Abstract
The enteric protozoa Entamoeba histolytica is the causative agent of amebiasis, which is one of the most common parasitic diseases in developed and developing countries. Entamoeba nuttalli is the genetically closest species to E. histolytica in current phylogenetic analyses of Entamoeba species, and is prevalent in wild macaques. Therefore, E. nuttalli may be a key organism in which to investigate molecules required for infection of human or non-human primates. To explore the molecular signatures of host-parasite interactions, we conducted de novo assembly of the E. nuttalli genome, utilizing self-correction of PacBio long reads and polishing corrected reads using Illumina short reads, followed by comparative genomic analysis with two other mammalian and a reptilian Entamoeba species. The final draft assembly of E. nuttalli included 395 contigs with a total length of approximately 23 Mb, and 9,647 predicted genes, of which 6,940 were conserved with E. histolytica. In addition, we found an E. histolytica-specific repeat known as ERE2 in the E. nuttalli genome. GO-term enrichment analysis of mammalian host-related molecules indicated diversification of transmembrane proteins, including AIG1 family and BspA-like proteins that may be involved in the host-parasite interaction. Furthermore, we identified an E. nuttalli-specific protein that contained 42 repeats of an octapeptide ([G,E]KPTDTPS). This protein was shown to be localized on the cell surface using immunofluorescence. Since many repeat-containing proteins in parasites play important roles in interactions with host cells, this unique octapeptide repeat-containing protein may be involved in colonization of E. nuttalli in the intestine of macaques. Overall, our draft assembly provides a valuable resource for studying Entamoeba evolution and host-parasite selection.
Author summary
Determination of host specificity is one of the most significant themes in the field of infectious diseases. Identification of molecules related to the host specificity of pathogens can lead to new treatment and prevention methods, and prediction of potential host shifts. Entamoeba histolytica is a human parasite that causes hemorrhagic diarrhea, amebic colitis, and liver abscess, which may result in death in severe cases. Entamoeba nuttalli is the closest species to E. histolytica and infects various wild macaques as natural hosts. E. nuttalli might also be a pathogen with a zoonotic hazard because severe inflammatory reactions in hamster livers and an asymptomatic case of human infection have been recorded. Here, we report E. nuttalli genomic data with a quality that allows comparison with genomes from other Entamoeba species. Comparative genomics revealed common proteins shared with other mammalian Entamoeba species, as well as E. nuttalli-specific proteins. Therefore, this study identifies candidate molecules required for host specificity and subsequent pathogenicity of Entamoeba species.
Introduction
The genus Entamoeba is an anaerobic protozoan lineage consisting of parasitic species that dwell in the digestive tract of various metazoan hosts, with a few species also isolated from the environment [1–4]. In this taxonomic group, Entamoeba histolytica, Entamoeba dispar, and Entamoeba invadens have a common life cycle of an infectious cyst and vegetative trophozoite, but have different virulence potentials and host specificity. E. histolytica is the causative agent of human amebic colitis and liver abscess, which results in up to 100,000 deaths annually [5]; E. dispar colonizes the intestine of humans and non-human primates without invasion [6–9]; and E. invadens is a pathogenic reptilian parasite and a good model organism for the study of encystation: the conversion process from trophozoite to cyst [10,11]. An E. histolytica-like amoeba that is virulent but genetically different from E. histolytica has been isolated from rhesus macaques, and revival of the name Entamoeba nuttalli was proposed for this amoeba [12]. E. nuttalli, as the species most closely related to E. histolytica, has been isolated from various species of wild macaques and captive non-human primates [12–21]. An asymptomatic case of human infection with E. nuttalli also occurred in a zoo caretaker. Therefore, E. nuttalli infection may be problematic for the health of non-human primates and may be a zoonotic hazard [22].
Comparative genomics is used in parasitology to identify virulence factors and functional molecules, to carry out evolutionary analyses, and to investigate molecules related to host range [23–27]. In Entamoeba species, this approach has identified AIG1 as a novel virulence factor [28], and has established a correlation between genomic diversity and virulence potential [29] and the contribution of repetitive elements to diversification [30]. However, the molecules responsible for host specificity in Entamoeba parasites remain to be identified.
The genome assembly of E. histolytica has the highest quality among Entamoeba genomic databases, and a 20 Mb assembly containing 1,496 scaffolds and 8,201 predicted genes has been proposed [31]. Moreover, analyses of the E. histolytica genome have revealed a high content of AT bases (approximately 75%) and repetitive elements (approximately 19.7%) [30], indicating that it is difficult to reconstruct the complete genome structure using a short-read sequencer only. Reflecting this, the ploidy of E. histolytica is still uncertain, but may be tetraploid [32]. Entamoeba genomic data generated by a long-read sequencer, such as the Pacific BioSciences platform [33], would allow complex genomic regions to be deciphered and provide a more refined assembly for comprehensive comparative genomics.
In this study, we conducted de novo assembly of the E. nuttalli genome and comparative analysis with published sequences of E. histolytica, E. dispar, and E. invadens. We report here mammalian host-related molecular signatures of Entamoeba species and an E. nuttalli-specific octapeptide-repeat surface protein (which we named PTORS). This draft genome of E. nuttalli aids in understanding of host specificity and evolution of Entamoeba species.
Materials and methods
Ethics statement
All animal experiments were performed in accord with the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology, Japan, and reviewed and approved by The Institutional Animal Care and Use Committee at Tokai University (Permit Number 185001).
Preparation of genomic DNA
Trophozoites of E. nuttalli P19-061405 strain clone 7 were cultured axenically in TYI-S-33 medium [34] supplemented with 15% adult bovine serum (Sigma-Aldrich, St. Louis, MO) at 37°C. Genomic DNA was isolated as previously described [35]. Briefly, nuclei were obtained by centrifugation after cell lysis in 1% Nonidet P-40. The pellet was lysed with 2% sodium dodecyl sulfate and proteinase K. DNA was extracted four times with phenol-chloroform-isoamyl alcohol and then precipitated with ethanol.
Whole genome sequencing using the PacBio RS system
For single molecule real-time (SMRT) sequencing, genomic DNA of E. nuttalli was sheared into 10 kb fragments using g-TUBE (Covaris, Woburn, MA) and the quantity and size distribution were measured using a Qubit Fluorometer (Life Technologies/Thermo Fisher Scientific, Palo Alto, CA) and an Agilent 2100 Bioanalyzer DNA12000 kit (Agilent Technologies, Santa Clara, CA). Double-stranded DNA fragments were end-repaired and hairpin adapters were added via blunt end ligation to produce SMRTbell templates using a PacBio DNA Template Prep Kit 2.0. These templates were then treated with exonucleases III and VII to remove failed ligation products and purified with a 0.45× volume of AMPure PB beads. Final SMRTbell libraries were again assessed using the DNA12000 kit (Agilent Technologies). The sequencing primer to polymerase ratio and loading concentration were determined using a PacBio binding calculator. The sequencing primer was annealed to the single-stranded loop of the SMRTbell template, and primer-annealed templates were then bound to DNA polymerase XL (or C2). MagBeads loading was conducted at 4°C for 20 min per the manufacturer’s guidelines, after which MagBeads-bound, polymerase-template complexes were loaded into zero-mode waveguides of SMRT Cells. Sequencing runs were performed with C2 sequencing chemistry with a 120-min movie (35 Cells) or 2 × 55-min (22 Cells) movies. Thus, in total, 57 SMRT Cells were used for sequencing.
Whole genome sequencing using the Illumina GAIIx system
Genomic DNA of E. nuttalli was also sequenced on the Illumina GAIIx platform. A paired-end library was prepared from 3 μg of genomic DNA following the protocol of the TruSeq library construction kit (Illumina Inc., San Diego, CA, USA) after fragmentation by sonication under standard conditions (Covaris). Cluster generation and sequencing were undertaken as per the manufacturer’s protocol for paired-end 114 bp sequence reads. The sequencing template was then loaded on one lane of a proprietary flow cell.
De novo assembly of the E. nuttalli genome
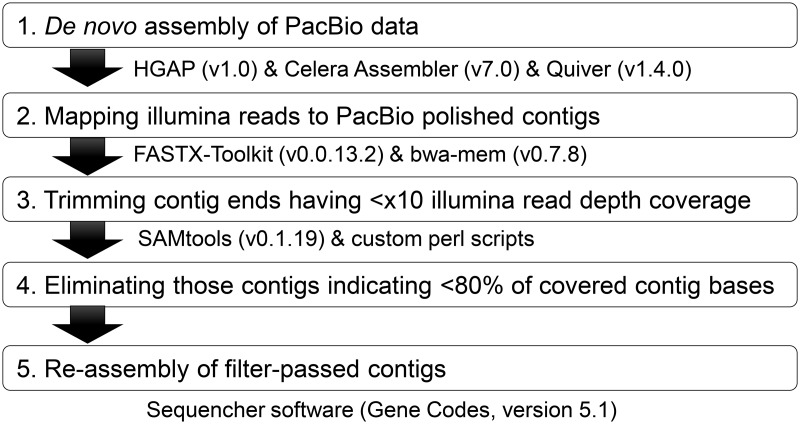
Long-read and short-read sequence data were utilized in the following procedures (Fig 1). (i) After automatically removing PacBio sub-reads with low accuracy (<80%) and/or short read length (< 500 bases), random errors of sub-reads were corrected by the PreAssembler pipeline of HGAP 1.0 using BLASR [36] with the following parameters: minMatch: 6, -minReadLength: 400, -maxScore: -1200, -bestn: 25, -maxLCPLength: 14, -nCandidates: 50, -allowAdjacentIndels, -indelRate: 0.5. Primary assembly was performed by Celera Assembler 7.0 with an 18 mer size parameter using over 5,000 bases error-corrected sub-reads that had at least 14 times sequence redundancy. The assembly errors within contigs were polished with Quiver [37] in SMRT Analysis v1.4.0. (ii) Raw sequence reads obtained from the Illumina GAIIx platform were quality trimmed to remove poor sequences using FASTX-Toolkit v.0.0.13 with the following parameters: minimum read length (-l): 70 bases, and quality cutoff (-t): 20. The quality-passed reads were mapped to the polished contigs using BWA-MEM v.0.7.8 [38] with default parameters. (iii) Quality assessments and control of poor supportive contigs were then performed with the following criteria: (a) trimming ends of contig with < 10-fold Illumina read coverage of depth, and (b) eliminating contigs with < 4 of 5 covered contig bases. The mapped data were manipulated and alignments were generated in a per-position (pileup) format using SAMtools v.0.1.19 [39]. (iv) The filter-passed contiguous sequences were assembled as >100 bp and 98% matched parameters using Sequencher v.5.1 DNA sequence assembly software (Gene Code Co., Ann Arbor, MI).
Fig 1. Procedure for reassembly of PacBio and Illumina data.
Assessment of genome assemblies
BUSCO v.3.0.2 [40] was utilized to assess the quality of genome assembly. BUSCO analysis used NCBI BLAST+ v.2.6.0, HMMER v.3.1b1 [41] and Augustus v.3.3.1 [42]. BUSCO was run with the following parameters: -l eukaryota_odb9, -m genome. E. histolytica HM-1:IMSS v.4.0 and E. nuttalli P19 4.0 were downloaded from AmoebaDB [43].
E. nuttalli genome annotation
Gene prediction was performed by GlimmerHMM with a training set as an E. histolytica gene model. Annotation of the predicted genes was performed by NCBI BLAST against the E. histolytica protein dataset (AmoebaDB v.4.0) and the NCBI non-redundant protein database (downloaded Jan 30, 2013). Protein signatures, motifs and Gene Ontology annotations were assigned using InterProScan v.5.6–48 [44]. Non-annotated and short amino acid sequences (< 50 amino acids) were removed from further analyses. Finally, predicted genes with overlap with repeat elements were excluded from the annotation after masking of the repeat sequences against the Entamoeba genus using RepeatMasker v.4.0.5. (http://www.repeatmasker.org/).
Search for orthologous clusters across multiple species
The protein dataset of E. invadens IP-1 v.4.0, E. histolytica HM-1:IMSS v.4.0, and E. dispar SAW760 v.4.0 were downloaded from AmoebaDB. The OrthoVenn server [45] was used for identification of mammalian host-related orthologous clusters with E. invadens IP-1 and E. histolytica HM-1:IMSS. The DAVID web server [46] was used for GO-term enrichment analysis with Benjamini adjusted P values < 0.05.
Prediction of cell surface proteins and subcellular localization
Cell surface proteins were predicted in silico using SOSUI [47], TMHMM [48,49], and SignalP [50]. Putative transmembrane helix-containing proteins were defined as those indicated by SOSUI as membrane proteins and those in which TMHMM predicted at least one transmembrane helix. Putative signal peptide-containing proteins were defined as those predicted by SignalP to include a signal peptide at the default D-cutoff. WoLF-PSORT [51] was used to predict subcellular localization, with the organism parameter as animal and fungi. WoLF-PSORT annotation with concordance between animal and fungi parameters was used.
Plasmid construction
To generate recombinant histidine-tagged PTORS without a putative signal peptide (rPTORS), PTORS gene was PCR-amplified from template E. nuttalli genomic DNA using sense (5′-GTC GCA TAT GAT TCT TTG TAT GGA ACA AGG AGT TAA AG-3′) and antisense (5′-GTC GTC TAG ATT AGA AGT AGA TAA ATG CAA TAA CAA TTG-3′) primers. Nde I and Xba I restriction sites are underlined in the respective primers. The PCR fragment and pCold I plasmid (TaKaRa, Otsu, Japan) were digested by Nde I and Xba I, and the digested products were ligated using a Ligation-Convenience Kit (Nippongene, Tokyo, Japan). After ligation, the plasmid was transformed in Competent Quick DH5α (Toyobo, Osaka, Japan) and the amplified plasmid was purified using a QIAprep Spin Miniprep Kit (Qiagen GmbH, Hilden, Germany).
Recombinant protein
The plasmid was transformed into BL21 (DE3) One Shot Chemically Competent E. coli (Life Technologies) and expression of rPTORS was induced by low temperature (15°C) with 1 mM IPTG. After induction, rPTORS was purified from bacterial lysates using a Ni-NTA system (Qiagen) under denaturing conditions with 6 M urea.
Antisera
Six-week-old male BALB/c mice were purchased from CLEA Japan, Inc. (Tokyo, Japan). Five mice were immunized subcutaneously with 100 μg of rPTORS emulsified in TiterMax Gold (TiterMax USA, Norcross, GA). Immunization was repeated twice at two-week intervals. Four weeks after the last injection, sera of mice were collected.
Immunoblot analysis
Immunoblot analysis was performed as previously described [52]. Briefly, antisera for PTORS were diluted 200-fold with PBST containing 5% skim milk (Wako, Osaka, Japan), and anti-mouse immunoglobulin F(ab′)2 fragment conjugated with horseradish peroxidase (Amersham) was diluted 3000-fold with PBST.
Immunofluorescence microscopy
Sample preparation for immunofluorescence microscopy was performed as previously described [53]. Briefly, antisera for PTORS were diluted 100-fold with 3% bovine serum albumin in PBS and Alexa Fluor 488 goat anti-mouse IgG (Life Technologies) was diluted 300-fold with PBS. Confocal fluorescence images were captured using a LSM880 confocal microscope (Carl Zeiss, Jena, Germany) in channel mode and analyzed with ZEN2 software.
Results
Sequence read information and draft genome assembly process
Sequence read information was initially obtained by single molecule real-time (SMRT) sequencing of E. nuttalli genomic DNA using the PacBio RS platform with 57 SMRT Cells. An initial filtering removed all subreads with low accuracy (< 80%) or short read length (< 500 bases), generating 1,094,547 subreads and 2,587,786,482 bases (subread length distributions are shown in S1 Fig). After random errors of subreads were corrected by PreAssembler pipeline in HGAP 1.0 using BLASR, a primary assembly of 1,172 contigs was constructed using Celera Assembler 7.0. Assembly errors within contigs were polished by Quiver in SMRT Analysis v1.4.0, resulting in a total assembly size of 32,595,857 bases, N50 size of 45,298 bases and the longest size of 306,099 bases (step 1 in Fig 1 and Table 1). Before reassembly of the primary assembly, short-reads obtained via the Illumina system were utilized for improvement of primary assembly completeness. Short-read sequencing of the E. nuttalli genomic DNA produced 36,954,093 paired-end 114-bp reads corresponding to >8,425,533,204 bases. Of 35,088,246 quality-passed paired-end reads, 34,921,637 were mapped to the primary assembly using bwa-mem v.0.7.8 with default parameters (step 2 in Fig 1). The mean read depth coverage of mapped data of the primary assembly was 238.83× coverage, and the percentages of contig sequence coverage above 1×, 50× and 100× sequenced were 91.7, 86.1 and 79.4%, respectively (S1 Table). The primary assembly was supported by most Illumina short-reads, but some 5′ and 3′ contig ends, which are approximately 10% of the sequence from each contig end, clearly had a lower read depth coverage (S2 Fig, upper panel). Therefore, we performed quality control filtration of the primary assembly using the Illumina mapped data, as shown in steps 3 and 4 of Fig 1 (detailed thresholds are given in the Methods). Most 5′ and 3′ contig ends were improved by the quality control filtration (S2 Fig, lower panel). Of 1,172 contigs, 743 that were quality-passed were reassembled using Sequencher v.5.1 with parameters of similarity = 98% and overlap = 100 bp. Finally, a total of 395 contigs (174 assembled and 221 singleton contigs) were obtained as a semi-hybrid assembly. The longest contig was 448,959 bases and N50 was 90,004 bases (Table 1).
Table 1. Genome assembly in E. nuttalli and comparison with public genome assembly.
| Item | Primary assemblya | Semi-hybrid assembly | Public genome assemblyb | |||
|---|---|---|---|---|---|---|
| Assembled | Singleton | Total | E. nuttalli | E. histolytica | ||
| Number of contigs | 1,172 | 174 | 221 | 395 | 5,233 | 1,496 |
| Number of bases | 32,595,857 | 13,591,796 | 9,619,065 | 23,210,861 | 14,399,953 | 20,799,072 |
| GC Content (%) | 24.19 | 24.20 | 24.28 | 24.24 | 25.02 | 24.28 |
| Mean contig size | 27,812 | 78,113 | 43,525 | 58,761 | 2,751 | 13,903 |
| N50 contig size | 45,298 | 100,804 | 75,353 | 90,004 | 7,707 | 49,118 |
| Longest contig size | 306,099 | 448,959 | 215,923 | 448,959 | 97,841 | 530,629 |
| Shortest contig size | 3,527 | 7,290 | 3,175 | 3,175 | 124 | 235 |
| BUSCO score (%)c | 12.2 | - | - | 48.5 | 46.6 | 53.1 |
a Genome assembly of PacBio data with an adapted assembly error correction using Quiver.
b E. nuttalli P19-061405 and E. histolytica HM-1:IMSS downloaded from AmoebaDB v.4.0.
c BUSCO score represented by the percentage classified as ‘Complete’.
The results of the new draft assembly of E. nuttalli showed similar metrics in GC content and estimated genome size to the current assembly of E. histolytica. The BUSCO algorithm was used for quality control assessment of the new draft genome assembly, in comparison to the primary assembly and public assembly (Table 1). Coverage of core protein hits was at a similar level to that in the well-annotated genome of E. histolytica. It is difficult to use BUSCO results as a measure of genomic completeness due to low coverage of core protein hits, but the reassembly process improved the quality of the primary assembly in terms of gene content.
Characteristics of the E. nuttalli genome
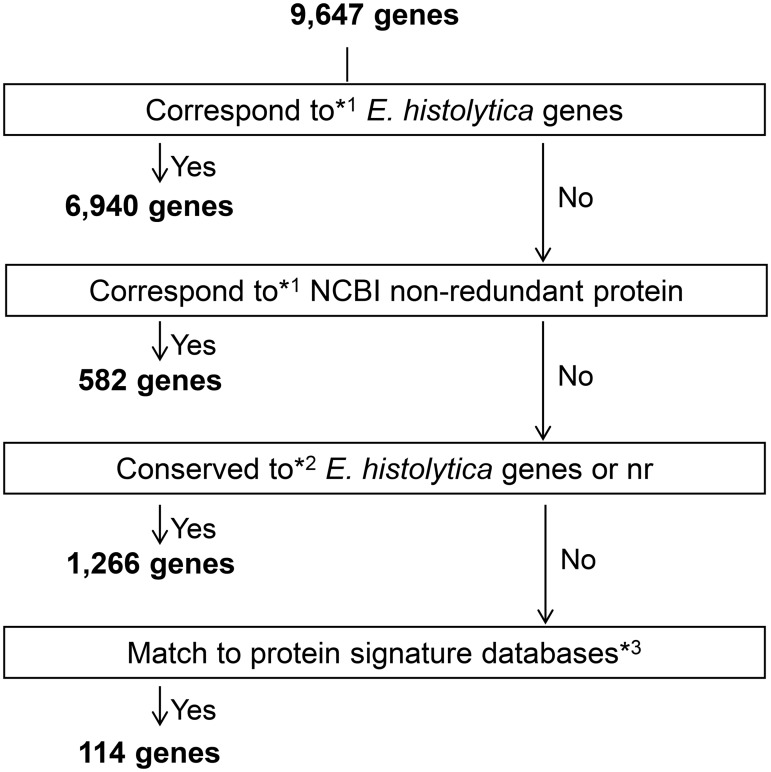
To assess the E. nuttalli genome features, we annotated the 9,647 predicted genes derived from the new assembly and compared the data with genome statistics of E. histolytica, E. dispar and E. invadens in AmoebaDB (Table 2). The results showed that the E. nuttalli genome had similar metrics to those of the mammalian Entamoeba species, including the percentage of coding regions and GC content, despite the larger total genome size and number of annotated genes compared to E. histolytica. Annotated genes were then classified into the following subsets: 6,940 and 582 genes with best sequence similarity to E. histolytica proteins and NCBI non-redundant proteins, respectively (≥80% identity, 90% coverage, e-value < 1×10−10); 1,266 genes with better sequence similarity to E. histolytica proteins or NCBI non-redundant proteins (≥50% identity, 70% coverage, e-value < 1×10−10); 114 genes assigned with the InterPro collection database and with poor sequence similarity (<50% identity, 70% coverage, and/or e-value > 1×10−10); and 745 genes that could not be assigned at the given thresholds (Fig 2).
Table 2. Comparison of genome statistics among four Entamoeba species.
| Item | E. nuttalli | E. histolytica* | E. dispar* | E. invadens* |
|---|---|---|---|---|
| Genome | ||||
| Size (bp) | 23,210,861 | 20,799,072 | 22,955,291 | 40,888,805 |
| Number of genes | 9,647 | 8,333 | 10,262 | 11,975 |
| GC content (%) | 24.23 | 24.20 | 23.54 | 29.92 |
| Mean gene length (bp) | 1,189.32 | 1,283.56 | 1,084.60 | 1,354.16 |
| Percent coding (%) | 49.43 | 51.43 | 48.49 | 39.66 |
| Exons | ||||
| Number | 12,727 | 10,891 | 13,932 | 17,958 |
| Mean number per gene | 1.32 | 1.31 | 1.36 | 1.50 |
| GC content (%) | 28.10 | 27.99 | 28.00 | 38.67 |
| Mean length (bp) | 880.05 | 965.15 | 777.99 | 869.2 |
| Total length (bp) | 11,200,448 | 10,511,492 | 10,838,988 | 15,609,079 |
* Numbers are data in AmoebaDB v.4.0.
Fig 2. Classification of E. nuttalli annotated genes.
Steps followed in categorization using AmoebaDB, the NCBI non-redundant protein database, and the InterProScan database. Categorization criteria for BLAST results were correspondence defined by blastp using coverage of ≥80% identity, 90% query coverage, and e-value < 1e-10; conservation defined by blastp using coverage of ≥50% identity, 70% query coverage and e-value < 1e-10; and InterProScan with preconfigured thresholds.
The repeat structure of the assembly was assessed using RepeatMasker software with the Entamoeba genus dataset (database 20140131 update). Approximately 21.57% of the genome assembly was classified as repetitive, comprising 5 Mb of DNA sequences. Transposable elements (TEs), such as short and long interspersed nuclear elements (SINEs and LINEs) [54] and Entamoeba-specific TEs (ERE1 and ERE2) [30] were also present (Table 3).
Table 3. Repeat elements in the E. nuttalli genome assembly identified by RepeatMasker.
| Name | Class/family | Number of sites | Total length (bp) | %Genome* |
|---|---|---|---|---|
| EHAPT2 | SINE | 343 | 139,869 | 0.60 |
| REP-1_ED | SINE | 157 | 65,024 | 0.28 |
| EdSINE1 | SINE | 134 | 29,462 | 0.13 |
| R4-1_ED | LINE | 805 | 1,002,819 | 4.32 |
| EhRLE3 | LINE | 441 | 577,331 | 2.49 |
| R4-N1_ED | LINE | 186 | 81,389 | 0.35 |
| EhRLE2 | LINE | 74 | 106,274 | 0.46 |
| ERE1_EH | Unknown | 960 | 1,220,105 | 5.26 |
| ERE2_EH | Unknown | 594 | 718,392 | 3.10 |
| EHINV1 | Unknown | 224 | 71,907 | 0.31 |
| ERE1_ED | Unknown | 101 | 42,317 | 0.18 |
| EHINV2 | Unknown | 68 | 10,226 | 0.04 |
| ERE1_EI | Unknown | 1 | 173 | < 0.01 |
| SCAI_EH | Repeat_region | 977 | 113,109 | 0.49 |
| LEAPFROG1_EI | DNA/PiggyBac | 1 | 108 | < 0.01 |
| Mogwai2_EI | DNA/TcMar-Mogwai | 1 | 93 | < 0.01 |
| Low_complexity | - | 3,832 | 231,577 | 1.00 |
| Simple_repeat | - | 10,618 | 597,899 | 2.58 |
| Total | 19,517 | 5,008,074 | 21.57 | |
* Expressed as a percentage of the assembly sequence length
Since surface-exposed proteins of parasites play an important role in the host-pathogen interaction, it is valuable to make a list of such proteins. An in silico analysis performed to classify the E. nuttalli annotated genes predicted that 2,070, 879, 237, and 500 genes coded for transmembrane-containing, signal peptide-containing, extracellular, and plasma membrane proteins, respectively, from the total of 9,647 genes in the E. nuttalli genome.
Identification of orthologous clusters among mammalian Entamoeba species
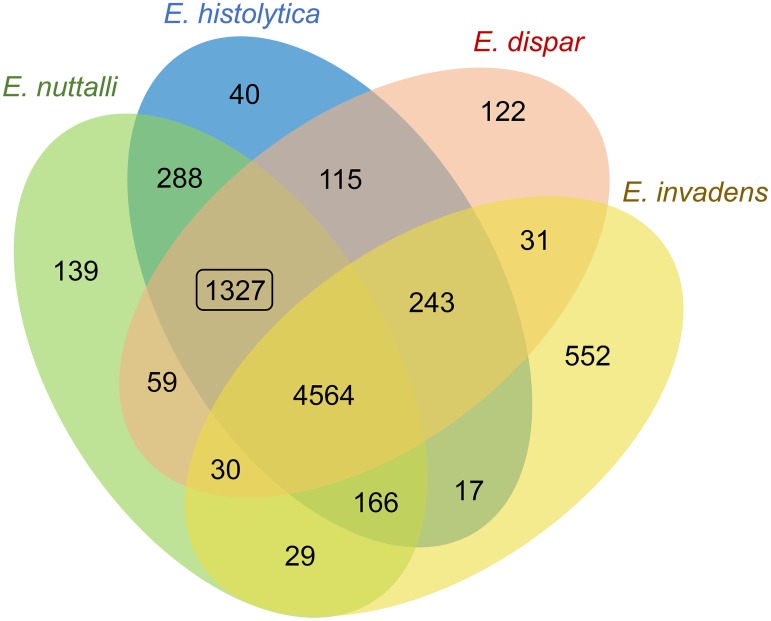
To identify specific and common orthologous genome clusters among E. nuttalli, E. histolytica, E. dispar, and E. invadens, comparative genome analysis was conducted using the OrthoVenn web server with default parameters. The 9,647 predicted proteins in the E. nuttalli genome were grouped into 6,602 clusters, of which 4,564 were shared with all other Entamoeba genomes and 1,327 were shared only with mammalian parasites, E. histolytica and E. dispar (Fig 3, S2 Table). To characterize the orthologous proteins shared among these mammalian Entamoeba species, GO-term enrichment analysis was performed using the DAVID web server. The 1,327 orthologous clusters comprised 1,591, 1,475, and 1,514 proteins of E. nuttalli, E. histolytica, and E. dispar, respectively. The E. histolytica orthologous proteins were used as a DAVID query because the E. histolytica genome is the most curated among Entamoeba species. GO-term enrichment analysis of the 1,475 E. histolytica proteins identified integral membrane components (cellular component, GO:0016021), including six AIG1 family proteins (EHI_022500, EHI_115160, EHI_144270, EHI_089670, EHI_195260 and EHI_195250) and 16 leucine rich repeat proteins as BspA-like proteins (EHI_062750, EHI_192600, EHI_139430, EHI_139390, EHI_054160, EHI_129870, EHI_110760, EHI_137910, EHI_013940, EHI_192250, EHI_082060, EHI_094080, EHI_020090, EHI_100700, EHI_147680 and EHI_127100) with an adjusted P-value < 0.05 (Table 4).
Fig 3. Characterization of mammalian host-related orthologous clusters.
Venn diagram of the orthologous gene family in four Entamoeba species. The rectangle indicates a possible mammalian host-related orthologous cluster.
Table 4. Enrichment of GO terms for ortholog clusters in mammalian Entamoeba species.
| GO ID | GO term | GO category | %Gene counts | Corrected P values* |
|---|---|---|---|---|
| GO:0016021 | Integral component of membrane | Cellular component | 12.8 | 0.0026626 |
| GO:0005525 | GTP binding | Molecular function | 4.0 | 0.0225842 |
| GO:0007264 | Small GTPase mediated signal transduction | Biological process | 3.6 | 0.0023422 |
| GO:0005643 | Nuclear pore | Cellular component | 0.8 | 0.0051100 |
| GO:0016788 | Hydrolase activity, acting on ester bonds | Molecular function | 0.5 | 0.0131763 |
* DAVID web server with adjusted P values < 0.05.
Refinement of candidates for E. nuttalli-specific surface proteins
Surface-exposed proteins are candidates for adhesion to host cells and defense or evasion from host immune attacks, while species-specific molecules may play an important role in host-specificity. A total of 114 annotated genes in the E. nuttalli genome had weak matches against a public database and were annotated only in the InterPro database (S3 Fig). Of these E. nuttalli-specific genes, three (ID; EN0317G0042, EN0144G0007 and EN0096G0007) were predicted to code for extracellular or plasma membrane proteins by WoLF-PSORT.
To examine whether these genes encode species-specific surface molecules, we manually curated the genes. In InterPro annotation, EN0317G0042 was assigned as a Sys1-family protein that functions in protein trafficking between the late Golgi and endosome [55]. Therefore, this protein was excluded from the list of surface-exposed proteins. The predicted amino acid sequence of EN0144G0007 had 94% sequence identity with E. histolytica SAPLIP6 (EAL50434), which is in the saposin-like protein family [56]. Sequence alignment of SAPLIP6 showed a conserved signal peptide and saposin-like structure (IPR011001) predicted by InterProScan (S4 Fig). A search in AmoebaDB (release 40, 15 Oct 2018) revealed no identical sequences with E. histolytica SAPLIP6. Therefore, EN0144G0007 was excluded from further analysis.
In contrast to EN0317G0042 and EN0144G0007 proteins, EN0096G0007 was not identified in other amoebozoan organisms, eukaryotes, archaea and bacteria, although some proteins in E. histolytica had <49% sequence identity with partial regions of EN0096G0007. Phylogenetic reconstruction of EN0096G0007 and its homologs in Entamoeba species showed that EN0096G0007 forms an isolated cluster from clusters of putative E. histolytica homologs, with strong bootstrap support (S5 Fig). Thus, EN0096G0007 may be a species-specific gene, and we conducted further characterization of its features in silico and in vitro.
A novel repeat protein specific for E. nuttalli
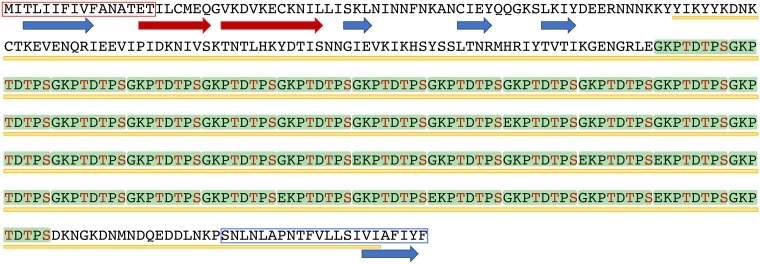
The function of EN0096G0007 was predicted using in silico analysis of the primary structure (Fig 4). The most remarkable feature of EN0096G0007 is the presence of 42 repeats of an octapeptide (NH2-[G,E]KPTDTPS-CO2H). Based on the primary structure prediction, we designated EN0096G0007 as PTORS (Proline and Threonine-rich Octapeptide-Repeat Surface protein). This repeat unit contains two threonines and one serine that were predicted to be phosphorylation and/or O-glycosylation sites by NetPhos 3.1 [57] and DictyOGlyc 1.1 [58]. Therefore, PTORS has 126 sites for putative modification in the octapeptide repeat region.
Fig 4. Secondary structure prediction for PTORS.
The predicted amino acid sequence of PTORS was subjected to secondary structure analysis by PSIPRED. This is shown as blue arrows for beta strands, red arrows for alpha helices, and yellow lines for disordered regions. For comparison, secondary structure prediction was also performed using Jpred (S6 Fig). A predicted signal peptide sequence (SignalP, red rectangle) and a transmembrane helix (SOSUI and TMPred, blue rectangle) are also shown. Octa-peptide repeat units of PTORS are shown as green rectangles.
In a secondary structure prediction, a putative signal peptide was detected by SignalP, and two α-helices, five β-strands, and 85.5% disordered regions were predicted by PSIPRED [59]. Almost all the α-helices and β-strands were within 80 residues from the N-terminus. In contrast, a transmembrane α-helix (NH2-SNLNLAPNTFVLLSIVIAFIYF-CO2H) near the C-terminus was predicted by SOSUI and TMPred (https://embnet.vital-it.ch/software/TMPRED_form.html), but not by TMHMM. GPI-anchor, myristoylation, and prenylation modifications of PTORS were not supported by PredGPI [60] NMT (http://mendel.imp.ac.at/myristate/SUPLpredictor.htm), and PrePS [61], respectively. However, Cys19 and Cys30 of PTORS were predicted as palmitoylation sites by CSS-Palm [62].
Expression and localization of the novel E. nuttalli-specific repeat protein
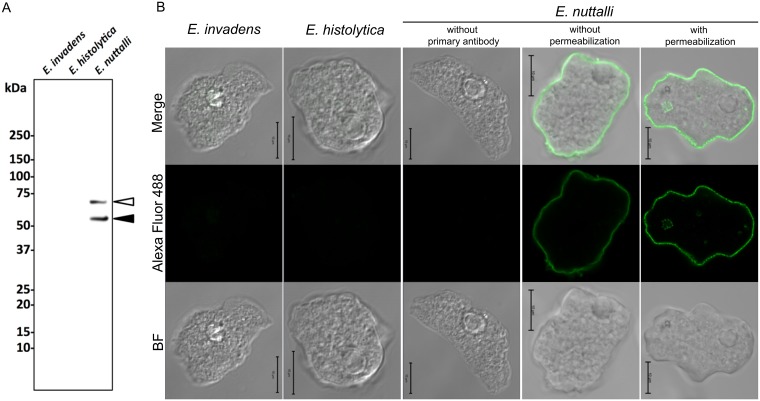
To confirm the localization of PTORS in E. nuttalli, we conducted immunoanalyses with murine antisera to recombinant PTORS. An immunoblot analysis using whole cell lysate of E. nuttalli showed a major band near the expected molecular mass of PTORS (55 kDa) (Fig 5), indicating that the antisera were reactive with a native PTORS. Another major band at approximately 70 kDa might have been due to phosphorylated and/or O-glycosylated PTORS modified post-translationally. Immunofluorescence staining using several Entamoeba species and antisera to PTORS (Fig 5 and S7 Fig) revealed fluorescence signals of PTORS on the surface of E. nuttalli trophozoites treated with Triton X-100. A similar result was observed using E. nuttalli treated without Triton X-100, strongly suggesting that most PTORS is exposed extracellularly. In addition, E. histolytica, E. invadens, E. dispar and E. moshkovskii had no proteins that were recognized by antisera to PTORS. These results indicate that PTORS is localized on the plasma membrane of E. nuttalli.
Fig 5. PTORS expression in Entamoeba species.
(A) Confirmation of expression of PTORS by immunoblot analysis with antisera specific for PTORS. Lanes contain 30 μg protein of whole cell lysates from E. nuttalli, E. histolytica HM-1:IMSS, and E. invadens IP-1. Closed and open arrowheads indicate PTORS bands without and with putative post-translational modification, respectively. (B) Immunofluorescence images of E. nuttalli, E. histolytica, and E. invadens using antisera specific for PTORS. Scale bar = 10 μm. Entamoeba species were stained by treatment with/without Triton X-100 and with/without antisera specific for PTORS. Merged bright field (BF) and fluorescence images are also shown (Merge). These images are approximately 0.9 μm in thickness per section.
Discussion
There are five important outcomes of this study: 1) a new gene catalog and contigs of E. nuttalli with a more refined assembly, 2) identification of key features of the E. nuttalli genome, 3) a new comparative analysis among Entamoeba genomes, 4) a list of candidate molecules associated with infection of mammalian hosts by Entamoeba species, and 5) identification of an E. nuttalli-specific surface protein that has not been found in other organisms.
Hybrid de novo genome assembly was useful for construction of a near-complete genome, especially using the SMRT sequencing platform combined with short-read sequencing in eukaryotic genomes [63–65]. We generated a good quality draft assembly of the E. nuttalli genome through self-correction of long reads and by polishing the corrected reads using short reads. The new genome assembly is much more complete than the public genome assembly of E. nuttalli as illustrated by the genomic properties shown in Table 1. Moreover, proteome coverage of our dataset indicated 5,594 orthologous clusters shared with the public dataset of E. nuttalli and only three orthologous clusters containing six protein sequences that were not included (S8 Fig). Although four of these six sequences were found in our dataset by BLAST search (e-value<1e-10), some of the contigs in the new genome assembly may include uncorrected bases due to sequence errors derived from SMART sequencing. However, the new assembly had an AT content and gene density in E. nuttalli similar to those in genomes of other Entamoeba species. This shows that our annotation dataset is comparable to other Entamoeba genomes.
The present analysis also confirms previous reports showing that E. nuttalli is the closest species to E. histolytica; indeed, approximately 70% of the E. nuttalli annotated genes were conserved in E. histolytica [66,67]. The repeat fraction of the E. nuttalli genome (21.57%) was more similar to that in E. histolytica (19.7%) compared to E. dispar (9.7%) and E. invadens (9.9%) [30]. Moreover, the population of repeat elements of E. nuttalli is enriched with non-LTR retroelements, in correspondence with those of other mammalian Entamoeba species, while those in non-mammalian species are enriched in class II transposons [30,68–70]. Notably, ERE2, an E. histolytica-specific repeat, was also found in the E. nuttalli genome [30], which also supports E. nuttalli as the closest species to E. histolytica. Since previous reports have demonstrated that chromosome rearrangements driven by transposable elements contribute to host adaptation [71,72], our findings also suggest that transposable elements have contributed to diversification of Entamoeba species, as well as to host adaptation. Nevertheless, we could not achieve chromosome-level sequence assembly. This result suggests that our dataset could not resolve genome complexity such as repetitive elements larger than SMRT sequencing reads and/or low complexity regions leading to misalignment of assembly [73]. Some of those complex regions might contribute to transcriptional silencing of the amoebapore gene [70,74] and diversification of gene families such as light and intermediate subunits of the galactose and N-acetyl-D-galactosamine-inhibitable adherence lectin [75]. It may be possible to improve the assembly utilizing methodologies such as Hi-C and the BioNano Genomics Irys System [76–79].
Our GO-term enrichment analysis revealed a high content of transmembrane components, such as AIG1 family proteins and BspA-like proteins, in the mammalian Entamoeba genomes (Table 4). An AIG1 family protein (EHI_176590) has recently been described as a virulence factor that was absent in an E. histolytica KU27 strain isolated from an asymptomatic cyst passer [28]. Moreover, the E. histolytica BspA-like protein (EHI_016490) seems to function as a chemoattractant receptor for tumor necrosis factor [80], and bacterial BspA-like proteins are involved in adherence, invasion of epithelial cells, and binding to fibronectin and fibrinogen [81–83]. Interestingly, a recent report has demonstrated that AIG1 and BspA families are undergoing lineage-specific expansion in E. histolytica [84]. These reports suggest that mammalian Entamoeba species have expanded the gene number for cell surface proteins to adapt to different environments in the host digestive tract and/or to develop a virulence mechanism for each host. This may also be supported by our data showing a correlation between the number of pathogenic Entamoeba genes for the BspA-like protein and host genes for fibronectin and fibrinogen (S9 Fig). Further analyses of these protein families might reveal the core set of surface proteins required for infection of a mammalian host. Incidentally, our phylogenetic analysis of the Entamoeba AIG1 protein family (S10 Fig) showed that E. histolytica AIG1 (EHI_176590) clustered with proteins of other Entamoeba species with moderate bootstrap support, suggesting that the quality, rather than the existence, of AIG1 is important for virulence associated with EHI_176590.
Repeat-containing proteins in intracellular parasites seem to contain a larger number of N- and O-glycosylation sites than those in extracellular parasites. In addition, extracellular parasites tend to contain degenerate repetitive motifs compared with intracellular parasites [85]. Therefore, PTORS found in the extracellular parasite in this study may be an exception because this protein has a large number of putative O-glycosylation sites and almost perfect repetitive motifs (Fig 4). The most important question is the function of this novel surface protein in E. nuttalli. Recently, O-glycosylated proteins on pathogens and tumors have been reported to contribute to immune evasion [86–88]. Moreover, repeat-containing proteins in parasites play important roles in interactions with host cells, such as adhesion, invasion, virulence, and evasion from the host immune system [89–94]. These reports suggest that E. nuttalli uses PTORS for evasion of the immune system of the host macaque. Infections with E. nuttalli have been observed in various species of wild macaques, but host macaques are asymptomatic, indicating a commensal host-parasite relationship in these natural hosts [13,15,17–19]. In contrast, fatal cases of liver abscess with E. nuttalli have been reported in an Abyssinian colobus and Geoffroy’s spider monkey in a zoo [95,96]; and severe inflammatory reactions have been found in livers of hamsters inoculated experimentally with E. nuttalli, which indicates pathogenicity for these host species [12,15,17,97]. These findings suggest that the surface molecules of E. nuttalli evolved to permit colonization in the intestine of natural hosts by keeping a balance with host immunity. The E. nuttalli-specific protein identified in this study may have an important role in this phenomenon. However, there is no experimental evidence of post-translational modifications or a contribution to parasitic adaptation at present, and further analyses are needed to determine the physiological function of PTORS.
A better understanding of host specificity would be obtained by analyses of host-parasite interactions, such as the mechanisms of evasion of the host defense system, adherence and/or invasion of host cells and tissue, and acquisition of nutrients from the host. Collectively, this study revealed common molecular signatures among mammalian Entamoeba species and an E. nuttalli-specific surface protein, based on refined assembly of the E. nuttalli genome and comparative genome analysis. The discovery of PTORS from E. nuttalli supports the validity of our catalog of molecular candidates related to host range. Our approach of host-driven comparative analysis of parasite molecules reflecting host specificity may be useful for prediction of possible host alternation, as well as understanding of parasite evolution and identification of new drug targets.
Supporting information
Filter-passed subreads were obtained from 57 SMRT Cells of raw data with automatic removal of subreads with low accuracy (< 80%) and/or short-read length (< 500 bases). A bar plot was constructed using R with the bin width set to 1.
(TIF)
After Illumina short-read data were mapped to the primary assembly and both contig ends of the primary assembly were trimmed, the mapped reads were counted at each position for each contig and aggregated per percentage of contig bases. The line plots indicate trimming of both contig ends before (upper) and after (lower).
(TIF)
Of 9,647 annotated genes derived from the E. nuttalli genome, 114 had weak matches against a public database and were only annotated in the InterPro collection database. Gene Ontology assignments of these 114 E. nuttalli-specific candidate genes were referred from the InterPro annotation using the interpro2go dataset.
(TIF)
(A) ClustalW alignment of SALIP6 in E. nuttalli (EN0144G0007) and E. histolytica (EAL50434.1). The amino acid sequence of EN0144G0007 was classified into a protein signature by InterPro. Signal peptide and saposin-like (IPR011001) regions are framed by green and orange rectangles, respectively. (B) SignalP 4.1 analysis of EN0144G0007.
(TIF)
Putative homologous genes were collected from AmoebaDB using a BLAST search based on alignment score, e-value and visual inspection of the sequence alignment. Additionally, the putative homologous genes of E. histolytica were searched against our E. nuttalli protein dataset because some putative homologous genes of E. nuttalli could not be identified in AmoebaDB. Multiple alignments of the sequences were obtained using MAFFT v.7 with the G-INS-i algorithm and without gap region realignment (“Leave gappy regions”) (Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30: 772–780.). The appropriate amino acid substitution model (JTT+G) for the reconstruction was selected using the “Find Best DNA/Protein Models” tool in MEGA7 (Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33: 1870–1874.). Unambiguously aligned positions were used in the Neighbor-Joining (NJ) and Maximum Likelihood (ML) methods with 1,000 bootstrap replications in MEGA7. The parameter of Rates among Sites was set as Gamma distributed (G) and value as 13. For construction of ML phylogeny, the parameter “Initial Tree File” was set as the NJ phylogeny that we constructed. The output best tree was further edited using FigTree v,1.4.3 (https://github.com/rambaut/figtree/). The support values at the nodes represent bootstrap values. EN0096G0007 was expressed as red text.
(TIF)
The predicted amino acid sequence of EN0096G007 was subjected to secondary structure analysis in Jpred and PSIPRED. Predicted α-helices, β-strands, coil and disordered regions are indicated as H, E, C and D, respectively. A predicted signal peptide sequence (SignalP, red rectangle) and transmembrane helix (SOSUI and TMPred, blue rectangle) are also shown.
(TIF)
Immunofluorescence images of E. nuttalli, E. dispar SAW1734RclAR and E. moshkovskii Laredo using antisera specific for PTORS. Scale bar = 10 μm. Entamoeba species were stained by treatment with Triton X-100 and with antisera specific for PTORS. Merged bright field and fluorescence images are also shown (Merge). These images are approximately 0.9 μm in thickness per section.
(TIF)
Venn diagram showing orthologous cluster coverage between the new genome assembly (in this study) and the public genome assembly (AmoebaDB ver.4.0) in E. nuttalli. Numbers are estimated by the OrthoVenn server. The number of orthologous clusters is shown in bold. Numbers given in brackets indicate the number of genes, including the orthologous clusters. The new genome assembly and public genome assembly have 1,090 and 530 singletons, respectively.
(TIF)
(A) Comparison of the number of gene candidates for BspA-like proteins with and without putative membrane association among pathogenic Entamoeba species. Entamoeba BspA-like proteins predicted to contain “LRR_5 family (PF13306)” by Pfam (El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2018;47: D427-D432.) were collected from AmoebaDB. Entamoeba BspA-like proteins with putative membrane association were extracted from Entamoeba BspA-like proteins using TMHMM for the transmembrane and/or GPS-Lipid (Xie Y, Zheng Y, Li H, Luo X, He Z, Cao S, et al. GPS-Lipid: a robust tool for the prediction of multiple lipid modification sites. Sci Rep. 2016;6: 28249.) and PrePS (Maurer-Stroh S, Eisenhaber F. Refinement and prediction of protein prenylation motifs. Genome Biol. 2005;6: R55.) for lipid modification. (B) Correlation between the number of BspA genes in pathogenic Entamoeba species and genes for host fibronectin. (C) Correlation between the number of BspA genes in pathogenic Entamoeba species and genes for host fibrinogen. Human (Homo sapiens), macaque (Macaca fascicularis), and snake (Python bivittatus) are hosts for E. histolytica, E. nuttalli, and E. invadens, respectively. Host genes were extracted from Genome Data Viewer (https://www.ncbi.nlm.nih.gov/genome/gdv/).
(TIF)
A multiple sequence alignment of Entamoeba AIG1 family proteins was obtained in MUSCLE (Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32: 1792–1797.) and corrected by manual inspection. With 59 proteins from 3 species (16, 15, and 28 from E. nuttalli, E. histolytica, and E. dispar, respectively), 201 aligned amino acid sites were used in the analysis. The maximum likelihood (ML) best tree inferred by the JTT+F model with four categories of among-site rate variation and the rate variation model allowed some sites to be evolutionarily invariable (+I, 2.7363% sites) in MEGA7. Bootstrap proportions in the ML method (100 replicates) are attached to the internal branches. Branches with <50% bootstrap support are unmarked. Alignments are available from the authors upon request.
(TIF)
Raw read data were obtained from the Illumina GAIIx platform with a 114 bp paired-end module. For trimming of low quality bases, raw read data were adapted with FASTX-Toolkit using a minimum read length (-l) of 70 bases and a quality cutoff (-t) of 20. The quality-passed reads were mapped to the primary assembly using bwa-mem with default parameters.
(XLSX)
Proteome data were downloaded from AmoebaDB v.4.0.
(XLSX)
Acknowledgments
We thank Dr. Primo Baybayan of Pacific Biosciences for advice on the SMRT sequencing strategy; Prof. Itsuro Inoue and Dr. Kazuyoshi Hosomichi of the National Institute of Genetics for advice on Illumina sequencing; and Prof. Atsushi Ogura of Nagahama Institute of Bioscience and Technology for technical advice of comparative genomics. We are grateful to Dr. Meng Feng of Tokai University of School Medicine for experimental support. We gratefully acknowledge the staff of the Support Center for Medical Research and Education of Tokai University for technical assistance. Computations were partially performed on the NIG supercomputer at ROIS National Institute of Genetics.
Data Availability
Short- and long-read sequences have been deposited in the DNA Data Bank of Japan (BioProject PRJDB4858, BioSample SAMD00053943, DRA accession no. DRA004867).
Funding Statement
This work was supported by Japan Society for the Promotion of Science (JSPS) (http://www.jsps.go.jp) KAKENHI Grant Numbers 23117009, 24406013, 26460516, JP16H05819, JP17K08811 to HT and JP18K07096 to TM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tawari B, Ali IKM, Scott C, Quail MA, Berriman M, Hall N, et al. Patterns of evolution in the unique tRNA gene arrays of the genus Entamoeba. Mol Biol Evol. 2007;25: 187–198. 10.1093/molbev/msm238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stensvold CR, Lebbad M, Victory EL, Verweij JJ, Tannich E, Alfellani M, et al. Increased sampling reveals novel lineages of Entamoeba: consequences of genetic diversity and host specificity for taxonomy and molecular detection. Protist. 2011;162: 525–541. 10.1016/j.protis.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 3.Shiratori T, Ishida K. Entamoeba marina n. sp.; a new species of Entamoeba isolated from tidal flat sediment of Iriomote Island, Okinawa, Japan. J Eukaryot Microbiol. 2016;63: 280–286. 10.1111/jeu.12276 [DOI] [PubMed] [Google Scholar]
- 4.Kawano T, Imada M, Chamavit P, Kobayashi S, Hashimoto T, Nozaki T. Genetic diversity of Entamoeba: Novel ribosomal lineages from cockroaches. PloS one. 2017;12: e0185233 10.1371/journal.pone.0185233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanley SL. Amoebiasis. The Lancet. 2003;361: 1025–1034. [DOI] [PubMed] [Google Scholar]
- 6.Tachibana H, Cheng X, Kobayashi S, Matsubayashi N, Gotoh S, Matsubayashi K. High prevalence of infection with Entamoeba dispar, but not E. histolytica, in captive macaques. Parasitol Res. 2001;87: 14–17. 10.1007/s004360000289 [DOI] [PubMed] [Google Scholar]
- 7.Tachibana H, Cheng X, Kobayashi S, Fujita Y, Udono T. Entamoeba dispar, but not E. histolytica, detected in a colony of chimpanzees in Japan. Parasitol Res. 2000;86: 537–541. 10.1007/s004360000205 [DOI] [PubMed] [Google Scholar]
- 8.Diamond LS, Clark CG. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J Eukaryot Microbiol. 1993;40: 340–344. 10.1111/j.1550-7408.1993.tb04926.x [DOI] [PubMed] [Google Scholar]
- 9.Bansal D, Ave P, Kerneis S, Frileux P, Boché O, Baglin AC, et al. An ex-vivo human intestinal model to study Entamoeba histolytica pathogenesis. PLoS Negl Trop Dis. 2009;3: e551 10.1371/journal.pntd.0000551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avron B, Stolarsky T, Chayen A, Mirelman D. Encystation of Entamoeba invadens IP-1 is induced by lowering the osmotic pressure and depletion of nutrients from the medium. J Protozool. 1986;33: 522–525. 10.1111/j.1550-7408.1986.tb05655.x [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Zapien AG, Hernandez-Gutierrez R, Mora-Galindo J. Simultaneous growth and mass encystation of Entamoeba invadens under axenic conditions. Arch Med Res. 1995;26: 257–262. [PubMed] [Google Scholar]
- 12.Tachibana H, Yanagi T, Pandey K, Cheng X, Kobayashi S, Sherchand JB, et al. An Entamoeba sp. strain isolated from rhesus monkey is virulent but genetically different from Entamoeba histolytica. Mol Biochem Parasitol. 2007;153: 107–114. 10.1016/j.molbiopara.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 13.Tachibana H, Yanagi T, Lama C, Pandey K, Feng M, Kobayashi S, et al. Prevalence of Entamoeba nuttalli infection in wild rhesus macaques in Nepal and characterization of the parasite isolates. Parasitol Int. 2013;62: 230–235. 10.1016/j.parint.2013.01.004 [DOI] [PubMed] [Google Scholar]
- 14.Levecke B, Dreesen L, Dorny P, Verweij JJ, Vercammen F, Casaert S, et al. Molecular identification of Entamoeba spp. in captive nonhuman primates. J Clin Microbiol. 2010;48: 2988–2990. 10.1128/JCM.00013-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tachibana H, Yanagi T, Akatsuka A, Kobayashi S, Kanbara H, Tsutsumi V. Isolation and characterization of a potentially virulent species Entamoeba nuttalli from captive Japanese macaques. Parasitology. 2009;136: 1169–1177. 10.1017/S0031182009990576 [DOI] [PubMed] [Google Scholar]
- 16.Feng M, Cai J, Min X, Fu Y, Xu Q, Tachibana H, et al. Prevalence and genetic diversity of Entamoeba species infecting macaques in southwest China. Parasitol Res. 2013;112: 1529–1536. 10.1007/s00436-013-3299-1 [DOI] [PubMed] [Google Scholar]
- 17.Tachibana H, Yanagi T, Feng M, Bandara KAT, Kobayashi S, Cheng X, et al. Isolation and molecular characterization of Entamoeba nuttalli strains showing novel isoenzyme patterns from wild toque macaques in Sri Lanka. J Eukaryot Microbiol. 2016;63: 171–180. 10.1111/jeu.12265 [DOI] [PubMed] [Google Scholar]
- 18.Feng M, Yanagi T, Putaporntip C, Pattanawong U, Cheng X, Jongwutiwes S, et al. Correlation between genotypes and geographic distribution of Entamoeba nuttalli isolates from wild long-tailed macaques in Central Thailand. Infect Genet Evol. 2019;70: 114–122. 10.1016/j.meegid.2019.02.030 [DOI] [PubMed] [Google Scholar]
- 19.Guan Y, Feng M, Cai J, Min X, Zhou X, Xu Q, et al. Comparative analysis of genotypic diversity in Entamoeba nuttalli isolates from Tibetan macaques and rhesus macaques in China. Infect Genet Evol. 2016;38: 126–131. 10.1016/j.meegid.2015.12.014 [DOI] [PubMed] [Google Scholar]
- 20.Wei M, Feng M, Guan Y, Guo C, Zhou H, Fu Y, et al. Correlation of genetic diversity between hosts and parasites in Entamoeba nuttalli isolates from Tibetan and rhesus macaques in China. Biosci trends. 2018;12: 375–381. 10.5582/bst.2018.01157 [DOI] [PubMed] [Google Scholar]
- 21.Takano J, Narita T, Tachibana H, Terao K, Fujimoto K. Comparison of Entamoeba histolytica DNA isolated from a cynomolgus monkey with human isolates. Parasitol Res. 2007;101: 539–546. 10.1007/s00436-007-0510-2 [DOI] [PubMed] [Google Scholar]
- 22.Levecke B, Dorny P, Vercammen F, Visser LG, Van Esbroeck M, Vercruysse J, et al. Transmission of Entamoeba nuttalli and Trichuris trichiura from Nonhuman Primates to Humans. Emerg Infect Dis. 2015;21: 1871–1872. 10.3201/eid2110.141456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponsuwanna P, Kochakarn T, Bunditvorapoom D, Kümpornsin K, Otto TD, Ridenour C, et al. Comparative genome-wide analysis and evolutionary history of haemoglobin-processing and haem detoxification enzymes in malarial parasites. Malaria J. 2016;15: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widmer G, Lee Y, Hunt P, Martinelli A, Tolkoff M, Bodi K. Comparative genome analysis of two Cryptosporidium parvum isolates with different host range. Infect Genet Evol. 2012;12: 1213–1221. 10.1016/j.meegid.2012.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon JL, Sibley LD. Comparative genome analysis reveals a conserved family of actin-like proteins in apicomplexan parasites. BMC Genomics. 2005;6: 179 10.1186/1471-2164-6-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tachibana S, Sullivan SA, Kawai S, Nakamura S, Kim HR, Goto N, et al. Plasmodium cynomolgi genome sequences provide insight into Plasmodium vivax and the monkey malaria clade. Nat Genet. 2012;44: 1051 10.1038/ng.2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doniger T, Katz R, Wachtel C, Michaeli S, Unger R. A comparative genome-wide study of ncRNAs in trypanosomatids. BMC Genomics. 2010;11: 615 10.1186/1471-2164-11-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakada-Tsukui K, Sekizuka T, Sato-Ebine E, Escueta-de Cadiz A, Tomii K, Kuroda M, et al. AIG1 affects in vitro and in vivo virulence in clinical isolates of Entamoeba histolytica. PLoS Pathog. 2018;14: e1006882 10.1371/journal.ppat.1006882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah PH, MacFarlane RC, Bhattacharya D, Matese JC, Demeter J, Stroup SE, et al. Comparative genomic hybridizations of Entamoeba strains reveal unique genetic fingerprints that correlate with virulence. Eukaryot Cell. 2005;4: 504–515. 10.1128/EC.4.3.504-515.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenzi H, Thiagarajan M, Haas B, Wortman J, Hall N, Caler E. Genome wide survey, discovery and evolution of repetitive elements in three Entamoeba species. BMC Genomics. 2008;9: 595 10.1186/1471-2164-9-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorenzi HA, Puiu D, Miller JR, Brinkac LM, Amedeo P, Hall N, et al. New assembly, reannotation and analysis of the Entamoeba histolytica genome reveal new genomic features and protein content information. PLoS Negl Trop Dis. 2010;4: e716 10.1371/journal.pntd.0000716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willhoeft U, Tannich E. The electrophoretic karyotype of Entamoeba histolytica. Mol Biochem Parasitol. 1999;99: 41–53. 10.1016/s0166-6851(98)00178-9 [DOI] [PubMed] [Google Scholar]
- 33.Rhoads A, Au KF. PacBio sequencing and its applications. Genomics, proteomics & bioinformatics. 2015;13: 278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72: 431–432. 10.1016/0035-9203(78)90144-x [DOI] [PubMed] [Google Scholar]
- 35.Tachibana H, Ihara S, Kobayashi S, Kaneda Y, Takeuchi T, Watanabe Y. Differences in genomic DNA sequences between pathogenic and nonpathogenic isolates of Entamoeba histolytica identified by polymerase chain reaction. J Clin Microbiol. 1991;29: 2234–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaisson MJ, Tesler G. Mapping single molecule sequencing reads using basic local alignment with successive refinement (BLASR): application and theory. BMC Bioinformatics. 2012;13: 238 10.1186/1471-2105-13-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chin C, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10: 563–569. 10.1038/nmeth.2474 [DOI] [PubMed] [Google Scholar]
- 38.Li H, Durbin R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics. 2009;25: 1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25: 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31: 3210–3212. 10.1093/bioinformatics/btv351 [DOI] [PubMed] [Google Scholar]
- 41.Eddy SR. A new generation of homology search tools based on probabilistic inference. In: Anonymous Genome Informatics 2009: Genome Informatics Series Vol. 23.: World Sci; 2009. pp. 205–211. [PubMed]
- 42.Stanke M, Waack S. Gene prediction with a hidden Markov model and a new intron submodel. Bioinformatics. 2003;19: ii215–ii225. 10.1093/bioinformatics/btg1080 [DOI] [PubMed] [Google Scholar]
- 43.Aurrecoechea C, Barreto A, Brestelli J, Brunk BP, Caler EV, Fischer S, et al. AmoebaDB and MicrosporidiaDB: functional genomic resources for Amoebozoa and Microsporidia species. Nucleic Acids Res. 2010;39: D612–D619. 10.1093/nar/gkq1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones P, Binns D, Chang H, Fraser M, Li W, McAnulla C, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30: 1236–1240. 10.1093/bioinformatics/btu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Coleman-Derr D, Chen G, Gu YQ. OrthoVenn: a web server for genome wide comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2015;43: W78–W84. 10.1093/nar/gkv487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dennis G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4: R60. [PubMed] [Google Scholar]
- 47.Hirokawa T, Boon-Chieng S, Mitaku S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics. 1998;14: 378–379. 10.1093/bioinformatics/14.4.378 [DOI] [PubMed] [Google Scholar]
- 48.Krogh A, Larsson B, Von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305: 567–580. 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 49.Sonnhammer EL, Von Heijne G, Krogh A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc Int Conf Intell Syst Mol Biol. 1998;6: 175–182. [PubMed] [Google Scholar]
- 50.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8: 785 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 51.Horton P, Park K, Obayashi T, Fujita N, Harada H, Adams-Collier C, et al. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35: W585–W587. 10.1093/nar/gkm259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makiuchi T, Santos HJ, Tachibana H, Nozaki T. Hetero-oligomer of dynamin-related proteins participates in the fission of highly divergent mitochondria from Entamoeba histolytica. Sci Rep. 2017;7: 13439 10.1038/s41598-017-13721-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kato K, Makiuchi T, Cheng X, Tachibana H. Comparison of hemolytic activity of the intermediate subunit of Entamoeba histolytica and Entamoeba dispar lectins. PLoS One. 2017;12: e0181864 10.1371/journal.pone.0181864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kögler A, Schmidt T, Wenke T. Evolutionary modes of emergence of short interspersed nuclear element (SINE) families in grasses. The Plant J. 2017;92: 676–695. 10.1111/tpj.13676 [DOI] [PubMed] [Google Scholar]
- 55.Liu YW, Lee SW, Lee FJ. Arl1p is involved in transport of the GPI-anchored protein Gas1p from the late Golgi to the plasma membrane. J Cell Sci. 2006;119: 3845–3855. 10.1242/jcs.03148 [DOI] [PubMed] [Google Scholar]
- 56.Winkelmann J, Leippe M, Bruhn H. A novel saposin-like protein of Entamoeba histolytica with membrane-fusogenic activity. Mol Biochem Parasitol. 2006;147: 85–94. 10.1016/j.molbiopara.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 57.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294: 1351–1362. 10.1006/jmbi.1999.3310 [DOI] [PubMed] [Google Scholar]
- 58.Gupta R, Jung E, Gooley AA, Williams KL, Brunak S, Hansen J. Scanning the available Dictyostelium discoideum proteome for O-linked GlcNAc glycosylation sites using neural networks. Glycobiology. 1999;9: 1009–1022. 10.1093/glycob/9.10.1009 [DOI] [PubMed] [Google Scholar]
- 59.Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol. 1999;292: 195–202. 10.1006/jmbi.1999.3091 [DOI] [PubMed] [Google Scholar]
- 60.Pierleoni A, Martelli PL, Casadio R. PredGPI: a GPI-anchor predictor. BMC Bioinformatics. 2008;9: 392 10.1186/1471-2105-9-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maurer-Stroh S, Eisenhaber F. Refinement and prediction of protein prenylation motifs. Genome Biol. 2005;6: R55 10.1186/gb-2005-6-6-r55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X. CSS-Palm 2.0: an updated software for palmitoylation sites prediction. Protein Eng, Des Sel. 2008;21: 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller JR, Zhou P, Mudge J, Gurtowski J, Lee H, Ramaraj T, et al. Hybrid assembly with long and short reads improves discovery of gene family expansions. BMC Genomics. 2017;18: 541 10.1186/s12864-017-3927-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pendleton M, Sebra R, Pang AWC, Ummat A, Franzen O, Rausch T, et al. Assembly and diploid architecture of an individual human genome via single-molecule technologies. Nat Methods. 2015;12: 780 10.1038/nmeth.3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakai H, Naito K, Ogiso-Tanaka E, Takahashi Y, Iseki K, Muto C, et al. The power of single molecule real-time sequencing technology in the de novo assembly of a eukaryotic genome. Sci Rep. 2015;5: 16780 10.1038/srep16780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacob AS, Busby EJ, Levy AD, Komm N, Clark CG. Expanding the Entamoeba universe: new hosts yield novel ribosomal lineages. J Eukaryot Microbiol. 2016;63: 69–78. 10.1111/jeu.12249 [DOI] [PubMed] [Google Scholar]
- 67.Elsheikha HM, Regan CS, Clark CG. Novel Entamoeba findings in nonhuman primates. Trends Parasitol. 2018;34: 283–294. 10.1016/j.pt.2017.12.008 [DOI] [PubMed] [Google Scholar]
- 68.Shire AM, Ackers JP. SINE elements of Entamoeba dispar. Mol Biochem Parasitol. 2007;152: 47–52. 10.1016/j.molbiopara.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 69.Pritham EJ, Feschotte C, Wessler SR. Unexpected diversity and differential success of DNA transposons in four species of Entamoeba protozoans. Mol Biol Evol. 2005;22: 1751–1763. 10.1093/molbev/msi169 [DOI] [PubMed] [Google Scholar]
- 70.Bakre AA, Rawal K, Ramaswamy R, Bhattacharya A, Bhattacharya S. The LINEs and SINEs of Entamoeba histolytica: comparative analysis and genomic distribution. Exp Parasitol. 2005;110: 207–213. 10.1016/j.exppara.2005.02.009 [DOI] [PubMed] [Google Scholar]
- 71.Blot M. Transposable elements and adaptation of host bacteria. Genetica. 1994;93: 5–12. 10.1007/bf01435235 [DOI] [PubMed] [Google Scholar]
- 72.Thomas MC, Macias F, Alonso C, López MC. The biology and evolution of transposable elements in parasites. Trends Parasitol. 2010;26: 350–362. 10.1016/j.pt.2010.04.001 [DOI] [PubMed] [Google Scholar]
- 73.Gilchrist CA. The E. histolytica Genome Structure and Virulence. Curr Trop Med Rep. 2016;3: 158–163. 10.1007/s40475-016-0088-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bracha R, Nuchamowitz Y, Mirelman D. Transcriptional silencing of an amoebapore gene in Entamoeba histolytica: molecular analysis and effect on pathogenicity. Eukaryot Cell. 2003;2: 295–305. 10.1128/EC.2.2.295-305.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weedall GD, Sherrington J, Paterson S, Hall N. Evidence of gene conversion in genes encoding the Gal/GalNac lectin complex of Entamoeba. PLoS Negl Trop Dis. 2011;5: e1209 10.1371/journal.pntd.0001209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cao H, Hastie AR, Cao D, Lam ET, Sun Y, Huang H, et al. Rapid detection of structural variation in a human genome using nanochannel-based genome mapping technology. GigaScience. 2014;3: 34 10.1186/2047-217X-3-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burton JN, Adey A, Patwardhan RP, Qiu R, Kitzman JO, Shendure J. Chromosome-scale scaffolding of de novo genome assemblies based on chromatin interactions. Nat Biotechnol. 2013;31: 1119 10.1038/nbt.2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bickhart DM, Rosen BD, Koren S, Sayre BL, Hastie AR, Chan S, et al. Single-molecule sequencing and chromatin conformation capture enable de novo reference assembly of the domestic goat genome. Nat Genet. 2017;49: 643 10.1038/ng.3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiao Y, Peluso P, Shi J, Liang T, Stitzer MC, Wang B, et al. Improved maize reference genome with single-molecule technologies. Nature. 2017;546: 524 10.1038/nature22971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silvestre A, Plaze A, Berthon P, Thibeaux R, Guillen N, Labruyere E. In Entamoeba histolytica, a BspA family protein is required for chemotaxis toward tumour necrosis factor. Microb Cell. 2015;2: 235–246. 10.15698/mic2015.07.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma A, Sojar HT, Glurich I, Honma K, Kuramitsu HK, Genco RJ. Cloning, expression, and sequencing of a cell surface antigen containing a leucine-rich repeat motif from Bacteroides forsythus ATCC 43037. Infect Immun. 1998;66: 5703–5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mengaud J, Lecuit M, Lebrun M, Nato F, Mazie JC, Cossart P. Antibodies to the leucine-rich repeat region of internalin block entry of Listeria monocytogenes into cells expressing E-cadherin. Infect Immun. 1996;64: 5430–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Inagaki S, Onishi S, Kuramitsu HK, Sharma A. Porphyromonas gingivalis vesicles enhance attachment, and the leucine-rich repeat BspA protein is required for invasion of epithelial cells by "Tannerella forsythia". Infect Immun. 2006;74: 5023–5028. 10.1128/IAI.00062-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilson IW, Weedall GD, Lorenzi H, Howcroft T, Hon C, Deloger M, et al. Genetic diversity and gene family expansions in members of the genus Entamoeba. Genome Biol Evol. 2019;11: 688–705. 10.1093/gbe/evz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mendes T, Lobo F, Rodrigues T, Rodrigues-Luiz G, Darocha W, Fujiwara R, et al. Repeat-enriched proteins are related to host cell invasion and immune evasion in parasitic protozoa. Mol Biol Evol. 2013;30: 951–963. 10.1093/molbev/mst001 [DOI] [PubMed] [Google Scholar]
- 86.Machiels B, Lété C, Guillaume A, Mast J, Stevenson PG, Vanderplasschen A, et al. Antibody evasion by a gammaherpesvirus O-glycan shield. PLoS Pathog. 2011;7: e1002387 10.1371/journal.ppat.1002387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cornelissen L, Van Vliet S. A bitter sweet symphony: immune responses to altered O-glycan epitopes in cancer. Biomolecules. 2016;6: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tan RM, Kuang Z, Hao Y, Lee F, Lee T, Lee RJ, et al. Type IV pilus glycosylation mediates resistance of Pseudomonas aeruginosa to opsonic activities of the pulmonary surfactant protein A. Infect Immun. 2015;83: 1339–1346. 10.1128/IAI.02874-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaillard J, Berche P, Frehel C, Gouln E, Cossart P. Entry of L. monocytogenes into cells is mediated by internalin, a repeat protein reminiscent of surface antigens from gram-positive cocci. Cell. 1991;65: 1127–1141. 10.1016/0092-8674(91)90009-n [DOI] [PubMed] [Google Scholar]
- 90.Fankhauser N, Nguyen-Ha T, Adler J, Mäser P. Surface antigens and potential virulence factors from parasites detected by comparative genomics of perfect amino acid repeats. Proteome science. 2007;5: 20 10.1186/1477-5956-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roditi I, Carrington M, Turner M. Expression of a polypeptide containing a dipeptide repeat is confined to the insect stage of Trypanosoma brucei. Nature. 1987;325: 272 10.1038/325272a0 [DOI] [PubMed] [Google Scholar]
- 92.Schofield L. On the function of repetitive domains in protein antigens of Plasmodium and other eukaryotic parasites. Parasitol Today. 1991;7: 99–105. 10.1016/0169-4758(91)90166-l [DOI] [PubMed] [Google Scholar]
- 93.Ramana J, Gupta D. ProtVirDB: a database of protozoan virulent proteins. Bioinformatics. 2009;25: 1568–1569. 10.1093/bioinformatics/btp258 [DOI] [PubMed] [Google Scholar]
- 94.Kędzierski Ł, Montgomery J, Curtis J, Handman E. Leucine-rich repeats in host-pathogen interactions. Arch Immunol Ther Exp. 2004;52: 104–112. [PubMed] [Google Scholar]
- 95.Suzuki J, Kobayashi S, Murata R, Tajima H, Hashizaki F, Yanagawa Y, et al. A survey of amoebic infections and differentiation of an Entamoeba histolytica–like variant (JSK2004) in nonhuman primates by a multiplex polymerase chain reaction. J Zoo Wildl Med. 2008;39: 370–380. 10.1638/2007-0171.1 [DOI] [PubMed] [Google Scholar]
- 96.Suzuki J, Kobayashi S, Murata R, Yanagawa Y, Takeuchi T. Profiles of a pathogenic Entamoeba histolytica-like variant with variations in the nucleotide sequence of the small subunit ribosomal RNA isolated from a primate (De Brazza’s guenon). J Zoo Wildl Med. 2007;38: 471–475. 10.1638/2006-0068.1 [DOI] [PubMed] [Google Scholar]
- 97.Guan Y, Feng M, Min X, Zhou H, Fu Y, Tachibana H, et al. Characteristics of inflammatory reactions during development of liver abscess in hamsters inoculated with Entamoeba nuttalli. PLoS Negl Trop Dis. 2018;12: e0006216 10.1371/journal.pntd.0006216 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Filter-passed subreads were obtained from 57 SMRT Cells of raw data with automatic removal of subreads with low accuracy (< 80%) and/or short-read length (< 500 bases). A bar plot was constructed using R with the bin width set to 1.
(TIF)
After Illumina short-read data were mapped to the primary assembly and both contig ends of the primary assembly were trimmed, the mapped reads were counted at each position for each contig and aggregated per percentage of contig bases. The line plots indicate trimming of both contig ends before (upper) and after (lower).
(TIF)
Of 9,647 annotated genes derived from the E. nuttalli genome, 114 had weak matches against a public database and were only annotated in the InterPro collection database. Gene Ontology assignments of these 114 E. nuttalli-specific candidate genes were referred from the InterPro annotation using the interpro2go dataset.
(TIF)
(A) ClustalW alignment of SALIP6 in E. nuttalli (EN0144G0007) and E. histolytica (EAL50434.1). The amino acid sequence of EN0144G0007 was classified into a protein signature by InterPro. Signal peptide and saposin-like (IPR011001) regions are framed by green and orange rectangles, respectively. (B) SignalP 4.1 analysis of EN0144G0007.
(TIF)
Putative homologous genes were collected from AmoebaDB using a BLAST search based on alignment score, e-value and visual inspection of the sequence alignment. Additionally, the putative homologous genes of E. histolytica were searched against our E. nuttalli protein dataset because some putative homologous genes of E. nuttalli could not be identified in AmoebaDB. Multiple alignments of the sequences were obtained using MAFFT v.7 with the G-INS-i algorithm and without gap region realignment (“Leave gappy regions”) (Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30: 772–780.). The appropriate amino acid substitution model (JTT+G) for the reconstruction was selected using the “Find Best DNA/Protein Models” tool in MEGA7 (Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33: 1870–1874.). Unambiguously aligned positions were used in the Neighbor-Joining (NJ) and Maximum Likelihood (ML) methods with 1,000 bootstrap replications in MEGA7. The parameter of Rates among Sites was set as Gamma distributed (G) and value as 13. For construction of ML phylogeny, the parameter “Initial Tree File” was set as the NJ phylogeny that we constructed. The output best tree was further edited using FigTree v,1.4.3 (https://github.com/rambaut/figtree/). The support values at the nodes represent bootstrap values. EN0096G0007 was expressed as red text.
(TIF)
The predicted amino acid sequence of EN0096G007 was subjected to secondary structure analysis in Jpred and PSIPRED. Predicted α-helices, β-strands, coil and disordered regions are indicated as H, E, C and D, respectively. A predicted signal peptide sequence (SignalP, red rectangle) and transmembrane helix (SOSUI and TMPred, blue rectangle) are also shown.
(TIF)
Immunofluorescence images of E. nuttalli, E. dispar SAW1734RclAR and E. moshkovskii Laredo using antisera specific for PTORS. Scale bar = 10 μm. Entamoeba species were stained by treatment with Triton X-100 and with antisera specific for PTORS. Merged bright field and fluorescence images are also shown (Merge). These images are approximately 0.9 μm in thickness per section.
(TIF)
Venn diagram showing orthologous cluster coverage between the new genome assembly (in this study) and the public genome assembly (AmoebaDB ver.4.0) in E. nuttalli. Numbers are estimated by the OrthoVenn server. The number of orthologous clusters is shown in bold. Numbers given in brackets indicate the number of genes, including the orthologous clusters. The new genome assembly and public genome assembly have 1,090 and 530 singletons, respectively.
(TIF)
(A) Comparison of the number of gene candidates for BspA-like proteins with and without putative membrane association among pathogenic Entamoeba species. Entamoeba BspA-like proteins predicted to contain “LRR_5 family (PF13306)” by Pfam (El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2018;47: D427-D432.) were collected from AmoebaDB. Entamoeba BspA-like proteins with putative membrane association were extracted from Entamoeba BspA-like proteins using TMHMM for the transmembrane and/or GPS-Lipid (Xie Y, Zheng Y, Li H, Luo X, He Z, Cao S, et al. GPS-Lipid: a robust tool for the prediction of multiple lipid modification sites. Sci Rep. 2016;6: 28249.) and PrePS (Maurer-Stroh S, Eisenhaber F. Refinement and prediction of protein prenylation motifs. Genome Biol. 2005;6: R55.) for lipid modification. (B) Correlation between the number of BspA genes in pathogenic Entamoeba species and genes for host fibronectin. (C) Correlation between the number of BspA genes in pathogenic Entamoeba species and genes for host fibrinogen. Human (Homo sapiens), macaque (Macaca fascicularis), and snake (Python bivittatus) are hosts for E. histolytica, E. nuttalli, and E. invadens, respectively. Host genes were extracted from Genome Data Viewer (https://www.ncbi.nlm.nih.gov/genome/gdv/).
(TIF)
A multiple sequence alignment of Entamoeba AIG1 family proteins was obtained in MUSCLE (Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32: 1792–1797.) and corrected by manual inspection. With 59 proteins from 3 species (16, 15, and 28 from E. nuttalli, E. histolytica, and E. dispar, respectively), 201 aligned amino acid sites were used in the analysis. The maximum likelihood (ML) best tree inferred by the JTT+F model with four categories of among-site rate variation and the rate variation model allowed some sites to be evolutionarily invariable (+I, 2.7363% sites) in MEGA7. Bootstrap proportions in the ML method (100 replicates) are attached to the internal branches. Branches with <50% bootstrap support are unmarked. Alignments are available from the authors upon request.
(TIF)
Raw read data were obtained from the Illumina GAIIx platform with a 114 bp paired-end module. For trimming of low quality bases, raw read data were adapted with FASTX-Toolkit using a minimum read length (-l) of 70 bases and a quality cutoff (-t) of 20. The quality-passed reads were mapped to the primary assembly using bwa-mem with default parameters.
(XLSX)
Proteome data were downloaded from AmoebaDB v.4.0.
(XLSX)
Data Availability Statement
Short- and long-read sequences have been deposited in the DNA Data Bank of Japan (BioProject PRJDB4858, BioSample SAMD00053943, DRA accession no. DRA004867).