With great interest we read the editorial by Kamson entitled “Hypometabolic Gliomas on FET-PET—Is There an Inverted U-Curve for Survival?” in Neuro-Oncology1 referring to our publication regarding the clinical relevance of photopenic defects on O-(2-[18F]fluoroethyl)-L-tyrosine (FET) PET in glioma patients.2 In that paper, we identified within 100 patients with FET-PET–negative gliomas a subgroup of patients who even exhibit photopenic defects and had, moreover, an unfavorable outcome compared with gliomas with indifferent FET uptake (isometabolic gliomas). From our point of view, the frequency of photopenic defects is even lower than 10% (ie, ~5%). In his editorial, the author raised valuable new thoughts and ideas that are worth discussing.
First of all, the hypothesis that photopenia may represent an early glioma stage (similar to a “status nascendi”) prior to the development of imaging features such as amino acid tracer uptake in PET or contrast enhancement on MRI is very interesting. Therefore, we reviewed again all cases with photopenic defects (n = 40) and isometabolic gliomas (n = 60) of our study2 regarding these imaging findings. Follow-up FET-PET imaging data of 44 patients were available, and the majority of patients (84%) exhibited increased FET tracer uptake after a median follow-up of 34 months (range, 3–65 mo). Remarkably, patients with photopenic World Health Organization (WHO) grade II astrocytomas and isocitrate dehydrogenase (IDH) gene mutation (n = 7) turned FET positive after a median time of 27 months (range, 15–65 mo), whereas isometabolic IDH-mutant WHO grade II astrocytomas (n = 10) turned FET positive only after 47 months (range, 30–105 mo). Most probably due to the low number of patients, the statistical significance of this difference was closely missed (P = 0.057). The rate of newly occurring contrast enhancement on MRI concurrent with increased FET uptake was similar (67% vs 63%).
In line with our previous study,2 these follow-up imaging changes suggest that photopenic defects have a more aggressive clinical course. Furthermore, the observation that the majority of FET-negative gliomas change their FET uptake behavior during the follow-up supports the hypothesis that both photopenia and isometabolism might represent an early stage of glioma development. However, it remains still unclear whether there is a sequence regarding the uptake behavior of FET-negative gliomas during follow-up, such as being initially isometabolic, then photopenic before turning FET positive, or whether photopenic and isometabolic gliomas simply represent different glioma entities with different clinical course.
Furthermore, the assumption that the progression-free survival (PFS) of glioma subgroups follows an inverted U-shaped curve—that is, isometabolic gliomas with the most favorable PFS and both photopenic gliomas and gliomas with increased FET uptake on the adverse end of the PFS spectrum—remains to be confirmed by the analysis of overall survival (OS). Up to now, gliomas with photopenic defects have had very little data available for OS. In the near future, OS data in this group of gliomas will be collected to verify this hypothesis.
The question of whether photopenic defects can be detected with amino acid PET tracers other than FET, such as 11C-methyl-L-methionine (MET) or 3,4-dihydroxy-6-[18F]-fluoro-L-phenylalanine (FDOPA), is also of great clinical relevance, because FET is not available in every center. This question is furthermore interesting because FET seems to have different properties than other radiolabeled amino acids. In particular, it has been demonstrated that the analysis of uptake dynamics of FET provides valuable additional diagnostic information in terms of prognostication, diagnosis of treatment-related changes, and the identification of malignant foci in gliomas.3,4 Controversially, other studies found up to now no additional diagnostic impact of dynamic MET- or FDOPA-PET,5,6 and it remains to be determined whether these amino acid tracers can characterize tumors in a similar manner to that of dynamic FET-PET imaging.
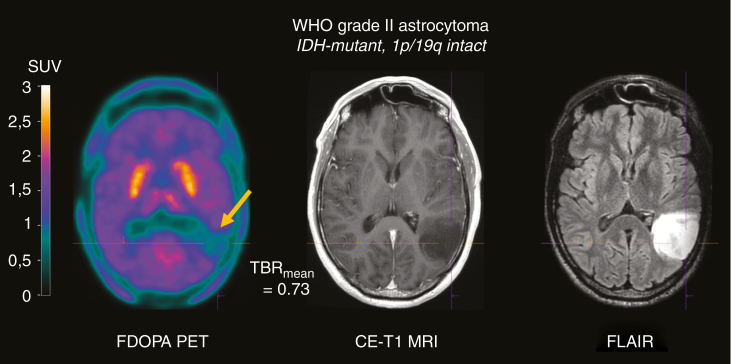
In collaboration with the Department of Nuclear Medicine, University Hospital Nancy, France, preoperative FDOPA-PET scans of 122 newly diagnosed glioma patients were reevaluated visually by 2 experienced raters (A.V., T.Z.). Interestingly, 7 of 15 patients with negative FDOPA-PET scans had photopenic defects (Fig. 1). Moreover, despite the low number of cases, patients with photopenic defects showed a tendency for a shorter PFS in comparison to patients with isometabolic FDOPA scans (P = 0.07). These findings suggest that photopenic defects do not occur exclusively in FET-PET and warrant further investigation regarding their prognostic relevance.
Fig. 1.
FDOPA-PET, contrast-enhanced (CE) T1-weighted, and fluid attenuated inversion recovery (FLAIR) MR images of a representative patient with a diffuse WHO grade II astrocytoma (IDH mutant, 1p/19q intact) without contrast enhancement and a hyperintense signal alteration. The FDOPA-PET image shows a photopenic defect in the left temporal lobe (arrow); the metabolic activity is considerably lower than the contralateral background activity (mean tumor/brain ratio [TBRmean] = 0.73). SUV = standardized uptake value.
Funding
This work was supported by the Wilhelm Sander-Stiftung, Munich, and the Else Kröner-Fresenius-Stiftung, Bad Homburg vor der Höhe, Germany.
Conflict of interest statement. Related to the present work, the authors disclose no potential conflicts of interest.
References
- 1. Kamson DO. Hypometabolic gliomas on FET-PET—is there an inverted U-curve for survival? Neuro Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galldiks N, Unterrainer M, Judov N, et al. Photopenic defects on O-(2-[18F]-fluoroethyl)-L-tyrosine PET—clinical relevance in glioma patients. Neuro Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13(5):279–289. [DOI] [PubMed] [Google Scholar]
- 4. Albert NL, Weller M, Suchorska B, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro Oncol. 2016;18(9):1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kratochwil C, Combs SE, Leotta K, et al. Intra-individual comparison of 18F-FET and 18F-DOPA in PET imaging of recurrent brain tumors. Neuro Oncol. 2014;16(3):434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moulin-Romsée G, D’Hondt E, de Groot T, et al. Non-invasive grading of brain tumours using dynamic amino acid PET imaging: does it work for 11C-methionine? Eur J Nucl Med Mol Imaging. 2007; 34(12):2082–2087. [DOI] [PubMed] [Google Scholar]