Abstract
What is the neural organization of the mental lexicon? Previous research suggests that partially distinct cortical networks are active during verb and noun processing, but what information do these networks represent? We used multivoxel pattern analysis (MVPA) to investigate whether these networks are sensitive to lexicosemantic distinctions among verbs and among nouns and, if so, whether they are more sensitive to distinctions among words in their preferred grammatical class. Participants heard 4 types of verbs (light emission, sound emission, hand-related actions, mouth-related actions) and 4 types of nouns (birds, mammals, manmade places, natural places). As previously shown, the left posterior middle temporal gyrus (LMTG+), and inferior frontal gyrus (LIFG) responded more to verbs, whereas the inferior parietal lobule (LIP), precuneus (LPC), and inferior temporal (LIT) cortex responded more to nouns. MVPA revealed a double-dissociation in lexicosemantic sensitivity: classification was more accurate among verbs than nouns in the LMTG+, and among nouns than verbs in the LIP, LPC, and LIT. However, classification was similar for verbs and nouns in the LIFG, and above chance for the nonpreferred category in all regions. These results suggest that the lexicosemantic information about verbs and nouns is represented in partially nonoverlapping networks.
Keywords: fMRI, lexicosemantic representations, multivoxel pattern analysis, nouns, verbs
Introduction
Words separate into classes based on the types of meanings they typically convey and the grammatical role they play in sentences. A key distinction that occurs across languages is the one between nouns and verbs (Sapir 2004). Nouns tend to refer to entities (e.g., “the swan,” “the barn”), whereas verbs tend to describe events situated in time and relations among entities (e.g., “to lick,” “to sparkle”), thus, reflecting the basic propositional acts of reference and predication (Sasse 1993; Bhat 2000; Peterson 2003; Croft 2005; Langacker 1987, 2008). Early neuropsychological studies have identified grammatical class as a candidate organizing principle of the mental lexicon and its neural basis: focal brain damage leads to a disproportionate impairments with verbs in a subset of patients, while others are more impaired in processing nouns (Goodglass et al. 1966; Luria and Tsvetkova 1967; McCarthy and Warrington 1985; Miceli et al. 1984, 1988; Zingeser and Berndt 1990; Caramazza and Hillis 1991; Damasio and Tranel 1993; Daniele et al. 1994; Hillis and Caramazza 1995; Shapiro et al. 2000; Collina et al. 2001; Luzzatti et al. 2002; Rapp and Caramazza 2002; Shapiro and Caramazza 2003a, 2003b; Laiacona and Caramazza 2004; Aggujaro et al. 2006). For example, when asked to describe a scene, some patients have difficulty naming the events depicted and fail to produce specific verbs for actions, either omitting the verbs altogether or using general verbs such as “be” or “do.” By contrast, patients with selective deficits for nouns have difficulty naming the objects in the scene and tend to use generic terms such as “things” and “stuff” (Mätzig et al. 2009).
Consistent with the dissociations documented in the neuropsychological literature, neuroimaging studies with healthy participants have identified cortical regions that are preferentially recruited during either verb or noun processing (for reviews, see Vigliocco et al. 2011; Crepaldi et al. 2013). Two regions that emerge across studies as specifically relevant to verb processing are the left middle temporal gyrus (LMTG+) and the left inferior frontal gyrus (LIFG). Both regions respond more to verbs than nouns, adjectives, and nonlinguistic stimuli, across a variety of tasks, including semantic similarity judgments, lexical decision, and synonym judgments (Martin et al. 1995; Fujimaki et al. 1999; Perani et al. 1999; Davis et al. 2004; Li et al. 2004; Kable et al. 2002, 2005; Tranel et al. 2005; Bedny and Thompson-Schill 2006; Thompson et al. 2007; Kemmerer et al. 2008; Liljeström et al. 2008; Tyler et al. 2003, 2008; Yu et al. 2011, 2012; Bedny, Caramazza, et al. 2008; Bedny, McGill, et al. 2008, 2011, 2014). Larger responses are observed not only for action verbs that involve motion (e.g., “to stroke” and “to give”), but also mental state verbs (e.g., “to think” and “to want”), changes of state verbs (e.g., “to rust”) and verbs of emission (e.g., “to glow”; Grossman et al. 2002; Davis et al. 2004, Kemmerer et al. 2008; Bedny, Caramazza, et al. 2008; Bedny, McGill, et al. 2008, 2011, 2014). Larger responses to verbs in the LMTG+ persist even when verbs and nouns are matched on imageability and are robust to changes in sensory experience, such as congenital blindness (Noppeney et al. 2003, Bedny and Thompson-Schill 2006, Bedny and Saxe 2012).
A separate set of cortical areas has been identified as preferentially responsive to nouns, including the left inferior parietal lobule (LIP) and angular gyrus, the left inferior temporal cortex (LIT), and the precuneus (PC) (Fujimaki et al. 1999; Li et al. 2004; Tyler et al. 2004; Bedny and Thompson-Schill 2006; Marangolo et al. 2006; Shapiro et al. 2005, 2006; Thompson et al. 2007; Berlingeri et al. 2008; Liljeström et al. 2008). Together these findings suggest that verbs and nouns are processed by partially nonoverlapping cortical networks.
The cognitive role of preferential responses to verbs and nouns remains an open question. One hypothesis is that some of the brain regions responding preferentially to one grammatical class over another are selectively involved in representing the lexicosemantic information associated with it. For example, one hypothesis is that the LMTG+ preferentially represents the lexicosemantics of verbs, whereas noun-responsive regions (e.g., LIP) are preferentially involved in representing the lexicosemantics of nouns. If so, we would expect such regions not only to show high levels of activity for their preferred grammatical class but also to show sensitivity to lexicosemantic distinctions within the preferred grammatical class.
In the case of the LMTG+, it has been hypothesized that it either represents the meanings of verbs, aspects of verb grammar that are closely related to verb meaning (e.g., argument structure), or both (Thompson et al. 2007; Hernández et al. 2014). Since verbs refer to the relations among entities, they differ amongst themselves according to the number and type of entities they relate (Jackendoff 1983; Tanenhaus et al. 1989; Frawley 1992). For instance, while a stroking event presupposes 2 entities (i.e., an agent doing the stroking and an object being stroked), sparkling only involves a sparkling object. This aspect of verb meaning is intimately intertwined with grammatical behavior: verbs that denote events involving 1 versus 2 entities also require 1 versus 2 arguments in the surface grammar, making “she stroked the cat” and “the jewel sparkled” well-formed, but “she stroked” and “the jewel sparkled the crown” not well formed (Chomsky 1981; Gleitman et al. 2005). Verbs also differ amongst themselves in more fine-grained ways. For example, emission verbs such as “glow” differ from each other according to the substance emitted (e.g., light “to glow” vs. sound “to clang”) as well the intensity of emission (e.g., “to blaze” vs. “to glow”, Faber and Usón 1999). Here, we investigate the hypothesis that the LMTG+ encodes the meanings of verbs and/or the grammatical information related to meaning (henceforth, lexicosemantic properties). If so, the LMTG+ should be sensitive to lexicosemantic distinctions among verbs.
Conversely, we would expect cortical areas that play a role in representing the meanings of nouns to distinguish among types of entities according to dimensions that are relevant to their meanings, such as their animacy (e.g., rock vs. bear) and, among inanimates, objects versus places (e.g., rock vs. desert), natural vs. manmade (e.g., desert vs. barn), and perhaps their physical characteristics such as size, shape, and color (Schachter and Shopen 1985; Frawley 1992; Smith and Mark 2003; Bromhead 2017; Si 2017).
An alternative possibility is that all or some of the cortical areas preferentially responding more to verbs than nouns, or vice versa, are not sensitive to lexicosemantic distinctions at all—or at least not more to distinctions within their preferred grammatical class. This could occur, for example, if responses to one grammatical class over the other reflect different processing demands imposed by verbs and nouns in a particular task. For instance, a region involved in morphological processing might respond more to verbs than nouns since, at least in English, verbs have a richer morphology (Tyler et al. 2001, 2003, 2004, 2008). Analogously, brain regions generally involved in retrieval of lexical information from long-term memory might respond more to verbs than nouns because verbs tend to have more variable or context-dependent meanings, making a single meaning more difficult to retrieve from memory (Gentner 1981; Tyler et al. 2001, 2004; Thompson-Schill et al. 1997, 1998, 2005). We might expect such a region to show larger responses to verbs on average but not to distinguish among lexicosemantic verb types more than among noun types.
The goal of the current study was to ask whether verb-responsive and noun-responsive cortical areas are preferentially involved in representing the lexicosemantic distinctions among members of their preferred grammatical class. To this end, we probed the informational content represented within verb- and noun-responsive cortical areas using multivoxel pattern analysis (MVPA). Specifically, we tested the prediction that verb-responsive cortical areas (i.e., the LMTG+) are more sensitive to lexicosemantic distinctions among verbs than nouns, whereas noun-responsive areas, including the inferior parietal cortex (IP), precuneus (PC), and inferior temporal cortex (IT), preferentially distinguish among nouns. As MVPA measures the spatial population code within a cortical area, this method is more sensitive than univariate analysis to the distinctions within broad stimulus categories (e.g., among verbs) and is thus specifically suited to address the current question (Haxby et al. 2001). Our approach here is analogous to the complementary use of univariate and MVPA analyses in the study of object recognition: while univariate methods identify regions within the ventral stream that are especially responsive to different stimulus categories (e.g., faces vs. places), MVPA shows that these regions contain neural population codes that distinguish among subtypes within these broad categories (e.g., forests vs. buildings within places, among faces of different identity; Walther et al. 2009, 2011; Anzellotti and Caramazza 2017; Kumar et al. 2017; Watson et al. 2014, 2017). Analogously, we hypothesized that verb- and noun-responsive regions would be more sensitive to differences among lexicosemantic subcategories of verbs and nouns, respectively.
Several prior studies have shown that the spatial patterns of activation within temporal, parietal, and prefrontal cortices, as measured by MVPA, are sensitive to lexicosemantic distinctions among words. Most studies thus far have focused on distinctions among entity nouns (Simanova et al. 2012; Fairhall and Caramazza 2013; Wang et al. 2013; Correia et al. 2014). For example, Fairhall and Caramazza (2013) showed that regions within the left temporal lobe, including the IP, PC, and IT, are sensitive to distinctions among nouns referring to fruit, tools, clothes, mammals, and birds (see also Simanova et al. 2012 and Kumar et al. 2017 for similar design with place nouns). As for verbs, one study found that in the angular gyrus (AG) the patterns of activation for 2-word phrases were more similar when these phrases shared the same verb (Boylan et al. 2015). Another recent study found that frontotemporal patterns of activation across action-verbs and object-nouns are correlated with semantic similarity structure, as measured by latent semantic analysis (Carota et al. 2017). However, whether cortical areas that preferentially respond to verbs as opposed to nouns, and vice versa, show sensitivity to lexicosemantic information and whether they do so differentially across grammatical classes has not yet been tested.
To answer this question, we first localized a set of brain regions that have previously been shown to respond more to verbs (LMTG+ and LIFG) and more to nouns (LIP, LPC, LIT) in each individual participant using a previously established localizer paradigm that compares univariate responses to verbs and nouns in a semantic similarity judgment task (Bedny, Caramazza, et al. 2008; Bedny, McGill, et al. 2008, 2014). Then, we tested whether the spatial patterns of activation within verb- and noun-responsive regions are more similar for verbs and nouns belonging to the same as opposed to different lexicosemantic categories—for instance, hand-action verbs (e.g., “to prod” and “to stroke”) should be more similar to each other than to light emission verbs (e.g., “to glow” and “to sparkle”).
Verb and noun stimuli were chosen from 2 broad lexicosemantic categories (verbs: actions vs. emission; nouns: animals vs. places), each including 2 narrower subcategories (action verbs: mouth actions, e.g., “to chew,” and hands actions, e.g., “to stroke;” emission verbs: sound, e.g., “to clang,” and light, e.g., “to sparkle;” animal nouns: birds, e.g., “the sparrow,” and mammals, e.g., “the fox;” place nouns: manmade, e.g., “the igloo” and natural, e.g., “the meadow”). A secondary question of interest was whether verb- and noun-responsive regions would be sensitive to broad lexicosemantic distinctions only (e.g., action vs. emission) or also to the narrow subcategories (e.g., hand vs. mouth actions). We might expect that cortical areas that are sensitive to lexicosemantic information should show more pronounced distinctions for the broader classes but should also distinguish between the fine-grained lexicosemantic categories.
Materials and Methods
Participants
Thirteen individuals participated in the study (9 women, age range 19–56, mean age = 34, SD = 10). All participants were native English speakers with no history of neurological conditions (screened through self-report). Informed consent was obtained in accordance with the Johns Hopkins Medicine Institutional Review Boards.
Stimuli
Word stimuli consisted of 144 words, 18 words in each of the following 8 lexicosemantic categories: light emission verbs (e.g., “to glow”—henceforth, light verbs for brevity), sound emission verbs (e.g., “to boom”—henceforth, sound verbs), hand-related action verbs (e.g., “to stroke”—henceforth, hand verbs), mouth-related action verbs (e.g., “to bite”—henceforth, mouth verbs), bird nouns (e.g., “the sparrow”), mammal nouns (e.g., “the deer”), manmade place nouns (e.g., “the dungeon”), and natural place nouns (e.g., “the creek”). All verb stimuli occurred in the infinitive and all noun stimuli were preceded by the article “the”, to mark grammatical category (see Supplementary Materials, Appendix 1 for a complete list of stimuli).
Words were matched across lexicosemantic categories in syllable length based on the CMU Pronouncing Dictionary (Weide 1998; one-way ANOVA with 8 lexicosemantic categories (bird, mammal, manmade pl., natural pl., hand, mouth, light, sound): F(7,136) = 0.38, P > 0.5) and on phonological neighborhood size using N-Watch (Davis 2005; one-way ANOVA: F(7,130) = 1.28, P > 0.1). Words were also matched in familiarity based on ratings collected using Amazon Mechanical Turk (AMT; repeated measures ANOVA: F(7,98) = 1.17, P > 0.1; see Supplementary Materials for details). Although familiarity and corpus frequency are often correlated, some words have low corpus frequency (especially in written corpora) while being highly familiar (Tanaka-Ishii and Terada 2011). While the current stimuli were matched on familiarity, they were not matched on frequency based on the Corpus of Contemporary American English (COCA—Davies 2009; one-way ANOVA: F(7, 136) = 3.92, P < 0.001). We choose to match our stimuli on judged familiarity rather than corpus frequency because familiarity has been shown to be a better psycholinguistic predictor of performance (Gernsbacher 1984; Kreuz 1987; Connine et al. 1990). Finally, based on AMT ratings (details in Supplementary Materials), words were also matched on concreteness (repeated measures ANOVA: F(7,98) = 0.91, P > 0.5) although, consistently with prior reports, nouns were more imageable than verbs (paired t-test(14) = −4.05, P < 0.005) and, among nouns, animals were more imageable than places (paired t-test t(14) = 2.91, P < 0.05). For a complete summary of word properties see Supplementary Table S1.
We quantified the overall semantic distance among nouns and among verbs by collecting semantic similarity judgments for all possible pairs within grammatical class on AMT (see Supplementary Materials and Supplementary Fig. S1 for details). Notably, verbs and nouns did not differ in their semantic distance within grammatical class (2-tailed t-test comparing verb- and noun-pairs similarity: t(9) = 0.34, P > 0.5). For the verb stimuli, we also quantified the similarity across verb subcategories according to semantic frames, syntactic frames and argument roles based on VerbNet (Schuler 2005; Kipper et al. 2006; see Supplementary Materials and Supplementary Fig. S2 for details).
Procedure
Participants heard pairs of words and judged how related in meaning they were on a scale from 1 “not at all similar” to 4 “very similar.” Words from each category were divided into 2 nonoverlapping sets (9 words per category per set) to be used separately in even/odd runs. Within each set, we generated all possible within category pairs (36 pairs per category per set). Word pairs were presented in blocks of 4. All blocks contained only words from a single lexicosemantic category and each word occurred only once per block (e.g., light verbs block: “to shine—to glisten,” “to flash—to gleam,” “to twinkle—to glare,” “to glimmer—to blaze”). Blocks were 16 s long, separated by 10 s of rest. Each trial within a block was 4 s long, including 2 words (0.9 s each), an interword interval (0.25 s) and the response period (1.95 s).
There was a total of 144 blocks (18 per word category) divided evenly into 8 runs. Blocks in even/odd runs contained stimuli exclusively from 1 of the 2 sets, ensuring that words were not repeated across even/odd runs (e.g., “to flash” occurred only in even runs). Word pairs, blocks and runs were presented in 1 of 2 pseudorandom orders, counterbalanced across participants. For every run, each word category was distributed evenly throughout the run to minimize position effects.
A female native English speaker recorded the word stimuli. The audio files were normalized to each other in volume with respect to root-mean square (RMS) amplitude and adjusted to have equal durations (0.9 s). The stimuli were presented over MRI-compatible earphones at the maximum comfortable volume for each participant. Participants indicated their responses by using an MRI-compatible button pad. Because data from the current participants were also used as a control group for a separate study with blind individuals, participants wore a light exclusion blindfold throughout the experiment.
fMRI Data Acquisition
MRI structural and functional data of the whole brain were collected on a 3 T Phillips scanner using a 32-channel head coil. T1-weighted 3D-MPRAGE structural images were collected using a pulse sequence in 170 sagittal slices with 1 mm isotropic voxels (TE/TR = 7.0/3.2ms, FoV = 240 × 240 mm2, 288 × 272 acquisition matrix, scan duration = 5:59′). Functional BOLD images were collected using parallel transverse ascending echo planar imaging (EPI) sequences in 36 axial slices with 2.5 × 2.5 × 2.5 mm3 voxels (TE/TR = 30/2000 ms, FoV = 192 × 172mm2, 76 × 66 acquisition matrix, 0.5 mm gap, flip angle = 70°, scan duration = 8:04′).
fMRI Data Analysis
Preprocessing
Data analyses were performed using FSL, Freesurfer, the Human Connectome Project workbench, and custom software (Dale et al., 1999; Smith et al. 2004; Glasser et al. 2013). Functional data were corrected for subject motion using FSL’s MCFLIRT algorithm (Jenkinson et al. 2002), high pass filtered to remove signal fluctuations at frequencies longer than 128 s/cycle, and then resampled to a cortical surface model and smoothed with a 2 mm FWHM Gaussian kernel to regularize the data on the cortical surface. All subsequent analyses were surface-based. Subject-specific cortical surface models were generated using the automated Freesurfer pipeline and visually inspected to assure accuracy.
Whole-Brain Univariate Analysis
The functional data were spatially smoothed on the cortical surface with a 6 mm FWHM Gaussian kernel and prewhitened to remove temporal autocorrelation. Each of the verb and noun categories were entered as a separate predictor in a general linear model (GLM) after convolving with a canonical hemodynamic response function. We also included the first temporal derivative as a covariate of no interest, to correct for small differences between the predicted and actual start of the hemodynamic response (Calhoun et al. 2004). Each run was modeled separately, and runs were combined within subject using a fixed-effects model. Group-level random-effects analyses were corrected for multiple comparisons at vertex level with P < 0.05 threshold false discovery rate (FDR) across the whole cortex (Genovese et al. 2002). Additionally, a nonparametric permutation test was used to cluster-correct at P < 0.01 family-wise error rate (FWER).
ROIs Definition
We defined individual subject functional ROIs to be used in the MVPA analysis. For each subject, we defined 2 verb-responsive ROIs and 4 noun-responsive ROIs (verb ROIs: LMTG extending into the superior temporal gyrus [STG] and into the most inferior aspect of the anterior parietal cortex [LMTG+], the LIFG; noun ROIs: left inferior parietal cortex [LIP], left inferior temporal cortex, both laterally [LlatIT] and medially [LmedIT], and left precuneus [PC]). These anatomical regions were chosen because they have been observed to respond preferentially to verbs or nouns in previous studies and showed verb/noun preferences in the univariate analysis in the current study (Crepaldi et al. 2013 for a review).
We employed a 2-step procedure to identify subject-specific functional ROIs. First, whole-brain results for the Verbs > Nouns contrast were used to identify group search spaces in the anatomical location of LMTG+, LIFG, LPC, LlatIT, and LmedIT. Next individual functional ROIs were defined for each subject by taking the top 300 active vertices for the Verbs > Nouns and Nouns > Verbs contrasts, for verb-responsive and noun-responsive ROIs, respectively. Note that although the focus of the current paper was on MVPA patterns within these ROIs, we also report the univariate signal for each verb/noun category in the Supplementary Materials (see also Supplementary Fig. S3).
MVPA ROI Analysis
MVPA was used to test whether verb- and noun-responsive regions are more sensitive to lexicosemantic differences among verbs and among nouns, respectively, using PyMVPA toolbox (Hanke et al. 2009). For each ROI in each participant, a linear support vector machine (SVM) classifier was used to separately decode among the 4 verb categories and among the 4 noun categories. In each vertex in each participant’s ROIs, we obtained one sample labeled by lexicosemantic category per block by averaging BOLD signal across time points over a block duration (16 s). Time points for each block were defined as block onset plus 4 s delay to account for the hemodynamic lag. Each vertex’s block sample was then normalized (z-scored) with respect to the mean signal for the vertex across the task blocks during a given run, such that the mean of each vertex across the run was set to 0 and standard deviation to 1. Normalization was applied to data from verbs and nouns separately, removing differences in mean signal across grammatical class. The classifier was then trained on half of the data (e.g., even runs) and tested on the other half (e.g., odd runs). Classification accuracy was then averaged across the 2 training/test splits. Importantly, individual verbs and nouns did not repeat across even and odd runs. Thus, the classifier was trained on one subset of words and tested on a different subset.
We compared classifier performance within each ROI to chance (25%) and across verbs and nouns. We also tested for an interaction between grammatical class (verbs vs. nouns) and ROI. Significance was evaluated against an empirically generated null distribution using a combined permutation and bootstrap approach (Schreiber and Krekelberg 2013; Stelzer et al. 2013). In this approach, t- and F-statistics obtained for the observed data are compared against an empirically generated null distribution. We report the t- and F-values obtained for the observed data and the nonparametric P-values, where P corresponds to the proportion of the shuffled analyses that generated a comparable or higher t/F value. Tests comparing 2 conditions (viz., accuracy for verbs and accuracy for nouns) used 2-tailed tests. One-tailed tests were used to test classifier’s accuracy against chance.
The null distribution was generated using a balanced block permutation test by shuffling the block labels within run 1000 times for each subject (Schreiber and Krekelberg 2013). Then, a bootstrapping procedure was used to generate an empirical null distribution for each statistical test across participants by sampling one permuted accuracy value from each participant’s null distribution 15 000 times (with replacement) and running each statistical test on these permuted samples, thus generating a null distribution of 15 000 statistical values for each test (Stelzer et al. 2013).
Confusion matrices were generated to describe how well the classifier performed on each pairwise distinction among verbs and nouns (e.g., manmade places vs. natural places). For any pair of categories or subcategories, the confusion matrix yields 2 measures of classification performance: the percentage of correctly classified trials, or hit rate (H), and the percentage of misclassified trials, or false alarm rate (F; Haxby et al. 2014). Classification and misclassification frequencies can be compared using a signal detection theory framework (Swets et al. 1961; Green and Swets 1966). Within each ROI, we computed A′, a nonparametric estimate of discriminability (; Pollack and Norman 1964; Grier 1971; Stanislaw and Todorov 1999), to assess the classifier’s ability to distinguish 1) between “major” lexicosemantic categories (i.e., animal vs. place nouns, action vs. emission verbs) and 2) between the lexicosemantic subcategories (i.e., birds vs. mammals, manmade vs. natural places; mouth vs. hand actions, sound vs. light emission verbs). An A′ of 0.5 corresponds to chance performance, while 1.0 indicates perfect discriminability. Paired student t-tests were used to compare A′ values to chance performance. Finally, to test whether the classifier was more likely to confuse words within than across major semantic categories, we used paired Student t-tests to compare the number of errors made by the classifier within a major subcategory (e.g., hand verbs mistaken for mouth verbs) and across a major subcategory (e.g., hand verbs mistaken for light or sound emission verbs).
Whole-Brain Searchlight MVPA Analysis
A whole-brain SVM classifier was used to decode independently among verbs and among nouns over the whole cortex using a 6 mm radius circular searchlight (according to geodesic distance, to better respect cortical anatomy over Euclidean distance; Glasser et al. 2013). This yielded for each participant 2 classification maps (1 for verbs and 1 for nouns) indicating the classifier’s accuracy in a neighborhood surrounding every vertex. Individual subject searchlight accuracy maps were averaged, and this group-wise map was thresholded using PyMVPA implementation of the 2-step cluster-thresholding procedure described in Stelzer et al. (2013) (Hanke et al. 2009). This procedure permutes block labels within participant to generate a null distribution within subject (100 times) and then samples from these (10 000) to generate a group-wise null distribution (as in the ROI analysis.) The whole-brain searchlight maps are then thresholded using a combination of vertex-wise threshold (P < 0.001 uncorrected) and cluster size threshold (FWER P < 0.05, corrected for multiple comparisons across the entire cortical surface).
Results
Behavioral Results
There was no difference in the semantic similarity of verbs and the semantic similarity of nouns as rated by the fMRI participants (verbs: mean = 2.08, SD = 0.55; nouns: mean = 2.03, SD = 0.47; paired t-test t(12) = 0.76, P > 0.1). Among verbs, light verbs were judged to be more similar than any other verb category (light mean = 2.74, SD = 0.6, sound mean = 1.8, SD = 0.29, hand mean = 1.86; SD = 0.31; mouth: mean = 1.9, SD = 0.26, repeated measures ANOVA F(3,36) = 43.15, P < 0.001). Among nouns, manmade places were judged to be less similar than the other categories (birds mean = 2.28, SD = 0.52, mammals mean = 2.19, SD = 0.44, manmade places mean = 1.6, SD = 0.13, natural places mean = 2.05, SD = 0.39, repeated measures ANOVA F(3,36) = 17.68, P < 0.001). These results closely resemble those obtained on AMT (see Supplementary Fig. S1). Participants’ reaction times (RTs) were not different for verbs and nouns (verbs mean = 1.57 s, SD = 0.17; nouns mean = 1.59 s, SD = 0.18; paired t-test t(12) = 1.45, P > 0.1). Among verbs, responses were slower for hand verbs than for other verbs (light mean = 1.57 s, SD = 0.21, sound mean = 1.58 s, SD = 0.16, hand mean = 1.61 s; SD = 0.17; mouth: mean = 1.52 s, SD = 0.16, repeated measures ANOVA F(3,36) = 3.69, P < 0.05). Among nouns, responses were slower for birds and natural places than for mammals and manmade places (birds mean = 1.60 s, SD = 0.22, mammals mean = 1.56 s, SD = 0.17, manmade places mean = 1.57 s, SD = 0.16, natural places mean = 1.64 s, SD = 0.19, repeated measures ANOVA F(3,36) = 3.61, P < 0.05).
To ascertain whether verb and noun categories could not be distinguished amongst themselves (i.e., among verbs and among nouns) based on differences in RTs, we trained 2 linear SVMs classifiers on the in-scanner RTs. One classifier was trained to distinguish among verbs, the other among of nouns, analogously to the classification of the fMRI data. The training/testing cross-validation split followed the even/odd run division of the fMRI design (i.e., training on word sets from odd runs and testing on word sets from even runs, and vice versa). Neither verb nor noun lexicosemantic categories could be classified based on in-scanner RTs (see Supplementary Materials for further details on the classification analyses). Thus, classification among verbs and among nouns based on the fMRI data is unlikely to be due to RTs differences.
fMRI Results
Verb and Noun Preferring Cortical Networks Identified in Univariate Mean Signal
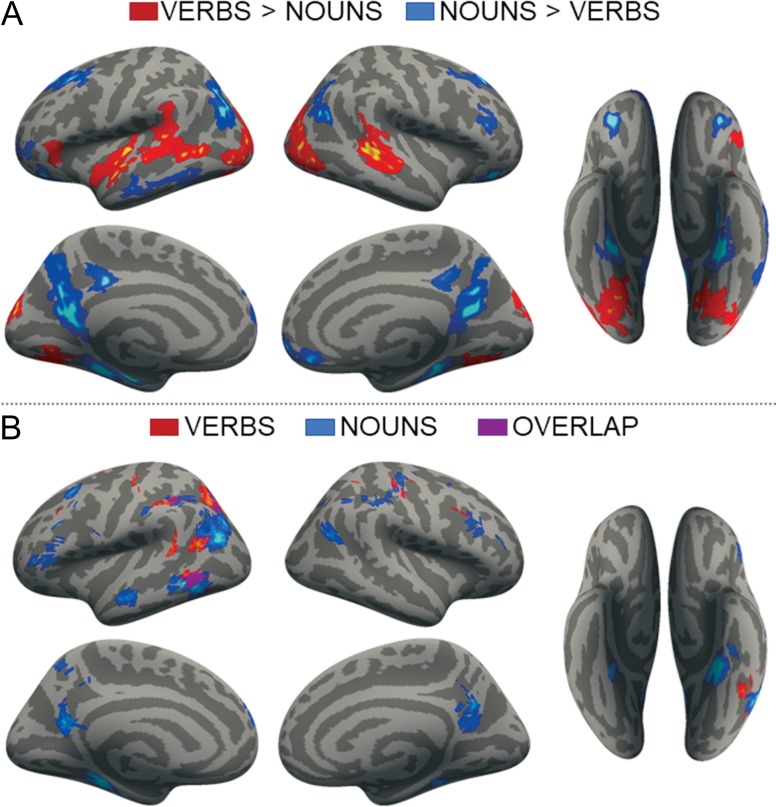
Group maps comparing the activation for verbs and nouns (P < 0.01 FWER) are shown in Figure 1A (see Supplementary Tables S2 and S3 for a complete list of activation peaks). Greater activation for verbs than for nouns was found bilaterally in the MTG+ and superior temporal sulcus (STS), extending in the left hemisphere into the STG, in the posterior aspect of the left IFG (pars triangularis) and bilaterally in occipital cortices (lateral, fusiform and lingual gyri). Activity was greater for nouns than verbs bilaterally in the IP and the PC/medial cingulate, in left medial inferior temporal cortex (LmedIT, parahippocampal and anterior-medial fusiform gyri), as well as lateral IT (LlatIT) and subregions of the superior and middle frontal gyri. Overall, responses to both nouns and verbs were larger in the left hemisphere.
Figure 1.
(A) whole-brain group maps, P < 0.01 FWER. Red: verbs, blue: nouns. (B) MVPA searchlight group maps, vertex-wise accuracy significance P < 0.001, cluster-thresholded FWER P < 0.05. Red: verbs, blue: nouns, purple: verbs/nouns overlap.
MVPA Distinctions Among Verb and Among Noun Types Within Verb- and Noun-Responsive ROIs
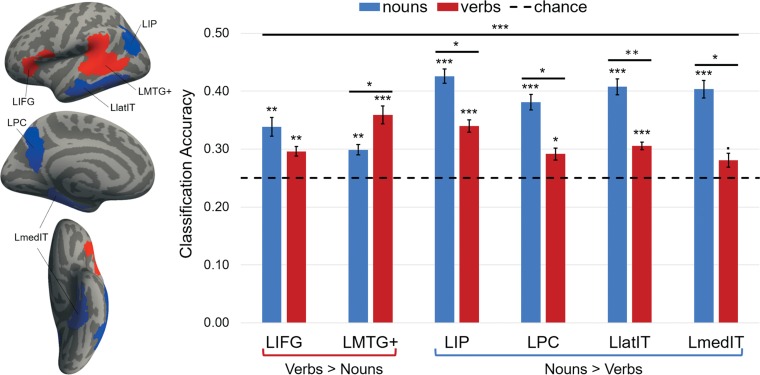
Classification was tested within 2 verb-responsive (LMTG+ and LIFG) and 4 noun-responsive (LIP, LPC, LmedIT, and LlatIT) ROIs within the left hemisphere. The classifier performance for both verbs and nouns was significantly above chance (25%) in all ROIs (all Ps < 0.05, Fig. 2; see Supplementary Table S4 for details). When classification was compared across ROIs (repeated measures ANOVA), we observed a grammatical class (verbs, nouns) by ROI (LMTG+, LIFG, LIP, LPC, LlatIT, LmedIT) interaction (F(5,60) = 7.06, permuted P < 0.0001), a main effect of ROI (F(5,60) = 3.29, permuted P < 0.05), and a main effect of grammatical class (F(1,12) = 6.03, permuted P < 0.05; Fig. 2). The LMTG+ was more sensitive to lexicosemantic distinctions among verbs than nouns (nouns vs. verbs paired t-test t(12) = −2.11, permuted P < 0.01), whereas all noun-responsive regions (LIP, LPC, LlatIT, and LmedIT) were more sensitive to lexicosemantic distinctions among nouns than verbs (nouns vs. verbs paired-t-tests, LIP t(12) = 2.67, permuted P < 0.05; LPC: t(12) = 2.65, permuted P < 0.05; LlatIT t(12) = 3.51, permuted P < 0.005; LmedIT: t(12) = 2.85, permuted P < 0.05). By contrast, verbs and nouns were equally decodable in the LIFG (t(12) = 1.12, permuted P > 0.1). When the 2 verb-responsive ROIs (LMTG+ and LIFG) were compared directly to each other, we observed a significant difference in the grammatical class effect across them (repeated measures ANOVA with grammatical class (verbs, nouns) by ROI (LMTG+, LIFG): interaction F(1,12) = 4.96, permuted P < 0.0005), but no main effects of either ROIs or grammatical class.
Figure 2.
Group search spaces used to define individual subject functional ROIs in the left hemisphere. Classifier accuracy in verb (LIFG and LMTG) and noun (LIP, LPC, LlatIT and LmedIT) selective regions. Chance: 25%. Signif. codes: 0 “***” 0.001 “**” 0.01 “*” 0.05 “.” 0.1 “” 1.
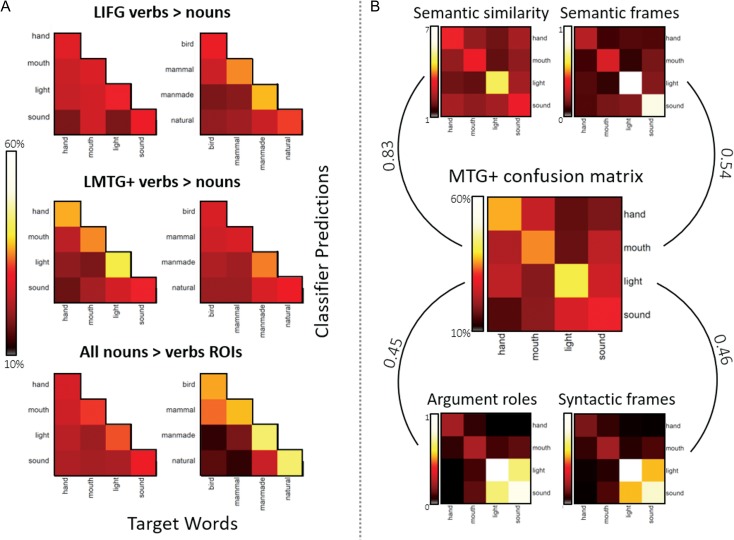
Then, we inspected each ROI’s confusion matrices to determine whether the classifier was able to discriminate only between major lexicosemantic categories or also between minor subcategories, as well as to determine whether errors were more likely within than across major lexicosemantic categories. Figure 3A shows the confusion matrices from the LMTG+, LIFG and the average of the noun-responsive ROIs with the classifier’s errors for mirroring misclassifications averaged across the diagonal (e.g., when the classifier confused hand verbs for mouth verbs, and mouth verbs for hand verbs; see Supplementary Fig. S4 for the complete confusion matrices in all ROIs). In the verb-responsive LMTG+, the classifier successfully discriminated between the major verb lexicosemantic categories of action and emission verbs (A′ = 0.67, t(12) = 4.46, P < 0.0001), as well as between the minor subcategories (mouth vs. hand actions A′ = 0.64, t(12) = 2.43, P < 0.05; sound vs. light emission A′ = 0.59, t(12) = 1.63, trending with P = 0.07). As for the errors, there was a trend for the classifier to more frequently improperly categorize verbs within a major lexicosemantic category (e.g., hand verbs mistaken for mouth verbs) than across major lexicosemantic categories (e.g., hand verbs mistaken for either light or sound emission verbs; number of errors: within mean = 4.56, SD = 1.57; across mean = 3.49, SD = 0.93; paired t-test t(12) = −1.86, P > 0.05).
Figure 3.
Confusion matrices for LMTG+, LIF and the average of the noun-responsive ROIs providing the percentage of predicted correct classifications (diagonals) and misclassifications (off diagonals). The confusion matrices show the classifier’s errors for mirroring misclassifications averaged across the diagonal.
In the verb-responsive LIFG, the only significant discrimination among verbs was between sound and light emission verbs (A′ = 0.66, t(12) = 3.5, P < 0.01). The classifier also successfully discriminated between the major, but not the minor, noun lexicosemantic categories in both verb-responsive regions (LMTG+: A′ = 0.61, t(12) = 2.45, P < 0.05; LIFG: A′ = 0.64, t(12) = 2.79, P < 0.005). Unlike in the LMTG+, in the LIFG the classifier was equally likely to make errors within and across major lexciosemantic categories (number of errors: within mean = 3.98, SD = 0.92; across mean = 4.35, SD = 0.6; paired t-test t(12) = 0.98, P > 0.1; repeated measures ANOVA with 2 ROI (LMTG+, IFG) by ErrorType (within, across): interaction F(1,12) = 5.03, P < 0.05).
In all the noun-responsive regions (LIP, LPC, LlatIT, and LmedIT), the classifier successfully discriminated between the major noun lexicosemantic categories of animals and places (LIP: A′ = 0.80, t(12) = 11.74, P < 0.0001; LPC: A′ = 0.72, t(12) = 4.2, P < 0.0001; LlatIT: A′ = 0.75, t(12) = 7.76, P < 0.0001; LmedIT: A′ = 0.82, t(12) = 15.06, P < 0.0001). Manmade and natural places were discriminated above chance in all but one noun-responsive ROI (LIP: A′ = 0.72, t(12) = 4.97, P < 0.0005; LPC: A′ = 0.68, t(12) = 3.51, P < 0.005; LlatIT: A′ = 0.71, t(12) = 4.8, P < 0.0005, LmedIT: A′ = 0.54; t(12) = 0.99, P > 0.1). By contrast, birds and mammals were not distinguishable in any ROI (LIP: A′ = 0.49, t(12) = −0.22, P > 0.5; LPC: A′ = 0.51, t(12) = 0.09, P > 0.1; LlatIT: A′ = 0.52, t(12) = 0.38, P > 0.1, LmedIT: A′ = 0.54; t(12) = 0.75, P > 0.1). Additionally, we checked whether the A′ results observed in the noun-responsive ROIs were consistent with the ones one would predict based on the noun semantic similarity judgments. We find that they are not (see Supplementary Materials and Supplementary Figs S5 and S6). As for the errors, in all the noun-responsive ROIs except for the LPC, the classifier more often miscategorized nouns within a major category (e.g., bird mistaken for mammal) than across major categories (e.g., bird mistaken for either manmade or natural places; all Ps < 0.01; see Supplementary Table S5 for details).
In 2 of the 4 noun-responsive regions, the classifier also discriminated between the major verb lexicosemantic categories (LIP: A′ = 0.62, t(12) = 4.08, P < 0.0005; trending in LPC: A′ = 0.55, t(12) = 1.52, P > 0.05; LlatIT: A′ = 0.59; t(12) = 3.1, P < 0.001, LmedIT: A′ = 0.55, t(12) = 1.25, P > 0.1). In the LIP, the classifier also discriminated successfully between mouth and hand verbs (A′ = 0.61; t(12) = 2.2, P < 0.05), and sound and light emission verbs (A′ = 0.62; t(12) = 1.81, P < 0.05). In the LPC, the classifier successfully distinguished between light and sound emission verbs (A′ = 0.63; t(12) = 1.94, P < 0.05). No other classifications were above chance.
Correlations Between LMTG+ Neural Confusion Matrix, Semantic Similarity Judgments and VerbNet Frames
We correlated the group average LMTG+ neural confusion matrix to the verb similarity matrices derived from VerbNet (syntactic frames, argument roles, and semantic frames) as well as to the semantic similarity matrix based on the AMT ratings (Fig. 3b). As the confusion matrix is not symmetric, to correlate it with the similarity matrices we used the average of the classifier’s errors for mirroring misclassifications.
There was a high correspondence between the LMTG+ neural confusion matrix and the verb semantic similarity matrix (Pearson’s r2 = 0.83, P < 0.0005). Correspondence with VerbNet frames was also high (VerbNet semantic frames: r2 = 0.54, P < 0.05; VerbNet syntactic frames: r2 = 0.46, P < 0.05; VerbNet argument roles: r2 = 0.45, P < 0.05; unpaired t-tests comparing Fisher-Z transformed r values: all Ps > 0.1). Since the semantic and grammatical matrices are highly correlated to each other (semantic similarity – semantic frames: r2 = 0.78 P < 0.001; semantic similarity – syntactic frames: r2 = 0.61 P < 0.005; semantic similarity – argument roles: r2 = 0.55 P < 0.05; semantic frames – syntactic frame: r2 = 0.85 P < 0.0005; semantic frames – argument roles: r2 = 0.78 P < 0.001; syntactic frame – argument roles: r2 = 0.98 P < 0.0001), it was impossible to statistically separate their effects.
MVPA Searchlight Results
Classification among verbs was significantly better than chance in the posterior LMTG+, although the searchlight classification peak was somewhat posterior and superior to the Verbs > Nouns peak observed in the univariate analysis (Fig. 1B). Verb classification was also above chance in LlatIT, as well as the posterior and superior LIP (peak in the interparietal sulcus). Classification among nouns was significantly better than chance in LIP (peak near the angular gyrus), LmedIT and LlatIT (an anterior and a posterior region), bilaterally in the PC, as well as in the LIFG. Consistent with the univariate analysis and the ROI results, the verb- and noun-responsive networks showed some overlap, but each had a distinctive neuroanatomical distribution.
Discussion
Consistent with the idea that verb- and noun-responsive regions are involved in representing lexicosemantic information, we find that the spatial patterns of activity within these areas are sensitive to lexicosemantic distinctions among nouns and verbs. Furthermore, we observe a double dissociation in the sensitivity of spatial patterns of activation to different lexical types across verb (LMTG+) and noun-responsive (LIP, LPC, LlatIT, and LmedIT) regions. These findings are consistent with the hypothesis that the lexicosemantic properties of verbs and nouns are represented in partially nonoverlapping neural systems.
The LMTG+ but not the LIFG is Preferentially Sensitive to the Lexicosemantics of Verbs
Consistent with prior evidence, we found that a region within the LMTG+ and a region within the LIFG respond more to verbs than nouns (Martin et al. 1995; Fujimaki et al. 1999; Perani et al. 1999; Davis et al. 2004; Li et al. 2004; Kable et al. 2002, 2005; Tranel et al. 2005; Bedny and Thompson-Schill 2006; Thompson et al. 2007; Liljeström et al. 2008; Tyler et al. 2003, 2008; Yu et al. 2011, 2012; Bedny, Caramazza, et al. 2008; Bedny, McGill, et al. 2008, 2011, 2014). However, only the verb-responsive LMTG+ was more sensitive to lexicosemantic distinctions among verbs than among nouns. Moreover, the neural confusion matrix of the LMTG+ was correlated with semantic similarity of verbs as measured by participant ratings outside the scanner and with VerbNet lexicosemantic and grammatical similarity.
This result is consistent with previous findings suggesting that the LIFG is not preferentially involved in representing lexicosemantic information associated with verbs (Cappelletti et al. 2008; Shapiro et al. 2012). Instead, LIFG may support general cognitive control functions that are particularly relevant to verb retrieval, such as selection among multiple alternatives (Thompson-Schill et al. 2005; Hoffman et al. 2015). Verbs tend to be more semantically malleable and influenced by the sentence context than object nouns, potentially increasing the demand for selection among possible alternative meanings, and the LIFG is sensitive to this property of words, that is, their semantic ambiguity (Gentner 1981; Thompson-Schill et al. 1997, 1999; Rodd et al. 2005; Bedny et al. 2007; Bedny, Caramazza, et al. 2008; Bedny, McGill, et al. 2008; Grindrod et al. 2008). Alternatively, the LIFG might contribute to morphosyntactic processing of verbs, since in English verbs have a richer morphology than nouns (Tyler et al. 2001, 2004). It is also possible that verb-responsive LIFG is involved in integrating verbs into a sentence frame. Since in the current study verbs and nouns were presented in isolation, greater differences between verbs and nouns might emerge when verbs and nouns are processed with a sentence context (Snijders et al. 2008; Zhu et al. 2012; Hagoort 2013).
By contrast, previous studies suggest that LMTG+ responses are not driven purely by grammatical factors. In particular, the LMTG+ responds not only to verbs but also to nouns that are similar in meaning to verbs, namely event nouns (e.g., “the hurricane,” “the rodeo”) (Bedny et al. 2014). The grammatical behavior of event nouns within sentences and their morphosyntactic properties are akin to those of object nouns (“the concert” but not “concerting”). Semantically, however, event nouns resemble verbs in that their referents are situated in time, in addition to space (e.g., “during the concert”) (Langacker 1987, 2008). The LMTG+ response to event nouns is intermediate between its response to object nouns and verbs (Collina et al. 2001; Tabossi et al. 2010; Garbin et al. 2012; Bedny et al. 2014; Lapinskaya et al. 2016). Some previous studies also suggest that the LMTG+ responds more to tool nouns than nouns referring to animals, possibly due to their association with actions (Hauk et al. 2008; Desai et al. 2009; Pillon and d’Honincthun 2011; Vannuscorps and Pillon 2011; Fernandino et al. 2015). However, responses to tools tend to be more posterior and inferior to those observed for verbs (Bedny and Caramazza 2011; Perini et al. 2014). These findings raise the possibility that the LMTG+ stores representations of lexicalized events and actions, including verbs and event nouns (Martin et al., 1995, Kable et al., 2002, Bedny, Caramazza, et al. 2008; Bedny, McGill, et al. 2008).
A nonmutually exclusive possibility is that the LMTG+ represents aspects of verb grammar that are intimately related to meaning, namely, the type and number of grammatical arguments that a verb takes when used in a sentence. As noted in the introduction, verbs denote events with specific numbers and types of participants (e.g., “give” entails a giver and a receiver) and tend to have a corresponding number of grammatical arguments. Children and adults are sensitive to this syntactic-semantic correspondence during verb acquisition and during comprehension (Gillette et al. 1999; Trueswell et al. 1999). Nevertheless, while argument structure is systematically related to verb meaning, it is a grammatical property distinct from it. Verbs with similar meanings can take different numbers of arguments (e.g., “to eat” vs. “to devour”; Chomsky 1981; Gleitman 1990; Jackendoff 1992; Levin and Hovav 1995; Pinker 1989; Tanenhaus et al. 1989, inter alia). LMTG+ could be sensitive to this aspect of verb grammar.
Consistent with this possibility, previous studies suggest that the LMTG+ is sensitive to argument number (i.e., transitivity) and responds more to 2-argument than one-argument verbs (Thompson et al. 2007; Hernández et al. 2014). In the current study, some of the verb types decoded in the LMTG+ differ in number of arguments: the action verbs were for the most part divalent, whereas the emission verbs were all monovalent. For example, the verb “to lick” requires 2 arguments, the agent doing the licking and the object being licked (e.g., “the dog licked the bone”), while emission verbs such as “to sparkle” only require one (e.g., “the diamond sparkled”) (Levin 1993; Levin and Hovav 1995). However, patterns of activity in the LMTG+ also distinguished among verb types that take the same number of arguments, that is, emission verbs, which differ in the substance being emitted (light vs. sound, e.g., “to glow” vs. “to boom”). The present results are therefore consistent with the idea that the LMTG+ represents either grammatical information related to verb meaning, semantic information associated with verbs or both of these types of information.
Many further questions remain open regarding LMTG+’s contribution to lexicosemantic representations and processing. In future work, it will be important to uncover the specific lexicosemantic dimensions that are represented in the LMTG+. Candidate dimensions include whether the verb refers to a state or an event (e.g., “to contain” vs. “to boil”), whether it describes an event that has a definite ending (i.e., telicity, e.g., “to run” vs. “to build”) or presupposes an event to be true (i.e., factivity, e.g., “she knew that John had left” vs. “she assumed that John had left”), the agentivity of the verb’s subject (animate/inanimate, e.g., “to run” vs. “to sparkle”), the path of the denoted event (e.g., “enter” vs. “exit”) or its manner (e.g., “to roll”, “to bounce”; Levin and Hovav 1995). Others have suggested that the LMTG+ represents sensory-motor dimensions such as the presence of visual motion and action-relatedness (Bird et al. 2000; Kable et al. 2002; Vigliocco et al. 2006; Hauk et al. 2008; Desai et al. 2009; Fernandino et al. 2015). Future studies could use MVPA to uncover which of these dimensions are explicitly coded by neural populations within the LMTG+ region. A further open question concerns the degree to which verb-responsive LMTG+ is language specific. Previous studies suggest that recognition and categorization of action images and videos depends on distinct right-lateralized STS and MTG regions (Vander Wyk et al. 2009; Pelphrey et al. 2004, 2005; Dravida et al. 2013; Kable et al. 2002). However, studies with matched verbal and nonverbal stimuli are needed to resolve this question. Finally, although the LMTG+ appears to contribute disproportionately to representations of verbs and event nouns as opposed to object nouns or concrete adjectives, its role in the representation of other semantic content (e.g., abstract non-event nouns such as “idea”) remains to be tested (Binder et al. 2005; Papagno et al. 2009; Wang et al. 2010; Hoffman et al. 2015).
Preferential Encoding of Object Nouns in LIP, LPC, and LIT
The present results suggest that a network of cortical areas, including the LIP, LPC, and LIT, contains spatial population codes that distinguish preferentially among entity nouns than among verbs. These cortical areas are similar to those identified by previous work as responding to nouns and being sensitive to distinctions among them. For example, Kumar et al. (2017) showed that patterns of activity in IP distinguish among different types of places (beaches, cities, highways, and mountains). Analogously, Fairhall and Caramazza (2013) found that a portion of lateral IT extending into MTG+ and the PC are sensitive to differences among mammals, birds, fruits, tools, and clothes. Interestingly, prior research suggests that activity in these noun-responsive areas is sensitive to object category not only when participants are presented with words, but also when they are presented with pictures of objects (Simanova et al. 2012; Devereux et al. 2013; Fairhall and Caramazza 2013; Kumar et al. 2017). Furthermore, classifiers trained on patterns of activity produced by object nouns successfully decode among images of the same objects, and vice versa (Simanova et al. 2012; Devereux et al. 2013; Fairhall and Caramazza 2013; Kumar et al. 2017). Together with this prior evidence, the present results are consistent with the hypothesis that this network contributes to representing the semantics of entities (Fujimaki et al. 1999; Li et al. 2004; Tyler et al. 2004; Bedny and Thompson-Schill 2006; Marangolo et al. 2006; Shapiro et al. 2005, 2006; Thompson et al. 2007; Berlingeri et al. 2008; Liljeström et al. 2008).
A potentially interesting pattern in the current data is that all entity-responsive regions showed less sensitivity to distinctions between mammals and birds than between manmade and natural places, as well as between places and animals. We successfully decoded animals from places, and manmade from natural places. By contrast, the seemingly salient distinction between mammals and birds did not lead to distinguishable patterns of activity in these regions, even though subjects judged birds to be more similar to other birds than to mammals and mammals to be more similar to other mammals than to birds to the same extent that they distinguished among places (see Supplementary Figs S5 and S6 and Supplementary Materials for details). These results are consistent with the idea that something other than overall semantic similarity is driving better performance for places than animals. Similarly, previous studies have found that patterns of activity within PC and IP distinguish between animals and inanimate objects (e.g., tools, places), as well as between manmade and natural places (Akama et al. 2012; Simanova et al. 2012; Devereux et al. 2013; Fairhall and Caramazza 2013; Correia et al. 2014; Kumar et al. 2017). We are, however, not aware of any study showing classification among animal types in the entity-responsive cortical areas or elsewhere when subjects are presented with verbal labels.
This could occur for several different reasons. The entity-responsive regions identified in the current study may be specialized for representing particular subtypes of entities. For instance, the medial IT region (LmeIT) is in a similar neuroanatomical location as the so called “parahippocampal place area” (PPA). The PPA was originally found to be responsive to images of scenes and thought to represent their spatial layout but has subsequently been shown to respond also to haptically presented Lego scenes and words referring to places (Epstein and Kanwisher 1998; Wolbers e al. 2011; Kumar et al. 2017). In the current study, LmedIT responded more to names of places than names of animals even in the univariate analysis and, like the other noun-responsive areas, patterns of activity within the medial IT distinguished among places but not among animals. Notably, while the “visual” scene responsive PPA is typically right lateralized (Epstein and Kanwisher 1998), in the current study the strongest decoding pattern was in the left hemisphere. Thus, an interesting possibility is that the left medial IT is particularly responsive to scene categories when accessed through verbal stimuli.
In contrast to the medial IT, other regions identified in the current study, such as the PC, did not respond more to names of places than names of animals in the univariate signal. Although the current study only included animal and place nouns, previous studies have found that the PC as well as the IP and the lateral IT respond to other noun types (e.g., names of objects; Fujimaki et al. 1999; Li et al. 2004; Tyler et al. 2004; Bedny and Thompson-Schill 2006; Marangolo et al. 2006; Shapiro et al. 2005, 2006; Thompson et al. 2007; Berlingeri et al. 2008; Liljeström et al. 2008). Furthermore, previous research has shown that activity patterns within these areas distinguish among noun categories other than places (e.g., animals vs. tools; Simanova et al. 2012, Fairhall and Caramazza 2013). The available evidence thus suggests that some of the identified noun-responsive cortical areas are involved in representing entities more generally.
As for the verb-responsive cortical systems, a key goal for future research is to uncover the distinctions among entities that are coded in the noun-responsive network. One hypothesis is that the neural code within entity-responsive IP and PC reflects more robustly those semantic distinctions that are cognitively primary: animate versus inanimate (e.g., animals vs. places) and artifacts versus natural kinds (e.g., manmade places vs. natural places; Gelman et al. 1983; Gelman and Markman 1986, 1987; Gelman 1988; Mandler et al. 1991; Bloom 1996; Gelman and Bloom 2000). Children distinguish animate and inanimate entities, as well as artifacts from natural kinds, early in development and many languages mark animacy grammatically, distinguishing between entities that can and cannot serve as agents (Gelman et al. 1983; Mandler et al. 1991; Frawley 1992; Bloom 1996; Medin et al. 2000; Diesendruck et al. 2003; Bromhead 2017; Kemmerer 2017). Only animate entities engage in volitional behavior and have mental states, whereas inanimate entities are characterized by their functions, shape, and compositional material (Frawley 1992).
By contrast, the distinction between birds and mammals is arguably less conceptually fundamental, although easily made. Indeed, children and adults often reason about living things based on knowledge of broad biological properties that are common to the animal class, such as breathes, eats, is born, sleeps, and dies (Carey 1985). It has also been suggested that Western adults living in urban settings have sparse knowledge about specific animal kinds (Medin and Atran 1999; Atran and Medin 2008; Medin and Bang 2014). Animals are often distinguished by their perceptual characteristics (e.g., size, shape, color) (Warrington and McCarthy 1983; Warrington and Shallice 1984; Farah and McClelland 1991). When making fine-grained distinctions among animals (e.g., elk vs. rhino), participants may rely on physical appearance information (e.g., size, shape, color), which is believed to be represented in ventral occipitotemporal cortices, rather than the IP/PC entity-responsive regions studied here (Thompson-Schill et al. 1999; Oliver and Thompson-Schill 2003; Connolly et al. 2012, 2016). Consistent with the possibility that appearance-related information is used to distinguish among animals, some studies also suggest larger differences between blind and sighted individuals’ representations for living as opposed to nonliving categories (Bi et al. 2016).
In future work it will be important to unconfound dimensions such as animacy from perceptual characteristics, such as size, to determine whether the IP/PC entity-responsive areas represent one or both types of information.
Distributed Representation of Verbs and Nouns Within the Lexical–Semantic Network
While we find that verb- and noun-responsive regions have a bias toward representing lexicosemantic information related to their preferred word class, decoding was also successful for the nonpreferred categories in every cortical area tested. Analogous sensitivity to the nonpreferred stimulus class has been observed in the visual object recognition literature. Images of objects from different classes (i.e., places, faces, and bodies) can be distinguished from each other based on patterns of activity outside the traditional areas that preferentially respond to those categories (Haxby et al. 2001; Spiridon and Kanwisher 2002; Kanwisher and Yovel 2006). What does this sensitivity to the nonpreferred lexical class reflect?
One possibility is that noun-related information is automatically retrieved during verb processing and vice versa. For example, retrieving a verb like “lick” may partially activate likely agents (e.g., dog) and objects (e.g., bone). Analogously, names of artifacts such as “the garage” could prime actions that typically occur in garages (e.g., parking) (Leshinskaya and Caramazza 2015). As noted above, the LMTG+ region does respond to some nouns, specifically ones that refer to events (Collina et al. 2001; Tabossi et al. 2010; Garbin et al. 2012; Bedny et al. 2014; Lapinskaya et al. 2016). Some studies have also suggested common neural responses to verbs and tools in the lateral temporal cortex, although others find that verbs and tools recruit neighboring but nonoverlapping regions (Bedny and Caramazza 2011; Vannuscorps and Pillon 2011; Perini et al. 2014; Pillon and d’Honincthun 2011). Verb- and noun-responsive areas could be involved in representing a type of lexicosemantic information that is particularly relevant for one grammatical class but is also relevant, to some degree, for the nonpreferred class. For example, the LMTG+ might respond to action verbs and also to some degree to names of tools because of both categories’ action-related semantic information (Hauk et al. 2008; Desai et al. 2009; Fernandino et al. 2015).
A further open question is whether and how activity in nonpreferred cortical areas is functionally relevant to behavior. As noted in the introduction, neuropsychological evidence shows that verbs and nouns can dissociate in the context of brain damage (Goodglass et al. 1966; Luria and Tsvetkova 1967). This observation suggests that some neural populations are more behaviorally relevant for one grammatical class over another. Supporting evidence for this hypothesis also comes from studies with transcranial magnetic stimulation (TMS) (Papeo et al. 2014). Disruption of activity in the verb-responsive posterior LMTG+ region with TMS interferes with participants’ performance on a synonym-judgment task with verbs, but not nouns (Papeo et al. 2014). This observation suggests that despite being sensitive to lexicosemantic distinctions among verbs and among nouns, verb-responsive LMTG+ is more behaviorally relevant for verb comprehension.
Conclusions
We observed a double dissociation in the neural representation of verbs’ and nouns’ lexicosemantic information. An LMTG+ region that responds more to verbs than nouns is more sensitive to lexicosemantic distinctions among verbs than among nouns. By contrast, several parietal and inferior temporal areas (LPC, LIP, and LIT) are more active during noun processing and more sensitive to lexicosemantic distinctions among nouns. However, all cortical areas tested were sensitive to the lexicosemantic distinctions of their preferred grammatical class as well as, to a lesser extent, their nonpreferred one. These results suggest that verb and noun lexicosemantic properties are represented in partially nonoverlapping neural networks.
Supplementary Material
Notes
We thank the F.M. Kirby Research Center for Functional Brain Imaging at the Kennedy Krieger Institute for their assistance with data collection; and the participants for making this research possible. Conflict of Interest: None declared. This work was supported by the National Institutes of Health (R01 EY027352 to M.B.) and the Johns Hopkins University Catalyst Grant (to M.B.).
References
- Aggujaro S, Crepaldi D, Pistarini C, Taricco M, Luzzatti C. 2006. Neuro-anatomical correlates of impaired retrieval of verbs and nouns: interaction of grammatical class, imageability and actionality. J Neurolinguistics. 19(3):175–194. [Google Scholar]
- Akama H, Murphy B, Na L, Shimizu Y, Poesio M. 2012. Decoding semantics across fMRI sessions with different stimulus modalities: a practical MVPA study. Front Neuroinform. 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzellotti S, Caramazza A. 2017. Multimodal representations of person identity individuated with fMRI. Cortex. 89:85–97. [DOI] [PubMed] [Google Scholar]
- Atran S, Medin DL. 2008. The native mind and the cultural construction of nature. Cambridge (Mass.): MIT Press. [Google Scholar]
- Azuma T. 1996. Familiarity and relatedness of word meanings: ratings for 110 homographs. Behav Res Methods Instrum Comput. 28(1):109–124. [Google Scholar]
- Bedny M, Caramazza A. 2011. Perception, action, and word meanings in the human brain: the case from action verbs. Ann N Y Acad Sci. 1224(1):81–95. [DOI] [PubMed] [Google Scholar]
- Bedny M, Caramazza A, Grossman E, Pascual-Leone A, Saxe R. 2008. Concepts are more than percepts: the case of action verbs. J Neurosci. 28(44):11347–11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M, Caramazza A, Pascual-Leone A, Saxe R. 2011. Typical neural representations of action verbs develop without vision. Cereb Cortex. 22(2):286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M, Dravida S, Saxe R. 2014. Shindigs, brunches, and rodeos: the neural basis of event words. Cogn Affect Behav Neurosci. 14(3):891–901. [DOI] [PubMed] [Google Scholar]
- Bedny M, Hulbert JC, Thompson-Schill SL. 2007. Understanding words in context: the role of broca’s area in word comprehension. Brain Res. 1146:101–114. [DOI] [PubMed] [Google Scholar]
- Bedny M, McGill M, Thompson-Schill SL. 2008. Semantic adaptation and competition during word comprehension. Cereb Cortex. 18(11):2574–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedny M, Saxe R. 2012. Insights into the origins of knowledge from the cognitive neuroscience of blindness. Cogn Neuropsychol. 29(1–2):56–84. [DOI] [PubMed] [Google Scholar]
- Bedny M, Thompson-Schill SL. 2006. Neuroanatomically separable effects of imageability and grammatical class during single-word comprehension. Brain Lang. 98(2):127–139. [DOI] [PubMed] [Google Scholar]
- Berlingeri M, Crepaldi D, Roberti R, Scialfa G, Luzzatti C, Paulesu E. 2008. Nouns and verbs in the brain: grammatical class and task specific effects as revealed by fMRI. Cogn Neuropsychol. 25(4):528–558. [DOI] [PubMed] [Google Scholar]
- Bhat DNS. 2000. Word classes and sentential functions. Empirical Approaches to Language Typology: pp. 47–64.
- Bi Y, Wang X, Caramazza A. 2016. Object domain and modality in the ventral visual pathway. Trends Cogn Sci. 20(4):282–290. [DOI] [PubMed] [Google Scholar]
- Binder JR, Westbury CF, McKiernan KA, Possing ET, Medler DA. 2005. Distinct brain systems for processing concrete and abstract concepts. J Cogn Neurosci. 17(6):905–917. [DOI] [PubMed] [Google Scholar]
- Bird H, Howard D, Franklin S. 2000. Why is a verb like an inanimate object? Grammatical category and semantic category deficits. Brain Lang. 72(3):246–309. [DOI] [PubMed] [Google Scholar]
- Bloom P. 1996. Intention, history, and artifact concepts. Cognition. 60(1):1–29. [DOI] [PubMed] [Google Scholar]
- Boylan C, Trueswell JC, Thompson-Schill SL. 2015. Compositionality and the angular gyrus: a multi-voxel similarity analysis of the semantic composition of nouns and verbs. Neuropsychologia. 78:130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromhead H. 2017. The semantics of standing-water places in English, French, and Pitjantjatjara/Yankunytjatjara In: Ye Z, editor. The semantics of nouns. New York, NY: Oxford University Press; p. 221. [Google Scholar]
- Brysbaert M, Warriner AB, Kuperman V. 2014. Concreteness ratings for 40 thousand generally known English word lemmas. Behav Res Methods. 46(3):904–911. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Stevens M, Pearlson GD, Kiehl K. 2004. fMRI analysis with the general linear model: removal of latency-induced amplitude bias by incorporation of hemodynamic derivative terms. Neuroimage. 22(1):252–257. [DOI] [PubMed] [Google Scholar]
- Cappelletti M, Fregni F, Shapiro K, Pascual-Leone A, Caramazza A. 2008. Processing nouns and verbs in the left frontal cortex: a transcranial magnetic stimulation study. J Cogn Neurosci. 20(4):707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A, Hillis AE. 1991. Lexical organization of nouns and verbs in the brain. Nature. 349(6312):788–790. [DOI] [PubMed] [Google Scholar]
- Carey S. 1985. Conceptual change in childhood. Cambridge, Mass.: MIT Press. [Google Scholar]
- Carota F, Kriegeskorte N, Nili H, Pulvermüller F. 2017. Representational similarity mapping of distributional semantics in left inferior frontal, middle temporal, and motor cortex. Cereb Cortex. 27(1):294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomsky N. 1981. Lectures on government and binding.
- Collina S, Marangolo P, Tabossi P. 2001. The role of argument structure in the production of nouns and verbs. Neuropsychologia. 39(11):1125–1137. [DOI] [PubMed] [Google Scholar]
- Connine CM, Mullennix J, Shernoff E, Yelen J. 1990. Word familiarity and frequency in visual and auditory word recognition. J Exp Psychol Learn Mem Cogn. 16(6):1084. [DOI] [PubMed] [Google Scholar]
- Connolly AC, Guntupalli JS, Gors J, Hanke M, Halchenko YO, Wu YC, Abdi H, Haxby JV. 2012. The representation of biological classes in the human brain. J Neurosci. 32(8):2608–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly AC, Sha L, Guntupalli JS, Oosterhof N, Halchenko YO, Nastase SA, di Oleggio Castello MV, Abdi H, Jobst BC, Gobbini MI, et al. 2016. How the human brain represents perceived dangerousness or “predacity” of animals. J Neurosci. 36(19):5373–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia J, Formisano E, Valente G, Hausfeld L, Jansma B, Bonte M. 2014. Brain-based translation: FMRI decoding of spoken words in bilinguals reveals language-independent semantic representations in anterior temporal lobe. J Neurosci. 34(1):332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepaldi D, Berlingeri M, Cattinelli I, Borghese NA, Luzzatti C, Paulesu E. 2013. Clustering the lexicon in the brain: a meta-analysis of the neurofunctional evidence on noun and verb processing. Front Hum Neurosci. 7:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft W. 2005. Word classes, parts of speech, and syntactic argumentation. Linguis Typol. 9(3):431–441. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 9(2):179–194. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D. 1993. Nouns and verbs are retrieved with differently distributed neural systems. Proc Natl Acad Sci USA. 90(11):4957–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele A, Giustolisi L, Silveri MC, Colosimo C, Gainotti G. 1994. Evidence for a possible neuroanatomical basis for lexical processing of nouns and verbs. Neuropsychologia. 32(11):1325–1341. [DOI] [PubMed] [Google Scholar]
- Davies M. 2009. The 385 million word corpus of contemporary American English (1990–2008): design, architecture, and linguistic insights. Int J Corpus Linguis. 14(2):159–190. [Google Scholar]
- Davis CJ. 2005. N-watch: a program for deriving neighborhood size and other psycholinguistic statistics. Behav Res Methods. 37(1):65–70. [DOI] [PubMed] [Google Scholar]
- Davis MH, Meunier F, Marslen-Wilson WD. 2004. Neural responses to morphological, syntactic, and semantic properties of single words: an fMRI study. Brain Lang. 89(3):439–449. [DOI] [PubMed] [Google Scholar]
- Desai RH, Binder JR, Conant LL, Seidenberg MS. 2009. Activation of sensory–motor areas in sentence comprehension. Cereb Cortex. 20(2):468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux BJ, Clarke A, Marouchos A, Tyler LK. 2013. Representational similarity analysis reveals commonalities and differences in the semantic processing of words and objects. J Neurosci. 33(48):18906–18916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesendruck G, Hammer R, Catz O. 2003. Mapping the similarity space of children and adults’ artifact categories. Cogn Dev. 18(2):217–231. [Google Scholar]
- Dravida S., Saxe R., & Bedny M. (2013). People can understand descriptions of motion without activating visual motion brain regions. Frontiers in psychology, 4, 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. 1998. A cortical representation of the local visual environment. Nature. 392(6676):598–601. [DOI] [PubMed] [Google Scholar]
- Faber PB, Usón RM. 1999. Constructing a lexicon of English verbs. Berlin, Germany: Walter de Gruyter. [Google Scholar]
- Fairhall SL, Caramazza A. 2013. Brain regions that represent amodal conceptual knowledge. J Neurosci. 33(25):10552–10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, McClelland JL. 1991. A computational model of semantic memory impairment: modality specificity and emergent category specificity. J Exp Psychol Gen. 120(4):339. [PubMed] [Google Scholar]
- Fernandino L, Binder JR, Desai RH, Pendl SL, Humphries CJ, Gross WL, Conant LL, Seidenberg MS. 2015. Concept representation reflects multimodal abstraction: a framework for embodied semantics. Cereb Cortex. 26(5):2018–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frawley W. 1992. Linguistic semantics. Hillsdale, NJ [u.a.]: Erlbaum. [Google Scholar]
- Fujimaki N, Miyauchi S, Pütz B, Sasaki Y, Takino R, Tamada T. 1999. Functional magnetic resonance imaging of neural activity related to orthographic, phonological, and lexico‐semantic judgments of visually presented characters and words. Hum Brain Mapp. 8(1):44–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbin G, Collina S, Tabossi P. 2012. Argument structure and morphological factors in noun and verb processing: an fMRI study. PLoS One. 7(9):e45091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman SA. 1988. The development of induction within natural kind and artifact categories. Cognit Psychol. 20(1):65–95. [DOI] [PubMed] [Google Scholar]
- Gelman SA, Bloom P. 2000. Young children are sensitive to how an object was created when deciding what to name it. Cognition. 76(2):91–103. [DOI] [PubMed] [Google Scholar]
- Gelman SA, Markman EM. 1986. Categories and induction in young children. Cognition. 23(3):183–209. [DOI] [PubMed] [Google Scholar]
- Gelman SA, Markman EM. 1987. Young children’s inductions from natural kinds: the role of categories and appearances. Child Dev. 58(6):1532–1541. [PubMed] [Google Scholar]
- Gelman R, Spelke ES, Meck E. 1983. What preschoolers know about animate and inanimate objects In: The acquisition of symbolic skills. New York: Springer; p. 297. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. 2002. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 15(4):870–878. [DOI] [PubMed] [Google Scholar]
- Gentner D. 1981. Some interesting differences between verbs and nouns. Cogn Brain Theory. 4:161–178. [Google Scholar]
- Gernsbacher MA. 1984. Resolving 20 years of inconsistent interactions between lexical familiarity and orthography, concreteness, and polysemy. J Exp Psychol Gen. 113(2):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhooly KJ, Logie RH. 1980. Age-of-acquisition, imagery, concreteness, familiarity, and ambiguity measures for 1,944 words. Behav Res Methods Instrum. 12(4):395–427. [Google Scholar]
- Gillette J, Gleitman H, Gleitman L, Lederer A. 1999. Human simulations of vocabulary learning. Cognition. 73(2):135–176. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR. 2013. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleitman L. 1990. The structural sources of verb meanings. Lang Acquis. 1(1):3–55. [Google Scholar]
- Gleitman LR, Cassidy K, Nappa R, Papafragou A, Trueswell JC. 2005. Hard words. Lang Learn Dev. 1(1):23–64. [Google Scholar]
- Goodglass H, Klein B, Carey P, Jones K. 1966. Specific semantic word categories in aphasia. Cortex. 2(1):74–89. [Google Scholar]
- Green DM, Swets JA. 1966. Signal detection theory and psychophysics. New York: Wiley. [Google Scholar]
- Grier JB. 1971. Nonparametric indexes for sensitivity and bias: computing formulas. Psychol Bull. 75(6):424. [DOI] [PubMed] [Google Scholar]
- Grindrod CM, Bilenko NY, Myers EB, Blumstein SE. 2008. The role of the left inferior frontal gyrus in implicit semantic competition and selection: an event-related fMRI study. Brain Res. 1229:167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Koenig P, DeVita C, Glosser G, Alsop D, Detre J, Gee J. 2002. Neural representation of verb meaning: an fMRI study. Hum Brain Mapp. 15(2):124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P. 2013. MUC (memory, unification, control) and beyond. Front Psychol. 4:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke M, Halchenko YO, Sederberg PB, Hanson SJ, Haxby JV, Pollmann S. 2009. PyMVPA: a python toolbox for multivariate pattern analysis of fMRI data. Neuroinformatics. 7(1):37–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauk O, Davis MH, Kherif F, Pulvermüller F. 2008. Imagery or meaning? evidence for a semantic origin of category‐specific brain activity in metabolic imaging. Eur J Neurosci. 27(7):1856–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Connolly AC, Guntupalli JS. 2014. Decoding neural representational spaces using multivariate pattern analysis. Annu Rev Neurosci. 37:435–456. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. 2001. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 293(5539):2425–2430. [DOI] [PubMed] [Google Scholar]
- Hernández M, Fairhall SL, Lenci A, Baroni M, Caramazza A. 2014. Predication drives verb cortical signatures. J Cogn Neurosci. 26(8):1829–1839. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. 1995. Representation of grammatical categories of words in the brain. J Cogn Neurosci. 7(3):396–407. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Binney RJ, Ralph MAL. 2015. Differing contributions of inferior prefrontal and anterior temporal cortex to concrete and abstract conceptual knowledge. Cortex. 63:250–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackendoff R. 1983. Semantics and cognition Cambridge, MA: MIT Press. MIT Press paperback ed. [Google Scholar]
- Jackendoff R. 1992. Semantic structures. Cambridge, MA: MIT press. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17(2):825–841. [DOI] [PubMed] [Google Scholar]
- Kable JW, Kan IP, Wilson A, Thompson-Schill SL, Chatterjee A. 2005. Conceptual representations of action in the lateral temporal cortex. J Cogn Neurosci. 17(12):1855–1870. [DOI] [PubMed] [Google Scholar]
- Kable JW, Lease-Spellmeyer J, Chatterjee A. 2002. Neural substrates of action event knowledge. J Cogn Neurosci. 14(5):795–805. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. 2006. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 361(1476):2109–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerer D. 2017. Categories of object concepts across languages and brains: the relevance of nominal classification systems to cognitive neuroscience. Lang Cogn Neurosci. 32(4):401–424. [Google Scholar]
- Kemmerer D, Castillo JG, Talavage T, Patterson S, Wiley C. 2008. Neuroanatomical distribution of five semantic components of verbs: Evidence from fMRI. Brain Lang. 107(1):16–43. [DOI] [PubMed] [Google Scholar]
- Kingsbury P, Kipper K.. 2003. Deriving verb-meaning clusters from syntactic structure. Proceedings of the HLT-NAACL 2003 Workshop on Text Meaning. Volume 9, Association for Computational Linguistics. 70 p.
- Kipper K, Korhonen A, Ryant N, Palmer M. 2006. Extending VerbNet with novel verb classes. Proceedings of LRECCiteseer. 1 p.
- Kreuz RJ. 1987. The subjective familiarity of english homophones. Mem Cognit. 15(2):154–168. [DOI] [PubMed] [Google Scholar]
- Kuhn M. 2008. Caret package. J Stat Softw. 28(5):1–26.27774042 [Google Scholar]
- Kumar M, Federmeier KD, Fei-Fei L, Beck DM. 2017. Evidence for similar patterns of neural activity elicited by picture- and word-based representations of natural scenes. Neuroimage. 155:422–436. [DOI] [PubMed] [Google Scholar]
- Laiacona M, Caramazza A. 2004. The noun/verb dissociation in language production: varieties of causes. Cogn Neuropsychol. 21(2–4):103–123. [DOI] [PubMed] [Google Scholar]
- Langacker RW. 1987. Nouns and verbs. Language. 63(1):53–94. [Google Scholar]
- Langacker RW. 2008. Cognitive grammar: a basic introduction. New York, NY: Oxford University Press. [Google Scholar]
- Lapinskaya N, Uzomah U, Bedny M, Lau E. 2016. Electrophysiological signatures of event words: dissociating syntactic and semantic category effects in lexical processing. Neuropsychologia. 93:151–157. [DOI] [PubMed] [Google Scholar]
- Leshinskaya A, Caramazza A. 2015. Abstract categories of functions in anterior parietal lobe. Neuropsychologia. 76:27–40. [DOI] [PubMed] [Google Scholar]
- Levin B. 1993. English verb classes and alternations: a preliminary investigation. Chicago, IL [u.a.]: Univ. of Chicago Press. [Google Scholar]
- Levin B, Hovav MR. 1995. Unaccusativity at the syntax-lexical semantics interface. Linguistic Inquiry Monographs 26:ALL.
- Li P, Jin Z, Tan LH. 2004. Neural representations of nouns and verbs in Chinese: an fMRI study. Neuroimage. 21(4):1533–1541. [DOI] [PubMed] [Google Scholar]
- Liljeström M, Tarkiainen A, Parviainen T, Kujala J, Numminen J, Hiltunen J, Laine M, Salmelin R. 2008. Perceiving and naming actions and objects. Neuroimage. 41(3):1132–1141. [DOI] [PubMed] [Google Scholar]
- Luria AR, Tsvetkova LS. 1967. Towards the mechanisms of “dynamic aphasia”. Acta Neurol Psychiatr Belg. 67(11):1045–1057. [PubMed] [Google Scholar]
- Luzzatti C, Raggi R, Zonca G, Pistarini C, Contardi A, Pinna G. 2002. Verb–noun double dissociation in aphasic lexical impairments: the role of word frequency and imageability. Brain Lang. 81(1):432–444. [DOI] [PubMed] [Google Scholar]
- Mandler JM, Bauer PJ, McDonough L. 1991. Separating the sheep from the goats: differentiating global categories. Cognit Psychol. 23(2):263–298. [Google Scholar]
- Marangolo P, Piras F, Galati G, Burani C. 2006. Functional anatomy of derivational morphology. Cortex. 42(8):1093–1106. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. 1995. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 270(5233):102. [DOI] [PubMed] [Google Scholar]
- McCarthy R, Warrington EK. 1985. Category specificity in an agrammatic patient: the relative impairment of verb retrieval and comprehension. Neuropsychologia. 23(6):709–727. [DOI] [PubMed] [Google Scholar]
- Medin DL, Atran S. 1999. Folkbiology. Cambridge, MA; London: MIT. [Google Scholar]
- Medin DL, Bang M. 2014. Who’s asking? Native science, western science, and science education. Cambridge, MA: MIT Press. [Google Scholar]
- Medin DL, Lynch EB, Solomon KO. 2000. Are there kinds of concepts? Annu Rev Psychol. 51(1):121–147. [DOI] [PubMed] [Google Scholar]
- Miceli G, Silveri MC, Nocentini U, Caramazza A. 1988. Patterns of dissociation in comprehension and production of nouns and verbs. Aphasiology. 2(3–4):351–358. [Google Scholar]
- Miceli G, Silveri MC, Villa G, Caramazza A. 1984. On the basis for the agrammatic’s difficulty in producing main verbs. Cortex. 20(2):207–220. [DOI] [PubMed] [Google Scholar]
- Mätzig S, Druks J, Masterson J, Vigliocco G. 2009. Noun and verb differences in picture naming: past studies and new evidence. Cortex. 45(6):738–758. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Friston KJ, Price CJ. 2003. Effects of visual deprivation on the organization of the semantic system. Brain. 126(7):1620–1627. [DOI] [PubMed] [Google Scholar]
- Nusbaum HC, Pisoni DB, Davis CK. 1984. Sizing up the hoosier mental lexicon. Research on Spoken Language Processing Report no. 10: pp. 357–376.
- Oliver RT, Thompson-Schill SL. 2003. Dorsal stream activation during retrieval of object size and shape. Cogn Affect Behav Neurosci. 3(4):309–322. [DOI] [PubMed] [Google Scholar]
- Paivio A, Yuille JC, Madigan SA. 1968. Concreteness, imagery, and meaningfulness values for 925 nouns. J Exp Psychol. 76(1):1–25. [DOI] [PubMed] [Google Scholar]
- Papagno C, Fogliata A, Catricalà E, Miniussi C. 2009. The lexical processing of abstract and concrete nouns. Brain Res. 1263:78–86. [DOI] [PubMed] [Google Scholar]
- Papeo L, Lingnau A, Agosta S, Pascual-Leone A, Battelli L, Caramazza A. 2014. The origin of word-related motor activity. Cereb Cortex. 25(6):1668–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Mccarthy G. 2004. Grasping the intentions of others: the perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. J Cogn Neurosci. 16(10):1706–1716. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G. 2005. Functional anatomy of biological motion perception in posterior temporal cortex: an fMRI study of eye, mouth and hand movements. Cereb Cortex. 15(12):1866–1876. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Schnur T, Tettamanti M, Collina S, Rosa MM, Fazio1 F. 1999. The neural correlates of verb and noun processing: a PET study. Brain. 122(12):2337–2344. [DOI] [PubMed] [Google Scholar]
- Perini F, Caramazza A, Peelen MV. 2014. Left occipitotemporal cortex contributes to the discrimination of tool-associated hand actions: FMRI and TMS evidence. Front Human Neurosci. 8:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. 2003. Languages without nouns and verbs? an alternative to lexical classes in Kharia. Old and new perspectives on south Asian languages. Grammar and semantics. Papers growing out of the Fifth International Conference on south Asian linguistics (ICOSAL-5), held at Moscow, Russia in July. 274 p.
- Pillon A, d’Honincthun P. 2011. A common processing system for the concepts of artifacts and actions? Evidence from a case of a disproportionate conceptual impairment for living things. Cogn Neuropsychol. 28(1):1–43. [DOI] [PubMed] [Google Scholar]
- Pinker S. (1989). Resolving a learnability paradox in the acquisition of the verb lexicon. In M. L. Rice & R. L. Schiefelbusch (Eds.), The teachability of language (pp. 13–61). Baltimore, MD, England: Paul H. Brookes Publishing. [Google Scholar]
- Pollack I, Norman DA. 1964. A non-parametric analysis of recognition experiments. Psychon Sci. 1(1–12):125–126. [Google Scholar]
- Rapp B, Caramazza A. 2002. Selective difficulties with spoken nouns and written verbs: a single case study. J Neurolinguistics. 15(3):373–402. [Google Scholar]
- Rodd JM, Davis MH, Johnsrude IS. 2005. The neural mechanisms of speech comprehension: FMRI studies of semantic ambiguity. Cereb Cortex. 15(8):1261–1269. [DOI] [PubMed] [Google Scholar]
- Sapir E. 2004. Language: an introduction to the study of speech. Mineola, NY: Dover Publications. [Google Scholar]
- Sasse H. 1993. Syntactic categories and subcategories. Syntax: An International Handbook of Contemporary Research 2:64686. Berlin, Germany: Mouton De Gruyter. [Google Scholar]
- Schachter P, Shopen T. 1985. Parts-of-speech systems. Lang Typol Syntactic Description. 1:3–61. [Google Scholar]
- Schreiber K, Krekelberg B. 2013. The statistical analysis of multi-voxel patterns in functional imaging. PLoS One. 8(7):e69328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler KK.. 2005. VerbNet: A BroadCoverage, Comprehensive Verb Lexicon. Ph.D. thesis, University of Pennsylvania. [Google Scholar]
- Shapiro K, Caramazza A. 2003. a. Grammatical processing of nouns and verbs in left frontal cortex? Neuropsychologia. 41(9):1189–1198. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Caramazza A. 2003. b. Looming a loom: evidence for independent access to grammatical and phonological properties in verb retrieval. J Neurolinguistics. 16(2):85–111. [Google Scholar]
- Shapiro KA, Moo LR, Caramazza A. 2006. Cortical signatures of noun and verb production. Proc Natl Acad Sci USA. 103(5):1644–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro KA, Moo LR, Caramazza A. 2012. Neural specificity for grammatical operations is revealed by content-independent fMR adaptation. Front Psychol. 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro KA, Mottaghy FM, Schiller NO, Poeppel TD, Flüß MO, Müller H, Caramazza A, Krause BJ. 2005. Dissociating neural correlates for nouns and verbs. Neuroimage. 24(4):1058–1067. [DOI] [PubMed] [Google Scholar]
- Shapiro K, Shelton J, Caramazza A. 2000. Grammatical class in lexical production and morphological processing: evidence from a case of fluent aphasia. Cogn Neuropsychol. 17(8):665–682. [DOI] [PubMed] [Google Scholar]
- Si A. 2017. The semantics of honeybee terms in Solega (Dravidian) In: Ye Z, editor. The semantics of nouns. New York, NY: Oxford University Press; p. 221. [Google Scholar]
- Simanova I, Hagoort P, Oostenveld R, Van Gerven MA. 2012. Modality-independent decoding of semantic information from the human brain. Cereb Cortex. 24(2):426–434. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE. 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith B, Mark DM. 2003. Do mountains exist? Towards an ontology of landforms. Environ Plan B Plan Design. 30(3):411–427. [Google Scholar]
- Snijders TM, Vosse T, Kempen G, Van Berkum JJ, Petersson KM, Hagoort P. 2008. Retrieval and unification of syntactic structure in sentence comprehension: an fMRI study using word-category ambiguity. Cereb Cortex. 19(7):1493–1503. [DOI] [PubMed] [Google Scholar]
- Spiridon M, Kanwisher N. 2002. How distributed is visual category information in human occipito-temporal cortex? An fMRI study. Neuron. 35(6):1157–1165. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. 1999. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 31(1):137–149. [DOI] [PubMed] [Google Scholar]
- Stelzer J, Chen Y, Turner R. 2013. Statistical inference and multiple testing correction in classification-based multi-voxel pattern analysis (MVPA): Random permutations and cluster size control. Neuroimage. 65:69–82. [DOI] [PubMed] [Google Scholar]
- Swets JA, Tanner WP Jr, Birdsall TG. 1961. Decision processes in perception. Psychol Rev. 68(5):301. [PubMed] [Google Scholar]
- Tabossi P, Collina S, Caporali A, Pizzioli F, Basso A. 2010. Speaking of events: the case of CM. Cogn Neuropsychol. 27(2):152–180. [DOI] [PubMed] [Google Scholar]
- Tanaka‐Ishii K, Terada H. 2011. Word familiarity and frequency. Studia Linguistica. 65(1):96–116. [Google Scholar]
- Tanenhaus MK, Carlson G, Trueswell JC. 1989. The role of thematic structures in interpretation and parsing. Lang Cogn Process. 4(3–4):SI211–SI234. [Google Scholar]
- Thompson CK, Bonakdarpour B, Fix SC, Blumenfeld HK, Parrish TB, Gitelman DR, Mesulam M. 2007. Neural correlates of verb argument structure processing. J Cogn Neurosci. 19(11):1753–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. 2005. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 15(2):219–224. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. 1997. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci U S A. 94(26):14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Kan IP. 1999. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 23(3):513–522. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. 1998. Verb generation in patients with focal frontal lesions: a neuropsychological test of neuroimaging findings. Proc Natl Acad Sci USA. 95(26):15855–15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Grabowski TJ, Lyon J, Damasio H. 2005. Naming the same entities from visual or from auditory stimulation engages similar regions of left inferotemporal cortices. J Cogn Neurosci. 17(8):1293–1305. [DOI] [PubMed] [Google Scholar]
- Trueswell JC, Sekerina I, Hill NM, Logrip ML. 1999. The kindergarten-path effect: studying on-line sentence processing in young children. Cognition. 73(2):89–134. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Bright P, Fletcher P, Stamatakis EA. 2004. Neural processing of nouns and verbs: the role of inflectional morphology. Neuropsychologia. 42(4):512–523. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Randall B, Stamatakis EA. 2008. Cortical differentiation for nouns and verbs depends on grammatical markers. J Cogn Neurosci. 20(8):1381–1389. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Russell R, Fadili J, Moss HE. 2001. The neural representation of nouns and verbs: PET studies. Brain. 124(8):1619–1634. [DOI] [PubMed] [Google Scholar]
- Tyler L, Stamatakis E, Dick E, Bright P, Fletcher P, Moss H. 2003. Objects and their actions: evidence for a neurally distributed semantic system. Neuroimage. 18(2):542–557. [DOI] [PubMed] [Google Scholar]
- Vander Wyk BC, Hudac CM, Carter EJ, Sobel DM, Pelphrey KA. 2009. Action understanding in the superior temporal sulcus region. Psychol Sci. 20(6):771–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannuscorps G, Pillon A. 2011. A domain-specific system for representing knowledge of both man-made objects and human actions. Evidence from a case with an association of deficits. Neuropsychologia. 49(9):2321–2341. [DOI] [PubMed] [Google Scholar]
- Vigliocco G, Vinson DP, Druks J, Barber H, Cappa SF. 2011. Nouns and verbs in the brain: a review of behavioural, electrophysiological, neuropsychological and imaging studies. Neurosci Biobehav Rev. 35(3):407–426. [DOI] [PubMed] [Google Scholar]
- Vigliocco G, Warren J, Siri S, Arciuli J, Scott S, Wise R. 2006. The role of semantics and grammatical class in the neural representation of words. Cereb Cortex. 16(12):1790–1796. [DOI] [PubMed] [Google Scholar]
- Walther DB, Caddigan E, Fei-Fei L, Beck DM. 2009. Natural scene categories revealed in distributed patterns of activity in the human brain. J Neurosci. 29(34):10573–10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DB, Chai B, Caddigan E, Beck DM, Fei-Fei L. 2011. Simple line drawings suffice for functional MRI decoding of natural scene categories. Proc Natl Acad Sci USA. 108(23):9661–9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Baucom LB, Shinkareva SV. 2013. Decoding abstract and concrete concept representations based on single‐trial fMRI data. Hum Brain Mapp. 34(5):1133–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Conder JA, Blitzer DN, Shinkareva SV. 2010. Neural representation of abstract and concrete concepts: a meta‐analysis of neuroimaging studies. Hum Brain Mapp. 31(10):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, McCarthy R. 1983. Category specific access dysphasia. Brain. 106(4):859–878. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. 1984. Category specific semantic impairments. Brain. 107(3):829–853. [DOI] [PubMed] [Google Scholar]
- Watson DM, Hartley T, Andrews TJ. 2014. Patterns of response to visual scenes are linked to the low-level properties of the image. Neuroimage. 99:402–410. [DOI] [PubMed] [Google Scholar]
- Watson DM, Hartley T, Andrews TJ. 2017. Patterns of response to scrambled scenes reveal the importance of visual properties in the organization of scene-selective cortex. Cortex. 92:162–174. [DOI] [PubMed] [Google Scholar]
- Weide RL. 1998. The CMU Pronouncing Dictionary. Available from: URL Http://www.Speech.Cs.Cmu.edu/cgibin/cmudict.
- Wolbers T, Klatzky RL, Loomis JM, Wutte MG, Giudice NA. 2011. Modality-independent coding of spatial layout in the human brain. Curr Biol. 21(11):984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Bi Y, Han Z, Zhu C, Law S. 2012. Neural correlates of comprehension and production of nouns and verbs in Chinese. Brain Lang. 122(2):126–131. [DOI] [PubMed] [Google Scholar]
- Yu X, Law SP, Han Z, Zhu C, Bi Y. 2011. Dissociative neural correlates of semantic processing of nouns and verbs in Chinese—a language with minimal inflectional morphology. Neuroimage. 58(3):912–922. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Hagoort P, Zhang JX, Feng G, Chen H, Bastiaansen M, Wang S. 2012. The anterior left inferior frontal gyrus contributes to semantic unification. Neuroimage. 60(4):2230–2237. [DOI] [PubMed] [Google Scholar]
- Zingeser LB, Berndt RS. 1990. Retrieval of nouns and verbs in agrammatism and anomia. Brain Lang. 39(1):14–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.