Abstract
Endocytosis has long been identified as a key cellular process involved in bringing in nutrients, clearing cellular debris in tissue, and maintaining cell membrane compositional homeostasis. While clathrin-mediated endocytosis (CME) has been the most extensively studied, a number of clathrin-independent endocytic (CIE) pathways are continuing to be delineated. Here we provide a current survey of the different types of endocytic pathways available at the cell surface and discuss a new classification and plausible molecular mechanisms for some of the less characterized ones. Along with an evolutionary perspective of the origins of some of these pathways, we provide an appreciation of the distinct roles these pathways play in various aspects of cellular physiology, including the control of signalling and membrane tension.
Keywords: clathrin-mediated endocytosis, clathrin-independent endocytosis, caveolae, CLIC/GEEC, dynamin, evolution, signalling
Introduction
Over a century ago, Elie Metchnikoff’s observations of phagocytosis laid the foundation to the field of endocytosis (Metschnikoff 1884). It has been proposed that the capacity for engulfment as a process has led to the acquisition of bacteria, allowing mitochondrial assimilation in the eukaryotic lineage (Cavalier-Smith 1987). Now, we understand that endocytosis is necessary for nutrient uptake, extracellular milieu sensing, maintaining and homeostasis of plasma membrane (PM) composition (chemical aspect), surface area and tension (physical aspect), by providing control mechanisms for these processes.
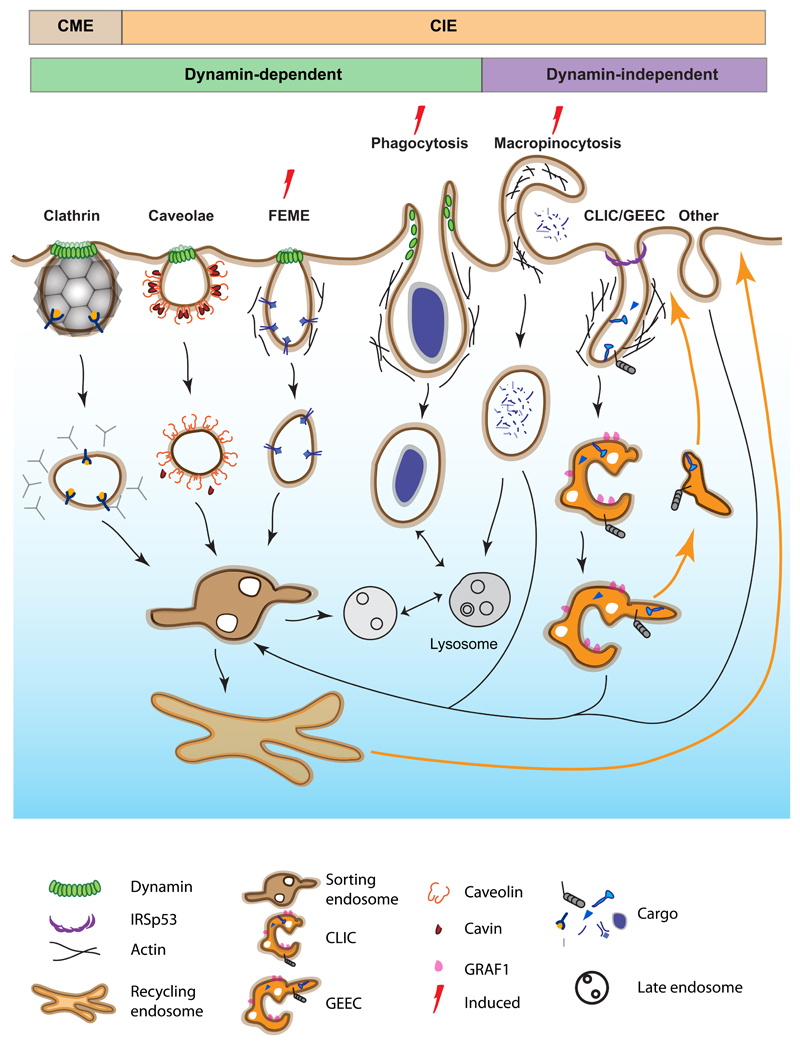
Several attempts to classify endocytic pathways have been made over the years (Conner & Schmid 2003, Doherty & McMahon 2009, Mayor et al. 2007, Schmid et al. 2014). Our knowledge of endocytosis has been framed mainly by early advances made in Clathrin Mediated Endocytosis (CME), where endocytosis is accomplished by the formation of a Clathrin coated pit and the endocytic vesicle is derived from the scission of the endocytic vesicle by a Dynamin-mediated process (Fig 1, 2). However, multiple endocytic mechanisms operate at the PM (Fig. 1), and endocytic pathways have been classified based on based on their requirement (or not) for Clathrin, therefore the term Clathrin Independent Endocytosis (CIE) (Kirkham & Parton 2005; Mayor et al. 2007, 2014). As depicted in Fig. 1, the presence or absence of dynamin encompasses a broader set of pathways and offers a different way to parse endocytic pathways. While dynamin-dependent mechanisms appear to accompany endocytic pathways that recruit membrane coats, dynamin-independent pathways rely on the association of actin with the membrane for their operation, and may represent a distinct class of membrane processes. Therefore, we propose an alternate way to categorize these pathways that distinguishes endocytic processes by the type of scission machinery utilized (Fig.1).
Figure 1. Different endocytic pathways and their itinerary.
Multiple clathrin independent endocytic pathways function at the cell surface. These can be divided based on their requirement for clathrin (brown, orange) or dynamin (green, purple) dependence. Some pathways are not constitutively active and are marked as induced. Cargoes for these pathways are listed in Supplementary Table S1. Endosomes formed from each of the dynamin-dependent pathways fuse with the sorting endosome from which material is sorted to recycling endosome. The sorting endosome matures to late endosomes which subsequently fuse with lysosome. Dynamin-independent pathways give rise to CLICs which fuse to form GEECs. GEECS also deliver some of their contents to the sorting endosome, but independently recycle content bypassing the sorting and recycling endosome. There are clathrin and dynamin independent pathways based on cargo (see Supplementary Table S1) which remain unclassified and are denoted as other (these are noted in the section - Types of endocytosis).
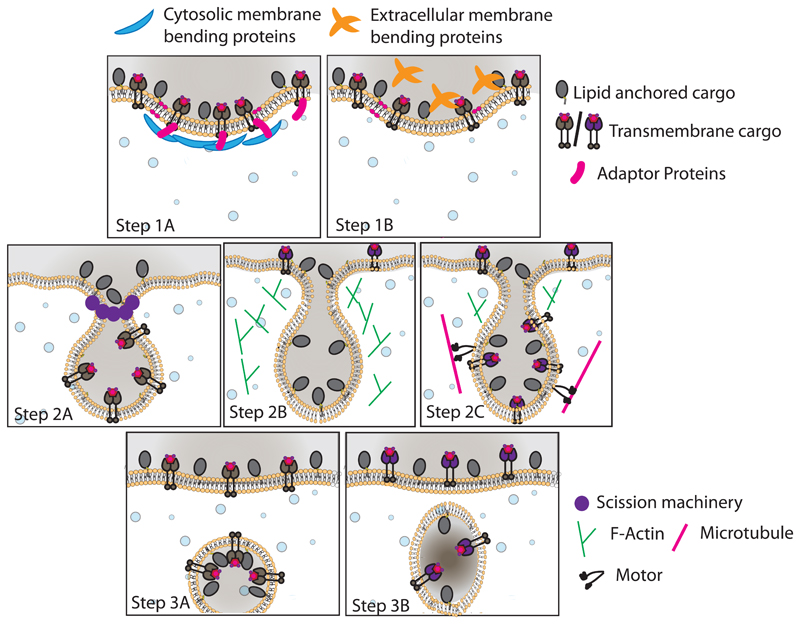
Figure 2. Steps in endocytosis.
The process is initiated with cargo selection and their recruitment to the forming endocytic site/pit. The cargo can come from the extracellular milieu or belong to the plasma membrane. Once selected, generation of a budding vesicle connected with the plasma membrane occurs by assembling proteins, which help with the bending and stabilization of the bud (Step 1). These proteins can be coat proteins such as clathrin/caveolin or curvature sensing/stabilization proteins. Regardless of the exact machinery used, it leads to the formation of an endocytic pit/invagination, which is, over time, sculpted to make a neck. The neck is constricted and eventually cut to release the endocytic vesicle inside the cell by a process of vesicle scission (Step 2). Here, most endocytic pathway deploys either dynamin alone (Step 2a) or dynamin in conjugation with actin or motor proteins (Step 2b). There are a class of processes that neither utilizes coat proteins nor requires dynamin for scission (Step 2c). These budding vesicles are then pinched to release an endocytic vesicle into the cell (Step 3).
The absence of precise mechanisms, a general medley of molecules with pleotropic functions that operate in regulating different CIEs, and the lack of codified information have helped fuel a perspective that CIE pathways are a consequence of perturbation of CME (Bitsikas et al. 2014). On the other hand, to understand these processes, there is no choice but to embrace the complexity that exists. Here, we will focus on a number of dynamin-dependent and independent pathways and provide evidence for their role in different physiological processes to highlight the importance of multiple endocytic pathways in a cell. We also provide an evolutionary argument for their existence. We list different mechanisms and provide a nomenclature that we believe will address some of the confusion. We also point out relevant features that help to distinguish the different pathways. We hope this will allow scientists in their areas of specialization to look at the role of the different endocytic pathways in multiple contexts and help in the interpretations of the phenomenon they observe.
Types of endocytic pathways
Clathrin-mediated Endocytosis
First described using Electron Microscopy (EM) (Pearse 1976, Roth & Porter 1964) (Fig.1), decades of work showed that CME governs endocytosis of receptors (Brown & Goldstein 1979) assisted by adaptor protein complex. This complex recognizes specific sequences in the cytoplasmic tails of receptors and also aids in Clathrin recruitment to the forming endocytic pit (Edeling et al. 2006, Robinson 2004). This begins with Clathrin recruitment and polymerization to form Clathrin-coated pits (CCP) (David et al. 1996, Neumann & Schmid 2013, Qualmann et al. 2011, Taylor et al. 2011), accompanied by local bending/invagination of membrane accomplished by curvature sensing/stabilising Bin-amphiphysin-Rvs (BAR) domain containing proteins such as amphiphysin, FBP17 and sortin nexin 9 (Taylor et al. 2011). Dynamin, a large GTPase, is subsequently recruited and oligomerizes at the neck of a CCP and powers vesicle scission via GTP hydrolysis (David et al. 1996, Shpetner & Vallee 1989). Following scission, these CCPs lose their coats and fuse with the early endosomal sorting compartment (Kirchhausen et al. 2014, Schmid 1997). The endocytosis paradigm of cargo recruitment via adaptor complexes, membrane coat formation, membrane bud formation and finally scission is derived from studies of CME (See Fig. 2; steps 1A, 2A, 3A). We refer the readers to several recent reviews for extensive details on CME (Kaksonen & Roux 2018, Mettlen et al. 2018, Robinson 2015).
Clathrin-independent (CI) endocytosis (CIE)
Inhibition of CCP formation failed to prevent Ricin intoxication and fluid phase uptake invoking the existence of CIE processes in a cell (Moya et al. 1985, Sandvig 1987). Several cargoes have been shown to be internalised in a similar fashion (Fig.1; (Mayor et al. 2014, Otto & Nichols 2011)), and via multiple pathways, making it difficult ascertain whether cargo chooses endocytic pathways or vice versa (Supplementary Table S1). Later, the fluid phase was also found to be internalized independent of dynamin function (Damke et al. 1994, 1995). Studies over the last three decades indicates a plethora of CIE pathways both dynamin-dependent or independent. CIE is an emerging field and we again refer the readers to excellent reviews on the different CIE pathways. Here we focus on only few of the CIEs whose molecular mechanisms have been worked out in detail, recently. For the others, we provide greater detail in Supplementary Figure S1.
Dynamin-dependent CIE pathways
Caveolar endocytosis
Caveolae, one of the earliest plasma membrane structures observed in EMs, are coated, flask-shaped invaginations (Fig. 1 and 3). They are implicated in a variety of cellular processes such as trans-cellular transport, membrane repair and mechanotransduction (Kirkham & Parton 2005, Sinha et al. 2011). In most cell types caveolae contain ~ 140 molecules of the integral protein caveolin 1, 30–70 molecules of caveolin-2, and a complex of membrane proteins termed cavins (Hansen & Nichols 2010, Hayer et al. 2010, Parton & Del Pozo 2013). Cavins 1, 2, and 3 associate with the caveolae of mammalian non-muscle cells. In skeletal muscle cells, caveolin-3 replaces the caveolin-1/2 complex and an additional cavin family member, cavin4/MURC, associates with the cavin complex (Bastiani et al. 2009, Ogata et al. 2008, Tagawa et al. 2008, Way & Parton 1996). Caveolae undergo a dynamin-dependent scission (Henley et al. 1998, Parton & Richards 2003). The molecular machinery involved in caveolar uptake also is emerging where the ATPase EHD2 acts as a negative regulator by interacting with pacsin, an F-BAR domain containing protein that promotes caveolar endocytosis (Moren et al. 2012).
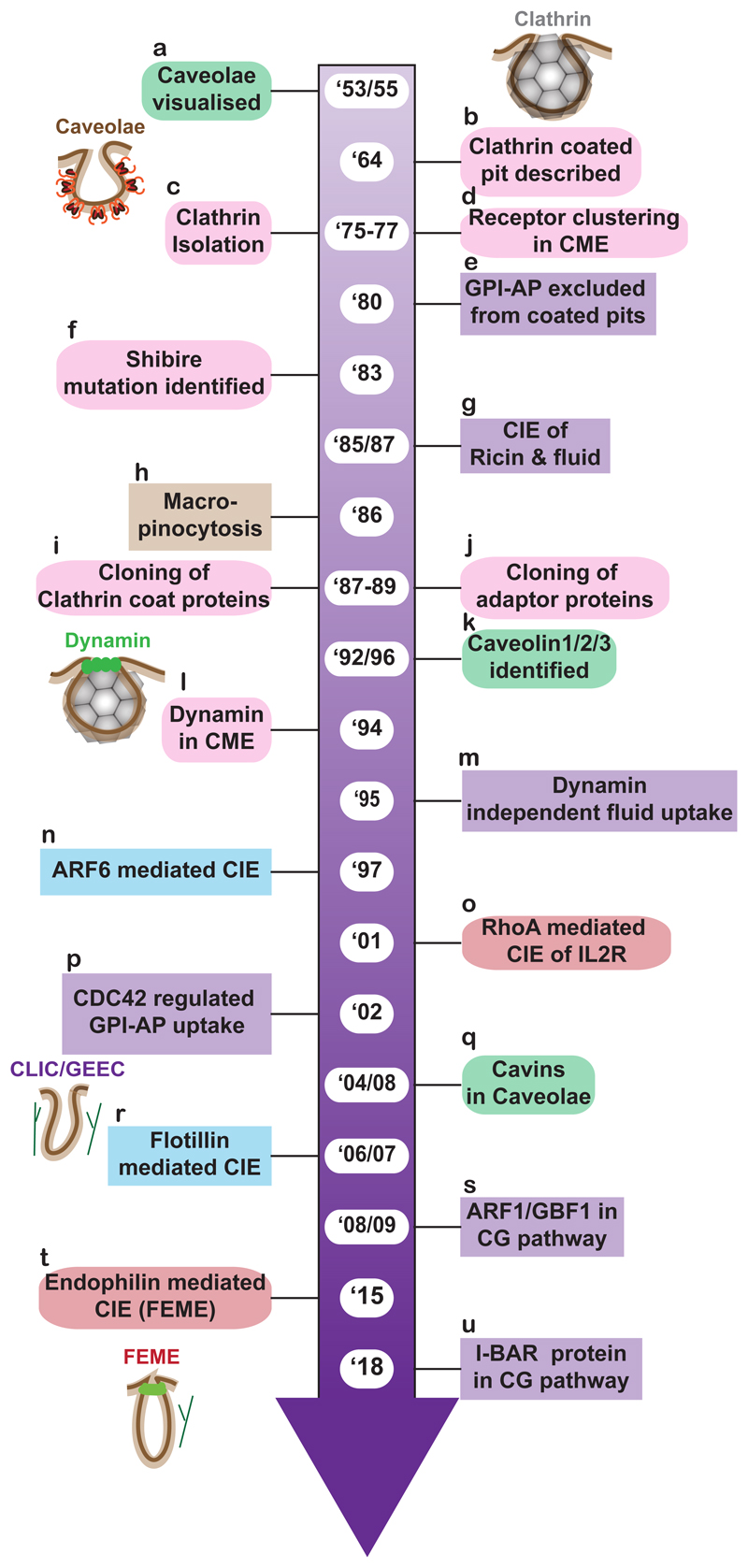
Figure 3. Timeline of key findings showing contrast between the study of CME and CIE.
The time line shows key findings in different endocytic pathways. EM based observations picked up clathrin dependent pathways due to the presence of electron dense coat (1964). It took another decade before clathrin was isolated. Existence of a clathrin independent pathway was hinted from observations where Thy1 (GPI-AP) was found excluded from coated pits (1980) and later the fluid-phase and ricin toxin were endocytosed even when clathrin mediated endocytosis was inhibited (1986/87). These molecules are now internalized via multiple clathrin and dynamin-independent mechanisms (1995/1997/2002). Clathrin independent pathways are still being discovered with the RhoA-dependent FEME being the most recent addition (2001/2015). Important milestones in the discovery of multiple endocytic pathways: a - (Palade & GE 1953, Yamada 1955), b- (Roth & Porter 1964), c- (Pearse 1975, 1976), d- (Anderson et al. 1977a,b), e- (Bretscher et al. 1980), f - (Kosaka & Ikeda 1983), g- (Moya et al. 1985, Sandvig 1987), h- (Bar-Sagi & Feramisco 1986), i - (Jackson et al. 1987; Kirchhausen et al. 1987a,b), j- (Robinson 1989, Thurieau et al. 1988), k-(Rothberg et al. 1992, Scherer et al. 1996, Tang et al. 1996), l- (Damke et al. 1994), m- (Damke et al. 1994, 1995), n- (Radhakrishna & Donaldson 1997), o- (Lamaze et al. 2001), p- (Sabharanjak et al. 2002), q- (Aboulaich et al. 2004, Hill et al. 2008), r-(Frick et al. 2007, Glebov et al. 2006) s- (Gupta et al. 2009, Kumari & Mayor 2008), t- (Boucrot et al. 2015, Renard et al. 2015), u- (Sathe et al. 2018).
Fast Endophilin A- Mediated Endocytosis (FEME)
Recently, an Endophilin A (EndoA) dependent pathway has been described that makes tubulo-vesicular compartments (<1μm) that derive from EndoA positive assemblies (EPAs) in a ligand, actin and dynamin-dependent fashion (Boucrot et al. 2015). EndoA is proposed as a versatile molecule, capable of cargo recognition (via its SH3 domain), inducing curvature with the aid of its BAR domain and driving scission by insertion of multiple amphipathic helices contained within its structure. Interestingly, high concentrations of epidermal growth factor (EGF) results in the down-regulation of CME but is needed for the full stimulation of EPAs. Cargoes such as GPCRs (α2a- and β1-adrenergic receptors), Interleukin-2, Epidermal Growth Factor Receptor (EGFR) and Shiga toxin (STx-B) traffic via FEME (Boucrot et al. 2015, Renard et al. 2015). FEME is downregulated by inhibitors of dynamin and phosphatidylinositol 3 Kinase (PI3K). The regulators of actin polymerisation, the small GTPases of the Rho family, RhoA, and Rac1 are also necessary for FEME functioning, while there are conflicting claims about whether the RhoGTPase, CDC42 activation, which positively or negatively regulates FEME (Boucrot et al. 2015, Chan Wah Hak et al. 2018).
Phagocytosis
Phagocytosis, one of the most important elements of innate immunity, mediates engulfment and destruction of invading microorganisms (>0.5μm) (Flannagan et al. 2012, Gordon 2016). The phagosome cup closes with the help of dynamin-2 and actin (Gold et al. 1999, Marie-Anaïs et al. 2016, Tse et al. 2003). Recently, in a mammalian genome-wide CRISPR screen, where Bassik and co-workers examined multiple phagocytic cargoes, many new regulators (such as NHLRC2 which participates in the Rac1 signalling cascade, and very long chain fatty acid synthesis essential albeit only for certain substrates) have been identified (Haney et al. 2018). Understanding the role of these regulators in phagocytic processing will undoubtedly become a focus of research in this area.
Dynamin-Independent CIE pathway
CLIC/GEEC (CG) endocytosis
Endocytic vesicles generated independent of Clathrin are called CLathrin-Independent Carriers (CLICs) and when they are formed independent of dynamin have a highly pleomorphic tubular structure. They are often formed in a polarised manner at the leading edge of migrating cells (Howes et al. 2010). The CG pathway is regulated by the small GTPases, ARF1 and CDC42 (Chadda et al. 2007, Guha et al. 2003, Gupta et al. 2009, 2014; Kumari & Mayor 2008, Sabharanjak et al. 2002) and internalises several cargoes including GPI-anchored proteins (GPI-APs), and a major fraction of the fluid-phase; several other cargo are detailed in Supplementary Table SI. The CG pathway is distinct from macropinocytosis (see next section), in that it is not sensitive to inhibitors of Na+/H+ exchange (NHE) inhibitors (Kalia et al. 2006). The CLICs/GEECs are high capacity endocytic carriers and can turn over the entire membrane surface of fibroblasts within 12 minutes (Howes et al. 2010). CG endocytosis is also postulated to generate a tubular vesicular endocytic network during cytokinesis (Kettle et al. 2015). GEECs are highly acidic (Kalia et al. 2006) and inhibiting vacuolar ATPase downregulates the CG pathway (Gupta et al. 2014). The CG pathway is sensitive to even slight changes in temperature, unlike CME (Thottacherry et al. 2018) and is reminiscent of the fast clathrin-independent response seen in neurons (Delvendahl et al. 2016).
The earliest events in the CG endocytic pathway begins with the recruitment of the GEF for ARF1, GBF1, to a membrane patch followed by the actin nucleator ARP2/3, CDC42 and filamentous actin (Sathe et al. 2018). The CG machinery also includes two BAR domain proteins (BDPs), PICK1 and IRSp53, which interact with ARP2/3. In the first phase, PICK1 inhibits ARP2/3 while in the second phase, CDC42/IRSp53 effector complex enables ARP2/3 activation and subsequent F-actin polymerisation. It is tempting to speculate that the initial repression of the ARP2/3 complex and therefore actin polymerization, allows membrane budding whereas its subsequent activation assist its pinching off.
Galectin-3 based CLIC biogenesis
The absence of a coat and an obvious adapter complex raises the question as to how are cargo proteins sorted and membrane bending is accomplished. In this context, Galectin-3 mediated CLIC biogenesis was put forward as one of the mechanisms in which sorting and membrane bending could be achieved (Johannes & Mayor 2010). Gal-3 is a member of N-glycan binding protein, capable of binding to carbohydrates via its C-terminus and other Gal-3 monomers via its N-terminus to form oligomers. In a recent study, Johannes and colleagues suggested that monomeric Gal-3 is recruited by glycosylated cargo to the membrane wherein it oligomerizes with neighbouring Gal-3 (Lakshminarayan et al. 2014). This co-clustering of cargo proteins with glycosphingolipids induces a local mechanical strain on the membrane, leading to buckling and subsequent generation of CLIC carriers. Further research is necessary at this point to establish if the Galectin-based mechanism is universal for initiation of CG vesicles or if it is part of a collection of mechanisms.
Macropinocytosis
Macropinocytosis is characterized by the formation of large endocytic vesicles (>0.5 μm) that originate from dorsal actin ruffles at the plasma membrane. Macropinosomes are generated by EGFR and PDGR based signaling and not found in resting cells. It is non-selective and is specifically inhibited by NHE inhibitors (West et al. 1989). Additionally, it is regulated by ARF6, Rac1 and requires PtdIns (4,5)P2 turnover (Brown et al. 2001, Koivusalo et al. 2010, West et al. 2000). CtBP1/BARS translocates to the macropinocytic cup and its surrounding membrane upon EGF stimulation and has been implicated in its scission (Bonazzi et al. 2005). Some reports implicate dynamin using dynasore perturbation (but not genetic perturbation) (Gold et al. 2010), that has many off target effects (Park et al. 2013, Preta et al. 2015). Thus, the role of dynamin in macropinocytosis should be interpreted with caution.
Other dynamin-independent pathways
ARF6 dependent endocytosis of MHC Class 1 and other receptors
Cargoes such as MHC Class I, CD1a, GPI-APs such as CD55 and CD59 are internalised via a pathway that requires ARF6 and cholesterol (Maldonado-Báez et al. 2013, Radhakrishna & Donaldson 1997). ARF6 activation and inactivation modulates recycling rather than internalisation. Other molecular machinery is detailed in Supplementary Figure S1, and in an extensive review (Grant & Donaldson 2009).
Rac1 and Src-dependent endocytosis
Nicotinic acetylcholine receptor (nAchR) internalisation is important for neuromodulation at neuromuscular junctions. When stimulated, nAChR internalisation occurs via narrow-tubular carriers formed independently of dynamin. Instead, it requires c-Src, Rac1 and actin polymerization (Kumari et al. 2008). A similar dynamin-independent mechanism involving c-Src and Rac1 activation is also utilized by the HIV-Nef protein in down-regulation of the costimulatory molecules CD80/CD86 (Chaudhry et al. 2005, 2007).
Flotillin mediated endocytosis
In HeLa cells, GPI-AP and Cholera Toxin B (a marker of cross-linked GM1) internalization occurs via a dynamin-independent manner (Glebov et al. 2006). Depletion of flotillin inhibits their uptake, suggesting that flotillins may outline yet another dynamin-independent pathway. However, contrasting reports suggests that flotillins regulated endocytosis of some cargoes is dynamin-dependent mechanism (Aït-Slimane et al. 2009, Compeer et al. 2018). It is therefore, suggested that flotillins could act as adaptors deciding the fate of specific cargo, (Otto & Nichols 2011).
General principles of endocytosis at the cell surface
An overview of multiple endocytic pathways in eukaryotic cells highlights the variety by which cargo can be internalized (Fig. 1), and suggests ways by which this process may be broken down into the steps delineated in Fig 2. A few general issues need to be taken on board and are listed below.
Membrane bending and vesicle scission
At the cell surface where different cytosolic coats assemble (clathrin, caveolin, endoA), the coats along with accessory proteins bend the membrane and subsequently dynamin is recruited for vesicle scission. However, to generate an endocytic carrier, neither a canonical coat nor dynamin as a scission protein is necessary. Physical mechanisms such as membrane domain formation can result in line tension driven membrane bending causing constriction and eventual budding in the absence of membrane coats (Johannes et al. 2015, Sens et al. 2008). Dynamin is not involved at most sites in intracellular membrane compartments where vesicles are generated (ER, Golgi, endosomes), and here dynamin-independent mechanisms operate where COPI and COPII coated vesicles bud and detach in the absence of dynamin (Campelo & Malhotra 2012). BDPs can also drive vesicle scission by inserting their amphipathic helix into the membrane. Recent in vitro experiments suggest that EndoA coated tubules facilitate scission by creating friction between rigid scaffolds and the membrane (Simunovic et al. 2017). The breakage occurs at the border of scaffolding and bare membrane. Such a mechanism provides an attractive alternative hypothesis for dynamin independent pinching. Scaffolding led membrane fission may also occur due to mechanisms like protein crowding (Snead et al. 2017), or by assisting in building up membrane bulges adjacent to sites of membrane scission, as observed in recent work on scission catalyzed by the ATP-driven EHD1 protein (Deo et al. 2018).
In the FEME pathway, STx-B induced tubules undergo scission in an actin-dependent fashion wherein actin polymerization on the STx-B induced tubules drives membrane reorganization (inferred from changes in the membrane order) and results in scission (Römer et al. 2010). Microtubules along with dynein and dynactin have also been implicated in facilitating tubulation and scission in CIE (Day et al. 2015). This may provide the pulling force necessary for the friction based scission mechanism proposed by (Simunovic et al. 2017). Thus, it is not surprising, that at the plasma membrane, multiple mechanisms, serve to help build and scission membrane buds (Fig 2).
Role of actin
While there is no generalizable mechanism for endocytosis, all endocytosis has to negotiate the cortical actin meshwork. Different endocytic processes depend on actin for different reasons. Actin polymerization helps counteract higher apical membrane tension relative to the basal side in CME (Boulant et al. 2011). Among the CIE pathways, CG endocytosis requires dynamic actin as both actin depolymerizing and stabilizing drugs inhibit it (Chadda et al. 2007). Other CIEs regulate the actin machinery required for endocytosis using small Rho family GTPases, RhoA (FEME), CDC42 (CG), and Rac1 (FEME and macropinocytosis) using known upstream regulators of the actin cytoskeleton (Boucrot et al. 2015, Koivusalo et al. 2010, Sabharanjak et al. 2002).
Mechanistic understanding for the role of actin in endocytosis comes primarily from studies in budding yeast where endocytosis is difficult to classify as either CME or CIE. The endocytic process begins with a membrane invagination followed by tubule elongation. This is exquisitely coordinated between actin polymerization and motor activity. Clathrin-recruitment precedes actin and is postulated to aid in the initial assembly of actin (Kaksonen et al. 2005). In the first phase, nucleation of actin and its crosslinking causes membrane invagination for which, a physical link between PM and the actin network is necessary. Crosslinking is speculated to tolerate larger stresses that occur during initial membrane deformation. During the second phase, nucleation ceases, while the actin network expands/rearranges. This rearrangement is thought to release the stress stored in the cross-linked actin mesh, thereby leading to vesicle scission (Picco et al. 2018). Hence, actin may play multiple roles in endocytosis depending upon the context. Actin may scaffold proteins, actin filaments may push against the membrane, and actin-based motors may pull the membrane.
Cargo sorting among different endocytic pathway
In CME, AP2 is traditionally known to act as a primary adaptor that recognises cargo via a specific sequence based motif on one hand while connects the cargo to clathrin assembly (Robinson 2004). Despite this, several CME cargoes can traffic independent of AP2 utilizing co-adaptors. For example, Epsin family proteins (ubiquitinated cargo) (Polo et al. 2002), β-arrestins (phosphorylated GPCR) (Laporte et al. 2000), Dab2 and ARH (Low Density Lipoprotein) (Maurer & Cooper 2006), Numb (Notch) (Santolini et al. 2000) are some of the diverse co-adaptors that are used by CME cargoes. Notably, GPCR are also internalised by FEME pathway, independent of β-arrestins that raises the question about the factors that govern cargo sorting to the different pathways (Boucrot et al. 2015). Ubiquitinylation may serve as a signal for CIE via the FEME, and the capacity to bind Galectins for the CG pathway. Identifying the sorting mechanisms for each of the CIEs must serve as a major focus.
Post-endocytic trafficking itineraries
Multiple endocytic pathways take in different cargoes and deliver them to different destinations within the cell. Endocytic vesicles derived from CME undergo fusion with a sorting endosome which in turn undergo homotypic fusion. Cargo is then directed towards recycling compartments or it matures to late endosomes and then fuses with lysosomes (Fig. 2). There is also a direct recycling route from the sorting endosome, and endosomes become progressively more acidic from early to late to lysosomes (Huotari & Helenius 2011). It appears that most cargo derived from dynamin-dependent endocytosis follow this itinerary, where different endocytic vesicles converge on sorting endosomes. On the other hand, dynamin-independent endocytic processes appear to form distinct early endosomal compartments which deliver only part of their material to the endosomal system that carries cargo derived from the dynamin-dependent system. CLICs eventually fuse to form an early endosomal pathway called GEECs. The resulting GEECs subsequently fuse with the sorting endocytic vesicles via a Rab5/phosphatidylinositol-3-kinase-dependent mechanism (Kalia et al. 2006) linking up with endosomal cargo from CME and caveolar pathway (Fig 1). The GEECs which are highly acidic, even more so than the sorting endosome (Kalia et al. 2006), recycles much of its membrane contents before fusing with the classical sorting endosome (Howes et al. 2010). Recycling of membrane from these compartments is likely to involve distinct recycling systems and machinery. This is an active area of research and the reader is referred to several excellent reviews in this area (Cullen & Steinberg 2018, Grant & Donaldson 2009, Huotari & Helenius 2011, Maxfield & McGraw 2004).
Endocytosis in HeLa cells, a cautionary note
It must be admitted that the CIE field has been plagued by contradictory data from different groups. For instance, as mentioned earlier, CG is a high capacity pathway as visualized using EM based quantitative imaging (Howes et al. 2010). However, in a study done primarily in a HeLa cell line, CIE(s) were found not to contribute significantly to endocytic flux (Bitsikas et al. 2014). Furthermore, it was argued that if CIE exists, it is a compensatory mechanism when CME is perturbed. This extreme view is unlikely since CIE is observed without perturbation of CME in many systems (Guha et al. 2003, Hemalatha et al. 2016, Howes et al. 2010, Renard et al. 2015, Sabharanjak et al. 2002) and in real time (Sathe et al. 2018). Moreover, removal of all three dynamin isoforms increases fluid uptake via the CG pathway in mouse embryonic fibroblasts (Park et al. 2013, Thottacherry et al. 2018), contrary to a reduction in fluid-uptake upon dynamin inhibition in HeLa cells (Bitsikas et al. 2014). Interestingly, while AP2 knock down inhibited CME in many different cell lines and upregulates fluid phase uptake, in HeLa cells it also reduces fluid uptake (Holst et al. 2017, Thottacherry et al. 2018). These observations along with previous studies (Kalia et al. 2006) indicate that HeLa cells do not have a functional CG pathway.
An important point to note is that many endocytic studies have been carried out in cell lines long adapted to tissue culture. In the case of HeLa cells, they appear to have diverged into unique cell lines in culture over the years, with different genomic, transcription and proteomic profiles (Frattini et al. 2015, Liu et al. 2018). An example highlighting this divergence is endocytosis of EGFR in HeLa cells. EGFR can traffic using CME and CIE and the discrepancy about the existence of a CIE route for EGFR was attributed to variation between different laboratory strains of HeLa cell line (Sigismund et al. 2013). Another example of variability in HeLa is the endocytic dependence on dynamin. On one hand, HeLa shows a predominant dynamin-dependent endocytosis (Bitsikas et al. 2014, Thottacherry et al. 2018) while on the other hand, one of the initial observations of dynamin-independent fluid uptake was made in a HeLa line (Damke et al. 1995). Hence, it is necessary to confirm inferences made in HeLa line, particularly, those pertaining to the role of CIE (Doherty et al. 2011, Francis et al. 2015, Holst et al. 2017, Krag et al. 2010, Lundmark et al. 2008), by doing experiments in other characterized cell systems.
Cross talk between Endocytic Pathways: A role for lipids?
Different endocytic pathways functioning at the cell surface appear to influence each other, indicating a cross-talk between the pathways. In the context of caveolae, the presence and expression of caveolins and cavins, regulates a number of CIEs by changing membrane fluidity and cholesterol levels at the plasma membrane, respectively (Chaudhary et al. 2014). Loss of dynamin function exhibits an increased activity of the CG pathway, indicating again cross talk between the dynamin-dependent and independent pathways (Guha et al. 2003, Thottacherry et al. 2018). However, the precise means for cross talk between different pathways is not understood.
Apart from the protein components, lipids in the plasma membrane are crucial for endocytosis. For example, cholesterol is important for the transbilayer interaction of membrane lipids (Raghupathy et al. 2015) and is necessary for making phosphatidylinositol phosphate (PIP)-rich domains (Jiang et al. 2014, Kwik et al. 2003). Cholesterol depletion affects almost all CIE pathways with varying efficacy (Boucrot et al. 2015, Chadda et al. 2007, Rodal et al. 1999, Subtil et al. 1999). Cholesterol, has been shown to regulate CDC42 activity and consequently CG endocytosis (Chadda et al. 2007); at extreme levels of depletion, it also affects CME (Subtil et al. 1999). It is unlikely that all CIE are affected by similar mechanisms upon cholesterol perturbations and the specific details need to be worked out.
Polyphosphoinositides are implicated in various aspects of cell physiology (Balla 2013). Phosphatidylinositol (PI) and its phosphorylated products, polyphosphoinositides (PIPs) are crucial signaling lipids that mark specific membrane domains and have been shown to differentially influence multiple endocytic pathways. There are seven PIP species depending on the phosphorylation status at 3-, 4-, and 5-OH positions and they are interconvertible by specific enzymes that phosphorylate or dephosphorylate them. Since many of these lipid modifying enzymes are localized to specific compartments, it is likely that their products are generated locally thereby providing a unique PIP identity to specific membrane compartments or sub-domains. During migration, for example, Class I PI3K is localized to the plasma membrane at the leading edge generating sub-domains of PI(3,4,5)P3 in a polarized fashion, helping in polarized migration (Funamoto et al. 2002, Servant et al. 2000). ClassII PI3Kβ on the late endosome or lysosomes in response to nutrient deprivation produces PI(3,4)P2 which clusters lysosomes and suppress mTOR activity (Marat et al. 2017). Class III PI3K (Phosphatidylinositol 3 kinase), Vps34, generates most of PI(3)P at the early endosomes that is important for early endosomal identity via regulating Rab5 recruitment (Simonsen et al. 1998). While the role for PI(3)P in endosomal maturation via a sequential cascade of recruitment of kinases and phosphatases by the Rab proteins is well appreciated (Wandinger-Ness & Zerial 2014), the role for different PIPs in regulating different forms of endocytosis is only recently being recognized. For an overview of PIPs in the endo-lysosomal system, the reader is referred to a recent review (Wallroth & Haucke 2018).
Turnover of PIPs is crucial for the initiation and completion of a Clathrin pit during CME. The generation of PI(4,5)P2 recruits Clathrin adaptors, FCHO, epsins and AP-2 complex. AP-2 can, in turn, recruit more PIP5K, creating a feed forward loop (Bairstow et al. 2006, Haucke 2005, Krauss et al. 2006). However, later stages require hydrolysis of the accumulated PI(4,5)P2 and production of PI(3,4)P2 from PI(4)P (Rusk et al. 2003). In the caveolar pathway our understanding of the role of PIs is limited. However, a distinct pool of PI(4,5)P2 is observed at the rim of caveolae (Fujita et al. 2009). The dynamin-related ATPase EHD2 is recruited to caveolae by binding to PI(4,5)P2 to negatively regulate internalization of caveolae (Daumke et al. 2007, Stoeber et al. 2012). The FEME pathway is initiated by PI(3,4)P2 produced by SHIP1/2 phosphatase from PI(3,4,5)P3. This helps in recruiting lammellipodin and thus EndoA2, which allows local deformation of the membrane to mediate FEME pathway (Renard et al. 2015). The CG pathway is also regulated by PI(3,4,5)P3 which recruits GBF1, a key regulator (Mazaki et al. 2012). Inhibition of Class 1 PI3K or its depletion abrogated the localization of Drosophila homolog of GBF1, Garz, to the plasma membrane, inhibiting the CG pathway (Hemalatha et al. 2016). These experiments, however, do not rule out a role for products of PI(3,4,5)P3 such as PI(3,4)P2. In addition, ARF1 is a potential activator of PI(4)P5K1 (Honda et al. 1999) and the resultant local PI(4,5)P2 (Di Paolo & De Camilli 2006) could indirectly activate CDC42 (Rohatgi et al. 2000), another regulator of CG pathway (Sabharanjak et al. 2002). Thus by-products of PI(3,4,5)P3 could potentially bring together two regulatory arms of CG pathway: GBF1 via PI(3,4,5)P3 and CDC42 via PI(4,5)P2 indicating a prominent role for PIPs in initiating the CG pathway, or regulating FEME.
PIPs along with other proteins help with the coincidence detection and provide compartment identities (Behnia & Munro 2005, Di Paolo & De Camilli 2006). This could work at the plasma membrane to give nano-scale identities for operating multiple endocytic pathways in adjacent regions of the membrane. Indeed, PI(4,5)P2 and PI(3,4,5)P3 are in separate nanoscale domains at the plasma membrane (Wang & Richards 2012). These domains by themselves could help to activate different endocytic pathways at the plasma membrane where signalling could spatially modulate endocytic levels by regulating the levels of the PIP species generated.
Endocytic pathways also compensate each other, resulting in substantial cross-talk among them. Could the nanodomain localization of PI-Kinases and phosphatases and feedback loops therein help coordinate this cross talk? Addressing this question will require looking at multiple pathways simultaneously, to get a system wide regulation of these pathways in the context of membrane lipid species generation and localization.
Evolutionary Antecedents of Endocytic pathways
Understanding the evolutionary history of each of the endocytic processes, will shed light on the function of each of these pathways, as well as answer questions about how ancient cells performed these tasks. Was there a primordial eukaryotic cell that lacked the capacity for specific forms of endocytosis? When did specific endocytic processes emerge? Was it operational in prokaryotes or Archaea or in FECA (first eukaryotic common ancestor) that arose out of merger?
Phylogenetic analyses are key to understanding evolutionary antecedents, since they allow us to compare molecular and other cellular processes within eukaryotes. If a trait is found in a representative species of all the super-groups, then the trait is presumed to be ancient and any absences are due to loss of these traits. On the other hand, if a trait is present in only a subset of super groups then it is considered a recent acquisition. Such genomic comparison indicates that several of endocytic proteins were present in last eukaryotic common ancestor (LECA) (Dacks et al. 2008, Field et al. 2007). A recent phylogenetic analysis of the dynamin superfamily members, revealed that there are three classes of dynamins. Class A and B were present in LECA while Class C appears to have arisen in amoebozoans and archaeplastids (Purkanti & Thattai 2015). Extant eukaryotes have two distinct Class A dynamin specialized for two distinct functions: mitochondrial division and vesicle scission. Several eukaryotic lineages, for instance Trypanosoma brucei have only a single Class A dynamin indicating that in these organisms both mitochondrial division and vesicle scission is performed by the same dynamin (Chanez et al. 2006). From the same excavata supergroup, Giardia lambia, an amitochondriate, has a single dynamin that colocalizes with CCP (Gaechter et al. 2008). Thus, it appears that LECA possessed a single bifunctional dynamin that duplicated and specialized into mitochondrial and vesicle variants three independent times (Purkanti & Thattai 2015). Looking at other key endocytic molecules in LECA, indicates that the endocytic system of LECA was quite sophisticated (Field et al. 2007). Rabs and Syntaxins are highly conserved and some of the key early, recycling and late Rabs, (5, 7 and 11) are present in almost all the taxa. In addition to the clathrin coat and the adaptin complex (Hirst et al. 2011), SNAREs and many other fusion and endocytic machineries are present in LECA and almost all taxa (Field et al. 2007). Thus, the endocytic system is not only ancient but LECA possessed a highly sophisticated endocytic system. A general scheme of endocytosis involves formation of the vesicles which become differentiated into early and subsequently late endosomes/lysosomes, and this network is closely linked with the exocytic traffic. Most organisms have all of these features, while some have lost one or more aspects.
So far evidence suggests that LECA possessed CME but what about CIEs? A phylogenetic analysis of caveolae and caveolin, shows that it appears very late in evolution (Field et al. 2007, Kirkham et al. 2008). Regardless, CIE is likely to occur in all eukarya, given that it is prevalent in single cell eukaryotes such as yeast, and metazoa such as both animals and plants (Baral et al. 2015). Many CIEs are regulated by small GTPases (RhoA, Rac1 and CDC42; Supplementary Table S1), which participate in several other crucial cellular processes and are likely to be present in the LECA. Recently, evidence has accumulated that IRSp53, an I-BAR domain protein specifically affects the CG endocytic pathway but leaves CME unperturbed (Sathe et al. 2018). I-BAR domain of IRSp53 that is speculated to stabilize the necks of forming CG endocytic tubules, apart from being present in metazoans (Scita et al. 2008), is also present in budding yeast with orthologs in filasterea and choanoflagellates (Itoh et al. 2016). Similarly, EndoA-dependent FEME pathway could exist in other organisms since BDPs are found not only in metazoans but also in unicellular organisms like yeast. Detailed phylogenetic analysis for IRSp53, EndoA and other recently characterized CIE molecules in a fashion similar to the analysis performed for dynamin should reveal insights about the prevalence of many of these pathways in eukaryotes.
Endocytic processes are also observed in some bacteria belonging to phylum Planctomycetes; Gemmata obscuriglobus carries out protein uptake via membrane encased vesicles (Fuerst & Sagulenko 2012). Lokiarchaeum postulated as the closest living ancestor of eukaryotes, encodes more eukaryotic signature proteins than any other Archaeon. Lokiarchaeum encodes small GTPases, BDPs, gelsolin and other proteins, and might display membrane trafficking (Dey et al. 2016). Unfortunately, members of this species are yet to be isolated and studied to confirm these exciting possibilities.
In addition to the phylogenetic analysis, it is instructive to study different cell systems that have diverged across different evolutionary time scales to provide inputs into how cells have evolved to construct endocytic processes. For example, understanding endocytosis in the budding yeast, Saccharomyces cerevisiae, has provided insights into what appears to be a strictly ARP2/3 (andLas17/N-WASP) dependent endocytic pathway but relies to a much lesser extent on clathrin and dynamin (Kaksonen & Roux 2018). By contrast, in another yeast, the pathogenic Candida albicans, membrane lipids and bulk fluid internalization were not perturbed in the absence of ARP2/3 whereas membrane receptor endocytosis via the CME machinery was compromised. This revealed the existence of two types of endocytic mechanisms that might resemble the CME and CIE processes, found in metazoan. Future comparative analysis of the endocytic mechanism among different eukaryotic organisms will provide further insights into the multiple design principles of endocytosis.
Functional roles of multiple endocytic pathways
High-throughput studies (Collinet et al. 2010, Gupta et al. 2014, Pelkmans et al. 2005) of multiple endocytic processes analyzed simultaneously, have not only advanced our understanding of the intricate molecular network involved in various endocytic pathways but have also elucidated the roles of endocytosis in maintaining cellular homeostasis. In this section, we focus on the roles of multiple endocytic pathways in nutrient uptake, setting plasma membrane tension, cell migration and cell signaling.
Membrane tension and homeostasis
Membrane tension has long been proposed to regulate endo-exocytic membrane trafficking (Sheetz & Dai 1996). The membrane responds passively and actively to changes in membrane tension. Passively by creating membrane tubules called ‘reservoirs’(Kosmalska et al. 2015), blebs (Norman et al. 2010), and flattening of caveolae (Sinha et al. 2011) and actively by gating mechanosensitive channels, and altering cytoskeleton dynamics and membrane trafficking (Apodaca 2002, Diz-Muñoz et al. 2013, Gauthier et al. 2012). In terms of endocytosis, different endocytic processes respond differently to the application of force on a cell. CME resists an increase in membrane tension by recruiting actin where actin and BDPs helps offset tension to allow internalization of the CCPs (Boulant et al. 2011, Ferguson et al. 2017, Hassinger et al. 2017, Schöneberg et al. 2018) while membrane tension is involved in transition of clathrin coats from flat to curved (Bucher et al. 2018). Caveolae help to passively buffer an increase in tension by rapidly flattening the cup shaped caveolae to provide excess membrane (Sinha et al. 2011), but is restored actively using cytoskeletal rearrangements. Upon deadhering, caveolae coated region of the membrane are endocytosed over a period of minutes to hours, resulting in an alteration of the membrane composition of the cell surface. Phosphorylation of caveolin is necessary to trigger internalization of membrane micro-domains enriched in cholesterol (del Pozo et al. 2005). This endocytosis is important for triggering anoikis and happens once the cell is in suspension. The CG pathway on the other hand rapidly and transiently responds to a decrease in tension by internalizing excess membrane (Thottacherry et al. 2018). This transient endocytic upregulation is also observed during deadhering (Thottacherry et al. 2018) potentially caused by a reduction in tension (Norman et al. 2010). It is important here to distinguish the difference between the caveolin-dependent endocytosis and CG pathway during deadhering. Contrary to the caveolar pathway, the CG pathway is downregulated in cells in suspension (Thottacherry et al. 2018) possibly due to the reduction in cholesterol at cell surface (del Pozo et al. 2004). Thus, different endocytic pathways respond differentially to changes in tension and this could be useful in different circumstances.
Integrin mediated cell-substrate detachment is accompanied by caveolar uptake of cholesterol enriched microdomains contributing to an alteration of the membrane composition as well as targeting of Rac to the plasma membrane (del Pozo et al. 2004). In parallel, the tension-regulated response of the CG pathway depends on the activation of vinculin, a key mechanotransducer downstream of cell-substrate and cell-cell adhesion (Thottacherry et al. 2018). The CG pathway acutely senses changes in tension and actively responds to the change. In addition, the CG pathway is also proposed to provide membrane during cell spreading from recycling of endocytic compartments in response to increase in tension (Gauthier et al. 2009). The high capacity and membrane tension sensitive features of CG endocytosis positions it to be a key component in maintaining membrane homeostasis.
Migration
During cell migration in 2D, multiple endocytic pathways are localized to spatially distinct sites. In migrating fibroblasts, CG endosomes are localized to the leading edge and transient ablation of these endosomes specifically inhibit efficient migration, while caveolae are at the rear end and CCPs are unpolarized (Howes et al. 2010). FEME, when induced, is observed at the leading edge of adherent BSC1 cells with CCPs remaining unpolarized (Boucrot et al. 2015).
Membrane tension is involved in coordinating multiple cellular processes during migration (Diz-Muñoz et al. 2013). For instance, the increase in the membrane tension at the leading edge maintains the polarity and helps in migration (Houk et al. 2012). The CG pathway helps in regulating membrane tension (Thottacherry et al. 2018) and key regulators of the CG pathway are localized to leading edge in neutrophils (Mazaki et al. 2012). Thus, the polarized CG pathway in migrating cells may be deployed to modulate membrane tension. In contrast, CCPs help in 3D migration of MDA-MB-231 by helping to pinch the collagen fibers (Elkhatib et al. 2017). In confined environments, migrating immune cells overcome hydraulic resistance by activating macropinocytosis at the leading edge to transport fluid across the cell (Moreau et al. 2018). Nevertheless, the deployment of a particular endocytic pathway will depend on the type of migration and/or the cell type and how regulation is achieved remains to be resolved in the context of both physical (membrane tension) and chemical (extracellular matrix or growth factor) cues.
Nutrient Uptake and Sensing
Historically the role of endocytosis has been associated with uptake of nutrients for the cell. In fact the uptake of iron (bound to Transferrin) and lipids (associated with lipoproteins) are perhaps the most celebrated aspects of the CME function (McMahon & Boucrot 2011). In addition, the CG pathway internalizes a variety of GPI-APs allowing for the uptake of its small molecule ligands. A prime example is the folate receptor that binds to the folate ligand in the neutral pH environment of the cell surface, and is endocytosed via CIE to robustly deliver folates to cells (Ritter et al. 1995). Folate dissociates from its receptors in the extremely low pH environment of the endosomes and is transported out to the cytosol. This pathway could be of vital importance for a number of cargoes where a low concentration bulk ligand is captured at the cell surface and is concentrated and delivered into the cell via GEECs.
Endocytic uptake of nutrients takes even more prominence in the case of tumors that are nutrient deprived. For instance, human pancreatic cancer tumors are nutrient poor because they lack the access to freely circulating amino acids due to poor vascularization (Kamphorst et al. 2015) and therefore need to actively scavenge extracellular proteins. In separate study, Ras-transformed cells upregulate macropinocytosis to provide an amino acid source by proteolytic degradation of endocytosed extracellular proteins, dependent on PTEN and AMPK (Commisso et al. 2013, Kim et al. 2018). Moreover, inhibition of macropinocytosis suppressed tumor growth (Commisso et al. 2013, Kim et al. 2018). The question then arises as to how the nutrient sensing machinery talks to the endocytic mechanisms. GAPDH comes up as an interesting candidate since it can act as a GAP for ARF1 in addition to its role in glycolysis, thereby connecting membrane trafficking to the energy state of the cell (Yang et al. 2018). Interestingly, upon GAPDH depletion, fluid-phase endocytosis increased while CME and other CIE remained unaffected. A second candidate that potentially connects nutrient sensing and endocytosis is AMPK. AMPK also phosphorylates GBF1 (Mao et al. 2013), the GEF for ARF1 and a key regulator of the CG pathway (Gupta et al. 2009). AMPK may even indirectly control ARF1, by regulating GAPDH localization to membrane compartments through its phosphorylation in response to nutrient status (Yang et al. 2018). Thus, GAPDH and AMPK open up ways to coordinate endocytic pathways with nutrient level within a cell. Nevertheless, the cell would need to take in key ligands and continue to exert basal signaling control. This can be achieved by allowing the CME to continues robustly whilst the fluid-phase uptake and secretion is blocked on GAPDH perturbation.
Endocytosis and signalling
Endocytosis of ligand-receptor pairs has been typically associated with downregulation of signalling. However, since the discovery of endosomal localization of EGFR and it’s signalling effectors (Di Guglielmo et al. 1994) upon EGF stimulation, numerous functions (positive, negative, and modulatory) of endocytosis have been identified in various signalling systems, and some excellent recent reviews have highlighted this (Di Fiore & von Zastrow 2014, Villaseñor et al. 2016). In this section, we focus on the roles of multiple endocytic pathways in one signalling module - Receptor Tyrosine Kinase (RTK) signalling.
Growth factors and their respective RTKs play pivotal roles in cellular functions during development as well as in pathogenesis (Lemmon & Schlessinger 2010). Typically, growth factor binding results in receptor dimerization, activation of the tyrosine kinase, rapid internalization and sorting of ligand-RTK complexes via CME into lysosomes for degradation. However, many RTKs deviate from this standard model (Goh & Sorkin 2013). Several factors, such as ligand concentration, types of ligands and receptors and cells, control the mode of ligand-RTK trafficking and subsequent downstream RTK signaling. The first evidence that EGFR is trafficked by CIEs came when it was noticed that clathrin heavy chain depletion did not affect EGF uptake in HeLa cells (Hinrichsen et al. 2003). Studies in HeLa cells showed that low concentrations of EGF (1.5 ng/ml) results in CME of EGFR while high concentrations of EGF (20 ng/ml) partitions EGFR via a cholesterol sensitive CIE pathway (Sigismund et al. 2005, 2008). The fate of EGFR internalized via the different pathways is also distinct: EGFR endocytosed via CME is recycled, thus aids in prolonged activation, while those internalized via CIE results in lysosomal degradation effecting signal attenuation. CIE trafficking is associated with Cbl mediated receptor ubiquitylation at the plasma membrane (Sigismund et al. 2013). However, the finding of CIE pathways for EGFR uptake is contested (Grandal et al. 2012, Kazazic et al. 2006, Rappoport & Simon 2009). The discrepancy could be attributed to cell specific or clone specific differences in HeLa cell lines used in these studies (Sigismund et al. 2008, 2013). The nature of the ligand in activating EGFR also directs EGFR endocytosis via different pathways: while EGF stimulated a CME uptake, more potent ligands such as Heparin binding EGF like growth factor (HB-EGF) and betacellulin (BTC) activate both CME and CIE routes (Henriksen et al. 2013). The specific CIE that EGFR utilizes requires dynamin (Henriksen et al. 2013, Sousa et al. 2012) and is identified as FEME (Boucrot et al. 2015). In cells lacking all isoforms of Endophilin, surface levels of EGFR were enhanced consistent with FEME aiding in receptor degradation.
In the Drosophila hematopoietic system, high concentrations of Spitz (Spi), one of the EGFR ligands in Drosophila, caused increased EGFR uptake via the CG pathway. Perturbation of molecules crucial for the CG pathway such as Arf1, Garz and GRAF-1 resulted in reduced EGFR uptake at high ligand concentrations and uptake was unaffected upon depletion of clathrin or by pharmacological inhibition of dynamin. EGFR ubiquitylation was also only observed only under high Spi and ubiquitylation defective EGFR failed to undergo CG endocytosis at high Spi, confirming the necessity of Cbl-mediated receptor ubiquitylation in directing EGFR to the CG pathway. This increased EGFR signalling resulting from depletion of GRAF-1 also caused over proliferation of plasmatocytes, one specific lineage of blood cells (Kim et al. 2017).
Other growth factors such as IGF, FGF and PDGF undergo CIE in different scenarios. IGF-I binding IGF-IR triggers CME or caveolar pathways based on the ligand concentration (Sehat et al., 2008; Salani et al., 2010). FGF when bound to FGFR1 undergoes uptake via CME whereas when bound to FGFR3 undergoes clathrin and dynamin independent uptake (Haugsten et al. 2011), suggesting a CG route. Trafficking of PDGFR-β also switches between CME and CIE depending on low or high concentrations of the ligand, PDGF-BB, respectively (De Donatis et al., 2008; Sadowski et al., 2013). A recent study shows that PDGFR-β internalization via CIE requires RhoA-ROCK, CDC42, CD44 and Galectin-3 (Jastrzębski et al. 2017). The involvement of the latter three molecules suggests that PDGFR-β may be internalized via the CG pathway. The different endocytic entry routes bias PDGFR-β signaling readouts towards cell proliferation or motility. Thus, various mechanisms of entry expand the scope for the regulation of downstream RTK signalling outputs.
In the context of Wingless signalling in Drosophila wing disc, Wingless is internalized via the CG pathway independent of its signalling receptor. Within merged endosomes, the CG internalized pool of wingless interacts with its signalling receptor that was endocytosed via the CME (Hemalatha et al. 2016). Therefore, both endocytic pathways are required for facilitating downstream signalling of Wingless, revealing yet another context where multiple endocytic mechanisms regulate signalling. Other classes of signalling systems, such as GPCRs (Boucrot et al. 2015, Delaney et al. 2002, Okamoto et al. 2000, Zhang et al. 1996), Integrins (Arjonen et al. 2012, De Franceschi et al. 2016, Paul et al. 2015), TGF-β (Di Guglielmo et al. 2003, Zwaagstra et al. 2001) and Wnts (Hemalatha et al. 2016, Yamamoto et al. 2006), also utilize similar design principles to modulate their signalling outcomes. Supplementary Table S1 summarizes some of these key findings in cargo trafficking.
In an extensive screen for modulators of the endosomal distribution of two endocytic cargoes, TfR and EGFR, the signalling pathways such as Wnt, integrin/cell adhesion, transforming growth factor (TGF)-? and Notch were found to regulate the endocytic system by altering the number, size, concentration of cargo and position of endosomes (Collinet et al. 2010). This indicates that not only does endocytosis influence signalling, but that signalling pathways also profoundly alter the endocytic machinery.
Conclusions
Different pathways operate at the cell surface helping cells integrate information from its surrounding and respond to it. These help the cell to sense and respond to forces, nutrients, coordinate migration and allow a cell to engage with its neighbors. A single endocytic pathway probably could not have made these complex and multiple decisions. From a functional perspective, multiple endocytic pathways appear to be responsible for distinct role(s) in specific contexts in a non-redundant fashion. Indeed, our understanding of these distinct pathways of endocytosis is crucial for understanding cell physiology as well as its role in a metazoan.
While we know the CME molecular machinery in greater temporal and spatial detail, details of CIE are emerging fast and furious. Key insights into mechanisms involved in cargo capture and scission in dynamin-independent pathways are also being acquired. It is clear that there is yet a dire need for a comprehensive parts list for the multiple CIE pathways, both dynamin-dependent and independent. This will occur using sophisticated genetic screens where multiple pathways are examined at the same time, and are capable of uncovering correlated perturbations in these pathways (Collinet et al. 2010, Dey et al. 2014, Gupta et al. 2014, Haney et al. 2018). This will also address relationships between the different CIE pathways. The cell invests a considerable amount of molecular wiring to calibrate cross talk between these pathways, and a systematic monitoring of the correlated expression of genes in different contexts may provide a handle to parse how endocytic networks are governed.
Endocytosis most likely drove eukaryotic evolution by ensnaring the major energy factory of the cell, the respiring bacterial ancestor of the mitochondrion. Endocytosis is definitely part of the core cellular plan and having multiple pathways help bring a sophistication to this plan by engaging in multiple processes like signalling, migration, nutrient sensing, cell membrane homeostasis (Scita et al. 2010, Sigismund et al. 2012). To understand how a cell works in its entirety, it would be important to have a systems view of different membrane trafficking pathways and study them in a physiological relevant context.
Supplementary Material
Acknowledgements
We thank Thomas S. van Zanten, Sowmya Jahnavi, Rajan Thakur, Sangeeta Nath and Muriel Grammont for their insightful comments about the manuscript and Sowmya Jahnavi for help with Figure 1 cartoon. We thank all the SM lab members for their valuable inputs towards material gathered for this manuscript. We also thank Mukund Thattai, Madan Rao, Rob Parton and Ludger Johannes for helping to frame some of the arguments presented here. J.J.T, M.S. and C.P. acknowledge pre-doctoral fellowship from CSIR (Government of India) (J.J.T), NCBS-TIFR (M.S, C.P), and research support from CCAMP from during the writing of this manuscript. S.M. acknowledges JC Bose Fellowship from DST (Government of India), a grant from HFSP (RGP0027/2012) and a Wellcome Trust-DBT India Alliance Margdarshi fellowship (IA/M/15/1/502018).
Contributor Information
Joseph Jose Thottacherry, Email: thottacherry@ncbs.res.in.
Mugdha Sathe, Email: mugdhas@ncbs.res.in.
Chaitra Prabhakara, Email: chaitrap@ncbs.res.in.
References
- Aboulaich N, Vainonen JP, Strålfors P, Vener AV, Stralfors P. Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase I and transcript release factor (PTRF) at the surface of caveolae in human adipocytes. Biochem J. 2004;383(Pt 2):237–48. doi: 10.1042/BJ20040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aït-Slimane T, Galmes R, Trugnan G, Maurice M. Basolateral internalization of GPI-anchored proteins occurs via a clathrin-independent flotillin-dependent pathway in polarized hepatic cells. Mol Biol Cell. 2009;20(17):3792–3800. doi: 10.1091/mbc.E09-04-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RG, Brown MS, Goldstein JL. Role of the coated endocytic vesicle in the uptake of receptor-bound low density lipoprotein in human fibroblasts. Cell. 1977a;10(3):351–64. doi: 10.1016/0092-8674(77)90022-8. [DOI] [PubMed] [Google Scholar]
- Anderson RG, Goldstein JL, Brown MS. A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature. 1977b;270(5639):695–99. doi: 10.1038/270695a0. [DOI] [PubMed] [Google Scholar]
- Apodaca G. Modulation of membrane traffic by mechanical stimuli. Am J Physiol Renal Physiol. 2002;282(2):F179–90. doi: 10.1152/ajprenal.2002.282.2.F179. [DOI] [PubMed] [Google Scholar]
- Arjonen A, Alanko J, Veltel S, Ivaska J. Distinct Recycling of Active and Inactive β1 Integrins. Traffic. 2012;13(4):610–25. doi: 10.1111/j.1600-0854.2012.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairstow SF, Ling K, Su X, Firestone AJ, Carbonara C, Anderson RA. Type Igamma661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J Biol Chem. 2006;281(29):20632–42. doi: 10.1074/jbc.M601465200. [DOI] [PubMed] [Google Scholar]
- Balla T. Phosphoinositides: Tiny Lipids With Giant Impact on Cell Regulation. Physiol Rev. 2013;93(3):1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233(4768):1061–68. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- Baral A, Irani NG, Fujimoto M, Nakano A, Mayor S, Mathew MK. Salt-Induced Remodeling of Spatially Restricted Clathrin-Independent Endocytic Pathways in Arabidopsis Root. Plant Cell. 2015;27(4):1297–1315. doi: 10.1105/tpc.15.00154. 1297(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiani M, Liu L, Hill MM, Jedrychowski MP, Nixon SJ, et al. MURC/Cavin-4 and cavin family members form tissue-specific caveolar complexes. J Cell Biol. 2009;185(7):1259–73. doi: 10.1083/jcb.200903053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438(7068):597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- Bitsikas V, Corrêa IR, Nichols BJ. Clathrin-independent pathways do not contribute significantly to endocytic flux. Elife. 2014;3:e03970. doi: 10.7554/eLife.03970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bökel C, Brand M. Endocytosis and signaling during development. Cold Spring Harb Perspect Biol. 2014;6(3) doi: 10.1101/cshperspect.a017020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonazzi M, Spanò S, Turacchio G, Cericola C, Valente C, et al. CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat Cell Biol. 2005;7(6):570–80. doi: 10.1038/ncb1260. [DOI] [PubMed] [Google Scholar]
- Boucrot E, Ferreira APA, Almeida-Souza L, Debard S, Vallis Y, et al. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature. 2015;517(7535):460–65. doi: 10.1038/nature14067. [DOI] [PubMed] [Google Scholar]
- Boulant S, Kural C, Zeeh J-C, Ubelmann F, Kirchhausen T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol. 2011;13(9):1124–31. doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher MS, Thomson JN, Pearse BM. Coated pits act as molecular filters. Proc Natl Acad Sci U S A. 1980;77(7):4156–59. doi: 10.1073/pnas.77.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown FD, Rozelle AL, Yin HL, Balla T, Donaldson JG. Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic. J Cell Biol. 2001;154(5):1007–17. doi: 10.1083/jcb.200103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc Natl Acad Sci U S A. 1979;76(7):3330–37. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher D, Frey F, Sochacki KA, Kummer S, Bergeest J-P, et al. Clathrin-adaptor ratio and membrane tension regulate the flat-to-curved transition of the clathrin coat during endocytosis. Nat Commun. 2018;9(1):1109. doi: 10.1038/s41467-018-03533-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campelo F, Malhotra V. Membrane Fission: The Biogenesis of Transport Carriers. Annu Rev Biochem. 2012;81(1):407–27. doi: 10.1146/annurev-biochem-051710-094912. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. The simultaneous symbiotic origin of mitochondria, chloroplasts, and microbodies. Ann N Y Acad Sci. 1987;503(1):55–71. doi: 10.1111/j.1749-6632.1987.tb40597.x. [DOI] [PubMed] [Google Scholar]
- Chadda R, Howes MT, Plowman SJ, Hancock JF, Parton RG, Mayor S. Cholesterol-sensitive Cdc42 activation regulates actin polymerization for endocytosis via the GEEC pathway. Traffic. 2007;8(6):702–17. doi: 10.1111/j.1600-0854.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadda R, Mayor S. PTRF triggers a cave in. Cell. 2008;132(1):23–24. doi: 10.1016/j.cell.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Chan Wah Hak L, Khan S, Di Meglio I, Law A, Lucken-Ardjomande Häsler S, et al. FBP17 and CIP4 recruit SHIP2 and lamellipodin to prime the plasma membrane for fast endophilin-mediated endocytosis. Nat Cell Biol. 2018;20(9):1023–31. doi: 10.1038/s41556-018-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanez A-L, Hehl AB, Engstler M, Schneider A. Ablation of the single dynamin of T. brucei blocks mitochondrial fission and endocytosis and leads to a precise cytokinesis arrest. J Cell Sci. 2006;119(14):2968–74. doi: 10.1242/jcs.03023. [DOI] [PubMed] [Google Scholar]
- Chaudhary N, Gomez GA, Howes MT, Lo HP, McMahon K-A, et al. Endocytic crosstalk: cavins, caveolins, and caveolae regulate clathrin-independent endocytosis. PLoS Biol. 2014;12(4):e1001832. doi: 10.1371/journal.pbio.1001832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry A, Das SR, Hussain A, Mayor S, George A, et al. The Nef protein of HIV-1 induces loss of cell surface costimulatory molecules CD80 and CD86 in APCs. J Immunol. 2005;175(7):4566–74. doi: 10.4049/jimmunol.175.7.4566. [DOI] [PubMed] [Google Scholar]
- Chaudhry A, Das SR, Jameel S, George A, Bal V, et al. A Two-Pronged Mechanism for HIV-1 Nef-Mediated Endocytosis of Immune Costimulatory Molecules CD80 and CD86. Cell Host Microbe. 2007;1(1):37–49. doi: 10.1016/j.chom.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Collinet C, Stöter M, Bradshaw CR, Samusik N, Rink JC, et al. Systems survey of endocytosis by multiparametric image analysis. Nature. 2010;464(7286):243–49. doi: 10.1038/nature08779. [DOI] [PubMed] [Google Scholar]
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497(7451):633–37. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compeer EB, Kraus F, Ecker M, Redpath G, Amiezer M, et al. A mobile endocytic network connects clathrin-independent receptor endocytosis to recycling and promotes T cell activation. Nat Commun. 2018;9(1) doi: 10.1038/s41467-018-04088-w. 1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003 Mar;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Cullen PJ, Steinberg F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat Rev Mol Cell Biol. 2018;19(11):679–96. doi: 10.1038/s41580-018-0053-7. [DOI] [PubMed] [Google Scholar]
- Dacks JB, Poon PP, Field MC. Phylogeny of endocytic components yields insight into the process of nonendosymbiotic organelle evolution. Proc Natl Acad Sci U S A. 2008;105(2):588–93. doi: 10.1073/pnas.0707318105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Baba T, Van Der Bliek AM, Schmid SL. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131(1):69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127(4):915–34. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumke O, Lundmark R, Vallis Y, Martens S, Butler PJG, McMahon HT. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature. 2007;449(7164):923–27. doi: 10.1038/nature06173. [DOI] [PubMed] [Google Scholar]
- David C, McPherson PS, Mundigl O, de Camilli P. A role of amphiphysin in synaptic vesicle endocytosis suggested by its binding to dynamin in nerve terminals. Proc Natl Acad Sci U S A. 1996;93(1):331–35. doi: 10.1073/pnas.93.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CA, Baetz NW, Copeland CA, Kraft LJ, Han B, et al. Microtubule motors power plasma membrane tubulation in clathrin-independent endocytosis. Traffic. 2015;16(6):572–90. doi: 10.1111/tra.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Donatis A, Comito G, Buricchi F, Vinci MC, Parenti A, et al. Proliferation versus migration in platelet-derived growth factor signaling: the key role of endocytosis. J Biol Chem. 2008;283(29):19948–56. doi: 10.1074/jbc.M709428200. [DOI] [PubMed] [Google Scholar]
- De Franceschi N, Arjonen A, Elkhatib N, Denessiouk K, Wrobel AG, et al. Selective integrin endocytosis is driven by interactions between the integrin α-chain and AP2. Nat Struct Mol Biol. 2016;23(2):172–79. doi: 10.1038/nsmb.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney KA, Murph MM, Brown LM, Radhakrishna H. Transfer of M2 muscarinic acetylcholine receptors to clathrin-derived early endosomes following clathrin-independent endocytosis. J Biol Chem. 2002;277(36):33439–46. doi: 10.1074/jbc.M205293200. [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Alderson NB, Kiosses WB, Chiang H-H, Anderson RGW, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303(5659):839–42. doi: 10.1126/science.1092571. [DOI] [PubMed] [Google Scholar]
- del Pozo MA, Balasubramanian N, Alderson NB, Kiosses WB, Grande-García A, et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat Cell Biol. 2005;7(9):901–8. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvendahl I, Vyleta NP, von Gersdorff H, Hallermann S. Fast, Temperature-Sensitive and Clathrin-Independent Endocytosis at Central Synapses. Neuron. 2016;90(3):492–98. doi: 10.1016/j.neuron.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo R, Kushwah MS, Kamerkar SC, Kadam NY, Dar S, et al. ATP-dependent membrane remodeling links EHD1 functions to endocytic recycling. Nat Commun. 2018;9(1) doi: 10.1038/s41467-018-07586-z. 5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey G, Gupta GD, Ramalingam B, Sathe M, Mayor S, Thattai M. Exploiting cell-to-cell variability to detect cellular perturbations. PLoS One. 2014;9(3):e90540. doi: 10.1371/journal.pone.0090540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey G, Thattai M, Baum B. On the Archaeal Origins of Eukaryotes and the Challenges of Inferring Phenotype from Genotype. Trends Cell Biol. 2016;26(7):476–85. doi: 10.1016/j.tcb.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore PP, von Zastrow M. Endocytosis, signaling, and beyond. Cold Spring Harb Perspect Biol. 2014;6(8) doi: 10.1101/cshperspect.a016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guglielmo GM, Baass PC, Ou W-J, Posner BI, Bergeron JJM. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J. 1994;13(18):4269–77. doi: 10.1002/j.1460-2075.1994.tb06747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol. 2003;5(5):410–21. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–57. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Diz-Muñoz A, Fletcher DA, Weiner OD. Use the force: membrane tension as an organizer of cell shape and motility. Trends Cell Biol. 2013;23(2):47–53. doi: 10.1016/j.tcb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty GJ, Åhlund MK, Howes MT, Morén B, Parton RG, et al. The endocytic protein GRAF1 is directed to cell-matrix adhesion sites and regulates cell spreading. Mol Biol Cell. 2011;22(22):4380–89. doi: 10.1091/mbc.E10-12-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Edeling MA, Smith C, Owen D. Life of a clathrin coat: insights from clathrin and AP structures. Nat Rev Mol Cell Biol. 2006;7(1):32–44. doi: 10.1038/nrm1786. [DOI] [PubMed] [Google Scholar]
- Elkhatib N, Bresteau E, Baschieri F, Rioja AL, van Niel G, et al. Tubular clathrin/AP-2 lattices pinch collagen fibers to support 3D cell migration. Science. 2017;356(6343) doi: 10.1126/science.aal4713. [DOI] [PubMed] [Google Scholar]
- Ferguson JP, Huber SD, Willy NM, Aygün E, Goker S, et al. Mechanoregulation of clathrin-mediated endocytosis. J Cell Sci. 2017;130(21):3631–36. doi: 10.1242/jcs.205930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MC, Gabernet-Castello C, Dacks JB. Reconstructing the evolution of the endocytic system: insights from genomics and molecular cell biology. Adv Exp Med Biol. 2007;607(1):84–96. doi: 10.1007/978-0-387-74021-8_7. [DOI] [PubMed] [Google Scholar]
- Flannagan RS, Jaumouillé V, Grinstein S. The Cell Biology of Phagocytosis. Annu Rev Pathol Mech Dis. 2012;7(1):61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- Francis MK, Holst MR, Vidal-Quadras M, Henriksson S, Santarella-Mellwig R, et al. Endocytic membrane turnover at the leading edge is driven by a transient interaction between Cdc42 and GRAF1. J Cell Sci. 2015;128(22):4183–95. doi: 10.1242/jcs.174417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frattini A, Fabbri M, Valli R, De Paoli E, Montalbano G, et al. High variability of genomic instability and gene expression profiling in different HeLa clones. Sci Rep. 2015;5:1–9. doi: 10.1038/srep15377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick M, Bright NA, Riento K, Bray A, Merrified C, Nichols BJ. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr Biol. 2007;17(13):1151–56. doi: 10.1016/j.cub.2007.05.078. [DOI] [PubMed] [Google Scholar]
- Fuerst JA, Sagulenko E. Keys to eukaryality: Planctomycetes and ancestral evolution of cellular complexity. Front Microbiol. 2012 May;3:1–12. doi: 10.3389/fmicb.2012.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Cheng J, Tauchi-Sato K, Takenawa T, Fujimoto T. A distinct pool of phosphatidylinositol 4,5-bisphosphate in caveolae revealed by a nanoscale labeling technique. Proc Natl Acad Sci U S A. 2009;106(23):9256–61. doi: 10.1073/pnas.0900216106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto S, Meili R, Lee S, Parry L, Firtel RA. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109(5):611–23. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Gaechter V, Schraner E, Wild P, Hehl AB. The single dynamin family protein in the primitive protozoan giardia lamblia is essential for stage conversion and endocytic transport. Traffic. 2008;9(1):57–71. doi: 10.1111/j.1600-0854.2007.00657.x. [DOI] [PubMed] [Google Scholar]
- Gauthier NC, Masters TA, Sheetz MP. Mechanical feedback between membrane tension and dynamics. Trends Cell Biol. 2012;22(10):527–35. doi: 10.1016/j.tcb.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Gauthier NC, Rossier OM, Mathur A, Hone JC, Sheetz MP. Plasma membrane area increases with spread area by exocytosis of a GPI-anchored protein compartment. Mol Biol Cell. 2009;20(14):3261–72. doi: 10.1091/mbc.E09-01-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glebov OO, Bright Na, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat Cell Biol. 2006;8(1):46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- Goh LK, Sorkin A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb Perspect Biol. 2013;5(5) doi: 10.1101/cshperspect.a017459. a017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold ES, Underhill DM, Morrissette NS, Guo J, McNiven Ma, Aderem A. Dynamin 2 is required for phagocytosis in macrophages. J Exp Med. 1999;190(12):1849–56. doi: 10.1084/jem.190.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold S, Monaghan P, Mertens P, Jackson T. A clathrin independent macropinocytosis-like entry mechanism used by bluetongue virus-1 during infection of BHK cells. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0011360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Phagocytosis: An Immunobiologic Process. Immunity. 2016;44(3):463–75. doi: 10.1016/j.immuni.2016.02.026. [DOI] [PubMed] [Google Scholar]
- Grandal MV, Grøvdal LM, Henriksen L, Andersen MH, Holst MR, et al. Differential Roles of Grb2 and AP-2 in p38 MAPK- and EGF-Induced EGFR Internalization. Traffic. 2012;13(4):576–85. doi: 10.1111/j.1600-0854.2011.01322.x. [DOI] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A, Sriram V, Krishnan KS, Mayor S. Shibire mutations reveal distinct dynamin-independent and -dependent endocytic pathways in primary cultures of Drosophila hemocytes. J Cell Sci. 2003;116(Pt 16):3373–86. doi: 10.1242/jcs.00637. [DOI] [PubMed] [Google Scholar]
- Gupta GD, Dey G, Swetha MG, Ramalingam B, Shameer K, et al. Population distribution analyses reveal a hierarchy of molecular players underlying parallel endocytic pathways. PLoS One. 2014;9(6):e100554. doi: 10.1371/journal.pone.0100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta GD, Swetha MG, Kumari S, Lakshminarayan R, Dey G, et al. Analysis of Endocytic Pathways in Drosophila Cells Reveals a Conserved Role for GBF1 in Internalization via GEECs. PLoS One. 2009;4(8):e6768. doi: 10.1371/journal.pone.0006768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney MS, Bohlen CJ, Morgens DW, Ousey JA, Barkal AA, et al. Identification of phagocytosis regulators using magnetic genome-wide CRISPR screens. Nat Genet. 2018;50(12):1716–27. doi: 10.1038/s41588-018-0254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CG, Nichols BJ. Exploring the caves: Cavins, caveolins and caveolae. Trends Cell Biol. 2010;20(4):177–86. doi: 10.1016/j.tcb.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Hassinger JE, Oster G, Drubin DG, Rangamani P. Design principles for robust vesiculation in clathrin-mediated endocytosis. Proc Natl Acad Sci. 2017;114(7):E1118–27. doi: 10.1073/pnas.1617705114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haucke V. Phosphoinositide regulation of clathrin-mediated endocytosis. Biochem Soc Trans. 2005;33(Pt 6):1285–89. doi: 10.1042/BST0331285. [DOI] [PubMed] [Google Scholar]
- Haugsten EM, Zakrzewska M, Brech A, Pust S, Olsnes S, et al. Clathrin- and Dynamin-Independent Endocytosis of FGFR3 – Implications for Signalling. PLoS One. 2011;6(7):e21708. doi: 10.1371/journal.pone.0021708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayer A, Stoeber M, Bissig C, Helenius A. Biogenesis of caveolae: Stepwise assembly of large caveolin and cavin complexes. Traffic. 2010;11(3):361–82. doi: 10.1111/j.1600-0854.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- Hemalatha A, Prabhakara C, Mayor S. Endocytosis of Wingless via a dynamin-independent pathway is necessary for signaling in Drosophila wing discs. Proc Natl Acad Sci. 2016;113(45):E6993–7002. doi: 10.1073/pnas.1610565113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JR, Krueger EWA, Oswald BJ, McNiven MA. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141(1):85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen L, Grandal MV, Knudsen SLJ, van Deurs B, Grøvdal LM. Internalization Mechanisms of the Epidermal Growth Factor Receptor after Activation with Different Ligands. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0058148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, et al. PTRF-Cavin, a Conserved Cytoplasmic Protein Required for Caveola Formation and Function. Cell. 2008;132(1):113–24. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen L, Harborth J, Andrees L, Weber K, Ungewickell EJ. Effect of clathrin heavy chain- and alpha-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J Biol Chem. 2003;278(46):45160–70. doi: 10.1074/jbc.M307290200. [DOI] [PubMed] [Google Scholar]
- Hirst J, Barlow LD, Francisco GC, Sahlender DA, Seaman MNJ, et al. The fifth adaptor protein complex. PLoS Biol. 2011;9(10):e1001170. doi: 10.1371/journal.pbio.1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst MR, Vidal-Quadras M, Larsson E, Song J, Hubert M, et al. Clathrin-Independent Endocytosis Suppresses Cancer Cell Blebbing and Invasion. Cell Rep. 2017;20(8):1893–1905. doi: 10.1016/j.celrep.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, et al. Phosphatidylinositol 4-Phosphate 5-Kinase Alpha Is a Downstream Effector of the Small G Protein ARF6. Cell. 1999;99:521–32. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- Houk AR, Jilkine A, Mejean CO, Boltyanskiy R, Dufresne ER, et al. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012;148(1–2):175–88. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes MT, Kirkham M, Riches J, Cortese K, Walser PJ, et al. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J Cell Biol. 2010;190(4):675–91. doi: 10.1083/jcb.201002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30(17):3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Kida K, Hanawa-Suetsugu K, Suetsugu S. Yeast Ivy1p Is a Putative I-BAR-domain Protein with pH-sensitive Filament Forming Ability in vitro. Cell Struct Funct. 2016;41(1):1–11. doi: 10.1247/csf.15014. [DOI] [PubMed] [Google Scholar]
- Jackson AP, Seow HF, Holmes N, Drickamer K, Parham P. Clathrin light chains contain brain-specific insertion sequences and a region of homology with intermediate filaments. Nature. 1987;326(6109):154–59. doi: 10.1038/326154a0. [DOI] [PubMed] [Google Scholar]
- Jastrzębski K, Zdżalik-Bielecka D, Mamińska A, Kalaidzidis Y, Hellberg C, Miaczynska M. Multiple routes of endocytic internalization of PDGFRβ contribute to PDGF-induced STAT3 signaling. J Cell Sci. 2017;130(3):577–89. doi: 10.1242/jcs.191213. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Redfern RE, Isler Y, Ross AH, Gericke A. Cholesterol stabilizes fluid phosphoinositide domains. Chem Phys Lipids. 2014;182(5):52–61. doi: 10.1016/j.chemphyslip.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes L, Mayor S. Induced Domain Formation in Endocytic Invagination, Lipid Sorting, and Scission. Cell. 2010;142(4):507–10. doi: 10.1016/j.cell.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Johannes L, Parton RG, Bassereau P, Mayor S. Building endocytic pits without clathrin. Nat Rev Mol Cell Biol. 2015;16(5):311–21. doi: 10.1038/nrm3968. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19(5):313–26. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123(2):305–20. doi: 10.1016/j.cell.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Kalia M, Kumari S, Chadda R, Hill MM, Parton RG, Mayor S. Arf6-independent GPI-anchored protein-enriched early endosomal compartments fuse with sorting endosomes via a Rab5/phosphatidylinositol-3’-kinase-dependent machinery. Mol Biol Cell. 2006;17(8):3689–3704. doi: 10.1091/mbc.E05-10-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75(3):544–53. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazic M, Roepstorff K, Johannessen LE, Pedersen NM, van Deurs B, et al. EGF-induced activation of the EGF receptor does not trigger mobilization of caveolae. Traffic. 2006;7(11):1518–27. doi: 10.1111/j.1600-0854.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Kettle E, Page SL, Morgan GP, Malladi CS, Wong CL, et al. A Cholesterol-Dependent Endocytic Mechanism Generates Midbody Tubules During Cytokinesis. Traffic. 2015;16(11):1174–92. doi: 10.1111/tra.12328. [DOI] [PubMed] [Google Scholar]
- Kim S, Nahm M, Kim N, Kwon Y, Kim J, et al. Graf regulates hematopoiesis through GEEC endocytosis of EGFR. Development. 2017;144(22):4159–72. doi: 10.1242/dev.153288. [DOI] [PubMed] [Google Scholar]
- Kim SM, Nguyen TT, Ravi A, Kubiniok P, Finicle BT, et al. PTEN deficiency and AMPK activation promote nutrient scavenging and anabolism in prostate cancer cells. Cancer Discov. 2018;8(7):866–83. doi: 10.1158/2159-8290.CD-17-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T, Harrison SC, Chow EP, Mattaliano RJ, Ramachandran KL, et al. Clathrin heavy chain: molecular cloning and complete primary structure. Proc Natl Acad Sci U S A. 1987a;84(24):8805–9. doi: 10.1073/pnas.84.24.8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T, Owen D, Harrison SC. Molecular structure, function, and dynamics of clathrin-mediated membrane traffic. Cold Spring Harb Perspect Biol. 2014;6(5) doi: 10.1101/cshperspect.a016725. a016725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T, Scarmato P, Harrison SC, Monroe JJ, Chow EP, et al. Clathrin light chains LCA and LCB are similar, polymorphic, and share repeated heptad motifs. Science. 1987b;236(4799):320–24. doi: 10.1126/science.3563513. [DOI] [PubMed] [Google Scholar]
- Kirkham M, Nixon SJ, Howes MT, Abi-Rached L, Wakeham DE, et al. Evolutionary analysis and molecular dissection of caveola biogenesis. J Cell Sci. 2008;121(12):2075–86. doi: 10.1242/jcs.024588. [DOI] [PubMed] [Google Scholar]
- Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim Biophys Acta. 2005;1745(3):273–86. doi: 10.1016/j.bbamcr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol. 2010;188(4):547–63. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka T, Ikeda K. Possible temperature-dependent blockage of synaptic vesicle recycling induced by a single gene mutation in Drosophila. J Neurobiol. 1983;14(3):207–25. doi: 10.1002/neu.480140305. [DOI] [PubMed] [Google Scholar]
- Kosmalska AJ, Casares L, Elosegui-Artola A, Thottacherry JJ, Moreno-Vicente R, et al. Physical principles of membrane remodelling during cell mechanoadaptation. Nat Commun. 2015;6 doi: 10.1038/ncomms8292. 7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krag C, Malmberg EK, Salcini AE. PI3KC2, a class II PI3K, is required for dynamin-independent internalization pathways. J Cell Sci. 2010;123(24):4240–50. doi: 10.1242/jcs.071712. [DOI] [PubMed] [Google Scholar]
- Krauss M, Kukhtina V, Pechstein A, Haucke V. Stimulation of phosphatidylinositol kinase type I-mediated phosphatidylinositol (4,5)-bisphosphate synthesis by AP-2mucargo complexes. Proc Natl Acad Sci U S A. 2006;103(32):11934–39. doi: 10.1073/pnas.0510306103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Borroni V, Chaudhry A, Chanda B, Massol R, et al. Nicotinic acetylcholine receptor is internalized via a Rac-dependent, dynamin-independent endocytic pathway. J Cell Biol. 2008;181(7):1179–93. doi: 10.1083/jcb.200709086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Mayor S. ARF1 is directly involved in dynamin-independent endocytosis. Nat Cell Biol. 2008;10(1):30–41. doi: 10.1038/ncb1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwik J, Boyle S, Fooksman D, Margolis L, Sheetz MP, Edidin M. Membrane cholesterol, lateral mobility, and the phosphatidylinositol 4,5-bisphosphate-dependent organization of cell actin. Proc Natl Acad Sci U S A. 2003;100(24):13964–69. doi: 10.1073/pnas.2336102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarayan R, Wunder C, Becken U, Howes MT, Benzing C, et al. Galectin-3 drives glycosphingolipid-dependent biogenesis of clathrin-independent carriers. Nat Cell Biol. 2014;16(6):592–603. doi: 10.1038/ncb2970. [DOI] [PubMed] [Google Scholar]
- Lamaze C, Dujeancourt A, Baba T, Lo CG, Benmerah A, Dautry-Varsat A. Interleukin 2 receptors and detergent-resistant membrane domains define a clathrin-independent endocytic pathway. Mol Cell. 2001;7(3):661–71. doi: 10.1016/s1097-2765(01)00212-x. [DOI] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Holt JA, Barak LS, Caron MG. The interaction of β-arrestin with the AP-2 adaptor is required for the clustering of β2-adrenergic receptor into clathrin-coated pits. J Biol Chem. 2000;275(30):23120–26. doi: 10.1074/jbc.M002581200. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Mi Y, Mueller T, Kreibich S, Williams EG, et al. Genomic, Proteomic and Phenotypic Heterogeneity in HeLa Cells across Laboratories: Implications for Reproducibility of Research Results. bioRxiv. 2018 307421. [Google Scholar]
- Lundmark R, Doherty GJ, Howes MT, Cortese K, Vallis Y, et al. The GTPase-Activating Protein GRAF1 Regulates the CLIC/GEEC Endocytic Pathway. Curr Biol. 2008;18(22):1802–8. doi: 10.1016/j.cub.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado-Báez L, Cole NB, Krämer H, Donaldson JG. Microtubule-dependent endosomal sorting of clathrin-independent cargo by Hook1. J Cell Biol. 2013;201(2):233–47. doi: 10.1083/jcb.201208172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Li N, Guo Y, Xu X, Gao L, et al. AMPK phosphorylates GBF1 for mitotic Golgi disassembly. J Cell Sci. 2013;126(6):1498–1505. doi: 10.1242/jcs.121954. [DOI] [PubMed] [Google Scholar]
- Marat AL, Wallroth A, Lo W-T, Müller R, Norata GD, et al. mTORC1 activity repression by late endosomal phosphatidylinositol 3,4-bisphosphate. Science. 2017;356(6341):968–72. doi: 10.1126/science.aaf8310. [DOI] [PubMed] [Google Scholar]
- Marie-Anaïs F, Mazzolini J, Herit F, Niedergang F. Dynamin-Actin Cross Talk Contributes to Phagosome Formation and Closure. Traffic. 2016;17(5):487–99. doi: 10.1111/tra.12386. [DOI] [PubMed] [Google Scholar]
- Maurer ME, Cooper JA. The adaptor protein Dab2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J Cell Sci. 2006;119(20):4235–46. doi: 10.1242/jcs.03217. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5(2):121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Mayor S, Pagano RE, Gtpase R. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603–12. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Parton RG, Donaldson JG. Clathrin-independent pathways of endocytosis. Cold Spring Harb Perspect Biol. 2014;6(6):a016758–a016758. doi: 10.1101/cshperspect.a016758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaki Y, Nishimura Y, Sabe H. GBF1 bears a novel phosphatidylinositol-phosphate binding module, BP3K, to link PI3K activity with Arf1 activation involved in GPCR-mediated neutrophil chemotaxis and superoxide production. Mol Biol Cell. 2012;23(13):2457–67. doi: 10.1091/mbc.E12-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12(8):517–33. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Metschnikoff E. Ueber die Beziehung der Phagocyten zu Milzbrandbacillen. Arch für Pathol Anat und Physiol und für Klin Med. 1884;97(3):502–26. [Google Scholar]
- Mettlen M, Chen P, Srinivasan S, Danuser G, Schmid SL. Regulation of Clathrin-Mediated Endocytosis. Annu Rev Biochem. 2018;87(1):871–96. doi: 10.1146/annurev-biochem-062917-012644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau HDHD, Blanch-Mercader C, Attia R, Alraies Z, Maurin M, et al. Macropinocytosis overcomes directional bias due to hydraulic resistance to enhance space exploration by dendritic cells. bioRxiv. 2018 doi: 10.1016/j.devcel.2019.03.024. [DOI] [PubMed] [Google Scholar]
- Moren B, Shah C, Howes MT, Schieber NL, McMahon HT, et al. EHD2 regulates caveolar dynamics via ATP-driven targeting and oligomerization. Mol Biol Cell. 2012;23(7):1316–29. doi: 10.1091/mbc.E11-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya M, Dautry-Varsat A, Goud B, Louvard D, Boquet P. Inhibition of coated pit formation in Hep2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin toxin. J Cell Biol. 1985;101(2):548–59. doi: 10.1083/jcb.101.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Schmid SL. Dual Role of BAR Domain-containing Proteins in Regulating Vesicle Release Catalyzed by the GTPase, Dynamin-2. J Biol Chem. 2013;288(35):25119–28. doi: 10.1074/jbc.M113.490474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman LL, Bruges J, Sengupta K, Sens P, Aranda-Espinoza H. Cell blebbing and membrane area homeostasis in spreading and retracting cells. Biophys J. 2010 Sep;99:1726–33. doi: 10.1016/j.bpj.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata T, Ueyama T, Isodono K, Tagawa M, Takehara N, et al. MURC, a Muscle-Restricted Coiled-Coil Protein That Modulates the Rho/ROCK Pathway, Induces Cardiac Dysfunction and Conduction Disturbance. Mol Cell Biol. 2008;28(10):3424–36. doi: 10.1128/MCB.02186-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Ninomiya H, Miwa S, Masaki T. Cholesterol oxidation switches the internalization pathway of endothelin receptor type A from caveolae to clathrin-coated pits in Chinese hamster ovary cells. J Biol Chem. 2000;275(9):6439–46. doi: 10.1074/jbc.275.9.6439. [DOI] [PubMed] [Google Scholar]
- Otto GP, Nichols BJ. The roles of flotillin microdomains - endocytosis and beyond. J Cell Sci. 2011;124(23):3933–40. doi: 10.1242/jcs.092015. [DOI] [PubMed] [Google Scholar]
- Palade GE. The fine structure of blood capillaries. J Appl Phys. 1953;24:1424. [Google Scholar]
- Park RJ, Shen H, Liu L, Liu X, Ferguson SM, De Camilli P. Dynamin triple knockout cells reveal off target effects of commonly used dynamin inhibitors. J Cell Sci. 2013;126(Pt 22):5305–12. doi: 10.1242/jcs.138578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton RG, Del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14(2):98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: New insights and common mechanisms. Traffic. 2003;4(11):724–38. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- Paul NR, Jacquemet G, Caswell PT. Endocytic Trafficking of Integrins in Cell Migration. Curr Biol. 2015;25(22):R1092–1105. doi: 10.1016/j.cub.2015.09.049. [DOI] [PubMed] [Google Scholar]
- Pearse BM. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci U S A. 1976;73(4):1255–59. doi: 10.1073/pnas.73.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BMF. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975;97(1):93–98. doi: 10.1016/s0022-2836(75)80024-6. [DOI] [PubMed] [Google Scholar]
- Pelkmans L, Fava E, Grabner H, Hannus M, Habermann B, et al. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436(7047):78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- Picco A, Kukulski W, Manenschijn HE, Specht T, Briggs JAG, Kaksonen M. The contributions of the actin machinery to endocytic membrane bending and vesicle formation. Mol Biol Cell. 2018;29(11):1346–58. doi: 10.1091/mbc.E17-11-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo S, Sigismund S, Faretta M, Guidi M, Capua MR, et al. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416(6879):451–55. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- Preta G, Cronin JG, Sheldon IM. Dynasore - Not just a dynamin inhibitor. Cell Commun Signal. 2015;13(1):1–7. doi: 10.1186/s12964-015-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purkanti R, Thattai M. Ancient dynamin segments capture early stages of host–mitochondrial integration. Proc Natl Acad Sci. 2015;112(9):2800–2805. doi: 10.1073/pnas.1407163112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualmann B, Koch D, Kessels MM. Focus Review Let’s go bananas: revisiting the endocytic BAR code Cellular membranes—between relaxation and defined topology Shaping membranes with BAR domains. EMBO J. 2011 Jul;30:3501–15. doi: 10.1038/emboj.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139(1):49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghupathy R, Anilkumar AA, Polley A, Singh PP, Yadav M, et al. Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell. 2015;161(3):581–94. doi: 10.1016/j.cell.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappoport JZ, Simon SM. Endocytic trafficking of activated EGFR is AP-2 dependent and occurs through preformed clathrin spots. J Cell Sci. 2009;122(9):1301–5. doi: 10.1242/jcs.040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard H-F, Simunovic M, Lemière J, Boucrot E, Garcia-Castillo MD, et al. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature. 2015;517(7535):493–96. doi: 10.1038/nature14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter TE, Fajardo O, Matsuet H, Andersont RGW, Lacey SW. Folate receptors targeted to clathrin-coated pits cannot regulate vitamin uptake. Proc Natl Acad Sci U S A. 1995 Apr;92:3824–28. doi: 10.1073/pnas.92.9.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. Cloning of cDNAs encoding two related 100-kD coated vesicle proteins (alpha-adaptins) J Cell Biol. 1989;108(3):833–42. doi: 10.1083/jcb.108.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14(4):167–74. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Robinson MS. Forty Years of Clathrin-coated Vesicles. Traffic. 2015;16(12):1210–38. doi: 10.1111/tra.12335. [DOI] [PubMed] [Google Scholar]
- Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of Cholesterol with Methyl-beta -Cyclodextrin Perturbs Formation of Clathrin-coated Endocytic Vesicles. Mol Biol Cell. 1999;10(4):961–74. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Ho HYH, Kirschner MW. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4,5-bisphosphate. J Cell Biol. 2000;150(6):1299–1309. doi: 10.1083/jcb.150.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer W, Pontani L-L, Sorre B, Rentero C, Berland L, et al. Actin dynamics drive membrane reorganization and scission in clathrin-independent endocytosis. Cell. 2010;140(4):540–53. doi: 10.1016/j.cell.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Roth TF, Porter KR. Yolk protein uptake in the oocyte of the mosquito Aedes aegypti.L. J Cell Biol. 1964;20:313–32. doi: 10.1083/jcb.20.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68(4):673–82. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Rusk N, Le PU, Mariggio S, Guay G, Lurisci C, et al. Synaptojanin 2 functions at an early step of clathrin-mediated endocytosis. Curr Biol. 2003;13(8):659–63. doi: 10.1016/s0960-9822(03)00241-0. [DOI] [PubMed] [Google Scholar]
- Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell. 2002;2(4):411–23. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- Sadowski L, Jastrzebski K, Kalaidzidis Y, Heldin CH, Hellberg C, Miaczynska M. Dynamin Inhibitors Impair Endocytosis and Mitogenic Signaling of PDGF. Traffic. 2013;14(6):725–36. doi: 10.1111/tra.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salani B, Passalacqua M, Maffioli S, Briatore L, Hamoudane M, et al. IGF-IR Internalizes with Caveolin-1 and PTRF/Cavin in Hacat Cells. PLoS One. 2010;5(11):e14157. doi: 10.1371/journal.pone.0014157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987;105(2):679–89. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolini E, Puri C, Salcini AE, Gagliani MC, Pelicci PG, et al. Numb is an endocytic protein. J Cell Biol. 2000;151(6):1345–51. doi: 10.1083/jcb.151.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathe M, Muthukrishnan G, Rae J, Disanza A, Thattai M, et al. Small GTPases and BAR domain proteins regulate branched actin polymerisation for clathrin and dynamin-independent endocytosis. Nat Commun. 2018;9(1):1835. doi: 10.1038/s41467-018-03955-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Okamoto T, Chun M, Nishimoto I, Lodish HF, Lisanti MP. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci U S A. 1996;93(1):131–35. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66(1):511–48. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Schmid SL, Sorkin A, Zerial M. Endocytosis: Past, Present, and Future. Cold Spring Harb Perspect Biol. 2014;6(12):a022509–a022509. doi: 10.1101/cshperspect.a022509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöneberg J, Dambournet D, Liu T-L, Forster R, Hockemeyer D, Betzig E, Drubin DG. 4D Cell Biology: Big Data Image Analytics and Lattice Light-Sheet Imaging Reveal Dynamics of Clathrin-Mediated Endocytosis in Stem Cell Derived Intestinal Organoids. MBoC Stem cell issue. 2018:1–24. doi: 10.1091/mbc.E18-06-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scita G, Confalonieri S, Lappalainen P, Suetsugu S. IRSp53: crossing the road of membrane and actin dynamics in the formation of membrane protrusions. Trends Cell Biol. 2008;18(2):52–60. doi: 10.1016/j.tcb.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Scita G, Paolo P, Fiore D, Di Fiore PP, Paolo P, et al. The endocytic matrix. Nature. 2010;463(7280):464–73. doi: 10.1038/nature08910. [DOI] [PubMed] [Google Scholar]
- Sehat B, Andersson S, Girnita L, Larsson O. Identification of c-Cbl as a New Ligase for Insulin-like Growth Factor-I Receptor with Distinct Roles from Mdm2 in Receptor Ubiquitination and Endocytosis. Cancer Res. 2008;68(14):5669–77. doi: 10.1158/0008-5472.CAN-07-6364. [DOI] [PubMed] [Google Scholar]
- Sens P, Johannes L, Bassereau P. Biophysical approaches to protein-induced membrane deformations in trafficking. Curr Opin Cell Biol. 2008;20(4):476–82. doi: 10.1016/j.ceb.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science. 2000;287(5455):1037–40. doi: 10.1126/science.287.5455.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz MP, Dai J. Modulation of membrane dynamics and cell motility by membrane tension. Trends Cell Biol. 1996;6(3):85–89. doi: 10.1016/0962-8924(96)80993-7. [DOI] [PubMed] [Google Scholar]
- Shpetner HS, Vallee RB. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989;59(3):421–32. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, et al. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15(2):209–19. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Sigismund S, Confalonieri S, Ciliberto A, Polo S, Scita G, Di Fiore PP. Endocytosis and signaling: cell logistics shape the eukaryotic cell plan. Physiol Rev. 2012;92(1):273–366. doi: 10.1152/physrev.00005.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S, Nappo G, Algisi V, Conte A, Pascolutti R, et al. Threshold-controlled ubiquitination of the EGFR directs receptor fate. EMBO J. 2013;32(15):2140–57. doi: 10.1038/emboj.2013.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci U S A. 2005;102(8):2760–65. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Lippé R, Christoforidis S, Gaullier JM, Brech A, et al. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394(6692):494–98. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Simunovic M, Manneville JB, Renard HF, Evergren E, Raghunathan K, et al. Friction Mediates Scission of Tubular Membranes Scaffolded by BAR Proteins. Cell. 2017;170(1):172–184.e11. doi: 10.1016/j.cell.2017.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, et al. Cells Respond to Mechanical Stress by Rapid Disassembly of Caveolae. Cell. 2011;144(3):402–13. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead WT, Hayden CC, Gadok AK, Zhao C, Lafer EM, et al. Membrane fission by protein crowding. Proc Natl Acad Sci. 2017;114(16):E3258–67. doi: 10.1073/pnas.1616199114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa LP, Lax I, Shen H, Ferguson SM, De Camilli P, Schlessinger J. Suppression of EGFR endocytosis by dynamin depletion reveals that EGFR signaling occurs primarily at the plasma membrane. Proc Natl Acad Sci U S A. 2012;109(12):4419–24. doi: 10.1073/pnas.1200164109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeber M, Stoeck IK, Hänni C, Bleck CKE, Balistreri G, Helenius A. Oligomers of the ATPase EHD2 confine caveolae to the plasma membrane through association with actin. EMBO J. 2012;31(10):2350–64. doi: 10.1038/emboj.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Gaidarov I, Kobylarz K, Lampson MA, Keen JH, McGraw TE. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci. 1999;96(12):6775–80. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa M, Ueyama T, Ogata T, Takehara N, Nakajima N, et al. MURC, a muscle-restricted coiled-coil protein, is involved in the regulation of skeletal myogenesis. Am J Physiol Cell Physiol. 2008;295(2):C490–8. doi: 10.1152/ajpcell.00188.2008. [DOI] [PubMed] [Google Scholar]
- Tang Z, Scherer PE, Okamoto T, Song K, Chu C, et al. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996;271(4):2255–61. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Perrais D, Merrifield CJ. A High Precision Survey of the Molecular Dynamics of Mammalian Clathrin-Mediated Endocytosis. PLoS Biol. 2011;9(3):e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thottacherry JJ, Kosmalska AJ, Kumar A, Vishen AS, Elosegui-Artola A, et al. Mechanochemical feedback control of dynamin independent endocytosis modulates membrane tension in adherent cells. Nat Commun. 2018;9(1):4217. doi: 10.1038/s41467-018-06738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurieau C, Brosius J, Burne C, Jolles P, Keen JH, et al. Molecular cloning and complete amino acid sequence of AP50, an assembly protein associated with clathrin-coated vesicles. DNA. 1988;7(10):663–69. doi: 10.1089/dna.1988.7.663. [DOI] [PubMed] [Google Scholar]
- Tse SML, Furuya W, Gold E, Schreiber AD, Sandvig K, et al. Differential role of actin, clathrin, and dynamin in Fcγ receptor-mediated endocytosis and phagocytosis. J Biol Chem. 2003;278(5):3331–38. doi: 10.1074/jbc.M207966200. [DOI] [PubMed] [Google Scholar]
- Villaseñor R, Kalaidzidis Y, Zerial M. Signal processing by the endosomal system. Curr Opin Cell Biol. 2016;39:53–60. doi: 10.1016/j.ceb.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Wallroth A, Haucke V. Phosphoinositide conversion in endocytosis and the endolysosomal system. J Biol Chem. 2018;293(5):1526–35. doi: 10.1074/jbc.R117.000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandinger-Ness A, Zerial M. Rab Proteins and the Compartmentalization of the Endosomal system. Cold Spring Harb Perspect Biol. 2014;6(11):1–25. doi: 10.1101/cshperspect.a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Richards DA. Segregation of PIP2 and PIP3 into distinct nanoscale regions within the plasma membrane. Biol Open. 2012;1(9):857–62. doi: 10.1242/bio.20122071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M, Parton RG. M-caveolin, a muscle-specific caveolin-related protein. FEBS Lett. 1996;378(1):108–12. doi: 10.1016/0014-5793(96)82884-5. [DOI] [PubMed] [Google Scholar]
- West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol. 1989;109(6 Pt 1):2731–39. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MA, Prescott AR, Eskelinen EL, Ridley AJ, Watts C. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr Biol. 2000;10(14):839–48. doi: 10.1016/s0960-9822(00)00595-9. [DOI] [PubMed] [Google Scholar]
- Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1(5):445–58. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A. Caveolin Is Necessary for Wnt-3a-Dependent Internalization of LRP6 and Accumulation of β-Catenin. Dev Cell. 2006;11(2):213–23. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Yang J-S, Hsu J-W, Park S-Y, Li J, Oldham WM, et al. GAPDH inhibits intracellular pathways during starvation for cellular energy homeostasis. Nature. 2018;561(7722):263–67. doi: 10.1038/s41586-018-0475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ferguson SSG, Barak LS, Ménard L, Caron MG. Dynamin and β-arrestin reveal distinct mechanisms for G protein- coupled receptor internalization. J Biol Chem. 1996;271(31):18302–5. doi: 10.1074/jbc.271.31.18302. [DOI] [PubMed] [Google Scholar]
- Zwaagstra JC, El-Alfy M, O’Connor-McCourt MD. Transforming Growth Factor (TGF)-β1 Internalization. J Biol Chem. 2001;276(29):27237–45. doi: 10.1074/jbc.M100033200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.