We read with interest the important study on vonoprazan in Helicobacter pylori eradication therapy.1 Vonoprazan likely represents a significant advance in antisecretory and H. pylori eradication therapy. Despite being technically well done, several issues related to study conduct and interpretation remain. Their sample size was based on 90% success with lansoprazole, which has not been achieved in Japan for several decades. When the cure rates proved to be lower, the sample size was increased to ‘maintain the statistical power of the study’. We question whether this was ethical. It is generally considered unethical to randomise against a regimen that provides unacceptably low cure rates; many journals reject papers that do this.2,3 We believe the ethical response would be to stop the study.
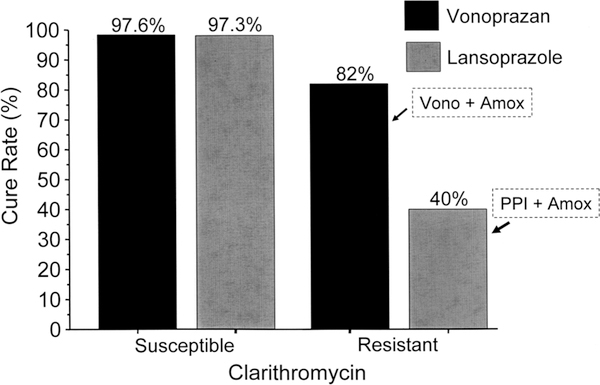
The study concluded that vonoprazan eradication therapy was superior. However, the cure rates of clarithromycin-susceptible infections with vonoprazan and lansoprazole were identical (eg, 97%) (figure 1). Differences in outcome were limited to those with clarithromycin resistance, which transforms triple therapy into antisecretory agent and amoxicillin dual therapies. The population cure rates reflect the prevalence of resistance and the cure rates with resistant infection (figure 2).4 Finally, the cure rate with vonoprazan among those with clarithromycin resistance was unacceptably low (82%). Amoxicillin–antisecretory dual therapy is sensitive to the antisecretory agent’s ability to maintain a high intragastric pH (reviewed in ref. 5). The failure of vonoprazan–amoxicillin to achieve >90% cure rates suggests that it failed to maintain the pH above 6, the duration of therapy was too short, twice-a-day amoxicillin was insufficient or a combination. The results with lansoprazole–amoxicillin were also study-specific or population-specific and could be improved. For example, in Japan, proton pump inhibitor–amoxicillin therapy has also been shown to achieve >90% cure rates when given four times daily for 14 days.5
Figure 1.
Results of vonoprazan and lansoprazole triple therapy with clarithromycin susceptible and resistant infections. Clarithromycin resistance effectively turns the regimens into a dual antisecretory drug-amoxicillin therapy. Amox, amoxicillin; PPI, proton pump inhibitor; vono, vonoprazan.
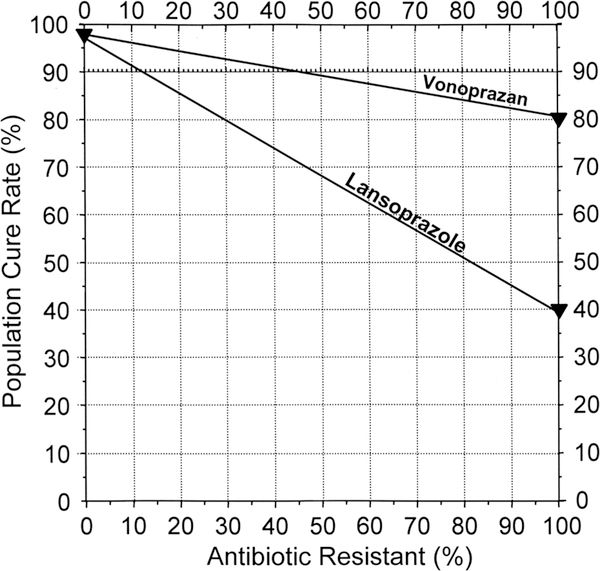
Figure 2.
Hp-normogram. This normogram plots the cure rate with susceptible infections on the left vertical axis and those with resistant infections on the right vertical axis. The proportion with resistance is shown on the horizontal axis.4 The cure rates are connected with a line allowing one to visualise the population cure rates for any prevalence of resistance. This plot shows the cure rates with vonoprazan and lansoprazole triple therapies (cure rate with susceptible strains of 97% for both and 82% vs 40% for vonoprazan and lansoprazole, respectively. Note that the lines diverge such that at 15% clarithromycin resistance the cure rates would differ by only 6%.
While vonoprazan is potentially an important addition to gastroenterology, this study failed to prove that it offered any advantage over lansoprazole with susceptible infections and it did not achieve acceptable cure rates for those with resistant strains. Nonetheless, the data suggest that with higher doses and longer duration, vonoprazan–amoxicillin dual therapy may prove to provide high cure rates and revolutionise H. pylori eradication therapy.
Acknowledgments
Funding DYG is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center.
Footnotes
Competing interests DYG is a paid consultant for RedHill Biopharma regarding novel H. pylori therapies and for BioGaia regarding use of probiotics for H. pylori infections.
Provenance and peer review Not commissioned; internally peer reviewed.
REFERENCES
- 1.Murakami K, Sakurai Y, Shiino M, et al. Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 2016;65: 1439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham DY. Editorial—Avoiding unethical Helicobacter pylori clinical trials: susceptibility-based studies and probiotics as adjuvants. Helicobacter 2015;20:321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pounder RE, Talley NJ. Letter: the ethics of using inferior regimens in H. pylori randomised trials—editors’ reply. Aliment Pharmacol Ther 2012;35:858. [DOI] [PubMed] [Google Scholar]
- 4.Graham DY. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: Effect of resistance, duration, and CYP2C19 genotype. Helicobacter 2016;21:85–90. [DOI] [PubMed] [Google Scholar]
- 5.Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut 2016;65:870–8. [DOI] [PubMed] [Google Scholar]