Abstract
Background
Polyunsaturated fatty acid (PUFA) supplements, involving omega‐3 and/or omega‐6 components, have been proposed as a therapy for dry eye. Omega‐3 PUFAs exist in both short‐ (alpha‐linolenic acid [ALA]) and long‐chain (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) forms, which largely derive from certain plant‐ and marine‐based foods respectively. Omega‐6 PUFAs are present in some vegetable oils, meats, and other animal products.
Objectives
To assess the effects of omega‐3 and omega‐6 polyunsaturated fatty acid (PUFA) supplements on dry eye signs and symptoms.
Search methods
CENTRAL, Medline, Embase, two other databases and three trial registries were searched in February 2018, together with reference checking. A top‐up search was conducted in October 2019, but the results have not yet been incorporated.
Selection criteria
We included randomized controlled trials (RCTs) involving dry eye participants, in which omega‐3 and/or omega‐6 supplements were compared with a placebo/control supplement, artificial tears, or no treatment. We included head‐to‐head trials comparing different forms or doses of PUFAs.
Data collection and analysis
We followed standard Cochrane methods and assessed the certainty of the evidence using GRADE.
Main results
We included 34 RCTs, involving 4314 adult participants from 13 countries with dry eye of variable severity and etiology. Follow‐up ranged from one to 12 months. Nine (26.5%) studies had published protocols and/or were registered. Over half of studies had high risk of bias in one or more domains.
Long‐chain omega‐3 (EPA and DHA) versus placebo or no treatment (10 RCTs)
We found low certainty evidence that there may be little to no reduction in dry eye symptoms with long‐chain omega‐3 versus placebo (four studies, 677 participants; mean difference [MD] ‐2.47, 95% confidence interval [CI] ‐5.14 to 0.19 units). We found moderate certainty evidence for a probable benefit of long‐chain omega‐3 supplements in increasing aqueous tear production relative to placebo (six studies, 1704 participants; MD 0.68, 95% CI 0.26 to 1.09 mm/5 min using the Schirmer test), although we did not judge this difference to be clinically meaningful. We found low certainty evidence for a possible reduction in tear osmolarity (one study, 54 participants; MD ‐17.71, 95% CI ‐28.07 to ‐7.35 mOsmol/L). Heterogeneity was too substantial to pool data on tear break‐up time (TBUT) and adverse effects.
Combined omega‐3 and omega‐6 versus placebo (four RCTs)
For symptoms (low certainty) and ocular surface staining (moderate certainty), data from the four included trials could not be meta‐analyzed, and thus effects on these outcomes were unclear. For the Schirmer test, we found moderate certainty evidence that there was no intergroup difference (four studies, 455 participants; MD: 0.66, 95% CI ‐0.45 to 1.77 mm/5 min). There was moderate certainty for a probable improvement in TBUT with the PUFA intervention relative to placebo (four studies, 455 participants; MD 0.55, 95% CI 0.04 to 1.07 seconds). Effects on tear osmolarity and adverse events were unclear, with data only available from a single small study for each outcome.
Omega‐3 plus conventional therapy versus conventional therapy alone (two RCTs)
For omega‐3 plus conventional therapy versus conventional therapy alone, we found low certainty evidence suggesting an intergroup difference in symptoms favoring the omega‐3 group (two studies, 70 participants; MD ‐7.16, 95% CI ‐13.97 to ‐0.34 OSDI units). Data could not be combined for all other outcomes.
Long‐chain omega‐3 (EPA and DHA) versus omega‐6 (five RCTs)
For long‐chain omega‐3 versus omega‐6 supplementation, we found moderate certainty evidence for a probable improvement in dry eye symptoms (two studies, 130 participants; MD ‐11.88, 95% CI ‐18.85 to ‐4.92 OSDI units). Meta‐analysis was not possible for outcomes relating to ocular surface staining, Schirmer test or TBUT. We found low certainty evidence for a potential improvement in tear osmolarity (one study, 105 participants; MD ‐11.10, 95% CI ‐12.15 to ‐10.05 mOsmol/L). There was low level certainty regarding any potential effect on gastrointestinal side effects (two studies, 91 participants; RR 2.34, 95% CI 0.35 to 15.54).
Authors' conclusions
Overall, the findings in this review suggest a possible role for long‐chain omega‐3 supplementation in managing dry eye disease, although the evidence is uncertain and inconsistent. A core outcome set would work toward improving the consistency of reporting and the capacity to synthesize evidence.
Plain language summary
Omega‐3 and omega‐6 polyunsaturated fatty acid supplements for dry eye disease
What is the aim of this review? Dry eye is a long‐term eye condition that can lead to eye discomfort and changes to vision. Omega‐3 and omega‐6 supplements, including the omega‐3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have been studied as a treatment for dry eye. This Cochrane Review summarizes the best available research evidence.
Key messages More research is needed to gain a full understanding of the role of omega‐3 and omega‐6 supplements in treating dry eye disease, particularly with how to use this therapy to treat dry eye due to different causes and severities. There is also a need for more research to provide information about how the supplement characteristics (eg, dose, form, composition) affect clinical outcomes.
What was studied in the review? The main outcome was improvement in dry eye symptoms, measured after at least one month of follow‐up. Secondary outcomes considered a range of clinical measures and side effects.
What are the main results of the review? We included 34 randomized controlled trials (RCTs) involving more than 4314 adult participants from 13 countries.
Although much of the evidence was uncertain, long‐chain omega‐3 supplements may have little to no benefit, relative to placebo, on dry eye symptoms, but did improve some clinical signs. There was a beneficial effect on dry eye symptoms when omega‐3 supplements were combined with standard dry eye treatments (eg, artificial tears, eyelid warm compresses, corticosteroid eye drops) compared to standard treatment alone, and when long‐chain omega‐3 supplements were compared with omega‐6 supplements. The most common side effect was temporary gastrointestinal problems.
For combined omega‐3 and omega‐6 supplements, relative to placebo, there was no benefit for tear production, and a small amount of improvement in the stability of the tears. Effects on other clinical measures, including dry eye symptoms and side effects, could not be clearly determined. It is also unclear whether other types of supplement combinations are effective for treating dry eye. We have low to moderate confidence in the evidence for all outcomes.
These findings suggest that long‐chain omega‐3 supplements may have a role in managing dry eye, however the evidence is currently inconsistent and more research is needed.
How up‐to‐date is this review? The Cochrane review authors searched for studies that had been published up to February 2018. A top‐up search was conducted in October 2019, but the results have not yet been incorporated.
Summary of findings
Background
Description of the condition
Dry eye disease, as recently defined in the Tear Film and Ocular Surface (TFOS) International Dry Eye WorkShop II (DEWS II) report, is a "multifactorial disease of the ocular surface characterized by loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neuro‐sensory abnormalities play aetiological roles” (Craig 2017). Dry eye disease is highly prevalent. Based upon epidemiological studies, the prevalence of dry eye worldwide varies considerably depending on the study population, the definition of dry eye, and the diagnostic criteria used. In adults, the prevalence of dry eye signs, with the presence or absence of symptoms, has been reported to range from approximately 5% to 50% (Stapleton 2017). It has been estimated that in the United States, more than five million adults aged 50 years or older have clinically significant dry eye disease (Schaumberg 2003; Schaumberg 2009). A range of risk factors for the condition have been identified and include advancing age, female sex, race (in particular, Asian ethnicity), certain medical conditions (eg, connective tissue disease, Sjögren's syndrome, androgen deficiency, diabetes), contact lens wear, some systemic medications (eg, estrogen replacement therapy, antihistamines, anxiolytics, antidepressants, diuretics), and certain environmental conditions (eg, computer use, low ambient humidity, air pollution) (Stapleton 2017).
Dry eye disease typically is associated with symptoms of ocular discomfort, including foreign body sensation, dryness, irritation, burning, and light sensitivity (Lemp 1995; Miljanovic 2005). The condition can have a major negative impact on quality of life (Schiffman 2003). Dry eye disease also is highly correlated with anxiety and depression (Li 2011). In 2011, the direct economic burden of dry eye disease in the United States was estimated to be $3.4B per annum (Yu 2011). With respect to indirect costs, the burden of disease is due primarily to losses in workplace productivity (Yu 2011).
In terms of its etiology, dry eye disease involves perturbation(s) to the lacrimal functional unit comprising the lacrimal gland and its accessory glands, ocular surface components (eg, cornea, conjunctiva), meibomian glands, and eyelids and their associated sensory and motor innervation (Stern 1998). Under physiologic conditions, the integrated lacrimal functional unit regulates tear secretion, distribution, and clearance to maintain ocular surface integrity. Disruption to one or more of its components promotes loss of tear homeostasis and tear film dysfunction. Dry eye disease can be primarily aqueous deficient or evaporative in etiology (Craig 2017); the former involves primarily reduced lacrimal grand secretion, whereas the latter is due predominantly to abnormalities in the lipid‐secreting meibomian glands.
Clinically, the diagnosis of dry eye can be challenging, as objective clinical signs and self‐reported symptoms are not well correlated (Downie 2015a; Johnson 2009; Nichols 2004). Although more recent studies indicate that this might be so because some ocular surface and tear film parameters are too dynamic for reliable measurement at rest, and that subjecting patients to a desiccating stress correlates better with "real‐life" tear film function (Karakus 2018). In 2017, the TFOS DEWS II Diagnostic Methodology subcommittee report outlined a contemporary approach to dry eye diagnosis, factoring in the quality of research evidence for current tests and their clinical accessibility (Wolffsohn 2017). This approach involves consideration of both patient symptoms and key clinical signs that are indicative of loss of tear film homeostasis (ie, tear instability, tear hyperosmolarity, and ocular surface damage) (Downie 2015c; Wolffsohn 2017).
In terms of management and therapy for dry eye disease, both pharmacologic and non‐pharmacologic therapeutic options are available (AAO 2013; Jones 2017). Although the mainstay of therapy involves artificial tear drops to supplement the tear film, this modality provides only temporary symptomatic relief, and up to two‐thirds of dry eye sufferers remaining symptomatic despite adherence to treatment (Downie 2015b). Depending on disease severity, other management options include devices or procedures for tear retention (eg, punctal occlusion, moisture‐conserving spectacles, contact lenses), devices or procedures for tear stimulation (eg, intranasal neurogenic tear stimulation, vectored thermal pulsation), pharmacologic tear stimulation (ie, secretagogues), biological tear substitutes (eg, serum and salivary gland autotransplantation), anti‐inflammatory medications (eg, cyclosporine, lifitegrast, corticosteroids, tetracyclines), surgical approaches, dietary interventions, environmental modifications, and complementary therapies. As recently reviewed, there is currently a paucity of high‐quality randomized controlled trial (RCT) evidence for many of these treatment options (Jones 2017), and this poses a challenge for implementing best practice clinical care, particularly with regard to instituting specific treatments for patients with different dry eye subtypes.
Description of the intervention
Fatty acids are composed of a methyl group at one end, a hydrocarbon chain in the middle, and a carboxyl group at the other end. Polyunsaturated fatty acids (PUFAs) are fatty acids that have two or more double bonds in the hydrocarbon chain. Omega‐3 and omega‐6 PUFAs have their first double bond located at the 3‐ or 6‐carbon from the methyl end, respectively. Because humans are unable to synthesize either omega‐3 or omega‐6 PUFAs in vivo, these fatty acids must be obtained from the diet or through supplementation.
Omega‐3 PUFAs exist as both long‐chain (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) and short‐chain (α‐linolenic acid [ALA]) subtypes. Short‐chain omega‐3s are present in relatively high amounts in certain vegetable oils (eg, flaxseed oil, canola oil) and terrestrial plants (eg, walnuts, chia seeds). Long‐chain omega‐3s derive predominantly from oily fish (eg, salmon, mackerel, anchovies, sardines) and to a lesser extent from other marine sources (eg, oysters, mussels, prawns) (James 2000; Kris‐Etherton 2007). Within the body, short‐chain ALA can be converted to the long‐chain omega‐3 PUFAs (EPA and DHA), although the efficiency of this conversion may be age‐dependent (Brenna 2002). Omega‐6 PUFAs, such as arachidonic acid and linoleic acid, are present in some vegetable oils (eg, corn, safflower, sunflower seed), meats (eg, poultry), and other animal products (James 2000; Kris‐Etherton 2007).
The American Dietetic Association (ADA), jointly with the Dietitians of Canada (DC), recommend that dietary fat for adults should provide 20% to 35% of daily energy requirements, with an intake of omega‐3 PUFAs of 500 mg per day, which is equivalent to consuming approximately 8 oz (227 g) of oily fish per week (Kris‐Etherton 2007). Adequate intakes (AIs) for omega‐6 PUFAs, which are estimated to cover the needs of individuals based on age and gender, are 17 g per day for men aged 19 to 50 years, 14 g per day for men older than 50 years, 12 g per day for women aged 19 to 50 years, and 11 g per day for women older than 50 years (IOM 2002). The recommended range for omega‐6 PUFAs is 3% to 10% of energy intake (Kris‐Etherton 2007). Table 6 summarizes the classification, structure, common food sources, and dietary recommendations for omega‐3 and omega‐6 PUFAs.
2. Table 1. Omega‐3 and omega‐6 polyunsaturated fatty acids ‐ structure, common food sources, and recommendations.
| Polyunsaturated fatty acid (PUFA) classification | Structure (number of carbons:number of double bonds) | Example food sources | Recommendations from the American Dietetic Association and Dietitians of Canada (Kris‐Etherton 2007) | |
| Omega‐3 | Alpha‐linolenic acid (ALA) | C18:3 | Flaxseed, canola oil, soybean oil | 0.6% to 1.2% of total energy intake for ALA 500 mg (approximately 8 oz [227 g] of cooked fish per week) for sufficient omega‐3 long‐chain PUFA (EPA or DHA) intake |
| Eicosapentaenoic acid (EPA) | C20:5 | Oily fish | ||
| Docosahexaenoic acid (DHA) | C22:6 | Oily fish | ||
| Omega‐6 | Linoleic acid (LA) | C18:2 | Soybean oil, safflower oil, corn oil | 3% to 10% of total energy intake; range of 11 to 17 g per day depending on age and sex |
| Arachidonic acid (AA) | C20:4 | Meat, poultry, eggs | ||
Based upon recommendations of the American Dietetic Association (ADA), jointly with the Dietitians of Canada (DC) (Kris‐Etherton 2007).
In addition, many omega‐3 and/or omega‐6 PUFA oral supplement products, intended to augment dietary intake, are commercially available, including some specifically marketed for dry eye disease (eg, Thera Tears Nutrition [Akorn Consumer Health, Ann Arbor, MI, USA], HydroEye [ScienceBased Health, Oak Ridge North, TX, USA], DRYeye Forte [MD Eyecare, Towson, MD, USA], and Lacritec [Stiltec, Eagle Farm, Australia]). The rationale for supplementation is that epidemiological evidence suggests that more than 80% of adults in developed countries may be omega‐3 deficient (Meyer 2016). It is important to recognize that these products can vary with respect to the following:
Form of omega‐3 PUFA (ie, ethyl ester, triacylglyceride, or phospholipid), given that form can affect the incorporation of DHA and EPA into plasma, and subsequent bioavailability (Dyerberg 2010).
Fatty acid content of the supplement, for example, most 1000‐mg fish oil capsules contain EPA 180 mg and DHA 120 mg; however different formulations may contain anywhere from one‐third to three times these levels, representing a 10‐fold difference in omega‐3 PUFA content.
Currently, one topical omega‐3 product (REMOGEN OMEGA, TRB Chemedica International, SA, Geneva, Switzerland) is commercially available; this product has a concentration of long‐chain omega‐3 fatty acids of 0.025% EPA and 0.0025% DHA.
How the intervention might work
Although the pathogenesis of dry eye disease is not fully understood, the condition involves loss of tear film homeostasis and is underwritten by an immune‐based inflammatory response in the anterior eye (Stevenson 2013). The concentrations of pro‐inflammatory cytokines, such as interleukin (IL)‐1, IL‐6, IL‐17A, tumour necrosis factor‐alpha (TNF‐α), and interferon‐gamma are upregulated in the tears of dry eye patients (Jackson 2016; Massingale 2008; Solomon 2001). Various mechanisms, including tear hyperosmolarity and tear film instability, are considered to contribute to the inflammatory response. Inflammation is considered to strongly contribute to the chronic irritation and pain experienced by people with dry eye disease (Pflugfelder 2008).
A potential strategy for modifying ocular surface inflammation involves modulating systemic cytokine production via dietary interventions, in particular, omega‐3 PUFAs. As omega‐3 and omega‐6 PUFAs compete for enzymes regulating their metabolism in vivo, the ratio of consumed omega‐3 to omega‐6 fatty acids is a determinant of the inflammatory status of the body (Simopoulos 2002). Most eicosanoids derived from the omega‐6‐dependent pathway (eg, prostaglandin‐E2, thromboxane‐A2, leukotriene‐B4) are pro‐inflammatory. In contrast, long‐chain omega‐3 PUFA metabolism biases the production of anti‐inflammatory eicosanoids (eg, resolvins, protectins), which can inhibit the production of pro‐inflammatory mediators, such as IL‐1 and TNF‐α (Caughey 1996; James 2000).
In current Western diets, the ratio of omega‐6 to omega‐3 intake is estimated to be approximately 15:1, whereas an ideal ratio is considered ≤ 4:1 (Simopoulos 2002). Increasing omega‐3 PUFA levels through dietary intervention to lower the omega‐6 to omega‐3 ratio could thus yield anti‐inflammatory effects, including within the eye (Rosenberg 2010). With respect to dry eye disease, the Women’s Health Study, involving 32,470 women aged 45 to 84 years, found an association between low dietary consumption of omega‐3 PUFAs and self‐reported dry eye disease (Miljanovic 2005). Specifically, a 30% reduction in dry eye risk was found with each additional gram of omega‐3 PUFAs consumed each day. In addition, a higher ratio of dietary omega‐6 to omega‐3 was associated with increased risk of dry eye disease (≥ 15:1 vs < 4:1; odds ratio [OR] 2.51, 95% confidence interval [CI] 1.13 to 5.58; P = 0.01) (Miljanovic 2005). However, these findings were not corroborated in a recent cross‐sectional study that investigated the relationship between dietary essential fatty acid intake and both dry eye disease and meibomian gland dysfunction in postmenopausal women (Ziemanski 2018). In this study, dietary consumption of omega‐3 and omega‐6 PUFAs was not significantly associated with dry eye disease, although both high omega‐3 PUFA intake (OR 0.22, 95% CI 0.06 to 0.78) and moderate omega‐6 consumption (OR 0.37, 95% CI 0.15 to 0.91) were reported to be protective against meibomian gland dysfunction (Ziemanski 2018).
In this respect, further potential benefit of omega‐3 PUFAs in individuals with dry eye disease may relate to their effects on meibomian gland lipid secretions. The ratio of omega‐6 to omega‐3 fatty acids in the tear film has been shown to be associated with tear film quality and ocular surface damage in individuals with dry eye disease (Walter 2016). In women with Sjögren's syndrome, the level of dietary omega‐3 consumption was shown to affect the polar lipid pattern of meibomian gland secretions (Sullivan 2002). Furthermore, experimental findings from in vitro studies demonstrate that the application of omega‐3 fatty acids to immortalized human meibomian gland epithelial cells results in upregulation of lipid production (Hampel 2015; Liu 2016). Together, these findings suggest that omega‐3 PUFAs may also act directly on meibomian glands to modify lipid synthesis, in addition to modulating ocular surface inflammation. It follows that preliminary evidence supports a role for resolvins (endogenous lipid‐derived immunomodulators derived from EPA, resolvin E1; and DHA, resolvin D1) in treating ocular surface disease. In a mouse model of dry eye, topical application of resolvin E1 was reported to enhance tear production and attenuate macrophage infiltration (Li 2010). Topically applied omega‐3 PUFAs with and without added 0.1% hyaluronic acid have been reported to improve corneal irregularity and reduce epithelial barrier disruption (Li 2014). Oral omega‐3 supplementation has also been found to impart corneal nerve regeneration in humans with dry eye disease (Chinnery 2017; Zhang 2019b). Recently, a low‐dose topical formulation containing EPA and DHA was found to reduce the concentration of pro‐inflammatory tear mediators in a RCT investigating anti‐inflammatory methods for modulating contact lens discomfort (Downie 2018a).
Why it is important to do this review
There is an urgent need to understand the role of omega‐3 and/or omega‐6 PUFA supplementation as treatment for dry eye disease, as informed by the best currently available research evidence. This is particularly the case given the widespread accessibility of omega‐3 and omega‐6 PUFA supplements, and the tendency for patients to self‐medicate based upon their claimed health benefits.
Over the past several years, numerous clinical trials have been undertaken with the intent of assessing the effects of omega‐3 and/or omega‐6 oral supplements in treating dry eye disease. Nevertheless, there remains significant debate about the role of essential fatty acid supplementation in the clinical management of dry eye disease; this relates, at least in part, to apparently contradictory findings between different trials and lack of consensus in relation to the dose, formulation, and duration of PUFA supplementation that might be required to impart clinical benefit (Jones 2017).
A further consideration relates to the safety of systemic omega‐3 PUFA supplementation. Individuals with atrial fibrillation, liver disease, or bleeding disorders should be cautioned against consuming omega‐3 PUFA supplements (Jones 2017). Although the intervention is generally considered low risk in healthy adults, high‐dose omega‐3 PUFA supplementation (> 2000 mg/d) may be associated with a modestly increased risk of bleeding in some populations (Buckley 2004). It is also possible that men with high serum concentrations of long‐chain omega‐3 PUFAs may have a heightened risk of prostate cancer (Brasky 2013), but this association is not definitive (Brenna 2002; Szymanski 2010). The Federal Drug Administration (FDA) in the USA, and the National Health and Medical Research Council (NHMRC) in Australia, recommend a limit of 3000 mg per day of omega‐3 PUFA consumption, including intake from both food and supplementation.
This systematic review intends to provide clarity on the use of PUFA supplementation for treating dry eye disease. This review also seeks to assess whether relevant factors, such as the formulation composition, dose (concentration and frequency), duration (how long to consume), and/or route of administration (diet, capsules and/or eye drops), are important for any therapeutic response.
Objectives
To assess the effects of omega‐3 and omega‐6 polyunsaturated fatty acid (PUFA) supplements on dry eye signs and symptoms, and to document any potential treatment‐related adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) only.
Types of participants
We included trials in which participants received the diagnosis of dry eye, as defined by the trial investigators. We included study populations with dry eye regardless of age, gender, severity of disease, or classification of dry eye (eg, not specifically aqueous deficient, evaporative tear deficiency).
Types of interventions
We included studies that compared omega‐3 and/or omega‐6 PUFA interventions versus other forms of dry eye treatment, such as artificial tears, placebo, or no treatment. We did not exclude studies that used artificial tears in combination with omega‐3 and/or omega‐6 PUFA interventions.
We also included head‐to‐head trials that compared omega‐3 and/or omega‐6 PUFA interventions of any form, dose (concentration and frequency), or route of administration (eg, dietary intake, supplements, eye drops) versus other forms, doses, or routes of administration. The main comparison was oral long‐chain omega‐3 PUFAs relative to placebo or no intervention.
Types of outcome measures
Primary outcomes
The prespecified primary outcome was subjective improvement in dry eye symptoms (eg, dryness, scratchiness, foreign body sensation, burning), as quantified by patient questionnaires, physician‐chosen scales, or assessments as reported in each included study. Outcomes were reported as mean changes in scores or symptoms from baseline and/or the proportion of participants who had improvement in subjective symptoms. The primary outcome time point was one‐month follow‐up, but we reported outcomes measured beyond one month when reported in the included studies.
Secondary outcomes
Secondary outcomes measured after at least one month of follow‐up comprised the following.
Ocular surface staining, defined by mean change in corneal fluorescein or conjunctival staining using lissamine green or rose bengal, from baseline to follow‐up.
Aqueous tear production, measured by mean change in Schirmer test scores (mm/5 min), performed with or without topical anesthesia.
Tear film stability, measured by mean change in tear film break‐up time (seconds).
Change in frequency of use of artificial tears, defined by included studies and reported as mean change or proportion of participants who increased or decreased their use.
Change in conjunctival goblet cell density, reported as mean change or proportion with changed clinical grade.
Change in the proportion of participants with improved blurred vision symptoms.
Change in ocular surface inflammatory biomarkers, including human leukocyte antigen‐DR (HLA‐DR) and lymphocytic cell infiltration.
Change in tear osmolarity, reported as mean change from baseline or proportion of participants who had an increase or decrease in tear osmolarity value.
We used longer time points for outcome assessments, as reported in included studies, in addition to our primary endpoint of one month.
Adverse outcomes
We documented adverse events as reported in the included studies. Specifically, we reported the incidence of cancer and gastrointestinal disorders, such as diarrhea (Brouwer 2004). We also reported ocular adverse events, such as an increase in blurred vision.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist searched the following electronic databases for RCTs and controlled clinical trials, while applying no restrictions to language or year of publication. We searched the electronic databases on February 27, 2018.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 2) (which contains the Cochrane Eyes and Vision Trials Register), in the Cochrane Library (searched February 27, 2018) (Appendix 1).
MEDLINE Ovid (1946 to February 27, 2018) (Appendix 2).
Embase.com (1947 to February 17, 2018) (Appendix 3).
PubMed (1948 to February 27, 2018) (Appendix 4).
Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to February 27, 2018) (Appendix 5).
metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com) (last searched March 19, 2014) (Appendix 6).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched February 27, 2018) (Appendix 7).
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched February 27, 2018) (Appendix 8).
Additionally, we conducted a top‐up search in October 2019. These results of this search have been added to the 'Characteristics of studies awaiting classification' section and will be incorporated into the review in the next update.
Searching other resources
We searched the reference lists of included studies to identify other potentially relevant studies. We did not handsearch conference abstracts and journals for this review, as Cochrane Eyes and Vision performs routine handsearching for clinical trials and adds these results to CENTRAL.
Data collection and analysis
Selection of studies
Two review authors (ie, two of SMN, KL and LED) independently screened the titles and abstracts within the search results. We classified each record as follows: include, unclear, or exclude; we retrieved the full‐text articles for reports and records classified as include or unclear by at least one review author. Two review authors then independently assessed the full‐text reports to classify each study as follows: definitely include, unclear, or definitely exclude. We resolved any discrepancies in study eligibility judgement between assessors by discussion, to reach consensus. We attempted to contact study authors via email when we needed clarification or more information than was available in the full texts. If we failed to receive a response from the study authors after one month, or when study authors could not provide further information, we used information available within the full‐text report. We documented reasons for excluding studies that underwent full‐text review. For studies written in languages not understood by the review authors, we identified colleagues to assist in assessing their eligibility for inclusion.
Data extraction and management
Two review authors (ie, two of LED, SMN or KL) independently extracted data with respect to study methodology, participants (including eligibility criteria), interventions, and outcomes for each included study, by using forms developed by Cochrane Eyes and Vision. For prespecified primary and secondary outcomes, we extracted all relevant quantitative data. When numeric data were not available, we presented the non‐numeric data reported in the study. We resolved any discrepancies in data extraction by discussion between the review authors. One review author exported all data into Cochrane's statistical software, Review Manager 2014, and a second review author independently verified the data. For the included trial in which one of the review authors (LED) was an author (Deinema 2017), a fourth (independent) person verified data extraction for each outcome.
Assessment of risk of bias in included studies
Two review authors (LED, SMN) independently evaluated the risk of bias of included studies according to the guidelines provided in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We assessed risk of bias in the following domains: selection bias (sequence generation and allocation concealment), performance and detection bias (masking of participants, study personnel, and outcome assessors), attrition bias (incomplete outcome data), reporting bias (selective outcome reporting), and other sources of bias. We judged each included study to be at "low risk," "unclear risk," or "high risk" of bias for each domain. We resolved discrepancies through discussion between the two review authors and a third review author, when necessary. We attempted to contact study authors for clarification when necessary. We used the information available within the full text when we were unable to contact or failed to receive any response from study authors after one month, or when study authors were unable to provide further information. For the included trial for which one of the review authors (LED) was an author (Deinema 2017), a third (independent) person verified the risk of bias assessments.
Measures of treatment effect
We undertook data analyses according to the methods described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). When appropriate, we presented results as summary risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes. We reported mean differences (MDs) with 95% CIs for continuous outcomes. We analyzed mean values at specified time points whenever MDs from baseline (to follow‐up) were not reported in the study. We assessed, to the best of our abilities, whether data reported as MDs may have been skewed. When we suspected that data were skewed, we analyzed data according to the methods provided in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017).
For the three studies with more than two intervention arms, we included all data relevant to the review question. For Deinema 2017, data from the two long‐chain omega‐3 supplementation arms (fish oil and krill oil), were mathematically combined into a single 'long‐chain omega‐3' intervention arm. No relevant extractable data were available from the two other trials with three intervention arms (Pinheiro 2007; Theander 2002).
Unit of analysis issues
The unit of analysis was the study participant. When both eyes of a single participant were eligible and the individual was randomized to a study intervention, we documented what was analyzed (eg, average of two eyes, right eye only, left eye only, one eye selected as the study eye). The unit of analysis was the eye for intracomparative studies, in which each eye of a participant received different study interventions. We extracted and included data in the analyses only when the intraperson correlation was considered in the analyses.
Dealing with missing data
We attempted to contact study authors to clarify study eligibility, to assess risk of bias, and/or to obtain missing outcome data. We used the information available whenever we were unable to contact or failed to receive any response from study authors after one month, or when study authors could not provide further information. When participant level outcome data were assumed to be missing at random, we analyzed the data as reported, while retaining the methods employed by the primary study investigators (eg, available case analysis, last observation carried forward method).
Assessment of heterogeneity
We examined clinical and methodological heterogeneity by examining the variability in design, risk of bias, and characteristics of participants, interventions, and outcomes among included studies. We used the I² statistic to assess statistical heterogeneity among studies. We interpreted an I² value greater than 60% as indicative of substantial statistical heterogeneity.
Assessment of reporting biases
We were unable to assess for potential publication biases or small‐study effects using a funnel plot, as none of the meta‐analyses included 10 or more studies. We assessed selective outcome reporting as part of the risk of bias assessment for each included study.
Data synthesis
We performed meta‐analyses only when there was absence of heterogeneity, in light of multiple potential sources of heterogeneity, including clinical (eg, different etiologies of dry eye disease), methodological (eg, unit of analysis issues), or statistical (with a threshold of I² ≤ 60%). We used a fixed‐effect model when fewer than three trials contributed to a specific outcome; otherwise we adopted a random‐effects model for meta‐analysis. We have presented a narrative or tabulated summary when we have not undertaken meta‐analyses due to significant heterogeneity or insufficient reporting of data (eg, no quantitative data provided in the study report).
Subgroup analysis and investigation of heterogeneity
We conducted subgroup analyses by etiology of dry eye to investigate significant heterogeneity, as prespecified in our protocol.
Sensitivity analysis
We did not perform a sensitivity analysis, as no individual meta‐analyses included a sufficient number of studies. For future iterations of this review, we will perform a sensitivity analysis to assess the impact of excluding studies with high risk of bias, including lack of allocation concealment, lack of masking, loss of a large proportion of participants to follow‐up (ie, 20% or more), industry funding, and lack of publication when adequate data are available.
"Summary of findings" tables
We have presented four "Summary of findings" tables, comparing (1) oral long‐chain omega‐3 PUFAs (EPA and DHA) versus oral placebo or no treatment (Table 1); (2) combined oral omega‐3 and omega‐6 PUFAs compared with placebo (Table 3); (3) oral omega‐3 PUFAs plus conventional therapy versus conventional therapy alone (Table 4); and (4) oral long‐chain omega‐3 PUFAs (EPA and DHA) compared with oral omega‐6 PUFAs, using the format recommended in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017). Outcomes included subjective improvement in dry eye symptoms, ocular surface staining, aqueous tear production, tear film stability, tear osmolarity, and adverse events. We followed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to assess the certainty of the body of evidence.
Summary of findings for the main comparison. Oral long‐chain omega‐3 PUFAs (ie, EPA and DHA) versus placebo or no treatment.
| Oral long‐chain omega‐3 PUFAs (EPA and DHA) compared with placebo or no treatment for dry eye | ||||||
|
Patient or population: people with dry eyea Settings: primary care setting Intervention: oral long‐chain omega‐3 PUFAs (EPA and DHA)b Comparison: placebo or no treatmentc | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Oral long‐chain omega‐3 PUFAs | |||||
| Change in dry eye symptoms, measured using the OSDI score (ranging from 0 to 100 units, with a reduction in scores indicating clinical improvement) at 1‐month (with actual follow‐up ranging from 1 to 12 months) | The pooled summary estimate from four studies (Asbell 2018; Deinema 2017; Goyal 2017; Kangari 2013) indicated little to no reduction in symptoms of dry eye (mean difference [MD] ‐2.47 OSDI units, 95% CI ‐5.14 to 0.19 units, n=677, I2 = 48%). Three additional studies (Bhargava 2015a; Bhargava 2016a; Bhargava 2016b) reported a significant difference in mean symptom score at the end of the follow‐up period, quantified using the Dry Eye Questionnaire and Scoring System (DESS). |
1785 (7 studies) | ⊕⊕⊝⊝ lowd | In addition, symptom score data were reported only as P‐values or in a non‐numeric form in two studies (Kawakita 2013; Pinazo‐Durán 2013), or included non‐dry eye participants (as in Kawashima 2016). | ||
| Change in ocular surface staining at 1‐month (with actual follow‐up ranging from 8 weeks to 12 months) | Two studies found no evidence of a difference between groups for change in corneal fluorescein staining score (Deinema 2017: MD ‐0.31, 95% CI ‐0.66 to 0.04 units, quantified using the Oxford scale; Asbell 2018: MD 0.1, 95% CI ‐0.2 to 0.4 units; P = 0.61, quantified using an unspecified grading scale). Goyal 2017 reported that the control group had a higher rate of conjunctival staining with lissamine green (43.4%) compared with the omega‐3 treatment group (14%), at 3‐months of follow‐up (P=0.009). Kawakita 2013 considered combined corneal and conjunctival staining using rose bengal, and reported that the fish oil (omega‐3) group were “significantly improved to those in the placebo group" after 8 and 12 weeks of treatment. |
681 (4 studies) |
⊕⊕⊝⊝ lowd | No relevant combinable data were available for this outcome. | ||
| Change in Schirmer test (aqueous tear production, measured in mm/5 min, with higher scores indicating more tear production) at 1‐month (with actual follow‐up ranging from 1 to 6 months) | The pooled summary estimate from six studies (Asbell 2018; Bhargava 2015a; Bhargava 2016a; Bhargava 2016b; Deinema 2017; Kangari 2013) showed an improvement in Schirmer test score with long‐chain omega‐3 supplementation relative to the control (MD 0.68, 95% CI 0.26 to 1.09 mm/5 min, n=1704, I² = 16%). In addition, one study (Kawakita 2013, n=26) described no significant inter‐group difference in Schirmer test score, but did not provide quantitative data. |
1730 (7 studies) | ⊕⊕⊕⊝ moderatee | For one additional study, we could not incorporate data into the analyses due to unit of analysis errors (Goyal 2017). Two other trials did not separately report data for dry eye participants versus non‐dry eye (healthy) controls (Kawashima 2016; Pinazo‐Durán 2013). | ||
| Change in tear film stability, measured using tear break‐up time (TBUT) with fluorescein (in seconds, with higher scores indicating greater tear film stability) at 1‐month (with actual follow‐up ranging from 45 days to 12 months) | Four studies (Bhargava 2015a; Bhargava 2016a; Bhargava 2016b; Deinema 2017) reported a significantly improved TBUT with the omega‐3 PUFA intervention compared with placebo, and 1 study (Asbell 2018) reported no significant difference between treatment groups. Meta‐analysis was not performed due to substantial heterogeneity (I² = 98%). | 1640 (5 studies) |
⊕⊕⊝⊝ lowd | The remaining studies were not included in the analysis due to insufficient data reporting (Kangari 2013; Kawakita 2013; Pinazo‐Durán 2013) or the presence of an unit of analysis error (Goyal 2017). | ||
| Change in tear osmolarity (measured in mOsmol/L, with reductions in osmolarity indicating clinical improvement) at 1‐month (with actual follow‐up at 3 months) | Deinema 2017 reported that the mean difference in tear osmolarity was significantly reduced (improved) relative to baseline in the omega‐3 group relative to the placebo group at day 90 (MD: ‐17.71, 95% CI ‐28.07 to ‐7.35 mOsmol/L). | 54 (1 study) |
⊕⊕⊝⊝ lowd | |||
|
Adverse event: gastrointestinal disorders at 1‐month (with actual follow‐up ranging from 3 to 12 months) |
Three studies (Asbell 2018; Bhargava 2016a; Deinema 2017) reported that gastrointestinal disorders were reported in between 5% and 19% of participants in the omega‐3 group and between 0% and 24% of participants in the placebo group. Meta‐analysis was not performed due to substantial heterogeneity (I² = 76%). | 719 (3 studies) | ⊕⊕⊝⊝ lowf | The presence or absence of adverse events was not indicated in three studies (Goyal 2017; Kawakita 2013; Pinazo‐Durán 2013). | ||
| *The basis for the assumed risk (ie, the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DESS: Dry Eye Questionnaire and Scoring System; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; MD: mean difference; OSDI: Ocular Surface Disease Index; PUFA: polyunsaturated fatty acid; RCT: randomized controlled trial; RR: risk ratio; TBUT: tear break‐up time. | ||||||
|
GRADE Working Group grades of evidence.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate. aDry eye associated with computer vision syndrome (Bhargava 2015a), rosacea (Bhargava 2016a), visual display terminal use (Bhargava 2016b), and post laser‐assisted in situ keratomileusis (LASIK) (Goyal 2017). The remaining studies included patients with non‐specific causes of dry eye. bDaily dose of EPA and DHA varied substantially between studies, ranging from a daily value of EPA of 85 mg to 2000 mg, and DHA of 108 mg to 1000 mg (see Table 2 for details). cAll studies used a placebo intervention, except for Pinazo‐Durán 2013 (no treatment). dDowngraded one level for each of risk of bias and inconsistency. eDowngraded one level for risk of bias. fDowngraded one level for each of inconsistency and imprecision. | ||||||
Summary of findings 2. Combined oral omega‐3 and omega‐6 PUFAs versus placebo.
| Combined oral omega‐3 and omega‐6 PUFAs compared with placebo for dry eye | ||||||
|
Patient or population: people with dry eyea Settings: primary care setting Intervention: combined oral omega‐3 and omega‐6 PUFAsb Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Combined oral omega‐3 and omega‐6 PUFAs | |||||
| Symptoms of dry eye at 1‐month (with actual follow‐up ranging from 3 to 6 months) | The Oral sea buckthorn oil study 2010 evaluated eight dry eye symptoms, and reported that the PUFA intervention was more efficacious in reducing ocular burning (P = 0.05) than placebo at 3 months of follow‐up. Creuzot 2006 assessed eight dry eye symptoms, and reported that the number of participants who had specific symptoms ameliorated was significantly greater in the PUFA supplement group compared with the placebo group for conjunctival hyperemia (P = 0.045), reflex lacrimation (P = 0.047), sensations of dryness (P = 0.059), and discomfort (P = 0.091). Creuzot‐Garcher 2011 assessed six dry eye symptoms and reported no significant inter‐group difference except in one sub‐domain (sensation of eye fatigue; P = 0.044). Brignole‐Baudouin 2011 quantified five dry eye symptoms to derive a 'global subjective dry eye score' and reported that neither the global score, nor the analysis of each symptom showed a significant difference between groups at the 3‐month study endpoint. |
Not explicitly reported (4 studies) |
⊕⊕⊝⊝ lowc | No relevant combinable data were available for this outcome, as studies either did not provide quantitative data or used different measurement scales. | ||
| Ocular surface staining at 1‐month (with actual follow‐up ranging from 3 to 6 months) | No significant intergroup difference was observed for corneal fluorescein staining at the study endpoint of 3 months (Brignole‐Baudouin 2011), and 6 months (Creuzot‐Garcher 2011). Creuzot 2006 reported that the PUFA supplement had an effect on corneal fluorescein staining, whereby at six months of follow‐up, 32% of participants had no corneal staining compared with 25% of participants in the placebo group. | Not explicitly reported (3 studies) |
⊕⊕⊕⊝ moderated | No relevant combinable data were available for this outcome, as studies either did not provide quantitative data or used different rating scales. | ||
| Schirmer test (aqueous tear production, measured in mm/5 min, with higher scores indicating more tear production) at 1‐month (with actual follow‐up ranging from 3 to 6 months) | The pooled summary estimate from four studies (Brignole‐Baudouin 2011; Creuzot 2006; Creuzot‐Garcher 2011; Oral sea buckthorn oil study 2010) showed no difference between the PUFA and placebo supplement groups (MD 0.66, 95% CI ‐0.45 to 1.77 mm/5 min, n=455, I² = 0%). | 455 (4 studies) | ⊕⊕⊕⊝ moderated | |||
| Change in tear film stability, measured using tear break‐up time (TBUT) with fluorescein (in seconds, with higher scores indicating greater tear film stability) at 1‐month (with actual follow‐up ranging from 3 to 6 months) | The pooled summary estimate from four studies (Brignole‐Baudouin 2011) indicated a significant improvement with combined omega‐3 and omega‐6 supplementation relative to placebo (MD 0.55, 95% CI 0.04 to 1.07 seconds, n=455, I² = 0%). | 455 (4 studies) |
⊕⊕⊕⊝ moderated | |||
| Change in tear osmolarity (measured in mOsmol/L, with reductions in osmolarity indicating clinical improvement) at 1‐month (with actual follow‐up at 3 months) | In Oral sea buckthorn oil study 2010, tear osmolarity was reported to increase in both groups, but the increase (worsening) was significantly greater in the placebo group after adjustment for "significant" covariates (P = 0.04). | 83 to 96 (1 study) |
⊕⊕⊝⊝ lowe | |||
| Adverse event: gastrointestinal disorders at 1‐month (with actual follow‐up at 3 months) | Brignole‐Baudouin 2011 reported that four (6.0%) participants in the PUFA intervention group and five (7.1%) participants in the placebo group experienced treatment‐related, non‐ocular adverse events. | 138 (1 study) |
⊕⊕⊝⊝ lowe | |||
| *The basis for the assumed risk (ie, the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ALA: alpha‐linolenic acid; CI: confidence interval; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; MD: mean difference; PUFA: polyunsaturated fatty acid; RCT: randomized controlled trial; TBUT: tear break‐up time. | ||||||
|
GRADE Working Group grades of evidence.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate. aDry eye in individuals with (non‐specific) dry eye (Oral sea buckthorn oil study 2010), mild to moderate dry eye disease (Brignole‐Baudouin 2011; Creuzot 2006), or moderate dry eye disease (Creuzot‐Garcher 2011). bDaily dose of EPA from 28 mg to 427.5 mg; DHA from 285 mg to 392 mg; "omega‐6" 15 mg; γ‐linolenic 82 mg; linoleic acid 126. cDowngraded one level for risk of bias and one level for inconsistency. dDowngraded one level for risk of bias. eDowngraded one level for risk of bias and one level for imprecision. | ||||||
Summary of findings 3. Oral omega‐3 PUFAs plus conventional therapy versus conventional therapy alone.
| Oral omega‐3 PUFAs plus conventional therapy versus conventional therapy alone, for dry eye | ||||||
|
Patient or population: people with dry eyea Settings: primary care setting Intervention: oral omega‐3 PUFAs plus conventional therapyb Comparison: conventional therapyc | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional therapy | Oral omega‐3 PUFAs plus conventional therapy | |||||
| Dry eye symptoms, measured using the OSDI score (ranging from 0 to 100 units, with a reduction in scores indicating clinical improvement) at 1‐month (with actual follow‐up ranging from 1 month to 3 months) | The pooled summary estimate from two studies (Korb 2015; Mohammadpour 2017) showed a significantly improved (lower) OSDI score at the study endpoint with omega‐3 supplementation plus conventional therapy, relative to conventional therapy alone (MD ‐7.16, 95% CI ‐13.97 to ‐0.34, n=70, I² = 0%). | 70 (2 studies) | ⊕⊕⊝⊝ lowd,e | Both studies reported OSDI at the study endpoint, rather than as change from baseline. | ||
| Change in ocular surface staining at 1‐month | ‐ | ‐ | ‐ | ‐ | ‐ | Neither study addressed this outcome. |
| Change in Schirmer test (aqueous tear production, measured in mm/5 min) at 1‐month of follow‐up | ‐ | ‐ | ‐ | ‐ | ‐ | There were no relevant combinable
data available for this outcome. One study (Mohammadpour 2017), with a unit of analysis error, reported no significant intra‐ or inter‐group differences in Schirmer test scores at the study endpoint. |
| Change in tear film stability, measured using tear break‐up time (TBUT) with fluorescein (in seconds) at 1‐month | ‐ | ‐ | ‐ | ‐ | ‐ | There were no relevant combinable
data available for this outcome. One study (Mohammadpour 2017), with a unit of analysis error, reported that the mean "TBUT improved in both (intervention) groups (p<0.001). However, TBUT was affected significantly more in the treatment group compared with the control group, p=0.038" (see Effects of interventions for further detail). |
| Change in tear osmolarity (measured in mOsmol/L, with reductions in osmolarity indicating clinical improvement) at 1‐month | ‐ | ‐ | ‐ | ‐ | ‐ | There were no relevant combinable
data available for this outcome. One study (Mohammadpour 2017), with a unit of analysis error, reported that the mean tear osmolarity significantly improved from 315.40 ± 17.06 to 296.90 ± 14.39 in the omega‐3 treatment group (P < 0.001) but not in the control group (P = 0.157) (see Effects of interventions for further detail). |
| Adverse events at 1‐month | ‐ | ‐ | ‐ | ‐ | ‐ | There were no relevant combinable data available for this outcome. One study (Korb 2015) reported that "a total of two adverse events (infectious mononucleosis and sinusitis) were reported for a single patient in the combination treatment group (omega‐3s plus conventional therapy); neither was considered to be treatment related." |
| *The basis for the assumed risk (ie, the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; MD: mean difference; OSDI: Ocular Surface Disease Index; PUFA: polyunsaturated fatty acid. | ||||||
|
GRADE Working Group grades of evidence.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate. aEvaporative dry eye ‐ Korb 2015 ‐ and dry eye secondary to cataract surgery ‐ Mohammadpour 2017. bIn Korb 2015, the "Intervention" consisted of lid hygiene with hypoallergenic eyelid cleansing wipes (Systane Lid Wipes; Alcon Laboratories, Inc., Fort Worth, TX, USA) once daily; instilled 1 drop of lipid emulsion eye drops formulated to restore lipid, aqueous, and mucin components of the tear film (Systane Balance; Alcon) 4 times daily; and 2 oral vitamin supplements containing 1000 mg of omega‐3 fatty acids (Systane Vitamin Omega‐3 Healthy Tears; Alcon), daily for 3 months (daily dose of 2000 mg of omega‐3 fatty acids). In Mohammadpour 2017, the "Intervention" consisted of standard therapy plus omega‐3 dietary supplement (1000 mg every 8 hours, Advanced Canada, each capsule containing 180 mg EPA and 120 mg DHA) every 8 hours (daily dose of 510 mg EPA and 360 mg DHA). cIn Korb 2015, the "Comparison" (conventional therapy) consisted of a warm wet microfiber compress (from Terry World Textiles, LLC, Santa Monica, CA, USA) to both eyelids for 8 minutes once daily, for 3 months. In Mohammadpour 2017, conventional therapy consisted of artificial tears (every 4 hours) and betamethasone 0.1% eye drops (every 8 hours). dDowngraded one level for risk of bias. eDowngraded one level for imprecision. | ||||||
Results
Description of studies
Results of the search
Electronic searches yielded a total of 1291 records, as of February 27, 2018 (Figure 1). One additional full‐text record was added in April 2018 (Asbell 2018), being the published findings of a RCT that was initially categorized as an 'ongoing study' (clinical trial registry: NCT02128763) in the electronic search. Of 1292 titles and abstracts that were screened independently by two review authors, we classified 73 records as relevant, or potentially relevant, and these proceeded to full‐text screening. After independent full‐text screening by two review authors, we included 38 reports of 34 completed trials, and excluded 23 studies (see Characteristics of excluded studies). Three trials (Gilbard 2008; Papas 2007; Reeder 2006) were published in abstract form only, and we identified no full‐text publication. We classified 12 reports of trials from clinical trial registries as "potentially relevant" ongoing trials (see Characteristics of ongoing studies); eight of these trials were marked as having completed participant recruitment but had not yet published their findings (ACTRN12610000991011; IRCT2013062413567N4; ISRCTN17233445; NCT00344721; NCT00357201; NCT01102257; NCT01733745; NCT02871440).
1.
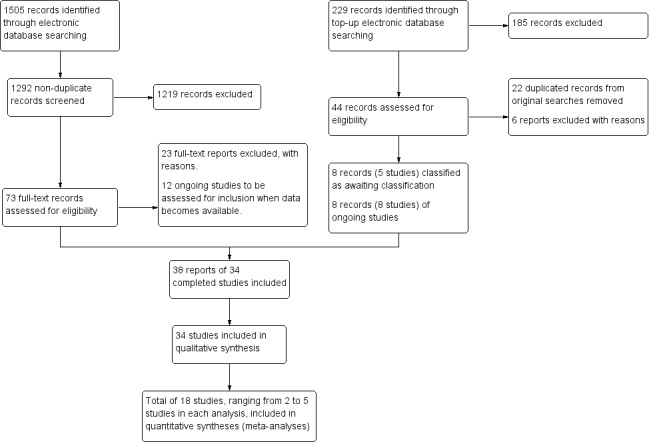
Study flow diagram.
Additional top‐up electronic searches were conducted on October 4, 2019, yielding 229 records. After the titles and abstracts were independently screened by two review authors, 44 potentially relevant full‐texts were retrieved. A total of 22 duplicate records (of already‐included studies) were removed. Of the remaining 22 records, six studies were excluded, eight clinical registries were ongoing trials (of these, four [IRCT201012265467N; ISRCTN10758297; NCT02014922; NCT02802150] had completed recruitment more than two years ago, yet no results were posted); and eight records of five studies that were recently published or were only reported in clinical trial registries, were classified as awaiting classification (see Studies awaiting classification). We will include additional information about these studies in the next update of this review.
In summary, we included a total of 38 reports (of 34 studies), excluded 29 reports (of 29 studies), classified 20 reports (of 20 studies) as ongoing, and classified 8 records (of 5 studies) as awaiting classification (Figure 1).
Included studies
We included 34 trials in this review. Table 2 presents a summary of study designs, participant population(s), interventions, and follow‐up periods for the included trials. We have provided further details for each trial in the Characteristics of included studies table.
1. Table 2. Summary of study design, participants, interventions, and follow‐up period.
|
Study ID Study design |
Condition(s) included | Total number of randomized participants | Intervention(s) studied | Follow‐up period | ||||
|
Aragona 2005 Randomized, parallel‐group, controlled trial |
Moderate to severe dry eye disease associated with primary Sjögren’s syndrome | 40 | Omega‐6 supplementation: oral sachets containing linoleic acid 112 mg and γ‐linolenic acid 15 mg, twice daily (daily dose of linoleic acid 224 mg and γ‐linolenic acid 30 mg) |
Placebo oral sachets, twice daily Each placebo sachet contained placebo with only non‐active excipients (matched to the omega‐6 arm), as follows ‐ Fructose 2383.3 mg ‐ Monohydrate citric acid 50 mg ‐ Aspartame 12.5 mg ‐ Silicon dioxide 6 mg ‐ Bigrade aroma 45 mg ‐ Citrus aroma 131 mg |
1 month | |||
|
Asbell 2018 Randomized, controlled trial |
Moderate to severe dry eye disease | 535 | Omega‐3 supplementationa: 5 soft gelatin oral capsules per day containing triglyceride omega‐3 PUFAs (400 mg EPA and 200 mg DHA) (daily dose of 2000 mg EPA and 1000 mg DHA) |
Placebo (5 oral 1000 mg olive oil capsules), comprising 68% oleic acid, 13% palmitic acid, and 11% linoleic acida (daily dose of 5000 mg olive oil) |
12 months | |||
|
Barabino 2003 Randomized, parallel‐group, controlled trial |
Dry eye disease | 26 | Omega‐6 supplementationb: oral tablets containing linoleic acid 28.5 mg and γ‐linolenic acid 15 mg, twice daily (daily dose of linoleic acid 57 mg and γ‐linolenic acid 30 mg) |
Placebo ("specially made tablets containing a low quantity of sugar at the same dose as the study group") oral tablets twice dailyb | 45 days | |||
|
Bhargava 2013 Randomized, parallel‐group, controlled trial |
Dry eye syndrome | 518 | Omega‐3 supplementation: oral 500 mg soft gel capsule containing EPA 325 mg and DHA 175 mg, twice daily (daily dose of EPA 650 mg and DHA 350 mg) |
Placebo oral capsules (500 mg), containing corn oil, twice daily | 3 months | |||
|
Bhargava 2015a Randomized, parallel‐group, controlled trial |
Dry eye associated with computer vision syndrome | 478 | Omega‐3 supplementation: oral capsule containing EPA 180 mg and DHA 120 mg, 2 capsules/time, twice daily (daily dose of 720 mg EPA and 480 mg DHA) |
Placebo capsules containing olive oil (dose not reported), twice daily | 3 months | |||
|
Bhargava 2015b Randomized, parallel‐group, controlled trial |
Dry eye associated with contact lens wear | 496 females | Omega‐3 supplementation: oral capsule containing EPA 180 mg and DHA 120 mg, 2 capsules/time, twice daily (daily dose of 720 mg EPA and 480 mg DHA) |
Placebo oral capsules containing corn oil (dose not reported), twice daily | 6 months | |||
|
Bhargava 2016a Randomized, parallel‐group, controlled trial |
Dry eye in rosacea patients | 130 | Omega‐3 supplementation: oral capsule containing EPA 180 mg and DHA 120 mg, 2 capsules/time, twice daily (daily dose of 720 mg EPA and 480 mg DHA) |
Placebo oral capsules containing olive oil (dose not reported), 2 capsules/time, twice daily | 6 months | |||
|
Bhargava 2016b Randomized, parallel‐group, controlled trial |
Dry eye in visual display terminal users | 522 | Omega‐3 supplementation: oral capsule containing EPA 180 mg and DHA 120 mg, 4 capsules/time, twice daily (daily dose of 1440 mg EPA and 960 mg DHA) |
Placebo oral capsules containing olive oil (dose not reported), 4 capsules/time, twice daily | 45 days | |||
|
Brignole‐Baudouin 2011 Randomized, parallel‐group, controlled trial |
Mild to moderate dry eye syndrome | 138 | Combined omega‐3 and omega‐6 supplementation: oral soft gel capsule containing fish oil (omega‐3 average of 285 mg, including EPA 142.5 mg and DHA 95 mg, and omega‐6 average of 5 mg), 3 capsules daily (daily dose of omega‐3: 855 mg including EPA 427.5 mg and DHA 285 mg, and omega‐6: 15 mg) |
Placebo oral soft gel capsule containing medium‐chain triglycerides (daily dose: 575 mg) |
3 months | |||
|
Creuzot 2006 Randomized, parallel‐group, controlled trial |
Mild to moderate dry eye syndrome | 71 | Combined omega‐3 and omega‐6 supplementation: oral capsule containing omega‐3 PUFAs (DHA 196 mg and EPA 14 mg), omega‐6 PUFA (γ‐linolenic acid 41 mg or linoleic acid 63 mg), various vitamins (C, E, B6, B9, B12), and a trace element (zinc) (Nutrilarm, Laboratoires Thea), twice daily (daily dose of EPA 28 mg and DHA 392 mg as omega‐3 PUFAs, and γ‐linolenic acid 82 mg or linoleic acid 126 mg as omega‐6 PUFAs) |
Placebo oral capsule containing oleic acid (dose not reported) twice daily (2 capsules per day) | 6 months | |||
|
Creuzot‐Garcher 2011 Randomized, parallel‐group, controlled trial |
Moderate dry eye | 181 | Combined omega‐3 and omega‐6 supplementation: oral capsule containing omega‐3 PUFAs (DHA 196 mg and EPA 14 mg), omega‐6 PUFA (γ‐linolenic acid 41 mg or linolenic acid 63 mg), various vitamins (C, E, B6, B9, B12), and a trace element (zinc) (Nutrilarm, Laboratoires Thea), twice daily (daily dose of EPA 28 mg and DHA 392 mg as omega‐3 PUFAs, and γ‐linolenic acid 82 mg or linolenic acid 126 mg as omega‐6 PUFAs) |
Placebo oral capsule (composition not reported) twice daily (2 capsules per day) | 6 months | |||
|
Deinema 2017 Randomized, parallel‐group, controlled trial |
Mild to moderate dry eye disease | 60 | Omega‐3 supplementation: fish oil capsules (triglyceride omega‐3 PUFAs) (daily dose of 1000 mg EPA and 500 mg DHA) |
Omega‐3 supplementation: krill oil capsules (phospholipid omega‐3 PUFAs) (daily dose of 945 mg EPA and 510 mg DHA) |
Placebo oral capsules (daily dose of 1500 mg) |
90 days | ||
|
Epitropoulos 2016 Randomized, parallel‐group, controlled trial |
Dry eye disease associated with meibomian gland dysfunction | 122 | Omega‐3 supplementation: oral capsules containing re‐esterified omega‐3 PUFAs: 420 mg EPA and 140 mg DHA (daily dose of 1680 mg EPA and 560 mg DHA) |
Control oral capsules containing 3136 mg linoleic acid (safflower oil, omega‐6 fatty acid) |
12 weeks | |||
|
Gilbard 2008 Randomized, parallel‐group, controlled trial |
Sjögren’s syndrome | 61 | Omega‐3 supplementation: TheraTears Nutrition (no other details reported, although this product contains EPA, DHA, and ALA) |
Placebo oral capsules containing wheat germ oil (dose not reported) | Not reported | |||
|
Goyal 2017 Randomized, controlled trial |
Dry eye associated with LASIK | 60 | Omega‐3 supplementation: oral capsule containing 180 mg EPA and 120 mg DHA, 2 capsules/time, twice daily (daily dose of 720 mg EPA and 480 mg DHA) |
Placebo oral capsule containing vitamin E (daily dose of 400 mg) |
13 weeks | |||
|
Kangari 2013 Randomized, parallel‐group, controlled trial |
Dry eye syndrome | 73 | Omega‐3 supplementation: oral capsule containing EPA 180 mg and DHA 120 mg, twice daily (daily dose of EPA 360 mg and DHA 240 mg) |
Placebo oral capsule (1 g) containing medium‐chain triglycerides, twice daily | 1 month | |||
|
Kawakita 2013 Randomized, controlled trial |
Dry eye | 27 | Omega‐3 supplementation: oral soft gel capsule containing EPA 83 mg and DHA 36 mg 3 times daily, 5 capsules/time, 3 times daily (15 capsules/d) (daily dose of EPA 1245 mg and DHA 540 mg) |
Placebo oral capsules containing mainly medium‐chain triglycerides (dose not reported) | 3 months | |||
|
Kawashima 2016 Randomized, controlled trial |
Dry eyec | 40c | Omega‐3 supplementation: oral capsules containing 40.5 mg EPA and 27 mg DHA, 2 capsules/time, once daily (daily dose of 81 mg EPA and 54 mg DHA) |
"Vehicle" (no further details provided), 2 capsules/time, once daily | 8 weeks | |||
|
Kokke 2008 Randomized, parallel‐group, controlled trial |
Dry eye associated with soft contact lens wear | 76 females | Omega‐6 supplementation: oral capsule containing evening primrose oil (linoleic acid about 57 mg and γ‐linolenic acid 50 mg, Equazen UK Ltd.), 6 capsules daily (daily dose of linoleic acid about 342 mg and γ‐linolenic acid 300 mg |
Placebo capsule containing olive oil (78.0% oleic acid, 11.2% palmitic acid, and 5.6% mainly linoleic acid), 6 capsules daily | 6 months | |||
|
Korb 2015 Randomized, controlled trial |
Lipid deficient/evaporative dry eye | 26 | Omega‐3 supplementationd: oral 1000 mg capsule of "omega‐3 fatty acids" (unspecified) |
No oral capsules; warm wet microfiber compress to both eyelids for 8 minutes once daily | 3 months | |||
|
Macsai 2008 Randomized, controlled trial |
Simple obstructive meibomian gland dysfunction and blepharitis | 38 | Omega‐3 supplementatione: flaxseed oil oral 1000 mg capsules 2 capsules/time, 3 times daily (daily dose of 6 g flaxseed oil, comprising approximately 55% ALA, 15% linoleic acid, and 19% oleic acid. Total daily dose of omega‐3 fatty acids: 3.3 g) |
Olive oil oral capsules (dose not reported), 6 capsules dailye | 1 year | |||
|
Manthorpe 1984 Randomized, cross‐over, controlled trial |
Keratoconjunctivitis sicca associated with primary Sjögren’s syndrome | 36 | Omega‐6 supplementation: oral capsule (500 mg) containing cis‐linoleic acid 365 mg and γ‐linolenic acid 45 mg, Efamol twice daily (3 capsules at a time) plus a tablet containing vitamin C 125 mg, pyridoxine 25 mg, niacin 25 mg, and ZnSo4 5 mg (Efavit) twice daily (3 tablets at a time) (daily dose of linoleic acid 2190 mg and γ‐linolenic acid 270 mg) |
"Placebo" oral 500 mg capsule (composition not reported), dosed twice daily (3 capsules at a time) | 3 weeks in each cross‐over phase | |||
|
Mohammadpour 2017 Randomized, controlled trial |
Post cataract surgery dry eye | 48 | Omega‐3 supplementationf: oral 1000 mg (Advanced Canada containing 180 mg EPA and 120 mg DHA), every 8 hours (daily dose of 510 mg EPA and 360 mg DHA) |
"Standard therapy" alone, comprising artificial tears every 4 hours and betamethasone 0.1% eye drops every 8 hours | 1 month | |||
| NCT01107964 | Dry eye syndrome | 27 | Omega‐3 supplementation of 4 g/d (1 g capsule, 4 times daily) containing omega‐3 acid ethyl esters (exact dose not specified) | Placebo oral capsule (1 g) containing corn oil, 4 times daily (dose not specified) | 45 days | |||
|
Oleñik 2013 Randomized, parallel‐group, controlled trial |
Symptomatic meibomian gland dysfunction, no tear instability | 64 | Omega‐3 supplementationg: oral capsule containing EPA 42.5 mg, DHA 350 mg, and DPA 30 mg (Brudysec 1.5 g, Brudy Lab SL), 1 capsule/time, 3 times daily (daily dose of EPA 127.5 mg, DHA 1050 mg, DPA 90 mg) |
Placebo oral 500 mg capsule containing sunflower oil, 1 capsule/time, 3 times daily | 3 months | |||
|
Oral sea buckthorn oil study 2010 Randomized, parallel‐group, controlled trial |
Dry eye symptoms | 100 | Combined omega‐3 and omega‐6 supplementation: oral capsule (1000 mg) containing sea buckthorn oil (Aromtech Ltd.), 1 capsule/time, twice daily |
Placebo oral capsule containing palm and coconut oil triacylglycerols of medium‐chain fatty acids, 1 capsule/time, twice daily | 3 months | |||
|
Oxholm 1986 Randomized, cross‐over, controlled trial |
Keratoconjunctivitis sicca associated with primary Sjögren’s syndrome | 28 | Omega‐6 supplementation: oral capsule (500 mg) containing evening primrose oil (primarily comprising linoleic acid 365 mg and γ‐linolenic acid 45 mg, Efamol), 6 capsules daily (daily dose of 3 g of Efamol containing linoleic acid 2190 mg and γ‐linolenic acid 270 mg) |
"Placebo" oral capsule (500 mg, composition not reported), 6 capsules daily | 8 weeks in each cross‐over phase | |||
|
Papas 2007 Randomized, parallel‐group, controlled trial |
Dry eye associated with Sjögren’s syndrome | 41 | Omega‐3 supplementation: omega‐3 supplement containing a flaxseed and fish oil blend (TheraTears Nutrition, Advanced Vision Research); daily dose not reported |
Germ seed oil oral capsule (composition and dose not reported) | 3 months | |||
|
Pinazo‐Durán 2013 Randomized controlled trial |
Mild to moderate dry eye | 30 | Omega‐3 supplementationg: oral 1.5 g capsule containing EPA 42.5 mg, DHA 350 mg, DPA 30 mg (Brudysec 1.5 g, Brudy Laboratories), 2 capsules daily (daily dose of EPA 85 mg, DHA 700 mg, DPA 60 mg) |
No treatment | 3 months | |||
|
Pinheiro 2007 Randomized, parallel‐group, controlled trial |
Keratoconjunctivitis sicca associated with rheumatoid arthritis or systemic lupus erythematosus | 38 | Omega‐3 supplementation: flaxseed oil capsules, 1 capsule plus 1 placebo capsule (daily dose of flaxseed oil 1 g) |
Omega‐3 supplementation: flaxseed oil, 2 capsules (daily dose of flaxseed oil 2 g) |
Placebo oral capsule containing 950 mg synthetic mineral oil and 50 mg evening primrose oil, 2 capsules daily | 6 months | ||
|
Reeder 2006 Randomized, parallel‐group, controlled trial |
Dry eye | 20 | Omega‐3 supplementation: omega‐3 supplement containing flaxseed oil and fish oil (TheraTears Nutrition); daily dose not reported |
Oral capsule containing flaxseed oil (1000 mg) | 2 months | |||
|
Sheppard 2013 Randomized, parallel‐group, controlled trial |
Moderate to severe keratoconjunctivitis sicca in postmenopausal women | 38 females | Combined omega‐3 and omega‐6 supplementationh: oral capsules containing omega‐6 PUFAs (γ‐linolenic acid 177.5 mg, linoleic acid 177.5 mg, and arachidonic acid < 0.75 mg) and omega‐3 PUFAs (EPA 31.5 mg, DPA 24.75 mg, DPA 9.75 mg), 2 capsules/time, twice daily (daily dose of omega‐3s: ALA 196 mg, EPA 126 mg, DHA 99 mg, and DPA 39 mg; and omega‐6s: linoleic acid 710 mg, γ‐linolenic acid 240 mg, and arachidonic acid < 3 mg) |
Placebo oral capsule, with a main ingredient of sunflower oil, 2 capsules/time, twice dailyh | 6 months | |||
|
Theander 2002 Randomized, parallel‐group, controlled trial |
Dry eye associated with primary Sjögren’s syndrome | 90 | Omega‐6 supplementation: oral γ‐linolenic acid 800 mg daily (Scotia Pharmaceutical Ltd.) |
Omega‐6 supplementation: oral γ‐linolenic acid 1600 mg daily (Scotia Pharmaceutical Ltd.) |
Placebo, containing mostly corn oil (dose not reported) | 6 months | ||
|
Wojtowicz 2011 Randomized, parallel‐group, controlled trial |
Dry eye, some with concomitant meibomitis or meibomian gland dysfunction | 36 | Omega‐3 supplementation: oral capsules containing fish oil 1600 mg (containing EPA 450 mg and DHA 300 mg) and flaxseed oil (1000 mg) daily (TheraTears Nutrition, Advanced Vision Research) |
Placebo oral capsules containing wheat germ oil (dose not reported) | 3 months | |||
aParticipants who were regularly using treatments for dry eye disease (including omega‐3 fatty acid supplements: eicosapentaenoic acid [EPA] plus docosahexaenoic acid [DHA] at a dose of < 1200 mg daily), systemic medications that are known to cause ocular dryness, systemic glucocorticoids, or other immunosuppressive agents were allowed to continue those treatments if they committed to using them for the 12‐month duration of the trial.
bThe omega‐6 oral supplement was administered in association with preservative‐free substitute tears, four times daily.
cNote: only 12 of the 40 enrolled participants had "confirmed dry eye disease," with the remainder classed as having probable dry eye (n = 22) or non‐dry eye (n = 5).
dThe omega‐3 oral supplement was administered in association with lid hygiene (Systane Lid Wipes) once daily and 1 drop of lipid emulsion eye drops (Systane Balance).
eParticipants were also counseled to continue their daily eyelash hygiene, which involved daily eyelash shampoo with a dilute non‐irritating baby shampoo on a washcloth in the shower, followed by a thorough rinse with the eyes closed.
fThe omega‐3 oral supplement was administered in association with "standard therapy for dry eye," as prescribed in the control arm, comprising Artelac artificial tears every 4 hours and betamethasone 0.1% eye drops every 8 hours.
gThe omega‐3 oral supplement also contained vitamin A 133.3 ug, vitamin C 26.7 mg, vitamin E 4 mg, tyrosine 10.8 mg, cysteine 5.83 mg, glutathione 2 mg, zinc 1.6 mg, copper 0.16 mg, manganese 0.33 mg, selenium 9.17 ug.
hThe combined omega‐6 and omega‐3 oral supplement also contained a daily dose of vitamin A 2180 IU, vitamin B6 12.8 mg, vitamin C 262.0 mg and vitamin E 13.7 mg, and magnesium (sulfate) 40.8 mg. Participants were provided with Refresh artificial tears (Allergan, Inc., Irvine, CA, USA) to use as needed for the duration of the study.
ALA: alpha‐linolenic acid; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; LASIK: laser in situ keratomileusis.
Types of studies
The 34 included trials were published between 1984 and 2018. Of these trials, 26 had a parallel‐group design (Aragona 2005; Barabino 2003; Bhargava 2013; Bhargava 2015a; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Brignole‐Baudouin 2011; Creuzot 2006; Creuzot‐Garcher 2011; Deinema 2017; Epitropoulos 2016; Gilbard 2008; Kangari 2013; Kawashima 2016; Kokke 2008; NCT01107964; Oleñik 2013; Oral sea buckthorn oil study 2010; Papas 2007; Pinazo‐Durán 2013; Pinheiro 2007; Reeder 2006; Sheppard 2013; Theander 2002; Wojtowicz 2011), two used a cross‐over design (Manthorpe 1984; Oxholm 1986), and in six trials participants were randomized to interventions and at least one eye of each participant was separately included in the analyses, with (as in Asbell 2018), or without appropriate statistical adjustment (Goyal 2017; Kawakita 2013; Korb 2015; Macsai 2008; Mohammadpour 2017).
Types of participants
In total, 4314 adult participants were enrolled in the 34 included studies. The sample size ranged from 20 to 535 participants in individual trials. Follow‐up periods ranged from one month to 12 months; for one trial (Gilbard 2008), the follow‐up period was not reported. The studies were conducted in 13 countries: one in Australia (Deinema 2017), one in Brazil (Pinheiro 2007), one in Denmark (Manthorpe 1984), one in Finland (Oral sea buckthorn oil study 2010), two in France (Creuzot 2006; Creuzot‐Garcher 2011), one in both France and Italy (Brignole‐Baudouin 2011), six in India (Bhargava 2013; Bhargava 2015a; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Goyal 2017), two in Iran (Kangari 2013; Mohammadpour 2017), two in Italy (Aragona 2005; Barabino 2003), two in Japan (Kawakita 2013; Kawashima 2016), two in Spain (Oleñik 2013; Pinazo‐Durán 2013), one in Sweden (Theander 2002), one in the United Kingdom (Kokke 2008), and six in the United States (Asbell 2018; Epitropoulos 2016; Korb 2015; Macsai 2008; NCT01107964; Sheppard 2013). Investigators for four trials did not report the country in which the trial was conducted (Gilbard 2008; Oxholm 1986; Reeder 2006; Wojtowicz 2011).
Participants in the included trials had dry eye symptoms and/or signs due to multiple etiologies, as follows.
Due to a non‐specific cause (severity not specified: Barabino 2003; Bhargava 2013; Kangari 2013; Kawakita 2013; Kawashima 2016; NCT01107964; Oral sea buckthorn oil study 2010; Reeder 2006; mild to moderate dry eye: Brignole‐Baudouin 2011; Creuzot 2006; Deinema 2017; Pinazo‐Durán 2013; moderate dry eye: Creuzot‐Garcher 2011; and moderate to severe dry eye: Asbell 2018).
Related to computer vision syndrome (Bhargava 2015a), or to visual display terminal use (Bhargava 2016b).
Associated with soft contact lens wear (Bhargava 2015b; Kokke 2008).
Secondary to laser‐assisted in situ keratomileusis (LASIK) (Goyal 2017).
Due to meibomian gland dysfunction (Epitropoulos 2016; Macsai 2008; Oleñik 2013; Wojtowicz 2011), or to lipid deficient/evaporative dry eye (Korb 2015).
Post cataract surgery (Mohammadpour 2017).
Post menopause (Sheppard 2013).
Associated with rosacea (Bhargava 2016a).
Associated with rheumatoid arthritis or systemic lupus erythematosus (Pinheiro 2007).
Associated with Sjögren’s syndrome (Aragona 2005; Gilbard 2008; Manthorpe 1984; Oxholm 1986; Papas 2007; Theander 2002).
In one trial (Kawashima 2016), which enrolled a total of 40 participants, only 12 had "confirmed dry eye disease," and the remainder were classed as having probable dry eye (n = 22) or non‐dry eye (n = 5); as data from the dry eye disease participants were not specifically detailed, we could not include these data in our analyses.
We noted considerable heterogeneity with respect to the participant eligibility criteria used in individual trials. Ten studies used a combination of dry eye signs and symptoms as eligibility criteria (Asbell 2018; Barabino 2003; Bhargava 2013; Brignole‐Baudouin 2011; Creuzot‐Garcher 2011; Deinema 2017; Kawakita 2013; Korb 2015; NCT01107964; Sheppard 2013). Eight trials defined dry eye on the basis of symptoms only (Bhargava 2015a; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Kawashima 2016; Kokke 2008; Mohammadpour 2017; Oral sea buckthorn oil study 2010). Ten studies used only objective clinical signs to define dry eye eligibility (Aragona 2005; Creuzot 2006; Epitropoulos 2016; Kangari 2013; Macsai 2008; Manthorpe 1984; Oleñik 2013; Oxholm 1986; Pinheiro 2007; Wojtowicz 2011), and it remains unclear how dry eye status was defined in six trials (Gilbard 2008; Goyal 2017; Papas 2007; Pinazo‐Durán 2013; Reeder 2006; Theander 2002).
A range of different questionnaires and surveys were used to define dry eye symptoms for participant inclusion. Eight trials used the Ocular Surface Disease Index (OSDI) questionnaire (Asbell 2018; Deinema 2017; Epitropoulos 2016; Goyal 2017; Kangari 2013; Oleñik 2013; Pinazo‐Durán 2013; Sheppard 2013). Five trials adopted the Dry Eye Scoring System (DESS) (Bhargava 2013; Bhargava 2015a; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b). One trial used the McMonnies Dry Eye Screening questionnaire (Kokke 2008), and another study used the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire (Korb 2015). Three studies used other (non‐specific) symptom questionnaires (Barabino 2003; Brignole‐Baudouin 2011; Creuzot‐Garcher 2011), and five trials did not specify the method used to quantify symptoms with respect to eligibility (Kawakita 2013; Kawashima 2016; Mohammadpour 2017; NCT01107964; Oral sea buckthorn oil study 2010).
Clinical signs typically used to define participant eligibility include the following:
Tear break‐up time (TBUT) with fluorescein (Aragona 2005; Asbell 2018; Barabino 2003; Brignole‐Baudouin 2011; Creuzot 2006; Creuzot‐Garcher 2011; Kangari 2013; Kawakita 2013; Manthorpe 1984; NCT01107964; Oxholm 1986; Pinheiro 2007; Sheppard 2013).
Corneal fluorescein staining (Aragona 2005; Asbell 2018; Brignole‐Baudouin 2011; Creuzot 2006; Creuzot‐Garcher 2011; Kawakita 2013; Pinheiro 2007; Sheppard 2013).
Lissamine green staining (Barabino 2003; Brignole‐Baudouin 2011; Creuzot 2006; Creuzot‐Garcher 2011; Manthorpe 1984; Oxholm 1986; Sheppard 2013; Wojtowicz 2011).
Schirmer test, with or without topical anesthesia (Aragona 2005; Asbell 2018; Barabino 2003; Brignole‐Baudouin 2011; Creuzot 2006; Creuzot‐Garcher 2011; Kawakita 2013; Manthorpe 1984; NCT01107964; Oxholm 1986; Pinheiro 2007).
Eyelid margin signs (Bhargava 2013; Epitropoulos 2016; Korb 2015; Macsai 2008; Oleñik 2013; Wojtowicz 2011).
Tear osmolarity (Deinema 2017; Epitropoulos 2016).
Types of interventions
The interventions investigated varied considerably across the included trials. Table 7 presents a summary of the treatment intervention(s) and comparator(s) examined. All of the omega‐3 and omega‐6 PUFA supplements were given orally.
3. Table 3. Summary of interventions.
| Main PUFA intervention | Daily dose | Comparison (daily dose) | Study | |
| Omega‐3, ‐6, or combined versus placebo/no treatment | Long‐chain omega‐3 versus placebo or no treatment (10 RCTs) |
EPA 2000mg DHA 1000mg (triglyceride form) |
Olive oil, 5000mg | Asbell 2018 |
| EPA 720mg DHA 480mg |
Olive oil (dose not reported) |
Bhargava 2015a (computer vision syndrome) |
||
| EPA 720mg DHA 480mg |
Olive oil (dose not reported) |
Bhargava 2016a (rosacea) |
||
| EPA 1440mg DHA 960mg |
Olive oil (dose not reported) |
Bhargava 2016b (visual display terminal users) |
||
| Fish oil EPA 1000mg DHA 500mg (triglyceride form) |
Olive oil (1500mg) | Deinema 2017 | ||
| Krill oil EPA 945mg DHA 510mg (phospholipid form) | ||||
| EPA 720mg DHA 480mg |
Vitamin E (400mg) |
Goyal 2017 (LASIK‐induced dry eye) |
||
| EPA 360mg DHA 240mg |
Medium‐chain triglycerides (2 x 1g capsules) | Kangari 2013 | ||
| EPA 1245mg DHA 540mg |
Medium‐chain triglycerides (does not reported) | Kawakita 2013 | ||
| EPA 81mg DHA 54mg |
"Vehicle" (no further details provided) |
Kawashima 2016 (note: also included non‐dry eye subjects) | ||
| EPA 85mg DHA 700mg DPA 60 mg |
No treatment | Pinazo‐Durán 2013 | ||
| Short‐chain omega‐3 (flaxseed oil) versus placebo (two RCTs) |
6000mg, equivalent to 3300mg omega‐3 fatty acids |
Olive oil (dose not reported) |
Macsai 2008 | |
| 1000mg flaxseed oil | Placebo (950mg synthetic mineral oil and 50mg evening primrose oil) |
Pinheiro 2007 (systemic lupus erythematosus or rheumatoid arthritis) |
||
| 2000mg flaxseed oil | ||||
| Omega‐6 versus placebo (six RCTs) | Linoleic acid 224 mg γ‐linolenic acid 30 mg |
Control (non‐active excipients only) |
Aragona 2005 (Sjögren’s syndrome) |
|
| Linoleic acid 57 mg γ‐linolenic acid 30 mg |
Control ("specially made tablets containing a low quantity of sugar at the same dose as the study group") |
Barabino 2003 | ||
| Linoleic acid about 342mg γ‐linolenic acid 300mg |
Olive oil (dose not reported) |
Kokke 2008 | ||
| Linoleic acid 2190mg γ‐linolenic acid 270mg |
"Placebo" 500mg capsule (composition not reported) |
Manthorpe 1984 (cross‐over trial) ‐ Sjögren’s syndrome |
||
| Linoleic acid 2190mg γ‐linolenic acid 270 mg (Efamol) |
“Placebo" 500mg capsule (composition not reported) |
Oxholm 1986 (cross‐over trial) ‐ Sjögren’s syndrome |
||
| γ‐linolenic acid 800mg | Corn oil (dose not reported) |
Theander 2002 (Sjögren’s syndrome) |
||
| γ‐linolenic acid 1600mg | ||||
| Combined omega‐3 and omega‐6 PUFAs versus placebo (four RCTs) | 2000 mg (comprising long‐chain omega‐3 and omega‐6 PUFAs) | Control (medium‐chain fatty acids) | Oral sea buckthorn oil study 2010 | |
| EPA 427.5 mg DHA 285 mg (total omega‐3 PUFAs 855 mg) Omega‐6 15 mg |
Medium‐chain triglycerides (575mg) |
Brignole‐Baudouin 2011 | ||
| Linoleic acid 126 mg γ‐linolenic acid 82 mg EPA 28 mg DHA 392 mg |
Oleic aid (dose not reported) |
Creuzot 2006 | ||
| linoleic acid 126 mg γ‐linolenic acid 82mg EPA 28mg DHA 392mg |
Placebo (composition and dose not reported) |
Creuzot‐Garcher 2011 | ||
| Omega with conventional treatment versus conventional treatment | Oral omega‐3 plus conventional therapy versus conventional therapy alone (two RCTs) | EPA 510mg DHA 360mg Plus artificial tears, and Betamethasone 0.1% eye drops (every 8 hours) |
Artificial tears (every 4 hours) and betamethasone 0.1% eye drops (every 8 hours) |
Mohammadpour 2017 (post‐cataract surgery) |
| 2000 mg of omega‐3 fatty acid (Systane Vitamin Omega‐3 Healthy Tears; Alcon), lid hygiene (once daily), and lipid emulsion eye drops (once daily) |
Warm compresses (8 minutes/time, once daily) | Korb 2015 | ||
| Comparison of different combinations of omega 3 and 6 with each other | Long‐chain omega‐3 versus omega‐6 (five RCTs) | EPA 650mg DHA 350mg |
Corn oil 1000mg | Bhargava 2013 |
| EPA 720mg DHA 480mg |
Corn oil (dose not reported) |
Bhargava 2015b (contact lens wearers) |
||
| EPA 1680mg DHA 560mg (re‐esterified omega‐3 PUFAs) |
Linoleic acid, safflower oil (3136 mg/d) |
Epitropoulos 2016 | ||
| 4 × 1 g capsules of "omega‐3‐ acid ethyl esters" (exact dose of EPA and DHA not reported) |
Corn oil (4 × 1g capsules; dose not reported) | NCT01107964 | ||
| EPA 127.5mg DHA 1050mg DPA 90mg |
Sunflower oil (3 × 500mg capsules; dose not reported) | Oleñik 2013 | ||
| Combined long‐ and short‐chain (flaxseed) omega‐3 versus combined omega‐3 and omega‐6 (three RCTs) | Fish oil 1600mg (containing EPA 450mg and DHA 300mg) Flaxseed oil 1000 mg (TheraTears Nutrition) |
Wheat germ oil (dose not reported) |
Gilbard 2008 (abstract only) ‐ Sjögren’s syndrome Wojtowicz 2011 |
|
| Fish oil and flaxseed oil blend (dose not reported) TheraTears Nutrition |
Germ seed oil (dose not reported) |
Papas 2007 (abstract only) ‐ Sjögren’s syndrome |
||
| Combined omega‐3 and omega‐6 PUFAs versus omega‐6 alone (one RCT) | Linoleic acid 710mg γ‐linolenic acid 240mg Arachidonic acid < 3mg EPA 126mg DHA 99mg DPA 39mg |
Sunflower oil (dose not reported) |
Sheppard 2013 (post‐menopausal women) |
|
| Combined long‐ and short‐chain (flaxseed) omega‐3 versus short‐chain omega‐3 alone (one RCT) | Fish oil and flaxseed oil blend (dose not reported) TheraTears Nutrition |
Flaxseed oil 1000mg |
Reeder 2006 (abstract only) |
|
Ten trials evaluated the efficacy of long‐chain omega‐3 fatty acids (combined EPA and DHA) for modifying signs and/or symptoms of dry eye disease, relative to an oral control or placebo supplement, to no treatment (Asbell 2018; Bhargava 2015a; Bhargava 2016a; Bhargava 2016b; Deinema 2017; Goyal 2017; Kangari 2013; Kawakita 2013; Kawashima 2016; Pinazo‐Durán 2013), or to an omega‐6 supplement (Bhargava 2013; Bhargava 2015b; Epitropoulos 2016; NCT01107964; Oleñik 2013). Collectively, in these studies (where dose was specified), the total daily dose of combined EPA and DHA ranged from 135 mg to 3000 mg, and a range of different comparators were adopted for both the placebo (eg, olive oil, medium‐chain triglycerides) and omega‐6 (eg, corn oil, safflower oil) interventions; in many cases, the dose of the comparator was not reported. Two trials considered a short‐chain omega‐3 fatty acid (ALA) intervention (dose range: flaxseed oil 1000 mg/d to 6000 mg/d) (Macsai 2008; Pinheiro 2007), and four trials examined combined long‐ and short‐chain omega‐3 fatty acid supplementation relative to combined omega‐3 and omega‐6 supplementation (Gilbard 2008; Papas 2007; Wojtowicz 2011) or a short‐chain omega‐3 supplement alone (Reeder 2006). In two trials, omega‐3 supplementation was combined with ocular therapies (artificial tears and topical corticosteroids: Mohammadpour 2017; artificial tears and eyelid hygiene: Korb 2015), which were compared to a standard dry eye therapy (rather than an oral supplement).
Six studies investigated omega‐6 fatty acid supplementation (linolenic acid, with or without γ‐linolenic acid) (Aragona 2005; Barabino 2003; Kokke 2008; Manthorpe 1984; Oxholm 1986; Theander 2002). In these trials, the total daily dose of omega‐6 fatty acids ranged from 87 mg to 2460 mg. Five trials evaluated a supplement that combined omega‐3 and omega‐6 fatty acids, in various forms and concentrations, relative to a placebo intervention (Brignole‐Baudouin 2011; Creuzot 2006; Creuzot‐Garcher 2011; Oral sea buckthorn oil study 2010) or omega‐6 supplementation (Sheppard 2013).
Outcome measures
Primary and secondary outcome measures were explicitly defined in about two‐thirds (n = 23) of the included trials (Aragona 2005; Asbell 2018; Barabino 2003; Bhargava 2013; Bhargava 2015a; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Brignole‐Baudouin 2011; Creuzot‐Garcher 2011; Deinema 2017; Epitropoulos 2016; Goyal 2017; Kangari 2013; Kawakita 2013; Korb 2015; Macsai 2008; NCT01107964; Oleñik 2013; Oral sea buckthorn oil study 2010; Papas 2007; Pinazo‐Durán 2013; Theander 2002). These trials adopted a heterogenous range of primary outcome measures, including subjective outcomes only (Asbell 2018, Bhargava 2013; Bhargava 2015a; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Creuzot‐Garcher 2011; NCT01107964; Theander 2002), clinician‐derived (as in Epitropoulos 2016,Goyal 2017,Kangari 2013,Korb 2015,Oleñik 2013, and Pinazo‐Durán 2013) or laboratory‐based (as in Aragona 2005,Barabino 2003, and Brignole‐Baudouin 2011) quantitative outcomes only, or a combination of objective and subjective (symptom‐based) measures (Deinema 2017; Kawakita 2013; Macsai 2008; Oral sea buckthorn oil study 2010; Papas 2007).
Primary outcome
As a frequent clinical measure of dry eye disease, all but two trials ‐ Creuzot 2006 and Oxholm 1986 ‐ assessed subjective symptoms (in some form). Patient‐reported symptoms were clearly nominated as a primary outcome measure in 12 trials, and were reported as the change from baseline (Asbell 2018;Bhargava 2013; Bhargava 2015a; Deinema 2017; Macsai 2008; NCT01107964; Oral sea buckthorn oil study 2010), or as endpoint data at the end of the follow‐up period (Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Kawakita 2013; Papas 2007). In these studies, the methods used to quantify dry eye symptoms were the OSDI questionnaire (Asbell 2018; Deinema 2017; Macsai 2008; NCT01107964), the modified OSDI (involving exclusion of item 11) (Oral sea buckthorn oil study 2010), or a study‐specific or unspecified survey (Aragona 2005; Barabino 2003; Bhargava 2013; Bhargava 2015a; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Kawakita 2013; Papas 2007).
Secondary outcomes
Objective physical examination and/or clinical diagnostic tests were performed in all trials except for Reeder 2006 (abstract only), which reported only outcomes related to dry eye symptoms.
Ocular surface staining
Twenty‐one studies evaluated ocular surface staining, defined as corneal fluorescein staining, and/or conjunctival staining with lissamine green or rose bengal (Aragona 2005; Asbell 2018; Barabino 2003; Bhargava 2013; Brignole‐Baudouin 2011; Creuzot 2006; Creuzot‐Garcher 2011; Deinema 2017; Epitropoulos 2016; Goyal 2017; Kawakita 2013; Kawashima 2016; Kokke 2008; Macsai 2008; Manthorpe 1984; NCT01107964; Oleñik 2013; Oxholm 1986; Sheppard 2013; Theander 2002; Wojtowicz 2011). In our protocol, we specified that we would report these data as the change in mean from baseline (at the specified follow‐up period); of the trials that measured this outcome, 10 reported data in this format (Asbell 2018; Brignole‐Baudouin 2011; Deinema 2017; Epitropoulos 2016; Kawakita 2013; Macsai 2008; Manthorpe 1984; NCT01107964; Oxholm 1986; Theander 2002), and 11 provided data at the end of the follow‐up period (Aragona 2005; Barabino 2003; Bhargava 2013; Creuzot 2006; Creuzot‐Garcher 2011; Goyal 2017; Kawashima 2016; Kokke 2008; Oleñik 2013; Sheppard 2013; Wojtowicz 2011). As summarized in Table 8, investigators used a range of different protocols and grading scales to quantify ocular surface staining.
4. Table 4. Summary of ocular surface staining procedures and grading scales.
| Study | Corneal fluorescein | Conjunctival fluorescein | Corneal lissamine green | Conjunctival lissamine green | Corneal rose bengal | Conjunctival rose bengal |
| Aragona 2005 | Scale not specified (scored from 0 to 15) | NA | NA | NA | NA | NA |
| Asbell 2018 | Scale not specified (scored from 0 to 15) | NA | NA | Scale not specified (scored from 0 to 6) | NA | NA |
| Barabino 2003 | NA | NA | NA | van Bijsterveld scale | NA | NA |
| Bhargava 2013 | NA | NA | NA | NA | van Bijsterveld scale (graded from 0 to 9) | |
| Brignole‐Baudouin 2011 | Oxford scale | NA | NA | van Bijsterveld scale | NA | NA |
| Creuzot 2006 | Oxford scale | NA | NA | van Bijsterveld scale | NA | NA |
| Creuzot‐Garcher 2011 | Oxford scale | NA | van Bijsterveld scale | NA | NA | |
| Deinema 2017 | Oxford scale | NA | NA | Oxford scale | NA | NA |
| Epitropoulos 2016 | Oxford scale | NA | NA | NA | NA | NA |
| Goyal 2017 | National Eye Institute/Industry workshop scale | NA | NA | National Eye Institute/Industry workshop scale | NA | NA |
| Kawakita 2013 | Scale not specified (scored from 0 to 9) | NA | NA | Scale not specified (scored from 0 to 9) | ||
| Kawashima 2016 | Japanese diagnostic criteria | NA | NA | NA | NA | |
| Kokke 2008 | CCLRU scale | CCLRU scale | NA | NA | van Bijsterveld scale | van Bijsterveld scale |
| NCT01107964 | NA | NA | Scale not specified (scored from 0 to 9) | NA | NA | |
| Macsai 2008 | National Eye Institute/Industry workshop scale | NA | NA | NA | NA | Six regions of conjunctiva, each graded from 0 to 3 |
| Manthorpe 1984 | NA | NA | NA | NA | van Bijsterveld scale | |
| Oleñik 2013 | Oxford scale | Oxford scale | NA | NA | NA | NA |
| Oxholm 1986 | NA | NA | NA | NA | van Bijsterveld scale | |
| Sheppard 2013 | Scale not specified | NA | NA | Scale not specified | NA | NA |
| Theander 2002 | NA | NA | van Bijsterveld scale | NA | NA | |
| Wojtowicz 2011 | NA | NA | National Eye Institute/Industry workshop scale | NA | NA | NA |
CCLRU, Cornea and Contact Lens Research Unit; NA, not applicable (not investigated in this study).
References to grading scales: CCLRU scale (Terry 1993); Japanese diagnostic criteria (Uchino 2012); National Eye Institute/Industry workshop scale (Lemp 1995); Oxford scale (Bron 2003); van Bijsterveld (van Bijsterveld 1969).
Aqueous tear production
The Schirmer test, as a measure of aqueous tear production, was included as an outcome in most trials, with the exception of Gilbard 2008,Kokke 2008,Korb 2015,Papas 2007, and Reeder 2006. Of the studies that included the Schirmer test as an outcome, 10 used topical anesthesia or described the procedure as the "Schirmer II" test (Aragona 2005; Asbell 2018; Bhargava 2013; Bhargava 2015a; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Deinema 2017; Epitropoulos 2016; Macsai 2008), 13 quantified tear production without topical anesthesia or described the procedure as the "Schirmer I" test (Barabino 2003; Creuzot 2006; Goyal 2017; Kangari 2013; Kawakita 2013; Kawashima 2016; Manthorpe 1984; Mohammadpour 2017; Oleñik 2013; Oxholm 1986; Pinheiro 2007; Theander 2002; Wojtowicz 2011), and six trials did not clearly specify how the technique was performed (Brignole‐Baudouin 2011; Creuzot‐Garcher 2011; NCT01107964; Oral sea buckthorn oil study 2010; Pinazo‐Durán 2013; Sheppard 2013). None of the included studies adopted an alternative clinical measure of aqueous tear secretion (eg, the phenol red thread test).
Tear film stability
As the most common clinical measure of tear film stability (Downie 2013), tear break‐up time (TBUT), which is typically quantified as the average of three repeat measures following the instillation of sodium fluorescein, was specified as an outcome in all but four of the included trials (Gilbard 2008; Korb 2015; Papas 2007; Reeder 2006). In our protocol, we specified that we would report these data as mean change from baseline (at the specified follow‐up period); of the trials that measured TBUT with fluorescein, 13 reported data in this format (Asbell 2018; Bhargava 2013; Brignole‐Baudouin 2011; Deinema 2017; Epitropoulos 2016; Goyal 2017; Kangari 2013; Macsai 2008; Manthorpe 1984; NCT01107964; Oral sea buckthorn oil study 2010; Oxholm 1986; Theander 2002), and 17 reported data at the study endpoint (Aragona 2005; Barabino 2003; Bhargava 2015a; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Creuzot 2006; Creuzot‐Garcher 2011; Kawakita 2013; Kawashima 2016; Kokke 2008; Mohammadpour 2017; Oleñik 2013; Pinazo‐Durán 2013; Pinheiro 2007; Sheppard 2013; Wojtowicz 2011). Consistent with the recent TFOS DEWS II recommendation that non‐invasive measures of tear stability are preferable for capturing the physiological integrity of the tear film, two studies ‐ Deinema 2017 and Kokke 2008 ‐ also included non‐invasive TBUT, quantified via Placido disc topography in Deinema 2017, and manually via the Tearscope in Kokke 2008, as an outcome measure.
Change in the frequency of use of artificial tears
Two studies considered the frequency of artificial tear use as an outcome measure (Brignole‐Baudouin 2011; Sheppard 2013). One trial evaluated the change in treatments used for dry eye disease over the study duration (Asbell 2018), and another study considered the average number of daily doses of tear substitutes (Theander 2002).
Change in conjunctival goblet cell density
Five trials incorporated measures of conjunctival goblet cell density, measured using Nelson grade, on a scale from 0 to 4 (with lower scores indicative of normal cellular appearance and goblet cell density) (Bhargava 2013; Bhargava 2015a; Bhargava 2015b; Bhargava 2016b; Pinheiro 2007).
Change in the proportion of participants with improved blurred vision symptoms
Although none of the included studies reported the percentage of participants with changes in blurred vision symptoms, three trials included visual acuity as an outcome measure (Asbell 2018; Deinema 2017; Sheppard 2013).
Change in ocular surface inflammatory biomarkers, including human leukocyte antigen‐DR (HLA‐DR) and lymphocytic cell infiltration
Four (12%) trials incorporated outcome measures related to HLA‐DR. In both Barabino 2003 and Brignole‐Baudouin 2011, the percentage of HLA‐DR positive cells was quantified in the intervention groups at the study endpoint, as the primary outcome. Two other trials measured HLA‐DR expression (Creuzot‐Garcher 2011; Sheppard 2013).
Adverse events
As summarized in Table 9, 18 (52.9%) trials assessed adverse events. Together, these studies considered a total of 2845 participants.
5. Table 5. Summary of adverse effects.
| Study ID | Number of participants and study population | Adverse event(s) | Serious adverse event(s) |
| Aragona 2005 | 40 participants with moderate to severe dry eye disease associated with primary Sjögren’s syndrome | None | None |
| Asbell 2018 | 535 participants with moderate to severe dry eye disease | "The percentage of patients with at least one non‐serious adverse event was similar in the active supplement group and the placebo group (61.9% and 60.8%, respectively; p=0.87), as was the percentage of patients with an episode of bleeding (2.0% and 1.6%, respectively; p=1.00). A higher percentage of patients reported diarrhoea in the active supplement group than in the placebo group (4.9% and 1.6%, respectively; p=0.09)" Based on data available in the online Appendix
|
"The percentage of patients with at least one serious adverse event was 6.0% in the active supplement group and 8.1% in the placebo group (p = 0.31)" Based on data available in the online Appendix
|
| Barabino 2003 | 26 participants with dry eye disease | "No obvious adverse effects associated with systemic LA and GLA were recorded" | Not reported |
| Bhargava 2015a | 478 participants with dry eye associated with computer vision syndrome | Adverse events were not explicitly reported, although it was stated that 6 participants in the omega‐3 intervention group dropped out due to gastric intolerance | Not reported |
| Bhargava 2015b | 496 participants with dry eye associated with contact lens wear | Adverse events were not explicitly reported, although non‐compliance and gastric intolerance were the reason for 14 participants dropping out of the omega‐3 intervention group | Not reported |
| Bhargava 2016a | 130 participants with dry eye due to rosacea | 4 participants in the placebo group (n = 65) experienced transient skin rashes that were not severe enough to warrant discontinuation from the study. Eight participants in the omega‐3 intervention group (n = 65) experienced gastric intolerance | Not reported |
| Bhargava 2016b | 522 visual display terminal users with dry eye | A total of 26 participants in the omega‐3 group (n = 256) discontinued the study due to gastrointestinal upset | Not reported |
| Brignole‐Baudouin 2011 | 137 participants with mild to moderate dry eye disease | "The number of participants with product‐related non‐ocular adverse events was similar in both groups (fatty acids n = 4 from 67 participants; placebo group n = 5 from 70 participants). In the fatty acid group, these were mainly mild gastrointestinal disorders. Four subjects were withdrawn prematurely because of adverse events, two in each group" | "No product‐related serious adverse events or product‐related ocular adverse events were reported. In the placebo group, there were two unrelated serious adverse events: hospitalization for moderate skin rash with complete recovery and pleural carcinoma. In the fatty acid group, one subject had moderate gastralgia and meteorism and another subject had mild nausea and diarrhoea, both completely recovered" |
| Deinema 2017 | 60 participants with mild to moderate dry eye disease | "The three (olive oil, fish oil and krill oil) interventions were generally well tolerated. Of the 53 adverse events noted, 51 were considered mild (i.e., awareness of symptoms or signs but well tolerated) and 2 were graded as moderate (i.e., discomfort interfering with normal activity)." "The most frequently reported adverse events were colds (24.5%), sore throat (11.3%), headache/migraine (11.3%), and gastrointestinal events (e.g., nausea, abdominal discomfort, bloating, stomach cramps, heartburn, and gastroenteritis; 13.2%). Based on the principal investigator’s (L.E.D.’s) assessment, 72% were deemed to have no potential association with the study supplements and 28% of the adverse events were considered to be potentially related to the study supplements" Based on data available in the online Supplementary Material: there was 1 event (from 19 participants) in the "fish oil" supplement group; 1 event (from 18 participants) in the "krill oil" supplement group; and 4 events (from 17 participants) in the "placebo group" related to gastrointestinal disorders |
None |
| Kangari 2013 | 73 participants with dry eye syndrome | Adverse events were not explicitly reported, although it was stated that "in the treatment group, 3 subjects stopped the medication because of digestion problems" | Not reported |
| Kawashima 2016 | 40 participants with dry eye | "None of the 40 subjects (0.0%) in either group reported any adverse events or side effects" | None |
| Korb 2015 | 26 participants with lipid deficient/evaporative dry eye | "A total of two adverse events (infectious mononucleosis and sinusitis) were reported for a single patient in the combination treatment group (omega‐3s + conventional therapy); neither was considered to be treatment related" | None |
| Manthorpe 1984 | 36 participants with keratoconjunctivitis sicca associated with primary Sjögren’s syndrome | "The following symptoms were evident in 47% of patients after intake of Efamol/Efavit: Sudden universal flushing which usually began in the face and throat, sensation of heat, increase in pulse frequency and fear. A few patients thought that their last minutes had arrived! The symptoms disappeared gradually within 15‐30 min. The symptoms are due to niacin and by changing the Efavit tablets to ZnSO4 and vitamin C tablets the symptoms disappeared. The type and frequency of other side‐effects were equal among the Efamol/Efavit and placebo treatment periods" | Not reported |
| NCT01107964 | 27 participants with dry eye syndrome | Two participants in the omega‐3 acid ethyl ester group (from n = 13) and 1 participant in the placebo group (from n = 14) experienced adverse events. These consisted of (a) omega‐3 group: diarrhea (n = 1) and breast tenderness (n = 1); (b) placebo group: dyspepsia (n = 1) (Note: "Events were collected by a non‐systematic assessment") |
None |
| Oleñik 2013 | 64 participants with symptomatic meibomian gland dysfunction and no tear instability | 2 participants from the omega‐3 + vitamin + mineral supplement group (n = 33) experienced "digestive upset." None of the participants in the placebo group (n = 31) experienced adverse events | None |
| Oxholm 1986 | 28 participants with keratoconjunctivitis sicca associated with primary Sjögren’s syndrome | "Three patients complained of transient nausea and softening of stools during the period of Efamol treatment" | None |
| Sheppard 2013 | 38 postmenopausal women participants with moderate to severe keratoconjunctivitis sicca | "There were no reported ocular adverse events directly attributed to either the study supplement or placebo. One supplement‐treated patient withdrew after a rash appeared ˜2 weeks after the initiation of treatment" | None. "(There were) no significant systemic adverse events were reported, including dyspepsia, dysgeusia, nausea, vomiting, anorexia, diarrhoea, constipation, bruising, or increased infections" |
| Theander 2002 | 90 participants with dry eye associated with primary Sjögren’s syndrome | "Only mild gastrointestinal side effects were reported, most often subsiding after some weeks despite continuous medication. 53% of the placebo‐treated and 56% of the GLA treated patients experienced some sort of gastrointestinal complaints. Some patients complained about weight gain. There was a tendency towards increased weight, especially in the GLA treated patients, who, however, did not reach statistical significance. No significant changes in any of the laboratory parameters were detected during the study" | Not reported |
Of the 10 trials that assessed serious adverse events, only two reported any such events (Asbell 2018; Brignole‐Baudouin 2011). Asbell 2018, which involved 535 participants with moderate to severe dry eye disease, reported that "the percentage of patients with at least one serious adverse event was 6.0% in the active (omega‐3) supplement group and 8.1% in the placebo group (p = 0.31)." Details of these events are provided in Table 9. Brignole‐Baudouin 2011 reported that "no product‐related serious adverse events or product‐related ocular adverse events were reported. In the placebo group, there were two unrelated serious adverse events: hospitalization for moderate skin rash with complete recovery, and pleural carcinoma."
The most commonly reported non‐serious adverse event was gastrointestinal upset. Detailed information related to these events is provided in the Effects of interventions section.
Excluded studies
After full‐text assessment, we excluded 23 studies. These trials are listed under Characteristics of excluded studies, along with the primary reason for exclusion. The most common reasons for excluding studies were that they involved a non‐dry eye study population (n=10), considered a different type of intervention (n=8), or were not randomized controlled trials (n=7). We have listed 12 'ongoing studies' under Characteristics of ongoing studies, although eight of the trials listed on clinical trial registries were listed to have completed participant recruitment, but had not yet published their findings.
Risk of bias in included studies
We have summarized risk of bias assessment in Figure 2 and Figure 3. We have provided additional information on risk of bias judgements for individual studies under Characteristics of included studies. We did not judge any of the 34 included studies to have low risk of bias in all seven domains. We judged one trial ‐ Deinema 2017 ‐ to have low risk of bias in six of the seven domains, and we determined that six studies had low risk of bias in five out of seven domains (Asbell 2018; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Kangari 2013; Sheppard 2013). The two most well‐reported domains were "random sequence generation" (selection bias) and "allocation concealment" (selection bias); we judged that approximately half of the included trials had low risk of bias in these domains. We judged over half of studies to have high risk of bias in the domains related to "attrition bias" and "other bias." The most frequent reason for categorizing a study as having high risk of "other" bias was industry funding or author affiliation with a commercial interest in the intervention.
2.
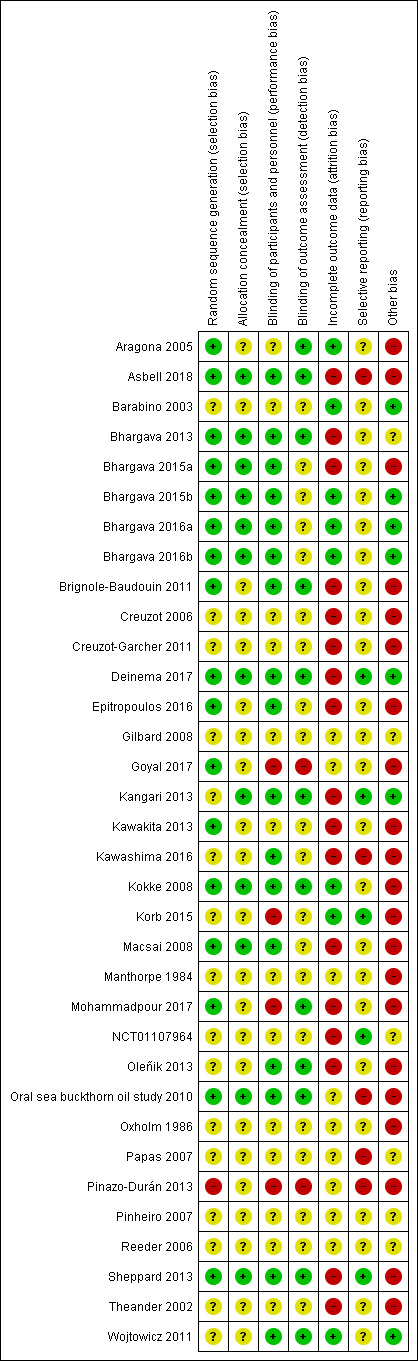
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
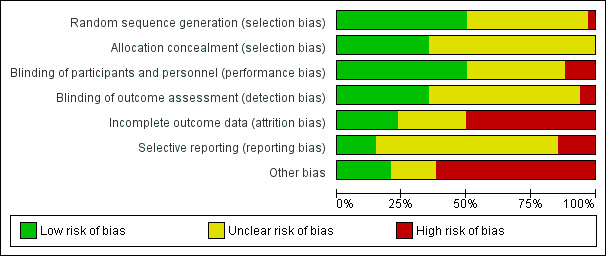
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
We assessed 17 (50.0%) trials as having low risk of bias in this domain, as they employed adequate methods of sequence generation. Thirteen trials used computer software to generate a random sequence (Asbell 2018; Bhargava 2013; Bhargava 2015a; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Brignole‐Baudouin 2011; Deinema 2017; Epitropoulos 2016; Kokke 2008; Macsai 2008; Oral sea buckthorn oil study 2010; Sheppard 2013), three trials employed random number tables (Aragona 2005; Goyal 2017; Kawakita 2013), and one study applied urn randomization (Mohammadpour 2017). Sixteen trials did not clearly report how the random sequence was generated despite the study having been described as "randomized", and we judged these studies as having unclear risk of bias in this domain (Barabino 2003; Creuzot 2006; Creuzot‐Garcher 2011; Gilbard 2008; Kangari 2013; Kawashima 2016; Korb 2015; Manthorpe 1984; NCT01107964; Oleñik 2013; Oxholm 1986; Papas 2007; Pinheiro 2007; Reeder 2006; Theander 2002; Wojtowicz 2011). We considered Pinazo‐Durán 2013 to have high risk of selection bias as there was a non‐random component in the sequence generation process.
Allocation concealment
We judged 12 (35.3%) trials as having low risk of bias in relation to allocation concealment, whereby trial personnel and participants were prevented from knowledge of the intervention allocation before participant assignment. Two studies used sequentially numbered sealed envelopes to conceal allocation (Bhargava 2013; Oral sea buckthorn oil study 2010), and five studies secured randomization codes in a sealed opaque envelope (Bhargava 2015a; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Sheppard 2013). In four studies, a third party performed coding and labeling of the supplement products (Asbell 2018; Deinema 2017; Kangari 2013; Kokke 2008), and the assignment code was not broken until after data analyses were completed (Deinema 2017; Kokke 2008). Macsai 2008 reported that the randomization list was held by research staff members who were not involved in patient care. The remaining 22 trials did not describe the method of allocation concealment or did not report sufficient detail to permit judgement of this parameter.
Blinding
Ten (30.3%) trials described adequate methods for masking participants, trial personnel, and outcome assessors to group allocations (Asbell 2018; Bhargava 2013; Brignole‐Baudouin 2011; Deinema 2017; Kangari 2013; Kokke 2008; Oleñik 2013; Oral sea buckthorn oil study 2010; Sheppard 2013; Wojtowicz 2011); therefore, we judged these trials as having low risk of both performance and detection bias.
Given that participant masking is likely to have a substantial effect on the subjective response to an intervention in dry eye disease, masking is considered a key risk of bias domain. Eleven (32.4%) trials were reported to be "double‐masked" (Barabino 2003; Creuzot 2006; Creuzot‐Garcher 2011; Kawakita 2013; Manthorpe 1984; NCT01107964; Oxholm 1986; Papas 2007; Pinheiro 2007; Reeder 2006; Theander 2002), but sufficient details were not provided as to who was masked and/or how masking was performed. We classified these trials as being at unclear risk of bias for this domain. Seven studies described how participants and trial personnel were masked but provided no details on masking of outcome assessors (Bhargava 2015a; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Epitropoulos 2016; Kawashima 2016; Macsai 2008). Outcome assessors were reported to be masked, but masking of participants and study personnel was unclear in two studies (Aragona 2005; Korb 2015). We assessed two trials as having high risk of both performance bias and detection bias, as they were open‐label studies (Goyal 2017; Pinazo‐Durán 2013). Mohammadpour 2017 performed masking for outcome assessors only, and Gilbard 2008 did not clearly describe masking.
Incomplete outcome data
We have presented the numbers of participants in each trial who were excluded after randomization or were lost to follow‐up in the Characteristics of included studies table. We rated risk of attrition bias to be low only when an intention to treat (ITT) analysis was performed. We judged whether the trial adopted an ITT analysis (or not) based on the following three principles: (1) keeping participants in the intervention groups to which they were randomized, regardless of the intervention they actually received; (2) measuring outcome data on all participants; and (3) including all randomized participants in the analysis (Higgins 2017). We judged eight (23.5%) trials to have low risk of bias in this domain (Aragona 2005; Barabino 2003; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Kokke 2008; Korb 2015; Wojtowicz 2011). We assessed nine trials as having unclear risk of attrition bias as the numbers of participants who were randomized, excluded, lost to follow‐up, and/or analyzed in each intervention group was not clearly reported (Gilbard 2008; Goyal 2017; Manthorpe 1984; Oral sea buckthorn oil study 2010; Oxholm 1986; Papas 2007; Pinazo‐Durán 2013; Pinheiro 2007; Reeder 2006). We assessed the remaining 17 trials to be at high risk of attrition bias as ITT analyses were not conducted (Asbell 2018; Bhargava 2013; Bhargava 2015a; Brignole‐Baudouin 2011; Creuzot 2006; Creuzot‐Garcher 2011; Deinema 2017; Epitropoulos 2016; Kangari 2013; Kawakita 2013; Kawashima 2016; Macsai 2008; Mohammadpour 2017; NCT01107964; Oleñik 2013; Sheppard 2013; Theander 2002) .
Selective reporting
Nine (26.5%) of the included trials had published protocols and/or were clearly prospectively registered on a clinical trials registry. Of these, six trials reported all of the prespecified outcomes; thus we judged these studies as having low risk of bias (Deinema 2017; Kangari 2013; Korb 2015; NCT01107964; Papas 2007; Sheppard 2013). Three studies did not report all of their prespecified outcome measures in the final published paper (Asbell 2018; Kawashima 2016; Oral sea buckthorn oil study 2010), and we judged them to have high risk of reporting bias. We considered two other studies to be at high risk of bias in this domain, as results were not fully reported for at least one outcome described in their methods sections (Pinazo‐Durán 2013), and as deviation was evident in the consistency of reporting of primary and secondary outcome measures (Papas 2007). In one trial ‐ Epitropoulos 2016 ‐ the clinical trial registry entry (https://clinicaltrials.gov/ct2/show/NCT02260960) was not referenced in the paper; the registry entry detailed a consistent primary outcome measure but did not list any of the reported secondary outcome measures; thus, we assessed the risk of reporting bias as unclear. We judged the remaining 22 included trials as having unclear risk of bias due to insufficient information within the papers to permit judgement (Aragona 2005; Barabino 2003; Bhargava 2013; Bhargava 2015a; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Brignole‐Baudouin 2011; Creuzot 2006; Creuzot‐Garcher 2011; Gilbard 2008; Goyal 2017; Kawakita 2013; Kokke 2008; Manthorpe 1984; Mohammadpour 2017; Oleñik 2013; Oxholm 1986; Pinheiro 2007; Reeder 2006; Theander 2002; Wojtowicz 2011) .
Other potential sources of bias
We found no other potential sources of bias in eight trials (Barabino 2003; Bhargava 2015b; Bhargava 2016a; Bhargava 2016b; Brignole‐Baudouin 2011; Deinema 2017; Kangari 2013; Wojtowicz 2011). We identified at least one of the following potential sources of bias in the remaining 26 trials.
Seventeen (50.0%) trials were funded by industry, had materials supplied by industry, and/or had authors who disclosed financial interests with industry that were potentially relevant to the intervention(s) (Aragona 2005; Asbell 2018; Brignole‐Baudouin 2011; Creuzot 2006; Creuzot‐Garcher 2011; Epitropoulos 2016; Kawakita 2013; Kawashima 2016; Kokke 2008; Korb 2015; Macsai 2008; Oleñik 2013; Oral sea buckthorn oil study 2010; Oxholm 1986; Pinazo‐Durán 2013; Sheppard 2013; Theander 2002).
Two trials used a cross‐over design. Manthorpe 1984 had a one‐week washout period between the two intervention phases, and Oxholm 1986 did not have a washout period (both of these time periods were considered insufficient to ensure an adequate washout period).
Five trials had unit of analysis errors (Goyal 2017; Kawakita 2013; Korb 2015; Macsai 2008; Mohammadpour 2017), in which the unit of randomization (individual participant) was different from the unit of analysis (eye), and the analysis did not take into account the within‐person correlation.
One study enrolled both patients with dry eye and healthy controls, and "two homogeneous subgroups were selected" (Pinazo‐Durán 2013); however how randomization was performed and the unit of randomization and the unit of analysis used were not specified.
One study included participants without dry eye disease in the analysis (Kawashima 2016).
Effects of interventions
See: Table 1; Table 3; Table 4; Table 5
Summary of findings 4. Oral long‐chain omega‐3 PUFAs versus oral omega‐6 PUFAs.
| Oral long‐chain omega‐3 PUFAs (EPA and DHA) compared with oral omega‐6 PUFAs for dry eye | ||||||
|
Patient or population: people with dry eyea Settings: primary care setting Intervention: oral long‐chain omega‐3 PUFAs (EPA and DHA)b Comparison: oral omega‐6 PUFAsc | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral omega‐6 PUFAs | Oral long‐chain omega‐3 PUFAs | |||||
| Change in dry eye symptoms, measured using the OSDI score (ranging from 0 to 100 units, with a reduction in score indicating clinical improvement) at 1‐month (with actual follow‐up ranging from 45‐days to 12 weeks) | The pooled summary estimate from two studies (Epitropoulos 2016; NCT01107964) indicated a significant reduction in dry eye symptoms with long‐chain omega‐3 PUFA supplementation relative to an omega‐6 PUFA intervention (MD ‐11.88, 95% CI ‐18.85 to ‐4.92 OSDI units, n=130, I² = 0%). In addition, two studies (Bhargava 2013; Bhargava 2015b) reported a difference in the mean symptom score at the end of the follow‐up period, measured using the Dry Eye Questionnaire and Scoring System (DESS), in favor of the omega‐3 PUFA intervention. |
1144 (4 studies) | ⊕⊕⊕⊝ moderated | |||
| Change in ocular surface staining at 1‐month |
Oleñik 2013 (n=61) reported that "in both (treatment) groups, corneal staining data presented no significant differences from baseline" at the 3‐month study endpoint. In NCT01107964, the change from baseline in lissamine green staining was not significantly different between the two intervention groups at day 45 of follow‐up (median [interquartile range] omega‐3 group 1.0 [‐1.5 to 1.0], n=12 vs omega‐6 group ‐1.0 [‐2.0 to 0.0] units, n=13; P = 0.44). Bhargava 2013 (n=518) reported that, relative to baseline, there was significant improvement in corneal and conjunctival rose bengal staining in the omega‐3 group (P < 0.001), but not in the omega‐6 group (P = 0.564), at 3‐months of follow‐up. Epitropoulos 2016 (n=105) reported a significant reduction in corneal fluorescein staining in the omega‐3 group relative to the omega‐6 group at 3 months of follow‐up (MD ‐0.10, 95% CI ‐0.18 to ‐0.03 Oxford units). |
709 (4 studies) | ⊕⊕⊝⊝ lowd,e | No relevant combinable data were available for this outcome. | ||
| Change in Schirmer test (aqueous tear production, measured in mm/5 min, with higher scores indicating more tear production) at 1‐month (with actual follow‐up ranging from 45 days to 6 months) |
Bhargava 2013 reported that omega‐3 supplementation improved tear production relative to the omega‐6 intervention at 3 months of follow‐up (omega‐3: mean ± SD: 0.62 ± 1.06 vs omega‐6: 0.15 ± 0.35 mm/5 min, P < 0.001, n=518). Bhargava 2015b reported a significantly higher Schirmer test score, in favor of the omega‐3 intervention, in individuals with contact lens associated dry eye at 6 months of follow‐up (MD: 2.60, 95% CI 2.21 to 2.99 mm/5 min, n=496). Two studies reported no significant change in Schirmer test score at 12‐weeks of follow‐up in individuals with meibomian gland dysfunction (Epitropoulos 2016, n=105), and at 45‐days of follow‐up in people with non‐specific dry eye (NCT01107964, n=25). Oleñik 2013 (n=61) reported no significant intergroup difference in Schirmer test score at the 3‐month study endpoint. |
1205 (5 studies) | ⊕⊕⊝⊝ lowe | Meta‐analysis was not performed due to substantial statistical heterogeneity (I² = 97%). | ||
| Change in tear film stability, measured using tear break‐up time (TBUT) with fluorescein (in seconds, with higher scores indicating greater tear film stability) at 1‐month (with actual follow‐up ranging from 45 days to 6 months) | Four of the included trials (Bhargava 2013; Bhargava 2015b; Epitropoulos 2016; Oleñik 2013) reported a significant improvement in TBUT with omega‐3 supplementation relative to the omega‐6 intervention, and one study (NCT01107964) reported no significant inter‐group difference. | 1205 (5 studies) |
⊕⊕⊝⊝ lowe | Meta‐analysis was not performed due to substantial statistical heterogeneity (I² = 94%). | ||
| Change in tear osmolarity (measured in mOsmol/L, with reductions in osmolarity indicating clinical improvement) at 1‐month (with actual follow‐up at 12 weeks) | Epitropoulos 2016 reported that tear osmolarity was reduced (improved) relative to baseline at the 12‐week study endpoint, in the omega‐3 group relative to the omega‐6 group (MD: ‐11.10, 95% CI ‐12.15 to ‐10.05 mOsmol/L, n=105). | 105 (1 study) |
⊕⊕⊝⊝ lowd,f | |||
|
Adverse event: gastrointestinal disorders at 1‐month (with actual follow‐up ranging from 45 days to 3 months) |
Combining the results from two trials (NCT01107964; Oleñik 2013) indicated that the relative risk of a gastrointestinal adverse event was unclear between omega‐3 and omega‐6 supplementation (two studies, 91 participants; RR 2.34, 95% CI 0.35 to 15.54, n=91, I² = 0%). | 91 (2 studies) | ⊕⊕⊝⊝ lowd,f | |||
| *The basis for the assumed risk (ie, the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DESS: Dry Eye Questionnaire and Scoring System; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; MD: mean difference; OSDI: Ocular Surface Disease Index; PUFA: polyunsaturated fatty acid; RCT: randomized controlled trial; RR: risk ratio; TBUT: tear break‐up time. | ||||||
|
GRADE Working Group grades of evidence.
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate. aDry eye associated with soft contact lens wear (Bhargava 2015b), meibomian gland dysfunction (Epitropoulos 2016; Oleñik 2013), and non‐specific causes (Bhargava 2013; NCT01107964). bDaily dose of EPA and DHA varied between studies, ranging from a daily value of EPA of 127.5 mg to 1680 mg, and DHA of 350 mg to 1050 mg (see Table 2 for details). cAll studies used a comparator intervention rich in omega‐6 PUFAs. dDowngraded one level for risk of bias. eDowngraded two levels for inconsistency. fDowngraded one level for imprecision. | ||||||
Oral long‐chain omega‐3 PUFAs (EPA and DHA) versus oral placebo or no treatment (10 RCTs)
Table 1 summarizes the effect of the intervention (long‐chain omega‐3 PUFAs) compared with the control (placebo or no intervention) for the prespecified outcomes.
Ten RCTs compared capsules containing long‐chain omega‐3 fatty acids ‐ EPA and DHA ‐ with a "placebo" intervention (Asbell 2018; Bhargava 2015a; Bhargava 2016a; Bhargava 2016b; Deinema 2017; Goyal 2017; Kangari 2013; Kawakita 2013; Kawashima 2016), or with no treatment for dry eye (Pinazo‐Durán 2013). Table 7 presents further details related to the interventions. Among studies that reported the adopted omega‐3 dosages, the daily dose of EPA and DHA varied substantially, ranging from a daily value of EPA of 85 mg to 2000 mg, and DHA of 108 mg to 1000 mg. Together, these 10 trials included 1955 participants; sample sizes ranged from 27 in Kawakita 2013 to 552 in Bhargava 2016b. Length of follow‐up ranged from one month to one year. In three studies, individuals were randomized to the intervention and both eyes of each individual were included in the analysis (Asbell 2018; Goyal 2017; Kawakita 2013), although Asbell 2018 adjusted for the within‐person correlation.The remaining studies used a parallel‐group design with either one eye, or the average of both eyes, used in the analyses. Participant eligibility criteria varied considerably across studies, and studies included participants with dry eye associated with computer vision syndrome (Bhargava 2015a), rosacea (Bhargava 2016a), visual display terminal use (Bhargava 2016b), and post laser in situ keratomileusis (LASIK) (Goyal 2017). Two studies included participants with mild to moderate dry eye disease (Deinema 2017; Pinazo‐Durán 2013), and one study investigated patients with moderate to severe dry eye (Asbell 2018). As noted above, two studies included non‐dry eye participants and did not report the results separately (Kawashima 2016; Pinazo‐Durán 2013); thus, we excluded these studies from the analyses.
Primary outcome
All eight studies included in the analysis evaluated subjective symptoms of dry eye and reported this parameter as final mean score, mean change from baseline, or number of participants with improved symptoms of dry eye.
In four studies (Asbell 2018; Deinema 2017; Goyal 2017; Kangari 2013), researchers evaluated subjective symptoms of dry eye using Ocular Disease Surface Index (OSDI) scores. Meta‐analysis (Analysis 1.1, Figure 4), based upon the availability of quantitative data, was performed by combining data from four studies, reported as mean change from baseline (Asbell 2018; Deinema 2017; Goyal 2017) or mean score at study endpoint (Kangari 2013). The summary estimate for these studies indicated little to no reduction in dry eye symptoms with long‐chain PUFA supplementation relative to placebo (four studies, 677 participants; mean difference (MD) ‐2.47, 95% confidence interval (CI) ‐5.14 to 0.19 OSDI units); however this result should be viewed with caution due to moderate statistical heterogeneity (I² = 48%) and wide confidence intervals (low certainty evidence).
1.1. Analysis.
Comparison 1 Oral long‐chain omega‐3 PUFAs (ie, EPA and DHA) versus placebo, Outcome 1 Dry eye symptoms, measured using the OSDI score [OSDI] units.
4.
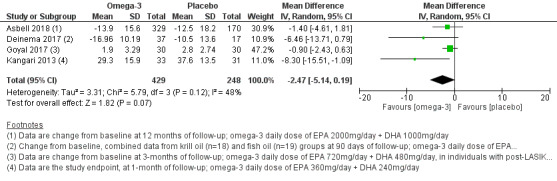
Forest plot of comparison: 1 Oral long‐chain omega‐3 PUFAs (ie, EPA and DHA) versus placebo, outcome: 1.1 Dry eye symptoms, measured using the OSDI score [OSDI] units.
Three trials led by the same study investigator adopted the Dry Eye Questionnaire and Scoring System (DESS) (Bhargava 2015a; Bhargava 2016a; Bhargava 2016b). Researchers evaluated five symptoms (ie, itching or burning, sandy or gritty sensation, redness, blurring of vision, ocular fatigue, and excessive blinking) on a scale ranging from 0 (absent) to 4 (always). Each of the three trials reported mean DESS scores at the study endpoint, and reported a significant difference in mean score at the end of the follow‐up period, in favor of the omega‐3 intervention. However, the summary estimate was not presented because of substantial statistical heterogeneity across studies (I² = 88%) (Analysis 1.2).
1.2. Analysis.
Comparison 1 Oral long‐chain omega‐3 PUFAs (ie, EPA and DHA) versus placebo, Outcome 2 DESS: mean score at study endpoint [DESS units].
Symptom score data were reported only as P values or in a non‐numeric form in two studies (Kawakita 2013; Pinazo‐Durán 2013), or included non‐dry eye participants (as in Kawashima 2016).
We used the GRADE classification to judge the certainty of the body of evidence for this outcome, and we downgraded the findings by two levels to low, as results were inconsistent between studies, and two of the four studies included for the OSDI parameter had received industry support (ie, judged to have high risk of "other bias").
Secondary outcomes
Ocular surface staining
As summarized in Table 8, four studies included in the analysis examined ocular surface staining as an outcome parameter (Asbell 2018; Deinema 2017; Goyal 2017; Kawakita 2013). We were unable to combine data for this outcome owing to lack of numeric data and/or use of different clinical grading scales.
For Deinema 2017 there was no significant inter‐group difference in corneal fluorescein staining, measured on the Oxford Scale, at three months of follow‐up (MD ‐0.31, 95% CI ‐0.66 to 0.04 units). Asbell 2018 reported no significant differences between the omega‐3 and placebo intervention arms for the mean change in corneal fluorescein staining score between six months and 12 months (MD 0.1, 95% CI ‐0.2 to 0.4 units; P = 0.61; quantified using an unspecified grading scale). Similar findings were reported for conjunctival lissamine green staining (MD 0.0, 95% CI ‐0.2 to 0.1 units; P = 0.77). Goyal 2017 quantified both corneal fluorescein staining and conjunctival lissamine green staining using the National Eye Institute/Industry Workshop Scale. These authors reported that "more eyes in the control group (43.4%) had conjunctival staining with lissamine green at 3 months compared with the (omega‐3) treatment group (14%), p=0.009."
Kawakita 2013 also considered combined corneal and conjunctival RBS as an outcome measure and graded this using an unspecified scoring system. This study reported RBS data in a graphical format and summarized them in the text such that "changes in the RBS score after 8 and 12 weeks of supplementation in the fish oil group were significantly improved to those in the placebo group."
We judged the certainty of evidence as low for this outcome, downgrading for each of inconsistency of results (as three studies reported no significant inter‐group differences and one study reported a significantly higher proportion of control participants having conjunctival lissamine green staining compared with the omega‐3 intervention group), and risk of bias (as two studies were judged to be of unclear or high risk of bias in the domain relating to blinding of outcome assessors).
Aqueous tear production
All 10 trials examined aqueous tear production using the Schirmer test (with or without topical anesthesia) and reported results as the mean change from baseline or final mean score at study endpoint. We extracted relevant quantitative data from six studies (Asbell 2018; Bhargava 2015a; Bhargava 2016a; Bhargava 2016b; Deinema 2017; Kangari 2013) (Analysis 1.3, Figure 5). Five of these trials performed the Schirmer test using topical anesthesia (Asbell 2018; Bhargava 2015a; Bhargava 2016a; Bhargava 2016b; Deinema 2017) and one study performed the test without topical anesthesia (Kangari 2013). Quantitative data for the Schirmer test was not reported in the full‐text paper, but instead was provided by the corresponding author for Deinema 2017.
1.3. Analysis.
Comparison 1 Oral long‐chain omega‐3 PUFAs (ie, EPA and DHA) versus placebo, Outcome 3 Schirmer test (tear production) (mm/5 min).
5.
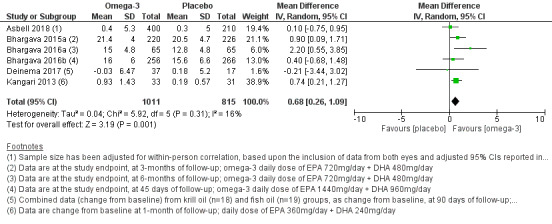
Forest plot of comparison: 1 Oral long‐chain omega‐3 PUFAs (ie, EPA and DHA) versus placebo, outcome: 1.3 Schirmer test (tear production) (mm/5 min).
The overall summary estimate for these studies showed a significant improvement in Schirmer test score with long‐chain omega‐3 supplementation relative to the control (six studies, 1704 participants; MD 0.68, 95% CI 0.26 to 1.09 mm/5 min), with low statistical heterogeneity (I² = 16%); however, we did not judge this difference to be clinically meaningful. For the analysis of data relating to Asbell 2018 in Analysis 1.3, the sample size was adjusted for the within‐person correlation, based upon the inclusion of data from both eyes and the adjusted 95% CIs reported in the manuscript.
One study reported, in descriptive terms, that they found no significant intergroup difference in Schirmer test score (Kawakita 2013). For one study, we could not incorporate data into the analyses due to (uncorrected) unit of analysis errors (Goyal 2017).
We judged the certainty of evidence for this outcome as moderate using GRADE, and we downgraded it by one level for risk of bias.
Tear film stability
All of the included studies assessed tear film break‐up time (TBUT) using fluorescein, to quantify tear film stability. As summarized in Figure 6 (Analysis 1.4), two studies reported quantitative TBUT data as mean change from baseline at the study endpoint (Asbell 2018; Deinema 2017), and three trials reported data as mean values at the end of the study follow‐up period (Bhargava 2015a; Bhargava 2016a; Bhargava 2016b). For the analysis of data relating to Asbell 2018 in Analysis 1.4, the sample size was adjusted for the within‐person correlation, based upon the inclusion of data from both eyes and the adjusted 95% CIs reported in the manuscript. Quantitative data for TBUT was not reported in the full‐text paper, but instead was provided by the corresponding author for Deinema 2017.
6.
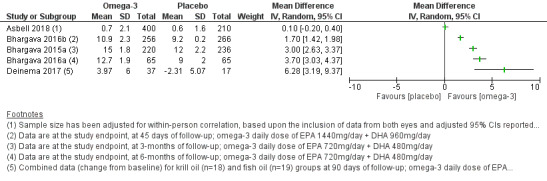
Forest plot of comparison: 1 Oral long‐chain omega‐3 PUFAs (ie, EPA and DHA) versus placebo, outcome: 1.4 Tear break‐up time (TBUT) measured using fluorescein (seconds).
1.4. Analysis.
Comparison 1 Oral long‐chain omega‐3 PUFAs (ie, EPA and DHA) versus placebo, Outcome 4 Tear break‐up time (TBUT) measured using fluorescein (seconds).
The overall summary estimate for these studies suggested an improvement in TBUT with long‐chain omega‐3 PUFA supplementation relative to the control (five studies, MD 2.53, 95% CI 1.17 to 3.94 seconds); however pooled data for this result are not shown in Figure 6 (Analysis 1.4) due to high levels of heterogeneity (I² = 98%).
We performed a subgroup analysis considering dry eye etiology (ie, participants defined with non‐specific "dry eye" (Asbell 2018;Deinema 2017) vs participants with "dry eye" secondary to another disease or condition (Bhargava 2015a; Bhargava 2016a; Bhargava 2016b)) to explore the potential source of heterogeneity. However, this did not adequately explain the heterogeneity (I² > 60% for both subgroup analyses; analyses not shown). Of the five studies included reporting on this outcome, four reported a significant improvement (increase) in TBUT with omega‐3 supplementation relative to placebo. The study that did not report a relative beneficial effect on TBUT was Asbell 2018 (MD 0.10, 95% CI ‐0.14 to 0.34 seconds).
The remaining studies were not included in the analysis due to insufficient data reporting (Kangari 2013; Kawakita 2013; Pinazo‐Durán 2013) or the presence of an unit of analysis error (Goyal 2017).
We judged the certainty of evidence for this outcome as low using GRADE, and we downgraded it by two levels, one for each of risk of bias and inconsistency.
Frequency of use of artificial tears
None of the studies in this category addressed this outcome.
Conjunctival impression cytology (CIC) score, as a measure of goblet cell density
Two studies, led by the same author, reported findings relevant to the outcome related to goblet cell density (Bhargava 2015a; Bhargava 2016b), and both reported an improvement with omega‐3 supplementation relative to placebo. However, we have not presented the meta‐analysis for this outcome (Analysis 1.5) due to substantial statistical heterogeneity (I² = 76%). Bhargava 2015a reported mean conjunctival impression cytology (CIC) scores (Nelson grade) and goblet cell density (cells/mm²) at three months of follow‐up. Relative to placebo, the omega‐3 intervention was found to improve goblet cell density (omega‐3: mean ± SD: 1018 ± 281, n = 220 vs 899 ± 375, n = 236 cells/mm²; P < 0.001). Bhargava 2016b reported an improvement in mean CIC score, based upon Nelson grade, in favor of the omega‐3 supplement at 45 days of follow‐up in middle‐aged visual display terminal users (MD ‐0.20, 95% CI ‐0.33 to ‐0.07).
1.5. Analysis.
Comparison 1 Oral long‐chain omega‐3 PUFAs (ie, EPA and DHA) versus placebo, Outcome 5 Conjunctival impression cytology (CIC) score: mean score at study endpoint (Nelson grade).
Proportion of participants with improved blurred vision symptoms
None of the 10 trials in this category reported on this outcome.
Ocular surface inflammatory biomarkers
No trials in this category considered changes to the human leukocyte antigen‐DR (HLA‐DR), or lymphocytic cell infiltration. Deinema 2017 analyzed changes from baseline in the levels of several pro‐inflammatory cytokines (ie, IL‐2, IL‐4, IL‐6, IL‐10, IL‐17A, interferon‐gamma, and TNF‐α) in the tear film. These study authors reported that at the day 90 study endpoint, basal tear concentrations of the pro‐inflammatory cytokine IL‐17A were significantly reduced relative to baseline in the krill oil (phospholipid omega‐3 intervention) group relative to the placebo (olive oil) group (krill oil: mean ± SEM: ‐27.1 ± 10.9, n = 18 vs placebo: 45.6 ± 30.4, n = 17 pg/mL; P = 0.02).
Tear osmolarity
Tear osmolarity data, reported as the change from baseline, were available from one trial (Deinema 2017). In this study, it was reported that at the day 90 study endpoint, tear osmolarity was significantly reduced relative to baseline in the omega‐3 group relative to the placebo group (MD: ‐17.71, 95% CI ‐28.07 to ‐7.35 mOsmol/L).
We used the GRADE assessment to judge the evidence as low certainty, after downgrading by two levels, one for each of imprecision and risk of bias.
Adverse outcomes
Table 9 summarizes adverse events as reported in the included studies.
Of the seven studies that investigated long‐chain omega‐3 fatty acid supplementation relative to placebo and reported adverse events, six trials reported that participants in the omega‐3 group experienced gastrointestinal disorders (including diarrhea) and one trial reported that participants in the comparator group experienced similar effects. Three of these studies provided sufficient data for a meta‐analysis related to gastrointestinal adverse effects (Analysis 1.6; Figure 7) (Asbell 2018; Bhargava 2016a; Deinema 2017). However, we have not presented the meta‐analysis for this outcome due to substantial statistical heterogeneity (I² = 76%). Gastrointestinal disorders were reported in between 5% and 19% of participants in the omega‐3‐treated group and between 0% and 24% of participants in the placebo group.
1.6. Analysis.
Comparison 1 Oral long‐chain omega‐3 PUFAs (ie, EPA and DHA) versus placebo, Outcome 6 Adverse event: gastrointestinal disorders.
7.
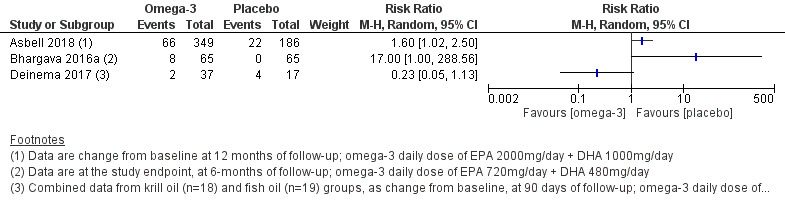
Forest plot of comparison: 1 Oral long‐chain omega‐3 PUFAs (ie, EPA and DHA) versus placebo, outcome: 1.6 Adverse event: gastrointestinal disorders.
With respect to cancer‐related events, Asbell 2018 reported four relevant events from 349 participants in the omega‐3 intervention group (n = 1 for each of chronic myeloid leukaemia, lymphoma, malignant melanoma, and squamous cell carcinoma) and two similar events (n = 1 for each of breast cancer and lymphoma) from 186 participants in the placebo supplement group. None of the other studies in this category reported cancer‐related events.
In terms of ocular adverse effects, Asbell 2018 reported 56 events (among 349 participants) in the omega‐3 supplement group and 33 events (among 186 participants) in the placebo group related to "eye disorders." None of the included studies reported ocular adverse events in this category.
The presence or absence of adverse events was not indicated in three studies (Goyal 2017; Kawakita 2013; Pinazo‐Durán 2013).
For this outcome, we downgraded the certainty of the body of evidence (according to the GRADE system) by two levels to low, as results were inconsistent among studies and few events accounted for the wide confidence intervals reported (imprecision).
Oral flaxseed oil (short‐chain omega‐3 PUFAs) versus placebo (two RCTs)
In a three‐arm RCT, Pinheiro 2007 compared the effects of flaxseed oil (administered in two different daily doses of 1 g and 2 g) versus a placebo intervention (daily dose of 1900 mg synthetic mineral oil and 100 mg of evening primrose oil) in women with keratoconjunctivitis sicca associated with rheumatoid arthritis or systemic lupus erythematosus. A total of 38 participants were enrolled in this six‐month follow‐up study. The other study in this category compared flaxseed oil (daily dose of 6000 mg) versus an oral olive oil (placebo) intervention (Macsai 2008). This trial involved 38 participants with simple, obstructive meibomian gland dysfunction and blepharitis over a follow‐up period of 12 months (Macsai 2008).
Primary outcome
Data were not combinable for the primary outcome measure related to dry eye symptoms, as the number of participants analyzed was not explicitly reported in Pinheiro 2007. This study reported that the mean reduction in dry eye symptoms was greater in both of the flaxseed oil groups compared with the placebo group. In the other trial, the mean OSDI score improved from baseline at 12 months in the flaxseed oil group (P = 0.02) but not in the placebo (olive oil) group (P = 0.17) (Macsai 2008). However, the mean change in OSDI score was not different between these groups (MD ‐4.05, 95% CI ‐13.12 to 4.12 OSDI units) (Macsai 2008). We graded the certainty of evidence as low after downgrading for imprecision and risk of bias.
Secondary outcomes
We were not able to combine data for any of the secondary outcomes due to lack of sufficient information about the participant numbers analyzed in Pinheiro 2007 and a unit of analysis error in Macsai 2008 (whereby individual participants were randomized to interventions, and analysis was performed "per eye" without appropriate statistical adjustment). Given these limitations, these data are not presented.
Adverse outcomes
Neither study reported adverse events.
Oral omega‐6 PUFAs versus placebo (six RCTs)
Six trials investigated omega‐6 PUFA supplements (as γ‐linolenic acid, or a combination of linoleic acid and γ‐linolenic acid) in people with dry eye (Barabino 2003), associated with primary Sjögren’s syndrome (Aragona 2005; Manthorpe 1984; Oxholm 1986; Theander 2002), or soft contact lens wear (Kokke 2008). The follow‐up period in these trials ranged from three weeks (using a cross‐over design: Manthorpe 1984; Oxholm 1986) to six months (Kokke 2008; Theander 2002). In total, these six trials enrolled 296 participants and ranged in sample size from 26 to 90 participants in individual studies. Two studies adopted a cross‐over design (Manthorpe 1984; Oxholm 1986); the other trials used a parallel‐group design.
Primary outcome
Aside from one trial (Oxholm 1986), all of the studies in this category measured dry eye symptoms.
In Aragona 2005, participants who received omega‐6 PUFA supplements had a greater improvement (reduction) in dry eye symptoms (measured via a custom grading scale, with scores ranging from 0 to 18) compared with those in the placebo group (MD ‐3.1, 95% CI ‐3.76 to ‐2.44). Barabino 2003 reported that there was no significant difference in mean symptom score (measured via the Rolando scoring system) between intervention groups after 45 days of treatment (MD ‐4.6, 95% CI ‐9.91 to 0.71). We could not combine data from these two studies due to the use of different grading scales. The remaining four trials reported results in non‐numeric formats or only with P values (Kokke 2008; Manthorpe 1984; Oxholm 1986; Theander 2002). In Kokke 2008, which involved the evaluation of contact lens wearers, no significant changes were evident for overall contact lens comfort over the trial duration in the placebo group. For the omega‐6 intervention group, results showed a reduction (relative to baseline) in the specific symptom of "dryness" at three and six months of follow‐up (P < 0.01), as measured on a 10‐point visual analogue scale. Manthorpe 1984,Oxholm 1986, and Theander 2002 all reported no significant change in subjective ocular dryness/ocular symptoms over the course of follow‐up. We graded the certainty of evidence as low after downgrading for imprecision and risk of bias.
Secondary outcomes
Ocular surface staining
Although all studies in this category assessed ocular surface staining, we were unable to combine findings due to interstudy differences in the clinical techniques used for assessment (ie, use of different staining agents, grading scales, regions of assessment, etc.). Details of the ocular surface staining procedures and the grading scales used are provided in Table 8.
Aragona 2005 used fluorescein to assess corneal staining via the National Eye Institute (NEI) grading scale, which examines five corneal areas (with scores ranging from 0 to 15). After one month of treatment, participants randomized to the omega‐6 PUFA group showed an improvement (reduction) compared with those in the control group (MD ‐2.5, 95% CI ‐3.37 to ‐1.63).
Three studies evaluated rose bengal staining (RBS) of both the cornea and the conjunctiva using the van Bijsterveld scoring system (Kokke 2008; Manthorpe 1984; Oxholm 1986). However, data from these studies could not be combined as the findings were described in terms of P values only. Kokke 2008 and Manthorpe 1984 reported that there was no significant change in RBS score after treatment. Although results showed no significant intergroup differences at the study endpoint, study authors in Oxholm 1986 reported an improvement at the study endpoint relative to the "start‐value" (baseline) in the omega‐6 PUFA group only (P < 0.05).
For Oxholm 1986, it should be noted that there was no apparent washout period between intervention phases, which would be necessary to account for the cross‐over design. Theander 2002 evaluated corneal and conjunctival lissamine green staining using the van Bijsterveld scale in individuals with primary Sjögren's syndrome and also reported no significant intragroup or intergroup differences over the six‐month study duration. Barabino 2003 reported both significant within‐group improvement (in the omega‐6 PUFA group only; P < 0.05) and between‐group differences favouring the omega‐6 supplementation group (P < 0.005) for conjunctival lissamine green staining score (measured on the van Bijsterveld scale) at day 45 of follow‐up.
Aqueous tear production
Five studies used the Schirmer test to measure aqueous tear production (Aragona 2005; Barabino 2003; Manthorpe 1984; Oxholm 1986; Theander 2002). Only one of these trials provided quantitative data and reported no significant intergroup differences after one month of intervention (MD ‐0.30, 95% CI ‐1.24 to 0.64) (Aragona 2005). Barabino 2003 reported no significant differences between treatment groups at 45 days of follow‐up. Manthorpe 1984 and Oxholm 1986 reported Schirmer test scores to be improved (relative to baseline) with Efamol (evening primrose oil) treatment at the study endpoints but reported no significant intergroup differences. In Theander 2002, no significant intragroup or intergroup differences were evident over the six‐month study duration. We graded the certainty of evidence as low after downgrading for inconsistency and risk of bias.
Tear film stability
All of the studies in this category measured tear film stability with TBUT, although study authors provided no quantitative data.
Kokke 2008 reported that compared to baseline, researchers noted no significant differences in TBUT at three or six months of follow‐up; they did not report intergroup differences. Barabino 2003 reported no significant differences between intervention groups at 45 days of follow‐up but noted a significant improvement in TBUT, relative to baseline, in the omega‐6 intervention arm only (P < 0.05). In Aragona 2005, TBUT did not differ within or between the two intervention groups at one month of follow‐up. Manthorpe 1984 and Oxholm 1986 reported no significant intergroup differences for TBUT over the duration of each study. Theander 2002 did not report TBUT findings.
Frequency of use of artificial tears
One study addressed the frequency of tear substitute use, measured as the average number of daily doses of tear substitutes (Theander 2002). These study authors reported that there was no significant intragroup or intergroup difference in tear substitute use over the six‐month study duration.
Ocular surface inflammatory biomarkers
One study assessed conjunctival HLA‐DR expression and showed a between‐group difference in favor of the omega‐6 group (MD ‐16.70, 95% CI ‐29.26 to ‐4.14) (Barabino 2003).
Adverse outcomes
Manthorpe 1984 reported that 47% of participants (n = 36) who were treated with Efamol/Efavit omega‐6 capsules experienced sudden universal flushing, sensation of heat, an increase in pulse frequency, and fear. The study authors suspected that these symptoms could be due to niacin, and the symptoms disappeared after participants changed from Efavit tablets to ZnSO₄ and vitamin C tablets. The study authors reported that the type and frequency of other adverse events were similar between the Efamol/Efavit and placebo groups.
Softening of stools and transient nausea were reported in three (10.7%) participants assigned to Efamol treatment in Oxholm 1986. Theander 2002 reported that gastrointestinal disorders were observed in 56% and 53% of participants in the omega‐6 and placebo supplement groups, respectively. The other three studies reported an absence of any significant adverse events (Aragona 2005; Barabino 2003; Kokke 2008).
Combined oral omega‐3 and omega‐6 PUFAs versus placebo (four RCTs)
Table 3 summarizes the effect of the intervention (combined omega‐3 and omega‐6 PUFAs) compared to the control (placebo) for the prespecified outcomes.
Four parallel‐group RCTs investigated combined omega‐3 and omega‐6 PUFA supplementation in individuals with (non‐specific) dry eye (Oral sea buckthorn oil study 2010), mild to moderate dry eye disease (Brignole‐Baudouin 2011; Creuzot 2006), or moderate dry eye disease (Creuzot‐Garcher 2011). Supplement content and dosage varied substantially between studies: daily dose of eicosapentaenoic acid (EPA) from 28 mg to 427.5 mg; docosahexaenoic acid (DHA) from 285 mg to 392 mg; "omega‐6" 15 mg; γ‐linolenic acid 82 mg; and linoleic acid 126 mg. Follow‐up periods ranged from three to six months. Overall, 490 participants were enrolled in this category, ranging from n = 71 in Creuzot 2006 to n = 181 in Creuzot‐Garcher 2011.
Primary outcome
All four studies in this category quantified dry eye symptoms, using a range of different rating scales. A meta‐analysis could not be performed due to lack of quantitative data or differences in the rating scales used.
Investigators in Oral sea buckthorn oil study 2010 examined eight dry eye symptoms (ie, soreness, foreign body sensation, dryness, grittiness, burning, redness, watery eyes, and blurry vision) using a 4‐point severity scale ranging from 0 (none) to 3 (severe). These study authors reported a trend toward improved (reduced) ocular redness (P = 0.04) and burning (P = 0.05) in the sea buckthorn supplement group, relative to placebo, after three months of treatment. Creuzot 2006 also assessed eight dry eye symptoms and reported that the number of participants who had specific symptoms ameliorated was significantly greater in the PUFA supplement group than in the placebo group for conjunctival hyperemia (P = 0.045), reflex lacrimation (P = 0.047), sensations of dryness (P = 0.059), and discomfort (P = 0.091). Creuzot‐Garcher 2011 measured ocular symptoms as related to sensations of eye fatigue, burning, prickling, sand/dust, light sensitivity, and reflex tearing and reported that the PUFA supplement group showed greater improvement than the placebo group in subjective symptoms of dry eye, but the difference between groups did not reach statistical significance, except in one sub‐domain (sensation of eye fatigue; P = 0.044). Brignole‐Baudouin 2011 used a four‐step rating scale to assess the symptoms of ocular burning, dryness, foreign body sensation, photophobia, and stinging, which were also used to derive a "global subjective dry eye score." Neither the global dry eye score nor the analysis of each individual symptom showed a significant difference between groups at the three‐month study endpoint.
We downgraded the certainty of evidence for this outcome, which we assessed using the GRADE approach, by two levels ‐ from high to low for risk of bias (one level) and inconsistency (one level).
Secondary outcomes
Ocular surface staining
Three trials in this category measured ocular surface staining as an outcome (Brignole‐Baudouin 2011; Creuzot 2006; Creuzot‐Garcher 2011), and quantified both corneal fluorescein staining (measured via the Oxford scale) and conjunctival lissamine green staining (measured using the van Bijsterveld scale). In Brignole‐Baudouin 2011, neither corneal staining with fluorescein (P = 0.70) nor conjunctival lissamine green staining (P = 0.09) showed a significant intergroup difference after three months of follow‐up. Creuzot‐Garcher 2011 reported the percentage of participants with different Oxford grades of corneal and conjunctival corneal staining, noting no significant intergroup difference at the six‐month study endpoint. Creuzot 2006 reported that the PUFA supplement had an effect on corneal fluorescein staining, whereby at six months of follow‐up, 32% of participants had no corneal staining compared with 25% of participants in the placebo group (P values were not provided).
We downgraded the certainty of the body of evidence by one level from high to moderate due to high risk of "other bias" from industry funding.
Aqueous tear production
All four studies used the Schirmer test to assess aqueous tear production. Data were reported as change from baseline (Brignole‐Baudouin 2011; Creuzot 2006), or as change at the study endpoint (Creuzot‐Garcher 2011; Oral sea buckthorn oil study 2010). For Oral sea buckthorn oil study 2010, participant numbers were provided as a range, and the lower value of the range was included in the analysis (ie, n = 45 in the PUFA intervention arm and n = 41 in the placebo arm). Creuzot 2006 reported performing the Schirmer test with anesthesia, and three trials did not specify how the technique was performed (Brignole‐Baudouin 2011; Creuzot‐Garcher 2011; Oral sea buckthorn oil study 2010). Combining data from these studies (Figure 8, Analysis 2.1) showed no difference between the PUFA and placebo supplement groups (four studies, 455 participants; MD 0.66, 95% CI ‐0.45 to 1.77 mm/5 min). The level of heterogeneity between these four studies was negligible (I² = 0%).
8.
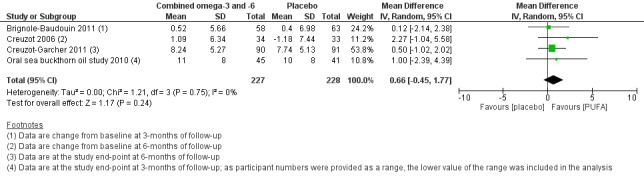
Forest plot of comparison: 2 Combined omega‐3 and omega‐6 PUFAs versus placebo, outcome: 2.1 Schirmer test (tear production) (mm/5 min).
2.1. Analysis.
Comparison 2 Oral combined omega‐3 and omega‐6 PUFAs versus placebo, Outcome 1 Schirmer test (tear production) (mm/5 min).
We assessed the certainty of evidence for this outcome using the GRADE approach, and we judged the certainty as moderate, as all of the studies for this outcome either were industry funded or included study authors with relevant pharmaceutical conflicts of interest.
Tear film stability
All four studies examined tear film stability by measuring TBUT with fluorescein. Combining data from these studies, for which TBUT was reported as change from baseline (Brignole‐Baudouin 2011; Creuzot 2006), or as change at the study endpoint (Creuzot‐Garcher 2011; Oral sea buckthorn oil study 2010), revealed significant improvement (increase) with combined omega‐3 and omega‐6 supplementation relative to placebo (four studies, 455 participants; MD 0.55, 95% CI 0.04 to 1.07 seconds) (Analysis 2.2; Figure 9). For Oral sea buckthorn oil study 2010, participant numbers were provided as a range, and the lower value of the range was included in the analysis (ie, n = 45 in the PUFA intervention arm and n = 41 in the placebo arm). The degree of heterogeneity between these four studies was negligible (I² = 0%).
2.2. Analysis.
Comparison 2 Oral combined omega‐3 and omega‐6 PUFAs versus placebo, Outcome 2 TBUT: Mean change from baseline (seconds).
9.
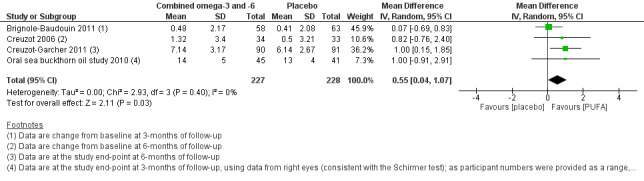
Forest plot of comparison: 2 Combined omega‐3 and omega‐6 PUFAs versus placebo, outcome: 2.2 TBUT: Mean change from baseline (seconds).
We used the GRADE approach to assess the certainty of evidence for this outcome as moderate because all of the studies for this outcome were industry funded or included study authors with relevant pharmaceutical conflicts of interest.
Frequency of use of artificial tears
One study (Brignole‐Baudouin 2011) provided data relevant to this outcome. This study reported that the "mean of daily tear substitute instillations was comparable for both (intervention) groups, with no significance difference" over the three‐month study duration.
Ocular surface inflammatory biomarkers
Two studies analyzed conjunctival HLA‐DR expression, but these data could not be meta‐analyzed due to differences in how they were reported. Brignole‐Baudouin 2011 reported a significant improvement (reduction) in fluorescence intensity of HLA‐DR in the PUFA supplement group relative to placebo at the study endpoint (P = 0.017). Creuzot‐Garcher 2011 also reported that HLA‐DR fluorescence was increased in the placebo group but observed no significant between‐group differences.
Tear osmolarity
Oral sea buckthorn oil study 2010 assessed tear osmolarity at monthly study visits over a three‐month intervention period and reported that osmolarity became worse (increased) in both the PUFA supplementation and placebo groups, but that this increase was significantly greater (P = 0.04) in the placebo group after adjustment for "significant" covariates (defined by study authors as baseline osmolarity, Schirmer test score, age, modified OSDI score, TBUT, and contact lens wear). The number of participants analyzed for each outcome was provided only as a range; therefore further analysis was not performed.
We judged the certainty of the evidence as low because only one study reported this outcome, and we assessed this study as having high risk of "other" bias.
Adverse outcomes
One trial reported data related to adverse events (Brignole‐Baudouin 2011). The authors reported treatment‐related, non‐ocular adverse events in four (6.0%) and five (7.1%) participants in the omega‐3 plus omega‐6 treatment group and in the placebo group, respectively. This study reported no product‐related serious adverse events and no product‐related ocular adverse events. The most common adverse event in the PUFA supplement group was a mild gastrointestinal disorder. Two participants in each intervention group discontinued the study due to adverse events.
We used GRADE to judge the certainty of the body of evidence as low because only one study reported this outcome and all study authors were industry funded or had relevant pharmaceutical conflicts of interest.
Oral omega‐3 PUFAs plus conventional therapy versus conventional therapy alone (two RCTs)
Table 4 summarizes the effects of omega‐3 PUFAs plus conventional therapy versus conventional therapy alone.
Omega‐3 supplementation was administered in association with once‐daily eyelid hygiene (Systane Lid Wipes, Alcon, Fort Worth, TX, USA) plus once‐daily lipid emulsion eye drops (Systane Balance, Alcon) (Korb 2015), or with "standard therapy" that comprised artificial tears every four hours and betamethasone 0.1% eye drops every eight hours (Mohammadpour 2017). In Korb 2015, the comparator was eyelid warm compresses (eight minutes, once daily), and in Mohammadpour 2017 , the comparator was the specified "standard therapy" alone. Twenty‐six participants with lipid deficient/evaporative dry eye were enrolled in Korb 2015.Mohammadpour 2017 analyzed 63 eyes from 48 participants with post‐cataract dry eye; these data from right and/or left eyes were incorporated into the analyses and were treated as independent samples, resulting in an unit of analysis errors for per eye data.
Primary outcome
Both studies reported data related to dry eye symptoms.
In Korb 2015, dry eye symptoms were a secondary outcome and were quantified using both the OSDI and SPEED questionnaires. This study reported that although both omega‐3 plus conventional therapy and conventional therapy intervention arms were significantly improved from baseline, researchers found no significant intergroup differences at three months of follow‐up (SPEED: MD ‐2.9, 95% CI ‐5.97 to 0.17 units; OSDI: MD ‐3.5, 95% CI ‐15.03 to 8.03 units). In Mohammadpour 2017, the OSDI score significantly improved (reduced) over the one‐month treatment duration in both omega‐3 plus conventional therapy (P < 0.001) and conventional therapy (P = 0.003) groups. In addition, the study authors reported that the "OSDI improvement was significantly higher in the (omega‐3) treatment group in comparison to the control group (p=0.026)."
Combining data from these two studies (Analysis 3.1; Figure 10), which had negligible heterogeneity (I² = 0%) revealed a significantly improved (lower) OSDI score at the study endpoint with omega‐3 supplementation with conventional therapy relative to conventional therapy alone (two studies, 70 participants; MD ‐7.16, 95% CI ‐13.97 to ‐0.34).
3.1. Analysis.
Comparison 3 Oral omega‐3 PUFAs plus conventional therapy versus conventional therapy alone (two RCTs), Outcome 1 OSDI score: mean score at study endpoint [OSDI units].
10.
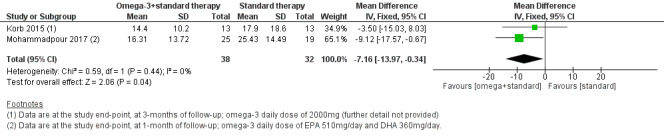
Forest plot of comparison: 3 Oral omega‐3 PUFAs plus conventional therapy versus conventional therapy alone (two RCTs), outcome: 3.1 OSDI score: mean score at study endpoint [OSDI units].
We used GRADE to judge the certainty of the body of evidence for this outcome; we downgraded the findings by two levels to low due to imprecision (ie, small sample size for each trial), and we determined that both trials had high risk of bias in one of the blinding domains.
Secondary outcomes
Data from Mohammadpour 2017 are not reported in detail here due to the unit of analysis error (as detailed above) for secondary outcomes related to per eye data. In brief, these study authors reported that the mean "TBUT improved (significantly) in both groups (p<0.001). However, TBUT was affected significantly more in the treatment group compared with the control group, p=0.038," and the mean tear osmolarity significantly improved from 315.40 ± 17.06 Osmol/L (although we suspect this should have been mOsmol/L units) to 296.90 ± 14.39 in the omega‐3 treatment group (P < 0.001) but not in the control group (P = 0.157). These investigators also found no significant intragroup or intergroup differences in Schirmer test scores over the study duration.
Korb 2015 reported none of the prespecified secondary outcomes for this review.
Adverse outcomes
Korb 2015 reported that "a total of two adverse events (infectious mononucleosis and sinusitis) were reported for a single patient in the combination treatment group (omega‐3s plus conventional therapy); neither was considered to be treatment related." Mohammadpour 2017 did not report adverse events.
Oral long‐chain omega‐3 PUFAs (EPA and DHA) versus oral omega‐6 PUFAs (five RCTs)
Table 5 summarizes the effect of the intervention (long‐chain omega‐3 PUFAs) compared with the omega‐6 PUFAs for the prespecified outcomes.
Five RCTs compared capsules containing long‐chain omega‐3 fatty acids ‐ EPA and DHA ‐ with an omega‐6 PUFA intervention (Bhargava 2013; Bhargava 2015b; Epitropoulos 2016; NCT01107964; Oleñik 2013). Table 7 presents further details related to the interventions. Among studies that reported the adopted omega‐3 dosages, the daily dose of EPA and DHA varied substantially, ranging from a daily value of EPA of 127.5 mg to 1680 mg, and DHA of 350 mg to 1050 mg. The omega‐6 interventions comprised of corn oil (Bhargava 2013; Bhargava 2015b, NCT01107964), linoleic acid (safflower oil 3136 mg/day) (Epitropoulos 2016) and sunflower oil (Oleñik 2013). Together, these five trials included 1227 participants; sample sizes ranged from n = 27 in NCT01107964 to n = 518 in Bhargava 2015b. Length of follow‐up ranged from 45 days to six months. All five studies used a parallel‐group design. Participant eligibility criteria varied across studies; for the studies that enrolled participants with specific etiologies of dry eye disease, these were specified as dry eye associated with contact lens wear (Bhargava 2015b), and meibomian gland dysfunction (Epitropoulos 2016; Oleñik 2013).
Primary outcome
All five studies evaluated subjective symptoms of dry eye and reported this parameter as the final mean score, mean change from baseline, or number of participants with improved symptoms of dry eye.
In three studies (Epitropoulos 2016; NCT01107964; Oleñik 2013), the researchers evaluated subjective symptoms of dry eye using the OSDI questionnaire. Meta‐analysis, based upon the availability of quantitative data, was performed by combining data from two studies, reported as mean change from baseline (Epitropoulos 2016; NCT01107964) (Analysis 4.1, Figure 11). The summary estimate for these studies indicated a significant reduction in dry eye symptoms with long‐chain omega‐3 PUFA supplementation relative to an omega‐6 PUFA intervention (two studies, 130 participants; MD ‐11.88, 95% CI ‐18.85 to ‐4.92 OSDI units). The level of heterogeneity between these two studies was negligible (I² = 0%). Symptom score data were reported only as P values in one study (Oleñik 2013).
4.1. Analysis.
Comparison 4 Oral long‐chain omega‐3 PUFAs versus oral omega‐6 PUFAs, Outcome 1 Dry eye symptoms, measured using the OSDI score [OSDI] units.
11.
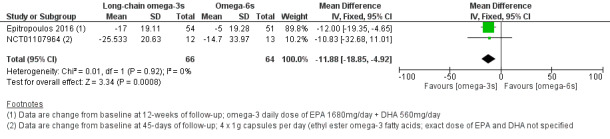
Forest plot of comparison: 4 Oral long‐chain omega‐3 PUFAs versus oral omega‐6 PUFAs, outcome: 4.1 Dry eye symptoms, measured using the OSDI score [OSDI] units.
Two trials led by the same study investigator adopted the DESS (Bhargava 2013; Bhargava 2015b). Bhargava 2013 reported data as change from baseline at the study endpoint (ie, at three months of follow‐up) and the other trial (Bhargava 2015b) reported mean DESS scores at the study endpoint. Both studies reported a difference in mean score at the end of the follow‐up period, in favor of the omega‐3 PUFA intervention. However the summary estimate was not presented because of substantial statistical heterogeneity between the studies (I² = 100%) (Analysis 4.2).
4.2. Analysis.
Comparison 4 Oral long‐chain omega‐3 PUFAs versus oral omega‐6 PUFAs, Outcome 2 DESS: mean score at study endpoint [DESS units].
Using GRADE to judge the certainty of the body of evidence, we downgraded the findings by one level to moderate, as one of the two studies included for the OSDI parameter had received industry support (ie, judged to have high risk of "other bias").
Secondary outcomes
Ocular surface staining
As summarized in Table 8, four studies examined ocular surface staining (Bhargava 2013; Epitropoulos 2016; NCT01107964; Oleñik 2013). We were unable to combine data for this outcome owing to lack of numeric data and/or use of different clinical grading scales.
Oleñik 2013 measured corneal and conjunctival fluorescein staining using the Oxford Scale. The study authors did not provide numeric data but reported that "in both (treatment) groups, corneal staining data presented no significant differences from baseline" at the three‐month study endpoint. Investigators in NCT01107964 assessed corneal and conjunctival staining using an unspecified scale, scored from 0 to 9. In this study, the change from baseline in lissamine green staining at day 45 of follow‐up was not significantly different between the two intervention groups (median [interquartile range] omega‐3 group 1.0 [‐1.5 to 1.0], n = 12 vs omega‐6 group ‐1.0 [‐2.0 to 0.0] units, n = 13; P = 0.44). Bhargava 2013 assessed corneal and conjunctival rose bengal staining (RBS), graded on the van Bijsterveld Scale from 0 to 9, and reported that (relative to baseline) there was significant improvement in staining in the omega‐3 group (P < 0.001) but not in the omega‐6 group (P = 0.564) after three months; study authors did not provide numeric data. Epitropoulos 2016 reported the mean change from baseline in corneal fluorescein staining, measured on the Oxford Scale, at three months of follow‐up. These authors reported a significant reduction in corneal fluorescein staining in the omega‐3 group relative to the omega‐6 group (MD ‐0.10, 95% CI ‐0.18 to ‐0.03 Oxford units).
We used the GRADE approach to judge the evidence as of low certainty, after downgrading by one level for inconsistency (due to the divergent findings across studies), and one level due to risk of bias (due to the presence of a high risk of attrition bias in three studies).
Aqueous tear production
All five trials examined aqueous tear production using the Schirmer test (with or without topical anesthesia) and reported results as the mean change from baseline or final mean score at study endpoint. We extracted relevant quantitative data from four studies (Bhargava 2013; Bhargava 2015b; Epitropoulos 2016; NCT01107964) (Analysis 4.3). Three of these trials performed the Schirmer test using topical anesthesia (Bhargava 2013; Bhargava 2015b; Epitropoulos 2016), and one trial did not report how the procedure was performed (NCT01107964). A meta‐analysis was not presented for this outcome due to substantial statistical heterogeneity (Analysis 4.3) (I² = 97%), and differences in dry eye etiology and supplementation dosing across the studies.
4.3. Analysis.
Comparison 4 Oral long‐chain omega‐3 PUFAs versus oral omega‐6 PUFAs, Outcome 3 Schirmer test (tear production) (mm/5 min).
Bhargava 2013 reported the mean change in Schirmer score (mm/5 min) at three months of follow‐up. Relative to the omega‐6 intervention, omega‐3 supplementation was found to improve tear production (omega‐3: mean ± SD: 0.62 ± 1.06 vs omega‐6: 0.15 ± 0.35 mm/5 min, P < 0.001). Bhargava 2015b reported a higher Schirmer test score in favor of the omega‐3 intervention in individuals with contact lens‐associated dry eye at six months of follow‐up (MD: 2.60, 95% CI 2.21 to 2.99 mm/5 min). The other two studies reported no significant change in Schirmer test score at 12‐weeks of follow‐up in individuals with meibomian gland dysfunction (Epitropoulos 2016), and at 45‐days of follow‐up in people with non‐specific dry eye (NCT01107964).
One study reported, in descriptive terms, no significant intergroup difference in Schirmer test score (Oleñik 2013).
We judged the certainty of evidence for this outcome as low using GRADE, and we downgraded it by two levels for inconsistency.
Tear film stability
All of the included studies assessed tear film break‐up time (TBUT) using fluorescein, to quantify tear film stability. As summarized in Analysis 4.4, three studies reported quantitative TBUT data as mean change from baseline at study endpoint (Bhargava 2013; Epitropoulos 2016; NCT01107964), and two trials reported data as mean values at the end of the study follow‐up period (Bhargava 2015b; Oleñik 2013). Four of the included trials (Bhargava 2013; Bhargava 2015b; Epitropoulos 2016; Oleñik 2013) reported an improvement in TBUT with omega‐3 supplementation relative to the omega‐6 intervention, and one study (NCT01107964) reported no significant inter‐group difference. A meta‐analysis (Analysis 4.4), however, was not presented for this outcome due to substantial statistical heterogeneity (I² = 94%), and differences in dry eye etiology and supplementation dosing across the studies.
4.4. Analysis.
Comparison 4 Oral long‐chain omega‐3 PUFAs versus oral omega‐6 PUFAs, Outcome 4 Tear break‐up time (TBUT) measured using fluorescein (seconds).
We judged the certainty of evidence for this outcome as low using GRADE, and we downgraded it by two levels for inconsistency.
Frequency of use of artificial tears
None of the studies in this category addressed this outcome.
Conjunctival impression cytology (CIC) score, as a measure of goblet cell density
Two studies, led by the same author, reported findings relevant to the outcome related to goblet cell density (Bhargava 2013; Bhargava 2015b). One trial (Bhargava 2015b) reported an improvement with omega‐3, relative to omega‐6, supplementation at six months of follow‐up in individuals with contact lens‐associated dry eye (MD ‐0.38, 95% CI ‐0.45 to ‐0.31 Nelson units). Bhargava 2013 performed CIC to assess for ocular surface changes and goblet cell density. Although this trial provided no quantitative data, the study authors reported that there was a reduction (relative to baseline) in the proportion of participants graded to have "abnormal" CIC scores (P = 0.000) at the study endpoint (day 90) and no change relative to baseline in the omega‐6 group (P = 0.250).
Proportion of participants with improved blurred vision symptoms
None of the five trials in this category reported on this outcome.
Ocular surface inflammatory biomarkers
None of the studies in this category considered changes to the human leukocyte antigen‐DR (HLA‐DR), or lymphocytic cell infiltration. Epitropoulos 2016 considered the proportion of participants with a positive test for matrix metalloproteinase‐9 (MMP‐9), assessed via the InflammaDry test (Rapid Pathogen Screening Inc., Sarasota, FL, USA), as a measure of ocular surface inflammation. It was reported that the number of participants who tested positive to MMP‐9 in the omega‐3 group decreased by 67.9% over 12 weeks, compared with a decrease in the proportion of participants in the omega‐6 group of 35.0% (Chi² testing indicated a significant intergroup difference; P = 0.024).
Tear osmolarity
Tear osmolarity data, reported as the change from baseline, were available from one trial (Epitropoulos 2016). In this study, it was reported that at the 12‐week study endpoint, tear osmolarity was reduced relative to baseline in the omega‐3 group relative to the omega‐6 group (MD: ‐11.10, 95% CI ‐12.15 to ‐10.05 mOsmol/L).
We used the GRADE assessment to judge the evidence as low certainty, after downgrading by one level for imprecision and one level for risk of bias, as data derived from a single study.
Adverse outcomes
Table 9 summarizes adverse events as reported in the included studies.
All three studies in this category that reported adverse events indicated that participants in the omega‐3 group experienced gastrointestinal disorders (such as diarrhea) and none of the participants in the comparator group experienced similar effects. Two of these studies provided sufficient data for a meta‐analysis (Analysis 4.5, Figure 12) related to gastrointestinal adverse effects (NCT01107964; Oleñik 2013). Combining the results from these two trials indicated that the relative risk of a gastrointestinal adverse event was unclear between omega‐3 and omega‐6 supplementation (two studies, 91 participants; RR 2.34, 95% CI 0.35 to 15.54). Negligible heterogeneity (I² = 0%) was noted.
4.5. Analysis.
Comparison 4 Oral long‐chain omega‐3 PUFAs versus oral omega‐6 PUFAs, Outcome 5 Adverse event: gastrointestinal disorders.
12.
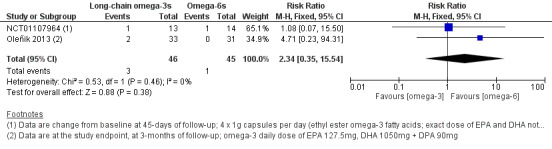
Forest plot of comparison: 4 Oral long‐chain omega‐3 PUFAs versus oral omega‐6 PUFAs, outcome: 4.5 Adverse event: gastrointestinal disorders.
Two studies (Bhargava 2013; Epitropoulos 2016) did not report on adverse events.
For this outcome, we downgraded the certainty of the body of evidence (according to GRADE) by two levels to low; one level related to there being few events, which accounted for the wide confidence intervals reported (imprecision), and the second level related to risk of bias.
Combined oral fish oil (long‐chain omega‐3 PUFAs) and flaxseed oil (short‐chain omega‐3 PUFAs) versus combined omega‐3 and omega‐6 PUFAs (three RCTs)
One trial evaluated a combination of fish oil (containing 450 mg EPA and 300 mg DHA) and flaxseed oil (1000 mg) in the commercially available formulation TheraTears Nutrition (Advanced Vision Research, Woburn, MA, USA) among participants with dry eye (Wojtowicz 2011) relative to a comparator of wheat germ oil (which contains both omega‐3 and omega‐6 PUFAs, but approximately eight‐fold more omega‐6 than omega‐3 PUFAs). Two trials evaluated the same TheraTears Nutrition formulation for treating dry eye disease associated with Sjögren’s syndrome (Gilbard 2008; Papas 2007) relative to wheat germ oil (Gilbard 2008) or germ seed oil (Papas 2007). In total these trials reported on a total of 138 participants, ranging from 36 to 61 in the individual studies included in this category. Two studies were reported in abstract format only (Gilbard 2008; Papas 2007).
Primary outcome
Due to lack of quantitative data, a meta‐analysis could not be performed for this outcome. Two studies stated that a greater number of participants in the "active" (omega‐3 only) group than in the comparator group improved in terms of dry eye symptoms (Gilbard 2008; Wojtowicz 2011). In contrast, Papas 2007 reported no significant intergroup differences in symptoms of dry eye between the active (omega‐3 only) and comparator groups.
Secondary outcomes
We found limited information related to the prespecified secondary outcomes. Two trials provided no relevant data (Gilbard 2008; Papas 2007). Wojtowicz 2011 reported that the Schirmer test score (measured without topical anesthesia) had improved in both active (omega‐3 only) and comparator (wheat germ oil) supplement groups after 90 days, but researchers observed no significant intergroup differences (MD 1.81, 95% CI ‐4.53 to 8.15 mm/5 min). We graded the certainty of evidence as low after downgrading for both imprecision and risk of bias.
Adverse outcomes
None of these studies provided information related to adverse events.
Combined omega‐3 and omega‐6 PUFAs versus omega‐6 PUFAs (one RCT)
One parallel‐group RCT (Sheppard 2013) investigated combined omega‐3 and omega‐6 PUFA supplementation, relative to an omega‐6 PUFA comparator (sunflower oil), in 45 women with post‐menopausal dry eye disease.
Primary outcome
Sheppard 2013 measured dry eye symptoms using the OSDI questionnaire. These trial authors reported a significant improvement from baseline in the combined omega‐3 and omega‐6 PUFA group (P = 0.004), which was significantly greater than the level of improvement in the omega‐6 PUFA group (P = 0.05).
Secondary outcomes
Ocular surface staining
Sheppard 2013 quantified corneal fluorescein staining and conjunctival lissamine green staining using an unspecified grading system in five regions (superior, inferior, temporal, nasal, and central), and reported that both interventions had no significant effect on either staining outcome in any region (P = 0.1 to 0.9).
Aqueous tear production
Aqueous tear production was assessed using the Schirmer test. Although no quantitative data were provided, Sheppard 2013 noted no significant intergroup differences in aqueous tear production over the study duration.
Tear film stability
Although no quantitative data were reported for this outcome, the study authors noted no significant intergroup differences in TBUT over the study duration.
Frequency of use of artificial tears
Sheppard 2013 reported that artificial tear use (as an adjunctive therapy) occurred approximately two times per day in both intervention groups, and this did not change throughout the six‐month study period.
Ocular surface inflammatory biomarkers
Sheppard 2013 reported that the fluorescent intensity of HLA‐DR‐positive dendritic cells in the conjunctiva was significantly greater in the sunflower oil supplement group than the combined omega‐3 and omega‐6 supplement group (P = 0.001).
Tear osmolarity
This outcome was not assessed in this study.
Adverse outcomes
It was reported that there no treatment‐related ocular adverse events and no significant systemic adverse events during the six‐month study period. One participant (5.2%) in the combined omega‐3 and omega‐6 PUFA treatment group withdrew from the study due to a rash that appeared about two weeks after treatment initiation.
Combined oral fish oil (long‐chain omega‐3 PUFAs) and flaxseed oil (short‐chain omega‐3 PUFAs) versus short‐chain omega‐3 PUFAs (one RCT)
One trial (Reeder 2006) evaluated a combination of fish oil (containing 450 mg EPA and 300 mg DHA) and flaxseed oil (1000 mg) in the commercially available formulation TheraTears Nutrition (Advanced Vision Research, Woburn, MA, USA) among participants with dry eye, relative to a comparator of short‐chain omega‐3 PUFAs (flaxseed oil, 1000 mg/day). This trial included 20 participants, and was reported in abstract form only.
Primary outcome
Reeder 2006 reported no significant intergroup differences in symptoms of dry eye between the two intervention arms.
Secondary outcomes
There were no relevant data relating to any of the secondary outcomes.
Adverse outcomes
Reeder 2006 did not provide information related to adverse events.
Discussion
Summary of main results
The primary objective of this review was to assess the effects of omega‐3 and omega‐6 polyunsaturated fatty acid (PUFA) supplements on the symptoms and signs of dry eye disease, while documenting any potential treatment‐related adverse events. We identified 34 relevant randomized controlled trials (RCTs) published between 1984 and 2018, involving 4314 adult participants with "dry eye" (as defined by study authors) from 13 countries. The number of participants ranged from 20 to 535 in individual trials. Study follow‐up periods ranged from one month to 12 months.
Overall, the included studies showed significant variation in quality. Only nine (26.5%) trials had published protocols and/or were clearly prospectively registered on a trial registry. The two best reported risk of bias domains were "random sequence generation" (selection bias) and "allocation concealment" (selection bias); we judged approximately half of the included trials to have low risk of bias in these domains. We judged more than half of the included studies to have high risk of bias in the domains related to "attrition bias" and "other bias." The most frequent reason for high risk of "other" bias was industry funding or author affiliation with a company having a potential commercial interest in the intervention. Given that participant masking is likely to have a substantial effect on the subjective response to an intervention in dry eye disease, masking is considered a key risk of bias domain; only 10 (30.3%) trials described adequate methods for masking participants, trial personnel, and outcome assessors to group allocations.
We subclassified the included studies based on type of oral PUFA supplement and provided summaries of their main results separately in the following nine categories:
Long‐chain omega‐3 PUFAs (ie, eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) versus placebo or no treatment (10 RCTs).
Flaxseed oil (short‐chain omega‐3 PUFAs) versus placebo (two RCTs).
Omega‐6 PUFAs versus placebo (six RCTs).
Combined omega‐3 and omega‐6 PUFAs versus placebo (four RCTs).
Omega‐3 PUFAs plus conventional therapy versus conventional therapy alone (two RCTs).
Long‐chain omega‐3 PUFAs (EPA and DHA) versus omega‐6 PUFAs (five RCTs).
Combined fish oil (long‐chain omega‐3 PUFAs) and flaxseed oil (short‐chain omega‐3 PUFAs) versus combined omega‐3 and omega‐6 PUFAs (three RCTs).
Combined omega‐3 and omega‐6 PUFAs versus omega‐6 PUFAs (one RCT).
Combined fish oil (long‐chain omega‐3 PUFAs) and flaxseed oil (short‐chain omega‐3 PUFAs) versus short‐chain omega‐3 PUFAs (one RCT).
Oral long‐chain omega‐3 PUFAs (ie, EPA and DHA) versus placebo or no treatment (10 RCTs)
Results for this comparison are summarized in Table 1.
For the primary outcome related to subjective improvement in symptoms, the summary estimate derived from pooling data from four studies indicated little to no clinically meaningful reduction in dry eye symptoms, quantified using the OSDI questionnaire. There was a moderate level of statistical heterogeneity amongst these studies (I² = 48%). The daily dose of combined EPA and DHA ranged from 600 mg to 3000 mg in these studies. In three other studies, symptom data were reported as P values or in a non‐quantitative format, or included non‐dry eye participants, and were not included in the analysis.
Three additional trials assessed dry eye symptoms using the Dry Eye Questionnaire and Scoring System (DESS) (Bhargava 2015a; Bhargava 2016a; Bhargava 2016b); however, again the level of heterogeneity was high and a summary estimate was not presented. In each of these studies, involving the same first author, participants assigned to the omega‐3 PUFA treatment had significantly lower symptom scores at the study endpoint than participants assigned to placebo. In these trials, the daily dose of combined EPA and DHA ranged from 1200 mg to 2400 mg.
Four trials reported data relating to ocular surface staining; however we were unable to combine data for this outcome due to a lack of quantitative data or use of different grading scales. Among trials for which data could not be analyzed, one study reported a significant improvement (reduction) in corneal and conjunctival rose bengal staining from baseline in the omega‐3 group only (Kawakita 2013). Two studies found no significant intergroup differences in the measured ocular surface staining parameters (Asbell 2018; Deinema 2017). In Goyal 2017, the proportion of eyes with lissamine conjunctival staining after three months of treatment was reported to be significantly greater in the omega‐3 PUFA group compared with the control group.
All 10 trials measured aqueous tear production; the overall a summary estimate, derived from pooling data from six trials that provided quantitative data, indicated a significant improvement in Schirmer test score with the omega‐3 supplement intervention, relative to control (MD 0.68, 95% CI 0.26 to 1.09 mm/5 min). However, this intergroup difference was judged not to be clinically meaningful (with an overall < 1 mm/5 min intergroup difference). For the other four studies, one described there to be no significant intergroup difference in Schirmer test score, one had an unit of an analysis error and could not be incorporated into the meta‐analysis, and two studies did not separately report data for dry eye and non‐dry eye (healthy) controls.
For tear break‐up time (TBUT) measured with fluorescein as a measure of tear stability, five trials reported quantitative data, however the meta‐analysis was not presented due to a high level of statistical heterogeneity (I² = 98%). Of these studies, four reported a significant improvement in TBUT with the omega‐3 PUFA intervention compared with placebo, and one study reported no significant intergroup difference. The remaining five studies were not included in the analysis due to insufficient data reporting, an unit of analysis error or inclusion of participants without dry eye disease.
For effects on conjunctival goblet cell density, two trials (Bhargava 2015a,Bhargava 2016b) reported a significant improvement with omega‐3 supplementation relative to placebo, although a meta‐analysis was not presented due to substantial statistical heterogeneity (I² = 76%).
One trial (Deinema 2017) considered tear osmolarity, and reported a significant improvement (reduction) with long‐chain omega‐3 PUFA supplementation relative to placebo.
None of the studies for this comparison considered the frequency of artificial tear use, nor the proportion of participants with improved blurred vision.
Among data from three trials for the safety outcome related to risk of a gastrointestinal adverse event, events were too few to show clear differences between the omega‐3 and placebo interventions (Asbell 2018; Bhargava 2016a; Deinema 2017).
Oral flaxseed oil (short‐chain omega‐3 PUFAs) versus placebo (two RCTs)
Meta‐analysis was not possible for any of the prespecified outcomes.
For the primary outcome measure, which was related to symptoms, Pinheiro 2007 reported a significant mean reduction with short‐chain omega‐3 PUFA supplementation relative to placebo, and Macsai 2008 found no significant intergroup difference.
Secondary outcome data are not presented due to incomplete data reporting. Pinheiro 2007 did not clearly state the number of participants per group, and Macsai 2008 had an unit of analysis error.
Neither study reported adverse events.
Oral omega‐6 PUFAs versus placebo (six RCTs)
Data could not be combined for the primary outcome related to symptoms due to the use of different grading scales and presentation of data in non‐numeric formats or with P values only.
Of the five RCTs that provided information relevant to this outcome, only Aragona 2005 reported that the omega‐6 PUFA intervention was significantly more efficacious, and four trials reported no significant intergroup differences (Kokke 2008; Manthorpe 1984; Oxholm 1986; Theander 2002).
Although all six trials reported findings related to ocular surface staining, our ability to combine data from these studies was limited due to lack of quantitative data and/or use of different grading scales. Corneal fluorescein staining was relatively reduced with the omega‐6 PUFA intervention relative to placebo in one trial (Aragona 2005). Barabino 2003 reported a similar intergroup difference for conjunctival lissamine green staining. However, these findings contrast with other studies, which reported no significant intergroup differences for ocular surface rose bengal staining (Kokke 2008; Manthorpe 1984; Oxholm 1986), nor for conjunctival lissamine green staining (Theander 2002).
For aqueous tear production (five trials), TBUT (five trials), and frequency of artificial tear use (one trial), all studies reported no statistically significant intergroup differences.
Only Barabino 2003 assessed conjunctival human leukocyte antigen‐DR (HLA‐DR) expression and reported a significant reduction in favor of the omega‐6 group relative to placebo.
Combined oral omega‐3 and omega‐6 PUFAs versus placebo (four RCTs)
Results for this comparison are summarized in Table 3.
Overall, trials provided mixed evidence related to the potential effects of combined omega‐3 and omega‐6 PUFA supplements on dry eye symptoms. Although data from the five trials could not be meta‐analyzed due to lack of provided quantitative data or differences in measurement methods, one RCT reported the PUFA intervention to be relatively more efficacious than placebo (Creuzot 2006), and three studies reported no significant intergroup differences in overall symptom scores (Brignole‐Baudouin 2011; Creuzot‐Garcher 2011; Oral sea buckthorn oil study 2010).
For TBUT, data were combined from all four trials (Brignole‐Baudouin 2011; Creuzot 2006; Creuzot‐Garcher 2011; Oral sea buckthorn oil study 2010). The overall effect estimate indicated a significant improvement (increase) in tear stability with the PUFA intervention relative to placebo. Whilst statistically significant, it should be noted that the mean difference and associated 95% confidence intervals may not be clinically significant (with an overall < 1 second change in TBUT).
Only Oral sea buckthorn oil study 2010 considered tear osmolarity and reported significantly greater worsening (increase) in the placebo group relative to the PUFA group. Two trials quantified ocular surface inflammatory markers (Brignole‐Baudouin 2011; Creuzot‐Garcher 2011); one study reported a significant improvement (reduction) in HLA‐DR‐positive conjunctival staining in the PUFA supplement group, and one trial reported no significant intergroup differences.
For the secondary outcomes of ocular surface staining (three trials), aqueous tear production (four trials), and frequency of use of artificial tears (one trial), all studies reported no significant intergroup differences.
One trial (Brignole‐Baudouin 2011) reported data relevant to adverse events; however the relative risk of gastrointestinal events could not be calculated due to lack of clarity surrounding the nature of specific events. In this study, four (6.0%) participants in the PUFA group and five participants (7.1%) in the placebo group experienced treatment‐related, non‐ocular adverse events.
Oral omega‐3 PUFAs plus conventional therapy versus conventional therapy alone (two RCTs)
Results for this comparison are summarized in Table 4.
For the primary efficacy outcome related to symptom improvement, combined data from the two trials in this category, both of which used the OSDI questionnaire (Korb 2015; Mohammadpour 2017) and had negligible heterogeneity (I² = 0%), showed a significant intergroup difference in symptoms favoring the omega‐3 intervention arm.
There was limited ability to draw conclusions in relation to secondary outcomes, as only one study ‐ Mohammadpour 2017 ‐ reported on relevant outcomes, and this study had an unit of analysis error.
Oral long‐chain omega‐3 PUFAs (EPA and DHA) versus oral omega‐6 PUFAs (five RCTs)
Results for this comparison are summarized in Table 5.
For the primary outcome related to improvement in dry eye symptoms, quantified using the OSDI questionnaire, the summary estimate derived from pooling data from two studies (Epitropoulos 2016; NCT01107964) indicated a significant reduction in dry eye symptoms with the omega‐3 intervention relative to the omega‐6 intervention. There was negligible heterogeneity between the studies (I² = 0%). An additional two studies (Bhargava 2013; Bhargava 2015b), performed by the same lead investigator, assessed symptoms of dry eye using the Dry Eye Questionnaire and Scoring System (DESS), but data were not combined due to high statistical heterogeneity; both studies reported a significant reduction in dry eye symptoms with omega‐3s, relative to the omega‐6 intervention. In the other study, symptom score data were reported only as P values, and there was no significant intergroup difference.
For ocular surface staining, meta‐analysis was not possible due to a lack of provided quantitative data or use the of different grading scales. Of the four studies that reported on this outcome, two studies reported a significant reduction in ocular surface staining in the omega‐3 group relative to the omega‐6 group (Bhargava 2013; Epitropoulos 2016) and two studies described no significant intergroup differences (Oleñik 2013; NCT01107964).
All five trials considered aqueous tear production, quantified using the Schirmer test, although a meta‐analysis was not presented due to considerable statistical heterogeneity (I² = 97%). Again, results were relatively divided across studies, with two studies reporting a significant increase in Schirmer test score with the omega‐3 intervention relative to the omega‐6 intervention (Bhargava 2013; Bhargava 2015b) and three studies (Epitropoulos 2016; NCT01107964; Oleñik 2013) reporting the absence of an intergroup difference.
For TBUT, there was again substantial statistical heterogeneity (I² = 94%) in the three trials that reported quantitative data. Overall, of the five trials in this category, four reported a significant improvement in tear stability in the omega‐3 group relative to the omega‐6 group and one study (NCT01107964) indicated that there was no significant intergroup difference.
Meta‐analysis was not possible for any of the other secondary efficacy outcomes.
Among data from two trials for the safety outcome related to risk of a gastrointestinal adverse event, events were too few to show clear differences between the omega‐3 and omega‐6 interventions (NCT01107964; Oleñik 2013).
Combined oral fish oil (long‐chain omega‐3 PUFAs) and flaxseed oil (short‐chain omega‐3 PUFAs) versus oral omega‐3 and omega‐6 PUFAs (three RCTs)
Meta‐analysis was not possible for any outcomes due to lack of available quantitative data.
For the primary outcome, two trials reported that a higher proportion of participants given long‐ and short‐chain omega‐3 PUFA supplementation improved in terms of dry eye symptoms relative to the comparator group (Gilbard 2008; Wojtowicz 2011). The other trial, which was available only in abstract format, reported no significant intergroup differences (Papas 2007).
Only Wojtowicz 2011 reported data relevant to the secondary outcome of tear production and described no significant intergroup difference.
None of the studies reported adverse event data.
Combined omega‐3 and omega‐6 PUFAs versus omega‐6 PUFAs (one RCT)
One trial (Sheppard 2013) considered this comparison, and reported a significant reduction in both dry eye symptoms and conjunctival inflammatory markers in the combined omega‐3 and omega‐6 PUFA group, relative to the omega‐6 PUFA group. This study also described no significant intergroup difference in ocular surface staining, aqueous tear production or tear film stability.
Combined oral fish oil (long‐chain omega‐3 PUFAs) and flaxseed oil (short‐chain omega‐3 PUFAs) versus short‐chain omega‐3 PUFAs (one RCT)
One trial (Reeder 2006) investigated this comparison, and reported no significant intergroup differences for dry eye symptoms. No relevant data were reported for any of the secondary outcomes or adverse events.
Overall completeness and applicability of evidence
The trials included in this systematic review considered the effects of a diverse range of oral PUFA supplement formulations (varying considerably in dose and/or composition) on clinical symptoms and/or signs in heterogeneous populations of individuals with "dry eye." These factors likely contributed to the high levels of statistical heterogeneity observed for several of the outcome measures. The choice of comparators also varied substantially, and included placebo, no treatment, omega‐6 PUFA supplements, short‐chain omega‐3 PUFAs, and combined omega‐3 and omega‐6 PUFA supplementation. A few factors proved challenging in terms of limiting our ability to synthesize the available evidence and our ability to draw general conclusions surrounding the efficacy and safety of PUFA supplementation for treating dry eye with high certainty, as follows.
Formulation of PUFA and comparator supplements and duration of the intervention
This review included RCTs in which omega‐3 and/or omega‐6 PUFA supplements were compared with other forms of dry eye treatment, placebo, or no treatment. Most of the included trials investigated long‐chain omega‐3 PUFA (ie. EPA and DHA) supplementation. Few trials considered the effects of combining oral PUFA supplements with other conventional forms of dry eye therapy (eg, warm compresses).
As summarized in Table 7, the PUFA intervention and comparator interventions varied considerably across the included trials. For the main efficacy and safety comparison of long‐chain omega‐3 PUFAs versus placebo or no treatment, among the 10 included trials the daily dose of combined EPA and DHA ranged from 135 mg/d to 3000 mg/d (ie, a 20‐fold dosing range). The "form" of the long‐chain omega‐3 PUFA (ie, triacylglyceride, phospholipid, or ethyl ester) was not reported in most of the included trials but is an additional factor that may affect efficacy by influencing tissue bioavailability. There is preclinical evidence for a difference in ocular tissue accretion with long‐chain omega‐3 PUFAs, depending upon the form of supplementation (Graf 2010). The relative composition and the ratio of EPA and DHA in these formulations also varied considerably, which may influence the nature of any biological effects, as a consequence of differences in the relative abundance of by‐products resulting from EPA versus DHA metabolism. Similar differences in supplement formulation characteristics were evident among trials that considered other forms of omega‐3 and/or omega‐6 PUFA interventions.
A range of different supplements were used for the oral comparators (including olive oil, corn oil, safflower oil, and medium‐chain triglycerides), which also differed in their daily dosing. The choice of comparator (as well as its relative neutrality at the adopted daily dose) is important, as it may affect the capacity to detect a relative difference with the active intervention. For instance, use of an omega‐6 supplement (such as corn oil) as a comparator, which is largely (although not entirely) pro‐inflammatory, may enhance the capacity to detect an intergroup difference with an omega‐3 PUFA intervention. On the contrary, the ability to identify a potential therapeutic effect could be impaired by the use of a non‐inert (potentially anti‐inflammatory) comparator. Indeed, this topic has recently received considerable attention, with debate in relation to the use of a 5000 mg/d olive oil placebo supplement in one of the trials included in this review (Asbell 2018), and varying viewpoints on its relative appropriateness as a non‐active comparator (Bistrian 2018; Maguire 2018). Supporting this concept, in relation to the results reported in this review for dry eye symptoms, the summary estimate for the effect on symptoms with omega‐3 PUFAs was not significantly different relative to a placebo intervention (four studies, 677 participants, MD ‐2.47, 95% CI ‐5.14 to 0.19 OSDI units). However, when omega‐3 PUFA supplementation was compared to omega‐6 supplementation, the summary estimate indicated a significant improvement with the omega‐3 PUFA intervention (two studies, 130 participants, MD ‐11.99 95% CI ‐18.85 to ‐4.92 OSDI units). These findings can also be interpreted to suggest that the relative ratio of omega‐3 to omega‐6 consumption in an individual's diet, at baseline, may be an important determinant of the relative treatment effect.
The vast majority of included trials did not survey (through food questionnaires) or quantify (e.g., through blood testing) baseline systemic fatty acid levels. Understanding these levels is important, as individuals already achieving sufficient levels of certain PUFAs from food sources may not demonstrate the same response to a given dose of fatty acid supplementation as those with a diet deficient in (some or all) PUFAs. The recent availability and validation of a simple food frequency questionnaire for quantifying baseline omega‐3 PUFAs in eye care practice, may be of value for this purpose (Zhang 2019a). Furthermore, the in vivo conversion of short‐chain omega‐3 fatty acids to the biologically‐active long‐chain forms (i.e., EPA and DHA), is dependent upon the concentration of omega‐6 and long‐chain omega‐3 PUFAs in the diet (Brenna 2002), and thus has implications for the expected biological effect(s) of this form of supplementation.
Most trials considered the effects of short‐term PUFA supplementation with intervention periods of one to six months. A three‐month intervention period is considered necessary to maximize tissue accumulation of omega‐3 fatty acids (Surette 2008). However, it is unclear whether longer intervention periods result in different treatment effects compared with shorter periods of supplementation.
We were unable to draw conclusions with respect to the potential effects of any of these factors on outcomes due to an insufficient number of trials reporting consistent outcome parameters to permit either subgroup analyses or meta‐regression. The relative (head‐to‐head) efficacy of different PUFA‐based formulations is also unclear. Three trials compared combined long‐ and short‐chain omega‐3 supplements to combined omega‐3 and omega‐6 supplements, however meta‐analysis was not possible for any outcomes due to lack of available quantitative data. One study compared combined omega‐3 and omega‐6 PUFA supplementation with omega‐6 supplements, and another evaluated combined long‐ and short‐chain omega‐3 supplements with omega‐6 supplementation; however, both were only reported in abstract form, with lack of numerical data. Thus there continues to be some uncertainty surrounding the optimal daily dose (of omega‐3 and/or omega‐6 PUFA supplementation) and composition, or intervention duration for treating dry eye disease.
Dry eye etiology and severity
To ensure the capture of all potentially relevant data, we included RCTs in which participants were given a diagnosis of "dry eye," as defined by trial investigators. Among the included studies, we observed considerable variation in terms of the participant eligibility criteria used to define dry eye status, including trials that used a combination of dry eye symptoms and clinician‐reported signs, objective clinical signs only, or subjective patient symptoms only, or did not explicitly define participant inclusion and exclusion criteria.
Lack of standardization of disease definition and classification is an established challenge in epidemiological dry eye research that has been suggested to (at least in part) account for vast differences in estimations for the prevalence of dry eye, over a 10‐fold range (ie, 5% to 50%) (Stapleton 2017). The absence of a single validated diagnostic test or combination of tests to define a "dry eye diagnosis" not only complicates the interpretation of past epidemiological study results, but also has implications for the comparability of "dry eye" study populations in clinical trials. In addition to adopting non‐uniform diagnostic criteria, consistent with the multi‐factorial nature of dry eye disease (Craig 2017), the studies in this review included participants with symptoms and/or signs of dry eye resulting from a diverse range of etiologies. Whilst most trials recruited individuals with non‐specific dry eye symptoms and/or signs, some targeted specific dry eye subpopulations (eg, dry eye associated with computer vision syndrome, contact lens wear, meibomian gland dsyfunction, post laser‐assisted in situ keratomileusis (LASIK), post cataract surgery, rosacea, rheumatoid arthritis or systemic lupus erythematosus, Sjögren’s syndrome). The primary etiological factor is likely to differ significantly amongst these conditions. For example, dry eye due to meibomian gland dysfunction would be expected to result in a primarily evaporative form, whereas dry eye associated with systemic autoimmune disease (eg, systemic lupus erythematosus, Sjögren’s syndrome) would typically manifest as aqueous deficiency, and once dry eye becomes clinically significant (ie, both signs and symptoms are present), most patients will have a mixed form of dry eye (Lemp 2012).
It follows that the potential effects of PUFA supplementation in modifying dry eye symptoms and/or signs may not have applicability across all dry eye subtypes, etiologies, or severities. Supporting this hypothesis, for our main comparison ‐ the effect of long‐chain omega‐3 PUFA supplements (relative to placebo) ‐ substantial heterogeneity (I² > 60%) was observed across multiple outcomes. A subgroup analysis, to consider only participants with non‐specific dry eye (ie, dry eye not secondary to another disease or condition) versus participants with dry eye secondary to another disease or condition was undertaken to explore the potential source of heterogeneity for TBUT. However, this did not adequately explain the heterogeneity (I² > 60% for both subgroup analyses).
Few studies reported the severity of dry eye in the study participant population or used severity as an eligibility criterion. The potential efficacy of PUFA supplementation and the capacity to detect change with an intervention may depend on the baseline severity of the condition. For example, recruitment of individuals with only mild dry eye disease (and potentially low‐grade symptoms and signs) may limit the dynamic range for detecting a between‐intervention difference.
Trial design, outcome measures, and outcome reporting
Most of the included trials used a parallel‐group design, although some adopted a cross‐over design or randomized individuals to interventions and then used at least one eye of each participant in the analyses.
Due to differences in measurement methods and data presentation (eg, providing only P values, presenting only graphical data) and unit of analysis errors (eg, including both eyes of participants without appropriate statistical adjustment), or a combination of these factors, capacity to pool data for prespecified outcome measures was often limited. These factors, in addition to observed substantial heterogeneity, limited our capacity to determine the efficacy of PUFA supplementation certainty.
In relation to adverse events, as summarized in Table 9, only about half of the included trials reported adverse events, which limited our ability to confidently ascertain the safety of PUFA supplementation for individuals with dry eye disease.
Quality of the evidence
Overall, we graded the evidence related to all prespecified outcomes as being of moderate or low certainty.
For the main comparison ‐ long‐chain omega‐3 PUFAs (iEPA and DHA) versus placebo or no treatment ‐ the two most common reasons for downgrading the certainty of the evidence were inconsistency (due to high levels of heterogeneity between studies) and risk of bias (primarily due to industry funding and/or author affiliations with relevant pharmaceutical companies). Specifically, we downgraded the certainty of the evidence to moderate for effects on aqueous tear production, due to risk of bias.
We judged the certainty of the evidence as low for all other outcome measures. For both the primary outcome (related to dry eye symptoms) and the secondary outcome relating to ocular surface staining, we downgraded the certainty of the evidence to low, in view of both risk of bias in these studies and high heterogeneity between studies (inconsistency). Substantial heterogeneity also reduced our confidence in the effect estimates for outcomes related to TBUT (which we downgraded to low). The certainty of the evidence for the effect on tear osmolarity was downgraded by two levels to low, for risk of bias and inconsistency.
We downgraded the certainty of the evidence to low for gastrointestinal adverse events, as results were inconsistent among studies and few events occurred (leading to wide confidence intervals and imprecision).
For the other main comparison ‐ combined omega‐3 and omega‐6 PUFAs versus placebo ‐ we downgraded the certainty of the evidence for all outcomes due to risks of bias, with all four studies in this category funded by industry or including at least one study author affiliated with a relevant pharmaceutical company.
Potential biases in the review process
We adopted the standard methodological procedures recommended by Cochrane, to minimize potential biases in the undertaking of this review.
One of the review authors (LED) is the senior author on two studies included in this review (Deinema 2017; Chinnery 2017). However, as all risk of bias assessments were independently performed by two review authors, who also had to reach consensus on these assessments, we consider the risk of bias induced by this association to be minimal.
We included one additional full‐text article (Asbell 2018) that was published in April 2018, after the electronic search strategies were run in February 2018. This trial was identified as an 'ongoing' study, as a clinical trial registry entry, in the initial search. We considered it important to include data from this trial in the analyses for this systematic review as it is the largest relevant single RCT undertaken to date. The study, which was published in a leading medical journal, reported largely negative findings and thus was not considered to represent a substantial risk of publication bias.
We conducted a top‐up search in October 2019; five studies classified as awaiting classification have not yet been incorporated into the review, which may represent a potential source of bias.
Agreements and disagreements with other studies or reviews
Over the past five years, several systematic reviews have considered the efficacy and/or safety of nutritional supplements containing omega‐3 and/or omega‐6 fatty acids for treating dry eye disease (Liu 2014; Molina‐Levya 2017; Zhu 2014). The TFOS DEWS II Management and Therapy sub‐committee (Jones 2017) also recently undertook a comprehensive narrative synthesis of relevant preclinical, epidemiological, and clinical trial evidence on this topic.
In contrast to our review, which subcategorized the PUFA interventions based on the supplement composition, the Zhu 2014 analysis pooled data from RCTs that evaluated any form of PUFA supplement. These authors identified a total of nine relevant studies and reported that PUFA supplementation appeared to provide no benefit with regard to tear volume (measured via the Schirmer test) nor tear stability (quantified via fluorescein TBUT) but imparted a significant improvement (relative to placebo) for each of dry eye symptoms (measured via the OSDI questionnaire), rate of conjunctival HLA‐DR‐positive cells, and HLA‐DR fluorescent intensity. Given that these authors pooled data from studies that evaluated omega‐3 PUFAs alone, omega‐6 PUFAs only, and combined omega‐3 and omega‐6 PUFAs, it is difficult to make direct comparisons with the present review (which subcategorized the comparisons based on the intervention and comparator formulation composition). As we noted in the present review, Zhu 2014 reported significant statistical heterogeneity for several outcomes and highlighted that these results should thus be considered with caution.
Molina‐Levya 2017, which involved a systematic review of RCTs published from 2005 to 2015 inclusive, did not attempt to meta‐analyze data. Instead, these authors summarized the number of studies, out of a total of 15 included trials, which reported significant and non‐significant effects with any form of omega‐3 or omega‐6 supplement. Considered outcomes included TBUT, Schirmer test, and dry eye symptoms. Given that this was a narrative review that did not attempt to differentiate between the different types of PUFA supplements, a direct comparison of its conclusions, relative to our findings, was not feasible.
Liu 2014 considered the specific effects of oral omega‐3 fatty acid supplements relative to placebo for the management of dry eye syndrome. This systematic review and meta‐analysis included seven RCTs and considered outcomes related to the change (from baseline) in dry eye symptoms (OSDI score), TBUT, and Schirmer test results. Pooling data from three trials showed considerable statistical heterogeneity (I² = 69%). Liu 2014 reported no significant difference in the change from baseline in OSDI score. This outcome is similar to the overall pooled estimate for the main comparison in our review (Analysis 1.1), where moderate levels of heterogeneity (I² = 48%) were observed. For TBUT, Liu 2014 pooled data from five RCTs, despite the presence of considerable statistical heterogeneity (I² = 91%), and reported a significant improvement with omega‐3 supplementation (weighted mean difference [WMD] 1.58, 95% confidence interval [CI] 0.60 to 2.25 seconds). The finding of substantial heterogeneity parallels the observation for the main comparison in our review (Analysis 1.4). Upon pooling of data from three trials for Schirmer test findings, the review authors of Liu 2014 reported a significant improvement with the omega‐3 PUFA intervention relative to placebo (WMD 0.75, 95% CI 0.29 to 1.19 mm/5 min). This finding parallels the reported estimate in our review, which identified a statistically significant, but not clinically significant, change in the Schirmer test score favoring the omega‐3 PUFA intervention (Analysis 1.3).
Thus, of the systematic reviews undertaken to date on this topic, our review is the most comprehensive (with respect to the breadth and systematic approach to the literature review and appraisal), has considered the most outcome measures (including subjective and objective clinical endpoints and safety outcomes), and is the only review to have evaluated the effects of PUFA supplements in dry eye disease based upon formulation subcategories. The conclusions of the present review, which highlight that many of the reported effect estimates are of low certainty due to heterogeneity and/or potential biases in the included studies, are consistent with those of Jones 2017, who concluded that the role of omega‐3 and/or omega‐6 PUFA supplements in treating dry eye disease remains incompletely understood.
Authors' conclusions
Implications for practice.
Based on our consideration of the best, current available clinical research evidence, this systematic review shows that long‐chain omega‐3 polyunsaturated fatty acid (PUFA) supplementation (with EPA and DHA) confers a benefit relative to placebo for both tear production, quantified via the Schirmer test (moderate certainty), and tear osmolarity (low certainty) among individuals with dry eye disease. There was no apparent effect on dry eye symptoms (low certainty), although results were variable across studies. Whether these findings are generalizable to all dry eye populations (eg, post cataract surgery, post laser‐assisted in situ keratomileusis (LASIK), contact lens‐associated dry eye) remains uncertain due to the limited number of studies that investigated these populations.
A beneficial effect on dry eye symptoms (low certainty) was evident when omega‐3 PUFA supplements were combined with conventional therapy and this combination was compared with conventional therapy alone (MD ‐7.16, 95% CI ‐13.97 to ‐0.34 OSDI units). A similar effect, of moderate certainty, was observed for the comparison of long‐chain omega‐3 PUFA supplements relative to omega‐6 PUFA supplementation (MD ‐11.88, 95% CI ‐18.85 to ‐4.92 OSDI units). For both of these comparisons, the effect size for the inter‐intervention improvement in Ocular Surface Disease Index (OSDI) score is clinically significant, given the established minimally clinically important difference (MCID) of 4.5 to 7.3 OSDI units for mild to moderate dry eye disease (Miller 2010). This latter finding contrasts with the outcome reported for the same intervention (long‐chain omega‐3 PUFA supplements) when compared to a placebo (rather than omega‐6 supplementation), for which there was no inter‐intervention difference for dry eye symptoms. Further research is necessary to clarify the association between baseline PUFA intake and the therapeutic response to omega‐3 PUFA supplementation.
Although the MCID for tear osmolarity is not established, the effect of long‐chain omega‐3 PUFA supplementation on this outcome (based on data from one trial that recruited individuals with tear hyperosmolarity at baseline) is also considered of clinical relevance (MD ‐17.71, 95% CI ‐28.07 to ‐7.35 mOsmol/L units), given that the mean baseline osmolarity in this study was only mildly elevated, and this degree of osmotic change is consistent with a high proportion of participants achieving relative normalization of their tear osmolarity. Similar tear osmolarity findings were reported in another study that compared oral long‐chain omega‐3 supplementation with omega‐6 supplementation (MD ‐11.10, 95% ‐12.15 to ‐10.05 mOsmol/L units).
In contrast, the relative improvement in tear production (Schirmer score: MD 0.68, 95% CI 0.26 to 1.09 mm/5 min) with long‐chain omega‐3 PUFAs relative to placebo is of uncertain clinical significance. We were unable to ascertain potential effects on tear break‐up time (TBUT) for this comparison with any certainty due to considerable statistical heterogeneity. Effects on ocular surface staining with long‐chain omega‐3 PUFAs were uncertain due to a lack of available combinable data.
For combined omega‐3 and omega‐6 PUFA supplementation, we found low certainty evidence for no benefit for tear production relative to placebo, and low certainty evidence for a modest improvement, in TBUT (MD 0.55, 95% CI 0.04 to 1.07 seconds). The effects of this category of PUFA intervention, relative to placebo, on other outcomes, including dry eye symptoms, ocular surface staining, and adverse events, could not be ascertained with certainty due to insufficient data and/or considerable statistical heterogeneity.
Due to an insufficient number of studies reporting quantitative data and/or inter‐trial variability in the methods used to quantify clinical outcomes, we are unable to ascertain (with any certainty) the effects of each of the following: combined fish oil (long‐chain omega‐3 PUFAs) and flaxseed oil (short‐chain omega‐3 PUFAs) versus any of combined omega‐3 and omega‐6 PUFAs, omega‐6 PUFAs alone or short‐chain omega‐3 PUFAs alone; flaxseed oil (short‐chain omega‐3 PUFAs) versus placebo; and omega‐6 PUFAs versus placebo, for treating dry eye disease.
There was low certainty evidence regarding the relative incidence of gastrointestinal adverse effects with PUFA supplementation due to the small number of included studies and low frequency of events. For the main comparison of long‐chain omega‐3 PUFA supplementation relative to placebo, a meta‐analysis was not performed due to substantial heterogeneity between the three studies that reported relevant data. There was also low level certainty regarding any effect on gastrointestinal disorders with long‐chain omega‐3 PUFAs relative to omega‐6 supplementation (two studies, 91 participants; RR 2.34, 95% CI 0.35 to 15.54).
Overall, the findings in this review suggest a potential role for long‐chain omega‐3 supplementation in managing dry eye disease. However, the evidence is currently inconsistent. We acknowledge the possibility that including the results of the five studies in 'Studies awaiting classification' may affect the conclusions of the review.
Implications for research.
Dry eye disease is of global public health importance, given its high prevalence and associated substantial economic burden. Yet, the dry eye field lags behind other fields in ophthalmology, such as cataract and glaucoma, in terms of core outcome set development. For clinical research study results to be considered in decision‐making, outcomes must be defined consistently across studies (Saldanha 2018). In addition, results should address the needs of stakeholders, including patients. However, as our review has indicated, these conditions often are not met in the field of dry eye disease.
The present review, which considered the best currently available clinical research evidence related to potential benefits of oral omega‐3 and/or omega‐6 PUFA supplementation for treating dry eye disease, identifies several areas for potential improvement in relation to research in this field, as follows.
Using best practice dry eye diagnostic criteria to define clinical trial study populations
Until recently, standardized clinical criteria for diagnosing dry eye disease have been lacking, leading to substantial variation in participant eligibility criteria for clinical trials, and thus in the clinical populations evaluated in intervention studies. With the relatively recent publication of the Tear Film and Ocular Surface (TFOS) International Dry Eye WorkShop II (DEWS II) Diagnostic Methodology subcommittee report (Wolffsohn 2017), future research studies now have an opportunity to recruit participants based on current best practice dry eye diagnostic criteria (to reduce potential clinical heterogeneity induced by recruitment of individuals with masquerading conditions that do not strictly meet the criteria for "dry eye disease"). Specifically, Wolffsohn 2017 recommends that the diagnosis of "dry eye disease" should be based on a combination of patient‐reported symptoms (quantified using the validated OSDI or Dry Eye Questionnaire (DEQ)‐5) and the presence of at least one dry eye clinical sign (related to measures of tear film stability, tear osmolarity, and ocular surface staining). Differentiation of aqueous deficient versus evaporative dry eye should also be undertaken.
Developing a set of core outcome measures for dry eye clinical trials
As noted, our ability to compare and meta‐analyze data for many clinical parameters (eg, dry eye symptoms, ocular surface staining) was limited by the use of different outcome measures and/or clinical grading scales in the included randomized controlled trials (RCTs) (eg, Table 8). As was recently identified (Saldanha 2018; Wang 2018), an agreed "core outcome set" for dry eye trials, as recommended by the Core Outcome Measures in Effectiveness Trials (COMET) initiative (Prinsen 2014), would work toward improving the consistency of outcome reporting, and subsequently the capacity to synthesize evidence (and draw more definitive conclusions) related to the effects of interventions. Ideally this core outcome set should specify preferred measurement tools and units for outcome measures, to reduce variability in outcome measures across different trials. Specifying the need to report quantitative outcome data in a readily extractable form (ie, in a numeric rather than a graphical format) would also enable more comprehensive pooling of data.
Consideration with respect to outcome measures specific to the two main subtypes of dry eye disease (ie, aqueous deficient and evaporative dry eye) would also assist in ascertaining the potential therapeutic effects of interventions on forms of dry eye related to specific etiologies. The core outcome set should also prioritize patient‐reported outcomes (Saldanha 2018), include measures of participant compliance, and emphasize the need to assess (and report) findings related to adverse events (which frequently were not reported in the studies included in this review).
Enhancing methodological rigor and reducing biases
As per the recommendations of the TFOS DEWS II Clinical Trial Design subcommittee (Novack 2017), trials designed to test the therapeutic efficacy of dry eye interventions should include an adequate sample size to detect a potential difference (relative to the comparator). Among the trials considered in this systematic review, only 15 (44.4%) reported a power calculation and the associated sample size. Sample size calculations and their reporting in the trial publication are thus urged for future studies.
It is also recommended that trialists report an appropriate level of detail regarding the PUFA intervention, including the form of the supplement (eg, triacylglyceride, phospholipid, ethyl ester), to enable replication of the intervention. Consideration and completion of the Template for Intervention Description and Replication (TIDieR) checklist (Hoffmann 2014), when trials are submitted for publication, would assist in ensuring a suitable level of detail within the final manuscript.
Prospective trial registration, including pre‐specification of primary and secondary outcome measures, represents another opportunity for improving the methodological rigour of future research in the field. Only nine (25.6%) of the included RCTs had published protocols and/or were included on a registry. For most trials, the potential for selective outcome reporting could not be ascertained as a result of our inability to access any a priori information about the study design. We also were unable to assess for potential publication bias due to an insufficient number of studies providing quantitative data relevant to the reported outcome measures. It has been shown that prospective clinical trial registration can improve the reporting of RCTs, in particular as this relates to sample size calculations, sequence generation, allocation concealment, and masking (Reveiz 2010).
Future clinical trials should ensure the use of appropriate statistical methods for analyzing data from both eyes. Only one trial adopted appropriate methods for considering within‐person correlations when including data from both eyes (Asbell 2018).
In the present review, 17 (50%) of the included trials were funded by industry, had materials supplied by industry, and/or had authors that disclosed financial commercial interests that were potentially relevant to the intervention(s). In medical and surgical intervention trials, industry funding has been shown to be associated with significant odds of pro‐industry findings (Bhandari 2004; Dalgado 2017), particularly when surrogate endpoints were used (Dalgado 2017). Non‐profit and mixed source funding RCTs are considered less inclined to demonstrate such effects and thus may be considered preferable sources of support for future research in the field.
The results of this review indicate that further research is required to comprehensively explore the role of PUFA supplementation in treating dry eye disease, particularly with respect to the role of this intervention in managing dry eye of different etiologies and severities, and the influence of specific formulation characteristics (eg, dose, form, composition) and baseline dietary PUFA intake on clinical outcomes.
Acknowledgements
We acknowledge Lori Rosman, Information Specialist, Cochrane Eyes and Vision (CEV), for developing the search strategies and conducting the electronic searches. We also acknowledge the CEV editorial team for their support during the development of this protocol, and Barbara Hawkins and Karla Zadnik for their comments on drafts of the protocol. We are grateful to the following peer reviewers for their time and comments on the review: Andrew Pucker (University of Alabama at Birmingham), Sezen Karakus (Wilmer Eye Institute), and Rebecca Petris (Dry Eye Company). We are also appreciative of the editorial feedback and advice provided by Jennifer Evans (Joint Co‐ordinating Editor for CEV).
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Dry Eye Syndromes] explode all trees #2 (dry near/2 eye*) #3 (ocular near/2 dry*) #4 MeSH descriptor: [Tears] explode all trees #5 tear* #6 MeSH descriptor: [Xerophthalmia] explode all trees #7 xerophthalmi* #8 MeSH descriptor: [Vitamin A Deficiency] explode all trees #9 (vitamin A near/3 deficien*) #10 (avitaminosis a or retinol deficien* or hypovitaminosis A) #11 MeSH descriptor: [Keratoconjunctivitis Sicca] explode all trees #12 (Keratoconjunctiv* or kerato conjunctivitis) #13 MeSH descriptor: [Sjogren's Syndrome] explode all trees #14 ((Sjogren* or Sjoegren*) near/1 (syndrom* or disease*)) #15 sicca syndrom* #16 MeSH descriptor: [Stevens‐Johnson Syndrome] explode all trees #17 (Steven* and Johnson and (syndrom* or disease*)) #18 MeSH descriptor: [Pemphigoid, Benign Mucous Membrane] explode all trees #19 Benign Muco* Pemphigoid* #20 cicatricial pemphigoid* #21 (Cicatricial near/2 Pemphigoid*) #22 blepharoconjunctiviti* #23 MeSH descriptor: [Meibomian Glands] explode all trees #24 (meibomian or tarsal) #25 MeSH descriptor: [Lacrimal Apparatus Diseases] explode all trees #26 (lacrima* or epiphora) #27 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 #28 MeSH descriptor: [Fatty Acids] this term only #29 (Fatty near/3 acid*) #30 MeSH descriptor: [Fatty Acids, Unsaturated] this term only #31 MeSH descriptor: [Fatty Acids, Essential] this term only #32 (PUFA* or LCPUFA*or polyunsaturated or poly‐unsaturated) #33 MeSH descriptor: [Fatty Acids, Omega‐3] explode all trees #34 (omega‐3 or omega 3 or ω‐3 or ω3 or "ω 3") #35 (alpha‐linolenic* or linol*) #36 (Docosahex* or docosapent*) #37 (eicosapen* or Timnodonic or icosapentaen*) #38 MeSH descriptor: [Fatty Acids, Omega‐6] explode all trees #39 (omega‐6 or omega 6 or ω‐6 or ω6 or "ω 6") #40 (gamma‐Linol* or linoic) #41 MeSH descriptor: [Arachidonic Acids] explode all trees #42 (Arachidonic Acid* or sodium arachidonate) #43 (EPA or E‐EPA or EFA or MaxEPA or DHA or DPA or ALA) #44 (Lipoplus or Lovaza or Omacor or Omega Rx or Omegaven or SMOFlipid) #45 MeSH descriptor: [Corn Oil] explode all trees #46 MeSH descriptor: [Linseed Oil] explode all trees #47 MeSH descriptor: [Safflower Oil] explode all trees #48 MeSH descriptor: [Soybean Oil] explode all trees #49 MeSH descriptor: [Flax] explode all trees #50 MeSH descriptor: [Fish Oils] explode all trees #51 (oil* near/3 (fish* or cod liver or halibut or squid or krill or mackerel or menhaden or salmon or seal or shark liver)) #52 (oil* near/3 (corn or maize or linseed or flax* or safflower or soy* or canola or hemp* or primrose)) #53 (oil* near/3 (algal or egg or seabuckthorn seed or berry or Sacha Inchi or Echium)) #54 #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 #55 #27 and #54
Appendix 2. MEDLINE Ovid search strategy
1. Randomized Controlled Trial.pt. 2. Controlled Clinical Trial.pt. 3. (randomized or randomised).ab,ti. 4. placebo.ab,ti. 5. drug therapy.fs. 6. randomly.ab,ti. 7. trial.ab,ti. 8. groups.ab,ti. 9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10. exp animals/ not humans.sh. 11. 9 not 10 12. exp dry eye syndromes/ 13. (dry adj2 eye*).tw. 14. (ocular adj2 dry*).tw. 15. exp tears/ 16. tear*.tw. 17. exp xerophthalmia/ 18. xerophthalmi*.tw. 19. exp vitamin A deficiency/ 20. (vitamin A adj3 deficien*).tw. 21. (avitaminosis a or retinol deficien* or hypovitaminosis A).tw. 22. exp keratoconjunctivitis sicca/ 23. (Keratoconjunctiv* or kerato conjunctivitis).tw. 24. exp Keratoconjunctivitis/ 25. limit 24 to yr="1966 ‐ 1985" 26. exp Sjogren's syndrome/ 27. ((Sjogren* or Sjoegren*) adj (syndrom* or disease*)).tw. 28. sicca syndrom*.tw. 29. exp Stevens Johnson syndrome/ 30. (Steven* and Johnson and (syndrom* or disease*)).tw. 31. exp Pemphigoid, Benign Mucous Membrane/ 32. Benign Muco* Pemphigoid*.tw. 33. (Cicatricial adj2 Pemphigoid*).tw. 34. blepharoconjunctiviti$.tw. 35. exp meibomian glands/ 36. (meibomian or tarsal).tw. 37. exp lacrimal apparatus diseases/ 38. (lacrima* or epiphora).tw. 39. or/12‐23,25‐38 40. Fatty Acids/ 41. (Fatty adj3 acid*).tw. 42. Fatty Acids, Unsaturated/ 43. exp Fatty Acids, Essential/ 44. (PUFA* or LCPUFA* or polyunsaturated or poly‐unsaturated).tw. 45. exp Fatty Acids, Omega‐3/ 46. (omega‐3 or omega 3).tw. 47. (alpha‐linol* or linol*).tw. 48. (Docosahex* or docosapent*).tw. 49. (eicosapen* or timnodonic or icosapentaen*).tw. 50. exp Fatty Acids, Omega‐6/ 51. (omega‐6 or omega 6).tw. 52. (gamma‐Linol* or linoic).tw. 53. exp Arachidonic Acids/ 54. (Arachidonic Acid* or sodium arachidonate).tw. 55. (EPA or E‐EPA or EFA or MaxEPA or DHA or DPA or ALA).tw. 56. (Lipoplus or Lovaza or Omacor or Omega Rx or Omegaven or SMOFlipid).tw. 57. exp corn oil/ 58. exp linseed oil/ 59. exp Safflower Oil/ 60. exp Soybean Oil/ 61. exp Flax/ 62. exp Fish Oils/ 63. (oil* adj3 (fish* or cod liver or halibut or squid or krill or mackerel or menhaden or salmon or seal or shark liver)).tw. 64. (oil* adj3 (corn or maize or linseed or flax* or safflower or soy* or canola or hemp* or primrose)).tw. 65. (oil* adj3 (algal or egg or seabuckthorn seed or berry or Sacha Inchi or Echium)).tw. 66. or/40‐64 67. 11 and 39 and 66
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase.com search strategy
#1 'randomized controlled trial'/exp #2 'randomization'/exp #3 'double blind procedure'/exp #4 'single blind procedure'/exp #5 random*:ab,ti #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'animal'/exp OR 'animal experiment'/exp #8 'human'/exp #9 #7 AND #8 #10 #7 NOT #9 #11 #6 NOT #10 #12 'clinical trial'/exp #13 (clin* NEAR/3 trial*):ab,ti #14 ((singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)):ab,ti #15 'placebo'/exp #16 placebo*:ab,ti #17 random*:ab,ti #18 'experimental design'/exp #19 'crossover procedure'/exp #20 'control group'/exp #21 'latin square design'/exp #22 #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 #23 #22 NOT #10 #24 #23 NOT #11 #25 'comparative study'/exp #26 'evaluation'/exp #27 'prospective study'/exp #28 control*:ab,ti OR prospectiv*:ab,ti OR volunteer*:ab,ti #29 #25 OR #26 OR #27 OR #28 #30 #29 NOT #10 #31 #30 NOT (#11 OR #23) #32 #11 OR #24 OR #31 #33 'dry eye'/exp #34 (dry NEAR/2 eye*):ab,ti #35 (ocular NEAR/2 dry*):ab,ti #36 'lacrimal fluid'/exp #37 tear*:ab,ti #38 'xerophthalmia'/exp #39 xerophthalmi*:ab,ti #40 'retinol deficiency'/exp #41 ('vitamin a' NEAR/3 deficien*):ab,ti #42 'avitaminosis a':ab,ti OR (retinol NEAR/1 deficien*):ab,ti OR 'hypovitaminosis a':ab,ti #43 'keratoconjunctivitis sicca'/exp #44 keratoconjunctiv*:ab,ti OR 'kerato conjunctivitis':ab,ti #45 'sjoegren syndrome'/exp #46 ((sjogren* OR sjoegren*) NEAR/2 (syndrom* OR disease*)):ab,ti #47 (sicca NEXT/1 syndrom*):ab,ti #48 'stevens johnson syndrome'/exp #49 steven*:ab,ti AND johnson:ab,ti AND (syndrom*:ab,ti OR disease*:ab,ti) #50 'mucous membrane pemphigoid'/exp #51 benign AND muco* AND pemphigoid*:ab,ti #52 (cicatricial NEAR/2 pemphigoid*):ab,ti #53 blepharoconjunctiviti*:ab,ti #54 'meibomian gland'/exp #55 meibomian:ab,ti OR tarsal:ab,ti #56 'lacrimal gland disease'/exp #57 lacrima*:ab,ti OR epiphora:ab,ti #58 #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 #59 'fatty acid'/exp #60 (fatty NEAR/3 acid*):ab,ti #61 'unsaturated fatty acid'/de #62 'polyunsaturated fatty acid'/exp #63 pufa*:ab,ti OR lcpufa*:ab,ti OR polyunsaturated:ab,ti OR 'poly unsaturated':ab,ti #64 'essential fatty acid'/exp #65 'omega 3 fatty acid'/exp #66 'omega‐3':ab,ti OR 'omega 3':ab,ti OR 'ω‐3':ab,ti or ω3:ab,ti or 'ω 3':ab,ti #67 'omega 6 fatty acid'/exp #68 'omega‐6':ab,ti OR 'omega 6':ab,ti OR 'ω‐6':ab,ti or ω6:ab,ti or 'ω 6':ab,ti #69 'docosahexaenoic acid'/exp #70 'icosapentaenoic acid'/exp #71 ('alpha linolenic'):ab,ti OR linol*:ab,ti #72 (Docosahex* OR docosapent*):ti,ab #73 (eicosapen* OR timnodonic OR icosapentaen*):ti,ab #74 ('gamma linolenic'):ab,ti OR (linoic):ab,ti #75 'arachidonic acid derivative'/exp #76 (arachidonic NEAR/1 acid*):ab,ti OR 'sodium arachidonate':ab,ti #77 epa:ab,ti OR 'e epa':ab,ti OR efa:ab,ti OR maxepa:ab,ti OR dha:ab,ti OR dpa:ab,ti OR ala:ab,ti #78 lipoplus:ab,ti OR lovaza:ab,ti OR omacor:ab,ti OR 'omega rx':ab,ti OR omegaven:ab,ti OR smoflipid:ab,ti #79 'canola oil'/exp #80 'cod liver oil'/exp #81 'corn oil'/exp #82 'fish oil'/exp #83 'linseed oil'/exp #84 'menhaden oil'/exp #85 'primrose oil'/exp #86 'safflower oil'/exp #87 'safflower oil plus soybean oil'/exp #88 'soybean oil'/exp #89 'linseed'/exp #90 (oil* NEAR/3 (fish* OR 'cod liver' OR halibut OR squid OR krill OR mackerel OR menhaden OR salmon OR seal OR 'shark liver')):ab,ti #91 (oil* NEAR/3 (corn OR maize OR linseed OR flax* OR safflower OR soybean OR soy* OR canola OR hemp* OR primrose)):ab,ti #92 (oil* NEAR/3 (algal OR egg OR 'seabuckthorn seed' OR berry OR 'sacha inchi' OR echium)):ab,ti #93 #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 OR #91 OR #92 #94 #32 AND #58 AND #93
Appendix 4. PubMed search strategy
#1 ((randomized controlled trial[pt]) OR (controlled clinical trial[pt]) OR (randomised[tiab] OR randomized[tiab]) OR (placebo[tiab]) OR (drug therapy[sh]) OR (randomly[tiab]) OR (trial[tiab]) OR (groups[tiab])) NOT (animals[mh] NOT humans[mh]) #2 dry[tiab] AND (eye[tiab] OR eyes[tiab] OR eyelid*[tiab]) NOT Medline[sb] #3 (ocular[tiab] AND dry*[tiab]) NOT Medline[sb] #4 tear*[tiab] NOT Medline[sb] #5 xerophthalmi*[tiab] NOT Medline[sb] #6 ("vitamin A"[tiab] AND deficien*[tiab]) NOT Medline[sb] #7 ("avitaminosis a"[tiab] OR retinol deficien*[tiab] OR "hypovitaminosis A"[tiab]) NOT Medline[sb] #8 (Keratoconjunctiv*[tiab] OR "kerato conjunctivitis"[tiab]) NOT Medline[sb] #9 ((Sjogren*[tiab] OR Sjoegren*[tiab]) AND (syndrom*[tiab] OR disease[tiab] OR diseases[tiab])) NOT Medline[sb] #10 sicca syndrom*[tiab] NOT Medline[sb] #11 (Steven*[tiab] AND Johnson[tiab] AND (syndrom*[tiab] OR disease[tiab] OR diseases[tiab])) NOT Medline[sb] #12 (Cicatricial [tiab] AND Pemphigoid*[tiab]) NOT Medline[sb] #13 Blepharoconjunctiviti*[tiab] NOT Medline[sb] #14 (meibomian[tiab] OR tarsal[tiab]) NOT Medline[sb] #15 (lacrima*[tiab] OR epiphora[tiab]) NOT Medline[sb] #16 #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 #17 (Fatty[tiab] AND (acid[tiab] OR acids[tiab])) NOT Medline[sb] #18 (PUFA*[tiab] OR LCPUFA*[tiab] OR polyunsaturated[tiab] OR poly‐unsaturated[tiab]) NOT Medline[sb] #19 (omega‐3[tiab] OR "omega 3"[tiab] OR "ω‐3"[tiab] OR ω3[tiab] OR "ω 3"[tiab]) NOT Medline[sb] #20 (alpha‐linol*[tiab] OR linol*[tiab]) NOT Medline[sb] #21 (Docosahex*[tiab] OR docosapent*) NOT Medline[sb] #22 (eicosapen*[tiab] OR Timnodonic[tiab] OR icosapentaen*[tiab]) NOT Medline[sb] #23 (omega‐6[tiab] OR "omega 6"[tiab] OR "ω‐6"[tiab] OR ω6[tiab] OR "ω 6"[tiab]) NOT Medline[sb] #24 (gamma‐Linol*[tiab] OR linoic[tiab]) NOT Medline[sb] #25 (Arachidonic Acid*[tiab] OR sodium arachidonate[tiab]) NOT Medline[sb] #26 (EPA[tiab] OR E‐EPA[tiab] OR EFA[tiab] OR MaxEPA[tiab] OR DHA[tiab] OR DPA[tiab] OR ALA[tiab]) NOT Medline[sb] #27 (Lipoplus[tiab] OR Lovaza[tiab] OR Omacor[tiab] OR "Omega Rx"[tiab] OR Omegaven[tiab] OR SMOFlipid[tiab]) NOT Medline[sb] #28 (oil*[tiab] AND (fish*[tiab] OR "cod liver"[tiab] OR halibut[tiab] OR squid[tiab] OR krill[tiab] OR mackerel[tiab] OR menhaden[tiab] OR salmon[tiab] OR seal[tiab] OR "shark liver")) NOT Medline[sb] #29 (oil*[tiab] AND (corn[tiab] OR maize[tiab] OR linseed[tiab] OR flax*[tiab] OR safflower[tiab] OR soy*[tiab] OR canola[tiab] OR hemp*[tiab] OR primrose[tiab])) NOT Medline[sb] #30 (oil*[tiab] AND (algal[tiab] OR egg[tiab] OR "seabuckthorn seed"[tiab] OR berry[tiab] OR "Sacha Inchi"[tiab] OR Echium[tiab])) NOT Medline[sb] #31 #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 #32 (#1 AND #16 AND #31)
Appendix 5. LILACS search strategy
("Dry Eye" OR "Síndromes de Ojo Seco" OR "Síndromes do Olho Seco" OR MH:C11.496.260$ OR Tear$ OR Lágrimas OR MH:A12.200.882$ OR Xerophthalmia OR Xeroftalmia OR MH:C11.187.810$ OR MH:C11.496.260.892$ OR "Vitamin A Deficiency" OR "Deficiencia de Vitamina A" OR MH:C18.654.521.500.133.628$ OR MH:SP6.016.052.063.109$ OR "avitaminosis a" OR "retinol deficiency" OR "hypovitaminosis A" OR Keratoconjunctivitis OR " kerato conjunctivitis" OR Queratoconjuntivitis OR Ceratoconjuntivite OR MH:C11.187.183.394$ OR MH:C11.204.564.585$ OR MH:C11.496.260.394$ OR "Sjogren's Syndrome" OR "Síndrome de Sjögren" OR MH:C05.550.114.154.774$ OR MH:C05.799.114.774$ OR MH:C07.465.815.929.669$ OR MH:C11.496.260.719$ OR MH:C17.300.775.099.774$ OR MH:C20.111.199.774$ OR "sicca syndrome" OR "Stevens Johnson Syndrome" OR "Síndrome de Stevens Johnson" OR MH:C07.465.864.500$ OR MH:C17.800.229.400.683$ OR MH:C17.800.865.475.683$ OR "Pemphigoid Benign Mucous Membrane" OR "Penfigoide Benigno de la Membrana Mucosa" OR "Penfigoide Mucomembranoso Benigno" OR "Cicatricial Pemphigoid" OR MH:C11.187.482$ OR MH:C17.800.865.670$ OR blepharoconjunctiviti$ OR "Meibomian Glands" OR "Glándulas Tarsales" OR "Glândulas Tarsais" OR MH:A09.371.337.614 OR MH:A10.336.827.600 OR "Lacrimal Apparatus Diseases" OR "Enfermedades del Aparato Lagrimal" OR "Doenças do Aparelho Lacrimal" OR MH:C11.496$ OR lacrima$ or epiphora) AND ("Fatty Acid" OR "fatty acids" OR "Ácidos Grasos" OR "Ácidos Graxos" OR MH:D10.251$ OR MH:SP4.011.097.039.704.390$ OR PUFA$ OR LCPUFA$ OR polyunsaturated OR poly‐unsaturated OR MH:D10.212.302.380.410$ OR MH:D10.627.430.450$ OR "omega‐3" OR "omega 3" OR alpha‐linol$ OR linol$ OR Docosahex$ OR docosapent$ OR eicosapen$ OR timnodonic OR icosapentaen$ OR "omega‐6" OR "omega 6" OR gamma‐Linol$ OR linoic OR "Arachidonic Acids" OR "Ácidos Araquidónicos" OR "Eicosatetraenoic Acids" OR MH:D10.251.355.096$ OR MH:D10.251.355.255.100$ OR MH:D10.251.355.310.166$ OR "sodium arachidonate" OR EPA OR E‐EPA OR EFA OR MaxEPA OR DHA OR DPA OR ALA OR Lipoplus OR Lovaza OR Omacor OR "Omega Rx" OR Omegaven OR SMOFlipid OR "Corn Oil" OR "Aceite de Maíz" OR "Óleo de Milho" OR "Maize Oil" OR MH:D10.212.302.380.370$ OR MH:D10.212.507.340$ OR MH:D10.627.700.240$ OR MH:D20.215.784.750.240$ OR MH:J02.500.375.400.250$ OR "Linseed Oil" OR "Aceite de Linaza" OR "Óleo de Semente do Linho" OR "Flaxseed Oil" OR MH:D10.212.507.550$ OR MH:D10.627.700.615$ OR MH:D20.215.784.750.615$ OR "Safflower Oil" OR "Aceite de Azafrán" OR "Óleo de Açafrão" OR MH:D10.212.302.380.750$ OR MH:D10.212.507.750$ OR MH:D10.627.700.840$ OR MH:D20.215.784.750.840$ OR MH:J02.500.375.400.700$ OR "Soybean Oil" OR "Aceite de Soja" OR "Óleo de Soja" OR "Soya Oil" OR MH:D10.212.302.380.800$ OR MH:D10.212.507.800$ OR MH:D10.627.700.880$ OR MH:D20.215.784.750.880$ OR MH:J02.500.375.400.750$ OR Senega OR MH:HP4.018.685.481$ OR "Aceites de Pescado" OR "Óleos de Peixe" OR MH:D10.627.430$ OR (oil$ AND (fish* OR "cod liver" OR halibut OR squid OR krill OR mackerel OR menhaden OR salmon OR seal OR "shark liver" OR corn OR maize OR linseed OR flax* OR safflower OR soy* OR canola OR hemp* OR primrose OR algal OR egg OR "seabuckthorn seed" OR berry OR "Sacha Inchi" OR Echium)))
Appendix 6. metaRegister of Controlled Trials search strategy
(dry eye OR Keratoconjunctivitis) AND (omega 3 OR omega 6 OR polyunsaturated OR fatty acid OR oil OR EPA or E‐EPA or EFA or MaxEPA or DHA or DPA or ALA OR PUFA OR LCPUFA)
Appendix 7. ClinicalTrials.gov search strategy
( dry eye OR Keratoconjunctivitis ) AND ( omega 3 OR omega 6 OR polyunsaturated OR fatty acid OR oil OR EPA or E‐EPA or EFA or MaxEPA or DHA or DPA or ALA OR PUFA OR LCPUFA OR docosahexaenoic OR icosapentaenoic OR alpha‐linolenic OR linoleic OR Eicosapentaenoic OR arachidonic )
Appendix 8. WHO ICTRP search strategy
dry eye AND omega 3 OR dry eye AND omega 6 OR dry eye AND polyunsaturated OR dry eye AND fatty acid OR dry eye AND oil OR dry eye AND epa OR dry eye AND e‐epa OR dry eye AND efa OR dry eye AND maxepa OR dry eye AND dha OR dry eye AND dpa OR dry eye AND ala OR dry eye AND pufa OR dry eye AND lcpufa OR dry eye AND docosahexaenoic OR dry eye AND icosapentaenoic OR dry eye AND alpha‐linolenic OR dry eye AND linoleic OR dry eye AND eicosapentaenoic OR dry eye AND arachidonic OR Keratoconjunctivitis AND omega 3 OR Keratoconjunctivitis AND omega 6 OR Keratoconjunctivitis AND polyunsaturated OR Keratoconjunctivitis AND fatty acid OR Keratoconjunctivitis AND oil OR Keratoconjunctivitis AND epa OR Keratoconjunctivitis AND e‐epa OR Keratoconjunctivitis AND efa OR Keratoconjunctivitis AND maxepa OR Keratoconjunctivitis AND dha OR Keratoconjunctivitis AND dpa OR Keratoconjunctivitis AND ala OR Keratoconjunctivitis AND pufa OR Keratoconjunctivitis AND lcpufa OR Keratoconjunctivitis AND docosahexaenoic OR Keratoconjunctivitis AND icosapentaenoic OR Keratoconjunctivitis AND alpha‐linolenic OR Keratoconjunctivitis AND linoleic OR Keratoconjunctivitis AND eicosapentaenoic OR Keratoconjunctivitis AND arachidonic
Data and analyses
Comparison 1. Oral long‐chain omega‐3 PUFAs (ie, EPA and DHA) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dry eye symptoms, measured using the OSDI score [OSDI] units | 4 | 677 | Mean Difference (IV, Random, 95% CI) | ‐2.47 [‐5.14, 0.19] |
| 2 DESS: mean score at study endpoint [DESS units] | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Schirmer test (tear production) (mm/5 min) | 6 | 1826 | Mean Difference (IV, Random, 95% CI) | 0.68 [0.26, 1.09] |
| 4 Tear break‐up time (TBUT) measured using fluorescein (seconds) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Conjunctival impression cytology (CIC) score: mean score at study endpoint (Nelson grade) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Adverse event: gastrointestinal disorders | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 2. Oral combined omega‐3 and omega‐6 PUFAs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Schirmer test (tear production) (mm/5 min) | 4 | 455 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐0.45, 1.77] |
| 2 TBUT: Mean change from baseline (seconds) | 4 | 455 | Mean Difference (IV, Random, 95% CI) | 0.55 [0.04, 1.07] |
Comparison 3. Oral omega‐3 PUFAs plus conventional therapy versus conventional therapy alone (two RCTs).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 OSDI score: mean score at study endpoint [OSDI units] | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐7.16 [‐13.97, ‐0.34] |
Comparison 4. Oral long‐chain omega‐3 PUFAs versus oral omega‐6 PUFAs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dry eye symptoms, measured using the OSDI score [OSDI] units | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | ‐11.88 [‐18.85, ‐4.92] |
| 2 DESS: mean score at study endpoint [DESS units] | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3 Schirmer test (tear production) (mm/5 min) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Tear break‐up time (TBUT) measured using fluorescein (seconds) | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Adverse event: gastrointestinal disorders | 2 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [0.35, 15.54] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aragona 2005.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single center Number of participants randomized (total and per group): 80 eyes of 40 participants (ie, 40 eyes of 20 participants per treatment group) Unit of randomization (individual or eye): individual Exclusions after randomization: none Losses to follow‐up: none Unit of analysis (individual or eye): individual (right eye) Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: Italy Age (mean ± SD, range): 36.6 ± 6.7 years for total; 36.9 ± 7.9 years for treatment group; 36.3 ± 5.5 years for control group Gender: 2 men and 18 women in the treatment group; 1 man and 19 women in the control group Inclusion criteria: 1. Age more than 18 years 2. Primary Sjögren’s syndrome, as defined by the American‐European Consensus Group 3. Ability and willingness to participate in the study 4. Stable disease and general therapy for at least 1 month 5. No local treatment other than preservative‐free cellulose‐derivative eye drops 6. Moderate to severe dry eye, as defined by tear basal secretion less than 5 mm/5 min, tear break‐up time (TBUT) less than 7 seconds, and signs of corneal damage demonstrated by corneal fluorescein staining (at least 2+ according to Lemp) Exclusion criteria: 1. Any systemic disease different from Sjögren’s syndrome 2. Menopausal status 3. Presence of systemic treatment with drugs such as β‐blocking agents, benzodiazepines, hormones, or antihistamine agents that could interfere with tear production 4. Use of non‐steroidal anti‐inflammatory drugs (NSAIDs) or steroids Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (treatment group): oral sachets containing linoleic acid 112 mg and γ‐linolenic acid 15 mg, twice daily Intervention #2 (control group'): oral placebo sachets, twice daily Length of follow‐up: 1 month of treatment and 15 days after suspension of treatment Notes: sachets were diluted in water; participants were instructed to follow their usual diets; participants were allowed to continue their general and local therapies |
|
| Outcomes |
Primary outcome(s): PGE1 content (mg/mL) of tears at study endpoint Secondary outcome(s): subjective dry eye symptoms (burning, itching, foreign body sensation, dryness, mucous discharge, and photophobia); TBUT; corneal fluorescein staining; tear basal secretion (Schirmer test with anaesthesia) at study endpoint Adverse events reported? (Y/N): Y Measurement time points (including intervals at which outcomes were assessed): baseline, 1 month of treatment, and 15 days after suspension of treatment Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): "supported by grants from the National Research Council and Bausch & Lomb" Conflicts of interest: 2 authors were affiliated with Bausch & Lomb (E, F); "The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked 'advertisement' in accordance with 18 U.S.C. §1734 solely to indicate this fact" Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Once recruited, patients were randomly divided into two groups, according to a table of random numbers, and assigned, in a double masked manner, to the treatments" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Once recruited, patients were randomly divided into two groups, according to a table of random numbers, and assigned, in a double masked manner, to the following treatments" Details of masking about participants and personnel were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The tear content of PGE1 was evaluated in a masked manner by enzyme immunoassay (EIA), using a commercial kit" (primary outcome. Self‐reported symptoms were graded by means of a questionnaire administered at the beginning of each examination by a different observer (RS) from the one who performed the examinations (PA)" "The statistical analysis of the results was performed in a masked manner" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | High risk | "Supported by grants from the National Research Council and Bausch & Lomb" Two of the authors were affiliated with Bausch & Lomb (E, F) "The publication costs of this article were defrayed in part by page charge payment. This article must therefore be marked 'advertisement' in accordance with 18 U.S.C. §1734 solely to indicate this fact" |
Asbell 2018.
| Methods |
Study design: randomized, controlled trial Study site(s): multicenter (27 sites) Number randomized (total and per group): 535 participants in total; 349 participants in the omega‐3 treatment group; 186 participants in the placebo group Unit of randomization (individual or eye): individual Exclusions after randomization: none Losses to follow‐up: 47 participants in total; 25 participants in the omega‐3 treatment group; 22 participants in the control group Unit of analysis (individual or eye): eye ("Generalized estimating equations were used for ocular measures to accommodate the correlation between eyes in the same person") Reported power calculation? (Y/N): Y (90% power) Reported subgroup analysis? (Y/N): Y (severity of dry eye) |
|
| Participants |
Baseline characteristics Country: United States of America Age (mean ± SD, range): 58.3 ± 13.5 years in the omega‐3 treatment group; 57.5 ± 12.6 years in the control group Gender: 65 men and 284 women in the omega‐3 treatment group; 36 men and 150 women in the control group Inclusion criteria: 1. Sign and date the informed consent form approved by the IRB 2. ≥ 18 years of age 3. Demonstrate at least 2 of the 4 following signs in the same eye at 2 consecutive visits. The same signs must be present in the same eye on both visits: (screening visit: 7 to 21 days before randomization, and visit 00 [baseline visit]: day of randomization) a. Conjunctival staining present ≥ 1 (out of possible score of 6 per eye) b. Corneal fluorescein staining present ≥ 4 (out of a possible score of 15 per eye) c. Tear film break‐up time (TBUT) ≤ 7 seconds d. Schirmer test ≥ 1 to ≤ 7 mm/5 min 4. Demonstrate symptoms of dry eye disease (OSDI score greater than 22 (≥ 23 to ≤ 80) at screening visit and at least 18 (≥ 18 to ≤ 80) at randomization visit 5. Have dry eye‐related ocular symptoms for at least 6 months before the screening visit and use or desire to use artificial tears on average 2 times per day in the 2 weeks preceding the screening visit 6. Intraocular pressure (IOP) ≥ 5 mmHg and ≤ 22 mmHg in each eye 7. Women of child‐bearing potential must agree to use a reliable method of contraception during study participation and must demonstrate a negative urine pregnancy test at the screening visit 8. Be willing/able to return for all study visits and to follow instructions from the study investigator and his/her staff 9. Be able to swallow large, soft gel capsules 10. Demonstrate compliance with taking soft gels as directed during the run‐in period (≥ 90% taken, by pill count) Exclusion criteria: 1. Allergic to ingredients of active or placebo pills (fish, shellfish, olive oil) 2. Contact lens wearers who are unwilling to discontinue use for 2 weeks before the baseline visit and for the duration of the study 3. Pregnant or nursing/lactating 4. Participation in a study of an investigational drug or device within the 30 days preceding the screening visit 5. Current diagnosis of any of the following ocular conditions: i) Infection (eg, bacterial, viral, protozoan, or fungal infection of the cornea, conjunctiva, lacrimal gland, lacrimal sac, or eyelids) or ii) Inflammation (eg, retinitis, macular inflammation, choroiditis, uveitis, scleritis, episcleritis, keratitis) 6. History of ocular herpetic keratitis 7. Ocular surgery (including cataract surgery) within 6 months of the screening visit 8. Previous LASIK surgery or any other corneal surgery 9. Use of glaucoma medication or history of surgery for glaucoma 10. Eyelid abnormalities that affect lid function (eg, lagophthalmos, blepharospasm, ectropion, entropion, severe trichiasis) 11. Extensive ocular surface scarring or condition that may compromise ocular surface integrity such as Stevens‐Johnson syndrome, prior chemical burn, recurrent corneal erosions, persistent corneal epithelial defects, prior ocular trauma, etc. 12. Dry eye due to seasonal allergic conjunctivitis, or other acute or seasonal diagnosis 13. Current use of EPA/DHA supplements in excess of 1200 mg/d 14. History of liver disease 15. Currently on anticoagulation therapy such as heparin and warfarin. Use of clopidogrel (Plavix) or aspirin does not exclude the patient 16. Patients with hemophilia, thrombocytopenia, or other bleeding tendencies 17. History of atrial fibrillation 18. Uncontrolled ocular or systemic disease 19. Cognitive or psychiatric deficit that precludes informed consent or ability to perform requirements of the investigation Equivalence of baseline characteristics? (Y/N): N (equivalence for all parameters, except for a higher mean EPA level in the active supplement group than in the placebo group [0.63% vs 0.56%; P = 0.047], but this was not a primary outcome measure.) The paper also states that "In accordance with the protocol, an analysis of the mean change in the OSDI score with adjustment for the baseline EPA level was performed because of an imbalance between trial groups in the EPA level (P<0.10)" |
|
| Interventions |
Intervention #1 (treatment group): 5 soft gelatin capsules per day (daily dose of 2000 mg EPA and 1000 mg DHA) Intervention #2 (control group): 5000 mg per day of refined olive oil Length of follow‐up: 12 months Notes: patients who were regularly using treatments for dry eye disease (including omega‐3 fatty acid supplements: eicosapentaenoic acid [EPA] plus docosahexaenoic acid [DHA] at a dose of <1200 mg daily), systemic medications that are known to cause ocular dryness, systemic glucocorticoids, or other immunosuppressive agents were allowed to continue those treatments if they committed to using them for the next 12 months. Patients with a history of thyroid disease, Sjögren’s syndrome, rheumatoid arthritis, or inflammatory diseases could be included in the trial if they were otherwise eligible |
|
| Outcomes |
Primary outcome(s): mean change from baseline in OSDI Secondary outcome(s): changes in the percentages of EPA and DHA in total fatty acids in red cells (by weight), changes in signs of dry eye disease (as assessed by conjunctival lissamine green staining score, corneal fluorescein staining score, tear break‐up time, and the result on Schirmer test with anesthesia), changes in scores on physical health and mental health subscales of the Medical Outcomes Study 36‐Item Short Form Health Survey (SF‐36; scores range from 0 to 100, with higher scores indicating better health‐related quality of life), changes in scores on the discomfort and pain interference subscales of the Brief Ocular Discomfort Index (BODI; scores range from 0 to 100, with higher scores indicating greater discomfort), changes in treatments used for dry eye disease, changes in visual acuity and intraocular pressure (safety outcomes) Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, 3 months, 6 months, and 12 months Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: October 2014 to July 2016 Funding source(s): "Supported by cooperative agreements (U10EY022879 and U10EY022881) from the National Eye Institute, National Institutes of Health (NIH), and by grant supplements from the NIH" Conflicts of interest: the lead author has multiple financial relationships, including personal fees, grants, and non‐financial support from multiple companies Publication language: English Registered on clinical trials registry? (Y/N): Y (clinicaltrials.gov, NCT02128763) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed with the use of a web‐based module and was stratified according to clinical center with a permuted block method with randomly chosen block sizes" |
| Allocation concealment (selection bias) | Low risk | "Personnel at the Investigational Drug Service, University of Pennsylvania, mailed the supplements directly to the patients" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All patients, clinical staff, and laboratory personnel were unaware of the trial‐group assignments" "The active and placebo capsules contained 3 mg of vitamin E (alpha‐tocopherol), as an antioxidant, as well as masking flavor and lemon flavor" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All patients, clinical staff, and laboratory personnel were unaware of the trial‐group assignments" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Although the paper states that "analyses were performed according to the intention‐to‐treat principle," it is evident that 36/535 (6.7%) who were randomized were not included in the final analysis. It is also stated that "propensity scores and the regression method of multiple imputation were used for missing OSDI scores at month 6 or 12 [ref 11]"; however imputation does appear to have been performed for the other outcome measures. |
| Selective reporting (reporting bias) | High risk | Some prespecified outcomes listed on the clinical trials registry entry (eg, frequency of artificial tears used, contrast sensitivity, tear cytokine level(s), ocular redness) were not reported in the paper |
| Other bias | High risk | The lead author (PA) has multiple financial relationships, including personal fees, grants, and non‐financial support from multiple companies |
Barabino 2003.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single center Number randomized (total and per group): 52 eyes of 26 participants; 26 eyes of 13 participants per group Unit of randomization (individual or eye): individual Exclusions after randomization: none Losses to follow‐up: none Unit of analysis (individual or eye): individual (worse eye) Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: Italy Age (mean ± SD, range): 58.8 ± 10.7 years for total; 63.4 ± 8.2 years for treatment group; 54.3 ± 11.3 years for control group Gender: 4 men and 9 women in the treatment group; 3 men and 10 women in the control group Inclusion criteria: 1. Age more than 18 years 2. Dry eye was identified on the basis of typical symptoms of dry eye (photophobia, burning, foreign body sensation, blurred vision improved by blinking, and pain) measured by a validated questionnaire, Schirmer test scores less than 5 mm/5 min, positive vital staining with 1% lissamine green (graded 0 to 9 according to the van Bijsterveld score system) less than 3.5, and fluorescein TBUT less than 7 seconds Exclusion criteria: 1. Infectious keratoconjunctivitis or inflammatory diseases unrelated to dry eye 2. Concomitant ocular pathologies 3. Previous ocular surgery 4. Eyelid or eyelash abnormalities 5. Alteration of the nasolacrimal apparatus 6. Treatment with drugs affecting tearing 7. Treatment with vitamin supplements 8. Concomitant ocular therapies 9. Topical ophthalmic steroids taken during the 4 weeks before the study 10. Pregnancy 11. Diabetes or other systemic, neurologic, or dermatologic disease affecting the health of the ocular surface Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (treatment group): oral tablets containing linoleic acid 28.5 mg and γ‐linolenic acid 15 mg (Medilar tablets, Fidia Oftal‐Bausch & Lomb Pharmaceuticals), twice daily Intervention #2 (control group): placebo tablets, twice daily Length of follow‐up: 45 days Notes: patients used preservative‐free artificial tears, 4 times daily; patients in both groups were instructed to follow a sensible diet of any kind of food without excess and to record changes in a diary |
|
| Outcomes |
Primary outcome(s): percentage of HLA‐DR‐positive cells at study endpoint Secondary outcome(s): subjective symptoms; conjunctival lissamine green staining; Schirmer I test; TBUT (paper indicates that these outcomes are measured as change from baseline, but these outcomes are reported as endpoint data) Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, day 45 Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): not reported Conflicts of interest: "The authors have no financial interest in any of the products or instruments used in this study" Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐masked" study, but details of masking about personnel were not reported" "we limited the length of the study to 45 days, and to avoid the well‐known placebo effect, we treated the control group with tablets similar to the one used in the study group, but without an active ingredient" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Slides were evaluated by optical microscopy; a researcher masked with respect to the study groups read the results" Masking of other outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | Low risk | No other apparent sources of bias |
Bhargava 2013.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): multi‐center (2 sites) Number randomized (total and per group): 518 eyes of 518 participants; 264 eyes of 264 participants in the treatment group; 254 eyes of 254 participants in the control group Unit of randomization (individual or eye): individual Exclusions after randomization: 6 participants, due to faulty impression cytology slides Losses to follow‐up: 12 participants Unit of analysis (individual or eye): individual (1 eye per participant was enrolled) Reported power calculation? (Y/N): Y (80% power) Reported subgroup analysis? (Y/N): Y (patients with Sjögren’s syndrome, contact lens users, video display terminals, chronic blepharitis, and acne rosacea) |
|
| Participants |
Baseline characteristics Country: India Age (mean ± SD, range): 38.8 ± 4.1 years in the treatment group; 40.1± 6.8 years in the control group Gender: 254 men and 268 women total Inclusion criteria: 1. Aged more than 16 years 2. Symptoms of dry eye 3. TBUT less than 10 s 4. Presence of lid margin scaling, telangiectasia, collarette and meibomian gland plugging on slit‐lamp examination Exclusion criteria: 1. Any pre‐existing ocular disease other than dry eye syndrome 2. Current treatment with oral tetracyclines or corticosteroids 3. Past history of herpetic eye disease, liver disease, diabetes, or laser keratomileusis (LASIK) 4. Pregnant or lactating mothers 5. Cognitive or psychiatric disorder 6. Postmenopausal women 7. HIV 8. Hepatitis B and C 9. Inability to swallow soft gel capsules 10. Current treatment with aspirin or anticoagulant therapy 11. Allergy to fluorescein 12. Malignancy or chronic infection of the lacrimal gland Equivalence of baseline characteristics? (Y/N): Y (personal communication) |
|
| Interventions |
Intervention #1 (treatment group): oral 500 mg capsule containing eicosapentaenoic acid (EPA) 325 mg and docosahexaenoic acid (DHA) 175 mg, twice daily Intervention #2 (control group): oral placebo capsules containing corn oil twice daily (daily dose: 1000 mg/d) Length of follow‐up: 3 months Notes: topical medications and contact lens use were discontinued before enrollment |
|
| Outcomes |
Primary outcome(s): change from baseline in subjective symptoms Secondary outcome(s): change from baseline in Schirmer test and TBUT; rose bengal score (RBS) and conjunctival impression cytology (CIC) scores (for cellular changes and goblet cell density, measured using Nelson grade) at study endpoint Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, months 1, 2, and 3 Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): not reported Conflicts of interest: not reported Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to one of two groups by parallel assignment. The allocation codes were generated by a DOS based computer software" |
| Allocation concealment (selection bias) | Low risk | "The allocation was concealed in green colored envelopes that were opened by health care staff not involved in patient care" "software (Disk operating system based) generates codes (sequentially numbered and coloured) which were sealed in envelopes and were opened by a third party who were not involved in patient care" (personal communication) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double blind" study "The two types of capsules and packs were similar to each other (omega‐3 dietary supplementation)" "The subjects were masked to the contents" Software (disk operating system based) generates codes (sequentially numbered and colored), which were sealed in envelopes and were opened by a third party who was not involved in patient care (personal communication) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "At each visit, each subject underwent a detailed ocular examination by an independent investigator (not a study ophthalmologist)" Independent investigator performed all tests (including tear film tests) and was masked to information obtained from the dry eye questionnaire (personal communication) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/518 (2.3%) participants were lost to follow‐up, and 6/518 (1.2%) were excluded from the analysis due to faulty impression cytology slides; it was not reported which treatment group these individuals belonged to |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | Unclear risk | Information regarding funding source and conflict of interest was not reported |
Bhargava 2015a.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): multi‐center (3 sites) Number randomized (total and per group): 478 participants in total Unit of randomization (individual or eye): individual Exclusions after randomization: 6 participants due to gastric intolerance (in the omega‐3 treatment group); and 6 participants due to faulty impression cytology slides Losses to follow‐up: 10 in total Unit of analysis (individual or eye): individual (1 eye selected at random) Reported power calculation? (Y/N): Y (90% power) Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: India Age (mean ± SD, range): 22.8 ± 2.5 years in the treatment group; 23.7 ± 6.8 years in the control group Gender: 219 men and 237 women, in total Inclusion criteria: 1. Symptomatic computer users (using computers for > 3 hours/d for minimum 1 year) on the basis of a questionnaire of dry eye‐related symptoms (Dry Eye Scoring System, DESS) Exclusion criteria: 1. Current ocular infection 2. Past history of laser in situ keratomileusis (LASIK) 3. Allergic conjunctivitis 4. Contact lens wear 5. Herpetic eye disease 6. Diabetes 7. Liver disease 8. Pregnant or lactating mothers 9. HIV and hepatitis B or C 10. Patients with inability to swallow soft gel capsules 11. Aspirin or anticoagulant therapy 12. Allergic to fluorescein 13. Systemic (tetracyclines and corticosteroids) or topical medications (other than artificial tear supplements) that could affect tear film or meibomian gland function Equivalence of baseline characteristics? (Y/N): N (symptoms of dry eye, P = 0.028; conjunctival impression cytology, P = 0.013) |
|
| Interventions |
Intervention #1 (treatment group): oral capsule containing EPA 180 mg and DHA 120 mg, 2 capsules/time, twice daily (daily dose of 720 mg EPA and 480 mg DHA) Intervention #2 (control group): olive oil (dose not reported) Length of follow‐up: 3 months Notes: participants were instructed not to use artificial tear preparations, 2 hours before testing |
|
| Outcomes |
Primary outcome(s): "decrease from baseline in" symptoms of dry eye Secondary outcome(s): Schirmer test with anesthesia; TBUT; Nelson grade (for cellular morphology and goblet cell density). Although these outcomes were described to be measured as "change from baseline," the manuscript reports endpoint data Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, 1, 2, and 3 months Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): "we do not have any financial interest" Conflicts of interest: "none" Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation codes were generated by a DOS based software" |
| Allocation concealment (selection bias) | Low risk | "The codes were sealed in blue coloured envelopes and were opened by health care personnel not involved in patient care" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The subjects as well as the investigators were masked to the contents. The two types of capsules and packs were similar to each other" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "the subjects underwent a detailed ocular examination by an independent investigator (SK), who was not a study surgeon" "The independent investigator (SK) was masked to the information obtained from the questionnaire" Study does not explicitly state that outcome assessors were masked to assignments |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Statistical analysis was performed on an intent to treat basis" However, we noted that 22/474 (4.6%) randomized participants were not included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | High risk | Participant treatment group baselines were not equivalent for the primary outcome measure (ie, dry eye symptom score; P = 0.028) |
Bhargava 2015b.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): multi‐center (3 sites) Number randomized (total and per group): 496 participants in total; 240 participants in the treatment group; 256 participants in the placebo group Unit of randomization (individual or eye): individual Exclusions after randomization: 8 participants, for impression cytology slides Losses to follow‐up: 14 participants dropped out of the treatment group due to gastric intolerance and non‐compliance. Unit of analysis (individual or eye): individual (1 eye selected at random) Reported power calculation? (Y/N): Y (90% power) Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: India Age (mean ± SD, range): not reported Gender: 496 women in total Inclusion criteria: 1. Female contact lens users experiencing dry eye symptoms and lens wear discomfort Exclusion criteria: 1. Current ocular infection 2. History of laser in situ keratomileusis 3. Allergic conjunctivitis 4. Herpetic eye disease 5. Diabetes 6. Liver disease 7. Pregnant or lactating mothers 8. HIV 9. Hepatitis B and C 10. Inability to swallow soft gel capsules 11. Taking aspirin or anticoagulant therapy 12. Allergic to fluorescein Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (treatment group): oral capsule containing EPA 180 mg and DHA 120 mg, 2 capsules/time, twice daily (daily dose of 720 mg EPA and 480 mg DHA) Intervention #2 (control group): corn oil (dose not reported) Length of follow‐up: 6 months Notes: systemic (tetracyclines and corticosteroids) or topical medications (other than artificial tear supplements) that could affect tear film or meibomian gland function were discontinued before the intervention. However, patients were instructed not to use artificial tear preparations 2 hours before testing. Computer work was not allowed during the course of the study as concurrent use of visual display terminals may independently influence ocular surface changes |
|
| Outcomes |
Primary outcome(s): "decrease from baseline" in symptoms of dry eye and contact lens wear discomfort, but reported as endpoint data Secondary outcome(s): Schirmer test; TBUT; Nelson grade (for cellular morphology and goblet cell density). Although these outcomes were described to be measured as "change from baseline," the manuscript reports endpoint data Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, 3 and 6 months Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): "the authors have no funding or conflicts of interest to disclose" Conflicts of interest: "the authors have no funding or conflicts of interest to disclose" Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation codes were generated by a DOS‐based software" |
| Allocation concealment (selection bias) | Low risk | "The codes were sealed in blue envelopes and were opened by health care personnel not involved in patient care" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The subjects were masked to the contents. The 2 types of capsules and packs were similar to each other" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The independent investigator (K.S.) was masked to the information obtained from the questionnaire" "A single examiner performed CIC and was masked to information obtained from the questionnaire" It is unclear whether outcome assessor was masked to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All dropouts were included for analysis based on the last‐observation‐carried‐forward method" |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | Low risk | No other apparent sources of bias |
Bhargava 2016a.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): multi‐center (3 sites) Number randomized (total and per group): 130 participants in total; 65 participants in each treatment group Unit of randomization (individual or eye): individual Exclusions after randomization: 8 in the omega‐3 treatment group (due to gastric intolerance) Losses to follow‐up: 6 participants in the omega‐3 treatment group Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): Y (90%) Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: India Age (mean ± SD, range): 47.7 ± 3.8 (range 24 to 68) years in the omega‐3 treatment group; 48.9 ± 4.5 (range 21 to 70) years in the control group Gender: 25 men and 40 women in the treatment group; 27 men and 38 women in the control group Inclusion criteria: 1. Patients with rosacea having dry eye symptoms were enrolled based on their response to (Dry Eye Scoring System, DESS) a questionnaire of common symptoms of dry eye. The minimum score for inclusion is 1 (ie, any patient having dry eye symptoms). A score of 0 to 3 was assigned to dry eye‐related symptoms Exclusion criteria: 1. Patients with corneal or episcleral/scleral involvement 2. Allergic conjunctivitis 3. Contact lens wear 4. Herpetic eye disease 5. Diabetes 6. Other skin disease 7. Inability to swallow soft gel capsules 8. Regular course of aspirin or anticoagulants (cyclo‐oxygenase‐2 inhibitors) 9. Allergic to fluorescein Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (treatment group): oral capsule containing EPA 180 mg and DHA 120 mg, 2 capsules/time, twice daily (daily dose of 720 mg EPA and 480 mg DHA) Intervention #2 (control group): olive oil (dose not reported) Length of follow‐up: 6 months Notes: systemic (tetracyclines and corticosteroids) or topical medications (other than artificial tear supplements) that could affect tear film or meibomian gland function (beta‐blockers, benzodiazepines, and antihistamines) were discontinued 3 weeks before the intervention. During this period, all participants were prescribed 0.5% carboxymethylcellulose eye drops 4 times a day. However, patients were instructed to not use tear supplements at least 2 hours before tear film testing |
|
| Outcomes |
Primary outcome(s): "change in" symptoms of dry eye, although data are reported as endpoint data Secondary outcome(s): meibomian gland score (MGS); Schirmer test with anesthesia; TBUT. Although these outcomes were described to be measured as "change from baseline," the manuscript reports endpoint data Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, 1, 3, and 6 months Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: January 2013 to June 2014 Funding source(s): "we do not have any financial interest in the copyrighted Dry Eye Scoring System (DESS)" Conflicts of interest: "the authors report no conflicts of interest" Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation codes were generated by a disk operating system‐based software" |
| Allocation concealment (selection bias) | Low risk | "The codes were sealed in blue‐colored envelopes that were opened by health care personnel not involved in patient care" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The subjects were masked about the contents. The two types of capsules and packs were similar to each other" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The independent investigator (KS) was masked to the information obtained from the questionnaire" It is unclear whether the outcome assessor was masked to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All dropouts were included for analysis based on the last observation carried forward method" |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | Low risk | No other apparent sources of bias |
Bhargava 2016b.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): multi‐center (3 sites) Number randomized (total and per group): 522 participants in total; 256 participants in the omega‐3 treatment group; 266 participants in the placebo group Unit of randomization (individual or eye): individual Exclusions after randomization: 26 in total; 22 (irregularly taking supplements because of bad taste and gastric intolerance) and 4 (inability to continue the trial) Losses to follow‐up: not reported Unit of analysis (individual or eye): individual (1 eye selected at random) Reported power calculation? (Y/N): Y (90% power) Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: India Age (mean ± SD, range): 28.9 ± 4.2 years in the omega‐3 treatment group; 29.6 ± 5.5 years in the control group Gender: not reported Inclusion criteria: 1. Symptomatic visual display terminal (VDT) users experiencing dry eye symptoms based on their response to the Dry Eye Scoring System questionnaire Exclusion criteria: 1. Allergic conjunctivitis 2. History of laser in situ keratomileusis 3. Contact lens wear 4. Other causes of dry eye in the office‐going population, software professionals, or university students 5. Inability to swallow soft gel capsules 6. On a regular course of aspirin or anticoagulants (cyclo‐oxygenase‐2 inhibitors) 7. Allergic to fluorescein Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (treatment group): oral capsule containing EPA 180 mg and DHA 120 mg, 4 capsules/time, twice daily (daily dose of 1440 mg EPA and 960 mg DHA) Intervention #2 (control group): olive oil (dose not reported) Length of follow‐up: 45 days Notes: systemic (tetracyclines and corticosteroids) or topical medications (other than artificial tear supplements) that could affect tear film or meibomian gland function (beta‐blockers, benzodiazepines, and antihistamines) were discontinued 3 weeks before the intervention commenced. Moreover, patients were instructed not to use artificial tear preparations 2 hours before testing |
|
| Outcomes |
Primary outcome(s): "improvement in" symptoms of dry eye, but reported as endpoint data Secondary outcome(s): "improvement in" Schirmer test with anesthesia, TBUT, and Nelson grade on conjunctival impression cytology, but reported as endpoint data in the manuscript Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, 30 and 45 days Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): "the authors have no funding or conflicts of interest to disclose" Conflicts of interest: "the authors have no funding or conflicts of interest to disclose" Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation codes were generated by disk operating system–based software" |
| Allocation concealment (selection bias) | Low risk | "The codes were sealed in green envelopes and were opened by a health care personnel not involved in patient care" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double masked" "The subjects were masked to the contents. The two types of capsules and packs were similar to each other" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "A detailed ocular examination was performed by an independent investigator (who was not a study surgeon, K.S.)" "The independent investigator (K.S.) was masked to the information obtained from the questionnaire" It is unclear whether the outcome assessor was masked to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All dropouts (n=26) were included for analysis based on the last‐observation‐carried‐forward method" |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | Low risk | No other apparent sources of bias |
Brignole‐Baudouin 2011.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): multi‐center (9 sites) Number randomized (total and per group): 138 participants in total Unit of randomization (individual or eye): individual Exclusions after randomization: 1 participant (unknown group) discontinued; 5 participants withdrew (2 due to adverse events; 1 lack of improvement; 2 withdrew consent) in the treatment group; 4 participants withdrew (2 due to adverse events; 1 because of their worsening condition; 1 withdrew consent) in the control group Losses to follow‐up: 1 participant in the treatment group Unit of analysis (individual or eye): individual (worse eye) Number analyzed: 58 participants in the treatment group; 63 participants in the control group (15 participants did not have 2 evaluable conjunctival impression cytologies, for reasons of early participant withdrawal, or lost or impaired samples) Reported power calculation? (Y/N): Y (80% power) Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Countries: France and Italy Age (mean ± SD, range): 60 ± 11.75 years in the omega‐3 treatment group; 59.7± 11.95 years in the control group Gender: 1 man and 57 women in the omega‐3 treatment group; 3 men and 60 women in the control group Inclusion criteria: 1. Scores for at least 2 of the following 4 objective tests: Schirmer test values < 10 mm/5 min; TBUT values < 10 s; corneal fluorescein staining score ≥ 1 and < 4; lissamine green by van Bijsterveld score > 3 and < 6 2. Score of at least 1 for at least 2 of the 5 following subjective tests (scored from 0 to 3): foreign body sensation; dryness; burning; stinging; photophobia 3. For those having systemic treatment, no change in treatment for at least a month before inclusion 4. Written informed consent Exclusion criteria: 1. Severe dry eye defined as lissamine green > 6 or corneal staining ≥ 4 2. Uncontrolled inflammatory disease 3. Drastic change in food and/or food supplements within the last month 4. Other food supplement with eicosapentaenoic acid and docosahexaenoic acid 5. Evidence of acute ocular infection and/or intraocular inflammation within 1 month before the start of this study 6. Ocular surgery within the last 6 months 7. Change in ocular treatment within the last month 8. Patients currently using any ophthalmic medication including any ocular ointment except artificial tear preparation and eye cleaning solution for treatment of dry eye syndrome 9. Patients treated with topical ocular, steroidal or non‐steroidal, anti‐inflammatory treatment within the last month 10. Patients treated with ocular topical cyclosporine within the last month 11. Occlusion therapy with lacrimal or punctum plugs within the last 3 months 12. Patients currently wearing contact lenses 13. Pregnant or lactating women Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (treatment group): oral capsule containing fish oil (omega‐3 fatty acids, average of 285 mg including eicosapentaenoic acid 142.5 mg and docosahexaenoic acid 95 mg) and omega‐6 average of 5 mg; Medilar, Bausch and Lomb, R. P. Scherer GmbH & Co., 3 capsules daily Intervention #2 (control group): placebo capsule containing medium‐chain triglycerides, 3 capsules daily (daily dose: 575 mg/d) Length of follow‐up: 3 months Notes: participants were instructed to take 3 capsules daily during meals with a glass of water; daily dosage of 855 mg of omega‐3 fatty acids and 15 mg of omega‐6 fatty acids |
|
| Outcomes |
Primary outcome(s): "reduction in" HLA‐DR expression (%) Secondary outcome(s): change from baseline in HLA‐DR arbitrary units of fluorescence (AUF); global subjective dry eye symptoms score (foreign body sensation, dry eye sensation, burning, stinging, and photophobia); subjective dry eye score for each symptom; Schirmer test; TBUT; corneal fluorescein staining; conjunctival lissamine green staining; QOL questionnaire; frequency of artificial tear usage (ie, "number of daily instillations of tear substitutes") Safety endpoints: occurrence of adverse events; changes in external eye exam and biomicroscopy Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, 6 weeks, 3 months Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): "this study was sponsored and funded by Bausch and Lomb Inc Montpellier, France" Conflicts of interest: 1 author is affiliated with a pharmaceutical firm Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The treatments were consecutively numbered and allocated to study participants in ascending order using the next available consecutive number from a randomization list (1:1) established prior to study enrolment (sas® 8.2, Cary, NC, USA) on Solaris 2.8 (Redwood Shores, CA, USA) and Framemaker 5.5.6 (San Jose, CA, USA)" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐masked" study "The treatments were identical in appearance, packaging and treatment regimen for the placebo to preserve masking" "The Investigator, patients, and Bausch and Lomb personnel involved in the monitoring or conduct of the study were blinded to the study drug codes. Study drug codes were not available to the above personnel until after the study was completed and the database was finalized" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐masked" study "The treatments were identical in appearance, packaging and treatment regimen for the placebo to preserve masking" "The Investigator, patients, and Bausch and Lomb personnel involved in the monitoring or conduct of the study were blinded to the study drug codes. Study drug codes were not available to the above personnel until after the study was completed and the database was finalized" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Analyses were performed on the full analysis set (FAS) and per‐protocol set (PPS). The FAS included all randomized patients who received the study treatment at least once, had at least one follow‐up visit available and had two assessable analyses of conjunctival IC (at day 0 and month 3)" "Fifteen patients did not have two evaluable conjunctival IC for reasons of early patient withdrawal or lost or impaired samples. Thus, only 121 patients were included in the FAS (n = 58 fatty acids; n = 63 placebo)" 15/138 (10.9%) patients who were randomized were not included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | High risk | One author is affiliated with a pharmaceutical firm |
Creuzot 2006.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported if single‐ or multi‐center Number randomized (total and per group): 71 participants; 36 participants in the treatment group; 35 in the placebo group Unit of randomization (individual or eye): individual Exclusions after randomization: 4 participants in the treatment group and 7 participants in the placebo group were excluded or were lost to follow‐up Losses to follow‐up: 4 participants in the intervention group and 7 participants in the placebo group were excluded or were lost to follow‐up Unit of analysis (individual or eye): individual Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: France Age (mean ± SD, range): 59.7 ± 14.7 years in the treatment group; 61.1 ± 11.1 years in the control group Gender: 2 men and 34 women in the treatment group; 1 man and 34 women in the control group Inclusion criteria: 1. Mild to moderate dry eye defined by lissamine green upper marking 4 according to van Bijsterveld and Schirmer test < 10 mm /5 min and/or TBUT< 10 s 2. Severe ocular surface disease that showed a superficial punctate keratitis strictly superior to 3 (of 5 by score of Oxford) or filamentary keratitis Exclusion criteria: not reported Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (treatment group): oral capsule containing omega‐3 PUFAs (DHA 196 mg and EPA 14 mg), omega‐6 PUFA (GLA 41 mg or LA 63 mg), various vitamins (C, E, B6, B9, B12), and a trace element (zinc) (Nutrilarm, Laboratoires Thea) twice daily (2 capsules per day) Intervention #2 (control group): placebo capsule (oleic acid), twice daily, 2 capsules per day (dose not reported) Length of follow‐up: 6 months |
|
| Outcomes | Primary and secondary outcome measures were not clearly distinguished Outcomes specified: Schirmer test; TBUT; corneal fluorescein staining; conjunctival lissamine green staining; reflex tearing; conjunctival hyperaemia; skin quality; "emotional quality" at study endpoint Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, months 1, 3, and 6 Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): not reported Conflicts of interest: 1 author was affiliated with a pharmaceutical company Publication language: French Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This study was reported as a "double‐masked" study, but details of masking were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This study was reported as a "double‐masked" study, but details of masking were not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four (11.1%) participants in the treatment group and 7 (20%) participants in the placebo group were excluded or were lost to follow‐up, and 2 participants in each group were not included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | High risk | 1 of the study authors was affiliated with a pharmaceutical company |
Creuzot‐Garcher 2011.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): multi‐center (number of sites not stated) Number randomized (total and per group): 181 participants; 90 participants in the treatment group; 91 participants in the placebo group. Unit of randomization (individual or eye): individual Exclusions after randomization: 11 participants in the treatment group and 5 participants in the placebo group were excluded or were lost to follow‐up Losses to follow‐up: 11 participants in the intervention group and 5 participants in the placebo group were excluded or were lost to follow‐up Unit of analysis (individual or eye): individual Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: France Age (mean ± SD, range): 61.54 ± 11.87 years total; 61.28 ± 12.15 years in the treatment group; 61.79 ± 11.64 years in the control group Gender: 8 men and 82 women in the treatment group; 7 men and 84 women in the control group Inclusion criteria: 1. 18 to 90 years of age 2. Moderate dry eye, defined as evocative symptoms of dry eye resulting in a bilateral upper dry eye sensation or equal to 2 (on a scale of severity from 0 to 3, 2 and 3 corresponding to moderate to severe), a Schirmer test less than or equal to 10 mm in 5 minutes or tear film break time (TBUT) lower 10 s, lissamine green test score > 3 according to the classification of van Bjisterveld, and corneal fluorescein staining equal to 1, 2, or 3 (Oxford scale) Exclusion criteria: not reported Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (treatment group): oral capsule containing omega‐3 PUFAs (DHA 196 mg and EPA 14 mg), omega‐6 PUFA (GLA 41 mg or LA 63 mg), various vitamins (C, E, B6, B9, B12), and a trace element (zinc) (Nutrilarm, Laboratoires Thea), twice daily, 2 capsules per day Intervention #2 (control group): placebo capsule (composition and dose not reported), twice daily, 2 capsules per day Length of follow‐up: 6 months Notes: participants were allowed to take their usual eye treatments throughout the study period |
|
| Outcomes |
Primary outcome(s): "dryness feeling" at study endpoint Secondary outcome(s): ocular symptoms (burning, stinging, sandy and/or gritty sensation, light sensitivity; reflex tearing; ocular fatigue); Schirmer test; TBUT; corneal fluorescein staining; conjunctival lissamine green staining; HLA‐DR expression, at study endpoint Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, months 1, 3, and 6 Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): Thea Laboratories funded this clinical study and drafted the article Conflicts of interest: the authors have no financial interest in Thea Laboratories and the product Nutrilarm Publication language: French Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | This study was reported as a "double‐masked" study, but details of masking were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This study was reported as a "double‐masked" study, but details of masking were not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11 (12.2%) participants in the treatment group and 5 (5.5%) participants in the placebo group were not included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | High risk | Thea Laboratories funded this clinical study and drafted the article |
Deinema 2017.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single center Number randomized (total and per group): 60 participants in total; 20 in each of the 3 intervention groups Unit of randomization (individual or eye): individual Exclusions after randomization: none Losses to follow‐up: 6 participants in total; 3 participants in the placebo group; 1 participant in the fish oil group; 2 participants in the krill oil group Unit of analysis (individual or eye): individual Reported power calculation? (Y/N): Y (90% power) Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: Australia Age (mean ± SE, range): 39.4 ± 3.4 years in the fish oil group; 42.3 ± 3.8 years in the krill oil group; 46.2 ± 4.5 years in the placebo group Gender: 47% (n = 9) female in the fish oil group; 72% female (n = 13) in the krill oil group; 82% female (n = 14) in the placebo group Inclusion criteria: 1. ≥ 18 years of age 2. Provision of written informed consent to participate 3. For females of child‐bearing potential, a negative pregnancy test result at baseline 4. OSDI score ≥ 18 and < 65 5. Tear osmolarity ≥ 316 mOsmol/L in at least 1 eye 6. Distance best corrected visual acuity ≥ 20/40 Snellen equivalent in each eye 7. IOP ≤ 21 mmHg in both eyes 8. Ability to follow study instructions, with the intention of completing all required visits Exclusion criteria: 1. Any of the following general medical conditions: diabetes, bipolar disorder, atrial fibrillation, implanted defibrillator, familial adenomatous polyposis, systemic immunocompromise, bleeding disorders, or history of liver disease 2. A major change to diet or dietary supplement intake in the 3 months before enrollment 3. Consumption of essential fatty acid oral supplements in the 3 months before enrollment 4. Current consumption of any systemic anticoagulants 5. Known allergy or sensitivity to study supplements or any of their components (eg, any of fish, seafood, peanuts, nuts, oil, gelatin) 6. Females of child‐bearing potential who were planning a pregnancy over the course of the study, or currently pregnant or breastfeeding 7. Current enrollment in another interventional drug or device study or participation in such a study within 30 days of anticipated entry into this study 8. Anticipated contact lens wear during the study or contact lens wear in the month before enrollment 9. Any scheduled or planned ocular or systemic surgery or procedure during the study 10. Presence of severe dry eye at baseline, defined as (1) OSDI score > 65, and/or (2) corneal or conjunctival fluorescein staining of Grade 5 (Oxford scheme) in any zone of either eye 11. Start date of any systemic medication (including over‐the‐counter, herbal, prescription, or nutritional supplements) that may affect tear film or vision; less than 3 months before enrollment; or change in dosage anticipated during the study 12. Presence of any of active ocular infection or non‐keratoconjunctivitis sicca ocular inflammation, active ocular allergy, history of recurrent herpes keratitis, or active disease within 6 months of baseline; a corneal disorder or abnormality that affects corneal sensitivity or normal spreading of the tear film (except superficial punctate keratitis); severe blepharitis that in the judgement of the investigator may interfere with interpretation of study results 13. Occlusion of the lacrimal puncta with punctal plugs or cauterization in the 3 months before enrollment 14. History of ocular surgery/trauma that could affect corneal sensitivity and/or tear distribution within 6 months of enrollment 15. Use of any of the following topical medications in the 3 months before baseline: corticosteroids, non‐steroidal anti‐inflammatories, or cyclosporine 16. A medical or ocular condition or a personal situation that in the principal investigator's opinion may put the patient at significant risk, may confound study results, or may interfere significantly with participation in the study 17. Cultural or religious beliefs that exclude the consumption of certain or all animal products Equivalence of baseline characteristics? (Y/N): N (krill oil group had a significantly shorter NaFl TBUT at baseline compared with placebo and fish oil groups [P = 0.02], but this was not a primary outcome measure) |
|
| Interventions |
Intervention #1 (treatment group #1): fish oil once daily (daily dose of 1000 mg EPA and 500 mg DHA) Intervention #2 (treatment group #2): krill oil once daily (daily dose of 945 mg EPA and 510 mg DHA) Intervention #3 (control group): 1500 mg of olive oil once daily Length of follow‐up: 90 days Notes: participants using topical lubricant eye drops at baseline (day 1) were allowed to continue to use these throughout the study. At each study visit, the investigator questioned participants with regard to how frequently they had used lubricant eye drops over the past month. There was no significant change in the frequency of eye drop utilization, compared with baseline, in any of the intervention arms over the study duration (data not shown) Participants were instructed to maintain their current dietary habits. Approximate dietary intake of omega‐3 essential fatty acids was determined by asking participants about their consumption of omega‐3‐rich foods over the preceding month. Participants were asked to quantify the approximate serving size (25, 50, 100, 150 g) and frequency of consumption of foods (including fish, oils, nuts, seeds, and spreads) containing greater than 1000 mg of combined EPA, DHA, docosapentaenoic acid, and alpha‐linolenic acid per 100 g edible portion (Australia New Zealand Food Authority, 2011; Nutrient Data Laboratory and Beltsville Human Nutrition Research Centre, 2011). At subsequent visits, participants were questioned about changes in their diet or medications and about compliance with taking the study supplements |
|
| Outcomes |
Primary outcome(s): change from baseline in tear osmolarity (mOsmol/L) and OSDI score Secondary outcome(s): change from baseline in tear film stability (measured using fluorescein TBUT and non‐invasive TBUT); bulbar and limbal redness; ocular surface staining; tear production (Schirmer test with anesthesia); tear volume; anterior blepharitis; meibomian gland capping; basal tear levels of inflammatory cytokines Safety outcomes: change from baseline in best‐corrected visual acuity and intraocular pressure; and monitoring for adverse events Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, 30, 60, and 90 days Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: October 29, 2014, to August 18, 2015 Funding source(s): "Rebecca L. Cooper Medical Foundation, Sydney, New South Wales, Australia (2015) and a University of Melbourne Early Career Researcher grant, Parkville, Victoria, Australia (2015)" Conflicts of interest: "L.E.D.: Grant from Rebecca L. Cooper Medical Foundation, Sydney, New South Wales, Australia (2015) and a University of Melbourne Early Career Researcher grant, Parkville, Victoria, Australia (2015). The sponsors had no role in the design or conduct of this research" Publication language: English Registered on clinical trials registry? (Y/N): Y ‐ ANZCTR (ACTRN12614001019695) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent data manager generated a participant randomization sequence using a random number generator in Microsoft Excel (2007; Microsoft Corporation, Redmond, WA)" |
| Allocation concealment (selection bias) | Low risk | "This randomization schedule was provided to an independent compounding pharmacist (Dartnell’s Pharmacy, Victoria, Australia), who repackaged the study supplements into identical, opaque containers using the randomization schedule. Supplement containers were labeled with the appropriate participant randomization code (from 001 to 060). Eligible participants were sequentially enrolled by a masked research optometrist (L.A.D.), who dispensed the investigational product labeled with the appropriate code" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants were masked to treatment allocation, as achieved by all investigational products being dispensed in identical opaque containers." ; "All study personnel, including the principal investigator (L.E.D.), clinical outcome assessor (L.A.D.), laboratory outcome assessors (C.Y.W., D.C.J.), and co‐investigators (H.R.C., A.J.V.), were masked to participant allocation. Following completion of all participant visits, data were analyzed only with knowledge of the simple randomization code (i.e., group A, B, or C allocation). Full unmasking of treatment allocation by the independent data manager only occurred after statistical analyses were complete" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All study personnel, including the principal investigator (L.E.D.), clinical outcome assessor (L.A.D.), laboratory outcome assessors (C.Y.W., D.C.J.), and co‐investigators (H.R.C., A.J.V.), were masked to participant allocation. Following completion of all participant visits, data were analyzed only with knowledge of the simple randomization code (i.e., group A, B, or C allocation). Full unmasking of treatment allocation by the independent data manager only occurred after statistical analyses were complete" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 out of 60 (10%) participants who were randomized were not included in the final analysis |
| Selective reporting (reporting bias) | Low risk | Reported outcomes are consistent with prespecified outcomes in the clinical trial registry |
| Other bias | Low risk | No other apparent sources of bias |
Epitropoulos 2016.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): multi‐center (number of sites not clearly specified) Number randomized (total and per group): 122 participants in total; 61 participants in the treatment group; 31 participants in the placebo group Unit of randomization (individual or eye): individual Exclusions after randomization: none Losses to follow‐up: 17 participants in total; 7 participants in the treatment group; 10 participants in the placebo group Unit of analysis (individual or eye): individual (eye with higher value in tear osmolarity was used for analysis but was not reported for other outcomes) Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: United States Age (mean ± SD, range): 57.0 ± 16.8 (range 21 to 85) years in the treatment group; 56.5 ± 17.3 (range 22 to 86) years in the control group Gender: 16 men and 38 women in the treatment group; 14 men and 37 women in the control group Inclusion criteria: 1. Over 18 years of age 2. Diagnosis of dry eye disease 3. Meibomian gland dysfunction (MGD) stage 1 or 2 4. Tear osmolarity of 312 mOsmol/L or greater in at least 1 eye using the TearLab Osmolarity System Exclusion criteria: 1. Using topical cyclosporine 0.05%, corticosteroids, non‐steroidal anti‐inflammatory drugs, glaucoma medications, or oral omega‐3 fatty acids within 3 weeks of screening, and anytime during participation in the study 2. Underwent LASIK or PRK surgery within 1 year of screening visit 3. Currently using a systemic medication that might affect the ocular surface Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (treatment group): 4 capsules per day (daily dose of 1680 mg EPA and 560 mg DHA) Intervention #2 (control group): 3136 mg/d linoleic acid (omega‐6) safflower oil Length of follow‐up: 12 weeks Notes: computer use and artificial tears were allowed during the study. Participants were instructed to discontinue contact lenses within 12 hours of any study visit |
|
| Outcomes |
Primary outcome(s): change from baseline in tear osmolarity Secondary outcome(s): change from baseline in each of TBUT; symptoms of dry eye; omega‐3 index; corneal fluorescein staining; MGD stage; Schirmer test with anesthesia; MMP‐9 (percentage of participants with a positive result on the InflammaDry test) Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, 6 and 12 weeks Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): "A. T. Epitropoulos, E. D. Donnenfeld, Z. A. Shah, E. J. Holland, M. Gross, W. J. Faulkner, C. Matossian, S. S. Lane, M. Toyos, and F. A. Bucci Jr received compensation from PRN Physician Recommended Nutraceuticals for participating in the study. The remaining author has no funding or conflicts of interest to disclose" Conflicts of interest: "A. T. Epitropoulos, E. D. Donnenfeld, Z. A. Shah, E. J. Holland, M. Gross, W. J. Faulkner, C. Matossian, S. S. Lane, M. Toyos, and F. A. Bucci Jr received compensation from PRN Physician Recommended Nutraceuticals for participating in the study. The remaining author has no funding or conflicts of interest to disclose" Publication language: English Registered on clinical trials registry? (Y/N): Y ‐ Although not referenced in the paper, this report appears to relate to the clinical trial registry entry: clinicaltrials.gov (NCT02260960) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned using a random number‐generated sequence to ingest 4 softgels daily with meals containing a total of either 1680 mg of EPA/560 mg of DHA re‐esterified omega‐3 group or 3136 mg linoleic acid safflower oil as the control group for 12 weeks" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both active and control softgels seemed identical and were supplied in identical containers for masking purposes" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17/122 (13.9%) participants who were randomized were not included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | Although not referenced in the paper, this study appears to relate to the clinical trial registry entry: https://clinicaltrials.gov/ct2/show/NCT02260960, which lists only "change in tear osmolarity" as the primary outcome measure but does not list any secondary outcome measures |
| Other bias | High risk | Study was funded by industry (Physician Recommended Nutraceuticals) |
Gilbard 2008.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported if single‐ or multi‐center Number randomized (total and per group): 61 participants Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: not reported Age (mean ± SD, range): not reported Gender: not reported Inclusion criteria: 1. Sjögren’s syndrome Exclusion criteria: not reported Equivalence of baseline characteristics? (Y/N): N |
|
| Interventions |
Intervention #1 (treatment group): omega‐3 supplement (TheraTears Nutrition), containing fish oil 1600 mg (with EPA 450 mg and DHA 300 mg) and flaxseed oil 1000 mg Intervention #2 (control group): placebo ‐ wheat germ oil (dose not reported) Length of follow‐up: not reported Notes: none |
|
| Outcomes | Primary and secondary outcome measures were not clearly distinguished Outcomes specified: percentage change from baseline in symptoms of dry eye; symptoms of dry mouth; unstimulated and stimulated salivary flow Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): not reported Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): not reported Conflicts of interest: not reported Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants and personnel was not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers randomized to each group, lost to follow‐up, and excluded were not reported |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | Unclear risk | This study was published in abstract form only, and further publication was not identified |
Goyal 2017.
| Methods |
Study design: randomized, controlled trial Study site(s): single center Number randomized (total and per group): 60 participants in total; 30 participants in each of the 2 intervention arms Unit of randomization (individual or eye): individual Exclusions after randomization: not reported Losses to follow‐up: not reported Unit of analysis (individual or eye): eye Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: India Age (mean ± SE, range): 23.6 ± 2.4 (range 20 to 32) years in the treatment group; 23.6 ± 3.4 (range 20 to 34) years in the control group Gender: 27 men and 33 women in total Inclusion criteria: 1. Participants attending the Cornea and Refractive Services of the Advanced Eye Centre, Post Graduate Institute of Medical Education and Research, Chandigarh, India, undergoing LASIK 2. Aged 20 years or greater 3. Stable refractive error for the last 1 year 4. Maximum manifest refraction spherical equivalent of 26.00 diopters (D) Exclusion criteria: 1. Presence of any inflammatory pathology of the lid margin/tarsal conjunctiva, which could be expected to adversely affect the tear film, that is, significant allergic conjunctivitis (symptoms of itching in the presence of papillae ≥ 0.3 mm over the upper tarsal conjunctiva), anterior blepharitis (hyperemia of lid margins, crusting around the base of lashes), or MGD (meibum quality total score ≥ 4, meibum expressibility score > 1, presence of lid margin signs, ie, meibomian gland dropout or displacement) 2. Lacrimal drainage abnormalities, for example, punctal stenosis or nasolacrimal duct obstruction 3. History of lacrimal gland pathology, such as dacryoadenitis or lacrimal gland surgery 4. Presence of structural or functional lid anomalies including lid laxity; lid laxity was assessed on a scale of 0 to 4 (0 = normal laxity, 4 = severe laxity), using the “snap back test” by pulling the lower lid away and down from the globe for several seconds and waiting to see the time taken before it returned to the original position without the patient blinking. Any patients having greater than grade 0 laxity (ie, normal lid springing back to its original position immediately) were excluded 5. Presence of pre‐existing dry eye due to any cause, defined for the purpose of the study as Schirmer test I readings without anesthesia < 10 mm/5 min and an ocular surface disease index (OSDI) questionnaire score ≥ 13 6. Current use of systemic corticosteroid or immunosuppressive therapy 7. Patients taking antidepressants, antihistamines, or anticoagulants 8. Presence of autoimmune diseases, collagen vascular diseases, diabetes mellitus 9. Pregnant, nursing, or lactating women 10. Patients having malabsorption syndromes 11. Allergy to fish oils Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (treatment group): oral capsule containing 180 mg EPA and 120 mg DHA, 2 capsules/time, twice daily (daily dose of 720 mg EPA and 480 mg DHA) Intervention #2 (control group): soft gel capsules of vitamin E (daily dose of 400 mg/d) Length of follow‐up: 13 weeks (1 week before LASIK, and 12 weeks post LASIK) Notes: contact lens use, if any, was stopped at least 2 weeks before the patient was recruited for the study |
|
| Outcomes |
Primary outcome(s): mean change from baseline in both of Schirmer test without anesthesia and TBUT Secondary outcome(s): corneal fluorescein staining and conjunctival lissamine green staining at study endpoint (measured as percentage of participants with abnormal ocular surface staining, defined as a score > 3 (of a total of 15 for corneal staining and of a total of 18 for conjunctival staining); change from baseline in symptoms of dry eye (OSDI score) Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline (presurgery); 1 week, 1 month, and 3 months post surgery Other issues with outcome assessment (eg, quality control for outcomes if any): this study has a unit of analysis issue, whereby both eyes from participants were included in the analyses without apparent statistical adjustment for within‐person (between‐eye) correlation |
|
| Notes |
Study dates: recruitment occurred between July 2014 and September 2015 Funding source(s): "the authors have no funding or conflicts of interest to disclose" Conflicts of interest: "the authors have no funding or conflicts of interest to disclose" Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sixty consecutive patients who were considered fit for the procedure were allocated either to a treatment group or a control group using a random number table, after obtaining written signed consent" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an "open‐label" study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "All examinations were performed by a single observer who was not masked to the intervention received by the patients" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data are not reported |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | High risk | Unit of analysis error: both eyes of a single participant were included in the analysis separately, without statistical adjustment |
Kangari 2013.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single center Number randomized (total and per group): 146 eyes of 73 participants; 72 eyes of 38 participants in the treatment group; 70 eyes of 35 participants in the control group Unit of randomization (individual or eye): individual Exclusions after randomization: 3 participants discontinued due to digestion problems, and 1 participant discontinued due to the advice of another doctor in the treatment group Losses to follow‐up: 1 participant in the treatment group; 4 participants in the control group Number analyzed (total and per group): 64 participants in total; 33 participants in the treatment group; 31 participants in the control group Unit of analysis (individual or eye): individual (average of both eyes) Reported power calculation? (Y/N): Y (80% power) Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics: Country: Iran Age (mean ± SD, range): 60.6 ± 8.7 years in the treatment group; 61.8± 8.0 years in the control group Gender: 15 men and 18 women in the treatment group; 11 men and 20 women in the control group Inclusion criteria: 1. Aged 45 to 90 years 2. TBUT < 10 seconds in both eyes 3. No use of artificial tears for the past 3 months Exclusion criteria: 1. Active allergy or infection at the ocular surface 2. Presence of pterygium or pinguecula 3. Treatment with ocular topical steroidal or non‐steroidal anti‐inflammatory treatment, glaucoma medication, or antiallergy eye drop in the past month 4. Positive history of refractive surgery or contact lens wear 5. Use of a systemic medication that may interfere with tear production, such as antianxiety, antidepressive, antihypertensive, and antihistamine medications 6. Positive history of blood or coagulation disorders 7. Positive history of gastric ulcers 8. Positive history of surgery in the past 3 months 9. Undergoing head and neck radiotherapy 10. Use of omega‐3 supplements in the past 3 months 11. Positive history of allergy to fish oil or gelatinous capsules Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (treatment group): oral capsule containing eicosapentaenoic acid 180 mg and docosahexaenoic acid 120 mg, twice daily (daily dose of eicosapentaenoic acid 360 mg and docosahexaenoic acid 240 mg) Intervention #2 (control group): placebo capsule containing medium‐chain triglyceride oil (Zahravi Pharmaceutical Company), 2 × 1 g capsules per day, twice daily Length of follow‐up: 1 month Notes: none |
|
| Outcomes |
Primary outcome(s): "increase from baseline" in TBUT; however both outcomes appear to be reported as endpoint data at the end of the follow‐up period Secondary outcome(s): "decrease from baseline" in OSDI score; "increase from baseline" in Schirmer test without anesthetic; however both outcomes appear to be reported as endpoint data at the end of the follow‐up period Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, 1 month Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): "this project is funded by the Vice Chancellor for Research of Shahid Beheshti University of Medical Sciences" Conflicts of interest: "the author(s) have no proprietary or commercial interest in any materials discussed in this article" Publication language: English Registered on clinical trials registry? (Y/N): Y ‐ clinical trial registry (IRCT201012265467N1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The method of randomization in this study was blocked randomization. Thirteen blocks of 6 were determined" However the method used to generate the random sequence is not reported |
| Allocation concealment (selection bias) | Low risk | "To avoid information bias, omega‐3 allocation was performed in a double‐blind fashion; the treatment group, the control group, and the examiners who performed the tests were all unaware of the allocation status. Patients were coded by a third person who applied the randomization protocol and provided them with the allocated type of capsules" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To avoid information bias, omega‐3 allocation was performed in a double‐blind fashion; the treatment group, the control group, and the examiners who performed the tests were all unaware of the allocation status. Patients were coded by a third person who applied the randomization protocol and provided them with the allocated type of capsules" "The placebo capsules were prepared by the Zahravi Pharmaceutical Company (Tehran, Iran) and appeared exactly like the omega‐3 capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "To avoid information bias, omega‐3 allocation was performed in a double‐blind fashion; the treatment group, the control group, and the examiners who performed the tests were all unaware of the allocation status. Patients were coded by a third person who applied the randomization protocol and provided them with the allocated type of capsules" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "A total of 9 subjects (5 in the treatment group, 4 in the control group) failed to complete the trial; in the treatment group, 3 subjects stopped the medication because of digestion problems, 1 subject stopped the medication because of the advice of another doctor, and the rest of the subjects were lost to follow‐up" 9/73 (12.3%) participants who were randomized were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes on the trial registry were reported in the paper |
| Other bias | Low risk | No other apparent sources of bias |
Kawakita 2013.
| Methods |
Study design: randomized, controlled trial Study site(s): not reported if single‐ or multi‐center Number randomized (total and per group): 27 participants Unit of randomization (individual or eye): individual Exclusions after randomization: 1 participant discontinued for a reason other than the present study Losses to follow‐up: none Number analyzed (total and per group): 26 participants in total; 30 eyes of 15 participants in the treatment group; 22 eyes of 11 participants in the control group Unit of analysis (individual or eye): individual (subjective symptoms); eye (TBUT; Schirmer test; fluorescein staining; rose bengal staining) Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics: Country: Japan Age (mean ± SD, range): 52.5 ± 2.5 years in the treatment group; 51.9 ± 2.2 years in the control group Gender: 5 men and 10 women in the treatment group; 1 man and 10 women in the control group Inclusion criteria: 1. Dry eye defined as (1) presence of symptoms of dry eye, (2) abnormality of tear production as determined by the Schirmer test (< 5 mm/5 min) or presence of tear film instability as determined by TBUT (< 5 s), and (3) positive ocular surface rose bengal score (> 3 points) or fluorescein vital staining (> 3 points) Exclusion criteria: 1. Severe disorder of the ocular surface such as Stevens‐Johnson syndrome or ocular pemphigoid 2. Systemic illness such as diabetes, hypertension, or autoimmune disease 3. Taking supplements containing eicosapentaenoic acid or docosahexaenoic acid 4. Hemophilia, gastrointestinal ulceration, urinary tract hemorrhage, hemoptysis, vitreous hemorrhage, or hemorrhagic tendency 5. Treated by an ophthalmologic surgery within the previous 6 months 6. Treated by punctal plug within the previous 1 month 7. Wearing contact lenses Equivalence of baseline characteristics? (Y/N): not reported |
|
| Interventions |
Intervention #1 (treatment group): oral soft gel capsule containing eicosapentaenoic acid 83 mg and docosahexaenoic acid 36 mg (Nippon Suisan Kaisha, Ltd.), 3 times daily, 5 capsules at a time (daily dose of eicosapentaenoic acid 1245 mg and docosahexaenoic acid 540 mg) Intervention #2 (control group): placebo capsules containing medium‐chain triglycerides (dose not reported), 3 times daily Length of follow‐up: 12 weeks, and 4 weeks additional after suspension of treatment Notes: taking of supplements other than experimental supplements was prohibited; participants were instructed to maintain their normal food and exercise habits; all usual dry eye treatments such as eye drops, but not supplementation, were maintained during this experiment in all participants |
|
| Outcomes |
Primary outcome(s): objective and subjective symptoms by the visual analogue scale (VAS) test and TBUT at the study endpoint; change from baseline in fluorescein staining and rose bengal staining Other outcome(s): Schirmer I test without anesthesia at study endpoint Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, 12 weeks of treatment, 4 weeks after suspension of treatment Other issues with outcome assessment (eg, quality control for outcomes if any): this study has a unit of analysis issue, whereby both eyes from participants were included in the analyses of ocular outcomes, without apparent statistical adjustment for within‐person (between‐eye) correlation |
|
| Notes |
Study dates: not reported Funding source(s): not reported Conflicts of interest: 2 authors were affiliated with the company that produced the fish oil products in the study Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "All eligible subjects were randomly allocated to the fish oil or placebo group by using a random number table" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind" study, but details of masking were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind" study, but details of masking were not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "One subject dropped out because of the reason other than the present experiment, and final data were calculated from 26 subjects" It is not clear which intervention arm the participant was assigned to |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | High risk | 2 authors were affiliated with the fishing company that produced the fish oil products in the study. In addition, the unit of randomization was the individual participant, but each eye of a single participant was separately included in the analysis for the Schirmer test and TBUT, without taking into account non‐independence (ie, unit of analysis error) |
Kawashima 2016.
| Methods |
Study design: randomized, controlled trial Study site(s): single center Number randomized (total and per group): 40 participants in total; 20 participants in each intervention arm Unit of randomization (individual or eye): individual Exclusions after randomization: 1 participant in the omega‐3 treatment group Losses to follow‐up: not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): Y (80% power) Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: Japan Age (mean ± SD, range): 41.75 ± 9.39 (range 22 to 57) years in the omega‐3 treatment group; 42.95 ± 6.74 (range 33 to 59) years in the control group Gender: 10 men and 10 women in the omega‐3 treatment group; 11 men and 9 women in the control group Inclusion criterion: 1. Between 20 and 60 years of age who reported subjective symptoms of dry eye Exclusion criteria: 1. Current or previous severe ocular disease(s) such as strabismus, cataract, or glaucoma 2. Risk of developing seasonal allergy between July and September 3. LASIK operation within the previous 3 months 4. Allergy to the test supplement 5. Routinely used other supplements that contained the same ingredients as those of the combined dietary supplement 6. Currently taking medicine to improve vision 7. Receiving medical treatment 8. Receiving long‐term medical treatment for their ocular condition 9. History of any other serious disease requiring medical treatment 10. Participation in another clinical trial within 1 month before the start of the present study 11. Pregnancy or lactation during the study period 12. Presence of any health disorders based on the questionnaire results Equivalence of baseline characteristics? (Y/N): N (Schirmer test was significantly higher in the placebo group compared with the treatment group; P = 0.039) |
|
| Interventions |
Intervention #1 (treatment group): oral capsule containing 40.5 mg eicosapentaenoic acid and 27 mg docosahexaenoic acid, 2 capsules/time, once daily (daily dose of 81 mg eicosapentaenoic acid and 108 mg docosahexaenoic acid) Intervention #2 (control group): "vehicle" capsules (dose not reported) Length of follow‐up: 8 weeks Notes: none |
|
| Outcomes | Primary and secondary outcome measures were not clearly distinguished Specified outcome(s): symptoms of dry eye; TBUT; Schirmer test without anesthesia; fluorescein staining of the cornea and conjunctiva; serum biochemical analysis, each measured at study endpoint Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, 4 and 8 weeks Other issues with outcome assessment (eg, quality control for outcomes, if any): participant population included individuals who did not have dry eye disease |
|
| Notes |
Study dates: July 2014 to September 2014 Funding source(s): "this work was supported by Wakamoto Pharmaceutical Co. Ltd. (funding support, supplement capsules, and placebo capsules)… The funding organization had no role in the design or conduct of this research" Conflicts of interest: not reported Publication language: English Registered on clinical trials registry? (Y/N): Y ‐ clinical trial registry (UMIN000014447) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind" study "Both capsule types were packaged in an opaque bag for blinding" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "One subject in the supplementation group was excluded from the efficacy analysis because of a lack of data" |
| Selective reporting (reporting bias) | High risk | The clinical trial registry entry (UMIN000014447) lists "dry eye symptoms" as the only outcome measure, and it is specified as the "primary outcome," without mention of the other reported outcomes (TBUT, Schirmer test, fluorescein staining score, and serum biochemical analysis, all of which are reported in the manuscript) |
| Other bias | High risk | Funded by industry; unit of analysis for ocular outcomes is unclear; participants with diagnosis of "non‐dry‐eye" were included in the analyses |
Kokke 2008.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported if single‐ or multi‐center Number randomized (total and per group): 76 participants; 38 participants randomized to each intervention group (personal communication) Unit of randomization (individual or eye): individual Exclusions after randomization: 26 participants discontinued Losses to follow‐up: none Number analyzed (total and per group): 52 participants in total; 28 participants in the fatty acid treatment group; 24 participants in the control group Unit of analysis (individual or eye): individual (average of both eyes) (personal communication) Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: United Kingdom Age (mean ± SD, range): 46.4 ± 12.6 years in the fatty acid treatment group; 37.3± 10.7 years in the control group Gender: 76 women Inclusion criteria: 1. Contact lens wearer 2. McMonnies dry eye history questionnaire indicating that they were suffering from dry eye or borderline dry eye (McMonnies score ≥ 10), or that they were experiencing symptoms of contact lens‐induced dry eye Exclusion criteria: not reported Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (treatment group): oral capsule containing evening primrose oil (linoleic acid about 57 mg and γ‐linolenic acid 50 mg, Equazen UK Ltd.), 6 capsules daily (daily dose of linoleic acid about 342 mg and γ‐linolenic acid 300 mg) Intervention #2 (control group): placebo capsule containing olive oil (Equazen UK Ltd.), 6 capsules daily (dose note reported) Length of follow‐up: 6 months Notes: participants were instructed not to change their diet and not to take any additional dietary supplements for the duration of the study |
|
| Outcomes | Primary and secondary outcome measures were not clearly distinguished Specified outcome(s): symptoms of dry eye and contact lens discomfort, tear meniscus height, corneal and conjunctival fluorescein staining, corneal and conjunctival rose bengal staining, meibomian gland assessment, lipid layer thickness and quality, TBUT (both non‐invasive with the Tearscope and with fluorescein) and ocular hyperemia; all outcomes were reported as data at study endpoint Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, months 3 and 6 Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): treatment and placebo formulations were provided by Equazen Ltd. Conflicts of interest: none Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The capsules were loaded into standard unmarked tablet jars with a four‐digit identifier code. Codes were allocated to one of two treatment groups (1 or 2), using an online random sequence generator" |
| Allocation concealment (selection bias) | Low risk | "Coding, labelling and randomisation were carried out by the supplier and codes were not broken until the analysis was complete" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind" study "The active and placebo capsules were prepared and supplied by the manufacturer (Equazen). They performed the randomisation and retained the allocation until the study and the analysis was complete. They then released the information on the allocation" (personal communication) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐blind" study "The active and placebo capsules were prepared and supplied by the manufacturer (Equazen). They performed the randomisation and retained the allocation until the study and the analysis was complete. They then released the information on the allocation" (personal communication) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Of the 76 contact lens wearers recruited, 52 completed the trial (placebo N = 24, EPO N = 28). The principal reason for dropout was non‐compliance. All subjects, including dropouts, were included in the statistical analysis. Missing data was incorporated using the 'last observation carried forward method'" A total of 26/76 participants (31.6%) discontinued the study |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol nor clinical trials registry |
| Other bias | High risk | Treatment and placebo formulations were provided by a supplier "The manufacturer had no involvement in the study other than supplying the capsules" (personal communication) |
Korb 2015.
| Methods |
Study design: randomized, controlled trial Study site(s): single center Number randomized (total and per group): 26 participants in total; 13 participants in each intervention arm Unit of randomization (individual or eye): individual Exclusions after randomization: none Losses to follow‐up: none Unit of analysis (individual or eye): eye Reported power calculation? (Y/N): Y (˜ 90% power) Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: United States Age (mean ± SD, range): 38.1 ± 19.9 years in the omega‐3 treatment group; 45.4 ± 19.8 years in the control group Gender: 2 men and 11 women in the omega‐3 treatment group; 3 men and 10 women in the control group Inclusion criteria: 1. Aged ≥ 18 years 2. Existing clinical diagnosis of lipid deficient/evaporative dry eye disease based on the following characteristics: having 6 or fewer functioning lower lid meibomian glands (≤ 6 MGYLS) and also symptomatic for dry eye (Standard Patient Evaluation of Eye Dryness [SPEED] questionnaire score ≥ 6 to ≤ 14) 3. Best‐corrected visual acuity (BCVA, Snellen) of 20/40 or better in each eye Exclusion criteria: 1. Ocular or systemic medical conditions that could, in the opinion of the investigator, preclude study participation 2. Ocular or intraocular surgery or serious ocular trauma in either eye ≤ 6 months before screening 3. Intolerance or hypersensitivity to any component of the study medications 4. Epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella disease of the cornea/conjunctiva, or bacterial/fungal or mycobacterial infection/disease of the eye 5. Use of contact lenses ≤ 1 week before screening 6. Concomitant use of topical ocular medications during the study 7. Use of systemic medications that may contribute to dry eye without a stable dosing regimen for ≥ 30 days before screening and throughout the study 8. Women who were pregnant, may have become pregnant, or were breastfeeding at the time of screening for the study Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (omega‐3 treatment group): lid hygiene with hypoallergenic eyelid cleansing wipes (Systane Lid Wipes; Alcon Laboratories, Inc., Fort Worth, TX, USA) once daily; instillation of 1 drop of lipid emulsion eye drops formulated to restore lipid, aqueous, and mucin components of the tear film (Systane Balance; Alcon) 4 times daily; and 2 oral vitamin supplements containing 1000 mg of omega‐3 fatty acids (Systane Vitamin Omega‐3 Healthy Tears; Alcon), daily for 3 months (daily dose of 2000 mg of omega‐3 fatty acids Intervention #2 (control group): a warm wet microfiber compress (from Terry World Textiles, LLC, Santa Monica, CA, USA) to both eyelids for 8 minutes once daily, for 3 months Length of follow‐up: 3 months Notes: participants were required to discontinue all other meibomian gland dysfunction management before screening and throughout the study |
|
| Outcomes |
Primary outcome(s): meibomian gland functionality, assessed by standardized diagnostic meibomian gland expression to determine the number of functional meibomian glands, at study endpoint Secondary outcome(s): OSDI and SPEED questionnaires at study endpoint Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, 1, 2, and 3 months Other issues with outcome assessment (eg, quality control for outcomes, if any): this study has a unit of analysis issue, whereby both eyes from participants were included in the analyses of ocular outcomes, without apparent statistical adjustment for within‐person (between‐eye) correlation. |
|
| Notes |
Study dates: not reported Funding source(s): "supported by Alcon Research, Ltd, Fort Worth, TX" Conflicts of interest: "D. R. Korb is the inventor or co‐inventor of and has financial interest in commercially marketed products including Systane Balance (Alcon Laboratories, Inc, Fort Worth, TX), Soothe XP, LipiView (TearScience, Morrisville, NC), LipiFlow (TearScience), and the Korb Meibomian Gland Evaluator (TearScience). C. A. Blackie and T. Douglass have financial interest in TearScience. V. M. Finnemore has no funding or conflicts of interest to disclose" Publication language: English Registered on clinical trials registry? (Y/N): Y ‐ clinical trial registry (clinicaltrials.gov ‐ NCT01733745) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an "open‐label" study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Investigator‐masked" study "Meibography was used to assess the percentage of partial meibomian glands. Results were graded by a single trained masked observer using a scale from 1 (no gland drop out) to 4 (>75% gland drop out)" It is unclear whether assessors of the other outcomes were masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes are consistent with those listed in the clinical trial registry (NCT01733745) |
| Other bias | High risk | Funded by industry; unit‐of‐analysis error: "right and left eyes were pooled and treated as independent cases for meibomian gland functionality, itching/ eye rubbing, lid status, and meibography endpoints" |
Macsai 2008.
| Methods |
Study design: randomized controlled trial Study site(s): single center Number randomized (total and per group): 38 participants in total; 18 participants in the flaxseed group; 20 participants in the control group Unit of randomization (individual or eye): individual Exclusions after randomization: 1 participant, because of a new diagnosis of Sjögren's syndrome Losses to follow‐up: 7 patients Unit of analysis (individual or eye): individual (OSDI); eye (other outcomes) Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: United States Age (mean ± SD, range): 46.9 ± 8.6 years in the flaxseed group; 54.5± 9.5 years in the control group Gender: 4 men and 14 women in the flaxseed group; 2 men and 18 women in the control group Inclusion criteria: 1. Over the age of 18 2. Diagnosis of moderate to severe chronic blepharitis and simple obstructive meibomian gland dysfunction and greater than 3 months' duration of ocular symptoms consistent with blepharitis, dry eye, and meibomian gland disease 3. Had not taken oral tetracycline drugs (including doxycycline and minocycline) or oral corticosteroids for at least 3 months and had discontinued all topical medications for at least 1 month before study enrollment Exclusion criteria: 1. Patients on a regular course of aspirin or COX‐2 inhibitors 2. Patients who were on anticoagulant therapies or who had blood disorders 3. Pre‐existing ocular disease or pathology 4. Systemic disease requiring anticoagulation 5. Long‐term use of non‐steroidal anti inflammatory agents or COX‐2 inhibitors 6. Use of dietary fatty acid (FA) supplementation, including omega‐3 or omega‐6 FAs, for 1 month before day 0 Equivalence of baseline characteristics? (Y/N): N (the flaxseed oil group had more visible meibomian gland orifices; participants in the flaxseed group were slightly younger) |
|
| Interventions |
Intervention #1 (flaxseed group): flaxseed oil (1000 mg) 6 capsules daily, equivalent to a daily dose of 3300 mg omega‐3 fatty acids Intervention #2 (control group): placebo (olive oil), 6 capsules daily (dose not reported) Length of follow‐up: 1 year Notes: participants were asked to maintain their normal dietary habits during the course of the study; patients were also counseled to continue their daily eyelash hygiene |
|
| Outcomes |
Primary outcome(s): "change (from baseline) in" TBUT; meibum score; OSDI Secondary outcome(s): "change (from baseline) in" Schirmer test with anesthesia; corneal fluorescein staining; conjunctival rose bengal staining; percentage of gland orifice blockage; grading of gross meibum character; blood fatty acid analysis; meibum lipid content. Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, every 3 month for a total period of 1 year Other issues with outcome assessment (eg, quality control for outcomes, if any): this study has a unit of analysis issue, whereby both eyes from participants were included in the analyses of ocular outcomes, without apparent statistical adjustment for within‐person (between‐eye) correlation |
|
| Notes |
Study dates: not reported Funding source(s): "supported by the Pearl Vision Foundation, Dallas, Texas; an unrestricted grant from Research for the Prevention of Blindness, Inc, to the Department of Ophthalmology, Northwestern University; and private contributions to the Ophthalmology Research Fund, Evanston Northwestern Healthcare, Division of Ophthalmology. The Natrol Corporation provided the flaxseed and olive oil capsules" Conflicts of interest: none Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subject numbers were preassigned to the control or study group with the aid of the random number generator in Microsoft Excel" |
| Allocation concealment (selection bias) | Low risk | "This (randomization) list was not incorporated into any documentation, and only research staff members not involved in patient care had access to these assignments" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This study was reported as a "double‐masked" trial "Subjects were masked to the contents of the oil capsule. Both flaxseed and olive oil have similar properties in appearance and texture" "This list was not incorporated into any documentation, and only research staff members not involved in patient care had access to these assignments" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This study was reported to be a "double‐masked" study, but details of masking of outcome assessors were not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "An intent‐to‐treat analysis has been done by assuming that patients lost to follow up had no change" However, ITT analysis was followed only for some outcomes, not for all analyses |
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available |
| Other bias | High risk | "The Natrol Corporation provided the flaxseed and olive oil capsules" Unit of randomization was the individual participant, but the analysis was performed by using the eye of single participants without taking into account the non‐independence of eyes (unit of analysis error); baseline data were not equivalent between groups |
Manthorpe 1984.
| Methods |
Study design: randomized, cross‐over, controlled trial Study site(s): not reported if single or multi‐center Number randomized (total and per group): 36 participants Unit of randomization (individual or eye): individual Exclusions after randomization: not explicitly reported, but 1 or 2 patients were not included in the analysis according to the graphs in the manuscript Losses to follow‐up: none reported Number analyzed (total and per group): 36 participants in total Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: Denmark Age (mean ± SD, range): median 39 years in men, and 51 years in women, range 34 to 76 Gender: 3 men and 33 women Inclusion criteria: 1. Primary Sjögren’s syndrome based on the Copenhagen criteria 2. Keratoconjunctivitis sicca defined as at least 2 of the following 3 objective tests for each organ proved abnormal; Schirmer test, TBUT, and lissamine green staining by the van Bijsterveld score 3. Xerostomia examined by lip biopsy; unstimulated sialometry values; salivary gland scintigraphy Exclusion criteria: not reported Equivalence of baseline characteristics? (Y/N): not applicable (cross‐over) |
|
| Interventions |
Intervention #1 (active treatment group): oral capsule containing cis‐linoleic acid 365 mg and γ‐linolenic acid 45 mg, Efamol twice daily (3 capsules at a time) plus tablet containing vitamin C 125 mg, pyridoxine 25 mg, niacin 25 mg, and ZnSo4 5 mg(Efavit), twice daily, 3 tablets at a time. Daily dose of linoleic acid 2190 mg and γ‐linolenic acid 270 mg Intervention #2 (control group): "placebo" (composition not reported) 500 mg capsule (dose not reported) and tablets, twice daily, 3 capsules at a time Length of follow‐up: 3 weeks in each phase, with 1‐week washout between phases; 7 weeks in total Notes: participants on NSAIDs were asked to reduce their usual intake, if possible, or to continue at the same dosage as usual. All other medications were kept constant during the trial, with the exception of bromhexine, which was discontinued at least 2 weeks before the start of the investigation |
|
| Outcomes | Primary and secondary outcome measures were not clearly distinguished Specified outcome(s): change from baseline in: Schirmer test; TBUT; van Bijsterveld score; subjective feeling of dryness in mouth and eyes; biscuit‐eating time; amount of snake‐like nuclear chromatin in conjunctival epithelial cells; tear lysozyme concentration; saliva Na+ and K+ concentrations Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, week 3 in each phase Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): not reported Conflicts of interest: not reported Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind" study but details of masking about personnel were not reported "All patients received three capsules of Egamol and three tablets of Efavit twice daily or placebo of identical appearance and number" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind" study, but details of masking about outcome assessors were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "All completed the investigation," but data in the graphs suggest that 1 or 2 participants were not included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | High risk | In this cross‐over design, there was only 1‐week washout period between intervention phases, which is likely inadequate; information regarding funding source and conflict of interest was not reported |
Mohammadpour 2017.
| Methods |
Study design: randomized, controlled trial Study site(s): single‐center Number randomized (total and per group): 63 eyes of 48 participants; 32 eyes in the omega‐3 treatment group; 31 eyes in the control group Unit of randomization (individual or eye): individual Exclusions after randomization: none Losses to follow‐up: 2 eyes in the control group Unit of analysis (individual or eye): eye Reported power calculation? (Y/N): Y (80% power) Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: Iran Age (mean ± SD, range): 59.75 ± 11.57 (range 37 to 82) years in the omega‐3 treatment group; 67.81 ± 9.62 (range 39 to 81) years in the control group Gender: 4 men and 21 women in the omega‐3 treatment group; 6 men and 13 women in the control group Inclusion criterion: 1. New‐onset dry eye symptoms (foreign body sensation, burning, itching, red eye, photophobia, or blurring of vision) with a history of recent cataract surgery via phacoemulsification Exclusion criteria: 1. History of ocular trauma, uveitis, other previous ocular surgeries 2. Contact lens wearing in the previous 2 years 3. Systemic diseases that are associated with dry eye syndrome including diabetes 4. Rheumatoid arthritis and Sjogren's syndrome 5. Existing dry eye symptoms before cataract surgery 6. Considerable change in lifestyle with an impact on ocular surface diseases (eg, computer use) during the postoperative period Equivalence of baseline characteristics? (Y/N): N (omega‐3 treatment group was significantly younger than the control group; P = 0.003) |
|
| Interventions |
Intervention #1 (treatment group): standard therapy plus omega‐3 dietary supplement (1000 mg every 8 hours, Advanced Canada, each capsule containing 180 mg eicosapentaenoic acid and 120 mg docosahexaenoic acid) every 8 hours (daily dose of 510 mg eicosapentaenoic acid and 360 mg docosahexaenoic acid) Intervention #2 (control group): standard therapy alone (artificial tears [every 4 hours] and betamethasone 0.1% eye drops [every 8 hours]) Length of follow‐up: 1 month Notes: standard therapy for dry eye (Artelac artificial tears every 4 hours, Dr. Gerhard Mann Company, Germany; and betamethasone 0.1% eye drops every 8 hours) |
|
| Outcomes | Primary and secondary outcome measures were not clearly distinguished Specified outcome(s): OSDI; TBUT; Schirmer test without anesthesia; tear osmolarity; each outcome was measured at study endpoint Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, 1 month Other issues with outcome assessment (eg, quality control for outcomes, if any): unit of analysis error, whereby 63 eyes of 48 participants were included without taking into account the non‐independence of eyes |
|
| Notes |
Study dates: recruitment occurred between September 2013 and May 2014 Funding source(s): "none of the authors has a financial or proprietary interest in any mentioned product, method, or material" Conflicts of interest: "none of the authors has a financial or proprietary interest in any mentioned product, method, or material" Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated into two groups using urn randomization" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Masking of participants was not performed due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All measurements were done by a single person (M.M), and the physician in charge of collecting outcome data was kept blinded to the allocation" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2/31 (6.5%) eyes in the control group were not included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | High risk | 63 eyes of 48 participants were included without taking into account the non‐independence of eyes (unit of analysis error); baselines were not equivalent for an important prognostic factor (age; P = 0.003) |
NCT01107964.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single center Number randomized (total and per group): 27 participants in total; 13 participants in the omega‐3 treatment group and 14 participants in the placebo group Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: 1 participant in each group Number analyzed (total and per group): 25 participants in total; 12 participants in the omega‐3 treatment group and 13 participants in the placebo group Unit of analysis (individual or eye): individual (worse eye) Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Participant characteristics Country: United States Age (mean ± SD, range): 58 years in total (interquartile range [IQR]: 49.5 to 67.5 years); 54 years [IQR: 44.5 to 65 years] in the omega‐3 treatment group and 60 years [IQR: 55 to 67 years] in the placebo group Gender: 4 men and 23 women in total; 1 man and 12 women in the omega‐3 treatment group, and 3 men and 11 women in the placebo group Inclusion criteria: 1. Age > 18 years 2. Typical symptoms of dry eye (photophobia, burning, foreign body sensation, blurred vision improved with blinking) 3. Schirmer test < 8 mm/5 min 4. Fluorescein tear break‐up time < 8 seconds 5. No current use of dry eye treatment (except artificial lubrication) 6. Signature on consent form Exclusion criteria: 1. Infectious keratoconjunctivitis or inflammatory disease unrelated to dry eye 2. Eyelid or eyelash abnormalities 3. Alteration in the nasolacrimal apparatus 4. Treatment with drugs affecting tearing 5. Concomitant ocular therapies 6. Topical ophthalmic steroids taken during the 4 weeks before the study 7. Pregnant/breast‐feeding women 8. History of liver disease 9. History of fish and/or shellfish allergy or hypersensitivity 10. History of corn allergy or hypersensitivity 11. Treatment with systemic anticoagulation therapy 12. Patients with bleeding disorders and those receiving anticoagulation (eg, warfarin, enoxaparin, dipyridamole, clopidogrel) Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (omega‐3 treatment group): omega‐3‐acid ethyl esters, 1 g capsule, 4 times daily Intervention #2 (control group): placebo (corn oil) capsules, 1 g capsule, 4 times daily Length of follow‐up: 45 days Notes: none |
|
| Outcomes |
Primary outcome(s): change from baseline in OSDI score Secondary outcome(s): change from baseline in each of Schirmer test; lissamine green staining; TBUT Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): not reported Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Recruitment status: completed Last post updated: December 2017 Results first posted: December 2017 Registered on clinical trials registry? (Y/N): Y (clinicaltrials.gov ‐ NCT01107964) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Masking reported to be: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" However method of masking is not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor)" However method of masking is not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Two participants (7.4%) (1 in each group) who were randomized were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes from the clinical trial registry are reported |
| Other bias | Unclear risk | Information obtained was from the clinical trial registry, and further publication was not identified |
Oleñik 2013.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single‐center Number randomized (total and per group): 64 participants in total; 33 participants in the omega‐3 treatment group; 31 participants in the control group Unit of randomization (individual or eye): individual Exclusions after randomization: 2 participants withdrew due to drug intolerance, and 1 participant did not pursue treatment in the omega‐3 treatment group Losses to follow‐up: none Number analyzed (total and per group): 61 participants in total; 30 participants in the omega‐3 treatment group; 31 participants in the control group Unit of analysis (individual or eye): individual (right eye) Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: Spain Age (mean ± SD, range): 58 years in the omega‐3 treatment group; 54 years in the control group Gender: 9 men and 24 women in the omega‐3 treatment group; 9 men and 22 women in the control group Inclusion criteria: 1. 18 to 85 years of age 2. Diagnosis of meibomian gland dysfunction based on criteria identified in the 2011 International Work Shop on meibomian gland dysfunction 3. Able to participate in the study 4. Provision of informed consent Exclusion criteria: 1. Atopy or allergic disorders 2. Contact lenses 3. Ophthalmic laser treatment less than 3 months before enrollment 4. Pregnancy 5. Systemic diseases and general treatments 6. Blepharitis without meibomian gland dysfunction diagnosis 7. Ocular disorders and eye drops other than artificial tears 8. Obvious infection Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (treatment group): oral capsule containing eicosapentaenoic acid 42.5 mg and docosahexaenoic acid 350 mg and 30 mg docosapentaenoic acid (Brudysec 1.5 g, Brudy Lab SL), 3 times daily, 1 capsule at a time. Daily dose of: 127.5 mg eicosapentaenoic acid, 1050 mg docosahexaenoic acid, and 90 mg docosapentaenoic acid Intervention #2 (control group): placebo capsule (500 mg) containing sunflower oil (dose not reported), 3 times daily, 1 capsule at a time Length of follow‐up: 3 months Notes: participants in both groups were instructed to apply a warm compress for 5 minutes and to scrub the eye with diluted baby shampoo and to use artificial tears without preservatives; participants were required to discontinue use of nutritional supplements and related treatments, such as antibiotics, non‐steroidal anti‐inflammatory drugs, corticosteroids, and tears with vitamins, for at least 15 days before the baseline visit; ocular lubricants without nutritional agents were not restricted |
|
| Outcomes |
Primary outcome(s): TBUT; corneal and conjunctival fluorescein staining, both measured as data at study endpoint Secondary outcome(s): Schirmer test without anesthesia; OSDI; meibomian gland expression and secretion; evaluation of lid margin inflammation, all measured as data at study endpoint Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, months 1, 2, and 3 Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: enrollment between March 2012 and December 2012 Funding source(s): "the food supplements used this trial were provided by Brudy Lab SL, Barcelona, Spain" Conflicts of interest: "the authors report no conflict of interest in this work" Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐masked" study "Throughout the randomized study, the investigators and patients were blinded to the treatment assignments" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐masked" study "Throughout the randomized study, the investigators and patients were blinded to the treatment assignments" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/64 (4.7%) participants in the treatment group withdrew (2 due to drug intolerance; 1 did not pursue the treatment), and they were not included in the analysis, even though the paper states that "both intra‐group and inter‐group comparisons were made with an intention‐to‐treat analysis" |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | High risk | Treatment supplements were provided by a supplier |
Oral sea buckthorn oil study 2010.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single‐center Number randomized (total and per group): 100 participants in total; 52 participants in the fatty acid treatment group; 48 participants in the control group Unit of randomization (individual or eye): individual Exclusions after randomization: 4 participants in total; 3 participants in the treatment group; 1 participant in the control group Losses to follow‐up: 10 participants in total; 4 participants in the fatty acid treatment group; 6 participants in the control group Number analyzed (total and per group): 100 participants in total; 52 participants in the fatty acid treatment group; 48 participants in the control group, in the intention‐to‐treat analysis Unit of analysis (individual or eye): eye (TBUT); individual (other outcomes) Reported power calculation? (Y/N): Y (˜ 80% power) Reported subgroup analysis? (Y/N): Y (contact lens status; age) |
|
| Participants |
Participant characteristics Country: Finland Age (mean ± SD, range): 45 ± 18 years in the fatty acid treatment group; 46 ±17 years in the control group Gender: 8 men and 44 women in the fatty acid treatment group; 7 men and 41 women in the control group Inclusion criteria: 1. 20 to 75 years of age 2. Subjective symptoms of dry eye Exclusion criteria: 1. Severe illness 2. Pregnancy or breastfeeding 3. Smoking 4. Regular use of strongly anticholinergic drugs Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (fatty acid treatment): oral capsule containing sea buckthorn oil (Aromtech Ltd.) twice daily, 1 capsule at a time. Daily dose of 2000 mg (comprising long‐chain omega‐3 and omega‐6 PUFAs) Intervention #2 (control treatment): placebo capsule containing medium‐chain fatty acids (dose not reported), twice daily, 1 capsule at a time Length of follow‐up: 3 months of intervention, and 1 to 2 months after suspension of treatment Notes: participants were advised not to use other oil supplements during the trial; linoleic acid 245 ± 34 mg/d and α‐linolenic acid 149 ± 21 mg/d |
|
| Outcomes |
Primary outcome(s): change from baseline in each of tear film osmolarity; TBUT; Schirmer test; modified OSDI Secondary outcome(s): subjective symptoms (as reported in a log book) Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, months 1 and 3, and 1 to 2 months after suspension of treatment Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: enrollment between October and November in 2008 Funding source(s): "supported by the ABS Graduate School, Aromtech Ltd., Finnish Agency for Technology and Innovation, Finnsusp Ltd., Niemi Foundation, Shinyhorse Ltd., TYKS Foundation, and Valioravinto Ltd." Conflicts of interest: 2 authors were affiliated with pharmaceutical firms "R. L. Järvinen is an employee of Finnsusp Ltd. During the trial execution, B. Yang was an employee of Aromtech Ltd." Publication language: English Registered on clinical trials registry? (Y/N): Y ‐ (clinicaltrials.gov ‐ NCT00739713) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Permuted block randomization was used" "A computer‐generated random generator was used" (personal communication) |
| Allocation concealment (selection bias) | Low risk | "Participants were interviewed for the inclusion and exclusion criteria. The included participants were randomized to treatment groups using age, sex, and contact lens wear as stratification factors in the randomization. Randomization was carried out by a statistician not otherwise involved in the study. The study products were given to the study personnel in boxes coded with the id‐numbers of the each participant. The code marking of the capsule boxes was done by a person not involved otherwise in the study and he kept the code in a sealed envelope during the study. The study personnel or the participants did not know the study groups or which participants were in the same groups with each other during the enrollment, or during the study" (personal communication) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both capsules had identical opaque gelatin shells. During the trial, the participants, study personnel, and researchers did not know who was getting the SB capsules. To study the success of blinding, the participants were asked to guess whether they were receiving the SB or placebo capsules at each study visit" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Both capsules had identical opaque gelatin shells. During the trial, the participants, study personnel, and researchers did not know who was getting the SB capsules. To study the success of blinding, the participants were asked to guess whether they were receiving the SB or placebo capsules at each study visit" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7/52 participants (13.5%) and 7/48 participants (14.6%) were excluded or were lost to follow‐up, and the reasons were not reported "The primary data analyses were done including all randomized participants [intention to treat (ITT) participants]. Unless otherwise noted, the presented results concern the ITT participants" |
| Selective reporting (reporting bias) | High risk | Outcomes prespecified in the clinical trial registration information, including tear cytokines and inflammation mediators, were not included in the final report |
| Other bias | High risk | Two authors were affiliated with pharmaceutical firms |
Oxholm 1986.
| Methods |
Study design: randomized, cross‐over, controlled trial Study site(s): not reported if single‐ or multi‐center Number randomized (total and per group): 28 participants Unit of randomization (individual or eye): individual Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): 28 participants in total Unit of analysis (individual or eye): individual Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Participant characteristics Country: not reported Age (mean ± SD, range): 51 years, range 32 to 71 years Gender: 4 men and 24 women Inclusion criteria: 1. Primary Sjögren’s syndrome based on the Copenhagen criteria 2. Keratoconjunctivitis sicca defined as at least 2 of the following 3 objective tests for each organ proved abnormal; Schirmer test values < 10 mm/5 min; TBUT< 10 s; lissamine green staining score > 4 (on a scale of 0 to 9) for each eye 3. Xerostomia examined by histopathologic changes by lip biopsy; unstimulated sialometry values < 1.5 mL saliva/15 min; salivary gland scintigraphy Exclusion criteria: not reported Equivalence of baseline characteristics? (Y/N): not applicable (cross‐over study) |
|
| Interventions |
Intervention #1 (fatty acid treatment group): oral capsule containing evening primrose oil (primarily including cis‐linoleic acid 365 mg and gamma linolenic acid 45 mg, Efamol), 6 capsules daily. Daily dose of linoleic acid 2190 mg and γ‐linolenic acid 270 mg (Efamol) Intervention #2 (control group): "placebo" (composition not reported) 500 mg capsule (dose not reported), 6 capsules daily Length of follow‐up: 8 weeks in each phase; 16 weeks in total Notes: participants were not allowed to take NSAID, bromhexine, or glucocorticosteroids for at least 2 weeks before the study or during the treatment period |
|
| Outcomes | Primary and secondary outcome measures were not clearly distinguished Specified outcome(s): Schirmer I test; TBUT; van Bijisterveld score; tear lysozyme concentration; unstimulated sialometry; fatty acid levels in plasma and erythrocytes Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, months 1 and 2, in each phase Other issues with outcome assessment (eg, quality control for outcomes, if any): cross‐over study design, with any apparent washout between intervention phases |
|
| Notes |
Study dates: not reported Funding source(s): not reported Conflicts of interest: 1 author was affiliated with Efamol Research Institute Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind" study, but details of masking were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind" study, but details of masking were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers of participants who were excluded or lost to follow‐up were not explicitly reported |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | High risk | Cross‐over design, without any apparent washout between phases; 1 author was affiliated with Efamol Research Institute |
Papas 2007.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported if single‐ or multi‐center Number randomized (total and per group): not reported Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): 41 participants in total Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: not reported Age (mean ± SD, range): not reported Gender: not reported Inclusion criterion: 1. Sjögren’s syndrome as defined by the European Criteria and a positive blood rest or lip biopsy Exclusion criteria: not reported Equivalence of baseline characteristics? (Y/N): Y (salivary flow) |
|
| Interventions |
Intervention #1 (treatment group): omega‐3 supplement containing a flaxseed and fish oil blend; dose not reported (TheraTears Nutrition, Advanced Vision Research) Intervention #2 (control group): placebo (germ seed oil); dose not reported Length of follow‐up: 3 months Notes: none |
|
| Outcomes |
Primary outcome(s): symptoms of dry eye; symptoms of dry mouth; unstimulated and stimulated salivary flow, all reported as data at study endpoint Secondary outcome(s): gingival index; plaque index Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): not reported Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): not reported Conflicts of interest: not reported Publication language: English Registered on clinical trials registry? (Y/N): Y ‐ although not referenced in the abstract, this report appears to relate to the clinical trial registry entry: http://www.isrctn.com/ISRCTN10758297 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐masked" study, but details of masking were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐masked" study, but details of masking were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers randomized, lost to follow‐up, and excluded were not reported |
| Selective reporting (reporting bias) | High risk | Although not referenced in the record (abstract), this report appears to relate to the clinical trial registry entry: http://www.isrctn.com/ISRCTN10758297, which lists only "increased oral and ocular comfort at 3 months" as the primary outcome measure, which is different from that reported in the abstract |
| Other bias | Unclear risk | This study was published in abstract form only, and a follow‐up publication was not identified |
Pinazo‐Durán 2013.
| Methods |
Study design: randomized controlled trial Study site(s): single‐center Number randomized (total and per group): 60 eyes of 30 participants with dry eye disease Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Participant characteristics Country: Spain Age (mean ± SD, range): median 52 ± 15 years Gender: 28% men and 72% women Inclusion criteria: 1. Age between 23 and 80 years 2. Diagnosis of mild to moderate dry eye disease Exclusion criteria: 1. Aged < 23 years or > 80 years 2. Atopy, allergic disorders 3. Wearing contact lenses 4. History of refractive surgery 5. Ophthalmic laser treatment less than 3 months before study entry 6. Systemic diseases and general treatments 7. Eyelid anomalies, severe blepharitis or meibomitis, punctual occlusion 8. Ocular disorders and eye drops other than artificial tears 9. Not able to participate in the study Equivalence of baseline characteristics? (Y/N): not reported |
|
| Interventions |
Intervention #1 (treatment group): oral 1.5 g capsule containing eicosapentaenoic acid 42.5 mg and docosahexaenoic acid 350 mg, docosahexaenoic acid 30 mg (Brudysec 1.5 g, Brudy Laboratories), 2 capsules daily. Daily dose of 85 mg eicosapentaenoic acid, 700 mg docosahexaenoic acid, and 60 mg docosapentaenoic acid Intervention #2 (control group): no treatment Length of follow‐up: 3 months Notes: before the baseline visit, participants were required to discontinue use of nutritional supplements, systemic antihistamines, and dry eye (or meibomian gland disorder)‐related treatments such as antibiotics, non‐steroidal and anti‐inflammatory drugs, and corticosteroids, as well as tears with vitamins, for at least 15 days, and participants were asked to strictly follow the recommendations of the ophthalmologists throughout the duration of the study |
|
| Outcomes |
Primary outcome(s): Schirmer test; TBUT, both at study endpoint Secondary outcome(s): OSDI; BCVA; expression of a set of cytokines/chemokines, at study endpoint Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, months 1, 2, and 3 Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: March 2011 and June 2011 Funding source(s): "the present work was partially financed by a research grant from the Junta de Andalucia, Servicio Andaluz de Salud, Sevilla, Spain (IP: Javier Benítez‐del‐Castillo; 2011–2012). Carmen Galbis‐Estrada received a research fellowship from Brudy Laboratories, Barcelona, Spain (2011–2012)" Conflicts of interest: none Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Two homogeneous subgroups (of DEDG and CG participants) were selected according to the oral intake of a supplement prescribed as two capsules a day (+S) or not receiving the oral supplement (−NS)" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Open‐label" study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Open‐label" study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers randomized to each group, lost to follow‐up or excluded, and analyzed were not reported |
| Selective reporting (reporting bias) | High risk | Not all outcomes prespecified in the methods section were reported in the results for each group |
| Other bias | High risk | In this study, patients with dry eye and healthy controls were enrolled and "Two homogeneous subgroups were selected according to the oral intake of a supplement prescribed as two capsules a day (+S) or not receiving the oral supplement (−NS)" How randomization was performed, the unit of randomization, and the unit of analysis were unclear; equivalence at baseline across groups was not reported; one author received a research fellowship from the company that produced the supplements |
Pinheiro 2007.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single‐center Number randomized (total and per group): 38 participants in total; 13 participants in the flaxseed 1 g group; 12 participants in the flaxseed 2 g group; 13 participants in the placebo group Unit of randomization (individual or eye): individual Exclusions after randomization: not reported Losses to follow‐up: not reported Unit of analysis (individual or eye): individual (average of both eyes) Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Participant characteristics Country: Brazil Age (mean ± SD, range): range 21 to 55 years Gender: 38 women Inclusion criteria: 1. TBUT ≤ 8 seconds 2. Schirmer test type ≤ 8 mm/5 min 3. van Bjisterveld with a score of 3 to 7 Exclusion criteria: not reported Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1: oral flaxseed oil capsule 1 g, 1 capsule (Douglas Laboratories, Pittsburgh, PA, USA, imported by "Langfor Import and Distribution" São Paulo, SP, Brazil) plus a placebo capsule (950 mg synthetic mineral oil, 50 mg of evening primrose oil) Intervention #2: oral flaxseed oil capsule 1 g, 2 capsules (2 g in total) Intervention #3 (control): placebo capsule (950 mg synthetic mineral oil, 50 mg evening primrose oil), 2 capsules (2 g) Length of follow‐up: 180 days Notes: none |
|
| Outcomes | Primary and secondary outcome measures not clearly distinguished Specified outcome(s): OSDI; conjunctival impression cytology; TBUT; Schirmer test with anesthesia Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, day 180 Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): not reported Conflicts of interest: not reported Publication language: Portuguese Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind" study; placebo capsule had identical appearance to treatment capsule; details of masking of personnel were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind" study, but details of masking of outcome assessors were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers of participants who were excluded, lost to follow‐up, and included in the final analysis were not reported |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | Unclear risk | Funding source(s) and conflicts of interest were not reported |
Reeder 2006.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported whether single‐ or multi‐center Number randomized (total and per group): 20 participants Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Participant characteristics Country: not reported Age (mean ± SD, range): not reported Gender: not reported Inclusion criteria: not reported Exclusion criteria: not reported Equivalence of baseline characteristics? (Y/N): not reported |
|
| Interventions |
Intervention #1: capsule containing flaxseed oil (dose not reported) Intervention #2: omega‐3 supplement containing flaxseed oil and fish oil (TheraTears Nutrition); dose not reported Length of follow‐up: 60 days Notes: none |
|
| Outcomes | Primary and secondary outcome measures not clearly distinguished Specified outcome(s): symptoms of dry eye Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): not reported Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): not reported Conflicts of interest: not reported Publication language: English Notes: abstract only Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double blind" study, but details of masking were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double blind" study, but details of masking were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers randomized to each group, lost to follow‐up, and excluded were not reported |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | Unclear risk | This study was published in abstract form only, and an associated publication was not identified |
Sheppard 2013.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): multi‐center (2 sites) Number randomized (total and per group): 90 eyes of 45 participants Unit of randomization (individual or eye): individual Exclusions after randomization: 1 participant withdrew because fish consumption exceeded the amount that was allowed; 1 participant enrolled in another study; 1 participant withdrew for health reasons; 1 participant withdrew because a rash developed Losses to follow‐up: 3 participants Number analyzed (total and per group): 38 participants in total; 19 participants in each intervention group Unit of analysis (individual or eye): individual Reported power calculation? (Y/N): Y (80% power) Reported subgroup analysis? (Y/N): N |
|
| Participants |
Participant characteristics Country: United States Age (mean ± SE, range): 62 ± 1 years, range 52 to 70 years in the fatty acid treatment group; 61 ± 2 years, range 44 to 86 years in the control group Gender: 45 women in total Inclusion criteria: moderate to severe dry eye defined as: 1. TBUT ≤ 8 s in at least 1 eye 2. At least grade 1 fluorescein superficial punctate keratitis in at least 1 corneal quadrant or at least grade 1 conjunctival lissamine green staining in at least 1 eye 3. OSDI score ≥ 16 4. Willing to discontinue use of any current dry eye treatment (except Refresh artificial tears) for 4 weeks before randomization and during the course of the 6‐month study 5. Postmenopausal women aged 40 years and older; postmenopause is defined as the absence of menstrual period for at least 1 year, or surgical hysterectomy with bilateral oophorectomy no less than 6 months previously 6. If using transdermal, vaginal, or systemic estrogen, progesterone, or estrogen derivatives, must be on a stable dose for at least 90 days, and must be planning on staying on the same stable dose for the duration of the study 7. Signature on the written informed consent form 8. Patient motivation and willingness to cooperate with the investigator by following the required medication regimen 9. Patient willingness and ability to return for all visits during the study Exclusion criteria: 1. Concurrent involvement in any other clinical trial involving an investigational drug or device 2. Compromised cognitive ability that may be expected to interfere with study compliance 3. Uncontrolled or poorly controlled systemic disease (eg, hypertension, diabetes) or the presence of any significant illness (eg, serious gastrointestinal, renal, hepatic, endocrine, pulmonary, cardiac, neurological disease, cancer, AIDS, or cerebral dysfunction) that could, in the judgement of the investigator, jeopardize subjects’ safety or interfere with interpretation of study results 4. Known hypersensitivity to any diagnostic components of the study or procedural drops or medications 5. Anticipated contact lens wear during the study 6. History of corneal transplant, active ocular infection, uveitis, or non‐KCS inflammation 7. History of recurrent herpes keratitis or active disease within the past 6 months 8. History of cataract surgery within 3 months before enrollment, history of ocular surface surgery (ie, refractive, laser in situ keratomileusis, pterygium) within 6 months before enrollment 9. Corneal disorder or abnormality that affects corneal sensitivity or normal spreading of the tear film except superficial punctate keratitis 10. Use of systemic cyclosporine within the previous 3 months. Initiation, discontinuation, or change in dosage of antihistamines, cholinergic agents, beta‐blocking agents, tricyclic or selective serotonin reuptake inhibitor antidepressants, phenothiazines, or topical or systemic acne rosacea medications in the 2 months before enrollment, or anticipated change in dosage during course of study 11. Topical ophthalmic medications within the previous 4 weeks or anticipated use of the same during the study (except artificial tears) 12. Use of Coumadin or Plavix within the previous 2 weeks or anticipated use of the same during study. Stable dosing of aspirin 325 or 85 mg/d was permitted 13. Use of supplemental fish, borage, evening primrose, flaxseed, or black current seed oils in the past 3 months. Routine, usual dietary intake of more than 12 ounces of cold water fatty fish (tuna, salmon, mackerel, sea bass, sardines, or herring) per week 14. Occlusion of the lacrimal puncta surgically or with temporary collagen punctal plugs within 1 month before the study, or anticipated use of the same during the study Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (fatty acid treatment group): oral capsule containing γ‐linolenic acid and omega‐3 polyunsaturated fatty acids twice daily, 2 capsules at a time. Daily dose linoleic acid 710 mg, γ‐linolenic acid 240 mg, arachidonic acid < 3 mg, EPA 126 mg, DHA 99 mg, and DPA 39 mg Intervention #2 (control group): placebo capsule containing sunflower oil (dose not reported), twice daily, 2 capsules at a time Length of follow‐up: 6 months Notes: participants were provided with Refresh artificial tears (Allergan, Inc., Irvine, CA, USA) to use as needed for the duration of the study. No other topical medications were allowed |
|
| Outcomes | Primary and secondary outcome measures were not clearly distinguished Specified outcome(s): OSDI; Schirmer test; TBUT; corneal fluorescein staining; conjunctival lissamine green staining; topographic corneal smoothness indexes; CD11c staining; HLA‐DR expression; frequency of artificial tear usage; facial expression discomfort scale; surface regularity and surface asymmetry corneal topography smoothness indexes; logMAR visual acuity, all reported at study endpoint Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, months 1, 3, and 6 Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: enrollment between September 2008 and December 2010 Funding source(s): "supported by an unrestricted research grant from ScienceBased Health and the Virginia Eye Foundation" Conflicts of interest: "J. D. Sheppard is a scientific advisor with Alcon (Fort Worth, TX), Allergan, Bausch + Lomb (Rochester, NY), Lux Biosciences (New Jersey), Merck (New Jersey), ScienceBased Health, and Vistakon (Jacksonville, FL). S. C. Pflugfelder is a consultant for Allergan, Alcon, Bausch & Lomb, GlaxoSmithKline, Mimetogen, and ScienceBased Health. He receives research funding from Allergan and GlaxoSmithKline. R. Singh, A. J. McClellan, M. P. Weikert, S. V. Scoper, T. J. Joly, W. O. Whitley, and E. Kakkar have no financial interests related to the current study" Publication language: English Registered on clinical trials registry? (Y/N): Y ‐ clinical trial registry (clinicaltrials.gov ‐ NCT00883649) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were randomized 1:1 using a computer generated randomization procedure"; "Patients were randomized using a permuted‐block randomization design with a block size of 4 for each center" |
| Allocation concealment (selection bias) | Low risk | "An independent statistician, who was also masked to the identity of the subjects until after the analyses were complete, generated these allocation sequences" "Codes linking the randomization number for each subject to the actual treatment were secured in a sealed opaque envelope and were maintained in a locked drawer in each research center" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐masked" study "Investigators, subjects, and staff were masked to the identity of the supplement and placebo" "The placebo was identical in appearance to the test supplement and contained sunflower oil as the main ingredient along with beeswax, lecithin, and calcium carbonate" "All subjects and research staff were masked to the identity of the subject treatment group until the end of the study. Supplements and placebo tablets were packaged uniformly. Codes linking the randomization number for each subject to the actual treatment were secured in a sealed opaque envelope and were maintained in a locked drawer in each research center" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐masked" study "Investigators, subjects, and staff were masked to the identity of the supplement and placebo" "All subjects and research staff were masked to the identity of the subject treatment group until the end of the study. Supplements and placebo tablets were packaged uniformly. Codes linking the randomization number for each subject to the actual treatment were secured in a sealed opaque envelope and were maintained in a locked drawer in each research center" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/45 (15.6%) participants discontinued the study (3 participants were lost to follow‐up; 1 participant due to deviation from the protocol; 1 participant entered another study; 1 participant for health reasons; 1 participant due to an adverse event), and these participants were not included in the analysis |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes in the trial registry were reported |
| Other bias | High risk | One author is a scientific advisor to pharmaceutical firms; one author is a consultant for pharmaceutical firms and receives research funding from a company |
Theander 2002.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single‐center Number randomized (total and per group): 90 participants in total; 30 participants in each intervention group Unit of randomization (individual or eye): individual Exclusions after randomization: 4 participants in the treatment group (1 participant due to a diagnosis of malignancy, 1 participant due to psychosocial problems, 2 participants due to gastrointestinal side effects); 1 participant in the control group (due to gastrointestinal side effects) Losses to follow‐up: none Number analyzed (total and per group): 85 participants in total; 29 participants in the low‐dose treatment group; 27 participants in the high‐dose treatment group; 29 participants in the control group Unit of analysis (individual or eye): individual Reported power calculation? (Y/N): Y (80% power) Reported subgroup analysis? (Y/N): Y (status of Sjögren’s syndrome) |
|
| Participants |
Baseline characteristics Country: Sweden Age (mean ± SD, range): median 62 years, interquartile range 50 to 68 years Gender: 8 men and 79 women Inclusion criterion: 1. Diagnosis of Sjögren’s syndrome according to the Copenhagen criteria or the European criteria Exclusion criteria: 1. Patients with unstable concomitant disease 2. Patients participating in other clinical trials 3. Patients already using commercially available omega‐6 fatty acids Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1: oral γ‐linolenic acid 800 mg daily (Scotia Pharmaceutical Ltd.) Intervention #2: oral γ‐linolenic acid 1600 m daily (Scotia Pharmaceutical Ltd.) Intervention #3 (control group): placebo (corn oil); dose not reported. Length of follow‐up: 6 months Notes: participants were allowed to reduce their analgesic or anti‐inflammatory medication and tear substitutes without being actively encouraged to do so |
|
| Outcomes |
Primary outcome(s): change from baseline in visual analogue scale (VAS) for fatigue, and average time staying in bed trying to rest, sleep, or relax Secondary outcome(s): change from baseline in VAS for dry eye, dry mouth, muscle pain, hand or finger joint pain, and feeling of depression; Schirmer I test; TBUT; lissamine green staining; average number of daily doses of tear substitutes Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, months 3 and 6 Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: between November 1996 and December 1997 Funding source(s): "this study was supported by Scotia Pharmaceuticals Ltd., Guilford, Surrey, UK" Conflicts of interest: not reported Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double‐blind" study, but details of masking were not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double‐blind" study, but details of masking were not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "An intention to treat analysis was not performed due to the small number of excluded patients" 3/90 participants (3.3%) were excluded (1 participant due to a diagnosis of malignancy, 1 participant due to psychosocial problems, 1 participant due to gastrointestinal side effects), and they were not included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | High risk | "Patients were only allowed to continue in the study when taking at least 50% of the prescribed amount of emulsion" "This study was supported by Scotia Pharmaceuticals Ltd., Guilford, Surrey, UK" |
Wojtowicz 2011.
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported whether single‐ or multi‐center Number randomized (total and per group): 36 participants in total; 21 participants in the omega‐3 treatment group; 15 participants in the control group Unit of randomization (individual or eye): individual Exclusions after randomization: none Losses to follow‐up: none Number analyzed (total and per group): 36 participants in total; 21 participants in the omega‐3 treatment group; 15 participants in the control group Unit of analysis (individual or eye): individual (left eye) Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
|
| Participants |
Baseline characteristics Country: not reported Age (mean ± SD, range): 61 years, range 29 to 84 years Gender: 16 men and 20 women Inclusion criteria: 1. Previous clinical diagnosis of dry eye 2. Positive vital dye staining with 1% lissamine green according to the National Eye Institute/Industry Workshop scale (1995) detected by slit‐lamp examination in the absence of concurrent disease including eyelid or ocular surface inflammation beyond 1+ bulbar conjunctival injection Exclusion criteria: 1. Patients with previous ocular surgery, alterations of the lacrimal drainage system, or eyelid abnormalities 2. Patients with any systemic disease that might affect the ocular surface 3. Pregnant or lactating women 4. Patients using ophthalmic medications or systemic medication affecting tear secretion or lipid metabolism or treatment with commercially available essential fatty acids or vitamin supplements Equivalence of baseline characteristics? (Y/N): Y |
|
| Interventions |
Intervention #1 (omega‐3 treatment group): oral capsules containing fish oil 1600 mg and flaxseed oil 1000 mg containing eicosapentaenoic acid (EPA) 450 mg, docosahexaenoic acid (DHA) 300 mg (TheraTears Nutrition, Advanced Vision Research) Intervention #2 (control group): placebo ‐ wheat germ oil (dose not reported) Length of follow‐up: 3 months Notes: preservative‐free TheraTears lubricant eye drops were supplied to standardize participants' topical therapy; all participants were instructed to follow their usual diet and to record changes in a diary |
|
| Outcomes | Primary and secondary outcome measures were not clearly distinguished Specified outcome(s): OSDI; Schirmer test without anesthesia; TBUT; corneal staining with lissamine green; meibomian gland expressibility; meibum appearance; fluorophotometry; aqueous tear evaporation; meibum lipid analysis, all reported at study endpoint Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, month 3 Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
|
| Notes |
Study dates: not reported Funding source(s): "supported in part by grants NIH EY12430 and EY016664; an unrestricted grant from the Research to Prevent Blindness, New York, NY; and Advanced Vision Research" Conflicts of interest: not reported Publication language: English Registered on clinical trials registry? (Y/N): N |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐masked" study "Both active and placebo soft gels appeared identical and were supplied in identical containers for masking purposes" "The patients and study personnel were masked with respect to group assignment to assure accuracy" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐masked" study "The patients and study personnel were masked with respect to group assignment to assure accuracy" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol or clinical trials registry |
| Other bias | Low risk | No other apparent sources of bias |
AUF: arbitrary units of fluorescence; BCVA: best‐corrected visual acuity; CIC: conjunctival impression cytology; COX: cyclo‐oxygenase; DESS: Dry Eye Scoring System; DHA: docosahexaenoic acid; EIA: enzyme immunoassay; EPA: eicosapentaenoic acid; FA: fatty acid; FAS: full analysis set; GLA: gamma linolenic acid; HLA‐DR: human leukocyte antigen‐DR; IC: impression cytology; IOP: intraocular pressure; IQR: interquartile ratio; IRB: institutional review board; ITT: intention‐to‐treat; LA: linoleic acid; LASIK: laser‐assisted in situ keratomileusis; MGD: meibomian gland dysfunction; MMP‐9: matrix metalloproteinase‐9; NSAID: non‐steroidal anti‐inflammatory drug; OSDI: Ocular Surface Disease Index; PGE: prostaglandin E; PPS: per‐protocol set; PRK: photorefractive keratectomy; PUFA: polyunsaturated fatty acid; RBS: rose bengal staining; SD: standard deviation; SPEED: Standard Patient Evaluation of Eye Dryness; TBUT: tear break‐up time; VAS: visual analogue scale; VDT: visual display terminal.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahluwalia 2001 | Wrong intervention |
| Arici 2006 | Wrong intervention |
| Costa 2012 | Wrong intervention |
| Downie 2018b | Wrong participant population |
| Gadaria‐Rathod 2013 | No relevant outcomes |
| Hom 2016 | Not a RCT |
| Jackson 2011 | Wrong intervention |
| Kawabata 2011 | Wrong participant population |
| Kaya 2016 | Not a RCT |
| Kwon 2017 | Not a RCT |
| Macrì 2001 | Wrong participant population |
| Macrì 2002 | Wrong participant population |
| Macrì 2003 | Wrong participant population |
| McMonnies 2019 | Wrong study design (commentary) |
| NCT00803452 | Wrong intervention |
| NCT01059019 | Wrong patient population |
| NCT01213342 | Study terminated (researchers left institution) |
| NCT01364311 | Study was withdrawn |
| NCT01630551 | Withdrawn with no participants enrolled |
| No author listed | Wrong study design (letter to editor) |
| No authors listed | Wrong study design (letter to editor) |
| Ong 2013 | Wrong participant population |
| Pinna 2007 | Wrong participant population (not explicitly defined as 'dry eye') |
| Querques 2007 | Wrong participant population |
| Scuderi 2012 | Wrong intervention |
| Sharma 2006 | Wrong intervention |
| Tellez‐Vazquez 2016 | Not a RCT |
| Wang 2016 | Wrong participant population |
| Yoon 2012 | Wrong intervention |
Characteristics of studies awaiting assessment [ordered by study ID]
Follow‐up reports from the DREAM study.
| Methods |
Study design: randomized, parallel‐group, controlled trial Number randomized (total and per group): 535 participants |
| Participants |
Country: United States of America Age (mean ± SD, range): 58.3 ± 13.5 years in the omega‐3 treatment group; 57.5 ± 12.6 years in the control group Gender: 65 men and 284 women in the omega‐3 treatment group; 36 men and 150 women in the control group Inclusion criteria: 1. Sign and date the informed consent form approved by the IRB 2. ≥ 18 years of age 3. Demonstrate at least 2 of the 4 following signs in the same eye at 2 consecutive visits. The same signs must be present in the same eye on both visits: (screening visit: 7 to 21 days before randomization, and visit 00 [baseline visit]: day of randomization) a. Conjunctival staining present ≥ 1 (out of possible score of 6 per eye) b. Corneal fluorescein staining present ≥ 4 (out of a possible score of 15 per eye) c. Tear film break‐up time (TBUT) ≤ 7 seconds d. Schirmer test ≥ 1 to ≤ 7 mm/5 min 4. Demonstrate symptoms of dry eye disease (OSDI score greater than 22 (≥ 23 to ≤ 80) at screening visit and at least 18 (≥ 18 to ≤ 80) at randomization visit 5. Have dry eye‐related ocular symptoms for at least 6 months before the screening visit and use or desire to use artificial tears on average 2 times per day in the 2 weeks preceding the screening visit 6. Intraocular pressure (IOP) ≥ 5 mmHg and ≤ 22 mmHg in each eye 7. Women of child‐bearing potential must agree to use a reliable method of contraception during study participation and must demonstrate a negative urine pregnancy test at the screening visit 8. Be willing/able to return for all study visits and to follow instructions from the study investigator and his/her staff 9. Be able to swallow large, soft gel capsules 10. Demonstrate compliance with taking soft gels as directed during the run‐in period (≥ 90% taken, by pill count) Exclusion criteria: 1. Allergic to ingredients of active or placebo pills (fish, shellfish, olive oil) 2. Contact lens wearers who are unwilling to discontinue use for 2 weeks before the baseline visit and for the duration of the study 3. Pregnant or nursing/lactating 4. Participation in a study of an investigational drug or device within the 30 days preceding the screening visit 5. Current diagnosis of any of the following ocular conditions: i) Infection (eg, bacterial, viral, protozoan, or fungal infection of the cornea, conjunctiva, lacrimal gland, lacrimal sac, or eyelids) or ii) Inflammation (eg, retinitis, macular inflammation, choroiditis, uveitis, scleritis, episcleritis, keratitis) 6. History of ocular herpetic keratitis 7. Ocular surgery (including cataract surgery) within 6 months of the screening visit 8. Previous LASIK surgery or any other corneal surgery 9. Use of glaucoma medication or history of surgery for glaucoma 10. Eyelid abnormalities that affect lid function (eg, lagophthalmos, blepharospasm, ectropion, entropion, severe trichiasis) 11. Extensive ocular surface scarring or condition that may compromise ocular surface integrity such as Stevens‐Johnson syndrome, prior chemical burn, recurrent corneal erosions, persistent corneal epithelial defects, prior ocular trauma, etc. 12. Dry eye due to seasonal allergic conjunctivitis, or other acute or seasonal diagnosis 13. Current use of EPA/DHA supplements in excess of 1200 mg/d 14. History of liver disease 15. Currently on anticoagulation therapy such as heparin and warfarin. Use of clopidogrel (Plavix) or aspirin does not exclude the patient 16. Patients with hemophilia, thrombocytopenia, or other bleeding tendencies 17. History of atrial fibrillation 18. Uncontrolled ocular or systemic disease 19. Cognitive or psychiatric deficit that precludes informed consent or ability to perform requirements of the investigation |
| Interventions |
Intervention #1: 5 soft gelatin capsules per day (daily dose of 2000 mg EPA and 1000 mg DHA) Intervention #2 (control): 5000 mg per day of refined olive oil Length of follow‐up: 12 months, with an extension of another 12 months |
| Outcomes |
Primary outcome(s): OSDI Secondary outcome(s): conjunctival staining; corneal staining; TBUT; Schirmer test; tear meniscus height Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, 3 months, 6 months, 12 months, and 24 months (extension study only) Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Notes |
Recruitment status: completed Actual completion date: not reported, but completed Last update posted: not reported |
Hom 2017.
| Methods |
Study design: randomized, parallel‐group, controlled trial Number randomized (total and per group): 242 participants |
| Participants |
Country: not reported Age (mean ± SD, range): not reported Gender: not reported Inclusion criteria: dry eye (details not reported) Exclusion criteria: not reported |
| Interventions |
Intervention #1: eye drop containing flaxseed oil and trehalose Intervention #2 (control): marketed lipid‐containing eye drop (Refresh Optive® Advanced, ROA) Length of follow‐up: 90 days Notes: followed 1 ‐week run‐in with a standard aqueous tear (Refresh Plus®) |
| Outcomes |
Primary outcome(s): change from baseline in Ocular Surface Disease Index (OSDI) score Secondary outcome(s): tear break‐up time (TBUT); ocular surface staining, Schirmer test; dry eye symptoms survey Adverse events reported? (Y/N): Y, ocular safety assessment Measurement time points (specify intervals at which outcomes were assessed): 90 days (other time points not reported in the abstract) Other issues with outcome assessment (eg, quality control for outcomes, if any): |
| Notes |
Recruitment status: completed Actual completion date: not reported, but completed Last update posted: not reported |
Laihia 2019.
| Methods |
Study design: randomized, parallel‐group, controlled trial Number randomized (total and per group): 52 participants total (Part 3); 26 each |
| Participants |
Country: Finland Age (mean ± SD, range):53.3 ± 12.6, range 26‐78 years Gender: 16 men and 36 women Inclusion criteria: 18–80 years of age;either (1) OSDI ≥20 and TBUT <10 seconds, or (2) OSDI ≥20 and positive ocular (corneal and conjunctival) staining pattern by Oxfordgrading; body weight ≥45 kg; under stable topical and/or systemic therapy for ≥4 weeks before study procedures and ability to abstain from other therapies during study; and ability and willingness to self‐administer eye drops Exclusion criteria: medical history of ocular surgery, trauma or refractive laser vision correction procedure <3 months earlier; corneal/conjunctival infection; Sjogren’s syndrome; contact lens use during study and <1 week earlier; ocular allergic symptoms; known allergy to eye drop constituents; current or planned pregnancy or nursing during study |
| Interventions |
Intervention #1: sacha inchi microemulsion (SIME; Finnsusp, Lieto, Finland), an eye drop formulation with 0.1% sacha inchi (Plukenetia volubilis) seed oil Intervention #2 (control): 0.2% medium‐molecular‐weight hyaluronic acid in isotonic phosphate saline buffer (Finnsusp) Length of follow‐up: 30 days Notes: none |
| Outcomes |
Primary outcome(s): tear osmolarity; TBUT Secondary outcome(s): blink rate; corneal and conjunctival staining; OSDI Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, 30 days Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Notes |
Recruitment status: completed Actual completion date: not reported, but completed Last update posted: published |
NCT02980224.
| Methods |
Study design: randomized, parallel‐group, controlled trial Number randomized (total and per group): 180 participants (planned) |
| Participants |
Country: United States of America Age (mean ± SD, range): 18 to 90 years (planned) Gender: both (planned) Inclusion criteria: subjects age ≥ 18 years and ≤ 90 years on the date of informed consent; all subjects must provide signed written consent prior to participation in any study related procedures; patient‐reported dry eye symptoms; clinical diagnosis of dry eye disease supported by global clinical assessment; presence of tear osmolarity in at least one eye ≥ 312 mOsm/L at both screening and baseline; presence of meibomian gland dysfunction as defined by a grade of 1 or 2 on the meibomian orifice size scale in at least one eye at both screening and baseline. The qualifying osmolarity level and meibomian orifice size grade must be present in the same eye at both screening and baseline if only one eye qualifies; female subjects of childbearing potential must have a negative urine pregnancy test atScreening. Women of childbearing potential (i.e., women who are not either postmenopausal for one year or surgically sterile) must use an acceptable form of contraception throughout the study. Exclusion criteria: allergy to fish oil or safflower oil (component of placebo softgels) or any component of the softgel material; Schirmer's test score < 5 mm at Screening in either eye; Tear break‐up time > 7 seconds at screening or baseline in either eye; clinically significant eyelid deformity or eyelid movement disorder that is caused by conditions such as notch deformity, incomplete lid closure, entropion, ectropion, hordeolum or chalazion; active seasonal and/or perennial allergic conjunctivitis or rhinitis; previous ocular disease leaving sequelae or requiring current topical eye therapy other than for dry eye disease, including, but not limited to: active corneal or conjunctival infection of the eye and ocular surface scarring; history or presence of abnormal nasolacrimal drainage; laser‐assisted in situ keratomileusis (LASIK) or photorefractive keratectomy (PRK) performed within one year prior to Screening and throughout the study period; ophthalmic drop use within 2 hours prior to any study visit. Any over‐the‐counter (OTC) artificial tear should be continued at the same frequency and with no change in drop brand; contact lens wear within 12 hours prior to any study visit; subjects determined to have worn contact lenses within 12 hours must be rescheduled; punctal cauterization or punctal plug placement within 60 days prior to screening and throughout the study period; started or changed the dose of systemic medications known to affect tear production within 30 days prior to Screening and throughout the study period. These include but are not limited to the following medications: |
| Interventions |
Intervention #1: dose of 2 OmegaD softgels twice daily Intervention #2 (control): placebo twice daily Length of follow‐up: 84 days Notes: none |
| Outcomes |
Primary outcome(s): OSDI; TBUT Secondary outcome(s): not listed Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline and 84 days Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Notes |
Recruitment status: completed Actual completion date: February 2017 Last update posted: September 11, 2019 |
NCT03569202.
| Methods |
Study design: randomized, parallel‐group, controlled group Number randomized (total and per group): 64 participants (planned) |
| Participants |
Country: Finland Age (mean ± SD, range): 18 to 80 years (planned) Gender: both (planned) Inclusion criteria: ability and willingness to give informed written consent prior to any screening procedure after explanation of the nature and possible consequences of the study; age between 18 and 80 years; at least two the following conditions (A and B):A. Symptomatic dry eye with OSDI score ≥20. AND B1. Tear film break‐up time (TBUT) <10 seconds. OR B2. Positive ocular (corneal and conjunctival) staining pattern; body weight at least 45 kg; under stable topical and/or systemic therapy for at least 4 weeks before the study procedures and apparent ability and willingness to abstain from other therapies until completion of the study period; ability and willingness to self‐administer eye drops; ability and willingness to understand and fill in the OSDI questionnaire; ability and willingness to comply with the study protocol and other study‐related procedures. Exclusion criteria: history of ocular surgery, trauma, or refractive laser vision correction procedure less than 3 months earlier; evidence of acute or chronic infection in the cornea or conjunctiva; diagnosis of Sjögren's syndrome; unwillingness or apparent disability to discontinue contact lens use during study period and at least one week before the first dosing day; current ocular allergy symptoms; known allergy to any constituent of the trehalose emulsion eye drops or control eye drops; currently pregnant, nursing or planning to become pregnant before completion of the study period; any other condition that may, in the Investigator's opinion, jeopardize the safety or availability of the subject or adherence to the study protocol or may interfere with the interpretation of the results and would thus make the subject inappropriate for entry in the study. |
| Interventions |
Intervention #1: topical application of preservative‐free multidose Piiloset trehalose emulsion eye drops 3 times daily Intervention #2 (control): topical application of preservative‐free multidose hyaluronic acid eye drops 3 times daily Length of follow‐up: 30 days Notes: none |
| Outcomes |
Primary outcome(s): OSDI; tear osmolarity; TBUT Secondary outcome(s): blink rate; corneal staining; conjunctival staining Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): baseline, 10 and 30 days Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Notes |
Recruitment status: completed Actual completion date: November 8, 2018 Last update posted: November 20, 2018 |
OSDI: Ocular Surface Disease Index
TBUT: tear break‐up time
Characteristics of ongoing studies [ordered by study ID]
ACTRN12610000991011.
| Trial name or title |
Public title: dietary supplements and ocular (eye) comfort Scientific title: a randomized placebo‐controlled double‐masked study to investigate the effects of dietary supplementation with a combination of omega oils on ocular comfort including symptoms of dry eye |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported Number randomized (total and per group): 80 participants (planned) Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: Australia Age (mean ± SD, range): not reported Gender: not reported Inclusion criteria: 1. Willing to comply with the dosage and study visit schedule 2. Contact lens wearers and non‐contact lens wearers 3. At least 18 years old Exclusion criteria: 1. Current consumption of omega oil dietary supplements 2. Moderate or severe dry eye disease 3. Systematic disease that would preclude participants from safely ingesting dietary supplementation with omega oils 4. No S3 or above ocular medications 5. No use of any anticoagulants or blood thinning medications Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: capsules containing eicosapentaenoic acid ˜ 550 mg, docosahexaenoic acid ˜ 120 mg, and liolenic acids per 3 capsules per day Intervention #2 (control): placebo capsules containing paraffin oil, 3 capsules per day Length of follow‐up: 3 months Notes: none |
| Outcomes |
Primary outcome(s): subjective ocular comfort assessed using validated questionnaires (OCI, OSDI, SESOD, numerical rating scales, WHS, DEQ) Secondary outcome(s): non‐invasive TBUT; tear osmometry; phenol red thread test; lipid layer thickness and meibum composition; cell and nerve morphology using impression cytology and confocal microscopy Adverse events reported? (Y/N): not reported Measurement time points (specify intervals at which outcomes were assessed): baseline, 1 and 3 months Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | March 2011 |
| Contact information | Dr. Isabelle Jalbert School of Optometry and Vision Science The University of New South Wales UNSW Sydney NSW, Australia 2052 |
| Notes |
Date of last participant enrollment: March 6, 2012 Date clinical trial registry last updated: November 12, 2013 Recruitment status: completed Other notes: study sponsored by Blackmores Limited |
IRCT201012265467N.
| Trial name or title |
Public title: the impact of omega‐3 on dry eye Scientific title: the impact of omega‐3 fatty acids supplements on dry eye |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported Number randomized (total and per group): 42 participants Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: Iran Age (mean ± SD, range): between 40 and 65 years (planned) Gender: both (planned) Inclusion criteria: subjects with tear break up time of less than 10 seconds Exclusion criteria: subjects with blood coagulation problems and those who take blood thinning medications such as warfarin or Coumadin; subject with the history of stomach ulcer, hemorrhage, or surgery in the past three months; history of allergies to fish oil or gelatinous capsules. Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: omega‐3 capsule twice daily Intervention #2 (control): placebo gelatinous capsule twice daily Length of follow‐up: 1 month Notes: none |
| Outcomes |
Primary outcome(s): OSDI; TBUT; Schimer test Secondary outcome(s): not listed Adverse events reported? (Y/N): not reported Measurement time points (specify intervals at which outcomes were assessed): baseline; 1 month Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | April 2012 |
| Contact information | Dr. Haleh Kangari College of Rehabilitation Corner of Bakhshifard Street, Damavand Ave. , Emam Hossein Square 1616913111, Tehran, Iran (Islamic Republic of) |
| Notes |
Date of last participant enrollment: not reported Date clinical trial registry last updated: February 22, 2018 Recruitment status: completed Actual completion date: not reported, but completed Other notes: study sponsored by Shahid Beheshti University of Medical Sciences |
IRCT2013062413567N4.
| Trial name or title |
Public title: impact of omega‐3 supplement on dry eye treatment Scientific title: clinical trial comparing the effects of omega‐3 supplements and artificial tear drops on dry eye symptoms after surgery, cataract emulsification methods |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single‐center Number randomized (total and per group): 60 eyes (planned) Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Baseline characteristics Country: Iran Age (mean ± SD, range): not reported Gender: not reported Inclusion criteria: 1. Dry eye symptoms following phacoemulsification cataract surgery 2. Index of 30 or more from the Ocular Surface Disease Questionnaire 3. Schirmer test of 10 millimeters or less 4. TBUTof 7 seconds or less 5. Lack of eye trauma 6. Absence of uveitis Exclusion criteria: 1. Historic eye surgeries 2. Using contact lenses 3. Suffering from systemic diseases such as Sjögren's syndrome and diabetic retinopathy that causes dry eye Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: omega‐3 capsules (Vita Natural Fish Oil Capsules) containing eicosapentaenoic acid 180 mg and docosahexaenoic acid 120 mg, 3 times daily Intervention #2 (control): no oral capsule Length of follow‐up: 3 months Notes: participants in both groups were instructed to instil a drop of artificial tears every 6 hours |
| Outcomes |
Primary outcome(s): OSDI; visual acuity; Schirmer test; TBUT Secondary outcome(s): none Adverse events reported? (Y/N): not reported Measurement time points (specify intervals at which outcomes were assessed): not reported Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | First enrollment: August 7, 2013 |
| Contact information | Dr. Mehrdad Mohammadpour Eye Research Center Farabi Hospital Ghazvin Square South Kargar Avenue, Enghelab Square, Tehran, Iran. |
| Notes |
Notes: reported to be "not blinded" Recruitment status: reported to be "complete" on February 22, 2018 (last update) Other notes: study sponsored by Vice Chancellor for research, Tehran University of Medical Science |
IRCT20180806040722N1.
| Trial name or title |
Public title: effect of complementary supplementation Camelina Oil on the improvement of dry eye disease in patients with dry eye Scientific title: effect of complementary supplementation Camelina Oil on the improvement of dry eye disease in patients with dry eye |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported Number randomized (total and per group): 60 participants (planned) Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: Iran Age (mean ± SD, range): between 18 and 65 years (planned) Gender: both (planned) Inclusion criteria: patients with moderate dry eye; ability to take oral capsules; fill in the consent form; ability to visit for examination Exclusion criteria: eye Schirmer test less than 5 mm; TBUT eyes less than 5 seconds; excessive corneal opacification; the presence of corneal ulcer at least in that eye; pregnancy or breastfeeding; corticosteroid use or systemic anti‐inflammatory treatment simultaneously; taking any oral supplement; a clear change in diet during the last month or during treatment; any eye surgery over the past 6 months in the affected eye Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: capsule containing paraffin oil, two grams, twice a day accompanied by breakfast and lunch Intervention #2 (control): capsule containing Comelina oil, 2 grams, twice a day accompanied by breakfast and lunch Length of follow‐up: 3 months Notes: none |
| Outcomes |
Primary outcome(s): Schirmer test; TBUT Secondary outcome(s): not listed Adverse events reported? (Y/N): not reported Measurement time points (specify intervals at which outcomes were assessed): not reported Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | November 2018 |
| Contact information | Mahdieh Reyhani Tabriz University of Medical Sciences Hospital Bina,Tehran, 1786521, Tehran, Iran (Islamic Republic of) |
| Notes |
Date of last participant enrollment : recruitment pending Date clinical trial registry last updated: May 21, 2019 Recruitment status: pending Other notes: study sponsored by Tabriz University of Medical Sciences |
IRCT20181028041487N1.
| Trial name or title |
Public title: evaluation the effect of intra nasal viola odorata almond oil drop on dry eye disease Scientific title: evaluation the effect of intra nasal viola odorata almond oil drop on dry eye disease |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported Number randomized (total and per group): 60 participants (planned) Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: Iran Age (mean ± SD, range): age between 30 to 70 years Gender: both (planned) Inclusion criteria: age between 30 to 70 years; Tear Break Up Time less than 10 seconds at least in one eye; Schirmer test less than 10 millimeter at least in one eye without Local anesthesia; 2 symptoms of feeling eye dryness, foreign body sensation, eye burning Exclusion criteria: any active eye infection; pregnancy or lactation; any anatomic disorder like entropion or ectropion; incoordination of patients; important surface diseases of eyes which affects test results; history of eye trauma during the past 2 months; moderate to severe blepharitis; refraction surgery or laser therapy of eyes during the past month |
| Interventions |
Intervention #1: daily treatment with artificial tear and viola odorata almond oil drop intranasal 2 drops in each nostril at night before sleeping category Intervention #2 (control): daily treatment with artificial tear and normal saline drop intranasal 2 drops in each nostril at night before sleeping Length of follow‐up: 6 weeks Notes: none Equivalence of baseline characteristics? (Y/N): not reported |
| Outcomes |
Primary outcome(s): eye dryness based on the score of questionnaire Secondary outcome(s): not listed Adverse events reported? (Y/N): not reported Measurement time points (specify intervals at which outcomes were assessed): not reported Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | January 2018 |
| Contact information | Dr Ali Davati Shahed University Beginning of Tehran Qom freeway‐Shahed university, 33191‐18651, Tehran, Iran (Islamic Republic of) |
| Notes |
Date of last participant enrollment: February 19, 2019 (recruitment) Date clinical trial registry last updated: January 28, 2019 Recruitment status: completed Other notes: study sponsored by Shahed University |
ISRCTN10758297.
| Trial name or title |
Public title: the effect of a unique omega‐3 supplement on dry mouth and dry eye in Sjogren's patients Scientific title: the effect of a unique omega‐3 supplement on dry mouth and dry eye in Sjogren's patients |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported Number randomized (total and per group): 65 participants (planned) Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: United States of America Age (mean ± SD, range): not reported Gender: not reported Inclusion criteria: subjects with Sjogren's syndrome as defined by the European Criteria and a positive blood test or lip biopsy Exclusion criteria: had less than 10 teeth; received periodontal therapy in the past 12 months or antibiotic therapy in the past 1 month; required pre‐medication with antibiotics had advanced periodontitis, an infectious or wasting disease; already supplementing with omega‐3s; participating in another clinical trial Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: omega‐3 supplement (TheraTears Nutrition, Advanced Vision Research, USA) containing 750 mg of long‐chain omega‐3s (450 mg of eicosapentaenoic acid [EPA] and 300 mg of docosahexaenoic acid [DHA]) and 1000 mg of flaxseed oil, once a day Intervention #2 (control): placebo Length of follow‐up: 3 months Notes: none |
| Outcomes |
Primary outcome(s): increased oral and ocular comfort Secondary outcome(s): increased salivary flow and improvement in gingival index Adverse events reported? (Y/N): not reported Measurement time points (specify intervals at which outcomes were assessed): 3 months Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | July 2005 |
| Contact information | Dr Athena Papas Tufts University School of Dental Medicine One Kneeland Street Room 508, Boston, United States of America 02111 |
| Notes |
Date of last participant enrollment: September 4, 2007 Date clinical trial registry last updated: April 11, 2008 Recruitment status: no longer recruited Other notes: study sponsored by Advanced Vision Research |
ISRCTN11797545.
| Trial name or title |
Public title: evaluation of combined Eyepeace and Eye Nutrients study Scientific title: Investigate the improvements in tear film/ocular surface and visual recovery in laser and intraocular lens (IOL) refractive surgery patients preoperative using the Eyepeace (Eyelid massager) and Eye Nutrients (omega fatty acid supplement) |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported Number randomized (total and per group): 225 participants (planned) Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: United Kingdom Age (mean ± SD, range): 18 years of age or older (planned) Gender: both (planned) Inclusion criteria: aged 18 or overHave otherwise healthy eyes; are prepared not to wear contact lens for 3 months of the trial; have a NITBUT <10s; OSDI score: greater than or equal to 12; symptom frequency at least "some of the time"; presence of cloudy fluid expressed from at least 1 of the central 8 glands on the lower/upper lid and/or presence of poor expressibility from at least 2‐3 of the central 8 glands on the lower lid Exclusion criteria: conjunctivitis; meibomian cysts; styes; damage to the cornea; ocular injury; cataract or laser refractive surgery in the past 6 months; increased intraocular pressure (primary or secondary); any chronic disease of the eye Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: Eyepeace eyelid massage: once a day, to be started 1 week prior to laser or intraocular lens surgery, to be stopped one day prior to surgery eye nutrients: two capsules a day, to be started 1 week prior to laser or intraocular lens surgery, to be stopped 3 months after surgery Intervention #2: eye nutrients: two capsules a day, to be started 1 week prior to laser or intraocular lens surgery, to be stopped 3 months after surgery Intervention #3: no Eyepeace or eye nutrients Length of follow‐up: 3 months Notes: none |
| Outcomes |
Primary outcome(s): tear film lipid layer thickness Secondary outcome(s): TBUT; meibomian gland function; ocular redness/hyperaemia; MMP‐9; corneal sensitivity; corneal staining; tear volume; dry eye symptoms; visual satisfaction; longitudinal progress of the patient’s dry eye symptoms and adherence to dry eye treatment, Adverse events reported? (Y/N): not reported Measurement time points (specify intervals at which outcomes were assessed):2 weeks, 1 month, 2 months and 3 months Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | March 2018 |
| Contact information | Proffesor Jonathan Moore Cathedral Eye Clinic 89‐91 Academy Street, Belfast, BT1 2LS, United Kingdom |
| Notes |
Date of last participant enrollment: April 1, 2018 Date clinical trial registry last updated: February 13, 2018 Recruitment status: no longer recruiting Other notes: study sponsored by Cathedral Eye Research |
ISRCTN17233445.
| Trial name or title |
Public title: oral supplementation of omega‐3 and omega‐6 in dry eye syndrome Scientific title: a 3‐month, multicenter, double‐masked, randomized, controlled clinical study to investigate the efficacy of Medilar™ in patients suffering from dry eye syndrome |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): multi‐center (2 sites) Number randomized (total and per group): 140 participants Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: France and Italy Age (mean ± SD, range): not reported Gender: males and females Inclusion criteria: 1. Legally adult outpatients, both males and females 2. Having given written informed consent 3. Suffering from dry eye syndrome as defined by the presence of 3.1. At least 2 of the following 4 objective tests corresponding to the scores below 3.1.1. Schirmer I values less than 10 mm/5 min 3.1.2. Break‐up time values less than 10 s 3.1.3. Fluorescein staining of the cornea score greater than or equal to 1 and less than 4 3.1.5. Van Bijsterveld score greater than or equal to 3 and less than or equal to 6 (Lissamine green) 3.2. Score of at least 1, for at least 2 of the following 5 subjective tests (scored 0 to 3) 3.2.1. Foreign body sensation 3.2.2. Dryness 3.2.3. Burning 3.2.4. Stinging 3.2.5. Photophobia 4. Stable systemic treatment (unchanged for 1 month or longer) Exclusion criteria: 1. Aged younger than 18 years 2. Severe dry eye (lissamine green greater than 6 or corneal staining greater than or equal to 4) 3. Uncontrolled evolutive systemic disease 4. Patients with an implantable cardioverter‐defibrillator (ICD) 5. Uncontrolled inflammatory disease (treated with varying doses of steroids or non‐steroidal anti‐inflammatory substances) 6. Change in systemic treatment within the last month 7. Expected change in treatment of concomitant disease 8. Patients treated with anticoagulants or predisposed to bleeding or hemorrhage 9. Drastic change in food and/or food supplements within the last month 10. Other food supplement with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) 11. Patients with a history of recurrent ocular herpes and/or recurrent uveitis 12. Evidence of acute ocular infection and/or intraocular inflammation within 1 month before the start of this study 13. Patients who have undergone ocular surgery within the last 6 months 14. Change in ocular treatment within the last month 15. Patients currently using any ophthalmic medication including any ocular ointment except artificial tear preparation and eye cleaning solution for treatment of dry eye syndrome 16. Patients treated with topical ocular, steroidal, or non‐steroidal anti‐inflammatory treatment within the last month 17. Patients treated with ocular topical cyclosporine within the last month 18. Occlusion therapy with lacrimal or punctum plugs within the last 3 months 19. Patients currently wearing contact lenses 20. Pregnant or lactating women 21. Women of child‐bearing potential considering becoming pregnant during the course of the study and those not taking precautions to avoid pregnancy 22. Patients for whom, in the physician's opinion, any of the protocol procedures may pose a special risk not outweighed by the potential benefits of participating in the study 23. Patients who are unlikely to comply with the study protocol, or who are likely to be moving and lost to follow‐up in the study period 24. Known contraindication, adverse reaction, or hypersensitivity to any constituents of this food supplement 25. Patients who have participated in any clinical investigation within the last 30 days or who are currently participating in a clinical study 26. Patients who are addicted to alcohol or drugs 27. Patients with neurotic, psychiatric disorders or suicidal tendencies 28. Patients who plan to start a diet or to change their diet during the course of the study Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: Medilar™: oral supplementation with 855 mg of omega‐3 and 15 mg of omega‐6, vitamins (C, E, B6, B12), and zinc per day (3 capsules per day) Intervention #2 (control): placebo (medium‐chain triglycerides), 3 capsules per day Length of follow‐up: 3 months Notes: none |
| Outcomes |
Primary outcome(s): percentage of conjunctival epithelial cells expressing human leukocyte antigen DR‐1 (HLA‐DR) inflammatory markers in the worst eye, measured at baseline and at month 3 Secondary outcome(s): efficacy (in worst eye) at baseline, week 6, and month 3 1. Global subjective dry eye score (foreign body sensation, dryness, burning, stinging, photophobia) 2. Subjective dry eye score for each symptom 3. Objective dry eye score for each test (fluorescein staining of the cornea, van Bijsterveld test, tear break‐up time [TBUT] test, Schirmer I) 4. Fluorescence intensity of conjunctival cells expressing HLA‐DR inflammatory marker 5. Quality of life questionnaire for ocular surface disease Safety outcome: adverse or unexpected events Adverse events reported? (Y/N): not reported Measurement time points (specify intervals at which outcomes were assessed): not reported Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | November 22, 2005 |
| Contact information | Prof. Catherine Creuzot‐Garcher Hôpital Général Service d'Ophtalmologie 3 Rue du Faubourg Raines BP 1519, Dijon 21034, France. |
| Notes |
Notes: retrospectively registered Overall trial end date: January 18, 2007 Other notes: industry sponsor (Laboratoire Chauvin, Bausch & Lomb Inc., France) |
NCT00344721.
| Trial name or title | A placebo controlled double masked clinical assessment study of essential fatty acid supplement and its effect on patients with apparent aqueous deficient dry eye syndrome |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single‐center Number randomized (total and per group): 42 participants Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: United States Age (mean ± SD, range): not reported Gender: not reported Inclusion criteria: 1. Patient over 18 years of age 2. Patient willing and able to comply with the protocol; no planned changes in diet, topical or systemic drugs during course of study 3. Ocular symptoms consistent with dry eye of insidious onset and greater than 3 months' duration 4. Ocular surface vital staining consistent with aqueous deficient dry eyes with less than +1 conjunctival injection and no more than minimal lid inflammation Exclusion criteria: 1. Patients who use topical eye drops other than artificial tears 2. Patients with punctal occlusion or punctal plugs 3. Patients with active ocular infection or inflammatory disease 4. History of herpetic keratitis 5. History of retinal detachment 6. Concurrent contact lens use during the trial period 7. Ocular surgery within the past 6 months 8. Patients with glaucoma, anterior membrane dystrophy, active trichiasis, or any eyelid globe malposition abnormality (eg, entropion, ectropion) 9. Patients with epiphora (excessive tearing) 10. Patients taking medications known to affect aqueous tear production or meibomian secretions 11. Patient must not have participated in (or be currently participating in) any investigational therapeutic drug or device trial within the previous 30 days before the start date for this trial 12. Patients suffering from organic brain syndromes or major psychiatric disorder that would interfere with compliance or subjective reporting Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: omega‐3 fatty acid supplement, containing a daily dose of EPA (eicosapentaenoic acid) 450 mg, DHA (docosahexaenoic acid) 300 mg, and flaxseed oil (organic) 1000 mg Intervention #2 (control): placebo supplements, containing wheat germ oil but not essential fatty acids Length of follow‐up: not reported Notes: patient's topical therapy will be standardized after identification exam, so that all patients will use TheraTears™ 4 times daily |
| Outcomes |
Primary outcome(s): meibomian gland secretion lipid biochemistry changes (before and after treatment) Secondary outcome(s): tear evaporation rate and tear fluorophometry (both measured before and after treatment) Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): not reported Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | September 2004 |
| Contact information | James McCulley University of Texas, Southwestern Medical Center at Dallas, USA |
| Notes |
Recruitment status: completed Actual completion date: October 2008 Last update posted: May 2018 |
NCT00357201.
| Trial name or title | Evaluation of the efficacy of T1675, a per os omega 3 and omega 6 polyunsaturated essential fatty acid dietary formulation versus placebo in patients with bilateral treated moderate dry eye syndrome |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single‐center Number randomized (total and per group): not reported Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): individual (right eye) Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: France Age (mean ± SD, range): not reported Gender: not reported Inclusion criteria: 1. Signed and dated informed consent 2. Male or female from 18 to 90 years old 3. Known treated bilateral dry eye 4. Dry eye syndrome confirmed by ocular examination with fluorescein and/or lissamine green staining, TBUT, and Schirmer, performed within the last 12 months before the inclusion visit, for both eyes 5. Bilateral symptoms suggestive of dry eye defined by at least 1 of the following ocular symptoms suggestive of dry eye (burning, stinging, dryness feeling, sandy and/or gritty sensation, light sensitivity, reflex tearing, ocular fatigue) and questioning on patient’s feeling (score ≥ 3) 6. Fulfilling the following criteria of dry eye syndrome in both eyes defined by keratoconjunctivitis defined by a lissamine green score ≥ 4 (van Bijsterveld score) and Schirmer test ≤ 10 mm in 5 min or TBUT < 10 s Exclusion criteria: 1. Severe dry eye symptoms 2. Eyelid dysfunction 3. Severe progressive rosacea 4. Any relevant ocular anomaly interfering with ocular surface 5. Best‐corrected far visual acuity ≤ 1/10 6. History of ocular allergy 7. Trauma, infection, inflammation within last 3 months 8. Ocular surgery and laser within last 3 months 9. LASIK, laser, PRK within last 12 months 10. Contact lenses 11. Any concomitant nutritive supplementation, vitamins 12. Any topical concomitant treatment Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: omega‐3 and omega‐6 polyunsaturated essential fatty acid (T1675) supplements Intervention #2 (control): placebo supplements Length of follow‐up: 6 months Notes: none |
| Outcomes |
Primary outcome(s): total score of lissamine green staining in the right eye Secondary outcome(s): global efficacy; tolerance Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, months 1, 3, 12, 18, 24, 30, and 36 Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | November 2004 |
| Contact information | Dr. Catherine Creuzot‐Garcher CHU of Dijon, France |
| Notes |
Recruitment status: completed (May 2005) Last post updated: October 2006 |
NCT01102257.
| Trial name or title | Clinical trial of essential fatty acids for dry eye disease: feasibility study |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported Number randomized (total and per group): 18 participants in total; 9 in each treatment group Unit of randomization (individual or eye): not reported Exclusions after randomization: 2 participants (due to adverse events), both in the omega‐3 intervention group Losses to follow‐up: none Number analyzed (total and per group): 16 participants in total; 7 in the omega‐3 intervention group; 9 in the placebo group Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: United States Age (mean ± SD, range): 56 ± 14 years in total; 55 ± 14 years in the omega‐3 intervention group; 57 ± 15 years in the placebo group Gender: 3 men and 15 women in total; 1 man and 8 women in the omega‐3 intervention group; 2 men and 7 women in the placebo group Inclusion criteria: 1. Signed and dated informed consent form approved by the IRB 2. ≥ 18 years of age 3. Demonstrated at least 2 of the 4 following signs in the same eye at 2 consecutive visits (visit 1: 7 to 21 days before randomization, and visit 2: day of randomization): (a) conjunctival staining present ≥ 1; (b) corneal fluorescein staining present ≥ 1; (c) tear film break‐up time (TBUT) ≤ 7 seconds; (d) Schirmer test ≤ 7 mm/5 min 4. Demonstrated symptoms of dry eye disease (OSDI score of at least 22 at screening visit and at least 15 at randomization visit) 5. Use or desire to use artificial tears on average 2 times per day in the 2 weeks preceding study entry (run‐in period). No newly diagnosed patients can be enrolled, and if a new patient wants to participate, she/he must be put on tears and re‐evaluated in 6 months 6. Intraocular pressure (IOP) ≥ 5 mmHg and ≤ 22 mmHg in each eye 7. Women of child‐bearing potential must agree to use a reliable method of contraception during study participation, and must demonstrate a negative urine pregnancy test at screening visit 8. Must be willing/able to return for all study visits and to follow instructions from the study investigator and his/her staff 9. Must be able to swallow large, soft gel caps Exclusion criteria: 1. Patients who are allergic to ingredients of active or placebo pills (fish, olive oil) 2. Current diagnosis of ocular infection (eg, bacterial, viral, fungal) 3. History of ocular herpetic keratitis 4. Eye surgery (including cataract surgery) within 6 months before randomization 5. Previous LASIK surgery 6. Pregnant or nursing/lactating 7. Participation in a study of an investigational drug or device within the past 30 days 8. Recent (≤ 3 months) initiation of use of systemic corticosteroids or other immunosuppressive agent and/or planning to change treatment during study participation 9. Cognitive or psychiatric deficit that precludes informed consent or ability to perform requirements of the investigation 10. Contact lens wearers 11. Use of glaucoma medication or history of surgery for glaucoma 12. Recent (≤ 3 months) insertion of punctal plugs 13. Use of punctal plugs but unwilling to commit to their use for the duration of the study 14. Unwilling to commit to same brand of artificial tears throughout the study 15. Current use of EPA/DHA supplements in excess of 1 gram/d 16. Recent (≤ 6 months) initiation of use of Restasis 17. Use of Restasis but unwilling to commit to use of Restasis for the duration of the study 18. Discontinued use of Restasis within the last 3 months Equivalence of baseline characteristics? (Y/N): Y |
| Interventions |
Intervention #1 (omega‐3 treatment group): total daily dose of eicosapentaenoic acid 2000 mg and docosahexaenoic acid 1000 mg (from 5 capsules) Intervention #2 (control group): placebo (olive oil), 5 capsules daily containing 3000 mg of olive oil Length of follow‐up: 3 months Notes: none |
| Outcomes |
Primary outcome(s): change in OSDI and red blood cell membrane fatty acid content Secondary outcome(s): change in each of Brief Ocular Discomfort Inventory (BODI); Impact of Dry Eye on Everyday Life (IDEEL); Quality of Life Associated With Chronic Pain; ocular surface; Schirmer test; HLA‐DR; MUC 5A; cytokines Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): not reported Other issues with outcome assessment (eg, quality control for outcomes if any): none |
| Starting date | January 2010 |
| Contact information | Penny Asbell, MD |
| Notes |
Recruitment status: completed Last post updated: August 2012 |
NCT01733745.
| Trial name or title | SYSTANE family efficacy in meibomian gland functionality for lipid deficient evaporative dry eye subjects |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single‐center Number randomized (total and per group): 26 participants in total; 13 participants in each group ("experimental" group and "active comparator" group) Unit of randomization (individual or eye): not reported Exclusions after randomization: none Losses to follow‐up: none Number analyzed (total and per group): 26 participants in total; 13 participants in each group ("experimental" group and "active comparator" group) Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: United States Age (mean ± SD, range): 41.7 ± 19.8 years total; 38.1 ± 19.9 years in the experimental group; 45.4 ± 19.8 years in the active comparator group Gender: 5 men and 21 women in total; 2 men and 11 women in the experimental intervention group; 3 men and 10 women in the active comparator group Inclusion criteria: 1. Clinical diagnosis of lipid deficient evaporative dry eye; meibomian gland functionality ‐ not more than 6 glands yielding liquid secretion 2. Willing to discontinue the use of all other meibomian glands 3. Dysfunction management before receiving the study test article at visit 1, up until the end of the study period 4. Best‐corrected visual acuity of 20/40 Snellen or better in each eye 5. Must be able to follow instructions and must be willing to attend required study visits 6. Read, sign, and date an ethics committee reviewed and approved informed consent form 7. Other protocol‐defined inclusion criteria may apply Exclusion criteria: 1. History or evidence of ocular or intraocular surgery or serious ocular trauma in either eye within the past 6 months 2. Current punctal occlusion of any type (eg, collagen plugs, silicone plugs) 3. History of intolerance or hypersensitivity to any component of study medications 4. History or evidence of epithelial herpes simplex keratitis (dendritic keratitis); vaccinia; active or recent varicella viral disease of the cornea and/or conjunctiva; chronic bacterial disease of the cornea and/or conjunctiva and/or eyelids; mycobacterial infection of the eye; and/or fungal disease of the eye 5. Pregnant or lactating at the time of enrollment 6. Not willing to take adequate precautions to avoid becoming pregnant during the study 7. Use of any concomitant topical ocular medications during the study period 8. Use of systemic medications that may contribute to dry eye unless on a stable dosing regimen for a minimum of 30 days before visit 1 9. Ocular conditions that may preclude safe administration of either drop under investigation 10. Unwilling to discontinue contact lens wear during the study period and for at least 1 week before visit 1 11. Participation in an investigational drug or device study within 30 days of entering this study 12. Other protocol‐defined exclusion criteria may apply Equivalence of baseline characteristics? (Y/N): Y |
| Interventions |
Intervention #1 (experimental group): SYSTANE Lid Wipes administered to treated eye(s) once a day; SYSTANE BALANCE Lubricant Eye Drops administered to treated eye(s), 1 drop, 4 times a day; SYSTANE Vitamins, 2 soft gels ingested daily Intervention #2 (active comparator group): microfiber towels as warm compress once daily for 8 minutes Length of follow‐up: 3 months Notes: none |
| Outcomes |
Primary outcome(s): number of meibomian glands yielding liquid secretion Secondary outcome(s): Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire; OSDI Adverse events reported? (Y/N): Y Measurement time points (specify intervals at which outcomes were assessed): not reported Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | February 2013 |
| Contact information | Donald R. Korb, O.D. Korb and Associates |
| Notes |
Recruitment status: completed First posted: November 2012 Last post updated: June 2018 |
NCT01880463.
| Trial name or title | Dry eye disease in the vitamin D and omega‐3 trial (VITAL) |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): not reported Number randomized (total and per group): 25,875 participants Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: United States Age (mean ± SD, range): not reported Gender: not reported (males and females) Inclusion criteria: 1. All participants in VITAL (NCT 01169259) are eligible to participate in this ancillary study Exclusion criteria: 1. Not reported Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: vitamin D (cholecalciferol, 2000 IU per day) plus fish oil placebo Intervention #2: vitamin D placebo, plus 1 capsule containing 840 mg of marine omega‐3 fatty acids (eicosapentaenoic acid 465 mg and docosahexaenoic acid 375 mg) Intervention #3: vitamin D placebo plus fish oil placebo Intervention #4: vitamin D (cholecalciferol, 2000 IU per day) plus 1 capsule containing 840 mg of marine omega‐3 fatty acids (eicosapentaenoic acid 465 mg and docosahexaenoic acid 375 mg) Length of follow‐up: 5 years Notes: none |
| Outcomes |
Primary outcome(s): dry eye disease (report of a diagnosis of dry eye disease confirmed by medical record review) Secondary outcome(s): none specified Adverse events reported? (Y/N): not reported Measurement time points (specify intervals at which outcomes were assessed): not reported Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | July 2010 |
| Contact information | None provided |
| Notes |
Recruitment status: unknown First posted: June 2013 Last post updated: January 2016 |
NCT02014922.
| Trial name or title | a study to determine the relief of dry eye symptoms with the use of TheraTears® products (DUNLIN) |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single‐center Number randomized (total and per group): 33 participants Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: Canada Age (mean ± SD, range): 18 to 65 years (planned) Gender: both (planned) Inclusion criteria: between 18 and 65 years of age and has full legal capacity to volunteer; read and signed an information consent letter; willing and able to follow instructions and maintain the appointment schedule; symptoms of dry eye for at least 3 months; OSDI score of ≥ 23; currently on a non‐omega 3 dry eye treatment regimen that, at the minimum consists of instilling artificial tears at least once a day for the past 3 months; an average non‐invasive tear breakup time ≤ 5.00 seconds in at least one eye. Exclusion criteria: participating in any concurrent clinical or research study; has any known active* ocular disease and/or infection and/or allergies;* For the purposes of study, active ocular disease is defined as infection or inflammation which requires therapeutic treatment. Lid abnormalities (blepharitis, meibomian gland dysfunction, papillae), corneal and conjunctival staining and dry eye are typical findings and are not considered active ocular disease. Neovascularization and corneal scars are the result of previous hypoxia, infection or inflammation and are therefore not active; a systemic condition that in the opinion of the investigator may affect a study outcome variable; Is using any systemic or topical medications that in the opinion of the investigator may affect a study outcome variable; known sensitivity to the diagnostic pharmaceuticals to be used in the study; pregnant, lactating or planning a pregnancy at the time of enrollment, as determined verbally; aphakic; undergone refractive error surgery; take part in another (pharmaceutical) research study within the last 30 days; worn contact lenses within the past 5 years; currently using or have used omega 3 supplements in the past 3 months. Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: TheraTears® lubricant eye drop, TheraTears® preservative‐free single‐use containers, TheraTears® Nutrition, and TheraTears® TheraLid® eyelid cleanser Intervention #2 (control): no treatment Length of follow‐up: 3 months Notes: none |
| Outcomes |
Primary outcome(s): OSDI; visual analogue scores; tear osmolarity; TBUT; corneal staining Secondary outcome(s): lid wiper epitheliopathy; meibomian gland expressibility; meibum quality; tear film lipid layer thickness; tear meniscus height, Schirmer test Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, 1 month and 3 months Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | December 2013 |
| Contact information | Centre for Contact Lens Research, University of Waterloo School of Optometry & Vision Science, Waterloo, Ontario, Canada, N2L3G1 |
| Notes |
Recruitment status: completed Actual completion date: November 2014 Last update posted: March 11, 2015 |
NCT02802150.
| Trial name or title | The effect of oral Zanthoxylum Schinifolium seed oil in individuals with dry eye disease |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single‐center Number randomized (total and per group): 20 participants (planned) Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: South Korea Age (mean ± SD, range): 20 to 70 years (planned) Gender: both (planned) Inclusion criteria: men and women; 20 to 70 years with dry eye disease low tear break‐up time (TBUT) (<10 seconds) or low Schirmer score (with application of local anesthetic) (<10mm for 5 min) or presence of corneal and conjunctival damage at the time of screening; blood level of triglyceride higher than 150 mg/dL or HDL‐cholesterol less than 40 and 50 mg/dL for men and women, respectively. Exclusion criteria: under the anti‐inflammatory eye drops for dry eye (topical steroid and topical cyclosporin); hypolipidemic medication within 3 months of study entry; history of chronic disease; any clinical trial using an investigative medicinal product within 2 months before the first dose of the present study allergic or hypersensitive to any of the ingredients in the test products; women who were pregnant or breast feeding; a history of alcoholism or drug abuse or medical or psychological conditions Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: Zanthoxylum Schinifolium Seed Oil(4g/day) Intervention #2 (control): placebo/soy bean oil(4g/day) Length of follow‐up: 10 weeks Notes: none |
| Outcomes |
Primary outcome(s): Schirmer test; TBUT; corneal staining; OSDI Secondary outcome(s): interleukin‐13; interferon‐gamma; interleukin‐1β; interleukin‐17; malondialdehyde; low‐density lipoprotein; total cholesterol; triglyceride; high‐density lipoprotein cholesterol; low‐density lipoprotein cholesterol Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, 5 weeks and 10 weeks Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | February 2015 |
| Contact information | Clinical Trial Center for Functional Foods; Chonbuk National University Hospital, Jeonju, Jeollabuk‐do, Korea, Republic of, 560‐822 |
| Notes |
Recruitment status: completed Actual completion date: February 2016 Last update posted: June 16, 2016 |
NCT02871440.
| Trial name or title |
Public title: a two comparator, controlled phase 3 study in patients with and without evaporative dry eye Scientific title: a two comparator, controlled phase 3 Study of OM3 tear formulation versus OPTIVE ADVANCED unit dose and OPTIVE unit dose eye drops in patients with and without evaporative dry eye |
| Methods |
Study design: randomized, cross‐over, controlled trial Study site(s): not reported Number randomized (total and per group): 57 participants Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: Australia Age (mean ± SD, range): not reported Gender: both sexes eligible to participate Inclusion criteria: 1. Able to read and comprehend English and give informed consent as demonstrated by signing a record of informed consent 2. Over 18 years of age 3. Not wearing contact lenses in the past 3 months before enrolling 4. Willing to use eye drops and comply with the study visit schedule as directed by the investigator 5. Habitual (corrected or uncorrected) visual acuity of 6/9.5 or better in each eye 6. At the screening visit (day ‐14), patients must have Ocular Surface Disease Index (OSDI) score > 18 (0 to 100 scale). At baseline (day 1) visits, patients must have OSDI score > 12 to continue in the study 8. TBUT≤ 10 s in at least 1 eye at screening visit and at baseline visit 9. Corneal fluorescein staining score ≥ 1 and < 4 (Oxford scheme) at screening and baseline visits Exclusion criteria: 1. Schirmer test (with anesthesia) ≤ 2 mm in either eye at screening 2. Patients who are currently using topical ocular medication or have used topical ocular medication within 2 weeks of the screening visit. Patients who are being treated bilaterally with a marketed artificial tear for dry eye can be considered, provided they discontinue use at the screening visit 3. Any active anterior segment disease excluding blepharitis 4. Any systemic disease that may affect ocular health (eg, Graves' disease, autoimmune diseases such as ankylosing spondylitis, multiple sclerosis, and systemic lupus erythematosus) 5. History of epilepsy or migraines exacerbated by flashing, strobe‐like lights 6. Rigid or soft contact lens wearer, including orthokeratology 7. History of eye surgery within 6 months before enrollment in the study 8. Previous corneal refractive surgery Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: omega‐3 eye drop Intervention #2: Optive Advanced eye drop Intervention #3: Optive eye drop Length of follow‐up: 4 weeks Notes: topical omega‐3 intervention |
| Outcomes |
Primary outcome(s): tear evaporation rate, measured by a vapometer (g ‐ 2 h) Secondary outcome(s): TBUT and subjective ocular comfort (measured using visual analogue scales from 0 to 100) Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): baseline, up to 4 weeks Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | September 19, 2016 |
| Contact information | Jacqueline Tan‐Showyin The University of New South Wales, Australia |
| Notes |
Actual study completion date: September 18, 2017 Last update posted: August 2, 2018 Other notes: single‐masked (investigator only) Study undertaken in collaboration with Allergan (industry) |
NCT02908282.
| Trial name or title | Topical omega‐3 fatty acids (REMOGEN OMEGA) in the treatment of dry eye (REMOTOP) |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): multi‐center (5 sites) Number randomized (total and per group): 100 participants Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: Germany Age (mean ± SD, range): not reported Gender: both sexes eligible to participate Inclusion criteria: 1. Male or female patient between 18 and 80 years of age and in good general health 2. Signed written informed consent 3. Existence of moderate to severe DES symptoms defined as break‐up time (TBUT) ≤ 10 s (mean of 3 consecutive measurements) and OSDI questionnaire score ≥ 20 Exclusion criteria: 1. Contraindication for the use of products (eg, hypersensitivity to constituents of the test products) or any study procedure 2. Concomitant or previous participation in a clinical investigation within the last 3 months 3. Concomitant therapies/manipulations that affect the tear film, tear secretion, or ocular surface integrity or would alter the effects of the devices being evaluated 4. Concurrent (systemic) DES‐associated diseases that are not on a stable therapy for at least 1 month (therapy not expected to change) 5. Glaucoma that is not on a stable dosage since at least 2 weeks (therapy not expected to change) 6. Any diseases or characteristics judged by the investigator to be incompatible with assessments and/or procedures for the study evaluation 7. Pregnant or lactating females 8. Participants of child‐bearing age who do not use adequate methods of birth control 9. Subjects unable to understand informed consent or having a high probability of non‐compliance with study procedures and/or non‐completion of the study according to investigator's judgement (eg, illiteracy, insufficient knowledge of local language) 10. Subjects not capable of contracting and of understanding the nature, risks, significance, and implications of the clinical investigation, and unable to form a rational intention in the light of these facts Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: REMOGEN OMEGA lubricant eye drops: preservative‐free, hypotonic microemulsion of polyunsaturated fatty acids and hydrating polymers provided in single‐dose containers of 0.25 mL Intervention #2 (control): povidone artificial tears: preservative‐free eye drops containing 2% povidone Length of follow‐up: 12 weeks Notes: topical omega‐3 intervention |
| Outcomes |
Primary outcome(s): OSDI and TBUT at week 4 Secondary outcome(s): OSDI (week 12), TBUT (week 12), best‐corrected visual acuity (weeks 4 and 12), tear osmolarity (weeks 4 and 12), MMP‐9 (weeks 4 and 12), lid parallel conjunctival folds (weeks 4 and 12), corneal staining ‐ Oxford scale (weeks 4 and 12), tear volume ‐ Schirmer test (weeks 4 and 12), and conjunctival staining ‐ Oxford scale (weeks 4 and 12) Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): day 0, week 4, and week 12 Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | Recruitment status: active, not recruiting (last updated: July 3, 2018) |
| Contact information | Thomas Kaercher, MD Heidelberg, Baden‐Württemberg, Germany, 69121 |
| Notes | Other notes: sponsored by TRB Chemedica AG (industry) |
NCT03141931.
| Trial name or title | The effects of dietary supplementation with a combination of flaxseed oil, borage oil and fish oil omega‐3 fatty acids on ocular comfort including symptoms of dry eye |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single site Number randomized (total and per group): 138 participants Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: Australia Age (mean ± SD, range): not reported Gender: both sexes eligible to participate Inclusion criteria: 1. Able to read and comprehend English and give informed consent as demonstrated by signing a record of informed consent 2. Must be at least 18 years old 3. Must have symptoms of ocular discomfort as measured by the Ocular Surface Disease Index (OSDI) score > 12 at the baseline visit 4. Willing to comply with the dosage and study visit schedule as directed by the investigator 5. No contact lens wear in the last 30 days and willing to refrain from contact lens wear for the duration of the study 6. No planned changes to diet and willing to not substantially alter usual diet for the duration of the study, including typical intake of fish 7. Willingness to notify the study investigator if instructed to alter diet by health/medical practitioner 8. Willing to continue using any artificial tear supplements at the same frequency throughout the study, as used before the study 9. Must have health and ocular health findings that would not prevent the participant from safely ingesting dietary supplementation with combination omega oils Exclusion criteria: 1. Any systemic disease that would preclude participants from safely ingesting dietary supplementation with combination omega oils 2. Self‐reported allergy/sensitivity to any of the study product ingredients 3. Use of any polyunsaturated fatty acid‐containing dietary supplements (such as fish oil, evening primrose oil, linseed oil) up to 12 weeks before the start of the study 4. Use of any of the following medications (including steroids) up to 12 weeks before the start of the study or during the course of the study: ocular medication, category S3 and above; any systemic or topical medications that will affect ocular physiology (eg, antiacne medications such as Roaccutane, corticosteroid, or immunosuppressant medications such as hydrocortisone, prednisolone, and antihistamine medications such as claritine) 5. Any systemic disease that may affect ocular health (eg, Graves' disease, autoimmune diseases such as ankylosing spondylitis, multiple sclerosis, and systemic lupus erythematosus) 6. Epilepsy or history of migraines exacerbated by flashing, strobe‐like lights 7. Eye surgery within 6 months immediately before enrollment for this study 8. Rigid or soft contact lens wearer, including orthokeratology in the last 30 days 9. Previous corneal refractive surgery 10. Pregnant or breastfeeding Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: Lacritec: concentrated omega‐3 triglycerides ‐ fish 332 mg equiv eicosapentaenoic acid (EPA), 134 mg equiv docosahexaenoic acid (DHA), 66.8 mg flaxseed oil (linseed oil), 334 mg equiv oleic acid, 58.5 mg equiv linoleic acid, 58.5 mg equiv linolenic acid, 192 mg borago officinalis seed oil fixed (borage), 434 mg equiv gamma‐linolenic acid 95.5 mg Intervention #2 (control): placebo polyethylene glycol (500 mg), oleic acid (659 mg), and propylene glycol (115 mg) Length of follow‐up: 3 months Notes: none |
| Outcomes |
Primary outcome(s): OSDI, ocular comfort index, DEQ‐5; each measured at 3 months of follow‐up Secondary outcome(s): non‐invasive tear break‐up time (Oculus Keratograph 5M), tear evaporation rate (vapometer), tear meniscus height (Oculus Keratograph 5M), tear volume (phenol red thread test), and tear film lipid layer thickness (LipiView ocular surface interferometer); each measured at 3 months of follow‐up Adverse events reported? (Y/N): not reported Measurement time points (specify intervals at which outcomes were assessed): baseline, 3 months Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | August 21, 2017 (currently recruiting) Estimated primary completion date: October 2018 |
| Contact information | Jacqueline Tan‐Showyin School of Optometry and Vision Science The University of New South Wales, Australia. |
| Notes |
Date of last participant enrollment: not reported Other notes: collaborators: Stiltec Pty Ltd. and the Australian Government |
NCT03265327.
| Trial name or title | Effect of an oral supplement containing omega‐3 and omega‐6 on dry eye symptoms (TURMERIC) |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): single site Number randomized (total and per group): 60 participants Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: Canada Age (mean ± SD, range): not reported Gender: both sexes eligible to participate Inclusion criteria: 1. Over 19 years of age with full legal capacity to volunteer 2. Has read and signed an informed consent letter 3. Is willing and anticipated able to comply with daily intake of liquid oil supplements (1 teaspoon per day for 3 months) 4. Is willing and able to follow instructions and maintain the appointment schedule 5. Exhibits moderate ocular dryness symptoms, defined as i. Score ≥ 23 on the Ocular Surface Disease Index (OSDI; Allergan Inc., Irvine, CA, USA) questionnaire ii. Currently using ocular lubricating drops at least once per day, at least for the past 3 months Exclusion criteria: 1. Is participating in any concurrent clinical or research study 2. Has any known active ocular disease and/or infection 3. Currently wears or has worn contact lenses in the past 3 months 4. Has sensitivity or an allergy to products that contain fish, soy, coconut oil, or olive oil 5. Has a systemic condition that in the opinion of the investigator may affect a study outcome variable 6. Is using any systemic or topical medications (including topical corticosteroids/NSAIDs or glaucoma medications) that in the opinion of the investigator may affect a study outcome variable 7. Is currently taking, or has used, any omega‐3 or omega‐6 supplements in the last 3 months 8. Has known sensitivity to the diagnostic pharmaceuticals to be used in the study 9. Is pregnant, lactating, or planning a pregnancy at the time of enrollment 10. Is aphakic 11. Has undergone refractive error surgery 12. Is an employee of the Centre for Contact Lens Research 13. Has taken part in another (pharmaceutical) research study within the last 30 days Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: oral supplement containing fish oil, evening primrose oil, and borage oil (omega‐3 and omega‐6) Intervention #2 (control): oral supplement containing coconut oil and light olive oil Length of follow‐up: 3 months Notes: none |
| Outcomes |
Primary outcome(s): OSDI, SANDE questionnaire, Schirmer test, objective non‐invasive tear film stability (NIKBUT), non‐invasive tear break‐up time (NITBUT); each measured at screening, 1 month, and 3 months Secondary outcome(s): change in each of bulbar hyperemia, limbal hyperemia, tear meniscus height, meiboscore (Arita's scale), visual acuity, tear osmolarity, and omega‐3 index; each measured at screening and at 3 months Adverse events reported? (Y/N): N Measurement time points (specify intervals at which outcomes were assessed): screening, 1 month, and 3 months Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | August 16, 2017 (currently recruiting) Estimated primary completion date: April 2018 |
| Contact information | Dr. Jill Woods Centre for Contact Lens Research Waterloo, Ontario, Canada, 2NL 3G1 |
| Notes | Sponsor: Nature's Way Canada |
NCT03460548.
| Trial name or title |
Public title: Remogen® Omega versus Cationorm® in the treatment of patients suffering from dry eye Scientific title: a multicentre randomized trial comparing the efficacy and safety of Remogen® omega versus Cationorm® in the treatment of patients suffering from dry eye |
| Methods |
Study design: randomized, parallel‐group, controlled trial Study site(s): multi‐center Number randomized (total and per group): 96 participants (planned) Unit of randomization (individual or eye): not reported Exclusions after randomization: not reported Losses to follow‐up: not reported Number analyzed (total and per group): not reported Unit of analysis (individual or eye): not reported Reported power calculation? (Y/N): N Reported subgroup analysis? (Y/N): N |
| Participants |
Participant characteristics Country: France Age (mean ± SD, range): 18 years and older (planned) Gender: both (planned) Inclusion criteria: at least a 3‐month documented history of bilateral dry eye; a score of ocular surface staining with fluorescein ≥ 4 and ≤ 9 on the Oxford scale; at least one objective sign of tear deficiency; OSDI score of ≥ 18 Exclusion criteria: refractive surgery within 12 months prior to selection; any other ocular surgery or ocular trauma within 6 months prior to selection; systemic or local use of one of the following medications: glucocorticosteroids, cyclosporine A, antibiotics, NSAIDs Equivalence of baseline characteristics? (Y/N): not reported |
| Interventions |
Intervention #1: Remogen 4 times daily Intervention #2 (control): Cationorm 4 times daily Length of follow‐up: 28 days Notes: none |
| Outcomes |
Primary outcome(s): fluorescein staining Secondary outcome(s): not listed Adverse events reported? (Y/N): not reported Measurement time points (specify intervals at which outcomes were assessed): 28 days Other issues with outcome assessment (eg, quality control for outcomes, if any): none |
| Starting date | April 9, 2018 |
| Contact information | Dr. Sabine Collaud Basset Quinze‐Vingts Hospital, Paris, France |
| Notes |
Recruitment status: recruiting Actual completion date: December 2019 (estimated) Last update posted: April 10, 2019 |
BODI: Brief Ocular Discomfort Index; DEQ: Dry Eye Questionnaire; DES: Dry eye syndrome; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; HLA‐DR: human leukocyte antigen‐DR; ICD: implantable cardioverter‐defibrillator; IDEEL: Impact of Dry Eye on Everyday Life; IOP: intraocular pressure; IRB: institutional review board; LASIK: laser‐assisted in situ keratomileusis; MMP‐9: matrix metalloproteinase‐9; MUC: mucin; NSAID: non‐steroidal anti‐inflammatory drug; OCI: Ocular Comfort Index; OSDI: Ocular Surface Disease Index; PRK: photorefractive keratectomy; SD: standard deviation; SESOD: Subjective Evaluation of Symptom of Dryness; SPEED: Standard Patient Evaluation of Eye Dryness; TBUT: tear break‐up time; WHS: Women's Health Study.
Differences between protocol and review
A new lead author, LED joined the review team.
We added tear osmolarity as a secondary outcome in this review, as it is currently recommended as a key component of the Tear Film and Ocular Surface Society (TFOS) International Dry Eye Workshop II (DEWS II) dry eye diagnostic work‐up (Wolffsohn 2017).
In the protocol for this review we had proposed that we would conduct subgroup analyses by prognostic factors (e.g., the severity of dry eye at baseline, gender, age, and history of diagnosis of Sjögren syndrome) and by potential effect modifiers (e.g. dose, duration, and route of administration of omega‐3 and/or omega‐6 PUFAs) when sufficient data were available. However, there were not a sufficient number of eligible studies to permit these analyses.
The protocol had also specified that we would include a Summary of Findings table comparing omega‐3 and/or omega‐6 PUFAs with placebo, for the following outcomes: subjective improvement in dry eye symptoms, ocular surface dye staining, aqueous tear production, tear film stability, change in conjunctival goblet cell density, change in inflammatory biomarkers and adverse events. In the present review, due to the large number of interventions and comparators identified, we included four Summary of Findings tables, for the following comparisons:
oral long‐chain omega‐3 PUFAs (EPA and DHA) versus placebo or no treatment;
combined oral omega‐3 and omega‐6 PUFAs versus placebo;
oral omega‐3 PUFAs plus conventional therapy versus conventional therapy alone;
oral long‐chain omega‐3 PUFAs (EPA and DHA) versus oral omega‐6 PUFAs.
Relative to the protocol, the outcomes included in each of the Summary of Findings tables involved the removal of both changes to conjunctival goblet cell density and inflammatory biomarkers, as few studies reported on these outcomes. The following additional outcomes, which were deemed of major clinical relevance to current dry eye clinical care, were also added: change in Schirmer test score and change in tear osmolarity.
Contributions of authors
SMN and LED drafted the review and responded to editorial and peer review comments. KL and EKA provided substantial feedback and edits to the review and responded to editorial and peer review comments. All authors provided final approval of the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Eye Institute, National Institutes of Health, USA.
Methodological support provided by the Cochrane Eyes and Vision US Project, which is funded by Grant UG1EY020522
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology
- This review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the CEV UK editorial base
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Declarations of interest
SMN and KL received salary support from Cochrane Eyes and Vision @ US Project, funded by the National Eye Institute, at the National Institutes of Health. LED is the senior author on two studies considered in this review (Chinnery 2017; Deinema 2017); in view of this, a third (independent) person verified data extraction and risk of bias assessment for these studies.
New
References
References to studies included in this review
Aragona 2005 {published data only}
- Aragona P, Bucolo C, Spinella R, Giuffrida S, Ferreri G. Systemic omega‐6 essential fatty acid treatment and pge1 tear content in Sjogren's syndrome patients. Investigative Ophthalmology and Visual Science 2005;46(12):4474‐9. [DOI] [PubMed] [Google Scholar]
Asbell 2018 {published data only}
- Dry Eye Assessment and Management Study Research Group, Asbell PA, Maguire MG, Pistilli M, Ying GS, Szczotka‐Flynn LB, et al. n‐3 Fatty acid supplementation for the treatment of dry eye disease. New England Journal of Medicine 2018;378(18):1681‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02128763. Dry Eye Assessment and Management Study (DREAM). https://clinicaltrials.gov/ct2/show/NCT02128763 (first received 1 May 2014).
Barabino 2003 {published data only}
- Barabino S, Rolando M, Camicione P, Ravera G, Zanardi S, Giuffrida S, et al. Systemic linoleic and γ‐linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea 2003;22(2):97‐101. [DOI] [PubMed] [Google Scholar]
Bhargava 2013 {published data only}
- Bhargava R, Kumar P, Kumar M, Mehra N, Mishra A. A randomized controlled trial of omega‐3 fatty acids in dry eye syndrome. International Journal of Ophthalmology 2013;6(6):811‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhargava 2015a {published data only}
- Bhargava R, Kumar P, Phogat H, Kaur A, Kumar M. Oral omega‐3 fatty acids treatment in computer vision syndrome related dry eye. Contact Lens and Anterior Eye 2015;38(3):206‐10. [DOI] [PubMed] [Google Scholar]
Bhargava 2015b {published data only}
- Bhargava R, Kumar P. Oral omega‐3 fatty acid treatment for dry eye in contact lens wearers. Cornea 2015;34(4):413‐20. [DOI] [PubMed] [Google Scholar]
Bhargava 2016a {published data only}
- Bhargava R, Chandra M, Bansal U, Singh D, Ranjan S, Sharma S. A randomized controlled trial of omega 3 fatty acids in Rosacea patients with dry eye symptoms. Current Eye Research 2016;41(10):1274‐80. [DOI] [PubMed] [Google Scholar]
Bhargava 2016b {published data only}
- Bhargava R, Kumar P, Arora Y. Short‐term omega 3 fatty acids treatment for dry eye in young and middle‐aged visual display terminal users. Eye and Contact Lens 2016;42(4):231‐6. [DOI] [PubMed] [Google Scholar]
Brignole‐Baudouin 2011 {published data only}
- Brignole‐Baudouin F, Baudouin C, Aragona P, Rolando M, Labetoulle M, Pisella PJ, et al. A multicentre, double‐masked, randomized, controlled trial assessing the effect of oral supplementation of omega‐3 and omega‐6 fatty acids on a conjunctival inflammatory marker in dry eye patients. Acta Ophthalmologica 2011;89(7):e591‐7. [DOI] [PubMed] [Google Scholar]
Creuzot 2006 {published data only}
- Creuzot C, Passemard M, Viau S, Joffre C, Pouliquen P, Elena PP, et al. Improvement of dry eye symptoms with polyunsaturated fatty acids [Amélioration de la symptomatologie chez des patients atteints de sécheresse oculaire et traités oralement par des acides gras polyinsaturés]. Journal Francais d'Ophthalmologie 2006;29(8):868‐73. [DOI] [PubMed] [Google Scholar]
Creuzot‐Garcher 2011 {published data only}
- Creuzot‐Garcher C, Baudouin C, Labetoulle M, Pisella PJ, Mouriaux F, Meddeb‐Ouertani A, et al. Efficacy assessment of Nutrilarm®, a per os omega‐3 and omega‐6 polyunsaturated essential fatty acid dietary formulation versus placebo in patients with bilateral treated moderate dry eye syndrome [Évaluation de l'efficacité du Nutrilarm®, complément nutritionnel à base d'acides gras essentiels polyinsaturés oméga 3 et oméga 6, versus placebo chez des patients atteints de sécheresse oculaire bilatérale modérée et traitée]. Journal Francais d'Ophthalmologie 2011;34(7):448‐55. [DOI] [PubMed] [Google Scholar]
Deinema 2017 {published data only}
- Chinnery HR, Naranjo Golborne C, Downie LE. Omega‐3 supplementation is neuroprotective to corneal nerves in dry eye disease: a pilot study. Ophthalmic and Physiological Optics 2017;37(4):473‐81. [DOI] [PubMed] [Google Scholar]
- Deinema LA, Vingrys AJ, Wong CY, Jackson DC, Chinnery HR, Downie LE. A randomized, double‐masked, placebo‐controlled clinical trial of two forms of omega‐3 supplements for treating dry eye disease. Ophthalmology 2017;124(1):43‐52. [DOI] [PubMed] [Google Scholar]
Epitropoulos 2016 {published data only}
- Epitropoulos AT, Donnenfeld ED, Shah ZA, Holland EJ, Gross M, Faulkner WJ, et al. Effect of oral re‐esterified omega‐3 nutritional supplementation on dry eyes. Cornea 2016;35(9):1185‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbard 2008 {published data only}
- Gilbard JP. Prospective, randomized, double‐masked, placebo controlled clinical trial of an omega‐3 supplement for dry eye and dry mouth in patients with Sjögren's disease. American Academy of Ophthalmology; 2008 Nov 8‐11; Atlanta (GA). 2008.
Goyal 2017 {published data only}
- Goyal P, Jain AK, Malhotra C. Oral omega‐3 fatty acid supplementation for laser in situ keratomileusis–associated dry eye. Cornea 2017;36(2):169‐75. [DOI] [PubMed] [Google Scholar]
Kangari 2013 {published data only}
- Kangari H, Eftekhari MH, Sardari S, Hashemi H, Salamzadeh J, Ghassemi‐Broumand M, et al. Short‐term consumption of oral omega‐3 and dry eye syndrome. Ophthalmology 2013;120(11):2191‐6. [DOI] [PubMed] [Google Scholar]
Kawakita 2013 {published data only}
- Kawakita T, Kawabata F, Tsuji T, Kawashima M, Shimmura S, Tsubota K. Effects of dietary supplementation with fish oil on dry eye syndrome subjects: randomized controlled trial. Biomedical Research 2013;34(5):215‐20. [DOI] [PubMed] [Google Scholar]
Kawashima 2016 {published data only}
- Kawashima M, Nakamura S, Izuta Y, Inoue S, Tsubota K. Dietary supplementation with a combination of lactoferrin, fish oil,and enterococcus faecium WB2000 for treating dry eye: a rat model and human clinical study. Ocular Surface 2016;14(2):255‐63. [DOI] [PubMed] [Google Scholar]
Kokke 2008 {published data only}
- Kokke KH, Morris JA, Lawrenson JG. Oral omega‐6 essential fatty acid treatment in contact lens associated dry eye. Contact Lens and Anterior Eye 2008;31(3):141‐6. [DOI] [PubMed] [Google Scholar]
Korb 2015 {published data only}
- Korb DR, Blackie CA, Finnemore VM, Douglass T. Effect of using a combination of lid wipes, eye drops, and omega‐3 supplements on meibomian gland functionality in patients with lipid deficient/evaporative dry eye. Cornea 2015;34(4):407‐12. [DOI] [PubMed] [Google Scholar]
Macsai 2008 {published data only}
- Macsai MS. The role of omega‐3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis). Transactions of the American Ophthalmological Society 2008;106:336‐56. [PMC free article] [PubMed] [Google Scholar]
Manthorpe 1984 {published data only}
- Manthorpe R, Hagen Petersen S, Prause JU. Primary Sjögren's syndrome treated with Efamol/Efavit. A double‐blind cross‐over investigation. Rheumatology International 1984;4(4):165‐7. [DOI] [PubMed] [Google Scholar]
Mohammadpour 2017 {published data only}
- Mohammadpour M, Mehrabi S, Hassanpoor N, Mirshahi R. Effects of adjuvant omega‐3 fatty acid supplementation on dry eye syndrome following cataract surgery: a randomized clinical trial. Journal of Current Ophthalmology 2017;29(1):33‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01107964 {published data only}
- NCT01107964. Oral omega‐3 fatty acids in the treatment of dry eye syndrome. clinicaltrials.gov/ct2/show/NCT01107964 (first received 21 April 2010).
Oleñik 2013 {published data only}
- Oleñik A, Jiménez‐Alfaro I, Alejandre‐Alba N, Mahillo‐Fernández I. A randomized, double‐masked study to evaluate the effect of omega‐3 fatty acids supplementation in meibomian gland dysfunction. Clinical Interventions in Aging 2013;8:1133‐8. [DOI: 10.2147/CIA.S48955] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleñik A, Mahillo‐Fernández I, Alejandre‐Alba N, Fernández‐Sanz G, Pérez MA, Luxan S, et al. Benefits of omega‐3 fatty acid dietary supplementation on health‐related quality of life in patients with meibomian gland dysfunction. Clinical Ophthalmology 2014;8:831‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oral sea buckthorn oil study 2010 {published data only}
- Järvinen RL, Larmo PS, Setälä NL, Yang B, Engblom JR, Viitanen MH, et al. Effects of oral sea buckthorn oil on tear film fatty acids in individuals with dry eye. Cornea 2011;30(9):1013‐9. [DOI] [PubMed] [Google Scholar]
- Larmo PS, Järvinen RL, Setälä NL, Yang B, Viitanen MH, Engblom JR, et al. Oral sea buckthorn oil attenuates tear film osmolarity and symptoms in individuals with dry eye. Journal of Nutrition 2010;140(8):1462‐8. [DOI] [PubMed] [Google Scholar]
Oxholm 1986 {published data only}
- Oxholm P, Manthorpe R, Prause JU, Horrobin D. Patients with primary Sjögren's syndrome treated for two months with evening primrose oil. Scandinavian Journal of Rheumatology 1986;15(2):103‐8. [DOI] [PubMed] [Google Scholar]
Papas 2007 {published data only}
- Papas A, Singh M, Singh M. The effect of a unique omega‐3 supplement on dry mouth and dry eye in Sjögren's patients. Investigative Ophthalmology and Visual Science 2007;48(13):377. [Google Scholar]
Pinazo‐Durán 2013 {published data only}
- Pinazo‐Durán MD, Galbis‐Estrada C, Pons‐Vázquez S, Cantú‐Dibildox J, Marco‐Ramírez C, Benítez‐del‐Castillo J. Effects of a nutraceutical formulation based on the combination of antioxidants and ω‐3 essential fatty acids in the expression of inflammation and immune response mediators in tears from patients with dry eye disorders. Clinical Interventions in Aging 2013;8:139‐48. [DOI: 10.2147/CIA.S40640] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinheiro 2007 {published data only}
- Pinheiro MN Jr, dos Santos PM, dos Santos RC, Barros Jde N, Passos LF, Cardoso Neto J. Oral flaxseed oil (Linum usitatissimum) in the treatment for dry‐eye Sjögren's syndrome patients [Uso oral do óleo de linhaça (Linum usitatissimum)no tratamento do olho seco de pacientesportadores da síndrome de Sjögren]. Arquivos Brasileiros de Oftalmologia 2007;70(4):649‐55. [DOI] [PubMed] [Google Scholar]
Reeder 2006 {published data only}
- Reeder RE, Blowe J, Mistry K. Effect of Theratears Nutrition versus flaxseed oil in managing dryness symptoms. www.aaopt.org/detail/knowledge‐base‐article/effect‐theratears‐nutrition‐versus‐flaxseed‐oil‐managing‐dryness‐symptoms (accessed July 31, 2018).
Sheppard 2013 {published data only}
- Sheppard JD Jr, Singh R, McClellan AJ, Weikert MP, Scoper SV, Joly TJ, et al. Long‐term supplementation with n‐6 and n‐3 PUFAs improves moderate‐to‐severe keratoconjunctivitis sicca: a randomized double‐blind clinical trial. Cornea 2013;32(10):1297‐304. [DOI] [PubMed] [Google Scholar]
Theander 2002 {published data only}
- Theander E, Horrobin DF, Jacobsson LT, Manthorpe R. Gammalinolenic acid treatment of fatigue associated with primary Sjögren's syndrome. Scandinavian Journal of Rheumatology 2002;31(2):72‐9. [DOI] [PubMed] [Google Scholar]
Wojtowicz 2011 {published data only}
- Wojtowicz JC, Butovich I, Uchiyama E, Aronowicz J, Agee S, McCulley JP. Pilot, prospective, randomized, double‐masked, placebo‐controlled clinical trial of an omega‐3 supplement for dry eye. Cornea 2011;30(3):308‐14. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahluwalia 2001 {published data only}
- Ahluwalia P, Anderson DF, Wilson SJ, McGill JI, Church MK. Nedocromil sodium and levocabastine reduce the symptoms of conjunctival allergen challenge by different mechanisms. Journal of Allergy and Clinical Immunology 2001;108(3):449‐54. [DOI] [PubMed] [Google Scholar]
Arici 2006 {published data only}
- Arici MK, Erdogan H, Toker I, Vural A, Topalkara A. The effect of latanoprost, bimatoprost, and travoprost on intraocular pressure after cataract surgery. Journal of Ocular Pharmacology and Therapeutics 2006;22(1):34‐40. [DOI] [PubMed] [Google Scholar]
Costa 2012 {published data only}
- Costa VP, Moreira H, Paolera MD, Moraes Silva MR. Efficacy and safety of travoprost 0.004%/timolol 0.5% fixed combination as transition therapy in patients previously on prostaglandin analog monotherapy. Clinical Ophthalmology 2012;6:699‐706. [DOI: 10.2147/OPTH.S30717] [DOI] [PMC free article] [PubMed] [Google Scholar]
Downie 2018b {published data only}
- Downie LE, Gad A, Wong CY, Gray JHV, Zeng W, Jackson DC, et al. Modulating contact lens discomfort with anti‐Inflammatory approaches: A randomized controlled trial. Investigative Ophthalmology & Visual Science 2018;59(8):3755‐66. [DOI] [PubMed] [Google Scholar]
Gadaria‐Rathod 2013 {published data only}
- Gadaria‐Rathod N, Dentone PG, Peskin E, Maguire MG, Moser A, Asbell PA. Red blood cell fatty acid analysis for determining compliance with omega3 supplements in dry eye disease trials. Journal of Ocular Pharmacology and Therapeutics 2013;29(9):837‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hom 2016 {published data only}
- Hom MM. Baseline characteristics in the dry eye assessment and management (DREAM) study. Investigative Ophthalmology and Visual Science 2016;57(12):2844. [Google Scholar]
Jackson 2011 {published data only}
- Jackson MA, Burrell K, Gaddie IB, Richardson SD. Efficacy of a new prescription‐only medical food supplement in alleviating signs and symptoms of dry eye, with or without concomitant cyclosporine A. Clinical Ophthalmology 2011;5:1201‐6. [DOI: 10.2147/OPTH.S22647] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawabata 2011 {published data only}
- Kawabata F, Tsuji T. Effects of dietary supplementation with a combination of fish oil, bilberry extract, and lutein on subjective symptoms of asthenopia in humans. Biomedical Research 2011;32(6):387‐93. [DOI] [PubMed] [Google Scholar]
Kaya 2016 {published data only}
- Kaya A, Aksoy Y. Omega‐3 fatty acid supplementation improves dry eye symptoms in patients with glaucoma: results of a prospective multicenter study. Clinical Ophthalmology 2016;10:911. [DOI: 10.2147/OPTH.S96433] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwon 2017 {published data only}
- Kwon JW, Han SB. Omega‐3 fatty acid supplementation can improve both symptoms and signs of dry eye disease. Clinical Interventions in Aging 2017;12:485‐6. [DOI: 10.2147/CIA.S132454] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macrì 2001 {published data only}
- Macrì A, et al. Effect of linoleic acid and gamma‐linolenic acid on tear production, clearance and the ocular surface after PRK. Investigative Ophthalmology and Visual Science 2001; Vol. 42:ARVO Abstract 2658.
Macrì 2002 {published data only}
- Macrì A, Amico V, Cro M, Giuffrida S. Effects and compliance of a new oral formulation containing linoleic acid and gamma‐linolenic acid on tear production, tear fluorescein clearance and ocular surface after PRK. Investigative Ophthalmology and Visual Science 2002; Vol. 43:ARVO E‐Abstract 2105.
Macrì 2003 {published data only}
- Macrì A, Giuffrida S, Amico V, Lester M, Traverso CE. Effect of linoleic acid and gamma‐linolenic acid on tear production, tear clearance and on the ocular surface after photorefractive keratectomy. Graefe's Archive for Clinical and Experimental Ophthalmology 2003;241(7):561‐6. [DOI] [PubMed] [Google Scholar]
McMonnies 2019 {published data only}
NCT00803452 {published data only}
- NCT00803452. Lipids of the human tear film and their effect on tear stability. clinicaltrials.gov/ct2/show/NCT00803452 (first received December 5, 2008).
NCT01059019 {published data only}
- NCT01059019. Epithelial healing and visual outcomes using omega‐3 therapy before and after Photorefractive Keratectomy (PRK) surgery (Omega‐3). https://clinicaltrials.gov/ct2/show/ NCT01059019 (first received January 29, 2010).
NCT01213342 {published data only}
- NCT01213342. Omega‐3 fatty acid supplements and dry eye. clinicaltrials.gov/ct2/show/NCT01213342 (first received October 4, 2010).
NCT01364311 {published data only}
- NCT01364311. Treatment of dry eye with supplements. https://clinicaltrials.gov/ct2/show/ NCT01364311 (first received June 2, 2011).
NCT01630551 {published data only}
- NCT01630551. Omega 3 supplementation and ocular surface disease in glaucoma. clinicaltrials.gov/ct2/show/NCT01630551 (first received June 28, 2012).
No author listed {published data only}
- Dry eye: fish oil capsules are not better than placebo. Pharmazeutische Zeitung 2018;163(16). [Google Scholar]
No authors listed {published data only}
- Omega‐3 fatty acid supplements do not improve symptoms of dry eye disease. Drug and Therapeutics Bulletin 2018;56(12):144. [DOI] [PubMed] [Google Scholar]
Ong 2013 {published data only}
- Ong NH, Purcell TL, Roch‐Levecq AC, Wang D, Isidro MA, Bottos KM, et al. Epithelial healing and visual outcomes of patients using omega‐3 oral nutritional supplements before and after photorefractive keratectomy: a pilot study. Cornea 2013;32(6):761‐5. [DOI] [PubMed] [Google Scholar]
Pinna 2007 {published data only}
- Pinna A, Piccinini P, Carta F. Effect of oral linoleic and gamma‐linolenic acid on meibomian gland dysfunction. Cornea 2007;26(3):260‐4. [DOI] [PubMed] [Google Scholar]
Querques 2007 {published data only}
- Querques G, Russo V, Barone A, Iaculli C, Delle Noci N. Efficacy of omega‐6 essential fatty acid treatment before and after photorefractive keratectomy [Efficacité du traitement avec des acides gras essentiels oméga‐6 administrés avant et aprés la kératectomie photoréfractive]. Journal Français d'Ophtalmologie 2008;31(3):282‐6. [DOI] [PubMed] [Google Scholar]
Scuderi 2012 {published data only}
- Scuderi G, Contestabile MT, Gagliano C, Iacovello D, Scuderi L, Avitabile T. Effects of phytoestrogen supplementation in postmenopausal women with dry eye syndrome: a randomized clinical trial. Canadian Journal of Ophthalmology 2012;47(6):489‐92. [DOI] [PubMed] [Google Scholar]
Sharma 2006 {published data only}
- Sharma AK, Raj H, Pandita A, Tandon VR, Mahajan A. Gamma linolenic acid in dry eye. JK Science 2008;8(1):24‐7. [Google Scholar]
Tellez‐Vazquez 2016 {published data only}
- Tellez‐Vazquez J. Omega‐3 fatty acid supplementation improves dry eye symptoms in patients with glaucoma: results of a prospective multicenter study. Clinical Ophthalmology 2016;10:617‐26. [DOI: 10.2147/OPTH.S96433] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2016 {published data only}
- Wang L, Chen X, Hao J, Yang L. Proper balance of omega‐3 and omega‐6 fatty acid supplements with topical cyclosporine attenuated contact lens‐related dry eye syndrome. Inflammopharmacology 2016;24(6):389‐96. [DOI] [PubMed] [Google Scholar]
Yoon 2012 {published data only}
- Yoon S, Gu N, Kim S, Soo Lim K. Ocular tolerability and safety assessment of DA‐6034 ophthalmic solution in healthy subjects. Clinical Pharmacology and Therapeutics 2012;91:S56. [Google Scholar]
References to studies awaiting assessment
Follow‐up reports from the DREAM study {published data only}
- Asbell PA, Maguire MG, Peskin E, Bunya VY, Kuklinski EJ. Dry Eye Assessment and Management (DREAM(c)) Study: Study design and baseline characteristics. Contemporary Clinical Trials 2018;71:70‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunya VY, Ying GS, Maguire MG, Kuklinski E, Lin MC, Peskin E, et al. Prevalence of novel candidate Sjogren syndrome autoantibodies in the Dry Eye Assessment and Management (DREAM) Study. Cornea 2018;37(11):1425‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M, Shtein RM, Pistilli M, Maguire MG, Oydanich M, Asbell PA, Dream Study Research Group. The Dry Eye Assessment and Management (DREAM) extension study ‐ A randomized clinical trial of withdrawal of supplementation with omega‐3 fatty acid in patients with dry eye disease. In: Ocular Surface; 2019;16:Pismo Beach (CA). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Schmucker E, Maguire MG, Sutphin JE, Asbell PA. Correlation of clinical and keratographic assessments of tear stability and tear production at baseline in the DRy Eye Assessment and Management (DREAM©) Study. Investigative Ophthalmology and Visual Science 2018;59(9). [Google Scholar]
Hom 2017 {published data only}
- Hom M, Berdy G, Downie L, El‐Harazi S, Verachtert A, Liu H, Carlisle‐Wilcox C, Simmons P, Vehige J. Clinical evaluation of a novel lipid‐containing lubricant eye drop with omega‐3 oil and trehalose. Investigative Ophthalmology & Visual Science 2017;58(8). [Google Scholar]
Laihia 2019 {published data only}
- Laihia J, Jarvinen R, Wylegala E, Kaarniranta K. Disease aetiology‐based design of multifunctional microemulsion eye drops for moderate or severe dry eye: a randomized, quadruple‐masked and active‐controlled clinical trial. Acta ophthalmologica 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02980224 {published data only}
- NCT02980224. Study of the safety and efficacy of OmegaD softgels in the treatment of dry eye disease. https://clinicaltrials.gov/ct2/show/ NCT02980224 (first received December 2, 2016).
NCT03569202 {published data only}
- NCT03569202. Piiloset trehalose emulsion eye drop study in moderate or severe dry eye. https://clinicaltrials.gov/ct2/show/ NCT03569202 (first received June 26, 2018).
References to ongoing studies
ACTRN12610000991011 {published data only}
- ACTRN12610000991011. Dietary supplements and ocular (eye) comfort [A randomised placebo‐controlled double‐masked study to investigate the effects of dietary supplementation with a combination of omega oils on ocular comfort including symptoms of dry eye]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610000991011 (first received November 16, 2010).
IRCT201012265467N {published data only}
- IRCT201012265467N. The impact of omega‐3 on dry eye. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201012265467N1 (first received April 3, 2012).
IRCT2013062413567N4 {published data only}
- IRCT2013062413567N4. Impact of omega‐3 supplement on dry eye treatment [Clinical trial comparing the effects of omega‐3 supplements and artificial tear drops on dry eye symptoms after surgery, cataract emulsification methods]. en.irct.ir/trial/13389 (first received January 11, 2013).
IRCT20180806040722N1 {published data only}
- IRCT20180806040722N1. Effect of complementary supplementation Camelina Oil on the improvement of dry eye disease in patients with dry eye. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20180806040722N1 (first received April 22, 2019).
IRCT20181028041487N1 {published data only}
- IRCT20181028041487N1. Evaluation the effect of intra nasal viola odorata almond oil drop on dry eye disease. https://en.irct.ir/trial/35071 (first received January 28, 2019).
ISRCTN10758297 {published data only}
- ISRCTN10758297. The effect of a unique omega‐3 supplement on dry mouth and dry eye in Sjogren's patients. http://www.isrctn.com/ISRCTN10758297 (first received February 7, 2007).
ISRCTN11797545 {published data only}
- ISRCTN11797545. Evaluation of combined Eyepeace and Eye Nutrients study. http://www.isrctn.com/ISRCTN11797545 (first received February 9, 2018).
ISRCTN17233445 {published data only}
- ISRCTN17233445. Oral supplementation of omega‐3 and omega‐6 in dry eye syndrome. isrctn.com/ISRCTN17233445 (first received November 28, 2008).
NCT00344721 {published data only}
- NCT00344721. A placebo controlled double masked clinical assessment study of essential fatty acid supplement and Its effect on patients with apparent aqueous deficient dry eye syndrome. clinicaltrials.gov/ct2/show/NCT00344721 (first received June 27, 2006).
NCT00357201 {published data only}
- NCT00357201. Efficacy of T1675 versus placebo in patients with bilateral treated moderate dry eye syndrome. clinicaltrials.gov/ct2/show/NCT00357201 (first received July 27, 2006).
NCT01102257 {published data only}
- NCT01102257. Dry eye assessment and management: feasibility study (DREAM). clinicaltrials.gov/ct2/show/NCT01102257 (first received August 23, 2012).
NCT01733745 {published data only}
- NCT01733745. SYSTANE family ‐ meibomian deficiency (M‐12‐077). clinicaltrials.gov/ct2/show/NCT01733745 (first received July 3, 2014).
NCT01880463 {published data only}
- NCT01880463. Dry eye disease in the vitamin D and omega‐3 Trial (VITAL). clinicaltrials.gov/ct2/show/NCT01880463 (first received June 19, 2013).
NCT02014922 {published data only}
- NCT02014922. A study to determine the relief of dry eye symptoms with the use of TheraTears® products (DUNLIN). https://clinicaltrials.gov/ct2/show/ NCT02014922 (first received December 18, 2013).
NCT02802150 {published data only}
- NCT02802150. The effect of oral Zanthoxylum Schinifolium seed oil in individuals with dry eye disease. https://clinicaltrials.gov/ct2/show/ NCT02802150 (first received June 16, 2016).
NCT02871440 {published data only}
- NCT02871440. A two comparator, controlled phase 3 study in patients with and without evaporative dry eye. clinicaltrials.gov/ct2/show/NCT02871440 (first received August 18, 2016).
NCT02908282 {published data only}
- NCT02908282. Topical omega‐3 fatty acids (REMOGEN OMEGA) in the treatment of dry eye (REMOTOP). clinicaltrials.gov/ct2/show/NCT02908282 (first received September 20, 2016).
NCT03141931 {published data only}
- NCT03141931. The effects of dietary supplementation with a combination of flaxseed oil, borage oil and fish oil omega‐3 fatty acids on ocular comfort including symptoms of dry eye. clinicaltrials.gov/ct2/show/NCT03141931 (first received May 5, 2017).
NCT03265327 {published data only}
- NCT03265327. Effect of an oral supplement containing omega‐3 and omega‐6 on dry eye symptoms (TURMERIC). clinicaltrials.gov/ct2/show/NCT03265327 (first received August 29, 2017).
NCT03460548 {published data only}
- NCT03460548. REMOGEN® omega versus Cationorm® in the treatment of patients suffering from dry eye. https://clinicaltrials.gov/ct2/show/ NCT03460548 (first received March 9, 2018).
Additional references
AAO 2013
- American Academy of Ophthalmology (AAO) Cornea/ External Disease Panel. Preferred practice pattern for dry eye syndrome. www.aao.org/preferred‐practice‐pattern/dry‐eye‐syndrome‐ppp‐2013 2013; Vol. (accessed July 14, 2018).
Bhandari 2004
- Bhandari M, Busse JW, Jackowski D, Montori VM, Schunemann H, Sprague S, et al. Association between industry funding and statistically significant pro‐industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004;170(4):477‐80. [PMC free article] [PubMed] [Google Scholar]
Bistrian 2018
- Bistrian BR. Letter to the Editor re: n‐3 fatty acid supplementation and dry eye disease. New England Journal of Medicine 2018;379:690‐1. [DOI] [PubMed] [Google Scholar]
Brasky 2013
- Brasky TM, Darke AK, Song X, Tangen CM, Goodman PJ, Thompson IM, et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. Journal of the National Cancer Institute 2013;105(15):1132‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brenna 2002
- Brenna JT. Efficiency of conversion of alpha‐linolenic acid to long chain n‐3 fatty acids in man. Current Opinion in Clinical Nutrition and Metabolic Care 2002;5(2):127‐32. [DOI] [PubMed] [Google Scholar]
Bron 2003
- Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003;22(7):640‐50. [DOI] [PubMed] [Google Scholar]
Brouwer 2004
- Brouwer IA, Katan MB, Zock PL. Dietary alpha‐linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta‐analysis. Journal of Nutrition 2004;134(4):919‐22. [DOI] [PubMed] [Google Scholar]
Buckley 2004
- Buckley MS, Goff AD, Knapp WE. Fish oil interaction with warfarin. Annals of Pharmacotherapy 2004;38(1):50‐2. [DOI] [PubMed] [Google Scholar]
Caughey 1996
- Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n‐3 fatty acids from vegetable oil or fish oil. American Journal of Clinical Nutrition 1996;63(1):116‐22. [DOI] [PubMed] [Google Scholar]
Chinnery 2017
- Chinnery HR, Naranjo Golborne C, Downie LE. Omega‐3 supplementation is neuroprotective to corneal nerves in dry eye disease: a pilot study. Ophthalmic and Physiological Optics 2017;37(4):473‐81. [DOI] [PubMed] [Google Scholar]
Craig 2017
- Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II Definition and Classification Report. Ocular Surface 2017;15(3):276‐83. [DOI] [PubMed] [Google Scholar]
Dalgado 2017
- Dalgado AF, Dalgado AF. The association of funding source on effect size in randomized controlled trials: 2013–2015 – a cross‐sectional survey and meta‐analysis. Trials 2017;18:125. [DOI 10.1186/s13063‐017‐1872] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JPT, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9. Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook..
Downie 2013
- Downie LE, Keller PR, Vingrys AJ. An evidence‐based analysis of Australian optometrists' dry eye practices. Optometry and Vision Science 2013;90(12):1385‐95. [DOI] [PubMed] [Google Scholar]
Downie 2015a
- Downie LE, Keller PR. A pragmatic approach to dry eye diagnosis: evidence into practice. Optometry and Vision Science 2015;92(12):1189‐97. [DOI] [PubMed] [Google Scholar]
Downie 2015b
- Downie LE, Keller PR. A pragmatic approach to the management of dry eye disease: evidence into practice. Optometry and Vision Science 2015;92(9):957‐66. [DOI] [PubMed] [Google Scholar]
Downie 2015c
- Downie LE. Automated tear film surface quality breakup time as a novel clinical marker for tear hyperosmolarity in dry eye disease. Investigative Ophthalmology and Visual Science 2015;56(12):7260‐8. [DOI] [PubMed] [Google Scholar]
Downie 2018a
- Downie LE, Gad A, Wong CY, Gray JH, Zeng W, Jackson DC, et al. Modulating contact lens discomfort with anti‐inflammatory approaches: a randomized controlled trial. Investigative Ophthalmology and Visual Science 2018;59(8):3755‐66. [DOI] [PubMed] [Google Scholar]
Dyerberg 2010
- Dyerberg J, Madsen P, Moller JM, Aardestrup I, Schmidt EB. Bioavailability of marine n‐3 fatty acid formulations. Prostaglandins, Leukotrienes and Essential Fatty Acids 2010;83(3):137‐41. [DOI] [PubMed] [Google Scholar]
Graf 2010
- Graf BA, Duchateau GS, Patterson AB, Mitchell ES, Bruggen P, Koek JH, et al. Age dependent incorporation of 14 C‐DHA into rat brain and body tissues after dosing various 14C‐DHA‐esters. Prostaglandins Leukotrienes and Essential Fatty Acids 2010;83(2):89‐96. [DOI] [PubMed] [Google Scholar]
Hampel 2015
- Hampel U, Kruger M, Kunnen C, Garreis F, Willcox M, Paulsen F. In vitro effects of docosahexaenoic and eicosapentaenoic acid on human meibomian gland epithelial cells. Experimental Eye Research 2015;140:139‐48. [DOI: 10.1016/j.exer.2015.08.024] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8. Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Milne R, Moher D, Barbour V, Johnston M, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
IOM 2002
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty acids, Cholesterol, Protein and Amino Acids. Washington, DC: National Academies Press, 2002. [DOI] [PubMed] [Google Scholar]
Jackson 2016
- Jackson DC, Zeng W, Wong C, Vingrys AJ, Downie LE. Tear interferon‐gamma as a biomarker for evaporative dry eye disease. Investigative Ophthalmology and Visual Science 2016;57:4824‐30. [DOI] [PubMed] [Google Scholar]
James 2000
- James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. American Journal of Clinical Nutrition 2000;71(1 Suppl):S343‐8. [DOI] [PubMed] [Google Scholar]
Johnson 2009
- Johnson ME. The association between symptoms of discomfort and signs in dry eye. Ocular Surface 2009;7(4):199‐211. [DOI] [PubMed] [Google Scholar]
Jones 2017
- Jones L, Downie LE, Korb D, Benitez‐Del‐Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II Management and Therapy Report. Ocular Surface 2017;15:575‐628. [DOI] [PubMed] [Google Scholar]
Karakus 2018
- Karakus S, Agrawal D, Hindman HB, Henrich C, Ramulu PY, Akpek E. Effects of prolonged reading on dry eye. Ophthalmology 2018;125(10):1500‐5. [DOI] [PubMed] [Google Scholar]
Kris‐Etherton 2007
- Kris‐Etherton PM, Innis S, American Dietetic Association, Dietitians of Canada. Position of the American Dietetic Association and Dietitians of Canada: dietary fatty acids. Journal of the American Dietetic Association 2007;107(9):1599‐611. [PubMed] [Google Scholar]
Lemp 1995
- Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO Journal 1995;21(4):221‐32. [PubMed] [Google Scholar]
Lemp 2012
- Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous‐deficient and evaporative dry eye in a clinic‐based patient cohort: a retrospective study. Cornea 2012;31(5):472‐8. [DOI] [PubMed] [Google Scholar]
Li 2010
- Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. Journal of Ocular Pharmacology and Therapeutics 2010;26(5):431‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2011
- Li M, Gong L, Sun X, Chapin WJ. Anxiety and depression in patients with dry eye syndrome. Current Eye Research 2011;36(1):1‐7. [DOI] [PubMed] [Google Scholar]
Li 2014
- Li Z, Choi JH, Oh HJ, Park SH, Lee JB, Yoon KC. Effects of eye drops containing a mixture of omega‐3 essential fatty acids and hyaluronic acid on the ocular surface in desiccating stress‐induced murine dry eye. Current Eye Research 2014;39(9):871‐8. [DOI] [PubMed] [Google Scholar]
Liu 2014
- Liu A, Ji J. Omega‐3 essential fatty acids therapy for dry eye syndrome: a meta‐analysis of randomized controlled studies. Medical Science Monitor 2014;20:1583‐9. [DOI: 10.12659/MSM.891364] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2016
- Liu Y, Kam WR, Sullivan DA. Influence of omega 3 and 6 fatty acids on human meibomian gland epithelial cells. Cornea 2016;35(8):1122‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maguire 2018
- Maguire MG, Asbell PA. Author's response to: Bistrian BR re: n‐3 fatty acid supplementation and dry eye disease. New England Journal of Medicine 2018;379:690‐1. [DOI] [PubMed] [Google Scholar]
Massingale 2008
- Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea 2008;28(9):1023‐7. [DOI] [PubMed] [Google Scholar]
Meyer 2016
- Meyer JB. Australians are not meeting the recommended intakes for omega‐3 long chain polyunsaturated fatty acids. Nutrients 2016;8(3):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miljanovic 2005
- Miljanovic B, Trivedi KA, Dana MR, Gilbard JP, Buring JE, Schaumberg DA. Relation between dietary n‐3 and n‐6 fatty acids and clinically diagnosed dry eye syndrome in women. American Journal of Clinical Nutrition 2005;82(4):887‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2010
- Miller KL, Walt JG, Mink DR, Satram‐Hoang S, Wilson SE, Perry HD, et al. Minimal clinically important difference for the ocular surface disease index. JAMA Ophthalmology 2010;128(1):94‐101. [DOI] [PubMed] [Google Scholar]
Molina‐Levya 2017
- Molina‐Leyva I, Molina‐Leyva A, Bueno‐Cavanillas A. Efficacy of nutritional supplementation with omega‐3 and omega‐6 fatty acids in dry eye syndrome: a systematic review of randomized clinical trials. Acta Ophthalmologica 2017;95(8):e677‐e85. [DOI] [PubMed] [Google Scholar]
Nichols 2004
- Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea 2004;23(8):762‐70. [DOI] [PubMed] [Google Scholar]
Novack 2017
- Novack GD, Asbell P, Barabino S, Bergamini MV, Ciolino JB, Foulks GN, et al. TFOS DEWS II clinical trial design report. Ocular Surface 2017;15(3):629‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pflugfelder 2008
- Pflugfelder S, Paiva C, Li D‐Q, Stern ME. Epithelial–immune cell interaction in dry eye. Cornea 2008;27(1):S9‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prinsen 2014
- Prinsen CA, Vohra S, Rose MR, King‐Jones S, Ishaque S, Bhaloo Z, Adams D, Terwee CB. Core Outcome Measures in Effectiveness Trials (COMET) initiative: protocol for an international Delphi study to achieve consensus on how to select outcome measurement instruments for outcomes included in a ‘core outcome set’. Trials 2014;15:247. [DOI: 10.1186/1745-6215] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reveiz 2010
- Reveiz L, Cortes‐Jofre M, Lobos CA, Nicita G, Ciapponi A, Garcia‐Dieguez M, et al. Influence of trial registration on reporting quality of randomized trials: study from highest ranked journals. Journal of Clinical Epidemiology 2010;63(11):1216‐22. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenberg 2010
- Rosenberg ES, Asbell PA. Essential fatty acids in the treatment of dry eye. Ocular Surface 2010;8(1):18‐28. [DOI] [PubMed] [Google Scholar]
Saldanha 2018
- Saldanha IJ, Petris R, Han G, Dickersin K, Akpek E. Research questions and outcomes prioritized by patients with dry eye. JAMA Ophthalmology 2018;136(10):1170‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schaumberg 2003
- Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. American Journal of Ophthalmology 2003;136(2):318‐26. [DOI] [PubMed] [Google Scholar]
Schaumberg 2009
- Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Archives of Ophthalmology 2009;127(6):763‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schiffman 2003
- Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology 2003;110(7):1412‐9. [DOI] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Akl E, Guyatt GH, on behalf of the Cochrane GRADEing Methods Group and the Cochrane Statistical Methods Group. Chapter 11. Completing ‘Summary of findings’ tables and grading the confidence in or quality of the evidence. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Simopoulos 2002
- Simopoulos AP. The importance of the ratio of omega‐6/omega‐3 essential fatty acids. Biomedicine and Pharmacotherapy 2002;56(8):365‐79. [DOI] [PubMed] [Google Scholar]
Solomon 2001
- Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro‐ and anti‐inflammatory forms of interleukin‐1 in the tear fluid and conjunctiva of patients with dry‐eye disease. Investigative Ophthalmology and Visual Science 2001;42(10):2283‐92. [PubMed] [Google Scholar]
Stapleton 2017
- Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. Ocular Surface 2017;15(3):334‐65. [DOI] [PubMed] [Google Scholar]
Stern 1998
- Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea 1998;17(6):548‐9. [DOI] [PubMed] [Google Scholar]
Stevenson 2013
- Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune‐mediated ocular surface disorder. Archives of Ophthalmology 2012;130(1):90‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sullivan 2002
- Sullivan BD, Cermak JM, Sullivan RM, Papas AS, Evans JE, Dana RM, Sullivan DA. Correlations between nutrient intake and the polar lipid profiles of meibomian gland secretions in women with Sjögren’s syndrome. In: Sullivan DA, Stern ME, Tsubota K, Dartt DA, Sullivan RM, Bromberg BB editor(s). Lacrimal Gland, Tear Film, and Dry Eye Syndromes 3. Advances in Experimental Medicine and Biology. Vol. 506, Boston, MA: Springer, 2002. [DOI] [PubMed] [Google Scholar]
Surette 2008
- Surette ME. The science behind dietary omega‐3 fatty acids. Canadian Medical Association Journal 2008;178(2):177‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Szymanski 2010
- Szymanski KM, Wheeler DC, Mucci LA. Fish consumption and prostate cancer risk: a review and meta‐analysis. American Journal of Nutrition 2010;92(5):1223‐33. [DOI] [PubMed] [Google Scholar]
Terry 1993
- Terry RL, Schnider CM, Holden BA, Cornish R, Grant T, Sweeney D, et al. CCLRU standards for success of daily and extended wear contact lenses. Optometry and Vision Science 1993;70(3):234‐43. [DOI] [PubMed] [Google Scholar]
Uchino 2012
- Uchino Y, Uchino M, Dogru M, Ward S, Yokoi N, Tsubota K. Changes in dry eye diagnostic status following implementation of revised Japanese dry eye diagnostic criteria. Japanese Journal of Ophthalmology 2012;56(1):8‐13. [DOI] [PubMed] [Google Scholar]
van Bijsterveld 1969
- Bijsterveld OP. Diagnostic tests in the sicca syndrome. Archives of Ophthalmology 1969;82(1):10‐4. [DOI] [PubMed] [Google Scholar]
Walter 2016
- Walter SD, Gronert K, McClellan AL, Levitt RC, Sarantopoulos KD, Galor A. ω‐3 tear film lipids correlate with clinical measures of dry eye. Investigative Ophthalmology and Visual Science 2016;57(6):2472‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2018
- Wang TM, Craig JP. Core outcome sets for clinical trials in dry eye disease. JAMA Ophthalmology 2018;136(10):1180‐1. [DOI] [PubMed] [Google Scholar]
Wolffsohn 2017
- Wolffsohn JS, Arita R, Chalmers R, Djalilian A, Dogru M, Dumbleton K, et al. TFOS DEWS II Diagnostic Methodology report. Ocular Surface 2017;15(3):539‐74. [DOI] [PubMed] [Google Scholar]
Yu 2011
- Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea 2011;30(4):379‐87. [DOI] [PubMed] [Google Scholar]
Zhang 2019a
- Zhang AC, Downie LE. Preliminary Validation of a Food Frequency Questionnaire to Assess Long‐Chain Omega‐3 Fatty Acid Intake in Eye Care Practice. Nutrients 2019;11(11):E817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2019b
- Zhang AC, Silva MEH, MacIsaac RJ, Roberts L, Kamel J, Craig JP, Busija L, Downie LE. Omega‐3 polyunsaturated fatty acid oral supplements for improving peripheral nerve health: a systematic review and meta‐analysis. Nutrition Reviews 2019:pii: nuz054. [DOI] [PubMed] [Google Scholar]
Zhu 2014
- Zhu W, Wu Y, Li G, Wang J, Li X. Efficacy of polyunsaturated fatty acids for dry eye syndrome: a meta‐analysis of randomized controlled trials. Nutrion Reviews 2014;72(10):662‐71. [DOI] [PubMed] [Google Scholar]
Ziemanski 2018
- Ziemanski J, Wolters LR, Jones‐Jordan L, Nichols JJ, Nichols KK. Relation between dietary essential fatty acid intake and dry eye disease and meibomian gland dysfunction in postmenopausal women. American Journal of Ophthalmology 2018;189:29‐40. [DOI: 10.1016/j.ajo.2018.01.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ng 2014
- Ng SM, Lindsley K, Akpek EK. Omega‐3 and omega‐6 polyunsaturated fatty acids for dry eye syndrome. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD011016] [DOI] [PMC free article] [PubMed] [Google Scholar]


