Abstract
Background
We developed a new model of hypertension (HTN) care for non-Hispanic black men that links health promotion by barbers to medication management by specialty-trained pharmacists and demonstrated efficacy in a 6-month randomized trial (Victor et al., NEJM, 2018). The marked reduction in systolic blood pressure (BP) seen at 6 months warranted continuing the trial through 12 months to test sustainability, a necessary precondition for subsequent implementation research.
Methods
We enrolled a cohort of 319 black male patrons with systolic BP ≥ 140 mm Hg at baseline, in a cluster-randomized trial. Fifty-two Los Angeles County barbershops were assigned to either a pharmacist-led intervention or an active control group. In the intervention group, barbers promoted follow-up with pharmacists who prescribed BP medication under a collaborative practice agreement with patrons’ primary care providers (PCPs). In the control group, barbers promoted follow-up with PCPs and lifestyle modification. After BP assessment at 6 months, the intervention continued with fewer in-person pharmacist visits to test if the intervention effect could be sustained safely for one year while reducing pharmacist travel time to and from barbershops. Final BP and safety outcomes were assessed in both groups at 12 months.
Results
At baseline, mean systolic BP was 152.4 mm Hg in the intervention group and 154.6 mm Hg in the control group. At 12 months, mean systolic BP fell by −28.6 mm Hg (to 123.8 mm Hg) in the intervention group and by −7.2 mm Hg (to 147.4 mm Hg) in the control group. The mean reduction was 20.8 mm Hg greater with the intervention (95% confidence interval, 13.9 to 27.7; p < 0.0001). A goal BP < 130/80 was achieved by 68.0% of the intervention group versus 11.0% of the control group (p < 0.02). These new 12-month efficacy data are statistically indistinguishable from our previously reported 6-month data. No treatment-related serious adverse events occurred in either group over 12 months. Cohort retention at 12 months was 90% in both groups.
Conclusion
Among black male barbershop patrons with uncontrolled HTN, health promotion by barbers resulted in large and sustained BP reduction when coupled with medication management by specialty-trained pharmacists. Broad-scale implementation research is both justified and warranted.
Clinical Trial Registration
INTRODUCTION
Undertreatment of hypertension is particularly devastating to non-Hispanic black men who are underrepresented in pharmacist-intervention trials in traditional healthcare settings.1–6 Health outreach to barbershops is common7, but programs have not evaluated efficacy with clinical trial methodology nor linked barber-based interventions to a community-partnered, team-based approach.
We created a new model of hypertension (HTN) care for non-Hispanic black men that links health promotion by barbers to medication management by specialty-trained pharmacists and demonstrated efficacy in a 6-month cluster-randomized trial.8
In this trial, barbershops were randomized to either a pharmacist-led intervention or an active control group. In the intervention group, barbers promoted follow up with pharmacists who met with intervention participants at least monthly in their barbershops and prescribed blood pressure (BP) medication under a collaborative practice agreement (CPA) with primary care providers (PCP). In the control group, barbers were trained to encourage lifestyle modification and doctor’s appointments.
The mean reductions in systolic and diastolic BP (−21.6 mm Hg and −14.9 mm Hg respectively) at 6 months were impressive for a community-based trial in a traditionally difficult-to-reach, mainly low-income male population. The intervention effect was also 3 times larger than the −7 mm Hg effect shown in other pharmacist-led HTN intervention trials with similar baseline systolic blood pressure levels (~150 mmHg).1–6
The results warranted a 6-month extension study as a means of testing sustainability, a necessary pre-condition for subsequent implementation research. Here we executed the same protocol for an additional 6-months for all participants with complete data at the end of the initial 6-month trial. The primary hypothesis was that the systolic blood pressure reduction achieved after 6-months would be sustained at 12-months and would continue to favor the pharmacist-led intervention.
METHODS
Study Design and Oversight
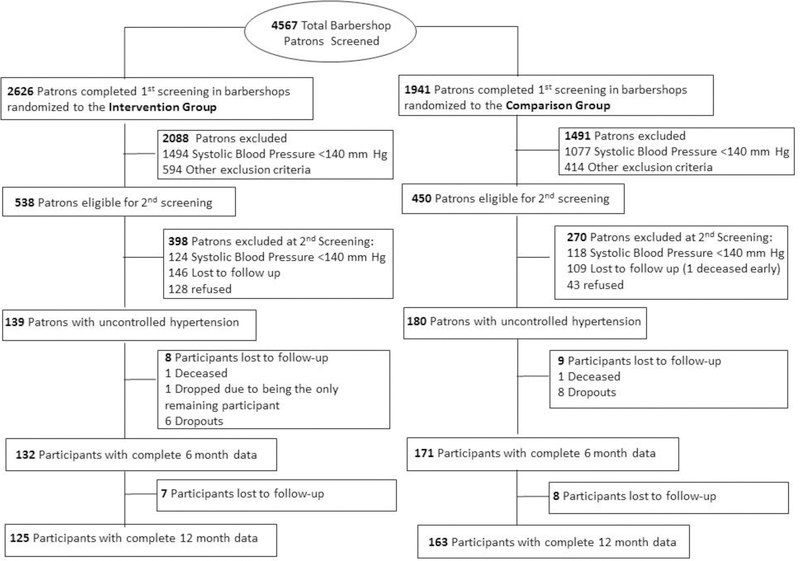
Barbershops were the unit of randomization. Participant arm was determined by barbershop (Fig. 1, Fig. S1 in Supplementary Appendix, and Protocol at NEJM.org) at baseline and did not change in the 6-month extension study. The study was approved by institutional review boards at Cedars-Sinai Medical Center, Kaiser-Permanente, and Westat (survey research company that conducted screening and enrollment and collected baseline and follow-up data), with an independent data safety and monitoring board.9 All participants gave informed written consent.
Figure 1. Screening, Enrollment, and Follow-Up of Barbershop Patrons.
Other exclusion criteria included: infrequent barbershop patronage (duration of less than 6 months or longer than every 6 weeks in between visits), age < 35 or >79 years old, receiving either dialysis or cancer chemotherapy, plans to relocate and incomplete 6-month data.
R.G.V. and R.E. designed the study, field interviewers, C.A.B. and K.L. gathered data, R.E., N.L., L.C.C. and R.G.V analyzed the data, R.G.V. and R.E. vouch for the data and analysis, R.G.V. and C.A.B. wrote the paper, and all coauthors decided to publish.
Study Population
A cohort of 319 self- identified non-Hispanic black men who had complete data at the end of our initial 6-month study were eligible to continue on to the 6-month extension phase. All men were 35–79 years of age, regular patrons of participating barbershops (≥ 1 haircut every 6 weeks for ≥ 6 months) and had systolic blood pressure ≥140 mm Hg on two screening days at baseline (Fig. 1). Men who planned to relocate, were on dialysis or chemotherapy, and women were excluded.
Randomization and Interventions
Randomization and intervention methods have been described previously.8 In brief, cluster randomization was necessary to avoid between-group contamination and to account for intra-class correlation (ICC).10,11 At baseline, barbershops were randomized 1:1 to intervention and comparison groups. Shop randomization occurred in equally balanced blocks of four using a pre-specified random-number sequence. Neither participants nor field interviewers could be blinded to barbershop condition assignment however, baseline and follow-up data were collected by independently-contracted field-interviewers who were not invested in study outcomes.
Barbers in shops randomized to the intervention were trained to encourage pharmacist follow-up and measure blood pressure. Before pharmacist intervention, participant’s PCP’s signed a CPA (Section S3 in the Supplementary Appendix). Two fulltime doctoral-level pharmacists (C.A.B, K.L.) received specialized training and certification as hypertension clinicians and regularly reviewed each participant’s treatment with physician hypertension specialists (R.G.V., J.H., J.B.). Pharmacists met regularly with participants in barbershops in the intervention arm and prescribed a combination antihypertensive drug regimen; measured blood pressure; encouraged lifestyle changes; and monitored plasma electrolytes and creatinine, with a CLIA-waived point-of-care device (i-STAT, Abbott Park, Illinois).12 The protocol required pharmacists to first prescribe a two-drug regimen that insurance would approve—preferably a dihydropyridine calcium-channel blocker (e.g. amplodipine) combined with either a long-acting angiotensin-converting–enzyme inhibitor (ACE-I) or angiotensin-receptor blocker (ARB). The long-acting thiazide-type diuretic indapamide was the preferred third-line drug13,14 followed by an aldosterone antagonist if a fourth drug was needed. Drug class substitutions were allowed when medically indicated. After each encounter with a participant, pharmacists sent progress notes with their contact information to the given participant’s healthcare provider.
In the control group participants received instruction about blood pressure and lifestyle modification (Fig. S2 in the Supplementary Appendix). Barbers were trained to discuss the instructional information with participants and encourage follow-up with primary care providers.
In the extension phase of the study, both groups received the following cohort retention tools that also fostered blood pressure reduction – 9-month follow-up calls on interval health changes; culturally-specific health lessons; and monthly haircut vouchers. In intervention shops only, participants received $25 per pharmacist visit to offset costs of generic drugs and pharmacy transportation.
Study Measurements
Field interviewers administered 30-minute structured in-person, computer-based health questionnaire to participants in both arms at baseline, 6 and 12 months. These interviewers recorded blood pressure and structured response data on demographic characteristics, patient-reported outcomes, and prescription information transcribed from pill bottles.
All blood pressures were measured in barbershops using a validated oscillometric monitor (AccutorrV, Mindray, Mahwah, NJ).15 To automate measurement and minimize operator-dependence, monitor readings were directly uploaded to a computer that electronically transmitted data to a secure website. Field interviewers, pharmacists, and barbers all used the same automated protocol, which required 5 sequential readings – the first 2 readings were discarded, and the last 3 readings produced a mean value.16 All parties were trained in proper measurement technique (5 minutes rest, arm at heart level, no conversation with participants, feet flat, back supported, and no urinary urgency). The correct arm cuff size was determined for each participant at the first screening and used throughout the trial. To reduce regression to the mean, the second screening blood pressure was taken as the baseline value.17
For 12 months, pharmacists and some barbers measured blood pressure monthly to monitor drug therapy in only the intervention arm. The final 12-month blood pressures were recorded by field interviewers in the control arm and by pharmacists in the intervention arm to minimize the alerting reaction evoked by an unfamiliar data collector.
The pre-specified blood pressure goal was <130/80 mmHg – 5/5 mm Hg lower than the conventional out-of-office blood pressure goal of <135/85 mmHg18 (prior to the release of the 2017 guidelines) – to account for blood pressure variability.
Study Outcomes
All study outcomes were taken as changes from baseline to 12 months. The pre-specified primary outcome was the change in systolic blood pressure. Secondary outcomes included the change in diastolic pressure, blood pressure goal attainment rates, number of antihypertensive drugs prescribed, adverse drug reactions, self-rated health19, and patient engagement by a validated instrument.20
Statistical Analysis
With an enrollment target of 10 barbershop clusters per study arm—25 participants per cluster, 70% cohort retention, and an estimated ICC of 0.0116 — the initial design yielded 90% power to detect a −6.9 mmHg greater reduction in systolic blood pressure at 6 months in the intervention versus control arm with a 2-sided alpha level of 0.05. Due to the total number of patrons per barbershop being lower than anticipated, we increased the number of shops and grouped low-enrolling shops into clusters by both enrollment date and geographic proximity, yielding 10 shop-clusters per arm with ≥10 participants per cluster.21,22 The number of dropouts was very small (Fig. 1) and thus considered random after extensive analysis.23
The intervention effect at 12 months was estimated by a linear mixed effects model, which included a random cluster effect. The primary predictor was an indicator for intervention versus control arm. Given the sample size, the model included three baseline covariates – baseline blood pressure, a doctor for routine medical care, and high cholesterol. These were either strongly correlated with the dependent variable or showed baseline imbalance between arms.
The linear mixed effects model and its assumptions were as follows:
where was the change in systolic or diastolic blood pressure from baseline to 12 months for patient j in cluster i, was the main intervention effect with if the i-th cluster was in the intervention group and if in the comparison group, and to were fixed effects of the baseline patient-level covariates. We also included the interaction between intervention and each of the covariates, routine doctor and high cholesterol. The random cluster effect was assumed to be , and the measurement error for the j-th individual in the i-th cluster was assumed to be . We further assumed that was independent of the measurement error and that ’s were mutually independent. The ICC was calculated as . For change in systolic blood pressure, the actual calculated ICC was 0.01. For binary secondary outcomes, we used generalized estimating equations with a compound symmetry working correlation matrix to estimate the invention effect while controlling for the above covariates.
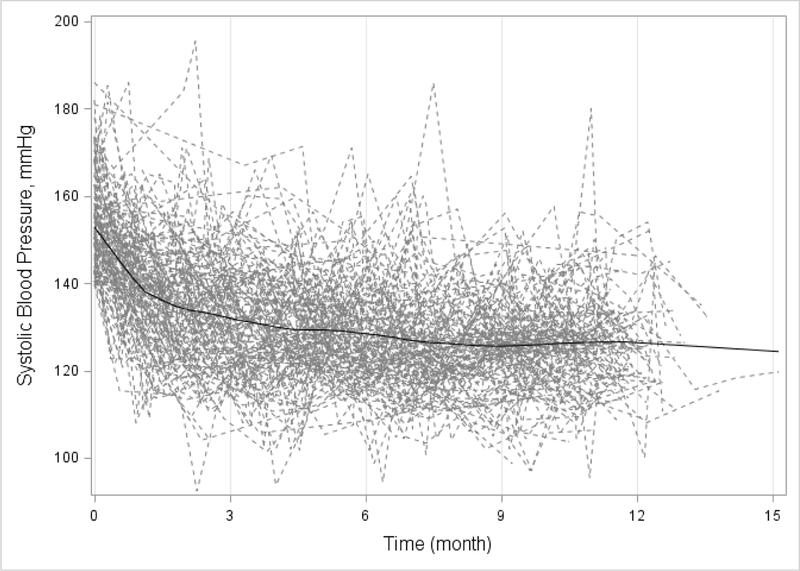
Longitudinal analysis was performed for the repeated measurements of systolic blood pressure on patients in the intervention arm. The profile plot of systolic blood pressure with the loess curve suggested a more rapid decline in the early stage of the intervention (Fig. 2). This non-linear trend was characterized by piece-wise linear splines with a knot at t0 in the following linear mixed effects model:
Figure 2.
Individual Profile Plot and Locally Weighted Polynomial Regression (LOESS) Curve of Systolic Blood Pressure in Intervention Group
where was the systolic blood pressure measured at time t for patient j in cluster i, t1 = t if t ≤ t0 and t1 = 0 if otherwise, and t2 = 0 if t ≤ t0 and t2 = t if otherwise, so that was the slope for t ≤ t0 and is the slope for t > t0. The analysis was adjusted for baseline age which was found to be associated with intervention systolic blood pressure in our preliminary analysis (p < 0.1). The random cluster effect was assumed to be , and the measurement error for the j-th individual in the i-th cluster was assumed to be . The random effects , , and characterized individual-level heterogeneity in the intercept and two piece-wise linear time trends and were posited to be . Finally, we assumed , , and (, , ) were mutually independent. We estimated the model for different locations of t0 and compared the goodness of fit using AIC. The knot at 6 months provided the best fit and thus was chosen as the final model.
RESULTS
Study Sites and Study Participants
Fifty-two Los Angeles County barbershops completed 12-month participation between February 2015 to December 2017 (Fig. S1 in Supplementary Appendix). The primary statistical analysis is based on 125 participants in 28 intervention shops and 163 participants in 24 control shops that completed 12-month follow-up (Fig. 1). An intention-to-treat (ITT) analysis also was performed, using the last measured blood pressure for 14 participants lost to follow-up in the intervention group and 8 participants lost to follow-up after 6 months in the control group; however, no adjustment for abbreviated treatment could be made for 9 participants lost to follow-up prior to 6 months in the control group who had only baseline data (Fig. 1).
The two groups remained well-balanced across most characteristics, except a higher percentage of participants in the intervention group had high cholesterol by self-report (Table 1 and Table S1 in Supplementary Appendix). Cohort retention at the end of 12 months was 90% in both groups (Fig. 1).
Table 1.
Baseline Characteristics of the Barbershops and Study Participants*
| Characteristic | Intervention | Control |
|---|---|---|
| Barbershops | ||
| No. of barbershops | 28 | 24 |
| Years in business | 17.3 ± 14.2 | 18.1 ± 8.3 |
| No. of Barbers per shop | 4 ± 2 | 4 ± 2 |
| No. of Patrons screened per shop | 90 ± 47 | 81 ± 43 |
| Participants | ||
| No. of participants | 139 | 180 |
| Age - yr | 54.4 ± 10.2 | 54.5 ± 9.4 |
| Married or living with a partner - no. (%) | 64 (46.4) | 88 (48.9) |
| Highest education - no. (%) | ||
| Less than high school | 6 (4.5) | 15 (8.6) |
| High school graduate (includes equivalency) | 30 (22.6) | 51 (29.1) |
| Some college, or Associate’s degree | 68 (51.1) | 76 (43.4) |
| Bachelor’s degree | 22 (16.5) | 23 (13.1) |
| Graduate or professional degree | 7 (5.3) | 10 (5.7) |
| Household Income, % of the federal poverty level - no. (%)† | ||
| <100% of Federal Poverty Limit | 41 (31.8) | 43 (24.4) |
| 100–300% of Federal Poverty Limit | 36 (27.9) | 48 (27.3) |
| 301–500% of Federal Poverty Limit | 26 (20.2) | 49 (27.8) |
| >500% of Federal Poverty Limit | 26 (20.2) | 36 (20.5) |
| Regular medical care provider - no. (%) | 106 (76.8) | 137 (77.0) |
| Any health insurance - no. (%) | 118 (84.9) | 155 (86.1) |
| Barbershop patronage | ||
| Duration of patronage - yr | 10.4 ± 9.9 | 11.4 ± 8.8 |
| Frequency of visits - every no. of weeks | 2.0 ± 0.9 | 2.1 ± 1.1 |
| Cardiac risk factors and history‡ | ||
| Body-mass index§ | 30.7 ± 5.5 | 31.2 ± 6.1 |
| Current smoker- no. (%) | 43 (31.4) | 55 (30.6) |
| Diabetes- no. (%) | 31 (22.3) | 38 (21.1) |
| High cholesterol - no. (%) | 49 (35.3) | 44 (24.4) |
Note: Unadjusted Data
Plus-minus values are means ± SD. There were no significant between-group differences (P<0.05).
The 2015 United States federal poverty guidelines are based on the total household income and family size, in 2015 the federal poverty threshold was $11,770 for a single person and $4,160 for each additional person.
Risk factors and history are by self-report.
The body-mass index is the weight in kilograms dived by the square of the height in meters, both height and weight were by self-report.
Primary Outcome
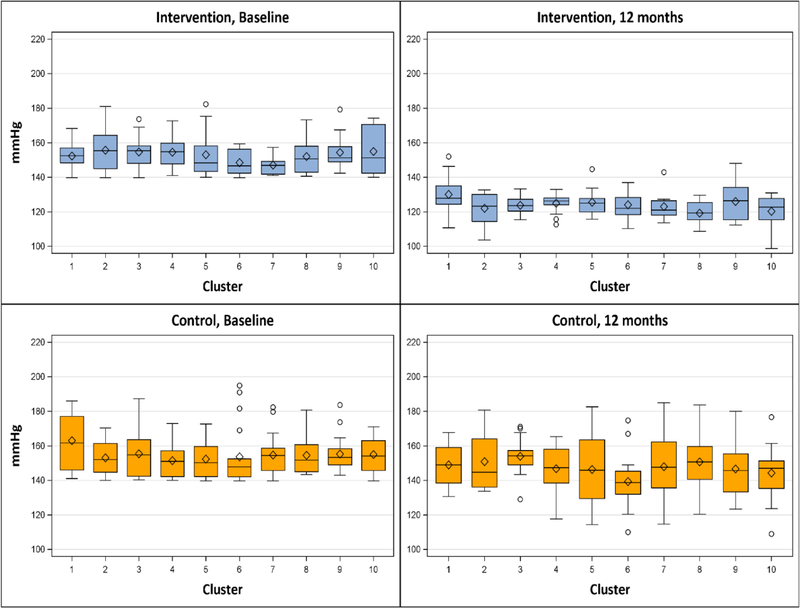
At baseline, mean systolic blood pressure was similar between intervention and control groups (152.4 mmHg and 154.6 mm Hg respectively; Table 2). At 12 months, mean systolic pressure fell −28.6 mm Hg (to 123.8 mm Hg) in the intervention group versus −7.2 mmHg (to 147.4 mm Hg) in the control group; mean systolic blood pressure reduction was −20.8 mmHg greater in the intervention group (95% confidence interval [CI], −13.9 to −27.7 mmHg; P <0.0001; Table 2). Intervention effect size was similar by ITT analysis: −20.6 mmHg (95% [CI], −13.8 to −27.3 mmHg; P <0.0001; Table 3). The intervention effect was also consistent across barbershop clusters (Fig. 3). The change in systolic BP from 6 months to 12 months was −1.9 ± 11.6 mm Hg in the intervention group and 2.2 ± 18.4 mm Hg the control group; the difference in mean change was 1.6 (95% confidence interval [CI], −6.6 to 9.8 mm Hg; P = 0.71; Table S2 in Supplementary Appendix). Longitudinal analysis of systolic BP in the intervention group estimated that the rate of change was −3.4 mmHg per month (95% [CI], −3.9 to −3.0 mmHg; p <0.0001) from baseline to 6 months and −2.0 mmHg per month (95% [CI], −2.2 to −1.8 mmHg; p <0.0001) after 6 months (Fig 2 and Table 4).
Table 2.
Primary and Secondary Blood Pressure Outcomes at 12 months*
| Intervention, N = 125 | Control, N =163 | Intervention Effect | ||
|---|---|---|---|---|
| Blood Pressure | Difference in Mean Change of BP (95% CI) | p-value† | ||
| Systolic Blood Pressure - mm Hg ‡ | ||||
| Baseline | 152.4 ± 10.1 | 154.6 ± 12.0 | ||
| 12-months | 123.8 ± 8.8 | 147.4 ± 15.7 | ||
| Change | −28.6 ± 12.7 | −7.2 ± 17.7 | −20.8 (−27.7, −13.9) | <0.0001 |
| Diastolic Blood Pressure - mm Hg | ||||
| Baseline | 91.9 ± 11.3 | 89.8 ± 11.3 | ||
| 12-month | 74.1 ± 8.2 | 86.5 ± 12.6 | ||
| Change | −17.8 ± 11.9 | −3.3 ± 11.2 | −14.5 (−19.5, −9.5) | <0.0001 |
| Hypertension Control Rate after 12 months - no. (%) | Odds Ratio (95% CI) | p-value§ | ||
| Blood Pressure <140/90 mm Hg | 118 (94.4%) | 47 (28.8%) | 3.3 (1.8, 6.1) | 0.0001 |
| Blood Pressure < 135/85 mm Hg | 110 (88.0%) | 24 (14.7%) | 6.7 (2.3, 18.9) | 0.0004 |
| Blood Pressure <130/80 mm Hg | 85 (68.0%) | 18 (11.0%) | 9.1 (1.5, 56.6) | 0.0177 |
Plus-minus values are means ± SD
p-values calculated from linear mixed effects models with random intercepts for clusters. The estimated intervention effect was controlled for baseline systolic blood pressure (or diastolic), routine doctor, and high cholesterol.
Pre-specified primary outcome. Intraclass Correlation Coefficient from the linear mixed effects model for change in SBP is 0.01.
p-values calculated from generalized estimating equations with a compound symmetry working correlation to account for cluster effects. The estimated intervention effect was controlled for baseline SBP, routine doctor, and high cholesterol.
Table 3. ITT analysis.
Primary and Secondary Blood Pressure Outcomes at 12 months*
| Intervention, N = 139 | Control, N =171 | Intervention Effect | ||
|---|---|---|---|---|
| Blood Pressure | Difference in Mean Change of BP (95% CI) | p-value† | ||
| Systolic Blood Pressure - mm Hg ‡ | ||||
| Baseline | 153.1 ± 10.6 | 154.6 ± 12.0 | ||
| 12-months | 125.1 ± 9.9 | 147.5 ± 16.0 | ||
| Change | −28.1 ± 13.7 | −7.2 ± 17.6 | −20.6 (−27.3, −13.8) | <0.0001 |
| Diastolic Blood Pressure - mm Hg | ||||
| Baseline | 92.6 ± 11.8 | 89.8 ± 11.2 | ||
| 12-month | 77.5 ± 17.1 | 89.4 ± 18.4 | ||
| Change | −15.2 ± 17.9 | −0.4 ± 17.5 | −18.9 (−27.2, −10.7) | <0.0001 |
| Hypertension Control Rate after 12 months - no. (%) | Relative Risk (95% CI) | p-value§ | ||
| Blood Pressure <140/90 mm Hg | 118 (84.9%) | 55 (32.2%) | 3.2 (2.3, 4.4) | <0.0001 |
| Blood Pressure < 135/85 mm Hg | 109 (78.4%) | 32 (18.7%) | 5.2 (2.4, 11.3) | <0.0001 |
| Blood Pressure <130/80 mm Hg | 84 (60.4 %) | 20 (11.7%) | 5.4 (2.4, 12.3) | <0.0001 |
Plus-minus values are means ± SD
p-values calculated from linear mixed effects models with random intercepts for clusters. The estimated intervention effect was controlled for baseline systolic blood pressure (or diastolic), routine doctor, and high cholesterol.
Pre-specified primary outcome. Intra-class Correlation Coefficient from the linear mixed effects model for change in SBP is 0.02.
p-values calculated from generalized estimating equations with a compound symmetry working correlation to account for cluster effects. The estimated intervention effect was controlled for baseline SBP, routine doctor, and high cholesterol.
Figure 3. Systolic Blood Pressure at Baseline and 12 months According to Barbershop Cluster.
Shown are box plots for systolic blood pressure according to barbershop cluster. The horizontal line inside each box indicates the median, the diamond indicates the mean, and the bottom and top of each box indicate the 25th percentile and 75th percentile, respectively. I bars indicate the upper adjacent value (75th percentile plus 1.5 times the interquartile range) and the lower adjacent value (25th percentile minus 1.5 times the interquartile range), and the circles outliers.
Table 4.
Longitudinal Analysis of Systolic Blood Pressure in Intervention Group (Baseline to 12 months)
| Effect | Estimate | 95% CI | p-value | |
|---|---|---|---|---|
| Rate of change (per month) from baseline to 6 months | −3.4 | (−3.9, −3.0) | <.0001 | |
| Rate of change (per month) after 6 months | −2.0 | (−2.2, −1.8) | <.0001 | |
| age | −0.1 | (−0.2, 0.01) | 0.09 |
Secondary Blood Pressure Outcomes
Mean diastolic blood pressure reduction was −14.5 mm Hg greater in the intervention group (95% [CI], −9.5 to −19.5 mm Hg; P <0.0001), with similar values by ITT (Table 2 and Table 3, Fig. S4 in Supplementary Appendix). A higher percentage of intervention participants achieved blood pressure goal of <130/80 (68.0% intervention group versus 11.0% control group; Table 2).
Changes in Medication and Doctors Visits
The intervention led to a greater number of antihypertensive drug classes per regimen and higher percentages of participants treated with preferred first-line, add-on drugs (Table 5 and Table S3 in Supplementary Appendix), and long-acting drugs (e.g., indapamide versus hydrochlorothiazide) (Table S4 in Supplementary Appendix). After 12 months, antihypertensive medication use increased from 57% to 100% in the intervention group and from 53% to 65% in the control group (P< 0.001) (Table S4 in Supplementary Appendix).
Table 5.
Blood Pressure Medications at 12 months*
| No. of Blood Pressure Medications per Participant | Intervention (N = 125 | Control (N = 163) | Difference at 12 months (95% CI) | p-value† |
|---|---|---|---|---|
| Mean | 2.7 ± 0.9 | 1.4 ± 1.3 | 2.0 (1.4, 2.6) | <0.0001 |
| Drug Class | Odds Ratio at 12 months (95% CI) | p-value‡ | ||
| First Line Drugs | ||||
| ACE inhibitor or ARB - no. (%) | 122 (97.6%) | 70 (42.9%) | 62.0 (19.2, 200.0) | <0.0001 |
| Calcium-channel blocker - no. (%) | 118 (94.4%) | 59 (36.2%) | 39.2 (17.4, 88.2) | <0.0001 |
| Diuretic - no. (%) | 60 (48.0%) | 48 (29.5%) | 2.5 (1.5, 4.0) | 0.0002 |
| Add On Drugs | ||||
| Aldosterone Antagonist - no. (%) | 15 (12.0%) | 2 (1.2%) | 15.5 (4.7, 51.1) | <0.0001 |
| Beta-blocker - no. (%) | 15 (12.0%) | 31 (19.0%) | 0.6 (0.4, 0.9) | 0.0183 |
| Alpha-blocker - no. (%) | 2 (1.6%) | 8 (4.9%) | 0.3 (0.1, 1.2) | 0.0981 |
| Central Sympatholytic- no. (%) | 1 (0.8%) | 6 (3.7%) | § | § |
| Direct Vasodilator - no. (%)¶ | 0 (0%) | 7 (4.3%) | § | § |
Plus-minus values are means ± SD. ACE denotes angiotensin-converting enzyme, ARB angiotensin-receptor blocker.
p-values calculated from linear mixed effects models with random intercepts for clusters. The estimated between-group difference was controlled for baseline SBP (or DBP), routine doctor, and high cholesterol.
p-values calculated from generalized estimating equations with a compound symmetry working correlation to account for cluster effects. The estimated between-group difference was controlled for baseline SBP, routine doctor, and high cholesterol.
odds ratio and p-value not available due to very low or zero counts.
The direct vasodilator was Hydralazine.
Intervention and control groups reported similar mean numbers of doctor visits in the past 3 months at baseline (1.0 ± 1.2 and 1.2 ± 1.4), however at 12 months the intervention group reported a greater number of doctors’ visits (1.5 ± 1.8 and 1.1 ± 1.5; p=0.0329). This suggests that the pharmacist intervention did not interfere with the patient-doctor relationship and perhaps enhanced it.
Safety Outcomes
There were no treatment-related serious adverse events or deaths related to trial participation in either group. Changes in medication side-effects were similar across groups, with few exceptions (Table S5 in Supplementary Appendix). There were no cases of acute kidney injury in the extension phase of the study, as compared to the 3 reversible cases documented in the first 6 months. We had no control group data on acute kidney injury.
Patient-Reported Outcomes
Self-rated health and patient engagement scores increased more in the intervention group (Tables S6, S7 in Supplementary Appendix) as judged by validated instruments.19,20
Process Data
Time from baseline to study completion was 12.0 ± 1.0 months in the control group and to 11.5 ± 0.9 months in the intervention group. In that time each intervention participant received an average of 11 in-person pharmacist visits (7 in months 0 to 6 and 4 in months 7 to 12). Barbers checked blood pressure in 6 of 28 intervention shops (4 checks /participant) and discussed health lessons in 10 of 24 comparison shops (4 lessons/participant).
DISCUSSION
Among black male barbershop patrons with uncontrolled HTN, health promotion by barbers resulted in large and sustained BP reduction when coupled with medication management conveniently delivered in their barbershops by specialty-trained pharmacists. The mean reductions in systolic and diastolic BP observed at 12 months are statistically indistinguishable from our previously reported 6-month data8 despite less interactions with the pharmacists in the second 6 months of the trial (7±2 visits versus 4±2). The observed 90% cohort retention, few treatment-related adverse events, improved patient satisfaction and self-rated health strongly suggest sustainability of our HTN detection and treatment model.
Major strengths of this study are the large intervention effect and notable cohort retention in both groups. We attribute the intervention potency to several factors. More intensive drug therapy using more combination regimens, more first-line blood pressure drugs, and more long-acting drugs largely explains the enhanced blood pressure reduction observed in our intervention group compared with standard treatment by community physicians. In a departure from most guidelines24,25 that recommend thiazide-type diuretics as first-line for black men, our starting regimen of amlodipine plus an ARB or ACE-I was well-tolerated and proved very effective with only 50% of regimens requiring three or more drugs.
Unlike other pharmacist intervention trials1–6 that required travel to traditional healthcare settings like clinics or pharmacies, our pharmacists made treatment more convenient by bringing drug therapy and monitoring to the patrons in their barbershops – a uniquely personal and readily accessible non-traditional setting. Our model was tailor-made for black men by addressing gender-specific issues of black men (i.e. underutilization of healthcare due to longstanding issues related to distrust of the medical profession) and enlisting barbers (trusted community members) to deliver health messages. Our trial differs from other NHLBI-funded hypertension trials that consider black men and women as one group.26 Finally, the participants loyal patronage (with average barbershop visit every 2 weeks for over a decade) facilitated frequent follow up and contributed to cohort retention.
As previously reported8, the study has several limitations. The lower participation rate in the intervention group may reflect lay misgivings about prescription drugs, but treatment rates were similar at baseline and the large effect on rates of antihypertensive drug treatment at 12 months (100% in the intervention group vs. 65% in the control group) and drug-regimen intensity (2 more antihypertensive drug classes per intervention participant than control-group participant bolster the validity of our primary outcome.27,28 Condition assignment could not be blinded; however, the intervention was evaluated by an independent survey research company and blood pressure was measured using an validated automated monitor and data capture software that eliminated human transcription error. The multiple reading blood pressure protocol was designed to reduce falsely high readings by habituation of the alerting reaction to arm cuff inflation; however, habituation was likely greater among the intervention participants for whom barbershop blood pressure measurement became routine. Financial incentives were used to off-set the cost of generic drugs used in the intervention. However, published data suggest that financial incentives have little effect on medication adherence.29 Finally, our blood pressure goal of <130/80 (which was influenced by the Systolic Blood Pressure Intervention Trial – SPRINT30) was likely lower than the <140/90 goal most community physicians would have targeted prior to the release of the 2017 ACC/AHA guidelines.25
The results presented herein successfully demonstrate both efficacy and sustainability, and now warrant broad scale implementation research. Towards that end, cost-effectiveness is being assessed to determine fiscally viable business models and to assess potential savings to public and private payors. An initial pilot study is also underway to assess whether these results can be replicated in a different city and with a different pharmacist-led team.
Beyond that, scalability will depend on our ability to adapt the model to create operational efficiencies while maintaining intervention potency. One of the most significant time-consuming aspects of in this trial was the amount of time pharmacists spent traveling to and from barbershops. While we found that the initial in-person visits between the pharmacist, barber, and patron were essential for establishing trust, once rapport was established and blood pressure control achieved the need for in-person pharmacist intervention decreased (as evidenced by the drop-in number of visits in the extension phase of the study). Telemonitoring, which has worked well in trials involving predominantly nonblack participants and shown some success in one trial involving exclusively black participants31–34, may constitute an appropriate means of maintaining/sustaining the intervention effect whilst also addressing this logistical inefficiency.
Perhaps the most critical first step towards widespread dissemination of our model is the expansion of collaborative practice between pharmacists and physicians, or the elimination of the requirement altogether (as in Canada and the UK)35. While team-based care models that include pharmacists have proven an effective way to manage chronic disease, many states have been slow to adopt broad collaborative practice authorities for pharmacists.
In conclusion, intensive medication management delivered in barbershops by specialty-trained pharmacists, as compared to standard management afforded by primary care practices, resulted in large and sustained blood pressure reduction in the shops’ hypertensive black male patrons. Our results indicate that our new model of HTN care can succeed in reaching high-risk hypertensive populations and markedly improve control rates with simple treatment algorithms, frequent follow-up and persistence in adjusting therapy when blood pressure remains above goal.36
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the participants for taking part in the study, the barbers and barbershop owners for allowing us to conduct the study in their barbershops. A special thanks to barbershop owner Eric Muhammad, who helped recruit our network of Los Angeles barbershops. Finally, we’d like to thank the Westat team for rigorous data collection.
SOURCES OF FUNDING
Supported by the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (R01HL117983 and 3R01HL117983-01A1S1), the NIH National Center for Advancing Translational Sciences UCLA Clinical and Translational Science Institute (UL1TR001881), the California Endowment (Grant 20131872 and 20162257), the Lincy Foundation, the Harriet and Steven Nichols Foundation, the Burns and Allen Chair in Cardiology Research at the Smidt Heart Institute, and the Division of Community Relations and Development at Cedars–Sinai Medical Center.
Footnotes
DISCLOSURES
Dr. Florian Rader is a consultant for Recor Medical. All other authors have nothing to disclose.
REFERENCES
- 1.Santschi V, Chiolero A, Colosimo AL, Platt RW, Taffé P, Burnier M, Burnand B, Paradis G. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. JAHA 2014;3:e000718. doi: 10.1161/JAHA.113.000718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheema E, Sutcliffe P, Singer DR. The impact of interventions by pharmacists in community pharmacies on control of hypertension: a systematic review and meta-analysis of randomized controlled trials. Brit J Clin Pharm 2014;78:1238–47. doi: 10.1111/bcp.12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter BL . Primary Care Physician-Pharmacist Collaborative Care Model: Strategies for Implementation. Pharmacotherapy 2016;36:363–73. doi: 10.1002/phar.1732 [DOI] [PubMed] [Google Scholar]
- 4.Tsuyuki RT, Houle SK, Charrois TL, Kolber MR, Rosenthal MM, Lewanczuk R, Campbell NR, Cooney D, McAlister FA. Randomized Trial of the Effect of Pharmacist Prescribing on Improving Blood Pressure in the Community: The Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation 2015;132:93–100. doi: 10.1161/CIRCULATIONAHA.115.015464 [DOI] [PubMed] [Google Scholar]
- 5.Anderegg MD, Gums TH, Uribe L, Coffey CS, James PA, Carter BL. Physician-Pharmacist Collaborative Management: Narrowing the Socioeconomic Blood Pressure Gap. Hypertension 2016;68:1314–20. doi: 10.1161/HYPERTENSIONAHA.116.08043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svarstad BL, Kotchen JM, Shireman TI, Brown RL, Crawford SY, Mount JK, Palmer PA, Vivian EM, Wilson DA. Improving refill adherence and hypertension control in black patients: Wisconsin TEAM trial. JAPhA 2013;53:520–9. doi: 10.1331/JAPhA.2013.12246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luque JS, Ross L, Gwede CK. Qualitative systematic review of barber-administered health education, promotion, screening and outreach programs in african-american communities. Journal of Community Health 2014; 39:181–190. doi: 10.1007/s10900-013-9744-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, Brettler J, Rashid M, Hsu B, Foxx-Drew D, Moy N, Reid AE, Elashoff RM. A Cluster-Randomized trial of blood pressure reduction in black barbershops. N Engl J Med 2018; 378:1291–1301. doi: 10.1056/NEJMoa1717250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter BL, Ardery G. Avoiding Pitfalls With Implementation of Randomized Controlled Multicenter Trials: Strategies to Achieve Milestones. JAHA 2016;5: e004432. doi: 10.1161/JAHA.116.004432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg 2011;9:672–7. doi: 10.1016/j.ijsu.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 11.Eldridge SM, Ashby D, Feder GS, Rudnicka AR, Ukoumunne OC. Lessons for cluster randomized trials in the twenty-first century: a systematic review of trials in primary care. Clin Trials 2004;1:80–90. doi: 10.1191/1740774504cn006rr [DOI] [PubMed] [Google Scholar]
- 12.Dashevsky M, Bernstein SL, Barsky CL, Taylor RA. Agreement Between Serum Assays Performed in ED Point-of-Care and Hospital Central Laboratories. West J Emerg Med 2017;18:403–9. doi: 10.5811/westjem.2017.1.30532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roush GC, Ernst ME, Kostis JB, Tandon S, Sica DA. Head-to-head comparisons of hydrochlorothiazide with indapamide and chlorthalidone: antihypertensive and metabolic effects. Hypertension 2015;65:1041–6. doi: 10.1161/HYPERTENSIONAHA.114.05021 [DOI] [PubMed] [Google Scholar]
- 14.Kaplan NM. Indapamide: is it the better diuretic for hypertension? Hypertension 2015;65:983–4. doi: 10.1161/HYPERTENSIONAHA.115.05138 [DOI] [PubMed] [Google Scholar]
- 15.Anwar YA, Tendler BE, McCabe EJ, Mansoor GA, White WB. Evaluation of the Datascope Accutorr Plus according to the recommendations of the Association for the Advancement of Medical Instrumentation. Blood Press Monit 1997;2:105–10. [PubMed] [Google Scholar]
- 16.Victor RG, Ravenell JE, Freeman A, Leonard D, Bhat DG, Shafiq M, Knowles P, Storm JS, Adhikari E, Bibbins-Domingo K, Coxson PG, Pletcher MJ, Hannan P, Haley RW. Effectiveness of a barber-based intervention for improving hypertension control in black men: the BARBER-1 study: a cluster randomized trial. Arch Intern Med 2011;171:342–50. doi: 10.1001/archinternmed.2010.390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess PL, Reingold JS, Jones J, Fellman MA, Knowles P, Ravenell JE, Kim S, Raju J, Ruger E, Clark S, Okoro C, Ogunji O, Knowles P, Leonard D, Wilson RP, Haley RW, Ferdinand KC, Freeman A, Victor RG. Barbershops as hypertension detection, referral, and follow-up centers for black men. Hypertension 2007;49:1040–6. doi: 10.1161/HYPERTENSIONAHA.106.080432 [DOI] [PubMed] [Google Scholar]
- 18.Mancia G, Fagard R, Narkiewicz K, Redán J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013;31:1281–357. doi: 10.1097/01.hjh.0000431740.32696.cc [DOI] [PubMed] [Google Scholar]
- 19.Victor RG1, Leonard D, Hess P, Bhat DG, Jones J, Vaeth PA, Ravenell J, Freeman A, Wilson RP, Haley RW. Factors associated with hypertension awareness, treatment, and control in Dallas County, Texas. Arch Intern Med 2008;168:1285–93. doi: 10.1001/archinte.168.12.1285 [DOI] [PubMed] [Google Scholar]
- 20.Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Med Care 2005;43:436–44. [DOI] [PubMed] [Google Scholar]
- 21.Hayes RJ, Moulton LH. Cluster Randomized Trials. Boca Raton, Fla.: Chapman & Hall/CRC; 2009. doi: 10.1016/B978-0-12-385075-1.00013-5 [DOI] [Google Scholar]
- 22.Everitt BS, Landau S, Leese M, Stahl D. Cluster Analysis. 5th Edition King’s College London, UK: John Wiley and Sons, Ltd.; 2011. [Google Scholar]
- 23.Elashoff RM, Li G, Li N. Joint modeling of longitudinal and time-to-event data. Boca Raton (FL): Chapman & Hall/CRC; 2017. [Google Scholar]
- 24.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20. doi: 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 25.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017. [Epub ahead of print]. doi: 10.1016/j.jash.2018.06.010 [DOI] [PubMed] [Google Scholar]
- 26.Einhorn PT. National Heart, Lung, and Blood Institute-initiated Program Interventions to Improve Hypertension Control Rates in African-Americans: Background and Implementation. Circ Cardiovasc Qual Outcomes 2009; 2:236–240. doi: 10.1161/CIRCOUTCOMES.109.850008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Victor RG, Blyler CA, Elsahoff RM. A trial of blood-pressure reduction in black barbershops. N Engl J Med 2018; 379:200–201. doi: 10.1056/NEJMc1806026 [DOI] [PubMed] [Google Scholar]
- 28.Yang R, Carter BL, Gums TH, Gryzlak BM, Xu Y, Levy BT. Selection bias and subject refusal in a cluster-randomized controlled trial. BMC Medical Research Methodology 2017; 17:94. doi: 10.1186/s12874-017-0368-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, Reisman L, Fernandes J, Spettell C, Lee JL, Levin R, Brennan T, Shrank WH. Full coverage for preventative medications after myocardial infarction. N Engl J Med 2011; 365:2088–2097. doi: 10.1056/NEJMsa1107913 [DOI] [PubMed] [Google Scholar]
- 30.The SPRINT Research Group. A randomized trial of intensive versus standard blood pressure control. N Engl J Med 2015; 373:2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, Carrell D, Tyll L, Larson EB, Thompson RS. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA 2008;299:2857–2867. doi: 10.1001/jama.299.24.2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Magid DJ, Olson KL, Billups SJ, Wagner NM, Lyons EE, Kroner BA. A pharmacist-led, American Heart Association Heart360 Web-enabled home blood pressure monitoring program. Circ Cardiovasc Qual Outcomes 2013;6:157–163. doi: 10.1161/CIRCOUTCOMES.112.968172 [DOI] [PubMed] [Google Scholar]
- 33.Margolis KL, Asche SE, Bergdall AR, Dehmer SP, Groen SE, Kadrmas HM, Kerby TJ, Klotzle KJ, Maciosek MV, Michels RD, O’Connor PJ, Pritchard RA, Sekenski JL, Sperl-Hillen JM, Trower NK. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA 2013;310:46–56. doi: 10.1001/jama.2013.6549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Artinian NT, Flack JM, Nordstrom CK, Hockman EM, Washington OG, Jen KL, Fathy M. Effects of nurse-managed telemonitoring on blood pressure at 12-month follow-up among urban African Americans. Nurs Res 2007;56:312–322. doi: 10.1097/01.NNR.0000289501.45284.6e [DOI] [PubMed] [Google Scholar]
- 35.Barragan NC, DeFosset AR, Torres J, Kuo T. Pharmacist-driven strategies for hypertension management in Los Angeles: a community and stakeholder needs assessment 2014–2015. Preventing Chronic Disease 2017; 14:E54. doi: 10.5888/pcd14.160423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Margolis KL. Inventing a new model of hypertension care for black men. N Engl J Med 2018; 378:1345–1347. doi: 10.1056/NEJMe1803106 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.