Abstract
Objective
Lifestyle factors, such as inactivity and obesity, contribute to cognitive decline in the general population, but little is known about how these factors may affect individuals with a chronic inflammatory condition such as rheumatoid arthritis (RA). We studied the clinical and functional risk factors related to a worsening of perceived cognitive function in patients with RA.
Methods
We collected clinical and functional questionnaire data over 10 years in a prospective RA cohort including yearly self‐reported memory, concentration, and word‐finding difficulties graded from “never” to “often.” Generalized estimating equation models examined the role of exercise (defined as those meeting the Department of Health and Human Services physical activity guidelines of 75 minutes of vigorous or 150 minutes of moderate aerobic activity per week), body mass index (BMI), sleep, depression (Mental Health Index‐Depression), Disease Activity Score (DAS)28–c‐reactive protein (CRP)3 score, disease‐modifying antirheumatic drug, and corticosteroid use from the previous year as predictors of cognitive complaints that progressed to “often” compared with the previous year (the first year (T i) progressed to “often” 1 year later (T i+1)).
Results
Of 1219 RA subjects, 127 (10.4%) described either poor memory, concentration, or word‐finding difficulties as affecting them “often” at study entry. RA patients (n = 1092, mean age = 56.5 years, 82% female, 58% college educated) were less likely to report word‐finding difficulties, poor memory, and concentration as “often” if they were physically active (p = 0.0001, P = 0.01, P < 0.0001, respectively). Female RA patients developed more concentration complaints compared with males (P = 0.03); patients taking an anti–tumor necrosis factor therapy were less likely to complain of poor memory (P = 0.01). Sleep, BMI, fatigue, depression, DAS28‐CRP3, methotrexate, and corticosteroid use were not independently associated with a worsening of any cognitive complaints.
Conclusion
RA patients who are physically active are less likely to report cognitive difficulties. Our study suggests potential modifiable risk factors for the prevention of cognitive dysfunction in RA.
INTRODUCTION
Individuals with inflammatory diseases such as rheumatoid arthritis (RA) have an increased prevalence of cognitive impairment, and recent articles suggest an increase in rates of Alzheimer's dementia 1, 2, 3, 4, 5. Measurable cognitive impairment is estimated to occur in 30% to 71% of patients with RA in several studies 1, 6; other studies have demonstrated an association between cognition and disease activity 7. A recent review of cognitive impairment in RA 5 demonstrated that RA patients underperformed on cognitive function tests compared with controls especially in areas of memory, attention, and verbal function. This impairment may limit self‐management of the disease and worsen functional outcomes 8. However, screening for cognitive impairment with neuropsychological testing presents challenges as its administration is costly, time intensive, and requires specialized personnel to administer and interpret tests.
In healthy cohorts, investigations of preclinical Alzheimer's type dementia have suggested that subjective cognitive complaints may precede abnormalities detected on neuropsychological testing 9, 10. However, in RA, cognitive impairment can co‐occur with markers of psychological distress 11, and self‐reported cognitive dysfunction can be influenced by symptoms of depression and fatigue 12. Appreciating the role that clinical and psychological factors play in the perception of cognition is important because cognitive complaints are clinically meaningful to patients and may portend cognitive decline.
Potentially modifiable lifestyle factors such as inactivity and obesity are risk factors for cognitive decline in the general population 13, 14. However, little is known about how these factors may contribute in the setting of a chronic inflammatory condition such as RA, although one study in systemic lupus erythematosus reported similar risk 15. In the present analysis, we studied RA patients followed since 2003 who reported yearly on their perceived cognition to better understand the risk factors for this outcome.
PATIENTS AND METHODS
Study design and patient population
The Brigham and Women's Arthritis Rheumatoid Arthritis Sequential Study (BRASS) registry is a large prospective, observational cohort at the Brigham and Women's Arthritis Center in Boston, Massachusetts. Enrollment for the registry began in March 2003 and is ongoing. Patients (ages 18 or older) with either new onset or established RA disease were recruited from the practices of rheumatologists. All diagnoses of RA were then verified according to either the American College of Rheumatology (ACR) criteria 16 or were the clinical opinion of a rheumatologist. Each patient signed an informed consent form that was obtained according to the Declaration of Helsinki and approved by the Partners Institutional Review Board at Brigham and Women's Hospital. Annual visits collected updated information on demographics, disease activity, medication use, comorbidities, psychosocial variables, and functional status. Additionally, serum was collected yearly. Details and information related to participation and the protocol in the BRASS registry has been reported elsewhere 17. In this analysis, BRASS participants were followed for up to 10 years. Individuals who completed at least two consecutive annual assessments were studied.
Measures
Patients’ self‐assessment of cognition
Subjects in the BRASS registry reported their cognitive complaints at their yearly visits. Patients were asked “Do you have any of the following symptoms NOW?” in the categories of poor memory, poor concentration, and word‐finding difficulties. Each question was rated as “not at all,” “sometimes,” or “often.” We defined worsening of a cognitive complaint if the description of difficulty changed from either “not at all” or “sometimes” to “often” for any of the three questions.
Other patient‐reported measures
Patients were asked to complete an annual questionnaire that inquired about their age, sex, education, ethnicity, and disease duration. Education was grouped into graduated high school, graduated college, and completed graduate school or higher. All other variables were analyzed as continuous variables and were updated yearly.
Each of the following measures was assessed at each annual visit. Sleep was rated using an item from the Multidimensional Health Assessment Questionnaire (MDHAQ): “Over the past week were you able to get a good night's sleep?” (range 0‐3) 18. A visual analog scale from the MDHAQ scale was used to rate fatigue severity on a scale of 0 (no fatigue) to 100 (maximum fatigue) over the previous week. The five‐item Mental Health Index‐5 (MHI‐5) was administered and scored to obtain the MHI‐D depression subscale; higher scores indicate worse depressive symptoms 19, 20.
Physical activity was measured with the Nurse's Health Study II physical activity scale, which is a self‐reported scale assessing the frequency, duration, and intensity of physical activity in a variety of different modes of exercise in the preceding week 21. Respondents were categorized active or inactive, based on whether they met the 2008 US Department of Health and Human Services (HHS) physical activity guideline (150 minutes or more of moderate intensity activity or 75 minutes or more of vigorous activity per week) 22.
Other variables
Disease activity was measured with the Disease Activity Score‐28 (DAS28)–c‐reactive protein 3 (CRP3) 23, a composite score consisting of a physician‐reported 28 joint count and CRP lab values. Subjects were categorized as having cardiovascular disease if they had a myocardial infarction, angioplasty, cardiac catheterization, coronary artery bypass grafting, congestive heart failure or stroke. Body mass index (BMI) and current medications were recorded at each study visit.
Statistical analyses
Demographic and clinical variables were tabulated for all patients with at least two consecutive yearly data points. We also tabulated the percentages of BRASS patients that had answered each question at each time point. To evaluate whether the frequency of cognitive complaints described as “often” increased over time, we ran a mixed effects logistic regression for trend for each question using the GLIMMIX procedure (Figure 1). We then ran an analysis for the main binary outcome variable, which was defined as a change from a description of “not at all” or “sometimes” to “often” for each of the cognitive complaints between consequent questionnaires, to identify predictors of new cognitive complaints. Covariates for this analysis came from the prior study visit and included age, gender, education, BMI, medication use (methotrexate, anti–tumor necrosis factor (TNF), corticosteroid use), DAS28‐CRP3 score, sleep, fatigue, MHI‐D depression score, and also required that physical activity guidelines were met, adjusted for days between visits. Generalized estimating equation (GEE) model analyses to identify predictors of developing a cognitive complaint were conducted using the GENMOD procedure for each of the three questions. Because subjects had multiple visits, they were able to make more than one transition to “often.” Study dropouts had data through their last visit included in the analyses. Any missing data remained as missing and was not imputed. A value of P < 0.05 was considered significant. All analyses were performed using SAS software, version 9.4 (SAS Institute).
Figure 1.
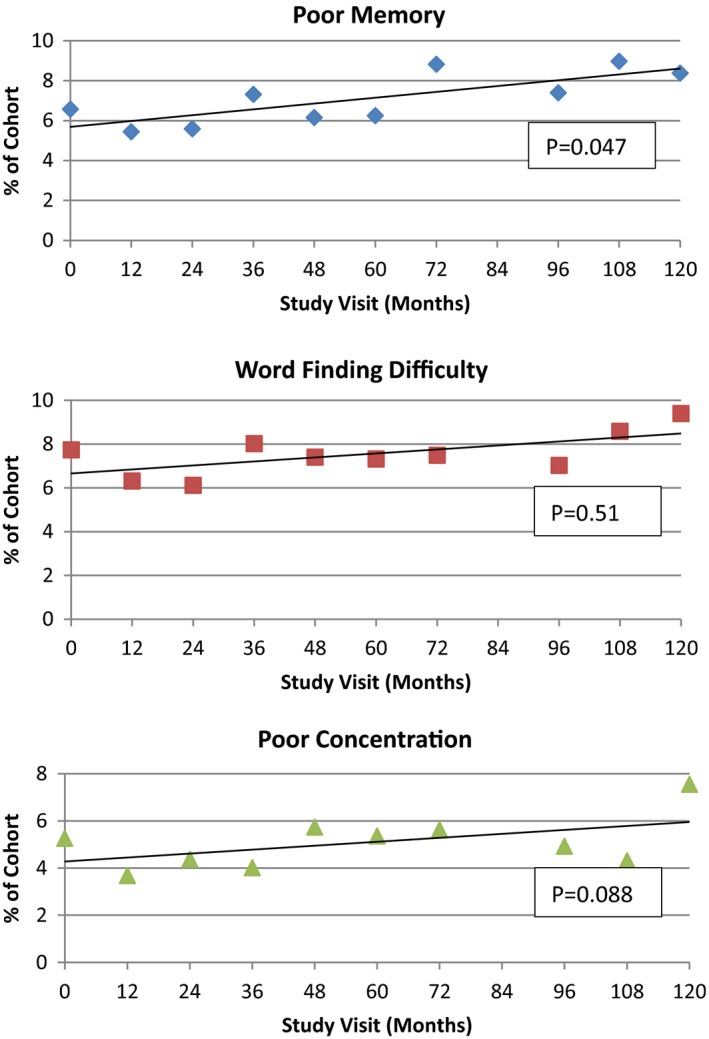
Percentage of study cohort members who each reported cognitive complaint of “often” at each study visit (n = 1219). Number of subjects in each study visit (months): 0, n = 1219; 12, n = 1122; 24, n = 926; 36, n = 817; 48, n = 723; 60, n = 598; 72, n = 404; 84, n = 350; 96, n = 326; 108, n = 279; 120, n = 258. P value reflects the mixed effects logistic regression trend for each question using the GLIMMIX procedure.
RESULTS
At study entry, of the 1219 members in the study, 127 (10.4%) described either poor memory, poor concentration, or word‐finding difficulties as occurring “often” and were excluded from further analysis. Among this group, 36 of 127 (28%) complained of all three symptoms. Nineteen (15%) complained of word‐finding difficulties and poor memory “often,” 11 (9%) complained of poor memory and concentration difficulties, and 7 complained “often” of word‐finding difficulties and concentration difficulties.
Table 1 delineates the clinical characteristics and demographics of the members of the BRASS cohort that contributed data to the analysis at the time of study entry (n = 1092). The cohort had a mean age of 56.5 years, they were mostly female, and more than half had a college degree. The study cohort's mean follow‐up duration was 5.4 years (range: 1‐10.9). The great majority of subjects were Caucasian. They had long‐standing disease with a mean disease duration of 12.8 years (±11.9). Their DAS28‐CRP3 scores averaged 3.6, indicating moderate disease activity. Approximately 28% currently used corticosteroids and one‐third (37.7%) were using anti‐TNF therapy at study entry. Thirteen percent (13%) of the cohort met the US Department of HHS guidelines for exercise, and 8.1% entered the cohort with a history of cardiovascular disease.
Table 1.
Demographics and clinical characteristics of the BRASS cohort at study entry (N = 1092)
| Variable | Numerical Values |
|---|---|
| Age, years (mean, SD) | 56.5 (13.7) |
| Gender (female) (N, %) | 894 (81.9) |
| Education (college or more) (N, %) | 632 (58.1) |
| Race (white) (N, %) | 1006 (92.8) |
| Corticosteroid use (N, %) | 306 (28.0) |
| Anti‐TNF use (N, %) | 411 (37.7) |
| Methotrexate use (N, %) | 562 (51.5) |
| Physically active (N,%) | 138 (12.7) |
| DAS28‐CRP3 (mean, SD) | 3.6 (1.6) |
| Depression score (MHI‐D) (3‐15)a (mean, SD) | 12.5 (2.1) |
| Fatigue (VAS) (0‐100)a (mean, SD) | 39.1 (28.7) |
| Sleep difficulties (MDHAQ) (0‐3)a (mean, SD) | 0.8 (0.8) |
| Cardiovascular disease (N, %) | 99 (8.1) |
| Disease duration, y (mean, SD) | 12.8 (11.9) |
| BMI (mean, SD) | 26.8 (5.7) |
Abbreviation: BMI, body mass index; MDHAQ, Multidimensional Health Assessment Questionnaire; MHI‐D, Mental Health Index‐Depression; TNF, tumor necrosis factor; VAS, visual analog scale.
aHigher score indicates worse value.
By the end of the 10‐year study period, 11.4% complained of at least one symptom as occurring “often” compared with 10.4% at baseline. The percentage of study subjects complaining that they “often” had the individual symptom complaint at each study visit is illustrated in Figure 1. Although trend lines demonstrated an increase in the frequency of all of the cognitive complaints, only memory complaints increased significantly over time, from 6.3% to 8.8% by study end (P = 0.047).
Table 2 demonstrates the GEE analysis of predictors of perceived cognitive complaints described as occurring “often.” RA patients who took TNF inhibitors and those who were active were less likely to report a worsening of memory (odds ratio [OR] (confidence interval [CI]), 0.69 (0.53,0.90) P = 0.01; 0.83 (0.72,0.96); P = 0.01, respectively). Those who were active were also less likely to report word‐finding difficulties occurring “often” (OR [CI], 0.45 [0.30,0.67], P = 0.0001). No other factor predicted an increase in reports of word‐finding difficulties. Worsened concentration was more likely to be reported as “often” 1 year later among those RA patients who were female (OR [CI],4.88 [1.13,21.12], P = 0.03), had a higher DAS28‐CRP3 score (OR [CI], 1.07 [0.99,1.17], P = 0.09), or those who were not active (OR [CI], 0.82 [0.77,0.87], P < 0.0001).
Table 2.
Generalized estimate equations multivariate models of demographic and clinical factors associated with worsened cognitive function (N = 1092)a
| Variable | Poor Memory | Word‐Finding Difficulty | Poor Concentration | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | ||||||||||
| OR | lower | upper | P value | OR | lower | upper | P value | OR | lower | upper | P value | |
| Physically activeb | 0.83 | 0.72 | 0.96 | 0.01* | 0.45 | 0.30 | 0.67 | 0.0001* | 0.82 | 0.77 | 0.87 | <0.0001* |
| Anti‐TNF use | 0.69 | 0.53 | 0.90 | 0.01* | 1.14 | 0.71 | 1.83 | 0.58 | 0.75 | 0.54 | 1.06 | 0.10 |
| DAS28‐CRP3 | 1.07 | 0.98 | 1.17 | 0.14 | 1.02 | 0.91 | 1.13 | 0.78 | 1.07 | 0.99 | 1.17 | 0.09 |
| Gender (female) | 2.67 | 0.80 | 8.90 | 0.11 | 1.21 | 0.46 | 3.22 | 0.70 | 4.88 | 1.13 | 21.12 | 0.03* |
| Depression score (MHI‐D) | 0.99 | 0.90 | 1.09 | 0.82 | 1.05 | 0.97 | 1.14 | 0.22 | 0.97 | 0.92 | 1.02 | 0.24 |
| Methotrexate use | 0.83 | 0.60 | 1.16 | 0.28 | 0.78 | 0.51 | 1.19 | 0.25 | 1.06 | 0.77 | 1.45 | 0.72 |
| Corticosteroid use | 0.92 | 0.62 | 1.37 | 0.69 | 0.99 | 0.62 | 1.56 | 0.95 | 1.22 | 0.96 | 1.54 | 0.11 |
| BMI | 1.00 | 0.96 | 1.04 | 0.82 | 1.00 | 0.96 | 1.03 | 0.92 | 0.98 | 0.93 | 1.03 | 0.46 |
| Education (college degree or more) | 0.83 | 0.44 | 1.55 | 0.55 | 0.83 | 0.42 | 1.66 | 0.60 | 0.84 | 0.41 | 1.72 | 0.63 |
| Sleep difficulties (MDHAQ) | 1.09 | 0.82 | 1.45 | 0.54 | 0.99 | 0.77 | 1.26 | 0.92 | 1.09 | 0.92 | 1.28 | 0.31 |
| Fatigue (VAS) | 1.00 | 0.99 | 1.01 | 0.70 | 1.00 | 1.00 | 1.01 | 0.70 | 1.00 | 0.99 | 1.00 | 0.36 |
| Age, y | 1.00 | 0.98 | 1.02 | 1.00 | 1.00 | 0.97 | 1.02 | 0.78 | 0.99 | 0.97 | 1.01 | 0.48 |
Abbreviation: CI, confidence interval; DAS28‐CRP, Disease Activity Score 28 joints–c‐reactive protein; MDHAQ, Multidimensional Health Assessment Questionnaire; MHI‐D, Mental Health Index‐Depression; OR, odds ratio; TNF, tumor necrosis factor; VAS, visual analog scale.
aAdjusted for time (days) between questionnaires. bMeeting the US Department of Health and Human Services recommendation for physical activity.
P < 0.05.
We evaluated whether individuals who dropped out of our study (approximately 5% per year) were more likely to have an increase in cognitive complaints prior to drop out. We evaluated the frequency of each of the cognitive complaints at a patient's last visit prior to dropping out and compared it with those who remained in the study. We did not find that the subjects who dropped had more cognitive complaints of poor memory, word‐finding difficulties, or trouble with concentration at multiple time points (data not shown).
DISCUSSION
In this study, we examined how exercise and lifestyle factors interacted with clinical factors as predictors of cognitive complaints in three domains: memory, concentration, and word retrieval difficulties. In middle‐aged RA patients followed since 2003 who reported yearly on their perceived cognition, we found that meeting guidelines for physical activity protected against the development of frequent complaints of word finding, memory, and concentration difficulties. Taking anti‐TNF therapy was also protective for the development of worsening memory. Additionally, female gender predicted an increase in concentration difficulties, indicating the complex role that clinical, demographic, and psychosocial factors can play in how RA patients assess their cognitive function in the setting of chronic inflammation.
Meeting physical activity guidelines protected against an increase in all three self‐reported cognitive difficulties. The finding that regular physical activity is beneficial for cognitive function and reduces longitudinal decline in cognition and risk of depression has been studied widely in healthy older adults 13, 24, 25, 26. Aerobic exercise has also recently been shown to be effective in improving cognition and increasing cortical thickness in younger healthy individuals 27. In systemic lupus erythematosus, physical inactivity and obesity were associated with impairment in cognitive function also adjusting for demographics, disease activity, and depression 15. Regular physical activity has been shown to decrease levels of inflammatory markers such as CRP, interleukin (IL)‐6, and TNF‐alpha in healthy individuals 28, 29, and IL‐6 and CRP have been shown to increase dementia incidence in mid‐life 30. This may explain, in part, how RA inflammation may contribute to worsened cognition and exercise may ameliorate this effect. In a recent systematic review of cognitive impairment in RA, several studies report a relationship with disease activity and reduced performance on cognitive function tests 5. In a study by Lee et al 7, cognitive function in RA was associated with disease activity rather than carotid atherosclerotic changes. Additionally, Chou et al 3 demonstrated an increased risk of Alzheimer's disease in the RA population that was lowered in those exposed to etanercept. In our study, higher disease activity showed a trend toward reports of poor concentration. Anti‐TNF use was protective against a worsening of memory complaints and showed a trend toward protection against concentration difficulties, suggesting that inflammation may also play a role in perceived cognitive function. In RA patients, there are many benefits to exercise. Physical activity has been shown to reduce vascular stiffness and increase small, high‐density lipoprotein particle concentration 31, improve cardiovascular risk by improving endothelial function 32, reduce fatigue, pain, and symptoms of depression 33, 34 as well as improve disease activity and reduce bone loss 35. All of these effects could contribute to reports of improved cognition in our participants. Of note, neither corticosteroids nor methotrexate use affected cognitive complaints in this study.
An increase in symptoms of depression influencing a report of worsened perceived cognition has been noted in other studies of individuals with chronic disease and in healthy subjects 36. Studies in patients with multiple sclerosis and cancer corroborated that depressive symptoms affect a patients’ report of cognitive difficulties 36, 37, 38, 39. In RA, Shin et al found that higher levels of depression and fatigue were associated with perceived cognitive dysfunction in 120 well‐characterized patients with RA 12. However, we found that after adjusting for multiple other known risk factors for cognitive function, higher depression scores did not affect the development of reports of frequent concentration, memory, or word‐finding difficulties. Our repeated measures of fatigue, sleep, BMI—independent of depression and other factors—did not influence cognitive complaints. Differences in study design, inclusion of disease severity, and inclusion of other clinical and demographic factors may account for these differences.
Patients with RA need adequate cognitive capacity, especially given their complex medication management and intermittent difficulties in performance of activities of daily living. Understanding what factors account for cognitive complaints is helpful in choosing who may need neuropsychological testing or brain imaging to document impairment. Several studies of healthy subjects report that subjective cognitive complaints may indicate early cognitive impairment not detectable on neurocognitive testing 9, 40. Others suggest that subjective cognitive complaints in healthy subjects relate more to depression 41, 42. Amariglio et al reported that even when the effects of depression are considered, there is a relationship between subjective cognitive complaints and objective cognitive impairment 43 and that subjective cognitive complaints were related to amyloid burden measure by positron emission tomography scanning 9. However, the study of persons with RA by Shin et al 12 revealed a gap between self‐reported cognitive complaints and objective cognitive impairment that was more influenced by depression and fatigue. Although we did not do neuropsychological assessments in our study to determine impairment, the report of frequent memory, word‐finding difficulties, or concentration difficulties was alarming to our patients and were presented to their doctors as a frequent complaint. Understanding how other risk factors for cognitive impairment, such as education level, BMI, exercise, medication use, disease activity, and psychosocial factors, contribute to this self‐assessed symptom is useful to a treating physician who may need to decide if further cognitive testing is necessary. If clinicians are interested in screening RA patients for early dementia and do not have the resources or time to perform neurocognitive testing, understanding the role that lifestyle and clinical factors play in the report of cognition difficulties is important.
Our study is of value in that it provides longitudinal data on the frequency of cognitive complaints in RA. There are few studies that document the prevalence of self‐reported cognitive difficulties over time in patients with RA. In the study by Shin et al 12, the mean Perceived Deficits Questionnaire (PDQ) score (a questionnaire used in multiple sclerosis patients that reports how often respondents have difficulty with attention, memory, and planning) was 5.8 out of a possible 16 in a group of 120 well‐studied patients with RA. This indicated an overall average of difficulties categorized between “rarely” and “sometimes.” In another study of 114 patients with a variety of rheumatic diseases including RA, up to 56% had at least one cognitive complaint in those with and without fibromyalgia at one time point 44. In the current study, we documented over 10 years that a variety of symptom complaints occur in RA patients and that the frequency of memory difficulties increases over time. Although subjects in the study were highly educated and cognitively well enough to fill out our questionnaires, we also found that subjects who dropped out did not do so because they more likely had cognitive complaints. Having data on the frequency of cognitive complaints in RA is useful and informative. It expands our understanding of the functional difficulties that patients with RA encounter every day.
Our study has several limitations. Although we are reporting data on patients’ perceived cognitive difficulties, we do not have neurocognitive testing as a comparison or gold standard of impairment and thus the relationship of perceived cognitive function to actual cognitive function is unclear. The risk factors we report for worsened cognitive complaints, therefore, may not directly link to dementia. However, the frequent complaints of the BRASS subjects appeared meaningful. Anecdotally, the BRASS patients who complained of frequent cognitive difficulties felt that this affected the quality of their lives. Although it did not appear that subjects who dropped out had an increased frequency of cognitive complaints, suggesting that this may be a cause for attrition, a stable cohort study could have provided more accurate data on the prevalence of cognitive complaints over time. Having access to a parallel analysis of a normal control group would also clarify the comparative prevalence of these complaints. Future studies should examine the functional implications of this increase in perceived cognitive difficulty, its relationship to objective impairment, and whether this difficulty is related to other diminished RA outcomes. We did not screen participants for fibromyalgia, which is associated with cognitive complaints. However, only 3 of the 127 who had cognitive complaints affecting them “often” at the study entry had a concurrent diagnosis of fibromyalgia, and only 15 of the entire cohort of 1219 subjects carried a diagnosis of fibromyalgia.
Although we queried subjects on a three‐point scale in the three cognitive domains previously reported as impaired in RA patients, our questions do not come from a validated questionnaire. We, therefore, do not have any data on validity, reliability, or sensitivity to change. Furthermore, although we chose the outcome transitioning to complaints described as “often” from either “never” or “sometimes,” these transitions are not equivalent; and although an increase in memory complaints reported “often,” for example, from 6.3% to 8.8%, appears to be small, many more subjects (40%‐48%) reported cognitive complaints that “sometimes” affected them. These complaints may also be clinically meaningful.
In summary, our study reported on the impact of exercise and other lifestyle and clinical factors on the development of frequent cognitive complaints in memory, concentration, and word retrieval domains. Clinical factors, such as anti‐TNF use, reduced the likelihood of worsened memory dysfunction. Both lower disease activity and anti‐TNF use showed trends toward fewer concentration difficulties. However, meeting the Department of HHS guidelines for exercise was the strongest protector against an increase in concentration, memory, and word retrieval difficulties. If perceived cognitive difficulties precede cognitive decline in RA, our study suggests potential risk factors for intervention that could be tested in a randomized controlled trial. Future studies should investigate the functional sequelae of these cognitive complaints, whether longitudinal follow‐up of these individuals predicts an increase in cognitive impairment or dementia, and whether an increase in exercise may ward off these debilitating outcomes.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual contact, and all authors approved the final version to be published. Dr. Shadick had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Shadick, Weinblatt, Cui, Iannaccone, Maica, Katz,
Acquisition of data
Iannaccone, Coblyn, Maica, Weinblatt, Shadick.
Analysis and interpretation of data
Cui, Iannaccone, Katz, Coblyn, Weinblatt, Shadick.
ACKNOWLEDGMENTS
We thank the dedicated rheumatoid arthritis participants and staff of the Brigham Rheumatoid Arthritis Sequential Study (BRASS) at Brigham and Women's Hospital for their continued participation in this longitudinal research study.
This study was supported by the funders of the Brigham and Women's Rheumatoid Arthritis Sequential Study (BRASS): Crescendo Biosciences, Bristol Myers Squibb, Sanofi‐Regeneron. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. Dr. Shadick received research grants from the National Institutes of Health (P30‐AR070253, P30‐AR072577, and R01‐HL127118), Mallinckrodt and Dr. Weinblatt received research grants from Amgen, Crescendo Biosciences, and Lilly, Bristol Myers Squibb, Sanofi/Regeneron.
Dr. Shadick received consulting fees from Bristol‐Myers Squibb. Dr. Weinblatt received consulting fees from Abbvie, Amgen, Bristol‐Myers Squibb, Corrona, Crescendo Bioscience, GlaxoSmithKline, Horizon, Lilly, Pfizer, Roche, Samsung, and Scipher; and owns stock options in Lycera, Canfite, Scipher, Vorso, and Inmedix. No other disclosures relevant to this article were reported.
REFERENCES
- 1. Shin SY, Katz P, Wallhagen M, Julian L. Cognitive impairment in persons with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:1144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wallin K, Solomon A, Kåreholt I, Tuomilehto J, Soininen H, Kivipelto M. Midlife rheumatoid arthritis increases the risk of cognitive impairment two decades later: a population‐based study. J Alzheimers Dis 2012;31:669–76. [DOI] [PubMed] [Google Scholar]
- 3. Chou RC, Kane M, Ghimire S, Gautam S, Gui J. Treatment for rheumatoid arthritis and risk of Alzheimer's disease: a nested case‐control analysis. CNS Drugs 2016;30:1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wotton CJ, Goldacre MJ. Associations between specific autoimmune diseases and subsequent dementia: retrospective record‐linkage cohort study, UK. J Epidemiol Community Health 2017;71:576–83. [DOI] [PubMed] [Google Scholar]
- 5. Meade T, Manolios N, Cumming SR, Conaghan PG, Katz P. Cognitive impairment in rheumatoid arthritis: a systematic review. Arthritis Care Res (Hoboken) 2018;70:39–52. [DOI] [PubMed] [Google Scholar]
- 6. Appenzeller S, Bertolo MB, Costallat LT. Cognitive impairment in rheumatoid arthritis. Methods Find Exp Clin Pharmacol 2004;26:339–43. [DOI] [PubMed] [Google Scholar]
- 7. Lee JH, Kim GT, Kim YK, Lee SG. Cognitive function of patients with rheumatoid arthritis is associated with disease activity but not carotid atherosclerotic changes. Clin Exp Rheumatol 2018;36:856–61. [PubMed] [Google Scholar]
- 8. Shin SY, Julian L, Katz P. The relationship between cognitive function and physical function in rheumatoid arthritis. J Rheumatol 2013;40:236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 2012;50:2880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Harten AC, Smits LL, Teunissen CE, Visser PJ, Koene T, Blankenstein MA, et al. Preclinical AD predicts decline in memory and executive functions in subjective complaints. Neurology 2013;81:1409–16. [DOI] [PubMed] [Google Scholar]
- 11. Medina LD, Hirshberg L, Taylor MJ, Gilbert PE, Heaton RK. Rates of neuropsychological dysfunction in fibromyalgia and rheumatoid arthritis: an automated clinical rating approach. J Clin Rheumatol 2019;25:252–7. [DOI] [PubMed] [Google Scholar]
- 12. Shin SY, Katz P, Julian L. Relationship between perceived cognitive dysfunction and objective neuropsychological performance in persons with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Middleton LE, Manini TM, Simonsick EM, Harris TB, Barnes DE, Tylavsky F, et al. Activity energy expenditure and incident cognitive impairment in older adults. Arch Intern Med 2011;171:1251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cournot M, Marquié JC, Ansiau D, Martinaud C, Fonds H, Ferrières J, et al. Relation between body mass index and cognitive function in healthy middle‐aged men and women. Neurology 2006;67:1208–14. [DOI] [PubMed] [Google Scholar]
- 15. Katz P, Julian L, Tonner MC, Yazdany J, Trupin L, Yelin E, et al. Physical activity, obesity, and cognitive impairment among women with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2012;64:502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 17. Iannaccone CK, Lee YC, Cui J, Frits ML, Glass RJ, Plenge RM, et al. Using genetic and clinical data to understand response to disease‐modifying anti‐rheumatic drug therapy: data from the Brigham and Women's Hospital Rheumatoid Arthritis Sequential Study. Rheumatology (Oxford) 2011;50:40–6. [DOI] [PubMed] [Google Scholar]
- 18. Pincus T, Swearingen C, Wolfe F. Toward a multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient‐friendly health assessment questionnaire format. Arthritis Rheum 1999;42:2220–30. [DOI] [PubMed] [Google Scholar]
- 19. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 20. Cuijpers P, Smits N, Donker T, ten Have M, de Graaf R. Screening for mood and anxiety disorders with the five‐item, the three‐item, and the two‐item Mental Health Inventory. Psychiatry Res 2009;168:250–5. [DOI] [PubMed] [Google Scholar]
- 21. Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self‐administered physical activity questionnaire. Int J Epidemiol 1994;23:991–9. [DOI] [PubMed] [Google Scholar]
- 22. Office of Disease Prevention and Health Promotion . 2008 Physical Activity Guidelines for Americans. 2008. URL: https://health.gov/paguidelines/2008/.
- 23. Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty‐eight‐joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995;38:44–8. [DOI] [PubMed] [Google Scholar]
- 24. Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA 2004;292:1454–61. [DOI] [PubMed] [Google Scholar]
- 25. Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev 2008;CD005381. [DOI] [PubMed] [Google Scholar]
- 26. Harvey SB, Øverland S, Hatch SL, Wessely S, Mykletun A, Hotopf M. Exercise and the prevention of depression: results of the HUNT cohort study. Am J Psychiatry 2018;175:28–36. [DOI] [PubMed] [Google Scholar]
- 27. Stern Y, MacKay‐Brandt A, Lee S, McKinley P, McIntyre K, Razlighi Q, et al. Effect of aerobic exercise on cognition in younger adults: a randomized clinical trial. Neurology 2019;92:e905–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the Health, Aging and Body Composition Study. J Am Geriatr Soc 2004;52:1098–104. [DOI] [PubMed] [Google Scholar]
- 29. Reuben DB, Judd‐Hamilton L, Harris TB, Seeman TE, MacArthur Studies of Successful Aging . The associations between physical activity and inflammatory markers in high‐functioning older persons: MacArthur Studies of Successful Aging. J Am Geriatr Soc 2003;51:1125–30. [DOI] [PubMed] [Google Scholar]
- 30. Singh‐Manoux A, Dugravot A, Brunner E, Kumari M, Shipley M, Elbaz A, et al. Interleukin‐6 and C‐reactive protein as predictors of cognitive decline in late midlife. Neurology 2014;83:486–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Byram KW, Oeser AM, Linton MF, Fazio S, Stein CM, Ormseth MJ. Exercise is associated with increased small HDL particle concentration and decreased vascular stiffness in rheumatoid arthritis. J Clin Rheumatol 2018;24:417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Metsios GS, Stavropoulos‐Kalinoglou A, Sandoo A, van Zanten JJ, Toms TE, John H, et al. Vascular function and inflammation in rheumatoid arthritis: the role of physical activity. Open Cardiovasc Med J 2010;4:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kelley GA, Kelley KS, Hootman JM. Effects of exercise on depression in adults with arthritis: a systematic review with meta‐analysis of randomized controlled trials. Arthritis Res Ther 2015;17:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weinstein AA, Drinkard BM, Diao G, Furst G, Dale JK, Straus SE, et al. Exploratory analysis of the relationships between aerobic capacity and self‐reported fatigue in patients with rheumatoid arthritis, polymyositis, and chronic fatigue syndrome. PM R 2009;1:620–8. [DOI] [PubMed] [Google Scholar]
- 35. Verhoeven F, Tordi N, Prati C, Demougeot C, Mougin F, Wendling D. Physical activity in patients with rheumatoid arthritis. Joint Bone Spine 2016;83:265–70. [DOI] [PubMed] [Google Scholar]
- 36. Singh‐Manoux A, Dugravot A, Ankri J, Nabi H, Berr C, Goldberg M, et al. Subjective cognitive complaints and mortality: does the type of complaint matter? [research support]. J Psychiatr Res 2014;48:73–8. [DOI] [PubMed] [Google Scholar]
- 37. Cull A, Hay C, Love SB, Mackie M, Smets E, Stewart M. What do cancer patients mean when they complain of concentration and memory problems? [original article]. Br J Cancer 1996;74:1674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Julian L, Merluzzi NM, Mohr DC. The relationship among depression, subjective cognitive impairment, and neuropsychological performance in multiple sclerosis. Mult Scler 2007;13:81–6. [DOI] [PubMed] [Google Scholar]
- 39. Middleton LS, Denney DR, Lynch SG, Parmenter B. The relationship between perceived and objective cognitive functioning in multiple sclerosis. Arch Clin Neuropsychol 2006;21:487–94. [DOI] [PubMed] [Google Scholar]
- 40. Reisberg B, Prichep L, Mosconi L, John ER, Glodzik‐Sobanska L, Boksay I, et al. The pre‐mild cognitive impairment, subjective cognitive impairment stage of Alzheimer's disease. Alzheimers Dement 2008;4 Suppl 1:S98–108. [DOI] [PubMed] [Google Scholar]
- 41. Smith GE, Petersen RC, Ivnik RJ, Malec JF, Tangalos EG. Subjective memory complaints, psychological distress, and longitudinal change in objective memory performance. Psychol Aging 1996;11:272–9. [DOI] [PubMed] [Google Scholar]
- 42. Minett TS, Da Silva RV, Ortiz KZ, Bertolucci PH. Subjective memory complaints in an elderly sample: a cross‐sectional study. Int J Geriatr Psychiatry 2008;23:49–54. [DOI] [PubMed] [Google Scholar]
- 43. Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc 2011;59:1612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Katz RS, Heard AR, Mills M, Leavitt F. The prevalence and clinical impact of reported cognitive difficulties (fibrofog) in patients with rheumatic disease with and without fibromyalgia. J Clin Rheumatol 2004;10:53–8. [DOI] [PubMed] [Google Scholar]


