Abstract
We tested the hypothesis that irregular menstruation predicts lower risk for ovarian cancer, possibly due to less frequent ovulation. We conducted a 50-year prospective study of 15,528 mothers in the Child Health and Development Studies cohort recruited from the Kaiser Foundation Health Plan from 1959 to 1966. Irregular menstruation was classified via medical record and self-report at age 26. We identified 116 cases and 84 deaths due to ovarian cancer through 2011 via linkage to the California Cancer Registry and Vital Statistics. Contrary to expectation, women with irregular menstrual cycles had a higher risk of ovarian cancer incidence and mortality over the 50-year follow-up. Associations increased with age (p <0.05). We observed a 2-fold increased incidence and mortality by age 70 (95% confidence interval [CI] = 1.1, 3.4) rising to a 3-fold increase by age 77 (95% CI = 1.5, 6.7 for incidence; 95% CI = 1.4, 5.9 for mortality). We also found a 3-fold higher risk of mortality for high-grade serous tumors (95% CI = 1.3, 7.6) that did not vary by age. This is the first prospective study to show an association between irregular menstruation and ovarian cancer—we unexpectedly found higher risk for women with irregular cycles. These women are easy to identify and many may have polycystic ovarian syndrome. Classifying high-risk phenotypes such as irregular menstruation creates opportunities to find novel early biomarkers, refine clinical screening protocols and potentially develop new risk reduction strategies. These efforts can lead to earlier detection and better survival for ovarian cancer.
Keywords: cohort study, irregular menstrual cycles, ovarian cancer, polycycstic ovarian syndrome, prospective
Ovarian cancer is the fifth leading cause of cancer death for women in the US, estimated to claim over 14,000 lives in 2015. The lack of specific early symptoms, aggressiveness of some of the histopathologic sub-types and need for more effective screening and treatment strategies is evidenced by a dismal 5-year overall survival rate (45%) for all stages.2 Ovarian cancer symptoms are nonspecific, and patients frequently come to diagnosis with advanced-stage disease.3 The need for identifiable risk factors and early markers of disease is critical for improving screening, treatment and survival and for providing insight about disease etiology and progression.
About 90% of ovarian cancer cases are epithelial and the remaining originate from sex cord-stromal cells (5–6%) or from germ cells (2–3%).4 Thus, most epidemiologic and clinical studies report findings that refer to epithelial ovarian cancer which is comprised of heterogeneous histologic subtypes, including serous (30–70%), endometrioid (10–20%), mucinous (5–20%) and clear cell (3–10%).4 Although epithelial ovarian tumors are situated in the ovary, recent molecular genetic and morphologic studies suggest they may originate primarily in the fallopian tube.5–7
The strongest risk factors for ovarian cancer are age, family history and germline mutations BRCA1, BRCA2 and other genes that have variable penetrance for disease. In a comprehensive screen of sequential ovarian cancer subjects, 24% of them carried a germline loss-of-function mutation, suggesting heritable ovarian cancer in just over one-fifth of cases.8 The remainder appear to be sporadic events. Several other risk characteristics, such as pregnancy, parity, infertility and other reproductive characteristics, are of more modest magnitude and have been identified with varying degrees of consistency.
A major focus of research has involved reproductive and hormonal factors. Nulliparity, infertility, early menarche and late menopause are associated with increased risk of ovarian cancer, although findings are not consistent.3,9–11 High parity, oral contraceptive use and bilateral prophylactic salpingooophorectomy are widely established as protective factors.9,12
Collectively, these epidemiological studies support the incessant ovulation hypothesis which suggests that chronic lifetime ovulation, uninterrupted by pregnancy, increases ovarian cancer risk. Adding confusion is contradictory epidemiologic evidence from a small study that showed women with polycystic ovarian syndrome, who experience chronic anovulation and irregular menstrual cycles, are at increased, rather than decreased, risk of ovarian cancer.13 Several studies have examined the influence of shorter, longer and irregular cycles on ovarian cancer risk. However, findings are inconclusive and show both increased and reduced risk associated with irregular cycles.14–16 The present study provides the first evidence in a large prospective study with long-term follow-up extending through the period of risk to help clarify the relation of menstrual irregularity to risk of ovarian cancer.
Material and Methods
Study population
The Child Health and Development Studies (CHDS) cohort, initiated in 1959, recruited nearly all pregnant women (over 98%) receiving prenatal care from the Kaiser Foundation Health Plan in the San Francisco East Bay area. In all, 15,528 women were enrolled during the 7-year recruitment period from 1959 until 1966, with deliveries extending into 1967.17 The CHDS cohort incorporated multiple race/ethnic groups with a broad socio-economic base and uniform access to health care. Pre-existing maternal conditions and clinical measures (e.g., prenatal and obstetric) were abstracted from medical records. Demographic information and health-related behavior were collected from in-person interviews for all pregnancies, generally early in the first trimester. The institutional review board of the Public Health Institute approved the study protocol and informed consent was obtained from all participants.
Cohort surveillance
Prospective surveillance has continued for 5 decades by annual linkage to: i) the California Department of Motor Vehicles, for a history of residence to identify the population at risk for morbidity and mortality, ii) the California Department of Vital Statistics, for identifying deaths and cause of death18,19 and iii) the California Cancer Registry, for identifying cancer diagnoses.20–23
CHDS mothers are regularly matched to these sources using an accumulated name and address history for each cohort member. This protects against establishing false matches and failing to identify true matches. Surveillance efforts routinely identify over 90% of CHDS mothers.
Ovary cancer mortality
Results from linkage to the California Vital Status files were used to append year of death and underlying cause. Since follow-up of the cohort spanned over 50 years, codes for the underlying cause of death from several ICD revisions were used to define ovarian cancer death, including 175 for ICD-7; 183 for ICD-8 and 29 and C56 for ICD-10. Linkages to the National Death Index to evaluate the completeness of mortality surveillance have yielded very few missed cases. California Vital Statistics linkage through 2011 yielded 84 ovarian cancer deaths.
Ovary cancer incidence
Results from linkage to the California Cancer Registry were used to append ovarian cancer diagnosis, year of diagnosis and tumor histology and grade. The California Cancer Registry has established that its cancer coverage is >99% complete after a lag time of about 2 years.24 Life table analyses estimating expected numbers of breast (unpublished) and testicular cancer cases20 show close comparability to observed numbers. Linkage to the California Cancer Registry through 2011 identified 116 cases of ovarian cancer.
Irregular menstruation was defined as self-report or physician report of irregular menstrual cycles, self or physician report of long cycles (>35 days); or physician coded oligomenorrhea, anovulatory cycles or irregular menses.18,25 The time frame captured by this measure refers to an assessment of menstrual cycle irregularity that encompasses the time between menarche and study enrollment. Thus, this measures estimates chronic menstrual irregularity in the CHDS over an extended time period, 14 years on average.
Covariates were derived from information provided during pregnancy interview and from medical records. Race was categorized as Caucasian, African-American, Hispanic, Asian or other. Age was based on reported age at entry to the study. Parity reflects number of live-born births prior to the observed pregnancy. Cigarette smoking during pregnancy was based on self-report at entry. BMI was calculated from weight (kg) divided by height (m) squared, measured at first prenatal visit.
Oral contraceptive use, age at menarche and subfertility were determined from interview and from medical record review. Subfertility was defined as physician-coded infertility or participant-reported “trouble getting pregnant”. Since all women in the cohort were pregnant, these characteristics were assessed as subfertility rather than infertility. Age at menarche was evaluated as early (≤11 years) and late (≥15 years) versus the reference Group (12–14 years). Oral contraceptive use, administration of fertility drugs and subfertility were coded as yes versus no and refer to the period just prior to the observed pregnancy.
Analysis methods
Kaplan-Meier curves of cumulative ovarian cancer survival and disease-free probability were created to examine the association of irregular menstrual cycles. Age at diagnosis and at death or follow-up was calculated based on year of diagnosis, death or last contact; and, year and age at entry. Hazard ratios (HRs) were calculated using Cox proportional hazards models to estimate ovarian cancer associations for irregular cycles accounting for potential confounding by covariates. The proportional hazards assumption was tested by including a cross-product term between irregular cycles and age at event. Analysis was performed using SAS 9.3.
Results
Among the 15,528 mothers enrolled in the CHDS, we identified 116 cases of ovarian cancer and 84 ovarian cancer deaths during the 501 years of cohort surveillance. The median age at diagnosis was 63 years, ranging from 27 to 86 years. The median age at case death was 69 years, ranging from 27 to 90 years. Cases were predominantly classified as epithelial tumors—only one was identified as a sex cord-stromal tumor and an additional four had unknown histological classification. The distribution of histologic subtype in the CHDS was similar to that reported elsewhere4: 45% serous, 7% mucinous, 12% endometrioid, 4% clear cell, 12% unclassified epithelial and 2% miscellaneous. Serous tumors represented 67% of classified histopathologic sub-types. This distribution was not different between women with regular and irregular cycles.
The median age at study pregnancy was 26 years, ranging from 14 to 48 years and most women were multiparous (60%) when they enrolled. The majority was Caucasian (67%), and there was also a large representation of African Americans (24%). The prevalence of women with irregular menstrual cycles was 13% and the prevalence of oral contraceptive use was low, slightly >4%, in keeping with the practice of the time. Table 1 provides the prevalence of these characteristics in the CHDS as well as age-adjusted rates of ovarian cancer incidence and mortality.
Table 1.
Distribution of study variables and rates of ovarian cancer mortality and incidence
| Study variable | Mean (SD) or percent | Ovarian cancer mortality, N=84 | Ovarian cancer incidence, N = 116 | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of case subjects | Person years | Age-adjusted mortality | No. of case subjects | Person years | Age-adjusted incidence | |||||||||||||||
| Rate per 100,0001 | 95% CI | Rate per 100,000] | 95% CI | |||||||||||||||||
| Lower | Upper | Lower | Upper | |||||||||||||||||
| Age at study entry (years)2 | ||||||||||||||||||||
| First quartile, <21 | 19 (1.6) | 6 | 103,552 | 5.79 | 2.12 | 12.61 | 16 | 103,456 | 15.47 | 8.83 | 25.12 | |||||||||
| Second quartile, 22–26 | 24 (1.4) | 15 | 151,938 | 9.87 | 5.52 | 16.28 | 25 | 151,840 | 16.46 | 10.65 | 24.31 | |||||||||
| Third quartile, 27–31 | 29 (1.4) | 23 | 115,636 | 19.89 | 12.60 | 29.85 | 32 | 115,486 | 27.71 | 18.95 | 39.12 | |||||||||
| Fourth quartile, >32 | 36 (3.2) | 40 | 121,123 | 33.02 | 23.59 | 44.97 | 42 | 120,971 | 34.72 | 25.02 | 46.93 | |||||||||
| Age at first pregnancy (years)2 | ||||||||||||||||||||
| First quartile, <19 | 18 (1.2) | 14 | 108,807 | 12.87 | 7.03 | 21.59 | 17 | 108,766 | 15.63 | 9.10 | 25.03 | |||||||||
| Second quartile, 20–22 | 21 (0.8) | 14 | 140,045 | 10.00 | 5.46 | 16.77 | 22 | 139,937 | 15.72 | 9.85 | 23.80 | |||||||||
| Third quartile, 23–25 | 24 (0.8) | 19 | 96,382 | 19.71 | 11.86 | 30.79 | 29 | 96,254 | 30.13 | 20.17 | 43.27 | |||||||||
| Fourth quartile, >26 | 29 (3.5) | 25 | 106,113 | 23.56 | 15.24 | 34.78 | 32 | 105,950 | 30.20 | 20.66 | 42.64 | |||||||||
| Parity (# prior liveborn) | ||||||||||||||||||||
| 0 | 39 | 23 | 189,817 | 17.24 | 9.79 | 27.41 | 39 | 189,669 | 25.95 | 16.84 | 37.52 | |||||||||
| 1–2 | 40 | 36 | 195,235 | 17.57 | 12.11 | 24.58 | 51 | 194,965 | 25.78 | 18.82 | 34.35 | |||||||||
| >3 | 21 | 25 | 104,786 | 11.57 | 7.33 | 17.28 | 25 | 104,709 | 11.92 | 7.54 | 17.84 | |||||||||
| Race/ethnicity | ||||||||||||||||||||
| African American | 24 | 16 | 119,979 | 13.80 | 7.87 | 22.44 | 23 | 119,926 | 19.03 | 12.01 | 28.62 | |||||||||
| Caucasian | 67 | 57 | 319,232 | 17.64 | 13.35 | 22.86 | 80 | 318,800 | 24.87 | 19.71 | 30.96 | |||||||||
| All other | 9 | 9 | 46,816 | 15.65 | 7.07 | 29.86 | 10 | 46,813 | 17.44 | 8.28 | 32.20 | |||||||||
| Age at menarche (years) | ||||||||||||||||||||
| <11 | 21 | 12 | 90,420 | 14.90 | 7.60 | 26.17 | 19 | 90,298 | 21.70 | 12.92 | 34.10 | |||||||||
| 12–14 | 70 | 52 | 304,672 | 16.47 | 12.30 | 21.61 | 73 | 304,335 | 23.48 | 18.39 | 29.53 | |||||||||
| >15 | 9 | 14 | 36,067 | 31.85 | 16.35 | 55.02 | 13 | 36,016 | 31.88 | 15.96 | 56.07 | |||||||||
| Body mass index (kg/m2) | ||||||||||||||||||||
| Underweight, <18.5 | 17.6 (0.7) | 3 | 27,223 | 14.73 | 2.89 | 43.42 | 6 | 27,207 | 23.41 | 7.96 | 52.14 | |||||||||
| Normal weight, 18.5–24.9 | 21.5 (1.6) | 50 | 325,448 | 15.67 | 11.62 | 20.67 | 73 | 325,037 | 22.66 | 17.75 | 28.50 | |||||||||
| Overweight, >25 | 28.5 (3.6) | 18 | 88,028 | 17.12 | 9.78 | 27.58 | 23 | 87,943 | 24.22 | 14.82 | 37.07 | |||||||||
| Gestational weight gain (lbs) | ||||||||||||||||||||
| First quartile, <15.8 | 10.9 (4.7) | 20 | 111,200 | 15.62 | 9.33 | 24.42 | 25 | 111,097 | 21.37 | 13.50 | 32.01 | |||||||||
| Second quartile, 15.8–20.5 | 18.3 (1.4) | 15 | 110,543 | 12.75 | 7.14 | 21.03 | 20 | 110,421 | 17.32 | 10.56 | 26.78 | |||||||||
| Third quartile, 20.6–25.4 | 22.9 (1.4) | 25 | 111,011 | 22.91 | 14.81 | 33.84 | 35 | 110,819 | 31.93 | 22.22 | 44.43 | |||||||||
| Fourth quartile, >25.4 | 30.8 (5.2) | 17 | 110,765 | 18.13 | 10.30 | 29.42 | 25 | 110,659 | 24.94 | 15.84 | 37.24 | |||||||||
| Smoking during pregnancy | ||||||||||||||||||||
| Yes | 36 | 20 | 144,771 | 14.03 | 8.55 | 21.69 | 29 | 144,655 | 19.92 | 13.31 | 28.64 | |||||||||
| No | 64 | 50 | 267,610 | 17.38 | 12.88 | 22.94 | 67 | 267,255 | 24.28 | 18.75 | 30.90 | |||||||||
| Trouble getting pregnant | ||||||||||||||||||||
| Yes | 16 | 11 | 61,199 | 14.82 | 6.69 | 27.65 | 13 | 61,122 | 17.62 | 8.71 | 31.15 | |||||||||
| No | 84 | 52 | 327,434 | 16.46 | 12.27 | 21.60 | 74 | 327,055 | 23.19 | 18.19 | 29.14 | |||||||||
| Oral contraception use | ||||||||||||||||||||
| Yes | 4 | 1 | 14,524 | 7.97 | 0.20 | 44.40 | 5 | 14,501 | 28.40 | 8.18 | 68.34 | |||||||||
| No | 96 | 61 | 352,032 | 16.75 | 12.79 | 21.53 | 81 | 351,606 | 22.74 | 18.04 | 28.30 | |||||||||
| Menstrual cycle variability | ||||||||||||||||||||
| Irregular | 13 | 15 | 63,434 | 24.95 | 13.90 | 41.23 | 18 | 63,363 | 29.30 | 17.29 | 46.41 | |||||||||
| Regular | 87 | 69 | 428,814 | 15.80 | 12.29 | 20.00 | 97 | 428,390 | 22.44 | 18.19 | 27.38 | |||||||||
Rates are age-adjusted according to the age distribution among all CHDS mothers.
Unadjusted, age-specific rates.
Abbreviations: SD: standard deviation; CI: confidence interval.
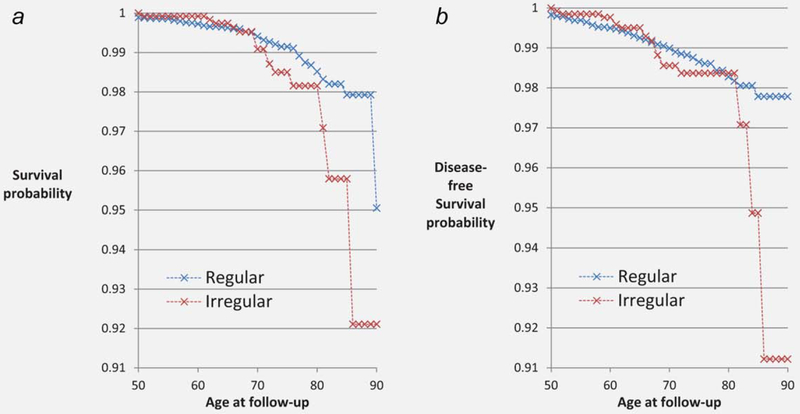
Figure 1 presents cumulative probability for ovarian cancer survival (Fig. 1a) and disease-free status (Fig. 1b) by cycle irregularity in CHDS mothers. Figure 1 shows markedly lower survival for women with irregular cycles beginning at age 70 and increasing with age.
Figure 1.
Cumulative probability of ovarian cancer survival (a) and disease-free status (b) in CHDS mothers, 1959–2011.
Table 2 provides HRs from age, parity and race-adjusted models; and, from fully adjusted models. We found significant and positive age dependence of similar magnitude for both mortality (β = 0.06, p 5 0.04) and incidence (β = 0.07, p = 0.01), demonstrating an increasing association for irregular cycles with advancing age. These associations were unchanged by adjustment for potential explanatory covariates.
Table 2.
Ovarian cancer associations for irregular menstrual cycles
| Age at follow-up (years)1 | Mortality | Incidence | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Lower | Upper | Lower | Upper | |||
| Age, parity and race adjusted | ||||||
| 65 | 1.41 | 0.74 | 2.67 | 1.42 | 0.84 | 2.38 |
| 70 | 1.90 | 1.08 | 3.37 | 1.97 | 1.14 | 3.40 |
| 75 | 2.58 | 1.36 | 4.90 | 2.74 | 1.38 | 5.43 |
| 80 | 3.49 | 1.54 | 7.91 | 3.81 | 1.58 | 9.18 |
| 85 | 4.73 | 1.66 | 13.49 | 5.30 | 1.76 | 15.98 |
| p values for age-dependence | 0.04 | 0.01 | ||||
| Fully adjusted2 | ||||||
| 65 | 1.39 | 0.66 | 2.94 | 1.55 | 0.86 | 2.80 |
| 70 | 1.97 | 1.02 | 3.83 | 2.26 | 1.20 | 4.26 |
| 75 | 2.80 | 1.31 | 6.00 | 3.29 | 1.47 | 7.37 |
| 80 | 3.98 | 1.48 | 10.71 | 4.78 | 1.68 | 13.61 |
| 85 | 5.65 | 1.57 | 20.34 | 6.94 | 1.86 | 25.92 |
| p values for age-dependence | 0.06 | 0.02 | ||||
Irregular menstrual cycle status is dichotomized as observed versus not observed at first study pregnancy. Age at follow-up is modeled as a continuous variable. Interactions between irregular cycles and age were significant and positive for both mortality (β 5 0.06, p 5 0.04) and incidence (β 5 0.07, p 5 0.01).
The fully adjusted models include: age (continuous), race (African-American and Asian versus all other), parity (continuous), subfertility (yes versus no) oral contraceptive use (yes versus no just prior to observed pregnancy), young (<12 years) and old (≥15 years) age at menarche (versus 12–14 years), smoking during pregnancy (yes versus no), gestational weight gain (continuous) and BMI at first prenatal visit (>25 kg/m2). Point estimates for the age-dependent term remain essentially the same regardless of adjustment. In the fully adjusted models, the interaction term is estimated as: β = 0.07 for mortality and β = 0.07 for incidence; and, in the age, parity and race adjusted models, the interaction is estimated as: β = 0.06 for mortality and β = 0.07 for incidence.
Abbreviations: 95% CI, confidence interval; HR, hazard ratio.
There were 30 diagnoses of high grade (grade ≥ 2) serous ovarian cancer and 23 deaths. The association with irregular cycles was substantial and significant for high grade serous death (HR = 3.1; 95% confidence interval [CI] = 1.3, 7.6) and still considerable but marginally significant for high grade serous incidence (HR = 2.1; 95% CI = 0.9, 5.0). This association was not age-dependent.
Discussion
Principal findings
Based on the incessant ovulation hypothesis, which postulates that less frequent ovulatory cycles reduce ovarian cancer risk, we expected that irregular cycles would be protective. Instead we found that irregular cycles were a marker for higher risk of ovarian cancer. Further, the increased risk of ovarian cancer observed for women with irregular cycles was significantly and positively age-dependent, demonstrating substantial risk beginning at age 70, suggesting that the effects of irregular cycles may require the susceptibility of advanced age to enhance risk.
Context
A number of studies have reported ovarian cancer associations with menstrual cycle variability,14,15,26–32 and four studies have reported associations with polycystic ovary syndrome (PCOS)33 (and reviewed in Refs. 16,34). Findings for these studies have been inconsistent showing negative, positive and null associations. Three of these studies observed statistically significant results—two showing decreased risk14,26 and one showing increased risk.13 All three were based on case-control study designs and may have been subject to errors in recall and recall bias, differences in participant age and parity and differences in how irregular cycles was defined, strictly as a specific length or as variable lengths, and whether anovulatory cycles were included.
Strengths
This is the first prospective study showing a significant and positive ovarian cancer association with irregular cycles. Information about menstrual cycle variability was captured at a young age during the peak of reproduction and long before diagnosis of ovarian cancer, minimizing the potential for recall bias. The prevalence of irregular cycles (13%) in the CHDS is comparable to that observed in other studies.35,36 The long follow-up period allowed for a sufficient accumulation of ovarian cancer diagnoses and deaths to examine risk by age. Since all the women in our study had achieved a pregnancy, we were able to rule out lifetime infertility as an explanation of the observed association. Infertility occurs with relatively low frequency (ranging from 11% in 1965% to 6% in 2010)37 and most women are able to achieve a pregnancy, including those with irregular cycles or PCOS, a condition associated with irregular cycles.38 Thus, our results apply to the many women with regular and irregular cycles who do conceive.
We were able to adjust for a number of important confounders measured prospectively from medical records (oral contraceptive use and history of infertility) and/or from self-report (age at menarche, difficulty becoming pregnant) assessed at entry during early pregnancy. Prior studies have demonstrated strong protection against ovarian cancer for oral contraceptive use and higher risk for infertility (reviewed in Refs. 9, 10, 14, 39). Oral contraceptive use (4% in our cohort) and infertility or difficulty becoming pregnant prior to entry (12% in our cohort) were infrequent in the CHDS as would be expected for a cohort of pregnant women, enrolled in the early 1960s just as the Food and Drug Administration approved use of oral contraception for birth control.40 Although CHDS women with irregular cycles were more likely to be subfertile (18% versus 11% in women with regular cycles), there was no evidence of interaction between irregular cycles and either oral contraception or subfertility in predicting ovarian cancer. Neither subfertility nor oral contraceptive use was a predictor; and, adjustment for these variables did not change the association with irregular cycles, likely due to their low prevalence in this cohort. Young age at menarche has also been observed to enhance ovarian cancer risk (reviewed in Ref. 9). However, young age at menarche (≤11 years) was not associated with ovarian cancer risk in the CHDS and did not confound the association with irregular cycles. None of the examined covariates—subfertility, timing of menarche, weight, age, race and parity—explained the observed increased risk for women with irregular cycles.
Limitations
It is possible that unmeasured factors account for the association we observed between menstrual irregularity and ovarian cancer. We do not have information about changes in menstrual cycle regularity or events (e.g., obstetric and gyneco-logic) and behavior after the study period, thus we were unable to examine the influence of these factors known to both increase (hormone replacement therapy [HRT]) and decrease (oral contraceptive pills [OCP] and tubal ligation) risk of ovarian cancer. Some of these unmeasured changes should have enhanced the association we observed rather than explain it, such as oral contraceptives and bilateral oophorectomy. Oral contraceptive use may have increased in this population over time regardless of menstrual cycle length as this form of birth control became increasingly more popular. And, it is likely that oral contraceptive use and possibly bilateral salpingo-oophorectomy (with hysterectomy) would have been more frequent in women with irregular cycles to regulate unpredictable bleeding. If so, the association we observed between irregular cycles and ovarian cancer may be larger than we were able to estimate.
More frequent use of HRT in later life by women with irregular cycles could have contributed to the associations we report, although it is unclear why HRT use would be higher among these women. The increased risk of ovarian cancer associated with HRT use is significant but moderate (HR 5 1.53; CI 1.4–1.66).41 Therefore, the frequency of HRT use would have to differ markedly for women with irregular cycles in order to account for the sizeable association we found.
Our definition of irregular cycles may capture heterogeneous biological and hormonal traits. Further, menstrual cycle status refers to the time between menarche and the observed pregnancy but we were unable to capture change in cycle variation after the study. Misclassifying women who became regular after the observed pregnancy would bias results toward the null. However, despite its lack of specificity, “irregular cycles”, as defined, is a strong predictor of ovarian cancer risk in the CHDS suggesting that cycle regularity in early reproductive life is the relevant risk factor.
Plausibility
It is possible that women with irregular cycles or PCOS have a hormonal profile that favors the neoplastic process through chronic unopposed estrogen, higher androgens or some other mechanism. The protection against ovarian cancer risk observed for oral contraception formulations containing only progestin and progestin-plus-estrogen supports the possibility that unopposed estrogen, rather than low progesterone, could be associated with increased risk.9,12 Alternatively, oral contraceptives may protect by reducing circulating androgen.12 Molecular evidence also suggests that androgen synthesis and action are involved in ovarian cancer etiology.42 Therefore, the increased risk of ovarian cancer among PCOS women who express symptoms of hyperandrogenism42 is consistent with an androgen-related mechanism.
Prevention
This study provides new prospective evidence that woman with irregular menstrual cycles, who are more likely to have PCOS, are at an increased risk of ovarian cancer. If confirmed this will add to the burden of liability for women with PCOS who have higher risk for endometrial cancer,43,44 metabolic syndrome45 and cardiovascular disease (CVD), including prospective evidence of higher CVD risk in this cohort.18 The cooccurrence of increased risk for multiple diseases in women with PCOS complicates the implications for prevention. Oral contraception could possibly provide greater protection against ovarian cancer for women with PCOS compared to the general population adding to the known benefit of oral contraceptive use for reducing risk of endome-trial cancer.46 However, the benefit of oral contraceptives for women with PCOS, must be considered against the cost of potential adverse effects on insulin resistance, glucose intolerance and vascular risk known to be more frequent in these women.45 The relatively higher frequency of CVD in women (lifetime risk for women with optimal risk factor levels is4.1%)47 compared to ovarian cancer (lifetime risk is 1.3%)2 and endometrial cancer (lifetime risk is 2.8%)2 suggests that it is essential to give significant consideration to the individual risk for cardiovascular consequences of OCP use. The risk reduction in ovarian cancer associated with metformin to control diabetes observed in a large case-control study33 may provide a promising alternative for women with PCOS who are at a higher risk of diabetes. However, more research from ongoing prospective clinical trials is needed to confirm whether metformin protects against ovarian cancer among women who do not have diabetes48 and whether there are other unforeseen, harmful consequences of metformin treatment.
Other factors to consider for women with PCOS are intrauterine devices (IUDs) and HRT use. Given the potential increased ovarian cancer risk associated with IUD use either directly or indirectly via increased inflammation,9 and with HRT,41 further research is needed to determine whether women with irregular cycles require special guidelines governing use of these.
Future research
Current screening procedures using trans-vaginal ultrasonography and serial assay for CA-125 have been relatively unsuccessful in reducing ovarian cancer mortality11,49 and have shown limited effectiveness for early detection.11,50,51 Developing a risk algorithm that integrates known risk markers such as germline mutations (e.g., BRCA), with phenotypes (e.g., irregular cycles) and behavioral characteristics (e.g., oral contraceptive and HRT use) may offer a way to improve screening targets until better biomarkers are identified. The identification of higher risk phenotypes such as irregular menstruation may provide opportunities to narrow the search for early biomarkers. However, additional work to understand the mechanisms underlying the association between irregular menstruation and ovarian cancer will be necessary in order to more specifically define the clinical phenotype driving this observed association.
What’s new?
The lack of specific early symptoms in ovarian cancer, aggressiveness of some of the histopathologic subtypes and need for more effective screening and treatment strategies are evidenced by a dismal 5-year survival rate for all stages. While several studies have reported ovarian cancer associations with menstrual cycle variability, the findings are inconsistent. This study provides the first prospective evidence that women with irregular menstrual cycles are at higher risk of ovarian cancer. Discovering high-risk phenotypes such as irregular menstruation creates opportunities to find novel early biomarkers, refine clinical screening protocols and potentially develop new risk reduction strategies for ovarian cancer.
Acknowledgements
We thank the CHDS families for their participation in this study. We acknowledge the late Jacob Yerushalmy who had the foresight to design and implement the CHDS; the late Barbara van den Berg, the second Director of the CHDS, whose steadfast allegiance and tireless efforts were responsible for granting the CHDS longevity; Nickilou Krigbaum for her meticulous assistance with file preparation; and Lauren Zimmermann for assistance with analysis and preparation of tables.
The point of view and conclusions expressed in this study are those of the authors and do not necessarily represent the official position or policies of the funding agency named above. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health, the National Cancer Institute and the Centers for Disease Control and Prevention or their Contractors and Subcontractors, or any of the funders of this research is not intended nor should be inferred
Grant sponsors: Eunice Kennedy Shriver National Institute of Child Health and Development, National Institutes of Health and Department of Health and Human Services; Grant number: HHSN275201100020C; Grant sponsor: California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885 (the collection of cancer incidence data used in this study); Grant sponsor: the National Cancer Institute’s Surveillance, Epidemiology and End Results Program awarded to the Cancer Prevention Institute of California; Grant number: HHSN261201000140C; Grant sponsor: the National Cancer Institute’s Surveillance, Epidemiology and End Results Program awarded to the University of Southern California; Grant number: HHSN261201000035C; Grant sponsor: National Cancer Institute’s Surveillance, Epidemiology and End Results Program awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries; Grant number: HHSN261201000034C; Grant sponsor: awarded to the California Department of Public Health; Grant number: U58DP003862-01
Abbreviations:
- BMI
body mass index
- CHDS
Child Health and Development Studies
- CI
confidence interval
- CVD
cardiovascular disease
- HR
hazard ratio
- HRT
hormone replacement therapy
- ICD
International Classification of Diseases
- OCP
oral contraceptive pill
- PCOS
polycystic ovarian syndrome
Footnotes
The authors report no conflict of interest.
References
- 1.American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society, 2015. [Google Scholar]
- 2.National Cancer Institute. Bethesda M, http://seer.cancer.gov/statfacts/html/ovary.html. Seer cancer statistics factsheets: ovary cancer. 1975–2012.
- 3.Stewart SL. Ovarian cancer incidence: current and comprehensive statistics. Atlanta, Georgia: INTECH Open Access Publisher, 2012. [Google Scholar]
- 4.Rosen DG, Yang G, Liu G, et al. Ovarian cancer: pathology, biology, and disease models. Front Biosci (Landmark Ed) 2009;14:2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson BK, Conner MG, Landen CN. The role of the fallopian tube in the origin of ovarian cancer. Am J Obstet Gynecol 2013;209:409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler M, Fotopoulou C, Meyer T. The molecular fingerprint of high grade serous ovarian cancer reflects its fallopian tube origin. Int J Mol Sci 2013;14:6571–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurman RJ, Shih I-M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer. Shifting the paradigm. Hum Pathol 2011;42: 918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh T, Casadei S, Lee MK, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci 2011;108: 18032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Permuth-Wey J, Besharat A, Sellers TA. Epidemiology of ovarian cancer: an update In: Farghaly SA, ed. Advances in diagnosis and management of ovarian cancer. New York: Springer, 2014. 1–21. [Google Scholar]
- 10.Schorge JO, Modesitt SC, Coleman RL, et al. SGO white paper on ovarian cancer: etiology, screening and surveillance. Gynecol Oncol 2010; 119:7–17. [DOI] [PubMed] [Google Scholar]
- 11.Clarke-Pearson DL. Screening for ovarian cancer. N Engl J Med 2009;361:170–7. [DOI] [PubMed] [Google Scholar]
- 12.Modugno F, Laskey R, Smith AL, et al. Hormone response in ovarian cancer: time to reconsider as a clinical target? Endocr Relat Cancer 2012;19: R255–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schildkraut JM, Schwingl PJ, Bastos E, et al. Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obst Gynecol 1996;88: 554–9. [DOI] [PubMed] [Google Scholar]
- 14.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst 1998;90:1774–86. [DOI] [PubMed] [Google Scholar]
- 15.Merritt MA, De Pari M, Vitonis AF, et al. Reproductive characteristics in relation to ovarian cancer risk by histologic pathways. Hum Reprod 2013;28:1406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barry JA, Azizia MM, Hardiman PJ. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2014;20:748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Berg BJ, Christianson RE, Oechsli FW. The California child health and development studies of the school of public health, University of California at Berkeley. Paediatr Perinat Epidemiol 1988;2:265–82. [DOI] [PubMed] [Google Scholar]
- 18.Wang ET, Cirillo PM, Vittinghoff E, et al. Menstrual irregularity and cardiovascular mortality. J Clin Endocrinol 2011;96:E114–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension 2010; 56:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohn BA, Cirillo PM, Christianson RE. Prenatal DDT exposure and testicular cancer: a nested case-control study. Arch Environ Occup Health 2010;65:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohn BA, Cirillo PM, Christianson RE, et al. Placental characteristics and reduced risk of maternal breast cancer. J Natl Cancer Inst 2001;93: 1133–40. [DOI] [PubMed] [Google Scholar]
- 22.Cohn BA, Wolff MS, Cirillo PM, et al. Timing of DDT exposure and breast cancer before age 50. Epidemiology 2002;13:S197. [Google Scholar]
- 23.Whittemore AS, Cirillo PM, Feldman D, et al. Prostate specific antigen levels in young adulthood predict prostate cancer risk: results from a cohort of black and white Americans. J Urol 2005;174:872–6; discussion 876. [DOI] [PubMed] [Google Scholar]
- 24.Kwong SL, Perkins CI, Morris CR, et al. Cancer in California, 1988–1999. Sacramento, CA: California Department of Public Health, California Cancer Registry, 2001. [Google Scholar]
- 25.Wang ET, Cirillo PM, Kao C, et al. Birth weight and childhood growth in daughters of women with irregular menstrual cycles. Gynecol Endocrinol 2013;29:615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parazzini F, La Vecchia C, Negri E, et al. Menstrual factors and the risk of epithelial ovarian cancer. J Clin Epidemiol 1989;42:443–8. [DOI] [PubMed] [Google Scholar]
- 27.Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst 1983;71:717–21. [PubMed] [Google Scholar]
- 28.Purdie D, Green A, Bain C, et al. Reproductive and other factors and risk of epithelial ovarian cancer: an Australian case-control study. Int J Cancer 1995;62:678–84. [DOI] [PubMed] [Google Scholar]
- 29.Kvåle G, Heuch I, Nilssen S, et al. Reproductive factors and risk of ovarian cancer: a prospective study. Int J Cancer 1988;42:246–51. [DOI] [PubMed] [Google Scholar]
- 30.Mori M, Kiyosawa H, Miyake H. Case–control study of ovarian cancer in Japan. Cancer 1984;53: 2746–52. [DOI] [PubMed] [Google Scholar]
- 31.Shu XO, Brinton LA, Gao YT, Yuan JM. Population-based case-control study of ovarian cancer in Shanghai. Cancer Res 1989;49:3670–4. [PubMed] [Google Scholar]
- 32.Chen Y, Wu PC, Lang JH, et al. Risk factors for epithelial ovarian cancer in Beijing, China. Int J Epidemiol 1992;21:23–9. [DOI] [PubMed] [Google Scholar]
- 33.Bodmer M, Becker C, Meier C, et al. Use of metformin and the risk of ovarian cancer: a case- control analysis. Gynecol Oncol 2011;123:200–4. [DOI] [PubMed] [Google Scholar]
- 34.Chittenden B, Fullerton G, Maheshwari A, et al. Polycystic ovary syndrome and the risk of gynaecological cancer: a systematic review. Reprod Biomed Online 2009;19:398–405. [DOI] [PubMed] [Google Scholar]
- 35.Franks S, Adams J, Mason H, et al. Ovulatory disorders in women with polycystic ovary syndrome. Clin Obstet Gynaecol 1985;12:605–32. [PubMed] [Google Scholar]
- 36.Taponen S, Martikainen H, Jarvelin M-R, et al. Hormonal profile of women with self-reported symptoms of oligomenorrhea and/or hirsutism: Northern Finland birth cohort 1966 study. J Clin Endocrinol Metab 2003;88:141–7. [DOI] [PubMed] [Google Scholar]
- 37.Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982–2010: data from the national survey of family growth. Natl Health Stat Report 2013;67:1–19. [PubMed] [Google Scholar]
- 38.Koivunen R, Pouta A, Franks S, et al. Fecundability and spontaneous abortions in women with self-reported oligo-amenorrhea and/or hirsutism: Northern Finland birth cohort 1966 study. Hum Reprod 2008;23:2134–9. [DOI] [PubMed] [Google Scholar]
- 39.Hennessy E, Alberman E. The effects of own fetal growth on reported hypertension in parous women aged 33. Int J Epidemiol 1997;26:562–70. [DOI] [PubMed] [Google Scholar]
- 40.Watkins ES. How the pill became a lifestyle drug: the pharmaceutical industry and birth control in the United States since 1960. Am J Public Health 2012;102:1462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cancer CGoESoO. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. The Lancet 2015;385:1835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson DA, Simitsidellis I, Collins F, et al. Evidence of androgen action in endometrial and ovarian cancers. Endocr Relat Cancer 2014;21: T203–18. [DOI] [PubMed] [Google Scholar]
- 43.Haoula Z, Salman M, Atiomo W. Evaluating the association between endometrial cancer and polycystic ovary syndrome. Hum Reprod 2012;27: 1327–31. [DOI] [PubMed] [Google Scholar]
- 44.Dumesic DA, Lobo RA. Cancer risk and pcos. Steroids 2013;78:782–5. [DOI] [PubMed] [Google Scholar]
- 45.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med 2005;352:1223–36. [DOI] [PubMed] [Google Scholar]
- 46.Mueck AO, Seeger H, Rabe T. Hormonal contraception and risk of endometrial cancer: a systematic review. Endocr Relat Cancer 2010;17: R263–71. [DOI] [PubMed] [Google Scholar]
- 47.Berry JD, Dyer A, Cai X, et al. Lifetime risks of cardiovascular disease. N Engl J Med 2012;366: 321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Febbraro T, Lengyel E, Romero IL. Old drug, new trick: repurposing metformin for gynecologic cancers? Gynecol Oncol 2014;135:614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen L, Cardenas-Goicoechea S, Gordon P, et al. Biomarkers for early detection of ovarian cancer. Women’s Health (Lond Engl) 2013;9:171. [DOI] [PubMed] [Google Scholar]
- 50.Menon U, Kalsi J, Jacobs I. The UKCTOCS experience—reasons for hope? Int J Gynecol Cancer 2012;22:S18–S20. [DOI] [PubMed] [Google Scholar]
- 51.Menon U, Gentry-Maharaj A, Hallett R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK collaborative trial of ovarian cancer screening (UKCTOCS). Lancet Oncol 2009;10:327–40. [DOI] [PubMed] [Google Scholar]