Abstract
Background
Glioblastoma (GBM) is the most common primary malignant adult brain tumor. Temozolomide (TMZ) is the standard of care and is most effective in GBMs that lack the DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT). Moreover, even initially responsive tumors develop a secondary resistance to TMZ and become untreatable. Since aberrant epidermal growth factor receptor (EGFR) signaling is widespread in GBM, EGFR inhibition has been tried in multiple clinical trials without success. We recently reported that inhibiting EGFR leads to increased secretion of tumor necrosis factor (TNF) and activation of a survival pathway in GBM. Here, we compare the efficacy of TMZ versus EGFR plus TNF inhibition in an orthotopic mouse model of GBM.
Methods
We use an orthotopic model to examine the efficacy of TMZ versus EGFR plus TNF inhibition in multiple subsets of GBMs, including MGMT methylated and unmethylated primary GBMs, recurrent GBMs, and GBMs rendered experimentally resistant to TMZ.
Results
The efficacy of the 2 treatments was similar in MGMT methylated GBMs. However, in MGMT unmethylated GBMs, a combination of EGFR plus TNF inhibition was more effective. We demonstrate that the 2 treatment approaches target distinct and non-overlapping pathways. Thus, importantly, EGFR plus TNF inhibition remains effective in TMZ-resistant recurrent GBMs and in GBMs rendered experimentally resistant to TMZ.
Conclusion
EGFR inhibition combined with a blunting of the accompanying TNF-driven adaptive response could be a viable therapeutic approach in MGMT unmethylated and recurrent EGFR-expressing GBMs.
Keywords: adaptive response, EGFR, glioblastoma, temozolomide, TNF
Key Points.
EGFR plus TNF inhibition is superior to TMZ in MGMT unmethylated primary GBM.
EGFR plus TNF inhibition is effective in TMZ-resistant recurrent GBM.
Importance of the Study.
GBM is a devastating and incurable cancer. The most effective drug currently available is TMZ. TMZ extends overall survival by a few months and is most effective in GBMs that lack the DNA repair protein MGMT. Resistance to TMZ in human GBM is inevitable, leading to recurrence of GBMs that now become untreatable. In this study, we compared TMZ with a recently described alternative treatment combining EGFR plus TNF inhibition in a preclinical mouse model of GBM. We found that the combination of EGFR and TNF inhibition is more effective compared with TMZ in GBMs that express MGMT and in TMZ-resistant recurrent GBMs. Since EGFR is expressed in the majority of GBMs, EGFR inhibition combined with a blunting of the accompanying TNF-driven adaptive response could be a broadly applicable and viable therapeutic approach in EGFR-expressing primary GBMs with non-methylated MGMT and in EGFR-expressing recurrent GBMs.
No targeted treatment is effective in glioblastoma (GBM), which remains a devastating disease. Amplification and mutation of the epidermal growth factor receptor (EGFR gene occurs in 40–50% of GBM patients.1,2 EGFR variant III (EGFRvIII), the most common EGFR mutant found in GBM, is constitutively active and oncogenic.2,3 EGFR wild type (EGFRwt) also plays an oncogenic role in GBM. In GBM, EGFRwt may be activated by ligand binding or signal constitutively even in the absence of ligand when overexpressed.4–7 EGFR expression has been detected in up to 81% of GBM by immunohistochemistry,8 suggesting that EGFR is an attractive therapeutic target in GBM. However, multiple clinical trials of EGFR inhibition have failed in GBM.9–11 While enthusiasm for EGFR inhibition in GBM may have diminished as a result, multiple mechanisms of resistance to EGFR inhibition in GBM have been described,11 including a dynamic downregulation of EGFRvIII in response to prolonged erlotinib treatment,12 de-repression of platelet derived growth factor receptor beta signaling,13 activation of a urokinase–B-cell lymphoma (Bcl)-2-like protein 11 (BIM) signaling axis,14 activation of an interleukin-6‒nuclear factor-kappaB (NF-κB)‒survivin pathway,15 and a glucose metabolism–linked p53 pathway.16
We have recently reported that EGFR inhibition in GBM or non-small-cell lung carcinoma triggers a rapid adaptive response driven by increased TNF levels.17–19 In GBM, a TNF‒c-Jun N-terminal kinase (JNK)‒Axl‒extracellular signal-regulated kinase (ERK) signaling axis mediates primary resistance to EGFR inhibition. EGFR inhibition when combined with TNF inhibition overcomes resistance to EGFR inhibition in an orthotopic model.17,18 While the heterogeneous expression of EGFRvIII may be a barrier for EGFRvIII-specific treatments,20 it does not impact the success of the therapeutic approach using EGFR plus TNF inhibition, since it is effective in GBM cells that express either EGFRwt or EGFRvIII,17 and EGFRwt is expressed diffusely in GBMs.21
Temozolomide (TMZ) is the standard of care in the treatment of GBM, even though its effect on overall survival remains modest.22 TMZ is an alkylating agent that induces toxicity by methylating the O6 position of guanine.23 O6-methylguanine-DNA methyltransferase (MGMT) directly reverses the O6-methylguanine lesions, opposing the effect of TMZ. Previous studies have shown that TMZ is most effective in the 45% of GBMs in which MGMT expression is suppressed through promoter methylation or other mechanisms.24 Also, TMZ is, by far, the most effective drug in animal models of GBM.25 However, TMZ resistance appears inevitable, resulting in GBM recurrence. There is no effective treatment for recurrent GBM.
In this study we compared the efficacy of a combination of EGFR plus TNF inhibition with standard TMZ in a preclinical model of various subsets of GBMs, including primary MGMT methylated and unmethylated GBMs, recurrent GBMs, and GBMs rendered experimentally resistant to TMZ. We also examined whether EGFR inhibition and TMZ target distinct and non-overlapping pathways in GBM.
Methods
Cell Culture of Patient-Derived Xenografts and Neurospheres
All Mayo patient-derived xenograft (PDX) lines used in this study were generated by the Mayo Clinic Brain Tumor Patient-Derived Xenograft National Resource and authenticated using short-tandem repeats.26 Mayo PDX cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum and 1% penicillin/streptomycin. Primary GBM neurosphere GBM9 was cultured in DMEM F12 supplemented with B27 without vitamin A, and with epidermal growth factor (EGF; 20 ng/mL) and basic fibroblast growth factor (20 ng/mL) as described previously.27 The murine glioma cell line GL261 was provided by Dr Maria Castro (University of Michigan). GL261 cells were cultured in DMEM with 10% fetal bovine serum and 1% penicillin/streptomycin. EGFRwt-HA in pcDNA 3.1 (Neo) was transfected into GL261 glioma cells followed by selection of stable clones with G418. Explant cultures were generated by culturing cells from subcutaneous tumors as previously described26 and used without repeated passaging.
Western Blotting
Before the addition of afatinib, Mayo PDX cells were serum starved overnight while primary GBM9 neurospheres were EGF starved overnight. Cells not treated with EGF or afatinib were also serum or EGF starved. Western blot was performed as we have described previously.17 Details of antibodies and reagents are provided in the Supplementary Methods.
Cell Viability Assay
Cell viability assay was conducted using the alamarBlue cell viability assay kit (ThermoFisher), and annexin assay was performed using the Annexin-V-FLUOS Staining Kit (Roche Applied Science) according to manufacturer’s protocols. Details are given in the Supplementary Methods.
Immunofluorescence, Immunohistochemistry, and TUNEL Staining
For immunofluorescence, cells were treated with drugs followed by immunostaining or immunohistochemistry with 53BP-1 or Ki67 antibody as described in the Supplementary Methods.
Staining for terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling (TUNEL) was performed using the TUNEL assay kit (4810-30-K) by R&D Systems according to the manufacturer’s instructions.
Enzyme-Linked Immunosorbent Assay
TNF protein concentration in supernatant and tissue extracts was determined by enzyme-linked immunosorbent assay (ELISA) using a commercial TNF detection kit (ThermoFisher) as described previously.17
Animal Studies
Female athymic nude mice 4 to 6 weeks old and 6-week-old female C57BL/6 mice were purchased from Charles River Laboratories. Neurosphere GBM9 or Mayo PDXs were injected into the right basal ganglia of 6- to 8-week-old nude mice using a stereotactic frame. Implanted were 1×105 GL261-EGFRwt cells into the right basal ganglia of 6-week-old C57BL6 mice.
Tumor-bearing nude mice were divided into 5 groups (6–8 mice per group) treated with vehicle, TMZ (50 mg/kg, oral gavage), afatinib (50 mg/kg, oral gavage), thalidomide (150 mg/kg, intraperitoneal injection), and afatinib plus thalidomide. C57BL/6 mice were treated with vehicle, thalidomide, afatinib, or thalidomide plus afatinib. The mice were treated for 10 consecutive days. Kaplan–Meier survival curves were calculated using GraphPad Prism 7.0 software. Mice were monitored visually or via bioluminescence imaging (BLI) and sacrificed when neurological signs appeared or after 3 months. Details of BLI are described in the Supplementary Methods.
To monitor in vivo expression of proteins after drug treatment, nude mice bearing intracranial tumor were dosed consecutively for 1, 2, or 7 days and then sacrificed, followed by protein extraction. Additionally, in orthotopic models, mice were divided into 5 groups (control group, afatinib group, thalidomide group, TMZ, and afatinib plus thalidomide, n = 3). After 48 hours of treatment, tumors were collected and subjected to TUNEL assay and immunostaining of Ki67 or 53BP1. All animal studies were done under Institutional Animal Care and Use Committee–approved protocols.
Statistical Analysis
All data were analyzed for significance using GraphPad Prism 7.0 software. Error bars represent the means ± SEM of 3 independent experiments. Two-tailed unpaired Student’s t-tests were used for comparison of 2 datasets. At least 3 independent experiments were performed unless otherwise indicated. P < 0.05 was considered statistically significant (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001).
Results
Comparable Efficacy of Temozolomide and EGFR Plus TNF Inhibition in MGMT Methylated GBMs
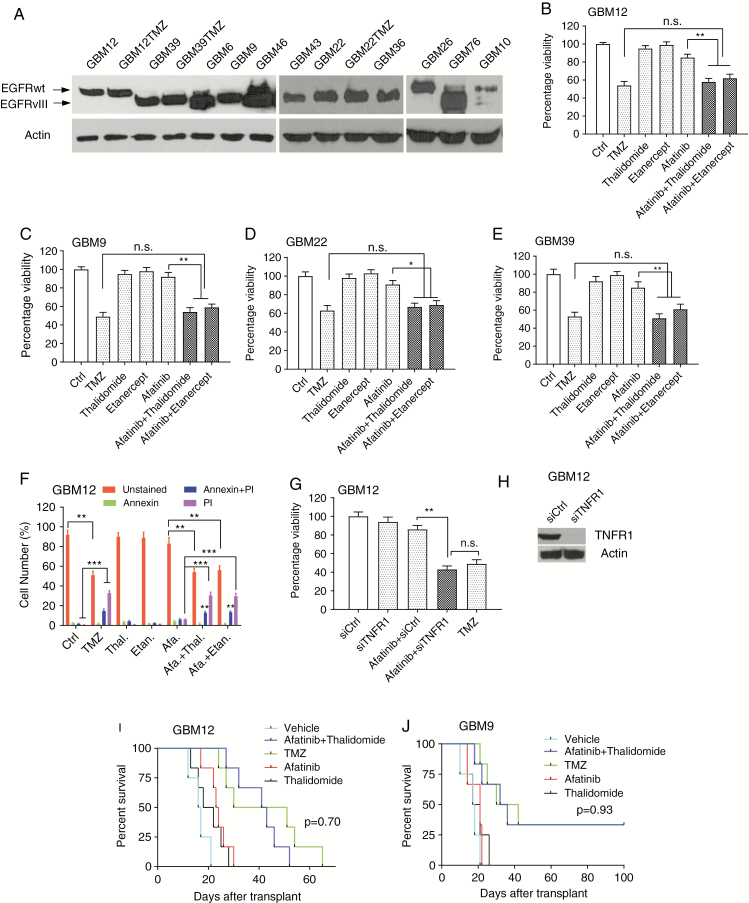
In this study, we compared EGFR plus TNF inhibition with treatment with TMZ using primary GBM neurospheres and the widely used Mayo PDX panel26 (Supplementary Table 1 and Fig. 1A). We started with a comparison of EGFR plus TNF inhibition in MGMT methylated GBMs using afatinib, an irreversible inhibitor of the EGFR kinase. Etanercept is a specific TNF blocker in clinical use and is a fusion protein of TNF receptor and immunoglobulin G1.28 We also used thalidomide, since it is a known potent inhibitor of TNF production and penetrates the blood–brain barrier.17,29–31 We confirmed that etanercept blocks TNF-induced NF-κB activation (Supplementary Fig. 1A–C) and that thalidomide inhibits EGFR inhibition-induced TNF upregulation (Supplementary Fig. 1D, E). We also determined that short-term culture of Mayo PDX cells in serum containing medium does not affect the EGFRwt or EGFRvIII levels (Supplementary Fig. 1F, G). To examine the effect of TMZ on survival of MGMT methylated GBM12 cells, we conducted a dose response and a time course experiment for TMZ in MGMT methylated GBM12 explant cultures (Supplementary Fig. 2A). Next, we tested the effect of afatinib combined with TNF inhibition using etanercept or thalidomide at different concentrations (Supplementary Fig. 2B–D). A time course experiment with EGFR plus TNF inhibition suggested that the maximum effect was detected at 72 h (Supplementary Fig. 2E, F). We found that TMZ and a combination of afatinib and etanercept or thalidomide decreased viability to a similar degree, while neither afatinib nor TNF inhibition alone had an effect (Fig. 1B). Similar results were detected in GBM9 neurospheres that lack MGMT expression32 (Fig. 1C) as well as in MGMT methylated Mayo PDX GBM22 and GBM39, but not in GBM14, which does not express EGFR (Fig. 1D, E; Supplementary Fig. 3B–C). A similar result was obtained using erlotinib, a first-generation EGFR inhibitor (Supplementary Fig. 3A). We confirmed that these treatments induced cell death by using a cell death/annexin assay (Fig. 1F). Additionally, small interfering (si)RNA knockdown of TNF receptor 1 resulted in enhanced sensitivity to EGFR inhibition (Fig. 1G, H).
Fig. 1.
A comparison of TMZ and a combination of EGFR plus TNF inhibition in GBMs lacking MGMT. (A) EGFR expression in the Mayo PDX lines. (B) GBM12 cells were exposed to dimethyl sulfoxide (vehicle control), TMZ (1 µM), or afatinib (1 µM) with or without etanercept (100 µg/mL), or thalidomide (1 µM) for 72 hours, followed by alamarBlue cell viability assay. (C–E) Similar experiments were conducted in GBM9, GBM22, and GBM39 cells. (F) GBM12 cells were treated with TMZ (1 μM), afatinib (1 µM) and/or etanercept (100 μg/mL), thalidomide (1μM) and/or afatinib for 72 hours followed by annexin–fluorescence activated cell sorting assay. (G) GBM12 cells were transfected with TNF receptor 1 siRNA, and after 48 hours cells were treated with afatinib for 72 hours followed by alamarBlue assay. (H) Western blotting confirming siRNA knockdown of TNF receptor 1. Data are presented as mean ± SEM of at least 3 independent experiments. (I) Both TMZ and combined treatment of afatinib plus thalidomide prolonged survival in a GBM12 orthotopic model. Ten days after intracranial injection, mice were divided into 5 groups: vehicle, afatinib (50 mg/kg), thalidomide (150 mg/kg), TMZ (50 mg/kg), afatinib plus thalidomide (n = 6). (J) A similar experiment was undertaken in a GBM9 orthotopic model (n = 6). Kaplan–Meier survival curves were calculated using GraphPad Prism 7. Statistical significance was verified by the log rank test. *P < 0.05, **P < 0.01,***P < 0.001; n.s., not significant.
Next, GBM12 cells were implanted intracranially in athymic mice. Ten days after tumor implantation, the mice were divided into 5 groups and treated with control gavage, TMZ, afatinib alone, thalidomide alone, or a combination of afatinib and thalidomide for 10 days. TMZ suppressed the growth of intracranial GBM12 tumors. The combination of afatinib plus thalidomide suppressed the growth of tumors to a similar degree (Fig. 1I; Supplementary Fig. 4A). A similar result was obtained with GBM9 tumors (Fig. 1J). GBM12 PDX expresses EGFRwt, while GBM9 expresses predominantly EGFRvIII with a lower level of EGFRwt. A longer duration of treatment for 4 weeks did not confer an additional survival advantage (Supplementary Fig. 4B).
Ki67 staining in GBM12 PDX or GBM9 neurosphere derived intracranial tumors from mice shows a significant decrease in proliferation in tumors treated with TMZ or a combination of afatinib plus thalidomide. Afatinib or thalidomide alone did not decrease proliferation (Supplementary Fig. 5A–D). TUNEL staining in GBM12 PDX or GBM9 intracranial tumors from mice shows a significant increase in apoptosis in tumors treated with TMZ or a combination of afatinib plus thalidomide. Afatinib or thalidomide alone did not induce apoptosis (Supplementary Fig. 5E–H).
EGFR Plus TNF Inhibition Is Superior to Temozolomide in MGMT Unmethylated GBMs
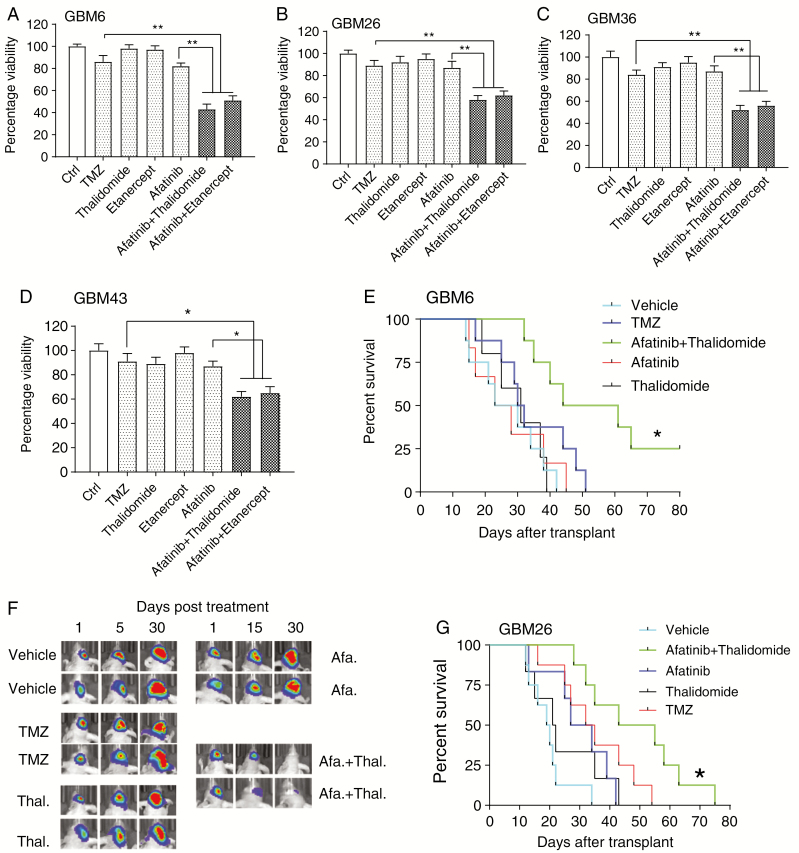
To examine the effect of TMZ on survival of MGMT unmethylated tumors, we conducted a dose response and a time course experiment for TMZ in MGMT unmethylated GBM6 explant cultures (Supplementary Fig. 2G). In GBM6 explant cultures, TMZ did not affect viability of GBM cells. Next, we tested the effect of afatinib combined with TNF inhibition using etanercept or thalidomide at different concentrations (Supplementary Fig. 2H–J). A time course experiment with EGFR plus TNF inhibition suggested that the maximum effect was detected at 72 h (Supplementary Fig. 2K, L). Under the optimal treatment concentrations and time conditions, we found that the combination of afatinib and etanercept or thalidomide significantly decreased viability of GBM6 cells (Fig. 2A). Similar results were found in additional MGMT unmethylated Mayo PDX tumors (Fig. 2B–D). Additionally, siRNA knockdown of TNF receptor 1 resulted in enhanced sensitivity to EGFR inhibition (Supplementary Fig. 3D–E). Finally, erlotinib, a first-generation EGFR tyrosine kinase inhibitor, also synergized with TNF inhibition to decrease cell viability in GBM6 cells (Supplementary Fig. 3F).
Fig. 2.
A comparison of TMZ and a combination of EGFR plus TNF inhibition in MGMT unmethylated GBMs. (A) GBM6 cells were exposed to dimethyl sulfoxide, TMZ (10 µM) or afatinib (1 µM) with or without etanercept (100 μg/mL) or thalidomide (1 µM) for 72 hours, followed by alamarBlue cell viability assay. (B–D) Similar experiments were also conducted in GBM26, GBM36, and GBM43 cells. Data are presented as mean ± SEM of at least 3 independent experiments, *P < 0.05, **P < 0.01. (E) Combined treatment with afatinib plus thalidomide prolonged survival in a GBM6 orthotopic model (n = 8). One week after intracranial injection, mice were divided into five 5 groups: vehicle, afatinib (50 mg/kg), thalidomide (150 mg/kg), TMZ (50 mg/kg), afatinib plus thalidomide (n = 8). Comparison was made between combination group and TMZ; P = 0.024. (F) Representative BLI from groups at days 1, 15, and 30 after treatment. (G) A similar experiment was conducted with GBM26 using an orthotopic model (n = 8) comparing EGFR plus thalidomide with TMZ; P = 0.041. Kaplan–Meier survival curves were calculated using GraphPad Prism 7. Statistical significance verified by the log rank test. *P < 0.05.
Next, we undertook an orthotopic experiment using GBM6 cells expressing a luciferase reporter. TMZ did not suppress the growth of intracranial GBM6 tumors, while the combination of afatinib plus thalidomide resulted in a significant suppression of tumor growth without significant change in mouse weight (Fig. 2E, F; Supplementary Fig. 4C). A similar result was obtained with GBM26 tumors (Fig. 2G). GBM26 PDX expresses EGFRwt, while the GBM6 PDX expresses both EGFRwt and EGFRvIII (Fig. 1A and Supplementary Table 1). Thus, a combined EGFR plus TNF inhibition is superior to TMZ in MGMT unmethylated GBMs.
Temozolomide and EGFR Plus TNF Inhibition Target Distinct Signal Transduction Pathways
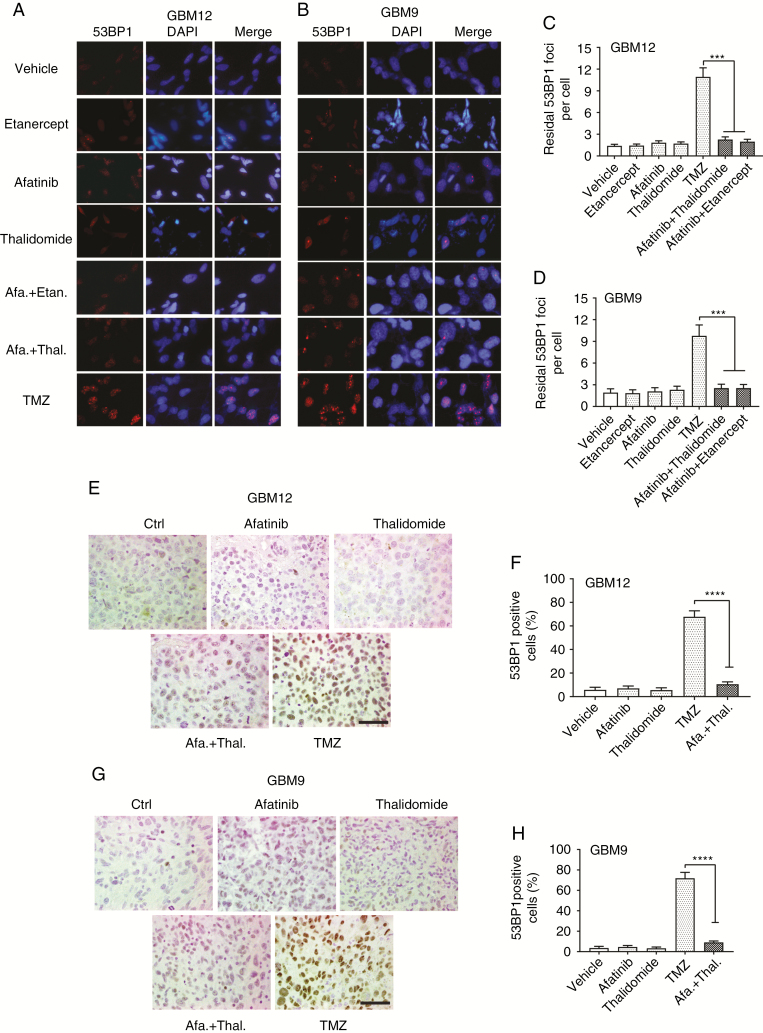
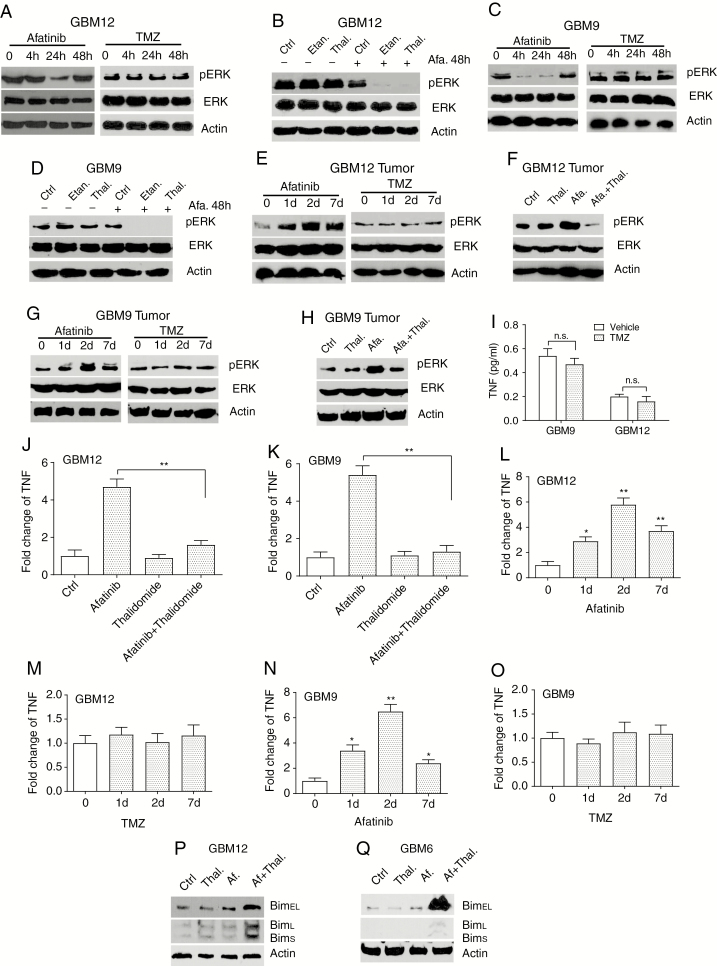
Treatment of MGMT methylated GBM12 explant cultures or GBM9 neurospheres resulted in increased numbers of 53BP1 foci, a surrogate marker for DNA double-stranded breaks, while treatment with afatinib and etanercept or thalidomide did not increase numbers of 53BP1 foci (Fig. 3A–D). A similar result was observed in mouse GBM12 and GBM9 tumors (Fig. 3E–H) and with a lower concentration of TMZ (Supplementary Fig. 6A–D). Thus, TMZ induces extensive DNA damage in GBMs, while the combination of EGFR plus TNF inhibition does not. We have reported that EGFR inhibition in GBMs results in increased TNF secretion and ERK activation.17 As previously reported, treatment of GBM12 or GBM9 cells with afatinib resulted in a downregulation of ERK activation in 24 hours (suggesting that ERK activation is driven by EGFR) and a feedback ERK reactivation at 48 h that can be blocked by TNF inhibition (Fig. 4A–D). No change in ERK activation was detected in TMZ-treated cells (Fig. 4A, C). We also see increased ERK activation in GBM12 or GBM9 intracranial tumors in mice treated with afatinib, but not TMZ (Fig. 4E, G). This ERK activation is blocked by a combination of afatinib plus thalidomide (Fig. 4F, H). We did not detect any increase in activation of Akt, mammalian target of rapamycin, or signal transducer and activator of transcription 3 in response to afatinib or TMZ in GBM12 or GBM6 (Supplementary Fig. 7A, B). TNF is upregulated in response to EGFR inhibition but not to TMZ in GBM9 or GBM12 cultures and in orthotopic tumors (Fig. 4L–O). Thalidomide blocks the EGFR inhibition–induced increase in TNF levels (Fig. 4J, K). These data indicate that TMZ and EGFR inhibition trigger distinct and non-overlapping signaling pathways. Furthermore, we have previously demonstrated that the TNF-ERK adaptive response could be interrupted at multiple nodes.17 Thus, a combination of EGFR inhibition plus ERK inhibition is also synergistic.17
Fig. 3.
TMZ but not afatinib plus thalidomide induce 53BP1 foci formation. (A) GBM12 cells were treated with TMZ (10 μM), afatinib (1 μM) plus etanercept (100 μg/mL), or thalidomide (1 μM) for 24 hours followed by immunofluorescence using antibody against 53BP1 (red). Nuclei are stained with 4′,6′-diamidino-2-phenylindole (blue). (B) Similar experiments were conducted in GBM 9 cells. (C–D) Double-strand breaks repair rates were determined by scoring 53BP1 foci. Data are presented as mean ± SEM of at least 3 independent experiments, ***P < 0.001. (E) Mice with GBM12 or GBM9 tumors were treated with drugs for 48 h followed by removal of tumors. Immunohistochemistry of 53BP1 in representative GBM12 orthotopic tumor sections from vehicle, afatinib, thalidomide, afatinib plus thalidomide, and TMZ group. (F) Quantification of 53BP1-positive in tumor sections. (G) Immunostaining of 53BP1 in brain sections from GBM9 orthotopic mice treated with indicated drugs. (H) Quantification of 53BP1-positive cells in tumor sections. Four random fields in 3 tissue blocks at x400 magnification were scored. Data are presented as mean ± SEM. ***P < 0.001, ****P < 0.0001. Scale bars represent 50 µm.
Fig. 4.
A combination of afatinib and thalidomide but not TMZ induces TNF dependent ERK activation. (A) GBM12 cells were treated with afatinib (1 μM) or TMZ (1 μM) for the indicated times followed by western blotting with phosphorylated ERK, ERK, or β-actin antibodies. (B) GBM12 cells were exposed to indicated drugs for 48 hours followed by western blot. (C–D) Similar experiments to that described in A and B were performed with GBM9 cells. (E) Mice were injected intracranially with GBM12 cells. After 10 days, mice were treated with afatinib or TMZ for 1, 2, or 7 days, followed by euthanasia (at 1, 2, or 7 days after treatment) and tumors were then harvested and western blot was conducted on tumor tissue to detect ERK activation. (F) Mice with GBM12 orthotopic tumors were treated with indicated drugs for 48 hours and brain tumor lysates were analyzed by western blot. (G–H) Similar experiments as performed in E and F in a GBM9 orthotopic model. (I) GBM9 and GBM12 cells were treated with TMZ for 48 hours followed by TNF ELISA. (J) Mice with GBM12 orthotopic tumors were treated with indicated drugs for 48 hours and brain tumor lysates were analyzed by TNF ELISA. (K) A similar experiment was conducted in a GBM9 orthotopic model. (L–M) Time course of TNF upregulation in mice with a GBM12 orthotopic tumor exposed to afatinib or TMZ for 1, 2, and 7 days. (N–O) A similar experiment was performed in a GBM9 orthotopic model. (P–Q) Western blot analysis of BIM protein expression in GBM12 and GBM6 cells treated with indicated drugs for 48 hours. Data are presented as mean ± SEM of at least 3 independent experiments. *P < 0.05, **P < 0.01; n.s., not significant.
EGFR inhibition leads to an apoptotic cell death that is mediated by BIM/Bcl2L11. In response to EGFR inhibition, there is an upregulation of 2 BIM isoforms, BIM-EL (BIM extra long) and BIM-L (BIM long) in lung cancer cells.33 We do not see an upregulation of BIM isoforms in response to afatinib alone. However, when it is combined with TNF inhibition, we see a sharp upregulation of BIM-EL and BIM-L, as shown in Fig. 4P, Q. Thus, combination treatment with EGFR and TNF inhibition prevents a reactivation of ERK, leading to increased BIM activation and apoptosis.
Efficacy of EGFR Plus TNF Inhibition in an Immunocompetent Model
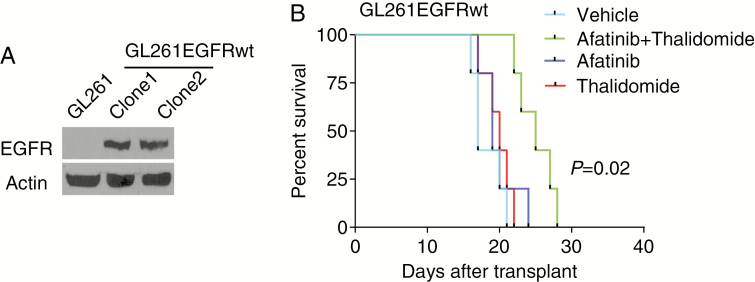
To examine whether EGFR plus TNF inhibition is effective in an immunocompetent model, we stably transfected the murine glioma line GL261 with EGFR (Fig. 5A). The parental GL261 line has low or absent EGFR. Next, we injected GL261 expressing EGFR intracranially into C57BL/6 mice followed by treatment with control vehicle, afatinib, thalidomide, or afatinib plus thalidomide. We found that a combination of afatinib plus thalidomide was effective in limiting growth of intracranial tumors, while afatinib or thalidomide alone had no effect (Fig. 5B).
Fig. 5.
Combination of afatinib and thalidomide prolongs survival of intracranial immune-competent tumor-bearing mice. (A) Western blot analysis of EGFR expression in GL261 and GL261EGFRwt cell lines. β-actin was used as a loading control. (B) Ten days after intracranial injection of GL261EGFRwt cells (clone 1) into C57BL/6 mice, the mice were divided into 4 groups: vehicle, afatinib (50 mg/kg), thalidomide (150 mg/kg), and afatinib plus thalidomide (n = 5). Comparison was made between combination group and afatinib group; P = 0.020.
EGFR Plus TNF Inhibition Is Effective in Temozolomide-Resistant Recurrent GBM
Recurrence of GBM is inevitable as the tumors become unresponsive to TMZ. EGFR amplification and EGFRwt expression remains relatively stable in recurrent GBMs, while there may be a loss of EGFRvIII at recurrence in some GBMs.34,35 We also confirmed expression of EGFR in recurrent GBMs used in our study (Fig. 1A).
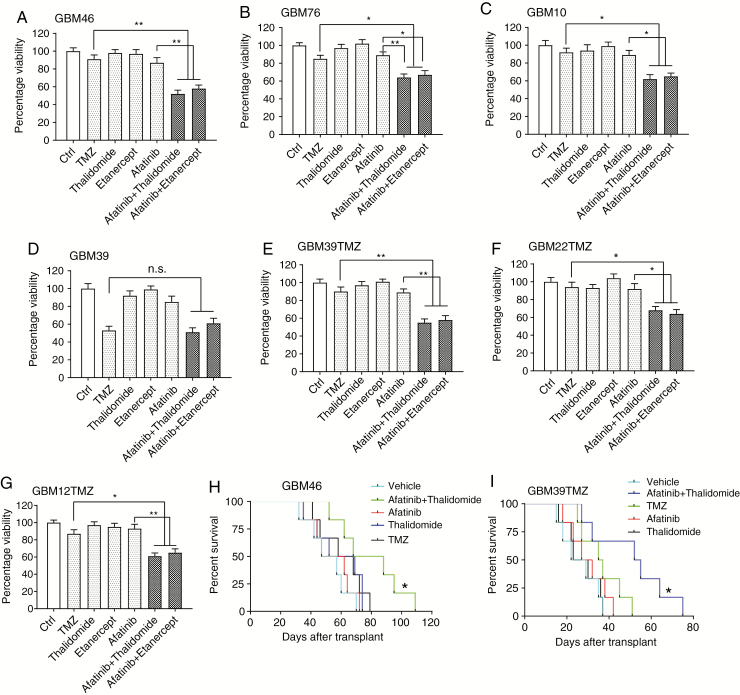
TMZ did not affect viability of recurrent GBM46 cells. However, the combination of afatinib and etanercept or thalidomide significantly decreased viability of GBM46 cells (Fig. 6A). Similar results were noted in the recurrent GBM76 and GBM10 tumors (Fig. 6B, C). We also examined the effect of a combined EGFR plus TNF inhibition in a GBM39TMZ that is rendered experimentally resistant to TMZ by exposing tumors to TMZ as described previously.36 While in parental GBM39 cells, both TMZ and a combination of EGFR plus TNF were equally effective, in GBM39TMZ cells TMZ did not affect cell viability, while the combination was effective (Fig. 6D, E). Similarly, GBM22TMZ and GBM12TMZ, which are Mayo PDX lines experimentally rendered resistant to TMZ, were sensitive to EGFR plus TNF inhibition (Fig. 6F, G). Next, we undertook an orthotopic experiment to compare the effects of TMZ versus the combined inhibition of TNF and EGFR in GBM46 tumors. TMZ did not suppress the growth of intracranial GBM46 tumors, while a combination of afatinib plus thalidomide resulted in a significant suppression of tumor growth (Fig. 6H). We also examined the effect of a combined EGFR plus TNF inhibition in a GBM39TMZ orthotopic experiment and found that the combined inhibition of EGFR and TNF resulted in a significant inhibition of tumor growth (Fig. 6I). These experiments demonstrate that a combination of EGFR plus TNF inhibition remains effective in TMZ-resistant recurrent GBMs.
Fig. 6.
EGFR plus TNF inhibition remains effective in recurrent GBMs and GBMs rendered experimentally resistant to TMZ. (A–C) AlamarBlue assay in recurrent GBM46, GBM76, and GBM10 cells. Cells were treated with TMZ (10 μM), afatinib (1 μM), etanercept (100 μg/mL), or thalidomide (1 μM), each drug alone or 2 drugs: afatinib plus thalidomide/etanercept for 72 hours, followed by alamarBlue cell viability assay. Dimethyl sulfoxide was used as a control (Ctrl). (D) A similar experiment was conducted in GBM39 cells, except that the afatinib concentration is 1 µM. (E–G) Acquired TMZ-resistant GBM39TMZ, GBM22TMZ, and GBM12TMZ cells were treated with TMZ (10 μM), afatinib (1 μM), etanercept (100 μg/mL), or thalidomide, each drug alone or 2 drugs: afatinib plus thalidomide/etanercept for 72 hours, followed by alamarBlue cell viability assay. Data are presented as mean ± SEM of at least 3 independent experiments. *P < 0.05, **P < 0.01; n.s., not significant. (H) Combined treatment of afatinib (50 mg/kg) and thalidomide (150 mg/kg) prolonged survival in GBM46 orthotopic model (n = 6). A comparison was done between EGFR plus TNF inhibition and TMZ (50 mg/kg). P = 0.045. (I) The same treatments were used in GBM39T orthotopic model (n = 8 mice per group). TMZ versus afatinib plus thalidomide: P = 0.019. Kaplan–Meier survival curves were calculated using GraphPad Prism 7. Statistical significance was verified by the log rank test. *P < 0.05.
Discussion
Targeted inhibition of receptor tyrosine kinase signaling pathways results in a feedback reprogramming of signaling pathways that leads to a resumption of previously suppressed signals or activation of alternative functionally similar signals.17,37 The adaptive response is triggered early, often within hours of targeted inhibition, and may be responsible for the primary resistance of cancer cells to targeted treatment. In GBM, EGFR inhibition results in a rapid upregulation of TNF that, in turn, leads to activation of a JNK-Axl-ERK signaling pathway that mediates resistance to EGFR inhibition.17,18
TMZ is the most effective drug available for GBM and the most effective drug in animal models of GBM.25 TMZ is effective as a pharmacological monotherapy and is more effective in GBMs with MGMT promoter hypermethylation.24 Importantly, secondary resistance to TMZ is inevitable, and there is no known effective treatment for TMZ-resistant recurrent GBMs. In this study, we compared the effectiveness of TMZ versus the combined inhibition of EGFR plus TNF in representative PDXs from primary MGMT methylated and unmethylated GBMs, recurrent TMZ-resistant GBMs, and GBMs that have been experimentally rendered TMZ resistant.
An important finding of our study is that the combination of EGFR and TNF inhibition is more effective than TMZ in large subsets of GBM in an orthotopic model. TMZ is highly effective in mouse models of GBM with methylated MGMT. TMZ is ineffective in GBM with unmethylated MGMT, and as expected, EGFR plus TNF inhibition is more effective than TMZ in primary GBMs with unmethylated MGMT in our mouse model. The efficacy of TMZ versus EGFR plus TNF inhibition is roughly equivalent in primary GBMs with hypermethylated MGMT. We also demonstrate that TMZ and EGFR inhibition target distinct and non-overlapping signaling pathways and that recurrent and TMZ-resistant GBMs remain responsive to a combination of EGFR plus TNF inhibition in our experimental model. Also, our data indicate that EGFR plus TNF inhibition is equally effective in MGMT methylated or MGMT unmethylated GBMs. While a combination of EGFR plus TNF inhibition improved overall survival in all groups tested, durable responses were detected in orthotopic models of some GBMs but not in others. It is likely that the adaptive response to EGFR inhibition is influenced by other genetic modifiers. As future studies improve our understanding of the multiple facets of the adaptive response to EGFR inhibition in GBM, it may become possible to blunt the adaptive response more effectively.
Our findings suggest that EGFR plus TNF inhibition could be an important new therapeutic approach for MGMT unmethylated primary GBMs as a primary treatment. In addition, and perhaps more importantly, EGFR plus TNF inhibition could be an effective treatment for recurrent TMZ-resistant GBMs. The ability of specific TNF inhibitors to cross the blood‒brain barrier is uncertain.38 Thalidomide is a potent TNF inhibitor30 and is known to cross the blood‒brain barrier, and indeed has been used in clinical trials of GBM, although not in combination with EGFR inhibition.29 Our current results suggest that a combination of an EGFR tyrosine kinase inhibitor such as afatinib and a blood‒brain barrier penetrable TNF inhibitor such as thalidomide could be rapidly tested in a clinical trial.
Funding
This work was supported in part by the Office of Medical Research, Departments of Veterans Affairs, and from the Dallas VA Research Corporation (AAH), by NIH grant 1R01CA194578 to DZ. SB is supported by grants from the National Institutes of Health (RO1CA197796 and R21CA202403) and NASA (NNX16AD78G).
Supplementary Material
References
- 1. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hatanpaa KJ, Burma S, Zhao D, Habib AA. Epidermal growth factor receptor in glioma: signal transduction, neuropathology, imaging, and radioresistance. Neoplasia. 2010;12(9):675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Sci Signal. 2009;2(87):re6. [DOI] [PubMed] [Google Scholar]
- 4. Acquaviva J, Jun HJ, Lessard J, et al. Chronic activation of wild-type epidermal growth factor receptor and loss of Cdkn2a cause mouse glioblastoma formation. Cancer Res. 2011;71(23):7198–7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chakraborty S, Li L, Puliyappadamba VT, et al. Constitutive and ligand-induced EGFR signalling triggers distinct and mutually exclusive downstream signalling networks. Nat Commun. 2014;5:5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo G, Gong K, Wohlfeld B, Hatanpaa KJ, Zhao D, Habib AA. Ligand-Independent EGFR Signaling. Cancer Res. 2015;75(17):3436–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Endres NF, Das R, Smith AW, et al. Conformational coupling across the plasma membrane in activation of the EGF receptor. Cell. 2013;152(3):543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan QW, Cheng CK, Gustafson WC, et al. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell. 2013;24(4):438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karpel-Massler G, Schmidt U, Unterberg A, Halatsch ME. Therapeutic inhibition of the epidermal growth factor receptor in high-grade gliomas: where do we stand? Mol Cancer Res. 2009;7(7):1000–1012. [DOI] [PubMed] [Google Scholar]
- 10. Weller M, Butowski N, Tran DD, et al. ; ACT IV trial investigators Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. [DOI] [PubMed] [Google Scholar]
- 11. Thorne AH, Zanca C, Furnari F. Epidermal growth factor receptor targeting and challenges in glioblastoma. Neuro Oncol. 2016;18(7):914–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nathanson DA, Gini B, Mottahedeh J, et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science. 2014;343(6166):72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akhavan D, Pourzia AL, Nourian AA, et al. De-repression of PDGFRβ transcription promotes acquired resistance to EGFR tyrosine kinase inhibitors in glioblastoma patients. Cancer Discov. 2013;3(5):534–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wykosky J, Hu J, Gomez GG, et al. A urokinase receptor-Bim signaling axis emerges during EGFR inhibitor resistance in mutant EGFR glioblastoma. Cancer Res. 2015;75(2):394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zanca C, Villa GR, Benitez JA, et al. Glioblastoma cellular cross-talk converges on NF-κB to attenuate EGFR inhibitor sensitivity. Genes Dev. 2017;31(12):1212–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mai WX, Gosa L, Daniels VW, et al. Cytoplasmic p53 couples oncogene-driven glucose metabolism to apoptosis and is a therapeutic target in glioblastoma. Nat Med. 2017;23(11):1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo G, Gong K, Ali S, et al. A TNF-JNK-Axl-ERK signaling axis mediates primary resistance to EGFR inhibition in glioblastoma. Nat Neurosci. 2017;20(8):1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Warta R, Herold-Mende C. Helping EGFR inhibition to block cancer. Nat Neurosci. 2017;20(8):1035–1037. [DOI] [PubMed] [Google Scholar]
- 19. Gong K, Guo G, Gerber DE, et al. TNF-driven adaptive response mediates resistance to EGFR inhibition in lung cancer. J Clin Invest. 2018;128(6):2500–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonavia R, Inda MM, Cavenee WK, Furnari FB. Heterogeneity maintenance in glioblastoma: a social network. Cancer Res. 2011;71(12):4055–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nishikawa R, Sugiyama T, Narita Y, Furnari F, Cavenee WK, Matsutani M. Immunohistochemical analysis of the mutant epidermal growth factor, deltaEGFR, in glioblastoma. Brain Tumor Pathol. 2004;21(2):53–56. [DOI] [PubMed] [Google Scholar]
- 22. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 23. Thomas A, Tanaka M, Trepel J, Reinhold WC, Rajapakse VN, Pommier Y. Temozolomide in the era of precision medicine. Cancer Res. 2017;77(4):823–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 25. Hirst TC, Vesterinen HM, Sena ES, Egan KJ, Macleod MR, Whittle IR. Systematic review and meta-analysis of temozolomide in animal models of glioma: was clinical efficacy predicted? Br J Cancer. 2013;108(1):64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carlson BL, Pokorny JL, Schroeder MA, Sarkaria JN. Establishment, maintenance and in vitro and in vivo applications of primary human glioblastoma multiforme (GBM) xenograft models for translational biology studies and drug discovery. Curr Protoc Pharmacol. 2011;Chapter 14:Unit 14 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li L, Chakraborty S, Yang CR, et al. An EGFR wild type-EGFRvIII-HB-EGF feed-forward loop regulates the activation of EGFRvIII. Oncogene. 2014;33(33):4253–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lethaby A, Lopez-Olivo MA, Maxwell L, Burls A, Tugwell P, Wells GA. Etanercept for the treatment of rheumatoid arthritis. Cochrane Database Syst Rev. 2013;5:CD004525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Groves MD, Puduvalli VK, Chang SM, et al. A North American brain tumor consortium (NABTC 99-04) phase II trial of temozolomide plus thalidomide for recurrent glioblastoma multiforme. J Neurooncol. 2007;81(3):271–277. [DOI] [PubMed] [Google Scholar]
- 30. Deng L, Ding W, Granstein RD. Thalidomide inhibits tumor necrosis factor-alpha production and antigen presentation by Langerhans cells. J Invest Dermatol. 2003;121(5):1060–1065. [DOI] [PubMed] [Google Scholar]
- 31. Tweedie D, Sambamurti K, Greig NH. TNF-alpha inhibition as a treatment strategy for neurodegenerative disorders: new drug candidates and targets. Curr Alzheimer Res. 2007;4(4):378–385. [DOI] [PubMed] [Google Scholar]
- 32. Gil Del Alcazar CR, Todorova PK, Habib AA, Mukherjee B, Burma S. Augmented HR repair mediates acquired temozolomide resistance in glioblastoma. Mol Cancer Res. 2016;14(10):928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Costa DB, Halmos B, Kumar A, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007;4(10):1669–1679; discussion 1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van den Bent MJ, Gao Y, Kerkhof M, et al. Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro Oncol. 2015;17(7):935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cioca A, Olteanu EG, Gisca MD, Morosanu CO, Marin I, Florian IS. Expression of EGFR in paired new and recurrent glioblastomas. Asian Pac J Cancer Prev. 2016;17(9):4205–4208. [PubMed] [Google Scholar]
- 36. Clarke MJ, Mulligan EA, Grogan PT, et al. Effective sensitization of temozolomide by ABT-888 is lost with development of temozolomide resistance in glioblastoma xenograft lines. Mol Cancer Ther. 2009;8(2):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun C, Bernards R. Feedback and redundancy in receptor tyrosine kinase signaling: relevance to cancer therapies. Trends Biochem Sci. 2014; 39(10):465–474. [DOI] [PubMed] [Google Scholar]
- 38. Banks WA, Plotkin SR, Kastin AJ. Permeability of the blood-brain barrier to soluble cytokine receptors. Neuroimmunomodulation. 1995;2(3):161–165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.