Abstract
Cocaine addiction afflicts nearly 1 million adults in the United States, and to date there are no known treatments approved for this psychiatric condition. Women are particularly vulnerable to developing a cocaine use disorder and suffer from more serious cardiac consequences than men when using cocaine. Estrogen is one biological factor contributing to the increased risk for females to develop problematic cocaine use. Animal studies have demonstrated that estrogen (17β-estradiol or E2) enhances the rewarding properties of cocaine. Although E2 affects the dopamine system, the molecular and cellular mechanisms of E2-enhanced cocaine reward have not been characterized. In this study, quantitative top-down proteomics was used to measure intact proteins in specific regions of the female mouse brain after mice were trained for cocaine conditioned place preference, a behavioral test of cocaine reward. Several proteoform changes occurred in the ventral tegmental area after combined cocaine and E2 treatment, with the most numerous proteoform alterations on myelin basic protein, indicating possible changes in white matter structure. There were also changes in histone H4, protein phosphatase inhibitors, cholecystokinin, and calmodulin proteoforms. These observations provide insight into estrogen signaling in the brain and may guide new approaches to treating women with cocaine use disorder.
Keywords: addiction, cocaine, estrogen, top-down mass spectrometry, label-free quantification, proteoforms
Graphical Abstract

Introduction
Approximately one-third of the nearly 1 million adults in the United States that have cocaine (COC) use disorder are women.1 Although more men than women abuse COC, women transition from casual COC use to abuse and dependence more rapidly than men.2 One factor that may play a role in this rapid transition to addiction is the hormone estrogen.3 Numerous studies in rats and mice have demonstrated that 17β-estradiol (E2), the predominant circulating form of estrogen produced by the ovaries, promotes several behaviors associated with COC addiction. For example, E2 treatment of ovariectomized (OVX) rats increases the acquisition of COC self-administration4–8 and the reinstatement of COC self-administration after abstinence.9–11 E2 also augments COC conditioned place preference (CPP), a behavioral test that measures drug reward, in OVX rats and mice.12, 13
The underlying molecular and cellular mechanisms responsible for the ability of E2 to enhance the rewarding and reinforcing properties of COC in females are not well-understood, although the neurotransmitter dopamine likely plays a role. COC increases synaptic dopamine levels in the nucleus accumbens (NAc, also known as the ventral striatum) through blockade of the dopamine transporter, and dopamine in the NAc is involved in COC CPP.14 One mechanism by which E2 might increase COC reward is by further augmenting COC-stimulated dopamine release in the NAc. A recent study demonstrated that E2 increases COC-induced dopamine release in the NAc through an estrogen-sensitive circuit involving neurons in the medial preoptic area that project to the ventral tegmental area (VTA), which contains dopamine neurons that project to and release dopamine in the NAc.15 E2 also acts through metabotropic glutamate receptors8 and cannabinoid type 1 receptors9 to promote COC self-administration and reinstatement of COC seeking, respectively, indicating that other complementary mechanisms contribute to E2-enhanced COC reinforcement. However, the intracellular proteins and signaling mechanisms responsible for the combined effects of COC and E2 on reward and reinforcement have not been identified.
To identify novel protein changes elicited by COC and E2 that might be involved in COC reward and could lead to new therapeutic targets for cocaine addiction, we employed top-down mass spectrometry (TDMS) on three estrogen-sensitive brain regions involved in COC CPP: the amygdala (AMY), NAc, and VTA.14, 16–18 OVX female mice were conditioned with COC using the CPP paradigm and treated with E2 throughout conditioning. Brain tissue was collected immediately after the CPP test, with the rationale that protein changes evident during the CPP test might be responsible for the E2-mediated increase in this behavior. Tissue was analyzed by TDMS to identify changes in intact proteins and their proteoforms (protein-forms).19
Over the past decade, bottom-up (BU) proteomics has been widely used to identify and quantify complete proteomes from a biological system such as a cell, tissue, or organism. However, due to the protein inference problem,20 BU proteomics has intrinsic limitations for identifying and characterizing intact proteoforms that underlie complex traits and molecular mechanisms in biology. In contrast to BU proteomics, which measures peptides produced from proteins by proteolytic digestion, TD proteomics allows for measuring intact forms of proteins without proteolysis, thus offering proteoform-resolved information on combinatorial post-translational modifications (PTMs), alternative splicing events, and coding polymorphisms on the same molecule present in vivo.21
In the context of large-scale proteomics investigations that include procedures for label-free quantitation,22 TDMS innovations have helped to quantify the relative abundance of biomarkers between two states,23 including examination of proteoform differences between patient derived xenografts,24 peripheral blood mononuclear cells associated with kidney and liver transplantation,25 and differential comparison of brain proteoforms between mouse strains.26 Here, we have used TDMS to identify several novel proteoforms that change in specific brain regions after COC CPP. These proteoforms may contribute to E2-enhanced COC reward. The protein and proteoform changes induced by E2 and COC in this study could provide new targets for the treatment of COC use disorder in females.
Experimental Section
Animals
A total of 80 female C57BL/6J mice were used in this study and were purchased at the age of 8 weeks from The Jackson Laboratory (stock number: 000664, Bar Harbor, ME). Mice underwent bilateral ovariectomy (OVX) with anesthesia within 1 week of arrival, as described below. Behavioral testing was performed at the age of 10–12 weeks. Mice were group-housed in a temperature- and humidity-controlled environment under a 12-hour light/dark cycle with lights on at 6 am and off at 6 pm. Behavioral testing was conducted during the light phase. All mice had access to food and water ad libitum for the duration of the study and were maintained and cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experimental procedures were approved by the University of Illinois at Chicago (UIC) Institutional Animal Care and Use Committee (protocol 15–034).
Materials and Reagents
Water (Optima HPLC Grade), acetonitrile (ACN, Optima HPLC Grade), acetone (Optima HPLC Grade), methanol (Optima HPLC Grade), and chloroform (Optima HPLC Grade) were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Formic acid (FA, 28905) and Halt™ Phosphatase & Protease Single-Use Inhibitor Cocktail (78443) were purchased from Thermo Scientific (Rockford, IL, USA). Trizma® base (T1503), hydrochloric acid (HCl, 320331), magnesium chloride (MgCl2, M8266), and benzonase nuclease (E1014) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium butyrate (A11079) was purchased from Alfa Aesar (Tewksbury, MA, USA). Sodium dodecyl sulfate (SDS) was purchased from Bio-Rad (Hercules, CA, USA). 1, 4-Dithiothreitol (DTT, 111474) was purchased from Merck KGaA (Darmstadt, Germany).
Surgery
Mice were anesthetized with an intraperitoneal (IP) injection of ketamine (100 mg/kg) and xylazine (8 mg/kg). Hair was shaved on the flank on both sides of the spine, and incisions were made through the skin and underlying muscle tissue to expose the ovary. The uterine horn was externalized from the abdominal cavity and the ovary was dissected away from the uterine horn by cauterization. The uterine horn was then placed back into the abdominal cavity. Muscle tissue was closed with silk sutures and skin was closed with stainless steel wound clips. The procedure was repeated on the opposite side of the spine to remove the second ovary. Mice received a subcutaneous injection of meloxicam (2 mg/kg) for analgesia immediately after surgery and 24 hours later. Mice recovered for 12–15 days prior to behavioral testing.
Drug Administration
Water-soluble E2 (encapsulated in [2-Hydroxypropyl]-β-cyclodextrin) and (2-Hydroxypropyl)-β-cyclodextrin vehicle (VEH) were purchased from Sigma-Aldrich (St. Louis, MO USA). E2 was dissolved in 0.9% NaCl (saline) to a final concentration of 0.02 mg/mL and administered IP at a dose of 0.2 mg/kg, 20 min before each conditioning session in the CPP experiment described below. The timing and dose of E2 was based on the pharmacokinetics of E2 such that conditioning was performed when the plasma concentration of E2 was peaking.27 This dose of E2 has been demonstrated to increase learning and memory in OVX mice and to be comparable to circulating E2 levels found during proestrus, when E2 levels are high.28 VEH-treated mice received the same amount of β-cyclodextrin as E2-treated mice. Cocaine hydrochloride (COC, Mallinckrodt Pharmaceuticals, St. Louis, MO) was dissolved in saline and administered IP at a dose of 5 mg/kg. This subthreshold dose of COC was chosen in order to observe an effect of E2 on the enhancement of COC CPP, since higher doses of COC on CPP rapidly plateau.12, 29
CPP
The CPP procedure is outlined in Figure 1A and was performed in a two-chambered apparatus with distinctive floor textures in each chamber as described in Satta et al.12 Briefly, mice were allowed free access to both chambers and tested for preference for 30 min on the first day (Test 1). Mice then underwent conditioning sessions in which they were injected with COC (5 mg/kg) and confined to the non-preferred chamber for 15 min. On alternate days, mice were injected with saline (SAL, 10 mL/kg) and confined to the opposite chamber. Six conditioning sessions were performed over 6 days, with one conditioning session per day, for a total of three COC and three SAL sessions (COC-conditioned group) or six SAL sessions (SAL- control group). Mice were randomly assigned to treatment groups. A 30-min drug-free preference test was performed on day 8 (Test 2). Preference scores were calculated as the difference between the time spent on the cocaine-paired side (or initially non-preferred, in the case of the saline control group) during Test 2 minus Test 1. For E2 treatments, mice were divided into 2 additional groups and given either a single E2 or VEH injection 20 min prior to each conditioning session. There were 4 treatment groups (SAL/VEH, SAL/E2, COC/VEH, and COC/E2) with 20 mice per group. Mice were tested in 4 independent cohorts of 20 mice per cohort (5 mice of each treatment group per cohort) over a period of two months. Experimenters were not blinded to treatment groups.
Figure 1. Cocaine conditioned place preference (CPP) in ovariectomized (OVX) mice treated with 17β-estradiol (E2).
(A) Schematic of the CPP procedure. Mice were OVX and allowed to rest for 12–15 days before undergoing the CPP procedure. On test day 1, mice were not treated and placed in the conditioning apparatus with access to both sides of the chamber (indicated as dark blue and white boxes). On days 2, 4, and 6, mice were conditioned with cocaine (COC) or saline (SAL) and restricted to one chamber (indicated as dark blue). On days 3, 5, and, 7, mice were conditioned with saline and restricted to the other chamber (indicated as white). Mice were tested for CPP on day 8. E2 or vehicle (VEH) treatments were given 20 min prior to each conditioning session on days 2–7. (B) Preference scores after conditioning. **p < 0.01 by post-hoc Tukey’s test, n = 20 mice per group.
Tissue Collection
Immediately after the CPP test on day 8, mice were euthanized with Somnasol (pentobarbital euthanasia solution) and transcardially perfused with ice-cold saline for 5 min to flush out blood. Mice were then rapidly decapitated and the brain removed from the cranium. Brains were sectioned on ice into 1 mm-thick coronal brain sections using a stainless-steel adult mouse brain matrix and razor blades (Zivic Instruments, Pittsburgh, PA). Punches of the amygdala (AMY, located at ~1.5 mm posterior to bregma30), nucleus accumbens (NAc, located at ~1.0 mm anterior to bregma), and ventral tegmental area (VTA, located at ~3 mm posterior to bregma) were obtained using 1 mm-diameter biopsy punches (Integra). Biopsies were heat stabilized using a Stabilizor™ Instrument (Denator, Inc.), transferred to 2 mL conical tubes, and stored at −80°C until tissue processing for proteomics. Bilateral biopsies from 5 mice within each treatment group within a cohort were combined in order to obtain sufficient material for proteomics (~150 μg/sample), thus giving 4 independent biological replicates per brain region.
Mouse Brain Tissue Preparation and Homogenization
Brain tissues from mice were homogenized in 100 μL of ice-cold lysis buffer (4% SDS, 15 mM Tris-HCl (pH 7.4), 10 mM sodium butyrate, 10 mM DTT, and 1X Halt™ Phosphatase & Protease Single-Use Inhibitor Cocktail) containing 1.4 mm ceramic beads (Omni International, Kennesaw, GA) for a total of 6 min using the Bullet Blender® (Next Advance, Inc., Averill Park, NY). All samples were kept ice-cold during homogenization. Next, the lysate was supplemented with 1 mM MgCl2 and benzonase nuclease to digest sample DNA and RNA, and incubated for 30 min at 37 °C.
Protein extraction and separation from mouse brain tissue homogenates
Sample preparation of mouse brain tissues was performed as previously described.26. Briefly, 300 μL of homogenized samples were precipitated with cold acetone at a ratio of 4:1 (acetone: sample) and the resulting protein precipitant was dissolved in 1% SDS by placing in a 95 °C heat block or thermal mixer for ~5 min.26, 31 The protein concentration was determined using a BCA assay (Pierce, Rockford, IL). Next, 140 μg of total protein was processed by gel-eluted liquid fraction entrapment electrophoresis (GELFrEE) using an 8% tris-acetate cartridge kit (catalog no. 42103; Expedeon, San Diego, CA) according to the manufacturer’s protocol (Expedeon, Cambridgeshire, UK, GELFrEE 8100 Fractionation System) to isolate proteins below 30 kDa. SDS was removed via methanol/chloroform/water extraction.32 Proteins were dissolved in 40 μL of Solvent A (95% H2O, 5% ACN, 0.2% FA), then centrifuged at 21,000 × g at 4 °C for 10 min prior to liquid chromatography with tandem mass spectrometry (LC-MS/MS) analysis.
LC-MS/MS analysis
Six μL of reconstituted GELFrEE protein fractions were injected onto a PepSwift RP-4H trap column (150 μm I.D. × 2 cm). A Dionex Ultimate 3000 RSLCnano system was operated at a flow rate of 10 μL/min for 10 min for loading onto trap column. The loaded proteins were separated on ProSwift RP-4H column (100 μm I.D. × 50 cm) and eluted into the mass spectrometer at flow rate of 1 μL/min. The LC gradient conditions were as follows: 5% B at 0 min.; 15% B at 5 min.; 55% B at 80 min.; 95% B from 83–102 min. Solvent A was described above, and Solvent B consisted of 5% H2O, 95% acetonitrile, and 0.2% formic acid. MS data were collected with an Orbitrap Elite (Thermo Scientific, Bremen, Germany) mass spectrometer operated in a top-2 data-dependent acquisition mode using previous instrument methods,23 with the following parameters: (1) FT scan, four microscans, m/z 500–2,000, and resolving power of 100,000 (at m/z 400) and (2) data-dependent MS/MS mode on the top two most abundant masses from scan event 1 using higher-energy collisional dissociation (HCD), which produces b- and y-ion types, with normalized collision energy of 25, four microscans, isolation width 15 m/z, and resolution 60,000 (at m/z 400). All raw data files are available in MassIVE repository (Center for computational Mass Spectrometry, University of California, San Diego, CA).
Experimental Design and Statistical Rationale
For a comparative TDMS analysis, we performed total 80 LC-MS runs (four treatments [SAL/VEH, SAL/E2, COC/VEH, and COC/E2], four biological replicates [5 mice per biological replicate, total N=80], and five technical replicates for LC-MS/MS), which were randomized to minimize bias, for each brain region. This study design is based on our previous TD quantitative experiment on brain proteoforms between mouse strains.26 Raw MS data files were processed via TDPortal software, a high-performance computing environment for high-throughput analysis of top-down proteomic data (freely available for academic collaborators at: http://nrtdp.northwestern.edu/tdportal-request/) by database searching, scoring, and quantification.25, 33–35 For proteoform identification and characterization, the linked set of deconvoluted MS1 and MS2 data were searched against a highly annotated mouse database (May 2016, 26,732 protein entries) created using UniProtKB PTM/Processing and Sequence features such as signal peptide removal, alternative splicing, and reduced disulfide bonds as fixed events, while most common PTMs (e.g., N-methionine, N-acetylation, phosphorylation, methylation, etc.) as variable events. Searches were performed using a three-tiered search tree (Tight Absolute Mass, BioMarker searches, and Find Unexpected Modifications).26 The first search was a Tight Absolute Mass search with a MS1 tolerance of 2.2 Da and MS2 tolerance of 10 ppm. The second search was a BioMarker search with both MS1 and MS2 tolerances of 10 ppm. The last search was a Find Unexpected Modifications search with a MS1 tolerance of 200 Da and a MS2 tolerance of 10 ppm, using Delta M mode, which allows the detection of proteoforms with unknown modifications. A tolerance of 200 Da was an optimal compromise between novel proteoform discovery potential and inflated false positive rates. It allows for the detection of common modifications such as one or two phosphorylation residues, but is small enough to keep a large number of incorrect results being returned. Many incorrect false positives can cause otherwise true positives to be filtered during our multiple testing correction. Search results were visualized with TDViewer software (available for download at http://topdownviewer.northwestern.edu). The p-cores, which measure the quality of the match between the spectral data and the theoretical proteoform,36 and characterization scores (C-scores), which measure the uniqueness of the matches relative to other entries in the database, were used to measure the confidence in the identification and characterization of proteoforms.37 For proteoform identification, a global false-discovery rate (FDR) was determined using a previous described procedure.38 All proteoform identifications used an FDR of 1%. The fully characterized proteoforms (C-Score > 40) were deposited into the Mouse Brain Proteoform Atlas (http://mousebrain.kelleher.northwestern.edu/) that has recently been established.
For each discovered proteoform, a hierarchical linear model was applied to allow variation in the technical replicates (random effect) to be nested within their biological replicates (also a random effect), before testing for the variation within treatments (fixed effect). For quantitative data processing in this study, proteoform-level extracted ion chromatograms were generated to determine an appropriate intensity value for each proteoform.26 Least square means estimates from the hierarchical linear model were used to estimate the size of the effect (i.e., fold-change) in the treatments. In addition, ANOVA analysis of normalized proteoform intensity across treatments was performed to determine differentially expressed proteoforms as previously described.23 All p-values were corrected for multiple testing at a false discovery rate of α = 0.05.39 Statistical analyses were performed using SAS 9.4 (SAS Institute; Cary, NC).
Results and Discussion
COC CPP is Increased in Mice Treated with E2
We previously found that OVX mice treated with E2 exhibit increased COC CPP when compared with VEH-treated mice.12 To discover proteins that are altered during the expression of CPP, we performed an experiment in which OVX mice (n = 20) were conditioned with a subthreshold dose of COC or SAL and treated with VEH or E2 prior to each conditioning session (Figure 1A). Preference scores were significantly different between treatment groups [one-way ANOVA, F (3, 76) = 3.69, p = 0.016]. Post hoc testing indicated that mice treated with COC/E2 had significantly higher preference scores (p = 0.0092) compared with the SAL/VEH group, whereas preference scores in the SAL/E2 and COC/VEH groups did not differ from the SAL/VEH group (Figure 1B). These results indicate that E2 enhances COC CPP in OVX female mice, similar to our previous finding.
Top-Down Proteomics as a Discovery-Based Approach for Proteoforms Associated with Cocaine Reward
We applied a label-free top-down proteomics approach in discovery mode to find novel proteins and posttranslational modifications that might underlie the enhanced COC reward observed in female mice treated with E212. Given that COC and E2 are known to rapidly regulate protein phosphorylation and signal transduction,14, 40 we hypothesized that proteoforms altered by the combination of COC and E2 could potentially be responsible for the behavioral effects. We examined three estrogen-sensitive brain regions (AMY, NAc, and VTA) that are known to be involved in COC reward.16–18 For comparative proteomic analysis, tissue samples of the AMY, NAc, and VTA were collected from mice immediately after testing for COC CPP. A 4 × 4 × 5 study design (4 treatment groups × 4 biological replicates × 5 technical replicates) was used, and each brain region was analyzed separately. Supplemental Table S1 lists the total proteoforms corresponding to the proteins identified in these three brain regions and Supplemental Tables S2–4 list the proteoforms detected within each brain region by treatment group. We next applied the hierarchical linear statistical model to quantify intact proteoforms within our top-down experiment. Supplemental Figure S1 shows the relative contributions of multiple levels of variation in signal intensity, which were grouped by four major sources: treatment, biological variation (biological replicates which were pooled samples from within each treatment group), technical variation (LC-MS injections), and residual, indicating that the contribution of the technical variation is quite small relative to the other sources.
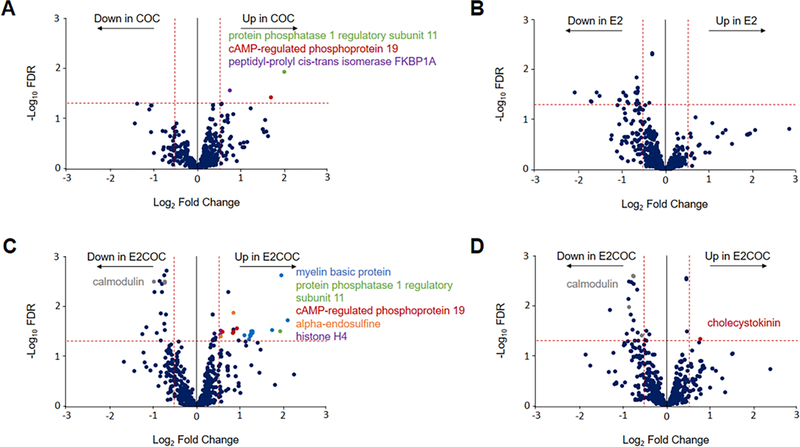
We performed a quantitative analysis of the proteoforms that were significantly altered between treatment groups within each brain region. We began by creating an overly generous list of differentially expressed proteoforms using a quantitation FDR of 10%. This list included 568, 684, and 618 proteoforms in AMY, NAc, and VTA, respectively. These proteoforms were next filtered by FDR (< 5%) and fold change threshold (1.5-fold change) between treatment groups. We found 98 unique proteoforms with statistically significant abundance differences between treatment groups in the VTA, whereas there were only 11 and 1 proteoforms differing between the treatments in the AMY and NAc, respectively, indicating that the VTA may be most impacted by COC and/or E2 at these doses. We therefore chose to focus on the COC- and E2-induced changes in the VTA. A list of the significantly differentially expressed proteoforms between treatment groups within the VTA from high-throughput analysis is depicted in Table 1. Volcano plots were generated which represent each proteoform as a function of estimated effect size (in log2 fold-change) and the statistical confidence (in −log10 FDR) and show that there was a difference in normalized signal intensity between the two treatment groups. Figure 2 and Supplemental Figures S2 and S3 show the four pairwise volcano plots for the VTA, NAc, and AMY, respectively, of mice treated with COC/VEH vs. SAL/VEH, SAL/E2 vs. SAL/VEH, COC/E2 vs. SAL/VEH, and COC/E2 vs COC/VEH, with selected significantly differentially expressed proteoforms indicated by colored text.
Table 1.
Differentially expressed proteoforms across comparisons within VTA (mice treated with COC/VEH vs. SAL/VEH, SAL/E2 vs. SAL/VEH, COC/E2 vs. SAL/VEH, and COC/E2 vs. COC/VEH) filtered by false discovery rate (< 5%) and fold change threshold (1.5-fold change between groups).
| PFRa | Accession | Description | Modifications | FCb |
|---|---|---|---|---|
| Up in COC/VEH over SAL/VEH | ||||
| 51988 | Q8K1L5 | Protein phosphatase 1 regulatory subunit 11 | N-terminal acetylation, 4 phospho residues | 4.0 |
| 73684 | P56212–2 | cAMP-regulated phosphoprotein 19 | N-terminal acetylation, 1 phospho residue, alternative splicing | 3.3 |
| 49975 | P26883 | Peptidyl-prolyl cis-trans isomerase FKBP1A | N6-acetyllysine@52 | 1.7 |
| Up in COC/E2 over SAL/VEH | ||||
| 244247 | P04370–8 | Myelin basic protein | N-terminal acetylation, 2 phospho residue, 1 methyl residue, alternative splicing | 4.4 |
| 244249 | P04370–8 | Myelin basic protein | N-terminal acetylation, O-phospho-L-threonine@33, O-phospho-L-serine@38, omega-N-methyl-L-arginine@127, alternative splicing | 3.9 |
| 244255 | P04370–8 | Myelin basic protein | N-terminal acetylation, 2 phospho residue, 1 methyl residue, alternative splicing | 3.9 |
| 51988 | Q8K1L5 | Protein phosphatase 1 regulatory subunit 11 | N-terminal acetylation, 4 phospho residues | 3.4 |
| 71909 | P04370–8 | Myelin basic protein | N-terminal acetylation, 1 phospho residue, alternative splicing | 2.4 |
| 71911 | P04370–8 | Myelin basic protein | N-terminal acetylation, 1 phospho residue, alternative splicing | 2.4 |
| 71914 | P04370–8 | Myelin basic protein | N-terminal acetylation, 1 phospho residue, alternative splicing | 2.4 |
| 50142 | P04370–8 | Myelin basic protein | N-terminal acetylation, O-phospho-L-tyrosine@65, alternative splicing | 2.4 |
| 71904 | P04370–8 | Myelin basic protein | N-terminal acetylation, O-phospho-L-serine@72, alternative splicing | 2.4 |
| 73746 | P04370–8 | Myelin basic protein | N-terminal acetylation, 1 phospho residue, alternative splicing | 2.4 |
| 71907 | P04370–8 | Myelin basic protein | N-terminal acetylation, 1 phospho residue, alternative splicing | 2.4 |
| 244241 | P04370–8 | Myelin basic protein | N-terminal acetylation, 1 phospho residue, alternative splicing | 2.4 |
| 71906 | P04370–8 | Myelin basic protein | N-terminal acetylation, O-phospho-L-threonine@63, alternative splicing | 2.4 |
| 56658 | P04370–8 | Myelin basic protein | N-terminal acetylation, alternative splicing | 2.4 |
| 50054 | P04370–8 | Myelin basic protein | N-terminal acetylation, alternative splicing | 2.4 |
| 49864 | P04370–8 | Myelin basic protein | N-terminal acetylation, alternative splicing | 2.3 |
| 49968 | P04370–6 | Myelin basic protein | N-terminal acetylation, alternative splicing | 2.2 |
| 49955 | P04370–6 | Myelin basic protein | N-terminal acetylation, omega-N-methyl-L-arginine@130, citrullinated L-arginine@153, alternative splicing | 2.0 |
| 56892 | P56212–1 | cAMP-regulated phosphoprotein 19 | 1.9 | |
| 49978 | P60840–2 | Alpha-endosulfine | N-terminal acetylation, alternative splicing | 1.8 |
| 244316 | P56212–1 | cAMP-regulated phosphoprotein 19 | N-terminal acetylation, 1 phospho residue, alternative splicing | 1.8 |
| 58648 | Q06185 | ATP synthase subunit e, mitochondrial | 2 acetyl residues | 1.8 |
| 58798 | P56212–1 | cAMP-regulated phosphoprotein 19 | 1 phospho residue, alternative splicing | 1.8 |
| 50071 | P43024 | Cytochrome c oxidase subunit 6A1, mitochondrial | N-terminal acetylation | 1.7 |
| 50002 | P56212–1 | cAMP-regulated phosphoprotein 19 | N-terminal acetylation, alternative splicing | 1.5 |
| 49991 | P60840–1 | Alpha-endosulfine | N-terminal acetylation, alternative splicing | 1.5 |
| 22557 | P62806 | Histone H4 | 2 acetyl residues, dimethylarginine@3 | 1.5 |
| 56803 | P62806 | Histone H4 | 3 acetyl residues, 1 methyl residue | 1.5 |
| Up in COC/E2 over COC/VEH | ||||
| 50255 | P09240 | Cholecystokinin | alternative splicing | 1.7 |
| Down in SAL/E2 under SAL/VEH | ||||
| 50251 | P60041 | Somatostatin | alternative splicing | 4.3 |
| 75356 | P31786 | Acyl-CoA-binding protein | N-terminal acetylation | 3.3 |
| 22651 | P31786 | Acyl-CoA-binding protein | N6-acetyl-L-lysine@8 | 3.3 |
| 71841 | Q91ZZ3 | Beta-synuclein | 1 acetyl residue, 1 phospho residue | 3.0 |
| 22482 | Q9CQR2 | 40S ribosomal protein S21 | 2.9 | |
| 57111 | Q9D855 | Cytochrome b-c1 complex subunit 7 | 1 acetyl residue, 2 succinyl residues | 2.1 |
| 223395 | Q9D855 | Cytochrome b-c1 complex subunit 7 | N6-acetyl-L-lysine@11, N6-succinyl-L-lysine@77, N6-succinyl-L-lysine@87 | 2.1 |
| 10169 | P18760 | Cofilin-1 | 2.0 | |
| 244314 | Q9JLR9 | HIG1 domain family member 1A, mitochondrial | O-phospho-L-serine@7 | 1.9 |
| 244332 | P56212–1 | cAMP-regulated phosphoprotein 19 | 2 phospho residues, alternative splicing | 1.9 |
| 22454 | P26350 | Prothymosin alpha | 1 acetyl residue | 1.8 |
| 49836 | Q91ZZ3 | Beta-synuclein | N-terminal acetylation | 1.8 |
| 50106 | P26350 | Prothymosin alpha | 1 phospho residue, 1 acetyl residue | 1.6 |
| 51972 | P62962 | Profilin-1 | N-terminal acetylation | 1.6 |
| 70070 | Q9EP89 | Serine beta-lactamase-like protein LACTB; mitochondrial | 1.6 | |
| 22738 | P56391 | Cytochrome c oxidase subunit 6B1 | N6-acetyl-L-lysine@61 | 1.6 |
| 22390 | P56391 | Cytochrome c oxidase subunit 6B1 | N-terminal acetylation | 1.6 |
| 49843 | O55042–1 | Alpha-synuclein | N-terminal acetylation, alternative splicing | 1.6 |
| 169159 | P26350 | Prothymosin alpha | N6-succinyl-L-lysine@14 | 1.6 |
| 56591 | P17742 | Peptidyl-prolyl cis-trans isomerase A | 3 acetyl residues | 1.6 |
| 51604 | P17742 | Peptidyl-prolyl cis-trans isomerase A | 3 acetyl residues | 1.6 |
| 244329 | P17742 | Peptidyl-prolyl cis-trans isomerase A | 3 acetyl residues | 1.5 |
| 22405 | P26350 | Prothymosin alpha | N-terminal acetylation, N6-acetyl-L-lysine@14 | 1.5 |
|
Down in COC/E2 under
SAL/VEH |
||||
| 244323 | O08997 | Copper transport protein ATOX1 | 2.4 | |
| 51771 | P63168 | Dynein light chain 1, cytoplasmic | 2.2 | |
| 51999 | P0DP26 | Calmodulin | N-terminal acetylation, N6,N6,N6-trimethyl-L-lysine | 2.0 |
| 69959 | Q9D3D9 | ATP synthase subunit delta, mitochondrial | N6-acetyl-L-lysine@143, alternative splicing | 2.0 |
| 49842 | Q91VR8 | Protein BRICK1 | N-terminal acetylation | 1.9 |
| 49897 | P15532 | Nucleoside diphosphate kinase A | N-terminal acetylation | 1.8 |
| 22436 | Q9CPQ8 | ATP synthase subunit g, mitochondrial | 3 acetyl residues | 1.8 |
| 51972 | P62962 | Profilin-1 | N-terminal acetylation | 1.8 |
| 51260 | P15532 | Nucleoside diphosphate kinase A | N6-acetyl-L-lysine@123 | 1.8 |
| 8655 | Q9D3D9 | ATP synthase subunit delta, mitochondrial | 1.7 | |
| 51344 | P61089 | Ubiquitin-conjugating enzyme E2 N | N-terminal acetylation | 1.7 |
| 51693 | Q8BVI4 | Dihydropteridine reductase | N-terminal acetylation | 1.7 |
| 203837 | P70296 | Phosphatidylethanolamine-binding protein 1 | 1 acetyl residue, 1 phospho residue | 1.7 |
| 244244 | P0DP26 | Calmodulin | N-terminal acetylation, N6-acetyl-L-lysine@22, 1 phospho residue, N6,N6,N6-trimethyl-L-lysine@116 | 1.7 |
| 244235 | P0DP26 | Calmodulin | 1 acetyl residue, 1 phospho residue, N6-acetyl-L-lysine@95, N6,N6,N6-trimethyl-L-lysine@116 | 1.7 |
| 22412 | P52760 | Ribonuclease UK114 | N-terminal acetylation | 1.6 |
| 244322 | P99029–1 | Peroxiredoxin-5, mitochondrial | 1 phospho residue, 1 succinyl residue, alternative splicing | 1.6 |
|
Down in COC/E2 under
COC/VEH |
||||
| 51771 | P63168 | Dynein light chain 1, cytoplasmic | 2.5 | |
| 49842 | Q91VR8 | Protein BRICK1 | N-terminal acetylation | 1.9 |
| 203837 | P70296 | Phosphatidylethanolamine-binding protein 1 | 1 acetyl residue, 1 phospho residue | 1.8 |
| 49897 | P15532 | Nucleoside diphosphate kinase A | N-terminal acetylation | 1.8 |
| 51999 | P0DP26 | Calmodulin | N-terminal acetylation, N6,N6,N6-trimethyl-L-lysine | 1.8 |
| 51972 | P62962 | Profilin-1 | N-terminal acetylation | 1.8 |
| 51260 | P15532 | Nucleoside diphosphate kinase A | N6-acetyl-L-lysine@123 | 1.8 |
| 69959 | Q9D3D9 | ATP synthase subunit delta, mitochondrial | N6-acetyl-L-lysine@143, alternative splicing | 1.7 |
| 244235 | P0DP26 | Calmodulin | 1 acetyl residue, 1 phospho residue, N6-acetyl-L-lysine@95, N6,N6,N6-trimethyl-L-lysine@116 | 1.7 |
| 244244 | P0DP26 | Calmodulin | N-terminal acetylation, N6-acetyl-L-lysine@22, 1 phospho residue, N6,N6,N6-trimethyl-L-lysine@116 | 1.7 |
| 51693 | Q8BVI4 | Dihydropteridine reductase | N-terminal acetylation | 1.7 |
| 49919 | P32848 | Parvalbumin alpha | N-terminal acetylation | 1.7 |
| 51344 | P61089 | Ubiquitin-conjugating enzyme E2 N | N-terminal acetylation | 1.6 |
| 22649 | Q01768 | Nucleoside diphosphate kinase B | N-terminal acetylation | 1.6 |
| 49931 | P0DP26 | Calmodulin | N-terminal acetylation, N6,N6,N6-trimethyl-L-lysine@115 | 1.5 |
Proteoform record,
FC, fold change
Figure 2. Quantitative comparison of proteoforms from treatment groups in ventral tegmental area.
The volcano plots show that for the mice treated with cocaine (COC) versus saline/vehicle control (A), estradiol (E2) versus control (B) combined COC and E2 versus control (C), and combined COC and E2 versus COC (D), 3, 23, 45, and 16 proteoforms were identified, respectively, below a 5% global FDR threshold (horizontal dotted red line) for quantitation and above a 1.5-fold change (vertical dotted red lines) in proteoform signal abundance between the treatments.
In the VTA, 262 proteoforms, which were fully characterized (C-score >40), were detected at <10% global FDR threshold using a Find Unexpected Modifications search on TDPortal. Of these detected proteoforms, 148 were uniquely identified unexpected modifications, of which 114 were related to proteoforms identified by Tight Absolute Mass searches. Of these unique proteoforms, 15 differentially abundant proteoforms were seen between treatment groups within the VTA from high-throughput analysis. One of them is a calmodulin proteoform (PFR49931), which was found to be decreased by 1.5-fold in the COC/E2 group versus COC/VEH group (Figure 2C–D and Table 1). Using ProSight Lite,41 we manually proposed a combination of modifications and their sites that were not confidently assigned by the other searches on the TDPortal. The proteoform was determined to be N-acetylated and tri-methylated at lysine 115 (C-score of 323), whose experimental mass matches the theoretical one within 5.5 ppm.
As mentioned above, we found that the combination of COC and E2 treatment more dramatically altered proteoforms below 30 kDa in the VTA compared with the NAc and AMY. The primary effect of COC is to inhibit reuptake of dopamine, serotonin, and norepinephrine by inhibiting their transporters. The reinforcing and rewarding properties of cocaine are attributed to the ability of COC to increase synaptic dopamine levels in VTA projection targets such as the prefrontal cortex, NAc and AMY through blockade of the presynaptic dopamine transporter.14 However, COC can also increase the firing of VTA dopamine neurons42, 43 and induce synaptic changes in the VTA.44 In addition, E2 increases COC-stimulated dopamine release, via a mechanism involving estrogen sensitive neurons projecting from the medial preoptic area to the VTA15. Because of the ability of both COC and E2 to increase dopamine neuron activity, we speculate that the combination of E2 and COC may have had more potent effects on signaling and post-translational modifications in the VTA compared with the NAc and AMY.
Our primary goal was to discover unique proteins and proteoforms showing significant differences in expression between mice that received COC/E2 during the CPP procedure compared with controls because this might illuminate novel mechanisms underlying the behavioral increase in COC CPP by E2. Therefore, we focused on proteoform differences in the VTA between the COC/E2 group and two of the control groups (SAL/VEH and COC/VEH) in more detail. In this study we found that combined COC and E2 had unique effects on several different functional categories of proteins in the VTA. Proteoforms of MBP, histone H4, protein phosphatase inhibitors (PPP1R11, ARPP19, and ENSA), and CCK were increased in the VTA, while proteoforms of calcium binding proteins (CaM, PVALB), proteins involved in cytoskeleton organization and synaptic signaling (BRICK1, DYNLL1, and PEBP1), and mitochondrial proteins (ATP5F1D, ATP5l, PRDX5) were decreased in the VTA after combined COC and E2 treatment. The potential behavioral consequences of these alterations have yet to be determined, but several hypotheses can be tested based on these findings.
In examining proteoforms that were higher in the COC/E2 group compared with the SAL/VEH group, we found a predominance of proteoforms (17) of myelin basic protein (MBP, P04370) that were increased by 2- to 4.4-fold (Figure 2C, Table 1, Supplemental Figure S4 and S5). We manually reanalyzed these MBP proteoforms reported by high-throughput analysis. These proteoforms were not altered in the SAL/E2 or the COC/VEH groups compared with the SAL/VEH group, suggesting that the combination of COC plus E2 had a distinct effect on MBP post-translational modifications in the VTA. MBP, an intrinsically disordered protein that manifests from numerous alternative splice variants and PTMs,45 is an important constituent of myelin and is involved in the formation and stability of white matter, in addition to other roles in neuronal signaling and transcription. Chronic COC abuse has repeatedly been demonstrated to be associated with deficits in white matter integrity in humans using brain imaging,46–50 and experiments in non-human primates and rats show that white matter integrity is decreased after chronic COC self-administration.51–53 Decreased expression of MBP is also observed in the brain of subjects with a history of COC abuse.54–56 We previously showed that even acute administration of cocaine leads to dramatic dose-dependent changes in levels of MBP-derived peptides in the hypothalamus of adolescent mice.57 Current results indicate that there is a significant increase in proteoforms of MBP modified by phosphorylation, acetylation, methylation, citrullination, and alternative splicing (P04370, specifically, isoform 8, 14 kDa form) in mice that have been treated with COC and E2. Phosphorylation of MBP is achieved by numerous kinases, including mitogen-activated protein kinases (MAPK), cAMP-dependent kinase (PKA), protein kinase C (PKC), and glycogen synthase kinase (GSK3β).58 Estrogen receptors at the plasma membrane are known to rapidly activate several kinase-mediated signaling cascades,59 thus the increase in phosphorylated MBP might be due to E2- and COC-mediated activation of one or more of these signaling pathways. Increased phosphorylation of MBP would decrease its positive charge and alter its interaction with the plasma membrane and ability to organize the myelin sheath.60
Higher levels of citrullinated MBP are found in neurodegenerative and demyelinating diseases such as multiple sclerosis. Citrullination of MBP also decreases the net positive charge of MBP, which is achieved by conversion of the positively charged amino acid arginine to neutral citrulline by peptidylarginine deiminases (PADs), and is hypothesized to alter protein folding and/or immunogenicity of MBP.60, 61 Therefore, increased citrullinated MBP might be predicted to trigger an inflammatory response and/or neurodegeneration. In this study, we used a low dose of COC and mice were only given 3 COC injections. In contrast, studies examining the effects of COC on white matter integrity and MBP expression often involve repeated, chronic COC exposure for weeks or months. It is interesting to speculate that even low doses of COC (when combined with E2) may initiate detrimental post-translational changes on MBP, and that what we have observed in this study are early events that might trigger an immune response and white matter degeneration. Even more intriguing is the potential role of E2 in this process. E2 is generally considered to be neuroprotective and to promote remyelination in conditions such as multiple sclerosis.62–65 However, here we demonstrate that the combination of COC plus E2 increased phosphorylation and citrullination of MBP. This has potentially important implications for the vulnerability of women to more rapidly progress from initial COC use to COC abuse,2 and suggests that women who use COC might be more susceptible to white matter damage and possibly impairments in cognitive function associated with COC abuse, even at low doses of COC.49, 66 The neuroimaging studies on individuals with COC use disorder were performed on a small number of women and have likely been underpowered to detect sex differences in white matter and cognitive function. Future studies should address this possibility as well as gain a better understanding of how COC and E2 affect MBP function and myelin structure.
Two different histone H4 (P62806) proteoforms (proteoform record PFR22557: acetylated and dimethylated; PFR56803: acetylated and monomethylated) were also significantly increased by 1.5-fold in the COC/E2 group compared with the SAL/VEH group (Figure 2C, Figure 3B, and Table 1). Like MBP, histone H4 proteoforms were not significantly altered in either the COC/VEH or E2/VEH groups when compared with the SAL/VEH group, suggesting that the increase in histone H4 proteoforms might be unique to combined COC and E2 treatment. Post-translational modifications of histone proteins alter the conformation of nucleosomes that assemble DNA into chromatin, the accessibility of transcription factors at gene promoter regions, and gene expression. It is now well accepted that histone modifications are important for controlling COC-induced neuroplasticity and the behavioral effects of COC.67 Several studies have demonstrated that a single COC injection increases histone H4 acetylation in the NAc.68–71 E2 treatment of neuronal cell lines has also been shown to increase histone H4 acetylation.72, 73 Notably, increased histone H4 dimethylation at arginine 3 (H4R3me2a) in the NAc is also observed after COC treatment.74 Levels of H4R3me2a are behaviorally important, because treatment with an inhibitor of protein arginine N-methyltransferase 1 (PRMT1), an enzyme that methylates arginine residues, or down-regulation of PRMT1 expression in the NAc, reduced COC CPP in male mice.74 To our knowledge, we are the first to demonstrate an increase in proteoforms of histone H4 modified by methylation and acetylation in the VTA after combined COC and E2 administration. Although we cannot precisely identify each of the modified residues on histone H4, it is likely that these modifications have significant effects on chromatin accessibility and gene expression in the VTA that may regulate COC reward in females.
Figure 3. Plots of specific proteoform changes in the VTA with cocaine (COC) and estradiol (E2) treatment.
Quantitation of proteoforms of (A) myelin basic protein (PFR71904), (B) histone H4 (PFR22557), (C) ARPP-19 (PFR73684), (D) ARPP-19 (PFR244316), (E) α-endosulfine (ENSA, PFR49978), (F) cholecystokinin, and (PFR50255) (G) calmodulin (PFR51999). Box and whisker plots are presented for each of proteoforms. The symbol ** and *** (p < 0.01 and p < 0.001, respectively) indicates statistically significant proteoform abundance changes. These proteoforms were reported by high-throughput analysis. Abbreviations: PFR, proteoform record; SAL, saline; VEH, vehicle.
Three protein phosphatase inhibitors were increased in the COC/E2 group compared with the SAL/VEH group: protein phosphatase 1 regulatory subunit 11 (PPP1R11, Q8K1L5), cAMP-regulated phosphoprotein 19 (ARPP19, P56212), and alpha endosulfine (ENSA, also known as ARPP-19e, P60840) (Figure 2C, Figure 3, and Table 1). An acetylated and phosphorylated PPP1R11 proteoform (PFR51988) was upregulated by 3.4-fold in the COC/E2 group versus the SAL/VEH group, but this same proteoform was also increased by 4-fold in the COC/VEH group vs. the SAL/VEH group, indicating that the increase in the PPP1R11 proteoform is probably solely due to COC treatment. Interestingly, one proteoform of ARPP19 (16 kDa form, PFR73684) was increased by 3.3-fold in the COC/VEH vs. SAL/VEH group (Figure 2A and Figure 3C), but 4 different proteoforms of ARPP19 were increased by 1.5 to 1.9-fold in the COC/E2 group versus the SAL/VEH group (PFR56892, PFR244316 [Figure 3D], PFR58798, and PFR50002), indicating that the combination of E2 and COC treatment differentially affected processing and post-translational modifications of ARPP19 compared with COC treatment alone. Finally, N-terminal acetylated and alternatively spliced ENSA proteoforms increased 1.5 to 1.8-fold (Figure 3E, PFR49978) in the COC/E2 group and not in the COC/VEH group compared with the SAL/VEH group, indicating that the increase in its proteoforms is likely due to the combination of COC and E2 and not COC alone. ARPP and ENSA are phosphorylated in response to increased cAMP and have highly conserved PKA phosphorylation sites (serine 104 in ARPP19 and serine 109 in ENSA).75, 76 In our experiment, these proteoforms were not phosphorylated at the PKA site, suggesting that another kinase is responsible for their phosphorylation in response to COC and E2. Interestingly, Greatwall kinase, which regulates cell cycle progression, phosphorylates serine 62 of ARPP19,77 and one of the proteoforms of ARPP19 that was increased by COC and E2 treatment (PFR244316), although not fully characterized, did appear to be phosphorylated at either serine 23 or serine 62. The functional significance of increasing these specific proteoforms of ARPP19 and ENSA is not known, but is important for future studies because PP2A and ARPP19 regulate neurite outgrowth.78, 79 Alterations in the PP2A pathway by COC and E2 may therefore regulate neural plasticity in the VTA.
Only one proteoform was significantly increased in the VTA when comparing the COC/E2 group with the COC/VEH group (Figure 2D, Figure 3F, and Table 1). This was cholecystokinin (CCK, P09240), a peptide hormone that regulates dopamine release and behavioral responses to cocaine.80 The predominantly expressed proteoform encoding CCK (PFR50255) increased by 1.7-fold in the COC/E2 vs. COC/VEH group. CCK is one obvious candidate for the increase in COC CPP mediated by E2. Dopamine neurons in the VTA that project to the NAc produce CCK,81 and administration of CCK directly into the VTA increases cocaine release in the NAc,82, 83 which is expected to increase CPP. Indeed, CCK infused directly into the VTA increased amphetamine CPP,84 and systemic administration of CCK receptor antagonists blocked the reinstatement of COC CPP induced by a cocaine injection or stress.85 COC and E2 can independently alter CCK levels in the brain.86, 87 Here, we found that levels of bioactive CCK were increased in the VTA of mice treated with COC/E2 over COC/VEH-treated mice. E2 may therefore potentiate the effects of a low dose of COC on dopamine release in the NAc by increasing CCK levels, thus enhancing the rewarding properties of COC.
Several proteoforms were decreased in the COC/E2 group compared with the SAL/VEH and COC/VEH groups. Perhaps one of the more intriguing and novel findings in this study was the decrease in CaM proteoforms in the VTA by combined COC and E2 treatment. Three proteoforms (PFR51999 [Figure 3G], PFR244244, and PFR244235) were decreased by 1.7 to 2-fold in the COC/E2 group compared with both the SAL/VEH and COC/VEH groups, and an additional fourth proteoform (PFR49931) was decreased by 1.5-fold in the COC/E2 versus COC/VEH group (Figure 2C–D and Table 1). None of these CaM proteoforms were significantly decreased in the COC/VEH or SAL/E2 groups versus the SAL/VEH group, indicating a specific effect of combined E2 and COC treatment on decreasing these proteoforms. Post-translational modifications on CaM included phosphorylation, acetylation, and trimethylation. The CaM proteoform (PFR49931) that was decreased only in the COC/E2 group compared with the COC/VEH group was identified with high confidence as modified by an N-terminal acetylated lysine and trimethylation at position 116. Trimethylation of calmodulin lysine 116 is catalyzed by calmodulin-lysine N-methyltransferase (CAMKMT).88 The functional consequences of CaM lysine trimethylation have been characterized in plants by deleting CAMKMT. These studies have demonstrated that CaM lysine trimethyation affects root length and differential binding of proteins with CaM.89 Less is known about the functional role of CaM lysine trimethylation in the mammalian brain, except that deletion of Camkmt results in sensory and motor deficits in mice.90 However, behaviors related to drug abuse and drug reward have not been tested. Although CaM regulates the activity of hundreds of target proteins, it is interesting to speculate on the potential functional consequences of CaM lysine trimethylation in the VTA on COC reward. One important binding partner of CaM in neurons is Ca2+/calmodulin-dependent protein kinase II (CaMKII). Since injection of an inhibitory peptide of CaMKII into the mouse VTA decreases COC CPP,16 it is therefore possible that altered lysine trimethylation of CaM in response to COC and E2 could affect the interaction of CaM with CaMKII and COC reward. Another calcium binding protein, parvalbumin alpha (PVALB, P32848, N-terminal acetylated [PFR49919]), was decreased by 1.5-fold in the COC/E2 group compared with the COC/VEH group (Table 1), but was not significantly altered in the COC/VEH or SAL/E2 groups compared with the SAL/VEH group. These results indicate that there are alterations in acetylation, phosphorylation, and trimethylation of calcium binding proteins with combined COC and E2 treatment.
Proteoforms related to cytoskeleton dynamics and synaptic plasticity were also decreased in the COC/E2 group compared with the SAL/VEH and COC/VEH groups (Table 1). These include an N-terminal acetylated version of protein BRICK1 (Q91VR8, PFR49842), involved in actin and microtubule cytoskeleton remodeling and endocytic trafficking, and cytoplasmic dynein light chain 1 (DYNLL1, P63168, PFR51771), a molecular motor involved in vesicle trafficking along microtubules. An N-terminal acetylated version of profilin-1 (P62962, PFR51972), which regulates actin cytoskeleton remodeling, was decreased by 1.8-fold in the COC/E2 group compared with both the SAL/VEH and COC/VEH groups, but was also decreased in the SAL/E2 group, indicating that this decrease was likely due to E2 treatment alone (Table 1). An acetylated and phosphorylated proteoform of phosphatidylethanolamine-binding protein 1 (PEPB1, P70296, PFR203837), a lipid-binding protein located at synapses, was decreased in the COC/E2 group by 1.7 to 1.8-fold when compared with the COC/VEH and SAL/VEH groups, respectively and was not altered by separate COC or E2 treatments.
Mitochondrial proteins were decreased in both the COC/E2 versus SAL/VEH and the COC/E2 versus COC/VEH conditions (Table 1), including ATP synthase subunit delta (ATP5F1D, Q9D3D9, PFR8655 and PFR69959, 1.7 to 2-fold) and ATP synthase subunit g (ATP5l, Q9CPQ8, PFR22436, 1.8-fold). In addition, a phosphorylated and succinylated version of peroxiredoxin-5 (PRDX5, P99029, PFR244322) was decreased by 1.6-fold. These alterations suggest that there are potential changes in energy expenditure and the response to oxidative stress after combined COC- and E2-treatment. In our prior work measuring brain peptide differences between low and high cocaine locomotor responder rats, we found a significant difference between groups in a shortened form of ATP synthase-coupling factor 6 in the medial prefrontal cortex,91 supporting our current observation of changes in ATP synthase proteoforms in the brain after cocaine exposure.
Finally, there were decreases in acetylated nucleoside diphosphate kinases A (NME1, P15532, PFR49897 and PFR51260, 1.8-fold) and B (NME2, Q01768, PFR22649 1.6-fold), which are involved in the synthesis of nucleoside triphosphates (other than ATP), and a 1.7-fold decrease in an N-terminal acetylated form of dihydropteridine reductase (QDPR, Q8VBI4, PFR51693) in the COC/E2 group compared with both the COC/VEH and SAL/VEH groups. This enzyme produces tetrahydrobiopterin (BH-4), a cofactor for the tyrosine hydroxylase enzyme that is involved in the biosynthesis of dopamine.
To the best of our knowledge, there have been no previous reports of proteomic or transcriptomic studies on in the VTA of the female mouse brain after the combination of COC and E2 treatment, where a large number of differentially expressed proteoforms were detected. However, transcriptomic profiling of the ventral midbrain has been done with postmortem tissue from human cocaine abusers and in the VTA from rhesus macaques that have self-administered cocaine.92, 93 Although there are differences in species and cocaine access conditions, we sought to explain some proteomic findings here from cocaine-treated mice by comparing with the transcriptomic data. We found significantly differentially abundant proteoforms of PPP1R11, ARPP19, and ENSA between either the COC/VEH or COC/E2 group and the control group (SAL/VEH) within the VTA in our analysis. As mentioned previously, these proteins are regulators of protein phosphatases (PP2A, PP1). In humans, PPP1R15A was upregulated in the midbrain of cocaine addicts and in the macaque, PPP1R32 was increased in animals that had chronically self-administered cocaine. Together, these findings indicate conserved dysregulation of protein phosphatase-mediated signaling in the VTA after cocaine exposure. Altered PP1 signaling has been well-documented in the NAc after cocaine exposure,94 but less is known in the VTA. We also found decreased PVALB in the COC/E2 group comparted with the COC/VEH group and the PVALB transcript was reported to be decreased in the midbrain of human cocaine abusers.93 PVALB is a calcium binding protein that is expressed in a subset of inhibitory interneurons,95 suggesting that inhibitory neurotransmission in the VTA may also be altered after cocaine exposure.
Conclusions
In this study, we applied a label-free quantitative platform to comparative TD experiments on proteoforms below 30 kDa having PTMs and alternatively splicing events in specific regions of the female mouse brain after mice were trained for CPP. We identified numerous proteoform alterations in the VTA of E2-treated female mice after the behavioral expression of COC CPP. These proteoform changes may be responsible for the ability of E2 to increase COC reward and thus may contribute to the increased vulnerability of women to develop COC use disorder. Increased CCK in the VTA is likely at least partially responsible for the increased COC CPP observed in E2-treated mice compared with controls. However, altered abundance of other novel proteoforms identified in this study could conceivably play a role in this addiction-related behavior. We found differences in proteoforms that can regulate white matter and neuronal structure, such as those of MBP and cytoskeleton-associated proteins, respectively. Altered PTMs on histone H4 are predicted to change gene expression, while different proteoforms of calcium binding proteins and protein phosphatase inhibitors would be expected to change intracellular signaling. Future work is necessary to determine the functional and behavioral significance of these PTMs.
Supplementary Material
Table S1. List of proteoforms identified at a 1% global FDR per brain region of C57BL/6J female mice
Table S2. List of proteoforms identified at a 1% global FDR within AMY brain region by treatment group
Table S3. List of proteoforms identified at a 1% global FDR within NAc brain region by treatment group
Table S4. List of proteoforms identified at a 1% global FDR within VTA brain region by treatment group
Figure S1. Sources of variation in differential top-down proteomics
Figure S2. Quantitative comparison of proteoforms from treatment groups in AMY
Figure S3. Quantitative comparison of proteoforms from treatment groups in NAc
Figure S4. LC/MS analysis of brain proteins in VTA region of the female mouse brain
Figure S5. Differential expression of MBP in VTA region of the female mouse brain
Acknowledgements
This work was supported by the National Institute on Drug Abuse (P30 DA018310 and R01 DA033429) and NIH P41 GM108569. All authors declare no actual or potential conflicts of interest.
Footnotes
SUPPORTING INFORMATION:
The following supporting information is available free of charge at ACS website http://pubs.acs.org
References
- 1.Grant BF; Saha TD; Ruan WJ; Goldstein RB; Chou SP; Jung J; Zhang H; Smith SM; Pickering RP; Huang B; Hasin DS, Epidemiology of DSM-5 Drug Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry 2016, 73 (1), 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker JB, Sex differences in addiction. Dialogues Clin Neurosci 2016, 18 (4), 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobzean SA; DeNobrega AK; Perrotti LI, Sex differences in the neurobiology of drug addiction. Exp Neurol 2014, 259, 64–74. [DOI] [PubMed] [Google Scholar]
- 4.Hu M; Becker JB, Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug Alcohol Depend 2008, 94 (1–3), 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson LR; Robinson TE; Becker JB, Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology 2006, 31 (1), 129–38. [DOI] [PubMed] [Google Scholar]
- 6.Larson EB; Anker JJ; Gliddon LA; Fons KS; Carroll ME, Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol 2007, 15 (5), 461–71. [DOI] [PubMed] [Google Scholar]
- 7.Lynch WJ; Roth ME; Mickelberg JL; Carroll ME, Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 2001, 68 (4), 641–6. [DOI] [PubMed] [Google Scholar]
- 8.Martinez LA; Gross KS; Himmler BT; Emmitt NL; Peterson BM; Zlebnik NE; Foster Olive M; Carroll ME; Meisel RL; Mermelstein PG, Estradiol Facilitation of Cocaine Self-Administration in Female Rats Requires Activation of mGluR5. eNeuro 2016, 3 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doncheck EM; Urbanik LA; DeBaker MC; Barron LM; Liddiard GT; Tuscher JJ; Frick KM; Hillard CJ; Mantsch JR, 17beta-Estradiol Potentiates the Reinstatement of Cocaine Seeking in Female Rats: Role of the Prelimbic Prefrontal Cortex and Cannabinoid Type-1 Receptors. Neuropsychopharmacology 2018, 43 (4), 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larson EB; Carroll ME, Estrogen receptor beta, but not alpha, mediates estrogen’s effect on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology 2007, 32 (6), 1334–45. [DOI] [PubMed] [Google Scholar]
- 11.Larson EB; Roth ME; Anker JJ; Carroll ME, Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacol Biochem Behav 2005, 82 (1), 98–108. [DOI] [PubMed] [Google Scholar]
- 12.Satta R; Certa B; He D; Lasek AW, Estrogen Receptor beta in the Nucleus Accumbens Regulates the Rewarding Properties of Cocaine in Female Mice. Int J Neuropsychopharmacol 2018, 21 (4), 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segarra AC; Torres-Diaz YM; Silva RD; Puig-Ramos A; Menendez-Delmestre R; Rivera-Bermudez JG; Amadeo W; Agosto-Rivera JL, Estrogen receptors mediate estradiol’s effect on sensitization and CPP to cocaine in female rats: role of contextual cues. Horm Behav 2014, 65 (2), 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson SM; Pierce RC, Cocaine-induced alterations in dopamine receptor signaling: implications for reinforcement and reinstatement. Pharmacol Ther 2005, 106 (3), 389–403. [DOI] [PubMed] [Google Scholar]
- 15.Tobiansky DJ; Will RG; Lominac KD; Turner JM; Hattori T; Krishnan K; Martz JR; Nutsch VL; Dominguez JM, Estradiol in the Preoptic Area Regulates the Dopaminergic Response to Cocaine in the Nucleus Accumbens. Neuropsychopharmacology 2016, 41 (7), 1897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X; Liu Y; Zhong P; Wilkinson B; Qi J; Olsen CM; Bayer KU; Liu QS, CaMKII activity in the ventral tegmental area gates cocaine-induced synaptic plasticity in the nucleus accumbens. Neuropsychopharmacology 2014, 39 (4), 989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li FQ; Xue YX; Wang JS; Fang Q; Li YQ; Zhu WL; He YY; Liu JF; Xue LF; Shaham Y; Lu L, Basolateral amygdala cdk5 activity mediates consolidation and reconsolidation of memories for cocaine cues. J Neurosci 2010, 30 (31), 10351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitra SW; Hoskin E; Yudkovitz J; Pear L; Wilkinson HA; Hayashi S; Pfaff DW; Ogawa S; Rohrer SP; Schaeffer JM; McEwen BS; Alves SE, Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology 2003, 144 (5), 2055–67. [DOI] [PubMed] [Google Scholar]
- 19.Smith LM; Kelleher NL; Consortium for Top Down, P., Proteoform: a single term describing protein complexity. Nat Methods 2013, 10 (3), 186–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nesvizhskii AI; Aebersold R, Interpretation of shotgun proteomic data: the protein inference problem. Mol Cell Proteomics 2005, 4 (10), 1419–40. [DOI] [PubMed] [Google Scholar]
- 21.Patrie SM, Top-Down Mass Spectrometry: Proteomics to Proteoforms. Advances in experimental medicine and biology 2016, 919, 171–200. [DOI] [PubMed] [Google Scholar]
- 22.Ntai I; Toby TK; LeDuc RD; Kelleher NL, A Method for Label-Free, Differential Top-Down Proteomics. Methods Mol Biol 2016, 1410, 121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ntai I; Kim K; Fellers RT; Skinner OS; Smith A. D. t.; Early BP; Savaryn JP; LeDuc RD; Thomas PM; Kelleher NL, Applying label-free quantitation to top down proteomics. Anal Chem 2014, 86 (10), 4961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ntai I; LeDuc RD; Fellers RT; Erdmann-Gilmore P; Davies SR; Rumsey J; Early BP; Thomas PM; Li S; Compton PD; Ellis MJ; Ruggles KV; Fenyo D; Boja ES; Rodriguez H; Townsend RR; Kelleher NL, Integrated Bottom-Up and Top-Down Proteomics of Patient-Derived Breast Tumor Xenografts. Mol Cell Proteomics 2016, 15 (1), 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toby TK; Abecassis M; Kim K; Thomas PM; Fellers RT; LeDuc RD; Kelleher NL; Demetris J; Levitsky J, Proteoforms in Peripheral Blood Mononuclear Cells as Novel Rejection Biomarkers in Liver Transplant Recipients. Am J Transplant 2017, 17 (9), 2458–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis RG; Park HM; Kim K; Greer JB; Fellers RT; LeDuc RD; Romanova EV; Rubakhin SS; Zombeck JA; Wu C; Yau PM; Gao P; van Nispen AJ; Patrie SM; Thomas PM; Sweedler JV; Rhodes JS; Kelleher NL, Top-Down Proteomics Enables Comparative Analysis of Brain Proteoforms Between Mouse Strains. Anal Chem 2018, 90 (6), 3802–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gresack JE; Frick KM, Effects of continuous and intermittent estrogen treatments on memory in aging female mice. Brain Res 2006, 1115 (1), 135–47. [DOI] [PubMed] [Google Scholar]
- 28.Gresack JE; Frick KM, Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav 2006, 84 (1), 112–9. [DOI] [PubMed] [Google Scholar]
- 29.Hilderbrand ER; Lasek AW, Sex differences in cocaine conditioned place preference in C57BL/6J mice. Neuroreport 2014, 25 (2), 105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paxinos G; Franklin KBJ, The Mouse Brain in Stereotaxic Coordinates. Second ed.; Academic Press: San Diego, 2001. [Google Scholar]
- 31.Toby TK; Fornelli L; Srzentic K; DeHart CJ; Levitsky J; Friedewald J; Kelleher NL, A comprehensive pipeline for translational top-down proteomics from a single blood draw. Nat Protoc 2019, 14 (1), 119–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wessel D; Flugge UI, A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem 1984, 138 (1), 141–3. [DOI] [PubMed] [Google Scholar]
- 33.Cleland TP; DeHart CJ; Fellers RT; VanNispen AJ; Greer JB; LeDuc RD; Parker WR; Thomas PM; Kelleher NL; Brodbelt JS, High-Throughput Analysis of Intact Human Proteins Using UVPD and HCD on an Orbitrap Mass Spectrometer. J Proteome Res 2017, 16 (5), 2072–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fornelli L; Durbin KR; Fellers RT; Early BP; Greer JB; LeDuc RD; Compton PD; Kelleher NL, Advancing Top-down Analysis of the Human Proteome Using a Benchtop Quadrupole-Orbitrap Mass Spectrometer. Journal of proteome research 2017, 16 (2), 609–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson LC; DeHart CJ; Kaiser NK; Fellers RT; Smith DF; Greer JB; LeDuc RD; Blakney GT; Thomas PM; Kelleher NL; Hendrickson CL, Identification and Characterization of Human Proteoforms by Top-Down LC-21 Tesla FT-ICR Mass Spectrometry. Journal of proteome research 2017, 16 (2), 1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng F; Cargile BJ; Miller LM; Forbes AJ; Johnson JR; Kelleher NL, Informatics and multiplexing of intact protein identification in bacteria and the archaea. Nature biotechnology 2001, 19 (10), 952–7. [DOI] [PubMed] [Google Scholar]
- 37.LeDuc RD; Fellers RT; Early BP; Greer JB; Thomas PM; Kelleher NL, The C-score: a Bayesian framework to sharply improve proteoform scoring in high-throughput top down proteomics. Journal of proteome research 2014, 13 (7), 3231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higdon R; Haynes W; Kolker E, Meta-analysis for protein identification: a case study on yeast data. OMICS 2010, 14 (3), 309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamini Y; Hochberg Y, Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met 1995, 57 (1), 289–300. [Google Scholar]
- 40.Tonn Eisinger KR; Larson EB; Boulware MI; Thomas MJ; Mermelstein PG, Membrane estrogen receptor signaling impacts the reward circuitry of the female brain to influence motivated behaviors. Steroids 2018, 133, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fellers RT; Greer JB; Early BP; Yu X; LeDuc RD; Kelleher NL; Thomas PM, ProSight Lite: graphical software to analyze top-down mass spectrometry data. Proteomics 2015, 15 (7), 1235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goertz RB; Wanat MJ; Gomez JA; Brown ZJ; Phillips PE; Paladini CA, Cocaine increases dopaminergic neuron and motor activity via midbrain alpha1 adrenergic signaling. Neuropsychopharmacology 2015, 40 (5), 1151–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koulchitsky S; De Backer B; Quertemont E; Charlier C; Seutin V, Differential effects of cocaine on dopamine neuron firing in awake and anesthetized rats. Neuropsychopharmacology 2012, 37 (7), 1559–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borgland SL; Malenka RC; Bonci A, Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci 2004, 24 (34), 7482–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plymire DA; Wing CE; Robinson DE; Patrie SM, Continuous Elution Proteoform Identification of Myelin Basic Protein by Superficially Porous Reversed-Phase Liquid Chromatography and Fourier Transform Mass Spectrometry. Anal Chem 2017, 89 (22), 12030–12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartzokis G; Beckson M; Lu PH; Edwards N; Bridge P; Mintz J, Brain maturation may be arrested in chronic cocaine addicts. Biol Psychiatry 2002, 51 (8), 605–11. [DOI] [PubMed] [Google Scholar]
- 47.Lim KO; Choi SJ; Pomara N; Wolkin A; Rotrosen JP, Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry 2002, 51 (11), 890–5. [DOI] [PubMed] [Google Scholar]
- 48.Lim KO; Wozniak JR; Mueller BA; Franc DT; Specker SM; Rodriguez CP; Silverman AB; Rotrosen JP, Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend 2008, 92 (1–3), 164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moeller FG; Hasan KM; Steinberg JL; Kramer LA; Dougherty DM; Santos RM; Valdes I; Swann AC; Barratt ES; Narayana PA, Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology 2005, 30 (3), 610–7. [DOI] [PubMed] [Google Scholar]
- 50.Ma L; Hasan KM; Steinberg JL; Narayana PA; Lane SD; Zuniga EA; Kramer LA; Moeller FG, Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend 2009, 104 (3), 262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narayana PA; Ahobila-Vajjula P; Ramu J; Herrera J; Steinberg JL; Moeller FG, Diffusion tensor imaging of cocaine-treated rodents. Psychiatry Res 2009, 171 (3), 242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narayana PA; Herrera JJ; Bockhorst KH; Esparza-Coss E; Xia Y; Steinberg JL; Moeller FG, Chronic cocaine administration causes extensive white matter damage in brain: diffusion tensor imaging and immunohistochemistry studies. Psychiatry Res 2014, 221 (3), 220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith HR; Beveridge TJ; Nader MA; Porrino LJ, Regionally-specific alterations in myelin proteins in nonhuman primate white matter following prolonged cocaine self-administration. Drug Alcohol Depend 2014, 137, 143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albertson DN; Pruetz B; Schmidt CJ; Kuhn DM; Kapatos G; Bannon MJ, Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem 2004, 88 (5), 1211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albertson DN; Schmidt CJ; Kapatos G; Bannon MJ, Distinctive profiles of gene expression in the human nucleus accumbens associated with cocaine and heroin abuse. Neuropsychopharmacology 2006, 31 (10), 2304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bannon M; Kapatos G; Albertson D, Gene expression profiling in the brains of human cocaine abusers. Addict Biol 2005, 10 (1), 119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Romanova EV; Rubakhin SS; Ossyra JR; Zombeck JA; Nosek MR; Sweedler JV; Rhodes JS, Differential peptidomics assessment of strain and age differences in mice in response to acute cocaine administration. J Neurochem 2015, 135 (5), 1038–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harauz G; Boggs JM, Myelin management by the 18.5-kDa and 21.5-kDa classic myelin basic protein isoforms. J Neurochem 2013, 125 (3), 334–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Micevych PE; Mermelstein PG; Sinchak K, Estradiol Membrane-Initiated Signaling in the Brain Mediates Reproduction. Trends Neurosci 2017, 40 (11), 654–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vassall KA; Bamm VV; Harauz G, MyelStones: the executive roles of myelin basic protein in myelin assembly and destabilization in multiple sclerosis. Biochem J 2015, 472 (1), 17–32. [DOI] [PubMed] [Google Scholar]
- 61.Yang L; Tan D; Piao H, Myelin Basic Protein Citrullination in Multiple Sclerosis: A Potential Therapeutic Target for the Pathology. Neurochem Res 2016, 41 (8), 1845–56. [DOI] [PubMed] [Google Scholar]
- 62.Khalaj AJ; Hasselmann J; Augello C; Moore S; Tiwari-Woodruff SK, Nudging oligodendrocyte intrinsic signaling to remyelinate and repair: Estrogen receptor ligand effects. J Steroid Biochem Mol Biol 2016, 160, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spence RD; Voskuhl RR, Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol 2012, 33 (1), 105–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiao Q; Luo Y; Lv F; He Q; Wu H; Chao F; Qiu X; Zhang L; Gao Y; Huang C; Wang S; Zhou C; Zhang Y; Jiang L; Tang Y, Protective Effects of 17beta-Estradiol on Hippocampal Myelinated Fibers in Ovariectomized Middle-aged Rats. Neuroscience 2018, 385, 143–153. [DOI] [PubMed] [Google Scholar]
- 65.He Q; Luo Y; Lv F; Xiao Q; Chao F; Qiu X; Zhang L; Gao Y; Xiu Y; Huang C; Tang Y, Effects of estrogen replacement therapy on the myelin sheath ultrastructure of myelinated fibers in the white matter of middle-aged ovariectomized rats. J Comp Neurol 2018, 526 (5), 790–802. [DOI] [PubMed] [Google Scholar]
- 66.Moreno-Lopez L; Catena A; Fernandez-Serrano MJ; Delgado-Rico E; Stamatakis EA; Perez-Garcia M; Verdejo-Garcia A, Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend 2012, 125 (3), 208–14. [DOI] [PubMed] [Google Scholar]
- 67.Rogge GA; Wood MA, The role of histone acetylation in cocaine-induced neural plasticity and behavior. Neuropsychopharmacology 2013, 38 (1), 94–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brami-Cherrier K; Valjent E; Herve D; Darragh J; Corvol JC; Pages C; Arthur SJ; Girault JA; Caboche J, Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci 2005, 25 (49), 11444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jordi E; Heiman M; Marion-Poll L; Guermonprez P; Cheng SK; Nairn AC; Greengard P; Girault JA, Differential effects of cocaine on histone posttranslational modifications in identified populations of striatal neurons. Proc Natl Acad Sci U S A 2013, 110 (23), 9511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar A; Choi KH; Renthal W; Tsankova NM; Theobald DE; Truong HT; Russo SJ; Laplant Q; Sasaki TS; Whistler KN; Neve RL; Self DW; Nestler EJ, Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 2005, 48 (2), 303–14. [DOI] [PubMed] [Google Scholar]
- 71.Levine AA; Guan Z; Barco A; Xu S; Kandel ER; Schwartz JH, CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci U S A 2005, 102 (52), 19186–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lalmansingh AS; Uht RM, Estradiol regulates corticotropin-releasing hormone gene (crh) expression in a rapid and phasic manner that parallels estrogen receptor-alpha and -beta recruitment to a 3’,5’-cyclic adenosine 5’-monophosphate regulatory region of the proximal crh promoter. Endocrinology 2008, 149 (1), 346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu JC; Baker RE; Chow W; Sun CK; Elsholtz HP, Epigenetic mechanisms in the dopamine D2 receptor-dependent inhibition of the prolactin gene. Mol Endocrinol 2005, 19 (7), 1904–17. [DOI] [PubMed] [Google Scholar]
- 74.Li Y; Zhu R; Wang W; Fu D; Hou J; Ji S; Chen B; Hu Z; Shao X; Yu X; Zhao Q; Zhang B; Du C; Bu Q; Hu C; Tang Y; Zhong L; Yang S; Zhao Y; Cen X, Arginine Methyltransferase 1 in the Nucleus Accumbens Regulates Behavioral Effects of Cocaine. J Neurosci 2015, 35 (37), 12890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dulubova I; Horiuchi A; Snyder GL; Girault JA; Czernik AJ; Shao L; Ramabhadran R; Greengard P; Nairn AC, ARPP-16/ARPP-19: a highly conserved family of cAMP-regulated phosphoproteins. J Neurochem 2001, 77 (1), 229–38. [DOI] [PubMed] [Google Scholar]
- 76.Girault JA; Shalaby IA; Rosen NL; Greengard P, Regulation by cAMP and vasoactive intestinal peptide of phosphorylation of specific proteins in striatal cells in culture. Proc Natl Acad Sci U S A 1988, 85 (20), 7790–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gharbi-Ayachi A; Labbe JC; Burgess A; Vigneron S; Strub JM; Brioudes E; Van-Dorsselaer A; Castro A; Lorca T, The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 2010, 330 (6011), 1673–7. [DOI] [PubMed] [Google Scholar]
- 78.White RE; Giffard RG, MicroRNA-320 induces neurite outgrowth by targeting ARPP-19. Neuroreport 2012, 23 (10), 590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu D; Zheng HY; Luo ZZ; Wang Q; Zhu LQ, Effect of PP-2A on neurite outgrowth in neuronal cells. In Vitro Cell Dev Biol Anim 2010, 46 (8), 702–7. [DOI] [PubMed] [Google Scholar]
- 80.Loonam TM; Noailles PA; Yu J; Zhu JP; Angulo JA, Substance P and cholecystokinin regulate neurochemical responses to cocaine and methamphetamine in the striatum. Life Sci 2003, 73 (6), 727–39. [DOI] [PubMed] [Google Scholar]
- 81.Lanca AJ; De Cabo C; Arifuzzaman AI; Vaccarino FJ, Cholecystokinergic innervation of nucleus accumbens subregions. Peptides 1998, 19 (5), 859–68. [DOI] [PubMed] [Google Scholar]
- 82.Hamilton ME; Freeman AS, Effects of administration of cholecystokinin into the VTA on DA overflow in nucleus accumbens and amygdala of freely moving rats. Brain Res 1995, 688 (1–2), 134–42. [DOI] [PubMed] [Google Scholar]
- 83.Reum T; Schafer U; Marsden CA; Fink H; Morgenstern R, Cholecystokinin increases extracellular dopamine overflow in the anterior nucleus accumbens via CCK(B) receptors in the VTA assessed by in vivo voltammetry. Neuropeptides 1997, 31 (1), 82–8. [DOI] [PubMed] [Google Scholar]
- 84.Pettit HO; Mueller K, Infusions of cholecystokinin octapeptide into the ventral tegmental area potentiate amphetamine conditioned place preferences. Psychopharmacology (Berl) 1989, 99 (3), 423–6. [DOI] [PubMed] [Google Scholar]
- 85.Lu L; Zhang B; Liu Z; Zhang Z, Reactivation of cocaine conditioned place preference induced by stress is reversed by cholecystokinin-B receptors antagonist in rats. Brain Res 2002, 954 (1), 132–40. [DOI] [PubMed] [Google Scholar]
- 86.Beinfeld MC; Connolly KJ; Pierce RC, Cocaine treatment increases extracellular cholecystokinin (CCK) in the nucleus accumbens shell of awake, freely moving rats, an effect that is enhanced in rats that are behaviorally sensitized to cocaine. J Neurochem 2002, 81 (5), 1021–7. [DOI] [PubMed] [Google Scholar]
- 87.Holm L; Liang W; Thorsell A; Hilke S, Acute effects on brain cholecystokinin-like concentration and anxiety-like behaviour in the female rat upon a single injection of 17beta-estradiol. Pharmacol Biochem Behav 2014, 122, 222–7. [DOI] [PubMed] [Google Scholar]
- 88.Magnani R; Dirk LM; Trievel RC; Houtz RL, Calmodulin methyltransferase is an evolutionarily conserved enzyme that trimethylates Lys-115 in calmodulin. Nat Commun 2010, 1, 43. [DOI] [PubMed] [Google Scholar]
- 89.Banerjee J; Magnani R; Nair M; Dirk LM; DeBolt S; Maiti IB; Houtz RL, Calmodulin-mediated signal transduction pathways in Arabidopsis are fine-tuned by methylation. Plant Cell 2013, 25 (11), 4493–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haziza S; Magnani R; Lan D; Keinan O; Saada A; Hershkovitz E; Yanay N; Cohen Y; Nevo Y; Houtz RL; Sheffield VC; Golan H; Parvari R, Calmodulin Methyltransferase Is Required for Growth, Muscle Strength, Somatosensory Development and Brain Function. PLoS Genet 2015, 11 (8), e1005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Romanova EV; Lee JE; Kelleher NL; Sweedler JV; Gulley JM, Mass spectrometry screening reveals peptides modulated differentially in the medial prefrontal cortex of rats with disparate initial sensitivity to cocaine. AAPS J 2010, 12 (3), 443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vallender EJ; Goswami DB; Shinday NM; Westmoreland SV; Yao WD; Rowlett JK, Transcriptomic profiling of the ventral tegmental area and nucleus accumbens in rhesus macaques following long-term cocaine self-administration. Drug Alcohol Depend 2017, 175, 9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bannon MJ; Johnson MM; Michelhaugh SK; Hartley ZJ; Halter SD; David JA; Kapatos G; Schmidt CJ, A molecular profile of cocaine abuse includes the differential expression of genes that regulate transcription, chromatin, and dopamine cell phenotype. Neuropsychopharmacology 2014, 39 (9), 2191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walaas SI; Hemmings HC Jr.; Greengard P; Nairn AC, Beyond the dopamine receptor: regulation and roles of serine/threonine protein phosphatases. Front Neuroanat 2011, 5, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu H; Gan J; Jonas P, Interneurons. Fast-spiking, parvalbumin(+) GABAergic interneurons: from cellular design to microcircuit function. Science 2014, 345 (6196), 1255263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of proteoforms identified at a 1% global FDR per brain region of C57BL/6J female mice
Table S2. List of proteoforms identified at a 1% global FDR within AMY brain region by treatment group
Table S3. List of proteoforms identified at a 1% global FDR within NAc brain region by treatment group
Table S4. List of proteoforms identified at a 1% global FDR within VTA brain region by treatment group
Figure S1. Sources of variation in differential top-down proteomics
Figure S2. Quantitative comparison of proteoforms from treatment groups in AMY
Figure S3. Quantitative comparison of proteoforms from treatment groups in NAc
Figure S4. LC/MS analysis of brain proteins in VTA region of the female mouse brain
Figure S5. Differential expression of MBP in VTA region of the female mouse brain