Abstract
Evidence suggests that age differences in associative memory are attenuated for associations that are consistent with prior knowledge. Such knowledge structures have traditionally been associated with the default network (DN), which also shows reduced modulation with age. In the present study, we investigated whether DN activity and connectivity patterns could account for this age-related effect. Younger and older adults underwent functional magnetic resonance imaging as they learned realistic and unrealistic prices of common grocery items. Both groups showed greater activity in the DN during the encoding of realistic, relative to unrealistic, prices. Moreover, DN activity at encoding and retrieval and its connectivity with an attention control network at encoding were associated with enhanced memory for realistic prices. Finally, older adults showed overactivation of control regions during retrieval of realistic prices relative to younger adults. Our findings suggest that DN activity and connectivity patterns (traditionally viewed as indicators of cognitive failure with age), and additional recruitment of control regions, might underlie older adults’ enhanced memory for meaningful associations.
Keywords: aging, associative memory, cognitive control, default network
An important and commonly reported finding in the cognitive literature is the age-related reduction in associative memory. This effect has been observed across a number of tasks and has been attributed to multiple factors including deficits in binding of individual elements at encoding (Chalfonte and Johnson 1996; Naveh-Benjamin 2000; Lyle et al. 2006; but see Campbell et al. 2010) and lack of strategic, control-based processes at retrieval (Light et al. 2004; Cohn et al. 2008; Dew and Giovanello 2010). Neurally, age differences in associative memory have typically been associated with age-related reductions in activity of hippocampal and frontoparietal regions at encoding (Dennis et al. 2008; Kim and Giovanello 2011), and lateral and anterior frontal control regions (dorsolateral prefrontal cortex—DLPFC and anterior prefrontal cortex—aPFC) at retrieval (Fandakova et al. 2014; Dulas and Duarte 2016). Yet, another set of findings suggests that age-related differences in associative memory are linked to reduced suppression of the default network (DN; Raichle et al. 2001) (Miller et al. 2008; Duverne et al. 2009; de Chastelaine et al. 2011)—a set of regions involved in internally based cognitive processes such as autobiographical memory, and storage and retrieval of semantic knowledge (Binder and Desai 2011; Rugg and Vilberg 2013; Andrews-Hanna et al. 2014). Critically, while associative memory, and related DN suppression, are reduced in advanced age, memory for associations that rely on prior knowledge tends to be preserved (Castel 2005; Amer et al. 2018). Despite evidence for the role of DN regions in structured, prior knowledge, and its lack of modulation with age, the relationship between DN activity and older adults’ enhanced memory for meaningful associations has not yet been investigated.
Research suggests that the DN, which is commonly active at rest and in memory retrieval, tends to be deactivated in young adults during externally oriented tasks, such as working memory (Anticevic et al. 2010) and memory encoding (Daselaar et al. 2004). Older adults, however, show reduced suppression of the DN, as well as increased coupling between the DN and control regions, during external tasks, suggesting decreased top-down modulation of the network (Lustig et al. 2003; Grady et al. 2006; Persson et al. 2007; Spreng and Schacter 2012; Turner and Spreng 2015; Rieck et al. 2017; Samu et al. 2017; for a review see Damoiseaux 2017). Although reduced DN modulation in older adults is typically associated with worse behavioral performance, the DN may support performance on tasks that rely on internally stored representations. For example, classic behavioral work (Bransford and Johnson 1972, 1973) has demonstrated that providing young adults with a semantic context before (but not after) hearing an ambiguous passage improved recall for details of the passage. Neuroimaging work using the same task provided evidence that the DN is involved in organizing incoming information in relation to prior knowledge (i.e., when a context is available), which possibly enhances the encoding of that information for better future recall (Maguire et al. 1999; Ames et al. 2015; see also Simony et al. 2016). Similarly, recent memory studies of young adults have provided strong evidence that areas of the DN are involved in encoding novel information when it is related to prior knowledge, and that the involvement of those areas is associated with enhanced memory for that information (Liu et al. 2017; Sommer 2017; see also Spreng et al. 2014 for similar evidence in a working memory context). Finally, and most relevant to the current study, increased interaction between DN and control regions in older adults has been linked to the use of prior knowledge and retrieval of familiar information (the default–executive coupling hypothesis of aging, DECHA; Turner and Spreng 2015; Spreng et al. 2018).
Taken together, the aforementioned findings point to a potentially beneficial role for the DN (and its connectivity with control regions) on tasks that rely on prior knowledge, which might provide a benefit for older adults. Indeed, behavioral studies support the benefits of prior knowledge use in older adults, particularly on memory tasks. For example, older adults show improved recognition memory (even better than younger adults) for words learned in the context of a meaningful sentence as opposed to individually (Matzen and Benjamin 2013). Similarly, older adults recall more episodic details (i.e., make more “Remember” judgments) of incoming information for which they have prior knowledge (e.g., names of famous actors from the 1950s vs. the 1990s; Toth et al. 2011). Finally, older adults show no age-related associative memory deficits when they learn information about a person that matches versus mismatches a stereotype (Mather et al. 1999), or when they learn realistic versus unrealistic prices for familiar grocery items (Castel 2005; Amer et al. 2018).
In the current study, we investigated the neural correlates of older adults’ enhanced associative memory performance for information that is consistent with prior knowledge. Older and younger adults underwent functional magnetic resonance imaging (fMRI) as they studied and retrieved realistic and unrealistic prices of common grocery items in a 2-alternative forced choice recognition task—a paradigm used by Amer et al. (2018) and adapted from Castel's (2005) (Supplementary Fig. S1). We hypothesized that increased DN activity and coupling with control regions (patterns that are more typical with old age) would be associated with older adults’ enhanced memory for realistic prices, consistent with the idea that DN engagement provides an advantage on tasks that benefit from prior knowledge. We also hypothesized that younger adults would show similar beneficial DN activation and coupling patterns in the realistic condition, given that they should also rely on prior knowledge and flexibly engage the DN when it aids performance.
In addition to the role of the DN, we investigated the influence of control regions, particularly at retrieval, on associative memory patterns. Namely, given the role of control regions, such as the DLPFC and superior parietal lobule (SPL), in associative memory and their reduced activity with age (e.g., Kim and Giovanello 2011; Fandakova et al. 2014), we predicted that those regions would show differential engagement across conditions in older adults. In particular, we predicted that those regions would show increased activity in the realistic, relative to the unrealistic price condition, consistent with the notion that meaningful conditions selectively engage cognitive control in older adults (see Rahhal et al. 2002; May et al. 2005; Hess 2014 for behavioral evidence of that theory). Additionally, we predicted that age-related reductions in control engagement would be more evident during the retrieval of unrealistic, relative to realistic, prices (see Amer et al. 2018). Our hypotheses were confirmed, illustrating for the first time that DN activity and connectivity patterns, traditionally viewed as a marker of cognitive decline, might actually confer an age-related benefit on memory tasks that rely on prior knowledge. Furthermore, additional recruitment of control regions on these tasks provides novel neural evidence of selective control engagement in older adults.
Materials and Methods
Participants
Twenty-five younger and 27 older adults were tested. Four older adults were removed prior to analysis due to incidental findings (n = 2), excess motion (n = 1), and failure to follow instructions (n = 1). Two younger adults were also removed due to failure to follow instructions (n = 1), and behavioral performance (memory accuracy on the realistic relative to the unrealistic condition was more than 2.5 SDs greater than the group mean; n = 1). The resulting final sample included 23 younger adults (19–29 years; M = 24.52, SD = 3.01; 8 males) and 23 older adults (63–85 years; M = 71.52, SD = 6.10; 9 males). All participants were right-handed, had normal or corrected to normal vision, had no history of any neurological or psychiatric illness, and were familiar with local grocery pricing and went grocery shopping a minimum of twice a month based on self-report. Older adults were cognitively intact, as indicated by their scores on Mini-Mental State Exam (MMSE; Folstein et al. 1975; M = 29.44, SD = 0.81) and Montreal Cognitive Assessment (MoCA; Nasreddine et al. 2005; M = 27.39, SD = 2.36). Older adults (M = 36.35, SD = 2.31) had higher vocabulary scores than younger adults (M = 33.54, SD = 4.81; data missing from one participant) on the Shipley-2 (Shipley et al. 2009), t(43) = 2.51, P < 0.05, as would be expected given the growth of vocabulary and knowledge with age (e.g., Park et al. 2002). The 2 groups (younger adults: M = 17.65, SD = 2.87; older adults: M = 17.39, SD = 2.48) were, however, matched on years of education, P > 0.7. Informed consent was obtained from all participants, and the study was approved by the Research Ethics Board of the Rotman Research Institute.
Experimental Design
Three encoding and 3 retrieval runs were scanned in alternating order. During encoding, participants viewed a series of grocery store items, presented individually with a realistic or an unrealistic price. Following each encoding run, participants retrieved the price of each item in a 2-alternative forced choice recognition task (see Supplementary Fig. S1). A 5-min baseline resting state scan with eyes open and an object localizer task performed at the end of the scanning session (both not analyzed in the current study) were also administered.
A mixed block/event-related design was used with 3 different block types: realistic, unrealistic, and fixation (the current study was analyzed as a block design with the block types concatenated across runs). Each run began with 10 s of fixation, followed by 8 task blocks (4 realistic and 4 unrealistic) of 30 s (encoding) or 40 s (retrieval) each, interleaved with 8 fixation blocks of 14 s each. Each task block contained 5 realistic or unrealistic items, thus there were 20 realistic and 20 unrealistic items per run (60 realistic and 60 unrealistic items in total). The task blocks were presented in alternating order (realistic always presented first), and the order of the blocks was fixed across participants. The items within each block were also fixed but presented in a random order for each participant. The items were counterbalanced, such that each item was equally likely to be presented with a realistic or unrealistic price. Realistic prices were selected on the basis of several local grocery stores (an average price was chosen), and unrealistic prices were selected by increasing each item’s price by a random value between $8 and $14 using a random number generator. Realistic prices ranged from $1.19 to $11.99, and unrealistic prices ranged from $9.49 to $23.99. As in Castel's (2005) study, all prices ended in the digit 9. Each item was presented for 4 s at encoding and 6 s at retrieval. The interstimulus interval varied randomly between 500 and 3500 ms.
Prior to encoding, participants were instructed to remember the exact price for each item, regardless of whether it was realistic or unrealistic and were informed of the nature of the recognition task. During retrieval, participants selected the price on the left or right below each item and additionally rated whether each choice was made with high or low confidence. Specifically, participants used a response box in their right or left hand to select the price on the right or left, respectively. The index finger was used to indicate a high-confidence response, and the middle finger was used to indicate a low-confidence response. Hence, on each retrieval trial, participants pressed one of the 4 keys. Participants practiced the task before entering the scanner.
fMRI Data Acquisition
Participants were scanned using a Siemens Trio 3 T scanner (Erlangen, Germany). The scanning session started with an anatomical scan acquired with a 3D magnetization-prepared rapid acquisition with gradient echo (MP-RAGE) sequence (repetition time (TR) = 2 s, echo time (TE) = 2.63 ms, field of view (FOV) = 256 mm2, 256 × 256 matrix, 160 slices of 1 mm thickness). Functional runs were acquired with an echo planar imaging (EPI) sequence, with 181 volumes for encoding runs and 221 volumes for retrieval runs (TR = 2 s, TE = 27 ms, flip angle = 62°, FOV = 192 mm2, 64 × 64 matrix, 40 slices of 3 mm thickness with 0.5 mm gap, 3.0 × 3.0 × 3.0 mm voxel size).
fMRI Data Preprocessing
Preprocessing of the data was performed with a combination of in house scripts (Churchill et al. 2015) and Analysis of Functional Neuroimages (Cox 1996). This included rigid motion and slice time correction, spatial normalization to Montreal Neurological Institute (MNI) space, and smoothing with a 6-mm Gaussian filter (the final voxel size was 4 × 4 × 4 mm). We also detrended the data to correct for low-frequency noise effects and regressed out the white matter, cerebral spinal fluid, vasculature, and motion–time series from each voxel–time series (Grady et al. 2010; Campbell et al. 2013). Finally, we used a motion-scrubbing procedure (described in detail by Campbell et al. 2013) to further reduce the impact of motion, given that standard motion correction procedures do not eliminate its influence on activity and connectivity measures (e.g., Power et al. 2012). This procedure uses a multivariate technique to identify outliers in both the motion–parameter estimates and fMRI signal intensity and replaces fMRI volumes where such outliers co-occur with adjacent values interpolated with cubic splines (never more than 3.4% of the total volumes in a single run in the current study). This method has the advantage of suppressing spikes, yet keeping the length of the time course intact across subjects. Less than 1% of the total volumes in each run were replaced for both age groups at encoding and retrieval (encoding: younger: 0.43%, older: 0.61%; retrieval: younger: 0.54%, older: 0.64%). The age difference in volumes replaced was statistically significant at encoding, U = 5299, z = 2.24, P < 0.05, but not retrieval, P > 0.4. One run from 3 older adults (2 encoding and one retrieval) was removed due to excess motion.
fMRI Data Analysis
Activation Analysis
Partial least squares (PLS; for full details and a review, see McIntosh et al. 1996; McIntosh et al. 2004; ; Krishnan et al. 2011) a multivariate, data-driven approach that identifies whole-brain patterns of activity associated with task conditions (task-PLS) or behavioral variables (behavioral-PLS) was used to analyze the data. PLS uses singular value decomposition to reduce the complexity of the dataset into orthogonal latent variables (LVs) that explain the maximum covariance between the task conditions or behavioral variables and the blood–oxygen-level-dependent (BOLD) signal. When analyzing data from different participant groups (e.g., age groups), the LVs can identify activation patterns for the different task conditions that are common across groups or patterns that are group-specific (i.e., identifies activation pattern differences between the groups). The significance of each LV was determined with a permutation test (McIntosh et al. 1996), using 500 permutations. For every LV, each brain voxel has a weight, known as a salience, which indicates how strongly the voxel contributes to the LV. The reliability of each voxel’s contribution to a particular LV was tested by submitting all saliences to a bootstrap estimation of the standard errors (SEs; Efron 1981), using 500 bootstraps. Peak voxels with a salience/SE ratio ≥3.0 (P < 0.001) are considered to be reliable (Sampson et al. 1989). Clusters containing at least 15 reliable contiguous voxels were extracted, with a local maximum defined as the voxel with a salience/SE ratio higher than any other voxel in a 2-cm cube centered on that voxel (the minimum distance between peaks was 10 mm). Coordinates of these locations are reported in MNI standard coordinate space (Mazziotta et al. 2001). To obtain summary measures of each participant’s expression of an LV spatial pattern, we calculated “brain scores” by multiplying each voxel’s salience by the BOLD signal in the voxel, and summing over all brain voxels for each participant. These brain scores were used to examine differences in expression of a brain pattern between conditions and groups in the task-PLS and to examine the correlation between brain pattern expression and memory accuracy in the behavioral-PLS. Because the extraction of the LVs and the corresponding brain images is done in a single step (i.e., the analysis can be considered as “one model”), no correction for multiple comparisons is required.
In order to examine activation patterns in older and younger adults associated with encoding and retrieval of realistic and unrealistic items, task-PLS was performed on the 4 conditions (realistic encoding, unrealistic encoding, realistic retrieval, unrealistic retrieval) for both age groups simultaneously. Additionally, behavioral-PLS was performed on the 4 conditions to identify regions at encoding and retrieval associated with better memory performance for the 2 item types in older and younger adults.
Connectivity Analysis
To investigate how network connectivity patterns are associated with memory accuracy for different item types, we focused on regions identified from the behavioral-PLS analysis, in which activity level was associated with behavioral performance. In particular, we applied an anatomical mask from the Schaefer et al.'s (2018) atlas on the activation pattern from the behavioral-PLS analysis and then determined peak voxels from the different regions of the analysis to use as coordinates for our nodes. Network affiliation for each node was determined based on the network membership of its corresponding parcel from the Schaefer et al. (2018) atlas (400 parcel resolution/7 network membership was used). Nodes from the DN and nodes from 3 control networks—dorsal attention, frontoparietal, and cingulo-opercular (also referred to as “ventral attention” in the atlas)—were identified (see Yeo et al. 2011). Then, each node was defined by a spherical ROI (5 mm radius) centered on the identified coordinate. Pairwise correlations between all the nodes across the fMRI time series of the realistic and unrealistic price conditions at encoding and retrieval were calculated for each participant using the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon 2012), and the correlation (r) values were transformed using Fisher’s z. Between-network and within-network connectivity measures were calculated for each participant by averaging the pairwise correlations between all nodes belonging to the different or same networks, respectively.
Recent work has demonstrated that increased between-network interaction for a frontoparietal control network is associated with reduced DN within-network interaction, particularly in older adults (Grady et al. 2016; see also Geerligs et al. 2015). Another study showed that a similar network interaction pattern (increased DN–frontoparietal control interaction and reduced global within-network connectivity) was associated with enhanced episodic memory retrieval, suggesting that greater between and reduced within-network interaction is associated with accessing internal representations (Westphal et al. 2017). Based on those findings, we calculated one score to characterize that interaction pattern. Specifically, for each participant, we subtracted the within-network DN–DN correlation from the between-network DN–control correlation (all correlations were group median-centered) for the realistic and unrealistic price conditions at encoding and retrieval. Hence, a larger score is indicative of greater between-network and less within-network connectivity. Finally, in order to examine if network interaction patterns at encoding and retrieval predicted memory for realistic and unrealistic item types, we used 2 bootstrapped multiple regressions with 1000 bootstrap resamples. The network interaction score at encoding (“encoding score”) and at retrieval (“retrieval score”), age group, age*encoding score, and age*retrieval score were entered simultaneously as predictors with realistic or unrealistic memory as the outcome variable.
In order to identify which control network to use in our interaction analyses, we assessed DN–control network interactions for each control network in relation to realistic memory performance, given our interest in the association between these interactions and memory for information consistent with prior knowledge. In particular, we ran separate bootstrapped multiple regressions (using all the predictors outlined above) for each control network with realistic memory as the outcome variable. Only the interaction between the DN and cingulo-opercular network (CON) showed a robust effect on memory, whereas interactions between the DN and the other control networks did not show a reliable association with realistic memory performance. Hence, nodes from the CON were used to define the control network in all the DN–control interaction analyses (see Table 1 and Supplementary Table S6 for coordinates of all nodes and Supplementary Tables S7 and S8 for interaction analyses results with the 2 other control networks).
Table 1.
Node coordinates from the DN, CON, and DAN
| Region | Hem | X (mm) | Y (mm) | Z (mm) |
|---|---|---|---|---|
| DN nodes | ||||
| Ventral medial prefrontal cortex | L | −4 | 48 | −4 |
| R | 4 | 52 | −8 | |
| Anterior temporal lobe | L | −52 | 0 | −20 |
| R | 48 | 8 | −24 | |
| Precuneus | R | 8 | −48 | 44 |
| Posterior cingulate cortex | L | −8 | −56 | 16 |
| Angular gyrus | L | −40 | −80 | 28 |
| CON nodes | ||||
| Dorsolateral prefrontal cortex | R | 28 | 40 | 24 |
| R | 44 | 12 | 20 | |
| Superior frontal gyrus | R | 12 | 8 | 68 |
| Cingulate cortex | R | 8 | 4 | 44 |
| Insula | L | −48 | 8 | 0 |
| R | 36 | 20 | 8 | |
| Anterior inferior parietal lobule | R | 64 | −24 | 36 |
| DAN nodes | ||||
| Inferior precentral sulcus | L | −48 | 0 | 24 |
| Middle temporal motion complex | L | −52 | −56 | −16 |
| Superior parietal lobule | R | 36 | −56 | 48 |
| R | 12 | −64 | 64 |
Note: Hem = hemisphere; R = right; L = left
Results
Behavioral Results
Trials with no responses and trials with a reaction time (RT) faster than 250 ms (unintentional responses) were first eliminated from all analyses (1.8% of trials for older adults and 0.80% for younger adults). Accuracy, RT for correct trials, and confidence ratings (i.e., the proportion of correct responses made with high confidence) were each analyzed by conducting a 2 × 2 mixed analysis of variance (ANOVA) with Age (old and young) as a between-subjects variables and Item Type (realistic and unrealistic) as a within-subjects variable. RT for correct trials was winsorized at the 90% level per subject and trial type by replacing the top and bottom 5% of trials with the 95th and 5th percentile, respectively. Confidence ratings from one older adult who failed to follow instructions on how to report confidence were removed from the analysis.
Accuracy
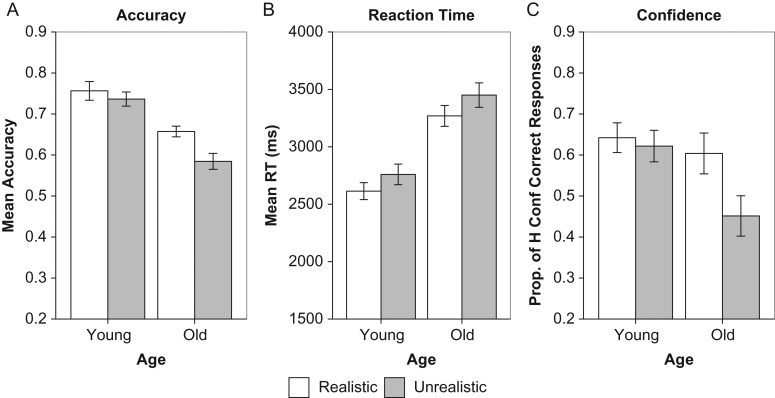
The ANOVA showed main effects of Age, F(1, 44) = 30.41, P < 0.0001, ηp2 = 0.41, with better performance by younger than older adults, and Item Type, F(1, 44) = 13.02, P < 0.001, ηp2 = 0.23, with better performance on realistic relative to unrealistic trials. The latter main effect was qualified by a significant interaction between the 2 variables, F(1, 44) = 4.21, P < 0.05, ηp2 = 0.09. As illustrated in Figure 1A, older, t(22) = 3.57, P < 0.005, d = 0.74, but not younger, P > 0.2, adults showed better memory for realistic than unrealistic items. Additionally, while younger adults outperformed older adults on the realistic items, t(44) = 3.77, P < 0.0005, d = 1.14, the difference was more pronounced for the unrealistic items, t(44) = 5.85, P < 0.0001, d = 1.76, consistent with previous work (Castel 2005; Amer et al. 2018).
Figure 1.
Performance on the behavioral task. The plots show (A) accuracy, (B) reaction time, and (C) the proportion of correct responses made with high confidence in younger and older adults. Error bars are standard errors of the mean.
Reaction Time
For RT (Fig. 1B), there were main effects of Age, F(1, 44) = 28.68, P < 0.0001, ηp2 = 0.39, with faster performance by younger than older adults, and Item Type, F(1, 44) = 13.02, P < 0.001, ηp2 = 0.23, with faster performance on realistic than unrealistic trials, but no Age × Item Type interaction, F < 1.
Confidence
The ANOVA on the proportion of correct responses made with high confidence showed no main effect of Age, F(1, 43) = 3.16, P = 0.08, but a main effect of Item Type, F (1, 43) = 19.42, P < 0.0001, ηp2 = 0.31, with a greater proportion of high-confidence correct responses for realistic than unrealistic items, which was qualified by a significant interaction, F(1, 43) = 13.02, P < 0.005, ηp2 = 0.21. As shown in Figure 1C, older, t(21) = 5.46, P < 0.0001, d = 1.16, but not younger, P > 0.4, adults showed a greater proportion of high-confidence correct responses for realistic than unrealistic items, and age differences were significant only for the unrealistic items, t(403) = 2.74, P < 0.01, d = 0.84. Thus, the confidence data augment the accuracy data and provide more support to the notion that age differences in memory are attenuated for realistic items.
Imaging Results
Activation Results
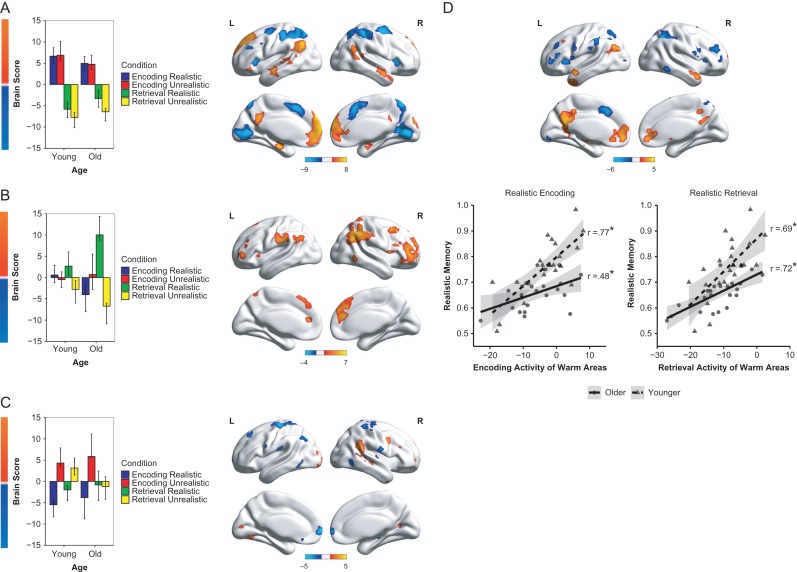
Task-PLS. The first LV from the task-PLS analysis (P < 0.001), which accounted for 37.99% of the covariance in the data, identified a pattern of activity that differentiated between encoding and retrieval (irrespective of item type) in older and younger adults (Fig. 2A). Areas active during encoding (shown in warm colors/assigned positive “brain scores”) included areas typically active during associative encoding such as the left hippocampus (HPC) and left inferior frontal gyrus, as well as other DN areas such as the medial PFC (mPFC), posterior cingulate cortex (pCC), and left angular gyrus (AG), possibly reflecting knowledge of the familiar grocery items (Binder and Desai 2011; Spreng et al. 2014). A different set of areas, which included areas associated with cognitive control (shown in cool colors/assigned negative “brain scores”), such as the bilateral frontal eye fields, medial superior PFC (msPFC), precuneus, and bilateral anterior insula were more active during retrieval (see Supplementary Table S1). A mixed ANOVA on the brain scores (a measure of the degree to which each individual expresses the identified brain pattern for each condition) with Age (old and young) as a between-subjects variable and Condition (realistic encoding, unrealistic encoding, realistic retrieval, unrealistic retrieval) as a within-subjects variable showed that there were no age differences in the activation of the identified regions at encoding or retrieval, as evidenced by the lack of a main effect of Age, F < 1, or Age × Condition interaction, F(3, 132) = 1.75, P > 0.1 (the main effect of Condition was significant as would be expected from the condition differences reflected in the positive and negative brain scores derived on this first LV).
Figure 2.
Activation results from the (A, B, C) task-PLS and (D) behavioral-PLS analyses. Task-PLS, which identifies patterns of brain activity in different task conditions and participant groups, indicated 3 significant LVs. The plots in A, B, and C indicate the set of regions that are active for different conditions and the extent of that activation in each LV. Positive brain scores indicate activity in warm colored regions, and negative brain scores indicate activity in cool colored regions (error bars are 95% CIs of the mean). (A) The first LV identified a pattern of activity that differentiated between encoding and retrieval in both age groups. (B) The second LV identified a set of regions that were more active during the retrieval of realistic, relative to unrealistic, prices, particularly in older adults. (C) The third LV identified a pattern that differentiated between realistic and unrealistic conditions (at encoding and retrieval in younger adults, but at encoding only in older adults). (D) The behavioral-PLS, which identifies patterns of brain activity associated with behavioral performance, produced an LV that differentiated between the realistic and unrealistic conditions in both age groups. The extent of activation (measured by brain scores from the behavioral-PLS analysis) in warm colored regions at encoding and retrieval was positively correlated with memory for realistic prices in younger and older adults. The extent of activation in cool colored regions was associated with memory for unrealistic prices (scatterplots in Supplementary Fig. S2). The color gradient bars in all panels indicate the bootstrap ratio of salience/SE for each voxel. The minimum thresholds used were 4 (or −4) for LV1 and 3 (or −3) for LVs 2 and 3 in the task-PLS, and 3 (or −3) in the behavioral-PLS. Brain images were visualized with BrainNet Viewer (Xia et al. 2013).
The second LV (P < 0.005) accounted for 23.73% of the covariance in the data and identified a set of regions that were more active during retrieval of realistic relative to unrealistic items, particularly in older adults (Fig. 2B). These regions (warm colors/positive scores) included a set of right-lateralized control regions, such as the right SPL and right middle frontal gyrus, and other control regions, such as bilateral aPFC, and anterior cingulate cortex (aCC) (Supplementary Table S2). The ANOVA on the brain scores showed a main effect of Age, F(1, 44) = 5.44, P < 0.05, with brain scores more positive in older adults than younger adults, and a significant Age × Condition interaction, F(3, 132) = 3.05, P < 0.05, indicating that older adults showed a larger difference between realistic and unrealistic retrieval conditions relative to younger adults. Planned comparisons between the 2 age groups showed that the only significant difference was under the realistic retrieval condition, t(44) = 4.60, P < 0.0001, with older adults showing more activity in the identified control regions, providing evidence that older adults selectively engage (and overactivate) control regions in meaningful conditions.
The third LV (P < 0.05) accounted for 15.17% of the covariance in the data and differentiated between the realistic and unrealistic conditions (Fig. 2C). Along with a few other regions (cool colors/negative scores), the mPFC showed activation for the realistic condition. In contrast, control (e.g., right temporal parietal junction and right DLPFC) and visual (bilateral inferior occipital gyrus) regions (warm colors/positive scores) showed activity for the unrealistic condition (Supplementary Table S3). Critically, this dissociation was present at both encoding and retrieval for younger adults, but only at encoding for older adults. The ANOVA showed a main effect of Age Group, F(1, 44) = 13.51, P < 0.001, with scores more positive in younger than older adults, and a significant Age × Condition interaction, F(3, 132) = 6.71, P < 0.0005, confirming that younger, but not older, adults showed a differentiation of the identified regions at retrieval. Planned comparisons showed that age differences were only present in the unrealistic retrieval condition, t(44) = 7.04, P < 0.0001, indicating that younger adults showed greater activation than older adults in the identified (warm colored) control and posterior visual regions. This is consistent with a recently reported behavioral finding suggesting that controlled retrieval, at least partly, accounts for age differences in arbitrary, but not meaningful, associations (Amer et al. 2018).
Behavioral-PLS. Behavioral-PLS was used to identify regions under the 4 conditions (encoding and retrieval of realistic or unrealistic items) that correlated with memory performance. The analysis yielded 2 significant LVs that accounted for a similar amount of covariance in the data. However, only the second LV (P < 0.05; accounted for 19.02% of the covariance in the data) reliably differentiated between the realistic and unrealistic conditions in both age groups and was of interest in the current study (see Supplementary Fig. S3 and Table S4 for results of the first LV). Consistent with our predictions, greater activity in a set of DN regions (warm colored regions in Fig. 2D) was correlated with better memory performance for realistic items, while greater activity in a set of control (cool colored) regions was associated with better memory performance for unrealistic items (Supplementary Table S5). Specifically, activity in DN areas implicated in prior knowledge, such as the pCC, ventral mPFC, and bilateral anterior temporal lobes (aTL), during encoding and retrieval was significantly associated with enhanced memory for realistic prices in younger and older adults (see Fig. 2D). Memory for realistic prices was also positively correlated with activity in the left HPC. Activity in control regions, however, such as the bilateral SPL, dorsal aCC, and bilateral aPFC, was significantly correlated with better memory for unrealistic prices. This effect was present only at encoding (r = 0.51, 95% CI [0.23, 0.82]) for younger adults, and only at retrieval (r = 0.41, 95% CI [0.18, 0.71]) for older adults (Supplementary Fig. S2).
Connectivity Results
Network interaction patterns between the DN and the CON were measured at encoding and retrieval and entered (along with age group and interactions with age) as predictors into 2 multiple regressions with realistic and unrealistic memory as the outcome variable. The estimates for all parameters (standardized coefficients) are provided in Table 2.
Table 2.
Parameter estimates for models assessing the relationship between DN–CON interactions and realistic and unrealistic memory
| β | 95% CI | |
|---|---|---|
| Realistic memory | ||
| Age group | 0.561 | [0.066, 0.158]a |
| Encoding score | 0.713 | [0.526, 1.647]a |
| Retrieval score | 0.065 | [−0.463, 0.601] |
| Encoding score*age | −0.382 | [−1.437, 0.259] |
| Retrieval score*age | 0.142 | [−0.538, 1.286] |
| Unrealistic memory | ||
| Age group | 0.718 | [0.118, 0.217]a |
| Encoding score | −0.35 | [−1.742, 0.161] |
| Retrieval score | 0.156 | [−0.39, 1.284] |
| Encoding score*age | 0.61 | [0.272, 2.308]a |
| Retrieval score*age | −0.063 | [−1.146, 0.71] |
Note: aindicates a robust effect.
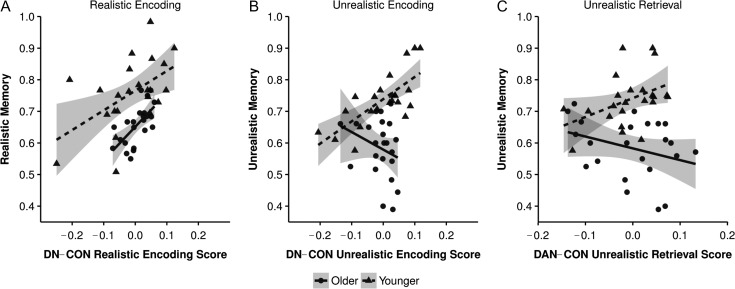
As illustrated in Table 2, only age group and encoding connectivity (95% CIs of the unstandardized beta coefficients do not include zero) were reliable predictors of realistic memory performance. This demonstrates that younger adults showed better memory than older adults for realistic prices. However, both age groups showed similar benefits (as suggested by the lack of significant interaction) from more connectivity between the DN and CON and less connectivity within the networks at encoding (i.e., “encoding score”; see Fig. 3A). With respect to unrealistic memory, the only reliable predictors were age group and the interaction between age and encoding score, suggesting age differences in how DN–CON network interaction patterns at encoding predict unrealistic memory. Additional bootstrapped regressions in younger and older adults, with encoding score as a predictor and unrealistic memory as the outcome variable, showed a robust relationship in younger (β = 0.725, 95% CI of b [0.406, 1.064]), but not older (β = −0.273, 95% CI of b [−1.485, 0.257]), adults (see Fig. 3B). Collectively, the results suggest that reliance on prior knowledge at encoding, reflected by greater interaction between default and control regions, was associated with enhanced memory for realistic prices in both groups. The same interaction pattern, however, also provided a memory advantage for unrealistic prices in younger adults only, suggesting that younger adults may be able to flexibly use prior knowledge, given the familiarity of the grocery items, to facilitate encoding of the unrealistic prices.
Figure 3.
Correlation between network interaction patterns and behavioral performance. (A) Greater interaction between the default network (DN) and cingulo-opercular network (CON) during encoding of realistic prices was correlated with better memory for those prices in both younger and older adults. (B) Greater DN-CON interaction during encoding of unrealistic prices was correlated with better memory for those prices in younger, but not older, adults. (C) Greater interaction the dorsal attention network (DAN) and CON during retrieval of unrealistic prices was correlated with better memory for those prices in younger, but not older, adults.
To examine whether the network interaction results were specific to the DN, we conducted a similar analysis that did not include the DN and instead examined the interaction between the CON and another control network. Given that regions of the CON are functionally and spatially interposed between the DN and dorsal attention network (DAN) (Vincent et al. 2008; Spreng et al. 2013; Grady et al. 2016), and that only the DN is associated with the use of prior knowledge, we hypothesized that the CON would show functionally relevant interactions with the DN, but not DAN, under conditions that engage prior knowledge (e.g., Spreng et al. 2010). To test that hypothesis, we examined whether DAN–CON interaction patterns (using DAN–DAN interactions as our measure of within-network connectivity) showed an association with memory performance (see Table 1 for DAN coordinates). The results of the bootstrapped multiple regressions are displayed in Table 3. As predicted, DAN–CON interaction scores in older and younger adults did not predict memory for realistic prices (only age group was a reliable predictor). Interestingly, for unrealistic price memory, the interaction between age and retrieval score was a reliable predictor, in addition to age group. Additional regressions in each age group, with retrieval score as the predictor and unrealistic memory as the outcome variable, showed a robust relationship in younger (β = 0.419, 95% CI of b [0.142, 1.095]), but not older (β = −0.318, 95% CI of b [−0.804, 0.06]) adults, although it was trending in the negative direction for older adults (see Fig. 3C). This suggests that interactions between control networks, possibly reflecting controlled retrieval (Iidaka et al. 2006; Jacques et al. 2011), facilitated the retrieval of unrealistic prices in younger adults only.
Table 3.
Parameter estimates for models assessing the relationship between DAN–CON interactions and realistic and unrealistic memory
| β | 95% CI | |
|---|---|---|
| Realistic Memory | ||
| Age group | 0.496 | [0.05, 0.153]a |
| Encoding score | 0.002 | [−0.772, 0.335] |
| Retrieval score | 0.101 | [−0.235, 0.518] |
| Encoding score*age | 0.236 | [−0.295, 1.509] |
| Retrieval score*age | −0.04 | [−0.803, 0.915] |
| Unrealistic Memory | ||
| Age group | 0.685 | [0.107, 0.219]a |
| Encoding score | −0.276 | [−1.095, 0.229] |
| Retrieval score | −0.096 | [−0.543, 0.266] |
| Encoding score*age | 0.239 | [−0.472, 1.433] |
| Retrieval score*age | 0.244 | [0.086, 1.569]a |
Note: aindicates a robust effect.
Discussion
Previous work has demonstrated reduced age differences in memory for meaningful associations that are consistent with prior knowledge, relative to arbitrary associations (Castel 2005; Amer et al. 2018). In the current study, we examined the neural basis of that effect, in younger and older adults who studied and retrieved realistic and unrealistic prices of common grocery items. Both younger and older adults showed activation of DN regions (particularly the mPFC) during the encoding of realistic, but not unrealistic, item prices, suggesting an involvement of these regions in organizing incoming information in relation to prior knowledge. Moreover, the extent of activation of DN regions at encoding and retrieval, as well as their interaction with CON regions at encoding, was associated with better memory for realistic prices. In contrast, activation of a set of control regions (at encoding for younger adults and at retrieval for older adults) was associated with better memory for unrealistic prices. Finally, the level of activation of control regions during retrieval in older adults varied as a function of item type. Relative to young adults, older adults showed overactivation of control regions during the retrieval of realistic prices but showed reduced activation during the retrieval of unrealistic prices.
Although activation of DN regions during memory encoding has typically been associated with worse memory (Daselaar et al. 2004; Miller et al. 2008), our findings provide evidence that such activity can enhance memory for new information that is related to prior knowledge, and at least partly account for older adults’ relatively preserved memory for that type of information. Suppression of DN regions, which possibly reduces interference from internal-based distraction (Anticevic et al. 2012), has commonly been associated with better performance on externally oriented tasks that do not engage stored knowledge representations (McKiernan et al. 2003; Daselaar et al. 2004; Shulman et al. 2007). In the context of memory, the activation profile of the DN for successfully learned information has been discussed in terms of an “encoding–retrieval flip”: DN areas are suppressed during the encoding of new information and activated during the retrieval of that information (Huijbers et al. 2012, 2013). Age-related deficits on attention and memory tasks have frequently been attributed, at least in part, to a lack of modulation of the DN (Miller et al. 2008; Spreng and Schacter 2012; Rieck et al. 2017). That is, older adults show reduced top-down suppression of the DN relative to younger adults, and the interpretation of this finding is that DN activity interferes with target task performance by introducing internally generated distraction or by disrupting the proper allocation of limited neural resources (Lustig et al. 2003; Grady et al. 2006; Persson et al. 2007). Here, we show that such DN activity can provide a benefit to older (and younger) adults when learning information that is related to prior knowledge. This finding is consistent with studies suggesting that older adults increasingly rely on crystallized, established forms of knowledge, which might compensate for the loss of efficiency of other basic cognitive functions (Li et al. 2013; Blanco et al. 2016; Spreng et al. 2018). Furthermore, our study illustrates that the relationship between DN activity at encoding and subsequent memory performance varies as a function of the type of information being learned (i.e., is not always detrimental), in accordance with recent work demonstrating that DN activity facilitates performance on tasks that engage internal representations (Spreng et al. 2014; Liu et al. 2017; Sommer 2017; see also van Kesteren et al. 2012).
In addition to the DN activity findings, our study also showed that during the encoding of realistic prices, the extent of interaction between DN and CON regions was positively correlated with memory for those prices in both age groups. This finding is notable considering the emphasis in the literature on how age-related coupling of control and default regions might be indicative of reduced top-down neuromodulation and increased dedifferentiation at the network level (e.g., Spreng & Schacter 2012; Geerligs et al. 2014, 2015; Amer, Anderson et al. 2016; Rieck et al. 2017). The current finding is, however, consistent with studies suggesting that such coupling patterns generally support internally focused goal-directed cognition (e.g., Spreng et al. 2010; Spreng & Schacter 2012) and is consistent with the DECHA model, which proposes that those patterns reflect an adaptive reliance on prior knowledge when task demands increase in older adults (Turner & Spreng 2015; Spreng et al. 2018). It is important to note that although the DECHA model focuses on interactions between the DN and lateral prefrontal regions in particular, the regions that define the CON in the current study include some lateral frontal regions, as well as other regions, such as the bilateral insula, which overlap with a larger frontoparietal network shown to increase its coupling with the DN with age (Spreng & Schacter 2012; Grady et al. 2016).
The positive relationship between DN–CON coupling and memory performance in the present study might reflect controlled access to relevant internal representations at encoding to facilitate “retrieval-mediated learning” (i.e., enhanced learning of new information related to prior knowledge; Preston and Eichenbaum 2013; Antony et al. 2017). Indeed, other studies have shown that similar network coupling patterns facilitate memory search during successful episodic retrieval, further suggesting a role for control networks in accessing internal representations in accordance with task demands (Fornito et al. 2012; Kragel and Polyn 2015; Westphal et al. 2017). Given that the relationship between DN–control interactions and memory was specific to the CON, our findings additionally suggest that memory benefits from prior knowledge, at least during encoding, depend not only on accessing internal representations but also on maintaining the task of relating incoming information to such representations (see Dosenbach et al. 2007, 2008 for evidence that the CON is involved in task-set maintenance). Interestingly, in addition to the prior knowledge benefit (through DN–CON coupling) seen for realistic memory in both age groups, younger adults showed a similar benefit during the encoding of unrealistic prices. Given the familiarity of the grocery items, this finding suggests that younger adults were able to use their knowledge of the items to organize or strategically encode the unrealistic prices, which is less likely to spontaneously occur in older adults (Schmitt et al. 1981; Naveh-Benjamin et al. 2007). Nonetheless, our findings demonstrate a memory benefit in older adults for new information that is consistent with prior knowledge, and importantly, demonstrate that connectivity patterns (e.g., increased default–control network coupling) typically characteristic of aging and cognitive decline can potentially be beneficial in certain learning contexts (see also Spreng et al. 2018 for how those patterns might influence the nature of autobiographical memory in older adults by increasing their tendency to recall semantic over episodic details).
To further examine whether memory benefits associated with DN–CON interactions were specific to the DN, we conducted an additional analysis that investigated the association between control network interactions (DAN–CON) and memory. Consistent with our predictions, DAN–CON interactions did not show the same associations with memory as DN–CON interactions, illustrating the importance of the DN and prior knowledge in explaining these associations. Interestingly, however, DAN–CON interactions during retrieval of unrealistic prices significantly predicted memory for those prices in younger, but not older, adults. These control network interactions may reflect controlled retrieval mechanisms that were beneficial to younger adults only. In support of that notion, previous studies have suggested that connectivity between control regions at retrieval is important for initializing and restricting memory search based on retrieval cues (Jacques et al. 2011; see also Iidaka et al. 2006).
Taken together, the network interaction findings suggest that memory for new, realistic information that can be readily integrated into a pre-existing knowledge network benefits from controlled access to schematic knowledge during encoding in younger and older adults. In contrast, memory for new, unrealistic information that is not consistent with prior knowledge may be more dependent on strategic encoding as well as controlled retrieval mechanisms in younger adults. The apparent age-related decrease in controlled retrieval, as indicated by the network interaction data, is consistent with the present activation results demonstrating reduced activity of control regions during the unrealistic retrieval condition in older adults. It is also consistent with recent behavioral evidence of a relationship between reduced controlled retrieval and worse memory for unrealistic, arbitrary associations in older adults (Amer et al. 2018).
Finally, our study demonstrated that while older adults showed reduced activity in a set of control regions during retrieval of unrealistic prices, they showed overactivation of right-lateralized control regions during retrieval of realistic prices. These brain areas, which have been shown to be vulnerable to aging (Rieck et al. 2017), overlap with regions that have been linked to successful retrieval (Fornito et al. 2012; Fandakova et al. 2014; Kragel and Polyn 2015; Dulas and Duarte 2016). Overactivation of these areas in the current study suggests that older adults selectively engaged control regions under the meaningful retrieval condition in which they showed better performance. It is important to note, however, that although these regions have previously been linked to successful retrieval, their overactivation might be indicative of greater task interest or engagement and not necessarily serve a memory function. Nonetheless, this finding provides neural support for the theory that, due to greater “cognitive effort” associated with task performance with age, older adults selectively engage cognitive control based on various factors, such as motivation and task meaningfulness/personal relevance (Rahhal et al. 2002; May et al. 2005; Hess 2014).
In conclusion, our findings demonstrate that brain patterns that are optimal for learning depend on the type of information being learned (see Amer, Campbell et al. 2016). While DN activity and connectivity with control networks during encoding have been associated with cognitive decline in older adults and with less effective learning, more generally, these patterns can provide an advantage on tasks that engage internal representations and benefit from a functional integration of intrinsically separate networks. These patterns also seem to, at least partly, account for older adults’ well-documented, improved performance on tasks that rely on prior knowledge or lifelong experiences. Future work will be important in exploring the trade-off between the benefits of prior knowledge and reduced DN modulation with old age, and how factors such as knowledge accumulation and type of task engagement influence this trade-off.
Supplementary Material
Notes
The authors thank Hasina Barrie, Elizabeth Howard, Kesho Wynn, and Ebtesam Moharam for their assistance in stimuli creation and data collection. The authors also thank Jordana Wynn for her thoughtful feedback on previous versions of the manuscript. Conflict of Interest: None declared.
Funding
Canadian Institutes of Health Research (Grant MOP89769 to L.H. and a Foundation Grant to C.G.) and Natural Sciences and Engineering Research Council of Canada (Grant 487235 to L.H.) and Alexander Graham Bell Canada Graduate Scholarship–Doctoral to T.A.
References
- Amer T, Anderson JAE, Campbell KL, Hasher L, Grady CL. 2016. Age differences in the neural correlates of distraction regulation: A network interaction approach. Neuroimage. 139:231–239. [DOI] [PubMed] [Google Scholar]
- Amer T, Campbell KL, Hasher L. 2016. Cognitive control as a double-edged sword. Trends Cogn Sci. 20:905–915. [DOI] [PubMed] [Google Scholar]
- Amer T, Giovanello KS, Grady CL, Hasher L. 2018. Age differences in memory for meaningful and arbitrary associations: a memory retrieval account. Psychol Aging. 33:74–81. [DOI] [PubMed] [Google Scholar]
- Ames DL, Honey CJ, Chow MA, Todorov A, Hasson U. 2015. Contextual alignment of cognitive and neural dynamics. J Cogn Neurosci. 27:655–664. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. 2014. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X, Krystal JH. 2012. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 16:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Shulman GL, Barch DM. 2010. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 49:2638–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony JW, Ferreira CS, Norman KA, Wimber M. 2017. Retrieval as a fast route to memory consolidation. Trends Cogn Sci. 21:573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH. 2011. The neurobiology of semantic memory. Trends Cogn Sci. 15:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco NJ, Love BC, Ramscar M, Otto AR, Smayda K, Maddox WT. 2016. Exploratory decision-making as a function of lifelong experience, not cognitive decline. J Exp Psychol Gen. 145:284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bransford J, Johnson M. 1972. Contextual prerequisites for understanding—some investigations of comprehension and recall. J Verbal Learn Verbal Behav. 11:717–726. [Google Scholar]
- Bransford JD, Johnson MK.. 1973. Considerations of some problems of comprehension. In:Chase W, editor. Visual information processing. New York: Academic Press; p. 383–438. [Google Scholar]
- Campbell KL, Grigg O, Saverino C, Churchill N, Grady CL. 2013. Age differences in the intrinsic functional connectivity of default network subsystems. Front Aging Neurosci. 5:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Hasher L, Thomas RC. 2010. Hyper-binding: a unique age effect. Psychol Sci. 21:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel A. 2005. Memory for grocery prices in younger and older adults: the role of schematic support. Psychol Aging. 20:718–721. [DOI] [PubMed] [Google Scholar]
- Chalfonte B, Johnson M. 1996. Feature memory and binding in young and older adults. Mem Cognit. 24:403–416. [DOI] [PubMed] [Google Scholar]
- Churchill NW, Spring R, Afshin-Pour B, Dong F, Strother SC. 2015. An automated, adaptive framework for optimizing preprocessing pipelines in task-based functional MRI. PLoS One. 10:e0131520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M, Moscovitch M, Emrich SM. 2008. Age-related deficits in associative memory: the influence of impaired strategic retrieval. Psychol Aging. 23:93–103. [DOI] [PubMed] [Google Scholar]
- Cox R. 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 29:162–173. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS. 2017. Effects of aging on functional and structural brain connectivity. Neuroimage. 160:32–40. [DOI] [PubMed] [Google Scholar]
- Daselaar S, Prince S, Cabeza R. 2004. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 23:921–927. [DOI] [PubMed] [Google Scholar]
- de Chastelaine M, Wang TH, Minton B, Muftuler LT, Rugg MD. 2011. The effects of age, memory performance, and callosal integrity on the neural correlates of successful associative encoding. Cereb Cortex. 21:2166–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. 2008. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol Learn Mem Cogn. 34:791–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dew ITZ, Giovanello KS. 2010. Differential age effects for implicit and explicit conceptual associative memory. Psychol Aging. 25:911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. 2008. A dual-networks architecture of top-down control. Trends Cogn Sci. 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RAT, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. 2007. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 104:11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulas MR, Duarte A. 2016. Age-related changes in overcoming proactive interference in associative memory: the role of PFC-mediated executive control processes at retrieval. Neuroimage. 132:116–128. [DOI] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. 2009. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb Cortex. 19:733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B. 1981. Nonparametric estimates of standard error—the jackknife, the bootstrap and other methods. Biometrika. 68:589–599. [Google Scholar]
- Fandakova Y, Lindenberger U, Shing YL. 2014. Deficits in process-specific prefrontal and hippocampal activations contribute to adult age differences in episodic memory interference. Cereb Cortex. 24:1832–1844. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. 1975. Mini-mental state—practical method for grading cognitive state of patients for clinician. J Psychiatr Res. 12:189–198. [DOI] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Zalesky A, Simons JS. 2012. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc Natl Acad Sci USA. 109:12788–12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM. 2015. A brain-wide study of age-related changes in functional connectivity. Cereb Cortex. 25:1987–1999. [DOI] [PubMed] [Google Scholar]
- Geerligs L, Saliasi E, Renken RJ, Maurits NM, Lorist MM. 2014. Flexible connectivity in the aging brain revealed by task modulations. Hum Brain Mapp. 35:3788–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Protzner AB, Kovacevic N, Strother SC, Afshin-Pour B, Wojtowicz M, Anderson JAE, Churchill N, McIntosh AR. 2010. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 20:1432–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C, Sarraf S, Saverino C, Campbell K. 2016. Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiol Aging. 41:159–172. [DOI] [PubMed] [Google Scholar]
- Grady C, Springer M, Hongwanishkul D, McIntosh A, Winocur G. 2006. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 18:227–241. [DOI] [PubMed] [Google Scholar]
- Hess TM. 2014. Selective engagement of cognitive resources: motivational influences on older adults’ cognitive functioning. Perspectives on. Psychol Sci. 9:388–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Schultz AP, Vannini P, McLaren DG, Wigman SE, Ward AM, Hedden T, Sperling RA. 2013. The Encoding/Retrieval flip: interactions between memory performance and memory stage and relationship to intrinsic cortical networks. J Cogn Neurosci. 25:1163–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Vannini P, Sperling RA, Pennartz CM, Cabeza R, Daselaar SM. 2012. Explaining the encoding/retrieval flip: memory-related deactivations and activations in the posteromedial cortex. Neuropsychologia. 50:3764–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Matsumoto A, Nogawa J, Yamamoto Y, Sadato N. 2006. Frontoparietal network involved in successful retrieval from episodic memory. spatial and temporal analyses using fMRI and ERP. Cereb Cortex. 16:1349–1360. [DOI] [PubMed] [Google Scholar]
- Jacques PL, Kragel PA, Rubin DC. 2011. Dynamic neural networks supporting memory retrieval. Neuroimage. 57:608–616. St. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Giovanello KS. 2011. The effects of attention on age-related relational memory deficits: FMRI evidence from a novel attentional manipulation. J Cogn Neurosci. 23:3637–3656. [DOI] [PubMed] [Google Scholar]
- Kragel JE, Polyn SM. 2015. Functional interactions between large-scale networks during memory search. Cereb Cortex. 25:667–679. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Williams LJ, McIntosh AR, Abdi H. 2011. Partial least squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 56:455–475. [DOI] [PubMed] [Google Scholar]
- Li Y, Baldassi M, Johnson EJ, Weber EU. 2013. Complementary cognitive capabilities, economic decision making, and aging. Psychol Aging. 28:595–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light L, Patterson M, Chung C, Healy M. 2004. Effects of repetition and response deadline on associative recognition in young and older adults. Mem Cognit. 32:1182–1193. [DOI] [PubMed] [Google Scholar]
- Liu Z, Grady C, Moscovitch M. 2017. Effects of prior-knowledge on brain activation and connectivity during associative memory encoding. Cereb Cortex. 27:1991–2009. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder A, Bhakta M, O’Brien K, McAvoy M, Raichle M, Morris J, Buckner R. 2003. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci USA. 100:14504–14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle K, Bloise S, Johnson M. 2006. Age-related binding deficits and the content of false memories. Psychol Aging. 21:86–95. [DOI] [PubMed] [Google Scholar]
- Maguire E, Frith C, Morris R. 1999. The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain. 122:1839–1850. [DOI] [PubMed] [Google Scholar]
- Mather M, Johnson M, De Leonardis D. 1999. Stereotype reliance in source monitoring: age differences and neuropsychological test correlates. Cogn Neuropsychol. 16:437–458. [Google Scholar]
- Matzen LE, Benjamin AS. 2013. Older and wiser: older adults’ episodic word memory benefits from sentence study contexts. Psychol Aging. 28:754–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C, Rahhal T, Berry E, Leighton E. 2005. Aging, source memory, and emotion. Psychol Aging. 20:571–578. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, et al. 2001. A probabilistic atlas and reference system for the human brain: International consortium for brain mapping (ICBM). Philos Trans R Soc B-Biol Sci. 356:1293–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A, Bookstein F, Haxby J, Grady C. 1996. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 3:143–157. [DOI] [PubMed] [Google Scholar]
- McIntosh A, Chau W, Protzner A. 2004. Spatiotemporal analysis of event-related fMRI data using partial least squares. Neuroimage. 23:764–775. [DOI] [PubMed] [Google Scholar]
- McKiernan K, Kaufman J, Kucera-Thompson J, Binder J. 2003. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 15:394–408. [DOI] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. 2008. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci USA. 105:2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine Z, Phillips N, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings J, Chertkow H. 2005. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 53:695–699. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. 2000. Adult age differences in memory performance: tests of an associative deficit hypothesis. J Exp Psychol Learn Mem Cogn. 26:1170–1187. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levy O. 2007. The associative memory deficit of older adults: the role of strategy utilization. Psychol Aging. 22:202–208. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. 2002. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 17:299–320. [PubMed] [Google Scholar]
- Persson J, Lustig C, Nelson JK, Reuter-Lorenz PA. 2007. Age differences in deactivation: a link to cognitive control? J Cogn Neurosci. 19:1021–1032. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. 2013. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 23:R764–R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahhal T, May C, Hasher L. 2002. Truth and character: sources that older adults can remember. Psychol Sci. 13:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M, MacLeod A, Snyder A, Powers W, Gusnard D, Shulman G. 2001. A default mode of brain function. Proc Natl Acad Sci USA. 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieck JR, Rodrigue KM, Boylan MA, Kennedy KM. 2017. Age-related reduction of BOLD modulation to cognitive difficulty predicts poorer task accuracy and poorer fluid reasoning ability. Neuroimage. 147:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Vilberg KL. 2013. Brain networks underlying episodic memory retrieval. Curr Opin Neurobiol. 23:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson P, Streissguth A, Barr H, Bookstein F. 1989. Neuro-behavioral effects of prenatal alcohol. 2. Partial least-squares analysis. Neurotoxicol Teratol. 11:477–491. [DOI] [PubMed] [Google Scholar]
- Samu D, Campbell KL, Tsvetanov KA, Shafto MA, Tyler LK, Cam-CAN consortium, Tyler LK. 2017. Preserved cognitive functions with age are determined by domain-dependent shifts in network responsivity. Nat Commun. 8:14743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X, Holmes AJ, Eickhoff SB, Thomas BT. 2018. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 28:3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt F, Murphy M, Sanders R. 1981. Training older adult free-recall rehearsal strategies. J Gerontol. 36:329–337. [DOI] [PubMed] [Google Scholar]
- Shipley WC, Gruber CP, Martin TA, Klein AM. 2009. Shipley-2 manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Shulman GL, Astafiev SV, McAvoy MP, Davossa G, Corbetta M. 2007. Right TPJ deactivation during visual search: functional significance and support for a filter hypothesis. Cereb Cortex. 17:2625–2633. [DOI] [PubMed] [Google Scholar]
- Simony E, Honey CJ, Chen J, Lositsky O, Yeshurun Y, Wiesel A, Hasson U. 2016. Dynamic reconfiguration of the default mode network during narrative comprehension. Nat Commun. 7:12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer T. 2017. The emergence of knowledge and how it supports the memory for novel related information. Cereb Cortex. 27:1906–1921. [DOI] [PubMed] [Google Scholar]
- Spreng RN, DuPre E, Selarka D, Garcia J, Gojkovic S, Mildner J, Luh W, Turner GR. 2014. Goal-congruent default network activity facilitates cognitive control. J Neurosci. 34:14108–14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Lockrow AW, DuPre E, Setton R, Spreng KAP, Turner GR. 2018. Semanticized autobiographical memory and the default - executive coupling hypothesis of aging. Neuropsychologia. 110:37–43. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Schacter DL. 2012. Default network modulation and large-scale network interactivity in healthy young and old adults. Cereb Cortex. 22:2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. 2013. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci. 25:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. 2010. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 53:303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth JP, Daniels KA, Solinger LA. 2011. What you know can hurt you: effects of age and prior knowledge on the accuracy of judgments of learning. Psychol Aging. 26:919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GR, Spreng RN. 2015. Prefrontal engagement and reduced default network suppression co-occur and are dynamically coupled in older adults: the default-executive coupling hypothesis of aging. J Cogn Neurosci. 27:2462–2476. [DOI] [PubMed] [Google Scholar]
- van Kesteren MTR, Ruiter DJ, Fernández G, Henson RN. 2012. How schema and novelty augment memory formation. Trends Neurosci. 35:211–219. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. 2008. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal AJ, Wang S, Rissman J. 2017. Episodic memory retrieval benefits from a less modular brain network organization. J Neurosci. 37:3523–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. 2012. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2:125–141. [DOI] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y. 2013. BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One. 8:e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zoeller L, Polimeni JR, et al. 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.