Abstract
Background
We integrated clinical, histopathological, and molecular data of central nervous system germ cell tumors to provide insights into their management.
Methods
Data from the Intracranial Germ Cell Tumor Genome Analysis (iGCT) Consortium were reviewed. A total of 190 cases were classified as primary germ cell tumors (GCTs) based on central pathological reviews.
Results
All but one of the cases that were bifocal (neurohypophysis and pineal glands) and cases with multiple lesions including neurohypophysis or pineal gland were germinomas (34 of 35). Age was significantly higher in patients with germinoma than other histologies. Comparison between tumor marker and histopathological diagnoses showed that 18.2% of histopathologically diagnosed germinomas were marker positive and 6.1% of non-germinomatous GCTs were marker negative, suggesting a limitation in the utility of markers or histopathology alone using small specimens for diagnosis. Comparison between local and central histopathological diagnoses revealed a discordance of 12.7%. Discordance was significantly less frequent in biopsy cases, implying difficulty in detecting all histopathological components of heterogeneous GCTs. Germinomas at the typical sites (neurohypophysis or pineal gland) showed a better progression-free survival than those at atypical sites (P = 0.03). A molecular clinical association study revealed frequent mitogen-activated protein kinase (MAPK) pathway mutations in males (51.4% vs 14.3%, P = 0.007), and phosphatidylinositol-3 kinase/mammalian target of rapamycin (PI3K/mTOR) pathway mutations in basal ganglia cases (P = 0.004). Basal ganglia cases also had frequent chromosomal losses. Some chromosomal aberrations (2q, 8q gain, 5q, 9p/q, 13q, 15q loss) showed potential prognostic significance.
Conclusions
The in-depth findings of this study regarding clinical and molecular heterogeneity will increase our understanding of the pathogenesis of this enigmatic tumor.
Keywords: germ cell tumor; pathological diagnosis; prognosis, tumor marker; rare disease
Key Points.
1. Clinical aspects of a large series of CNS germ cell tumors were studied in association with molecular and histopathological features.
2. Central nervous system GCT is best diagnosed based on both tumor markers and pathology.
3. Biology and clinical course of CNS GCTs are different depending on sex or the location of occurrence.
Importance of the Study.
We report an integrated clinical, histopathological, and molecular analysis of 190 primary CNS GCT cases registered in the iGCT Consortium. The study revealed that germinomas occurring at atypical sites had worse prognoses than those at typical sites (neurohypophysis or pineal gland) (P = 0.03). Comparison between tumor markers and histopathological diagnoses showed that 18.2% of histopathologically diagnosed germinomas were marker positive and 6.1% of non-germinomatous GCTs were marker negative, suggesting a limitation in the utility of either tumor markers or histopathology alone for making diagnosis. Discordance between local and central histopathological diagnoses was found to be 12.7%, implying difficulty in detecting all histopathological components in large heterogeneous specimens, and advantage of performing a central review. The study showed that MAPK pathway mutations were significantly more common in males, that basal ganglia cases were enriched in PI3K/mTOR pathway mutations and chromosomal losses, and that some chromosomal aberrations had prognostic significance.
Central nervous system (CNS) germ cell tumors (GCTs) predominantly occur in pediatric and young adult males.1 They arise preferentially in midline structures, including pineal gland and neurohypophysis. The World Health Organization (WHO) classification system recognizes 5 major histological subtypes: germinoma (G), teratoma, choriocarcinoma (CC), yolk sac tumor (YST), and embryonal carcinoma (EC).2 GCTs often consist of more than one of these components. Germinoma has a relatively good prognosis, with a 5-year progression-free survival (PFS) rate of approximately 90%. However, it is still challenging to achieve long-term survival in patients with non-germinomatous GCTs (NGGCTs) (5-year PFS/overall survival [OS] of 68–72/75–82%),3,4 although improved outcomes using established treatment regimens have recently been reported by treatment groups in the United States (5-year PFS/OS of 84/93%).5
CNS GCTs are rare in Western countries. According to the Central Brain Tumor Registry of the United States, the US frequency was 3.9% among brain tumor patients under the age of 20.6 By comparison, CNS GCTs are more common in East Asia. The frequency in Japan was 16.9% among brain tumor patients under the age of 20, making them the second most common brain tumor type after astrocytoma.1 In Japan, the treatment regimen is generally based on the histopathology of surgically obtained specimens. Patients are treated based on placement in one of 3 prognostic groups based on histopathological classification.7,8 The details of the treatment regimen are described in the Materials and Methods section.
In North America and Europe, CNS GCTs are divided into the risk groups germinoma and NGGCTs. According to the latest Children’s Oncology Group (COG) study protocol, localized germinoma and NGGCT are treated with platinum-based chemotherapy, followed by whole ventricle irradiation (WVI), with additional irradiation of the tumor, on the latest protocol and study. In Europe, germinoma is treated with either low-dose craniospinal irradiation (CSI) alone or chemotherapy.9 NGGCT is treated with chemotherapy followed by focal radiation or CSI depending on the metastasis status according to the European International Society of Paediatric Oncology (SIOP) CNS GCT 96 trial.3 A promising recent report by SIOP indicates that localized NGGCTs can be safely treated with focal radiation and chemotherapy, avoiding CSI.3 In North America and Europe, the common practice is to diagnose GCTs based on clinical manifestations and tumor markers, rather than on histopathology.
In the fourth international CNS Germ Cell Tumor Symposium, 34 consensus statements regarding clinical managements were agreed upon.10 However, significant geographical differences still exist in diagnosis and treatment, and clinical questions remain to be addressed.10
The Intracranial GCT Genome Analysis (iGCT) Consortium was established in Japan in 2011 to facilitate biological research. It has so far collected tissue samples from more than 200 CNS GCTs and has published a number of papers reporting a high frequency of mutations in the mitogen-activated protein kinase/phosphatidylinositol-3 kinase/mammalian target of rapamycin (MAPK/PI3K/mTOR) pathway,11,12 aberrant copy number alterations in germinomas,11 and global hypomethylation in germinoma.13 Here we report a retrospective analysis of clinical data from 190 primary CNS GCTs from the iGCT Consortium. This report provides a snapshot of current clinical management of CNS GCTs in Japan based on therapeutic classification regimen,14 centrally reviewed histopathological diagnoses, and clinical/molecular associations.
Materials and Methods
Patients and Histopathological Diagnoses
A total of 217 locally diagnosed CNS GCTs from 24 institutions had been curated by the consortium prior to March 2016, including tissue samples from all histological subtypes; pathological specimens were centrally reviewed by an expert neuropathologist (Y.N.) (Supplementary Table 1). Clinical information was recorded, including age, sex, tumor location, treatment (surgery, radiation and chemotherapy), laboratory results (tumor markers), and follow-up data. Of the total 217, diagnosis of primary GCT was made in 190, while 12 were metastatic and 15 were non-GCTs. Metastasis was diagnosed when a primary lesion(s) was identified outside the extracraniospinal regions, such as in testis, ovary, or mediastinum. This paper focuses on the clinical features of the 190 primary GCTs. Tumor markers were measured in serum and/or cerebrospinal fluid (CSF) pre- or intraoperatively. CSF was obtained by preoperative lumbar puncture or intraoperative ventricular drainage. Multifocal cases were defined as cases with tumors presented at more than one site, including bifocal tumors (neurohypophysis and pineal gland). Cases with atypical sites were classified as harboring a lesion outside of the pineal gland or neurohypophysis, such as basal ganglia and cerebral cortex.
The investigation was approved by the ethical committee of the National Cancer Center, Tokyo, Japan, and the respective local institutional review boards.
Treatment
In Japan, treatment regimens are generally based on histopathological diagnoses that categorize patients into 3 risk groups.8,14 The good prognosis group consists of germinomas and mature teratomas (MTs). The intermediate prognosis group includes germinomas with syncytiotrophoblastic giant cells (STGCs), immature teratomas (ImTs), teratomas with malignant transformation, and mixed tumors composed mainly of germinoma or teratoma. The poor prognosis group includes CC, YST, EC, and mixed tumors mainly composed of these malignant types. Platinum-based chemotherapy is administered to all groups, followed by WVI of 24 Gy for the good prognosis group, WVI of 30 Gy with boosted tumor irradiation (20 Gy) for the intermediate prognosis group, and CSI of 30 Gy with boosted tumor irradiation (30 Gy) for the poor prognosis group. The chemotherapy regimen also varies with prognosis group; 3 courses of carboplatin + etoposide (CARE) for the good prognosis group, 3 courses of CARE followed by 5 maintenance courses for the intermediate prognosis group, and 1 course of ifosfamide + carboplatin + etoposide (ICE) followed by 5 courses as maintenance for the poor prognosis group. MTs are treated by surgical resection alone. Germinoma with STGC is currently treated similarly to germinoma.
Molecular Data
Somatic mutation data were obtained by whole-exome or targeted sequencing. Chromosomal aberrations were identified by array-comparative genomic hybridization, as described previously.11,12 Mutational and chromosomal analyses were performed in 123 and 74 cases (74 were analyzed for both) of the total 190 cases, respectively, which mostly overlap with the cases from the previous studies.11,12
Statistical Analysis
Nonparametric values were compared using Wilcoxon’s test. Categorized data were compared between subgroups using the Pearson chi-square test. Multivariate analyses were performed with logistic or least square regression methods. Survival data were analyzed using the log-rank test and multivariate Cox regression analysis, and the results were shown as Kaplan–Meier curves. All statistical analyses were carried out using JMP 13 (SAS Institute). A P-value below 0.05 was considered significant.
Results
Patient Demographics
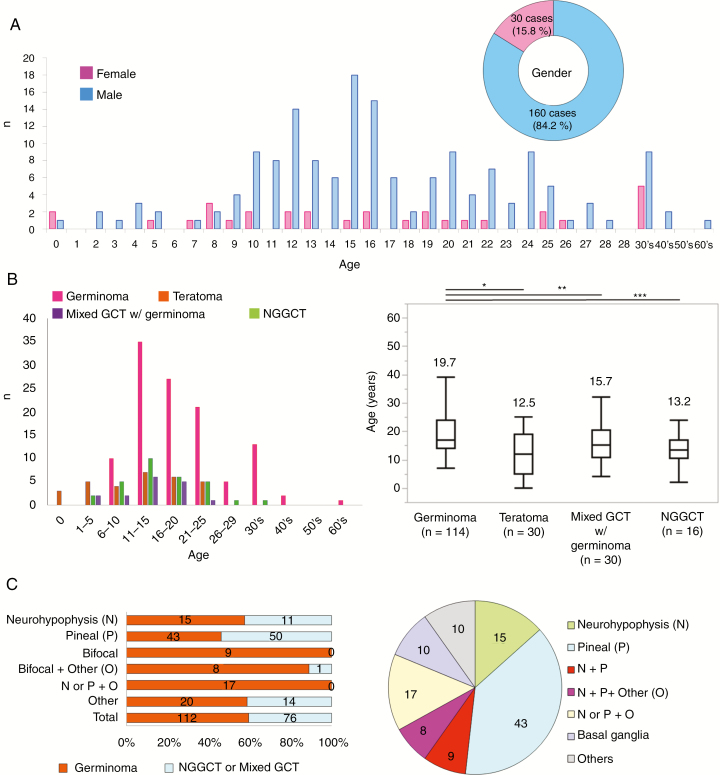
Among the 190 cases, 160 of the patients (84.2%) were male and 30 (15.8%) were female (Fig. 1 inset). The majority of patients were teens (10–19 y, n = 102, 53.7%), and the mean and median ages were 17.5 and 16 years, respectively. Germinoma occurred at a significantly older age than other histologies, and patients at or older than 30 years of age mostly had germinomas (16/17, 94.1%). The majority of bifocal or other multifocal tumors (34/35 cases, 97.1%) were germinomas, and bifocal germinomas constituted 15.0% of total germinomas (Supplementary Table 2). Forty-eight patients (25.3%) were in their 20s. Twenty-three patients (12.1%) were under 10 years old. Fourteen patients (7.4%) were in their 30s, two (1.05%) in their 40s, and one (0.5%) in their 60s. As a surgical procedure, biopsy, subtotal resection, and gross total resection were performed for 81, 55, and 48 cases, respectively (data not available for 6 cases) (Supplementary Table 3).
Fig. 1.
(A) Age distribution of GCT patients is shown in a bar graph (blue, male; pink, female). Mean and median ages were 17.5 and 16 years. The pie chart shows the male to female ratio (approximately 5:1). (B) Left: The distribution of histological subtypes according to age. Of note, patients <6 years (n = 12) had mostly teratomas and yolk sac tumors (n = 10, 83.3%). Patients ≥30 years (n = 17) mostly had germinomas (n = 16, 94.1%). Right: Germinomas (19.7 ± 9.0) occurred in significantly older patients than did teratomas (12.5 ± 7.7, *P < 0.0001), mixed germinomas (15.7 ± 6.7, **P = 0.03), or non-germinomatous GCTs (13.2 ± 5.8, ***P = 0.004). (C) Left: The distribution of histology according to tumor locations. The majority of bifocal or other multifocal tumors (34/35 cases, 97.1%) were germinomas. About half of cases with solitary lesions were germinoma. Half were non-germinoma (NGGCT) or mixed GCTs with a germinoma component (mixed GCTs). Right: The pie chart shows the distribution of tumor locations in germinoma cases. Bifocal germinomas constitute 15.0% of total germinomas.
Histopathological Diagnoses
Central histopathological reviews identified 114 (60.0%) germinomas, 30 (15.8%) mixed GCTs with a germinoma component (“mixed GCT” hereafter), 17 (8.9%) MTs, 12 ImTs (6.3%), 1 teratoma with malignant transformation (0.5%), 8 YSTs (4.2%), 3 CCs (1.6%), 2 ECs (1.1%), and 3 mixed GCTs without a germinoma component or high grade GCTs (1.6%). In young children less than 6 years old (n = 12), teratoma (MT and ImT) and YST were dominant (10 out of 12 cases). Two other cases had teratoma in their mixed components. By contrast, 18 of the 19 adult patients over 30 years had germinoma. The single remaining case was a mixed GCT with a germinoma component (Fig. 1B, left). Age distribution based on histology is shown in Figure 1B, right, revealing that germinoma patients were significantly older than the others.
Correlation Between Local and Central Histopathological Diagnoses
Of the 190 cases diagnosed locally (in each institution) and centrally, there were 157 newly diagnosed (not recurrent) cases for which detailed information on their histopathological components was available (for example, not just labeled as “mixed GCT” [see Supplementary Table 1]). Complete concordance between the 2 diagnoses was seen in 137 cases (87.3%). Discordances were observed in 20 cases (12.7%). Central diagnoses could have changed the classification from “good” to “intermediate” in 2 cases (1.3%) due to identification of new components, including “malignant transformation of teratoma” and “ImT” in one case and change of “germinoma” to “non-germinoma” in the other (Table 1). Whereas 48.5% of the 137 concordant cases were biopsied, only 20.0% of the 20 discordant cases were biopsied (P = 0.017). There was no statistical difference in prognosis between the concordant and discordant cases in NGGCTs (data not shown).
Table 1.
The 20 cases in which pathological diagnoses showed difference between local and central reviews
| Sample Name | Local Diagnosis | Central Diagnosis | Newly Found Components | Tx | HCG | AFP | Treatment Received | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histology | Prognosis Group | Histology | Prognosis Group | Serum (IU/L) | CSF (IU/L) | Serum (ng/mL) | CSF (ng/mL) | Radiation | Chemotherapy (number of cycles) | |||
| GCT04 | Mixed GCT (ImT + YST + EC) | Poor | Mixed GCT (YST + EC + ImT + G) | Poor | G | No change | ND | ND | 2066 | ND | CSI+L | ICE (8) |
| GCT09 | Immature teratoma | Intermediate | Mixed GCT (ImT + G) | Intermediate | Good | ND | ND | 1492.5 | ND | L+ventricle | PE (8) | |
| GCT43 | Mixed GCT (G + CC + EC + MT) | Intermediate | Mixed GCT (G + ImT + CC + EC) | Intermediate | ImT | 9550 | ND | 316 | ND | CSI+L | ICE (8) | |
| GCT58 | Teratoma with malignant component | Intermediate | Mixed GCT (MT + G) | Intermediate | Good | ND | ND | ND | ND | EL | PE | |
| GCT61 | Teratoma | Intermediate | Mixed GCT (MT + G) | Intermediate | Good | ND | ND | 1.87 | ND | WBRT | PE | |
| GCT64 | Choriocarcinoma | Poor | Mixed GCT (MT + CC + G) | Intermediate (or Poor) | MT, G | 16632 | ND | ND | ND | CSI+EL | ICE | |
| GCT66 | Choriocarcinoma | Poor | Immature teratoma | Intermediate (or Poor) | ImT | 9359 | ND | 6.9 | ND | CSI+EL | ICE | |
| GCT70 | Mixed GCT (MT + G) | Intermediate | Mixed GCT (MT + G + Hemangioma) | Intermediate | Hemangioma | ND | <0.5 | ND | ND | WVI | ICE | |
| GCT73 | Choriocarcinoma | Poor | Mixed GCT (CC + G + ImT) | Poor | G, ImT | 9550 | ND | 317.2 | ND | ND | ND | |
| GCT77 | Yolk sac tumor | Poor | Mixed GCT (G + YST) | Intermediate (or Poor) | Good | ND | ND | 693.89 | 42.36 | CSI | ICE (4) | |
| GCT86 | Teratoma | Intermediate | Mixed GCT (MT + G + YST + EC) | Intermediate | G, YST, EC | ND | ND | 223 | ND | L | ICE (6) | |
| GCT90 | Germinoma | Good | Mixed GCT (MT + G) | Good | MT | <1.0 | ND | 1.2 | <0.4 | L | PE (5) | |
| GCT99 | Germinoma | Good | Germinoma with STGC | Intermediate | STGC | <1.0 | ND | <5.0 | ND | WVI | CARE (8) | |
| GCT113 | Embryonal carcinoma | Poor | Mixed GCT (EC + G) | Poor | Good | 39.2 | ND | <5.0 | ND | CSI+L | CARE (1), ICE (2) | |
| GCT153 | Mixed GCT (MT + YST + G) | Poor | Mixed GCT (ImT + YST + G) | Poor | ImT | 2.9 | 12.6 | ND | ND | WVI+L | PE (3) | |
| GCT155 | Immature teratoma | Intermediate | Mixed GCT (T + G) | Intermediate | Good | 92.8 | 33.4 | ND | ND | WVI+L | PE (3) | |
| GCT174 | Mixed GCT (EC + YST) | Poor | Mixed GCT (YST + EC + G) | Poor | Good | ND | ND | 70.4 | ND | ND | ICE (2) | |
| GCT176 | Immature teratoma | Intermediate | Mixed GCT (ImT + G) | Intermediate | Good | <2.0 | <2.0 | 372.8 | 20.2 | WVI+L | PE (3) | |
| GCT71 | Mature teratoma | Good | Teratoma with somatic-type malignancy (rhabdomyosarcoma) | Intermediate | Malignancy | Change | 35.7 | 76.3 | 21.4 | 0.4 | WBRT | ICE |
| GCT211 | Germinoma | Good | Immature teratoma | Intermediate | ImT | 4.9 | ND | ND | ND | EL | PE | |
Abbreviations: AFP: alpha fetoprotein; CARE: carboplatin + etoposide; CC: choriocarcinoma; CSF: cerebrospinal fluid; CSI: craniospinal irradiation; EC: embryonal carcinoma; EL: extended local radiation therapy; Good: good prognosis group; G: germinoma; GCT: germ cell tumor; HCG: human chorionic gonadotropin; Intermediate: intermediate prognosis group; ICE: ifosfamide + carboplatin + etoposide; ImT: immature teratoma; L: local radiation therapy; MT: mature teratoma; ND: no data; Poor: poor prognosis group; PE: cisplatin + etoposide; STGC: syncytiotrophoblastic giant cell; WBRT: whole brain radiation therapy; WVI: whole ventricular irradiation; YST: yolk sac tumor.
Bifocal Tumors
Among the 188 cases with information on tumor locations, 9 cases occurred bifocally (neurohypophysis and pineal gland). Another 9 cases with bifocal with additional lesions included one YST (Fig. 1C, Table 2). Multifocal tumors that included either neurohypophysis or pineal gland (n = 17) were all germinomas. Tumor markers in serum and/or CSF were measured in 15 bifocal cases. Most of these (12 cases, 80.0%) showed normal values. Two cases showed mild elevation of human chorionic gonadotropin (HCG) (GCT185: 16 IU/L in CSF, GCT206: 27.2 IU/L in serum). One case showed exceedingly high serum alpha-fetoprotein (AFP) (12 200 ng/mL), which conferred a histopathological diagnosis of YST (Table 2).
Table 2.
Total 18 cases of bifocal GCTs and their tumor markers
| GCT No. | Histology | Age | Sex | Tumor Location | Tumor Marker | HCG Serum (IU/L) | HCG CSF (IUL) | AFP Serum (ng/mL) | AFP CSF (ng/mL) | Site of CSF |
|---|---|---|---|---|---|---|---|---|---|---|
| GCT13 | Germinoma | 8 | M | Bifocal | Negative | <0.1 | ND | <5.0 | ND | NA |
| GCT14 | Germinoma | 25 | M | Bifocal | Negative | <0.1 | ND | <5.0 | ND | NA |
| GCT34 | Germinoma | 45 | M | Bifocal | Negative | 0.11 | 0.8 | 3.2 | <0.4 | L |
| GCT50 | Germinoma | 12 | M | Bifocal | Negative | <0.1 | ND | 2 | ND | NA |
| GCT133 | Germinoma | 15 | M | Bifocal | Negative | 1.2 | ND | 6 | ND | NA |
| GCT185 | Germinoma | 15 | M | Bifocal | Mild | 1.6 | 16 | 6.4 | 5 | V |
| GCT206 | Germinoma | 20 | M | Bifocal | Mild | 27.2 | ND | ND | ND | NA |
| GCT210 | Germinoma | 15 | M | Bifocal | ND | ND | ND | ND | ND | NA |
| GCT215 | Germinoma | 15 | M | Bifocal | Negative | 0.8 | ND | ND | ND | NA |
| GCT10 | Germinoma | 13 | M | Bifocal + V | Negative | <0.1 | ND | <5.0 | ND | NA |
| GCT35 | Germinoma | 15 | M | Bifocal + V | Negative | <0.1 | 0.14 | 1.5 | <0.4 | L |
| GCT45 | Germinoma | 20 | M | Bifocal + V | Negative | <0.1 | <0.1 | 1 | 1 | V |
| GCT54 | Germinoma with STGC | 12 | M | Bifocal + V | Negative | ND | ND | 1 | ND | NA |
| GCT96 | Germinoma | 16 | M | Bifocal + V | Negative | <0.1 | ND | 4.5 | ND | NA |
| GCT100 | Yolk sac tumor | 12 | M | Bifocal + O | Severe | ND | ND | 12200 | ND | NA |
| GCT157 | Germinoma | 14 | M | Bifocal + V | Negative | <0.1 | ND | <5.0 | <0.6 | V |
| GCT238 | Germinoma | 39 | M | Bifocal + V | ND | ND | ND | ND | ND | NA |
| GCT241 | Germinoma | 15 | M | Bifocal + O | ND | ND | ND | ND | ND | NA |
Abbreviations: AFP: alpha fetoprotein; CSF: cerebrospinal fluid; HCG: human chorionic gonadotropin; LP: lumbar puncture; M: male; NA: not applicable; ND: no data; O: other locations; V: ventricle; VD: ventricular drainage.
Correlation Between Tumor Markers and Histopathological Diagnoses
A total of 124 cases with tumor marker (“marker” hereafter) data available were analyzed. We analyzed only newly diagnosed (not recurrent) cases to avoid potential treatment effects. There were 85 cases which were examined for HCG (29 for both serum and CSF, 51 for only serum, and 5 for only CSF) and 108 cases for AFP (69 cases for only serum and 39 cases for both). Cases were stratified into 4 categories: normal, mild, moderate, and severe elevation. Since cases were from multiple institutions, we chose 5 IU/L and 10 ng/mL as cutoff values for normal HCG and AFP levels, and 50 IU/L and 25 ng/mL as cutoff levels distinguishing mild and moderate HCG and AFP levels, respectively, by referring to representative standards and the SIOP guideline.9 We used concentrations 10 times higher than the cutoffs between mild and moderate as boundaries between moderate and severe. Table 3 shows the cases of each histopathological subtype stratified according to tumor marker levels. Of the 25 cases with elevated serum HCG, 6 cases were germinoma, and 2 cases were MT. Of the 14 cases with elevated CSF HCG, 5 were germinoma and 1 was MT. Of the 33 cases with elevated serum AFP, 4 were germinoma and 1 was MT. Of the 11 cases with elevated CSF AFP, 1 was germinoma.
Table 3.
Tumor markers and histopathological diagnoses
| Serum total HCG | Value (IU/L) | n | Germinoma | Germinoma with STGC | Mature teratoma | Immature teratoma | Malignant teratoma | Mixed GCT | Chorio carcinoma | Yolk sac tumor | Embryonal carcinoma | Others | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G+MT | G+other NGGCT | Without G | ||||||||||||
| Normal | ‒5 | 55 | 38 | 2 | 3 | 4 | 0 | 2 | 3 | 0 | 0 | 1 | 1 | 1 |
| Mild | 5–50 | 11 | 4 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| Moderate | 50–500 | 6 | 2 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 |
| Severe | 500‒ | 8 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 3 | 0 | 1 | 0 |
| CSF total HCG | Value (IU/L) | n | Germinoma | Germinoma with STGC | Mature teratoma | Immature teratoma | Malignant teratoma | Mixed GCT | Chorio carcinoma | Yolk sac tumor | Embryonal carcinoma | Others | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G+MT | G+other NGGCT | Without G | ||||||||||||
| Normal | ‒5 | 20 | 9 | 1 | 2 | 2 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 1 |
| Mild | 5–50 | 7 | 2 | 0 | 1 | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Moderate | 50–500 | 4 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Severe | 500‒ | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Serum AFP | Value (ng/mL) | n | Germinoma | Germinoma with STGC | Mature teratoma | Immature teratoma | Malignant teratoma | Mixed GCT | Chorio carcinoma | Yolk sac tumor | Embryonal carcinoma | Others | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G+MT | G+other NGGCT | Without G | ||||||||||||
| Normal | ‒10 | 75 | 62 | 2 | 3 | 1 | 0 | 3 | 2 | 0 | 2 | 0 | 0 | 0 |
| Mild | 10–25 | 4 | 1 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Moderate | 25–250 | 14 | 3 | 0 | 1 | 4 | 0 | 0 | 4 | 0 | 0 | 1 | 1 | 0 |
| Severe | 250–2500 | 15 | 0 | 0 | 0 | 1 | 0 | 0 | 7 | 1 | 0 | 4 | 1 | 1 |
| CSF AFP | Value (ng/mL) | n | Germinoma | Germinoma with STGC | Mature teratoma | Immature teratoma | Malignant teratoma | Mixed GCT | Chorio carcinoma | Yolk sac tumor | Embryonal carcinoma | Others | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G+MT | G+other NGGCT | Without G | ||||||||||||
| Normal | ‒10 | 28 | 20 | 0 | 2 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 0 |
| Mild | 10–25 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Moderate | 25–250 | 6 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| Severe | 250–2500 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
Abbreviations: AFP: alpha fetoprotein; CSF: cerebrospinal fluid; G: germinoma; GCT: germ cell tumor; HCG: human chorionic gonadotropin; MT: mature teratoma, NGGCT: non-germinomatous GCT; STGC: syncytiotrophoblastic giant cell.
Of 69 cases in which both markers were examined, 38 cases had normal levels of both markers. The majority of these were germinoma (n = 34) or MT (n = 1), although this group also included mixed GCT (n = 3; 2 G+MT, G+ImT). There were 3 additional cases in which both markers were below the threshold for NGGCT (ie, below moderate elevation). These included 2 germinomas and 1 mixed GCT (EC>>G) (Supplementary Table 4).
In 22 cases, one marker showed severe elevation. Most of these (n = 21) were NGGCT, but also included one germinoma.
We then investigated correlations between tumor location and markers, incorporating histology (germinoma vs NGGCT) as a parameter in multivariate analyses. Tumors at the neurohypophysis were significantly associated with elevated serum/CSF HCG levels and tumors at the pineal gland were associated with decreased serum/CSF HCG levels, irrespective of their histology (Supplementary Table 5).
Prognosis Analyses
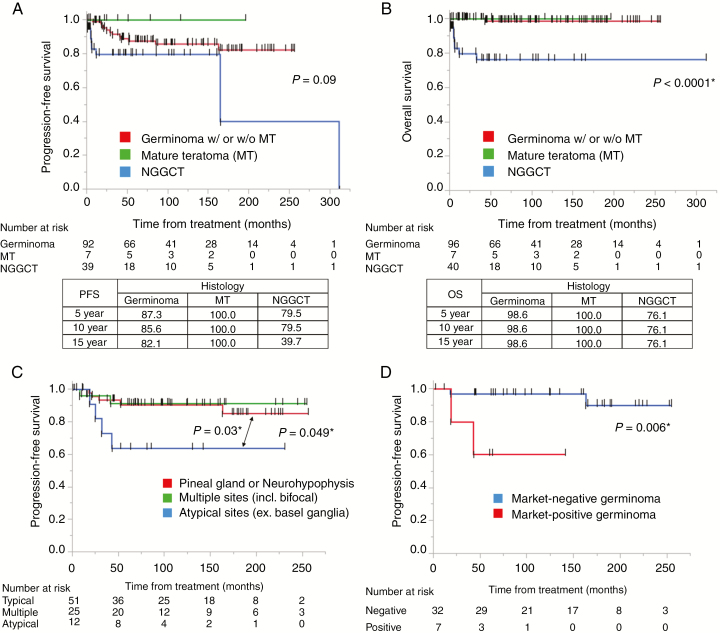
Survival rates between each of the 3 histological categories (germinoma, MTs, and NGGCTs) were compared (Fig. 2A, B). Five-year PFS/OS rates for the 96 germinoma, 7 MTs, and 40 NGGCTs were 87.3 ± 12.7/98.6 ± 1.5, 100.0 ± 0.0/100.0 ± 0.0, and 79.5 ± 20.6/76.1 ± 24.0%, respectively.
Fig. 2.
(A, B) Kaplan–Meier curves showing PFS and OS of GCT patients. Tables in the lower panel show 5-, 10-, and 15-year PFS and OS values. PFS of germinomas and NGGCTs were not statistically different, although all 7 cases of mature teratoma did not show recurrence. OS of these 3 histological classifications were markedly statistically significant. (C) Kaplan–Meier curves of PFS for germinomas at different locations show that those at a typical single site (neurohypophysis or pineal gland) (n = 51) had significantly longer PFS than those at atypical sites (n = 12) (P = 0.03). (D) Kaplan–Meier curves of PFS for marker-positive germinoma and marker-negative germinoma. Marker-positive germinoma cases showed significantly worse PFS than marker-negative germinoma cases (P = 0.006).
We next compared the PFS of germinoma cases in the following tumor locations: (i) typical sites (pineal gland or neurohypophysis), (ii) multiple sites (including bifocal tumors), and (iii) atypical sites (including basal ganglia and cerebral cortex). We limited the cases in this analysis to newly diagnosed (not recurrent) cases in which patients had received chemotherapy and radiation therapy. Those at typical sites showed significantly longer PFS than those at atypical sites, which had the shortest PFS (P = 0.03); however, there was no significant difference between cases with tumors at typical sites and those with tumors at multiple sites (Fig. 2C).
Finally, PFS of marker-positive germinoma (AFP >25 ng/mL or HCG >50 IU/L) and marker-negative germinoma (both being below the above criteria) were compared using the above criteria (chemotherapy and radiation therapy) for eligible cases. All these cases received chemotherapy and radiation therapy at least covering the whole ventricles. Marker-positive germinoma showed shorter PFS than marker-negative germinoma (P = 0.006) (Fig. 2D).
Clinical and Molecular Association
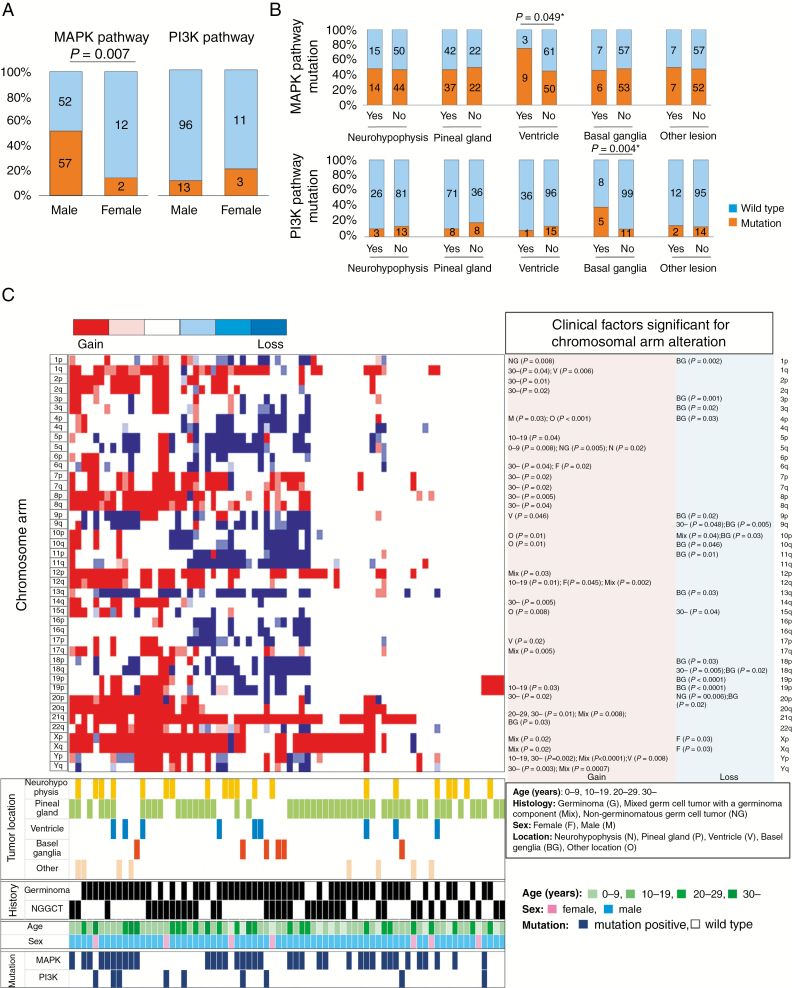
Most of the following genomic data are from previous studies.11,12 MAPK pathway mutations (KRAS, NRAS, HRAS, RRAS2, KIT, NF1, CBL, FGD6, FGFR2, TRAF6, or F2R)12 were significantly more frequent in males than in females (52.3%, vs 14.3%, P = 0.007; Fig. 3A). Cases with ventricular lesions and those with basal ganglia lesions had a higher frequency of mutations in the MAPK and PI3K/mTOR pathways (MTOR, PTEN, PIK3C2B, and PIK3R2), respectively (Fig. 3B). The latter correlation (PI3K/mTOR pathway mutation–basal ganglia lesion) remained significant in multivariate analyses after incorporating sex and histology as parameters (Supplementary Table 6).
Fig. 3.
(A) Percent presence of MAPK pathway and PI3K/mTOR pathway mutations is shown partitioned by sex. MAPK pathway mutations were significantly more frequent in male cases. There was no statistical difference in frequency of PI3K/mTOR pathway mutations (female: 21.4%, vs male: 11.9%, P = 0.32). (B) MAPK pathway mutations (top row) and PI3K/mTOR pathway mutations (bottom row) were analyzed for association with tumor locations. MAPK pathway mutations were more common in cases with ventricular lesions, whereas PI3K/mTOR pathway mutations were more common in cases with basal ganglia lesions. (C) Distribution of chromosome aberrations in 74 cases was described in a heatmap, with clinical and molecular (mutation) information at the bottom. Two major clusters were created based on chromosomal instability data, one with abundant alterations (left) and one with scant alterations (right). Of note, cases with basal ganglia lesions, cases in older patients, and cases with PI3K/mTOR mutations obviously preferentially aggregated in the left cluster with abundant chromosomal aberrations. Detailed multivariate analyses of clinical factors significantly associated with each chromosomal arm aberration are listed to the right of the heatmap.
A trend was observed toward a direct correlation between increasing age and chromosomal instability (P = 0.08), which was significant with regard to gain of 1q, 2p/q, 3q, 6q, 7p/q, 8p/q, 14q, 20p, 21q, and Yp/q, and loss of 9q, 15q, and 18q (P < 0.05).
Cases with basal ganglia lesions were characterized by loss of numerous chromosome arms, especially 1p, 3p/q, 4p, 9p/q, 10p/q, 11p, 13q, 18p/q, 19p/q, and 20p (P < 0.05). On average, 12.3 chromosomal arms were lost per case with a basal ganglia lesion, compared with 4.7 arms lost in cases with lesions at other locations (P = 0.017). Supplementary Figure 1 presents a heatmap of chromosomal aberrations found in 74 cases. Cases with significant aberrations cluster on the left and are enriched in basal ganglia cases, cases in older patients, and cases with PI3K/mTOR pathway mutations.
We also looked for correlations between tumor markers and mutations by multivariate analyses of parameters, including histology and tumor locations. This showed that MAPK pathway mutations were positively correlated with increased serum AFP (P = 0.04) (Supplementary Table 7).
Finally, using the Cox proportional hazards model and incorporating tumor location as a covariate, we analyzed chromosomal instability to assess its prognostic significance in germinoma (PFS). Among all observed aneuploidies, gain of 2q and 8q, and loss of 5q, 9p/q, 13q, and 15q were found to significantly worsen prognosis in multivariate analysis (Fig. 3D, Supplementary Table 8).
Discussion
General Clinical Profiles and Perspectives
Here we report an overview of clinical features of CNS GCTs from a large retrospective cohort collected through the iGCT consortium. A strength of this study was that all 190 cases underwent surgical resection and central pathological review. This enabled precise and detailed investigations into various clinical features and clinical-molecular associations.
The histopathology of GCTs in infants and young children <6 years old were mostly (83.3%) YST or teratoma (mature and immature). This finding suggests that CNS GCTs in this age group may belong to a biologically different subset that corresponds to type I GCTs as proposed by Oosterhuis et al,15 which may require intensive treatment, at least for yolk sac tumors. This may coincide with the therapeutic strategy of the SIOP protocol, in which stem cells are harvested, and intensive chemotherapy is administered in patients aged less than 6 years and/or serum/CSF AFP >1000 ng/mL.16 In contrast, GCTs in older adults (>30 y) were almost always germinoma (94.1%). This suggests that pathogenesis of GCTs differs depending on age of onset. This is congruent with reports on gonadal GCTs, which suggest that GCTs from different age groups are biologically distinct; those occurring in neonates and children are classified as type I, represented by YST and teratoma, while GCTs in older patients (>50 y) are classified as type III, described as spermatocytic seminoma.15 Types of chromosomal instability among these classifications were diverse.15 Age dependence of chromosomal instability will be discussed below.
Bifocal GCTs
One statement that did not achieve consensus at the fourth CNS GCT symposium was that bifocal lesions with typical imaging and negative tumor markers could be assumed to be germinomas without biopsy.10 In our series, all 9 cases with bifocal origins, and 8 of 9 bifocal cases with additional locations, were germinoma. The only exception was one YST case, which showed distinctly elevated AFP. Thus, all bifocal GCTs lacking tumor marker elevation were germinomas.
By contrast, Aizer et al reported that 3 of 9 bifocal GCTs were NGGCTs histologically, one of which showed a normal AFP level.17 Diabetes insipidus usually accompanies germinoma at neurohypophysis,18 but this did not help in their series to rule out NGGCT. Further study to find methods, including imaging, to completely differentiate NGGCT is warranted.
Histopathological Diagnostic Difficulties of GCTs
Another ongoing argument is whether pathological review should be made by secondary independent pathologists.10 Here, while 87.3% of local and central pathological diagnoses were made in complete concordance, including histological components, 12.7% were discordant. Among discordant cases, histological diagnoses rendered by the central review recategorized tumors into different prognostic groups in 2 cases (1.3%). Furthermore, there was a case in which histopathological diagnosis was totally changed (choriocarcinoma to immature teratoma in GCT66) and a case in which important NGGCT components were missed (YST and EC in GCT86). In terms of “germinoma or NGGCT,” 1 case would have been recategorized. As described, 94.3% of cases where specimens were obtained by biopsy were concordant. Concordance dropped to 81.4% in cases with “bigger” specimens. This suggests that experienced neuropathologists can take advantage of generous availability of pathological slides to find every component. The results of our systematic central review highlighted the difficulties in making diagnoses of GCTs. Accordingly, central pathology review is recommended to achieve accurate diagnoses and proper treatment for this rare disease.
Tumor Markers and Diagnoses
Another unsettled matter concerned the reliability of tumor markers for differentiating NGGCTs from germinoma or MT.10 Our study produced 41 “clinical” diagnoses of NGGCT (ie, secreting GCTs; AFP >25 ng/mL or HCG >50 IU/L, excluding MT) and 41 non-secreting GCTs which were not supposed to be diagnosed as NGGCT (in which both markers were less than the above criteria, excluding MT). Concordance between “clinical” and “histopathological” diagnoses was achieved for 70 cases. Diagnoses were discordant for 12 cases. Interestingly, the biopsy rates in concordance/discordance cases were similar; 37.1% and 41.7%, respectively (P = 0.77). This suggests that malignant components are not necessarily missed due to a small size of specimens, but that there exist marker-positive germinomas (8 out of 44 germinomas) and marker-negative NGGCT cases (2 out of 33 NGGCTs). Marker-positive MT cases were often diagnosed in cases with elevated tumor markers as well, accounting for 2 out of 15 in serum HCG, 5 out of 33 in serum AFP, and 2 out of 11 in CSF AFP.
We have previously shown that HCG is universally expressed in GCT cells.19 By ultra-sensitive enzyme immunoassay, HCG was also detected in most germinoma cases.20 In testicular GCTs, serum markers have been reported to be elevated in approximately 20–30% of seminoma cases at diagnosis.21 Here we showed cases of mismatch between marker and histopathology in a large series. This suggests that diagnosis should not rely solely on markers or histology alone. Surgical specimens, especially biopsy samples, inevitably carry the possibility of not representing a whole tumor, considering that GCTs are inherently heterogeneous. Of note, germinoma with elevated tumor marker was shown to have significantly shorter PFS than those without tumor marker elevation, suggesting that marker-positive germinoma needs extra caution during treatment, especially when the histopathological diagnosis is based on a small specimen, which risks underdiagnosis (5 of 7 cases were diagnosed based on biopsy specimen). At the same time, this does not necessarily support the idea that germinoma with mildly or moderately elevated tumor markers should be treated as NGGCTs. Thus, we recommend collecting all information, including tumor markers and histology, to make an integrated clinical diagnosis to guide appropriate treatment, especially for those cases without high levels of tumor markers. Supplementary Table 8 provides the findings from the current study lined up with the statements which reached consensus or did not reach consensus in fourth international CNS Germ Cell Tumor Symposium.
Prognosis
Five-year PFS and OS rates for germinoma are 87.3% and 98.6%, respectively (Fig. 2A), which are relatively consistent with data from the Brain Tumor Registry Japan, reporting 89.8% and 97.6%, respectively.1 The SIOP study reported good results for management of germinoma, with 5-year PFS of 88% or 97% depending on choice of focal radiation therapy with chemotherapy, or CSI for localized diseases. The 10-year OS of 98.6% was also similar to the 92.3% reported in the Surveillance, Epidemiology, and End Results database.22
For NGGCTs, the 5-year PFS and OS were 79.5% and 76.1%, respectively. Although it is difficult to interpret Brain Tumor Registry Japan results because they are categorized by detailed histopathological classification, the 5-year PFS and OS in NGGCTs were 51.9–100% and 73.3–100%, respectively (5-year PFS/OS for YST were 68.8/74.5%, respectively, for example). In the recent studies by SIOP, the 5-year PFS and OS were 68–72% and 75–82%, respectively.3 The Children’s Oncology Group presents the 5-year PFS and OS as 84% and 93%, respectively.5 Of note, these clinical studies did not require histopathological diagnoses when markers were elevated; thus, NGGCTs likely included germinoma with elevated markers, mixed GCTs with a germinoma component, and MT. The effect of those “mixed NGGCTs” and germinomas with elevated markers (especially HCG) on the prognosis among the NGGCT population is unclear.14,20,23
Although it is known that GCTs rarely occur in locations such as basal ganglia, thalamus, the cerebral hemispheres, or the cerebellum,24 the clinical behavior of GCTs at these atypical sites has not been unraveled. Here, prognosis of germinoma appeared to be different depending on location and distribution. This difference in prognosis also raises a concern that uniform radiation coverage for germinoma, regardless of its location, may not be appropriate, although this is confounded by discrepancies in treatment courses. This finding needs to be further investigated, and treatment intensity or regimen may need to be reconsidered according to location.
Clinical-Molecular Correlations
We found complex but significant relationships between clinical and molecular features of GCT. One of these was a difference in mutation profiles between sexes; mutations in the MAPK pathway were found in half of the male cases, but they were rare in female cases. Considering the possible acquisition of MAPK alterations at a very early stage of embryogenesis,13 this suggests that the key component in tumorigenesis may be different between sexes, and female GCTs may mostly develop independently of MAPK pathway alterations. Contribution of chromosome Y or genomic imprinting may play a role. Further molecular research is necessary to unravel the potential sex-specific pathogenesis of GCTs.
As previously described, the MAPK pathway mutation frequency was significantly associated with histology (ie, mutations were more frequent in germinoma than in other tumors).12 Basal ganglia GCTs are not common, often accompany various neurological deficits, including cognitive decline, and are deeply seated in the brain, which all make management difficult.25 We found that basal ganglia lesions were positively correlated with PI3K/mTOR pathway mutations and had numerous chromosomal instabilities, especially losses (1p, 3p/q, 4p, 9p/q, 10q, 11p, 13q, 18p, 19p/q). Elevated serum HCG was also associated with cases with basal ganglia lesions. These show that basal ganglia GCTs have clinically and molecularly distinct characteristics. Furthermore, some chromosomal alterations correlated significantly with worse prognosis in germinoma cases. Although our study is preliminary and retrospective, the findings suggest that GCTs are highly heterogeneous, most likely develop through complex and diverse mechanisms of pathogenesis and may require different treatment strategies depending on clinical/molecular factors.
Limitations
As the principal aim of the iGCT Consortium is genomic investigations, cases without specimens were not registered. This potentially creates a bias by excluding cases with typical imaging and/or elevated markers. However, considering that histopathological diagnoses are the standard in many Japanese neurosurgical institutions, the influence of this factor is deemed relatively small. Another limitation is related to the inherent spatial and temporal heterogeneity of GCTs. A specimen may not reflect the overall histology of the case, which can result in underdiagnosis. A safe strategy to avoid undertreatment in case of a mismatch between histopathology and markers is to consider those factors that point to poorer prognosis and plan treatment accordingly. Finally, this retrospective study examined cases collected from 24 centers, which inevitably subsumed a variety of diagnosis and treatment practices, including inconsistent investigation of spinal dissemination. An advantage is that many of these cases had a very long clinical follow-up, which allowed us to follow a natural history of these very rare tumors. The data presented reflect general clinical practices for CNS GCTs in neurosurgical centers in Japan and will hopefully provide insights to guide future treatment strategies and prospective clinical studies of CNS GCTs.
Funding
This study was supported by a research program of the Project for Development of Innovative Research on Cancer Therapeutics (P-Direct), Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan.
Supplementary Material
Acknowledgments
The following individuals in the iGCT Consortium made significant contributions to the study but are not included in the author list due to the limitation of the number of coauthors. We here acknowledge their contributions as being equivalent to other coauthors’ and express gratitude for their dedication: Yuko Matsushita, Kai Yamasaki, Tomonari Suzuki, Toshihiro Kumabe, Kaoru Tamura, Masahiro Nonaka, Kiyotaka Yokogami, Keiichi Kobayashi, Masahide Matsuda, Ryosuke Matsuda.
Conflict of interest statement.
Koichi Ichimura received a research grant from EPS Corporation. The other authors declare no conflicts of interest related to this work.
Authorship statement.
Conceptualization: H.T., K.F., K.I. Manuscript writing: H.T., K.I.Final editing and approval of the manuscript: H.T., K.F., S.F., T.N., A.M., N.S., Y.Y., H.N., K.S., M.K., T.T., T.M., M.N., Y.K., A.A., H.T., Y.H., T.I., M.N., K.Y., A.M., K.K., H.N., K.S., T.T., S.S., Y.N., Y.N., R.N., M.M., K.I.
References
- 1. Shibui S. Report of brain tumor registry of Japan (2001–2004). Neurol Med Chir. 2014;54(suppl. 1) :1–102. [Google Scholar]
- 2. Louis D, Ohgaki H, Wiestler O, Cavenee W.. WHO Classification of Tumours of the Central Nervous System. Revised 4th ed. Lyon, France: International Agency for Research on Cancer; 2016. [Google Scholar]
- 3. Calaminus G, Frappaz D, Kortmann RD, et al. Outcome of patients with intracranial non-germinomatous germ cell tumors-lessons from the SIOP-CNS-GCT-96 trial. Neuro Oncol. 2017;19(12):1661–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al-Hussaini M, Sultan I, Abuirmileh N, Jaradat I, Qaddoumi I. Pineal gland tumors: experience from the SEER database. J Neurooncol. 2009;94(3):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldman S, Bouffet E, Fisher PG, et al. Phase II trial assessing the ability of neoadjuvant chemotherapy with or without second-look surgery to eliminate measurable disease for nongerminomatous germ cell tumors: a Children’s Oncology Group Study. J Clin Oncol. 2015;33(22):2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matsutani M; Japanese Pediatric Brain Tumor Study Group Combined chemotherapy and radiation therapy for CNS germ cell tumors–the Japanese experience. J Neurooncol. 2001;54(3):311–316. [DOI] [PubMed] [Google Scholar]
- 8. Sawamura Y, Ikeda J, Shirato H, Tada M, Abe H. Germ cell tumours of the central nervous system: treatment consideration based on 111 cases and their long-term clinical outcomes. Eur J Cancer. 1998;34(1):104–110. [DOI] [PubMed] [Google Scholar]
- 9. Calaminus G, Kortmann R, Worch J, et al. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. 2013;15(6):788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murray MJ, Bartels U, Nishikawa R, Fangusaro J, Matsutani M, Nicholson JC. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. 2015;16(9):e470–e477. [DOI] [PubMed] [Google Scholar]
- 11. Fukushima S, Otsuka A, Suzuki T, et al. ; Intracranial Germ Cell Tumor Genome Analysis Consortium (iGCT Consortium) Mutually exclusive mutations of KIT and RAS are associated with KIT mRNA expression and chromosomal instability in primary intracranial pure germinomas. Acta Neuropathol. 2014;127(6):911–925. [DOI] [PubMed] [Google Scholar]
- 12. Ichimura K, Fukushima S, Totoki Y, et al. ; Intracranial Germ Cell Tumor Genome Analysis Consortium Recurrent neomorphic mutations of MTOR in central nervous system and testicular germ cell tumors may be targeted for therapy. Acta Neuropathol. 2016;131(6):889–901. [DOI] [PubMed] [Google Scholar]
- 13. Fukushima S, Yamashita S, Kobayashi H, et al. ; Intracranial Germ Cell Tumor Genome Analysis Consortium (The iGCTConsortium) Genome-wide methylation profiles in primary intracranial germ cell tumors indicate a primordial germ cell origin for germinomas. Acta Neuropathol. 2017;133(3):445–462. [DOI] [PubMed] [Google Scholar]
- 14. Matsutani M, Sano K, Takakura K, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86(3):446–455. [DOI] [PubMed] [Google Scholar]
- 15. Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5(3):210–222. [DOI] [PubMed] [Google Scholar]
- 16.ClinicalTrials.gov.https://clinicaltrials.gov/ct2/show/NCT01424839. Accessed July 23, 2019.
- 17. Aizer AA, Sethi RV, Hedley-Whyte ET, et al. Bifocal intracranial tumors of nongerminomatous germ cell etiology: diagnostic and therapeutic implications. Neuro Oncol. 2013;15(7):955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reddy AT, Wellons JC 3rd, Allen JC, et al. Refining the staging evaluation of pineal region germinoma using neuroendoscopy and the presence of preoperative diabetes insipidus. Neuro Oncol. 2004;6(2):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takami H, Fukushima S, Fukuoka K, et al. Human chorionic gonadotropin is expressed virtually in all intracranial germ cell tumors. J Neurooncol. 2015;124(1):23–32. [DOI] [PubMed] [Google Scholar]
- 20. Fukuoka K, Yanagisawa T, Suzuki T, et al. Human chorionic gonadotropin detection in cerebrospinal fluid of patients with a germinoma and its prognostic significance: assessment by using a highly sensitive enzyme immunoassay. J Neurosurg Pediatr. 2016;18(5):573–577. [DOI] [PubMed] [Google Scholar]
- 21. Trigo JM, Tabernero JM, Paz-Ares L, et al. Tumor markers at the time of recurrence in patients with germ cell tumors. Cancer. 2000;88(1):162–168. [DOI] [PubMed] [Google Scholar]
- 22. Acharya S, DeWees T, Shinohara ET, Perkins SM. Long-term outcomes and late effects for childhood and young adulthood intracranial germinomas. Neuro Oncol. 2015;17(5):741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ogino H, Shibamoto Y, Takanaka T, et al. CNS germinoma with elevated serum human chorionic gonadotropin level: clinical characteristics and treatment outcome. Int J Radiat Oncol Biol Phys. 2005;62(3):803–808. [DOI] [PubMed] [Google Scholar]
- 24. Kim DI, Yoon PH, Ryu YH, Jeon P, Hwang GJ. MRI of germinomas arising from the basal ganglia and thalamus. Neuroradiology. 1998;40(8):507–511. [DOI] [PubMed] [Google Scholar]
- 25. Zhang S, Liang G, Ju Y, You C. Clinical and radiologic features of pediatric basal ganglia germ cell tumors. World Neurosurg. 2016;95:516–524 e511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.