Abstract
Background
Diagnostic accuracy in previous studies of O-(2-[18F]-fluoroethyl)-L-tyrosine (18F-FET) PET in patients with suspected recurrent glioma may be influenced by prolonged dynamic PET acquisitions, heterogeneous populations, different non–standard-of-care therapies, and PET scans performed at different time points post radiotherapy. We investigated the diagnostic accuracy of a 20-minute 18F-FET PET scan in MRI-suspected recurrent glioblastoma 6 months after standard radiotherapy and its ability to prognosticate overall survival (OS).
Methods
In total, 146 glioblastoma patients with 168 18F-FET PET scans were reviewed retrospectively. Patients with MRI responses to bevacizumab or undergoing re-irradiation or immunotherapy after 18F-FET PET were excluded. Maximum and mean tumor-to-background ratios (TBRmax, TBRmean) and biological tumor volume (BTV) were recorded and verified by histopathology or clinical/radiological follow-up. Thresholds of 18F-FET parameters were determined by receiver operating characteristic (ROC) analysis. Prognostic factors were investigated in Cox proportional hazards models.
Results
Surgery was performed after 104 18F-FET PET scans, while clinical/radiological surveillance was used following 64, identifying 152 glioblastoma recurrences and 16 posttreatment changes. ROC analysis yielded thresholds of 2.0 for TBRmax, 1.8 for TBRmean, and 0.55 cm3 for BTV in differentiating recurrent glioblastoma from posttreatment changes with the best performance of TBRmax (sensitivity 99%, specificity 94%; P < 0.0001) followed by BTV (sensitivity 98%, specificity 94%; P < 0.0001). Using these thresholds, 166 18F-FET PET scans were correctly classified. Increasing BTV was associated with shorter OS (P < 0.0001).
Conclusion
A 20-minute 18F-FET PET scan is a powerful tool to distinguish posttreatment changes from recurrent glioblastoma 6-month postradiotherapy, and predicts OS.
Keywords: glioblastoma, 18F-FET PET, tumor recurrence, posttreatment changes
Key Points.
TBRmax is a powerful imaging biomarker to detect recurrent glioblastoma.
BTV is independently and inversely correlated with overall survival.
Importance of the Study.
Results from published literature regarding the diagnostic accuracy of 18F-FET PET for distinguishing glioma recurrence from posttreatment changes vary substantially due to inclusion of mixed patient populations with different tumor histopathology/grades, different treatment modalities, and 18F-FET PET being performed at different time points postradiotherapy also within 6 months, which may increase the risk of including patients with pseudoprogression. This study investigated the issue in a large group of glioblastoma patients with MRI-suspected recurrent disease later than 6 months after standard radiotherapy and evaluated the prognostic value of 18F-FET. The results show that 18F-FET PET serves as an excellent imaging biomarker for identification of recurrent glioblastoma with a diagnostic accuracy of 99%, and particularly increasing tumor burden is a strong predictor for OS. Supplemental 18F-FET PET in cases of ambiguous MRI findings is advantageous, especially in cases of recurrent glioblastoma, providing neuro-oncologists and neurosurgeons with a longer time window for subsequent treatment management.
Glioblastoma is the most malignant brain tumor in adults, with an annual incidence of approximately 6 per 100 000 individuals worldwide.1 Despite standard-of-care treatment comprising maximal tumor resection followed by radiation and chemotherapy, glioblastoma remains incurable, with a median survival of about 14 months.2,3 Magnetic resonance imaging (MRI) with gadolinium contrast is the standard surveillance tool in the setting of posttreatment care. However, distinguishing glioblastoma recurrence from posttreatment changes using conventional MRI remains diagnostically challenging, as both entities occur frequently at the primary site and often share similar characteristics such as nonspecific contrast enhancement and vasogenic edema following mass effect due to blood–brain barrier (BBB) breakdown.4 The ability to differentiate recurrent disease from posttreatment changes is pivotal, since these two entities differ significantly in subsequent treatment management and prognosis. Posttreatment changes often resolve spontaneously or after administration of corticosteroids, but the inability to identify such changes may lead to unfavorable and harmful premature interruption of an efficacious standard or experimental treatment, thus introducing subsequent errors in response assessment in clinical routine and trials, respectively.5 Conversely, the reliable detection of recurrent disease at an early stage is crucial to optimize the treatment strategy in the individual patient and thus improve prognosis and overall survival (OS). Finally, considerable morbidity is associated with surgical interventions in order to obtain tissue sampling, which remains the gold standard for verification of recurrent or progressive disease. Hence, non-invasive imaging biomarkers that reliably differentiate residual or recurrent disease from posttreatment changes and provide timely opportunity to select alternative treatments are therefore warranted.
Gliomas overexpress a variety of L-amino acid transporters (LATs), and thus, O-(2-[18F]-fluoroethyl)-L-tyrosine (18F-FET) as a LAT substrate is one of the increasingly used PET amino acid tracers for imaging brain tumors. Unlike 2-[18F]-fluoro-2-deoxy-D-glucose (18F-FDG), 18F-FET exhibits high uptake in brain tumors and low uptake in healthy brain tissue, resulting in high contrast between malignant and normal brain tissue. 18F-FET PET has already shown a proven utility in treatment planning, identification of glioma in newly diagnosed cerebral lesions, and detection of malignant transformation in low-grade gliomas, and is recommended at every stage of management of brain tumors in the recently published joint professional practice guidelines for PET image, acquisition, interpretation, and clinical use.6–8 Increasing clinical evidence suggests that 18F-FET PET could overcome the diagnostic dilemma in cases of ambiguous MRI findings, since 18F-FET uptake is independent of BBB disruption, as opposed to contrast enhancement on MRI.9,10
Several studies have evaluated the diagnostic role of 18F-FET for differentiation between recurrent glioblastoma and posttreatment changes.11–17 The reported diagnostic accuracies are generally above 90% but are possibly influenced by the composition of the study population, with different histologic glioma types and malignancy grades; lack of subsequent histologic confirmation; additional dynamic PET metrics; inclusion of nonstandard treatment modalities; and/or differences in data processing, which may all affect the generalizability of results and the transfer into clinical practice. Furthermore, some studies include 18F-FET PET scans performed at a wide range of time points, also within 6-month postradiotherapy where approximately 30% of MRI scans with suspected disease progression may represent pseudoprogression,17–19 which is a quite different pathological phenomenon than late posttreatment changes.4,20–22 The objectives of this retrospective study were to evaluate the diagnostic accuracy of a 20-minute static 18F-FET PET for differentiating recurrent glioblastoma from posttreatment changes later than 6 months after the completion of standard radiotherapy, and its ability to prognosticate OS.
Materials and Methods
Through a computerized database established in 2011 prospectively including all 18F-FET PET scans performed at our institution (Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark), we identified all adult patients with glioblastoma who had undergone 18F-FET PET for evaluation of MRI-suspected disease recurrence between November 2011 and March 2019. The Danish Patient Safety Authority approved the retrospective review of imaging and clinical data from patients (reference no. 3-3013-1957/1). Medical records contained oral or written informed consent prior to the 18F-FET PET assessment from all patients as a part of their clinical management.
Patient Selection Criteria
Study inclusion criteria included (i) histologically proven glioblastoma World Health Organization (WHO) grade IV; (ii) previous tumor resection or stereotactic biopsy followed by standard-of-care oncological treatment consisting of first-line radiotherapy or radiochemotherapy or second-line chemotherapy at first or subsequent recurrences; (iii) progressive or new contrast-enhancing lesion(s) on T1 or non-enhancing lesion(s) on T2/fluid-attenuated inversion recovery (FLAIR) MRI later than 6 months after radiotherapy, where the distinction between disease recurrence and posttreatment changes was uncertain; (iv) 20-minute static 18F-FET PET for supplementary assessment; and (v) histologic verification by tumor re-resection or stereotactic biopsy within 3 months after 18F-FET PET, or clinical/radiological follow-up up to 6 months after 18F-FET PET.
Subsequent disease courses were recorded only if the time interval between disease courses was at least 3 months. A disease course was defined as a period starting from the time of MRI-suspected disease recurrence and supplemental 18F-FET PET followed by a subsequent intervention comprising surgical resection with or without chemotherapy, or chemotherapy alone with MRI surveillance.
Figure 1 provides a complete overview of included patient cases, as well as excluded cases.
Fig. 1.
Inclusion and exclusion criteria for patients referred for18F-FET PET in the time period of November 2011 until March 2019.
Standard of Care
Information regarding standard-of-care therapy according to Danish national guidelines is available in the Supplementary Material.
MRI Protocol
MRI protocol was identical over the study course from 2011 to 2019. The contrast agent was gradually altered from Mulihance (Bracco Imaging) 0.1 mmol/kg body weight to Gadovist (Bayer) 0.1 mmol/kg body weight during the period. The postcontrast protocol was a sagittal T1-weighted MPRAGE (magnetization-prepared rapid acquisition with gradient echo), 1 mm slice thickness with 3 mm reconstructions in the axial and coronal plane, performed on a 1.5 or 3.0T MRI scanner with a standard head coil.
PET Imaging
The acquisition and reconstruction protocols have been previously published in detail.7,23 Briefly, 18F-FET PET scans were performed as static PET acquisitions obtained 20 minutes after injection of ~200 MBq of 18F-FET and evaluated and co-registered to postcontrast T1-weighted and T2/FLAIR-weighted MRI. Maximal and mean tracer uptake activity for tumor-to-background ratio (TBRmax, TBRmean) were calculated as the maximum and mean tissue activity concentration (kilobecquerel per milliliter [kBq/mL]) in the tumor divided by the mean activity concentration in a normal-appearing cortical region in the contralateral hemisphere. Biological tumor volume (BTV), in cubic centimeters (cm3), was defined as tumor activity concentration ≥1.6 mean background activity concentration according to the current international guidelines.8 The image analyses were performed consecutively and thus blinded to follow-up histopathology and MRI findings.
Diagnosis of Primary and Recurrent Glioblastoma or Postoperative Changes by Histopathology
All glioblastomas were originally histologically classified as WHO grade IV according to the WHO 2007 or 2016 classification of tumors of the central nervous system.24,25 Isocitrate dehydrogenase 1 (IDH1) immunostaining had been performed since 2010.26O-6-methylguanine-DNA-methyltransferase (MGMT) was determined by immunohistochemistry until 2014, and thereafter gradually replaced by methylation-specific polymerase chain reaction (PCR) with a threshold set at 9%. To ensure uniformity, the tumor tissue was considered as negative for methylation with stained tumor cell nuclei, and vice versa.27 During data collection, all but 6 tumor tissue blocks prior to 2016 were available for reevaluation and verified to ensure that the glioblastoma diagnosis was in accordance with the WHO 2016 classification. As the exclusion of the last 6 patients did not change the final results below, they remained in the analysis.
Recurrent glioblastoma was diagnosed as significant amount of tumor cells in areas of high cellularity that was similar to primary glioblastoma prior to treatment. Posttreatment changes were defined as necrotizing treatment effects with the complete absence or an insignificant amount of viable tumor cells. In cases of mixed histopathology with glioblastoma tissue and posttreatment changes, the results were reclassified based on the presence or absence of significant amount of tumor tissue by a board-certified neuropathologist (H.B.). All pathologies were interpreted blinded to the 18F-FET PET results.
Diagnosis of Preliminary and Confirmed Glioblastoma Recurrence or Posttreatment Changes by MRI
Identification of preliminary disease recurrence/progression (pre-PET MRI) was based on modified Response Assessment in Neuro-Oncology (RANO) criteria as a ≥25% increase in size of the existing contrast-enhancing lesion or an appearance of any new contrast-enhancing lesion of at least 10 mm of 2 perpendicular dimensions on postcontrast T1-weighted MRI, or a significant increase in the non-contrast-enhancing lesion on T2/FLAIR-weighted MRI.21 Nonmeasurable lesions (<10 mm of at least 1 perpendicular diameter) with a punctate configuration (eg, at the surgical cavity) were interpreted as preliminary disease recurrence/progression as well, but not all lesions necessarily required a subsequent confirmatory MRI exhibiting further enlargement, as suggested by modified RANO criteria, prior to 18F-FET PET assessment.21
Follow-up MRI (post-PET MRI) as a reference for confirmed glioblastoma recurrence/progression or posttreatment changes was performed at least 2 weeks after 18F-FET PET in cases without subsequent histopathology. Progression was assessed as lesion-based and defined by sustained growth of contrast- and non-contrast-enhancing lesions, despite corticosteroid increase and/or ongoing chemotherapy.21 Nonmeasurable lesions still below 10 mm regardless of preceding 18F-FET uptake were not interpreted as a confirmed disease progression/recurrence, but necessitated a closer imaging surveillance. Posttreatment changes were defined by stabilization or regression of previously contrast- and non-contrast-enhancing lesions without initiation or change in therapy with at least 6 months follow-up to minimize the possibility of misclassification. Finally, clinical deterioration attributed to tumor growth that prevented follow-up MRI was interpreted as disease progression as well.21
Survival
OS was measured from the date of 18F-FET PET until the date of death or last follow-up in which the patient was recorded alive (date for last status update: March 30, 2019).
Statistical Analysis
Data analysis was performed with the statistical software package SPSS v25 (IBM). Continuous data were presented as median values with ranges. Receiver operating characteristic (ROC) curves were calculated for TBRmax, TBRmean, and BTV with the histologic verification or clinical/radiological follow-up as a reference, separately and combined, in order to determine the optimal threshold of parameters. For each parameter, the threshold that resulted in the highest sum of sensitivity and specificity was considered optimal for differentiation between tumor recurrence and posttreatment changes. Using these thresholds, 18F-FET PET was classified as true-positive/negative or false-positive/negative according to concordance or discordance with final diagnosis at histopathology or clinical/radiological follow-up. The corresponding area under the curve was calculated as well. The nonparametric Mann–Whitney U-test was used to assess differences between patient groups. Univariate and multivariate Cox proportional hazards models with the enter method were applied to test the relationship of OS with 18F-FET parameters and prognostic factors, including age, sex, MGMT methylation, IDH1 mutation, performance status (PS), corticosteroid use, extent of previous tumor resection, and second-line chemotherapy. Corresponding hazard ratios (HRs) were provided with 95% confidence intervals (CIs). For variables with skewed distribution with or without 0-values, a log2 (x + 1 or x) transformation was used to obtain normal distribution, and the log-transformed data were used subsequently. A Kaplan–Meier plot was generated to obtain survival estimates using the log-rank test. All statistical tests were performed 2-sided with a significance level less than 0.05.
Results
Patient Population
The inclusion criteria were met by 146 patients (96 males, 50 females), of median age of 59.5 years (range, 21–80 y), with a total of 168 disease courses with 18F-FET PET scans. Eight patients (5.5%) had undergone radiotherapy as monotherapy up to 60 Gy after primary surgical resection, while the rest of the patients (94.5%) had received radiotherapy with concurrent and adjuvant temozolomide (Stupp). Sixty patients (41.1%) had already experienced disease recurrence, and had been exposed to second-line chemotherapy (Supplementary Table 1). Patients were divided into 4 subgroups dependent on first-line or second-line treatment at the time of 18F-FET PET assessment and according to diagnostic reference (histopathology or MRI follow-up). Clinical and imaging data are presented in Table 1.
Table 1.
Patient characteristics in the groups with subsequent histopathology, clinical/radiological follow-up, and first-line and second-line treatment
| Variables | Histopathology | Clinical/MRI Follow-up | P-value | First-line Treatment | Second-line Treatment | P-value |
|---|---|---|---|---|---|---|
| N | 104 | 64 | – | 108 | 60 | – |
| Age, y, median (range) | 60 (29–80) | 59 (21–80) | 0.654 | 60 (24–80) | 59 (21–78) | 0.988 |
| Sex, n.(%) | ||||||
| Male | 69 (66) | 39 (61) | 0.479 | 69 (64) | 39 (65) | 0.886 |
| Female | 35 (34) | 25 (39) | 39 (36) | 21 (35) | ||
| Tumor location, n (%) | ||||||
| * Peripheral | 103 (99) | 55 (86) | 0.001 | 103 (95) | 55 (92) | 0.332 |
| ** Central | 1 (1) | 9 (14) | 5 (5) | 5 (8) | ||
| Previous tumor resection, n (%) | ||||||
| Biopsy/partial resection | 14 (13) | 19 (30) | 0.010 | 18 (17) | 15 (25) | 0.194 |
| Subtotal/total resection | 90 (87) | 45 (70) | 90 (83) | 45 (75) | ||
| Prognostic histologic factors, n (%) | ||||||
| MGMT methylation | 52 (50) | 28 (44) | 0.766 | 57 (53) | 23 (38) | 0.229 |
| IDH1 mutation | 9 (9) | 9 (14) | 0.240 | 11 (10) | 7 (12) | 0.700 |
| Corticosteroid intake, n (%) | ||||||
| Dosage ≤10 mg | 76 (73) | 37 (58) | 0.041 | 76 (70) | 37 (62) | 0.251 |
| Dosage >10 mg | 28 (27) | 27 (42) | 32 (30) | 23 (38) | ||
| 18F-FET parameters, median (range) | ||||||
| TBRmax | 3.3 (1.4–5.6) | 2.7 (0.6–6.2) | 0.001 | 3.0 (0.6–6.2) | 3.1 (1.5–5.6) | 0.226 |
| TBRmean | 2.0 (1.2–2.7) | 1.9 (0.6–2.5) | <0.0001 | 2.0 (0.6–2.7) | 2.0 (1.5–2.7) | 0.326 |
| BTV (cm3) | 10.9 (0.0–181) | 16 (0.0–147) | 0.765 | 9.5 (0.0–107) | 19.6 (0.0–181) | 0.005 |
| Performance status, n (%) | ||||||
| 0–1 | 100 (96) | 53 (83) | 0.003 | 101 (94) | 52 (87) | 0.137 |
| 2–4 | 4 (4) | 11 (17) | 7 (7) | 8 (13) | ||
| Subsequent tumor management, n (%) | ||||||
| Biopsy/re-resection | – | – | <0.0001 | 76 (73) | 25 (42) | <0.0001 |
| Follow-up MRI | – | – | 29 (27) | 35 (58) | ||
| Second-line chemotherapy | 66 (64) | 25 (39) | – | – |
* Peripheral locations: frontal, parietal, temporal, and occipital.
** Central locations: butterfly configuration, basal ganglia, thalamus, and brainstem.
Radiological Findings at the Time of Suspected Disease Recurrence
At the time of suspected disease recurrence, a total of 134 MRI scans demonstrated new or progressive measurable lesions (79.8%), while 34 MRI scans showed new nonmeasurable lesions (20.2%) with a median size of 7 mm (range, 4–9 mm). Supplemental 18F-FET PET scans demonstrated increased metabolic activity (TBRmax ≥ 1.6) in 126 MRI scans with measurable lesions (94%) and in all MRI scans with nonmeasurable lesions (100%). There was no significant difference with regard to 18F-FET uptakes between measurable and nonmeasurable lesions (TBRmax, 3.0 vs 3.2; TBRmean, 2.0 vs 2.0; and BTV, 12.0 cm3 vs 10.5 cm3; P > 0.05), respectively. Detailed radiological course at the time of suspected disease progression and subsequent management is illustrated in Supplementary Figure 1.
Patient Management Following 18F-FET PET
Subsequent surgical interventions were performed in 105 (62.5%) of the disease courses, of which histopathology showed recurrent glioblastoma in 102 cases (97.1%) and posttreatment changes in 3 cases (1.8%). However, a strong suspicion of sampling error was raised in one of the cases with posttreatment changes, and thus the patient was instead evaluated by clinical/radiological follow-up (Fig. 2). In addition to this case, 63 disease courses (37.5%) were evaluated with clinical/radiological follow-up, where 29 MRI scans (45.3%) were classified as glioblastoma recurrence and 14 (21.9%) as posttreatment changes. In the remaining 21 disease courses (32.8%), follow-up MRI scans were not available because of severe clinical deterioration among patients following 18F-FET PET, interpreted as disease progression. 18F-FET parameters were significantly higher in patients with recurrent glioblastoma compared with patients with posttreatment changes (TBRmax, 3.2 vs 1.6; TBRmean, 2.0 vs 1.6; and BTV, 14.8 cm3 vs 0.01 cm3; P < 0.0001) (Table 2).
Fig. 2.
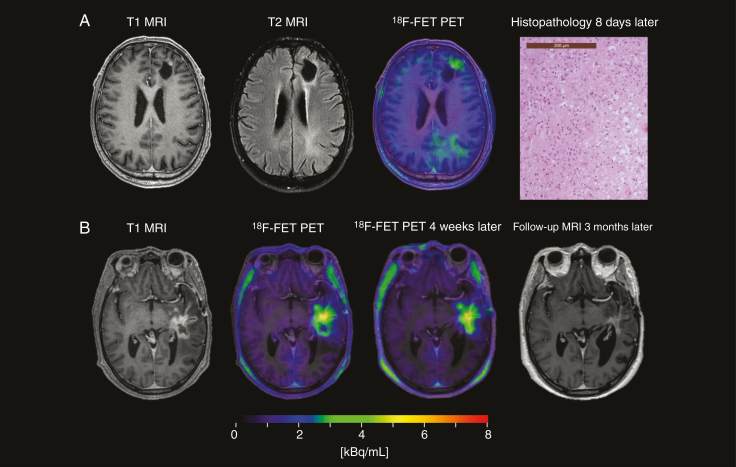
Case study: Suspected sampling error. A 62-year-old patient with glioblastoma, treated with subtotal tumor resection (STR) and Stupp. Eight months after radiotherapy, MRI showed a mixed response with regression of the contrast-enhancing necrotic tumor process (shown) and increased T2/FLAIR changes (not shown) in the left-sided frontal lobe, overall suggesting tumor progression.18F-FET PET showed marked uptake in the left orbitofrontal region, indicating tumor recurrence with TBRmax of 2.7 and BTV of 29 cm3. Patient underwent an STR a month later with a histopathology revealing predominantly reactive changes and only insignificant amount of glioblastoma tissue. Patient had an unfavorable outcome with clinical progression only 2 months and death 6.5 months after18F-FET PET. (Left) Preoperative MRI, (middle)18F-FET PET, and (right) postoperative MRI. As brain shift was insignificant, postoperative MRI allowed the resected tissue area to be delineated (red border) and projected to preoperative PET (with previous resection delineated with yellow/orange border) to estimate the volume of active tissue resected, which was below 5%, and might not have been in the tissue specimen at histopathological examination.
Table 2.
ROC analyses for18F-FET PET following histopathology and 6-month follow-up MRI, for first- and second-line treated patient groups, and for all cases
| FET Parameters | Histology, n = 104 | Clinical/MRI Follow-up, n = 64 | First-line Treatment, n = 108 | Second-line Treatment, n = 60 | All cases, n = 168 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TBRmax | TBRmean | BTV | TBRmax | TBRmean | BTV | TBRmax | TBRmean | BTV | TBRmax | TBRmean | BTV | TBRmax | TBRmean | BTV | |
| Recurrent glioblastoma, median (range) |
3.3 (1.9–5.6) |
2.0 (1.7–2.7) |
11.3 (0.2–181) |
3.0 (2.0–6.2) |
1.9 (1.6–2.5) |
20.5 (1.4–147) |
3.2 (1.9–6.2) |
2.0 (1.6–2.7) |
11.5 (0.2–107) |
3.1 (2.0–5.6) |
2.0 (1.8–2.7) |
19.8 (1.4–181) |
3.2 (1.9–6.2) |
2.0 (1.6–2.7) |
14.8 (0.2–181) |
| Posttreatment changes, median (range) |
1.6 (1.4–1.7) |
1.4 (1.2–1.6) |
0.05 (0.0–0.10) |
1.6 (0.6–3.1) |
1.6 (0.6–1.9) |
0.01 (0.0–28.0) |
1.6 (0.6–3.1) |
1.6 (0.6–1.9) |
0.01 (0.0–28.0) |
1.7 (1.5–1.9) |
1.6 (1.5–1.7) |
0.2 (0.0–0.4) |
1.6 (0.6–3.1) |
1.6 (0.6–1.9) |
0.01 (0.0–28.0) |
| Threshold | 1.8 | 1.7 | 0.15 | 2.0 | 1.8 | 0.95 | 1.8 | 1.8 | 0.55 | 2.0 | 1.8 | 0.90 | 2.0 | 1.8 | 0.55 |
| Sensitivity | 100% | 100% | 100% | 100% | 96% | 100% | 100% | 94% | 97% | 100% | 98% | 100% | 99% | 96% | 98% |
| Specificity | 100% | 100% | 100% | 93% | 93% | 93% | 93% | 93% | 93% | 100% | 100% | 100% | 94% | 94% | 94% |
| PPV | 100% | 100% | 100% | 98% | 98% | 98% | 99% | 99% | 99% | 100% | 100% | 100% | 99% | 99% | 99% |
| NPV | 100% | 100% | 100% | 100% | 87% | 100% | 100% | 68% | 81% | 100% | 100% | 100% | 94% | 71% | 83% |
| AUC | 1.000 | 1.000 | 1.000 | .954 | .962 | .958 | .966 | .973 | .945 | 1.000 | 1.000 | 1.000 | .970 | .976 | .955 |
| Accuracy | 100% | 100% | 100% | 98% | 95% | 98% | 99% | 94% | 96% | 100% | 100% | 100% | 99% | 96% | 98% |
| P-value | 0.016 | 0.016 | 0.016 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.017 | 0.017 | 0.017 | <0.0001 | <0.0001 | < .0001 |
Abbreviations: PPV = positive predictive value; NPV = negative predictive value; AUC = area under the curve.
Diagnostic Accuracy of 18F-FET PET
ROC analysis yielded the optimal thresholds of 2.0 for TBRmax, 1.8 for TBRmean, and 0.55 cm3 for BTV for differentiation between posttreatment changes and recurrent glioblastoma with the best performance of TBRmax (sensitivity 99%, specificity 94%, accuracy 99%; P < 0.0001) followed by BTV (sensitivity 98%, specificity 94%, accuracy 98%; P < 0.0001) (Table 2). Using a threshold of 2.0 for TBRmax, PET-based classifications of recurrent glioblastoma or posttreatment changes were confirmed in 166 cases (98.8%) (151 true-positive and 15 true-negative), while 2 18F-FET PET scans (1.2%) were false-positive and false-negative, verified by subsequent follow-up MRI and histopathology, respectively (Fig. 3).
Fig. 3.
False-negative and false-positive18F-FET PET scans. (A) A 62-year-old patient with a left-sided frontal glioblastoma, treated with gross tumor resection and Stupp. Eleven months after adjuvant TMZ, MRI showed a nonmeasurable solitary contrast enhancement at the anterior part of the surgical cavity, identified by 18F-FET PET with TBRmax of 1.9 and BTV of 0.5 cm3. A suspicion of disease recurrence was raised due to the nodular configuration. Subsequent histopathology showed recurrent glioblastoma (magnification x200). (B) A 48-year-old patient with a left-sided temporal glioblastoma, treated with subtotal tumor resection and Stupp, and re-resection 5 months later with histopathological findings of posttreatment changes. Nine months after radiotherapy, MRI demonstrated progressive contrast-enhancing lesion near the surgical cavity. Two consecutive 18F-FET PET with a 4-week window showed increased uptakes, with TBRmax of 3.1 and 3.5, respectively. Three months later, a spontaneous regression was observed.
Overall Survival and Prognostic Factors
Median follow-up time after 18F-FET PET was 6 months (range, 1–71.5 mo). During follow-up, 124 deaths (84.9%) occurred. In univariate analyses, increasing TBRmax (HR 1.328, 95% CI: 1.116–1.582; P = 0.001), increasing log BTV (HR 1.303, 95% CI: 1.179–1.439; P < 0.0001), corticosteroid use ≥10 mg per day (HR 3.202, 95% CI: 2.158–4.751; P < 0.0001), and PS >1 (HR 1.856, 95% CI: 1.060–3.249; P = 0.030) were significantly associated with a shorter OS, while subsequent second-line chemotherapy (HR 0.650, 95% CI: 0.450–0.938; P = 0.021) showed a significant association with a longer OS. Older age (HR 1.009, 95% CI: 0.993–1.026; P = 0.253), sex (HR 1.113, 95% CI: 0.772–1.606; P = 0.567), extent of previous tumor resection (HR 0.767, 95% CI: 0.505–1.165; P = 0.214), MGMT methylation (HR 0.699, 95% CI: 0.479–1.019; P = 0.063), and IDH1 mutation (HR 0.979, 95% CI: 0.525–1.824; P = 0.947) did not show any significant association with OS. In multivariate analysis, the factors that remained significantly and independently associated with OS were log BTV (HR 1.339, 95% CI: 1.196–1.498; P < 0.0001), corticosteroid use (HR 2.035, 95% CI: 1.321–3.134; P = 0.001), and subsequent second-line chemotherapy (HR 0.448, 95% CI: 0.295–0.681; P < 0.0001), while TBRmax was unable to predict OS (HR 1.095, 95% CI: 0.821–1.459; P = 0.537). When investigating the subgroup with recurrent glioblastoma only (n = 130), increasing log BTV (HR 1.185, 95% CI: 1.048–1.340; P = 0.007) remained associated with a shorter OS. Kaplan–Meier curves are shown in Figure 4.
Fig. 4.
Kaplan–Meier plot of OS. Kaplan–Meier survival analysis plot for prediction of OS, stratified by TBRmax and BTV with thresholds set at 2.0 and 0.55 cm3, respectively.
Discussion
To the best of our knowledge, this is the largest study to date to systematically evaluate the diagnostic value of 18F-FET PET limited to investigation of suspected recurrent glioblastoma later than 6 months after conventional radiotherapy. We demonstrated that 18F-FET PET was able to reliably distinguish tumor recurrence from late posttreatment changes with a discriminatory accuracy of up to 99%. Moreover, 18F-FET PET, especially BTV, was highly prognostic of OS, as it was independently and inversely associated with OS.
The published literature is scarce investigating the diagnostic accuracy of 18F-FET PET for distinguishing tumor recurrence from late posttreatment changes based on the abovementioned rigid criteria. The previous studies include patient populations with a variety of WHO grades (II–IV) with histopathology of both astroglial and oligodendroglial origin and with the largest glioblastoma subpopulation of only 49 patients.11–17 The expressed morphology and pathophysiology of these gliomas are influenced by the underlining molecular biomarkers, and thus both the clinical course and the tumor management are different.28,29 In the present study, all patients had undergone conventional radiotherapy, while previous studies have also included patients with non–standard of care such as brachytherapy with 125I-seed implantation and intracavitary radio-immunotherapy12,14 leading to a high local radiation exposure that may modify the imaging expression of posttreatment changes. Secondly, an observational time period of more than 6 months after radiotherapy was chosen to exclude the majority of cases with pseudoprogression. The pathophysiology of pseudoprogression is quite different from late posttreatment changes, and involves an interaction of radiotherapy and temozolomide leading to a toxic BBB breakdown and contrast leakage, while the latter is thought to have an ischemic pathophysiology.20,22 Finally, a 40- to 50-minute dynamic acquisition permits evaluation of the dynamic activity uptake time course of the tumor. One study reported a significant increase in diagnostic sensitivity from 68% using 20-minute static metrics alone to 93% when including dynamic metrics.15 Similarly, it has been shown that the dynamic imaging metrics are of value in detecting possible malignant transformation of low-grade gliomas.7 In the present study, owing to the focus on a more selective study population, we were able to achieve much higher diagnostic accuracies based on a short 20-minute static PET scan alone, which is an overall more beneficial setup for routine clinical use in terms of scan time, costs, and patient and caregiver convenience.
Two 18F-FET PET scans (1.2%) were false-negative and false-positive, respectively (Fig. 3). None of the patients were receiving any therapy at the time of PET assessment. In the first case of false-negative 18F-FET PET (Fig. 3A), the patient had a nonmeasurable lesion of 6 mm on MRI, which turned out to be purely glioblastoma tissue in the surgical specimen 16 days later. A possible contributing factor could be the partial volume effect on a small BTV of less than 0.5 cm3 reducing the metabolic activity below the diagnostic threshold. However, the majority of patients with nonmeasurable lesions (91%) had 18F-FET uptake above the calculated thresholds with subsequent histology and follow-up MRI scans confirming disease progression (Supplementary Fig. 1). 18F-FET PET thus allows for earlier identification of progression, presumably because of an increased accuracy in detection of tumor infiltration. In addition, a glioblastoma in its most aggressive forms may arise from no visible disease to devastating progression within a few months, thus reflecting the natural course of the disease when in its most severe form. In the case of false-positive 18F-FET PET (Fig. 3B), increased 18F-FET uptakes were found in 2 consecutive PET scans within a 4-week window preceding a progressive contrast-enhancing lesion on MRI performed 9-month postradiotherapy. The patient was clinically stable and declined surgery, with a spontaneous regression of the lesion on follow-up MRI 3 months later. Only 4 months prior to the first PET scan, the patient had been resected for pseudoprogression, indicating that the inflammatory process was continuing even after the completion of temozolomide. Thus, a recent history of verified posttreatment changes could warrant a conservative approach.
The influence of treatment-related alterations of BBB caused by anti-angiogenic drugs such as bevacizumab on 18F-FET uptake may be a matter of concern.30 Bevacizumab is reported to reduce tumor neovascularization, causing a concomitant decrease in contrast enhancement on MRI in 30–60% of cases that may not always reflect a genuine tumor regression, referred to as pseudoresponse.20,31 Experimental and clinical studies have reported that 18F-FET uptake is highly specific for tumor tissue and mainly explained by increased system LAT expression representing a carrier-mediated facilitated transport in the glioblastoma tissue, and is thus independent of disruption of the BBB.31–3318F-FET PET has been reported to be predictive for treatment failure at an early stage in patients with recurrent high-grade gliomas who received anti-angiogenic treatment, further supporting the potential of tracer.22,34 In the present study, the thresholds for 18F-FET uptake were comparable in both first- and second-line treated groups with diagnostic accuracies above 93%, emphasizing that the semiquantitative metrics of 18F-FET PET are reliable for differential diagnosis regardless of previous or ongoing chemotherapy.
Studies evaluating the prognostic value of 18F-FET PET on OS in cases with suspected disease recurrence/progression are increasing.23,35–37 In the study by Galldiks et al,3518F-FET PET using both static and kinetic metrics predicted OS better than MRI. Niyazi et al36 also demonstrated a significant relationship between kinetic PET metrics and OS in recurrent glioblastoma after re-irradiation. Using only static PET metrics in the present study, increasing TBRmax was unable to predict OS in the multivariate analysis, while a twofold increase in BTV was significantly associated with a ~34% increased risk of death, a slightly higher risk than previously reported in a Danish study with 18F-FET PET performed prior to radiotherapy planning of residual BTV.37 In contrast, prognostic factors for newly diagnosed glioblastoma patients, such as MGMT and IDH1 status, did not show any significant association with OS in the present study, suggesting that these prognostic factors have limited impact in recurrent glioblastoma patients.38
Some notable limitations are worth mentioning. Firstly, there are indications of verification bias in the data. Much clinical emphasis is placed on avoiding resection of posttreatment changes, which were found in only 2 of 104 surgical resections when excluding the case with sampling error. Hence, it is likely that the result of the preceding 18F-FET PET directly influenced the decision to perform surgery. However, both cases with resection of posttreatment changes had 18F-FET uptakes below the calculated threshold of 2.0 (TBRmax of 1.4 and 1.7). Patients with negative or only moderately positive 18F-FET PET were less likely to undergo surgery, as well as patients with poor PS or glioblastomas that were inoperable due to central locations. Thus, it necessitates the inclusion of a broader patient sample in the analysis of diagnostic accuracy of 18F-FET PET by using clinical/radiological follow-up as well, despite the inadequacies of MRI as standard. This reflects the clinical management of these patients, but nevertheless the results should be interpreted with caution. Secondly, the patients with MRI-based radiological response following second-line treatment were excluded, giving rise to concerns about selection bias. This was done because a radiological response cannot discern spontaneous resolution of treatment-related inflammation and treatment effects of a genuine tumor recurrence. Second-line treatment with especially bevacizumab yields high response rates independent of antitumor effects complicating the evaluation of treatment response. The assumption is that tumor recurrence/progression can always be evaluated on MRI even during second-line treatment, while posttreatment changes can only be evaluated without initiation or change in treatment. In pre-statistical analysis, we found no significant differences between responders and nonresponders with regard to clinical/radiological data, as has been reported previously.38 Thus, the exclusion of this patient group has not induced a study bias. Thirdly, the time interval between 18F-FET PET assessment and surgery was set to within 3 months, which may seem a long time considering glioblastoma's tendency to rapid growth. However, only 3 cases were included with the time interval close to 3 months, where 1 case demonstrated TBRmax of 1.7, and histopathology 88 days later revealed posttreatment changes, while 2 other cases had TBRmax ≥ 3.3, and histopathology 89 days later showed recurrent glioblastoma. The fourth, only semiquantitative 18F-FET PET metrics at a single time point were evaluated. In clinical practice, the development in activity uptake over time is an independent supportive feature, which we did not make use of here. However, use of these simplified metrics, even when performed at a single time point, offers a more robust approach, and increases the generalizability of the presented results. Finally, contrary to previous studies, an important strength of this study is the large number of included patients, who are homogeneous with regard to the diagnosis of glioblastoma exclusively, and with subsequent histopathology available in 60% of cases and MRI surveillance up to 6 months following 18F-FET PET.
Conclusion
A 20-minute static 18F-FET PET is a powerful method for differentiating late posttreatment changes from tumor regrowth in patients with suspected recurrent glioblastoma, with a diagnostic accuracy up to 99%, and for predicting OS. However, management of recurrent glioblastoma is complex, and in a few patient cases, 18F-FET PET at a single time point may not always be adequate to guide further tumor management due to a low risk of false-negative or false-positive results. Therefore, the entire disease course, including known prognostic factors, in each individual patient at the time of progression should continue to be considered when determining further patient management.
Funding
This work was supported by a grant from the Danish Cancer Society (R146-A9508-16-S2).
Conflict of interest statement.
The authors declare that they have no conflicts of interest.
Authorship statement.
All authors conceived and designed the study. Asma Bashir and Sofie Mathilde Jacobsen collected the data. Asma Bashir, Otto Mølby Henriksen, and Ian Law wrote the first and subsequent drafts of the manuscript. Asma Bashir conducted the statistical analysis with guidance from a senior statistician. All authors have participated in critical revision and writing of the article. All authors have seen and approved the final version.
Declarations statement.
Portions of this retrospective material have been presented at the Scandinavian Neuro-Oncology (SNOG) Meeting, Trondheim, Norway, on May 4, 2018, and European Association of Neuro-Oncology (EANO) XIII in Stockholm, Sweden, on October 12, 2018. Besides this, the manuscript is not under consideration, in press, or being published elsewhere.
Supplementary Material
References
- 1. Ostrom QT, Gittleman H, Fulop J, et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17Suppl 4:iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weller M, van den Bent M, Tonn JC, et al. ; European Association for Neuro-Oncology (EANO) Task Force on Gliomas European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017;18(6):e315–e329. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 4. Wen PY, Macdonald DR, Reardon DA, et al. . Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 5. Okada H, Weller M, Huang R, et al. . Immunotherapy Response Assessment in Neuro-Oncology: a report of the RANO working group. Lancet Oncol. 2015;16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albert NL, Winkelmann I, Suchorska B, et al. . Early static (18)F-FET-PET scans have a higher accuracy for glioma grading than the standard 20-40 min scans. Eur J Nucl Med Mol Imaging. 2016;43(6):1105–1114. [DOI] [PubMed] [Google Scholar]
- 7. Bashir A, Brennum J, Broholm H, Law I. The diagnostic accuracy of detecting malignant transformation of low-grade glioma using O-(2-[18F]fluoroethyl)-l-tyrosine positron emission tomography: a retrospective study. J Neurosurg. 2018;130(2):451–464. [DOI] [PubMed] [Google Scholar]
- 8. Law I, Albert NL, Arbizu J, et al. . Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46(3):540–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Götz I, Grosu AL. [(18)F]FET-PET imaging for treatment and response monitoring of radiation therapy in malignant glioma patients—a review. Front Oncol. 2013;3:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stegmayr C, Bandelow U, Oliveira D, et al. . Influence of blood-brain barrier permeability on O-(2-18F-fluoroethyl)-L-tyrosine uptake in rat gliomas. Eur J Nucl Med Mol Imaging. 2017;44(3):408–416. [DOI] [PubMed] [Google Scholar]
- 11. Pöpperl G, Götz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K. Value of O-(2-[18F]fluoroethyl)- L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging. 2004;31(11):1464–1470. [DOI] [PubMed] [Google Scholar]
- 12. Rachinger W, Goetz C, Pöpperl G, et al. . Positron emission tomography with O-(2-[18F]fluoroethyl)-L-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57(3):505–511. [DOI] [PubMed] [Google Scholar]
- 13. Pöpperl G, Götz C, Rachinger W, et al. . Serial O-(2-[18F ]fluoroethyl)-L-tyrosine PET for monitoring the effects of intracavitary radioimmunotherapy in patients with malignant glioma. Eur J Nucl Med Mol Imaging. 2006;33(7):792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehrkens JH, Pöpperl G, Rachinger W, et al. . The positive predictive value of O-(2-[18F]fluoroethyl)-L-tyrosine (FET) PET in the diagnosis of a glioma recurrence after multimodal treatment. J Neurooncol. 2008;88(1):27–35. [DOI] [PubMed] [Google Scholar]
- 15. Galldiks N, Stoffels G, Filss C, et al. . The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 2015;17(9):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kebir S, Fimmers R, Galldiks N, et al. . Late pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET. Clin Cancer Res. 2016;22(9):2190–2196. [DOI] [PubMed] [Google Scholar]
- 17. Kebir S, Khurshid Z, Gaertner FC, et al. . Unsupervised consensus cluster analysis of [18F]-fluoroethyl-L-tyrosine positron emission tomography identified textural features for the diagnosis of pseudoprogression in high-grade glioma. Oncotarget. 2017;8(5):8294–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abbasi AW, Westerlaan HE, Holtman GA, Aden KM, van Laar PJ, van der Hoorn A. Incidence of tumour progression and pseudoprogression in high-grade gliomas: a systematic review and meta-analysis. Clin Neuroradiol. 2018;28(3):401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mihovilovic MI, Kertels O, Hänscheid H, et al. . O-(2-(18F)fluoroethyl)-L-tyrosine PET for the differentiation of tumour recurrence from late pseudoprogression in glioblastoma. J Neurol Neurosurg Psychiatry. 2019;90(2):238–239. [DOI] [PubMed] [Google Scholar]
- 20. Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22(6):633–638. [DOI] [PubMed] [Google Scholar]
- 21. Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellingson BM, Chung C, Pope WB, Boxerman JL, Kaufmann TJ. Pseudoprogression, radionecrosis, inflammation or true tumor progression? Challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. J Neurooncol. 2017;134(3):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moller S, Law I, Munck Af Rosenschold P, et al. . Prognostic value of 18F-FET PET imaging in re-irradiation of high-grade glioma: results of a phase I clinical trial. Radiother Oncol. 2016;121(1):132–137. [DOI] [PubMed] [Google Scholar]
- 24. Louis DN, Ohgaki H, Wiestler OD, et al. . The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 26. Capper D, Weissert S, Balss J, et al. . Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010;20(1):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kristensen LS, Michaelsen SR, Dyrbye H, et al. . Assessment of quantitative and allelic MGMT methylation patterns as a prognostic marker in glioblastoma. J Neuropathol Exp Neurol. 2016;75(3):246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Capper D, Jones DTW, Sill M, et al. . DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson BE, Mazor T, Hong C, et al. . Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahluwalia MS, Wen PY. Antiangiogenic therapy for patients with glioblastoma: current challenges in imaging and future directions. Expert Rev Anticancer Ther. 2011;11(5):653–656. [DOI] [PubMed] [Google Scholar]
- 31. Galldiks N, Rapp M, Stoffels G, Dunkl V, Sabel M, Langen KJ. Earlier diagnosis of progressive disease during bevacizumab treatment using O-(2-18F-fluorethyl)-L-tyrosine positron emission tomography in comparison with magnetic resonance imaging. Mol Imaging. 2013;12(5):273–276. [PubMed] [Google Scholar]
- 32. Stegmayr C, Oliveira D, Niemietz N, et al. . Influence of bevacizumab on blood-brain barrier permeability and O-(2-18F-fluoroethyl)-L-tyrosine uptake in rat gliomas. J Nucl Med. 2017;58(5):700–705. [DOI] [PubMed] [Google Scholar]
- 33. Miyagawa T, Oku T, Uehara H, et al. . “Facilitated” amino acid transport is upregulated in brain tumors. J Cereb Blood Flow Metab. 1998;18(5):500–509. [DOI] [PubMed] [Google Scholar]
- 34. Hutterer M, Nowosielski M, Putzer D, et al. . O-(2-18F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med. 2011;52(6):856–864. [DOI] [PubMed] [Google Scholar]
- 35. Galldiks N, Rapp M, Stoffels G, et al. . Response assessment of bevacizumab in patients with recurrent malignant glioma using [18F]fluoroethyl-L-tyrosine PET in comparison to MRI. Eur J Nucl Med Mol Imaging. 2013;40(1):22–33. [DOI] [PubMed] [Google Scholar]
- 36. Niyazi M, Jansen N, Ganswindt U, et al. . Re-irradiation in recurrent malignant glioma: prognostic value of [18F]FET-PET. J Neurooncol. 2012;110(3):389–395. [DOI] [PubMed] [Google Scholar]
- 37. Poulsen SH, Urup T, Grunnet K, et al. . The prognostic value of FET PET at radiotherapy planning in newly diagnosed glioblastoma. Eur J Nucl Med Mol Imaging. 2017;44(3):373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jakobsen JN, Urup T, Grunnet K, et al. . Toxicity and efficacy of lomustine and bevacizumab in recurrent glioblastoma patients. J Neurooncol. 2018;137(2):439–446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.