Abstract
The hippocampus displays a complex organization and function that is perturbed in many neuropathologies. Histological work revealed a complex arrangement of subfields along the medial–lateral and the ventral–dorsal dimension, which contrasts with the anterior–posterior functional differentiation. The variety of maps has raised the need for an integrative multimodal view. We applied connectivity-based parcellation to 1) intrinsic connectivity 2) task-based connectivity, and 3) structural covariance, as complementary windows into structural and functional differentiation of the hippocampus. Strikingly, while functional properties (i.e., intrinsic and task-based) revealed similar partitions dominated by an anterior–posterior organization, structural covariance exhibited a hybrid pattern reflecting both functional and cytoarchitectonic subdivision. Capitalizing on the consistency of functional parcellations, we defined robust functional maps at different levels of partitions, which are openly available for the scientific community. Our functional maps demonstrated a head–body and tail partition, subdivided along the anterior–posterior and medial–lateral axis. Behavioral profiling of these fine partitions based on activation data indicated an emotion–cognition gradient along the anterior–posterior axis and additionally suggested a self-world-centric gradient supporting the role of the hippocampus in the construction of abstract representations for spatial navigation and episodic memory.
Keywords: anterior–posterior, gradient, map, medial temporal lobe, structural covariance
Introduction
The hippocampus is involved in a variety of tasks ranging from memory, learning, navigation, and emotion (Moser and Moser 1998; Prince et al. 2005; Fanselow and Dong 2010; Poppenk et al. 2013; Strange et al. 2014). However, an integrative conceptual framework is currently lacking to account for this diversity of behavioral findings. To progress in that direction, first, a better understanding of the hippocampus’ organization and function is crucially needed to shed light on its role in a range of behavioral aspects and second, a common generic map would be highly useful to further support cross-studies comparison and integration.
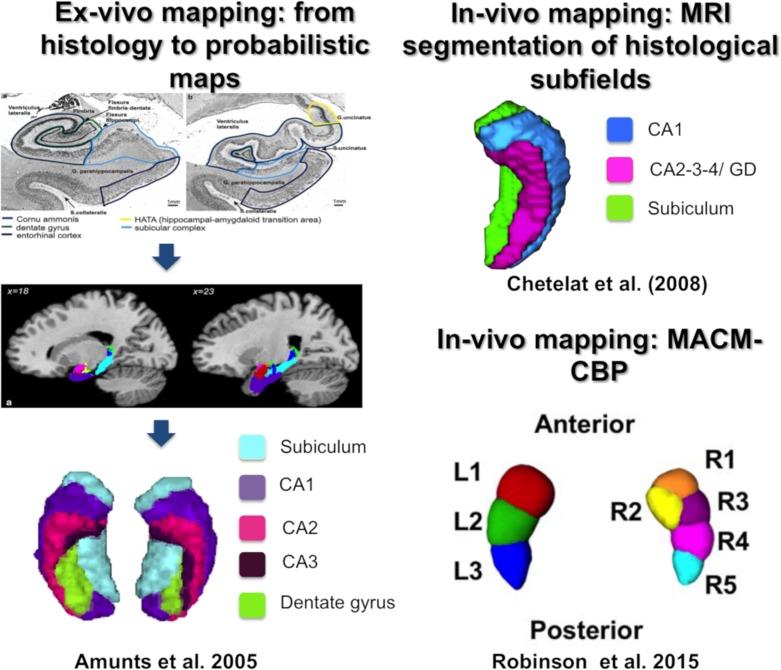
As far, 2 opposing organizational patterns were introduced in the past. The first mapping based on cytoarchitecture has evidenced a subdivision into subfields (CA1-4, dentate gyrus, subiculum) along the medial–lateral and ventro–dorsal axes as illustrated in Figure 1 (Amunts et al. 2005). In parallel to this organization, an organization into subregions (head, body, tail) along the anterior–posterior (longitudinal) axis (Lepage et al. 1998; Moser and Moser 1998; Fanselow and Dong 2010; Poppenk et al. 2013; Strange, et al. 2014) commonly emerged across a variety of in vivo approaches such as electrophysiology (Komorowski et al. 2013) and connectivity-based parcellation (CBP) (see Fig. 1, Robinson et al. 2015; Chase et al. 2015).
Figure 1.
Hippocampal mapping based on histology, structural MRI segmentation, and CBP method. (Images reproduced with permission from publishers.)
In line with the histological work and despite evidence of functional anterior–posterior differences, many in vivo and ex vivo studies in the human hippocampus used a priori segmentation into subfields based on either an automated or a manually delineation in the anatomical MRI scans (see Fig. 1;Adler et al. 2014, 2018; de Flores et al. 2015). Such segmentation into subfields has the advantage of using the histological “ground truth” as an a priori representation but has the disadvantage of neglecting higher order features, such as the rich long-range connectivity of the hippocampus, which contributes to its functional organization. Thus, the knowledge from this one-sided perspective should be complemented by a CBP approach, which now allows the combination of different MRI measurements within the whole hippocampus hence potentially probing different aspects of its organization.
CBP is an in vivo brain-mapping method that characterizes the organization of the brain based on connectivity estimates, usually derived from MRI (Eickhoff et al. 2018). CBP can be applied on any estimates of connectivity from MRI data (functional or structural) with different types of connectivity measurements being usually referred to as different CBP modalities. Across the previous years, evidence have been brought that CBP can capture organizational aspects that were previously revealed by tracing studies, as well as by histological work (Behrens et al. 2003; Lambert et al. 2017). Additionally, CBP was shown to be sensitive to functional distinction, for example, replicating the supplementary motor area (SMA) and pre-SMA differentiation evidenced by functional signal during task (Johansen-Berg et al. 2004). Hence this approach appears to identify regional differentiation supported by local microarchitecture, connectivity and local functional signal to some extent.
In the present study, we focused on the functional connectivity between hippocampus’ voxels and all gray matter voxels. In other words, we examined long-range (whole brain) connectivity by computing for every hippocampal voxel its individual connectivity fingerprint with all other gray matter voxels. Based on the (dis-)similarity of connectivity fingerprints, the voxels were clustered into either same or different partitions. CBP has the advantage to be model-free and unsupervised hence offering maps that optimally represent the data on hand. It has already been used in previous studies to examine hippocampal organization. Nevertheless, previous work focused mainly on a single CBP modality, either structural connectivity (Adnan et al. 2016) or meta-analytic connectivity modeling (MACM) (Chase et al. 2015; Robinson et al. 2015, 2016). Examining structural connectivity, Adnan et al. (2016) proposed a bipartite anterior–posterior subdivision of the hippocampus, which contrasted with MACM parcellations revealing a more detailed architecture. This latter modality yielded a 3-part organization for the left hippocampus and a 5-partite structure for the right hippocampus along the anterior–posterior axis (Robinson et al. 2015, 2016), while the subiculum subfield was subdivided into 5 modules (Chase et al. 2015). In sum, unimodal CBP has thus far provided evidence for an anterior–posterior organization of the hippocampus, but at different levels of partition across studies and even across hemispheres. This variety of partition schemes hinders a deep investigation of the functional relevance of the anterior–posterior differentiation and also complicates the study of hippocampus dysfunction in brain pathology.
In this latter perspective, a common set of maps of the hippocampus for MRI investigation would be highly useful. Across the previous years, one major avenue of neuroimaging research of brain pathology has developed from phenotype (such as cognitive performance or symptoms) prediction approaches based on multivariate pattern analyses applied to large-scale clinical datasets (Zhang et al. 2016). In this promising avenue of research, individual voxels have to be compressed into homogeneous subregions in which the measurements (e.g., fMRI signal) can be summarized. This compression is most of the time required not only for computational purposes but also for post hoc investigations of subregions contributing to the predictions. In this framework, the compression should be based on robustly defined maps that would represent a universal framework for comparison and integration of results across studies.
In this study, we investigated hippocampal functional organization using a multimodal CBP approach to generate robust functional hippocampal maps based on hippocampus– whole-brain connectivity profiles (Eickhoff et al. 2015, 2018). To do so, we focused on 2 purely functional modalities: MACM-CBP and resting-state functional connectivity (RSFC-CBP). Despite showing convergence (Reid et al. 2016) and being conceptually related, these 2 modalities are based on very different types of data and methods. MACM reflects functional organization during task and is computed from whole-brain coactivation peaks in activation databases such as BrainMap. For each hippocampal voxel, we obtained a whole-brain coactivation profile. RSFC, on the other hand, reflects the functional connectivity estimated in the unconstrained function of the brain and is computed at the subject level. For each individual hippocampal voxel, we obtained its functional connectivity profile to all the other gray matter voxels in the brain. RSFC is based on resting-state functional MRI (RS-fMRI), which is known to be prone to noise due to various artifacts (Van Dijk et al. 2012; Satterthwaite et al. 2013, 2017; Power et al. 2015), but the choice of an optimal denoising strategy has remained relatively unexplored in the particular framework of CBP. For that reason, as a preliminary step in the present study, we performed a systematic evaluation of different denoising methods in order to favor stable partitions with high biological validity for RSFC-CBP. We aimed to generate a functional subdivision of the hippocampus that would be stable across subjects and CBP modalities offering a representation that would be optimal for any type of functional signal (such as task-based fMRI activations, RS-fMRI or PET).
Nevertheless, to complement this purely functional parcellation and to start building a scientific bridge with previous structural mapping modalities, we additionally examined the subdivision of the hippocampus based on structural covariance (SC-CBP), which represents on the group-level covariation of hippocampal voxels with all the other brain voxels. SC stands at an ambiguous place in the mapping approaches. On the one hand, it is assumed to reflect functional dependencies between regions through synchronous firing of neurons reflecting functional neuroplasticity (Alexander-Bloch et al. 2013; Evans 2013). Accordingly, SC and RSFC are conceptually related to each other (Kotkowski et al. 2018), as indicated by structural changes through function (Seeley et al. 2009), although both are technically 2 distinct modalities. However, on the other hand, SC is based on structural changes (Mechelli et al. 2005; Alexander-Bloch et al. 2013) and thus should be influenced by the underlying structural organization like gene expression during neurodevelopment and direct structural connectivity through monosynaptic connection as indicated in a recent rodent study (Yee et al. 2017). In sum, SC is assumed to reflect common influences of certain factors on microstructure be it synaptogenesis based on functional synchronous firing, connectivity as direct monosynaptic connection, or gene expression in synapses development. Therefore, we expected that SC-CBP would to some extent confirm functional organization, and additionally, provide anatomical information conveyed in brain structure to complement our understanding of the hippocampal functional topography and provide an alternative map for studies capitalizing on structural MRI data.
Our final objective was to characterize the obtained cross-modal functional maps in terms of associated behavioral functions via a quantitative approach of activation studies (e.g., using the BrainMap or NeuroSynth databases). Importantly, our conceptual objective here was not to identify specific behavioral functions segregated into different subregions of the hippocampus but rather to assess the functional relevance and integration of the organization pattern in terms of cognitive information processing. Several hypotheses have been proposed in the past to describe the anterior–posterior differentiation in terms of psychological functions. But these hypotheses usually pertain to a specific psychological or neuroscientific research domain and hence could not account for pluripotency of the engagement of the hippocampus across psychological domains. As far, 2 main hypotheses derived from psychological ontologies have been proposed in that regard: an emotional–cognitive dimension (Moser and Moser 1998) and an encoding–retrieval dimension (Lepage et al. 1998; Prince et al. 2005; Kim 2015). Further investigations have proposed a novelty–familiarity (Strange et al. 1999) and an imagination–perception differentiation along the anterior–posterior axis (Zeidman and Maguire 2016). However, a common framework accounting for the relevance of the organization of the hippocampus across domains of human behavior is still lacking. The current study aimed to fill this gap by performing behavioral profiling (Genon, Reid, Langner et al. 2018) of hippocampus subregions using thousands of activation studies collected across 2 different databases using different behavioral taxonomies. Such a quantitative approach offers an overview, which can be used as a starting point to build an integrative theory.
Thus, the objectives of the present study were 2-fold 1) a conceptual objective of understanding hippocampal organization as revealed across different neurobiological properties and its relevance in terms of cognitive information processing and 2) a mapping objective to provide robust and fine-grained partitions of the hippocampus. While current high-level parcellations (Glasser et al. 2016; Schaefer et al. 2017) have focused on the cerebral cortex they neglected crucial subcortical structures. A consensual robust map of the bilateral hippocampus is still missing which in turn can help to study its structure and function across the lifespan as well as in disease. Our study was designed to offer such partitions and their patterns of associations with behavioral functions. These resources are openly available to the scientific community via ANIMA database (http://anima.fz-juelich.de/).
Materials and Methods
The bilateral hippocampi were parcellated using different connectivity modalities. Task-based connectivity was examined with MACM performed on reported activation peaks across paradigms in the BrainMap database. RSFC-CBP was performed at the subject level for a sample of participants from the Human Connectome Project (HCP), while SC-CBP was performed at the group level using the structural MRI data of the same HCP sample (Smith et al. 2013; Ugurbil et al. 2013). The main methodological differences between these 2 CBP modalities are illustrated in Supplemental Material Methods I.5.
After computing parcellations for each modality, we established a functional map of the hippocampus by merging the functional parcellations (i.e., RSFC and MACM, that showed the highest convergence) into one hippocampal map, hence representing a cross-modal consensus map. Finally, we characterized our cross-modal consensus map at high granularity with regard to behavioral functions using BrainMap and NeuroSynth databases.
Volume of Interest
We defined our VOI as a consortium of the cytoarchitectonic maps, available in the SPM Anatomy Toolbox 2.0 (Eickhoff et al. 2005), and the macro anatomically-defined Harvard-Oxford Structural Probability Atlas (http://neuro.imm.dtu.dk/wiki/Harvard-Oxford_Atlas) (Desikan et al. 2006). The hippocampal formation included the following subfields: CA1–3, dentate gyrus, and subiculum. The total number of voxels in a 2 mm × 2 mm × 2 mm space in the right hippocampus was 865 (6920 mm3) and that of the left hippocampus was 831 (6648 mm3) voxels.
Sample
The sample was obtained from the longitudinal study of the HCP (Van Essen and Ugurbil 2012) representing one of the best openly accessible MRI datasets. We included unrelated participants in order to avoid heritability effects. The sample consisted of n = 323 young adults (age: 22–37 years, mean age: 28.2 years, 50.7% females). All participants gave their written statement of agreement, and the analyses of the data were approved by the ethical committee of the Heinrich Heine University Düsseldorf.
MRI Measurements
Structural MRI
All scans were acquired on a 3 T MRI scanner of Siemens Skyra (Siemens AG, Erlangen, Germany) with a 32-channel coil (Van Essen and Ugurbil 2012). The 3D structural T1-weighted MRI scans were performed with a MPRAGE sequence (256 sagittal slices in a single slab, TR = 2400 ms, TE = 2.14 ms, TI = 1000 ms, FoV = 224 × 224 mm, flip angle = 8◦, voxel size = 0.7 × 0.7 × 0.7 mm3). Preprocessing of the MRI data was performed with SPM8 (Statistical Parametric Mapping) and the VBM8 toolbox, running on Matlab R2014a. Structural images were normalized with the DARTEL algorithm to the ICBM-152 template using both affine and nonlinear spatial normalization. Images were bias-field corrected and segmented into gray matter, white matter (WM), and cerebrospinal fluid (CSF) tissues. The gray matter segments were then modulated for nonlinear transformations only and subsequently smoothed with an isotropic Gaussian kernel (full-width-half-maximum = 8).
Resting-state Functional MRI
The acquisition of resting-state fMRI with opposite phase encoding directions (L/R and R/L) was performed with an EPI sequence for a duration of 30 min (eyes open and fixated on a hair cross), 72 slices covering the whole brain (TR = 720 ms, using a multiband factor of 8, TE = 33 ms, FoV = 208 × 180 mm, flip angle = 52°, voxel resolution = 2 × 2 × 2 mm3; Smith et al. 2013; Ugurbil et al. 2013). During preprocessing, we corrected for movements by affine 2-pass registration and aligned the images to the first volume and to the mean of the volumes. Variance explained by 6 motion parameters from the realignment and their first derivatives were regressed out. Spatial normalization to the Montreal Neurological Institute (MNI) was carried out for the average EPI scans for each subject using the unified segmentation approach (Ashburner and Friston 2005). We applied a band-pass filter with the cut-off frequencies of 0.01 and 0.08 Hz. The images were smoothed with the isotropic Gaussian kernel (full-width-half-maximum = 5).
Connectivity-based Parcellation
Parcellation Based on Structural Covariance (SC-CBP)
For each subject, structural covariance was measured by computing the Pearson’s correlation coefficient between gray matter volume values of the hippocampus’ VOI voxels (seed voxels) and all other brain gray matter voxels across the whole sample. This procedure yielded a seed voxels by target voxels connectivity matrix at the group level that was then used for clustering (see Supplemental material I.5 Fig. 4).
Parcellation Based on Resting-state Functional Connectivity (RSFC-CBP)
Resting-state functional connectivity between 2 brain regions was estimated by computing Pearson’s correlation between time series of blood oxygen-level-dependent signal (BOLD) at the subject level (Biswal et al. 1995; Buckner et al. 2013). For each seed voxel in the VOI, we calculated the correlation with every other gray matter voxels of the brain (see Supplemental Material I.5 Fig. 4). Correlation values were then standardized using the Fisher’s Z-transformation.
Temporal MRI preprocessing. The goal of denoising is to remove or at least to reduce the contribution of any artifacts and confounds that contaminate neurally generated BOLD signal. Noise in RSFC can result from scanner artifacts (Ojemann et al. 1997), subject movement (Van Dijk et al. 2012; Satterthwaite et al. 2013; Power et al. 2014), and physiological processes (Birn et al. 2006). Standard denoising approaches aim to regress out variance that is driven by noise in the measured BOLD signal. One simple approach to do so relies on the calculation of global signal or/and signal in 2 specific nongray matter tissues (i.e., WM and CSF), which are assumed to reflect artifacts. An alternative approach capitalizes on machine-learning techniques (e.g., FIX) to automatically identify potential noise in the data. With these approaches, the part of variance related to noise is typically first estimated and then regressed out from time series. Several variants have been developed over the previous years and we described in the following the most commonly used strategies.
Global signal regression. In global signal regression (GSR), the mean fMRI signal across all brain voxels is regressed out (Desjardins et al. 2001; Macey et al. 2004). The underlying axiom is that any fluctuations that are measured globally are not attributable to neural activity but have physiological or mechanical origin (Wise et al. 2004; Birn et al. 2006; Bianciardi et al. 2009; Caballero-Gaudes and Reynolds 2017). Although it is unclear what exactly is reflected in the global signal and to what extent signal or nuisance is regressed out, it is still widely used. In this context of scientific uncertainty, the consequences of GSR on RSFC should be considered.
WM–CSF signal regression. Another alternative is to estimate nuisance regressors from WM and CSF signal (Weissenbacher et al. 2009; Jo et al. 2010, 2013; Anderson et al. 2011; Power et al. 2012; Hallquist et al. 2013; Yan et al. 2013). The signal’s fluctuations in these parts of the brain are assumed to reflect drifts mainly caused by cardiac and respiratory effects (Dagli et al. 1999; Windischberger et al. 2002; de Munck et al. 2008; Van Dijk et al. 2010). To measure the mean signal across these regions, we created subject-specific masks by coregistering the WM and CSF templates to each individuals’ space and subsequently, regressed out the mean signal computed within these masks. Note that the subject-specific templates were eroded in order to remove voxels on the edge of the mask that relate to gray matter and do not contain pure WM/CSF tissue (Jo et al. 2010; Caballero-Gaudes and Reynolds 2017).
FMRIB’s ICA-based X-noiseifier. FMRIB’s ICA-based X-noiseifier (FIX) is based on a machine-learning approach, in which RS-fMRI signal has been decomposed into components of neural and non-neural sources by applying an independent component analysis (ICA) method (Beckmann and Smith 2004; Cole et al. 2010; Salimi-Khorshidi et al. 2014). FIX classifies the ICA components into relevant signal and noise-related components (Cole et al. 2010; Salimi-Khorshidi et al. 2014). We used the default classifier trained on a standard fMRI dataset, which has been shown to achieve 95% accuracy (Salimi-Khorshidi et al. 2014). For each participant, spatial ICA was performed using FSL’s MELODIC toolbox (Beckmann and Smith 2004) and subsequently noise-variance components were regressed out.
In this study, we evaluated the denoising techniques both individually or in combination as recently suggested by Burgess et al. (2016) (see Supplemental Material I.4 methods).
We investigated 6 different strategies:
Standard motion regression with 24 regressors without additional explicit denoising, termed as “no denoising” emphasizing no additional transformations.
Regression of the averaged fMRI signal across all voxels of the brain (GSR).
Regression of WM and CSF-related variance (WM/CSF).
Neutralization of “bad” components of fMRI signal decomposed by ICA (FIX).
A combination of FIX and GSR regression (FIX + GSR).
A combination of FIX and WM/CSF regression (FIX + WM/CSF).
Parcellation Based on Meta-analytic Connectivity Modeling (MACM-CBP)
From a computational point of view, MACM substantially differs from the aforementioned approaches since connectivity is not computed from collected MRI data as done for RSFC and SC but meta-analytically across activation foci of neuroimaging studies and paradigms archived in the BrainMap database (Laird et al. 2011) (http://www.brainmap.org). All experiments in BrainMap that were associated with each seed voxel or in the immediate vicinity of activation were considered. To account for spatial uncertainty, a spatial filter was systematically varied by including the closest 20–200 experiments in steps of 5 (for more details, see Clos et al. 2013, 2014; Genon et al. 2017; Genon, Reid, Li et al. 2018). For each seed voxel, a meta-analytical coactivation likelihood profile for every other brain voxel given each of the 25 filter sizes was computed with revised ALE algorithm (Eickhoff et al. 2012). The final CBP analysis was performed in the filter range of 100–148 experiments for the right hippocampus and in the filter range of 82–130 for the left hippocampus. An optimal filter range was defined based on the consistency of each voxels’ cluster assignment across all the filter sizes (see Clos et al. 2013; Clos et al. 2013; Chase et al. 2015; Genon et al. 2017) (see in Supplementary Methods I.3).
Clustering Method
In line with previous studies, we used k-means (using MATLAB software 2014a) clustering, which showed good agreement with spectral clustering and outperformed hierarchical clustering (Arslan et al. 2018). The repetition number was set to 500, which almost doubled the recommended number of 256 repetitions (Nanetti et al. 2009), and the iteration number was set to 255. We examined 6 levels of granularity (levels of partitions) ranging from k = 2 to 7 since previous work has reported stable cluster solutions at different level of partitions (2, 3, and 5 (Chase et al. 2015; Robinson et al. 2015, 2016; Adnan et al. 2016)). The clustering was performed at the subject level for RSFC and at the experiments range (filter range) for MACM, while it was performed at the group level with average across bootstrap resampling for SC. Modality-specific and group-specific parcellations were achieved by assigning the hippocampal voxels to its most frequent cluster’s label (i.e., by using the mode) across subjects, filter sizes, and bootstrapping samples.
Measurement of Stability and Consistency of Parcellations
In this work, we estimated stability and consistency of the partitions yielded by RSFC-CBP since this modality is particularly challenging in terms of its sensitivity to noise, interindividual variability (Mueller et al. 2013) and its dynamic nature (Hutchison et al. 2013). We examined both criteria in this CBP framework, but emphasized consistency over stability, as we aimed for biological validity in addition to stability by capturing convergent organizational characteristics across CBP modalities. In line with previous study (Varikuti et al. 2017), we considered the possibility that high stability within RSFC could be influenced by “structured noise,” which when regressed out might result in apparently lower stability but preserving biological relevance or even enhancing it.
We used 2 procedures in order to cross-validate our findings: 1) split-half (Strother et al. 2002; LaConte et al. 2003) to estimate the stability within a CBP modality (i.e., RSFC) and 2) bootstrap resampling with replacement to assess consistency between CBP modalities (i.e., RFSC vs. MACM) (Efron 1979; Bellec et al. 2010).
In contrast to previous studies, we here assumed that multiple partitions at different granularities might be valid representations of the hippocampus organization, but at different levels. Accordingly, we focused on split-half and bootstrap resampling instead of internal validity criteria such as the silhouette value or the percentage of misclassified voxels as these latter metrics probe optimal data representation within the specific modality at hand, while we here aimed for stability within and reproducibility across modalities. Stability was estimated by splitting the sample into halves 10 000 times. The similarity between the 2 halves was examined by computing the Adjusted Rand Index (ARI) between the 2 split partitions. To assess consistency, we generated 10 000 bootstrap samples for each modality and compared these samples between CBP modalities using the ARI (RSFC vs. MACM, RSFC vs. SC, MACM vs. SC). An ARI value of 1 indicates that the clusterings are identical and a value of 0 suggests that the clusterings are not similar to each other, whereas negative values indicate a dissimilarity of clusterings higher than chance (see Supplemental Material I.6 methods for detailed information).
Evaluating Denoising Performance
To better understand the actual effect of denoising strategies on the stability and consistency of RSFC-CBP partitions, we investigated the effect of denoising on seed voxels’ time course similarity and on connectivity profile dissimilarity. We assumed that structured noise influences the BOLD response in the measured time series in such a way that the voxels become more artificially similar (higher time series similarity) and show higher similarity in their connectivity profiles. Accordingly, we can expect that a denoising method, which reduced structured noise successfully, will decrease time series similarity and increase dissimilarity of the connectivity fingerprint. Since this latter marker directly drives the clustering pattern, its sensitivity to denoising is crucially relevant in the CBP application perspective. We could indeed expect that efficient denoising would to some degree enhance voxels' dissimilarity facilitating the assignment of voxels to clusters. We therefore first examined voxels similarity regarding their time series as measured by correlations of the time series (Pearson’s correlation), but we also examined the dissimilarity of the seed voxels regarding their pattern of connectivity with all other brain gray matter voxels by computing the Euclidean distance between seed voxels’ connectivity fingerprint.
Consensus Clustering
In order to create a cross-modal and stable map of the hippocampus from functional modalities, we used the bootstrap resampling method (Bellec et al. 2010). The basic idea was to simulate the replication process of parcellation a large number of times to preserve stable features and to reduce the occurrence of unlikely or unstable individual patterns (Bellec et al. 2010). After having created 10 000 bootstrap samples containing a matrix with all seed voxels and their cluster’ labels (assigning each voxel to a cluster for each modality), we pooled these samples. After pooling, we identified for each seed voxel the most frequent assignment to a cluster by computing the mode. This procedure allowed each modality to be represented in the same regard independent of group or filter size, but only the most stable partition across both modalities was retained. Accordingly, if one modality provides unstable partitions, which is particularly likely for RSFC-CBP, the stable partitions from the other modality (here MACM) will determine the final clustering. Thus, this procedure promotes a final general parcellation, which is both, functionally cross-modal, and stable.
Cluster Characterization with BrainMap and NeuroSynth Databases
To characterize the clusters of our cross-modal consensus parcellation behaviorally, we used 2 different databases, BrainMap (http://www.brainmap.org/) and NeuroSynth (http://neurosynth.org/). Both databases are complementary so that we expect their combination to provide novel insights into the behavioral association and the profile of a brain region (Genon, Reid, Langner et al. 2018). Furthermore, using both databases circumvents a circularity limitation (see Supplementary Methods I.2). In the BrainMap protocol, each activation peak has been individually labeled according to a predefined taxonomy of behavioral domains such as cognition.memory.working (see (Genon et al. 2017). Behavioral profiling was performed with a reverse inference approach (Genon, Reid, Langner et al. 2018), which identifies the posterior probability P(Task|Activation), that is, the probability of task given activation in that cluster.
In contrast, studies in NeuroSynth were labeled according to terms occurrence in the paper by using a text-mining approach so that behavioral associations were determined by the terms used in the corresponding article text (Yarkoni et al. 2011). This automated strategy resulted in the inclusion of 11 406 studies (tripled the number of archived studies in BrainMap) but can suffer from a lack of behavioral precision. We also used the reverse inference approach with NeuroSynth, that is, P(Term|Activation). The decoding with BrainMap was region of interest (ROI)-based, whereas it was coordinate-based with NeuroSynth requiring the usage of centroid coordinates of each cluster in MNI152 space (see Supplementary Methods I.7, Table 1). The lexical meta-analysis approach of NeuroSynth required decision criteria for selecting functional associated terms so that we excluded all noncontent words (e.g., “addressed,” “abstract,” or “reliable”) and all brain terms that did not refer to function (e.g., “hippocampus” and “middle temporal lobe”). Additionally, we pooled terms with the same phonological root (e.g., “memory” was used as a generic term for “memories”). All terms that reached a z-score higher than zero were included.
Results
To optimize first the reliability of RSFC-CBP, we examined the stability of its yielded partitions dependent on the application of different denoising strategies. After having identified the most reliable denoising method for RSFC, we investigated hippocampal organization across CBP modalities to determine consistency between modalities and levels of partition in order to establish a stable cross-modal functional map, which we characterized behaviorally in the last step.
Stable RSFC Parcellations as a Function of Denoising
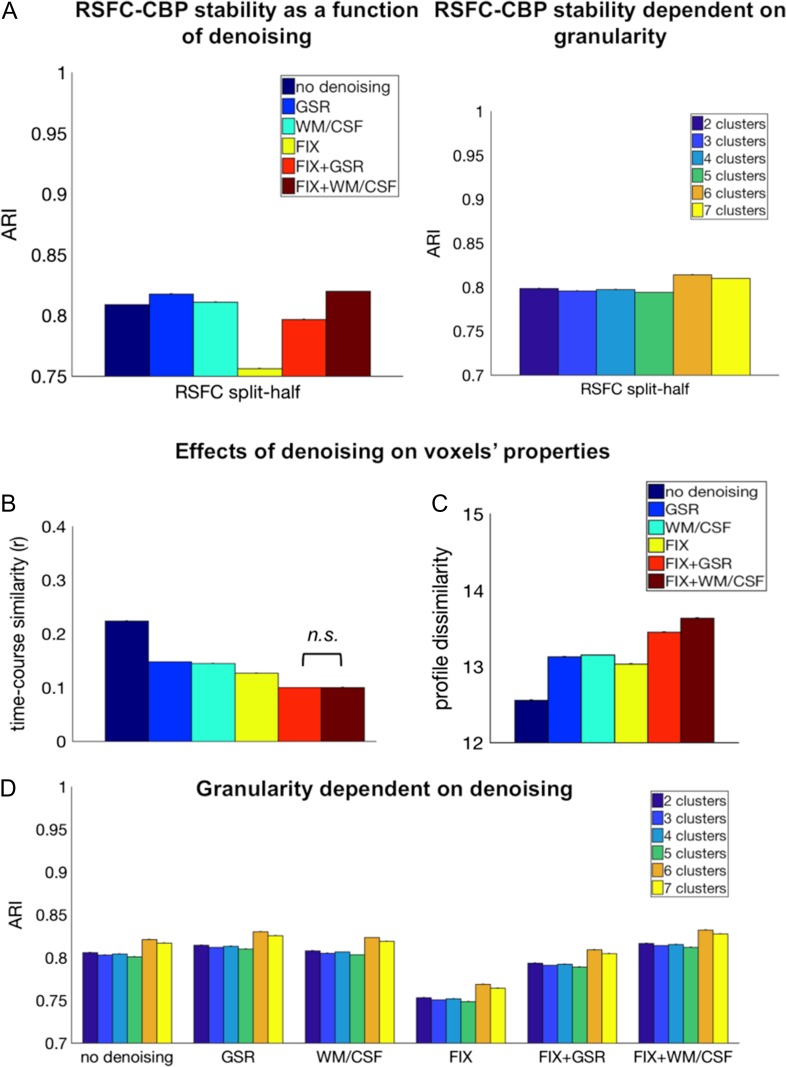
We measured stability within RSFC-CBP with split-half cross-validation and computed a 2-way ANOVA with denoising as one factor (no denoising, GSR, WM/CSF, FIX, FIX + GSR vs. FIX + WM/CSF), levels of partition as a second factor (k = 2–7), and ARI as a dependent variable.
The analysis showed that the most robust RSFC parcellation was achieved when using FIX + WM/CSF (M = 0.82 ARI, SE = 0.03), GSR (M = 0.82 ARI, SE = 0.03), or WM/CSF regression (M = 0.81 ARI, SE = 0.04). The least stable parcellation was achieved when using FIX only (M = 0.76 ARI, SE = 0.005); main effect denoising [F(5, 719 999) = 60 220, P < 0.0001] (see Fig. 2A).
Figure 2.
The effect of denoising on RSFC-CBP and on voxel functional properties. (A) Most stable hippocampal parcellations across all levels of partition (k = 2–7) were obtained with FIX + WM/CSF, GSR, and WM/CSF regression as denoising approaches. Bars indicate mean ARI (±standard errors). Independent of denoising technique the highest stability was acquired for 6 clusters. All comparisons were statistically significant. (B) Seed voxels’ time course similarity was reduced after the application of denoising. No significant difference was observed between FIX + GSR and FIX + WM/CSF, whereas all the other comparisons were significant. (C) Denoising resulted in an increase in seed voxels’ dissimilarity in comparison to uncleaned data. FIX-related strategies demonstrated the strongest effect of connectivity profile dissimilarity. (D) FIX + WM/CSF showed the highest stability across all levels of partition (k = 2–7) compared with other denoising techniques. Mean ARI (±standard errors). All comparisons were statistically significant.
The main effect of partition levels was also significant [F(5, 719 999) = 7377.6, P < 0.0001] demonstrating highest stability for 6 clusters (M = 0.81 ARI, SE = 0.03), followed by 7 clusters (M = 0.81 ARI, SE = 0.03). The ANOVA yielded also a significant interaction effect between denoising and levels of partition, [F(25, 719 999) = 6812.48, P < 0.0001] (see Fig. 2D). All comparisons between denoising methods and between levels of partition were significant according to a post hoc Bonferroni-corrected analysis (P < 0.001).
However, the small magnitude of the differences between denoising approaches suggested that, overall, they all offered high stability. In the following section, we further investigated the effect of denoising at the voxel level to better understand how different methods influence voxels’ properties and thereby the stability of the clustering.
Effects of Denoising on Voxels’ Time Course Similarity and Connectivity Profile Dissimilarity
We computed 2 separated ANOVAs in order to examine the effect of variance regression performed with denoising on each type of voxel measure separately: voxels’ time course similarity and voxels’ connectivity profile dissimilarity.
The ANOVA with averaged seed voxels’ time course similarity as a dependent variable revealed a significant main effect denoising [F(5, 4 311 269) = 78 078.56, P < 0.0001], demonstrating a decrease in time course similarity for denoised data. Post hoc Bonferroni-corrected multiple comparisons revealed no significant difference between FIX + GSR and FIX + WM/CSF regression (P = 0.40), but all the other comparisons were significant (P < 0.0001). The combination of a model-based (FIX) and model-free (GSR, WM/CSF) denoising strategy resulted in highly reduced seed voxels’ time course similarity compared with other techniques (Fig. 2B).
The second ANOVA with averaged seed voxels’ connectivity profile dissimilarity as a dependent variable revealed an increase in dissimilarity of seed voxels with every additional denoising technique [significant main effect denoising: F(5, 4 311 269) = 113 132.16, P < 0.0001] (Fig. 2C). Our results showed that following denoising, seed voxels’ connectivity profiles were more discriminable and especially, FIX + WM/CSF led to the highest dissimilarity between seed voxels’ connectivity profiles. All post hoc Bonferroni-corrected comparisons were significant (P < 0.0001).
Thus, overall our analyses supported the use of FIX + WM/CSF as a denoising strategy and accordingly this procedure was retained for the following multimodal functional parcellation.
Robust Levels of Subdivisions Across CBP Modalities
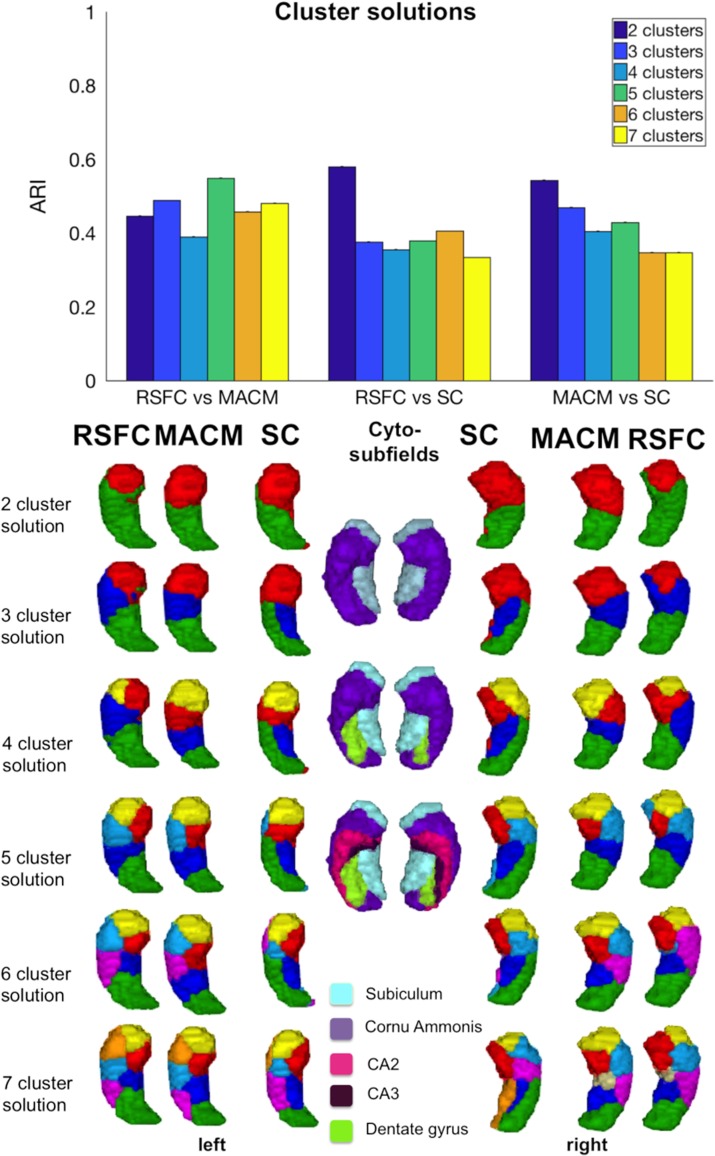
After defining the optimal denoising strategy (FIX + WM/CSF) from the voxels’ properties, as well as from the parcellation’s stability perspective, we examined which level of partition, in other words, granularity, promotes consistency across CBP modalities. We focused on the consistency measure instead of stability, since we aimed to promote biological validity estimated through comparisons across modalities. To examine consistency across partition’s levels, we computed one-way ANOVAs with number of clusters (k = 2–7) as factor and ARI index measuring similarity as the dependent variable, separately for each pair of modality (RSFC vs. MACM, RSFC vs. SC, and MACM vs. SC).
Cross-modal comparisons between SC and functional modalities (RSFC, MACM) resulted in less consistency than between the 2 functional CBP modalities (RSFC vs. MACM). The highest similarity between RSFC and SC was achieved for a partition of 2 (M = 0.58, SE = 0.03) and 6 clusters (M = 0.41, SE = 0.05) [F(5, 119 999) = 167 974.16, P < 0.0001] (see Fig. 3). The comparison between MACM and SC revealed that the highest consistency occurred at a 2- (M = 0.54, SE = 0.15), 3- (M = 0.47, SE = 0.04), and 5-cluster partition (M = 0.43, SE = 0.06), [F(5, 119 999) = 17 685.1, P < 0.0001]. All post hoc Bonferroni-corrected comparisons were significant (P < 0.0001).
Figure 3.
Hippocampus partitions based on SC, MACM, RSFC, and cytoarchitecture mapping. Mean ARI (±standard error). All comparisons between cluster solutions showed significant differences.
Visual examination suggested that the highest convergence between SC and functional modalities could be observed at low granularity, that is, for 2-cluster partition in which all modalities subdivided the hippocampus into an anterior and a posterior cluster. At the next subdivision level, partitions already differed markedly between modalities. Namely, the pattern of subdivisions based on SC is dominated by a medial–lateral organization that strikingly mimics cytoarchitecture differentiation between subiculum and CA subfields. Such a medial–lateral subdivision first concerns the posterior portion of the hippocampus (at 3-cluster partition) but extends into the hippocampus head at 4-cluster partition (see Fig. 3).
In turn, functional convergence between RSFC and MACM showed a significant effect on the ARI [F(5, 719 999) = 12 506.39, P < 0.0001], with the highest convergence being observed for partitions of 5 (M = 0.55, SE = 0.10), 3 (M = 0.49, SE = 0.03), and 7 clusters (M = 0.48, SE = 0.02) (see Supplementary Results II.2, Table 2). Consequently, partitions into 3, 5, and 7 subregions were considered as an optimal level of partitions for defining robust functional maps of the hippocampus.
In addition to the quantitative analysis, the visual examination of the partition schemes also proposed high convergence for 3, 5, and 7 clusters, revealing that the 3-cluster partition divided the hippocampus into anterior, intermediate, and posterior subregions in both modalities (Fig. 3). In turn, the 5-cluster partition divided the hippocampus into one anterior cluster (head), 3 intermediate clusters (intermediate caudal, intermediate lateral and medial, for the body) and finally, a posterior cluster (tail). Finally, the 7-cluster partition included 3 anterior, 3 intermediate clusters, and one posterior subregion (see Fig. 3).
Cross-modal Functional Consensus Map
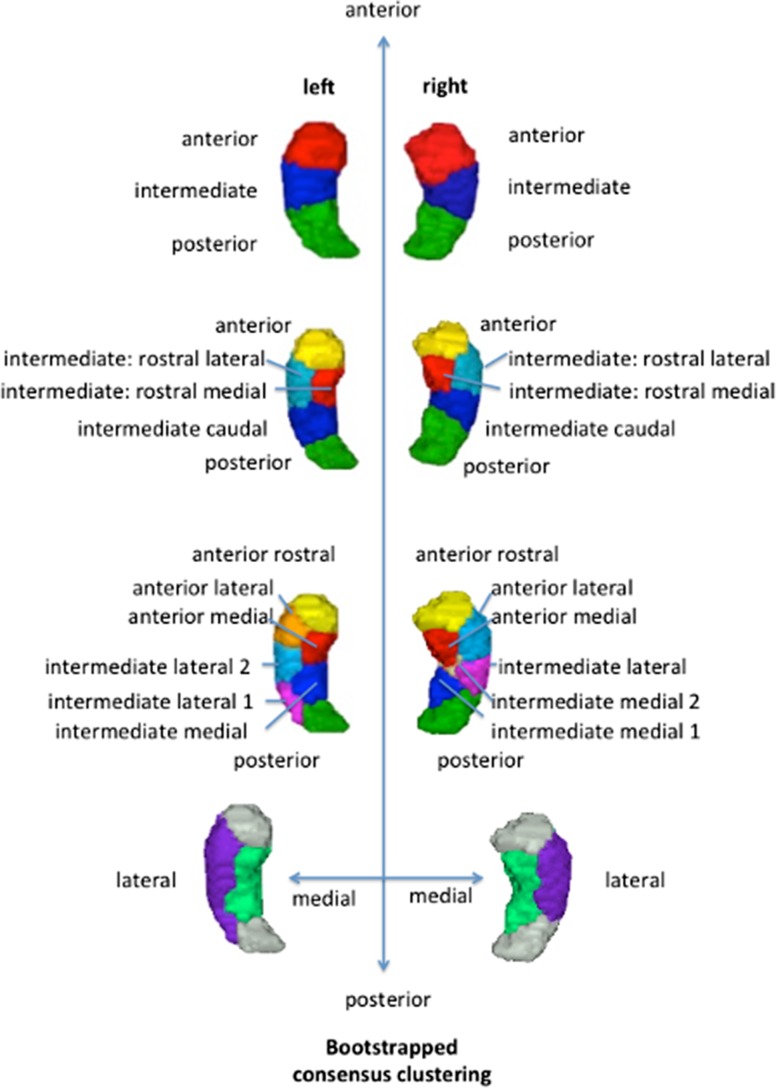
Cross-modal comparisons of CBP modalities revealed high convergence between RSFC and MACM, especially at higher granularities, whereas SC showed an idiosyncratic subdivision that deviated from pure functional modalities. For this reason, we established a pure functional cross-modal map of the hippocampus using bootstrap resampling as described in section Consensus Clustering and based on RSFC and MACM parcellations while excluding SC. Importantly, we created functional maps at different granularity levels (3, 5, and 7 clusters) reflecting convergence between modalities as an approximation of biological validity. These functional maps at different partition levels should allow the community to investigate hippocampus’ function and dysfunction at various levels of organization. In the present study, we focused on the 7-cluster partition to study hippocampus function as this high level of partition offers a detailed architecture along the anterior–posterior axis with small functional units.
As illustrated in Figure 4, each and every retained level of granularity revealed a specific aspect of hippocampal functional organization. The cross-modal 3-cluster partition subdivided the left and right hippocampi into an anterior (head), intermediate (body), and a posterior (tail) subregion. At the next subdivision (5-cluster granularity), bilateral hippocampi were partitioned into a posterior, intermediate part including 3 subregions—intermediate caudal, intermediate lateral rostral, and intermediate medial rostral—and finally an anterior subregion. The 7-cluster cross-modal partition showed hemispheric asymmetry. The body of the right hippocampus was subdivided into one intermediate lateral and 2 intermediate medial clusters. In contrast, the left hippocampus was partitioned into 2 intermediate lateral clusters and one intermediate medial cluster (see Fig. 4). However, the posterior tail cluster as well as the head, which was subdivided into 3 clusters (anterior rostral, anterior lateral and anterior medial), were found in both hemispheres.
Figure 4.
Functional multimodal maps across different granularities showing differentiation along the anterior–posterior and the medial–lateral dimensions.
We hypothesized that the subdivision into medial versus lateral subregions could partially reflect the already known cytoarchitectonic subdivision. The lateral segments in the body of the hippocampus corresponded mainly to the CA1–3 subfields and the medial clusters mainly to the subiculum. This hypothesis was supported by a quantitative comparison of our clusters with cytoarchitecture from the Anatomy Toolbox (see Table 1).
Table 1.
Consensus hippocampus in comparison to cytoarchitecture
| Consensus cluster | Cluster size | x | y | z | Overlap with cytoarchitectonic subfields |
|---|---|---|---|---|---|
| Right hippocampus | |||||
| Anterior rostral cluster (yellow) | 161 | 22 | −10 | −24 | CA1, subiculum |
| Anterior lateral cluster (light blue) | 181 | 31 | −16 | −20 | CA1, DG, subiculum, CA2 |
| Anterior medial cluster (red) | 139 | 21 | −17 | −17 | Subiculum, CA3 |
| Intermediate lateral cluster (purple) | 128 | 33 | −26 | −12 | DG, CA1, CA2 |
| Intermediate medial 2 clusters (ocher) | 34 | 26 | −22 | −16 | Subiculum |
| Intermediate medial one cluster (dark blue) | 86 | 24 | −31 | −9 | Subiculum, DG |
| Posterior cluster (green) | 129 | 26 | −37 | −2 | DG, CA1 |
| Left hippocampus cluster | |||||
| Anterior rostral cluster (yellow) | 166 | −23 | −11 | −24 | CA1, subiculum |
| Anterior lateral cluster (orange) | 122 | −31 | −15 | −21 | CA1, DG, CA2 |
| Anterior medial cluster (red) | 149 | −24 | −20 | −18 | Subiculum, CA3 |
| Intermediate lateral 2 clusters (light blue) | 133 | −33 | −24 | −14 | DG, CA1, CA2 |
| Intermediate lateral one cluster (purple) | 76 | −31 | −35 | −7 | DG, CA1 |
| Intermediate medial cluster (dark blue) | 112 | −26 | −30 | −9 | Subiculum, DG |
| Posterior cluster (green) | 73 | −20 | −35 | 1 | DG, CA1 |
Cluster Characterization
After having defined a functional parcellation map of the hippocampus, we characterized the subregions with regard to behavioral functions using BrainMap and NeuroSynth activation databases. We focused on the finer partitions (7 subregions) since an examination of changes in behavioral associations across subregions at this high partition level could provide novel insights into the functional dimensions, which has not been investigated previously. More concretely, at this high level of partitions, we could both track behavioral associations across the anterior–posterior gradient and explore medial–lateral differentiation.
Anterior Versus Posterior Functional Differentiation
The characterization with BrainMap and NeuroSynth revealed a functional gradient along the anterior–posterior axis of the hippocampus that, on the one hand, supported the hypothesis of an emotion–cognition gradient, and on the other hand, suggested a self-world-centric processing gradient.
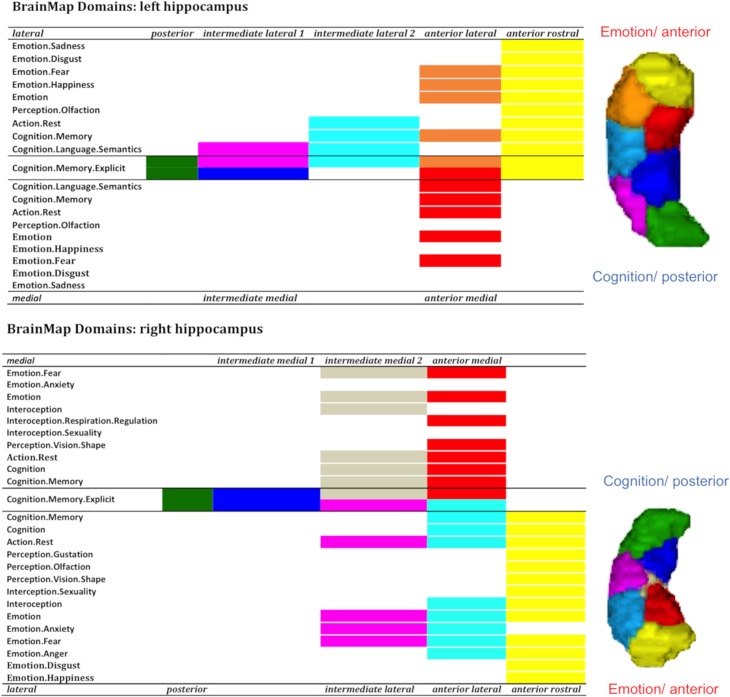
In BrainMap, anterior clusters were more likely engaged in emotion processing, whereas posterior subregions displayed a sparse functionality emphasizing higher cognition functions requiring abstract representations (e.g., Cognition.memory, Cognition.memory.explicit, Cognition.memory.language.semantics). (see Fig. 5). In addition, the anterior clusters were associated with various behavioral domains such as perception, interoception, and cognition, demonstrating a diverse behavioral spectrum.
Figure 5.
Anterior to posterior characterization with BrainMap.
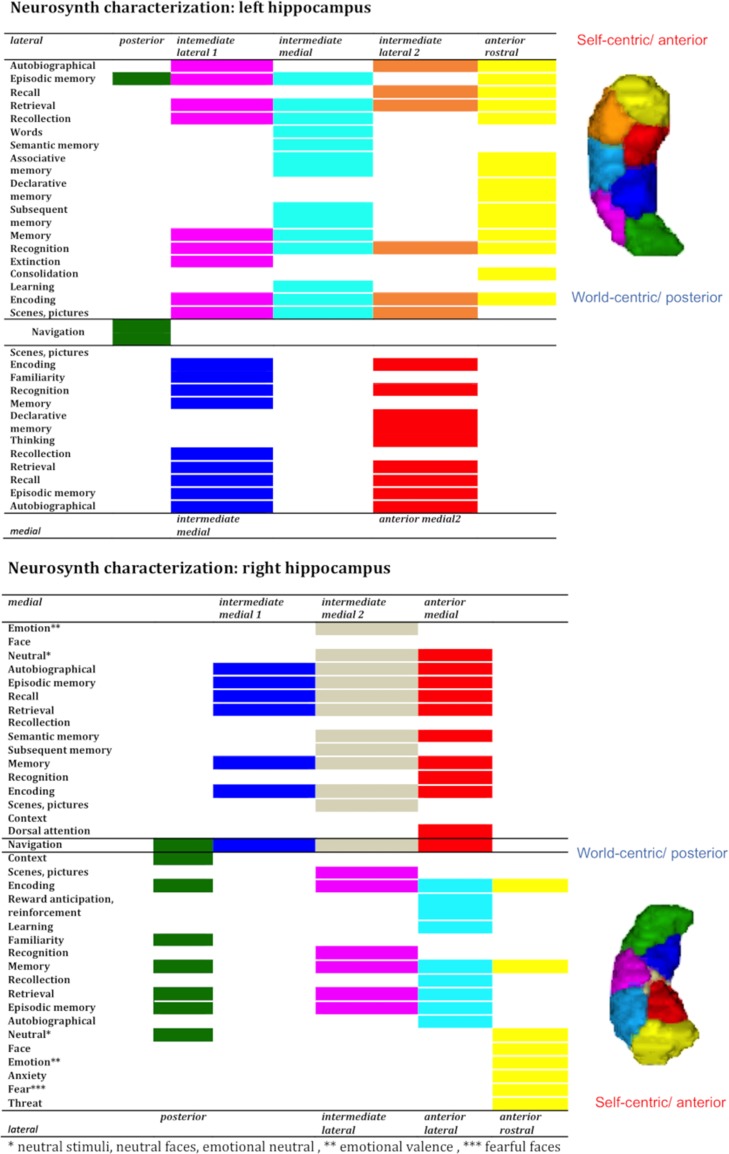
NeuroSynth provided a more detailed functional distinction along the anterior–posterior axis suggesting a gradient from self-centric (anterior parts) to more world-centric processing (posterior parts) as represented in Figure 6. The more anterior head clusters were engaged in cognitive and emotional processes related to personal experiences (e.g., episodic memory, experiences, autobiographical memory), whereas the more posterior clusters were associated with behavior like navigation (which requires the use of an abstract representation) and the processing of information in its environmental context. All the other intermediate body and head subregions showed a graduated profile within this qualitative behavioral gradient (see Fig. 6) and all clusters, independent of their position along the anterior–posterior axis, were associated with encoding, memory and retrieval processes. Interestingly, the gradients were especially evident in the lateral clusters (green, purple, light blue (orange), and yellow) of the hippocampus that were associated with CA subfields along the anterior–posterior axis as illustrated in Figures 5 and 6.
Figure 6.
Anterior to posterior characterization with NeuroSynth.
Medial Versus Lateral Functional Differentiation
In addition to an anterior–posterior organization, the parcellation yielded a medial versus lateral differentiation predominantly in the body and partly in the head. In order to explore the functional relevance of the medial–lateral axis, we merged the intermediate clusters into one medial VOI segment and one lateral VOI segment, while anterior rostral and posterior subregions were not integrated (see Fig. 4). Along the medial–lateral dimension, the functional differentiation was less obvious with only slight differences between the 2 segments (see Figs 5 and 6). The medial segments were engaged in perception (visual shape discrimination), interoception (respiration regulation), dorsal attention, and familiarity. Navigation, declarative memory, and thinking were also associated with medial parts. In contrast, the lateral segments seemed to assimilate information into the hippocampal memory system hence being engaged in associative memory, learning, reinforcement, and extinction. Finally, we observed a lateralization effect in the sense that left lateral parts were involved in words and language processing and the right lateral subregions in emotion processing of happiness, anger, and anxiety.
Discussion
In this study, we investigated the hippocampal organization in humans bridging the gap between hippocampal architecture and function using multimodal CBP and 2 complementary databases for functional characterization. In contrast to other CBP modalities, RSFC, which is especially sensitive to noise, required a preliminary optimal denoising, which we evaluated in this study in regard to clustering stability and voxels’ properties. Our results showed that the combination of a model-based (FIX) and a model-free (WM/CSF regression) denoising technique resulted in stable and biological plausible parcellations estimated through convergence across modalities. Especially, both pure functional modalities, MACM and RSFC, displayed high convergence at lower and higher parcellation granularities and could therefore be combined to derive a cross-modal functional map. We excluded SC from the cross-modal map as this modality demonstrated a relatively specific organization partly reflecting functional, as well as micro-architectonic characteristics. We emphasized and characterized the cross-modal 7-cluster hippocampus yielding a subdivision into one posterior cluster, 3 head clusters and depending on lateralization 2 or 3 intermediate clusters along the anterior–posterior and medial–lateral dimension. Following this, our behavioral profiling of the clusters revealed a functional emotion–cognition and a self-world-centric gradient along the anterior–posterior dimension, which seemed to be particularly evident along the lateral subregion and more pronounced in the right hemisphere. In the following sections, after briefly discussing our new methodological findings regarding denoising for RSFC-CBP, we discuss the new insight into hippocampus organization and function brought by the current study with regard to previous literature.
Optimal Denoising for RSFC-CBP
Our preliminary goal was to find a stable and consistent RSFC parcellation. But in the absence of unanimous guidelines of denoising approaches for RSFC-CBP in the scientific literature, we investigated various strategies with regard to stability, and voxels’ properties such as time course similarity and connectivity profile dissimilarity.
FIX + WM/CSF was found to contribute to highly stable partitions of RSFC-CBP, although other denoising techniques showed likewise high stability of parcellations. The subsequent examination of voxels’ properties on which the clustering builds suggested 2 potential mechanisms underlying higher stability of FIX + WM/CSF. First, the part of variance neutralized by FIX + WM/CSF seemed to contain structured noise, as seed voxels’ time course similarity highly decreased when this strategy was applied (when compared to not denoised data and other denoising strategies, except for FIX + GSR). Second, and more importantly in the application-driven perspective, FIX + WM/CSF increased the discrimination between voxels as reflected by the significant improvement of seed voxels’ connectivity profile dissimilarity. These influences eventually resulted in a better assignment of voxels to clusters. Overall our investigation promoted the combination of a model-based (FIX) and a model-free (WM/CSF regression) technique as an optimal denoising method, both from voxel-wise properties and partition-wise clustering. Burgess et al. (2016) already proposed to use FIX and GSR simultaneously in order to eliminate both local spatial artifacts and global drifts in fMRI data. Our results also suggested that the combination of FIX and GSR successfully removed structured noise outperforming FIX or GSR applied separately. FIX + GSR also led to stable parcellations in a similar extent than FIX + WM/CSF, but the use of FIX + WM/CSF was further supported by its improvement of voxels connectivity fingerprint discriminability, which is especially important in the clustering context. The reason why FIX + GSR performed less efficiently might be that GSR on one hand effectively neutralized motion artifacts, but on the other hand, distorted distance relationships (Murphy et al. 2009; Satterthwaite et al. 2013, 2017; Yan et al. 2013; Power et al. 2014) that influenced connectivity measures. Based on these considerations, we can assume that GSR can have detrimental effects for parcellation and that WM/CSF represents a better alternative for combination with FIX. It has been suggested that WM/CSF regression eliminates more effectively respiration and cardiac effects (Jo et al. 2010; Anderson et al. 2011; Liu 2016) and more generally, any slow undulations compared with GSR or FIX. FIX, in turn, could catch local or spatial-related artifacts, which cannot be captured by WM/CSF in the same way. For these reasons, we here suggest that the combination of FIX with WM/CSF represents the most sophisticated double-approach for denoising, in particular in the context of CBP.
A Convergent Functional Topography of the Hippocampus Across Different Measures of Functional Connectivity
In our study, 2 functional CBP modalities, task-independent (RSFC) and task-dependent (MACM), yielded CBP results with high convergence at the granularity of 3-, 5-, and 7-cluster partitions, despite divergent methodological procedures. This high similarity between conceptually related methods, but based on completely different procedures and independent objects of investigation (i.e., coactivations across paradigms vs. participants’ RS-fMRI) argued for biological relevance of the revealed topographical pattern. Importantly, the convergence in partition scheme between the 2 modalities cannot be attributed to an artifact intrinsic to the k-means clustering as a similar clustering procedure applied to structural covariance data revealed a different partition scheme. Indeed, SC-CBP parcellations deviated substantially from functional organizations already at low granularity even though at high granularity, this modality also contained a functional head separation, the medial–lateral differentiation within body and tail seemed to mirror cytoarchitectonic differentiation between cornu ammonis and subiculum. Although our goal was not to elucidate the relationship between functional aspects, microstructure, and SC, our parcellation work suggested that SC pattern could to a greater extent than functional connectivity be influenced by microstructural aspects. Future studies should further investigate the relationships between SC, microstructure, and functional connectivity across the human brain.
Overall, our findings converged with previous literature reporting studies in different methods and species, which further supported the biological validity of the obtained parcellations. In this context, one of the most prominent subdivisions for the hippocampus along the anterior–posterior axis is the tripartite model found in human and non-human segmentations. According to that model, the hippocampus is subdivided in an anterior (ventral, head), intermediate (body), and posterior (dorsal, tail) subregions, shown by anatomical (Swanson and Cowan 1977) and gene expression data in rodents (Dong et al. 2009), and CBP research in humans (Robinson et al. 2015, 2016).
Furthermore, Robinson et al. (2015) and Chase et al. (2015) extended the subdivision of the hippocampus and revealed an organization into 5 subregions for the entire hippocampus as well as for subiculum subfield using CBP. Robinson’s (2015) MACM parcellation yielded 4 serial clusters along the anterior–posterior axis and a fifth anterior head cluster tilted medially (see Fig. 1). Of note, this pattern was replicated in the current study by RSFC parcellation but does not appear as a prominent pattern retained in the cross-modal map. Beyond these minor differences between studies, nonspecies studies divided the CA1 subfield in rodents in 5 serial segments along the dorsoventral axis (Risold and Swanson 1996; Petrovich et al. 2001), supporting the observation and potential biological meaningfulness of serially aligned clusters in our and Robinson’s work.
To investigate hippocampus’ function, we capitalized on the consensual 7-cluster partition scheme as it provides a very detailed functional architecture representing anterior–posterior gradient into small units, which was never achieved before. Our cross-modal map of the hippocampus at this level exhibited 3 head clusters, 3 or 2 intermediate clusters dependent on lateralization and one tail cluster. Interestingly, the posterior cluster in the tail remained as a relatively homogeneous functional region across functional modalities and granularities. This level of fine parcellation also contains a medial–lateral differentiation, which seemed to reflect differences between cornu ammonis and subiculum, respectively. According to the current parcellation, these 2 regions could be partitioned into serially positioned clusters along the anterior–posterior axis, which is in line with other studies (Dong et al. 2009; Fanselow and Dong 2010). In other words, our clustering of 7 subregions seemed to reflect on the one hand the differentiation between cornu ammonis and subiculum and on the other hand further subdivisions along the anterior–posterior axis.
Functional Organization of the Hippocampus and Human Behavior
Based on the high convergence between RSFC and MACM, we computed a fine consensual parcellation combining both modalities. We then drew up the behavioral profile of each subregion using BrainMap and NeuroSynth as 2 complementary databases. We hence behaviorally characterized the anterior–posterior gradient and the medial–lateral differentiation taking an overarching view with activation databases.
Medial–Lateral Differentiation
We hypothesized that our organization along the medial–lateral axis reflected the differentiation between the subiculum (medial) and the CA subfields (lateral) evidenced by cytoarchitecture. Our behavioral profiling suggested that the medial segments participated in navigation, declarative memory, and familiarity, whereas the lateral segments were associated with reinforcement, learning, and extinction. Overall functional differences along the medial–lateral axis were sparse. Based on these behavioral descriptions we can only speculate that the lateral clusters were functionally involved in storing potentially integrating information into other systems and networks, whereas the functional specificity of the medial subregion was less evident. The conceptual inferences of the present study are limited on the one hand by the spatial precision of standard MRI measurements and on the other hand, by current cognitive ontologies which have been derived by the study of human behavior and mind. By making all our partitions openly available to the scientific community, we invite future studies to further complement these first integrative findings on hippocampus organization and function. Nevertheless and importantly, the medial–lateral differentiation in the current fine parcellation has revealed that the functional gradient proposed in previous studies is mainly evident along the lateral segment. This aspect of functional organization of the hippocampus has presumably complicated or obscured the characterization and understanding of the gradient. In the current study, extensive behavioral profiling of fine subdivisions has allowed us to discuss new hypothesis beyond common psychology distinctions of behavioral functions.
Anterior–Posterior Organization
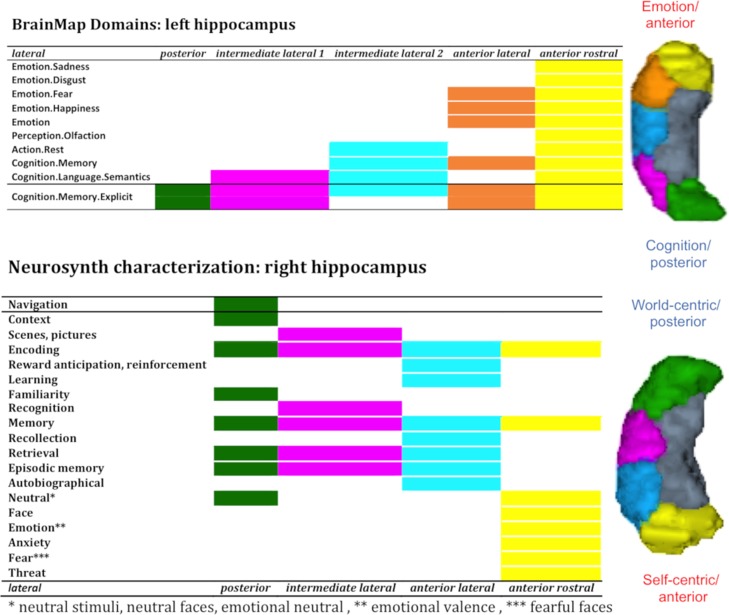
Evidence for an emotion–cognition and self-world-centric gradient. The present study brought new integrative insights across research fields on the longitudinal functional differentiation of the hippocampus previously demonstrated in rats (Moser et al. 1993; Jung et al. 1994; Moser and Moser 1998; Vann et al. 2000; de Hoz et al. 2003), monkeys (Colombo et al. 1998) and humans (Small et al. 2001; Poppenk et al. 2013; Robinson et al. 2015, 2016) by revealing an emotion–cognition gradient within the broad behavioral domains of BrainMap and a self-world-centric gradient based on more specific behavioral concepts in NeuroSynth. For reader’s convenience the main pattern is illustrated in Figure 7 in which we focused on lateral clusters. We discuss in the following these patterns with regard to previous hypotheses proposed in the literature.
Figure 7.
Emotion–cognition and self-world-centric functional gradient along the anterior–posterior axis. Lateral clusters display an emotion–cognition gradient yielded with BrainMap and a self-world-centric gradient found with NeuroSynth.
Besides the already often discussed differentiation of emotion–cognition along the anterior–posterior dimension that we replicated with both databases integrating the scientific knowledge of thousands of studies, we also speculated that hippocampal organization along the anterior–posterior axis could be better explained with a self-world-centric gradient. As actually almost all subregions are associated with memory processes, we speculate a self-centric information processing mode in the most anterior cluster with psychological functions such as autobiographical memory and emotion (see Fig. 7) contrasting with a world-centric processing of information calling concepts such as navigation, scene, and context processing associated with the most posterior hippocampal subregions. In other words, the overall pattern of behavioral concepts along the anterior–posterior axis suggest, in our view, a latent or underlying functional change from one pole of self-related processing to another pole of world-related processing. Importantly, general processes like encoding, and retrieval appeared equally distributed along the longitudinal axis. Thus, altogether our findings are more in favor of a self-world-centric information processing gradient rather than a behavioral domain-wise (imagination–perception (Zeidman and Maguire 2016) or encoding–retrieval organization (Lepage et al. 1998; Prince et al. 2005; Kim 2015)) along the anterior–posterior axis of the hippocampus. Importantly, this self- and world-centric distinction may be reminiscent of egocentric versus allocentric distinction suggested by studies of spatial processing in rodents (Morris et al. 1986) and is particularly evident in the right hemisphere, but their meaning in the human cognitive system should nevertheless be considered beyond spatial representation, that is, also in relation to memory and decision-making domains. If this hypothesis holds true, it has important implications for our understanding of psychiatric and neurological diseases but its validity remains to be further evaluated in future studies using hypothesis-based experiments.
Limitations. The large-scale data aggregation on which the current study capitalized also comes with specific limitations. First, we focused on MRI, a method, which has a relatively limited spatial resolution and a relatively limited signal-to-noise ratio in the subcortical structures. Therefore, the clusters we have obtained can only be considered as homogeneous regions with respect to the usual MRI signal. Accordingly, we assume that our lateral segment actually represents an aggregation of the known different CA subfields showing different cytoarchitecture and function. In particular, rodent studies suggested that CA1 and CA3 differ in their involvement in processes such as pattern separation and pattern completion (Guzowski et al. 2004). While these differentiations remain debated in humans (Deuker et al. 2014; Koster et al. 2018) in whom processes such as encoding, retrieval, and association between unrelated items have been additionally proposed to differentiate distinct subfields (Bakker et al. 2008; Deuker et al. 2014; Dimsdale-Zucker et al. 2018). These differentiations could not be investigated in the present study due to a lack of behavioral precision in the representative concepts of both activation databases, in addition to the aforementioned limited spatial resolution. Future studies should therefore investigate how the anterior–posterior functional differentiation could be integrated with the subfields functional specialization in the hippocampus.
Another relevant limiting point refers to the complex structure of the hippocampus itself and its consequences for the optimal number of clusters. The human hippocampus is characterized by angulation and a variable number of digitations (Wisse et al. 2012; Ding and Van Hoesen 2015; Treit et al. 2018). Both features could have influenced our results in terms of the optimal number of clusters. Due to the limited spatial resolution of MRI data, we may have missed differences in connectivity profiles of conflated subfields in the posterior hippocampus hence leading to a single-tail cluster in the present study. Additionally, hippocampal gyrification, known as digitations, vary between individuals and have been previously discussed as a possible factor for interindividual variability in the hippocampus (Ding and Van Hoesen 2015; Chang et al. 2018; DeKraker et al. 2018; Treit et al. 2018). How this affects cognition and psychopathology (Oppenheim et al. 1998) and whether different digitations have different connectivity profiles and hence could influence clustering pattern is still unclear. This question should be addressed in future studies with high-spatial precision techniques. Overall, the maps and conceptual findings reported in the present study are useful for the specific mapping modality they have been derived from, that is, conventional field MRI in humans.
Conclusions
In the present study, we established for the first time a robust and stable RSFC hippocampal parcellation by applying a combination of a model-free and a model-based denoising framework. By combining partitions based on spontaneous connectivity with partitions based on task-based connectivity, we built the first cross-modal generic hippocampal map at different levels of partition. Extensive behavioral profiling of the finest partition allowed inferences regarding the nature of information processing principles along the anterior–posterior axis in the hippocampus, beyond the concepts derived from psychological studies in specific fields. Importantly, while previous characterization of the anterior–posterior differentiation based on these concepts cannot be refuted and were partially supported, they could not account for the range of associations observed by our quantitative approaches. In turn, we proposed a self-world-centric processing mode gradient along the anterior–posterior axis in humans, a data-based hypothesis that should be further investigated with specific model-based approaches. Further functional decoding allowed us to speculate that the medial–lateral distinction represented an assimilating process for the lateral part integrating information across different systems. Importantly, our medial–lateral distinction for the first time evidenced that the anterior–posterior gradient is predominantly observed in the lateral part of the hippocampus and an independent mapping approach based on structural data (structural covariance) further evidenced a medial–lateral distinction. Finally, the pattern of separation revealed by structural covariance appeared as a hybridization of functional connectivity and microstructure hence bringing new light into this relatively understudied mapping modality and offering an alternative and potentially better partition for compression of structural data (cfr. (Varikuti et al. 2018)). All our unimodal and cross-modal maps are available in the ANIMA database (http://anima.fz-juelich.de/) to support future hippocampal investigations of hippocampal function in healthy or pathological populations.
Supplementary Material
Notes
The authors also thank Dr Nicola Palomero-Gallagher and Shahrzad Kharabian for fruitful discussions. Conflict of Interest: None declared.
Funding
Deutsche Forschungsgemeinschaft (DFG, GE 2835/1-1, EI 816/4-1), the National Institute of Mental Health (R01-MH074457), the Helmholtz Portfolio Theme “Supercomputing and Modelling for the Human Brain,” and the European Union’s Horizon 2020 Research and Innovation Programme under Grant Agreement No. 720270 (HBP SGA1) and No. 785907 (HBP SGA2).
References
- Adler DH, Pluta J, Kadivar S, Craige C, Gee JC, Avants BB, Yushkevich PA. 2014. Histology-derived volumetric annotation of the human hippocampal subfields in postmortem MRI . Neuroimage. 84:505–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler DH, Wisse LEM, Ittyerah R, Pluta JB, Ding SL, Xie L, Wang J, Kadivar S, Robinson JL, Schuck T, et al. . 2018. Characterizing the human hippocampus in aging and alzheimer’s disease using a computational atlas derived from ex vivo MRI and histology. Proc Natl Acad Sci USA. 115(16):4252–4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnan A, Barnett A, Moayedi M, McCormick C, Cohn M, McAndrews MP. 2016. Distinct hippocampal functional networks revealed by tractography-based parcellation. Brain Struct Funct. 221(6):2999–3012. [DOI] [PubMed] [Google Scholar]
- Alexander-Bloch A, Raznahan A, Bullmore E, Giedd J. 2013. The convergence of maturational change and structural covariance in human cortical networks. J Neurosci. 33(7):2889–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K.. 2005. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol. 210(5–6):343–352. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong EK, Desai K, Yurgelun-Todd D. 2011. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum Brain Mapp. 32(6):919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan S, Ktena SI, Makropoulos A, Robinson EC, Rueckert D, Parisot S. 2018. Human brain mapping: a systematic comparison of parcellation methods for the human cerebral cortex. Neuroimage. 170:5–30. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. 2005. Unified segmentation. Neuroimage. 26(3):839–851. [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. 2008. Pattern separation in the human hippocampal ca3 and dentate gyrus. Science. 319(5870):1640–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. 2004. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 23(2):137–152. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, et al. . 2003. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 6(7):750–757. [DOI] [PubMed] [Google Scholar]
- Bellec P, Rosa-Neto P, Lyttelton OC, Benali H, Evans AC. 2010. Multi-level bootstrap analysis of stable clusters in resting-state fMRI. Neuroimage. 51(3):1126–1139. [DOI] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Shmueli K, Duyn JH. 2009. Sources of functional magnetic resonance imaging signal fluctuations in the human brain at rest: a 7 t study. Magn Reson Imaging. 27(8):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. 2006. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 31(4):1536–1548. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 34(4):537–541. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BT. 2013. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 16(7):832–837. [DOI] [PubMed] [Google Scholar]
- Burgess GC, Kandala S, Nolan D, Laumann TO, Power JD, Adeyemo B, Harms MP, Petersen SE, Barch DM. 2016. Evaluation of denoising strategies to address motion-correlated artifacts in resting-state functional magnetic resonance imaging data from the human connectome project. Brain Connectivity. 6(9):669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero-Gaudes C, Reynolds RC. 2017. Methods for cleaning the bold FMRI signal. Neuroimage. 154:128–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Huang C, Zhou N, Li SX, Ver Hoef L, Gao Y. 2018. The bumps under the hippocampus. Hum Brain Mapp. 39(1):472–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Clos M, Dibble S, Fox P, Grace AA, Phillips ML, Eickhoff SB. 2015. Evidence for an anterior-posterior differentiation in the human hippocampal formation revealed by meta-analytic parcellation of fMRI coordinate maps: focus on the subiculum. Neuroimage. 113:44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos M, Amunts K, Laird AR, Fox PT, Eickhoff SB. 2013. Tackling the multifunctional nature of broca’s region meta-analytically: co-activation-based parcellation of area 44. Neuroimage. 83:174–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos M, Rottschy C, Laird AR, Fox PT, Eickhoff SB. 2014. Comparison of structural covariance with functional connectivity approaches exemplified by an investigation of the left anterior insula. Neuroimage. 99:269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. 2010. Advances and pitfalls in the analysis and interpretation of resting-state fMRI data. Front Syst Neurosci. 4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Fernandez T, Nakamura K, Gross CG. 1998. Functional differentiation along the anterior-posterior axis of the hippocampus in monkeys. J Neurophysiol. 80(2):1002–1005. [DOI] [PubMed] [Google Scholar]
- Dagli MS, Ingeholm JE, Haxby JV. 1999. Localization of cardiac-induced signal change in fMRI. Neuroimage. 9(4):407–415. [DOI] [PubMed] [Google Scholar]
- de Flores R, La Joie R, Chetelat G. 2015. Structural imaging of hippocampal subfields in healthy aging and Alzheimer’s disease. Neuroscience. 309:29–50. [DOI] [PubMed] [Google Scholar]
- de Hoz L, Knox J, Morris RG. 2003. Longitudinal axis of the hippocampus: both septal and temporal poles of the hippocampus support water maze spatial learning depending on the training protocol. Hippocampus. 13(5):587–603. [DOI] [PubMed] [Google Scholar]
- de Munck JC, Goncalves SI, Faes TJ, Kuijer JP, Pouwels PJ, Heethaar RM, Lopes da Silva FH. 2008. A study of the brain’s resting state based on alpha band power, heart rate and fMRI. Neuroimage. 42(1):112–121. [DOI] [PubMed] [Google Scholar]
- DeKraker J, Ferko KM, Lau JC, Kohler S, Khan AR. 2018. Unfolding the hippocampus: an intrinsic coordinate system for subfield segmentations and quantitative mapping. Neuroimage. 167:408–418. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. . 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31(3):968–980. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF. 2001. Removal of confounding effects of global signal in functional MRI analyses. Neuroimage. 13(4):751–758. [DOI] [PubMed] [Google Scholar]
- Deuker L, Doeller CF, Fell J, Axmacher N. 2014. Human neuroimaging studies on the hippocampal CA3 region—integrating evidence for pattern separation and completion. Front Cell Neurosci. 8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimsdale-Zucker HR, Ritchey M, Ekstrom AD, Yonelinas AP, Ranganath C. 2018. CA1 and CA3 differentially support spontaneous retrieval of episodic contexts within human hippocampal subfields. Nat Commun. 9(1):294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen GW. 2015. Organization and detailed parcellation of human hippocampal head and body regions based on a combined analysis of cyto- and chemoarchitecture. J Comp Neurol. 523(15):2233–2253. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW, Chen L, Fanselow MS, Toga AW. 2009. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc Natl Acad Sci USA. 106(28):11794–11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B. 1979. Bootstrap methods: another look at the jackknife. Ann Statist. 7(1):1–26. [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. 2012. Activation likelihood estimation meta-analysis revisited. Neuroimage. 59(3):2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. 2005. A new spm toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 25(4):1325–1335. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Thirion B, Varoquaux G, Bzdok D. 2015. Connectivity-based parcellation: critique and implications. Hum Brain Mapp. 36(12):4771–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Yeo T, Genon S. 2018. Imaging-based parcellations of the human brain. Nat Rev Neurosci. 19(11):672–686. [DOI] [PubMed] [Google Scholar]
- Evans AC. 2013. Networks of anatomical covariance. Neuroimage. 80:489–504. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Dong HW. 2010. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 65(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genon S, Li H, Fan L, Muller VI, Cieslik EC, Hoffstaedter F, Reid AT, Langner R, Grefkes C, Fox PT, et al. . 2017. The right dorsal premotor mosaic: Organization, functions, and connectivity. Cereb cortex. 27(3):2095–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genon S, Reid A, Langner R, Amunts K, Eickhoff SB. 2018. How to characterize the function of a brain region. Trends Cogn Sci. 22(4):350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genon S, Reid A, Li H, Fan L, Muller VI, Cieslik EC, Hoffstaedter F, Langner R, Grefkes C, Laird AR, et al. . 2018. The heterogeneity of the left dorsal premotor cortex evidenced by multimodal connectivity-based parcellation and functional characterization. Neuroimage. 170:400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, et al. . 2016. A multi-modal parcellation of human cerebral cortex. Nature. 536(7615):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. 2004. Ensemble dynamics of hippocampal regions ca3 and ca1. Neuron. 44(4):581–584. [DOI] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, Luna B. 2013. The nuisance of nuisance regression: spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. Neuroimage. 82:208–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS. 2013. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp. 34(9):2154–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Gotts SJ, Reynolds RC, Bandettini PA, Martin A, Cox RW, Saad ZS. 2013. Effective preprocessing procedures virtually eliminate distance-dependent motion artifacts in resting state fMRI. J Appl Math. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. 2010. Mapping sources of correlation in resting state fMRI, with artifact detection and removal. Neuroimage. 52(2):571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM. 2004. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA. 101(36):13335–13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Wiener S, McNaughton B. 1994. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 14(12):7347–7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. 2015. Encoding and retrieval along the long axis of the hippocampus and their relationships with dorsal attention and default mode networks: the hernet model. Hippocampus. 25(4):500–510. [DOI] [PubMed] [Google Scholar]
- Komorowski RW, Garcia CG, Wilson A, Hattori S, Howard MW, Eichenbaum H.. 2013. Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. J Neurosci. 33(18):8079–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster R, Chadwick MJ, Chen Y, Berron D, Banino A, Duzel E, Hassabis D, Kumaran D. 2018. Big-loop recurrence within the hippocampal system supports integration of information across episodes. Neuron. 99(6):1342–1354.e1346.. [DOI] [PubMed] [Google Scholar]
- Kotkowski E, Price LR, Mickle Fox P, Vanasse TJ, Fox PT. 2018. The hippocampal network model: a transdiagnostic metaconnectomic approach. Neuroimage Clin. 18:115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaConte S, Anderson J, Muley S, Ashe J, Frutiger S, Rehm K, Hansen LK, Yacoub E, Hu X, Rottenberg D, et al. . 2003. The evaluation of preprocessing choices in single-subject bold fMRI using npairs performance metrics. Neuroimage. 18(1):10–27. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ Jr., McKay DR, Bzdok D, Laird RW, Robinson JL, et al. . 2011. The brainmap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res Notes. 4:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert C, Simon H, Colman J, Barrick TR. 2017. Defining thalamic nuclei and topographic connectivity gradients in vivo. Neuroimage. 158:466–479. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. 1998. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus. 8(4):313–322. [DOI] [PubMed] [Google Scholar]
- Liu TT. 2016. Noise contributions to the fMRI signal: an overview. Neuroimage. 143:141–151. [DOI] [PubMed] [Google Scholar]
- Macey PM, Macey KE, Kumar R, Harper RM. 2004. A method for removal of global effects from fMRI time series. Neuroimage. 22(1):360–366. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Friston KJ, Frackowiak RS, Price CJ. 2005. Structural covariance in the human cortex. J Neurosci. 25(36):8303–8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Hagan JJ, Rawlins JN. 1986. Allocentric spatial learning by hippocampectomised rats: a further test of the “spatial mapping” and “working memory” theories of hippocampal function. Q J Exp Psychol B. 38(4):365–395. [PubMed] [Google Scholar]
- Moser MB, Moser EI. 1998. Functional differentiation in the hippocampus. Hippocampus. 8(6):608–619. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. 1993. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 13(9):3916–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Wang D, Fox MD, Yeo BT, Sepulcre J, Sabuncu MR, Shafee R, Lu J, Liu H. 2013. Individual variability in functional connectivity architecture of the human brain. Neuron. 77(3):586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. 2009. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 44(3):893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanetti L, Cerliani L, Gazzola V, Renken R, Keysers C. 2009. Group analyses of connectivity-based cortical parcellation using repeated k-means clustering. Neuroimage. 47(4):1666–1677. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. 1997. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 6(3):156–167. [DOI] [PubMed] [Google Scholar]
- Oppenheim C, Dormont D, Biondi A, Lehericy S, Hasboun D, Clemenceau S, Baulac M, Marsault C. 1998. Loss of digitations of the hippocampal head on high-resolution fast spin-echo mr: a sign of mesial temporal sclerosis. AJNR Am J Neuroradiol. 19(3):457–463. [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. 2001. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 38(1–2):247–289. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. 2013. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 17(5):230–240. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. 2014. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE. 2015. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 105:536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. 2005. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. J Neurosci. 25(5):1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AT, Bzdok D, Langner R, Fox PT, Laird AR, Amunts K, Eickhoff SB, Eickhoff CR. 2016. Multimodal connectivity mapping of the human left anterior and posterior lateral prefrontal cortex. Brain Struct Funct. 221(5):2589–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risold PY, Swanson LW. 1996. Structural evidence for functional domains in the rat hippocampus. Science. 272(5267):1484–1486. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Barron DS, Kirby LA, Bottenhorn KL, Hill AC, Murphy JE, Katz JS, Salibi N, Eickhoff SB, Fox PT. 2015. Neurofunctional topography of the human hippocampus. Hum Brain Mapp. 36(12):5018–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JL, Salibi N, Deshpande G. 2016. Functional connectivity of the left and right hippocampi: Evidence for functional lateralization along the long-axis using meta-analytic approaches and ultra-high field functional neuroimaging. Neuroimage. 135:64–78. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. 2014. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 90:449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Ciric R, Roalf DR, Davatzikos C, Bassett DS, Wolf DH. 2017. Motion artifact in studies of functional connectivity: characteristics and mitigation strategies. Hum Brain Mapp. https://www.ncbi.nlm.nih.gov/pubmed/29091315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, et al. . 2013. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage. 64:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, Eickhoff SB, Yeo BTT. 2017. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 28(9):3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. 2009. Neurodegenerative diseases target large-scale human brain networks. Neuron. 62(1):42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Nava AS, Perera GM, DeLaPaz R, Mayeux R, Stern Y. 2001. Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation. Nat Neurosci. 4(4):442–449. [DOI] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, Duff E, Feinberg DA, Griffanti L, Harms MP, et al. . 2013. Resting-state fMRI in the human connectome project. Neuroimage. 80:144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ. 1999. Segregating the functions of human hippocampus. Proc Natl Acad Sci USA. 96(7):4034–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. 2014. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 15(10):655–669. [DOI] [PubMed] [Google Scholar]
- Strother SC, Anderson J, Hansen LK, Kjems U, Kustra R, Sidtis J, Frutiger S, Muley S, LaConte S, Rottenberg D. 2002. The quantitative evaluation of functional neuroimaging experiments: the NPAIRS data analysis framework. Neuroimage. 15(4):747–771. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. 1977. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 172(1):49–84. [DOI] [PubMed] [Google Scholar]
- Treit S, Steve T, Gross DW, Beaulieu C. 2018. High resolution in-vivo diffusion imaging of the human hippocampus. Neuroimage. 182:479–487. [DOI] [PubMed] [Google Scholar]
- Ugurbil K, Xu J, Auerbach EJ, Moeller S, Vu AT, Duarte-Carvajalino JM, Lenglet C, Wu X, Schmitter S, Van de Moortele PF, et al. . 2013. Pushing spatial and temporal resolution for functional and diffusion MRI in the human connectome project. Neuroimage. 80:80–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. 2010. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 103(1):297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. 2012. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 59(1):431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K. 2012. The future of the human connectome. Neuroimage. 62(2):1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Brown MW, Erichsen JT, Aggleton JP. 2000. Fos imaging reveals differential patterns of hippocampal and parahippocampal subfield activation in rats in response to different spatial memory tests. J Neurosci. 20(7):2711–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varikuti DP, Genon S, Sotiras A, Schwender H, Hoffstaedter F, Patil KR, Jockwitz C, Caspers S, Moebus S, Amunts K, et al. . 2018. Evaluation of non-negative matrix factorization of grey matter in age prediction. Neuroimage. 173:394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varikuti DP, Hoffstaedter F, Genon S, Schwender H, Reid AT, Eickhoff SB. 2017. Resting-state test-retest reliability of a priori defined canonical networks over different preprocessing steps. Brain Struct Funct. 222(3):1447–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. 2009. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 47(4):1408–1416. [DOI] [PubMed] [Google Scholar]
- Windischberger C, Langenberger H, Sycha T, Tschernko EM, Fuchsjager-Mayerl G, Schmetterer L, Moser E. 2002. On the origin of respiratory artifacts in BOLD-EPI of the human brain. Magn Reson Imaging. 20(8):575–582. [DOI] [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. 2004. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in bold signal. Neuroimage. 21(4):1652–1664. [DOI] [PubMed] [Google Scholar]
- Wisse LE, Gerritsen L, Zwanenburg JJ, Kuijf HJ, Luijten PR, Biessels GJ, Geerlings MI. 2012. Subfields of the hippocampal formation at 7 T MRI: In vivo volumetric assessment. Neuroimage. 61(4):1043–1049. [DOI] [PubMed] [Google Scholar]
- Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo XN, Castellanos FX, Milham MP. 2013. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 76:183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. 2011. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 8(8):665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee Y, Fernandes DJ, French L, Ellegood J, Cahill LS, Vousden DA, Spencer Noakes L, Scholz J, van Eede MC, Nieman BJ, et al. . 2017. Structural covariance of brain region volumes is associated with both structural connectivity and transcriptomic similarity. bioRxiv. [DOI] [PubMed]
- Zeidman P, Maguire EA. 2016. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 17(3):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mormino EC, Sun N, Sperling RA, Sabuncu MR, Yeo BT. 2016. Bayesian model reveals latent atrophy factors with dissociable cognitive trajectories in Alzheimer’s disease. Proc Natl Acad Sci USA. 113(42):E6535–E6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.