Abstract
The neural substrates of intelligence represent a fundamental but largely uncharted topic in human developmental neuroscience. Prior neuroimaging studies have identified modest but highly dynamic associations between intelligence and cortical thickness (CT) in childhood and adolescence. In a separate thread of research, quantitative genetic studies have repeatedly demonstrated that most measures of intelligence are highly heritable, as are many brain regions associated with intelligence. In the current study, we integrate these 2 streams of prior work by examining the genetic contributions to CT–intelligence relationships using a genetically informative longitudinal sample of 813 typically developing youth, imaged with high-resolution MRI and assessed with Wechsler Intelligence Scales (IQ). In addition to replicating the phenotypic association between multimodal association cortex and language centers with IQ, we find that CT–IQ covariance is nearly entirely genetically mediated. Moreover, shared genetic factors drive the rapidly evolving landscape of CT–IQ relationships in the developing brain.
Keywords: cortical thickness, genetics, intelligence, MRI, neurodevelopment
Introduction
Intelligence represents a defining characteristic of the human species. The emergence of our intelligence parallels the rapid evolutionary expansion of the cerebral cortex in primates generally and in Homo sapiens specifically (Gilbert et al. 2005; Roth and Dicke 2005). Perhaps not surprisingly, genetic studies have established that intelligence quotient (IQ), the predominant psychologic construct of intelligence, is highly heritable. For example, twin studies have estimated that between 60% and 80% of the observed individual differences in full scale IQ are attributable to additive genetic factors (i.e., a2 of 0.60–0.80). Metrics of intelligence thus rank among the most heritable of all cognitive measures (Posthuma et al. 2002; Deary et al. 2006; Polderman et al. 2015). Although IQ itself is a relatively stable measure over the lifespan, there is evidence that the influence of genetic factors on intelligence may change over time, particularly from infancy to childhood (Bartels et al. 2000; Deary et al. 2006).
The advent of neuroimaging has enabled the search for the neural substrates of human intelligence in vivo. Initial studies using magnetic resonance imaging (MRI) reported modest correlations between brain volumes and intelligence (Andreasen et al. 1993; Reiss et al. 1996; Gur et al. 1999). A subsequent meta-analysis (N = 1530) estimated that the correlation between total brain volume and IQ is 0.33, or approximately 11% of the total phenotypic covariation (Mcdaniel 2005). Although small, the association between intelligence and brain volumes appears almost entirely under genetic control (Posthuma et al. 2002; Koenis et al. 2017).
More recent neuroimaging studies have investigated the neural correlates of human intelligence with increased regional specificity. For example, voxel-level analyses of gray matter have found that IQ correlates with gray matter density in the dorsolateral prefrontal cortex, anterior prefrontal cortex, orbitofrontal cortex, precuneus, cingulate, and the lateral temporal lobes (Frangou et al. 2004; Haier et al. 2004). When considered together, the preponderance of structural and functional neuroimaging data suggests that general intelligence is most associated with a frontoparietal network involving language centers, association cortex, and frontopolar cortex (Jung and Haier 2007; Gläscher et al. 2010; Basten et al. 2015). Brain structure in these regions also is among the most heritable in both children (Lenroot et al. 2009) and adults (Thompson et al. 2001; Rimol et al. 2010).
Intelligence is a trait that has remarkable stability over the lifespan, with IQ in early childhood highly predictive of IQ measured in the eighth decade of life (Deary et al. 2004). Nevertheless, there is converging evidence that the associations between brain morphology and intelligence are influenced by neurodevelopmental changes. In children, the prefrontal cortex and anterior cingulate cortex are most associated with intelligence (Reiss et al. 1996; Wilke et al. 2003), while in adolescence there is an emergence of novel associations with the orbitofrontal and middle frontal cortices (Frangou et al. 2004). In a longitudinal study of 307 children and adolescents, Shaw et al. (2006) found that the correlations between IQ and cortical thickness (CT) were highly dynamic, with phenotypic correlations peaking during middle childhood. Moreover, the trajectory of CT was more closely related to intelligence in youth than were static measures of CT, with the dorsolateral and superior frontal cortices having the strongest associations. There is evidence that intelligence and measures of cortical thickness change are influenced by common genes (Brans et al. 2010).
The extant literature suggests that genetics, neuroanatomic specificity, and age are all critical to understanding the neural substrates of intelligence. In the current study, we report results from analyses examining these factors simultaneously via multivariate latent growth structural equation models in a large, genetically informative neuroimaging dataset.
Materials and Methods
Subjects
A total of 813 typically developing children, adolescents and young adults from 410 families were recruited by the Child Psychiatry Branch of the National Institute of Mental Health (NIMH). The sample included pediatric, adolescent, and young adult monozygotic twins (MZ, N = 252), dizygotic twins (DZ, N = 133), siblings of twins (N = 110), and singleton (N = 318) family members (summarized in Table 1). Details of this sample have been described elsewhere (Schmitt et al. 2014b). Parents of prospective participants were interviewed by phone and asked to report their child’s developmental, educational, and health history. Subjects were excluded if they had been diagnosed with a psychiatric disorder, taken psychiatric medications, had experienced brain trauma, or had any condition known to affect gross brain development. Inclusion criteria were a minimum gestational age of 29 weeks and a minimum birth weight of 1500 g. Approximately 80% of families responding to the ads met inclusion criteria.
Table 1.
Demographic characteristics of the sample
| MZ | DZ | Siblings of twins | Singletons | Total | |
|---|---|---|---|---|---|
| Sample size | 252 | 133 | 110 | 318 | 813 |
| Mean age at first scan (years ) | 11.3 (3.8) | 9.6 (3.5) | 12.0 (4.4) | 11.7 (5.1) | 11.3 (4.4) |
| Mean scan interval (years ) | 2.4 (0.66) | 2.4 (0.67) | 2.3 (0.86) | 2.3 (0.85) | 2.4 (1.1) |
| Gender | 117 F (46%) | 62 F (47%) | 61 F (55%) | 143 F (45%) | 383 F (47%) |
| 135 M (54%) | 71 M (53%) | 49 M (45%) | 175 M (55%) | 430 M (53%) | |
| SES (Hollingshead index) | 44.2 (18.6) | 43.0 (15.1) | 43.0 (17.9) | 40.5 (20.4) | 42.6 (18.7) |
| Handedness | 218 R (87%) | 108 R (81%) | 88 R (82%) | 284 R (89%) | 698 R (87%) |
| 16 M (6%) | 15 M (11%) | 7 M (7%) | 18 M (6%) | 56 M (7%) | |
| 14 L (5%) | 10 L (8%) | 12 L (11%) | 14 L (5%) | 50 L (6%) | |
| FSIQ | 110.3 (12.1) | 110.7 (11.5) | 113.4 (13.4) | 115.4 (12.9) | 112.7 (12.7) |
For each subject, age-appropriate versions of a Wechsler Intelligence scale were administered. Full scale IQ data were available for 794 (98%) of the participants. In total, 712 subjects (88%) were administered the Abbreviated Intelligence Scale (WASI), 52 (6%) were administered the Intelligence Scale for Children-Revised (WISC-R), and the remaining 6% of subjects undergoing either a version of the Preschool and Primary Scale of Intelligence (WPPSI) or the Adult Intelligence Scale (WAIS).
For twin subjects, zygosity was determined by DNA analysis of buccal cheek swabs (BRT Laboratories and Proactive Genetics) using 9–21 unlinked short tandem repeat loci for a minimum certainty of 99%. We obtained verbal or written assent from the child and written consent from the parents (or adult participants) for their participation in the study. The Combined Neurosciences Institutional Review Board (CNS-IRB) at the National Institutes of Health approved the protocol.
MRI Acquisition
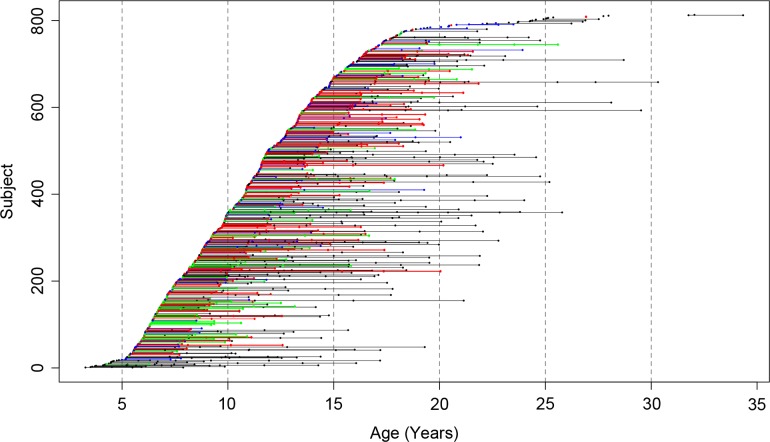
All MRI images were acquired on the same General Electric 1.5 T Signa Scanner located at the National Institutes of Health Clinical Center in Bethesda, Maryland. A 3-D spoiled gradient recalled echo sequence in the steady state sequence was used to acquire 124 contiguous 1.5-mm thick slices in the axial plane (TE/TR = 5/24 ms; flip angle = 45°, matrix = 256 × 192, NEX = 1, FOV = 24 cm, acquisition time 9.9 min). A Fast Spin Echo/Proton Density weighted imaging sequence was also acquired for clinical evaluation. A total of 1748 MRI datasets were used in the current study (Fig. 1). Up to 8 MRI scans were performed per individual, with sibships containing up to 5 members. The mean interval between scans was 2.4 years.
Figure 1.
Age distribution of the sample. Each point represents a high-resolution MRI, with connected points indicating data from the same subject. Subjects are color-coded based on study group (MZ = red, DZ = green, siblings of twins = blue, singletons = black).
Image Analysis
All MR images were imported into the CIVET pipeline for automated structural image processing (Ad-Dab’bagh et al. 2006). Briefly, the native MRI scans were registered into standardized stereotaxic space using a linear transformation (Collins et al. 1994) and corrected for nonuniformity (Sled et al. 1998). The registered and corrected volumes were segmented into white matter, gray matter, cerebrospinal fluid, and background using a neural net classifier (Zijdenbos et al. 2002). The gray and white matter surfaces were fitted using deformable surface-mesh models and nonlinearly aligned toward a template surface (MacDonald et al. 2000; Robbins et al. 2004; Kim et al. 2005). The gray and white matter surfaces were resampled into native space. Cortical thickness was measured in native-space using the linked distance between the white and pial surfaces (MacDonald et al. 2000; Lerch and Evans 2005). This data has been made publicly available (Schmitt et al. 2014a).
Statistical Analysis
Each subject’s neuroanatomic measures were imported into the R statistical environment for analysis (R Core Team 2006). The data were reformatted such that each record represented family-wise (rather than individual-wise) data. The subsequent dataset contained up to 8 MRI scans per individual, up to 5 individuals per family, and 81 924 measures of cortical thickness per subject. Latent growth curve models were then used to decompose the phenotypic covariance between cortical thickness and IQ. A traditional longitudinal growth curve model uses repeated measures to estimate changes in means and variances with time (Duncan and Duncan 2004). Compared with other longitudinal methods, latent growth curve models have the advantage that they allow for direct age-based predictions, are robust to missing data cells, and are customizable to unique data structures (Mcardle and Epstein 2013).
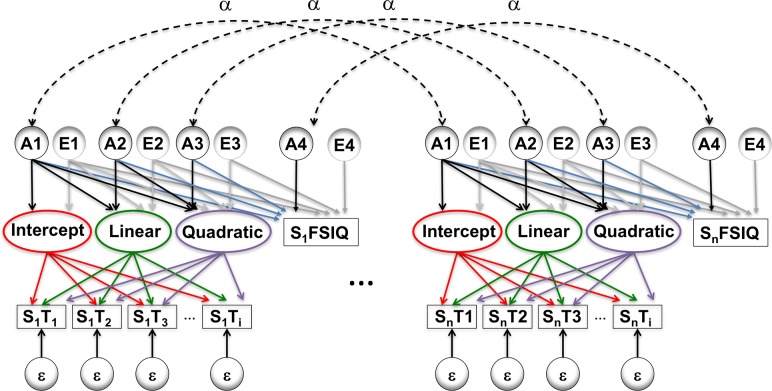
Genetic modeling was performed in OpenMx, a structural equation modeling package fully integrated into the R environment (Boker et al. 2011; Neale et al. 2016). First, variance decomposition of IQ was performed using the classic ACE model with an extended twin design (Posthuma and Boomsma 2000). Then at each vertex, a genetically informative quadratic latent growth curve model was constructed (Neale and McArdle 2000; McArdle et al. 2004) similar in structure to a model previously used to decompose covariance between lobar morphology and demonstrate its changes with time (Schmitt et al. 2018). In the current study, rather than simultaneously analyzing 2 neuroanatomic regions of interest, a vertex-level measure of cortical thickness and full scale IQ were included and iterated over all vertices (Fig. 2). This model represents a “bivariate” extension of the single-ROI model described previously to quantify changes in brain heritability with age (Schmitt et al. 2014b). In order to understand changes in the patterns of genetic relationships between neuroanatomic structures, the variances and covariances between the 3 latent growth curve factors per individual were decomposed into additive genetic (A) and environment components (E). Each of these components was specified as a Cholesky decomposition, which factors any symmetric positive definite matrix into a lower triangular matrix of free parameters postmultiplied by its transpose (Neale and Cardon 1992).
Figure 2.
Simplified path diagram. Rectangles denote observed measures of full scale IQ and cortical thickness (measured at up to 8 timepoints), with circles indicating latent variables. Changes with time are modeled with a latent growth curves, allowing for both linear and nonlinear effects with age. Variance and CT–IQ covariance were decomposed into genetic (A), environmental (E), and error (ε) components. Paths in red were constrained to unity, green paths were defined as age at timepoint i, purple paths were set to age2 at timepoint i, and α represents the degree of kinship between 2 family members. The remaining paths were freely estimated. The paths in blue contribute to genetically mediated CT–IQ covariance. While only 2 related individuals are shown, the model included up to 5 members per family.
The rich family structure in the present data made it possible to employ an extended twin design (Posthuma and Boomsma 2000; Posthuma et al. 2000). Because the study design acquired panel rather than cohort longitudinal data, the age at scan was integrated into the model as a dynamic (e.g., definition) variable to individualize growth curve predictions (Mehta and West 2000). Models were fitted by maximum likelihood, which is efficient and yields asymptotically unbiased parameter estimates. In order to test the statistical significance of genetic factors on CT–IQ covariance, the original model was compared with a submodel in which genetic covariance paths were removed; differences in log-likelihood between these models generally follow a χ2 distribution with degrees of freedom equal to number of parameters removed (Neale and Cardon 1992; Visscher 2004; Dominicus et al. 2006). A similar approach was performed for environmental covariance, as well as for testing for changes over time (i.e., while retaining main effects). Because several prior studies have shown little role of the shared environment (c2) on the variance of brain structures in children (Wallace et al. 2006; Peper et al. 2007; Lenroot et al. 2009; Schmitt et al. 2018), it was excluded from the model in order to reduce the computational burden. Similarly, since prior studies have shown little effect of sex on variance in cortical thickness (Schmitt et al. 2014b), sex moderators on variance components were not estimated. Control of multiple comparisons was performed via false discovery rate (Genovese et al. 2002).
For each timepoint–vertex pair, ROI genetic covariance matrices with full scale IQ were calculated based on parameter estimates from the full model. Genetic correlations (rG) were then calculated by standardizing the genetic covariance matrix, mathematically defined for vertex i as follows:
Since rG can be misleading when heritabilities are low, as an alternative metric we also calculated the genetic contribution to covariance (pcorG), which adjusts rG by the heritability (a2):
This statistic is also sometimes referred to as the “bivariate heritability.” In order to visualize how these parameters change over time, rG, pcorG, and the phenotypic correlation (rP) were projected on to the brain surface at multiple timepoints from ages 6 to 18.
CT–IQ Relationships Beyond Adolescence
In order to examine differences between CT and IQ relationships between adolescents and adults, we analyzed data from the genetically informative subsample of the Human Connectome Project (HCP). This publicly available dataset includes cross-sectional cognitive and neuroimaging measures for 188 MZ and 298 DZ adult twins (191 males, 291 females). Precise subject ages are not available, but the sample included 56 subjects ages 22–25 years, 240 from 26 to 30, 186 ages 31–35, and 4 subjects over 35 years. Intelligence was assessed via the NIH Toolbox’s “Total Cognition” composite score (NTC); this score correlates highly (r = 0.95) with traditional constructs of cognition in adults (Heaton et al. 2014). All subjects also had high-resolution structural neuroimaging data postprocessed with Freesurfer; details have been described elsewhere (Van Essen et al. 2012). We used the 32k vertex-level measures of cortical thickness smoothed with a 5 mm kernel via the “cifti-smoothing” command from the Connectome Workbench (Van Essen et al. 2012). Traditional univariate ACE models for CT and NTC were implemented while simultaneously regressing on gender and age level, followed by bivariate CT–NTC Cholesky decomposition to assess for shared genetic influences.
Results
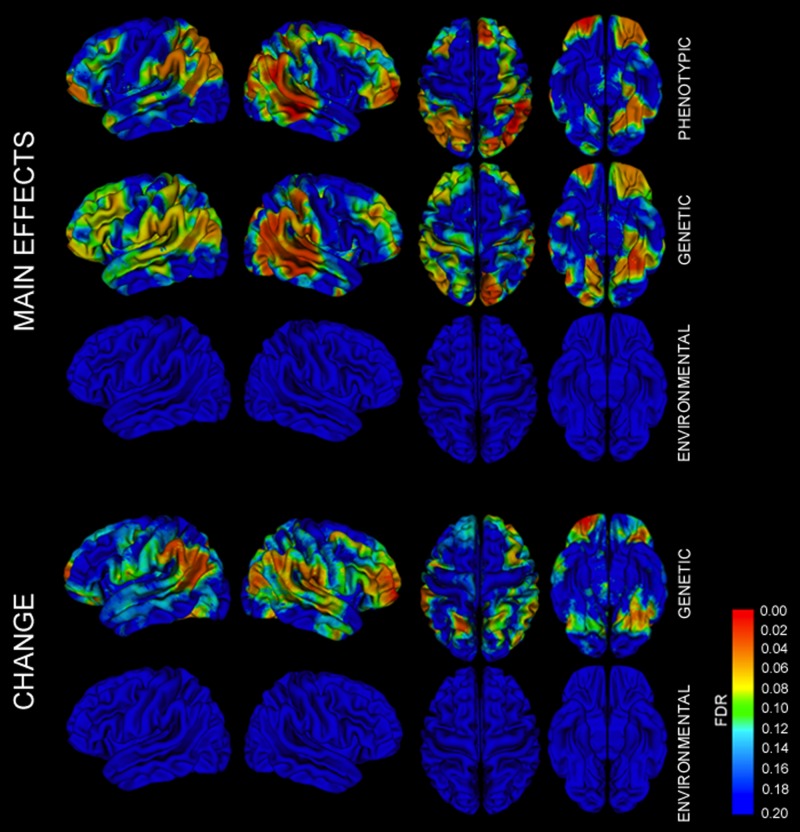
There were significant phenotypic correlations between CT and IQ in broad regions of the dorsolateral prefrontal cortex, orbitofrontal cortices, parietal lobes (particularly supramarginal gyri), superior temporal gyri, and left lingual and parahippocampal gyri (Figs 3 and S1). Probability maps testing for the significance of genetic contributions to CT–IQ covariance had a very similar pattern, with the most significant regions including dorsolateral prefrontal cortex, orbitofrontal cortex, perisylvian temporal lobes, and lingual gyri (Figs 3 and S1). Genetic influences on dorsolateral prefrontal cortex were more widespread in the left hemisphere. While the CT of Broca’s area was significantly associated with IQ via genetic factors, its homolog in the right inferior frontal gyrus was not. Similar patterns were observed when examining how shared genetic factors influence changes in CT–IQ covariance over time, with regions of the bilateral inferior parietal lobes, left greater than right orbital cortex, left dorsolateral frontal cortex, and bilateral lingual gyri. These findings were in sharp contrast to probability maps testing for contributions of the environmental influence on CT–IQ covariance, which were not significant after correction for multiple testing.
Figure 3.
FDR-corrected probability maps of CT–IQ covariance. Main effects of phenotypic, genetic, and environmental factors on CT–IQ covariance, as well as effects of genetic and environmental factors on changes in covariance over time. Similar maps with discrete significance thresholds are also provided in Figure S1.
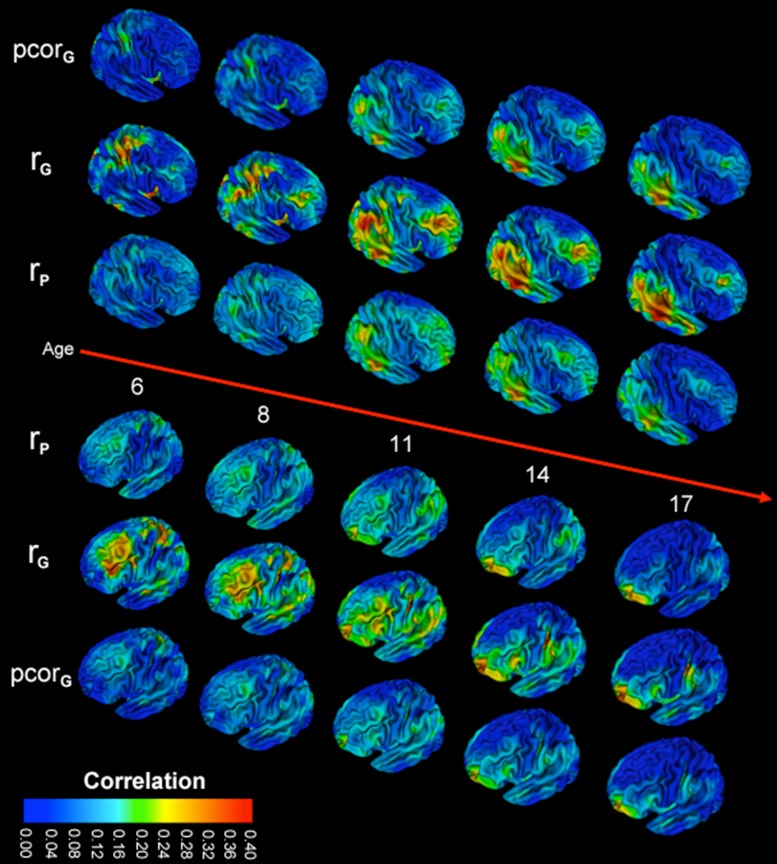
Correlations between IQ and global mean CT were generally weak and peaked near 10 years of age (Fig. S2). Consistent with prior studies, vertex-level phenotypic correlations between CT and IQ were of modest magnitude, generally ranging from −0.10 to 0.40. There was substantial regional variation; the regions of the brain with the strongest correlations changed substantially over childhood and adolescence (Fig. 4). In children under 10, there were relatively homogeneous but weak global correlations. During middle childhood, a wave of increased correlational strength spread over the dorsolateral prefrontal cortex and the parietal lobes, peaking between 10 and 11 years of age. With the approach of adulthood, phenotypic correlations generally decreased (even below that seen in early childhood). However, in the frontopolar cortex and left lingual gyrus, progressive increases in phenotypic correlations continued throughout adolescence and were among the strongest correlations seen at any time point.
Figure 4.
Dynamic changes between cortical thickness and full scale IQ over childhood and adolescence. Maximum likelihood estimates of the phenotypic correlation (rP), genetic correlations (rG), and the genetic contribution to covariance (pcorG) shown for ages 6–17. Changes with time can be viewed dynamically in the Supplementary Movies. Additional views are also provided in Figure S1.
IQ was highly heritable in our sample, with approximately 66% of its variance attributable to additive genetic (a2) factors; variance attributable to shared environmental (c2) and individual-specific (e2) sources was substantially lower (a2 = 0.66 [95% CI: 0.48–0.85], c2 = 0.17 [0.00–0.34], e2 = 0.17 [0.01–0.22]). The contribution of genetic factors to individual differences in IQ was statistically significant (χ2 = 46.23, df = 1, P-value <0.0001), while contributions from the shared environment were not significant. The heritability of CT generally increased over time (Fig. S3). Genetic correlations between IQ and CT largely paralleled the phenotypic correlations (Figs 4 and S3). Genetic correlations were somewhat higher, but similarly were strongest in mid-childhood and involved dorsolateral prefrontal, inferior parietal cortex, and right greater than left posterior–superior temporal lobes, with the emergence of strong correlations in the orbitofrontal cortex and left lingual gyrus in late adolescence. The proportion of the phenotypic covariance attributable to genetic factors (pcorG) mirrored phenotypic correlations and were similar in magnitude, indicating that a large proportion of CT–IQ covariance was mediated via genetic factors. The dynamic nature of these relationships can be appreciated in Supplementary Movies (Supplementary Movies 1–11).
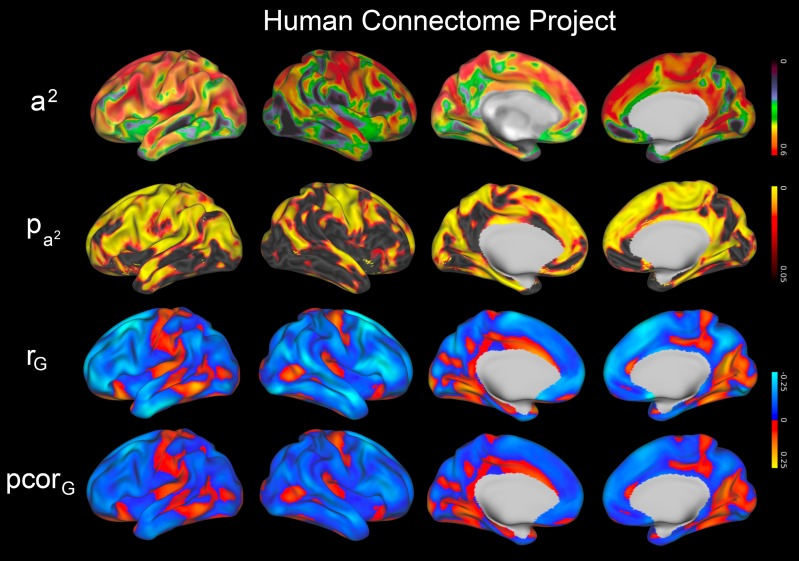
In the HCP data, heritability of CT in adults was high throughout most of the brain, similar in pattern to the late adolescents from the NIH sample (Fig. 5). Lower heritability estimates were seen in the right inferior frontal lobe and inferior temporal lobes, although most regions were statistically significant. Heritability for NTC was also high, with approximately 70% of the phenotypic variance attributable to genetic effects (a2 = 0.71 [95% CI: 0.45–0.78]; c2 = 0.00 [0.00–0.08]; e2 = 0.29 [95% CI: 0.20–0.38]); genetic effects on NTC were highly significant (χ2 = 22.6, df = 1, P-value <0.0001). CT–NTC correlations were weak in adults and did not reach statistical significance after correction for multiple testing. However, the regions of strongest correlation largely mirrored those seen in late adolescence in the NIH sample, with orbitofrontal, parahippocampal, and fusiform gyri among the regions with the strongest shared genetic influences (Figs 5 and S4).
Figure 5.
Genetics of adult cortical thickness and cognition in the Human Connectome Project (HCP) dataset. Heritability of cortical thickness (a2) and corresponding FDR-corrected probability maps () are provided, as well as the genetic correlation (rG) and genetic contribution to covariance (pcorG) between cortical thickness and the NIH Toolbox construct of total cognition; genetic covariances were not statistically significant after correction for multiple testing.
Discussion
The current study supports prior work suggesting modest but significant correlations between IQ and several regions of the cerebral cortex, most notably superior frontoparietal cortex, language centers, and the frontopolar cortex. We observed that the strength of these correlations changed rapidly in youth, with the strongest associations for most cortical regions seen during middle childhood. Our data also suggest that similar to prior analyses using brain volumes (Posthuma et al. 2002; Wallace et al. 2010), the relationships between IQ and CT are largely genetically mediated. Moreover, the genetic influences on CT–IQ covariance were highly dynamic in childhood and adolescence.
Most prior studies investigating the phenotypic relationships between intelligence and CT have implicated similar neuroanatomic regions. For example, in 216 typically developing children and adolescents ages 6–18 (mean age 12.1), Karama et al. (2009) found associations in the left greater than right multimodal association, lateral prefrontal, parahippocampal/fusiform, and extrastriate cortex. In a sample of 181 older children and young adults (mean age 16.31), Menary et al. (2013) found statistically significant correlations in the rostral frontal, posterior temporal, parahippocampal/fusiform gyri, and left inferior parietal lobes. In 225 young adults (mean age 20.9), Choi et al. (2008) found strongest associations between IQ and CT in the left hemisphere, primarily in the anterior temporal cortex, temporal operculum, and lateral parietal cortex. Overall our results are similar to these prior studies and largely support the hypothesis that human intelligence is dependent on distributed networks primarily involving multimodal association cortex (Jung and Haier 2007; Deary et al. 2010).
Our longitudinal findings provide additional evidence that neurodevelopmental timing is critical in understanding CT–IQ relationships, and support the hypothesis that discrepancies in the existing literature may largely be owed to differences in sample age. Not surprisingly, the results in the current study are therefore most concordant with prior studies when age is considered during comparison. For example, using data acquired at 2 timepoints (mean ages 11.59 and 13.55 years), Burgaleta et al. (2014) investigated how rates of change in CT influence CT–IQ associations in 431 children. They found that CT–IQ relationships were strongest in the left posterior frontal lobe. We similarly observe prominent CT–IQ correlations in the left posterior frontal lobe during this age range; however, we also find that these relationships substantially decrease in strength with the onset of adolescence. Menary et al. (2013) also observed that the strength of CT–IQ relationships decrease in adolescence when their sample was split into age groups (ages 9.0–16.45 vs. 16.46–24.0 years), as did Shaw et al. (2006). Yet despite these global decreases in the second decade of life, we observe regional increases in CT–IQ correlations in the frontopolar cortex in late adolescence. The emergence of robust associations between frontopolar (BA 10) cortex and IQ have also been reported in older samples of adolescents (Frangou et al. 2004) and young adults (Narr et al. 2007), as well as via lesion mapping in older adults (Gläscher et al. 2010). We also observed that frontopolar cortex had among the highest genetic correlations with intelligence in the adult HCP data, although overall strength of associations with were weak in this sample, possibly owed its broad age range.
Intelligence represents one of the most stable psychological traits over the human life span (Plomin and Stumm 2018), a somewhat paradoxical observation considering the rapid and regionally specific changes of its correlates with the brain. It seems highly implausible that the most important neural substrates of intelligence “migrate” throughout the cerebrum with age. Rather, the observed CT–IQ relationships may represent a proxy for a wave of maturational changes in brain regions that are closely coupled to intellectual ability. To our knowledge, this hypothesis was first implied by Shaw et al. (2006), who identified that differences in CT trajectories were strongly related to levels of intelligence. Our results indicate that the observed dynamic changes in CT–IQ relationships in youth are nearly entirely genetically mediated. Recent large genome wide association (GWAS) studies have begun to identify the genes associated with intelligence (Sniekers et al. 2017; Hill et al. 2018). Although to date only a small fraction (<10%) of the variance in intelligence can be attributed to a specific genetic variant, the candidate genes thus far identified have established roles in neurodevelopmental regulation, neurogenesis, synapse formation, and other neurodevelopmental processes (Hill et al. 2018). Given that neurodevelopment continues well into adulthood and is dependent on multiple factors including cellular proliferation, synaptogenesis, and myelination (Huttenlocher 1979; Petanjek et al. 2011; Miller et al. 2012), it is therefore not surprising that we see evidence of changes in neurogenetic influences on IQ with time. Although genotypes themselves are fixed, gene expression in the brain changes throughout childhood, adolescence, and early adulthoods in both nonhuman primates (Bakken et al. 2016) and humans (Kang et al. 2011).
The extant literature on how genetic factors mediate associations between cortical thickness and intelligence is limited. To our knowledge, there is only one similar prior study in children (Brouwer et al. 2014). In this study, Brouwer et al. examined a large sample of pediatric twins scanned at approximately 9 (N = 190) and 12 (N = 124) years of age. They found decreases in CT–IQ phenotypic correlations of the superior and middle frontal gyri between the 2 timepoints. Bivariate variance decomposition at the second timepoint (age 12) suggested that the observed correlations between CT and IQ were largely driven by shared genetic factors. Our study is similar in that we also identified that this age range is a crucial timepoint for CT–IQ covariance, correlations are rapidly changing during this interval, and that genetics appears to be the dominant factor driving these relationships. The studies differ in that Brouwer et al. reports largely negative CT–IQ correlations. The exact reason for this discrepancy is unclear, but there are differences in design that make the 2 studies largely complementary. The narrow age ranges of Brouwer et al. provides a unique perspective on changes occurring near the onset of puberty, while the larger sample, broad age range, vertex-level image processing, and longitudinal statistical design of the current study provides a comprehensive overview of the links between neurodevelopment and intelligence in youth. Our findings are similar to a study of gray matter density by Hulshoff Pol et al. (2006), which also found modest but positive genetically mediated correlations with intelligence in medial frontal cortex, occipital cortex, and parahippocampal gyrus.
Limitations
There are several limitations of the current study that must be considered when interpreting these findings. First, although a convenient and reproducible metric, full scale IQ represents an imperfect proxy for general intelligence (g). Second, we assume that IQ is stable with time and measurement invariant with age; although the literature generally supports this assumption (Wicherts 2016), there is some evidence that small temporal changes in intelligence correlate with subtle structural and functional MRI changes in adolescence (Ramsden et al. 2011). Furthermore, there is some evidence that the heritability of IQ changes with age (Deary et al. 2006). Third, given the computational demands of our analyses, we only report findings on full scale IQ. Although examining components of IQ remains a potential avenue for future investigation, prior work has suggested that brain–behavior correlates with components of IQ is largely mediated by g (Karama et al. 2011). Fourth, although standard quality control techniques were employed, we did not explicitly investigate the role of motion artifacts on our data; differences in motion related to zygosity or age could potentially influence our results (Couvy-Duchesne et al. 2014; Reuter et al. 2015). It is therefore reassuring that our findings integrate well into the extant literature. Finally, given the observed changes in the role of genetic factors with time, our findings are unlikely to extrapolate to other populations outside of our studied age range. Further investigations on neonatal/perinatal and adult populations will be required to fully understand the dynamic nature of gene–brain–intelligence relationships throughout the life cycle.
Supplementary Material
Funding
National Institute of Mental Health (NIMH) grant MH-20030, Big Data to Knowledge (BD2K) grant K01-ES026840 through the National Institute of Environmental Health Sciences (NIEHS) and the National Cancer Institute (NCI), and the intramural program of the National Institutes of Health (Clinical trial reg. no. NCT00001246, clinicaltrials.gov; NIH Annual Report Number, ZIA MH002949-01). Data were provided in part by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
References
- Ad-Dab’bagh Y, Lyttelton O, Muehlboeck J, Lepage C, Einarson D, Mok K, Ivanov O, Vincent R, Lerch J, Fombonne E, et al. 2006. The CIVET image-processing environment: a fully automated comprehensive pipeline for anatomical neuroimaging research In: Corbetta M, editor. Proceedings of the 12th annual meeting of the organization for human brain mapping. Florence, Italy. [Google Scholar]
- Andreasen NC, Flaum M, Swayze VW II, O’Leary D, Alliger R, Cohen G, Ehrhardt J, Yuh WT. 1993. Intelligence and brain structure in normal individuals. Am J Psychiatry. 150:130–134. [DOI] [PubMed] [Google Scholar]
- Bakken TE, Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, Dalley RA, Royall JJ, Lemon T, et al. 2016. A comprehensive transcriptional map of primate brain development. Nature. 535:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M, Reitveld M, van Baal G, Boomsma D. 2000. Genetic and environmental influences on the development of reproductive strategies during adolescence. Behav Genet. 32:237–249. [DOI] [PubMed] [Google Scholar]
- Basten U, Hilger K, Fiebach CJ. 2015. Where smart brains are different: a quantitative meta-analysis of functional and structural brain imaging studies on intelligence. Intelligence. 51:10–27. [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M. 2011. OpenMx: an open source extended structural equation modeling framework. Psychometrika. 76:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brans RGH, Kahn RS, Schnack HG, van Baal GCM, Posthuma D, van Haren NEM, Lepage C, Lerch JP, Collins DL, Evans AC, et al. 2010. Brain plasticity and intellectual ability are influenced by shared genes. J Neurosci. 30:5519–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer RM, Soelen ILC Van, Swagerman SC, Kahn S, Schnack HG, Ehli EA, Pol HEH, Boomsma DI. 2014. Genetic associations between intelligence and cortical thickness emerge at the start of puberty. Hum Brain Mapp. 3773:3760–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgaleta M, Johnson W, Waber DP, Colom R, Karama S. 2014. Cognitive ability changes and dynamics of cortical thickness development in healthy children and adolescents. Neuroimage. 84:810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YY, Shamosh NA, Cho SH, Deyoung CG, Lee MJ, Lee J, Kim SI, Cho Z, Kim K, Gray JR, et al. 2008. Multiple bases of human intelligence revealed by cortical thickness and neural activation. J Neurosci. 28:10323–10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D, Neelin P, Peters T, Evans A. 1994. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 18:192–205. [PubMed] [Google Scholar]
- Couvy-Duchesne B, Blokland GAM, Hickie IB, Thompson PM, Martin NG, de Zubicaray GI, McMahon KL, Wright MJ. 2014. Heritability of head motion during resting state functional MRI in 462 healthy twins. Neuroimage. 102:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Penke L, Johnson W. 2010. The neuroscience of human intelligence differences. Nat Rev Neurosci. 11:201. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Spinath FM, Bates TC. 2006. Genetics of intelligence. Eur J Hum Genet. 14:690–700. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Whiteman MC, Starr JM, Whalley LJ, Fox HC. 2004. The impact of childhood intelligence on later life: following up the Scottish Mental Surveys of 1932 and 1947. J Pers Soc Psychol. 86:130–147. [DOI] [PubMed] [Google Scholar]
- Dominicus A, Skrondal A, Gjessing HK, Pedersen NL, Palmgren J. 2006. Likelihood ratio tests in behavioral genetics: problems and solutions. Behav Genet. 36:331–340. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC. 2004. An introduction to latent growth curve modeling. Behav Ther. 35:333–363. [Google Scholar]
- Frangou S, Chitins X, Williams SCR. 2004. Mapping IQ and gray matter density in healthy young people. Neuroimage. 23:800–805. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. 2002. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 15:870–878. [DOI] [PubMed] [Google Scholar]
- Gilbert SL, Dobyns WB, Lahn BT. 2005. Genetic links between brain development and brain evolution. Nat Rev Genet. 6:581–590. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Rudrauf D, Colom R, Paul LK, Tranel D, Damasio H, Adolphs R. 2010. Distributed neural system for general intelligence revealed by lesion mapping. Proc Natl Acad Sci USA. 107:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. 1999. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 19:4065–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. 2004. Structural brain variation and general intelligence. Neuroimage. 23:425–433. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Akshoomoff N, Tulsky D, Mungas D, Weintraub S, Dikmen S, Beaumont J, Casaletto KB, Conway K, Slotkin J, et al. 2014. Reliability and validity of composite scores from the NIH toolbox cognition battery in adults. J Int Neuropsychol Soc. 20:588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WD, Marioni RE, Ritchie OMSJ, Hagenaars SP, Mcintosh AM, Davies G, Deary IJ. 2018. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol Psychiatry. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl CW, Baare WF, Van Oel C, Van Haren NE, Collins DL, Evans AC, Amunts K, et al. 2006. Genetic contributions to human brain morphology and intelligence. J Neurosci. 26:10235–10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. 1979. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 163:195–205. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. 2007. The Parieto-Frontal Integration Theory ( P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 30:135–187. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kawasawa YI, Cheng F, Zhu Y, Xu X, Li M, Sousa AMM, Pletikos M, Meyer KA, Sedmak G, et al. 2011. Spatio-temporal transcriptome of the human brain. Nature. 478:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Ad-dab Y, Haier RJ, Deary IJ, Lyttelton OC, Lepage C, Evans AC. 2009. Intelligence positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 37:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Colom R, Johnson W, Deary IJ, Haier R, Waber DP, Lepage C, Ganjavi H, Jung R, Evans AC, et al. 2011. Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. Neuroimage. 55:1443–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab’bagh Y, MacDonald D, Lee JM, Kim SI, Evans AC. 2005. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 27:210–221. [DOI] [PubMed] [Google Scholar]
- Koenis MMG, Brouwer RM, Swagerman SC, Van Soelen IL, Boomsma DI, Hulshoff HE. 2017. Association between structural brain network efficiency and intelligence increases during adolescence. Hum Brain Mapp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN. 2009. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp. 30:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch J, Evans A. 2005. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 24:163–173. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans AC. 2000. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 12:340–356. [DOI] [PubMed] [Google Scholar]
- Mcardle AJJ, Epstein D. 2013. Latent growth curves within developmental structural equation models. Child Dev. 58:110–133. [PubMed] [Google Scholar]
- McArdle JJ, Hamgami F, Jones K, Jolesz F, Kikinis R, Spiro A, Albert MS. 2004. Structural modeling of dynamic changes in memory and brain structure using longitudinal data from the normative aging study. J Gerontol B Psychol Sci Soc Sci. 59:P294–P304. [DOI] [PubMed] [Google Scholar]
- Mcdaniel MA. 2005. Big-brained people are smarter: a meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 33:337–346. [Google Scholar]
- Mehta P, West S. 2000. Putting the individual back into individual growth curves. Psychol Methods. 5:23–43. [DOI] [PubMed] [Google Scholar]
- Menary K, Collins PF, Porter JN, Muetzel R, Olson EA, Kumar V, Steinbach M, Lim KO, Luciana M. 2013. Intelligence associations between cortical thickness and general intelligence in children, adolescents and young adults. Intelligence. 41:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, Mcarthur MJ. 2012. Prolonged myelination in human neocortical evolution. Proc Natl Acad Sci. 109:16480–16485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, Gurbani M, Toga AW, Bilder RM. 2007. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb Cortex. 17:2163–2171. [DOI] [PubMed] [Google Scholar]
- Neale M, Cardon L. 1992. Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluver. [Google Scholar]
- Neale MC, Hunter MD, Pritikin JN, Zahery M, Brick TR, Kirkpatrick RM, Estabrook R, Bates TC, Maes HH, Boker SM. 2016. OpenMx 2.0: extended structural equation and statistical modeling. Psychometrika. 81:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale M, McArdle J. 2000. Structured latent growth curves for twin data. Twin Res. 3:165–177. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn S, Pol HEH. 2007. Genetic influences on human brain structure: a review of brain imaging studies in twins. Hum Brain Mapp. 473:464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HBM, Rakic P, Kostovic I. 2011. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci. 108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Von Stumm S. 2018. The new genetics of intelligence. Nat Rev Genet. 19:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polderman TJC, Benyamin B, De Leeuw CA, Sullivan PF, Van Bochoven A, Visscher PM, Posthuma D. 2015. Meta-analysis of the heritability of human traits based on fifty years of twin studies. Nat Genet. 47:702–709. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Boomsma DI. 2000. A note on the statistical power in extended twin designs. Behav Genet. 30:147–158. [DOI] [PubMed] [Google Scholar]
- Posthuma D, de Geus EJ, Hulshoff Pol HE, Kahn RS, Boomsma DI. 2002. The association between brain volume and intelligence is of genetic origin. Nat Neurosci. 5:83–84. [DOI] [PubMed] [Google Scholar]
- Posthuma D, de Geus EJ, Neale MC, Hulshoff Pol HE, Baaré WEC, Kahn RS, Boomsma D. 2000. Multivariate genetic analysis of brain structure in an extended twin design. Behav Genet. 30:311–319. [DOI] [PubMed] [Google Scholar]
- R Core Development Team 2006. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Ramsden S, Richardson FM, Josse G, Thomas MSC, Ellis C, Shakeshaft C, Seghier ML, Price CJ. 2011. Verbal and non-verbal intelligence changes in the teenage brain. Nature. 479:113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. 1996. Brain development, gender and IQ in children. A volumetric imaging study. Brain. 119:1763–1774. [DOI] [PubMed] [Google Scholar]
- Reuter M, Tisdall MD, Qureshi A, Buckner RL, van der Kouwe AJW, Fischl B. 2015. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage. 107:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Panizzon MS, Fennema-notestine C, Eyler LT, Fischl B, Franz CE, Hagler DJ, Lyons MJ, Neale MC, Pacheco J, et al. 2010. Cortical thickness is influenced by regionally specific genetic factors. Biol Psychiatry. 67:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins S, Evans AC, Collins DL, Whitesides S. 2004. Tuning and comparing spatial normalization methods. Med Image Anal. 8:311–323. [DOI] [PubMed] [Google Scholar]
- Roth G, Dicke U. 2005. Evolution of the brain and intelligence. Trends Cogn Sci. 9:250–257. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Giedd JN, Raznahan A, Neale MC. 2018. The genetic contributions to maturational coupling in the human cerebrum: a longitudinal pediatric twin imaging study. Cereb Cortex. 28:3184–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JE, Neale M, Fassassi B, Perez J, Lenroot R, Wells E, Giedd J.. 2014. a. Data from: The dynamic role of genetics on cortical patterning during childhood and adolescence. Dryad Digit Repos. [DOI] [PMC free article] [PubMed]
- Schmitt JE, Neale MC, Fassassi B, Perez J, Lenroot RK, Wells EM, Giedd JN. 2014. b. The dynamic role of genetics on cortical patterning during childhood and adolescence. Proc Natl Acad Sci USA. 111:6774–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. 2006. Intellectual ability and cortical development in children and adolescents. Nature. 440:676–679. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. 1998. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 17:87–97. [DOI] [PubMed] [Google Scholar]
- Sniekers S, Stringer S, Watanabe K, Jansen PR, Coleman JRI, Krapohl E, Taskesen E, Hammerschlag AR, Okbay A, Zabaneh D, et al. 2017. Individuals identifies new loci and genes influencing human intelligence. Nat Genet. 49:1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P, Cannon TD, Narr KL, Van Erp T, Poutanen V, Huttunen M, Lönnqvist J, Kaprio J, Khaledy M, Dail R, et al. 2001. Genetic influences on brain structure. Nat Neurosci. 4:1253–1258. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TEJ, Bucholz R, Chang A, Chen L, Corbetta M, Curtiss SW, et al. 2012. The Human Connectome Project: a data acquisition perspective. Neuroimage. 62:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher PM. 2004. Power of the classical twin design revisited. Twin Res. 7:505–512. [DOI] [PubMed] [Google Scholar]
- Wallace G, Raitano N, Prom-Wormley E, Schmitt JE, Neale M, Giedd J. 2010. A bivariate twin study of regional brain volumes and verbal and nonverbal intellectual skills during childhood and adolescence. Behav Genet. 40:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Schmitt JE, Lenroot R, Viding E, Ordaz S, Rosenthal MA, Molloy EA, Clasen LS, Kendler KS, Neale MC, et al. 2006. A pediatric twin study of brain morphometry. J Child Psychol Psychiatry. 47:987–993. [DOI] [PubMed] [Google Scholar]
- Wicherts JM. 2016. The importance of measurement invariance in neurocognitive ability testing. Clin Neuropsychol. 30:1006–1016. [DOI] [PubMed] [Google Scholar]
- Wilke M, Sohn J, Byars AW, Holland SK. 2003. Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. Neuroimage. 20:202–215. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. 2002. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 21:1280–1291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.