Abstract
Context
Heterozygous frameshift variants in PLIN1 encoding perilipin-1, a key protein for lipid droplet formation and triglyceride metabolism, have been implicated in familial partial lipodystrophy type 4 (FPLD4), a rare entity with only six families reported worldwide. The pathogenicity of other PLIN1 null variants identified in patients with diabetes and/or hyperinsulinemia was recently questioned because of the absence of lipodystrophy in these individuals and the elevated frequency of PLIN1 null variants in the general population.
Objectives
To reevaluate the pathogenicity of PLIN1 frameshift variants owing to new data obtained in the largest series of patients with FPLD4.
Methods
We performed histological and molecular studies for patients referred to our French National Reference Center for Rare Diseases of Insulin Secretion and Insulin Sensitivity for lipodystrophy and/or insulin resistance and carrying PLIN1 frameshift variants.
Results
We identified two heterozygous PLIN1 frameshift variants segregating with the phenotype in nine patients from four unrelated families. The FPLD4 stereotypical signs included postpubertal partial lipoatrophy of variable severity, muscular hypertrophy, acromegaloid features, polycystic ovary syndrome and/or hirsutism, metabolic complications (e.g., hypertriglyceridemia, liver steatosis, insulin resistance, diabetes), and disorganized subcutaneous fat lobules with fibrosis and macrophage infiltration.
Conclusions
These data suggest that some FPLD4-associated PLIN1 variants are deleterious. Thus, the evidence for the pathogenicity of each variant ought to be carefully considered before genetic counseling, especially given the importance of an early diagnosis for optimal disease management. Thus, we recommend detailed familial investigation, adipose tissue-focused examination, and follow-up of metabolic evolution.
We have described novel heterozygous PLIN1 frameshift variants in the largest cohort of FPLD4 reported to date and provide a set of arguments for their pathogenicity.
Familial partial lipodystrophy (FPLD) syndromes are rare diseases characterized by a limited capacity of peripheral fat to store triglycerides, which results in metabolic abnormalities including insulin resistance, hypertriglyceridemia, liver steatosis, and polycystic ovary syndrome (1). Heterozygous frameshift variants in the PLIN1 gene encoding perilipin-1 have been identified in six families (2–4), thereby defining the FPLD type 4 (FPLD4) subtype.
Perilipin-1 is a structural lipid droplet protein that facilitates triglyceride storage or initiates lipolysis, depending on the hormonal stimuli. We have shown that several FPLD4-associated PLIN1 frameshift variants disrupt the ability of perilipin-1 to inhibit basal lipolysis in adipocytes (2, 3, 5). However, the pathogenicity of other heterozygous PLIN1 null variants, identified in patients referred for evaluation of maturity onset diabetes of the young, hyperinsulinemic hypoglycemia, or type 2 diabetes, has recently been questioned by Laver et al. (6).
Since proper interpretation of PLIN1 variants is crucial for genetic counseling, the aim of the present study was to determine the pathogenicity of PLIN1 null variants in the light of the new genotype–phenotype data obtained in the largest series of patients with FPLD4 reported to date.
Subjects and Methods
Sequencing of a panel of lipodystrophy genes (AGPAT2, AKT2, BSCL2, CAV1, CIDEC, LIPE, LMNA, PLIN1, POLD1, PPARG, PTRF, and ZMPSTE24) was performed in 237 independent index cases, investigated in our French National Reference Network for Rare Diseases of Insulin Secretion and Insulin Sensitivity, for manifestations evocative of a lipodystrophic syndrome. Capture (SeqCap EZ enrichment protocol, Roche NimbleGen, Roche Sequencing, Pleasanton, CA) was followed by massively parallel sequencing on a MiSeq platform (Illumina Inc., San Diego, CA). The data were analyzed using the Sophia Genetics DDM pipeline®. PLIN1 frameshift variants were confirmed by Sanger sequencing. Affected relatives were identified after familial investigations. Abdominal subcutaneous adipose tissue biopsy specimens were obtained from two patients. Western blot and histological analyses were performed as previously described (2). All the subjects provided written informed consent in accordance with the legal procedures for molecular and histological investigations and publication of photographs. The Comité de Protection des Personnes Ile-de-France 5 (Paris, France) approved the present study.
Results
Molecular diagnosis
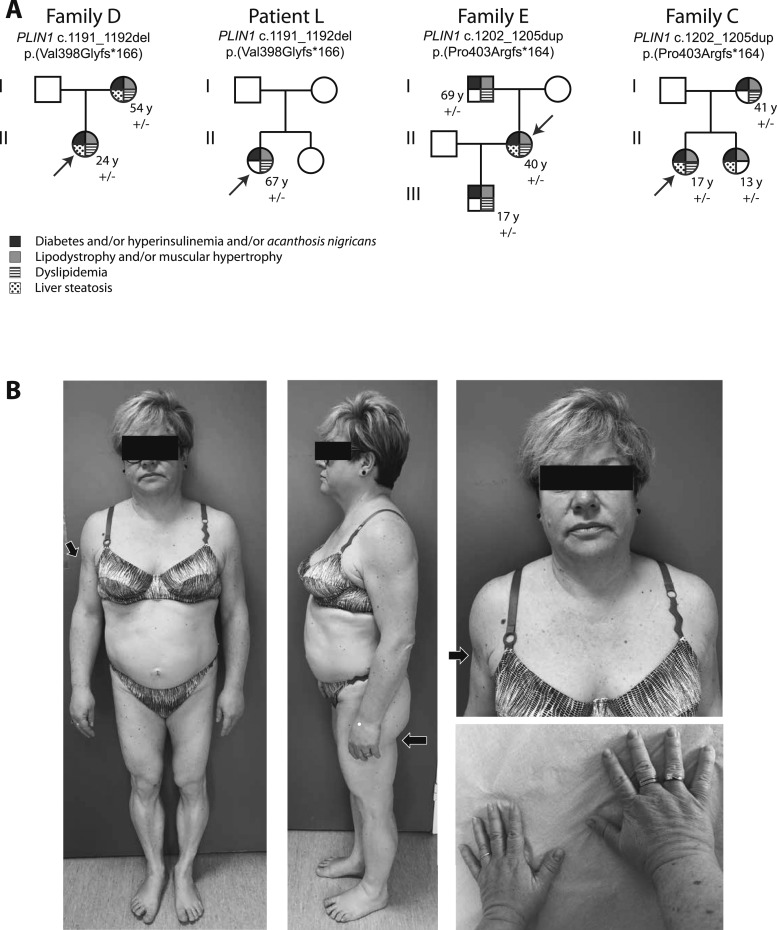
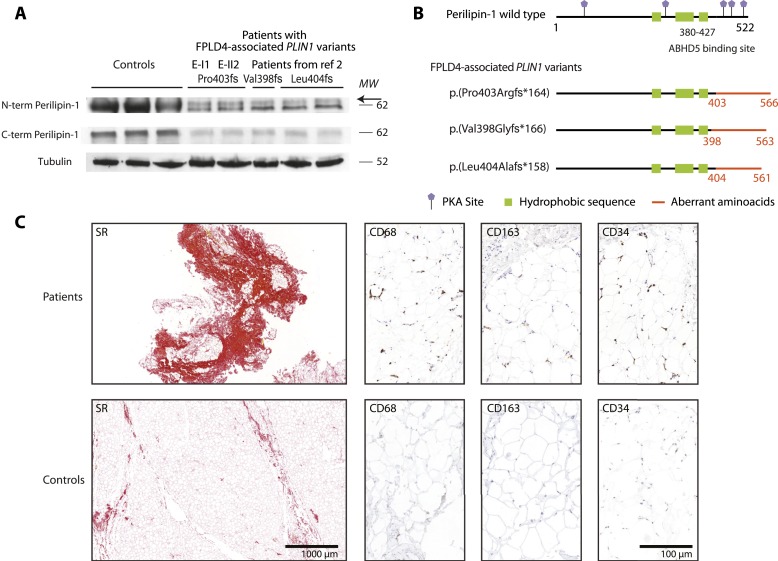
A heterozygous PLIN1 frameshift variant was identified in four index cases and five affected relatives (Fig. 1A). Patients from family D and L carried the c.1191_1192del deletion, previously shown to be expressed as an abnormal p.(Val398Glyfs*166) elongated form of perilipin-1, leading to constitutive activation of basal lipolysis (2, 5). In family E and C, we identified a 4bp-duplication in exon 8 (c.1202_1205dup), leading to the synthesis of a p.(Pro403Argfs*164) mutant protein, whose expression in adipose tissue was confirmed by Western blot (Fig. 2). These variants were not found in public databases (Exome Aggregation Consortium, Genome Aggregation Database) and cosegregated with the disease within each family (Fig. 1A). In all patients, we did not find any other molecular defect in known lipodystrophy genes.
Figure 1.
(A) Molecular and clinical investigations of patients with heterozygous PLIN1 null variants. Arrows indicate index cases. Genealogical trees show a cosegregation of PLIN1 variants with the disease phenotype. The nomenclature of PLIN1 variants is based on the RefSeq accession numbers NM_002666.5 and NP_002657.3. (B) Morphotype of patient E-II2 with partial lipodystrophy and acromegaloid features. Subcutaneous lipoatrophy of the upper and lower limbs can be observed, with muscle hypertrophy (arrows). Compared with patients with FPLD type 2 (LMNA-linked FPLD), the neck is less broad, and the breast and subcutaneous abdominal fat are not affected by lipoatrophy. The acromegaloid features include face infiltration, a slightly enlarged nose, deep wrinkles, thick lips and hands, and enlarged feet.
Figure 2.
Study of subcutaneous adipose tissue from patients. (A) Perilipin-1 expression in abdominal subcutaneous adipose tissue from patients E-II2 and E-I1 compared with controls and previously described patients with FPLD4 (2). Western blot of whole cell extracts was performed using antibodies directed against the N-terminal and C-terminal parts of wild-type perilipin-1, as previously described (2). The mutant isoform was recognized by the N-terminal antibody as an additional band (arrow) just above the 62-kD molecular weight (MW) marker, which was not detected by the C-terminal antibody. Tubulin (antibody T5168, Sigma-Aldrich, St. Quentin-Fallavier, France) was used as a loading control. (B) Consequences on perilipin-1 protein expression of FPLD4-associated PLIN1 frameshift variants studied in (A). (C) Histological and immunohistological analyses of abdominal subcutaneous adipose tissue from patients E-I1 and E-II-2 compared with controls. Adipose tissue samples from patients displayed disorganized fat lobules of heterogeneous size, with increased fibrosis [Sirius red (SR) staining, 16% to 35% of the total sample surface vs 0.2% to 3% in controls], increased vascularization (density of CD34 staining, 0.13% to 0.44% in adipose lobules from patients vs 0.001% to 0.004% in controls), and increased macrophage infiltration with crown-like structures (assessed by CD163 and CD68 staining, density per 10−5 μm2: 1.9 to 2.2 and 3.6 to 4.3 in patients vs 0.0 and 0.0 to 0.01 in controls, respectively).
Disease phenotype
The clinical and biological features of the nine investigated family members carrying a PLIN1 variant recapitulated the FPLD4 cardinal signs (2) (i.e., lipoatrophy, muscular hypertrophy, facial acromegaloid features, insulin resistance-related ovarian dysfunction, and metabolic complications (e.g., hyperinsulinemia or insulin-resistant diabetes, hypertriglyceridemia, liver steatosis) (Table 1). The index cases had initially been referred for ovarian hyperandrogenism with lipodystrophy (patient D-II3), suspicion of acromegaly (patients L and E-II2), or early-onset nonautoimmune diabetes (patient C-II1). In several individuals (patients D-I2, C-I2, C-II2), the disease manifestations were only detected after family studies.
Table 1.
Characteristics of Patients Investigated in the Present Study
| Characteristic | Family D | Family L | Family E | Family C | Mean ± SD | Median (Range) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient number | D-I2 | D-II3* | L* | E-I1 | E-II2* | E-III1 | C-I2 | C-II1* | C-II2 | NA | NA |
| Sex | Female | Female | Female | Male | Female | Male | Female | Female | Female | NA | NA |
| Age, y | 54 | 24 | 67 | 69 | 40 | 17 | 41 | 17 | 13 | 38 ± 21.7 | 40 (13–69) |
| BMI, kg/m2 | 23 | 25.5 | 22.7 | 25.7 | 30.7 | 23.8 | 29.8 | 25.4 | 19.6 | 25.1 ± 3.5 | 25.4 (19.6–30.7) |
| Origin | France | France | France | France | France | France | France | France | France | ||
| Age at diagnosis, y | 54 | 15 | 40 | 58 | 38 | 17 | 41 | 15 | 13 | 32.3 ± 17.7 | 38 (13–58) |
| Lipodystrophy | Android habitus; four limb lipoatrophy | Android habitus; four limb lipoatrophy | Generalized lipoatrophy | Four limb, gluteal and trunk lipoatrophy; cervicofacial fat accumulation | Lower limb and gluteal lipoatrophy | Mild lower limb, gluteal and trunk lipoatrophy | Android habitus; four limb and gluteal lipoatrophy; cervicofacial fat accumulation | Android habitus; lower limb and gluteal lipoatrophy; cervicofacial fat accumulation | Mild lower limb lipoatrophy | NA | NA |
| Acromegaloid features | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | NA | NA |
| Muscular hypertrophy | Present | Present | Present | Present | Present | Present | Present | Present | Not obvious | NA | NA |
| Acanthosis nigricans | Absent | Present | Absent | Absent | Present | Absent | Present | Present | Absent | NA | NA |
| Total fat (DEXA), % | 23.1 | NA | 12 | 16.4 | 21 | 13 | NA | NA | NA | 17.1 ± 4.9 | 16.4 (12–23.1) |
| Serum leptin, ng/mL | 1.7 | 1.1 | 1.9 | 6.5 | 7.0 | 3.2 | NA | 12 | NA | 4.8 ± 3.9 | 3.2 (1.1–12) |
| Glucose tolerance | Gestational diabetes at age 20, 23, and 30 y; permanent diabetes at age 34 y | Impaired glucose tolerance at age 15 y; diabetes at age 20 y | Diabetes diagnosed at age 40 y | Diabetes diagnosed at age 58 y | Gestational diabetes at age 30 y; permanent diabetes at age 38 y | Normal glucose tolerance with increased fasting insulin | Impaired fasting glucose; antecedent of gestational diabetes | Diabetes diagnosed at age 15 y | Normal fasting glucose with increased fasting insulin | NA | NA |
| Serum fasting glucose/insulin, mmol/L/pmol/L | 8/NA (insulin therapy); preserved C-peptide (1.2 nmol/L) | 6.1/402 at age 15 y with BMI 24 kg/m2 | 3.9/NA (insulin therapy) | 8.3/NA | 7.4/112 | 4.5/65.2 | 6.1/456 | 7.2/NA (insulin therapy); high C-peptide (3.3 nmol/L) | 4.6/257 | 6.2 ± 1.6/258.4 ± 172.1 | 6.1 (3.9–8.3)/257 (65.2–456) |
| HbA1c, % | 8 | 5.8 | 7.1 | 7.1 | 6.5 | 4.9 | 5.6 | 12.2 | 5.4 | 7.0 ± 2.2 | 6.5 (5.4–12.2) |
| Triglycerides (≥2 mmol/L) | Present | Present | Present | Present | Present from age 18 y | Present | Present | Present | Absent | NA | NA |
| Serum fasting triglycerides/HDL-C, mmol/L | 2/1.17 | 3.4/0.55 | 3.3/0.98 | 4.7/1.0 | 5.7/0.82 | 6.2/0.82 | 6.4/0.57 | 6.1/NA | 0.5/0.98 | 4.3 ± 2.1/0.86 ± 0.22 | 4.7 (0.5–6.4)/0.9 (0.55–1.17) |
| Liver steatosis | Present | Present | NA | NA | Present | NA | NA | Present | Present | NA | NA |
| AST/ALT, mIU/L | 18/29 | 50/78 | 45/63 | 22/38 | 24/23 | 19/10 | 30/56 | 171/216 | 36/36 | 46.1 ± 48.1/61 ± 61.8 | 30 (18–171)/38 (10–216) |
| PCOS or hirsutism | Hirsutism; oligomenorrhea | PCOS | Absent | NA | Mild hirsutism | NA | PCOS | Oligomenorrhea | Absent | NA | NA |
| Glucose and lipid lowering therapy | Metformin, insulin (>2 U/kg/d), statin | Metformin, iDPP4 | Metformin, insulin (>3 U/kg/d) | Metformin, HS, GLP1R-A, insulin | Metformin, GLP1R-A, statin | None | None | Metformin, GLP1R-A, insulin | None | NA | NA |
| Other signs | Fatigue; unexplained recurrent vomiting | Becker nevus of the shoulder; muscle fatigability | Hypertension; myocardial infarction and rhythm disturbances | None | Severe fatigue; hypertension | None | NA | NA | NA | NA | NA |
| PLIN1 variant | c.1191_1192del; p.(Val398Glyfs*166) | c.1191_1192del; p.(Val398Glyfs*166) | c.1191_1192del; p.(Val398Glyfs*166) | c.1202_1205dup; p.(Pro403Argfs*164) | c.1202_1205dup; p.(Pro403Argfs*164) | c.1202_1205dup; p.(Pro403Argfs*164) | c.1202_1205dup; p.(Pro403Argfs*164) | c.1202_1205dup; p.(Pro403Argfs*164) | c.1202_1205dup; p.(Pro403Argfs*164) | NA | NA |
Patient numbers refer to those shown in Fig. 1; the nomenclature of PLIN1 variants is based on RefSeq accession numbers NM_002666.5 and NP_002657.3.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; DEXA, dual energy X-ray absorptiometry; GLP1R-A, GLP1R agonist; HDL-C, high-density lipoprotein cholesterol; HS, hypoglycemic sulfonamide; iDPP4, dipeptidyl peptidase-4 inhibitors; NA, not available/not applicable; PCOS, polycystic ovary syndrome.
Proband.
Lipoatrophy had mainly affected the trunk, limbs, and femorogluteal regions and was associated with muscular hypertrophy predominantly in the calves (Fig. 1B). Lipoatrophy could be mild, especially in young patients (patients E-III1 and C-II2). Cervicofacial fat accumulation was observed in three patients (patients E-I1, C-I2, and C-II1). Low serum leptin levels and a decrease in the total fat mass, as assessed by dual energy X-ray absorptiometry, were consistent with lipoatrophy. Abdominal subcutaneous adipose tissue, studied in patients E-I1 and E-II2, displayed disorganized fat lobules of heterogeneous size, with macrophage infiltrates, increased fibrosis, and increased vascularization, consistent with previous findings (2) (Fig. 2). Seven patients showed a facial acromegaloid appearance with enlarged hands and feet. All investigated patients had presented with hyperinsulinemia or diabetes, frequently accompanied by acanthosis nigricans. Five of the seven women had polycystic ovary syndrome and/or hirsutism and oligomenorrhea. Hypertriglyceridemia was present in all investigated adult patients. No history of acute pancreatitis was reported. All examined patients had liver steatosis. Patient L-II1 had experienced major complications, including neuropathy, hypertension, and myocardial infarction with rhythm disturbances, which required the implantation of a cardioverter defibrillator.
Discussion
The increasing use of next generation sequencing in clinical practice highlights the need for accurate interpretation of variants. When large population exome data became available, the pathogenicity of several genes involved in Mendelian disorders was questioned (7). This issue was recently raised for PLIN1 by Laver et al. (6). Because of the importance of early genetic counseling for appropriate disease management (1), the clues arguing for and against a pathogenic effect of PLIN1 null variants should be considered carefully (Table 2).
Table 2.
Criteria for Evaluating Pathogenicity of PLIN1 Null Variants
| Criteria supporting evidence of pathogenicity |
| Absence of FPLD4-associated PLIN1 variants in controls (Genome Aggregation Database, Exome Aggregation Consortium databases) |
| Enrichment of PLIN1 frameshift variants in cohorts of patients with FPLD compared with general population |
| Segregation of FPLD4-associated PLIN1 frameshift variants with the disease in eight families including 13 informative relatives |
| Absence of other molecular explanations for the disease in all patients with FPLD4-associated PLIN1 frameshift variants |
| Homogeneity of the clinical and biological phenotype in patients with FPLD4-associated PLIN1 frameshift variants |
| Demonstration of the deleterious effect of three frameshift variants in several cellular models expressing the wild-type and mutated forms of the protein |
| Criteria supporting evidence of benign effect |
| Elevated frequency of PLIN1 null variants in the general population |
| Absence of manifestations evocative of FPLD in several individuals carrying PLIN1 null variants |
First, the allele frequency of PLIN1 null variants in the general population, estimated at ∼4.10−4 in public databases, is higher than the prevalence of all forms of FPLD, estimated at ∼3.10−6 (8). Even if FPLD remains underdiagnosed, this suggests that certain PLIN1 null variants are not pathogenic. However, we observed a marked enrichment of PLIN1 frameshift variants in patients with FPLD. The allele frequency of PLIN1 frameshift variants was estimated at 1.9% in one of our previous studies (three heterozygotes among 78 independent patients with clinically ascertained FPLD) (2) and at ∼0.8% in the present study (four positive probands for 237 tested patients).
Strikingly, the FPLD4-associated PLIN1 variants cosegregated with the disease within the eight families available to date [Gandotra et al. (2), Kozusko et al. (3), Chen et al. (4), and the present study]. If these variants were all polymorphisms, the probability to see such a segregation by chance in the 13 informative affected relatives would be extremely low [(1/2)13; i.e., 1.10−4).
The pathogenicity of PLIN1 null variants was also questioned because the patients reported by Laver et al. (6) did not have overt lipoatrophy. As acknowledged by the authors, it can be difficult to exclude the presence of lipodystrophy in young patients, and the lack of adipose tissue-focused examinations in large cohort studies hamper the recognition of subtle lipodystrophic morphotypes. In this regard, one study underlined that FPLD remains underdiagnosed and could affect >3% of patients investigated for metabolic syndrome (9). Accordingly, lipodystrophy was diagnosed a posteriori in several patients in the present study, owing to the familial investigations.
Four FPLD4-associated frameshift variants lead to the synthesis of aberrant perilipin-1 isoforms [Gandotra et al. (2), Kozusko et al. (3), and the present study]. Expression in adipocyte models of three of these mutant forms of perilipin-1 decreases the size of lipid droplets and increases basal lipolysis (2, 3, 5). All but one FPLD4-associated PLIN1 variants [p.(Val398Glyfs*166), p.(Tyr401Leufs*165), p.(Pro403Argfs*164), p.(Leu404Alafs*158)] alter the interaction domain of perilipin-1 with ABHD5 (amino acids 380 to 427). Consistently, the p.(Val398Glyfs*166) and p.(Leu404Alafs*158) mutants fail to interact with ABHD5, leading to constitutive activation of adipocyte triglyceride lipase (5). The p.(Pro439Valfs*125) mutant, which is located in the vicinity of this binding domain, fails to inhibit basal lipolysis by an alternate mechanism (3). It would be interesting to determine the functional consequences of PLIN1 null variants identified in the general population or in those with maturity onset diabetes of the young.
At present, the classification of genetic variants follows the guidelines of the American College of Medicals Genetics and uses a five-class score (10). According to these criteria, FPLD4-associated PLIN1 frameshift variants that disrupt protein function should be classified as “pathogenic.” Laver et al. (6) suggested that PLIN1 null variants should not be reported as causative of lipodystrophy. Our study provides a set of arguments supporting the pathogenicity of several PLIN1 frameshift variants. It is thus important to evaluate each of these variants carefully for genetic counseling. We would recommend detailed familial investigations, adipose tissue-focused examination, and careful follow-up of metabolic evolution in patients carrying such variants.
Acknowledgments
The PLIN1-Study Group includes Fabrice Devemy (Department of Endocrinology, Lens Hospital, Lens, France), Wassila Karrouz (Department of Endocrinology, Diabetes and Metabolism, Lille University Hospital, Lille, France), Sophie Haye (Department of Endocrinology, Béthune Hospital, Béthune, France), Sonja Janmaat (Department of Endocrinology, Assistance Publique-Hôpitaux de Paris, Reference Center for Rare Diseases of Insulin Secretion and Insulin Sensitivity, Paris, France), Brigitte Delemer (Department of Endocrinology, Reims University Hospital, Reims, France), Hilary Meggison (Department of Endocrinology and Metabolism, Ottawa Hospital, Ottawa Ontario, Canada), and Michael T. Geraghty (Department of Pediatrics, Children’s Hospital of Eastern Ontario, University of Ottawa, Ottawa, Ontario, Canada).
We thank the patients who participated in these studies, Drs. Jean-Philippe Bastard and Soraya Fellahi (Department of Biochemistry, Tenon Hospital, Assistance Publique-Hôpitaux de Paris, Paris, France) for leptin measurements, and Dr. Jian Wang and Professor Robert A. Hegele (Robarts Research Institute, Western University, London, Ontario, Canada) for fruitful discussions.
Financial Support: The present study was supported by the French Ministry of Solidarity and Health, Assistance-Publique Hôpitaux de Paris, Sorbonne Université, the Institut National de la Santé et de la Recherche Médicale (Inserm), and the Institut Hospitalo-Universitaire de Cardiométabolisme et Nutrition (ICAN), France. D.B.S. is supported by the Wellcome Trust (grant WT 107064), the MRC Metabolic Disease Unit (grant MRC_MC_UU_12012.1), and the National Institute for Health Research (NIHR) Cambridge Biomedical Research Centre and NIHR Rare Disease Translational Research Collaboration. M.C.V. is supported by the French Ministry of Health (grant PHRC 2009).
Glossary
Abbreviations:
- FPLD
familial partial lipodystrophy
- FPLD4
familial partial lipodystrophy type 4
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References and Notes
- 1. Brown RJ, Araujo-Vilar D, Cheung PT, Dunger D, Garg A, Jack M, Mungai L, Oral EA, Patni N, Rother KI, von Schnurbein J, Sorkina E, Stanley T, Vigouroux C, Wabitsch M, Williams R, Yorifuji T. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101(12):4500–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gandotra S, Le Dour C, Bottomley W, Cervera P, Giral P, Reznik Y, Charpentier G, Auclair M, Delépine M, Barroso I, Semple RK, Lathrop M, Lascols O, Capeau J, O’Rahilly S, Magré J, Savage DB, Vigouroux C. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med. 2011;364(8):740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kozusko K, Tsang V, Bottomley W, Cho YH, Gandotra S, Mimmack ML, Lim K, Isaac I, Patel S, Saudek V, O’Rahilly S, Srinivasan S, Greenfield JR, Barroso I, Campbell LV, Savage DB. Clinical and molecular characterization of a novel PLIN1 frameshift mutation identified in patients with familial partial lipodystrophy. Diabetes. 2015;64(1):299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen RX, Zhang L, Ye W, Wen YB, Si N, Li H, Li MX, Li XM, Zheng K. The renal manifestations of type 4 familial partial lipodystrophy: a case report and review of literature. BMC Nephrol. 2018;19(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gandotra S, Lim K, Girousse A, Saudek V, O’Rahilly S, Savage DB. Human frame shift mutations affecting the carboxyl terminus of perilipin increase lipolysis by failing to sequester the adipose triglyceride lipase (ATGL) coactivator AB-hydrolase-containing 5 (ABHD5). J Biol Chem. 2011;286(40):34998–35006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laver TW, Patel KA, Colclough K, Curran J, Dale J, Davis N, Savage DB, Flanagan SE, Ellard S, Hattersley AT, Weedon MN. PLIN1 haploinsufficiency is not associated with lipodystrophy. J Clin Endocrinol Metab. 2018;103(9):3225–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Piton A, Redin C, Mandel JL. XLID-causing mutations and associated genes challenged in light of data from large-scale human exome sequencing. Am J Hum Genet. 2013;93(2):368–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chiquette E, Oral EA, Garg A, Araujo-Vilar D, Dhankhar P. Estimating the prevalence of generalized and partial lipodystrophy: findings and challenges. Diabetes Metab Syndr Obes. 2017;10:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dutour A, Roll P, Gaborit B, Courrier S, Alessi MC, Tregouet DA, Angelis F, Robaglia-Schlupp A, Lesavre N, Cau P, Lévy N, Badens C, Morange PE. High prevalence of laminopathies among patients with metabolic syndrome. Hum Mol Genet. 2011;20(19):3779–3786. [DOI] [PubMed] [Google Scholar]
- 10. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]