Abstract
Objective
Identifying individuals at risk for cognitive decline, Mild Cognitive Impairment (MCI), and dementia due to Alzheimer’s disease (AD) is a critical need. Functional decline is associated with risk and can be efficiently assessed by participants and study partners (SPs). We tested the hypothesis that SP‐reported functional decline is an independent predictor of dementia risk and cognitive decline.
Methods
In 1048 older adults in the Alzheimer’s Disease Neuroimaging Initiative (ADNI), we measured associations between Everyday Cognition Scale scores (ECog, self‐ and SP‐reported versions) and (1) baseline and longitudinal change in neuropsychological test (NPT scores) across multiple cognitive domains; (2) diagnostic conversion to MCI or dementia. Models included Mini Mental Status Exam (MMSE) score and ApoE ε4 genotype (APOE) as predictors. Model fits were compared with and without predictors of interest included.
Results
SP‐reported ECog was the strongest predictor of cognitive decline across multiple domains, as well as diagnostic conversion. Self‐reported ECog was associated with baseline NPT scores in some cognitive domains, and diagnostic conversion to MCI in participants with biomarker evidence for AD (elevated brain β‐amyloid, Aβ). Models including SP‐reported ECog were significantly stronger at predicting outcomes.
Conclusions
SP‐reported functional decline is an independent indicator of cognitive decline and dementia risk, even when accounting for cognitive screening, genetic risk, demographics, and self‐report decline. The results provide a rationale for greater utilization of SP‐reported functional decline to identify those at risk for dementia due to AD and other causes.
Introduction
Identification of individuals at risk for cognitive decline, Mild Cognitive Impairment (MCI) and dementia, including dementia due to Alzheimer’s disease (AD), is a critical need. In older adults, functional decline is associated with AD pathology,1, 2, 3,and predicts future decline and disease progression.4 Furthermore, preservation of everyday functioning is important to patients and their families.5 Subjective functional decline can be measured by asking the individual him/herself or a study partner (SP) about recent changes in activities of daily living.
A number of validated instruments that measure subjective decline are used in AD clinical trials and research studies.6, 7, 8, 9, 10, 11 SP report of decline is associated with magnitude of cognitive impairment determined from neuropsychological test (NPT) assessment,12, 13 and with diagnosis of MCI and dementia due to AD.10, 13, 14, 15, 16 SP report of impairment is associated moderately with abnormal levels of AD biomarkers, such as Positron Emission Tomography (PET) β‐amyloid (Aβ) burden, and regional brain volume1, 17, 18, 19; although some studies fail to find such a relationship.20 Thus, there is accumulating evidence that the SP report of functional and cognitive abilities can provide important information for early identification of preclinical AD and for predicting objective cognitive status. The same relationship is not observed as consistently for self‐reported subjective cognitive and functional decline, where factors such as depressed mood21, 22, 23 and lack of awareness associated with dementia13, 24, 25 limit its usefulness.
Although previous studies have examined the association between SP‐reported cognitive and functional information and various outcomes related to cognitive decline and progression along the dementia disease continuum, the additional predictive power of SP‐report compared to other predictors has not been established. Understanding this is important because it will indicate whether the SP report, in the absence of additional clinical measures, can help identify those at risk for or with disease. Such findings would support the approach of several initiatives to identify people at risk remotely, such as through registries,26 including remotely collected, SP‐reported Everyday Cognition scale (SP‐ECog).27 Determining the predictive value of SP‐reported functional decline is likely to facilitate screening and recruitment for AD clinical trials, screening of older adults at risk for AD dementia and other dementias in various healthcare settings, and identification of individuals who are good candidates to receive future novel treatments. We investigated this issue using SP report of functional decline in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Novel aspects of our approach include (1) use of the ECog, which measures SP‐ and self‐reported subjective decline in instrumental activities of daily living that map to multiple cognitive domains; (2) inclusion of cognitive screening tool (Mini Mental Status Exam, MMSE) and genetic risk (APOE) in our multivariable models; and (3) analysis of the association between baseline SP report of subjective decline and future decline in NPT scores across multiple domains. We tested the a priori hypotheses that: SP‐ECog is significantly associated with baseline and longitudinal change in NPT scores, and diagnostic conversion from cognitively unimpaired (CU) to MCI or MCI to dementia. (2) SP‐ECog is significantly associated with outcomes, even when accounting for self‐reported ECog (Self‐ECog), MMSE, APOE, and participant demographics; (3) Models without SP‐ECog are significantly less powerful than those with SP‐ECog at predicting outcomes; (4) SP‐ECog is associated with cognitive decline and diagnostic conversion in Aβ+ participants. Analyses of associations in Aβ+ participants allow us to address the extent to which SP‐report subjective decline, measured by ECog, can specifically identify cognitive decline, MCI, and dementia that is likely due to AD.
Methods
Participants
Data used in the preparation of this manuscript were obtained from the ADNI database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public‐private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), PET, other biological markers, and clinical and NPT assessment can be combined to measure the progression of MCI and early AD. For up‐to‐date information, see http://www.adni-info.org. All ADNI data were downloaded from http://www.loni.usc.edu/ADNI between September–November 2018. We included all participants with Aβ PET imaging results, and completion of ECog (Table 1). Further details regarding ADNI inclusion and exclusion criteria can be found at http://adni.loni.usc.edu/methods/documents/.
Table 1.
Participants included in this study
| CU | MCI | Dementia | Total | P‐value | |
|---|---|---|---|---|---|
| Total N | 420 | 482 | 146 | 1048 | |
| Age, mean ± SD (range) | 73.8 ± 5.79 (56.2–90.0) | 72.4 ± 7.35 (55–91.4) | 74.6 ± 8.21 (55.7–90.3) | 73.3 ± 6.95 (55–91.4) | CU> MCI: 0.016 |
| MCI < DEM: 0.002 | |||||
| Female, n (%) | 220 (52.4%) | 197 (40.9%) | 60 (41.1%) | 477 (45.5%) | CU> MCI: <0.001 |
| MCI‐DEM: 0.96 | |||||
| Education, mean ± SD (range) | 16.5 ± 2.61 (6–20) | 16.1 ± 2.83 (6–20) | 15.7 ± 2.68 (9–20) | 16.2 ± 2.74 (6–20) | CU> MCI: 0.04 |
| MCI‐DEM: 0.12 | |||||
| MMSE | 29 ± 1.18 (24–30) | 27.8 ± 1.76 (24–30) | 23.1 ± 2.13 (19–28) | 27.6 ± 2.5 (19–30) | CU> MCI: <0.001 |
| MCI> DEM: <0.001 | |||||
| ApoE ε4+ | 117 (27.9%) | 238 (49.4%) | 97 (66.4%) | 452 (43.1%) | CU < MCI: <0.001 |
| MCI < DEM: <0.001 | |||||
| Aβ+, N (%) | 135 (36.1%) | 264 (61.7%) | 125 (85.6%) | 524 (50.0%) | CU < MCI: <0.001 |
| MCI < DEM: <0.001 | |||||
| SP‐ECog, mean ± SD (range) | 1.23 ± 0.335 (1–3.35) | 1.95 ± 0.759 (1–3.97) | 2.73 ± 0.662 (1.13–3.95) | 1.77 ± 0.794 (1–3.97) | CU < MCI: <0.001 |
| MCI < DEM: <0.001 | |||||
| Self‐ECog, mean ± SD (range) | 1.41 ± 0.336 (1–2.74) | 1.81 ± 0.56 (1–3.82) | 1.89 ± 0.6 (1–3.66) | 1.66 ± 0.531 (1–3.82) | CU < MCI: <0.001 |
| MCI‐DEM: 0.14 |
CU, Cognitively Unimpaired; MCI, Mild Cognitive Impairment; DEM, Dementia; MMSE, Mini Mental Status Exam. P‐values represent differences between indicated diagnostic groups, based on Mann–Whitney text for continuous variables or Chi‐square test for categorical variables. P values less than 0.05 are indicated by bold italics.
Everyday cognition scale
The ECog includes 39 questions, asked of both the participant and the SP, that measure changes in functional activities compared to 10 years ago using a five point severity scale. Functional activities map to four cognitive domains.7 Participants and SPs completed the ECog as part of their ADNI protocol: at baseline, month 6, and ongoing annual visits (new ADNI2 participants), or initial ADNI2 visit and ongoing annual visits (ADNI1 and ADNIGO follow‐up participants). For analyses using longitudinal ECog, 844 participants with at least two self‐ and SP‐ECog scores were included.
β‐Amyloid (Aβ)
Full details of acquisition and analysis of ADNI florbetapir PET image data were previously described28 and can be found at http://adni.loni.usc.edu/methods/. Briefly, 18F‐AV45 (florbetapir) images were collected at multiple sites. Mean cortical standardized uptake value ratio (SuVR) was derived by normalizing average retention values of cortical regions to retention value of whole cerebellum. Aβ was dichotomized as positive or negative (SuVR> 1.11 or less than < 1.11, respectively) using previously established thresholds to identify the presence of Aβ pathology.28, 29
Clinical diagnosis
Cognitively Unimpaired (CU) participants met ADNI inclusion criteria: MMSE scores of 24–30,31 Clinical Dementia Rating (CDR) of 0 and memory box score of 06; absence of major depressive disorder, memory dysfunction,32 impairment in activities of daily living, MCI, or dementia; no memory complaints aside from those common to other normal subjects of that age range. MCI was defined using Petersen criteria.33 Patients were required to demonstrate objective impairment on NPTs without evidence of impaired activities of daily living or dementia; scores of 24–30 on the MMSE; and 0.5 on the global CDR and CDR memory box score ≥ 0.5. Impairment on NPTs was defined as scores of 0.5‐1.5 standard deviations below education‐adjusted norms for early MCI and >1.5 standard deviations below education‐adjusted norms for late MCI on the Logical Memory II subscale (Delayed Paragraph Recall) of the Wechsler Memory Scale–Revised,32 Early MCI and late MCI participants were considered to be part of the MCI group for our analyses. Dementia was defined as memory complaint by the participant or SP that was confirmed by the SP, abnormal memory function documented by scoring below the education adjusted cutoff on the Logical Memory II subscale (Delayed Paragraph Recall) from the Wechsler Memory Scale–Revised, MMSE between 20 and 26 (inclusive), CDR of 0.5 or 1, and NINCDS/ADRDA criteria34 for probable AD.
Diagnostic conversion
Conversion from CU to MCI, and from MCI to dementia, were defined by the ADNI protocol. Briefly, ADNI site physicians reviewed current visit measures. When a conversion event was triggered, the site’s clinical monitor reviewed the CDR for that visit. The monitor resolved any issues with the site Principal Investigator and instructed the site to reverse the conversion if incorrectly reported. Ron Petersen, clinical core Principal Investigator for ADNI, then reviewed scores or asked for the clinical monitor to resolve scoring issues. The Conversion Committee then reviewed the participant’s diagnosis summary, progress report, and consensus diagnosis form. Differences in diagnosis were resolved.
Neuropsychological tests
Cognitive measures assessed included the MMSE,30 Alzheimer's Disease Assessment Scale‐cognitive subscale, 13‐item version (ADAS13), delayed memory score from the ADAS13 (ADAS dMem),33 immediate and delayed memory recall from the Wechsler Memory Scale (iLogMem, dLogMem), immediate and delayed Rey Auditory Verbal Learning Test (iAVLT, dAVLT),34 Trail Making Test parts A and B (Trails A and Trails B),35 Category Fluency,36 and, Boston Naming Test.37 Participants took NPTs according to the ADNI protocol (http://adni.loni.usc.edu/study-design/). Briefly, in ADNI1, all participants took NPTs at baseline; at months 3, 6, and 18; and at annual follow‐up visits thereafter. Additionally, ADNI1 CU participants took NPTs at months 24 and 36; MCI participants took NPTs at months 12 and 24. In ADNIGO, eMCI participants took NPTs at screening, baseline, months 3 and 6, and annual follow‐up thereafter. In ADNI2, CU and MCI participants took NPTs at baseline and 3 months, and annual follow‐up thereafter. Dementia participants in ADNI 2 took NPTs at baseline and months 3, 6, and 18 months. In ADNI3, all participants took NPTs at baseline and months 24 and 48. Additionally, MCI and dementia participants took NPTs at months 12 and 36; and MCI participants took NPTs at annual follow‐up thereafter.
APOE
APOE ε4 genotype was determined by blood test at ADNI screening visit. APOE was dichotomized as positive (at least one APOE ε4 allele) or negative (absence of any APOE ε4 alleles).
Statistical analysis
The objectives of the statistical analysis were to assess and compare magnitudes of the associations of age, education, MMSE, SP‐ECog, Self‐ECog, gender, and APOE with change in repeated NPTs, and, diagnostic conversion. Variables were compared between diagnostic groups using Mann–Whitney test for continuous variables and chi‐square tests for categorical variables.
Associations with NPTs
We fit separate linear mixed effects models to each NPT score, stratified by diagnostic group. Each model included random intercepts and time effects, main effect terms for Self‐ECog, SP‐ECog, age, education, MMSE, gender and APOE status, and interactions of each predictor with time. The time by predictor interaction terms were used to assess the magnitude of the association of each predictor with change in NPT score. Trails A and B scores were log transformed and estimates back transformed to percentage effect. We also performed similar analyses in a cohort limited to Aβ+ participants. We accounted for multiple comparisons (multiple NPTs) using false discovery rate analysis.
Associations with diagnostic conversion
Weibull survival regression models were used to accommodate interval censored observations.38 We did not observe the exact dates of conversion, only that the conversion happened between two study visits. To assess the associations of longitudinal changes in ECog with conversion, we obtained participant‐specific changes in each ECog variable by fitting linear mixed effects models with random intercepts and slopes and time as the single predictor with each ECog variable as an outcome.
Likelihood ratio tests
For the Weibull survival models described above, three variations of each model were run (1) including all variables: Age, education, MMSE, SP‐ECog, Self‐ECog, gender, APOE; (2) excluding SP‐ECog; (3) excluding Self‐ECog, APOE, and MMSE. The relative predictive strength of the various models was assessed using Likelihood Ratio (LR) tests. All analyses were completed using SAS Version 9.4.
Results
Associations between subjective decline and neuropsychological test scores
Overall results
We measured associations between ECog and NPT scores (baseline and longitudinal change in scores) in CU and MCI participants. In both CU and MCI, higher (worse) SP‐ECog scores were associated with greater decline in ADAS13, LogMem, and AVLT. In MCI, higher SP‐ECog scores were also associated with greater decline in Boston Naming and Trails B. (Fig. 1A). We also found associations between ECog (self‐ and SP‐reported) and baseline NPT test scores, summarized in Figure 1B. Besides ECog, additional variables were significantly associated with decline in some NPTs (Table 2).
Figure 1.
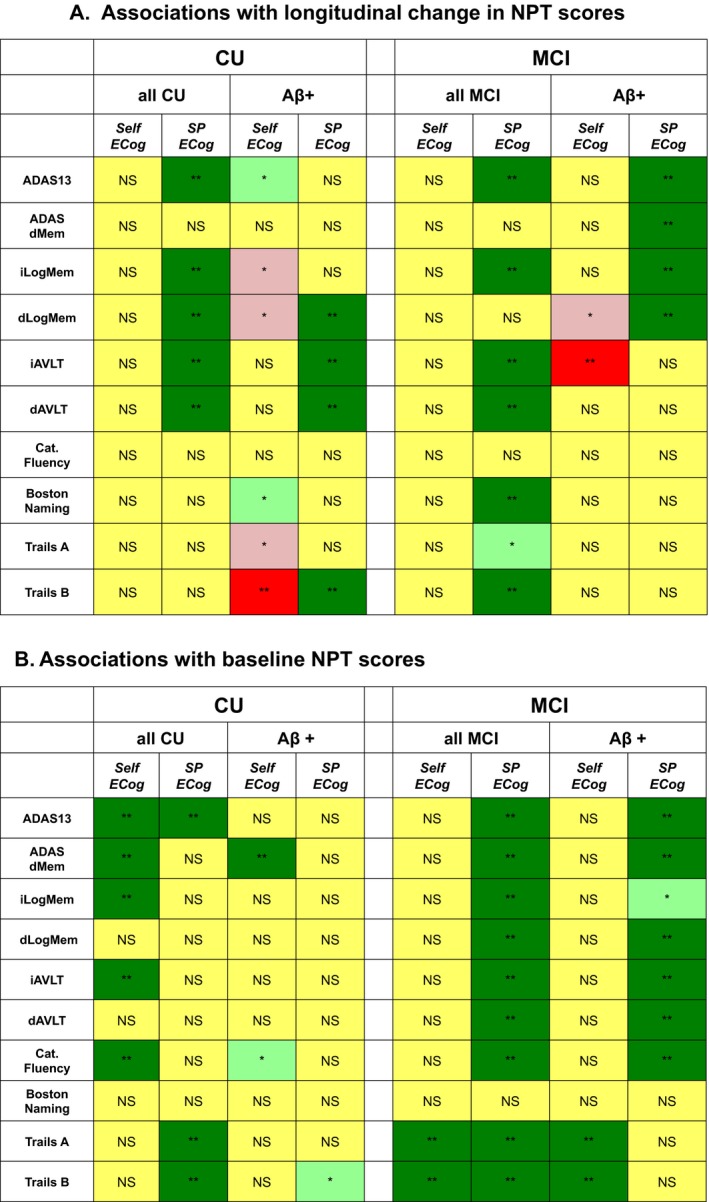
Associations between Neuropsychological test scores and ECog. Associations with longitudinal change in NPT scores (A) or baseline NPT scores (B) are color‐coded according to statistical significance in linear mixed effects models. Dark green (**): Higher (worse) ECog score associated with worse NPT score, P < 0.001, significant after multiple comparison correction. Light green (*): Higher (worse) ECog score associated with worse NPT score, P < 0.05, not significant after multiple comparison correction. Yellow (NS): No significant association between NPT score and ECog, P> 0.05. Pink (*): Higher (worse) ECog score associated with better NPT score, not significant after multiple comparison correction. Red (**): Higher (worse) ECog score associated with better NPT score, P < 0.001, significant after multiple comparison correction
Table 2.
Associations between predictors and longitudinal change in NPT scores
| CU | MCI | |
|---|---|---|
| ADAS13 | Age, Female | |
| ADAS dMem | Age, Female | |
| iLogMem | Age | |
| dLogMem | Age | |
| iAVLT | Edu | Age, Female |
| dAVLT | Age | |
| Category fluency | ||
| Trails A | Male | Age |
| Trails B |
Results of linear mixed effects models measuring the associations between the variables of age, gender, education, APOE, and MMSE; and change in NPT scores (columns) broken down by diagnostic group (CU, MCI). Variables indicated in each cell are those that had a significant variable by time from baseline score interaction term for a given NPT, P < 0.015 (remained significant after multiple comparison adjustment). Edu, Education; Age, advanced age was associated with greater decline in NPT scores; APOE, APOE+ was associated with greater decline in NPT scores; MMSE, lower MMSE scores were associated with greater decline in NPT scores; Male, greater decline in NPTs in males; Female, greater decline in NPTs in females.
Associations between subjective decline and NPT scores in Aβ+ participants
In order to determine whether ECog predicts cognitive decline in those at high risk for developing dementia due to AD, we examined associations between ECog and NPT scores in Aβ+ participants. In CU Aβ+ participants, higher SP‐ECog scores were associated with greater decline in dLogMem, iAVLT, dAVLT, and Trails B. Conversely, higher Self‐ECog scores were associated with lower levels of decline in Trails B (Fig. 1A). In MCI Aβ+ participants, higher SP‐ECog scores were associated with greater decline in ADAS13, ADAS dMem, iLogMem, and dLogMem. Higher Self‐ECog scores were associated with lower levels of decline in iAVLT. We also found associations between ECog (self‐ and SP‐reported) and baseline NPT test scores in Aβ+ participants, summarized in Figure 1B.
Associations between subjective decline and diagnostic conversion
Overall results
SP‐ECog was significantly associated with conversion from CU to MCI, and MCI to dementia (Table 3). In both cases, SP‐ECog was the strongest predictor of conversion based on magnitude of Hazard Ratios. In contrast, Self‐ECog was significantly associated with CU to MCI conversion, but not MCI to dementia conversion. Removing SP‐ECog from the model resulted in a significant decrease in the predictive value of the model predicting CU to MCI conversion (LR = 27, P < 0.001) and of the model predicting MCI to dementia conversion (LR = 108, P < 0.001). Removing Self‐ECog, MMSE, and APOE from the model, but retaining SP‐ECog in the model, resulted in a significant decrease in the model predictive power for predicting CU to MCI conversion (LR = LR = 14.8, P = 0.002), and MCI to dementia conversion (LR = 239, P < 0.001).
Table 3.
Associations with Diagnostic conversion
| Variable | Hazard ratio | 95% CI |
|---|---|---|
| CU to MCI: All participants | ||
| SP‐ECog | 3.21* | 1.83–6.83 |
| Self‐ECog | 3.10† | 1.57–7.80 |
| APOE+ | 1.27 | 0.80–2.44 |
| Age | 1.03 | 0.98–1.10 |
| MMSE | 1.00 | 0.85–1.25 |
| Edu | 0.90‡ | 0.85–0.97 |
| Female | 0.72 | 0.51–1.20 |
| CU to MCI: Aβ+ participants | ||
| Self‐ECog | 4.30† | 1.54–20.49 |
| SP‐ECog | 1.79 | 0.96–4.68 |
| APOE+ | 1.56 | 0.73–2.44 |
| Gender | 1.12 | 0.56–3.42 |
| Age | 1.07 | 0.99–1.21 |
| Edu | 0.96 | 0.88–1.11 |
| MMSE | 0.77 | 0.64–1.05 |
| MCI to dementia: All participants | ||
| SP‐ECog | 3.11* | 2.23–4.69 |
| APOE+ | 2.32† | 1.54–3.87 |
| Female | 1.67† | 1.16–2.65 |
| Edu | 1.06‡ | 1.00–1.14 |
| Age | 1.02 | 1.00–1.05 |
| Self‐ ECog | 0.97 | 0.76–1.34 |
| MMSE | 0.84† | 0.79–0.91 |
| MCI to dementia: Aβ+ participants | ||
| SP‐ECog | 2.49* | 1.72–4.02 |
| Female | 2.07* | 1.31–3.79 |
| APOE+ | 1.67* | 1.04–2.33 |
| Self‐ ECog | 1.16 | 0.84–1.79 |
| Edu | 1.10† | 1.02–1.21 |
| Age | 1.03 | 1.00–1.08 |
| MMSE | 0.80* | 0.75–0.89 |
Variables are listed in order of magnitude of Hazards Ratio. Edu, Education; CI, Confidence interval. Bolded values indicate significant associations, *P < 0.001, † P < 0.01, ‡ P < 0.05.
Associations between subjective decline and conversion in Aβ+ participants
In Aβ+ participants, Self‐ECog but not SP‐ECog was significantly associated with CU to MCI conversion (Table 3). Removing SP‐ECog from the model did not result in a significant decrease in the predictive value of the model (LR = 3.0, P = 0.082). Removing Self‐ECog, MMSE, and APOE from the model, but retaining SP‐ECog in the model, resulted in a significant decrease in the model predictive power (LR = 11.9, P = 0.008). In Aβ+ participants, SP‐ECog was significantly associated with MCI to dementia conversion (Table 3). A model excluding SP‐ECog was significantly less powerful at predicting conversion to dementia than the full model (LR = 27, P < 0.001). A model excluding Self‐ECog, MMSE, and APOE; but including SP‐ECog, was not significantly different than the full model (LR = 3.9, P = 0.27).
Longitudinal ECog
Models including longitudinal change in Self‐ECog and SP‐ECog instead of baseline measures yielded similar results for all outcomes measured. The one exception was for the analysis of CU to MCI conversion. In this case, removing longitudinal Self‐ECog, MMSE, and APOE from the model, but retaining longitudinal SP‐ECog in the model, resulted in no significant decrease in the model predictive power (LR = 4.2, P = 0.24).
Discussion
The major findings of this study are: (1) SP‐reported functional decline, measured using ECog, is associated with baseline cognition and cognitive decline across multiple domains; as well as diagnostic conversion to MCI or to dementia. The associations remain significant in multivariable models accounting for self‐reported functional decline (Self‐ECog), cognitive screening test (MMSE), genetic risk (APOE), and participant demographics. These results demonstrate that SP‐reported functional decline adds predictive power over and above more objective measures. (2) Conversely, self‐reported ECog is not significantly associated with cognitive decline across most domains, or with conversion from MCI to Dementia. Therefore, SP‐reported decline is superior to self‐report for identifying cognitive decline and dementia risk. (3) Associations between ECog and dementia risk‐related outcomes vary by Aβ status. This includes domain‐specific associations between ECog and cognitive decline in Aβ+ participants, as well as greater predictive value of Self‐ECog to identifying diagnostic conversion from CU to MCI in the Aβ+ subgroup. Taken together, these novel results provide a rationale for greater utilization of functional decline to identify those at risk for or with AD, and for development of improved methods to efficiently capture SP‐ and self‐reported data.
Associations with NPTs
Previous studies found associations between subjective decline and baseline cognition,13 or between cognitive complaints and NPT decline in CU and MCI.20, 39 Novel aspects of our study were identification of associations between subjective decline and decline in NPT scores across multiple cognitive domains; inclusion of many years of longitudinal NPT scores; use of the ECog, which measures changes in activities of daily living that map to multiple cognitive domains; and the use of multivariable models accounting for APOE, MMSE, and participant demographics.
Several interesting findings emerged from this approach. In CU, participants themselves more accurately identified their current memory and verbal fluency abilities compared to their SPs (Fig. 1B), but SPs were better than participants in identifying low baseline attention and executive function (Trails A and B), as well as predicting decline in overall cognition and memory (Fig. 1A). These results are novel and surprising, and do not support our a priori hypothesis that in CU participants, who are not likely to lack insight about their own functioning level, participant self‐report and SP‐report would be equally predictive of cognitive status and decline. Our findings contrast those of,40 which found that self‐reported subjective cognitive concerns predict future cognitive decline in the Preclinical Alzheimer’s Cognitive Composite (PACC). The differences may stem from differences in instruments used (ECog vs. Subjective Cognitive Complaints; individual NPTs vs. PACC) and in the study population.
In MCI, SP‐ECog scores were significantly associated with most baseline and longitudinal scores. Thus, in MCI SP‐report outperforms self‐report in identifying cognitive status across multiple cognitive domains, including memory. Surprisingly, there were no significant associations between participants’ self report of decline and actual decline in any NPTs for CU or MCI. (Fig. 1A). Our findings argue for greater reliance on SPs to recognize early changes in everyday functioning that map to future cognitive decline. Such information can be used to identify CU individuals at risk for cognitive decline for clinical trials and in various healthcare settings.
To determine the extent to which subjective decline, measured by ECog, specifically identifies cognitive decline associated with increased risk for AD, we measured the associations between ECog and NPT scores in Aβ+ participants. There were three major findings from these analyses. First, associations between SP‐ECog and cognitive decline in Aβ+ CU closely matched the associations found in the entire MCI group. This can be explained by the fact that Aβ+ CU have worse prognoses in terms of cognitive decline, and therefore more closely resemble MCI participants.
Second, in Aβ+ participants, for some NPTs, participants who self‐reported greater decline had significantly less objective decline in NPT scores over time. In MCI, this inverse relationship between self‐reported decline and actual decline in MCI is likely due to loss of insight about one’s own level of functioning. However, the inverse relationship between self‐report decline and actual decline in Aβ+ CU is surprising, and its cause requires further exploration. Aβ+ CU older adults are unlikely to have impairments affecting their insight about recent changes in their functional abilities. Their failure to report changes that reflect actual changes in cognition may therefore be due to other factors, such as anxiety or denial about recent changes in function.
Third, in Aβ+ MCI, significant associations between SP‐reported ECog and decline in NPT scores were restricted to ADAS13 and a few memory tests. This contrasts with findings in the overall MCI cohort, in which SP‐ECog is also significantly associated with decline in executive function (Trails) and lexical retrieval (Boston Naming). This finding may reflect the fact that the Aβ+ MCI groups are more amnestic than the overall MCI cohort. This idea is described in more detail below.
A major goal of this work was to assess the predictive power of SP‐ECog in relation to other variables. We found that in MCI, advanced age was significantly associated with greater decline in multiple NPTs, and females had significantly greater decline in some NPTs (Table 2). Conversely, MMSE, APOE, and education had limited predictive power, as shown by the lack of significant associations between these variables and longitudinal change in NPTs in our multivariable models. Therefore, in predicting future cognitive decline, subjective decline eclipses the contribution of genetic risk and cognitive screening instrument in identifying older adults at risk. These findings, argue compellingly for the use of SP‐reported decline to identify older adults at risk for cognitive decline, including CU individuals.
Associations with diagnostic conversion
Previous reports demonstrate that SP report of memory problems could distinguish CU from dementia due to AD and predict future diagnosis of AD dementia.41, 42 Few studies have explored the relationship between ECog and diagnostic conversion.14 In addition to the use of the ECog, the novelty of our multivariable approach was to identify the relative predictive power of subjective decline compared to other variables. We found that SP‐ECog was the strongest predictor of diagnostic conversion from CU to MCI or from MCI to dementia, compared to other variables. Furthermore, removing SP‐ECog from the models resulted in a significant decrease in the predictive power of both models.
In order to determine the ability of SP‐ECog and other predictors to specifically identify risk of conversion to MCI or dementia likely due to AD, we performed the same analyses in only Aβ+ participants. In the Aβ+ subgroup, only Self‐ECog significantly predicted conversion to MCI, whereas, SP‐ECog was significantly associated with conversion from MCI to dementia. Therefore, self report of subjective decline has a unique ability to predict conversion in Aβ+ CU. No variables other than ECog were significantly associated with CU to MCI conversion. The results argue for greater utilization of both self‐reported and SP‐reported subjective decline to identify older adults at risk for MCI likely due to AD.
What is the underlying cause of different results when we consider only Aβ+ individuals? A simple interpretation is that, since Aβ+ participants decline more than Aβ‐ participants, the association between predictors and outcomes is stronger in Aβ+ participants. As described above, this could explain why SP‐ECog is more strongly associated with cognitive decline in Aβ+ CU. But why, then, is SP‐ECog more strongly associated with diagnostic conversion to MCI in the overall cohort versus the Aβ+ subgroup? SP‐ECog may be detecting changes that lead to decline in cognition and conversion to MCI that is not due to AD, but is instead due to other causes. A related idea is that the ability of study partners to detect functional decline is somewhat cognitive domain specific. Although all ADNI MCI participants meet criteria for amnestic MCI, Aβ‐ participants are likely to be less amnestic than those who are Aβ+. SPs may be better able to predict conversion from CU to MCI in Aβ‐ participants because SPs can detect decline in domains other than memory, such as language, and attention. Some recent evidence supports the idea of domain specificity in subjective decline.43, 44 Further studies exploring the relationship between SP‐reported subjective decline across different domains and AD related outcomes, including biomarkers other than Aβ, are necessary to address these issues.
Possibility for remote collection
These results are especially important in the light of recent evidence for feasibility and validity of remotely collected ECog.26, 27 Since remote assessments can be administered efficiently to a large number of individuals using few resources, they could be used for longitudinal monitoring in clinical trials. Furthermore, use of SP‐reported decline provides the opportunity to identify decline in older adults reluctant to seek care themselves, has low influence from cultural or educational backgrounds, and can be applied widely in the community without requiring any formal clinical setting.41, 45 A crucial next step is to validate remote assessments against traditional in‐clinic assessments; such studies are ongoing using an online platform for remote assessments of participants and SPs.27
Limitations
We limited our subjective measure to ECog. In future studies, it will be important to include additional subjective measures, such as cognitive complaints, memory concerns, and behavioral and neuropsychiatric symptoms. These may confer additional predictive power compared to subjective decline.46, 47 Clinical diagnosis of CU and MCI in ADNI relies on Clinical Dementia Rating (CDR) scores, which are derived in part from SP‐ and self report of functional impairment. This could confound the association between ECog and diagnostic conversion. We did not explore the ability of ECog to predict other important biomarkers and risk factors, such as Tau and functional connectivity, which have been found to be significantly associated with self‐reported subjective cognitive decline, concerns, and complaints.48, 49 Finally, lack of diversity and the specific inclusion/exclusion criteria in ADNI somewhat limit generalizability of our results.
Conclusions
SP‐ECog significantly predicts cognitive decline across multiple domains, and diagnostic conversion to MCI or to dementia. SP‐ECog confers additional, independent predictive power over and above Self‐ECog, cognitive screening (MMSE), APOE, and demographics. Analyses limited to Aβ+ older adults confirm the predictive power of subjective decline in those at risk for AD, and suggest some cognitive‐domain specificity associated with subjective decline. These findings have important implications for using self‐ and SP‐reported measures to identify those at risk for cognitive decline, MCI, dementia, and dementia due to AD in clinical research and healthcare settings.
Author Contributions
RLN, JN, PSI, RSM, and MWW contributed to the conception and design of the study. RLN, CJ, and JN contributed to the analysis of data. RLN drafted a significant portion of the manuscript and figures. Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Conflicts of Interest
RLN, JN, PSI, JN, and RSM have no potential conflicts of interest to disclose related to the work in this manuscript. MWW reports personal fees from Bioclinica, personal fees from Cerecin/Accera, personal fees from Genentech, grants and personal fees from NIH, personal fees from Indiana University, personal fees from CHU Toulouse, personal fees from St. George University, personal fees from Eli Lilly, personal fees from Roche, personal fees from Lynch Group GLC, personal fees from Dolby Family Ventures, personal fees from Nestec/Nestle, personal fees from Health & Wellness Partners for American Academy of Neurology Conference, personal fees from AC Immune, personal fees from Alzheimer's Association, personal fees from Merck, personal fees from Bionest Partners, personal fees from Alzheon, Inc., personal fees from University of Tokyo, personal fees from Australian Catholic University, personal fees from University of Melbourne, personal fees from National Cntr for Geriatrics & Gerontology (Japan), grants from Department of Defense (DOD), grants from Johnson & Johnson (J&J), grants from Connie & Kevin Shanahan, grants from General Electric Research Labs (GE), grants from PCORI, grants from California Dept. of Public Health, grants from VUmc Vanderbilt University Medical Center, grants from University of Michigan, grants from Ray & Dagmar Dolby Fund, grants from Biogen, grants from Hillblom Foundation, grants from Stroke Foundation, grants from Siemens, grants from Veterans Administration. All relationships stated above are outside the submitted work.
Acknowledgments
This work was funded by the National Institutes of Health [K01AG055692]. Data collection and sharing for this project was funded by the ADNI [National Institutes of Health Grant U01 AG024904] and DOD ADNI [Department of Defense award number W81XWH‐12‐2‐0012]. ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol‐Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Funding information
This work was funded by the National Institutes of Health [K01AG055692]. Data collection and sharing for this project was funded by the ADNI [National Institutes of Health Grant U01 AG024904] and DOD ADNI [Department of Defense award number W81XWH‐12‐2‐0012]. ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol‐Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann‐La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co. Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org).
Funding Statement
This work was funded by Department of Defense grant W81XWH‐12‐2‐0012; National Institute on Aging grants K01AG055692 and U01 AG024904; National Institute of Biomedical Imaging and Bioengineering grant ; AbbVie grant ; Alzheimer’s Association grant ; Alzheimer’s Drug Discovery Foundation grant ; Araclon Biotech grant ; BioClinica, Inc. grant ; Biogen grant ; Bristol‐Myers Squibb Company grant ; CereSpir, Inc. grant ; Cogstate; Eisai Inc. grant ; Elan Pharmaceuticals, Inc. grant ; Eli Lilly and Company grant ; EuroImmun grant ; F. Hoffmann‐La Roche Ltd grant ; Genentech, Inc. grant ; Fujirebio grant ; GE Healthcare grant ; IXICO Ltd. grant ; Janssen Alzheimer Immunotherapy Research & Development, LLC. grant ; Johnson & Johnson Pharmaceutical Research & Development LLC. grant ; Lumosity grant ; Lundbeck; Merck & Co. Inc. grant ; Meso Scale Diagnostics, LLC. grant ; NeuroRx Research grant ; Neurotrack Technologies grant ; Novartis Pharmaceuticals Corporation grant ; Pfizer Inc. grant ; Piramal Imaging grant ; Servier grant ; Takeda Pharmaceutical Company grant ; Transition Therapeutics grant ; The Canadian Institutes of Health Research grant ; ADNI grant .
References
- 1. Okonkwo OC, Alosco ML, Griffith HR, et al. Cerebrospinal fluid abnormalities and rate of decline in everyday function across the dementia spectrum: normal aging, mild cognitive impairment, and Alzheimer disease. Arch Neurol 2010;67:688–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marshall GA, Lorius N, Locascio JJ, et al. Regional cortical thinning and cerebrospinal biomarkers predict worsening daily functioning across the Alzheimer's disease spectrum. J Alzheimer's Dis 2014;41:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lilamand M, Cesari M, del Campo N, et al. Brain amyloid deposition is associated with lower instrumental activities of daily living abilities in older adults. Results from the MAPT study. J Gerontol Ser A: Biol Sci Med Sci 2016;71:391–397. [DOI] [PubMed] [Google Scholar]
- 4. Farias ST, Mungas D, Reed BR, et al. Progression of mild cognitive impairment to dementia in clinic‐ vs community‐based cohorts. Arch Neurol 2009;66:1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dauphinot V, Delphin‐Combe F, Mouchoux C, et al. Risk factors of caregiver burden among patients with Alzheimer's disease or related disorders: a cross‐sectional study. J Alzheimer's Dis 2015;44:907–916. [DOI] [PubMed] [Google Scholar]
- 6. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2412. [DOI] [PubMed] [Google Scholar]
- 7. Farias ST, Mungas D, Reed BR, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology 2008;22:531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pfeffer RI, Kurosaki TT, Harrah CH Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol 1982;37:323–329. [DOI] [PubMed] [Google Scholar]
- 9. Jorm AF, Scott R, Cullen JS, MacKinnon AJ. Performance of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychol Med 1991;21:785–790. [DOI] [PubMed] [Google Scholar]
- 10. Amariglio RE, Donohue MC, Marshall GA, et al. Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer's Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol 2015;72:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology 2005;65:559–564. [DOI] [PubMed] [Google Scholar]
- 12. Dong Y, Pang WS, Lim LB, et al. The informant AD8 is superior to participant AD8 in detecting cognitive impairment in a memory clinic setting. J Alzheimer's Dis 2013;35:159–168. [DOI] [PubMed] [Google Scholar]
- 13. Rueda AD, Lau KM, Saito N, et al. Self‐rated and informant‐rated everyday function in comparison to objective markers of Alzheimer's disease. Alzheimers Dement 2015;11:1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marshall GA, Zoller AS, Kelly KE, et al. Everyday cognition scale items that best discriminate between and predict progression from clinically normal to mild cognitive impairment. Curr Alzheimer Res 2014;11:853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donovan NJ, Amariglio RE, Zoller AS, et al. Subjective cognitive concerns and neuropsychiatric predictors of progression to the early clinical stages of Alzheimer disease. Am J Geriatr Psychiatry 2014;22:1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomaszewski Farias S, Mungas D, Harvey DJ, et al. The measurement of everyday cognition: development and validation of a short form of the Everyday Cognition scales. Alzheimers Dement 2011;7:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vidoni ED, Honea RA, Burns JM. Neural correlates of impaired functional independence in early Alzheimer's disease. J Alzheimer's Dis 2010;19:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marshall GA, Olson LE, Frey MT, et al. Instrumental activities of daily living impairment is associated with increased amyloid burden. Dement Geriatr Cogn Disord 2011;31:443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perrotin A, Mormino EC, Madison CM, et al. Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol 2012;69:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gifford KA, Liu D, Hohman TJ, et al. A mutual self‐ and informant‐report of cognitive complaint correlates with neuropathological outcomes in mild cognitive impairment. PLoS ONE 2015;10:e0141831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoon JS, Charness N, Boot WR, et al. Depressive Symptoms as a Predictor of Memory Complaints in the PRISM Sample. J Gerontol B Psychol Sci Soc Sci 2017;00:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yates JA, Clare L, Woods RT, et al. Subjective memory complaints are involved in the relationship between mood and mild cognitive impairment. J Alzheimer's Dis 2015;48(Suppl 1):S115–S123. [DOI] [PubMed] [Google Scholar]
- 23. Jimenez‐Huete A, Del Barrio A, Riva E, et al. Subjective evaluation of mood and cognitive functions in a general neurology clinic: patients versus informants. J Clin Neurol 2017;13:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other‐reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry 2005;20:827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scherling CS, Wilkins SE, Zakrezewski J, et al. Decreased self‐appraisal accuracy on cognitive tests of executive functioning is a predictor of decline in mild cognitive impairment. Front Aging Neurosci 2016;8:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weiner MW, Nosheny R, Camacho M, et al. The Brain Health Registry: an internet‐based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement 2018;14:1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nosheny RLCM, Insel PS, Flenniken D, et al. Online study partner‐reported cognitive decline in the Brain Health Registry. Alzheimer's Dement. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Landau SM, Mintun MA, Joshi AD, et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol 2012;72:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Joshi AD, Pontecorvo MJ, Clark CM, et al. Performance characteristics of amyloid PET with florbetapir F 18 in patients with Alzheimer's disease and cognitively normal subjects. J Nucl Med 2012;53:378–384. [DOI] [PubMed] [Google Scholar]
- 30. Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 31. Wechsler D. WMS‐R Wechsler memory scale ‐ revised manual. New York: Harcourt Brace Jovanovich, Inc, 1987. [Google Scholar]
- 32. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 33. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–1364. [DOI] [PubMed] [Google Scholar]
- 34. Mungas D. Differential clinical sensitivity of specific parameters of the Rey Auditory‐Verbal Learning Test. J Consult Clin Psychol 1983;51:848–855. [DOI] [PubMed] [Google Scholar]
- 35. Gaudino EA, Geisler M, Squires NK. Construct validity in the Trail Making Test: what makes Part B harder? J Clin Exp Neuropsychol 1995;17:529–535. [DOI] [PubMed] [Google Scholar]
- 36. Welsh KA, Butters N, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology 1994;44:609–614. [DOI] [PubMed] [Google Scholar]
- 37. Williams BW, Mack W, Henderson VW. Boston naming test in Alzheimer's disease. Neuropsychologia 1989;27:1073–1079. [DOI] [PubMed] [Google Scholar]
- 38. Allison PD. Survival analysis using the SAS system: a practical guide, 2nd ed Cary, NC: SAS Press, 2011. [Google Scholar]
- 39. Hohman TJ, Beason‐Held LL, Lamar M, Resnick SM. Subjective cognitive complaints and longitudinal changes in memory and brain function. Neuropsychology. 2011;25:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Amariglio RE, Buckley RF, Mormino EC, et al. Amyloid‐associated increases in longitudinal report of subjective cognitive complaints. Alzheimers Dement (N Y) 2018;4:444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carr DB, Gray S, Baty J, Morris JC. The value of informant versus individual's complaints of memory impairment in early dementia. Neurology 2000;55:1724–1727. [DOI] [PubMed] [Google Scholar]
- 42. Tierney MC, Szalai JP, Snow WG, Fisher RH. The prediction of Alzheimer disease. The role of patient and informant perceptions of cognitive deficits. Arch Neurol. 1996;53:423–427. [DOI] [PubMed] [Google Scholar]
- 43. Shokouhi S, Conley AC, Baker SL, et al. The relationship between domain‐specific subjective cognitive decline and Alzheimer's pathology in normal elderly adults. Neurobiol. Aging 2019;81:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Valech N, Tort‐Merino A, Coll‐Padros N, et al. Executive and language subjective cognitive decline complaints discriminate preclinical Alzheimer's disease from normal aging. J Alzheimer's Dis. 2018;61:689–703. [DOI] [PubMed] [Google Scholar]
- 45. Razavi M, Tolea MI, Margrett J, et al. Comparison of 2 informant questionnaire screening tools for dementia and mild cognitive impairment: AD8 and IQCODE. Alzheimer Dis Assoc Disord 2014;28:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van Harten AC, Mielke MM, Swenson‐Dravis DM, et al. Subjective cognitive decline and risk of MCI: the Mayo Clinic Study of Aging. Neurology 2018;91:e300–e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Masters MC, Morris JC, Roe CM. "Noncognitive" symptoms of early Alzheimer disease: a longitudinal analysis. Neurology 2015;84:617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verfaillie SCJ, Pichet Binette A, Vachon‐Presseau E, et al. Subjective cognitive decline is associated with altered default mode network connectivity in individuals with a family history of Alzheimer's disease. Biol Psychiatry Cogn Neurosci Neuroimaging 2018;463–472. [DOI] [PubMed] [Google Scholar]
- 49. Swinford CG, Risacher SL, Charil A, et al. Memory concerns in the early Alzheimer's disease prodrome: regional association with tau deposition. Alzheimers Dement (Amst) 2018;10:322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]


