]
- 28.Bowsher D. Allodynia in relation to lesion site in central post-stroe pain. J Pain. 2005;6(11):736–740. doi: 10.1016/j.jpain.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 29.Corbett DB, Simon CB, Manini TM, George SZ, JLr R, Fillingham RB. Movement-evoked pain: transforming the way we understand and measure pain. Pain. 2018. doi: 10.1097/00006396-900000000-98824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leijon G, Boivie J. Central post-stroke pain–the effect of high and low frequency TENS. Pain. 1989;38(2):187–191. doi: 10.1016/0304-3959(89)90237-6 [DOI] [PubMed] [Google Scholar]
- 31.Anderson RJ, Craggs JG, Bialosky JE, et al. Temporal summation of second pain: variability in responses to a fixed protocol. Eur J Pain. 2013;17(1):67–74. doi: 10.1002/ejp.2013.17.issue-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012;13(10):936–944. doi: 10.1016/j.jpain.2012.07.005 [DOI] [PubMed] [Google Scholar]