Abstract
Objective
We sought to evaluate the efficacy and tolerability of intranasal midazolam (in‐MDZ) as first‐line inhospital therapy in patients with status epilepticus (SE) during continuous EEG recording.
Methods
Data on medical history, etiology and semiology of SE, anticonvulsive medication usage, efficacy and safety of in‐MDZ were retrospectively reviewed between 2015 and 2018. Time to end of SE regarding the administration of in‐MDZ and ß‐band effects were analyzed on EEG and with frequency analysis.
Results
In total, 42 patients (mean age: 52.7 ± 22.7 years; 23 females) were treated with a median dose of 5 mg of in‐MDZ (range: 2.5–15 mg, mean: 6.4 mg, SD: 2.6) for SE. The majority of the patients suffered from nonconvulsive SE (n = 24; 55.8%). In total, 24 (57.1%) patients were responders, as SE stopped following the administration of in‐MDZ without any other drugs being given. On average, SE ceased on EEG at 05:05 (minutes:seconds) after the application of in‐MDZ (median: 04:56; range: 00:29–14:53; SD:03:13). Frequency analysis showed an increased ß‐band on EEG after the application of in‐MDZ at 04:07 on average (median: 03:50; range: 02:20–05:40; SD: 01:09). Adverse events were recorded in six patients (14.3%), with nasal irritations present in five (11.9%) and prolonged sedation occurring in one (2.6%) patient.
Conclusions
This pharmaco‐EEG–based study showed that in‐MDZ is effective and well‐tolerated for the acute treatment of SE. EEG and clinical effects of in‐MDZ administration occurred within 04:07 and 5:05 on average. Intranasal midazolam appears to be an easily applicable and rapidly effective alternative to buccal or intramuscular application as first‐line treatment if an intravenous route is not available.
Introduction
Status epilepticus (SE) is a major medical emergency associated with high mortality and morbidity.1, 2, 3 While the outcome of SE depends mainly on its underlying etiology,4 further predictors known to influence patient prognosis include age, consciousness, seizure semiology, and time of initial treatment.5, 6, 7, 8, 9 The latter is the only factor that can be addressed by the treating physician via the administration of a sufficient dose of a benzodiazepine such as clonazepam, diazepam, lorazepam, or midazolam by way of a route that results in a short brain entry time. Intravenous lorazepam is considered the drug of choice in hospitals and its efficacy and safety have been proven in different settings.10, 11, 12 However, the Rapid Anticonvulsant Medication Prior to Arrival Trial (RAMPART) showed, impressively, that obtaining intravenous access delays the onset of treatment and the time to SE control using intravenous lorazepam as compared with the use of intramuscular midazolam applied via a ready‐to‐use applicator.13 Since this applicator is currently not available in many countries, alternative nonintravenous application routes should be taken into consideration in clinical practice.14, 15, 16, 17, 18, 19 Various meta‐analyses concluded that intramuscular, buccal, or intranasal applications of benzodiazepines are reliable and safe options in acute seizure management and may be preferable over rectal and intravenous application.20, 21, 22, 23 Recently, a midazolam nasal spray of 5 mg/0.5 mL was approved by the United States Food and Drug Administration for the acute treatment of intermittent, stereotypic episodes of frequent seizure activity (e.g., seizure clusters, acute repetitive seizures) that are distinct from a patient's usual seizure pattern in patients with epilepsy aged 12 years or older.24
We have used a concentrated midazolam nasal spray (in‐MDZ) since 2008 for the prevention of seizure clusters and management of prolonged seizures in clinical routine during video‐electroencephalography (EEG) monitoring.25, 26 The aim of this study was to investigate the efficacy of in‐MDZ for first‐line inhospital treatment of SE and to assess the time to SE control and increase in ß activity in the EEG, respectively, as surrogate markers for midazolam brain entry time.
Methods
Study settings and design
This study was performed at the University Hospital Frankfurt, Germany. In clinical routines, in‐MDZ was used in patients with SE in whom the intravenous application of benzodiazepines was not possible without treatment delay. This applies to patients without intravenous access, with nonfunctional intravenous access, expected delay for preparation of intravenous lorazepam, nonpresence of a physician and expected delay to obtain a physician's order for intravenous treatment. This approach is in line with the current guidelines of the German Society for Neurology regarding SE.27, 28
This study was approved by the local ethics committee of Goethe‐University Frankfurt (Az.: 247/18). Due to the retrospective study design, informed consent for analysis of the data used was not necessary. This research was not sponsored or funded by any company. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed to enhance the quality and standardization of the reporting of this observational study.29
A standardized questionnaire was used to ascertain data for each patient regarding SE etiology; epilepsy syndrome; anticonvulsant treatment; Charlson Comorbidity Index (CCI); SE Severity Score (STESS);30 modified Rankin Scale (mRS); EEG, video, and electrocardiogram (ECG) findings; treatment success; and side effects upon treatment with in‐MDZ.
Definition of SE, inclusion and exclusion criteria
The latest International League against Epilepsy (ILAE) definitions for seizures, epilepsy syndromes, and SE including classification into convulsive, focal motor, and nonconvulsive with and without coma were applied.31, 32, 33 Furthermore, the subcategory of typical absence status was provided as this diagnosis carries an excellent prognosis. Regarding seizure duration, we followed the SE ILAE definition and considered all tonic‐clonic seizures lasting for more than five minutes and focal seizures with impaired consciousness or absence seizures for more than 10 min as SE.33 All patients were included in whom the initial clinical diagnosis of SE was confirmed by EEG. We analyzed all patients with SE who were treated with in‐MDZ during EEG recording between August 2015 and March 2018. Patients showing SE due to acute hypoxic‐ischemic encephalopathy from cardiorespiratory arrest were excluded.
EEG analysis and outcome parameters
EEG and ECG data were collected in all patients, while additional video data were recorded in selected cases. Patients were monitored with scalp electrodes according to the 10–20 international system. To evaluate the efficacy of in‐MDZ, we analyzed the duration of SE after the administration of in‐MDZ by two board‐certified epileptologists who were blinded to both the patient outcomes and the therapies applied. Response to in‐MDZ was defined as a cessation of EEG SE pattern with no further administration of other benzodiazepines or anticonvulsants before SE cessation. Additionally, the duration of SE prior to the start of treatment with in‐MDZ was recorded based on EEG data (in cases where SE started during EEG recording) or based on clinical evidence of seizure onset.
The pharmacodynamic effects of in‐MDZ were assessed using quantitative EEG. The EEG data were averaged across all EEG sensors and a high‐pass filter (1 Hz) and a low‐pass filter (70 Hz) were applied. Spectral analysis for the frequency range 1 of 30 Hz in 0.5‐Hz frequency steps was performed using a Hanning window and frequency‐dependent window length (seven cycles per frequency bin) for each 50‐ms time bin. The absolute power was plotted for each patient individually and visually examined by two experienced investigators for the time(s) to onset of significant EEG effects in the ß‐band as previously described in healthy probands.34 The times of interest were set at 4 min prior and 14 min after the intranasal application of midazolam, respectively. For plotting we additionally applied Savitzky–Golay filtering, as implemented in MATLAB (first polynomial order, with a frame length of 25) to smooth out the noisy signal. EEG processing was performed using MATLAB 2012b (The MathWorks Inc., Natick, MA, USA) and the open‐source Matlab toolbox FieldTrip.35
We quantified the beta‐band power increase in one subject (with the best visible MDZ beta‐band effect and least amount of noise). We computed the MDZ‐related beta‐band increase as the relative change:
whereas the time before MDZ was set as the 4 min before MDZ application and the time after MDZ application was set as the 12 min after MDZ application. The beta‐band was chosen as 15–25 Hz. From this data, we determined the average amount of beta‐band increase as well as the peak frequency (within the beta‐band) and the time at which the beta‐band power increase starts.
Preparation of intranasal midazolam
The concentrated midazolam nasal spray was manufactured and supplied by the central pharmacy of the University Hospital Frankfurt. The formulation was composed as previously described 36 and adapted for in‐house use.25 The nasal spray contained midazolam hydrochloride in a mixture of water at a pH of 3.3 (adjusted with 1 N of hydrochloride acid). A ready‐to‐use nasal spray applicator (Zscheile & Klinger, Hamburg, Germany) delivered an equivalent dose of 2.5 mg of midazolam per puff (140 µL).
Statistical analysis
Data were analyzed using IBM SPSS Statistics, version 25.0 (IBM Corp., Armonk, NY, USA). Nominal data were characterized by number and percentage, while metric data were characterized by mean, median, range, and standard deviation. In the case of time specifications, data were presented as follows: hours:minutes:seconds. Univariate comparisons of proportions were performed using the chi‐squared test. The Mann–Whitney U test was used for comparisons of variables with nonnormal distribution. Two‐sided P‐values of less than 0.05 were considered to be statistically significant. We did not adjust for multiple comparisons.
Results
Patient characteristics
Forty‐two patients were treated with in‐MDZ as first‐line therapy for SE during continuous EEG recording. Their mean age was 52.7 years [range: 5–92 years; standard deviation (SD): 22.7] and 54.8% (n = 23) of them were women. Twenty‐five patients (59.5%) were diagnosed with epilepsy prior to their hospitalization, whereas 17 patients (40.5%) had no history of previous seizures or epilepsy. In a subset of 10 patients, SE occured during video‐EEG‐monitoring and withdrawal of AEDs to localize the seizure onset zone for potential surgical treatment,37 or to characterize the type of seizures.
Half of the patients (n = 21; 50%) had been diagnosed with a remote‐symptomatic (structural) cause, while seven patients (16.7%) had an acute symptomatic cause for SE. In the remaining patients, the SE etiology was unknown due to nonstructural epilepsy or a progressive disease (n = 14; 33.3%).
At admission, 18 patients (42.9%) were not taking any AEDs. In 10 patients (23.8%), AEDs were reduced during video‐EEG‐monitoring before onset of SE. The mean number of AEDs was 1.3 (range: 0–3 AEDs; SD: 1.3) with four patients on AED monotherapy (9.5%) and 20 (47.6%) on polytherapy. Levetiracetam (n = 15), lamotrigine (n = 8), valproate (n = 6), brivaracetam (n = 6) and lacosamide (n = 4) were the five mainly used AEDs before onset of SE.
A mild, moderate, or severe disability as measured by the mRS (score: 2–5 points) was present in 21 patients on admission, while the CCI showed a low to moderate (score: 1–4 points) number of comorbidities in 25 patients and multiple or more severe diseases in five patients. STESS score was favorable (0–2 points) in 23 patients (54.8%) and unfavorable (3–6 points) in 19 patients (45.2%). For further details, please refer to Table 1.
Table 1.
Clinical and status epilepticus characteristics in all patients, responders, and nonresponders.
| Results | All patients (n = 42) | Responders (n = 24) | Nonresponders (n = 18) | P‐value | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | ||
| Age in years | 52.7 ± 22.7 | 5–92 | 53.7 ± 25.5 | 5–92 | 51.3 ± 19 | 24–82 | 0.75 |
| Duration of SE (if onset < 10 h) | 00:46:15 ± 00:55:53 | 00:05:49–02:30:00 | 00:53:36 ± 01:00:01 | 00:05:49–02:30:00 | 00:16:49 ± 00:13:54 | 00:06:46–00:32:41 | 0.07 |
| Dose of in‐MDZ | 6.4 ± 2.6 | 2.5–15 | 5.6 ± 1.8 | 2.5–10 | 7.5 ± 3.1 | 5–15 | 0.02 |
| Time to cessation of SE after in‐MDZ | ‐‐ | ‐‐ | 00:05:05 ± 00:03:10 | 00:00:29–00:14:53 | ‐‐ | ‐‐ | |
| Recording time after in‐MDZ | 5:06:13 ± 09:02:37 | 00:02:17–24:00:00 | 06:18:00 ± 09:48:13 | 00:02:17–24:00:00 | 03:30:30 ± 7:54:22 | 00:04:59–24:00:00 | 0.33 |
| Length of stay in days | 16.3 ± 14.4 | 2–80 | 13 ± 11.4 | 2–50 | 20.7 ± 16.8 | 3–80 | 0.12 |
| Number | Percentage | Number | Percentage | Number | Percentage | ||
|---|---|---|---|---|---|---|---|
| n | 42 | 100 | 24/42 | 57.1 | 18/42 | 42.9 | |
| Male gender | 19 | 45.2 | 8 | 33.3 | 11 | 61.1 | 0.07 |
| Preexisting epilepsy | 25 | 59.5 | 13 | 54.2 | 12 | 66.7 | 0.42 |
| Charlson Comorbidity Index | 0.81# | ||||||
| 0 | 12 | 28.6 | 6 | 25 | 6 | 33.3 | |
| 1–4 | 25 | 59.5 | 14 | 58.3 | 11 | 61.1 | |
| >4 | 5 | 11.9 | 4 | 17.7 | 1 | 5.6 | |
| mRS (admission) | 1.00# | ||||||
| 0 | 15 | 35.7 | 9 | 37.5 | 6 | 33.3 | |
| 1 | 6 | 14.3 | 3 | 12.5 | 3 | 16.7 | |
| 2–5 | 21 | 50 | 12 | 50 | 9 | 50 | |
| Etiology of SE | 0.80# | ||||||
| Structural epilepsy | 21 | 50 | 13 | 54.2 | 8 | 44.4 | |
| Nonstructural epilepsy | 7 | 16.7 | 2 | 8.3 | 5 | 27.8 | |
| Acute symptomatic | 7 | 16.7 | 5 | 20.8 | 2 | 11.1 | |
| Progressive tumor | 5 | 11.9 | 3 | 12.5 | 2 | 11.1 | |
| Unknown | 2 | 4.8 | 1 | 4.2 | 1 | 5.6 | |
| STESS | 0.47 | ||||||
| Favorable (0–2) | 23 | 54.8 | 12 | 50 | 11 | 61.1 | |
| Nonfavorable (3–6) | 19 | 45.2 | 12 | 50 | 7 | 38.9 | |
| AED | 0.56# | ||||||
| No AED | 18 | 42.9 | 12 | 50 | 6 | 33.3 | |
| Monotherapy | 4 | 9.5 | 2 | 8.3 | 2 | 11.1 | |
| 2 AEDs | 10 | 23.8 | 7 | 29.2 | 3 | 16.7 | |
| 3 or more AEDs | 10 | 23.8 | 3 | 12.5 | 7 | 38.9 | |
| SE semiology | 0.62# | ||||||
| NCSE | 24 | 55.8 | 15 | 62.5 | 9 | 50 | |
| FMSE | 13 | 31 | 7 | 29.2 | 6 | 33.3 | |
| ASE | 3 | 7.1 | 2 | 8.3 | 1 | 5.6 | |
| GTCSE | 2 | 4.8 | 0 | 0 | 2 | 11.1 | |
| EEG lateralization | 0.86# | ||||||
| Bilateral | 24 | 55.8 | 14 | 58.3 | 10 | 55.6 | |
| Left | 11 | 26.2 | 7 | 29.2 | 4 | 22.2 | |
| Right | 7 | 16.7 | 3 | 12.5 | 4 | 22.2 | |
| SE duration> 10 h or unknown | 27 | 64.3 | 12 | 50 | 15 | 83.3 |
0.056 |
| Nasal irritation | 5 | 11.9 | 5 | 20.8 | 0 | 0 | 0.113 |
| mRS (discharge) | 0.89# | ||||||
| 0 | 16 | 38.1 | 9 | 37.5 | 7 | 38.9 | |
| 1 | 2 | 4.8 | 2 | 8.3 | 0 | 0 | |
| 2–5 | 19 | 45.2 | 9 | 37.5 | 10 | 55.6 | |
| 6 | 5 | 11.9 | 4 | 17.7 | 1 | 5.6 | |
| Disposition | 0.75# | ||||||
| Home | 21 | 0.5 | 13 | 54.2 | 8 | 44.4 | |
| Rehabilitation | 6 | 14.3 | 4 | 16.7 | 2 | 11.1 | |
| Nursery home | 4 | 9.5 | 2 | 8.3 | 2 | 11.1 | |
| Palliative care | 5 | 11.9 | 1 | 4.2 | 4 | 22.2 | |
| Death | 5 | 11.9 | 4 | 16.7 | 1 | 5.6 | |
| ICU of another hospital | 1 | 2.4 | 0 | 0 | 1 | 5.6 |
SD, standard deviation; SE, status epilepticus; in‐MDZ, intranasal midazolam; mRS, modified Rankin Scale; STESS, Status Epilepticus Severity Score; AEDs, antiepileptic drugs; NCSE: nonconvulsive SE; FMSE: focal motor SE; ASE: typical absence SE; GTCSE: generalized tonic‐clonic SE; ICU: intensive care unit; # (chi‐squared test: CCI 0 vs ≥ 1; mRS 0–1 vs. ≥ 2; AEDs 0–1 vs. ≥ 2; Semiology nonconvulsive vs other; etiology acute vs. other; EEG bilateral vs. unilateral; discharge home vs. other)
Characteristics of SE and of in‐MDZ applications
In 15 patients (35.7%), seizure onset was recorded on EEG and SE lasted 46 min on average (mean: 46:15; median: 18:48; range: 00:05:49–02:30:00; SD: 55:35) prior to the administration of in‐MDZ. In 27 patients (64.3%), SE onset was not recorded on EEG; based on clinical evidence of seizure onset, SE lasted for at least 10 h or an exceeding unknown timespan in these individuals.
The majority of patients suffered from nonconvulsive SE (n = 24; 55.8%). Thirteen patients presented with focal motor SE (31%), three patients presented with typical absence SE (7.1%), and two patients presented with generalized tonic‐clonic SE that continued as nonconvulsive SE (4.8%). In EEG, seizure activity was localized bilaterally in 24 patients (55.8%) and was restricted to either the left or right hemisphere in 18 patients (left: n = 11; 26.2% and right: n = 7; 16.7%).
In all patients, in‐MDZ was used as first‐line inhospital therapy, as it was assumed that intravenous administration would have caused a treatment delay. On average, 6.4 mg of in‐MDZ was administered (median: 5 mg; range 2.5–15 mg; SD: 2.6). The initial dose was mostly 5 mg (n = 38; 90.5% and n = 4: 2.5 mg; 9.5%), corresponding to one puff of the nasal spray per each nostril.
Outcomes
In total, 24 (57.1%) episodes of SE ceased on continuous EEG recording after the administration of in‐MDZ without any other drug being given prior to such. In these in‐MDZ responders, SE ceased on EEG at 05:05 after the application of in‐MDZ (median: 04:56; range: 00:29–14:53; SD: 03:10).
In three cases (7.1%), seizure activity stopped within 10 min after administering in‐MDZ, but, within this time, other anticonvulsants such as lorazepam, brivaracetam, or lacosamide were given, so these patients were considered as nonresponders to in‐MDZ. In 15 cases (35.7%), seizure activity was ongoing after the administration of in‐MDZ and EEG data were monitored for a median time of 13:20 (mean: 03:25:12; range: 00:04:59–24:00:00; SD 08:21:25). In the nonresponder group, 10 patients were treated with a higher dose of 7.5 or more mg of in‐MDZ (P = 0.02), whereas this was only the case in eight patients of the responder group. For details of responders and nonresponders, please refer to Table 1. Using the Kaplan–Meier method, we depicted the time to SE cessation (Fig. 1).
Figure 1.
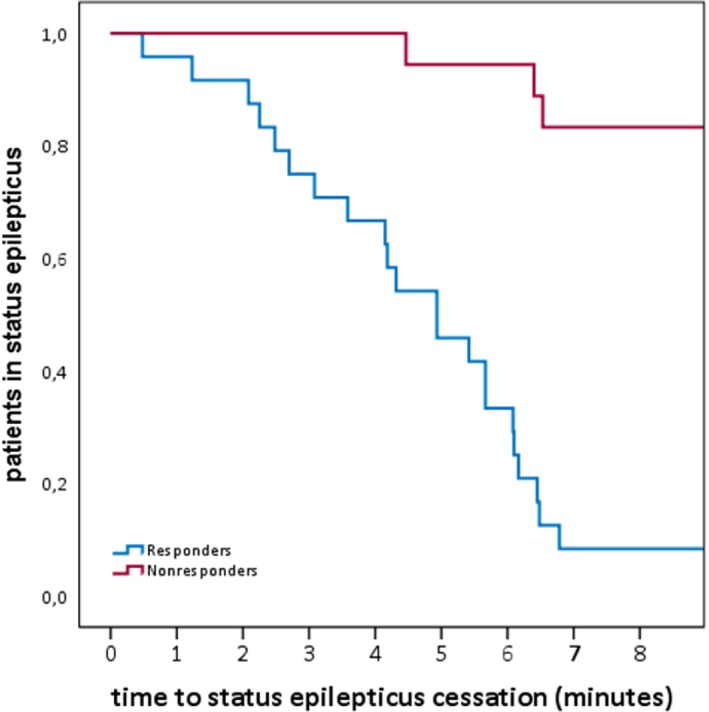
Kaplan–Meier graph showing the time from in‐MDZ application to the cessation of SE in patients rated as responders and nonresponders to in‐MDZ. In three cases rated as nonresponders, other anticonvulsants such as lorazepam, brivaracetam, or lacosamide were given prior to the end of SE.
After the administration of in‐MDZ, 15 patients showed an increase in diffuse slowing on EEG (35.7%), while, in 24 patients, the baseline activity remained stable (57.1%) and three patients showed an improvement in EEG background frequency (7.1%).
Adverse treatment‐emergent events were recorded in six patients (14.3%)—specifically, nasal irritations occurred in five patients (11.9%) and one patient (2.6%) presented with prolonged sedation. We did not observe any respiratory, circulation, or ECG abnormalities due to the administration of in‐MDZ.
Most of the patients (n = 21; 50%) were discharged to their home. Six patients were transferred to rehabilitation (14.3%), while nursing homes (n = 4; 9.5%) and palliative care (n = 5; 11.9%) were the destinations for nine patients. At the time of discharge, 18 patients showed no disability (mRS: 0–1 points; 42.9%), whereas 19 patients were disabled (mRS: 2–5 points; 45.2%). Five patients died during their hospital stay (case fatality rate at discharge: 11.9%).
Pharmaco‐EEG
Time‐frequency analysis of the EEG data recordings during an instance of SE and the application of MDZ was performed for all cases. In total, 24 complete EEG datasets were analyzed (55.8%), while 18 datasets (42.9%) had to be excluded due to artifacts. An in‐MDZ–related ß‐band increase was visible in the EEG of 10 out of 24 patients starting at 04:07 on average (median: 03:50; range: 02:20–05:40; SD: 01:09), one example is presented in Figure 2. The in‐MDZ–related ß‐band increase did not differ between the responder (n = 6, 13 patients with sufficient EEG quality) and the nonresponder (n = 4, 11 patients with sufficient EEG quality) groups. The quantification of the beta‐band increase was performed for one subject with best visible MDZ beta effect and least amount of noise in the EEG (Fig. 3). In this example subject, we found the MDZ‐related relative change in beta‐band power (15–25 Hz) to be on average 15.7%, whereas the maximum increase was 18.3% and found at 21 Hz. The beta‐band increase started roughly 04:30 min after the application of MDZ, stayed on a plateau for about 3 min, until 7.5 min after MDZ application and then diminishes back to baseline within 2 min (Fig. 3).
Figure 2.
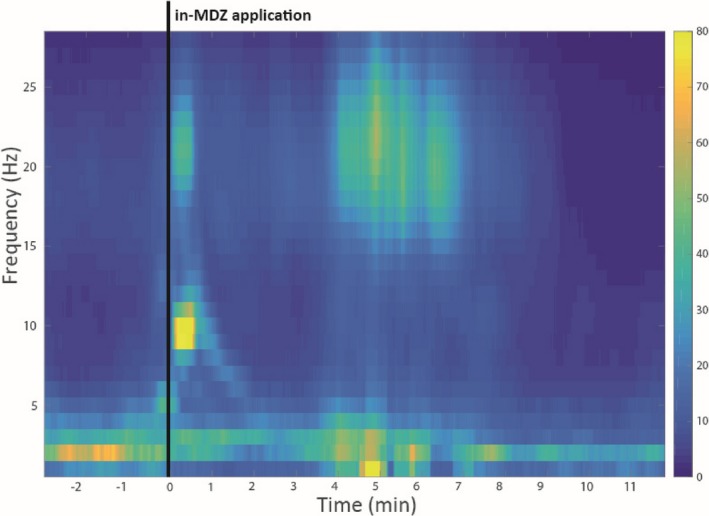
Beta‐band power increase in one example subject that had the least amount of noise. Beta‐band power increase is visible within a narrow band (18–25 Hz), starting 4 min after in‐MDZ application.
Figure 3.
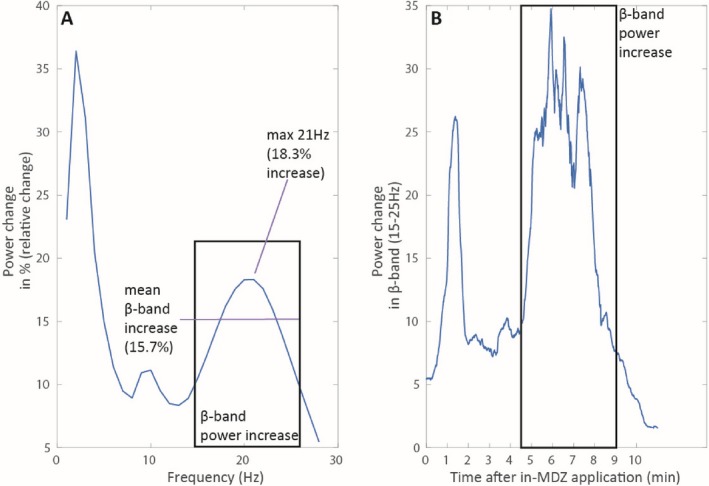
(A) Quantification of the signal increase over the whole frequency band (1‐30) Hz. For this, we calculated the relative change ([time_after MDZ – time_before MDZ]/ time_before MDZ) and plotted this relative change. The average increase in beta‐band power was 15.7%, the maximum increase was found at 21Hz at 18.3%. (B) Quantification of the beta‐band increase over time. The beta‐band power increase starts at 4.5 min after in‐MDZ application. It then stays on a plateau for about 3 min (until 7.5 min after in‐MDZ application) and diminishes back to baseline.
Discussion
This EEG‐based study showed that the administration of a median of 5 mg of concentrated in‐MDZ terminates an EEG‐proven SE within a median time of 5 min in 57% of cases. Our results are consistent with the Prehospital Treatment of Status Epilepticus (PHTSE) trial findings, which revealed efficacy rates of 59.1% for intravenous lorazepam and 42.6% for intravenous diazepam, as well as with the RAMPART study, which presented efficacy rates of 63.4% for intravenous lorazepam and 73.4% for intramuscular midazolam. For further details, please refer to Table 2.
Table 2.
Comparison of different studies regarding nonintravenous benzodiazepines.
|
Kay et al., current study |
Silbergleit et al., NEJM 2012 |
Lahat et al., BMJ 2000 |
Alldredge et al., NEJM 2001 |
Thakker et al., J Neurol 2013 | Holsti et al., Arch Pediatr Adolesc Med 2010 | de Haan et al., Epilepsia 2010 | Nakken et al., Acta Neurol Scand 2011 | Hardmeier et al., Clin Pharmacol Ther 2012 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Design | EEG‐based, single arm, r | RCT, p | RCT, p | RCT, p | RCT, p | RCT, p | randomized crossover, p | randomized, partly crossover, p | EEG‐based, healthy probands, p | |||||||||
| Year | 2015–2018 | 2009 –2011 | n.a.; 12 months | 1994–1999 | 2006 | 2006–2008 | n.a. | n.a.; 8 months | n.a. | |||||||||
| Country (city) |
Germany (Frankfurt) |
USA (multicenter) | Israel (Zerifin) | USA (multicenter) | India (Mumbai) | USA (Salt Lake City) | Netherlands (Heemstede) | Norway (Baerum) | Switzerland (Basel) | |||||||||
| Inclusion criteria | Children or adults with EEG‐proven SE (>5 minb,> 10 minc) | Children or adults with convulsions lasting> 5 min | Children with prolonged febrile seizures (> 10 min) | Adults with prolonged (>5 min) or repetitive generalized convulsions | Children with seizures> 10 min | Pediatric patients who were prescribed a home rescue medication | Adults with refractory epilepsy, who require rescue medication | Patients with seizures lasting more than 5 min | Healthy volunteers | |||||||||
| Primary outcome measure | Cessation of SE in EEG | Termination of seizures before arrival in the ED | Cessation of seizures | SE termination on arrival in the ED | Cessation of seizures and time from treatment to cessation | Total seizure time after medication administration | No clinical visible seizure activity within 15 minutes | Cessation of seizure activity within 10 minutes | Pharmacokinetic and pharmacodynamic measures | |||||||||
| Setting | IH | OH and ED | OH and ED | ED | OH | REC | REC | REC | IH | |||||||||
| Drug investigated | in‐MDZ | im‐MDZ | iv‐LZP | in‐MDZ | iv‐DZP | iv‐DZP | iv‐LZP | Placebo | in‐MDZ | iv‐DZP | in‐MDZ | r‐DZP | in‐MDZ | r‐DZP | r‐DZP | b‐MDZ | in‐MDZ | iv‐MDZ |
| Dose | 6.4 ± 2.6 mg (2.5–15) | 5 or 10 mg | 2 or 4 mg | 0.2 mg/kg | 0.3 mg/kg | 5 mg | 2 mg | 0.2 mg/kg | 0.3 mg/kg | 0.2 mg/kg | 0.3–0.5 mg/kg | 10 mg | 10 mg | 26 mg* (10–30 mg | 16 mg* (10–20 mg) | 3 or 6 mg | 5 mg | |
| Patients included | 42 | 448 | 445 | 21 | 23 | 68 | 66 | 71 | 27 | 23 | 50 | 42 | 21 (61)a | 21 (63)a | 18 (37)a | 16 (43)a | 12 (24)a | 12 |
|
Mean age in years (range) |
52.7 ± 22.7 (5–92) |
43 ± 22 (0–102) |
44 ± 22 (1v94) |
16* (6–38) months | 18* (6–40) months | 50.4 ± 19.1 | 49.9 ± 20.1 | 52.0 ± 18.2 | 3.8 ± 2.9 | 4.0 ± 3.3 | 5.6* (2.5–0.7)# | 6.9* (3.8–10.8)# | 40.2 | 40.2 | 42.4 | 29 (20–45) | ||
| Male gender, n (%) | 19; 45% | 198; 44% | 238; 53% | 13; 62% | 12; 52% | 41; 60% | 46; 70% | 42; 59% | 15; 56% | 12; 52% | 24; 48% | 22; 52% | 13; 62% | 13; 62% | 12; 55% | 12; 100% | ||
| History of seizures | 25; 60% | 293; 65% | 295; 66% | 17; 81% | 17; 74% | 48; 70% | 36; 55% | 47; 66% | 7; 26% | 6; 26% | 41; 82% | 32; 76% | 21; 100% | 21; 100% | 22; 100% | n.a. | ||
| Convulsive SE | 33% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | 100% | n.a. | n.a. | 100% | 100% | 46% | ‐ | ||
| Efficacy rate BZP | 24; 57% | 329; 73% | 282; 63% | 23/26; 88% | 24/26; 92% | 29; 43% | 39; 59% | 15; 21% | 18; 67% | 15; 65% | n.a. | n.a. | 50; 82% | 56; 89% | 30; 81% | 32; 74% | n.a. | |
| ß‐band increase | 04:07 mins (±01:09) | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 6.9 and 5.5 mins | 1.2 mins |
| Time to SE end | 05:05 mins (± 03:10) | n.a. | n.a. | 3.1 mins (± 1.8) | 2.5 mins (± 1.9) | n.a. | n.a. | n.a. | 3.0 mins (± 2.8) | 2.7 mins (± 2.3) | 3.0* mins (1.0–10)# | 4.3* mins (2.0–14.5)# | 4.6 mins (± 3.4) | 4.3 mins (± 3.4) | 5.0 min and 9 min | 2.8 min and 9.3 min | n.a. | |
| Adverse events (%) | 6; 14.3% nasal irritation | No acute side effects documented; total SAE in study: | None | None | Out‐of‐hospital complications: (hypotension, cardiac dysrhythmia, respiratory intervention) | Respiratory depression | Intubation |
Drowsiness, local irritation |
Tiredness, bitter taste, numbness in mouth | Local irritation | ||||||||
|
137/ 514; 26.7% |
156/ 509; 30.6% |
7; 10.3% | 7; 10.6% | 16; 22.5% | None | 1; 4.3% | 1; 0.02% | None |
40; 68% 17 (29%) |
34; 55% None |
18; 49% None |
9; 21% 9; 21% |
17; 71% | None | ||||
| Case fatality rate | 5; 11.9% | n.a. | 0; 0% | 0; 0% | 5; 7.7% | 3; 4.5% | 11; 15.7% | n.a. | n.a. | 0; 0% | 0; 0% | n.a. | None | |||||
Data are presented in the format of mean ± SD or as “number; percentage”; deviations are displayed as follows: *median # interquartile range
R,retrospective; RCT, randomized controlled trial; p, prospective; n.a., not available; IH, inhospital; OH, out‐of‐hospital; ED, emergency department; REC, residential epilepsy center; SE, status epilepticus; BZP, benzodiazepines; in‐, intranasal; r‐, rectal; b‐, buccal; im‐, intramuscular; iv‐, intravenous; MDZ, midazolam; LZP, lorazepam; DZP: diazepam.
Number of seizure episodes.
For convulsive SE.
For nonconvulsive SE.
The strength of our analysis is the investigation of in‐MDZ under continuous EEG monitoring, which allowed for not only the exact determination of time to SE control but also of the occurrence of ß‐band increase as an early surrogate marker for midazolam brain entry time. All technicians and physicians in our department have been well‐trained in the administration of the midazolam nasal spray since 2008,25 ensuring a high reliability of the in‐MDZ delivery. We were able to record and exactly define the SE cessation on EEG in all responders; furthermore, in 35.7% of the patients, the SE onset could be defined. This contrasts with the results of other studies on emergency treatment of prolonged seizures performed in an out‐of‐hospital setting that rely on observations made by laypeople and paramedics. Furthermore, the observed median time of 5 min to the cessation of SE in responders was supported by pharmaco‐EEG effects of in‐MDZ, which showed an increase in the ß‐band at a median time of 4 min. Out‐of‐hospital, non‐EEG–based studies show a cessation of convulsions between a mean of 2.5 and 5 min. For details, please refer to Table 2.
Like other benzodiazepines, midazolam enhances the efficacy of gamma‐aminobutyric acid (GABA). Intranasally administered, in‐MDZ bypasses the liver’s first‐pass effect and reaches the bloodstream via the nasal mucosa. Thus, its bioavailability is relatively high.38 In healthy probands, pharmacological studies have shown that in‐MDZ reaches its maximal plasma concentration after 7 to 15 min. As compared with intravenous application, the bioavailability of the nasal form is approximately 72.5% to 83%.38, 39, 40, 41 In addition to the rather favorable pharmacological profile, previous studies have shown that in‐MDZ is well‐tolerated and does not lead to serious adverse events. In our cohort, 11.9% suffered from nasal irritations (n = 5) and one patient (2.6%) experienced prolonged sedation. The rather low median dose of 5 mg in‐MDZ is an underdosage and might explain the low rate of relevant adverse events. Use of 10 mg of intramuscular MDZ in the RAMPART trial suggests that higher doses could have been used with good tolerability, which may have resulted in even better efficacy. Local irritations present as an expected adverse event due to the acidity of the in‐MDZ solution. In comparison with another study showing local irritations in 29% of patients (n = 17),42 11.9% is a relatively low rate and may be due to reduced awareness during SE and to the retrospective design of this study, which may account for an underdetection of adverse events. This is supported by the fact that nasal irritations were only reported in the responder group with cessation of SE.
Our study alleviates the lack of high‐quality data regarding the initial treatment of SE, especially nonconvulsive SE with benzodiazepines. This lack of data was due to benzodiazepines being predominantly used in the prehospital setting for prolonged and convulsive seizures, which do not necessarily match the criteria for SE and for which no EEG‐controlled data are available.10, 21, 43, 44, 45 On the other hand, the initial therapy of SE often involves benzodiazepines in combination with other anticonvulsants.46 Such a treatment approach makes it more difficult to determine if the benzodiazepine, the other anticonvulsants, or the combination of both terminated the SE.
Although our efficacy rates for in‐MDZ are consistent with results from the PHTSE trial10 and the RAMPART study,13 the patient populations probably differ significantly. Our study was conducted in the hospital, and it is likely that, in out‐of‐hospital studies, the duration of SE before drug administration is shorter than in our mixed patient cohort. Furthermore, the RAMPART trial and other first‐line therapy trials randomized patients with convulsive SE, which can be diagnosed easily on in a prehospital setting, while the majority of SE patients actually has nonconvulsive SE, requiring EEG for detection and therefore usually lasting longer at the time of treatment initiation.47 This may explain why prehospital studies reported slightly higher seizure termination rates of 63% to 83% for benzodiazepines.48, 49
Our study provides important findings for everyday clinical practice, as different studies have shown that nonintravenous benzodiazepines can be applied faster in comparison with intravenous solutions.13, 18 The rapid application of anticonvulsants is crucial for the treatment of SE, as the drug effectiveness is inversely proportional to the seizure duration.50, 51 Especially considering the time taken to prepare the intravenous solution or an intravenous line, the administration of intranasal midazolam may prevent treatment delay, which is an important risk factor in the context of a refractory SE.6 Taken together with the recommendations of the authors of the RAMPART study,52 the available evidence is generally supportive of nonintravenous midazolam administration in cases of SE.
Due to its retrospective design, this study has inherent limitations, as we were not able to randomize or blind the administration of in‐MDZ. Although we protocolled in detail the delivery of in‐MDZ and the administration of other anticonvulsants, we did not systematically obtain serum levels. However, this is offset by the analysis of the effect of in‐MDZ on the EEG power spectrum. The application of midazolam also has effects on oscillatory neuronal activity, and these effects are visible in the spectral representation of the EEG data. Such spectral power changes related to midazolam application were reported by Hardmeier et al.34 in healthy volunteers showing significant effects in the ß2‐band (18–25 Hz) at 5.5 and 6.9 min after receiving 6 and 3 mg of in‐MDZ, respectively. This is in line with our findings in SE patients, in whom we were able to detect the ß‐band at a median time of 4 min in 10 out of 42 patients. Difficulties in the detection of the ß‐band effects in a relatively high proportion of patients are common and are usually due to quality issues with the clinical EEG recordings in the emergency setting. Hardmeier et al. had to exclude three out of nine datasets despite studying healthy probands in a controlled environment, while Knoester et al. were only able to show an increase in EEG ß‐band power after in‐MDZ administration in one of five healthy probands.40 As the absence of the ß‐effect was equally distributed between responders and nonresponders, we can assume that differences are explained by interindividual differences within subjects and not related to responder status.
Conclusions
To our knowledge, this is the first pharmaco‐EEG–based study investigating the use of a nonintravenous benzodiazepine for the initial treatment of SE. Administration of a relatively low median dose of 5 mg in‐MDZ terminated an EEG‐proven SE within a median time of 05:05 in 57% of the cases, while relevant sedation was documented in only one of 42 patients. These data suggest that in‐MDZ may constitute a first‐line treatment alternative to buccal and intramuscular application if intravenous access is not in place. Further controlled trials should compare intranasal, buccal, and intramuscular administration to elucidate the fastest and most effective route.
Conflict of Interests
Dr. Kay reports nonfinancial support from Eisai, nonfinancial support from UCB, outside the submitted work. Dr. Merkel has nothing to disclose. A. von Blomberg has nothing to disclose. Dr. Bauer has nothing to disclose. Dr. Willems has nothing to disclose. Dr. Reif received personal fees from Eisai, outside the submitted work. Dr. Schubert‐Bast reports personal fees from UCB Pharma, Eisai, Desitin Pharma, LivaNova, and Zogenix, outside the scope of the submitted work. Dr. Rosenow reports personal fees and nonfinancial support from UCB Pharma, personal fees from Shire, personal fees from EISAI, personal fees from Desitin Arzneimittel, personal fees from Bial, personal fees from cerbomed, nonfinancial support from Novartis Japan, personal fees from Bayer Vital, personal fees from Sandoz, personal fees from Verband der forschenden Arzneimittleindustrie, grants from European Union, FP7, grants from Hessisches Ministerium für Wissenschaft und Kunst (LOEWE‐Programme), personal fees from GW Pharma, grants from Detlev‐Wrobel Fonds for Epilepsy Research, outside the submitted work. Dr. Strzelczyk reports grants and personal fees from Desitin Arzneimittel, personal fees from Eisai, grants and personal fees from GW pharmaceuticals, grants and personal fees from LivaNova, personal fees from Marinus pharmaceuticals, personal fees from Medtronic, grants and personal fees from Sage therapeutics, grants and personal fees from UCB pharma, grants and personal fees from Zogenix, outside the submitted work.
Acknowledgments
We are grateful to our patients, colleagues, and hospital staff for assistance in conducting this study and for the excellent technical support received for the EEG recordings.
Funding Information
This work was supported by the State of Hessen with a LOEWE‐Grant to the CePTER‐Consortium (https://www.uni-frankfurt.de/67689811).
References
- 1. Strzelczyk A, Ansorge S, Hapfelmeier J, et al. Costs, length of stay, and mortality of super‐refractory status epilepticus: a population‐based study from Germany. Epilepsia 2017;58:1533–1541. [DOI] [PubMed] [Google Scholar]
- 2. Knake S, Rosenow F, Vescovi M, et al. Incidence of status epilepticus in adults in Germany: a prospective, population‐based study. Epilepsia 2001;42:714–718. [DOI] [PubMed] [Google Scholar]
- 3. Schubert‐Bast S, Zöllner JP, Ansorge S, et al. Burden and epidemiology of status epilepticus in infants, children, and adolescents: a population‐based study on German health insurance data. Epilepsia 2019;60:911–920. [DOI] [PubMed] [Google Scholar]
- 4. Neligan A, Shorvon SD. Frequency and prognosis of convulsive status epilepticus of different causes: a systematic review. Arch Neurol 2010;67:931–940. [DOI] [PubMed] [Google Scholar]
- 5. Shorvon S. Super‐refractory status epilepticus: an approach to therapy in this difficult clinical situation. Epilepsia 2011;52(Suppl 8):53–56. [DOI] [PubMed] [Google Scholar]
- 6. Ferlisi M, Shorvon S. The outcome of therapies in refractory and super‐refractory convulsive status epilepticus and recommendations for therapy. Brain 2012;135(Pt 8):2314–2328. [DOI] [PubMed] [Google Scholar]
- 7. Kortland LM, Alfter A, Bähr O, et al. Costs and cost‐driving factors for acute treatment of adults with status epilepticus: a multicenter cohort study from Germany. Epilepsia 2016;57:2056–2066. [DOI] [PubMed] [Google Scholar]
- 8. Kellinghaus C, Rossetti AO, Trinka E, et al. Factors predicting cessation of status epilepticus in clinical practice: data from a prospective observational registry (SENSE). Ann Neurol 2019;85:421–432. [DOI] [PubMed] [Google Scholar]
- 9. Kortland LM, Knake S, von Podewils F, et al. Socioeconomic outcome and quality of life in adults after status epilepticus: a multicenter, longitudinal, matched case-control analysis from Germany. Front Neurol 2017;8:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out‐of‐hospital status epilepticus. N Engl J Med 2001;345:631–637. [DOI] [PubMed] [Google Scholar]
- 11. Treiman DM, Walker MC. Treatment of seizure emergencies: convulsive and non‐convulsive status epilepticus. Epilepsy Res 2006;68(Suppl 1):S77–82. [DOI] [PubMed] [Google Scholar]
- 12. Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med 1998;339:792–798. [DOI] [PubMed] [Google Scholar]
- 13. Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med 2012;366:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lahat E, Goldman M, Barr J, et al. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ 2000;321:83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thakker A, Shanbag P. A randomized controlled trial of intranasal‐midazolam versus intravenous‐diazepam for acute childhood seizures. J Neurol 2013;260:470–474. [DOI] [PubMed] [Google Scholar]
- 16. Holsti M, Dudley N, Schunk J, et al. Intranasal midazolam vs rectal diazepam for the home treatment of acute seizures in pediatric patients with epilepsy. Arch Pediatr Adolesc Med 2010;164:747–753. [DOI] [PubMed] [Google Scholar]
- 17. de Haan GJ, van der Geest P, Doelman G, et al. A comparison of midazolam nasal spray and diazepam rectal solution for the residential treatment of seizure exacerbations. Epilepsia 2010;51:478–482. [DOI] [PubMed] [Google Scholar]
- 18. Silbergleit R, Lowenstein D, Durkalski V, Conwit R. Neurological Emergency Treatment Trials I: RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial): a double‐blind randomized clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the prehospital treatment of status epilepticus by paramedics. Epilepsia 2011;52(Suppl 8):45–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ashrafi MR, Khosroshahi N, Karimi P, et al. Efficacy and usability of buccal midazolam in controlling acute prolonged convulsive seizures in children. Eur J Paediatr Neurol 2010;14:434–438. [DOI] [PubMed] [Google Scholar]
- 20. Arya R, Kothari H, Zhang Z, et al. Efficacy of nonvenous medications for acute convulsive seizures: a network meta‐analysis. Neurology 2015;85:1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haut SR, Seinfeld S, Pellock J. Benzodiazepine use in seizure emergencies: a systematic review. Epilepsy Behav 2016;63:109–117. [DOI] [PubMed] [Google Scholar]
- 22. Jain P, Sharma S, Dua T, et al. Efficacy and safety of anti‐epileptic drugs in patients with active convulsive seizures when no IV access is available: Systematic review and meta‐analysis. Epilepsy Res 2016;122:47–55. [DOI] [PubMed] [Google Scholar]
- 23. Kadel J, Bauer S, Hermsen AM, et al. Use of emergency medication in adult patients with epilepsy: a multicentre cohort study from Germany. CNS Drugs 2018;32:771–781. [DOI] [PubMed] [Google Scholar]
- 24. U.S. Food and Drug Administration : NAYZILAM (midazolam) Approved by FDA. 2019: https://www.accessdata.fda.gov/‐ New Drug Application (NDA): 211321.
- 25. Kay L, Reif PS, Belke M, et al. Intranasal midazolam during presurgical epilepsy monitoring is well tolerated, delays seizure recurrence, and protects from generalized tonic‐clonic seizures. Epilepsia 2015;56:1408–1414. [DOI] [PubMed] [Google Scholar]
- 26. Strzelczyk A, Kay L, Kellinghaus C, et al. Concepts for prehospital and initial in‐hospital therapy of status epilepticus. Neurol Int Open 2017;01:E217–E223. [Google Scholar]
- 27. Rosenow F. Status epilepticus im Erwachsenenalter In: Diener H. C., Weimar C.eds. Leitlinien für Diagnostik und Therapie in der Neurologie. Stuttgart: Thieme Verlag, 2012. [Google Scholar]
- 28. Möddel G, Kellinghaus C, Strzelczyk A. Initial treatment of status epilepticus. Z Epileptol 2018;31:245–249. [Google Scholar]
- 29. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 30. Rossetti AO, Logroscino G, Milligan TA, et al. Status epilepticus severity score (STESS): a tool to orient early treatment strategy. J Neurol 2008;255:1561–1566. [DOI] [PubMed] [Google Scholar]
- 31. Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia 2017;58:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International league against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia 2017;58:522–530. [DOI] [PubMed] [Google Scholar]
- 33. Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus – Report of the ILAE task force on classification of status epilepticus. Epilepsia 2015;56:1515–1523. [DOI] [PubMed] [Google Scholar]
- 34. Hardmeier M, Zimmermann R, Ruegg S, et al. Intranasal midazolam: pharmacokinetics and pharmacodynamics assessed by quantitative EEG in healthy volunteers. Clin Pharmacol Ther 2012;91:856–862. [DOI] [PubMed] [Google Scholar]
- 35. Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011;2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neues Rezeptur‐Formularium: Midazolam‐Rezepturen. Bundesvereinigung Deutscher Apothekerverbände e. V. , 2002.
- 37. Rosenow F, Bast T, Czech T, et al. Revised version of quality guidelines for presurgical epilepsy evaluation and surgical epilepsy therapy issued by the Austrian, German, and Swiss working group on presurgical epilepsy diagnosis and operative epilepsy treatment. Epilepsia 2016;57:1215–1220. [DOI] [PubMed] [Google Scholar]
- 38. Wermeling DP, Record KA, Kelly TH, et al. Pharmacokinetics and pharmacodynamics of a new intranasal midazolam formulation in healthy volunteers. Anest Analg 2006;103:344–349. [DOI] [PubMed] [Google Scholar]
- 39. Haschke M, Suter K, Hofmann S, et al. Pharmacokinetics and pharmacodynamics of nasally delivered midazolam. Br J Clin Pharmacol 2010;69:607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knoester PD, Jonker DM, Van Der Hoeven RT, et al. Pharmacokinetics and pharmacodynamics of midazolam administered as a concentrated intranasal spray. A study in healthy volunteers. Br J Clin Pharmacol 2002;53:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wermeling DP. Intranasal delivery of antiepileptic medications for treatment of seizures. Neurotherapeutics 2009;6:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mahmoudian T, Zadeh MM. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav 2004;5:253–255. [DOI] [PubMed] [Google Scholar]
- 43. Corrigan M, Wilson SS, Hampton J. Safety and efficacy of intranasally administered medications in the emergency department and prehospital settings. Am J Health Syst Pharm 2015;72:1544–1554. [DOI] [PubMed] [Google Scholar]
- 44. Lagae L. Overview of clinical efficacy and risk data of benzodiazepines for prolonged seizures. Epileptic Disorders 2017;16(1):44–49. [DOI] [PubMed] [Google Scholar]
- 45. Sofou K, Kristjansdottir R, Papachatzakis NE, et al. Management of prolonged seizures and status epilepticus in childhood: a systematic review. J Child Neurol 2009;24:918–926. [DOI] [PubMed] [Google Scholar]
- 46. Lowenstein DH, Alldredge BK. Status epilepticus at an urban public hospital in the 1980s. Neurology 1980s;43(3 Pt 1):483–488. [DOI] [PubMed] [Google Scholar]
- 47. Leitinger M, Trinka E, Giovannini G, et al. Epidemiology of status epilepticus in adults: a population‐based study on incidence, causes, and outcomes. Epilepsia 2019;60:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakken KO, Lossius MI. Buccal midazolam or rectal diazepam for treatment of residential adult patients with serial seizures or status epilepticus. Acta Neurol Scand 2011;124:99–103. [DOI] [PubMed] [Google Scholar]
- 49. Trinka E, Höfler J, Leitinger M, Brigo F. Pharmacotherapy for status epilepticus. Drugs 2015;75(13):1499–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen JW, Wasterlain CG. Status epilepticus: pathophysiology and management in adults. Lancet Neurol 2006;5:246–256. [DOI] [PubMed] [Google Scholar]
- 51. Smith RA, Martland T, Lowry MF. Children with seizures presenting to accident and emergency. J Accid Emerg Med 1996;13:54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Silbergleit R, Lowenstein D, Durkalski V, et al. Lessons from the RAMPART study–and which is the best route of administration of benzodiazepines in status epilepticus. Epilepsia 2013;54(Suppl 6):74–77. [DOI] [PMC free article] [PubMed] [Google Scholar]


