BRAF V600E mutation (BRAFV600E) has been identified in a number of glioma subtypes, including ganglioglioma,1 and the use of BRAFV600E inhibitors may significantly improve the clinical outcome of patients.2,3 Here, we report a successful treatment with dabrafenib of a patient with BRAFV600E ganglioglioma, whose treatment has been monitored by MRI and by liquid biopsy to identify BRAFV600E in plasma.
In March 2015, a 31-year-old woman was referred to the University Hospital due to a motor aphasia of recent onset. The MRI displayed a fronto-parietal tumor with intense contrast enhancement. The patient underwent a subtotal tumor resection with a histologic diagnosis of World Health Organization grade III ganglioglioma. Immunohistochemistry documented focal expression of p53, CD34, and Ki-67 proliferation index of 3%; BRAFV600E was detected by pyrosequencing.
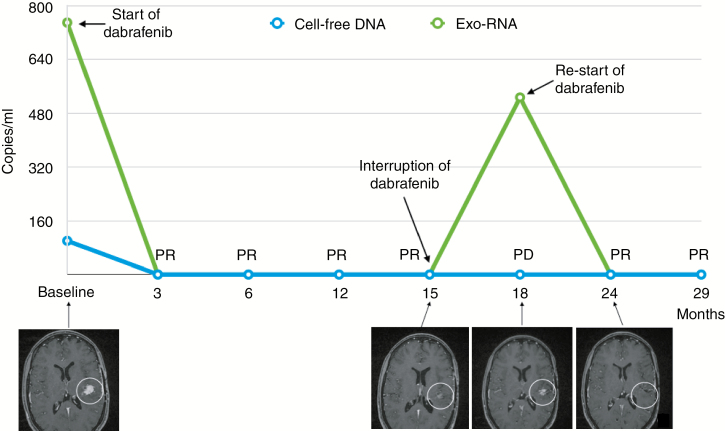
The patient received adjuvant radiotherapy with temozolomide. Ten months after the end of radiochemotherapy, progressive disease (PD) was documented by MRI. A second subtotal surgery was performed without any neurological impairment, and the pathology confirmed the original diagnosis. Based on the presence of the BRAFV600E, dabrafenib (75 mg twice per day for the first 21 days, followed by 150 mg twice per day) was administered. During treatment, 6 mL of plasma were taken at each clinical evaluation, to assess BRAFV600E both in cell-free DNA (cfDNA) and in cancer mRNA from plasma-derived exosomes (exo-RNA), which were extracted with QIAamp circulating free nucleic acids and the exoRNeasy Maxi kit (Qiagen), respectively. The One-Step RT-ddPCR Advanced Kit for Probes (Bio-Rad) was used to obtain cDNA from exo-RNA, and both cfDNA and cDNA were analyzed by a digital droplet PCR (BioRad). The baseline (pretreatment) plasma sample confirmed the presence of BRAFV600E at 100 copies/mL in cfDNA and at 750 copies/mL in exo-RNA. MRI, performed 2 months after the start of dabrafenib and then every 3 months, showed a major partial response (PR; Figure 1). After 15 months of treatment, due to the persistent PR, the administration of dabrafenib was discontinued. BRAFV600E in plasma rapidly declined from baseline to the the third month and remained negative until the fifteenth month, thus confirming the MRI finding. However, 3 months after the interruption of dabrafenib, PD was detected at MRI, although the patient was asymptomatic; for this reason, dabrafenib was restarted at the same schedule. Of note, the analysis of the cfDNA was negative for BRAFV600E, while in the exo-RNA the BRAFV600E appeared again at PD (Figure 1). At data closure, after 11 months of dabrafenib rechallenge, the treatment is still ongoing and well tolerated and BRAFV600E is still undetectable in plasma.
Fig. 1.
Assessment of BRAF V600E mutation in cell-free DNA and exo-RNA during treatment with dabrafenib and its correlation with MRI and clinical response. White circles indicate the tumor in the MR images. PR: partial response; PD: progressive disease.
While the administration of BRAF inhibitors, including dabrafenib and trametinib, is the treatment of choice for BRAFV600E melanoma, dabrafenib has been used in a limited series of patients with primary brain cancers.2–5 At the best of our knowledge, this is the first case of a ganglioglioma in which changes in plasma levels of exo-RNA were able to document disease response to the rechallenge with dabrafenib, which was also documented by MRI. According to the data on the use of dabrafenib in BRAFV600E brain tumors,3–5 this case provides evidence that this drug can be active in patients with BRAFV600E ganglioglioma. Moreover, the analysis of circulating tumor DNA/RNA demonstrated that exosomes may be a better vehicle of mutated allele in cerebral tumors compared with cfDNA, allowing the prediction of tumor progression and resistance to treatment.6
Additional prospective studies are needed to evaluate whether exo-RNA, integrated with sequential imaging analysis, may improve the management of patients with malignant brain tumors treated with drugs targeting actionable mutations, as in this case of BRAFV600E ganglioglioma.
Funding
This study was funded by research grants from the University of Pisa, Pisa, Italy, to Romano Danesi.
Conflict of interest statement.
The authors declare no conflict of interest.
References
- 1. Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. [DOI] [PubMed] [Google Scholar]
- 2. Brown NF, Carter T, Mulholland P. Dabrafenib in BRAFV600-mutated anaplastic pleomorphic xanthoastrocytoma. CNS Oncol. 2017;6(1):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burger MC, Ronellenfitsch MW, Lorenz NI, et al. Dabrafenib in patients with recurrent, BRAF V600E mutated malignant glioma and leptomeningeal disease. Oncol Rep. 2017;38(6):3291–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marks AM, Bindra RS, DiLuna ML, et al. Response to the BRAF/MEK inhibitors dabrafenib/trametinib in an adolescent with a BRAF V600E mutated anaplastic ganglioglioma intolerant to vemurafenib. Pediatr Blood Cancer. 2018;65(5):e26969. [DOI] [PubMed] [Google Scholar]
- 5. Kaley T, Touat M, Subbiah V, et al. BRAF inhibition in BRAF(V600)-mutant gliomas: results from the VE-BASKET study. J Clin Oncol. 2018;36(35):3477–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mrowczynski OD, Zacharia BE, Connor JR. Exosomes and their implications in central nervous system tumor biology. Prog Neurobiol. 2019;172:71–83. [DOI] [PubMed] [Google Scholar]