Abstract
Exposure to inflammation during pregnancy has been linked to adverse neurodevelopmental consequences for the offspring. One common route through which a developing fetus is exposed to inflammation is with intrauterine inflammation. To that end, we utilized an animal model of intrauterine inflammation (IUI; intrauterine lipopolysaccharide (LPS) administration, 50 μg, E15) to assess placental and fetal brain inflammatory responses, white matter integrity, anxiety-related behaviors (elevated zero maze, light dark box, open field), microglial counts, and the CNS cytokine response to an acute injection of LPS in both males and females. These studies revealed that for multiple endpoints (fetal brain cytokine levels, cytokine response to adult LPS challenge) male IUI offspring were uniquely affected by intrauterine inflammation, while for other endpoints (behavior, microglial number) both sexes were similarly affected. These data advance our understanding of sex-specific effects of early life exposure to inflammation in a translationally-relevant model.
1. Introduction
Maternal bacterial or viral infections during pregnancy increase the risk for development of certain mental health disorders including schizophrenia [1, 2], autism [3], and intellectual disabilities [4]. The most widely used models of prenatal infection and inflammation involves systemic (intraperitoneal (IP) or subcutaneous (SC)) administration of bacterial (lipopolysaccharide, LPS) or viral (poly I:C) mimetics to a pregnant dam [5]. These animal models of systemic prenatal inflammation have been shown to induce behavioral and neurochemical endophenotypes reminiscent of schizophrenia, mood/anxiety disorders, and autism. Specifically, dopaminergic dysfunction [6, 7], deficits in prepulse inhibition, exaggerated locomotor response to amphetamine [7], decreased “cognitive flexibility” [8], social behavior deficits [9], hyperactivity and difficulty with sustained attention [10] and anxiety [5, 11] have all been documented in offspring exposed to inflammation during pregnancy. The present work was designed to examine the long term consequences of offspring exposure to local inflammation during pregnancy.
Clinically, a fetus is exposed to localized intrauterine inflammation in a spontaneous preterm birth or with the diagnosis of chorioamnionitis at term (inflammation of the fetal membranes). Chorioamnionitis is diagnosed with clinical criteria (maternal fever, tachycardia) or histological endpoints (presence of infiltrating leukocytes in placental tissues and fetal membranes) and occurs in approximately 10–15% of term births [12]. However, in spontaneous preterm births, most women do not mount a fever or leukocytosis that would be indicative of chorioamnionitis. Yet, most women who have a spontaneous preterm birth will have evidence of local inflammation. This inflammation is noted by histological examination of the placenta, termed histological chorioamnionitis. In fact, in babies born at less than 28 weeks, approximately 85% will have histological chorioamnionitis, suggesting that intrauterine inflammation is present [13]. Other studies have demonstrated elevated cytokines in the amniotic fluid of women who ultimately have a spontaneous preterm birth despite negative bacterial cultures [14], again suggesting the presence of intrauterine inflammation in a preterm birth. Since the spontaneous preterm birth rate ranges from 10–15% in the United States [15], the number of children exposed to intrauterine inflammation is of great clinical significance.
Considering these clinical scenarios, the goal of the present work was to use an animal model to specifically address whether local (intrauterine) inflammation would adversely affect brain development, through evaluation of behavior, white matter integrity, and CNS monocyte and cytokine responses. Additionally, as there are well-documented sex differences in the prevalence of many neurodevelopmental and psychiatric disorders, such as autism, ADHD, anxiety/mood disorders and schizophrenia, we sought to determine if there were sex-specific differences in the response to intrauterine inflammation.
2. Methods
2.1. Intrauterine inflammation (IUI).
All animals were cared for according to the guidelines of both the University of Cincinnati and the University of Pennsylvania Institutional Animal Care and Use Committees and all procedures were in compliance with the NIH guidelines for the Care and Use of Animals. Details of the intrauterine inflammation model have been published [16], and will be briefly summarized here. CD-1 out-bred mice (Charles River, Wilmington, MA) were used in the present study. These mice have a gestational period of 19 days, and the preterm period is defined as ~70% of gestation which would be embryonic day 15 (E15). To induce intrauterine inflammation, timed-pregnant CD-1 dams were placed under isoflurane anesthesia on E15. A mini-laparotomy was performed and the lower right uterine horn was identified. In separate groups of dams, lipopolysaccharide (LPS; 50 μg/100 μl/mouse, serotype 055:B5, Sigma-Aldrich, St. Louis, MO) or sterile saline (100 μl) was infused between the first two gestational sacs, with care not to inject into the amniotic cavity. Dams were exposed to LPS or saline on E15 and allowed to deliver at term (any dams that delivered prior to E18 were not used). Litters were culled to equal sizes on postnatal day 2 (PN2). At weaning (P21), animals were weighed, and then group housed (4–5/cage) with house chow and water available ad libitum. Only one animal/sex/litter was used in any one experiment.
2.2.1. Tissue collection: Early (E17).
To collect matched placenta and embryo tissue, dams were killed 48 hr following intrauterine LPS (n=10) or saline exposure (n=5) (E17) (see Figure 1 for experimental timeline). The frontal pole was dissected from the embryonic brain, and DNA and RNA were extracted using Qiagen AllPrep DNA/RNA Mini kit (Qiagen, Valencia, CA). Sex determination was done by assessment of the presence or absence of SRY (sex-determining region Y, expressed only on the Y chromosome) using embryo DNA.
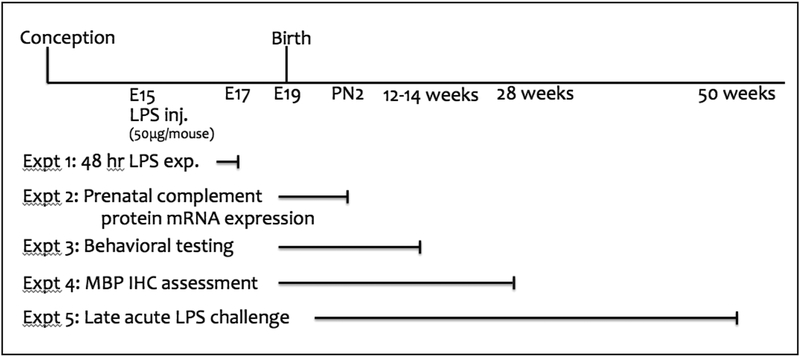
Figure 1.
Experimental timeline. In all groups, timed-pregnant dams received injections of either LPS or saline into the right uterine horn at E15. The offspring in experiment 1 were culled at E17 (48 hr following LPS or saline injection) to assess cytokine mRNA expression. The offspring in experiments 2–5 were used to assess mRNA expression of immune factors, anxiety-like behaviors and MPB protein expression. Only one offspring per sex per litter was used for each assessed endpoint.
2.2.2. Late (adult)-Acute LPS challenge.
Adult offspring (n=5/group, 50 weeks of age) were given intraperitoneal injections (at 10am) of either saline or LPS (10 μg/mouse). Animals were killed 2 hr later and brains were removed and placed immediately into RNA later. The prefrontal cortex (PFC), hypothalamus (HYP) and amygdala (AMYG) were dissected as previously described [17, 18] and RNA was extracted using Qiagen AllPrep DNA/RNA Mini kit (Qiagen, Valencia, CA).
2.3. Flow cytometry.
Brains were removed from saline-perfused animals (28 weeks of age), weighed, minced, transferred to Medicon inserts, and ground in a MediMachine (BD Biosciences, San Jose, CA) for 20–30 s. The cell suspension was washed with HBSS, and cells were resuspended in 70% Percoll (GE Healthcare Life Sciences, Pittsburgh, PA) and overlaid with 30% Percoll. The gradient was centrifuged at 2,250 g for 30 min at 4°C without brake. A total of 106 cells were incubated for 30 min on ice with saturating concentrations of labeled antibodies with 40 μg/ml unlabeled 2.4G2 mAb (BD Biosciences, San Jose, CA), to block binding to Fc receptors, and then washed 3 times with 1% BSA in PBS. After surface staining with antibodies against CD45, CD11b, and CD11c, cell suspensions were fixed and permeabilized by Cytofix/Cytoperm solution (BD Biosciences, San Jose, CA), followed by staining with anti–tumor necrosis factor alpha (TNF-α) antibodies. Fluorochrome-labeled antibodies against CD45 (fluorochrome: PerCP), CD11b (fluorochrome: phycoerythin) and appropriate isotype controls were purchased from BD. Fluorochrome-labeled antibody against CD11c (fluorochrome: FITC), and TNF-α (fluorochrome: APC) were purchased from eBioscience (San Dieg, CA). Isotype controls were the following: CD11b: PE Rat IgG2b, K Isotype Control Clone A95–1, CD45: PerCP Rat IgG2b, K Isotype Control Clone A95–1, CD11c: FITC Hamster IgG1, λ1 Isotype Control, TNF-α: APC Rat IgG1, K Isotype Control. Cell staining was acquired on a FACSCalibur or LSRII (BD Biosciences, San Jose, CA) and analyzed with FlowJo (Tree Star, Ashland, OR) software version 5.4.5.
2.4. Behavior.
Behavior procedures were performed during the first 3 hr of the light phase (7:00–10:00 am). The same animals were tested in open field, elevated zero maze, and light dark box (in this order) with 1 week between testing days. Testing occurred between 12–14 weeks of age. All tests were performed in dim light conditions.
2.4.1. Open Field Activity:
Spontaneous activity in an open field was measured with a Photobeam Activity System (PAS)-Open Field (San Diego Instruments, San Diego, CA). Mice were individually placed in the arena (40.64 cm × 40.64 cm × 38.1 cm) for a single 10 min trial. The arena was fitted with a scaffold of infrared light emitting diodes and photo detectors that registered beam breaks. Horizontal and vertical beam breaks were collected to assess general locomotion and rearing activity.
2.4.2. Elevated Zero Maze:
Mice were individually placed on a 6.35 cm wide circular track with an external diameter of 50.80 cm, raised 60.96 cm above the floor (San Diego Instruments, San Diego, CA). The track had two open and two walled quadrants of equal dimensions. Mice were placed in the center of a closed quadrant to begin a 5 min trial. A highly trained scorer, blind to group designation, graded the digitally recorded trials for time spent in the open quadrants. Mice were scored as within a segment when all four paws were within that segment. Transitions between quadrants were also noted as a measure of general locomotor activity.
2.4.3. Light-dark box:
Mice were initially placed in the bright side of a 2-compartment light-dark box. Light intensity was 5 lux in the dark compartment and 300 lux in the light compartment, and test duration was 10 min. Time spent in the light side as well as transitions between light and dark sides of the test were recorded.
2.5. Immunohistochemistry for myelin basic protein (MBP).
Animals were deeply anesthetized with choral hydrate (35 mg/kg, ip) and perfused via the ascending aorta with saline followed by 4% paraformaldehyde in 0.1% borate buffer pH 9.5. Brains were postfixed for 4 hr and then cryoprotected in 10% sucrose in 0.1 M phosphate buffer. Brains were frozen on dry ice and sectioned using a sliding microtome. Five series of 30 μm-thick frozen sections were collected in cold ethylene glycol-based cryoprotectant and stored at −20˚C until histochemical processing. Endogenous peroxidase activity was neutralized by treating tissue for 10 min with 0.3% hydrogen peroxide, followed by 8 min in 1% sodium borohydride to reduce free aldehydes. Tissue was incubated with primary antibody rat anti-mouse myelin basic protein (MBP; [1:100] Abcam, Cambridge, UK; cat. # ab7349) for 24 hr in PBS/2% blocking serum. Localization was performed using a conventional avidin-biotin immunoperoxidase method.
Slices were then dry mounted and images were obtained via a digital video camera (Carl Zeiss Microimaging, Thornwood, NY). A uniform threshold was applied to all images within a given brain region. Image analyses were performed by semi-quantitative densitometry using Scion Image software (Scion, Frederick, MD). Intensities of gray level were determined within the corpus callosum and anterior commissure. Background gray level signal was taken from a similarly sized brain region showing no MBP signal, and was subtracted from each gray level measure to yield a normalized gray level measurement. Images of the corpus callosum were taken between Bregma 1.10 mm to 0.62 mm, while images of the anterior commissure were taken between Bregma 0.20 mm to 0.10 mm.
2.6. cDNA synthesis and high-throughput qPCR
RNA concentration was determined using a NanoDrop spectrophometer (Thermo Scientific, Waltham, MA) and 1μg was used in each High Capacity cDNA (Applied Biosystems, Foster City, CA) synthesis reaction. Concentrated cDNA was used in a specific target preamplification reaction following the manufacturer’s recommendations (Fluidigm Corp. South San Francisco, CA). Briefly, Taqman assays for all probes were pooled to a concentration of 0.2x and 1.25 μl of the pooled assay mix was added to 1.25μl cDNA and 2.5μl 2x Taqman PreAmp Master Mix (Life Techologies, Carlsbad, CA). The preamplification reaction was cycled as follows: 10 min at 95˚C, then 14 cycles of 95˚C for 15 sec followed by 60˚C for 4 min. Preamplified samples were diluted 1:5, 1:10 and 1:50 with 1×TE. Diluted samples were run with GAPDH on RT-qPCR to test if the specific target amplification worked (~9 cycles), and to determine the best dilution to send for Fluidigm analysis. 1:50 dilution samples were then delivered to the Molecular Profiling Core at the University of Pennsylvania, where they were run on a 96.96 Dynamic Array IFC on the Biomark HD machine (Fluidigm Corp, San Francisco, CA). Expression of targets was normalized to the geometric mean of the housekeeping genes (ACTNB, GAPDH).
2.7. Gene expression analysis by quantitative Real-Time PCR.
For each individual sample, 500 ng of total RNA was used in reverse transcription using High Capacity Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Expression of target genes was determined by quantitative RT-PCR using gene specific Taqman Probes with Taqman gene expression Master Mix (Applied Biosystems, Foster City, CA) on the ABI7900HT Real-Time PCR Cycler. Relative amount of each transcript was determined using delta CT values as previously described in (Pfaffl, 2001). Changes in gene expression were calculated against an unchanged GAPDH standard.
2.8. Statistics.
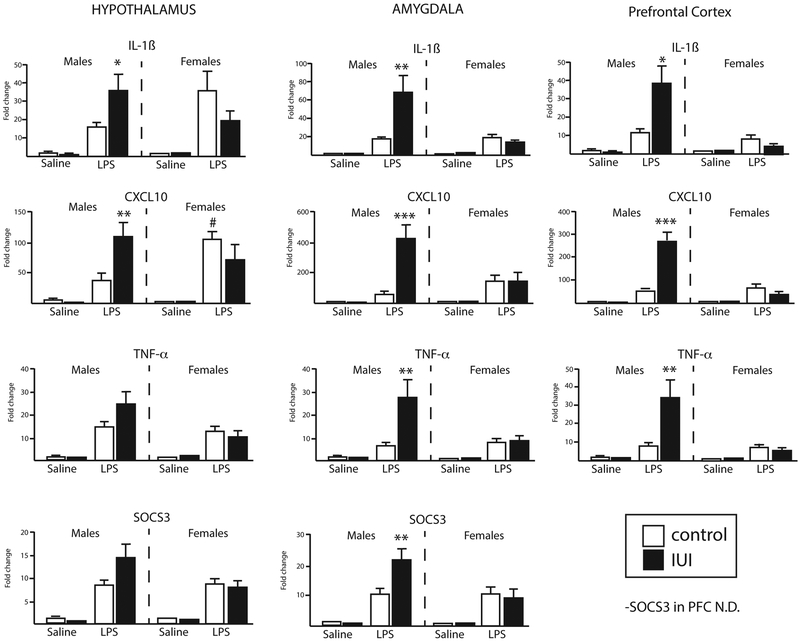
For most experiments, two way ANOVAs were run using Prism to examine the interaction of prenatal exposure (LPS vs saline) and sex. Planned post-hoc comparisons evaluated differences within sex in the response to IUI or male-female control differences when a significant interaction was detected. For the adult exposure to LPS, three-way ANOVAs were run in JMP to evaluate the interaction of offspring prenatal exposure (IUI vs control), adult exposure (LPS vs saline) and sex. Planned posthoc comparisons were used to interpret significant interactions. In Figure 6, posthoc comparisons were Bonferonni corrected to an α-level of p=0.0167 (0.05/3) to account for testing in multiple brain regions.
Figure 6.
Expression of inflammation-related transcripts in the hypothalamus (left), amygdala (middle) and prefrontal cortex (right) (saline: n=4–5; LPS: n=4–5) in response to an acute injection of adult animals with either saline or LPS (10 μg/mouse) in control (white bar) and IUI exposed (black bars) mice of both sexes. For all genes shown, 3-way interactions (sex x IUI x injection) were identified; significant post-hocs (LPS control vs LPS IUI) are indicated. *p<.02, **p<.007, ***p<.0005. #p=0.01, male control LPS vs female control LPS.
3. Results
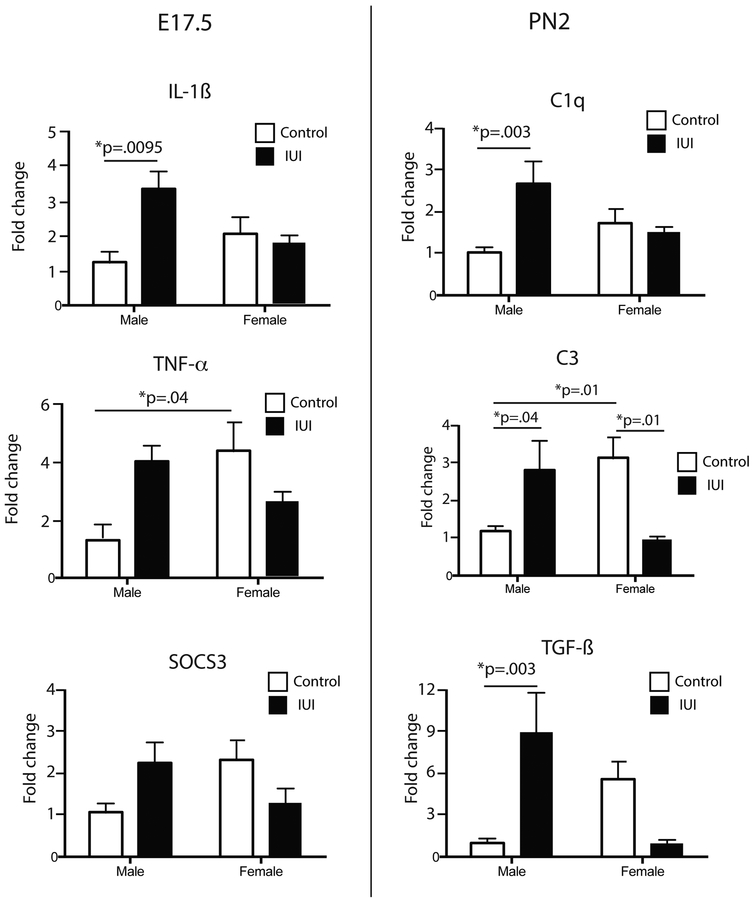
To determine the early (acute) response to intra-uterine LPS exposure, placenta and fetal brains (frontal pole) were collected 48 hr post-injection of LPS (Figure 1). Our previous work has demonstrated a robust induction of cytokines 6 hr after LPS administration [16], and the rationale for studying the response at 48 hr was to examine whether the inflammatory response persisted beyond 6 hr. For placenta samples, 32 genes including both pro- and anti-inflammatory cytokines as well as epigenetic-related genes (see Supplemental Table 1) were evaluated. Aside from a single chemokine (CCL2, see below), no sex x IUI interactions or main effects for IUI were observed, suggesting that the local inflammatory response was largely resolved by 48 hr. The chemokine CCL2 was significantly increased at 48 hr, with a main effect for LPS exposure (F(1, 49)=7.34, p=0.009), and mean (s.e.) fold change increases of 5.0 (0.9) and 4.9 (0.7), for males and females respectively. In fetal brain tissue (E17), four proinflammatory (CXCL10, IL-1ß, SOCS3 and TNF-α,) and three anti-inflammatory (IL-10, IL1ra, TGF-ß) cytokines/chemokines were measured. For IL-1ß, TNF-α and SOCS3, a significant interaction was observed (IL-1ß: F(1,45)=5.52, p=0.02; TNF-α: F(1,46)=4.62, p=0.04; SOCS3 (1,48)=4.83, p=0.03), such that male offspring showed an elevation that was absent or reversed in female offspring (Fig 2, left). Additionally, for TNF-α, there was a significant sex difference in the baseline levels, such that control females had significantly higher levels of TNF-α as compared to control males. There were no differences observed in CXCL10 or any of the anti-inflammatory transcripts (data not shown).
Figure 2.
mRNA transcripts for immune (left) and complement-related (right) molecules were measured in the frontal pole from E17 brains (left) (saline: n=4–6; LPS: n=8–11) or whole brain at PN2 (right) (saline: n=9; LPS: n=7–9) in control (white bar) and IUI exposed (black bars) mice. Significant IUI x sex interactions were observed in all genes shown, with an increased expression in the male IUI-exposed mice, and unchanged or decreased levels in females. Significant posthoc comparisons are shown with the stated p-value.
Whole brains were also available from animals that had been culled at PN2 to equalize the litter sizes for the survival studies (see below). These brains were used to examine the expression of transcripts for complement-related proteins, C1q, C3 and TGF-ß, known to play a role in synaptic pruning/neurodevelopment. Significant interactions between sex and prenatal condition were noted for each of these transcripts (C1q: F(1,29)=5.92, p=0.02; C3: F(1,30)=11.57, p=0.0019; TGF-ß: (1,30)=12.46, p=0.0014), and in a pattern similar to that seen for the cytokines at E17, male IUI exposed animals had increased levels of these transcripts, while the female levels were unchanged or reduced (Fig 2, right). Additionally, for C3, there was a significant sex difference in the baseline levels, such that control females had significantly higher levels of C3 as compared to control males.
For survival studies, forty-five pregnant dams were injected prenatally (22 saline, 23 LPS). Of those, litters were born to 19 saline-injected dams and 12 LPS-injected dams. Litter sizes (saline: n=10; LPS: n=11.2 pups) did not differ between the groups, litters with fewer than 9 pups were not used and all litters were culled to n=9 on postnatal day 2, to ensure equal access to maternal care and nutrition. At PN21, pup weights (averaged across the litter) were significantly different, with a main effect for sex (F(1,47)= 10.03, p=0.0027) and prenatal condition (F(1,47)= 8.92, p=0.0045), with females and LPS-exposed pups weighing less (grams, mean (se); Male/Control: 17.01 (0.53), Male/IUI: 15.4 (0.29), Female/Control: 15.34 (0.27), Female/IUI: 14.87 (0.20)).
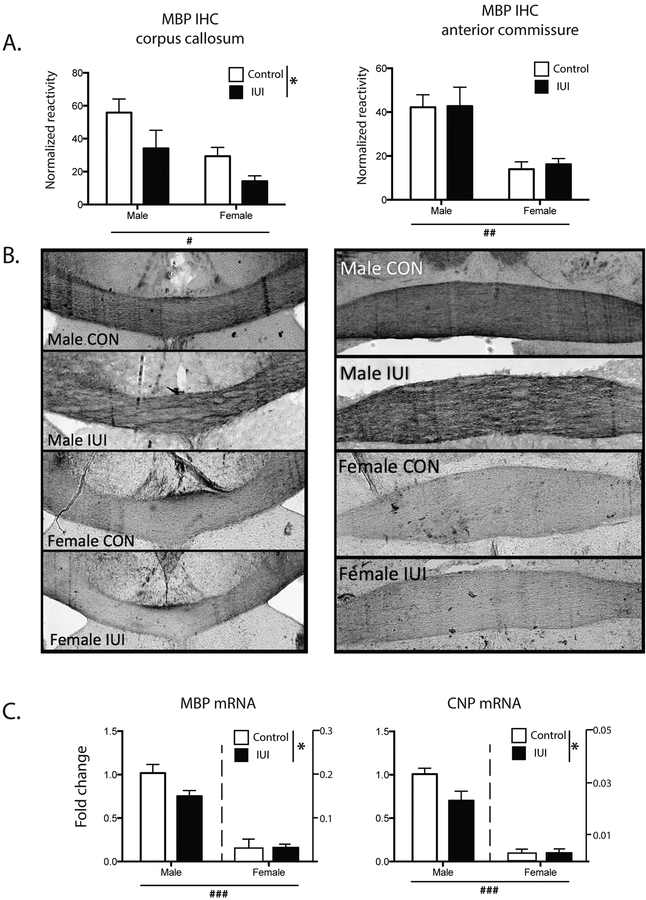
Animals were then assessed as adults, to determine whether there were any enduring consequences of the early life exposure to inflammation. As white matter damage has been associated with prenatal inflammation [19], two markers of white matter integrity were assessed, myelin basic protein (MBP), a major protein component of the myelin sheath and 2’,3’-cyclic-nucleotide 3’-phosphodiesterase (CNP), which is expressed exclusively by oligodendrocytes and is also a significant component of myelin. In adults, MBP was measured in two white matter tracts, corpus callosum (CC) and anterior commissure (AC). In the corpus callosum, there was a significant main effect of IUI exposure, with IUI significantly reducing the expression of MBP in both males and females (F(1,16)= 5.96, p<0.05, Fig 3A, left), while IUI did not affect MBP expression in the anterior commissure (Fig 3A, right). In both regions females had significantly decreased levels of MBP (main effect for sex-CC: F(1,16=9.42. p=0.007, AC: F(1,16)=23.85, p=0.0002, representative images in 3B). mRNA expression of two oligodendrocyte markers, MBP and CNP, was then evaluated in whole brains from animals at PN2 to determine whether early changes could be detected. For both genes, there was a significant IUI x sex interaction (F(1,16)=5.17, p<0.05, F(1,16)=5.83, p<0.05, respectively), as well as both main effects for IUI (F(1,16)=4.93, p<0.05, F(1,16)=5.82, p<0.05, respectively), and sex (F(1,16)=207, p<0.0001, F(1,16)=180, p<0.0001, respectively), with IUI and female sex resulting in significantly lower expression of both MBP and CNP mRNA (Fig 3C). While the IUI x sex interactions were not significant for either gene, in both cases the IUI effect appeared to be largely driven by the differences in the males.
Figure 3.
White matter damage was assessed through the measurement MBP in control (white bar) and IUI exposed (black bars) mice in adult corpus callosum (left A and B), and adult anterior commissure (right A and B). (A) shows normalized reactivity of immunohistochemistry for MBP, with representative images shown in B (C) MBP and CNP mRNA from whole brain PN2 mice. *p<.05, main effect for IUI; #p<.005, ##p<.0005, ###p<.0001, main effect for sex (saline: n=4–5; LPS: n=4–5).
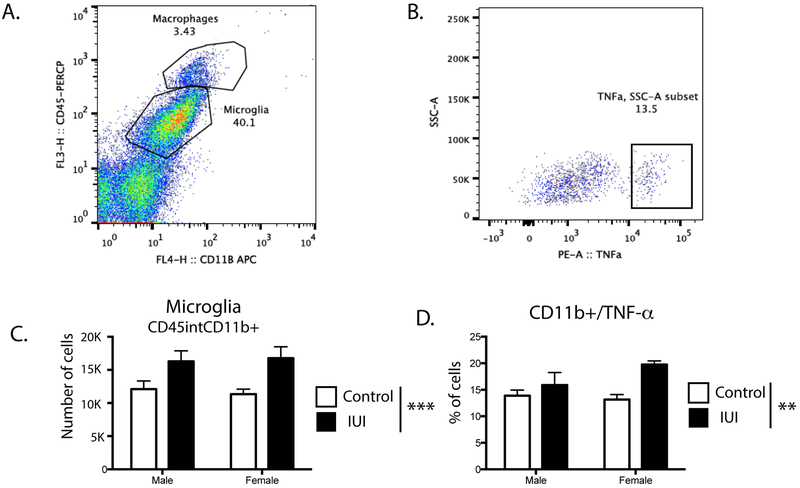
One causative factor in oligodendrocyte damage involves neuroinflammation, therefore, whole brains were analyzed using flow cytometric quantification of myeloid cells in the brain (Fig 4A). IUI exposed animals had significantly more microglia per gram tissue from brain (CD45intCD11b+) (F (1,15) =11.25, p= 0.0043, Fig 4B), as well as a significant increase in the percent of microglia that were positive for TNF-α (CD45int/CD11b+/TNF-α+; F (1,15) = 8.37, p= 0.011) (Fig 4C). There were no main effects for sex nor significant interactions.
Figure 4.
(A) Representative flow cytometry gating of CD45high CD11b+ (macrophages) and CD45 int CD11b+ (microglia) from brains harvested from male IUI exposed mice. (B) CD45 int CD11b+ (microglia) from brains harvested from male IUI exposed mice display detectable levels of TNF-α C) Number of CD45 int CD11b+ (microglia) cells per gram tissue from brain in males and females of control (white bars) or IUI exposed (black bars) mice. D) Quantification of percentage of CD45 int CD11b+(microglia) that are TNF-α+ from brain in males and females of control (white bars) or IUI exposed (black bars) mice. Main effect for IUI; **p=.01, ***p=.004; n=10 per group.
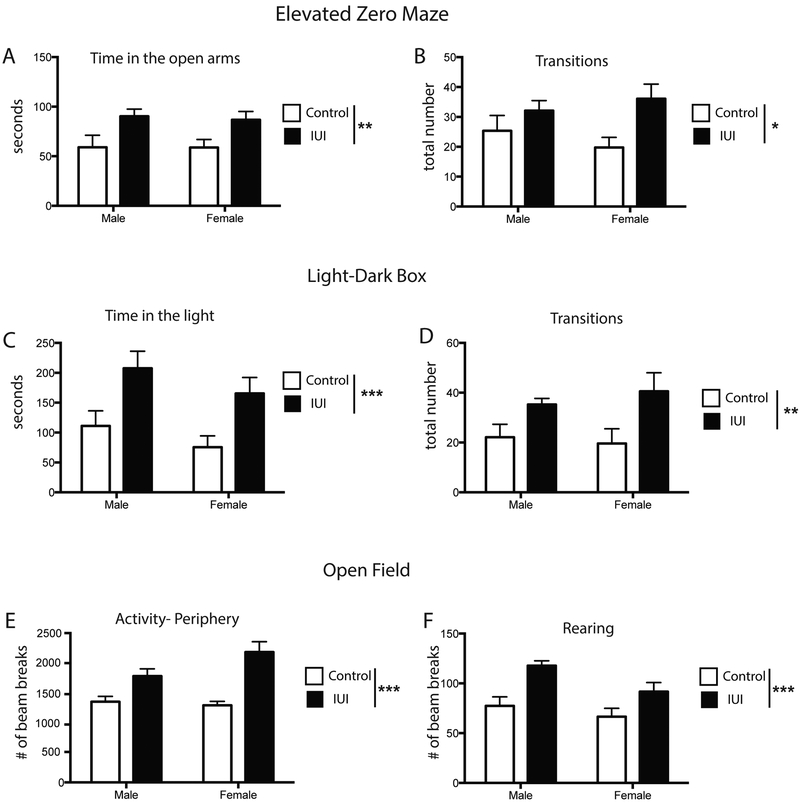
To determine whether prenatal exposure to intrauterine inflammation (IUI) affected locomotor and anxiety-related behaviors, adult animals were tested in the elevated zero maze (EZM), light-dark box (LD), and open field (OF). Both males and females performed similarly across the tasks. In the EZM, IUI animals spent significantly more time in the open arms (F(1,33) = 10.54, p = 0.0027), and had significantly more transitions (F(1,33) = 7.065, p= 0.012) (Fig 5A, 5B). This behavioral pattern was also observed in the LD test, with IUI animals spending more time in the light side of the box (F(1,36) = 13.86, p= 0.0007) and registering more transitions (F(1,36) = 13.86, p= 0.0007) (Fig 5C, 5D). In the OF, both male and female IUI offspring were more active in the peripheral zone, registering significantly more beam breaks ((F(1,35)=29.5, p<0.0001, Fig 5E), while total activity or activity in the center did not differ between the groups. IUI animals also showed a significant increase in the number of rearings (F (1,36) = 16.57, p= 0.0002) (Fig 5F).
Figure 5.
Anxiety-related behaviors were tested in the elevated zero maze (A,B), light-dark box (C, D) and open field (E,F) in adult control (white bar) and IUI exposed (black bars) male and female mice. IUI exposed mice spent more time in the open arms (EZM) and in the light (LD), with an increase in transitions in both assays. In the open field, IUI-exposed animals were more active in the periphery and showed a significant increase in rearing. *p=.01, **p<.005, ***p<.001; main effect for IUI. n=10 per group.
To examine whether prenatal exposure to inflammation alters the CNS response to later life immune challenges, adult animals were challenged with an acute injection of LPS (or saline). Gene expression profiling of 15 genes (Table 1) was used to examine the transcriptional response within the hypothalamus (HYP), amygdala (AMYG), and prefontal cortex (PFC), as these regions are known to participate in the response to peripheral inflammation. Notably, a number of three-way interactions were revealed (p<0.05), and these interactions followed a similar general pattern; male animals exposed to IUI had a potentiated response to the acute LPS injections, while IUI did not differentially affect the response to LPS in females (Fig 6). This pattern is seen in IL-1ß, CXLC10, TNF-α, and SOCS3 in HYP, AMYG, and PFC (statistical details are listed in Table 2).
Table 1:
Gene list for acute LPS experiment
| Gene name | Probe ID |
|---|---|
| ACTB | Mm00607939_s1 |
| CXCL10 | Mm00445235_m1 |
| GAD1 | Mm04207432_g1 |
| GAPDH | Mm99999915_g1 |
| IL-1B | Mm01336189_m1 |
| IL-1R | Mm00434237_m1 |
| TNFα | Mm00443258_m1 |
| D1R | Mm01353211_m1 |
| D2R | Mm00438545_m1 |
| DNMT1 | Mm00599763_m1 |
| DNMT3a | Mm00432881_m1 |
| Gadd45b | Mm00435123_m1 |
| MeCP2 | Mm01193537_g1 |
| MTR | Mm01340053_m1 |
| 0PRD1 | Mm00443063_m1 |
| PENK | Mm01212875_m1 |
| TET1 | Mm01169087_m1 |
Table 2.
Statistical analyses for 3 way ANOVAs in Figure 5.
| HYPOTHALAMUS | AMYGDALA | PREFRONTAL CORTEX |
|
|---|---|---|---|
| IL-1β | F(7,32) = 6.47* | F(7,34) = 7.67 | F(7,33) = 6.47 |
| CXCL10 | F (7,32) = 7.89 | F(7,34) = 8.96 | F(7,33) = 15.81 |
| TNF-α | F (7,32) = 7.27 | F(7,34) = 6.60 | F(7,33) = 7.64 |
| S0CS3 | F97,32) = 7.32 | F(7,34) = 12.44 | n.d. |
all p<.0001, except
p=.0002
n.d. = not done
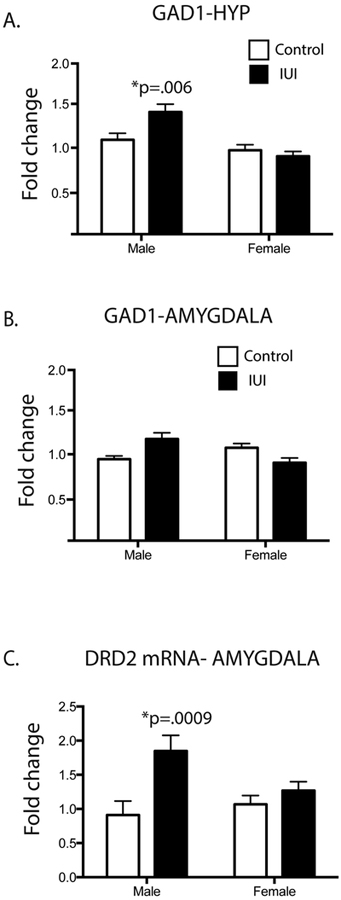
In addition to the immune transcripts discussed above, two neurotransmitters had notable expression patterns. A significant IUI x sex interaction was observed for GAD1 mRNA in the HYP and AMYG (F(1,30)=5.52, p=0.03, F(1,31)=16.3, p=0.0003, respectively) (Fig 7A, 7B). In both regions, IUI exposure increased expression of GAD1 mRNA in male offspring, an effect that was absent in the females. Further, DRD2, the dopamine type 2 receptor, demonstrated a similar interaction (F(1,31)=4.28, p<0.05), with a significant increase in the male exposed IUI mice and no observed difference in the female exposed mice (Fig 7C). Posthoc analyses revealed a significant difference between male control and IUI offspring in both GAD1 (HYP) and DRD2 (AMYG).
Figure 7.
Neurotransmitter differences in control (white bar) and IUI exposed (black bars) male and female mice (saline: n=4–5; LPS: n=4–5). Significant sex x IUI interactions were detected in adult animals for GAD1 in (A) hypothalamus and (B) amygdala and DRD2 in (C) amygdala. Significant posthoc values are represented on the graphs, such that *p values represent a significant difference between male control and male IUI animals.
4. Discussion
The goal of this work was to determine if exposure to intrauterine inflammation significantly impacted brain development and behavior, both very early as well as into adulthood, and to determine whether these effects varied by sex. Using a model of intrauterine inflammation that does not elevate maternal circulating cytokines [16], we found this exposure is more than sufficient to alter gene expression, inflammatory profiles, behavior and long term immune responses. We find that males are more vulnerable to some effects of intrauterine exposure to inflammation; including early baseline and adult LPS-induced neuroinflammation, as well as increased GAD1 mRNA. Additionally, a number of the effects were found to occur equally in males and females, including white matter damage, increased microglia number and behavioral changes consistent with novelty-seeking. Collectively, these data demonstrate significant acute and long-term adverse consequences as a result of exposure to intra-uterine inflammation in an animal model with high translational relevance.
Initial characterizations were done of the placenta and fetal brain 48 hr after intrauterine exposure to LPS. IL-1ß and TNF-α were increased in the brains of male, but not female, mice exposed to intrauterine inflammation. At this timepoint, inflammation in the placental tissue had largely resolved, aside from CCL2 levels which remained elevated, consistent with the finding in human placenta that increased CCL2 levels are associated with births complicated by infection [20]. The early elevation of IL-1 may be particularly relevant to later outcomes, as peripheral administration of an IL-1R antagonist following gestational exposure to LPS was able to normalize cytokine levels in brain and placenta [21]. Previously, we have shown that 6 hr after exposure to intrauterine inflammation, expression of IL-1, IL-6 and TNF-α mRNA increased in the placenta and IL-1 β, TNF-α and cyclooxygenase-2 (COX-2) mRNA expression increased in the brain at E15 [16]. These results expand upon these findings by showing that the LPS exposure induced a prolonged (48 hr) up-regulation of the pro-inflammatory cytokines IL-1 and TNF-α in the brain, but only in male pups. A number of other reports in the literature have indicated that effects of early life immune challenge or other brain insults vary by sex and tend to affect males more than females [22–25]. Intraperitoneal administration of LPS has also been used to examine the effect of maternal inflammation on offspring brain development. A recent report using 100μg/kg LPS IP on GD15 found no effect on litter size or composition, with a decrease in body weight in the LPS-exposed offspring [26], in a pattern similar to the current study. Interestingly, they also report a male-specific effect of LPS exposure on synaptic pruning in the hippocampus, while the observed LPS-induced behavioral deficits (increased vocalizations and repetitive movements) did not differ between the sexes. Mechanisms explaining these sex differences are not clear, but could involve sex differences in how the early brain is colonized by microglia [24] or perhaps differences in prenatal exposure to sex hormones [27]. Importantly, our data indicate that the sex differences are apparent in the initial response to the intrauterine inflammation, and exist well prior to the onset of puberty-related changes in hormone levels.
Complement-related proteins were also evaluated in the early fetal brains (PN2). In the CNS, certain complement proteins play an important role in synaptic pruning. Astrocyte-derived TGF-ß specifically signals neurons to produce C1q [28]. Subsequently, C1q and C3 are expressed on weak synapses that are then targeted for phagocytosis and clearance by microglia, a process first identified in the developing visual system [29]. Beyond synaptic pruning, complement proteins may play a role in neurodevelopment. C1q and C3 expression are dynamically regulated in the cortex throughout development, with C1q expression increasing throughout the lifespan, and C3 expression peaking in early development (P6), and declining to a stable level at P35 [30]. Mice with a global deletion of C1q show enhanced excitatory synaptic connections within the neocortex [31], supporting the idea that C1q is involved in preferentially pruning excitatory, but not inhibitory, synapses during development. In contrast, work in the adult mouse cortex, has shown that C1q is selectively expressed only on GABA (inhibitory) neurons [30]. The current data are the first to demonstrate that exposure to early life inflammation affects the expression of these complement-related transcripts, an effect seen only in males. This represents another potential explanation for the increased susceptibility of males to the adverse effects of early life inflammation.
As adults, the number of microglia was significantly elevated in adult animals, however the activational state of these cells is unknown. Given that cytokine expression was no longer elevated in the brain at baseline, this suggests an absence of active, ongoing neuroinflammation. However, acute immune challenge with a peripheral injection of LPS revealed a male-specific potentiation of the cytokine response. Across hypothalamus, amygdala, and prefrontal cortex, IL-1ß, CXCL10, TNF-α and SOCS3 responses in the IUI males were potentiated compared to control males, while females displayed no such potentiation. Several studies utilizing a “double-hit” model have reported an exacerbated immune response to LPS in adult rodents which were exposed to neonatal infection [32, 33], however this appears to be the first report to identify this sex-specific potentiation in response to exposure to in utero inflammation. Cytokine action in the brain has a well-characterized role in the acute behavioral responses to illness (sickness behavior, fever [34, 35]). However, increasingly, cytokine action in the brain has also been associated with numerous adverse mental health outcomes, including HPA dysregulation, depression, anxiety, and substance use/abuse [36–39]. The observed male potentiation suggests that males exposed to early life inflammation may harbor a lingering vulnerability to cytokine-mediated adverse mental health outcomes, particularly in response to a challenge (stress or immune-related) known to increase central levels of cytokines.
Estradiol has been shown to have anti-inflammatory effects, lowering cytokine levels in response to traumatic injury [40] as well as in response to stress [41]. Therefore, one potential explanation for the lack of potentiation in the females would be the anti-inflammatory effects of circulating estrogen. But it is important to note that at 50 weeks of age (when these studies were completed), mice are considered middle aged and estrogen levels are significantly lower as compared to young adult mice (ages 12–28 weeks) [42]. Choosing a timepoint in which estrogen levels are likely lower minimized, though certainly did not eliminate, the potential of circulating estrogen to reduce the female neuroinflammatory response to an acute immune stimulus (to note, estrogen levels were not directly measured in the current study). Our data support this conclusion, as control male and female mice had largely identical cytokine responses to the acute LPS injection, save for CXCL10 levels, which were significantly higher in the control females. It remains possible that IUI animals were differentially responsive to estrogen, which may have contributed to the lack of potentiation in the cytokine response that was observed in the male offspring. Future studies manipulating these hormones and examining specific stages of the estrus cycle will provide additional important information. With regard to the increased levels of CXCL10 in the females, all animals received the same dose of LPS (10μg/mouse), however the females likely weighed less than the males at this timepoint (although body weight was not measured at this time), resulting in a larger LPS dose per body weight. Given that this sex difference was only observed in a single cytokine (CXCL10), this sex difference is more likely related to an underlying biological sex difference rather than a differential effective dose of LPS.
Perhaps equally important, a number IUI-driven phenotypes did not differ between the sexes. White matter damage, as evidenced by reduced MBP reactivity in the adult corpus callosum was seen equally in both male and female IUI mice. Increases in whole brain microglia and TNF-α positive microglia were also evident in both male and female IUI-exposed animals. Microglia activation has been associated with damage to both neurons and white matter [43–45]; therefore, the IUI-induced increase in microglia may be related to the reduction of myelin in both males and females exposed to IUI. When stimulated, microglia release nitric oxide (NO) and TNF-α, which can promote oligodendrocyte cell death [45–47]. In vitro, LPS-induced microglia activation reduced oligodendrocyte progenitor cells [36–39], while other studies have found connections between activation of microglia during development and white matter damage [45]. In rabbits, intrauterine injection of LPS three to four days before delivery increases the total number of activated microglia in white matter structures, and this corresponds with the degree of motor deficits in the offspring [48]. Similarly, a single low dose of LPS in midgestation fetal sheep increased total microglia number and induced white matter damage [49, 50]. These findings indicate that the reduction of white matter can be related to increased activation and/or number of microglia during an innate inflammatory response.
Further, a significant sex difference in white matter markers was also evident, such that females had lower overall levels of MBP and CNP mRNA at PN2, as well as lower MBP reactivity in adulthood. While data is limited in neonatal rodents, a study in neonatal humans found total white matter and cortical gray matter volumes to be larger in males versus females [51]. With regard to adults, sex differences in white matter have been documented, such as a larger genu of the corpus callosum in males [52], and greater white matter volume relative to total cerebral size in men compared to women [53, 54]. Similar to human studies, rodent studies have shown increased white matter volume in males, particularly in the corpus callosum [55–58], including an increase in the number of oligodendrocytes and increased expression of MBP in adult male rats compared to females. [58]. These sex differences in white matter, either in early life or in adulthood, could be related to the exposure to sex hormones, specifically estrogen and testosterone [58]. Estrogen and androgen receptors are expressed on oligodendrocytes [59], and cortical regions, including the corpus callosum, are enriched with sex steroids receptors during early life [60], with expression levels declining with age [58, 61]. Emerging evidence suggests that steroid hormones are involved in both the proliferation of myelin and the protection of myelin during development [62–64].
The IUI-induced effects on behavior were also independent of sex. Both male and female IUI mice showed a significant increase in open arm time (elevated zero maze), time in the light (light dark box), transitions (both tests) and rearing (open field). While an increase in open arm time and time in the light is often interpreted as an anxiolytic phenotype, this conclusion is confounded somewhat by the increase in transitions in both of these tasks. The open field revealed an increase in activity in the periphery, as well as an increase in rearing, a behavior which can be considered a risk assessment behavior [65]. Collectively, these behaviors are consistent with hyperlocomotion and increased “novelty seeking” in the IUI males and females. Dopamine, and specifically the D2 receptor have been implicated in coding risk/novelty assessment. Recently, the importance of DRD2 in the nucleus accumbens was shown with regard to risk seeking behavior, as a D2/D3/D4 agonist increased risk seeking behavior, while activation of D2-receptor bearing neurons increased risk preference [66]. Therefore, it is interesting to note that an increase in DRD2 was observed in the amygdala of IUI mice (although only in males). Future pharmacological intervention studies will be necessary to fully interpret these behavioral data and to evaluate the potential importance of DRD2 in the amygdala to IUI-induced increases in novelty-seeking. Work in other models of maternal immune activation have documented an increase in anxiety-like behaviors, however, each of these models have used peripheral administration of LPS (IP or SC), at gestational day 9, 15 or 18 [5, 11, 67], highlighting the fact that different models of prenatal inflammation can lead to divergent findings.
GAD1 mRNA codes for GAD67, an enzyme which provides the majority of basal GABA for inhibitory neurotransmission. It was notable that IUI differentially affected GAD1 expression, leading to increased expression only in the male IUI mice in both hypothalamus and amygdala. Given that DRD2 was also increased in the amygdala of IUI-exposed male mice, it is interesting to note that DRD2 in amygdala is expressed on GABAergic cells [68]. GABA in the amygdala has been linked to anxiety [69] and stress responses [70], so dysregulation of GAD1 mRNA could lead to alterations in GABA tone, which may affect anxiety and stress-related behaviors. As mentioned previously, IUI-exposed males had a potentiated neuroinflammatory response to peripheral immune activation. Future studies will examine whether the observed male-specific increases in the expression of GAD1 and/or DRD2 could be important mechanistically, either upstream or downstream of the potentiated neuroinflammatory response.
A comparison with other models of maternal immune activation during pregnancy reveals certain similarities between the models. Literature on the use of the viral mimetic poly I:C is extensive and will be only briefly addressed here. Maternal immune activation with poly I:C has demonstrated adverse effects on adult white matter [71, 72], with an absence of microglial activation [73], while behavioral deficits are largely reminiscent of endophenotypes associated with schizophrenia and autism (see reviews [74, 75]). When compared to models involving intraperitoneal administration of LPS, other similarities are noted. Similar to our findings of increased locomotor activity, IP LPS (300 μg/kg at GD15-GD17) resulted in increased activity in the LPS-exposed offspring [76]. Further, IP LPS at 120 μg/kg on GD17 potentiated the LPS-induced cytokine response in a mixed neonatal neuron/glial culture and potentiated the cytokine response (IL-6 and TNF) in animals with experimental autoimmune encephalitis [77]. And lastly, with a dose of 80 μg/kg at E16, an increase in the number of oligodendrocytes was observed in cortex and hippocampus, while microglia numbers were also increased in hippocampus, but only when combined with neonatal hyperoxia for 14 days [78].
Our previous work with this model has shown an elevation in placental and fetal brain cytokines at 6 hr post-LPS administration, and importantly, consistent with the clinical observations in preterm birth, no detectable cytokine elevation in the maternal circulation [16]. Immediate neonatal brain injury had been documented [16, 79], however, long-term consequences were unknown. As IUI is one of the most frequent causes for preterm delivery [12, 80–82] in a clinical population, it is difficult to disentangle the adverse effects of prematurity versus exposure to inflammation, factors that would inform therapeutic approaches. Therefore, the present model holds significant interest as a translationally relevant model in which to examine the adverse effects of prenatal exposure to inflammation in the absence of prematurity.
Supplementary Material
Highlights.
Use of a translationally relevant model of early life exposure to inflammation
Intrauterine inflammation absent systemic maternal response affects offspring brain
Male offspring show heightened cytokine responses to acute challenge
No sex difference in microglia, white matter and behavioral changes
Acknowledgments.
The authors would like to acknowledge the services of Dr. W. Timothy O’Brien, Director of the Neurobehavior Testing Core at the University of Pennsylvania for running the open field and elevated zero maze assays.
Funding was provided by the NIH: MH087978 (TMR) and MH100828 (TMR & ME), HD076032 (M.E.), NS37570 (to Z.F), as well as the American Heart Association award #15PRE25500022 (to A.R.), and the Neuroscience Training Program T32-GM007507 (to A.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited.
- 1.Buka SL, et al. , Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav Immun, 2001. 15(4): p. 411–20. [DOI] [PubMed] [Google Scholar]
- 2.Brown AS, et al. , Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry, 2004. 61(8): p. 774–80. [DOI] [PubMed] [Google Scholar]
- 3.Fatemi SH, et al. , Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse, 2005. 57(2): p. 91–9. [DOI] [PubMed] [Google Scholar]
- 4.Rantakallio P and von Wendt L, Risk factors for mental retardation. Arch Dis Child, 1985. 60(10): p. 946–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arsenault D, et al. , The different effects of LPS and poly I:C prenatal immune challenges on the behavior, development and inflammatory responses in pregnant mice and their offspring. Brain Behav Immun, 2014. 38: p. 77–90. [DOI] [PubMed] [Google Scholar]
- 6.Baharnoori M, Bhardwaj SK, and Srivastava LK, Effect of maternal lipopolysaccharide administration on the development of dopaminergic receptors and transporter in the rat offspring. PLoS One, 2013. 8(1): p. e54439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguilar-Valles A, et al. , Leptin and interleukin-6 alter the function of mesolimbic dopamine neurons in a rodent model of prenatal inflammation. Psychoneuroendocrinology, 2012. 37(7): p. 956–69. [DOI] [PubMed] [Google Scholar]
- 8.Bitanihirwe BK, et al. , Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology, 2010. 35(12): p. 2462–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machado CJ, et al. , Maternal immune activation in nonhuman primates alters social attention in juvenile offspring. Biol Psychiatry, 2015. 77(9): p. 823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vuillermot S, et al. , Prenatal immune activation interacts with genetic Nurr1 deficiency in the development of attentional impairments. J Neurosci, 2012. 32(2): p. 436–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penteado SH, et al. , Prenatal lipopolysaccharide disrupts maternal behavior, reduces nest odor preference in pups, and induces anxiety: studies of F1 and F2 generations. Eur J Pharmacol, 2014. 738: p. 342–51. [DOI] [PubMed] [Google Scholar]
- 12.Mueller-Heubach E, Rubinstein DN, and Schwarz SS, Histologic chorioamnionitis and preterm delivery in different patient populations. Obstet Gynecol, 1990. 75(4): p. 622–6. [PubMed] [Google Scholar]
- 13.Yoon BH, et al. , The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol, 2000. 183(5): p. 1124–9. [DOI] [PubMed] [Google Scholar]
- 14.Romero R, et al. , Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol, 2015. 213(6): p. 836 e1–836 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statistics, N.C.f.H. April 12, 2017]; Available from: http://www.marchofdimes.org/peristats.
- 16.Elovitz MA, et al. , Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci, 2011. 29(6): p. 663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grissom NM, George R, and Reyes TM, The hypothalamic transcriptional response to stress is severely impaired in offspring exposed to adverse nutrition during gestation. Neuroscience, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Grissom NM, et al. , Dissociable deficits of executive function caused by gestational adversity are linked to specific transcriptional changes in the prefrontal cortex. Neuropsychopharmacology, 2015. 40(6): p. 1353–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuypers E, et al. , White matter injury following fetal inflammatory response syndrome induced by chorioamnionitis and fetal sepsis: lessons from experimental ovine models. Early Hum Dev, 2012. 88(12): p. 931–6. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton SA, Tower CL, and Jones RL, Identification of chemokines associated with the recruitment of decidual leukocytes in human labour: potential novel targets for preterm labour. PLoS One, 2013. 8(2): p. e56946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nadeau-Vallee M, et al. , Antenatal Suppression of IL-1 Protects against Inflammation-Induced Fetal Injury and Improves Neonatal and Developmental Outcomes in Mice. J Immunol, 2017. 198(5): p. 2047–2062. [DOI] [PubMed] [Google Scholar]
- 22.Nunez JL, Alt JJ, and McCarthy MM, A novel model for prenatal brain damage. II. Long-term deficits in hippocampal cell number and hippocampal-dependent behavior following neonatal GABAA receptor activation. Exp Neurol, 2003. 181(2): p. 270–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos-Galindo M, et al. , Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol Sex Differ, 2011. 2: p. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz JM, Sholar PW, and Bilbo SD, Sex differences in microglial colonization of the developing rat brain. J Neurochem, 2012. 120(6): p. 948–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wynne O, et al. , Early life infection alters adult BALB/c hippocampal gene expression in a sex specific manner. Stress, 2011. 14(3): p. 247–61. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez de Cossio L, et al. , Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav Immun, 2017. 63: p. 88–98. [DOI] [PubMed] [Google Scholar]
- 27.Hutchison JB, Gender-specific steroid metabolism in neural differentiation. Cell Mol Neurobiol, 1997. 17(6): p. 603–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bialas AR and Stevens B, TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci, 2013. 16(12): p. 1773–82. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Stevens B, et al. , The classical complement cascade mediates CNS synapse elimination. Cell, 2007. 131(6): p. 1164–78. [DOI] [PubMed] [Google Scholar]
- 30.Stephan AH, et al. , A dramatic increase of C1q protein in the CNS during normal aging. J Neurosci, 2013. 33(33): p. 13460–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bochukova EG, et al. , Large, rare chromosomal deletions associated with severe early-onset obesity. Nature, 2010. 463(7281): p. 666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bilbo SD, et al. , Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci, 2005. 25(35): p. 8000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilbo SD and Schwarz JM, Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci, 2009. 3: p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konsman JP, et al. , Central nervous action of interleukin-1 mediates activation of limbic structures and behavioural depression in response to peripheral administration of bacterial lipopolysaccharide. Eur J Neurosci, 2008. 28(12): p. 2499–510. [DOI] [PubMed] [Google Scholar]
- 35.Gourine AV, et al. , Anterior hypothalamic interleukin-1 receptors are involved in mediation of fever during bacterial sepsis in rats. Ann N Y Acad Sci, 1998. 856: p. 266–9. [DOI] [PubMed] [Google Scholar]
- 36.Goldsmith DR, et al. , Inflammatory markers are associated with decreased psychomotor speed in patients with major depressive disorder. Brain Behav Immun, 2016. 56: p. 281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bekhbat M and Neigh GN, Sex differences in the neuro-immune consequences of stress: Focus on depression and anxiety. Brain Behav Immun, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarz JM, Hutchinson MR, and Bilbo SD, Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci, 2011. 31(49): p. 17835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crews FT, et al. , The role of neuroimmune signaling in alcoholism. Neuropharmacology, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedersen AL, Nelson LH, and Saldanha CJ, Centrally Synthesized Estradiol Is a Potent Anti-Inflammatory in the Injured Zebra Finch Brain. Endocrinology, 2016. 157(5): p. 2041–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arakawa K, et al. , Effects of the estrous cycle and ovarian hormones on central expression of interleukin-1 evoked by stress in female rats. Neuroendocrinology, 2014. 100(2–3): p. 162–77. [DOI] [PubMed] [Google Scholar]
- 42.Danilovich N, Maysinger D, and Sairam MR, Perspectives on reproductive senescence and biological aging: studies in genetically altered follitropin receptor knockout [FORKO] mice. Exp Gerontol, 2004. 39(11–12): p. 1669–78. [DOI] [PubMed] [Google Scholar]
- 43.Bilbo SD and Schwarz JM, The immune system and developmental programming of brain and behavior. Front Neuroendocrinol, 2012. 33(3): p. 267–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frick LR, Williams K, and Pittenger C, Microglial dysregulation in psychiatric disease. Clin Dev Immunol, 2013. 2013: p. 608654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baburamani AA, et al. , Microglia toxicity in preterm brain injury. Reprod Toxicol, 2014. 48: p. 106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zajicek JP, et al. , Interactions between oligodendrocytes and microglia. A major role for complement and tumour necrosis factor in oligodendrocyte adherence and killing. Brain, 1992. 115 (Pt 6): p. 1611–31. [PubMed] [Google Scholar]
- 47.Merrill JE, et al. , Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol, 1993. 151(4): p. 2132–41. [PubMed] [Google Scholar]
- 48.Kannan S, et al. , Magnitude of [(11)C]PK11195 binding is related to severity of motor deficits in a rabbit model of cerebral palsy induced by intrauterine endotoxin exposure. Dev Neurosci, 2011. 33(3–4): p. 231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mallard C, et al. , White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res, 2003. 28(2): p. 215–23. [DOI] [PubMed] [Google Scholar]
- 50.Dean JM, et al. , Cerebellar white matter injury following systemic endotoxemia in preterm fetal sheep. Neuroscience, 2009. 160(3): p. 606–15. [DOI] [PubMed] [Google Scholar]
- 51.Gilmore JH, et al. , Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci, 2007. 27(6): p. 1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witelson SF, Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain, 1989. 112 (Pt 3): p. 799–835. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein JM, et al. , Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex, 2001. 11(6): p. 490–7. [DOI] [PubMed] [Google Scholar]
- 54.Filipek PA, et al. , The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex, 1994. 4(4): p. 344–60. [DOI] [PubMed] [Google Scholar]
- 55.Gur RC, et al. , Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci, 1999. 19(10): p. 4065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fitch RH, et al. , Corpus callosum: effects of neonatal hormones on sexual dimorphism in the rat. Brain Res, 1990. 515(1–2): p. 111–6. [DOI] [PubMed] [Google Scholar]
- 57.Yang S, et al. , Sex differences in the white matter and myelinated nerve fibers of Long-Evans rats. Brain Res, 2008. 1216: p. 16–23. [DOI] [PubMed] [Google Scholar]
- 58.Cerghet M, et al. , Sexual dimorphism in the white matter of rodents. J Neurol Sci, 2009. 286(1–2): p. 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santagati S, et al. , Estrogen receptor is expressed in different types of glial cells in culture. J Neurochem, 1994. 63(6): p. 2058–64. [DOI] [PubMed] [Google Scholar]
- 60.MacLusky NJ, et al. , Estrogen formation in the mammalian brain: possible role of aromatase in sexual differentiation of the hippocampus and neocortex. Steroids, 1987. 50(4–6): p. 459–74. [DOI] [PubMed] [Google Scholar]
- 61.Miranda RC and Toran-Allerand CD, Developmental expression of estrogen receptor mRNA in the rat cerebral cortex: a nonisotopic in situ hybridization histochemistry study. Cereb Cortex, 1992. 2(1): p. 1–15. [DOI] [PubMed] [Google Scholar]
- 62.Nunez JL, et al. , Myelination in the splenium of the corpus callosum in adult male and female rats. Brain Res Dev Brain Res, 2000. 120(1): p. 87–90. [DOI] [PubMed] [Google Scholar]
- 63.Jung-Testas I and Baulieu EE, Steroid hormone receptors and steroid action in rat glial cells of the central and peripheral nervous system. J Steroid Biochem Mol Biol, 1998. 65(1–6): p. 243–51. [DOI] [PubMed] [Google Scholar]
- 64.Gerstner B, et al. , Estradiol attenuates hyperoxia-induced cell death in the developing white matter. Ann Neurol, 2007. 61(6): p. 562–73. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi A, et al. , Systematic analysis of emotionality in consomic mouse strains established from C57BL/6J and wild-derived MSM/Ms. Genes Brain Behav, 2008. 7(8): p. 849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zalocusky KA, et al. , Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature, 2016. 531(7596): p. 642–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Depino AM, Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience, 2015. 299: p. 56–65. [DOI] [PubMed] [Google Scholar]
- 68.De Bundel D, et al. , Dopamine D2 receptors gate generalization of conditioned threat responses through mTORC1 signaling in the extended amygdala. Mol Psychiatry, 2016. 21(11): p. 1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heldt SA, Mou L, and Ressler KJ, In vivo knockdown of GAD67 in the amygdala disrupts fear extinction and the anxiolytic-like effect of diazepam in mice. Transl Psychiatry, 2012. 2: p. e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Di S, et al. , Acute Stress Suppresses Synaptic Inhibition and Increases Anxiety via Endocannabinoid Release in the Basolateral Amygdala. J Neurosci, 2016. 36(32): p. 8461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farrelly L, et al. , Maternal immune activation induces changes in myelin and metabolic proteins, some of which can be prevented with risperidone in adolescence. Dev Neurosci, 2015. 37(1): p. 43–55. [DOI] [PubMed] [Google Scholar]
- 72.Richetto J, et al. , Genome-Wide Transcriptional Profiling and Structural Magnetic Resonance Imaging in the Maternal Immune Activation Model of Neurodevelopmental Disorders. Cereb Cortex, 2017. 27(6): p. 3397–3413. [DOI] [PubMed] [Google Scholar]
- 73.Missault S, et al. , The risk for behavioural deficits is determined by the maternal immune response to prenatal immune challenge in a neurodevelopmental model. Brain Behav Immun, 2014. 42: p. 138–46. [DOI] [PubMed] [Google Scholar]
- 74.Estes ML and McAllister AK, Maternal immune activation: Implications for neuropsychiatric disorders. Science, 2016. 353(6301): p. 772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meyer U, Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry, 2014. 75(4): p. 307–15. [DOI] [PubMed] [Google Scholar]
- 76.Al-Amin MM, et al. , Astaxanthin ameliorates prenatal LPS-exposed behavioral deficits and oxidative stress in adult offspring. BMC Neurosci, 2016. 17: p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zager A, et al. , Maternal immune activation in late gestation increases neuroinflammation and aggravates experimental autoimmune encephalomyelitis in the offspring. Brain Behav Immun, 2015. 43: p. 159–71. [DOI] [PubMed] [Google Scholar]
- 78.Graf AE, et al. , Perinatal inflammation results in decreased oligodendrocyte numbers in adulthood. Life Sci, 2014. 94(2): p. 164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burd I, et al. , A mouse model of term chorioamnionitis: unraveling causes of adverse neurological outcomes. Reprod Sci, 2011. 18(9): p. 900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andrews WW, et al. , Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol, 2008. 198(4): p. 466 e1–466 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Romero R, Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol, 2007. 30(5): p. 675–86. [DOI] [PubMed] [Google Scholar]
- 82.Seong HS, et al. , The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. Am J Obstet Gynecol, 2008. 199(4): p. 375 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.