Abstract
Age-associated cognitive impairments affect an individual’s quality of life and are a growing problem in society. Therefore, therapeutic strategies to treat age-related cognitive decline are needed to enhance the quality of life among the elderly. Activation of the Nr4a family of transcription factors has been closely linked to memory formation and dysregulation of these transcription factors is thought to be associated with age-related cognitive decline. Previously, we have shown that Nr4A transcription can be activated by synthetic bis-indole derived compounds (C-DIM). C-DIM compounds enhance synaptic plasticity and long-term contextual fear memory in young healthy mice. In this study, we show that activation of Nr4a2 by 1,1-bis(3′-Indolyl)-1-(p-chlorophenyl) methane (C-DIM12), enhances long-term spatial memory in young mice and rescues memory deficits in aged mice. These findings suggest that C-DIM activators of Nr4a transcription may be suitable to prevent memory deficits associated with aging.
Keywords: Nr4a, spatial memory, C-DIM drugs, aging
Introduction
Aging is associated with a decline in cognitive ability. These deficits are particularly noticeable in hippocampus-dependent spatial and episodic memory (Gallagher et al., 2010; Wimmer et al., 2012). Studies using rodent models have demonstrated that age-associated cognitive decline may be due to deficits in transcriptional activity within the hippocampus that typically follows learning (Kwapis et al., 2019; Spiegel et al., 2014). Transcription is required for memory consolidation as well as the long-term stability and maintenance of hippocampal memories (Alberini and Kandel, 2014; Peixoto and Abel, 2013). Age-related impairments in transcription may prevent newly learned events from being stored properly as long-term memory and may explain some of the cognitive deficits that accompany aging. The Nr4a family of transcription factors are among the genes whose expression is downregulated in aged mice (Kwapis et al., 2018; Kwapis et al., 2019; Rowe et al., 2007). The Nr4a family of transcription factors consists of three members: Nr4a1/Nur77/HZF-3, Nr4a2/Nurr1/NGFI-B, and Nr4a3/NOR-1/TEC (Maxwell and Muscat, 2006), Nr4a1 and Nr4a2 are critical for hippocampus-dependent long-term memory (Hawk and Abel, 2011; Hawk et al., 2012; McNulty et al., 2012; Pena de Ortiz et al., 2000). Further, restoring the expression level of Nr4a1 or Nr4a2 is sufficient to prevent memory deficits in aged mice (Kwapis et al., 2019).
The members of the Nr4a family are orphan receptors lacking endogenous ligands (Hawk and Abel, 2011), but para-phenyl substituted diindolylmethane analogs—or ‘C-DIM’ compounds have been designed to specifically and selectively activate members of the Nr4a family (Bridi et al., 2017; Hammond et al., 2018; Li et al., 2012). C-DIM5, an activator of Nr4a1, has been found to increase long-term fear memory in young adult mice and enhance hippocampal synaptic plasticity (Bridi et al., 2017). A recently developed Nr4a ligand, 1,1-bis(3′-indolyl)-1-(p-chlorophenyl)methane (C-DIM12/ DIM-C-pPhCl), predominantly activates Nr4a2, but also shows some affinity towards Nr4a1 (Hammond et al., 2018). Although the cognitive enhancing effects of Nr4A activation have been demonstrated in young mice (Bridi et al., 2017), the question remains as to whether the select activation of endogenous Nr4a in aged mice would be sufficient to rescue memory deficits. In this study, we administered the Nr4a2 selective agonist, C-DIM12, via oral gavage to young and aged mice, resulting in enhanced long-term memory in a spatial object recognition task (SOR).
Materials and Methods
Animals:
Mice were maintained under standard conditions consistent with National Institute of Health guidelines and approved by the Institutional Animal Care and Use Committee of the University of Iowa. Mice were individually housed and kept on a 12 h light/12 h dark cycle. Food and water were available ad libitum, and all experiments performed during the light cycle between zeitgeber time (ZT) 0 to 3. Young male C57BL/6J (2–4 months old) were from Jackson Labs and aged male C57BL/6NIA (20–22 months old) were provided by the National Institute of Aging. Male mice were used for all experiments to aid in the interpretation of the results from published literature. Each mouse was handled for 2 minutes and habituated to oral gavage with corn oil in the experimental room for 5 days prior to the training day. Handling and gavage habituation were performed between ZT0 to ZT2.
Drugs:
C-DIM12/ DIM-C-pPhCl was synthesized in Dr. Stephen Safe’s laboratory at Texas A&M University. For behavioral experiments using oral gavage on young mice, C-DIM12 was dissolved in corn oil (Sigma) and administered to a final dose of 10, 35 or 100 mg/kg for dose response analysis. For aged mice, C-DIM12 was prepared at a concentration of 7mg/ml and injected at a dose of 35 mg/kg. The dose and timepoint of C-DIM12 administration was estimated based on previous publication (Hammond et al., 2018).
Spatial object recognition (SOR) task:
To test spatial memory enhancement in young mice, weak SOR learning protocols are typically used which do not result in long-term memory (McQuown and Wood, 2011; Stefanko et al., 2009). On the training day, one hour following C-DIM12 or vehicle administration, we performed the weak training protocol. Training consisted of one three-minute habituation to the open field arena without any objects, followed by three 3-minute training trials where the mice could explore two distinct glass objects in the arena. There was an intersession interval of 3–4 minutes, during which mice were returned to their home cage. Between each trial, objects were wiped with 70% ethanol. Testing was performed either 1 hour or 24 hours after training in the same arena, but with one object displaced to a new location (displaced object, DO), while the other object remained in its previous location (Non displaced object, NDO). As aging is accompanied with cognitive decline, even strong training protocols do not lead to memory in aged mice (Wimmer et al., 2012). Therefore, we used a strong training protocol for drug treatment in aged mice as reported previously by our group (Wimmer et al., 2012). For aged mice, C-DIM12 was delivered once daily via oral gavage for 5 consecutive days including the day of training. Strong training consisted of one 10 minute habituation to the open field arena without objects, followed by three 10 mins sessions with two distinct glass objects. Exploration was recorded during training and testing, and object exploration was scored by an experimenter blind to the treatment groups. The relative exploration time was recorded and expressed by percent discrimination towards the displaced object (percent discrimination=time with DO - time with NDO)/(time with DO + time with NDO) × 100. From the habituation sessions, the open-field arena was quantified as two equal sections, outer edge from the walls (outer zone) and the center square (inner zone). Distance travelled, and time spend in each zone were measured using Ethovision software (Noldus Instruments).
Statistical analysis:
Statistical analyses conducted as indicated in text and figure legends using Prism 8 (Graphpad) were performed using either two-tailed Student’s t-test or two-way ANOVA followed by Sidak’s multiple comparisons post hoc tests. Two-way ANOVAs had factors of Session (Training and Test) and Treatment. Training was analyzed as the mean across all the three training trials. In all cases, differences were considered significant when p ≤ 0.05. Error bars in all figures represent ±SEM.
Results
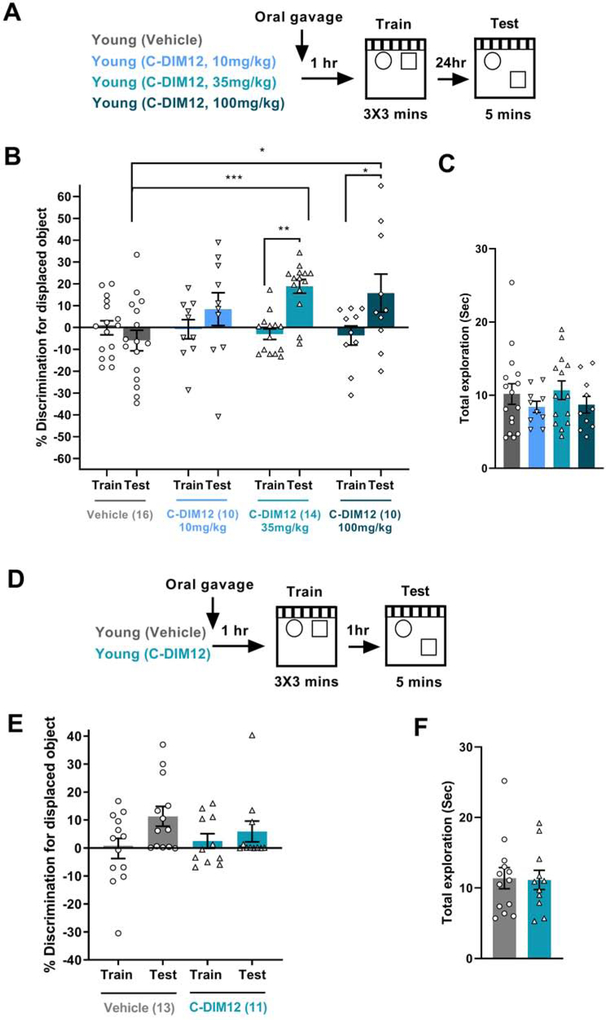
To test whether Nr4a activation by C-DIM12 enhances memory in young adult mice (2 to 4 months old) using a weak SOR protocol, we administered C-DIM12 or vehicle via oral gavage one hour prior to SOR training (Fig 1A). We examined the efficacy of C-DIM12 using a dose response analysis (10 mg/kg, 35 mg/kg or 100 mg/kg) to assess the impact on long-term memory. Vehicle-treated animals failed to show any discrimination towards the DO during the test session compared to the training session (Fig 1B, % discrimination= −0.11 (Training) and −5.93 (Test)) confirming that the weak learning protocol does not result in long-term retention. Mice administered 10 mg/kg C-DIM12 failed to show memory enhancement (Fig 1B, % discrimination= −0.77 (Training) and +8.42 (Test)). However, mice administered 35 mg/kg or 100 mg/kg C-DIM12 showed significant discrimination towards the DO in the test session compared to the training session (Fig 1B, % discrimination with 35 mg/kg C-DIM12= −3.06 (Training) and +18.86 (Test) and % discrimination with 100 mg/kg C-DIM12= −3.66 (Training) and +15.74 (Test)) and showed significant enhancement in long-term memory compared to the vehicle-treated animals (Fig 1B). Importantly, all the groups of mice showed similar exploration towards the objects during the test session (Fig 1C). 35 mg/kg dose of C-DIM12 was selected for all subsequent experiments. We next investigated the effect of Nr4A activation by C-DIM12 on short-term retention. Like the long-term memory experiment, we administered C-DIM12 or vehicle to young adult mice one hour prior to training with the weak learning SOR paradigm. Short-term retention was assessed one hour following the last training session (Fig 1D). C-DIM12 treated mice failed to show memory enhancement during the 1-hour test session compared to the vehicle-treated mice (Fig 1E, % discrimination during test= +11.2 (Vehicle) and +5.9 (C-DIM12)). In conjunction with the long-term memory findings, these results indicate that C-DIM12 selectively enhances long-term memory in young mice. Both groups of animals showed similar exploration of objects during the one hour test session (Fig 1F).
Figure 1. Activation of Nr4a transactivation by C-DIM12 enhances hippocampus-dependent long-term memory in young adult mice.
Preference for the displaced object (DO) in a weak-learning spatial object recognition (SOR) task in young mice receiving either vehicle (n=16) or different doses of C-DIM12 drug (10 mg/kg n=10, 35 mg/kg n=14, and 100 mg/kg n=10) is shown as % discrimination for the displaced object compared to the non-displaced object. (A) Long term memory was assessed 24-hours after initial training, with C-DIM12 or vehicle administered one hour before training. (B) Mice treated with 35 mg/kg and 100 mg/kg C-DIM12 displayed higher % discrimination for the displaced object, while vehicle or 10 mg/kg C-DIM12 treated mice showed no apparent preference (Two way ANOVA: Significant Treatment × Session interaction F(3, 46)= 4.155, p=0.011, Sidak’s post-hoc tests, **p < 0.01 comparing 35 mg/kg C-DIM12 train and test, *p < 0.05comparing 100 mg/kg C-DIM12 train and test, ***p < 0.001 comparing vehicle test and C-DIM12 35 mg/kg test, *p < 0.05 comparing vehicle test and C-DIM12 100 mg/kg test. (C) Total exploration for both objects during the 24 hr test session (D) Assessment of short-term retention following C-DIM12 administration in young mice. (E) Short-term retention assessed 1-hour after the training revealed no significant memory enhancement by C-DIM12 (n=11) compared to vehicle (n=13) treatment. (Two way ANOVA: no significant Treatment × Session interaction, F (1, 22) = 1.784, p=0.1954). (F) Total exploration for both objects during the 1 hr test session (t(22)= t=0.1204, p= 0.9053).
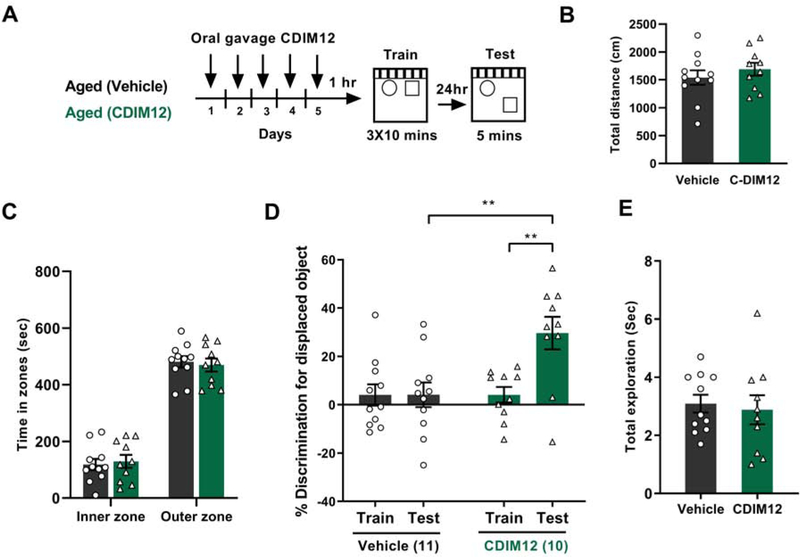
We have shown that aged mice have long-term memory deficits in SOR following a strong training protocol (Wimmer et al., 2012). Therefore, we explored the possibility of memory rescue in aged mice (20–22 months old) by activating Nr4a proteins using C-DIM12. C-DIM12 was administered for 5 consecutive days to have a stronger effect in aged mice, which otherwise might have weaker sensitivity towards the memory enhancing drug due to reduced expression of Nr4a2 (Kwapis et al., 2019). Long-term memory was assessed 24 hours after the last training trial (Fig 2A). Five consecutive days of C-DIM12 administration did not induce any alterations in locomotion or anxiety as evidenced by total distance travelled (Fig 2B) and time spent in the inner or outer zone of the open-field during habituation (Fig 2C). Vehicle-treated aged mice failed to discriminate the displaced object during the test session (Fig 2D, black bars, Mean % discrimination= +4.05 (Training) and +4.1 (Test)), whereas C-DIM12-treated aged mice showed significant long-term memory retention (Fig 2D, green bars, Mean % discrimination= +4.07 (Training) and +29.63 (Test)). Both groups of mice showed equal amounts of exploration during the test session (Fig 2E). This finding indicates that C-DIM12-treated aged mice show significant memory rescue at the 24-hour test compared to vehicle-treated aged mice. C-DIM12 treatment had no impact on short-term memory in aged mice (Supplementary Fig 2A). Both vehicle and C-DIM12 treated mice performed similarly during the one-hour test (Supplementary Fig 2B and 2C).
Figure 2. C-DIM12 mediated activation of Nr4a rescues long-term memory deficits in aged mice.
(A) Strong memory procedure to test memory rescue by C-DIM12 (n=10) compared to vehicle (n=11) in aged mice, with C-DIM12 drug delivery 4 days preceding training and on the training day. (B) Total distance travelled in the open-field during habituation (t(19)=0.8397, p=0.4115). (C) Total time spent in inner and outer zones of the open-field during habituation session (Two-way ANOVA: Treatment × zones interaction F (1, 19) = 0.1406, p=0.7119. (D) Preference for displaced object (DO) in a strong-training SOR task in aged mice receiving either vehicle or C-DIM12 drug is shown as % discrimination for the displaced object compared to the non-displaced object. Long-term memory was assessed 24-hours after initial training, and aged mice in the C-DIM12 treatment group showed greater discrimination for the displaced object, whereas the vehicle treated mice displayed nearly no % discrimination for the displaced object (Two-way ANOVA: Treatment × Sessions interaction F (1, 19) = 6.716, p= 0.0179, Sidak’s post-hoc tests: **p = 0.01 comparing C-DIM12 Vs vehicle during the 24hr test, **p < 0.01 comparing C-DIM12 training Vs C-DIM12 24hr test). (E) Total exploration was similar during the test for both the groups (t (19)= 0.3681, p=0.7168).
Discussion
Our main finding is that C-DIM12 enhances long-term memory, but not short-term memory, in young and aged mice. This result is consistent with our earlier work demonstrating that Nr4a transcription is a critical regulator of long-term memory (Bridi et al., 2017; Hawk et al., 2012). Even though we do not have direct evidence that the memory enhancement by C-DIM drugs are due directly to activation of Nr4a transcription factors in vivo, previous studies show that C-DIM compounds activate Nr4a mediated transcription (Hammond et al., 2018; Hedrick et al., 2019; Li et al., 2012). Electrophysiological studies show that Nr4a dominant negative mouse model have impaired synaptic plasticity, which C-DIM drugs fail to rescue (Bridi and Abel, 2013; Bridi et al., 2017). Notably, C-DIM12 fails to activate Nr4a downstream gene expression following Nr4a2 knockdown in cultured cell lines (Hammond et al., 2015; Inamoto et al., 2008; Li et al., 2012). Additionally, in vitro reporter assays and computational modeling further support the idea that C-DIM12 directly activates Nr4a (Hammond et al., 2018; Inamoto et al., 2008). Finally, C-DIM compounds fail to enhance synaptic plasticity in an Nr4a dominant negative mouse model, which otherwise are impaired in synaptic plasticity.
Age-related memory decline is linked with Nr4a dysregulation (Kwapis et al., 2018; Kwapis et al., 2019), and in humans, aging is accompanied by reduced expression of Nr4a2 (Chu et al., 2002). A recent study showed that overexpression of Nr4a1 or Nr4a2 is sufficient to rescue memory decline in aged mice (Kwapis et al., 2019). Nr4a1 and Nr4a2 dysregulation has also been observed in diseases associated with memory loss including Alzheimer’s disease (Chatterjee et al., 2018; Montarolo et al., 2016; Moon et al., 2015), Parkinson’s disease (Montarolo et al., 2016) and schizophrenia (Buervenich et al., 2000; Corley et al., 2016; Xing et al., 2006). Therefore, Nr4a could be controlling critical pathways during memory consolidation that are otherwise downregulated in cognitive impairment. Previous reports (Kwapis et al., 2019) showed memory rescue by increasing Nr4a using viral approaches, while the pharmacological approach used here to activate Nr4a function offers a novel and non-invasive strategy to treat age-related cognitive deficits. Additionally, pharmacological activation of Nr4a1 may also rescue memory in aged mice as the Nr4a1 activator C-DIM5 enhances memory and synaptic plasticity in young mice (Bridi et al., 2017).
In conclusion, our results show that pharmacological activation of Nr4a can rescue cognitive deficits observed in aged mice. These results are consistent with the literature and indicate that activation of the Nr4a pathway is a potential therapeutic approach to treat age-associated memory decline and cognitive impairment associated with neurodegenerative and neuropsychiatric disorders. As the Nr4a family of transcription factors regulate downstream gene expression following learning, future experiments seeking to identify downstream targets of Nr4a would increase our understanding of the mechanism of memory enhancement by C-DIM related Nr4a ligands. Future studies should seek to investigate the dose response to the C-DIM drug in aged mice to find the optimal dose required to reverse memory deficits as well as extending our study to other hippocampus and non-hippocampus dependent memory tasks.
Supplementary Material
Pharmacological activation of Nr4a using C-DIM drugs enhance long-term memory in young mice
C-DIM drug reverses long-term memory deficits in aged mice
Nr4a could be a potential therapeutic target to treat age-related cognitive impairment.
Acknowledgements
This work was funded by NIH grants P50 AG 017628, RO1 MH 087463 and R01 MH 087463-08S1 to TA and Nellie Ball trust to SC. TA is supported by the Roy J. Carver Charitable Trust. We thank Tania Chatterjee and Joseph Lederman for technical assistance. We also thank the University of Iowa Neural Circuits and Behavior Core for use of the facility.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors declare no competing financial interests.
References
- Alberini CM, and Kandel ER (2014). The regulation of transcription in memory consolidation. Cold Spring Harb Perspect Biol 7, a021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridi MS, and Abel T (2013). The NR4A orphan nuclear receptors mediate transcription-dependent hippocampal synaptic plasticity. Neurobiol Learn Mem 105, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridi MS, Hawk JD, Chatterjee S, Safe S, and Abel T (2017). Pharmacological Activators of the NR4A Nuclear Receptors Enhance LTP in a CREB/CBP-Dependent Manner. Neuropsychopharmacology 42, 1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buervenich S, Carmine A, Arvidsson M, Xiang F, Zhang Z, Sydow O, Jonsson EG, Sedvall GC, Leonard S, Ross RG, et al. (2000). NURR1 mutations in cases of schizophrenia and manic-depressive disorder. Am J Med Genet 96, 808–813. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Cassel R, Schneider-Anthony A, Merienne K, Cosquer B, Tzeplaeff L, Halder Sinha S, Kumar M, Chaturbedy P, Eswaramoorthy M, et al. (2018). Reinstating plasticity and memory in a tauopathy mouse model with an acetyltransferase activator. EMBO Mol Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Kompoliti K, Cochran EJ, Mufson EJ, and Kordower JH (2002). Age-related decreases in Nurr1 immunoreactivity in the human substantia nigra. J Comp Neurol 450, 203–214. [DOI] [PubMed] [Google Scholar]
- Corley SM, Tsai SY, Wilkins MR, and Shannon Weickert C (2016). Transcriptomic Analysis Shows Decreased Cortical Expression of NR4A1, NR4A2 and RXRB in Schizophrenia and Provides Evidence for Nuclear Receptor Dysregulation. PLoS One 11, e0166944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Bakker A, Yassa MA, and Stark CE (2010). Bridging neurocognitive aging and disease modification: targeting functional mechanisms of memory impairment. Curr Alzheimer Res 7, 197–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SL, Popichak KA, Li X, Hunt LG, Richman EH, Damale P, Chong E, Backos DS, Safe S, and Tjalkens RB (2018). The Nurr1 ligand,1,1-bis(3’-indolyl)-1-(p-chlorophenyl)methane, modulates glial reactivity and is neuroprotective in MPTP-induced parkinsonism. J Pharmacol Exp Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SL, Safe S, and Tjalkens RB (2015). A novel synthetic activator of Nurr1 induces dopaminergic gene expression and protects against 6-hydroxydopamine neurotoxicity in vitro. Neurosci Lett 607, 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk JD, and Abel T (2011). The role of NR4A transcription factors in memory formation. Brain Res Bull 85, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk JD, Bookout AL, Poplawski SG, Bridi M, Rao AJ, Sulewski ME, Kroener BT, Manglesdorf DJ, and Abel T (2012). NR4A nuclear receptors support memory enhancement by histone deacetylase inhibitors. J Clin Invest 122, 3593–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick E, Li X, Cheng Y, Lacey A, Mohankumar K, Zarei M, and Safe S (2019). Potent inhibition of breast cancer by bis-indole-derived nuclear receptor 4A1 (NR4A1) antagonists. Breast Cancer Res Treat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamoto T, Papineni S, Chintharlapalli S, Cho SD, Safe S, and Kamat AM (2008). 1,1-Bis(3’-indolyl)-1-(p-chlorophenyl)methane activates the orphan nuclear receptor Nurr1 and inhibits bladder cancer growth. Mol Cancer Ther 7, 3825–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Alaghband Y, Kramar EA, Lopez AJ, Vogel Ciernia A, White AO, Shu G, Rhee D, Michael CM, Montellier E, et al. (2018). Epigenetic regulation of the circadian gene Per1 contributes to age-related changes in hippocampal memory. Nat Commun 9, 3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Alaghband Y, Lopez AJ, Long JM, Li X, Shu G, Bodinayake KK, Matheos DP, Rapp PR, and Wood MA (2019). HDAC3-mediated repression of the Nr4a family contributes to age-related impairments in long-term memory. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lee SO, and Safe S (2012). Structure-dependent activation of NR4A2 (Nurr1) by 1,1-bis(3’-indolyl)-1-(aromatic)methane analogs in pancreatic cancer cells. Biochem Pharmacol 83, 1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell MA, and Muscat GE (2006). The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal 4, e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty SE, Barrett RM, Vogel-Ciernia A, Malvaez M, Hernandez N, Davatolhagh MF, Matheos DP, Schiffman A, and Wood MA (2012). Differential roles for Nr4a1 and Nr4a2 in object location vs. object recognition long-term memory. Learn Mem 19, 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, and Wood MA (2011). HDAC3 and the molecular brake pad hypothesis. Neurobiol Learn Mem 96, 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarolo F, Perga S, Martire S, Navone DN, Marchet A, Leotta D, and Bertolotto A (2016). Altered NR4A Subfamily Gene Expression Level in Peripheral Blood of Parkinson’s and Alzheimer’s Disease Patients. Neurotox Res 30, 338–344. [DOI] [PubMed] [Google Scholar]
- Moon M, Jeong I, Kim CH, Kim J, Lee PK, Mook-Jung I, Leblanc P, and Kim KS (2015). Correlation between orphan nuclear receptor Nurr1 expression and amyloid deposition in 5XFAD mice, an animal model of Alzheimer’s disease. J Neurochem 132, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto L, and Abel T (2013). The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology 38, 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena de Ortiz S, Maldonado-Vlaar CS, and Carrasquillo Y (2000). Hippocampal expression of the orphan nuclear receptor gene hzf-3/nurr1 during spatial discrimination learning. Neurobiol Learn Mem 74, 161–178. [DOI] [PubMed] [Google Scholar]
- Rowe WB, Blalock EM, Chen KC, Kadish I, Wang D, Barrett JE, Thibault O, Porter NM, Rose GM, and Landfield PW (2007). Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J Neurosci 27, 3098–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel AM, Sewal AS, and Rapp PR (2014). Epigenetic contributions to cognitive aging: disentangling mindspan and lifespan. Learn Mem 21, 569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, and Wood MA (2009). Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci U S A 106, 9447–9452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer ME, Hernandez PJ, Blackwell J, and Abel T (2012). Aging impairs hippocampus-dependent long-term memory for object location in mice. Neurobiol Aging 33, 2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing G, Zhang L, Russell S, and Post R (2006). Reduction of dopamine-related transcription factors Nurr1 and NGFI-B in the prefrontal cortex in schizophrenia and bipolar disorders. Schizophr Res 84, 36–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.