Abstract
Introduction:
Concurrent prescribing of opioids and benzodiazepines is discouraged by evidence-based clinical guidelines because of the known risks of taking these medications in combination.
Methods:
This study analyzed concurrent opioid and benzodiazepine prescribing in 9 states using the 2015 Prescription Behavior Surveillance System, a multistate database of de-identified prescription drug monitoring program data. Concurrent prescribing rates were examined among individuals with both an opioid and a benzodiazepine prescription. Among patients with concurrent prescribing, total days of opioid supply, daily dosage of opioids, and total days of concurrent prescriptions were examined. Analyses were stratified by whether concurrent prescribing was from a single prescriber or multiple prescribers. Opioid prescribing and concurrent opioid and benzodiazepine prescribing rates were examined by age and sex. Analyses were conducted in 2018.
Results:
Among 19,977,642 patients that were prescribed an opioid, 21.6% (4,324,092) were also prescribed a benzodiazepine, of which 54.9% (2,375,219) had concurrent prescriptions. More than half of patients with concurrent opioids and benzodiazepines received prescriptions from 2 or more distinct prescribers. Mean total opioid days, daily opioid dosage, and days of concurrent prescribing were higher among patients when multiple prescribers were involved compared with concurrent prescriptions from the same prescriber. Concurrent prescribing was more common among adults aged ≥50 years and female patients.
Conclusions:
Public health interventions are needed to reduce concurrent prescribing of opioids and benzodiazepines. Evidence-based guidelines can help reduce concurrent prescribing when one prescriber is involved, and utilization of prescription drug monitoring programs and improved care coordination could help address concurrent prescribing when multiple prescribers are involved.
INTRODUCTION
Although opioid analgesics are the most common drugs involved in prescription drug overdose deaths, benzodiazepines often play a contributing role, and are involved in approximately one third of all opioid overdose deaths in the U.S.1 When taken together, opioids and benzodiazepines can result in synergistic respiratory depression and substantially increase the risk of drug overdose.1–5 In an effort to decrease the use of opioids and benzodiazepines together, the U.S. Food and Drug Administration added a boxed warning to the drug labeling of prescription opioids and benzodiazepines in 2016.6
Despite reductions in opioid prescribing in the U.S.,7 concurrent prescribing of opioids and benzodiazepines remains common.4,8–10 From 2002 to 2014, the proportion of opioid recipients in the U.S. who were dispensed a benzodiazepine concurrently rose by 41%, with approximately half of patients receiving both prescriptions from the same prescriber on the same day.8 Further, a retrospective analysis of privately insured claims data from 2001 to 2013 found that concurrent opioid and benzodiazepine prescribing increased from 9% in 2001 to 17% in 2013.4 Nearly 3% of all emergency room encounters receiving an opioid prescription also received a benzodiazepine prescription.10 In addition, a study using national laboratory test results indicated concurrent use of opioids and benzodiazepines in more than 25% of patients in 2015.11
The trend in concurrent opioid and benzodiazepine prescribing also parallels a rising trend in emergency department visits and drug overdose deaths in which both opioids and benzodiazepines are implicated.1 From 2004 to 2011, the rate of emergency department visits involving nonmedical use of both opioid analgesics and benzodiazepines rose from 11.0 to 34.2 visits per 100,000, and during this same period, drug overdose deaths involving both opioids and benzodiazepines increased from 0.6 to 1.7 per 100,000.1
Although previous studies provide information on the trends and frequency of concurrent opioid and benzodiazepine prescribing,8 limited information exists on how frequently these overlapping episodes result from a single provider versus multiple providers. In addition, limited information exists on the patient characteristics, frequency, dosage, and duration of concurrent prescribing.12 Such information could be helpful in understanding the patterns of concurrent prescribing of opioids and benzodiazepines and help inform prevention efforts. Implications for prevention efforts could be different based on the number of prescribers involved in overlapping opioid/benzodiazepine episodes. For example, evidence-based guidelines, such as the “CDC Guideline for Prescribing Opioids for Chronic Pain” (Centers for Disease Control [CDC] Prescribing Guideline),13 which recommends a high degree of caution when prescribing an opioid in combination with a benzodiazepine, could be more helpful in reducing concurrent prescribing when one prescriber is involved, as opposed to multiple providers unaware of each other’s prescriptions. Meanwhile, prescription drug monitoring programs (PDMPs), electronic databases used to monitor the prescribing and dispensing of controlled substance, and care coordination may be more effective when concurrent prescribing is the result of multiple prescribers. Understanding the characteristics of patients with an overlapping episode can help identify effective interventions and better target efforts to reduce the concurrent prescribing of opioids and benzodiazepines. The objectives of this study are the following: (1) to examine the number of distinct prescribers involved in concurrent opioid and benzodiazepine prescribing episodes; (2) to investigate the days of opioid use, daily dosage of opioids, and duration of concurrent prescribing; and (3) to analyze the demographic characteristics of patients with concurrent prescriptions.
METHODS
Data from PDMPs were obtained through the Prescription Behavior Surveillance System (PBSS) at Brandeis University. PBSS is a longitudinal database of de-identified PDMP data from multiple states that have collected statewide PDMP data. PBSS was created to serve as an early warning public health surveillance tool and a database for evaluation of state and local policies and interventions aimed at improving the prescribing of controlled substances. CDC determined this study to be exempt from human subject regulations and IRB approval.
The PBSS contains information regarding all in-state filled controlled substance prescriptions that are received by a state PDMP. Information includes de-identified patient ID, de-identified prescriber ID, de-identified pharmacy ID, dispensing date, days of supply, quantity dispensed, drug name, National Drug Code, patient birthday, patient sex, and geographic information of the patient and the pharmacy. Availability of this information varies by state. Brandeis University calculated the total dosage and daily dosage of all opioid prescriptions as morphine milligram equivalents (MMEs). MME conversion factors have been published elsewhere.14
Study Population
The current study includes 2015 PDMP data from 9 of the 11 states participating in PBSS: California, Delaware, Florida, Idaho, Kentucky, Maine, Ohio, Virginia, and West Virginia. Two states were excluded because prescription information needed (e.g., prescriber ID) for the analysis was not available. Data from 2015 were used, as they were the most current data available for analysis. Out-of-state residents who filled opioid or benzodiazepine prescriptions in 1 of the 9 states were excluded from the analysis.
Measures
Individuals with at least one opioid prescription were identified. Overall opioid prescribing rates were calculated using 2015 population estimates from the U.S. Census Bureau for each of the 9 states. Among individuals with an opioid prescription, the percentage with at least one benzodiazepine prescription in 2015 was examined. Opioid and benzodiazepine prescriptions were identified using their National Drug Code codes. Buprenorphine products, typically used for substance use disorder treatment, were excluded from the analysis.7 Methadone used for the treatment of opioid use disorder at methadone clinics is not available in PDMP data.
The rate of concurrent prescribing was examined among individuals who had both an opioid and a benzodiazepine prescription. Concurrent prescribing was defined as having any opioid and benzodiazepine prescriptions, which overlapped for at least 7 consecutive days.8,15–17 Concurrent prescriptions did not have to be dispensed on the same day. De-identified prescriber ID was used to identify the source of prescriptions. If an individual had concurrent prescriptions from the same prescriber during the year, the source of concurrent prescriptions was defined as one prescriber; otherwise, the source of concurrent prescriptions was defined as multiple prescribers (i.e., different prescribers).
Three characteristics of concurrent opioid and benzodiazepine prescriptions were examined among individuals who had concurrent opioid and benzodiazepine prescriptions: total days of opioid supply, daily dosage of opioids, and total days of concurrent prescribing. Total days of opioid supply were calculated as the total number of calendar days during the year that an individual had an opioid supply. Individuals with overlapping opioid prescriptions were assumed to take them concurrently. Thus, given the definition, total days of opioid supply could only be equal to or greater than the total days of concurrent prescribing, and it could not exceed 365 days. Daily dosage of opioids was defined as the total dosage of opioid prescriptions of an individual divided by total days of opioid supply. High daily dosage of opioids was defined as daily dosage ≥90 MMEs per day.13 Total days of concurrent prescribing was defined as total days of overlapping opioid and benzodiazepine prescriptions. When an individual had more than one occurrence of concurrent prescribing during the year, total days of concurrent prescribing was obtained by adding up each occurrence. If multiple opioid prescriptions overlapped with a single benzodiazepine or multiple benzodiazepines prescriptions overlapped with a single opioid, an overlapping day was only counted once to avoid double counting.
Statistical Analysis
Means were calculated for total days of opioid supply, daily dosage of opioids, and total days of concurrent prescriptions stratified by prescriber sources of concurrent opioid and benzodiazepine prescriptions (one prescriber versus multiple prescribers). To test for differences across the measures, t-tests were used. Rates of opioid prescribing and concurrent opioid and benzodiazepine prescribing were examined by age and sex. All analyses were conducted in SAS, version 9.4, and Stata, version 14.2. Analyses were conducted in 2018.
RESULTS
In 2015, across the 9 states, 20.0 million patients (22.3% of their total population) were dispensed an opioid (Table 1). The rate of opioid prescriptions varied at the state level from 20.8% in Delaware to 24.9% in Virginia and West Virginia. In 2015, among patients prescribed an opioid, 21.6% were also prescribed at least one benzodiazepine. The rate of benzodiazepine prescriptions among opioid patients ranged from 19.5% in Virginia to 26.5% in West Virginia. Among patients prescribed both an opioid and a benzodiazepine, 54.9% had concurrent prescriptions. The rate of concurrent prescribing varied at the state level from 46.1% in Idaho to 65.1% in West Virginia.
Table 1.
Rate of Opioid and Benzodiazepine Prescriptions by State, 2015
| Variable | California | Delaware | Florida | Idaho | Kentucky | Maine | Ohio | Virginia | West Virginia | 9 states |
|---|---|---|---|---|---|---|---|---|---|---|
| State population | 39,144,818 | 945,934 | 20,271,272 | 1,654,930 | 4,425,092 | 1,329,328 | 11,613,423 | 8,382,993 | 1,844,128 | 89,611,918 |
| Population with at least one opioid prescription | ||||||||||
| Number | 8,153,894 | 197,234 | 4,728,318 | 356,806 | 1,070,725 | 298,516 | 2,680,067 | 2,089,185 | 402,888 | 19,977,642 |
| Percentage | 21.5 | 20.8 | 23.3 | 21.5 | 24.2 | 22.4 | 23.1 | 24.9 | 24.9 | 22.3 |
| Patients prescribed an opioid, population with at least one benzodiazepine | ||||||||||
| Number | 1,677,950 | 41,553 | 1,143,916 | 73,749 | 234,021 | 66,996 | 572,275 | 406,814 | 106,818 | 4,324,092 |
| Percentage | 20.6 | 21.1 | 24.2 | 20.7 | 21.9 | 22.4 | 21.4 | 19.5 | 26.5 | 21.6 |
| Patients prescribed both, population with concurrent prescriptions | ||||||||||
| Number | 948,189 | 20,652 | 635,356 | 33,976 | 144,909 | 32,532 | 289,546 | 200,563 | 69,496 | 2,375,219 |
| Percentage with concurrent prescriptions, % | 56.5 | 49.7 | 55.5 | 46.1 | 61.9 | 48.6 | 50.6 | 49.3 | 65.1 | 54.9 |
Note: State population estimates were obtained from the U.S. Census Bureau.
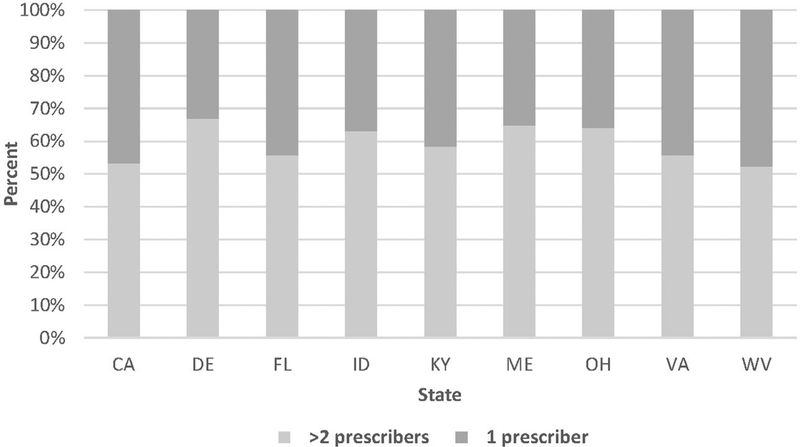
In each of the 9 examined states, more than half of patients with concurrent opioids and benzodiazepines received prescriptions from 2 or more distinct prescribers (Figure 1). The involvement of 2 or more prescribers in a concurrent episode was most common in Delaware (66.9%), Maine (64.8%), and Ohio (64.1%), and least common in West Virginia (52.4%) and California (53.2%).
Figure 1.
Distribution of concurrent opioid and benzodiazepine prescribing by the number of prescribers involved. CA, California; DE, Delaware; FL, Florida; ID, Idaho; KY, Kentucky; ME, Maine; OH, Ohio; VA, Virginia; WV, West Virginia.
The characteristics of concurrent opioid and benzodiazepine prescriptions varied by state and the number of prescribers involved. Across the 9 states, mean total days of opioid supply were higher when the concurrent prescriptions were from 2 or more prescribers (153.1 days) compared with when 1 prescriber was involved (118.8 days, p<0.001). Kentucky had the highest number of mean total days of opioid supply across both categories, an average of 218.8 days when the concurrent prescriptions were from 2 or more prescribers and 201.9 days when 1 prescriber was involved.
The mean daily dosage of opioids was higher among patients when the concurrent prescriptions were from 2 or more prescribers (71.4 MMEs/day) compared with concurrent prescriptions from the same prescriber (63.9 MMEs/day, p<0.001) (Table 2). The mean daily dosage of opioids was highest in Maine when the concurrent prescriptions were from multiple prescribers (83.1 MMEs/day) and highest in California when 1 prescriber was involved (70.5 MMEs/day).
Table 2.
Characteristics of Concurrent Opioid and Benzodiazepine Prescriptions by State
| Variable | Mean total opioid days | Mean daily dose of opioids (MME) | Mean days of overlap | ||||||
|---|---|---|---|---|---|---|---|---|---|
| One prescriber | Two+ prescribers | p-value | One prescriber | Two+ prescribers | p-value | One prescriber | Two+ prescribers | p-value | |
| Total | 118.8 | 153.1 | <0.0001 | 63.9 | 71.4 | <0.0001 | 77.6 | 101.2 | <0.0001 |
| State | |||||||||
| California | 91.3 | 127.8 | <0.0001 | 70.5 | 80.2 | <0.0001 | 56.5 | 81.0 | <0.0001 |
| Delaware | 138.8 | 179.4 | <0.0001 | 66.4 | 77.8 | <0.0001 | 80.2 | 114.1 | <0.0001 |
| Florida | 125.9 | 154.7 | <0.0001 | 62.9 | 68.8 | <0.0001 | 85.0 | 104.3 | <0.0001 |
| Idaho | 147.8 | 186.6 | <0.0001 | 69.3 | 75.7 | <0.0001 | 80.1 | 114.8 | <0.0001 |
| Kentucky | 201.9 | 218.8 | <0.0001 | 52.1 | 75.7 | <0.0001 | 148.7 | 157.0 | <0.0001 |
| Maine | 129.7 | 174.0 | <0.0001 | 69.4 | 83.1 | <0.0001 | 72.8 | 115.0 | <0.0001 |
| Ohio | 156.1 | 181.2 | <0.0001 | 49.0 | 57.7 | <0.0001 | 99.6 | 120.7 | <0.0001 |
| Virginia | 112.3 | 147.6 | <0.0001 | 61.4 | 66.2 | <0.0001 | 71.7 | 95.0 | <0.0001 |
| West Virginia | 155.2 | 163.0 | <0.0001 | 55.0 | 59.4 | <0.0001 | 112.2 | 115.0 | 0.001 |
Note: Boldface indicates statistical significance (p<0.05).
MME, morphine milligram equivalent.
The mean total days of concurrent prescribing was higher among patients when the concurrent prescriptions were from multiple prescribers (101.2 days) compared with concurrent prescriptions involving one prescriber (77.6 days, p<0.001). Kentucky had the highest mean days of concurrent prescribing across both categories, an average of 157.0 days when the concurrent prescriptions were from multiple prescribers and 148.7 days when the concurrent prescriptions were from the same prescriber.
The rates of opioid prescribing and concurrent prescribing of opioids and benzodiazepines varied by age and sex (Table 3). The greatest percentages of patients with opioid prescriptions were aged 50–64 years (28.4%) and ≥65 years (25.9%). Similarly, the greatest share of patients with concurrent opioid and benzodiazepine prescriptions were aged 50–64 years (37.6%) and ≥65 years (34.6%).
Table 3.
Demographic Characteristics Among Patients With Opioids and Patients With Concurrent Opioid and Benzodiazepine Prescriptions
| Variable | Patients with an opioid, % | Patients with concurrent prescriptions, % |
|---|---|---|
| Age, years | ||
| <18 | 3.1 | 0.3 |
| 18–34 | 20.5 | 7.4 |
| 35–49 | 22.1 | 20.1 |
| 50–64 | 28.4 | 37.6 |
| ≥65 | 25.9 | 34.6 |
| Sex | ||
| Male | 41.2 | 34.1 |
| Female | 58.8 | 65.9 |
DISCUSSION
Concurrent prescribing of opioids and benzodiazepines puts individuals at greater risk for overdose.1–5 This study shows that among individuals prescribed an opioid in 2015 in 9 PBSS states, nearly 1 in 4 were also prescribed a benzodiazepine during the year. Among those prescribed both medications during the year, 55% were prescribed opioids and benzodiazepines concurrently.
Almost half of patients with concurrent prescriptions for opioids and benzodiazepines obtained both medications from the same prescriber. When one prescriber is involved, evidence-based guidelines, such as the CDC Prescribing Guideline, which discourages the concurrent prescribing of opioids and benzodiazepines,18 may be effective in reducing this prescribing behavior. In fact, research has shown that the release of the CDC Prescribing Guideline was associated with a reduction in the percentage of patients receiving overlapping opioid and benzodiazepine prescriptions.18 Given the greater risks of benzodiazepine withdrawal relative to opioid withdrawal, when patients receiving both benzodiazepines and opioids require tapering to reduce overdose risk, it might be safer and more practical to taper opioids first.13 Therefore, clinicians should communicate with mental health professionals managing the patient to discuss the patient’s needs, prioritize patient goals, weigh risks of concurrent benzodiazepine and opioid exposure, and coordinate care.13 Despite the lower risk of opioid withdrawal, it is important to avoid abrupt tapering or sudden discontinuation of opioids, as these practices can result in severe opioid withdrawal symptoms and some patients seeking other sources of opioids.19
More than half of concurrent opioid and benzodiazepine prescribing instances resulted from prescriptions involving 2 or more distinct prescribers. Furthermore, when patients obtained concurrent prescriptions from 2 or more prescribers, they tended to have more total days of opioid supply, higher daily dosages of opioids, and more total days of concurrent prescriptions, compared with patients who obtained concurrent prescriptions from one prescriber. These findings have several important implications. First, among patients prescribed opioids, higher opioid doses are associated with an increased risk of opioid overdose death.20–22 Second, patients receiving opioids for longer durations are at higher risk for long-term use and developing opioid use disorder.23,24 Third, higher daily dosage and longer duration of concurrent prescriptions might indicate that prescribers were not aware of all other opioids or benzodiazepines that their patients were prescribed, thus reducing the likelihood of engaging in risk mitigation strategies. The concurrent prescribing of opioids and benzodiazepines by multiple prescribers highlights the importance of PDMPs in avoiding concurrent prescribing, and the importance of reviewing PDMPs before prescribing controlled substances to mitigate risk and improve coordination of care. PDMPs allow prescribers to view a patient’s prescribing history to determine if a patient has a current prescription from a different prescriber. Some states, including California in October 2018, have mandated checking the PDMP before prescribing any controlled substance. Evidence has shown that PDMPs, particularly those mandating provider review before prescribing opioids, are associated with lower rates of opioid prescribing and lower opioid overdose rates.25–27
Although there was little variation across the states in opioid prescribing rates and patients receiving both opioids and benzodiazepines, more variation was observed in the rate of concurrent prescribing and the characteristics of concurrent prescriptions. For instance, the rate of concurrent prescribing varied from 65% in West Virginia to 46% in Idaho. The number of prescribers involved in concurrent prescribing also varied substantially across states. For example, in Delaware, 67% of patients concurrently prescribed opioid and benzodiazepines obtained them from 2 or more prescribers, compared with 52% in West Virginia. Among patients with concurrent prescriptions, the mean total days of opioid supply and mean days of concurrent prescribing in Kentucky were twice that of California. However, although California had the lowest mean days of concurrent prescribing, it had the second highest mean daily dosage of opioids among patients with concurrent prescribing. These variations indicate that, in addition to national efforts to address the high-risk concurrent prescribing of opioids and benzodiazepines, tailored and targeted intervention efforts, such as educational outreach to opioid prescribers (academic detailing), could be impactful given the heterogeneous patterns of concurrent prescribing at the state level.
Female patients were more likely to be prescribed opioids, as well as concurrent opioid and benzodiazepine prescriptions, than male patients. These findings are consistent with evidence showing that female patients seek medical care more often than male patients and have a higher prevalence of chronic pain, mental health conditions, and anxiety—conditions for which these medications are commonly prescribed.8,28–31 The largest percentage of individuals with concurrent opioid and benzodiazepine prescriptions were older adults, aged 50–64 years (37.6%) and ≥65 years (34.6%). This finding is consistent with previous research showing that benzodiazepine prescribing increases with age.32 Although drug overdose rates tend to be lowest among older adults, the concurrent use of opioids and benzodiazepines among older adults is associated with other risk factors, including falls and injuries.33–36
Though this study provides important insights into the concurrent prescribing of opioids and benzodiazepines, it also highlights important areas for future research. Given the association between PDMP characteristics and prescribing behaviors,25,26 future research is needed to examine how PDMP characteristics, such as mandatory use, are associated with the concurrent prescribing of opioid and benzodiazepines. In addition, future research could examine patient characteristics associated with the concurrent prescribing of opioid and benzodiazepines. Together, this information could help identify effective interventions and target efforts to reduce the number of individuals concurrently prescribed an opioid and a benzodiazepine.
Limitations
This study is subject to several limitations. First, the clinical context and indications for the concurrent prescribing of opioids and benzodiazepines cannot be determined. Second, the data used in this study represent dispensed prescriptions, and information is not available on actual medication use. Third, data are not available on medication use without a prescription. Previous research has indicated that in more than half of patients with positive laboratory test results for concurrent opioid and benzodiazepine use, one drug was prescribed, whereas the other was nonprescribed.11 Fourth, prescriptions dispensed out-side of the state are not captured. Fifth, the cross-sectional nature of the study limits the ability to examine factors associated with cross-state variation in concurrent prescribing rates. Lastly, the data used in the present study examine prescribing behaviors occurring before the release of the CDC Prescribing Guideline in March 2016. Although the percentage of patients with overlapping opioid and benzodiazepine prescriptions has been declining in recent years, particularly after the release of the CDC Prescribing Guideline,18 it is important to continue monitoring changes in concurrent prescribing.
CONCLUSIONS
Concurrent prescribing of opioids and benzodiazepines is common, despite the known risks of taking these medications in combination. The findings in this study highlight the need for public health actions to reduce concurrent prescribing. Evidence-based guidelines, such as the CDC Prescribing Guideline, could be helpful in reducing this prescribing behavior when one prescriber is involved. Meanwhile, the utilization of PDMPs and care coordination could help address concurrent prescribing when multiple prescribers are involved.
ACKNOWLEDGMENTS
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.Jones CM, McAninch JK. Emergency department visits and overdose deaths from combined use of opioids and benzodiazepines. Am J Prev Med. 2015;49(4):493–501. 10.1016/j.amepre.2015.03.040. [DOI] [PubMed] [Google Scholar]
- 2.Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert ASB. Benzodiazepine prescribing patterns and deaths from drug overdose among U.S. veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasgupta N, Funk MJ, Proescholdbell S, et al. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med. 2016;17(1):85–98. 10.1111/pme.12907. [DOI] [PubMed] [Google Scholar]
- 4.Sun EC, Dixit A, Humphreys K, et al. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760 10.1136/bmj.j760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones JD, Mogali S, Comer SD. Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Depend. 2012;125(1–2):8–18. 10.1016/j.drugalcdep.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA Drug Safety Communication. FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. Silver Spring, MD: U.S. Food and Drug Administration; 2016. www.fda.gov/drugs/drugsafety/ucm518473.htm. Accessed June 20, 2019. [Google Scholar]
- 7.Guy GP Jr., Zhang K, Bohm MK, et al. Vital Signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697–704. 10.15585/mmwr.mm6626a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang CS, Kang EM, Kornegay CJ, et al. Trends in the concomitant prescribing of opioids and benzodiaepines, 2002–2014. Am J Prev Med. 2016;51(2):151–160. 10.1016/j.amepre.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Hirshtritt ME, Delucchi KL, Olfson M. Outpatient, combined use of opioid and benzodiazepine medications in the United States, 1993–2014. Prev Med Rep. 2017;9:49–54. 10.1016/j.pmedr.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HS, McCarthy DM, Hoppe JA, Mark Courtney D, Lambert BL. Emergency department provider perspectives on benzodiazepine-opioid coprescribing: a qualitative study. Acad Emerg Med. 2018;25 (1):15–24. 10.1111/acem.13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClure FL, Niles JK, Kaufman HW, Gudin J. Concurrent use of opioids and benzodiazepines: evaluation of prescription drug monitoring by a United States laboratory. J Addict Med. 2017;11(6):420–426. 10.1097/adm.0000000000000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez I, He M, Brooks MM, Zhang Y. Exposure–response association between concurrent opioid and benzodiazepine use and risk of opioid-related overdose in Medicare part D beneficiaries. JAMA Netw Open. 2018;1(2):e180919 10.1001/jamanetworkopen.2018.0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. 10.15585/mmwr.rr6501e1er. [DOI] [PubMed] [Google Scholar]
- 14.National Center for Injury Prevention and Control. CDC compilation of benzodiazepines, muscle relaxants, stimulants, zolpidem, and opioid analgesics with oral morphine milligram equivalent conversion factors, 2017 version. Atlanta, GA: CDC; 2017. www.cdc.gov/drugoverdose/resources/data.html. [Google Scholar]
- 15.Zhu Y, Coyle DT, Mohamoud M, et al. Concomitant use of buprenorphine for medication-assisted treatment of opioid use disorder and benzodiazepines: using the prescription behavior surveillance system. Drug Alcohol Depend. 2018;187:221–226. 10.1016/j.drugalcdep.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strickler GK, Zhang K, Halpin JF, et al. Effects of mandatory prescription drug monitoring program (PDMP) use laws on prescriber registration and use on risky prescribing. Drug Alcohol Depend. 2019;199:1–9. 10.1016/j.drugalcdep.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19(8):648–658. [PubMed] [Google Scholar]
- 18.Bohnert ASB, Guy GP Jr., Losby JL. Opioid prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 Opioid Guideline. Ann Intern Med.. 2018;169(6):367–375. 10.7326/m18-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowell D, Haegerich CR, Chou R. No shortcuts to safer opioid prescribing. N Engl J Med. 2019;380(24):2285–2287. 10.1056/NEJMp1904190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn KM, Saunders KW, Rutter CM. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bohnert ASB, Valenstein M, Bair MJ. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305 (13):1315–1321. 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 22.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):886–891. 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- 23.Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use - United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(10):265–269. 10.15585/mmwr.mm6610a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin BC, Fan MY, Edlund MJ, et al. Long-term chronic opioid therapy discontinuation rates from the TROUP study. J Gen Intern Med. 2011;26(12):1450–1457. 10.1007/s11606-011-1771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowell D, Zhang K, Noonan RK, Hockenberry JM. Mandatory provider review and pain clinic laws reduce the amounts of opioids prescribed and overdose death rates. Health Aff (Millwood). 2016;35 (10):1876–1883. 10.1377/hlthaff.2016.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winstanley EL, Zhang Y, Mashni R, et al. Mandatory review of a prescription drug monitoring program and impact on opioid and benzodiazepine dispensing. Drug Alcohol Depend. 2018;188:169–174. 10.1016/j.drugalcdep.2018.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brandeis University Prescription Drug Monitoring Program Center of Excellence. PDMP prescriber use mandates: characteristics, current status, and outcomes in selected states. www.pdmpassist.org/pdf/COE_documents/Add_to_TTAC/COE%20briefing%20on%20mandates%203rd%20revision.pdf. Published 2016. Accessed June 20, 2019. [Google Scholar]
- 28.O’Brien PL, Karnell LH, Gokhale M, et al. Prescribing of benzodiazepines and opioids to individuals with substance use disorders. Drug Alcohol Depend. 2017;178:223–230. 10.1016/j.drugalcdep.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Stein MD, Anderson BJ, Kenney SR, Bailey GL. Beliefs about the consequences of using benzodiazepines among persons with opioid use disorder. J Subst Abus Treat. 2017;77:67–71. 10.1016/j.jsat.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulozzi LJ, Strickler GK, Kreiner PW, Koris CM. Controlled substance prescribing patterns–Prescription Behavior Surveillance System, eight states, 2013. MMWR Surveill Summ. 2015;64(9):1–14. 10.15585/mmwr.ss6409a1. [DOI] [PubMed] [Google Scholar]
- 31.Kessler RC, McGonagle KA, Zhao S. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National comorbidity Survey. Arch Gen Psychiatry. 1994;51 (1):8–19. 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 32.Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136–142. 10.1001/jamapsychiatry.2014.1763. [DOI] [PubMed] [Google Scholar]
- 33.Huang AR, Mallet L, Rochefort CM, et al. Medication-related falls in the elderly: causative factors and preventive strategies. Drugs Aging. 2012;29(5):359–376. 10.2165/11599460-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Woolcott JC, Richardson KJ, Wiens MO. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952–1960. 10.1001/archinternmed.2009.357. [DOI] [PubMed] [Google Scholar]
- 35.Hampton LM, Daubresse M, Chang HY, Alexander GC, Budnitz DS. Emergency department visits by adults for psychiatric medication adverse events. JAMA Psychiatry. 2014;71(9):1006–1014. 10.1001/jamapsychiatry.2014.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zint K, Haefeli WE, Glynn RJ, et al. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf. 2010;19(12):1248–1255. 10.1002/pds.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]