Abstract
Transplant recipients have an elevated risk of skin cancer, with a 65 - 250 fold increase in squamous cell carcinoma. Usage of the immunosuppressant cyclosporine A (CsA) is associated with the development of skin cancer. We hypothesized that the increased incidence of skin cancer was due to the action of CsA within keratinocyte mitochondria where it can inhibit mitochondrial permeability transition pore (MPTP) opening. Normally, MPTP opening is induced by oxidative stress such as that caused by UV light and leads to cell death, thereby eliminating a cell that has been exposed to genotoxic insult. However, in the presence of CsA, damaged cells may survive and consequently form tumors. To test this hypothesis, we treated keratinocytes with levels of CsA used therapeutically in transplant patients and assessed their viability following UVA-irradiation. CsA prevented cell death by inhibiting MPTP opening, even though the levels of oxidative stress were increased markedly. Nim811, a non-immunosuppressive drug that can block the MPTP had a similar effect while the immunosuppressive drug tacrolimus that does not interact with the mitochondria had no effect. These findings suggest that CsA may promote skin cancer in transplant patients by allowing keratinocyte survival under conditions of increased genotoxic stress.
Keywords: Cyclosporine A, oxidative stress, skin cancer, UVA, organ transplantation, cell death
1. Introduction
Currently there are over 1 million people living with solid organ transplants with numbers rising steadily (Randle 2004). Cyclosporine A (CsA), the catalyst for the field of organ transplantation, has proven to be effective in preventing graft rejection and has increased patient survival following transplantation. However, organ transplant recipients are now confronting the complications associated with long-term immunosuppressive therapy. Transplant recipients have an overall incidence of de novo malignancies greater than non-transplant individuals, with the incidence of squamous cell carcinoma (SCC) dramatically elevated a reported 65 to 250-fold (Hartevelt, Bavinck et al. 1990; Penn 1998; Jensen, Hansen et al. 1999; Lindelof, Sigurgeirsson et al. 2000; Euvrard, Kanitakis et al. 2003). These SCCs are also associated with a younger age of onset, a greater tendency to metastasize, and increased aggressiveness (reviewed in (Berg and Otley 2002; Euvrard, Kanitakis et al. 2003)). As in the general population, the primary risk factor for the development of SCC is exposure to ultraviolet radiation (UVR). SCCs usually develop in sun-exposed areas of the body and incidence is highest in countries with a high UV-index. Although the contribution of individual immunosuppressive drugs to SCC development in transplant recipients is unclear, epidemiological studies have shown that immunosuppressive regimens which include CsA are associated with a higher rate of skin cancer (Jensen, Hansen et al. 1999). However, the mechanism by which CsA contributes to malignancy remains unclear, and the risk of SCC in individuals taking newer immunosuppressive drugs is unknown due to the latency of skin cancer presentation following transplantation.
CsA exerts its action through binding to cyclophilins. The immunosuppressive activity of CsA is exerted through the complex formed between CsA and Cyclophilin A (CypA) (Walsh, Zydowsky et al. 1992). The CsA-CypA complex binds and inhibits the function of calcineurin. Calcineurin inhibition prevents the expression of multiple cytokine genes involved in T-cell activation (Stepkowski 2000). Cyclophilin D (CypD), located on the matrix side of the inner mitochondrial membrane, is a critical regulator of mitochondrial permeability transition pore (MPTP) opening (Baines, Kaiser et al. 2005; Basso, Fante et al. 2005; Nakagawa, Shimizu et al. 2005). The MPTP is a complex, multiprotein conductance channel which opens in response to oxidative stress (Zamzami, Larochette et al. 2005). MPTP opening results in mitochondrial swelling, collapse of mitochondrial membrane potential, and outer mitochondrial membrane rupture with subsequent release of proapoptotic proteins into the cytosol (Lemasters, Nieminen et al. 1998)). Upon CsA binding to CypD, opening of the MPTP is inhibited (Halestrap, Connern et al. 1997). Inhibition of MPTP opening by CsA has been reported to suppress cell death in various models of apoptotic and necrotic cell death (Zamzami, Larochette et al. 2005).
MPTP opening is particularly sensitive to increases in reactive oxygen species (ROS) (Lemasters, Nieminen et al. 1998). Environmental exposure of the skin to UVR makes it a primary target for UVR-generated ROS. The UVA component (320nm-400nm) of solar radiation penetrates the basal layer of the epidermis and into the dermis where it generates ROS which cause myriad cellular effects including oxidation of proteins, lipids, and nucleic acids (Sander, Chang et al. 2002; Matsumura and Ananthaswamy 2004; Sander, Chang et al. 2004). Evidence has shown UVA-induced ROS-mediated cellular damage to be associated with human skin carcinogenesis. ROS-mediated premutagenic photoproducts, 8-oxo-7,8-dihydro-2’-deoxyguanosine adducts, which are induced by wavelengths in the UVA range were found in the basal layer of human SCCs (Agar, Halliday et al. 2004). Additionally, F2-Isoprostanes (F2-IsoPs), stable molecules produced by cellular lipid peroxidation, have been found to be increased in UVA-irradiated skin and human skin cancer specimens suggesting that they may also serve as a marker of UVA-induced cellular damage in skin cancer (Belli, Amerio et al. 2005). Recently, He et al. confirmed the potential of UVA to induce skin cancer by demonstrating that repetitive UVA-irradiation of HaCaT keratinocytes at environmentally relevant doses can induce malignant transformation (He, Pi et al. 2006). UVR-induced oxidative damage is also associated with mitochondrial dysfunction and characteristic mitochondrial DNA deletions in the skin (Berneburg, Grether-Beck et al. 1999; Gonzalez Maglio, Paz et al. 2005)). However, data have shown that treatment with CsA can protect cells with pathogenic mtDNA mutations from oxidant-induced cell death (Wong and Cortopassi 1997).
In the present study, we examine the contribution of CsA to cutaneous carcinogenesis by evaluating its ability to suppress UVA-induced cell death in human keratinocytes. Although originally thought to contribute to cancer via impairment of immune surveillance, emerging evidence suggests that CsA increases the likelihood of a cell becoming cancerous independent of its immunosuppressive action. Data indicate that CsA may promote tumorigenesis directly by stimulating TGF-β production (Hojo, Morimoto et al. 1999), increasing angiogenesis (Guba, von Breitenbuch et al. 2002), and impairing DNA damage repair (Herman, Weinstein et al. 2001; Yarosh, Pena et al. 2005). Since inhibition of MPTP opening by CsA is implicated in suppressing apoptosis, a fundamental safeguard against neoplasia, it is conceivable that antiapoptotic effects of CsA may be involved in tumorigenesis. Our data show that clinically relevant doses of CsA suppress UVA-induced cell death even in the presence of increased UVA-induced oxidative stress as a result of CsA’s inhibition of MPTP opening. Our results provide a potential mechanism for the increased incidence of skin cancer in transplant recipients taking CsA, and suggest that therapeutic strategies for immunosuppression that do not include CsA or pharmacological strategies to modulate MPTP opening in patients taking CsA may diminish the burden of skin cancer in transplant recipients.
2. Materials and methods
2.1. Cell Culture and Treatment of Cells with CsA, NIM811, and Tacrolimus
HaCaT keratinocytes, a non-transformed, spontaneously immortalized human keratinocyte cell line derived from adult back skin, were obtained from the Vanderbilt Skin Diseases Research Center Phenotype Core (Boukamp, Petrussevska et al. 1988). We chose to perform the study in the HaCaT keratinocyte cell line due of the difficulty of maintaining and performing experiments with primary keratinocytes which senesce rapidly in culture. HaCaT keratinocytes were maintained in DMEM containing 10% FBS and 50 μg/ml penicillin and 50 μg/ml streptomycin and grown in a humidified CO2 incubator at 37°C with 5% CO2. CsA and NIM811 were provided by Novartis (Basel, Switzerland), tacrolimus was from BioAge Pharmaceuticals, Inc. (San Diego, CA). All drugs were solubilized in 95% ethanol and added to the cells at a final concentration in medium that did not exceed 0.1% of solvent. The final CsA concentrations in culture medium of 125 nM and 250 nM were chosen to approximate the target blood serum levels of CsA in transplant recipients. Final concentrations of NIM811 and tacrolimus in culture medium of 125 nM and 250 nM were chosen to provide comparisons to CsA treatment.
2.2. UVA-Irradiation
Cells were grown in culture dishes with removable lids and exposed to either single or serial doses of UVA from our panel (Ultralite Inc.) of ultraviolet bulbs (six F72T12-BL-HO UVA) that deliver broadband UVA with peak output between 340 and 368 nm. The UVA dose was monitored with a UVA meter (National Biological Corporation, Beachwood, OH). The UVA-dose of 12 J/cm2 was chosen to mimic the UVA exposure of 15-30 minutes in midday sun in a temperate climate. Control samples were mock irradiated in the same conditions.
2.3. Flow Cytometry
A total of 10,000 events were counted for each sample using a FACScan flow cytometer (Becton–Dickinson, Franklin Lakes, NJ) equipped with a 488 nm argon laser with appropriate filters. Data were collected from gated cells of appropriate size. Data were analyzed using Cellquest software (Becton-Dickinson).
2.4. Cell Viability and Cell Death Measurements Following UVA-Irradiation
HaCaT keratinocytes were seeded at 200,000 cells per well in 6-well dishes. Cells were treated with media containing CsA, NIM811, tacrolimus, or vehicle for 24 h prior to the first irradiation. Cells were irradiated with 12 J/cm2 of UVA at 12 h intervals for a total of 3 irradiations. The irradiation dose of 12 J/cm2 took between 17-18 minutes. We chose to repeatedly irradiate the cells with physiologic doses to of UVA to simulate chronic UVA exposure in skin and also to eliminate thermal stimulation from long irradiations. In initial studies, medium was removed and cells were washed and then covered with a thin layer of PBS. To eliminate the loss of cells from washing steps, cells were allowed to remain in the drug containing media during all irradiations. After the last irradiation, cells were incubated for 4 h, media was collected, and then cells were trypsinized and added to collected media. Cells were centrifuged, washed, stained with Ann V-PE and 7-AAD (BD Pharmingen, San Diego, CA) and analyzed by flow cytometry. Cells positive for both Ann V-PE and 7-AAD, were defined as necrotic, and cells negative for 7-AAD and positive for Ann V-PE were defined as apoptotic. Cells negative for both markers were considered viable.
2.5. Toxicity and Proliferation Assays
Toxic effects of CsA were assessed by plating cells at 200,000 cells per well in 6-well dishes and allowing cells to grow in untreated media or media containing vehicle or CsA over a range of doses and measuring viability at 24 h, 48 h, and 72 h. Viability measurements, as determined by the exclusion of Ann V-PE and 7-AAD, were performed by flow cytometry. For proliferation studies, cells were seeded at 5,000 cells per well in a 96-well plates. Cells were allowed to attach, then untreated media or media containing vehicle or CsA over a range of doses was added to cells for 24 h, 48 h, or 72 h. At 72 h cell proliferation was assessed using the CyQUANT NF Cell Proliferation Assay Kit (Molecular Probes, Eugene, OR). Cell proliferation was measured by incorporation of the CyQUANT fluorescent dye into cellular DNA using a fluorescent microplate reader.
2.6. Mitochondrial Membrane Potential Measurements
HaCaT keratinocytes were seeded at 1 × 106 cells per 100 mm dish and treated with media containing CsA, NIM811, tacrolimus, or vehicle for 24 h prior to irradiation. Cells were irradiated with 12 J/cm2 of UVA at 12 h intervals for a total of 3 irradiations and remained in drugged media throughout. Following irradations, cells were incubated for 4 h, media collected, and cells were trypsinized and added to collected media. Then cells were centrifuged, resuspended in PBS and loaded with JC-1 (Molecular Probes) at a final concentration of 2 μM and incubated at 37°C, 5% CO2 for 30 min. As controls, non-irradiated cells were loaded with JC-1 or simultaneously loaded with JC-1 and CCCP (carbonyl cyanide 3-chlorophenylhydrazone), a mitochondrial depolarizing agent. After incubation, cells were centrifuged, and cell pellets were resuspended in 200 μl of PBS and analyzed for JC-1 fluorescence by flow cytometry.
2.7. Assessment of Mitochondrial Permeability Transition Pore Activity
MPTP opening was measured using the MitoProbe Transition Pore Assay Kit (Molecular Probes). HaCaT keratinocytes were seeded at 1 × 106 cells per 100 mm dish and treated with media containing CsA, NIM811, tacrolimus, or vehicle. Cells were maintained in drug containing media for 24 h, then the media was removed and replaced with warm Hank’s balanced salt solution containing calcium (HBSS/Ca) and cells were irradiated with a single dose of UVA at 12 J/cm2. Following irradiation, cells were trypsinized and resuspended in HBSS/Ca and loaded with calcein-AM and cytosolic calcein fluorescence quencher CoCl2 for 15 min at 37°C according to manufacturer’s instructions. In parallel experiments, cells were loaded with calcein-AM alone or calcein-AM, CoCl2, and the ionophore, ionomycin (which causes MPTP opening) for 15 min at 37°C. Cells were centrifuged, washed, and cell pellets were resuspended in 200 μl of HBSS/Ca and analyzed for mitochondrial calcein fluorescence by flow cytometry analysis. Fluorescence intensity was calculated as a percentage by gating upon the positive and negative controls.
2.8. Determination of Oxidant Injury
MitoSOX Red mitochondrial superoxide indicator (Molecular Probes) was used to detect superoxide, as a general measure of cellular oxidative stress, in the mitochondria of live cells. HaCaT keratinocytes were seeded at 200,000 per well in 6-well dishes and treated with media containing CsA, NIM811, tacrolimus, or vehicle. Cells were maintained in drug containing media for 24 h prior to UVA-irradiation. Just prior to irradiation, the media was removed and replaced with Hank’s balanced salt solution containing calcium and magnesium (HBSS/Ca/Mg) and cells were irradiated with a single dose of UVA at 12 J/cm2. After irradiation, HBSS/Ca/Mg was removed and HBSS/Ca/Mg containing 5 μM MitoSox Red was added to the cells. Cells were incubated in the dark at 37°C for 30 min, washed with warm PBS, trypsinized, centrifuged and resuspended in PBS, and MitoSox Red fluorescence was analyzed by flow cytometry. To quantify F2-IsoPs, HaCaT keratinocytes were seeded at 5 × 106 per well in 150 mm plates. Cells were treated with media containing CsA, Nim811, tacrolimus, or vehicle for 24 h prior to the first irradiation. Cells were given 12 J/cm2 of UVA 3 times at 12 h intervals. Following the last irradiation cells were trypsinized, centrifuged, and resuspended in 1 ml of PBS. The cells were then rapidly frozen in liquid nitrogen and stored at −70°C until processed. To quantify F2-IsoPs in cells, the phospholipids were first extracted from the cells and subjected to alkaline hydrolysis to release free F2-IsoPs. Free F2-IsoPs were then quantified using a gas chromatography-negative ion chemical ionization- mass spectrometry (GC-NICI-MS) approach employing a stable isotope dilution (detailed protocol described in Milne, Sanchez et al. 2007).
2.9. Statistical analyses
Continuous variables were summarized by the mean and standard deviation when normally distributed. The Shapiro-Wilk test was used to determine if continuous variables were normally distributed. One-way analysis-of-variance (ANOVA) models were constructed to compare values for continuous variables across multiple categories. Bartlett’s test was used to confirm for equal variances prior to each ANOVA analysis. Subset comparison analysis utilized the Bonferroni multiple-comparison test. The level of significance for all analyses was set at 0.05. All statistical analyses were done using STATA 9.0 (College Station, TX).
3. Results
3.1. CsA Suppresses Keratinocyte Cell Death Following UVA-Irradiation
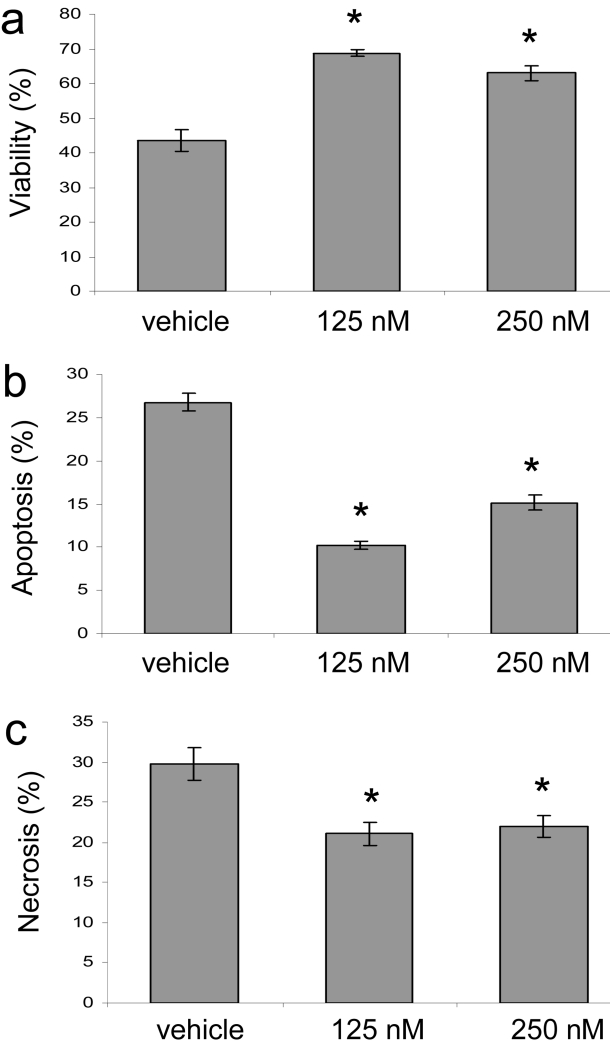
To determine the effect of CsA on keratinocyte cell death, we UVA-irradiated HaCaT keratinocytes incubated with doses of CsA at therapeutic levels (target blood serum levels) similar to those used in organ transplantation of 125 nM and 250 nM (Vincenti, Monaco et al. 2003; Ekberg, Grinyo et al. 2007). To simulate chronic UVA damage in human skin, cells were repeatedly exposed to environmentally relevant UVA light doses. Flow cytometric analysis showed that CsA-treatment significantly prevented cell death at therapeutic levels of 125 nM and 250 nM (Fig. 1a). To discern whether the cells were undergoing apoptotic or necrotic cell death we measured Annexin V-PE (Ann V-PE) staining as a marker of apoptosis and 7-AAD staining for necrosis. Both apoptosis and necrosis were significantly suppressed at CsA doses of 125 nM and 250 nM, with a more significant effect at 125 nM (Fig. 1b and c). It is possible that some of the ‘necrotic’ cells underwent apoptotic death but had moved into end stage apoptosis and were stained with both markers. Thus, in our UVA irradiation scheme, CsA suppressed both apoptotic and necrotic cell death at low, therapeutic doses. These low doses were not toxic to cells incubated for up to 72 h, but CsA was cytotoxic at higher concentrations (Supplemental Fig. 1a).
Fig. 1.
CsA represses UVA-induced cell death. HaCaT cells treated with CsA at 125nM and 250nM or vehicle (95% ethanol) were subjected to repetitive UVA irradiation (3 irradiations at 12 J/cm2 at 12 hour intervals) and analyzed by flow cytometry. (a) Viable cells excluded Ann V-PE and 7-AAD. (b) Apoptotic cells stained with Ann V-PE and, (c), necrotic cells were stained with Ann V-PE and 7-AAD or 7-AAD. Data represent means ± s.e.m. n=3. Asterisks indicate significant differences compared to vehicle treated cells, *P < 0.05.
To examine if CsA influenced cell growth, HaCaT keratinocytes were left untreated or treated with media containing CsA for 24 h, 48 h, or 72 h out of the 72 h the cells were allowed to grow. Cell proliferation was measured based upon total DNA content. Our results showed that at 125 nM and 250 nM of CsA there was no difference in cell proliferation at 24 h, 48 h, or 72 h, confirming that the cell death effects were not influenced by proliferative effects of CsA (Supplemental Fig. 1b). Higher doses of CsA inhibited cell proliferation.
3.2. CsA and Nim811 Suppress UVA-Induced Cell Death through CypD-Binding
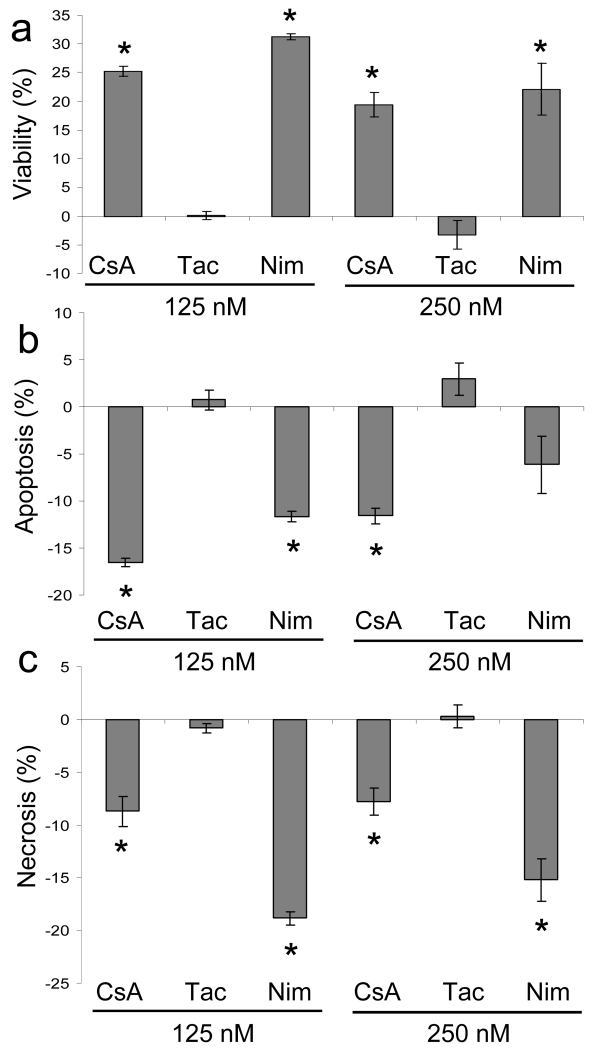
To determine if the cytoprotective effects were attributable to CsA’s inhibition of MPTP opening by binding to CypD at the mitochondria, we tested the ability of the non-immunosuppressive CypD-binding CsA analog, NIM811, to alter the sensitivity of HaCaT keratinocytes to UVA-induced cell death. Nim811 has been shown to inhibit MPTP opening in isolated mitochondria from brain and liver tissue (Waldmeier, Feldtrauer et al. 2002; Hansson, Mattiasson et al. 2004). To confirm that the cell death effects were not the result of calcineurin inhibition, we also treated cells with tacrolimus. Tacrolimus, a commonly used immunosuppressant, exerts its action via calcineurin inhibition, identical to CsA, but does not bind CypD. Cells were treated with drugs, subjected to repetitive UVA-irradiation, and evaluated for apoptotic and necrotic responses. Our data show that cells treated with the CypD-binding drugs, CsA and NIM811, exhibit less cell death at both 125 nM and 250 nM (Fig. 2a). Tacrolimus treated cells showed no difference in viability. Apoptosis measurements showed that CsA suppressed apoptotic cell death at 125 nM and 250 nM, NIM811 suppressed cell death at 125 nM but not at 250 nM, and tacrolimus had no effect on apoptosis (Fig. 2b). Necrotic cell death analysis showed that CsA and NIM811 inhibited necrotic cell death at both 125 nM and 250 nM, whereas tacrolimus had no effect (Fig. 2c). Thus, our results indicate that the critical events involved in CsA’s cytoprotective effect are occurring as a result of its CypD-binding properties.
Fig. 2.
CypD-binding drugs repress UVA-induced cell death. HaCaT cells were subjected to repetitive UVA irradiation in the presence of vehicle, CsA, tacrolimus (Tac), or NIM811 (NIM) at 125nM and 250nM and analyzed by flow cytometry. The data are expressed as values relative to vehicle, which is set at 0. (a) Viable cells excluded Ann V-PE and 7-AAD. (b) Apoptotic cells stained with Ann V-PE and, (c), necrotic cells stained with Ann V-PE and 7-AAD or 7-AAD. Data represent means ± s.e.m. n=3. Asterisks indicate significant differences compared to vehicle treated cells, *P < 0.05.
3.3. MMP (Mitochondrial Membrane Potential) and MPTP activity following UVA-Irradiation are Modulated by CypD-Binding drugs
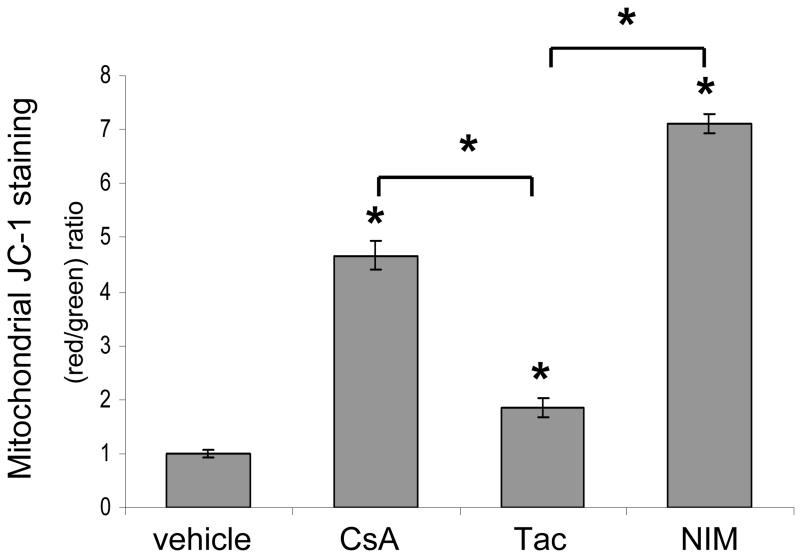
To analyze the mitochondrial effects of CypD-binding compounds, we measured MMP and MPTP activity in HaCaT keratinocytes treated with CypD-binding drugs, CsA and NIM811, and non-CypD-binding tacrolimus following UVA-irradiation. Prolonged MPTP opening results in dissipation of the MMP, so it can be used as a marker of MPTP activity. We used the fluorescent dye JC-1, a specific indicator of MMP, to evaluate the ability of CypD-binding drugs to preserve MMP in cells subjected to repetitive UVA-irradiation. JC-1 accumulates in mitochondria where it forms aggregates in cells with high MMP and emits a red fluorescence. When mitochondrial membrane potential collapses there is a shift in JC-1 fluorescence from red to green. Unirradiated control cells showed high MMP with red JC-1 staining and treatment with CCCP, a mitochondrial membrane disrupter, caused a complete shift from red to green fluorescence (data not shown). In UVA-irradiated cells, our JC-1 flow cytometric measurements showed that treatment with CsA, NIM811, or tacrolimus all increased the ratio of red to green fluorescence as compared to vehicle, but that the ratio of red to green fluorescence was dramatically increased in cells treated with CypD-binding drugs, NIM811 and CsA, as compared to tacrolimus treated cells (Fig. 3). Our data show that CsA’s interaction with CypD and inhibition of MPTP opening prevented the dissipation of MMP. The ability of the CypD-binding drugs, CsA and NIM811, to sustain or to allow restoration of MMP in keratinocytes with high levels of oxidative stress induced by UVA light suggests that cells which would normally undergo cell death due to MPTP opening would be able to survive under toxic conditions.
Fig. 3.
Mitochondrial membrane potential is maintained in UVA-irradiated cells treated with CypD-binding drugs. HaCaT cells were treated with vehicle and CsA, Tac, or NIM at 125 nM and repetitively irradiated with UVA. Mitochondrial depolarization was assessed as the fluorescence shift of JC-1 from red to green by flow cytometry analysis. Data are represented as a ratio of red to green fluorescence. Data represent means ± s.e.m. n=3. Asterisks directly above error bars indicate significant differences compared to vehicle treated cells, asterisks above brackets indicate significant differences between drug groups, *P < 0.05.
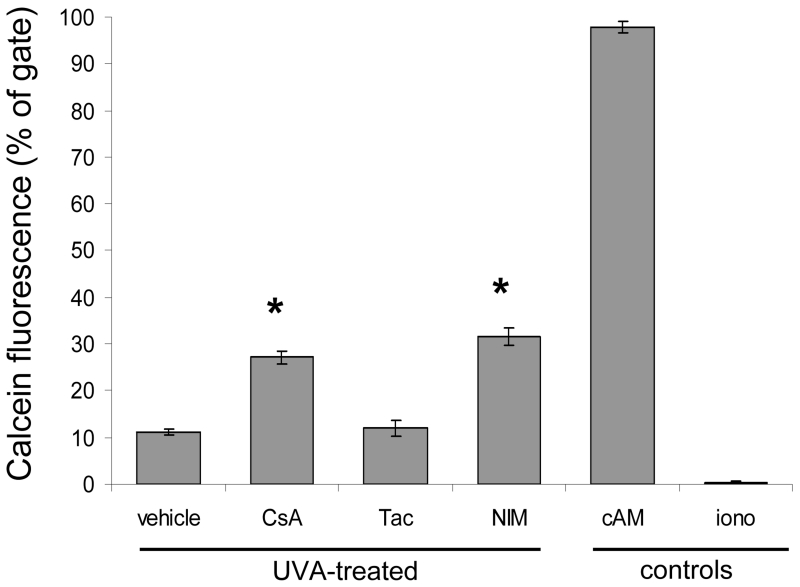
To further evaluate MPTP opening in cells treated with CypD binding drugs we monitored MPTP activity using calcein AM (cAM). Following UVA-irradiation at 12 J/cm2, HaCaT keratinocytes were incubated with the acetoxymethyl (AM) ester of calcein which passively diffuses across the plasma membrane and accumulates in cytosolic compartments, including mitochondria. Intracellularly, cytosolic esterases cleave the acetoxylmethyl esters which liberate the fluorescent dye calcein. Calcein fluorescence is retained inside the cytosolic and mitochondrial compartments. Upon MPTP opening, calcein moves out of the mitochondria into the cytosol. We measured MPTP activity by specifically examining calcein fluorescence in the mitochondria. Cytosolic calcein fluorescence was quenched by cobalt chloride (CoCl2). Flow cytometric analysis showed that in UVA-irradiated HaCaT cells treated with CypD-binding drugs, CsA and NIM811, there was less loss of calcein fluorescence, as compared to vehicle and tacrolimus treated cells (Fig. 4). In our assay, maintenance of calcein fluorescence indicates less active MPTP opening, as more calcein is being trapped in the mitochondria. Inhibition of MPTP opening by CsA may allow mitochondria to resist depolarization and prevent proapoptotic molecules from exiting the mitochondria.
Fig. 4.
Mitochondrial Permeability Transition Pore activity is diminished in UVA-irradiated cells treated with CypD-binding drugs. The MPTP was monitored by quantifying the fluorescence of calcein in the mitochondria of HaCaT cells by flow cytometry. Cells were treated with vehicle, CsA, Tac, or NIM at 125 nM and irradiated with a single dose of 12 J/cm2 of UVA. The cells were then loaded with calcein AM (cAM) and CoCl2 (cytosolic calcein quencher) to determine the calcein fluorescence in the mitochondria. Control cells were loaded with cAM alone cAM, CoCl2, and ionomycin (iono) which triggers pore opening and loss of mitochondrial calcein fluorescence. Data represent means ± s.e.m. n=3. Asterisks indicate significant differences compared to vehicle treated cells, *P < 0.05.
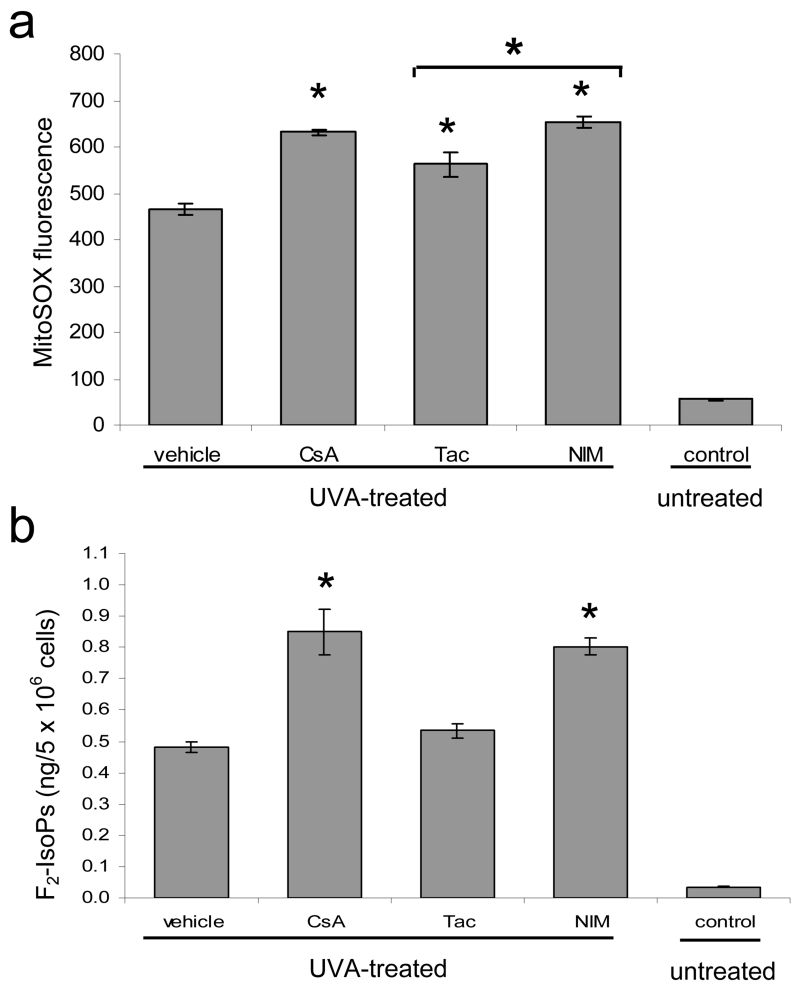
3.4. UVA-Induced Mitochondrial Superoxide and F2-IsoPs are elevated in keratinocytes treated with CypD-Binding Drugs
Because UVA light is known to stimulate intracellular ROS, we examined the ability of CypD-binding drugs to modulate the amount of mitochondrial superoxide and F2-isoprostanes (F2-IsoPs), stable molecules produced by cellular lipid peroxidation, in UVA-irradiated HaCaT keratinocytes. In mitochondria, free electrons released during oxidative phosphorylation interact with molecular oxygen to form superoxide anion. Increases in superoxide anion, the predominant ROS in mitochondria, are associated with many pathological conditions (Lenaz 2001). We used the fluoroprobe, MitoSox, for the specific detection of superoxide in the mitochondria of cells. MitoSox localizes to the mitochondria where it is oxidized by superoxide and subsequently fluoresces. Cells were treated with CsA, NIM811, and tacrolimus and subjected to an acute exposure of UVA at 12 J/cm2 of UVA. Although our results showed an increase in mitochondrial superoxide levels in CsA and NIM811 treated cells as compared to tacrolimus treated cells, the difference was not significant between CsA and tacrolimus. This finding indicates that CypD-binding may causes either an increase in mitochondrial superoxide production or a decrease in the clearing of mitochondrial superoxide species (Fig. 5a). Furthermore, all drugs exhibit higher levels of mitochondrial superoxide than vehicle treated cells indicating CypD binding cannot solely account for increased mitochondrial superoxide levels.
Fig. 5.
Oxidative stress is elevated in UVA-irradiated cells treated with CypD-binding drugs. (a) HaCaT cells were treated with vehicle and CsA, Tac, or NIM811 at 125 nM and irradiated with a single dose of 12 J/cm2 of UVA. Control cells were not treated with UVA or drug. Data represent the amount of mitochondrial superoxide as measured by the linear mean of MitoSox Red fluorescence by flow cytometry analysis. (b) F2-IsoPs were quantified in HaCaT cells treated with vehicle and CsA, Tac, and NIM at 125 nM and subjected to repetitive UVA irradiation as described in the methods section. Control cells were not treated with UVA or drug. Data represent means ± s.e.m. n=3. Asterisks directly above error bars indicate significant differences compared to vehicle treated cells, asterisks above brackets indicate significant differences between drug groups, *P < 0.05.
To examine a stable marker of oxidative damage we quantified F2-IsoPs. Quantification of F2-IsoPs in human cells reflects their endogenous production and provides an index of cellular lipid peroxidation and oxidant stress (Milne, Sanchez et al. 2007). F2-IsoPs were quantified in cells treated with CsA, NIM811, and tacrolimus, and repeatedly UVA-irradiated. Cells treated with CsA and NIM811 exhibited higher levels of F2-IsoPs than vehicle and tacrolimus treated cells post-irradiation (Fig. 5b). In unirradiated cells incubated with CsA, NIM811, and tacrolimus there was no significant differences in F2-IsoPs levels. Using F2-IsoPs as a biomarker of oxidative stress, our results suggest that CsA may cause increased oxidative damage in response to UVA-irradiation by inhibiting oxidative stress induced MPTP opening and cell death.
4. Discussion
Our results demonstrate that CsA suppresses cell death in UVA-irradiated cultured HaCaT keratinocytes as a result of CypD-binding and inhibition of MPTP opening. Interestingly, these cytoprotective effects were achieved at CsA doses within the therapeutic range used in immunosuppression. Furthermore, CsA-treated cells exhibit higher levels of F2-IsoPs following UVA-irradiation suggesting that cells are evading cell death even in the face of increased oxidative damage. These results indicate that mitochondrial effects of CsA may directly contribute to skin tumorigenesis, and provide a model whereby UVA and CsA, combined, may promote skin cancer in transplant recipients.
Other investigators have sought to define the contribution of CsA to malignancy by exploring the ability of CsA to alter cellular behavior independent of its immunosuppressive effects. Data have shown that CsA directly contributes to increased aggressiveness of cancer cells. Hojo et al. reported that CsA treatment of a human pulmonary adenocarcinoma cell line resulted in marked morphological changes and induced anchorage-independent growth by a cell-autonomous mechanism (Hojo, Morimoto et al. 1999). In vivo results of increased tumor development in immunoincompetent mice injected with CsA-treated cells corroborated that CsA directly promoted increased tumor development (Hojo, Morimoto et al. 1999). Based upon the finding of increased TGF-ß levels, the authors proposed that CsA-induced TGF-ß production is a likely mechanism involved in neoplasia. However, other reports have not identified an increase in TGF-ß in the media of CsA treated cells, indicating that TGF-ß effects may be cell specific and that other mechanisms may be involved in CsA’s carcinogenic effects (Tanaka, Takahara et al. 2002). Duncan et al. found that mice which were immunosuppressed by CsA and repeatedly UVB-irradiated developed more malignant tumors than mice treated with other immunosuppressants, including tacrolimus, and showed increased tumor angiogenesis and inflammation (Duncan, Wulff et al. 2007). Another study found that CsA treatment promoted tumor growth in a liver metastasis model by stimulating angiogenesis (Guba, von Breitenbuch et al. 2002). These studies strongly promote a role for CsA in contributing to cancer progression, but do not elucidate the mechanism whereby a non-cancer cell would become malignant. We propose that CsA’s inhibition of MPTP opening and subsequent suppression of cell death is potential mechanism whereby a damaged cell may evade cell death and initiate carcinogenesis.
Although the role of CsA in the inhibition of cell death has been extensively studied in certain models of cell injury and disease, there has been little investigation in CsA-mediated cell death in skin cells. The cytoprotective effects of CsA have primarily been viewed as having a potential therapeutic benefit in decreasing cardiac ischemia/reperfusion injury and protecting neurons in pathological conditions such as traumatic brain injury and Alzheimer’s disease and Parkinson’s disease (Matsuura, Kabuto et al. 1997; Friberg and Wieloch 2002; Kim, He et al. 2003). However, in skin cells exposed to UVA, cell death is a necessary safeguard against neoplasia and its suppression is likely to have pathological consequences. Evidence shows that CsA is capable of inhibiting apoptosis in UVA-exposed Jurkat cells (Godar 1999) and inhibiting the repair of cyclobutane pyrimidine dimers in UVB-irradiated HaCaT keratinocytes (Yarosh, Pena et al. 2005). Additionally, in a SCC cell line CsA blocked mitochondrial translocation of p53 and apoptosis induced by CP-31398, a molecule shown to reduce the growth of UVB-induced skin tumors in mice (Tang, Zhu et al. 2007).
Our data demonstrate pharmacologically that CypD-binding and subsequent inhibition of MPTP opening is the mechanism by which CsA-treated skin cells evade UVA-induced cell death. In healthy cells, the MPTP normally fluxes between open and closed states (Petronilli, Miotto et al. 1999). However, Ca2+ overload and oxidative stress cause prolonged MPTP opening which is responsible for the mitochondrial permeability transition (MPT) (Petronilli, Miotto et al. 1999). The MPT causes mitochondrial swelling and dissipation of MMP resulting in cell death (Zamzami, Larochette et al. 2005). CypD has been implicated in MPT regulated cell death because the MPT is inhibited by CsA, but the specific role of CypD in cell death remained unclear until recent genetic studies. The creation of CypD null mice confirmed that CypD is the pharmacological target of CsA in mitochondria and corroborate that MPTP opening plays a critical role in cell death regulation (Baines, Kaiser et al. 2005; Basso, Fante et al. 2005; Nakagawa, Shimizu et al. 2005). CypD null fibroblasts were more resistant to Ca2+ and hydrogen peroxide-induced cell death, but not from cell death induced by common apoptotic stimuli such as TNF-α, Bax, or staurosporine (Baines, Kaiser et al. 2005; Nakagawa, Shimizu et al. 2005). The investigators concluded that CypD-dependent (CsA-inhibitable) MPTP opening regulates Ca2+ and oxidative stress induced cell death but not apoptotic cell death (Baines, Kaiser et al. 2005; Nakagawa, Shimizu et al. 2005).
However, physiologic stimuli such as UVA are known to induce both apoptotic and necrotic cell death mediated by oxidative stress (Morley, Rapp et al. 2006). In our study, UVA-induced apoptotic and necrotic death were suppressed by CsA, suggesting that MPTP opening may be involved in both pathways. In pathological conditions such as ischemia/reperfusion injury, MPTP inhibition via CsA has also been demonstrated to provide cytoprotection from both death fates (Kim, He et al. 2003). One proposal to explain the inhibition of both apoptosis and necrosis is that the MPT is a shared component both cell death pathways and that mode of cell death varies depending upon the magnitude of the cellular insult, duration of pore opening, and maintenance of cytosolic ATP levels (Kim, He et al. 2003). Our observations of CsA cytoprotection of HaCaT keratinocytes in response to UVA support a shared role for the MPT.
Our results also indicate that mitochondrial effects of CsA increase UVA-induced oxidant damage. Cells treated with CypD-binding drugs, CsA and Nim811, exhibited significantly elevated F2-IsoPs post-irradiation. Due to CsA’s inhibition of MPTP opening it is likely that cells are tolerating higher levels of UV-induced ROS and accruing increased oxidative damage. Data have shown that transplant recipients with SCC have elevated levels of urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine, a biomarker of oxidative stress, as compared to transplant recipients without SCC indicating that oxidative burden is a risk factor for the development of these cancers (Cooke, Osborne et al. 2007).
The ability of CsA to modulate the immune response has broadened its usage beyond transplantation to treat certain autoimmune diseases and inflammatory skin conditions, such as psoriasis. Treatment for psoriasis, a disease of the skin and joints, may include CsA and PUVA (psoralen + UVA) photochemotherapy. Long-term studies have shown an increase in SCCs in patients treated with both PUVA and CsA (Marcil and Stern 2001). Our identification of a mitochondrial mechanism by which CsA inhibits UVA-induced cell death and increases oxidative stress in HaCaT keratinocytes has strong implications into the development of aggressive SCCs in both OTRs and psoriasis patients taking CsA. Our findings support the concept that non-CypD binding immunosuppressants such as tacrolimus or rapamycin may offer fewer complications of skin cancers in OTRs due to their lack of interaction with CypD and MPTP opening. In the future, studies to pharmacologically control CypD-CsA binding or to chemically sensitize MPTP opening may allow for novel treatment mechanisms of skin cancers including the possible use of photodynamic therapy to induce cell death in cancer cells.
Supplementary Material
Supplementary Fig. 1. Modulation of cellular proliferation and toxicity by CsA. HaCaT cells were treated with vehicle and CsA at 0.15, 0.30, 1.0, and 10.0 μg/mL for 24 h, 48 h, or 72 h. (a) In the proliferation assay, all cells were allowed to grow for 72 h. Cell number was determined by fluorescent dye binding to cellular DNA and was measured in relative fluorescent units (RFU). Data represent the amount of time the cells were exposed to CsA out of 72 h and are expressed as percentages relative to untreated cells. (b) In toxicity studies, HaCaT cells viability was measured at 24 h, 48 h, and 72 h. Data represent means + s.e.m. n=3. Asterisks indicate significant differences compared to vehicle treated cells, *P < 0.05.
Acknowledgements
This work was supported by a VA Merit Review and Career Development Award (J.E.S.) and NIH grant AR0501552 (J.E.S.). F2-IsoPs quantification was supported by grants GM15431, ES00267, and DK48831 (J.D.M.). Vanderbilt Skin Diseases Research Center is supported by NIH Center Grant AR41943. We would like to thank the Skin Diseases Research Center and the VA Flow Cytometry Resource Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agar, et al. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc Natl Acad Sci U S A. 2004;101:4954–4959. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Basso, et al. Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- Belli, et al. Elevated 8-isoprostane levels in basal cell carcinoma and in UVA irradiated skin. Int J Immunopathol Pharmacol. 2005;18:497–502. doi: 10.1177/039463200501800309. [DOI] [PubMed] [Google Scholar]
- Berg, Otley Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. J Am Acad Dermatol. 2002;47:1–17. doi: 10.1067/mjd.2002.125579. [DOI] [PubMed] [Google Scholar]
- Berneburg, et al. Singlet oxygen mediates the UVA-induced generation of the photoaging-associated mitochondrial common deletion. J Biol Chem. 1999;274:15345–15349. doi: 10.1074/jbc.274.22.15345. [DOI] [PubMed] [Google Scholar]
- Boukamp, et al. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke, et al. Evidence that oxidative stress is a risk factor for the development of squamous cell carcinoma in renal transplant patients. Free Radic Biol Med. 2007;43:1328–1334. doi: 10.1016/j.freeradbiomed.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Duncan, et al. Clinically relevant immunosuppressants influence UVB-induced tumor size through effects on inflammation and angiogenesis. Am J Transplant. 2007;7:2693–2703. doi: 10.1111/j.1600-6143.2007.02004.x. [DOI] [PubMed] [Google Scholar]
- Ekberg, et al. Cyclosporine sparing with mycophenolate mofetil, daclizumab and corticosteroids in renal allograft recipients: the CAESAR Study. Am J Transplant. 2007;7:560–570. doi: 10.1111/j.1600-6143.2006.01645.x. [DOI] [PubMed] [Google Scholar]
- Euvrard, et al. Skin cancers after organ transplantation. N Engl J Med. 2003;348:1681–91. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- Friberg, Wieloch Mitochondrial permeability transition in acute neurodegeneration. Biochimie. 2002;84:241–250. doi: 10.1016/s0300-9084(02)01381-0. [DOI] [PubMed] [Google Scholar]
- Godar UVA1 radiation triggers two different final apoptotic pathways. J Invest Dermatol. 1999;112:3–12. doi: 10.1046/j.1523-1747.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez Maglio, et al. Skin damage and mitochondrial dysfunction after acute ultraviolet B irradiation: relationship with nitric oxide production. Photodermatol Photoimmunol Photomed. 2005;21:311–317. doi: 10.1111/j.1600-0781.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- Guba, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128–35. doi: 10.1038/nm0202-128. [DOI] [PubMed] [Google Scholar]
- Halestrap, et al. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem. 1997;174:167–72. [PubMed] [Google Scholar]
- Hansson, et al. The nonimmunosuppressive cyclosporin analogs NIM811 and UNIL025 display nanomolar potencies on permeability transition in brain-derived mitochondria. J Bioenerg Biomembr. 2004;36:407–413. doi: 10.1023/B:JOBB.0000041776.31885.45. [DOI] [PubMed] [Google Scholar]
- Hartevelt, et al. Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation. 1990;49:506–509. doi: 10.1097/00007890-199003000-00006. [DOI] [PubMed] [Google Scholar]
- He, et al. Chronic UVA irradiation of human HaCaT keratinocytes induces malignant transformation associated with acquired apoptotic resistance. Oncogene. 2006;25:3680–3688. doi: 10.1038/sj.onc.1209384. [DOI] [PubMed] [Google Scholar]
- Herman, et al. Effect of cyclosporin A on DNA repair and cancer incidence in kidney transplant recipients. J Lab Clin Med. 2001;137:14–20. doi: 10.1067/mlc.2001.111469. [DOI] [PubMed] [Google Scholar]
- Hojo, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- Jensen, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol. 1999;40:177–186. doi: 10.1016/s0190-9622(99)70185-4. [DOI] [PubMed] [Google Scholar]
- Kim, et al. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun. 2003;304:463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- Kim, et al. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med. 2003;3:527–535. doi: 10.2174/1566524033479564. [DOI] [PubMed] [Google Scholar]
- Lemasters, et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- Lenaz The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52:159–64. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- Lindelof, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol. 2000;143:513–519. [PubMed] [Google Scholar]
- Marcil, Stern Squamous-cell cancer of the skin in patients given PUVA and ciclosporin: nested cohort crossover study. Lancet. 2001;358:1042–1045. doi: 10.1016/S0140-6736(01)06179-7. [DOI] [PubMed] [Google Scholar]
- Matsumura, Ananthaswamy Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Matsuura, et al. Initial cyclosporin A but not glucocorticoid treatment promotes recovery of striatal dopamine concentration in 6-hydroxydopamine lesioned mice. Neurosci Lett. 1997;230:191–194. doi: 10.1016/s0304-3940(97)00515-6. [DOI] [PubMed] [Google Scholar]
- Milne, et al. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- Morley, et al. UVA-induced apoptosis studied by the new apo/necro-Comet-assay which distinguishes viable, apoptotic and necrotic cells. Mutagenesis. 2006;21:105–14. doi: 10.1093/mutage/gel004. [DOI] [PubMed] [Google Scholar]
- Nakagawa, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Penn Occurrence of cancers in immunosuppressed organ transplant recipients. Clin Transpl. 1998:147–158. [PubMed] [Google Scholar]
- Petronilli, et al. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J. 1999;76:725–734. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle The historical link between solid-organ transplantation, immunosuppression, and skin cancer. Dermatol Surg. 2004;30:595–597. doi: 10.1111/j.1524-4725.2004.30142.x. [DOI] [PubMed] [Google Scholar]
- Sander, et al. Role of oxidative stress and the antioxidant network in cutaneous carcinogenesis. Int J Dermatol. 2004;43:326–335. doi: 10.1111/j.1365-4632.2004.02222.x. [DOI] [PubMed] [Google Scholar]
- Sander, et al. Photoaging is associated with protein oxidation in human skin in vivo. J Invest Dermatol. 2002;118:618–625. doi: 10.1046/j.1523-1747.2002.01708.x. [DOI] [PubMed] [Google Scholar]
- Stepkowski Molecular targets for existing and novel immunosuppressive drugs. Expert Rev Mol Med. 2000;2000:1–23. doi: 10.1017/S1462399400001769. [DOI] [PubMed] [Google Scholar]
- Tanaka, et al. A novel immunosuppressive drug, FTY720, prevents the cancer progression induced by cyclosporine. Cancer Lett. 2002;181:165–171. doi: 10.1016/s0304-3835(01)00799-6. [DOI] [PubMed] [Google Scholar]
- Tang, et al. CP-31398 restores mutant p53 tumor suppressor function and inhibits UVB-induced skin carcinogenesis in mice. J Clin Invest. 2007;117:3753–3764. doi: 10.1172/JCI32481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti, et al. Multicenter randomized prospective trial of steroid withdrawal in renal transplant recipients receiving basiliximab, cyclosporine microemulsion and mycophenolate mofetil. Am J Transplant. 2003;3:306–311. doi: 10.1034/j.1600-6143.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- Waldmeier, et al. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol Pharmacol. 2002;62:22–29. doi: 10.1124/mol.62.1.22. [DOI] [PubMed] [Google Scholar]
- Walsh, et al. Cyclosporin A, the cyclophilin class of peptidylprolyl isomerases, and blockade of T cell signal transduction. J Biol Chem. 1992;267:13115–13118. [PubMed] [Google Scholar]
- Wong, Cortopassi mtDNA mutations confer cellular sensitivity to oxidant stress that is partially rescued by calcium depletion and cyclosporin A. Biochem Biophys Res Commun. 1997;239:139–145. doi: 10.1006/bbrc.1997.7443. [DOI] [PubMed] [Google Scholar]
- Yarosh, et al. Calcineurin inhibitors decrease DNA repair and apoptosis in human keratinocytes following ultraviolet B irradiation. J Invest Dermatol. 2005;125:1020–1025. doi: 10.1111/j.0022-202X.2005.23858.x. [DOI] [PubMed] [Google Scholar]
- Zamzami, et al. Mitochondrial permeability transition in apoptosis and necrosis. Cell Death Differ. 2005;12:1478–80. doi: 10.1038/sj.cdd.4401682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Modulation of cellular proliferation and toxicity by CsA. HaCaT cells were treated with vehicle and CsA at 0.15, 0.30, 1.0, and 10.0 μg/mL for 24 h, 48 h, or 72 h. (a) In the proliferation assay, all cells were allowed to grow for 72 h. Cell number was determined by fluorescent dye binding to cellular DNA and was measured in relative fluorescent units (RFU). Data represent the amount of time the cells were exposed to CsA out of 72 h and are expressed as percentages relative to untreated cells. (b) In toxicity studies, HaCaT cells viability was measured at 24 h, 48 h, and 72 h. Data represent means + s.e.m. n=3. Asterisks indicate significant differences compared to vehicle treated cells, *P < 0.05.