Abstract
Background
Community-acquired pneumonia (CAP) is the leading cause of death in children. Identification of reliable biomarkers offers the potential to develop a severity quantitative score to assist in clinical decision-making and improve outcomes.
Methods
A systematic review and meta-analysis was performed in PubMed and EMBASE on November 13, 2018, to examine the association between host inflammatory biomarkers and CAP severity in children. The inclusion criteria were case–control, cross-sectional, and cohort studies that examined candidate serum biomarkers. We extracted outcomes of interest, means, and standardized mean differences (SMDs) of plasma and serum levels of biomarkers together with information on disease severity. Meta-analysis was performed. This review was registered in the PROSPERO international registry (CRD42019123351).
Results
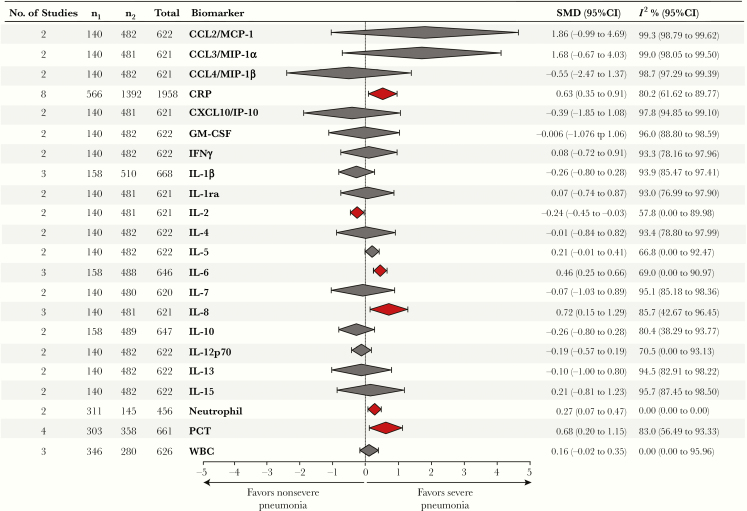
Two hundred seventy-two abstracts were identified, and 17 studies were included. Among the biomarkers evaluated, levels of C-reactive protein (CRP; SMD, 0.63; 95% confidence interval [CI], 0.35 to 0.91), interleukin (IL)-6 (SMD, 0.46; 95% CI, 0.25 to 0.66), IL-8 (SMD, 0.72; 95% CI, 0.15 to 1.29), neutrophil count (SMD, 0.27; 95% CI, 0.07 to 0.47), and procalcitonin (SMD, 0.68; 95% CI, 0.20 to 1.15) were substantially increased in severe CAP. In contrast, IL-2 concentrations (SMD, –0.24; 95% CI, –0.45 to –0.03) were higher in nonsevere CAP. Study heterogeneity was reported to be high (I2 > 75%), except for IL-2, IL-5, IL-6, and IL-12p70, which were classified as moderate (I2 = 50%–74%). Only neutrophil and white blood cell counts were described by studies exhibiting a low level of heterogeneity.
Conclusions
Our results suggest that host biomarkers, and especially CRP, IL-6, IL-8, and procalcitonin levels, have the potential to predict severe CAP in pediatric populations.
Keywords: biomarkers, children, inflammation, pneumonia, severity
Community-acquired pneumonia (CAP) is the leading cause of death in children [1]. In pediatric populations, especially in children under 5 years old, CAP-associated mortality is substantially higher than HIV/AIDS, malaria, and measles combined [1]. Severe CAP and lethal CAP are more frequent in low-resource settings, resulting in significant disease burden [2]. Nevertheless, such conditions are also very common in well-developed countries in Europe and North America [3]. These observations demonstrate that the morbimortality linked to CAP has a devastating impact on the public health system globally, irrespective of the economic status of the affected populations.
Assessment of CAP severity is critical for improving site-of-care decisions and implementation of adequate clinical management in a timely fashion [3–5]. In adults, several prognostic scores are available for patients with pneumonia, and studies of these models have indicated that its use may reduce broad-spectrum antibiotic use and decrease hospitalization among low-risk individuals [3–5]. Unfortunately, absence of a standardized and validated pediatric CAP severity quantitative score remains one of the biggest challenges in the field [3–5].
The most used guidelines for the management of CAP worldwide are from the World Health Organization (WHO) [6], the British Thoracic Society [7], and the Pediatric Infectious Diseases Society/Infectious Diseases Society of America [8]. Importantly, such guidelines do not consider markers in the blood to assess severity, but only clinical parameters. The parameters found in all 3 guidelines are difficulty breathing (grunting, chest retraction) and elevated respiratory rate. Other parameters are also used, such as cyanosis, peripheral oxygen saturation <90%, tachycardia, capillary refill time ≥2 seconds, and fever (temperature >38.58°C). However, these parameters also have their limitations and are highly dependent on the physician’s capability to perform correct assessment [9] and consequently are not sufficient to determine disease severity. Therefore, identification of reliable biomarkers offers the potential to improve the decision-making around CAP severity beyond clinical scoring systems [3, 5].
Biomarkers of inflammation, such as white blood cell counts (WBCs), procalcitonin (PCT), and C-reactive protein (CRP), have been described in children as useful markers associated with severe pneumonia [5]. Such biomarkers may be useful in the management of patients with CAP [3, 5]. Additional studies are necessary to test whether a panel of combined biomarkers in addition to clinical assessment can substantially improve the management of pediatric CAP and reduce the unfavorable outcomes associated with this disease. Thus, a better understanding of the utility of immune and inflammatory markers can potentially lead to development of innovative prediction tools that could be implemented as point-of-care approaches to optimize clinical decisions. To contribute to advances in this field, we performed a meta-analysis examining the association of circulating host biomarkers with disease severity in pediatric CAP.
METHODS
Search Strategy
We performed a systematic literature search in the PubMed and EMBASE medical literature databases using the following consecutive terms in PubMed: (“Biomarkers”[MeSH] AND “Pneumonia”[MeSH]) AND “Child”[MeSH]; (“cytokines”[MeSH Terms] OR “cytokines”[All Fields] OR “cytokine”[All Fields]) AND (“community-acquired”[All Fields] AND (“pneumonia”[MeSH Terms] OR “pneumonia”[All Fields])) AND (“child”[MeSH Terms] OR “child”[All Fields] OR “children”[All Fields]); ((“pediatrics”[MeSH Terms] OR “pediatrics”[All Fields] OR “pediatric”[All Fields]) AND (“pneumonia”[MeSH Terms] OR “pneumonia”[All Fields])) AND (“severity”[All Fields] AND (“Markers”[Journal] OR “markers”[All Fields])). In EMBASE, we used the following search: (“child”[MeSH Terms] OR “child”[All Fields]) AND (“residence characteristics”[MeSH Terms] OR (“residence”[All Fields] AND “characteristics”[All Fields]) OR “residence characteristics”[All Fields] OR “community”[All Fields]) AND “acquired”[All Fields] AND “severity”[All Fields] AND (“pneumonia”[MeSH Terms] OR “pneumonia”[All Fields]). We did an additional manual search of the reference lists from selected articles to identify eligible publications that may not have appeared in our electronic search strategy. The search was conducted on November 13, 2018.
The selection of the studies was divided into 4 steps: (1) reading of the titles, (2) reading of the article abstracts, (3) evaluation of the full articles selected from the previous step and inclusion of other studies present in the reference lists of the selected articles, (4) selection of the studies to include in the meta-analysis. Two reviewers (C.F. and P.M.) independently did the search, and any disagreement was resolved through consulting a third author (M.B.A.), who was more experienced in conducting meta-analyses.
We selected case–control, cross-sectional, and cohort studies that examined the candidate serum biomarkers able to predict the severity of CAP in children. Studies needed to include children (aged 0 months to 18 years). Exclusion criteria included systematic or narrative literature reviews, letters, editorials, and comments; patients with respiratory comorbidities such as asthma and cystic fibrosis; and hospital-acquired and ventilator-associated pneumonia. We selected studies in which the reference standard for diagnosis of pneumonia was based on a chest radiograph and/or clinical features. The criteria considered for diagnosis of severity of CAP were the classification of the British Thoracic Society, the WHO classification, and clinical (chest x-ray, length of hospitalization, difficulty in breathing, presence of pleural effusion/empyema, and others) and laboratory (positive blood culture, altered markers) parameters. Studies that did not define the disease according to severity parameters were excluded. Other exclusion criteria were if (1) the studies were clearly not related to the theme; (2) the studies were designed to examine the biomarkers present in the bronchoalveolar lavage or fluids different than blood; (3) the studies analyzed the biomarkers for diagnosis or disease severity of pathogen-specific pneumonia; (4) the biomarker was studied only to verify its ability to diagnose CAP, not to predict the severity of the disease; (5) it was not possible to obtain the full article. Articles with incomplete data were not considered for the meta-analysis. This review was registered in the PROSPERO International Registry (registration number: CRD42019123351).
Quality Assessment
The quality of selected studies and potential risk of bias were accessed with the Newcastle-Ottawa quality assessment scale using 2 different versions: a version for cohort studies and another version for cross-sectional studies [10]. Each article was given a score in number of stars from 3 perspectives: (a) selection (maximum, 4 stars in the original version and 5 stars in the adapted version); (b) comparability (maximum, 2 stars); and (c) exposure/outcome (maximum, 3 stars). Thus, in the processing of the article quality analysis, a maximum of 9 or 10 stars could be obtained. In this way, an article quality classification was created: 8–9/10 stars = excellent; 6–7 stars = good; 4–5 stars = fair; 0–3 stars = poor. Article quality assessment was done independently by 3 authors (C.D.F., M.Cl.M.C., and M.Ca.M.C.), and any disagreement was resolved consulting a third author (M.B.A.). Low methodological quality was not an exclusion criterion.
Data Extraction
Data extraction was performed using a standardized model. We recorded the following data from all studies: (1) overall characteristics of the study (design, publication year, city and county of origin); (2) sample (size, age range, inclusion and exclusion criteria); (3) severity criteria; (4) biomarkers studied; (5) statistical analyses performed, including median, interquartile range (IQR), mean, and SD. Data were extracted by 3 authors (C.D.F., M.Cl.M.C., and M.Ca.M.C.). A fourth author (M.B.A.) cross-checked all extracted data compiled in a table (Microsoft Excel 2010), comparing them with the original data available from the selected articles to ensure accuracy. Identified errors were corrected.
Data Analysis
Biomarker measurements compared between severe pneumonia and nonsevere pneumonia were extracted from studies as mean ± SD. Studies with data reported as median (IQR) and mean ± SD were calculated using methods outlined by Wan et al. (Supplementary Table 1) [11].
Heterogeneity and the I2 statistical analyses were performed using Cochran’s Q statistics across all studies, the latter describing the percentage of variation across studies that is due to heterogeneity, rather than chance, presented with a 95% confidence interval (CI). An I2 of 25%–49% was considered to represent a low level of heterogeneity, 50%–74% a moderate level, and 75%–100% a high level. Meta-analysis of mean differences was pooled using random (moderate and high heterogeneity) or fixed-effects models (low heterogeneity). P < .05 was considered statistically significant.
Statistical analyses were performed using the Comprehensive Meta-Analysis software package (version CM 2.2; Biostat, Englewood, NJ, USA). Network meta-analyses of severe pneumonia were performed using the netmeta 0.9–6 package in R (version 3.4.0).
Role of the Funding Source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
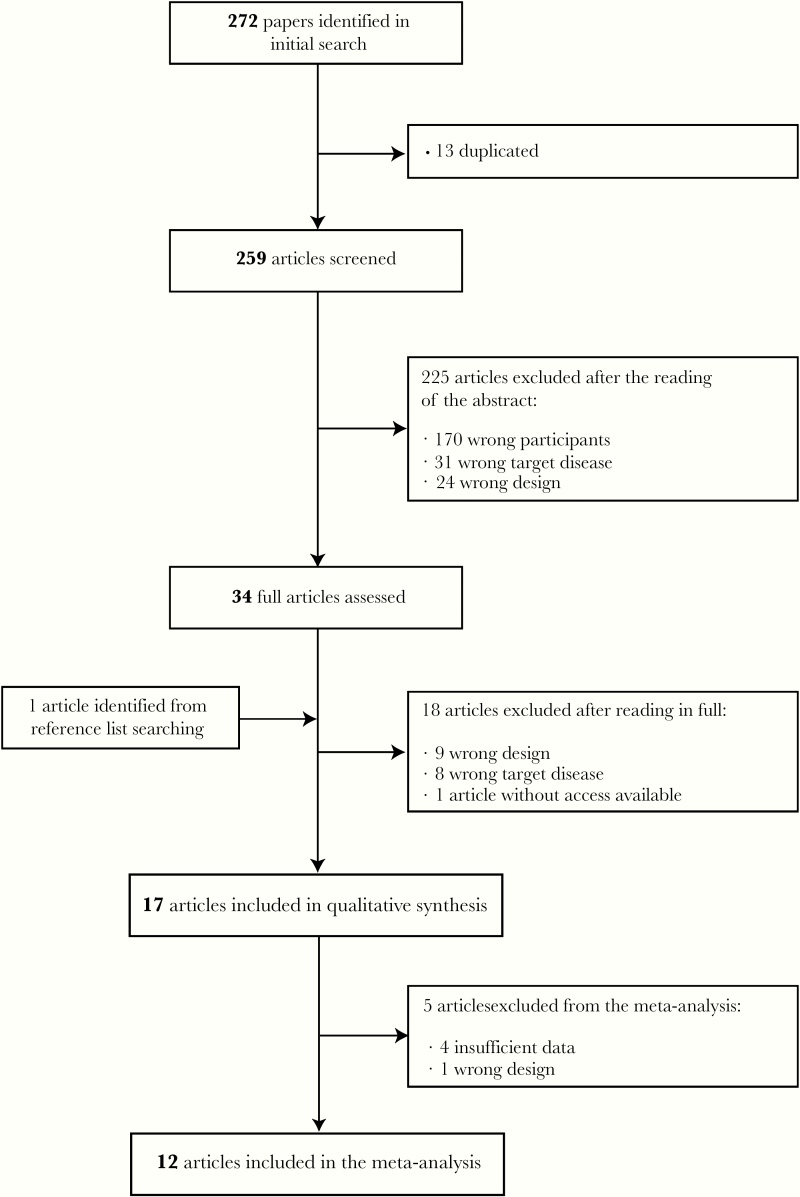
Our search identified 272 papers. After removing duplications, we assessed a total number of 259 articles. Through the study selection process (Figure 1), 17 articles were included in the review and 12 in the meta-analysis. All 17 studies underwent quality assessment using the Newcastle-Ottawa quality assessment scale.
Figure 1.
Flowchart of the study selection process study characteristics. The characteristics of the included studies are summarized in Table 1. The majority of the studies (n = 13) were cross-sectional, and 2 of them were a secondary analysis of a previous randomized controlled trial, both using the same sample but evaluating different biomarkers. The remaining studies included 4 cohorts, 2 of which were secondary analyses of previous cohorts.
In this systematic review, data on 3192 patients were examined, whereas 2269 were assessed in the meta-analysis. The median number of patients with severe CAP per study (IQR) was 43 (10–312), whereas 116 (7–387) individuals were identified as having nonsevere CAP. Nine studies were performed in inpatient settings, 4 in emergency departments. An additional 4 studies evaluated both outpatient (for nonsevere cases) and inpatient (for severe cases) settings. Four studies recruited healthy subjects to compare with CAP patients: 3 at the community level, and 1 recruited healthy children who had blood collected as part of well-child care at the hospital; these individuals were defined as controls and represented a total of 438 subjects (Table 1).
Table 1.
Characteristics of Included Studies
| No. | Study (Author, Year) | Study Type | Country | Criteria for Diagnosis of Severe CAP | Sample Size | Age of Patients | Inclusion Criteria | Exclusion Criteria | Biomarkers Studied |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Agnello et al. (2015) | Cross- sectional | Italy | Extent of chest x-ray infiltration (lobar vs segmental consolidation) and presence of pleural effusion | 119 | 1 to 14 y | Radiographically confirmed diagnosis of CAP | If the patient received antibiotics for >48 h before admission, if they were suffering from a chronic respiratory disease, or if they were hospitalized for >48 h | CRP and PCT |
| 2 | Alcoba et al. (2015) | Secondary analysis from a previous cohort study | Switzerland | CAP with bacteremia (positive blood culture) or empyema (positive pleural culture) | 88 | 0 to 16 y | Age, fever (>38°C), cough, increased respiratory rate or distress, and infiltrates on chest radiographs | Children with immunodeficiency, chronic lung or heart diseases, and hospital-acquired pneumonia | Pro-ADM, CRP, WBC, CoPEP, BC |
| 3 | Alcoba et al. (2017) | Cross-sectional | Switzerland | CAP with bacteremia, pleural effusion, or empyema | 142 | 2 mo to 16 y | Age, fever (>38°C), cough, increased respiratory rate or distress, and infiltrates on chest radiographs | Children with immunodeficiency, chronic lung or heart diseases, and hospital-acquired pneumonia | CRP, PCT |
| 4 | Brito et al. (2016) | Cross-sectional | Brazil | Based on the WHO criteria | 25 | 0 to 18 ya | Patients admitted to 3 hospitals in the municipality of Recife, Northeastern Brazil, from June to December 2012 | History of immunodeficiency, suspicion of TB, chronic conditions, children transferred from other hospitals, and use of antibiotic therapy for >3 d at the time of admission | Cytokines e |
| 5 | Don et al. (2007) | Cross- sectional | Italy | Need for hospital treatment and presence of an alveolar infiltration on chest radiograph | 100 | 1 mo to 18 yb | Signs and/or symptoms compatible with respiratory infection (fever >38°C, tachypnea, cough and/or findings of crackles, bronchial breathing or diminished breath sounds on auscultation) and radiological infiltrations consistent with pneumonia | Infants <1 mo of age, presence of severe and chronic diseases, wheezing, and hospital-acquired pneumonia | PCT |
| 6 | Du et al. (2013) | Cross-sectional | China | Presence of pleural effusion, pneumothorax, pneumatocele, or lung abscess | 165 cases and 100 controls | Preschool children (2 to 6 y) | During the period from January 2010 to January 2012, all preschool children with CAP were initially assessed | Children with genetic diseases (including cystic fibrosis), heart disease with hemodynamic repercussions, pulmonary malformations, and neurological disorders | CRP, CoPEP |
| 7 | Esposito et al. (2016) | Cross-sectional | Italy | Followed the criteria indicated for children by the British Thoracic Society | 110 | 0 to 14 y | Children with clinical signs (tachypnea and abnormal breath sounds) that suggested CAP who required hospitalization | Not reported | LIP-2, WBC, CRP, SYN4 |
| 8 | Esposito et. al (2016) | Cross-sectional | Italy | Followed the criteria indicated for children by the British Thoracic Society | 433 | 4 mo to 14 y | Healthy children consecutively hospitalized for clinical signs suggestive of CAP (tachypnea and abnormal breath sounds) and radiological confirmation of CAP | Patients with underlying chronic disease or an antibiotic treatment of any type in the 48 h before admission | CRP, PCT, WBC, N%, MR-proADM, MR-proANP, sTREM-1 |
| 9 | Haugen et al. (2015) | Cross-sectional (secondary analysis from a previous RCT) | Nepal | WHO-defined severe pneumonia | 430 | 2 to 35 mo | Children with severe or nonsevere pneumonia according to WHO definitions attending the study clinic because of cough and/or difficulty breathing | Lack of consent, not planning to live in the area for the next 6 mo, requiring care for very severe disease, severe malnutrition, presence of congenital heart disease, documented tuberculosis, documentation of any oral antibiotic treatment in the past 48 h, children whose symptoms resolved after receiving 2 doses of salbutamol, cough for >14 d, severe anemia or dysentery | CRP, cytokines, chemokines, and growth factorsf |
| 10 | Haugen et al. (2017) | Cross-sectional (secondary analysis from a previous RCT) | Nepal | WHO-defined severe pneumonia | 430 | 2 to 35 mo | Children with severe or nonsevere pneumonia according to WHO definitions attending the study clinic because of cough and/or difficulty breathing | Lack of consent, not planning to live in the area for the next 6 mo, requiring care for very severe disease, severe malnutrition, presence of congenital heart disease, documented tuberculosis, documentation of any oral antibiotic treatment in the past 48 h, children whose symptoms resolved after receiving 2 doses of salbutamol, cough for >14 d, severe anemia or dysentery | Plasma-25(OH)D |
| 11 | Huang et al. (2014) | Secondary analysis from a previous cohort study | Gambia | Cough or difficulty in breathing plus respiratory distress (lower chest wall indrawing or nasal flaring) | 204 cases and 186 controls | 2–59 mo | Children from the Greater Banjul and Basse areas taking part in a study of childhood pneumonia between June 2007 and June 2010 at 5 health facilities in this area | Not reported | LIP-2, CRP, Hap, vWF |
| 12 | Korkmaz et al. (2018) | Cross-sectional | Turkey | Patients were classified into severity groups according to the respiratory clinical scorec | 66 | 3 to 181 mo | Presence of fever, respiratory symptoms, and at least 1 abnormality on physical examination or chest radiograph according to the WHO definition | Congenital heart disease, neuromuscular disease, immunodeficiency, or any chronic disease (cystic fibrosis, asthma, bronchopulmonary dysplasia, tuberculosis, etc.) | Pro-ADM, IL-1β, CRP, WBC, NC, LC |
| 13 | Ning et al. (2016) | Cross-sectional | China | Defined by the extent of consolidation on chest x-ray and presence of pleural effusion | 174 cases and 33 controls | 3 mo to 12.4 y (cases) 8 mo to 9.8 y (controls) | Presence of signs and symptoms of pneumonia and pulmonary consolidation on chest radiography in a previously healthy child caused by an infection that was acquired outside the hospital | Children who had received antibiotic treatments for >48 h before admission or those suffering from an underlying chronic respiratory disease | N%, NC, WBC, ESR, CRP, PCT, M%, PA |
| 14 | Ramakrishna et al. (2015) | Cross-sectional | Malawi | WHO-defined severe and very severe pneumonia | 233 | 2 mo to 14 y | Children with severe or very severe pneumonia who had serum lactate concentration measured at the time of admission | Children who had clinical features consistent with the WHO criteria for pneumonia but found to have other diagnoses characterized by respiratory distress | Lactate |
| 15 | Saghafian-Hedengren et al. (2017) | Cohort | India | WHO-defined severe pneumonia | 196 | 1 mo to 12 y | Patients with pneumonia based on WHO criteria of tachypnea with cough or difficulty breathing | Children with chronic conditions, illness duration >1 wk, use of antibiotics for >24 h at presentation, previous hospitalization within the preceding 30 d, and children whose symptoms resolved after receiving a single dose of salbutamol | Cytokines, chemokines, and growth factorsg |
| 16 | Sanchez et al. (2012) | Cohort | Spain | Presence of pleural effusion, cavitation, lung abscess or necrosis, air escape, massive atelectasis, or signs of systemic infection (sepsis) | 50 | 1.9 mo to 13.6 y | Patients assessed between January and October 2009 in the ES and hospitalized with CAP as the main diagnosis, with blood work completed on admission | Children with immunocompromising or chronical medical condition, patients with complicated CAP at the time of admission, and those receiving antibiotic treatment for a previous diagnosis of CAP | Pro-ADM, CRP |
| 17 | Wrotek et al. (2014) | Cross-sectional | Poland | Clinical and laboratory indicatorsd | 227 cases and 119 controls | 8 d to 18 y (cases) 7 d to 18 y (controls) | Children who were diagnosed with pneumonia up to 48 hours after hospital admission with radiologic confirmation of CAP and hsCRP levels within the normal range | Previously diagnosed proliferative disease, hsCRP levels above normal, diabetes mellitus and organ insufficiency, medical interventions, musculoskeletal defects, and lack of full information on the CAP course | su-PAR |
Abbreviations: BC, band cell; CAP, community-acquired pneumonia; CoPEP, copeptin; CRP, C-reactive protein; ES, emergency service; ESR, erythrocyte sedimentation rate; Hap, haptoglobin; hsCRP, high sensitive C-reactive protein; IFN, interferon; IL, interleukin; LC, lymphocyte count; LIP-2, lipocallin-2; hsCRP, high sensitive C-reactive protein; MR-proADM, midregional proadenomedullin; MR-proANP, midregional proatrial natriuretic peptide; N%, percentage of neutrophils; NC, neutrophil count; PCT, procalcitonin; Plasma-25(OH)D, plasma 25-hydroxy vitamin D; Pro-ADM, proadenomedullin; RCT, randomized controlled trial; sTREM-1, soluble triggering receptor expressed on myeloid cells-1; su-PAR, soluble urokinase plasminogen activator receptor; SYN4, syndecan-4; vWF, von Willembrand factor; TNF, tumor necrosis factor; WBC, white blood cell count; WHO, World Health Organization.
aThe article does not specify the age group, but as it followed WHO criteria, we assumed this range.
bWhich age cuts were used was not detailed, only that children <1 month were excluded, so we assumed this range.
cThe respiratory clinical score includes 4 clinical parameters: respiratory rate, retractions, dyspnea, and auscultation. Patients were classified into 3 severity groups according to this classification; low (1–3 points), moderate (4–6 points), and high (7–12 points) severity.
dClinical indicators: fever, time to defervescence, heart and breath rate, saturation, and length of antibiotic treatment and hospitalization. Laboratory indicators: CRP, procalcitonin, white blood cell count, and sodium.
eCytokines studied: IL-8, IL-1β, IL- 6, IL-10, TNF, IL-12p70, IL-17A, IL-2, IL-4, IL-5, and IFN-γ.
fCytokines, chemokines, and growth factors studied: IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17A, bFGF, eotaxin, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1 MIP-1α, MIP-1β, RANTES, TNF-α, PDGF-BB, and VEGF.
gCytokines, chemokines, and growth factors studied: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IL-15, IL-17, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, CCL17, CCL22, GM-CSF, and IL-1RA.
As observed in Supplementary Figure 1, the majority of studies originated from Europe (n = 8), with Italy being the most frequent study site (n = 4). Asia also composed a notable part of the studies (n = 6): 2 in China, 2 in Nepal, 1 in Turkey, and 1 in India. Only 2 studies were set in Africa—1 in Gambia and 1 in Malawi. Just 1 study took place in South America, in Brazil. No study was identified in North America or Oceania.
Quality Assessment
Table 2 shows quality scores for the studies, assessing risk of bias. Twelve studies were of excellent quality, 4 had good quality, and 1 was of fair quality (Table 2).
Table 2.
Quality Assessment of Studies Included in the Systematic Review and Meta-analysis by Newcastle Ottawa Scale
| Selection | Comparability | Outcome/Exposure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Source | Newcastle Ottawa Scale | Qualitative Synthesis/Meta-analysis | 1 | 2 | 3 | 4 | 5A | 5B | 6 | 7 | 8 | Overall Scorea |
| 1 | Agnello et al. (2015) | Cross-sectional | QS | * | * | * | ** | * | NA | ** | * | NA | 9 |
| 2 | Alcoba et al. (2015) | Secondary analysis from a previous cohort study | QS | * | * | * | * | * | NA | * | * | * | 8 |
| 3 | Alcoba et al. (2017) | Cross-sectional | QS/MA | * | * | * | ** | * | NA | ** | * | NA | 9 |
| 4 | Brito et al. (2016) | Cross-sectional | QS/MA | * | 0 | * | ** | 0 | NA | ** | * | NA | 7 |
| 5 | Don et al. (2007) | Cross-sectional | QS/MA | * | * | 0 | ** | * | NA | ** | * | NA | 8 |
| 6 | Du et al. (2013) | Cross-sectional | QS/MA | * | * | * | ** | * | NA | ** | * | NA | 9 |
| 7 | Esposito et. al (2016) | Cross-sectional | QS | * | * | * | ** | * | NA | ** | * | NA | 9 |
| 8 | Esposito et. al (2016) | Cross-sectional | QS/MA | * | * | * | ** | 0 | NA | ** | * | NA | 8 |
| 9 | Haugen et al. (2015) | Cross-sectional (secondary analysis from a previous RCT) | QS/MA | * | * | * | * | * | NA | * | * | NA | 7 |
| 10 | Haugen et al. (2017) | Cross-sectional secondary analysis from a previous RCT) | QS/MA | * | * | * | * | * | NA | * | * | NA | 7 |
| 11 | Huang et al. (2014) | Secondary analysis from a previous cohort study | QS/MA | * | * | * | * | * | NA | * | * | * | 8 |
| 12 | Korkmaz et al. (2018) | Cross-sectional | QS/MA | * | * | * | ** | * | NA | * | * | NA | 8 |
| 13 | Ning et al. (2016) | Cross-sectional | QS/MA | * | 0 | * | ** | * | NA | ** | * | NA | 8 |
| 14 | Ramakrishna et al. (2015) | Cross-sectional | QS | * | * | * | ** | * | NA | * | * | NA | 8 |
| 15 | Saghafian-Hedengren et al. (2017) | Cohort | QS/MA | 0 | * | * | 0 | 0 | NA | * | * | * | 5 |
| 16 | Sanchez et al. (2012) | Cohort | QS | * | * | * | 0 | 0 | NA | * | * | * | 6 |
| 17 | Wrotek et al. (2014) | Cross-sectional | QS/MA | * | * | * | ** | 0 | NA | ** | * | NA | 8 |
*Indicates the score given to the study according to the NOS quality assessment scale.
Abbreviations: MA, meta-analysis; NA, not applicable; QS, qualitative synthesis; RCT, randomized controlled trial.
aDetermined by the total number of stars assigned to the study: 0–3 stars = poor; 4–5 stars = fair; 6–7 stars = good; 8–9/10 stars = excellent.
Potential Biomarkers of Disease Severity
Meta-analyses were not performed for some biomarkers (copeptin, lymphocyte count, lipocalin-2, and proadenomedullin [pro-ADM]) because the published data were not sufficient. The authors attempted to contact the corresponding authors of the articles, without success. Moreover, plasma 25-hydroxy vitamin D, serum lactate, soluble urokinase plasminogen activator receptor, CCL17, CCL22, eotaxin, G-CSF bFGF, RANTES, tumor necrosis factor alpha (TNF-α), PDGF-BB, VEGF, CCL17, CCL22, interleukin (IL)-17, IL-17a, IL-9, TNFA, midregional proadenomedullin, midregional proatrial natriuretic peptide, lymphocyte count, Strem-1, syndecan-4, white blood cell band-forms, PA, erythrocyte sedimentation rate (ESR), and monocyte count each appeared in only 1 study, making their participation in the meta-analysis not possible (Supplementary Table 1).
Among the biomarkers evaluated and included in the meta-analysis from the 13 studies (Figure 2), circulating levels of CRP (standard mean difference [SMD], 0.63; 95% CI, 0.35 to 0.91), IL-6 (SMD, 0.46; 95% CI, 0.25 to 0.66), IL-8 (SMD, 0.72; 95% CI, 0.15 to 1.29), and PCT (SMD, 0.68; 95% CI, 0.20 to 1.15) and neutrophil counts (SMD, 0.27; 95% CI, 0.07 to 0.47) were significantly increased in patients presenting with severe CAP compared with those with nonsevere pneumonia. In contrast, levels of IL-2 in peripheral blood (SMD, –0.24; 95% CI, –0.45 to –0.03) were found to be higher in children with nonsevere CAP. The other 16 markers did not show statistically significant associations with either severe or nonsevere CAP, including WBC count and a number of pro- and anti-inflammatory cytokines and chemokines (Figure 2).
Figure 2.
Summary of biomarker meta-analysis of severe pneumonia. Red coloration of the diamond represents a significant difference. Abbreviations: CI, confidence interval; SMD, standardized mean difference.
Heterogeneity analysis revealed that the vast majority of the reports evaluated were not homogeneous by means of associations described and weight of the studies. As shown in Figure 2, for most inflammatory markers, the study heterogeneity was reported to be high (I2 > 75%), except for IL-2, IL-5, IL-6, and IL-12p70, for which heterogeneity was classified as moderate (I2 = 50%–74%). Of note, only cellular parameters (neutrophil and WBC counts) exhibited a low level of heterogeneity. The meta-analysis for each biomarker is shown in Supplementary Figure 2.
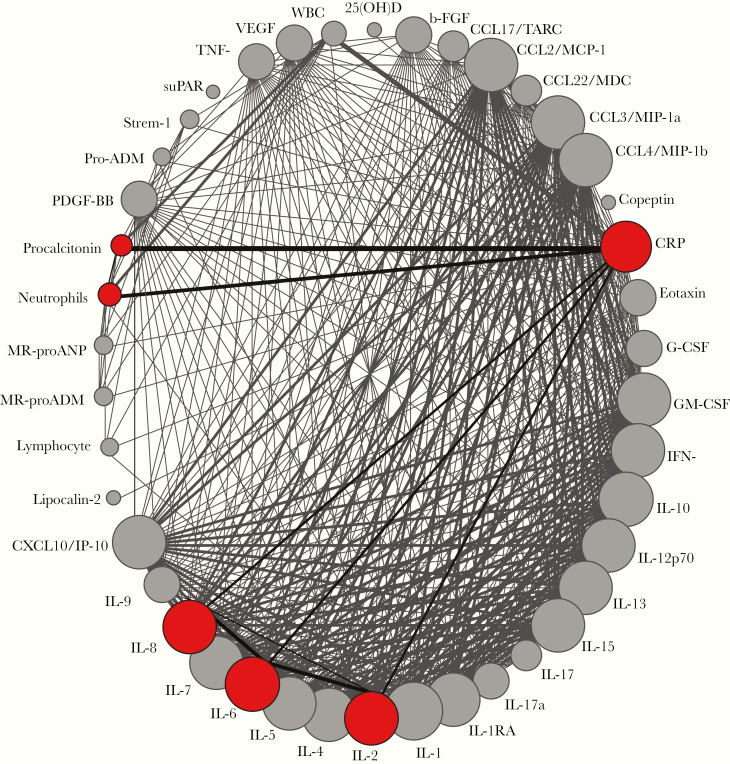
To visualize the number of publications investigating biomarkers in the context of severe CAP, we employed a model adapted from a network plot (Figure 3), previously used in a meta-analysis of clinical trials, using all the 13 studies included in the present study [12]. Using this approach, we observed that most of the markers found herein that were associated with severe CAP (CRP, PCT, IL-6, and neutrophil count) were investigated by several studies and were often studied together. Thus, these markers were more commonly studied in most of the reports included in the meta-analysis.
Figure 3.
Network analysis of the frequency of articles reporting each biomarker in the context of severe CAP. Each biomarker reported in the studies evaluated is shown as a circle. Colored circles highlight biomarkers that were significantly associated with severe CAP. The circle size is proportional to the number of patients who presented with severe CAP and had this marker measured (see n1 values). Lines connecting the circles highlight biomarkers that were studied together. The width of the lines is proportional to the number of studies reporting each pair of biomarkers.
DISCUSSION
To our knowledge, the present study is the first systematic review and meta-analysis to investigate host inflammatory biomarkers of disease in pediatric CAP. An important strength of our study was the comprehensive search strategy, which used detailed inclusion and exclusion criteria. Furthermore, methodological quality was assessed in duplicate using the Newcastle-Ottawa scale [10], which reduced the subjectivity of the selection of studies and allowed for precise evaluation of the risk of bias in several domains. After the application, we noted that most studies were of excellent quality according to the Newcastle-Ottawa scale, and there was no report that was categorized as poor quality. Thus, we concluded that the high quality of the selected studies resulted in a reliable meta-analysis with reduced risk of bias.
Our analyses identified the most frequently reported host inflammatory biomarkers in peripheral blood that were associated with CAP severity in pediatric populations. In severe pneumonia, the activation of immune responses culminates in unbalanced systemic inflammation and increased immunopathology [13]. Such dysregulated host responses lead to lung tissue injury and unfavorable clinical outcomes, such as acute respiratory failure or death [13]. In this scenario, 2 cytokines described to be relevant in our analyses, IL-6 and IL-8, are found in high concentrations in the plasma of critically ill children and generally represent hyperactivation of innate immune responses [13]. In our selected articles, only 3 studied the correlation between IL-6 and pediatric CAP severity ([14–16], and in all of these, IL-6 was significantly higher in children with severe pneumonia. Consistently, 2 studies evaluated IL-8, with the same results [14, 15].
One study in children with CAP found that IL-6 was elevated and revealed significant associations with markers of severity, as measured by white blood cell band-forms, and unequivocal consolidation in chest radiographs [17]. Two studies of cytokines that focused on specific etiologic agents, 1 on Mycoplasma pneumoniae and the other on influenza H1N1, verified that IL-6 concentrations were significantly higher in children with symptoms of severity [14, 15]. There are no additional studies reporting IL-6 or IL-8 and its relationship with CAP severity in children. Thus, additional studies are still needed to validate such biomarkers as prediction tools of disease severity.
Of note, IL-8 is a well-known chemoattractant for neutrophils, and elevated levels in blood are linked to neutrophilia [13]. As expected, our results reinforce the idea that augmented neutrophil counts, probably resulting from high IL-8 levels, hallmark severe CAP. Indeed, neutrophilia is an established criterion of hospitalization in children, especially in febrile infants [18]. However, the elevated neutrophil counts had a low predictive value in assessing the severity of the disease, as reported by Esposito et al. [19]. Neutrophilia is a parameter that is more often studied as a prognostic marker in other settings such as severe asthma [20], and more evidence is warranted to establish its utility in the context of pediatric CAP.
Whether measurements of cytokines such IL-8 and IL-6 can add value to the prediction power of neutrophil counts in children with acute pneumonia is a topic of interest. Interestingly, IL-6 and IL-8 were analyzed together in 2 studies included in our analyses [14, 15], which used the same criteria of clinical severity (WHO), although an additional investigation to specifically test if the 2 cytokines together with neutrophil counts increase the predictive power of severe CAP was not performed. Importantly, it is possible that severe CAP shares features of inflammation with sepsis and/or shock, which develop later, and thus such markers could be used as early predictive markers of these outcomes [21]. Future studies are warranted to test this hypothesis.
Another relevant biomarker identified here is CRP, an acute-phase protein that is triggered in response to nonspecific inflammation and/or tissue damage [22, 23]. Production of CRP occurs mostly in the liver and is induced by IL-6 and TNFα [22, 23]. CRP has great clinical utility, as shown by the meta-analysis results reported here. In pediatric CAP, many studies have shown that elevated CRP levels are associated with unfavorable outcomes, such as lobar consolidation, fever, long hospital length of stay, pleural effusion, and even death [22, 24, 25]. Our analysis showed that children with low CRP levels were at a low risk of severe pneumonia, reinforcing the possibility of using this marker to predict severity at admission to the emergency department. Moreover, this biomarker is very cost-effective, readily available, and accessible in most emergency departments [16]. Nevertheless, this biomarker does not seem to have a role in predicting secondary outcomes (ICU admission, vasopressor use, or the need for mechanical ventilation), as Lee et al. verified in their study [26], restricting its use for the assessment of disease severity at hospital admission.
PCT is one of the most well-studied biomarkers in the context of bacterial infections in both pediatric and adult populations. Heightened levels of serum PCT suggest invasive and bacteremic infections during hospitalization (especially in CAP) [27]; however, the use of this biomarker is more frequently reported in adults [24]. PCT has proven to be a good predictor of sepsis, being frequently used as an indicator of whether to continue, consider changing, or stop antibiotic therapy [28]. Some studies have concluded that PCT is superior to other nonspecific inflammatory markers such as CRP with respect to diagnosis and assessment of clinical severity of bacterial pneumonia [19, 27, 29]. Here, we report that PCT levels are consistently higher in children with severe CAP, thereby reinforcing its importance as a prognostic marker, which should be considered for systematic implementation in the clinical setting. Measuring PCT could reduce costs during hospitalization by decreasing the need for multiple bacterial cultures, the proportion of children with CAP receiving antibiotics, and the duration of antibiotic therapy [30, 31].
Curiously, PCT and CRP were studied in combination in 2 studies included in the present meta-analysis [19, 32], but the reported results were not sufficient to demonstrate a combined effect; rather, the authors only tested whether 1 parameter is a better marker than the other. In 1 study included here, performed in Italy, the authors identified a significant positive correlation between serum PCT levels and the main markers of inflammation such as CRP, percentage of neutrophils, and WBC [24]. In agreement with these findings, another study, from South Korea, that analyzed data from 76 hospitalized patients with CAP and 18 healthy controls demonstrated that serum PCT levels positively correlate with values of CRP, ESR, and WBC count [33]. These results reinforce the need for studies analyzing if these biomarkers in combination can increase the power to predict CAP severity.
One intriguing finding was the observation that high levels of IL-2 in peripheral blood are robustly linked to reduced odds of severe CAP. IL-2 has pleiotropic functions in the immune system, including activation and proliferation of effector T cells and induction of T-regulatory cells (Treg) that dampen the intensity of immune responses [34, 35]. In a Chinese study that examined the associations of IL-2 and IL-4 polymorphisms and their expression with regard to the risks of asthma and M. pneumoniae infection in children [33], it was observed that the average concentration of IL-2 in subjects not infected with M. pneumoniae was significantly higher than in the infected subjects. It is already known that this bacteria can lead to an atypical community‑acquired pneumonia with predominantly respiratory symptoms, but it can also induce autoimmune hemolytic anemia and other diseases in the blood, gastrointestinal tract, and skin, including pericarditis, myocarditis, nephritis, and meningitis [36], possibly increasing morbimortality. Thus, our meta-analysis is consistent with data from the literature in which IL-2 appears to have a protective effect on CAP. Also, as there are not many data from IL-2 in severe pneumonia, further studies are still needed to strengthen this association.
The study of this cytokine is of tremendous interest in the clinical immunology field, as the manipulation of IL-2 could directly impact the profiles of adaptive immune responses [34]. Immunotherapy using IL-2 has been employed in a number of scenarios, such as cancer and mycobacterial infections [37, 38]. There are no published data in humans indicating that recombinant IL-2 therapy brings therapeutic benefits in severe CAP.
Our study has some limitations. Some studies lacked the information necessary to be properly evaluated (eg, they reported median instead of mean values). For this reason, markers such as pro-ADM, which has been reported as a robust marker of severe CAP, could not be assessed. Furthermore, a high level of heterogeneity was recorded for most studies reporting the biomarkers analyzed here. Regardless of the heterogenous body of studies, the paucity of published evidence in this field justifies the importance of the present systematic investigation.
The findings of this meta-analysis show that the implementation of biomarker measures has the potential to optimize the clinical management of children with suspected severe CAP, adding value to the current guidelines for the diagnosis and management of severe CAP in children. The services that have the measurement of these biomarkers, especially CRP and PCT, available can possibly benefit now from this feature to improve the suspicion of severe pneumonia. This could potentially reduce the burden of pediatric CAP and the unnecessary use of antibiotics, decreasing antimicrobial resistance and improving clinical outcomes. Still, additional planned, rigorous studies are needed to clarify whether the evaluation of the significant biomarkers found in our meta-analysis can be used pragmatically in a clinical setting, mostly those that have not yet been extensively studied in pediatric severe CAP, such as IL-6, IL-8, neutrophils, and IL-2, and the ones that could not be included in our analysis (pro-ADM, copepetin, and lipocalin-2). It is necessary to evaluate whether the measurement of these biomarkers, alone and together, in combination with the clinical parameters, is financially viable, if it increases the power of detection of severe cases, and if its systematic application reduces occurrence of unfavorable outcomes.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors acknowledge Dr. Melissa Schechter for helping with the final version of this paper.
Financial support. This work was supported by the financial grants of the authors. C.D.F. is supported by scholarship from the Fundação de Amparo à Pesquisa da Bahia (FAPESB). M.B.A. and P.M. receive a fellowship from the Fundação de Amparo à Pesquisa da Bahia (FAPESB). M.Cl.M.C. and C.L.V. are supported by CNPq (Brazilian National Council for Scientific and Technological Development). M.Ca.M.C. receives a scholarship from the Faculdade de Tecnologia e Ciências (FTC). B.B.A. and K.F. are supported by the intramural research program of FIOCRUZ. B.B.A. receives a senior fellowship from CNPq.
Potential conflicts of interest. The authors declare no competing interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. C.D.F. and M.B.A. conceived the study. C.D.F., P.M., M.Cl.M.C., M.Ca.M.C., M.H.M.C., and M.B.A. were responsible for the data collection. K.F. and M.B.A. did the statistical analysis. C.D.F., M.Ca.M.C., C.L.V., M.B.A., K.F., and B.B.A. did the figures and tables. C.D.F., M.B.A., C.L.V., P.M., and K.F. did the scientific literature search. C.D.F., M.B.A., and C.L.V. participated in the writing of the report. B.B.A. revised the article critically and approved the version to be published.
References
- 1. Wardlaw T, Salama P, Johansson EW, Mason E. Pneumonia: the leading killer of children. Lancet 2006; 368:1048–50. [DOI] [PubMed] [Google Scholar]
- 2. Dagan R, Bhutta ZA, de Quadros CA, et al. The remaining challenge of pneumonia: the leading killer of children. Pediatr Infect Dis J 2011; 30:1–2. [DOI] [PubMed] [Google Scholar]
- 3. Uwaezuoke SN, Ayuk AC. Prognostic scores and biomarkers for pediatric community-acquired pneumonia: how far have we come? Pediatric Health Med Ther 2017; 8:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams DJ, Zhu Y, Grijalva CG, et al. Predicting severe pneumonia outcomes in children. Pediatrics 2016; 138:e20161019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Florin TA, Ambroggio L. Biomarkers for community-acquired pneumonia in the emergency department. Curr Infect Dis Rep 2014; 16:451. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. Guidelines for the management of common childhood illnesses. In: WHO, ed. Pocket Book of Hospital Care for Children. 2nd ed. Vol. 2 Geneva, Switzerland: World Health Organization; 2013:438. [PubMed] [Google Scholar]
- 7. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 2011; 66(Suppl 2):ii1–23. [DOI] [PubMed] [Google Scholar]
- 8. Bradley JS, Byington CL, Shah SS, et al. ; Pediatric Infectious Diseases Society and the Infectious Diseases Society of America The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis 2011; 53:e25–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dean P, Florin TA. Factors associated with pneumonia severity in children: a systematic review. J Pediatric Infect Dis Soc 2018; 7:323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25:603–5. [DOI] [PubMed] [Google Scholar]
- 11. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu Z, Zhang J, Liu Y, et al. Efficacy and toxicity of external-beam radiation therapy for localised prostate cancer: a network meta-analysis. Br J Cancer 2014; 110:2396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bordon J, Aliberti S, Fernandez-Botran R, et al. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int J Infect Dis 2013; 17:e76–83. [DOI] [PubMed] [Google Scholar]
- 14. Saghafian-Hedengren S, Mathew JL, Hagel E, et al. Assessment of cytokine and chemokine signatures as potential biomarkers of childhood community-acquired pneumonia severity: a nested cohort study in India. Pediatr Infect Dis J 2017; 36:102–8. [DOI] [PubMed] [Google Scholar]
- 15. Haugen J, Chandyo RK, Brokstad KA, et al. Cytokine concentrations in plasma from children with severe and non-severe community acquired pneumonia. PLoS One 2015; 10:e0138978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Brito RC, Lucena-Silva N, Torres LC, et al. The balance between the serum levels of IL-6 and IL-10 cytokines discriminates mild and severe acute pneumonia. BMC Pulm Med 2016; 16:e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ning J, Shao X, Ma Y, Lv D. Valuable hematological indicators for the diagnosis and severity assessment of Chinese children with community-acquired pneumonia: prealbumin. Medicine (Baltimore) 2016; 95:e5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghani S, Baaker R, Akram N. Significance of extreme leukocytosis in evaluation of febrile children aged 3–36 months: a single center experience. Iraqi J Hematol 2016; 5:e167. [Google Scholar]
- 19. Esposito S, Di Gangi M, Cardinale F, et al. ; Ita-CAP Study Group Sensitivity and specificity of soluble triggering receptor expressed on myeloid cells-1, midregional proatrial natriuretic peptide and midregional proadrenomedullin for distinguishing etiology and to assess severity in community-acquired pneumonia. PLoS One 2016; 11:e0163262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ray A, Kolls JK. Neutrophilic inflammation in asthma and association with disease severity. Trends Immunol 2017; 38:942–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ceccato A, Torres A. Sepsis and community-acquired pneumonia. Ann Res Hosp 2018; 2:e7. [Google Scholar]
- 22. Wu J, Jin YU, Li H, et al. Evaluation and significance of C-reactive protein in the clinical diagnosis of severe pneumonia. Exp Ther Med 2015; 10:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003; 111:1805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agnello L, Bellia C, Di Gangi M, et al. Utility of serum procalcitonin and C-reactive protein in severity assessment of community-acquired pneumonia in children. Clin Biochem 2016; 49:47–50. [DOI] [PubMed] [Google Scholar]
- 25. Esposito S, Bianchini S, Gambino M, et al. Measurement of lipocalin-2 and syndecan-4 levels to differentiate bacterial from viral infection in children with community-acquired pneumonia. BMC Pulm Med 2016; 16:e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee JH, Kim J, Kim K, et al. Albumin and C-reactive protein have prognostic significance in patients with community-acquired pneumonia. J Crit Care 2011; 26:287–94. [DOI] [PubMed] [Google Scholar]
- 27. Don M, Valent F, Korppi M, et al. Efficacy of serum procalcitonin in evaluating severity of community-acquired pneumonia in childhood. Scand J Infect Dis 2007; 39:129–37. [DOI] [PubMed] [Google Scholar]
- 28. Trásy D, Molnár Z. Procalcitonin – assisted antibiotic strategy in sepsis. EJIFCC 2017; 28:104–13. [PMC free article] [PubMed] [Google Scholar]
- 29. Moulin F, Raymond J, Lorrot M, et al. Procalcitonin in children admitted to hospital with community acquired pneumonia. Arch Dis Child 2001; 84:332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Westwood M, Ramaekers B, Whiting P, et al. Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in intensive care settings and for suspected bacterial infection in emergency department settings: a systematic review and cost-effectiveness analysis. Health Technol Assess 2015; 19:v–xxv, 1–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Díez-Padrisa N, Bassat Q, Morais L, et al. Procalcitonin and C-reactive protein as predictors of blood culture positivity among hospitalised children with severe pneumonia in Mozambique. Trop Med Int Health 2012; 17:1100–7. [DOI] [PubMed] [Google Scholar]
- 32. Alcoba G, Keitel K, Maspoli V, et al. A three-step diagnosis of pediatric pneumonia at the emergency department using clinical predictors, C-reactive protein, and pneumococcal PCR. Eur J Pediatr 2017; 176:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee JY, Hwang SJ, Shim JW, et al. Clinical significance of serum procalcitonin in patients with community-acquired lobar pneumonia. Korean J Lab Med 2010; 30:406–13. [DOI] [PubMed] [Google Scholar]
- 34. Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013; 38:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Banchereau J, Pascual V, O’Garra A. From IL-2 to IL-37: the expanding spectrum of anti-inflammatory cytokines. Nat Immunol 2012; 13:925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. He J, Liu M, Ye Z, et al. Insights into the pathogenesis of Mycoplasma pneumoniae (review). Mol Med Rep 2016; 14:4030–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology 2016; 5:e1163462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang R, Xi X, Wang C, et al. Therapeutic effects of recombinant human interleukin 2 as adjunctive immunotherapy against tuberculosis: a systematic review and meta-analysis. PLoS One 2018; 13:e0201025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.