Abstract
Objective
Quality of life (QOL) and quality of care (QOC) in systemic lupus erythematosus (SLE) remains poor. Satisfaction with care (SC), a QOC surrogate, correlates with health behaviors and outcomes. This study aimed to determine correlates of SC in SLE.
Methods
A total of 1262 patients with SLE were recruited from various countries. Demographics, disease activity (modified Systemic Lupus Erythematosus Disease Activity Index for the Safety of Estrogens in Lupus Erythematosus: National Assessment trial [SELENA‐SLEDAI]), and QOL (LupusPRO version 1.7) were collected. SC was collected using LupusPRO version 1.7. Regression analyses were conducted using demographic, disease (duration, disease activity, damage, and medications), geographic (eg, China vs United States), and QOL factors as independent predictors.
Results
The mean (SD) age was 41.7 (13.5) years; 93% of patients were women. On the univariate analysis, age, ethnicity, current steroid use, disease activity, and QOL (social support, coping) were associated with SC. On the multivariate analysis, Asian participants had worse SC, whereas African American and Hispanic patients had better SC. Greater disease activity, better coping, and social support remained independent correlates of better SC. Compared with US patients, patients from China and Canada had worse SC on the univariate analysis. In the multivariate models, Asian ethnicity remained independently associated with worse SC, even after we adjusted for geographic background (China). No associations between African American or Hispanic ethnicity and SC were retained when geographic location (Canada) was added to the multivariate model. Canadian patients had worse SC when compared with US patients. Higher disease activity, better social support, and coping remained associated with better SC.
Conclusion
Greater social support, coping, and, paradoxically, SLE disease activity are associated with better SC. Social support and coping are modifiable factors that should be addressed by the provider, especially in the Asian population. Therefore, evaluation of a patient's external and internal resources using a biopsychosocial model is recommended. Higher disease activity correlated with better SC, suggesting that the latter may not be a good surrogate for QOC or health outcomes.
Significance & Innovations.
Social support and coping are positively correlated with satisfaction with care in systemic lupus erythematosus (SLE). Addressing these topics could be an opportunity to improve patients' satisfaction with care (SC), particularly among those at risk (eg, Asian patients), because both social support and coping are modifiable factors.
Paradoxically, higher disease activity was correlated with better SC. This may reflect greater health care use associated with greater disease activity rather than quality of care or overall health outcomes. Therefore, SC may not be a good index for tracking quality of care in SLE.
Geographic factors, cultural factors, socioeconomic status, and health care systems may influence SC, besides ethnicity or race. Canadian patients had worse SC compared with US patients but did not show differences by ethnicity or race. In contrast, Asian patients showed worse SC, even after we adjusted for geographic location of Asian patients in China, compared with those living in the United States. Education, a surrogate measurement for socioeconomic status, was not associated with SC.
Introduction
Systemic lupus erythematosus (SLE) has the potential to interfere with many important aspects of a patient's life, including their personal life as well as their physical, emotional, financial, and mental well‐being 1. With advances in diagnosis and treatment, the prognosis of SLE has significantly improved; however, patient‐reported outcomes (PROs) indicate that patients with SLE have significantly worse quality of life (QOL) compared with their age‐ and sex‐matched peers. Furthermore, the cumulative effect of SLE on patients' QOL may be worse than that of other common chronic diseases, such as congestive heart failure and depression 2, 3.
Quality of care (QOC) and patient experience are top priorities in health care, with the goal of improving QOL, which is considered an independent predictor of mortality in SLE 4. Unfortunately, QOC (evaluated as process‐based care) in SLE is significantly lagging in several areas 5 and is tied with damage accrual 6, 7. Patient satisfaction with care (SC) relates to how well our service meets the patients' expectations 8. SC is increasingly used as a surrogate for QOC because it may correlate with adherence, outcomes, and the patient‐physician relationship 9, 10, 11. For example, better patient satisfaction has been linked to higher regimen adherence in young, mostly ethnic minority patients with type 1 diabetes 10. Higher SC has also been shown to reduce hospital readmission rates 11.
SC may be affected by factors related to patients, providers, care processes, and the disease itself 12, 13. Given the propensity of SLE to cause a wide range of clinical manifestations with unpredictable flares 1, management can be challenging and involves collaboration between patients and their health care providers. There is a paucity of studies investigating SC in SLE. SC is associated with better health and vice versa 14, 15, but knowledge of the factors that drive SC in SLE is still lacking.
The purpose of this study was to determine the modifiable and nonmodifiable correlates of SC for patients with SLE and to identify areas for improvement. With the results, we aim to inform clinicians of these correlates, recognize those at higher risk for poor SC, and target any modifiable variables.
Patients and Methods
This study was approved by the site's institutional review board.
Patient characteristics
Cross‐sectional data were used from the Study of Outcomes in Lupus (SOUL) data repository. A total of 1262 consenting patients were recruited consecutively between 2009 and 2016 from multiple centers, including three sites in the United States as well as centers in Mexico, Argentina, Canada, the Philippines, Turkey, and China. All patients were 18 years of age or older and met the American College of Rheumatology (ACR) 1997 revised criteria for classification of SLE 16, 17.
Individuals completed assessments of demographics (age, sex, education, marital status), disease (duration), and PROs (LupusPROv1.7) at a single routine clinic visit. Consecutive patients seen in the rheumatology outpatient clinics were approached for participation in the LupusPRO study at each of the centers, and consenting patients were subsequently enrolled. The LupusPROv1.7 tool was validated at each of the participating sites for data collection. Treating rheumatologists provided information on comorbidities (diabetes, hypertension, coronary artery disease, psychiatric conditions, and fibromyalgia), the ACR classification criteria for SLE, current SLE medications (including steroids), disease activity (modified Systemic Lupus Erythematosus Disease Activity Index for the Safety of Estrogens in Lupus Erythematosus: National Assessment trial [SELENA‐SLEDAI]), and damage (Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index [SDI]).
The SELENA‐SLEDAI is a cumulative scoring index used to assess disease activity across a combination of clinical features and laboratory test results. It is composed of 24 items with descriptors that must be present at the time of visit or in the preceding 10 days. A higher score represents greater disease activity 18.
The SDI is a validated tool used to quantify damage that has occurred since the onset of SLE and has been present for at least 6 months, ascertained by clinical assessment. A higher score indicates greater damage and has been shown to correlate with higher mortality 19.
LupusPROv1.7 is a disease‐specific PRO questionnaire, comprised of 43 questions, that measures health‐related quality of life (HRQOL) and non‐HRQOL. Responses are ranked on a five‐point Likert scale. The HRQOL construct includes lupus symptoms/flares, lupus medications, cognition, procreation, physical and emotional health, pain, vitality, sleep, and body image domains. The non‐HRQOL construct includes desires/goals, planning, coping, social support, and SC domains. A higher total domain score indicates better QOL 20.
Outcome measure
SC was the primary outcome of interest and was measured using the corresponding domain (“satisfaction with care”) on LupusPROv1.7. This was assessed via four items: “My doctor was accessible when I had a question regarding my lupus,” “My doctor understood the impact of lupus on my life,” “My doctor provided me with the information I needed to understand my lupus,” and “My doctor discussed/monitored the side effects of lupus medicine/s.” Each item was ranked on a five‐point Likert scale ranging from 0, indicating “none of the time,” to 4, denoting “all of the time.” A sixth option of “not applicable” was available. These were pertinent to a 3‐month recall period 20. Because various domains of LupusPRO (including SC) may be correlated, for all reported analyses, we excluded SC domain scores from the other QOL domains on the LupusPRO.
Statistical analyses
Statistical analyses were performed using the IBM Statistical Package for the Social Sciences software (SPSS version 19; IBM Corporation). Descriptive summary statistics were obtained for the group. Univariate and multivariate linear regressions were performed; SC was used as as the dependent variable, whereas patient demographic (age, sex, ethnicity, education, comorbidities), disease (duration, disease activity, damage, medications), and self‐reported QOL (LupusPRO) variables were used as independent predictors. Education was used as a surrogate for socioeconomic status. Given the potential impact of different health care systems, culture, and socioeconomic status between the various countries where participants were recruited, we conducted a secondary analysis. We coded geographic locations for China, Canada, and the Philippines using US patients as a reference category and performed a univariate analysis for SC using geographic variables as predictor variables. Next, we performed the same multivariate analyses as mentioned above but with the addition of the geographic variable for China in model 1 and the geographic variable for Canada in model 2 to assess for independent predictors (including ethnicity and country) for SC. R 2 was used to determine the variance in SC attributed to each independent predictor. P ≤ 0.05 was considered significant on two‐tailed tests.
Results
Patient characteristics
Data were available for 1262 patients with SLE. The mean (SD) age at recruitment was 41.7 (13.5) years, and the majority (93.2%) of patients were women (Table 1). Ethnicities were as follows: Asian (48%), white (23%), Hispanic (18%), African American (10%), and other (1%). According to the ACR classification criteria, more than 70% of patients met arthritis and immunologic criteria. Hematologic manifestations were noted in 67% of patients; history of malar rash, in 56%; lupus nephritis, in 43%; and serositis, in 26%. A significant proportion of patients had comorbidities such as hypertension, psychiatric conditions, and fibromyalgia (33%, 23.8%, and 13.7%, respectively). Less than 40% of patients were receiving corticosteroids. Nearly 75% of participants were on hydroxychloroquine at the time of the study. Azathioprine (31.3%), mycophenolate mofetil (14.2%), methotrexate (4.8%), and cyclosporine (3.2%) were some of the other medications being used for SLE. The mean (SD) SELENA‐SLEDAI and SDI scores were 3.47 (4.12) and 0.90 (0.49), respectively. Summary scores are shown in Table 1.
Table 1.
Description of patients
| Demographics | Result |
|---|---|
| Age, mean (SD), y | 41.70 (13.46) |
| Female sex, % | 93.2 |
| Ethnicity, % | |
| White | 22.9 |
| African American | 9.7 |
| Asian | 48 |
| Hispanic | 17.8 |
| Other | 1.6 |
| Education level, % | |
| Less than high school | 20 |
| High school | 41.7 |
| College or university degree | 30.5 |
| Graduate degree or higher | 7.7 |
| Medications, % | |
| Currently using steroids | 37.3 |
| Ever used steroids | 74.2 |
| Hydroxychloroquine | 62.6 |
| Methotrexate | 4.8 |
| Azathioprine | 31.3 |
| Mycophenolate | 14.2 |
| Cyclophosphamide | 0.9 |
| Cyclosporine | 3.2 |
| Comorbidities, % | |
| Diabetes mellitus | 5 |
| Hypertension | 33 |
| Coronary artery disease | 4.8 |
| Psychiatric condition, including depression | 23.8 |
| Fibromyalgia | 13.7 |
| ACR criteria, % | |
| Malar rash | 55.9 |
| Discoid rash | 15.7 |
| Photosensitivity | 49.3 |
| Oral or nasal sores | 31.2 |
| Arthritis | 73.2 |
| Serositis | 26.1 |
| Renal disorder | 42.8 |
| Neurology disorder | 9.4 |
| Hematology disorder | 67.1 |
| Positive immunology | 78.8 |
| Positive ANA antibodies | 98.6 |
| Disease severity | |
| PGA, mean (SD) | 0.75(0.83) |
| Total SELENA‐SLEDAI score | 3.47(4.12) |
| Total SDI score | 0.90(1.49) |
Abbreviation: ACR, American College of Rheumatology; ANA, antinuclear; PGA, Physician Global Assessment; SDI, Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index; SELENA‐SLEDAI, modified Systemic Lupus Erythematosus Disease Activity Index for the Safety of Estrogens in Lupus Erythematosus: National Assessment trial.
SC and QOL
The mean (SD) SC was 64.02 (33.54), and 63% to 66% of patients responded favorably (“most” or “all of the time”) to each of the four SC domain items. The distribution of LupusPRO scores (mean [SD]) on other domains is shown in Figure 1.
Figure 1.
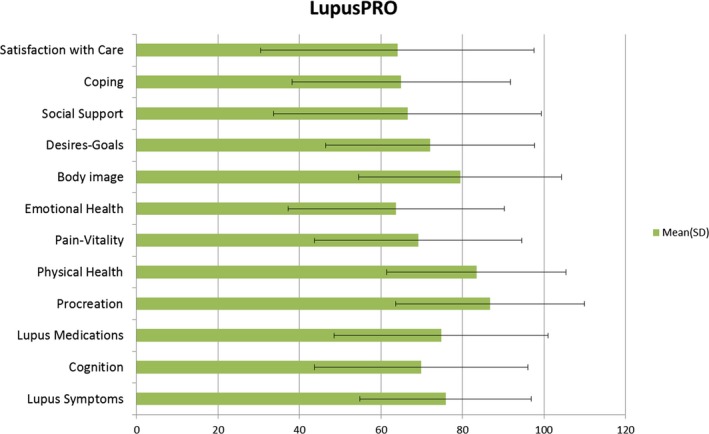
Quality of Life (including satisfaction with care) by using LupusPRO.
Correlates of SC
By using the univariate linear regression analyses, greater age was associated with worse SC. Education, a surrogate for socioeconomic status, and sex were not associated with SC. Asian patients had worse SC, whereas African American patients had better SC. Disease duration and damage did not correlate with SC, but current steroid use was associated with worse SC. Contrary to our expectation, greater disease activity and worse functioning of desires/goals on the QOL domain were associated with better SC. Better social support and coping on the QOL domain correlated with better SC (Table 2). Of all the variables, most variability in SC was explained by coping (R 2 0.20) and social support (R 2 0.14).
Table 2.
Predictors of satisfaction with care
| Variable | Univariate Analyses | Multivariate Analyses | ||||||
|---|---|---|---|---|---|---|---|---|
| R 2 | β | 95% CI | P | R 2 | β | 95% CI | P | |
| Age | 0.005 | −0.07 | −0.31 to −0.03 | 0.018 | 0.30 | −0.008 | −0.15 to −0.11 | 0.772 |
| Ethnicity or racea | ||||||||
| African American | 0.01 | 0.1 | 5.11 to 17.99 | <0.001 | 0.30 | 0.06 | 0.55 to 16.70 | 0.036 |
| Asian | 0.08 | −0.28 | −22.32 to −15.14 | <0.001 | 0.30 | −0.12 | −11.81 to −3.58 | <0.001 |
| Hispanic | 0.08 | 0.28 | 20.05 to 29.44 | <0.001 | 0.30 | 0.18 | 10.61 to 21.27 | <0.001 |
| Disease features | ||||||||
| Steroid use currently | 0.02 | −0.13 | −12.42 to −4.77 | <0.001 | 0.30 | −0.034 | −5.71 to 1.13 | 0.19 |
| Total SLEDAI score | 0.02 | 0.15 | 0.72 to 1.65 | <0.001 | 0.30 | 0.09 | 0.35 to 1.18 | <0.001 |
| QOL | ||||||||
| LupusPRO‐COG | 0.004 | 0.06 | 0.01 to 0.15 | 0.028 | 0.30 | 0.03 | −0.03 to 0.11 | 0.27 |
| LupusPRO‐DG | 0.01 | −0.12 | −0.22 to −0.08 | <0.001 | 0.30 | −0.09 | −0.19 to −0.05 | 0.001 |
| LupusPRO‐SS | 0.14 | 0.37 | 0.33 to 0.43 | <0.001 | 0.30 | 0.14 | 0.08 to 0.20 | <0.001 |
| LupusPRO‐CO | 0.20 | 0.44 | 0.49 to 0.62 | <0.001 | 0.30 | 0.29 | 0.29 to 0.44 | <0.001 |
Abbreviation: CI, confidence interval; CO, Coping; COG, Cognition; DG, Desires‐Goals; QOL, quality of life; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SS, Social Support.
Other ethnicity or race as a reference.
On the multivariate regression analysis, independent predictors of SC were ethnicity, disease activity, and QOL. Directions of these correlations were the same as those in the univariate analysis. Age and current steroid use did not retain association with SC in this multivariate model (Table 2). Collectively, these accounted for 30% of variability in SC.
In our secondary analysis, US patients fared better than non‐US patients on SC in the univariate analysis (β 0.34; 95% confidence interval [CI] 21.19‐28.94; P < 0.001; R 2 0.12). Patients from China (β −0.53; 95% CI −39.44 to −31.72; P < 0.001; R 2 0.29) and Canada (β −0.28; 95% CI −24.62 to −13.86; P < 0.001; R 2 0.08) fared worse on SC compared with US patients in the univariate analysis. When we performed the multivariate analysis (as in Table 2) using an additional variable for geographic location for China (model 1) and Canada (model 2) (Table 3), Asian ethnicity remained a significant independent inverse predictor of SC, whereas the geographic variable for China and disease activity did not. In model 1, the only independent predictors for SC were Asian ethnicity, social support, and coping. The whole model (model 1) explained 41% of variance of SC. In model 2, with the addition of the geographic variable for Canada, the only significant independent predictors for SC that remained were disease activity, social support, coping, and the geographic location. Canadian patients had worse SC compared with US patients after we accounted for age, ethnicity, disease activity, steroid use, and QOL. Collectively, model 2 explained 48% variance in SC (Table 3).
Table 3.
Secondary analyses for SC with geographic region
| Variable | Multiple Regression Models | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 (China vs United States) | Model 2 (Canada vs United States) | |||||||
| R 2 | β | 95% CI | P | R 2 | β | 95% CI | P | |
| Age | 0.41 | 0.05 | −0.03 to 0.27 | 0.120 | 0.48 | −0.003 | −0.22 to 0.21 | 0.95 |
| Ethnicity or racea | ||||||||
| African American | 0.41 | −0.01 | −17.33 to 13.28 | 0.800 | 0.48 | 0.02 | −8.33 to 12.82 | 0.68 |
| Asian | 0.41 | −0.77 | −89.12 to −15.12 | 0.006 | 0.48 | 0.04 | −6.14 to 15.94 | 0.38 |
| Hispanic | 0.41 | −0.02 | −15.16 to 11.61 | 0.800 | 0.48 | 0.03 | −9.92 to 14.16 | 0.73 |
| Disease features | ||||||||
| Steroid use currently | 0.41 | 0.03 | −2.18 to 5.78 | 0.380 | 0.48 | 0.04 | −3.74 to 8.75 | 0.43 |
| Total SLEDAI score | 0.41 | 0.03 | −0.30 to 0.82 | 0.370 | 0.48 | 0.10 | 0.15 to 1.56 | 0.02 |
| QOL | ||||||||
| LupusPRO‐COG | 0.41 | −0.01 | −0.09 to 0.07 | 0.750 | 0.48 | 0.02 | −0.09 to 0.14 | 0.66 |
| LupusPRO‐DG | 0.41 | −0.02 | −0.10 to 0.06 | 0.560 | 0.48 | −0.07 | −0.20 to 0.03 | 0.15 |
| LupusPRO‐SS | 0.41 | 0.18 | 0.11 to 0.26 | <0.001 | 0.48 | 0.17 | 0.07 to 0.26 | 0.001 |
| LupusPRO‐CO | 0.41 | 0.17 | 0.12 to 0.30 | <0.001 | 0.48 | 0.22 | 0.16 to 0.41 | <0.001 |
| Geographic regionb | 0.41 | 0.26 | −17.53 to 52.36 | 0.33 | 0.48 | −0.22 | −25.68 to −3.49 | 0.01 |
Abbreviation: CI, confidence interval; CO, Coping; COG, Cognition; DG, Desires‐Goals; QOL, quality of life; SC, satisfaction with care; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index; SS, Social Support.
Other ethnicity or race as a reference.
United States as a reference.
Discussion
Improving QOC and patient experience remains one of the top priorities in health care despite being a challenging task for both physicians and health care administrators. This is especially important in SLE because the disease has multiple manifestations, is associated with significant diagnostic delays, and lacks effective yet safe treatment options. Furthermore, SLE requires long‐term, comprehensive care, which is often multidisciplinary.
QOC may be judged in various ways. These may include patients' evaluation of access to care, efficiencies and safety of health care processes, quality of patient‐physician interactions, and health care facility, among others. QOC may also be inferred by the physician meeting certain standard‐of‐care metrics or by tracking patients' health outcomes (eg, remission, flares, morbidity, and mortality). Health care centers and third parties may judge QOC by balancing services provided against metrics of avoidable urgent and emergent health care use by the patients.
QOC in SLE is suboptimal, and poor QOC may lead to irreversible damage 6, 7. SC is one way to judge QOC, and it may influence patients' adherence to treatment, patient‐physician relationships, and health outcomes (eg, number of medications, flares, and hospitalizations) 21, 22, 23, 24. Health care services routinely track patients' SC with individual physicians, practices, and hospitals (eg, Press Ganey scores) 25. We undertook the study to enhance our understanding of SC and, specifically, its drivers because the health status of patients with SLE may be improved by increasing patients' social support and SC 14.
Little is known about SC in SLE. Our overall understanding of SC relies on data from the general inpatient setting or secondary care interface. The studies pertaining to SC in SLE are focused more on treatment satisfaction rather than exploring other facets that may contribute to SC, such as psychosocial factors, coping strategies, and the patient‐provider relationship 26, 27. One of the main reasons cited by patients with SLE for treatment adherence is trust in their rheumatologist, whereas the reason for nonadherence originates from difficulty in accepting a chronic disease diagnosis that requires lifelong therapy 28. Both of these factors may be related to the QOC provided, and thereby, patients' SC. Internal and external resources available to the patient (eg, social support and coping) may also influence patients' understanding and acceptance of the diagnosis as well as impact management and shared decision‐making.
Both social support and coping were independently associated with better SC. Because both social support and coping are modifiable factors, we suggest evaluation of these QOL domains in all patients SLE by using comprehensive, disease‐specific PRO tools, such as LupusPRO. In fact, it has been found that better self‐reported physical and mental health status and social support are associated with higher patient satisfaction among patients with SLE 14. Patients with SLE with poorer social networks may have greater unmet needs, which consequently reflects onto their health care needs and increases their satisfaction threshold. Alternatively, patients with better social support may be equipped with the skills needed to ensure that their health care expectations are met and, hence, are more likely to be satisfied 29. Similarly, social support as a correlate of SC has been previously reported in fibromyalgia and other diseases 30. Because one‐third of patients with SLE may have fibromyalgia, social support may be of additional relevance.
Because SLE commonly affects younger patients, it poses unique challenges, including coping with a diagnosis of chronic disease, need for lifelong medical care, unpredictable flares, organ damage, changes in body function, functional abilities, appearance, poor external or internal reserves (financial and social resources), and disruptions in normative milestones (eg, vocation, dating, pregnancy, parenting). Stress is known to contribute to systemic inflammation and flares in autoimmune diseases. Without access to appropriate coping resources, patients may resort to maladaptive coping techniques. Resilience, catastrophizing, and helplessness are known predictors of health outcomes in SLE 31, 32, 33. Coping, a modifiable driver for SC, may be overlooked by health professionals 34. We recommend integration of a biopsychosocial model of health care for SLE, whereby patients' external and internal resources (social support and coping) are evaluated, and appropriate referrals and resources are provided to address these issues. Interventions may include offering educational materials to patients and their caregivers and incorporating social work services.
Disparities in health care access and outcomes arising from socioeconomic status and ethnicity remain a predicament. SLE has a higher prevalence in ethnic minorities and demonstrates worse outcomes. It is difficult to distinguish whether this discrepancy exists because of variability in the lupus phenotype among different ethnicities versus a consequence of different health behaviors, communication barriers 35, access to care, and socioeconomic status. In this study, we did not find an association between SC and education, which was used as a surrogate for socioeconomic status. However, we found a significant association between patients' ethnicity and SC. African American and Hispanic patients reported better SC, whereas Asian patients reported worse SC. This may be explained by an imbalance in expectations and appraisal between various ethnic groups 36. Negative evaluations of care may also stem from communication issues between patients of the ethnic minority and their health care providers. These communication exchanges may be challenging to both parties and, for the patient, may be built on past negative experiences involving miscommunication, disrespect, or lack of trust.
Because health care systems may differ across various geographic areas, it may confound the relationship between ethnicity and SC. We did note differential SC across US patients compared with non‐US patients and among Chinese and Canadian patients compared with US patients. When accounting for the geographic location (China vs United States), we still found Asian ethnicity to be associated with worse SC. This finding suggests that health behaviors, cultural beliefs 8, appraisal, and different expectations may play a role within the Asian population rather than a difference in health care systems and delivery. Such an association with ethnicity and SC was not noted among patients in Canada versus the United States when geographic location was explored. It is known that expenditure for care is significantly higher for patients with SLE in the United States compared with Canada, without significant gains in health outcomes. In the Tri‐Nation study, patients with SLE from Canada had better health status compared with US patients, and no significant differences in patient satisfaction were noted 37. These issues need to be further studied.
Another modifiable variable is disease activity. Hypothetically, one would expect patients with low disease activity or in remission to have better SC because disease activity is linked with damage accrual, hospitalization, QOL, and mortality. The use of a standardized disease activity measurement as a surrogate for QOC would thus be justifiable. Paradoxically, we found greater disease activity to be associated with better SC. This could be potentially due to greater health care use and frequent interactions with the health care providers given the acuity or flare activity. In a nationally representative cohort, higher patient satisfaction was associated with greater inpatient use and higher overall health care and prescription drug expenditures. Greater satisfaction was also associated with greater mortality 38. As a result, patient satisfaction surveys (eg, Press Ganey scores) may not be a good outcome to track as a surrogate for QOC. This may especially be true during acute health episodes when a patient's expectations may be directed at short‐term gains such as reversibility, stabilization of acute health issues, discharge to home, and immediate survival.
There are several limitations to our study. First and foremost, we used existent data on four items of the SC LupusPRO domain to estimate SC. This was a limited scope to assess SC. Use of other comprehensive measurement tools for SC would have been ideal. Previously used SC tools are the Treatment Satisfaction Questionnaire for Medication 39 and the Treatment Satisfaction with Medicines Questionnaire 40. More recently, the Lupus Satisfaction Questionnaire was developed to comprehensively measure treatment satisfaction specifically in lupus 27. The second limitation was the cross‐sectional design, which could only suggest associations and not causality. Thirdly, SC is affected by many confounders and modifiers, such as socioeconomic status, education, access to care, cultural and health beliefs, health behaviors, and health care systems. Education was used as a singular surrogate for socioeconomic status. We did not take into account other variables, such as income or health insurance, to measure socioeconomic status, which might have contributed to a lack of association. Although we tried to undertake a secondary analysis by adjusting for the geographic location of patients, it is possible that differences attributed to ethnicity or geographic regions were due to other factors not studied. Lastly, we had a greater representation of Asian patients and lower disease activity and damage, which limits the generalizability. Studies that include a more comprehensive evaluation of SC in SLE are indicated.
Our study has several strengths. These include a large, ethnically diverse, international patient cohort that provides generalizability and confidence in the main findings. The analyses were adjusted for age, ethnicity, steroid use, disease activity, and QOL. This is the first study to investigate the drivers of SC using a disease‐specific tool (LupusPROv1.7) to measure QOL and SC. The tool has also been previously validated in these various patient groups, languages, and countries 20.
In conclusion, social support and coping are major independent and modifiable determinants of SC. Our results emphasize the need to use comprehensive PRO tools (such as LupusPRO) that evaluate social support, coping, and SC during routine medical care visits. It is also important to specifically address these modifiable variables in high‐risk patients (eg, Asian patients) by using a biopsychosocial model of care for chronic diseases to improve overall outcomes. Lastly, our study suggests that SC may not be a good QOC surrogate to follow in patients with active SLE.
Author Contributions
All authors drafted the article, revised it critically for important intellectual content, approved the final version to be published, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Jolly.
Acquisition of data
Jolly, Sequeira, Block, Toloza, Bertoli, Blazevic, Vila, Moldovan, Torralba, Cicognani, Mazzoni, Hasni, Goker, Haznedaroglu, Bourre‐Tessier, Navarra, Mok, Clarke, Weisman, Wallace.
Analysis and interpretation of data
Jolly, Block, Clarke, Sethi, O'Brien.
Supported in part through (a) philanthropic support from Brewer Foundation, and (b) Intramural Research Program of the National Insitute of Arthritis and Musculoskeletal and Skin Diseases of the National Institute of Health.
Rush University owns copyrights to LupusPRO; as a coinventor, Dr. Jolly receives a portion of the proceeds from intellectual property use per Rush University regulations. No other disclosures relevant to this article were reported.
References
- 1. Jolly M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol 2005;32:1706–8. [PubMed] [Google Scholar]
- 2. Kulczycka L, Sysa‐Jedrzejowska A, Robak E. Quality of life and satisfaction with life in SLE patients: the importance of clinical manifestations. Clin Rheumatol 2010;29:991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmeding A, Schneider M. Fatigue, health‐related quality of life and other patient‐reported outcomes in systemic lupus erythematosus. Best Pract Res Clin Rheumatol 2013;27:363–75. [DOI] [PubMed] [Google Scholar]
- 4. Azizoddin DR, Jolly M, Arora S, Yelin E, Katz P. Patient‐reported outcomes predict mortality in lupus. Arthritis Care Res (Hoboken) 2019;71:1028–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arora S, Nika A, Trupin L, Abraham H, Block J, Sequeira W, Yazdany J, Jolly M. Does systemic lupus erythematosus care provided in a lupus clinic result in higher quality care than that provided in a general rheumatology clinic? Arthritis Care Res (Hoboken) 2018;70:1771–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yazdany J, Trupin L, Schmajuk G, Katz PP, Yelin EH. Quality of care in systemic lupus erythematosus: the association between process and outcome measures in the Lupus Outcomes Study. BMJ Qual Saf 2014;23:659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yazdany J, Trupin L, Tonner C, Dudley RA, Zell J, Panopalis P, et al. Quality of care in systemic lupus erythematosus: application of quality measures to understand gaps in care. J Gen Intern Med 2012;27:1326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kupfer JM, Bond EU. Patient satisfaction and patient‐centered care: necessary but not equal. JAMA 2012;308:139–40. [DOI] [PubMed] [Google Scholar]
- 9. Manary MP, Boulding W, Staelin R, Glickman SW. The patient experience and health outcomes. N Engl J Med 2013;386:201–3. [DOI] [PubMed] [Google Scholar]
- 10. Taylor CJ, La Greca A, Valenzuela JM, Hsin O, Delamater AM. Satisfaction with the health care provider and regimen adherence in minority youth with type 1 diabetes. J Clin Psychol Med Settings 2016;23:257–68. [DOI] [PubMed] [Google Scholar]
- 11. Boulding W, Glickman SW, Manary MP, Schulman KA, Staelin R. Relationship between patient satisfaction with inpatient care and hospital readmission within 30 days. Am J Manag Care 2011;17:41–8. [PubMed] [Google Scholar]
- 12. Sanchez‐Piedra CA, Prado‐Galbarro FJ, Garcia‐Perez S, Santamera AS. Factors associated with patient satisfaction with primary care in Europe: results from the EUprimecare project. Qual Prim Care 2014;22:147–55. [PubMed] [Google Scholar]
- 13. Batbaatar E, Dorjdagya J, Luvsannyam A, Savino MM, Amenta P. Determinants of patient satisfaction: a systematic review. Perspect Public Health 2016;137:89–101. [DOI] [PubMed] [Google Scholar]
- 14. Da Costa D, Clarke AE, Dobkin PL, Senecal JL, Fortin PR, Danoff DS, et al. The relationship between health status, social support and satisfaction with medical care among patients with systemic lupus erythematosus. Int J Qual Health Care 1999;11:201–7. [DOI] [PubMed] [Google Scholar]
- 15. Sutcliffe N, Clarke AE, Levinton C, Frost C, Gordon C, Isenberg DA. Associates of health status in patients with systemic lupus erythematosus. J Rheumatol 1999;26:2352–6. [PubMed] [Google Scholar]
- 16. Hochberg MC, for the Diagnostic and Therapeutic Criteria Committee of the American College Of Rheumatology . Updating the American College Of Rheumatology revised criteria for the classification of systemic lupus erythematosus [letter]. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 17. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 18. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH, and the Committee on Prognosis Studies in SLE . Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum 1992;35:630–40. [DOI] [PubMed] [Google Scholar]
- 19. Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College Of Rheumatology Damage Index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 20. Jolly M, Pickard AS, Block JA, Kumar RB, Mikolaitis RA, Wilke CT, et al. Disease‐specific patient reported outcome tools for systemic lupus erythematosus. Semin Arthritis Rheum 2012;42:56–65. [DOI] [PubMed] [Google Scholar]
- 21. Chrystyn H, Small M, Milligan G, Higgins V, Gil EG, Estruch J. Impact of patients' satisfaction with their inhalers on treatment compliance and health status in COPD. Respir Med 2014;108:358–65. [DOI] [PubMed] [Google Scholar]
- 22. Amin AN, Ganapathy V, Roughley A, Small M. Confidence in correct inhaler technique and its association with treatment adherence and health status among US patients with chronic obstructive pulmonary disease. Patient Prefer Adherence 2017;11:1205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams B. Patient satisfaction: a valid concept? Soc Sci Med 1994;38:509–16. [DOI] [PubMed] [Google Scholar]
- 24. Deyo RA, Inui TS. Dropouts and broken appointments: a literature review and agenda for future research. Med Care 1980;18:1146–57. [DOI] [PubMed] [Google Scholar]
- 25. Dempsey C. Patient satisfaction scores: optimizing the patient and clinician experience. URL: http://www.pressganey.com/About/News/Patient-Satisfaction-Scores-Optimizing-The-Patient-And-Clinician-Experience.
- 26. Mozaffarian N, Lobosco S, Lu P, Roughley A, Alperovich G. Satisfaction with control of systemic lupus erythematosus and lupus nephritis: physician and patient perspectives. Patient Prefer Adherence 2016;10:2051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mathias SD, Berry P, Pascoe K, de Vries J, Askanase AD, Colwell HH, et al. Treatment satisfaction in systemic lupus erythematosus: development of a patient‐reported outcome measure. J Clin Rheumatol 2017;23:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Farinha F, Freitas F, Agueda A, Cunha I, Barcelos A. Concerns of patients with systemic lupus erythematosus and adherence to therapy: a qualitative study. Patient Prefer Adherence 2017;11:1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carpenter DM, Thorpe CT, Alexander DS, Sage AJ, Lewis M, Hogan SL, et al. The relationship between social support, social constraint, and psychological adjustment for patients with rare autoimmune disease. Curr Rheumatol Rev 2016;12:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Serber ER, Cronan TA, Walen HR. Predictors of patient satisfaction and health care costs for patients with fibromyalgia. Psychol Health 2003;18:771–87. [Google Scholar]
- 31. Fischin J, Chehab G, Richter JG, Fischer‐Betz R, Winkler‐Rohlfing B, Willers R, et al. Factors associated with pain coping and catastrophising in patients with systemic lupus erythematosus: a cross‐sectional study of the LuLa‐cohort. Lupus Sci Med 2015;2:e000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown S. Coping with SLE: just in case vs. just in time: nurse's perspective. Lupus 2013;22:1320–3. [DOI] [PubMed] [Google Scholar]
- 33. Cal SF, Santiago MB. Resilience in systemic lupus erythematosus. Psychol Health Med 2013;18:558–63. [DOI] [PubMed] [Google Scholar]
- 34. Yen JC, Abrahamowicz M, Dobkin PL, Clarke AE, Battista RN, Fortin PR. Determinants of discordance between patients and physicians in their assessment of lupus disease activity. J Rheumatol 2003;30:1967–76. [PubMed] [Google Scholar]
- 35. Pinder RJ, Ferguson J, Møller H. Minority ethnicity patient satisfaction and experience: results of the National Cancer Patient Experience Survey in England. BMJ Open 2016;6:e011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mead N, Roland M. Understanding why some ethnic minority patients evaluate medical care more negatively than white patients: a cross sectional analysis of a routine patient survey in English general practices. BMJ 2009;339:b3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clarke AE, Petri M, Manzi S, Isenberg DA, Gordon C, Senécal JL, et al. The systemic lupus erythematosus Tri‐Nation Study: absence of a link between health resource use and health outcome. Rheumatology (Oxford) 2004;43:1016–24. [DOI] [PubMed] [Google Scholar]
- 38. Fenton JJ, Jerant AF, Bertakis KD, Franks P. The cost of satisfaction: a national study of patient satisfaction, health care utilization, expenditures, and mortality. Arch Intern Med 2012;172:405–11. [DOI] [PubMed] [Google Scholar]
- 39. Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ruiz MA, Pardo A, Rejas J, Soto J, Villasante F, Aranguren JL. Development and validation of the “Treatment Satisfaction with Medicines Questionnaire” (SATMED‐Q). Value Health 2008;11:913–26. [DOI] [PubMed] [Google Scholar]


