Abstract
Distributed neural dysconnectivity is considered a hallmark feature of schizophrenia (SCZ), yet a tension exists between studies pinpointing focal disruptions versus those implicating brain-wide disturbances. The cerebellum and the striatum communicate reciprocally with the thalamus and cortex through monosynaptic and polysynaptic connections, forming cortico-striatal-thalamic-cerebellar (CSTC) functional pathways that may be sensitive to brain-wide dysconnectivity in SCZ. It remains unknown if the same pattern of alterations persists across CSTC systems, or if specific alterations exist along key functional elements of these networks. We characterized connectivity along major functional CSTC subdivisions using resting-state functional magnetic resonance imaging in 159 chronic patients and 162 matched controls. Associative CSTC subdivisions revealed consistent brain-wide bi-directional alterations in patients, marked by hyper-connectivity with sensory-motor cortices and hypo-connectivity with association cortex. Focusing on the cerebellar and striatal components, we validate the effects using data-driven k-means clustering of voxel-wise dysconnectivity and support vector machine classifiers. We replicate these results in an independent sample of 202 controls and 145 patients, additionally demonstrating that these neural effects relate to cognitive performance across subjects. Taken together, these results from complementary approaches implicate a consistent motif of brain-wide alterations in CSTC systems in SCZ, calling into question accounts of exclusively focal functional disturbances.
Keywords: connectivity, CSTC circuits, fMRI, machine learning, resting-state
Introduction
A fundamental challenge in clinical neuroscience is the search for robust neuroimaging biomarkers reflecting disease-related alterations in large-scale neural systems. Advances in human neuroscience offer opportunities for clinical translations and biomarker development. For instance, emerging human neuroimaging studies have identified the presence of large-scale cortico-striatal-thalamic-cerebellar (CSTC) functional pathways that are stable and replicable across hundreds of individuals (Buckner et al. 2011; Yeo et al. 2011; Choi et al. 2012). Such pathways may be particularly sensitive to disruptions in psychiatric illnesses such as schizophrenia (SCZ), which has been conceptualized as a disorder of synaptic communication affecting distributed neural systems. Leading theoretical models of SCZ propose that disruptions in local microcircuit excitation (E) and inhibition (I) neuronal balance, due to altered synaptic signaling, may underlie noted alterations across neural systems (Stephan et al. 2006; Murray, Anticevic, et al. 2014; Yang, Murray, Wang, et al. 2016). One possibility is that such alterations occur only in select CSTC functional pathways if such pathways are more vulnerable to the upstream microcircuit perturbations. Alternatively, such synaptic perturbations may generalize across distributed CSTC pathways. Hence, a major gap in knowledge concerns whether neural disturbances in SCZ manifest brain-wide across CSTC functional pathways, or if they occur in only select regions of these circuits. Addressing this tension is critical to informing neuro-marker development that can guide treatment for either targeted neuropathology in specific areas or diffuse alterations that span CSTC pathways.
One widely replicated effect in SCZ neuroimaging is the disruption in functional relationships involving the thalamic resting-state blood-oxygen-level-dependent (BOLD) signal (Ramsay and MacDonald 2018). The thalamus consists of topographically organized nuclei that are densely connected with other brain areas (Ray and Price 1993; Haber and McFarland 2001; Klein et al. 2010), making it highly sensitive to widespread disturbances neural disturbances. Specifically, several independent replications have established a bi-directional pattern of dysconnectivity with the thalamus, characterized by elevated thalamic coupling with sensorimotor brain regions and reduced thalamic coupling with associative cortical areas (Welsh et al. 2010; Woodward et al. 2012; Anticevic, Cole, et al. 2014). These effects generalize across patients with chronic SCZ and prodromal individuals at ultra-high-risk for developing SCZ (Anticevic et al. 2015). Critically, these effects are most pronounced along higher-order associative thalamic functional subdivisions (e.g., mediodorsal nucleus). Multiple theoretical models have proposed that these disruptions in thalamic functional coupling may be a hallmark of the illness (Lisman et al. 2010). Yet, it remains unknown if these disrupted patterns manifest across the entire CSTC functional pathway or if they are exclusive to the thalamus. If the thalamic effect is indeed specific, then similar patterns would not be expected to emerge across other CSTC systems as robustly. However, if SCZ involves synaptic neural alterations that are not exclusive to the thalamus and affect other CSTC components, then we hypothesize that the described bi-directional effects may emerge across brain-wide CSTC systems, reflecting shared disruption. Previous studies have identified alterations in isolated regions of the basal ganglia and cerebellum in SCZ (Andreasen et al. 1996; Holt et al. 2005; Konarski et al. 2005; Rusch et al. 2007; Collin et al. 2011; Liu et al. 2011; Tu et al. 2012; Wang et al. 2014; Sarpal et al. 2015, 2016), but to date none have examined whether disturbances in functional relationships are unified across these neural systems.
A way to close this knowledge gap involves leveraging existing resting-state findings in healthy humans that have defined large-scale functional networks across cortical, striatal, and cerebellar neural territories (Buckner et al. 2011; Yeo et al. 2011; Choi et al. 2012). This work implicated a shared functional architecture across the CSTC systems that can be used to assay patterns of changes across each functional CSTC subdivision in SCZ. We focus here on the striatum and cerebellum, as using the striatal or cerebellar elements of each functional network allows us to characterize altered functional relationships across CSTC systems while bypassing the thalamus as a starting “seed” point. Notably, the cerebellum does not share monosynaptic anatomical connections with cortex, thalamus or striatum (hence is not a part of the anatomically-defined cortico-striatal-thalamic-cortical loop). However, it is highly functionally connected these areas via multiple pathways, as evidenced by stable and cohesive large-scale functional networks across these neural territories (Buckner et al. 2011; Yeo et al. 2011; Choi et al. 2012). Hence, the cerebellum serves as an important “control” in our study: if cerebellar effects are seen, it would support the hypothesis that disruptions in SCZ are pervasive across functional brain-wide networks, and not limited by thalamic or striatal anatomy per se. Even if such patterns are revealed, it is unknown known if these “bi-directional” dysconnectivity CSTC effects may relate to psychosis symptoms or cognitive deficits. Prior work has demonstrated that thalamic dysconnectivity may present a robust cognitive remediation target in SCZ (Ramsay et al. 2017). Therefore, we tested the hypothesis that, if observed, CSTC dysconnectivity may be predictive of cognitive impairment in patients.
In summary, we report robust bi-directional CSTC dysconnectivity patterns that are prominent across the cerebellar and striatal functional subdivisions, including the thalamus and cortex. This CSTC dysconnectivity in SCZ is inconsistent with the possibility of exclusive thalamic disruptions. Results replicated and revealed most pronounced alterations along higher-order associative divisions of the CSTC networks, using both an independently defined a priori parcellation and a data-driven clustering approaches. The severity of CSTC dysconnectivity was related to cognition but not psychosis symptoms. Collectively, these effects implicate distributed associative CSTC alterations in SCZ in support of brain-wide disruptions in information flow that may preferentially relate to severity of cognitive deficits.
Materials and Methods
Primary Dataset
For the primary analyses, we analyzed data from 159 patients with chronic SCZ and 162 demographically-matched healthy controls (Table 1) recruited from the Olin Neuropsychiatry Research Center and from a publicly distributed dataset provided by the Center for Biomedical Research Excellence (COBRE). All subjects met identical methodological stringency criteria and underwent identical analyses. Of note, because each independent sample included both patients and matched controls, we collapsed the 2 samples for our analyses. Additional details are given in Supplementary Materials and Methods.
Table 1.
Discovery sample—clinical and demographic characteristics
| Characteristic | CON (N = 162) | SCZ (N = 159) | Significance | |||
|---|---|---|---|---|---|---|
| M | SD | M | SD | T Value/Chi -Square | P Value (two-tailed) | |
| Age (years) | 32.92 | 12.08 | 35.15 | 12.78 | 1.60 | 0.11 |
| Gender (% male) | 67 | 77 | 1.62 | 0.20 | ||
| Parental education | 4.71 | 1.82 | 4.33 | 1.96 | 1.80 | 0.07 |
| Participant’s education | 5.09 | 1.47 | 4.02 | 1.45 | 6.56* | <0.0001 |
| Handedness (% right) | 92.00 | 83.65 | 5.21* | 0.02 | ||
| Signal-to-noise (SNR) | 215.67 | 63.30 | 204.56 | 77.60 | 1.41 | 0.16 |
| % Frames Flagged | 14.21 | 15.79 | 23.53 | 23.60 | 4.17* | <0.0001 |
| Medication (CPZ equivalents) | — | — | 289.16 | 255.23 | — | — |
| PANSS Positive Symptoms | — | — | 15.38 | 4.76 | — | — |
| PANSS Negative Symptoms | — | — | 14.42 | 5.10 | — | — |
| PANSS General Psychopathology | — | — | 29.75 | 7.63 | — | — |
| PANSS Total Psychopathology | — | — | 59.57 | 13.78 | — | — |
Demographics for discovery sample. CON, control subjects; SCZ, Patients diagnosed with schizophrenia; PANSS, Positive and Negative Syndrome Scale; M, Mean; SD, Standard Deviation. Education level for the COBRE sample was determined based on the following scale: Grade 6 or less=1; Grade 7–11 = 2; high school graduate=3; attended college=4; graduated 2-year college=5; graduated 4-year college=6; attended graduate or professional school=7; Completed graduate or professional school=8. Olin education data were converted from “years of education” to the COBRE education scale in order to permit combining of education demographic data. Parental education for the Olin set is the average of the mother and father’s education. CPZ, Chlorpromazine equivalents were calculated according to latest validated approaches (Andreasen et al. 2010). SNR (signal-to-noise ratio) was determined by obtaining the mean signal and standard deviation for a given slice across the relevant BOLD run, while excluding all non-brain voxels across all frames (Anticevic, Repovs, et al. 2012). An * denotes a significant T statistic for the between-group t-test, uncorrected for multiple comparisons.
Replication Dataset
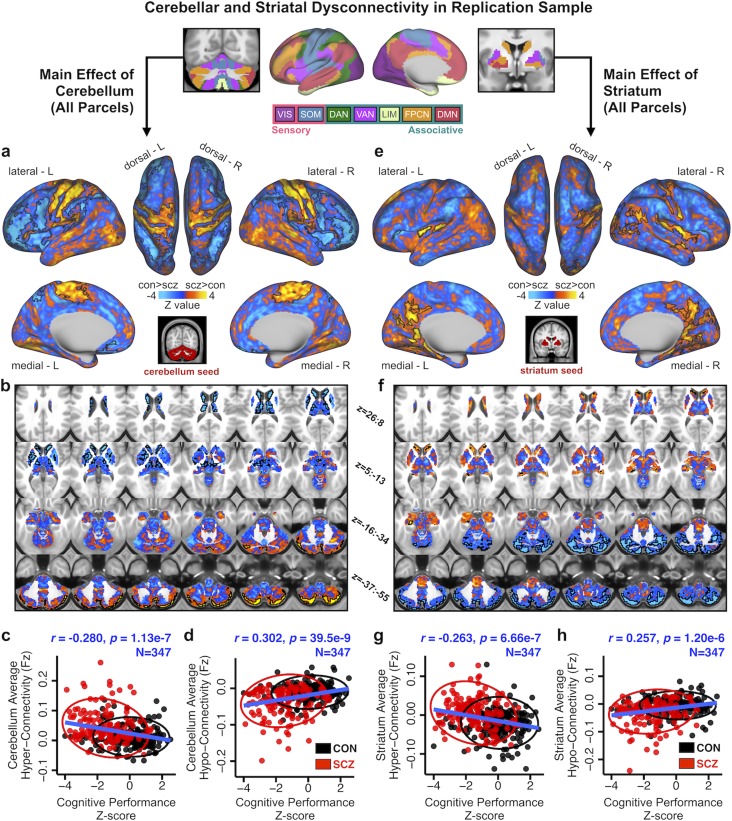
The replication and extension sample was obtained from the Bipolar–Schizophrenia Network on Intermediate Phenotypes (B-SNIP) dataset in collaboration with the BSNIP Principal Investigators. B-SNIP participants were collected across 6 different sites in the USA. Full details on the B-SNIP data acquisition and characterization of site effects have been previously rigorously characterized (Tamminga et al. 2013; Meda et al. 2015; Sheffield et al. 2017). Briefly, subjects were excluded if they had (i) a history of seizures or head injury resulting in >10 min loss of consciousness, (ii) positive drug screen on the day of testing, (iii) a diagnosis of substance abuse in the past 30 days or substance dependence in the past 6 months, (iv) history of serious medical or neurological disorder that would likely affect cognitive functioning, (v) history of serious medical or neurological disorder that would likely affect cognitive functioning, (vi) insufficient English proficiency, or (vii) an age-corrected Wide-Range Achievement Test (4th edition) reading test standard score <65. Additionally, patients were required to have had no change in medication and been clinically stable over the past month. Demographic information for this dataset is given in Supplementary Table S1. Details on acquisition parameters across the 6 sites are given in Supplementary Table S2. After preprocessing and quality control (described below), data from 145 patients with SCZ and 202 healthy controls were included. Critically, participants in the B-SNIP dataset underwent the Brief Assessment of Cognition in Schizophrenia (BACS) battery, which provided an assessment of cognitive functioning (Keefe et al. 2004). The composite scores used here are presented as standardized Z-scores normalized to mean and standard deviation of the control group and were used to test the hypothesis that, if observed, CSTC dysconnectivity may be predictive of cognitive impairment in patients.
Symptoms and Medication
The severity of SCZ symptoms was assessed using the Positive and Negative Syndrome Scale (PANSS; Kay et al. 1987), which includes positive, negative, and general psychopathology symptom dimensions (Kay et al. 1987) (Table 1). Seventy-five of the 90 SCZ patients in the Olin sample were receiving antipsychotic treatment, and all patients in the COBRE sample were receiving stable doses of antipsychotic medication with no medication changes for at least 1 month. All medications were converted to chlorpromazine equivalents and used as covariates (Andreasen et al. 2010).
Neuroimaging Data Acquisition
Neuroimaging data acquired at the Olin Neuropsychiatry Research Center were obtained using a Siemens–Allegra 3-T scanner, with axial slices parallel to the anterior–posterior commissure (AC–PC) using a T2*-weighted gradient-echo, echo-planar sequence [time repetition (TR)/time echo (TE) = 1500/27 ms, flip angle = 60°, field of view = 24 × 24 cm, acquisition matrix = 64 × 64, voxel size = 3.43 × 3.43 × 4 mm], ensuring whole-brain coverage. The acquisition lasted 5.25 min and produced 210 volumetric images per subject (29 slices/volume, inter-slice gap = 1 mm). Subjects were instructed to lay awake in the scanner and keep their eyes open. Subjects were monitored on a video camera to ensure that they stayed awake and were removed from analyses if they fell asleep during the scan, or if their head movement >1 mm along any axis. Structural images were acquired using a T1-weighted, 3D magnetization-prepared rapid gradient-echo sequence (TR/TE/time to inversion = 2200/4.13/766 ms, flip angle = 13°, voxel size (isotropic) = 0.8 mm, image size = 240 × 320 × 208 voxels), with axial slices parallel to the AC–PC line. Subjects that comprised the COBRE sample underwent data collection at Center for Biomedical Research Excellence using a Siemens Tim-Trio 3 T scanner. Full acquisition details for the COBRE SCZ sample and CON have been detailed previously (Yang, Murray, Wang, et al. 2016). Briefly, BOLD signal was collected with 32 axial slices parallel to the AC–PC using a T2*-weighted gradient-echo, echo-planar sequence (TR/TE = 2000/29 ms, flip angle = 75°, acquisition matrix = 64 × 64, voxel size = 3 × 3 × 4 mm). The acquisition lasted 5 min and produced 150 volumetric images per subject. Structural images were acquired using a 6-min T1-weighted, 3D MPRAGE sequence (TR/TE/TI = 2530/[1.64, 3.5, 5.36, 7.22, 9.08]/900, flip angle = 7°, voxel size (isotropic) = 1 mm, image size = 256 × 256 × 176 voxels) with axial slices parallel to the AC–PC line. Details on acquisition parameters across the 6 sites comprising the replication B-SNIP sample are given in Supplementary Table S2.
Data Preprocessing and Analysis
For the primary dataset, all preprocessing followed previously validated approaches (Repovs et al. 2011; Anticevic, Gancsos, et al. 2012; Anticevic, Repovs, et al. 2012; Repovs and Barch 2012) to ensure continuity with reported thalamic effects. Critically, the B-SNIP “replication” dataset was preprocessed in accordance with the Human Connectome Project (HCP) minimal preprocessing pipeline (Glasser et al. 2013). We made the choice to use HCP pipelines for the replication dataset (as opposed to processing methods in the primary dataset following published procedures) to ensure generalizability of effects across pipelines choices. This allowed us to verify 2 key observations: (i) if the prior thalamic generalizes across the CSTC systems using prior methods; (ii) if the effects replicate and remain robust to preprocessing pipeline choices. Details on the HCP adaptation for “legacy” B-SNIP data are comprehensively described in Supplementary data.
Irrespective of pipeline choice, all data underwent consistent standard practices processing procedures. BOLD data underwent: (i) slice-time correction, (ii) first 5 images removed from each run, (iii) rigid-body motion correction, (iv) transform of the structural images to the standard template, and (v) co-registration of BOLD volumes to the standard structural image template. All structural data underwent FreeSurfer’s recon-all pipeline to compute brain-wide segmentation of grey and white matter, which was used to define anatomical nuisance regressors and brain-wide grey matter masks. Additionally, all BOLD images had to pass stringent quality assurance criteria to ensure that all functional data were of comparable and high quality. We excluded images with signal-to-noise ratios (SNR) <90 (1.5 standard deviations below the mean SNR), computed by obtaining the mean signal and standard deviation (SD) for a given slice across the BOLD run, while excluding all non-brain voxels across all frames (Anticevic, Brumbaugh, et al. 2012) (see Table 1). To remove sources of spurious correlations present in resting-state BOLD data, all time-series were high-pass filtered (only signal below 0.08 Hz was retained). The following regressors were used in additional BOLD de-noising: (i) nuisance signal removal from ventricles, (ii) deep white matter, (iii) global grey matter mean signal (GMS), (iv) 6 rigid-body motion correction parameters, and their first derivatives. Finally, data were additionally low-pass temporal filtered (only signal above 0.009 Hz was retained). In addition, we implemented “movement scrubbing” as recommended by (Power et al. 2012). Movement scrubbing refers to the practice of removing BOLD volumes that have been flagged for high motion in order to minimize movement artifacts. Specifically, all frames with possible movement-induced artifactual fluctuations in intensity were identified via 2 criteria: (i) frames in which the sum of the displacement across all 6 rigid-body movement correction parameters exceeded 0.5 mm (assuming 50-mm cortical sphere radius) were identified; (ii) root mean square (RMS) of differences in intensity between the current and preceding frame was computed across all voxels divided by mean intensity and normalized to time-series median. Frames in which normalized RMS exceeded the value of 3 were identified. The frames flagged by either criterion were marked for exclusion (logical or), as well as the one preceding and 2 frames following the flagged frame. Importantly, levels of motion and SNR did not relate to reported effects (Supplementary Figs S7–S9).
Network and Parcel Terminology
Throughout our analyses we make a distinction between the sensory networks (VIS, visual; SOM, somatomotor) and the associative networks (DAN, dorsal attention; VAN, ventral attention; LIM, limbic; FPCN, frontoparietal control; and DMN, default mode). We use the term “parcel” to refer to sets of voxels in the cerebellum or striatum that are defined by their membership to one of the functional networks. For instance, the cerebellar FPCN parcel is the set of voxels that comprises the FPCN network subdivision in the cerebellum. Put differently, the FPCN network refers to the brain-wide set of voxels that belong to this network, whereas the cerebellum FPCN “parcel” is the set of voxels within the cerebellum that belong to the FPCN, as defined by Buckner and colleagues. Similarly, the striatum FPCN “parcel” is the set of striatal voxels that belong to the FPCN, as defined by Choi and colleagues.
Seed-Based Functional Connectivity (fcMRI) analyses
Throughout, parallel analyses were conducted independently for the cerebellum and the striatum parcels. In-house tools (Repovs et al. 2011) were used to compute whole-brain correlation maps by extracting average time-series across all voxels within a given subject for each of the 7 cerebellar and 6 striatal parcels as defined by Buckner et al. (2011) and Choi et al. (2012). Importantly, we did not seed each possible isolated region from the cerebellar and striatal solution, but rather treated all the FPCN-belonging voxels in the cerebellum (i.e., cerebellum FPCN parcel) as a “seed”. The same logic applies to the striatum.
This average striatal or cerebellar signal for each element/parcel was then correlated with each grey matter voxel in the brain. The computed Pearson correlation values were transformed to Fisher Z-values (Fz) using a Fisher’s r-to-Z transform. This yielded 7 cerebellar and 6 striatal whole-brain Fz maps for each subject, one for each parcel seed. For every such map each voxel’s value represents connectivity with the original cerebellar or striatal parcel.
We then computed group average connectivity maps for each parcel for each group. To test the central hypotheses, the 7 cerebellum parcel-seeded maps were entered into a 2 × 7 mixed model ANOVA with one between-group factor (SCZ vs. CON) and one within-subject factor (7 cerebellar parcels). We evaluated whole-brain type I error protected effects using non-parametric techniques implemented with the Permutation Analysis of Linear Models package (PALM, see Smith and Nichols 2009; Winkler et al. 2014). The entire general linear model was permuted 2 000 times with tail-approximation (Winkler et al. 2016) to obtain null distributions for every test. To circumvent the need to define clusters using arbitrary thresholds for cluster size, we used threshold-free cluster-enhancement (TFCE, see (Smith and Nichols 2009)). Resulting statistical images were then thresholded at whole-brain corrected P < 0.05 (controlled for family-wise error). A similar analysis was performed for striatum parcel-seeded maps but with a 2 × 6 mixed model ANOVA design given that the striatum contained 6 parcels. Results were visualized using the Caret 5.5 software (http://brainvis.wustl.edu/wiki/index.php/Caret), NeuroLens software (http://www.neurolens.org) and the Connectome Workbench software (https://www.humanconnectome.org/software/connectome-workbench.html).
Quantifying the Overlap Between Cerebellar and Striatal Main Effects of Group
We performed a conjunction analysis by testing for regions that were significantly hyper- or hypo-connected (P < 0.05) in both the cerebellar and the striatal main effects of parcel (Fig. 2c and d). We tested for the significance of this overlap using the hypergeometric test, given the total number of voxels in the brain and the number of significant voxels in the striatal and cerebellar maps (referred to as Map 1 and Map 2 below, as the order is arbitrary and interchangeable). We performed the test separately for the 2 hyper-connectivity maps and the 2 hypo-connectivity maps. The hypergeometric distribution describes the probability of drawing k successes in n sequential trials without replacement, in a population of size N containing K successes. The hypergeometric distribution is similar to the binomial distribution, which is used in overlap analyses in the neuroimaging field (Ramsey et al. 1996; Gazzola and Keysers 2009; Anticevic, Cole, et al. 2014). However, the binomial distribution assumes that samples are replaced after drawing and therefore assumes that trials are independent, whereas the hypergeometric does not. Hence, the probability of obtaining k overlapping significant voxels between Map 1 and Map 2 can be found using the formula:
| (1) |
where k is the number of overlapping voxels, n is the number of significant voxels in Map 1, K is the number of significant voxels in Map 2, and N is the total number of voxels in the brain. is a binomial coefficient and is calculated using:
| (2) |
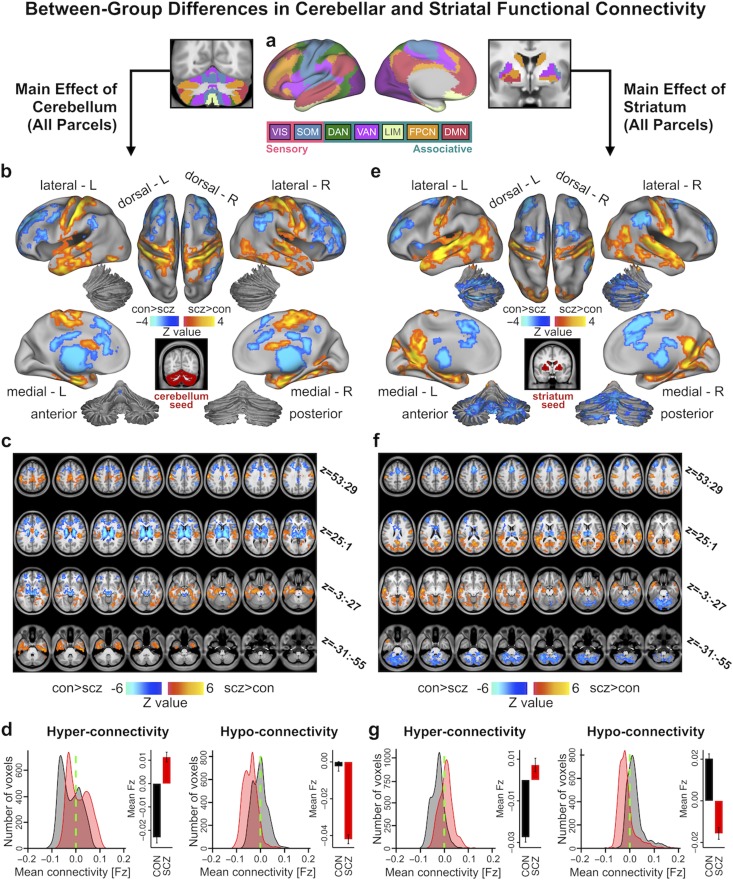
Figure 2.
Main effect of cerebellar and striatal network parcel connectivity in SCZ. (a) Functionally-defined networks obtained from 1000 resting-state scans (first defined and independently replicated in 500 scans, respectively) in the cerebral cortex, cerebellum and striatum (Buckner et al. 2011; Yeo et al. 2011; Choi et al. 2012). Whole-brain functional connectivity was computed from each of these 7 network parcels in the cerebellum (6 in striatum) and quantified in a Group (SCZ vs. CON group) x Parcel (VIS, SOM, DAN, VAN, LIM, FPCN, and DMN) ANOVA. Networks were broadly grouped as either sensory or associative. (b) Cortical surface view of areas showing significant main effect of Group in whole-brain connectivity with the cerebellar parcels (P < 0.05 TFCE whole-brain corrected) between 159 patients with chronic schizophrenia (SCZ) and 162 healthy controls (CON). Orange/yellow areas indicate regions where patients exhibited stronger cerebellar connectivity, whereas blue areas indicate regions where patients exhibited reduced cerebellar connectivity, relative to controls. Inset shows coverage of all cerebellar parcels. (c) Volume-based axial view of cerebellar connectivity group differences in b with Z-coordinate ranges (each slice in each row increments by 3 mm). (d) Distribution of connectivity strength (Fz) values within voxels showing significant hyper- and hypo-connectivity in SCZ and CON. Bar plots show mean connectivity averaged across all voxels in hyper- and hypo-connected areas. (e–g) Results for identical independent analysis conducted with the 6 functionally-defined striatal network parcels. Note that there was no representation of the visual network in striatum (Choi et al. 2012) and therefore that network was omitted. Abbreviations: VIS, visual network; SOM, somatosensory; DAN, dorsal attention network; VAN, ventral attention network; LIM, limbic network; FPCN, frontoparietal control network; DMN, default mode network.
The expected value for the hypergeometric distribution, here the expected number of voxels in the overlap between Maps 1 and 2 due to chance, is:
| (3) |
When k is greater than , the probability of observing k or more voxels is obtained from the cumulative distribution function:
| (4) |
We then calculated exact 95% confidence intervals for the expected value of the hypergeometric distribution using the Clopper–Pearson approach (Clopper and Pearson 1934). The upper limit at confidence level is given such that:
| (5) |
and the lower limit is given such that:
| (6) |
Furthermore, to account for the dependence between brain voxels, we estimated the smoothness of the data (using AFNI’s 3dFWHMx program and the autocorrelation function (ACF) estimate). This yielded the effective full-width half-maximum (FWHM) of the data, which we rounded up to 24 mm, or 8 voxels to remain conservative. We then computed spheres of radii equivalent to this FWHM as voxels beyond the edge of the sphere cannot have any spatial dependency on a voxel in the center. We then re-computed the significance of overlap using the hypergeometric distribution by randomly sampling spheres rather than individual voxels. We show that even with this extremely conservative approach, the overlap is greater than expected (see Supplementary Fig. S52).
K-means Clustering of Group Dysconnectivity
We first identified all grey matter voxels using FreeSurfer segmentation and computed the functional connectivity of every cerebellar and striatal “seed” voxel with all other grey matter voxels in the brain. The grey matter mask was defined based on FreeSurfer segmentation codes. To ensure adequate grey matter signal, we included only voxels where at least 25% of all subjects had grey matter in the cortex and thalamus and 10% in the cerebellum and striatum (Cole et al. 2011; Anticevic, Brumbaugh, et al. 2012; Anticevic, Cole, et al. 2014). Hence, a whole-brain connectivity map was computed for each “seed” voxel in the striatum and the cerebellum. Three subjects were excluded from the clustering analyses due to incomplete coverage at the most inferior slices of the cerebellum. Next, we applied a Fisher’s r-to-Z transform to all of the subject-specific voxel-seeded connectivity maps and then computed group-level maps by averaging each seed voxel’s maps across SCZ and CON subjects. We then computed a group difference whole-brain “dysconnectivity” map for each seed voxel (note that every seed voxel in the cerebellum/striatum is still associated with a whole-brain map, and all of these maps are of the same dimensions). The k-means clustering algorithm was then applied on these difference maps to obtain clusters of seed voxels with the most similar patterns of whole-brain disturbance. Specifically, the distance measure used was the 1 − r, where r is the Pearson correlation in BOLD time-series between each seed cerebellar or striatal voxel and all other voxels in the brain. Hence, when the k-means clustering algorithm was applied it identified k clusters that group together the voxels with the most similar patterns of whole-brain dysconnectivity. To minimize the possibility of the algorithm being caught in a local minimum, the clustering for each k was repeated 10 times with different random starting values and the solution with the smallest within-cluster distances was accepted (Nanetti et al. 2009; Cauda et al. 2011; Anticevic, Cole, et al. 2014). See Supplementary Figure S18 for a schematic of the k-means clustering workflow. Notably, because there is no known a priori number of cerebellar or striatal subdivisions with distinct patterns of SCZ-related dysconnectivity, we examined a range of k values and highlight the k = 7 solution (Supplementary Figs S19c and S20) so that comparisons can be made with the Yeo/Buckner parcellation, which contains 7 functional networks. Alternative solutions (k = 4 and k = 6) are provided in Supplementary Figure S19a–b. For similar reasons, we highlight the k = 6 solution for the striatum (Supplementary Figs S19e and S21). Alternative striatal clustering solutions (k = 4 and k = 7) are presented in Supplementary Figure S19d and f.
Dissimilarity in Cerebellar and Striatal Voxel-wise Connectivity
We also examined the voxels in the cerebellum and striatum with the greatest difference between groups in coherence of BOLD fluctuations with the rest of the brain. Here, we used the eta2 index, which quantifies the pattern of similarity between 2 signals. Eta2 is calculated by:
| (7) |
where and correspond to connectivity at position i (in this case, a given voxel in the cerebellum or striatum) for Maps a and b, respectively (in this case, SCZ and CON connectivity maps); corresponds to the mean value of the 2 images at position i, i.e., and represents the grand mean value across the mean image (designated by m). We converted the index to 1 − eta2 in order to show the degree of dissimilarity in functional connectivity in each voxel. Hence, a 1 − eta2 value of 1 indicates no similarity between the 2 signals, and a 1 − eta2 of 0 indicates perfect signal similarity. While 1 − r reflects dissimilarities in the overall patterns of connectivity between the 2 maps, 1 − eta2 also reflects differences in the connectivity strength for voxels with similar patterns of connectivity.
Single-Feature Support Vector Machine Binary Classification of Diagnostic Status
We used support vector machine (SVM) binary classifiers to test whether individual cerebellar and striatal connectivity features could be used to distinguish between patients and controls. We masked each subject’s 13 individual seed-based connectivity maps with the significantly hyper-connected and hypo-connected regions from the main effect of group. We then calculated each subject’s average connectivity strength (Fz) within the hyper-connected mask and hypo-connected mask for each parcel’s functional connectivity map, resulting in 26 values per subject. The average hyper-connectivity and hypo-connectivity Fz for each network were then linearly combined, resulting in 13 values per subject, and each of these was used as a single feature to train an SVM (i.e., the 13 parcel connectivity features were used to train 13 separate classifiers; Supplementary Figs S23 and S24). We also trained a classifier on the average connectivity across all cerebellar parcels and on the average connectivity across all striatal parcels. Lastly, we trained classifiers using a linear combination of both the cerebellar and striatal parcel connectivity from each of the 6 networks (VIS was excluded as it had no striatal component) as well as the average connectivity of all 13 parcels (Supplementary Fig. 25). Hence, there were a total of 13 individual parcel + 1 average cerebellum parcels + 1 average striatum parcels + 6 combined + 1 average all parcels = 22 single-feature classifiers. For each classifier, we ran 1 000 cross-validation runs. In each run, a subset of subjects (50%) was randomly selected and used to train the classifier, which was then tested on the remaining subset of subjects.
We employed receiver operating characteristic (ROC) curves to quantify the performance of the obtained classifiers. The ROC curve plots the true positive rate (TPR) against the false positive rate (FPR). TPR, or sensitivity, was calculated as the proportion of SCZ subjects correctly diagnosed as SCZ by the classifier. FPR, which is equal to (1–specificity), was calculated as the proportion of CON subjects incorrectly diagnosed as SCZ by the classifier. A “perfect” classifier that can accurately predict all new cases will thus have a point at (0,1) where TPR = 1.00 (100% sensitivity) and FPR = 0.00 (100% specificity), whilst a classifier operating at chance will fall along the 45° diagonal (“line of no discrimination”). We also calculated the Area Under the Curve (AUC), the area between the ROC curve and the X-axis, for all 1000 runs of each classifier. The AUC is a summary statistic that can be interpreted as the probability that the classifier will accurately classify a randomly drawn pair of SCZ and CON subjects.
Finally, we performed hyperparameter optimization of the SVM by repeating the process reported above using a range of parameters. Specifically, we tested soft margins (C) from 1.0e-5 to 1.0e5 at incrementing orders of magnitude. We also tested linear and nonlinear (Gaussian radial basis function with sigma=1) kernels. Additionally, we ran cross-validation using a 80%/20% split (as opposed to 50%/50%) between training and test samples. These parameters were first validated within the discovery dataset; notably, no other set of model parameters performed significantly better than the version reported here. This SVM, which was trained and optimized within the discovery dataset, was then tested on the independent replication dataset.
Multi-feature SVM
To test if interactive effects between specific combinations of features contribute additional diagnostically relevant information, we used all 13 cerebellar and striatal parcel features to train one classifier in a multidimensional feature space (i.e., there were 13 values per subject). To perform feature selection, we used stepwise backward elimination, as follows: we ran 10 000 cross-validation runs on the 13-feature classifier and extracted the weights of each individual feature. We then removed the feature with the smallest absolute weight (i.e., the least discriminatory feature between SCZ and CON, here the striatal SOM); reran the 12-feature classifier; and calculated the mean AUC. We repeated this stepwise process, each time removing the least discriminatory network feature from the remaining features, until only one (here the striatal FPCN) was left. We also explicitly tested the interactive effect between the 2 features that were assigned the strongest weights in the multi-feature classifier, by adding the 4-way interaction ([striatum FPCN] × [cerebellum DAN]; [striatum DAN] × [cerebellum FPCN]; [striatum FPCN] × [striatum DAN]; [cerebellum FPCN] × [cerebellum DAN]) as 4 additional features.
Data Sharing and Availability
Given the extent of our results, we have not presented our complete analyses in this manuscript. For access to analyses and images not displayed in full here, please contact jielisa.ji@yale.edu.
Results
Bi-directional Brain-Wide Cerebellar and Striatal Dysconnectivity is Observed in SCZ
Due to the length and scope of this study, a summary of our main hypotheses and findings, as well as the supporting figures and Supplementary data, is provided in Figure 1. First, to test whether SCZ is associated with uniform disturbances across CSTC networks, we quantified the functional connectivity of functionally distinct subdivisions in the cortex, thalamus, cerebellum and striatum, defined by a parcellation by Yeo et al. (2011); Buckner et al. (2011) and Choi et al. (2012). This parcellation, which was identified in 1000 healthy adults and shown to be robust and replicable (Buckner et al. 2011; Yeo et al. 2011; Choi et al. 2012), contains both sensory networks (visual, VIS and somatomotor, SOM) and associative networks (dorsal attention, DAN; ventral attention, VAN; limbic, LIM; frontoparietal control, FPCN; and default mode, DMN) (Supplementary Fig. S1). Below, we use the term “parcel” to refer to a subdivision of the cortex, thalamus, striatum, or cerebellum belonging to 1 of the 7 functional networks. Of note, no representation of the visual network was discovered in the striatum (Choi et al. 2012); hence only 6 striatal parcels were used for all striatal analyses, and no representation of the limbic network was found in the thalamus. Figure 2a shows the cortical (middle panel), cerebellar (left), and striatal (right) parcels of the 7 functional networks. Thalamic results are shown in Supplementary Figure S10.
Figure 1.
Summary of core questions and findings. Table of contents outlining the main questions studied in the present paper, the relevant figures in the main text and Supplemental data, and the core results.
We studied a well-powered sample of patients diagnosed with chronic SCZ (N = 159) and matched healthy controls (CON, N = 162; see Table 1 for detailed demographics). First, we computed a 2 × 7 Group x Parcel ANOVA to test for differences in functional connectivity across the 7 cerebellar network parcels between SCZ and CON groups (see Materials and Methods). Supplementary Fig. S2 shows the unthresholded Z-maps of all seeds, which show the expected general patterns of connectivity (e.g., VIS with primary visual cortex and FPCN with frontal cortex). All reported whole-brain effects survived non-parametric threshold-free-cluster-enhancement (TFCE) correction (Smith and Nichols 2009) with 2 000 permutations (see Materials and Methods) and were not driven by site effects (Supplementary Figs S42–S43, Supplementary Materials and Methods). Henceforth, we use the term “dysconnectivity” to denote the difference between SCZ and CON groups in the statistical covariation of BOLD signals over time across regions. Between-group differences revealed cerebellar hyper-connectivity with somatomotor cortex in SCZ relative to CON, and hypo-connectivity with prefrontal cortex, thalamus and striatum (Fig. 2b–c; see Supplementary Fig. S4a–b for unthresholded maps). Figure 2d highlights the shift in the distribution of cerebellar connectivity strengths in SCZ for both hyper-connected and hypo-connected regions and the group difference in mean connectivity of these regions. An independent 2 × 6 Group x Parcel ANOVA was computed for the 6 functional striatal parcels (see Supplementary Fig. S3 for unthresholded Z-maps of all parcels). Between-group differences revealed qualitatively similar dysconnectivity patterns as with cerebellar parcels, namely hyper-connectivity with somatomotor cortex and hypo-connectivity with frontal cortex, thalamus, and cerebellum (Fig. 2e–g; see Supplementary Fig. S4c–d for unthresholded map). Repeating these analyses without global signal regression did not alter key effects (Supplementary Fig. S5). As expected, differences across parcels (i.e., main effect of Parcel irrespective of group) revealed widespread differential coupling for both cerebellar and striatal analyses, indicating that connectivity patterns differ across functional parcels (Supplementary Fig. S6). Critically, the reported between-group effects were not explained by smoking status, head motion, signal-to-noise ratio, or medication dose (Supplementary Figs S7, S46). Between-group effects adjusted for motion and signal-to-noise, as well as the effects of these covariates, are reported in Supplementary Figs S8–S9.
To verify that the bi-directional pattern of dysconnectivity is in fact pervasive across CSTC systems, we conducted a similar analysis for functional connectivity of thalamic parcels (Supplementary Fig. S10, Supplementary Materials and Methods). These results replicate earlier findings of bi-directional thalamic connectivity in SCZ (including results previously reported in a subset of the same subjects used in present analyses) (Welsh et al. 2010; Woodward et al. 2012; Anticevic, Cole, et al. 2014). Additionally, we studied the dysconnectivity of the entire CSTC functional pathway, simultaneously seeding from all parcels of the same network across cortex, striatum, thalamus, and cerebellum. Here, we examined the effects of specific circuits, such as the CSTC-wide FPCN (Supplementary Fig. S11) because combining all networks across CSTC systems lacks specificity (i.e., it includes virtually all grey matter). As hypothesized, the bi-directional pattern of dysconnectivity was again observed, suggesting that these disturbances in functional relationships are indeed unified across neural systems. As noted, thalamic and cortical dysconnectivity in SCZ has been previously well-characterized (Welsh et al. 2010; Woodward et al. 2012; Anticevic, Cole, et al. 2014; Baker et al. 2014) but never extended across CSTC. Therefore, we focus on the cerebellar and striatal components of the CSTC systems to examine similarity of disturbances.
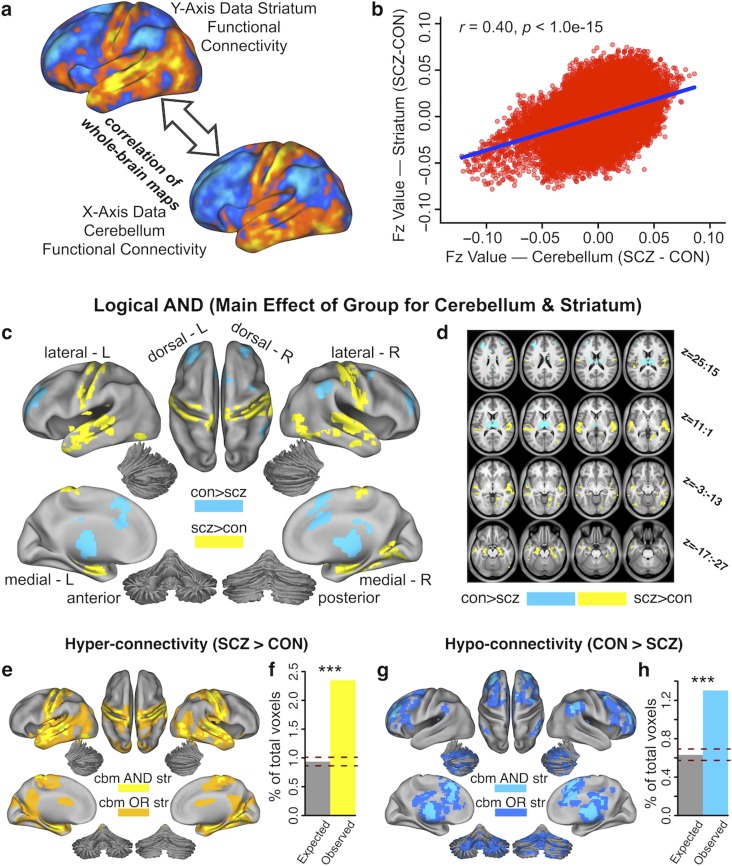
Given the marked qualitative similarity between the cerebellar and striatal dysconnectivity maps (Fig. 3a, Supplementary Fig. S4), we formally quantified the overlap between the 2 independent a priori analyses. This was achieved by computing the correlation between the 2 group difference maps (expressed as difference in Fz values), but after removing cerebellar and striatal voxels from their respective maps. A significant positive relationship (r = 0.40, P < 0.001) indicated high similarly between striatal and cerebellar dysconnectivity in SCZ (Fig. 3b). We also quantified the cerebellar and striatal dysconnectivity overlap for areas showing significant hyper-connectivity (yellow) and hypo-connectivity (blue) (Fig. 3c and d). Fig. 3e indicates high overlap across both cerebellar and striatal hyper-connectivity effects (orange areas indicate overlapping effects, logical OR; yellow areas indicate joint effects, logical AND). Notably, the number of voxels in the overlapping areas was above chance (hypergeometric test for probability of overlap, P < 0.001, given the total number of voxels in the brain) (Fig. 3f). Similarly, Figure 3g indicates the same result for hypo-connectivity effects (dark blue areas indicate overlapping effects, logical OR; light blue areas indicate joint effects, logical AND). Again, the number of hypo-connected voxels that overlapped between cerebellar and striatal analyses was above chance (P < 0.001, Fig. 3h). Overall, quantitatively and spatially high similarity between cerebellar and striatal dysconnectivity suggests highly comparable brain-wide perturbations.
Figure 3.
Similarity between perturbed cerebellar and striatal connectivity in SCZ. (a) Surface unthresholded Z-maps showing cerebellar and striatal between-group connectivity differences. Qualitatively, regions that exhibited an increase in cerebellar connectivity in SCZ also exhibited an increase in striatal connectivity, and vice versa. (b) Scatterplot showing a highly significant positive relationship between-group differences in cerebellar and striatal whole-brain connectivity (r = 0.40, P < 1.00e-15). The plot shows Fz values for all voxels in cerebellar (X-axis) and striatal (Y-axis) between- group connectivity maps. (c) Surface maps of conjunction analysis showing areas that are significantly hyper-connected (yellow) and hypo-connected (blue) with both cerebellar and striatal parcels. (d) Volume-based axial view of a with Z-coordinate ranges (each slice in each row increments by 3 mm) (e) Surface view of areas showing both significant cerebellar (cbm) and striatal (str) hyper-connectivity (logical AND, bright yellow) and areas significantly hyper-connected to one or the other (logical OR, orange). (f) The number of voxels overlapping between observed cerebellar and striatal hyper- connectivity maps is significantly greater than chance (P < 0.001), given the total number of voxels in the brain. (g) Surface view of areas showing both significant cerebellar (cbm) and striatal (str) hypo- connectivity (logical AND, bright blue) and areas significantly hypo-connected to one or the other (logical OR, dark blue). (h) Number of voxels that overlap between observed cerebellar and striatal hypo-connectivity maps is significantly greater than chance (P < 0.001), given the total number of voxels in the brain. Dashed red lines in f and h indicate 95% Clopper–Pearson confidence intervals for chance.
Dysconnectivity in SCZ is Driven by Associative Network Disruptions
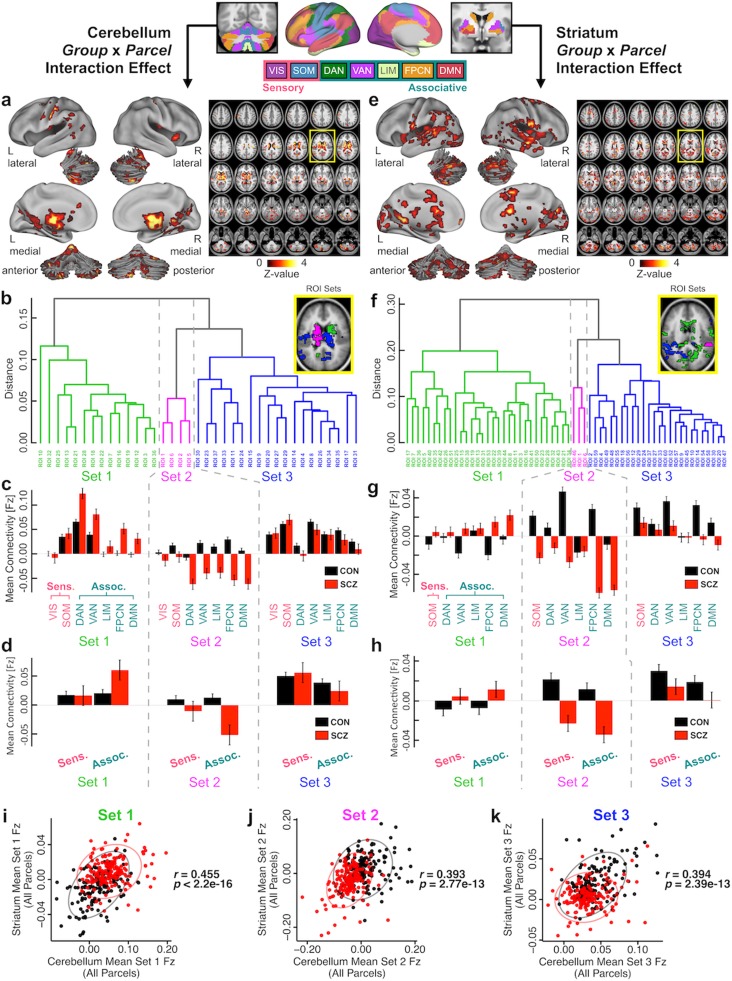
Prior analyses are highly consistent with the hypothesis that CSTC disruptions in SCZ are robust and highly conserved across both the cerebellar and striatal systems. However, it remains unknown if these alterations are preferential to some functional networks that span both cerebellum and striatum. For instance, prior work strongly implicates associative network disturbances in SCZ in the cerebral cortex (Meyer-Lindenberg et al. 2005; Baker et al. 2014; Yang, Murray, Wang, et al. 2016). Yet, to our knowledge, no study has tested if such disruptions persist in a uniform pattern across the associative networks for both cerebellum and striatum. To test this we computed a Group x Parcel interaction, which would explicitly reveal differential between-group disruptions across distinct cerebellum and striatum parcels. The cerebellar Group x Parcel interaction effect revealed 37 areas throughout the brain (Fig. 4a). In turn, the striatal Group x Parcel interaction effect revealed 60 areas throughout the brain (Fig. 4e). These effects suggest that between-group dysconnectivity was not uniform across all parcels for either the cerebellar or striatal analyses. Importantly, these whole-brain interaction effects did not solely depend on within-cerebellum or within-striatum interactions (see Supplementary Materials and Methods and Supplementary Figs S12–S14). Next, we tested for the source of the interaction and examined if the interaction effect was consistent for both cerebellum and striatum. Specifically, given the number of identified regions, we performed a hierarchical average-linkage clustering algorithm on the between-group connectivity of regions identified across the 2 interaction maps (Materials and Methods). For the cerebellum analysis, we identified 3 sets of areas that showed quantitatively similar patterns of dysconnectivity (Fig. 4b). The inset shows the distribution of areas in the 3 sets an axial slice (color-coded green, fuchsia and blue, as in the dendrogram). Next, we characterized the interaction effects for each set of areas produced by the clustering algorithm. Specifically, for a given striatal or cerebellar parcel/seed we averaged connectivity for all areas within a set produced by the clustering algorithm (Fig. 4c). We did this separately for both SCZ (red bars) and CON (black bars) groups. Each of the 3 sets of areas by definition shows quantitatively similar interaction effects (i.e., they are grouped by the clustering algorithm). The goal here is to characterize how the 3 sets of areas produced by the clustering algorithm differ. In Set 1, areas exhibited higher mean connectivity in SCZ compared with CON (i.e., hyper-connected on average). In Set 2, areas exhibited lower mean connectivity in SCZ (i.e., hypo-connected on average). The difference between SCZ and CON was less pronounced in Set 3 ROIs and exhibited a “mixed” motif. Next, we averaged exclusively across sensory (VIS and SOM; pink) and associative (DAN, VAN, LIM, FPCN, DMN; teal) parcels to test if the observed alterations are preferential to higher-order networks. This analysis indicated that the between-group effect in each set is much more pronounced for associative parcels than sensory parcels (Fig. 4d). In essence, the entire source of the Group x Parcels interaction was driven by a more pronounced associative network alteration in the cerebellum.
Figure 4.
Group x Parcel interaction effect for cerebellar and striatal connectivity. Parallel analyses were conducted independently for the cerebellum and striatum, using the functionally-defined 7 cerebellar and 6 striatal parcels. (a) Surface view (left) and volume slice view (right) of regions of interest (ROI) revealing a significant Group x Parcel interaction effect for the cerebellar analysis. Slice outlined in yellow is magnified in inset in panel b. (b) Each of the 37 cerebellar interaction ROIs were assigned to 1 of 3 sets based on the between-group differences in functional connectivity of all 7 cerebellar functional parcels using an average-linkage hierarchical clustering algorithm, such that ROIs within a set share the most similar patterns of group differences in seed-based connectivity. Inset shows location of ROIs color-coded for each set. (c) To illustrate the sources of the interaction effect, the mean functional connectivity for each set of ROIs is shown separately for SCZ and CON subjects in each of the cerebellar parcels, averaged across all ROIs in the set. Error bars indicate standard error. ROIs in Set 1 exhibit higher mean connectivity in patients compared with controls, whereas ROIs in Set 2 exhibit lower mean connectivity in patients. (d) This effect is more pronounced for associative parcels (pink) than for sensory parcels (teal). ROIs in Set 3 are less differentiated between patients and controls. (e–h) Parallel analysis conducted for the 60 interaction ROIs from 6 striatal functional parcels. (i–k) Correlations between mean functional connectivity (Fz) of all cerebellar and striatal parcels in (i) Set 1, (j) Set 2, and (k) Set 3 interaction ROIs, across SCZ and CON subjects. Displayed r values are across all subjects (N = 320, 1 outlier removed). Abbreviations: Sens., sensory networks; Assoc., associative networks; VIS, visual network; SOM, somatomotor network; DAN, dorsal attention network; VAN ventral attention network; LIM, limbic network; FPCN, frontoparietal control network; DMN, default mode network.
Next, we repeated the analyses for the striatal interaction effects. Strikingly, as with the cerebellum, a hierarchical clustering algorithm revealed 3 sets of areas with similar patterns of connectivity (Fig. 4f). The group differences in connectivity in each of these sets driving the striatal interaction effect was similar to those in the cerebellar interaction effect—namely, higher mean connectivity in SCZ than CON for ROIs in Set 1, lower mean connectivity in SCZ than CON for ROIs in Set 2, and a less ostensible difference for ROIs in Set 3 (Fig. 4g). These between-group differences were not as pronounced in striatal associative versus sensory parcels than in the analogous cerebellar analysis (Fig. 4h) because there was only 1 sensory parcel in the striatum. Finally, we computed the statistical similarity of these sets of ROIs between the cerebellum and striatum (Fig. 4i–k). Specifically, for each subject, we averaged Fz values across all ROIs in each cerebellar and striatal set. We did so for each parcel within the striatum and the cerebellum (e.g., the FPCN parcel). This revealed a robust and consistent relationship between cerebellar and striatal values across all sets. This strongly supports consistency of interactive effects for cerebellar and striatal ROIs for individual subjects. This relationship held for all networks (Set 1: r = 0.455, P < 2.20e-16; Set 2: r = 0.393, P = 2.77e-13; Set 3: r = 0.394, P = 2.39e-13) and particularly for the FPCN (Set 1: r = 0.538, P < 2.20e-16; Set 2: r = 0.475, P < 2.20e16; Set 3: r = 0.367, P = 1.16e-11; see Supplementary Fig. S15 for all individual parcels/networks). Collectively, these analyses suggest that interactive brain-wide network disturbances are highly similar between the cerebellum and striatum. However, they are particularly obvious and highly consistent in certain functional networks, especially those involved in higher-order executive tasks.
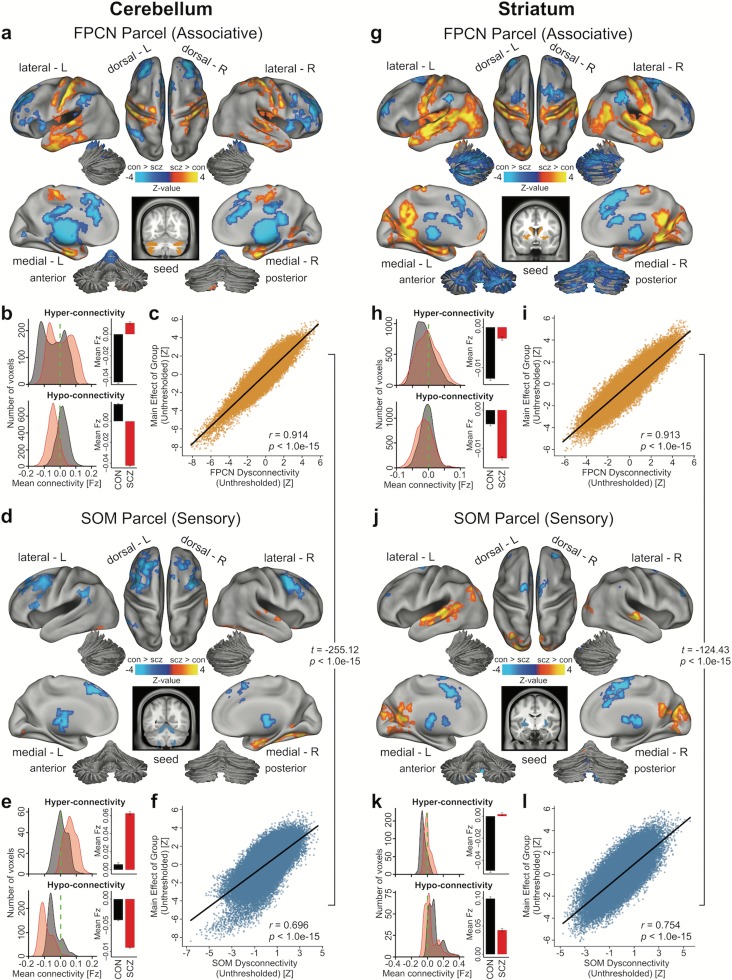
Next, to further establish that the SCZ dysconnectivity was more pronounced in associative than in sensory networks, we examined seed-based functional connectivity maps of each parcel. Qualitatively, the between-group dysconnectivity for associative parcels closely matched the main group dysconnectivity effect (i.e., across all parcels, Fig. 2b and 2e). This was markedly less prominent for sensory parcels across both cerebellum and striatum. To quantitatively verify this, we examined the similarity of the FPCN parcel (Fig. 5a–b, g–h) and the SOM parcel between-group effects (Fig. 5d–e, j–k) in relation to the main effects across both cerebellum and striatum. Specifically, we correlated unthresholded whole-brain between-group maps for the FPCN parcel with the unthresholded overall dysconnectivity maps (i.e., maps in Fig. 2b and 2e). Fig. 5c highlights the similarity between FPCN and overall effects across all parcels (Cerebellum: r = 0.914, P < 1.0e-15; Striatum: r = 0.913, P < 1.0e-15). In contrast, the SOM effect dysconnectivity was substantially less similar to the main effects for the cerebellum and striatum (r = 0.696, P < 1.0e-15; r = 0.754, P < 1.0e-15). This constituted a highly significant difference between correlations for the FPCN and SOM similarity (Cerebellum: t = −255.12, P < 1.0e-15, Striatum: t = −124.43, P < 1.0e-15, William’s test for dependent correlations). The full expansion of all pair-wise thresholded and unthresholded maps is shown in Supplementary Figs S16 and S17. Collectively, these effects buttress prior analyses by showing pervasive disruptions across brain-wide networks that preferentially affect associative functional systems in SCZ.
Figure 5.
Group connectivity differences for associative versus sensory cerebellar and striatal parcels. (a) Surface view of regions for which connectivity with the cerebellar FPCN parcel, an exemplar “associative” parcel, showed significant between-group differences, P < 0.05. (b) Density plots show distribution of connectivity strength (Fz) values within voxels showing significant hyper- and hypo-connectivity. Bar plots show mean connectivity averaged across all voxels in hyper- and hypo-connected areas. (c) Correlation between the voxel-wise unthresholded whole-brain group dysconnectivity maps from the cerebellar FPCN parcel and the cerebellar main effect of group. Similar analyses are shown for the (d–f) cerebellar SOM parcel, (g–i) striatal FPCN parcel, and (j–l) striatal SOM parcel. Note the high resemblance between the associative parcel effect and the main effect of group for both the cerebellar and striatal analyses. Contrarily, the sensory parcel effects are significantly less similar (Cerebellum: t = −255.12, P < 1.0e-15, Striatum: t = −124.43, P < 1.0e-15, William’s test for dependent correlations). Abbreviations: FPCN, frontoparietal control network; SOM; somatosensory network.
Relationship Between Hyper-connectivity and Hypo-connectivity Across Individuals
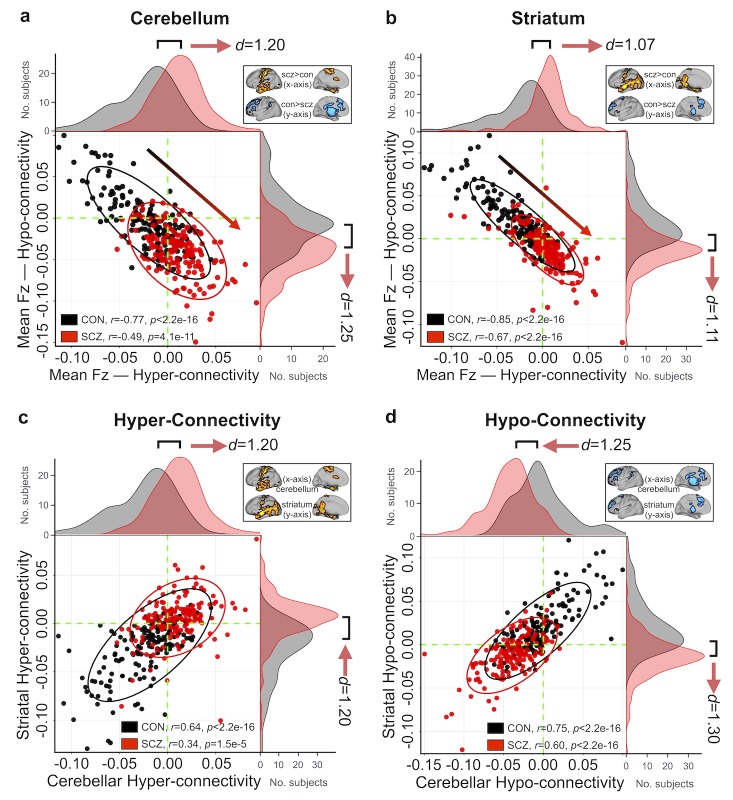
Presented analyses highlight that the alterations are strongly driven by associative networks within the cerebellum and striatum. However, more broadly, it is unknown if the observed hyper-connectivity and hypo-connectivity effects that dominate all presented analyses arise are linked. Alternatively, the bi-directional disruption may result from functionally independent perturbations: distinct groups of subjects could be driving the hyper- vs. hypo-effects across all analyses. To arbitrate between these possibilities, we quantified the connectivity strengths across subjects for areas showing hyper- and hypo-connectivity generally. There was a significant negative relationship between cerebellar hypo-and hyper-connectivity in CON (r = −0.77, P < 2.2e-16), indicating that control subjects with the weakest coupling between cerebellar–sensorimotor regions also show the strongest coupling between cerebellar-thalamo-striatal-prefrontal regions (Fig. 6a). This effect replicated in the SCZ sample (r = −0.49, P < 4.1e-11), but it was significantly attenuated compared with CON (Z = −4.29, P < 0.001). Similarly, CON subjects with the weakest striatal-sensorimotor coupling showed the strongest striatal-thalamo-prefrontal-cerebellar coupling (r = −0.85, P < 2.2e-16), and an attenuated effect was seen in SCZ subjects (r = −0.67, P < 2.2e-16; Z = −3.95, P < 0.001; Fig. 6b). Additionally, CON subjects that showed the strongest coupling with cerebellum also exhibited the strongest coupling with striatum, for both the hyper-connectivity (r = 0.64, P < 2.2e-16, Fig. 6c) and hypo-connectivity (r = 0.75, P < 2.2e-16; Fig. 6d) effects. This relationship was also present but attenuated in SCZ subjects (hyper-connectivity: r = 0.34, P < 1.5e-5, Z = 3.58, P < 0.001; hypo-connectivity: r = 0.60, P < 2.2e-16, Z = 2.48, P = 0.013). Note that in all Fig. 6 panels, SCZ subjects show a diagonal “shift” relative to CON across both zero-lines (dashed green), suggesting that the differences in hyper- and hypo-connectivity in SCZ may share a source of disturbance. Notably, this shift is clearly present in the subsample of unmedicated patients relative to demographically-matched controls (Supplementary Fig. S46), suggesting that our core results are not driven by medication effects. Collectively, these analyses support the hypothesis that perturbations in information flow across these networks may stem from related systems-level phenomena, as opposed to functionally independent perturbations.
Figure 6.
Relationship between cerebellar and striatal hyper- and hypo-connectivity across subjects. (a) Significant negative relationship evident between average hyper- and hypo-whole-brain connectivity with the cerebellum across all CON (black data points). SCZ (red data points) showed a “shift” across the zero lines, indicating weaker prefrontal-striatal-thalamic-cerebellar coupling but stronger somatomotor–cerebellar coupling. Ellipses for each group mark the 95% confidence intervals. Distributions of average connection strengths for each subject show a shift in cerebellar coupling in SCZ, highlighting increased cerebellar coupling with somatomotor regions and decreased coupling with prefrontal-striatal and thalamic regions for patients. Inset shows regions of hyper- and hypo-connectivity with the cerebellum in SCZ, from Figure 2b. (b) A similar effect is seen in an independently conducted analysis on whole-brain striatal connectivity. Again, SCZ showed a shift across the zero lines relative to CON, suggesting that the differences in hyper- and hypo-connectivity observed in SCZ may share a source of disturbance. Inset shows regions of hyper- and hypo-connectivity with the striatum in SCZ, from Figure 2e. (c) CON subjects with the highest cerebellar connectivity in hyper-connected regions also show highest connectivity in striatal hyper-connected regions. Shift in SCZ subjects relative to CON is evident for both cerebellar and striatal hyper-connectivity (identical data as those plotted along X-axes in panels a and b), suggesting the underlying disruption is linked across these 2 systems. (d) Similarly, SCZ subjects show a shift in both cerebellar and striatal hypo-connectivity.
Data-Driven Clustering of Cerebellar and Striatal Dysconnectivity
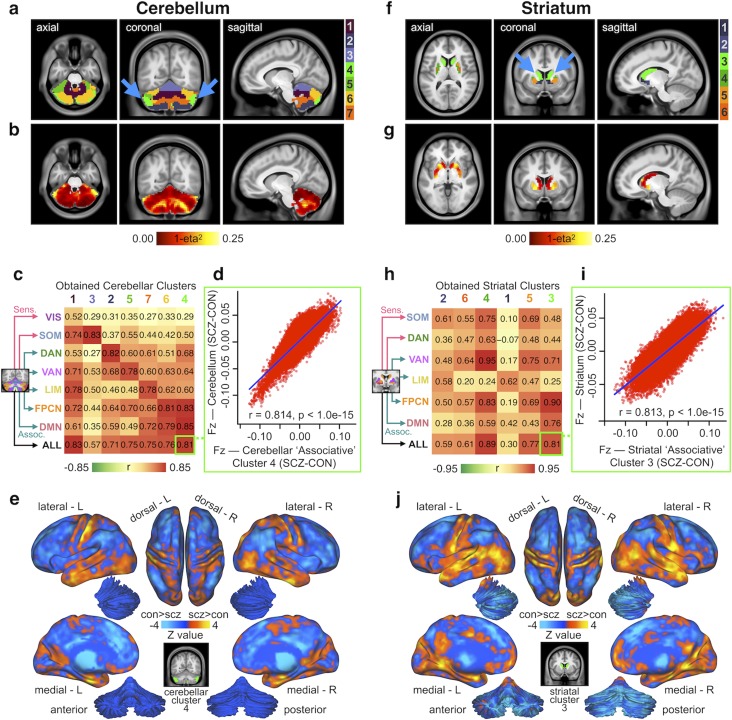
While robust, all presented analyses have used a priori functional networks to reveal shared CSTC perturbations in SCZ. However, there may be important functional differences in the spatial distribution of these network parcels in patients—in other words, patients may not show a clean separation of cerebellum and striatum parcels that is assumed to be present in healthy individuals. It is therefore important to test if the bi-directional dysconnectivity effects hold without any a priori network assumptions. Hence, we tested whether we could “recapture” functionally distinct subdivisions of the cerebellum and striatum that were differentially affected in SCZ, by performing data-driven k-means clustering on voxel-wise group dysconnectivity (see Materials and Methods and Supplementary Fig. S18 for details). We report several cluster solutions of between-group differences (see Supplementary Fig. S19 for full 4-, 6-, and 7-cluster solutions). While the pattern is highly similar irrespective of cluster choices, here we highlight the 7-cluster solution for the cerebellum and the 6-cluster solution for the striatum to allow a direct comparison with the a priori network parcellations.
The clustering solution for the cerebellum revealed well-defined and bilaterally symmetrical clusters, which is not consistent with the effects being driven by artifact (Fig. 7a). A voxel-wise measure of dissimilarity (1-eta2; see Materials and Methods) shows regions with the numerically greatest degree of dysconnectivity between SCZ and CON. These foci were also bilaterally symmetrical (Fig. 7b). Next, we tested if these data-driven clustering solutions map onto the a priori network parcellations. Here we quantified the relationship between the group dysconnectivity for each of the obtained clusters and each a priori cerebellar parcel (top 7 rows) as well as the average across all parcels (bottom row). This yields a 8 × 7 matrix of Pearson’s r values shown in Figure 7c, where the columns of the matrix are ordered such that the trace of the parcel-by-cluster square matrix is maximized. As illustrated by the diagonal, there was a selective mapping between data-driven and a priori dysconnectivity patterns. Notably, 6 of the 7 clusters have their strongest correlation value along the diagonal, suggesting that these clusters mapped selectively to existing parcels. Collectively, this indicates qualitatively similar disruptions irrespective of analytic methods used to define cerebellar dysconnectivity in SCZ. This is corroborated, for instance, by examining Cluster 4 (Fig. 7a, bright green), which is most similar in dysconnectivity to the associative parcels (r = 0.81, P < 0.001, Fig. 7d), indicating that in the context of SCZ-related disturbances it exhibits a functionally “associative-like” profile. We show the threshold-free between-group differences in whole-brain connectivity of this “associative-like” cerebellar cluster in Figure 7e (see Supplementary Fig. S20 for maps of all clusters).
Figure 7.
Voxel-wise clustering of cerebellar and striatal group difference in connectivity. (a) Results for k-means 7-cluster solution identifying cerebellar voxels with the most similar patterns of between-group connectivity differences. Note the cluster solution is highly symmetrical (blue arrows). (b) Cerebellar dysconnectivity based on group dissimilarity. Brightest voxels indicate greatest between- group differences. (c) Matrix of correlations (Pearson’s r) between dysconnectivity of obtained clusters and dysconnectivity of functional parcels. Cluster numbers (column headings) are colored as in a. Functional parcels (row headings) are labeled and colored as in Figure 2a and original parcellation (Yeo et al. 2011). Columns have been ordered such that the trace of the parcel-by-cluster square matrix is maximal among all permutations. Cluster-seeded dysconnectivity shows a range of correlation strengths with functional parcel-seeded dysconnectivity. Cluster 4 (bright green) is strongly correlated with associative parcels, as well as with the overall main effect of group, suggesting that it is a functionally “associative-like” cluster. (d) Scatterplot showing high degree of correlation between voxel-wise whole-brain group dysconnectivity (Fz values) of cerebellar cluster 4 (the “associative” cluster) and voxel-wise whole-brain group dysconnectivity (Fz values) across all cerebellum parcels. (e) Surface unthresholded Z-maps showing between-group connectivity for cerebellar executive cluster (shown in inset). (f–j) Identical analyses conducted independently for between-group differences in whole-brain striatal connectivity. Striatal cluster 3 (bright green) is strongly correlated with associative parcels, as well as with the overall main effect of group, suggesting that it is a functionally “associative-like” cluster.
Importantly, k-means clustering with k = 6 on striatal voxel-wise dysconnectivity yielded a consistent and bilaterally symmetrical solution (Fig. 7f). The most dissimilar voxels are again indicated in bright yellow in Fig. 7g, suggesting a non-uniform pattern of striatal alterations. As with the cerebellum, there was a selective mapping between data-driven and a priori dysconnectivity patterns for the striatum (Fig. 7h). Cluster 3 (Fig. 7f, bright green) exhibited a pattern of dysconnectivity most similar to those of associative functional parcels (r = 0.81, P < 0.001, Fig. 7i). Finally, threshold-free between-group differences in whole-brain connectivity of this “associative-like” striatal cluster highlight a pattern that in line with the a priori association parcel analysis (Fig. 7j, see Supplementary Fig. S21 for maps of all clusters). Additionally, we report the same data-driven clustering solutions for cerebellum and striatum in CON only (Supplementary Fig. S22), which fully replicated the functional parcels identified previously by Bucker et al. and Choi et al. (Buckner et al. 2011; Choi et al. 2012). Together these results suggest that the observed disruptions are not attributable to spatial network assumptions in the cerebellum and striatum.
Cerebellar and Striatal Dysconnectivity Features Predict Diagnostic Group Status
Above, we show consistent and robust alterations in BOLD functional relationships across striatal and cerebellar functional subdivisions in SCZ. Next, we examined if these effects may yield a sensitive and specific binary classification of diagnostic group status. Specifically, we used a SVM binary classifier to test if cerebellar and striatal parcel connectivity features reliably distinguish between groups. In turn, we tested 3 secondary questions related to the brain-wide patterns of disruptions: first, we tested if any of the identified features selectively “drive” the classification performance or if the classifier performance remains stable irrespective of parcel feature used. Put differently, if SCZ is associated with brain-wide alterations across CSTC systems, then cerebellar and striatal parcel features should yield comparable classification performance, as there should be overlap in the disruptions. Second, a corollary of this hypothesis is that specific parcels within the striatum or cerebellum may drive classifier performance. In particular, given that our previous analyses show preferential SCZ disruptions in associative parcels, we hypothesized that associative parcel features would yield the highest classification performance. However, if the brain-wide disruptions are shared across CSTC systems, then the same network parcels should contribute information to the classifier irrespective of whether they are selected from the striatum or the cerebellum. Third, we examined whether classifier performance could be improved by combining select subsets of cerebellar and striatal parcel features. We hypothesized that if there exists considerable overlap in diagnostically relevant information across cerebellar and striatal parcels, a classifier using a combination of all parcel features would not markedly outperform the most discriminatory single-feature classifier. Alternatively, a multi-feature classifier would yield better performance if interactive effects across specific cerebellar and striatal parcel features contribute unique diagnostically relevant variance.
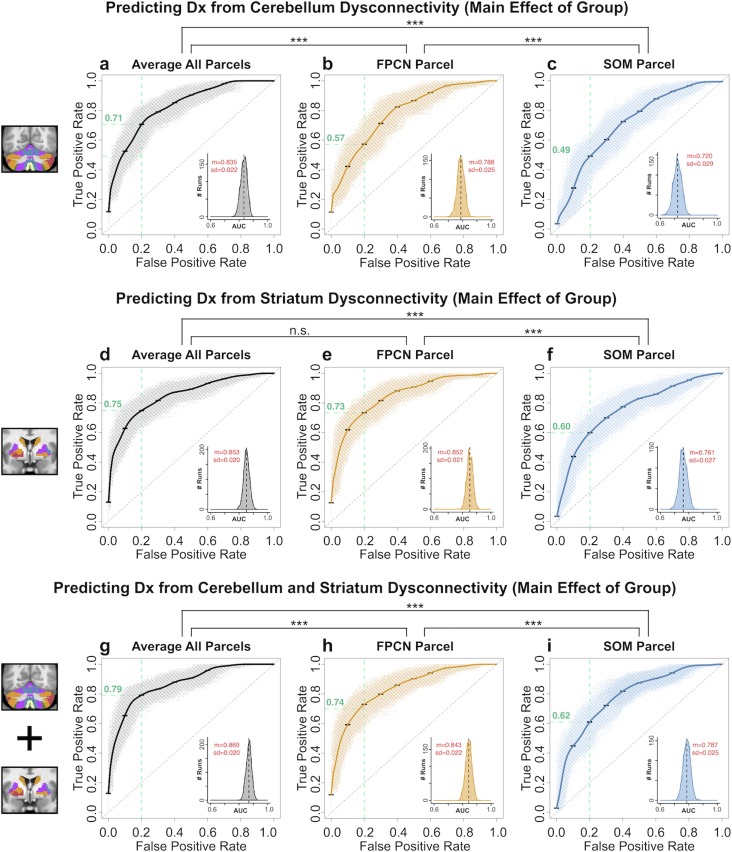
First, to test if cerebellar and striatal features are comparable for classification, we used the connectivity strengths (Fz values) of hyper- and hypo-connected areas from each of the seed-based functional connectivity analyses as input features. Each classifier was trained on a randomly selected subset (50%) of subjects and tested on the remaining subjects, repeated 1 000 times (cross-validation runs, see Materials and Methods). Classifier performance across specific exemplar parcel features is shown in Fig. 8 (see Supplementary Figs S23–S25 for all classifiers). Cerebellar and striatal classifiers yielded highly comparable performance, supporting the hypothesis of shared brain-wide disruptions across both the cerebellum and striatum. Specifically, the classifier trained on the average dysconnectivity across all parcels (AVERAGE) was similar for the cerebellum (accuracy = 75.0%, sensitivity = 76.9% & specificity = 73.2%, mean area under the receiver operating characteristic curve (AUC) = 0.835, Fig. 8a) and the striatum (accuracy = 75.7%, sensitivity = 79.0% and specificity = 72.5%, AUC = 0.853, Fig. 8d). Collectively, these measures suggest that the cerebellum and striatum may exhibit shared disruptions. Consequently, cerebellum and striatum do not outperform one another.
Figure 8.
Receiver operating characteristic (ROC) curves from binary classifiers of diagnostic status trained on cerebellar and striatal dysconnectivity features. Exemplar associative (FPCN) and sensory (SOM) parcels, as well as the average combined across all parcels (AVERAGE), are shown. (a) Performance of classifier using AVERAGE dysconnectivity across all cerebellar parcels. Bold black curve plots the mean true positive rate (TPR) at each false positive rate (FPR) across 1 000 cross-validation runs (standard error bars shown in black). Gray curves show individual results from all cross-validation runs. Inset plots the distribution of the Area Under the Curve (AUC) for all 1 000 runs; black dashed line shows the mean AUC. Mean (m) and standard deviation (sd) of the AUC distribution are displayed in red. (b) Performance of classifier using cerebellar FPCN dysconnectivity. (c) Performance of classifier trained using cerebellar SOM parcel dysconnectivity. Green dashed lines show the TPR for each classifier at FPR = 0.20. The cerebellar FPCN TPR = 0.71, indicating that this classifier achieves a higher sensitivity than the SOM classifier (TPR = 0.49) at the same “cost” of specificity. Similarly, the AUC performance is significantly higher for the FPCN than the SOM classifier (t = 55.692, df = 1953.4, P < 1.0e-15). Asterisks denote significance at P < 0.001 in a two-tailed t-test between the indicated AUC distributions. (d–f) Performance of classifiers using striatal AVERAGE, SOM, and FPCN features. Again, the FPCN classifier outperformed the SOM classifier, as indicated by sensitivity at FPR = 0.20 (FPCN TPR = 0.73, SOM TPR = 0.60) and AUC (t = 83.869, df = 1871.2, P < 1.0e-15). (g–i) Performance of classifiers using combined cerebellar and striatal AVERAGE, FPCN, and SOM features. See Supplementary Figs S34–S36 for ROC curves of all parcels.
As noted, our second classifier hypothesis was to test if disruptions in SCZ are more pronounced in associative parcels relative to other parcels within cerebellar and striatal systems. This would result in better performance for classifiers trained on associative parcel features relative to sensory parcel features. Both the cerebellar and striatal FPCN classifiers outperformed the SOM classifiers (Cerebellum-FPCN Classifier: accuracy = 71.2%, sensitivity = 80.6% & specificity = 61.8%, AUC = 0.788, Fig. 8b; Striatum-FPCN Classifier: accuracy = 75.7%, sensitivity = 71.3% & specificity = 71.0%, AUC = 0.852, Fig. 8e; Cerebellum-SOM Classifier: accuracy = 66.1%, sensitivity = 76.9% & specificity = 61.0%, AUC = 0.720, Fig. 8c; Striatum-SOM Classifier: accuracy = 68.3%, sensitivity = 77.1% & specificity = 59.7%, AUC = 0.761, Fig. 8f). This was supported by a formal test of differences in FPCN vs. SOM AUC parameters across the cerebellum (t = 55.692, df = 1953.4, p < 1.0e-15) and striatum (t = 83.869, df = 1871.2, p < 1.0e-15). This result is consistent with the hypothesis of shared CSTC disruptions being preferential to associative parcels in SCZ.
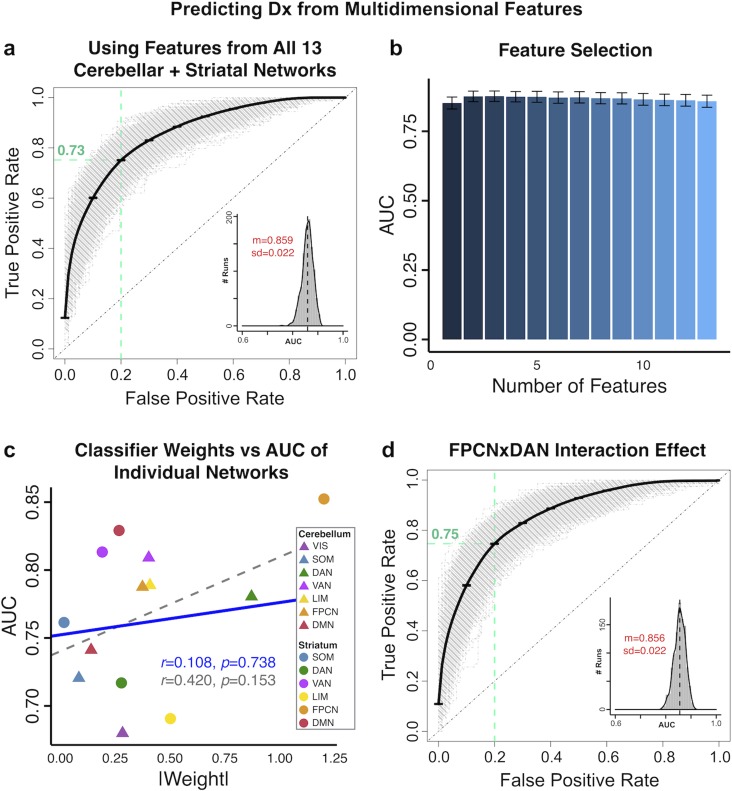
In turn, our third classifier hypothesis tested if combining cerebellar and striatal information would improve classifier performance relative to the single best feature. We first trained classifiers on a linear combination of both cerebellar and striatal parcel features (referred to as “combined” classifiers below). The combined AVERAGE classifier yielded the highest performance (mean accuracy of 78.7%, with 80.7% sensitivity and 76.7% specificity, AUC = 0.869, Fig. 8g). This was comparable to the combined FPCN classifier (accuracy = 74.2%, sensitivity = 80.8%, specificity = 67.6%, AUC = 0.843, Fig. 8h). Again, the combined classifier trained with SOM yielded the lowest performance (accuracy = 70.8%, sensitivity = 75.7%, specificity = 66.0%, AUC = 0.787, Fig. 8i). We then examined if interactive effects between these striatal and cerebellar parcel features contribute information that improves classification. Here we trained a 13-feature classifier using connectivity from all 7 cerebellar and 6 striatal parcels as predictors. This multi-feature classifier did not perform better than the classifiers trained on one parcel feature per subject (accuracy = 77.3%, sensitivity = 78.6% & specificity = 76.0%, AUC = 0.859, Fig. 9a), suggesting that interactions between parcel features did not contribute unique additional information. In further support of this effect, no added number of additional features appreciably improved classifier performance compared with the classifier trained on the single most discriminatory feature (Fig. 9b). Additionally, the relationship between the AUC of individual features and the weight assigned to them in the multi-feature classifier was not significant, suggesting that no informational tradeoffs were made by selecting certain parcel features over others in the 13 multi-feature classifier (Fig. 9c; all features: r = 0.420, P = 0.153, dashed gray line; excluding most discriminatory feature: r = 0.11, P = 0.74, blue line). Lastly, classifier performance was not improved by including the interactions between the 2 most strongly weighted parcel features—namely cerebellar and striatal FPCN and DAN—as additional predictors (accuracy = 77.1%, sensitivity = 79.1% and specificity = 75.1%, AUC = 0.856, Fig. 9d).
Figure 9.
Binary classifiers using multidimensional predictors. (a) We trained a linear kernel SVM to distinguish between SCZ and CON subjects using connectivity features from all 7 cerebellar and 6 striatal parcels, in a 13-dimensional feature space. This multi-feature classifier did not perform significantly better than classifiers trained on individual features (Fig. 8, Supplementary Figs S34–S36). ROC curves show the TPR plotted against the FPR of the classifier trained on all 13 parcel features. Gray curves show ROC curves for 1 000 individual cross-validation runs; bold black curve shows mean ROC curve (standard error bars in black). Green dashed lines indicate TPR = 0.73 at FPR = 0.20. Inset shows the distribution of the AUC across all 1 000 runs (black dashed line shows mean), with mean AUC (m) and standard deviation (sd) displayed in red. (b) Comparison of AUC performance across classifiers with 1 through 13 features. There were no significance differences in AUC between any of these classifiers, indicating that no additional number of features improved performance compared with the classifier trained on the single most discriminatory feature. Bar plots show the mean AUC of each classifier; error bars show standard deviation. (c) Plotting the mean AUC from classifiers trained on each individual parcel feature (from Supplementary Figs S34–S35) against the absolute weight of these parcels in the 13-feature classifier shows that there is no linear dependence between the two. Dashed gray line shows correlation between all points (r = 0.42, P = 0.15); solid blue line shows correlation between all points excluding the striatal FPCN, the most discriminatory feature (r = 0.11, P = 0.74). (d) ROC curves of a classifier trained with interaction effects between the 2 strongest-weighted seeds. In addition to the individual parcel features, we added the 4-way interaction between the cerebellar FPCN and DAN and the striatal FPCN and DAN as features. Again, the classifier performance was not improved, indicating that no additional information is contributed by the interactive effect.
Importantly, the classifier was not driven by group differences in head motion or SNR (Supplementary Fig. 26). Furthermore, the SVMs performed with comparable, though slightly attenuated, results when tested in an independent Replication dataset (Supplementary Figs S49–S51), suggesting these effects are robust and generalizable across datasets. These analyses suggest that diagnostically informative patterns are highly shared across cerebellar and striatal features, and no additional information is contributed by their interactions across these analyses.
Collectively, we show that SVM binary classifiers using cerebellar and striatal connectivity features can distinguish between groups with high sensitivity and specificity. These classifier results are consistent with prior analyses indicating that neither cerebellum nor striatum as a whole drives classifier performance, suggesting that network disruptions in SCZ persists across both systems. Furthermore, associative parcels in both cerebellum and striatum are particularly discriminatory between groups, supporting both a priori and data-driven results presented above. That is, brain-wide disturbance in SCZ appear most pronounced for higher-order associative subdivisions across cerebellar and striatal functional parcels.
Cerebellar and Striatal Dysconnectivity Relationship with Symptoms
While the observed effects reflected robust between-group differences, we also tested if CSTC alterations relate to symptom measures across subjects. Notably, there were no significant correlations between the degree of cerebellar or striatal dysconnectivity across patients (hyper-connectivity, hypo-connectivity, or a an aggregate measure of the two) and SCZ symptom severity (as measured by the Positive and Negative Symptom Scale (PANSS)). This absence held for individual symptoms (Supplementary Figs S27 and S28) as well as when using the classic 3-factor model of the Positive and Negative Syndrome Scale—a well-established measure of symptoms across the psychosis spectrum (PANSS, positive, negative, and general symptoms (Kay et al. 1987); see Supplementary Figs S29 and S30, P > 0.05 with Bonferroni correction). To provide a comprehensive characterization of symptom-related effects, we additionally investigated the relationship between identified dysconnectivity and an alternative 5-factor model of PANSS, shown to be stable in an external sample of 5 789 subjects (van der Gaag et al. 2006). These additional analyses again revealed weak correlations between the degree of dysconnectivity and the 5-factor model symptom solution (Supplementary Figs S31–S32). These comprehensive symptom analyses suggest that the identified CSTC dysconnectivity likely constitutes a trait-like effect, as opposed to a robust state-like marker, in line with prior observations that revealed modest relationships with thalamic connectivity (Anticevic, Cole, et al. 2014). As such, these brain-wide differences do not seem to strongly track patients’ current symptom status, but may perhaps map onto a more sustained disease-associated change.
Cerebellar and Striatal Dysconnectivity Relationship with Cognition
Above we tested whether CSTC dysconnectivity relates to symptom measures. We observed weak or no statistical relationships despite adequate power. One alternative possibility is that the observed dysconnectivity may reflect cognitive deficits—a possibly in line with recent reports showing that thalamic connectivity may provide a cognitive remediation target in SCZ (Ramsay et al. 2017). To test this in relation to CSTC dysconnectivity, we first replicated the cerebellar and striatal connectivity analyses in an independent sample of 145 patients with SCZ and healthy controls from the Bipolar–Schizophrenia Network on Intermediate Phenotypes (B-SNIP) dataset, which also included measures of cognition (Fig. 10; see Materials and Methods and Supplementary Tables S1 and S2 for details) (Tamminga et al. 2013). The similarity between the discovery and replication dysconnectivity effects was highly significant for both cerebellar and striatal seeds (Supplementary Methods, Supplementary Fig. S48). Dysconnectivity in the replication dataset was not driven by site or sex confounds (Supplementary Figs S44–S45). We quantified the relationship between the degree of neural dysconnectivity and PANSS symptom severity as well as cognitive performance across individual subjects. As with the discovery sample, cerebellar and striatal dysconnectivity showed only weak correlations with symptom severity measures (Supplementary Figs S36–S41). However, Fig. 10c shows a significant negative correlation between cerebellar hyper-connectivity and a measure of cognitive performance (the Brief Assessment of Cognition in Schizophrenia (BACS) summary score (Keefe et al. 2004)), indicating that individuals with a higher degree of hyper-connectivity performed more poorly on the BACS (r = −0.280, P = 4.47e-8). Similarly, individuals with the greatest cerebellar hypo-connectivity also had the lowest BACS scores (r = 0.301, P = 3.62e-9, Fig 10d). These relationships held for striatal hyper-connectivity (r = −0.282, P = 3.38e-8, Fig. 10g) and hypo-connectivity (r = 0.271, P = 1.17e–7, Fig. 10h). Notably, the preferentially stronger dysconnectivity with associative cerebellar and striatal subdivisions replicated and this FPCN dysconnectivity was significantly correlated with cognitive performance (Supplementary Figs S33 and S35). The relationship between cognition and CSTC dysconnectivity (either cerebellum or striatum for either hypo- or hyper-directions) accounted for close to 10% of variance across subjects, whereas the top relationships across all summary measures for symptom scores accounted for ~2.3% of the variance (see Supplementary Figs S27–S32). This observation is consistent with the possibility that the brain-wide CSTC dysconnectivity reflects cognitive disturbances in SCZ.
Figure 10.
Main effect of cerebellar and striatal network parcel connectivity in replication sample and relationship with cognition. (a) Cortical surface view of areas showing main effect of Group in whole-brain connectivity with the cerebellar parcels between 167 patients with schizophrenia with psychosis (SCZ) and 162 healthy controls (CON) in an independent dataset. Regions that are significantly different between groups (P < 0.05 with TFCE family-wise error protection) are outlined in black (see also Supplementary Fig. S47). Orange/yellow areas indicate regions where patients exhibited stronger cerebellar connectivity, whereas blue areas indicate regions where patients exhibited reduced cerebellar connectivity, relative to controls. Inset shows coverage of all cerebellar parcels. (b) Subcortical group differences in cerebellar connectivity shown in volume-based axial view, with Z-coordinate ranges (each slice in each row increments by 3 mm). (c) Relationship between mean cerebellar hyper-connectivity and a measure of cognitive performance is highly significant across subjects. (d) Relationship between mean cerebellar hypo-connectivity and cognitive performance is also highly significant across subjects. (e–h) Results for identical independent analysis conducted with the 6 functionally-defined striatal network parcels. Note that there was no representation of the visual network in striatum (Choi et al. 2012) and therefore that network was omitted. Abbreviations: VIS, visual network; SOM, somatosensory; DAN, dorsal attention network; VAN, ventral attention network; LIM, limbic network; FPCN, frontoparietal control network; DMN, default mode network.
Discussion
Identifying robust, replicable and neurobiologically-grounded neuroimaging markers for neuropsychiatric illness in general and SCZ in particular remains a major knowledge gap and an obstacle for rational treatment development. An ongoing debate concerns a tension between hypotheses suggesting that SCZ neural disturbances manifest across large-scale functional networks and those suggesting they occur in select regions (Anticevic and Lisman 2017). Informing this question is important to facilitate biomarker development that can guide treatments for either targeted neuropathology in specific areas or diffuse alterations that span brain-wide pathways. We show that robust and replicable bi-directional dysconnectivity is present across CSTC, which were more pronounced in higher-order associative areas than in sensory areas. Using complementary data-driven approaches, we demonstrate that the cerebellum and striatum can be divided into functionally distinct disrupted circuits. Additionally, we demonstrate, using SVM-based machine learning, that “core” associative alterations appear across both cerebellum and striatum and yield robust classification tools. Finally, we replicate the CSTC effects and we show that observed dysconnectivity weakly correlates with psychosis symptom severity but more strongly relates to cognitive traits across individual subjects. Collectively, these data are consistent with brain-wide bi-directional alterations in CSTC circuits in SCZ that manifest preferentially across associative neural networks.
Brain-wide Disruptions Occur Across CSTC Functional Circuits in SCZ
Numerous lines of evidence implicate localized regional disruptions in SCZ. For instance, the dopamine hypothesis of SCZ points to a striatal disruption in DA signaling. This is supported by the clinical potency of first-generation antipsychotics (and to some extent, second-generation “atypical” (Seeman 2002)), whereby blockade of striatal dopamine D2 receptors correlates with therapeutic efficacy for psychotic symptoms (Seeman and Lee 1975; Seeman et al. 1975). In humans, these receptors are primarily expressed in the dorsal striatum (Lee and Seeman 1980; Mackay et al. 1982; Howes et al. 2009). Complementary work centered on the DA hypothesis implicates hippocampal, thalamic and focal ventral tegmental area (VTA) alterations as central in the formation of positive symptoms of SCZ (Grace et al. 2007; Lodge and Grace 2011). Conversely, other hypotheses regarding SCZ neurobiology suggest a widespread synaptic disruption, such as the glutamatergic NMDAR hypo-function model, which is centered on cortical microcircuits (Krystal et al. 2003; Gonzalez-Burgos and Lewis 2012). Although it has been challenging to link such a broad-ranging behavioral disturbances across the SCZ spectrum to a single or a few punctate brain regions, it should be noted that many studies have also provided evidence suggesting that selective striatal dopaminergic dysfunction may lead to widespread changes in cortical function (Kellendonk et al. 2006), and it is possible that selective and localized neurobiological disruptions may be leading to persistent and distributed neural abnormalities that underlie behavioral symptoms at a longer time scale in SCZ. Behaviorally, the SCZ spectrum is associated with broad deficits, including impaired executive cognitive functioning, deficits in perception, belief formation, as well as severe affective deficits marked by amotivation and anhedonia (Kay et al. 1987; Berenbaum and Oltmanns 1992; Barch 2005; Stephan et al. 2009; Leitman et al. 2010; Calderone et al. 2013).
To date, neuroimaging studies have provided support for both “local hotspot” and “widespread” alterations, largely as a consequence of analytic method used. For instance, the thalamus has featured prominently in theoretical models of SCZ, both as a region of core putative disruption and part of a broader dysfunctional cortico-striato-thalamic loop, and as a locus of perturbation in functional coupling (Woodward et al. 2012; Anticevic, Cole, et al. 2014; Anticevic and Lisman 2017). However, examining if such perturbations appear across neural systems, or if they are localized to select regions, necessitates the consideration of large-scale brain-wide network. We leveraged existing findings in healthy individuals that have identified large-scale functional networks across the cerebral cortex, basal ganglia, and cerebellum (Buckner et al. 2011; Yeo et al. 2011; Choi et al. 2012). If SCZ involves synaptic alterations that extend beyond thalamus, then the bi-directional dysconnectivity seen with the thalamus may also be present across other systems as a shared pattern of disruption. Presented results combined complimentary analytic approaches, namely a priori subcortical network parcel seeds, voxel-wise clustering of dysconnectivity, and binary classifiers of group status, to show that bi-directional disruptions in SCZ are indeed present across the striatum and cerebellum. Moreover, these alterations are particularly pronounced along associative higher-order functional subdivisions of these 2 systems. These 3 complementary results point to robust bi-directional alterations across both the striatum and cerebellum, extending more focused reports in SCZ subjects (Andreasen et al. 1996; Konarski et al. 2005; Collin et al. 2011; Tu et al. 2012; Fornito et al. 2013; Sarpal et al. 2015; Horga et al. 2016), as well as in related psychiatric disorders (Anand et al. 2009; Harrison et al. 2009; Cubillo et al. 2010; Di Martino et al. 2011; Anticevic, Hu, et al. 2014; Dandash et al. 2014; Shinn et al. 2015).
A number of neurobiological models for SCZ spectrum disease feature a central dysfunction in a given pathway or region. Importantly, many if not all of these theories acknowledge that even a focal disruption (e.g., associative striatum, hippocampus, etc.) could have numerous downstream consequences affecting functioning of various cortico-subcortical systems. Thus, future work that goes beyond observational cross-sectional resting-state fMRI in chronic patients will be critical to ascertain if indeed there are people along the psychosis spectrum who manifest a “hotspot” dysfunction that then “spreads” or exhibit a brain-wide dysfunction that then preferentially affects some pathways more than others.
Treatment and Diagnostic Implications of Disturbances Across CSTC
While SCZ patients exhibited changes across both striatum and cerebellum, the associative higher-order subdivisions across the CSTC systems exhibited the most robust disturbances, in line with prior studies focused on thalamic and cortical networks (Barch et al. 2001; Camchong et al. 2011; Woodward et al. 2012; Anticevic, Cole, et al. 2014; Baker et al. 2014). This preferentially more severe associative disruption may reflect pervasive brain-wide synaptic deficits (e.g., NMDAR dysfunction), which in turn affects the most “vulnerable” circuits (i.e., associative cortex). Recent biophysically-informed computational modeling work showed that widespread cellular-level glutamatergic deficits can yield preferentially more severe associative network dysconnectivity due to higher recurrent excitation in associative (versus sensory) cortex (Murray, Bernacchia, et al. 2014; Scholtens et al. 2014; Yang, Murray, Wang, et al. 2016). Such differences in neuronal structure and functional coupling may extend throughout CSTC systems and may underlie the preferentially patterns of dysconnectivity observed across the cerebellum and striatum.
Critically, these findings present a challenge for treatment design. There appears to be some final converging regional expression of more severe dysconnectivity in association networks across cortex, thalamus, striatum, and cerebellum. Consequently, do therapeutic effects need to occur somewhere (i.e., hotspot of disturbance if it indeed exists or emergent associative cortex disruption), anywhere (i.e., at any point of the affected CSTC circuit) or everywhere (i.e., global tuning of excitation/inhibition balance across all circuits), and at what stage of disease progression? A way to address this question is to longitudinally study duration-of-untreated psychosis (DUP) in early-course illness as a predictor of currently reported CSTC alterations and to map the neurobiology of at-risk clinical populations.
Notably, cerebellar and striatal features yielded high between-group single-subject classification accuracy (comparable to other classifiers in the literature (Davatzikos et al. 2005; Kawasaki et al. 2007; Ardekani et al. 2011; Iwabuchi et al. 2013; Schnack et al. 2014)). Nevertheless, there are key considerations for clinical applicability of the neuroimaging effects. First, the observed dysconnectivity effects did not relate to state-like measures of psychosis symptom severity, across several well-powered analyses for both the cerebellum and the striatum. This modest symptom effect was observed in the context of highly robust individual differences in hyper-/hypo-connectivity relationships across both the cerebellum and the striatum (see Fig. 6), which was replicated in an independent sample (i.e., not a floor or ceiling effect in neural data). Critically, in an independent SCZ replication sample we observed a significant relationship between cognition and CSTC dysconnectivity (either cerebellum or striatum for both hypo- or hyper-directions in cognition) across individuals. This is consistent with the possibility that CSTC dysconnectivity reflects cognitive disturbances as opposed to psychosis symptoms. These findings illustrate the complexity in generating the mapping between reliable group-level markers and clinically-meaningful behavioral dimensions. Future studies will need to develop a unified “geometry” that integrates both categorical disturbances while concurrently capturing unique dimensions of clinical symptom variation.
Limitations, Pitfalls and Future Approaches
As most of our patient sample was medicated at the time of scanning, we cannot fully rule out the possibility that antipsychotic effects may be confounding the relationships between symptoms, cognition, and neural dysconnectivity. It is also possible that a relationship with medication may indeed be present in a SCZ sample with more severe symptoms (e.g., active hallucinations). Additionally, although we did not find subject sex/gender to affect our key results, it is possible that differences in CSTC connectivity will emerge in a more thorough set of analyses, given the body of literature reporting differences between male and female patients with SCZ and prodromal adolescents (Satterthwaite et al. 2015; Mendrek and Mancini-Marie 2016). Another limitation of this (and virtually all clinical) resting-state datasets is the relatively short duration of the resting-state BOLD runs. Although our core results are robust and replicable across multiple analytic approaches (including classification) and datasets, it will be important to validate these findings in a clinical dataset with longer resting-state runs. A vital corollary of this consideration is just how long do the BOLD runs need to be to capture “state”/‘trait’ symptom/syndrome variation across the neuropsychiatric spectrum. Future work will be critical to address this problem more definitively. It is also worth noting that although we found that our results are replicable across 2 datasets preprocessed with different pipelines, a more principled approach to test whether the results are generalizable across pipeline choice would be to assess both datasets with multiple pipelines. Such a comparison would be important for evaluating the robustness of the CSTC effect as well as the differences between fMRI preprocessing options.
Importantly, while these results strongly indicate that disruptions in SCZ are pervasive across cerebellar and striatal systems, they do not rule out the possibility that these disturbances emerge over time as a consequence of a local “hotspot” of disruption. Addressing these questions will require prospective longitudinal clinical design that starts from the prodrome and examines if specific regional markers of dysfunction yield superior prospective classifier performance that in turn predict symptom severity. As discussed, leveraging diagnostically relevant information across different neuroimaging modalities may increase classifier performance in relation to state/trait predictive power. Another outstanding question concerns whether this observed dysconnectivity is consistent across different DSM-defined disorders. Studying dysconnectivity across CSTC systems using matched cross-diagnostic samples may reveal patterns of bi-directional CSTC disruption in some patients exclusively (i.e., DSM-like categorical pattern) or perhaps may appear in all patients as a function of cognitive deficit severity (RDoC-like dimensional pattern) (Insel et al. 2010); alternatively, it may show marked differences across non-DSM group boundaries (e.g., biomarker-defined biotypes (Clementz et al. 2016)). This will be an important direction for future study that can be tested in a cross-diagnostic “psychosis spectrum” sample, such as the full B-SNIP dataset (Tamminga et al. 2013).
Conclusion
SCZ research faces a tension between hypotheses proposing “focal hotspot” disturbances affecting neural communication versus pervasive alterations that span distributed neural circuits. We demonstrate that similar bi-directional disruptions in functional coupling are robustly present across both cerebellar and striatal subdivisions of the CSTC system, with preferentially more pronounced alterations along “associative” CSTC subdivisions. This shared and widespread CSTC dysconnectivity in SCZ is inconsistent with the possibility of exclusive “localized” thalamic disruptions (at least in chronic states). Notably, results replicated across multiple complementary analytic approaches and independent datasets, linking alterations across CSTC systems with cognition in SCZ.
Supplementary Material
Notes
Data were provided by the Centers of Biomedical Research Excellence (COBRE), the Olin Neuropsychiatric Research Center, and the Bipolar–Schizophrenia Network on Intermediate Phenotypes consortium. Conflict of Interest: None declared.
Funding
This work was supported by National Institutes of Health (DP50D012109 to A.A and 5R01MH077862 to J.S.); the National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to A.A.); and the Yale Center for Clinical Investigation (A.A. and P.I.).
Disclosures
G.R. and J.M. consult for BlackThorn Therapeutics. A.A. consults and is a SAB member for BlackThorn Therapeutics Inc. J.H.K. is a co-inventor for the following approved or pending patents: (1) Seibyl J.P., Krystal J.H., Charney D.S.: Dopamine and noradrenergic reuptake inhibitors in treatment of schizophrenia, US Patent No. 5447,948, 5 September 1995; (2) Coric V., Krystal J.H., Sanacora G.: Glutamate modulating agents in the treatment of mental disorders, US Patent No. 8778,979, B2 patent issue date 15 July 2014; (3) Charney D., Krystal J.H., Manji H., Matthew S., Zarate C.: Intranasal administration of ketamine to treat depression, US Application No. 14/197,767 filed on 5 March 2014, US Application or PCT International Application No. 14/306,382 filed on 17 June 2014; (4) Arias A, Petrakis I, Krystal JH: Composition and methods to treat addiction, Provisional Use Patent Application No. 61/973/961, 2 April 2014, filed by Yale University Office of Cooperative Research; and (5) Chekroud A, Gueorguieva R, Krystal, JH: Treatment selection for major depressive disorder, US Patent and Trademark Office Docket No. Y0087.70116US00, filed on 3 June 2016, provisional patent submitted by Yale University. Over the past year, he has received more than $5000 in compensation related to consulting or licensed patents from Janssen Pharmaceuticals. All other authors report no conflicts of interest.
References
- Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M. 2009. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 171:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD. 1996. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA. 93:9985–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. 2010. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, Kober H, Gruber J, Repovs G, Cole MW, et al. 2012. a. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 73:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, Savic A, Krystal JH, Pearlson GD, Glahn DC. 2014. a. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 24:3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, Niciu MJ, Morgan PT, Surti TS, Bloch MH, et al. 2012. b. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci USA. 109:16720–16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, McEwen SC, Bearden CE, Addington J, Goodyear B, et al. 2015. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA. Psychiatry. 72:882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, Repovs G, Cole MW, Bednarski S, Krystal JH, et al. 2014. b. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry. 75:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Lisman J. 2017. How can global alteration of excitation/inhibition balance lead to the local dysfunctions that underlie schizophrenia? Biol Psychiatry. 81:818–820. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Barch DM. 2012. c. Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophr Bull. 38:967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani BA, Tabesh A, Sevy S, Robinson DG, Bilder RM, Szeszko PR. 2011. Diffusion tensor imaging reliably differentiates patients with schizophrenia from healthy volunteers. Hum Brain Mapp. 32:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, Ongür D. 2014. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 72:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM. 2005. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophr Bull. 31:875–881. [DOI] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A 3rd, Noll DC, Cohen JD. 2001. Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry. 58:280–288. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Oltmanns TF. 1992. Emotional experience and expression in schizophrenia and depression. J Abnorm Psychol. 101:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 106:2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone DJ, Martinez A, Zemon V, Hoptman MJ, Hu G, Watkins JE, Javitt DC, Butler PD. 2013. Comparison of psychophysical, electrophysiological, and fMRI assessment of visual contrast responses in patients with schizophrenia. Neuroimage. 67:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Macdonald AW, Bell C, Mueller BA, Lim KO. 2011. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 37:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. 2011. Functional connectivity of the insula in the resting brain. Neuroimage. 55:8–23. [DOI] [PubMed] [Google Scholar]
- Choi EY, Yeo BT, Buckner RL. 2012. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 108:2242–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA. 2016. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 173:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clopper CJ, Pearson ES. 1934. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 26:404–413. [Google Scholar]
- Cole MW, Anticevic A, Repovs G, Barch DM. 2011. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 70:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin G, Hulshoff Pol HE, Haijma SV, Cahn W, Kahn RS, van den Heuvel MP. 2011. Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Front Psychiatry. 2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. 2010. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood Attention-Deficit Hyperactivity Disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. J Psychiatr Res. 44:629–639. [DOI] [PubMed] [Google Scholar]
- Dandash O, Fornito A, Lee J, Keefe RS, Chee MW, Adcock RA, Pantelis C, Wood SJ, Harrison BJ. 2014. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr Bull. 40:904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davatzikos C, Shen D, Gur RC, Wu X, Liu D, Fan Y, Hughett P, Turetsky BI, Gur RE. 2005. Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch Gen Psychiatry. 62:1218–1227. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, Lord C, Castellanos FX, Milham MP. 2011. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry. 69:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Goodby E, Dean A, Ooi C, Nathan PJ, Lennox BR, Jones PB, Suckling J, Bullmore ET. 2013. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA psychiatry. 70:1143–1151. (Chicago, Ill). [DOI] [PubMed] [Google Scholar]
- Gazzola V, Keysers C. 2009. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cereb Cortex. 19:1239–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, et al. 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. 2012. NMDA receptor hypofunction, parvalbumin-positive neurons and cortical gamma oscillations in schizophrenia. Schizophr Bull. 38:950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. 2007. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 30:220–227. [DOI] [PubMed] [Google Scholar]
- Haber S, McFarland NR. 2001. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist. 7:315–324. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Soriano-Mas C, Pujol J, Ortiz H, López-Solà M, Hernández-Ribas R, Deus J, Alonso P, Yücel M, Pantelis C, et al. 2009. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry. 66:1189–1200. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Bachus SE, Hyde TM, Wittie M, Herman MM, Vangel M, Saper CB, Kleinman JE. 2005. Reduced density of cholinergic interneurons in the ventral striatum in schizophrenia: an in situ hybridization study. Biol Psychiatry. 58:408–416. [DOI] [PubMed] [Google Scholar]
- Horga G, Cassidy CM, Xu X, Moore H, Slifstein M, Van Snellenberg JX, Abi-Dargham A. 2016. Dopamine-related disruption of functional topography of striatal connections in unmedicated patients with schizophrenia. JAMA Psychiatry. 73:862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, et al. 2009. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 66:13–20. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 167:748–751. [DOI] [PubMed] [Google Scholar]
- Iwabuchi SJ, Liddle PF, Palaniyappan L. 2013. Clinical utility of machine-learning approaches in schizophrenia: improving diagnostic confidence for translational neuroimaging. Frontiers in psychiatry. 4:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Kherif F, Takahashi T, Zhou SY, Nakamura K, Matsui M, Sumiyoshi T, Seto H, Kurachi M. 2007. Multivariate voxel-based morphometry successfully differentiates schizophrenia patients from healthy controls. Neuroimage. 34:235–242. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 13:261–276. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. 2004. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 68:283–297. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, Moore H, Kandel ER. 2006. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 49:603–615. [DOI] [PubMed] [Google Scholar]
- Klein JC, Rushworth MFS, Behrens TEJ, Mackay CE, de Crespigny AJ, D’Arceuil H, Johansen-Berg H. 2010. Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. Neuroimage. 51:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH. 2005. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci. 30:178–186. [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. 2003. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology (Berl). 169:215–233. [DOI] [PubMed] [Google Scholar]
- Lee T, Seeman P. 1980. Elevation of brain neuroleptic/dopamine receptors in schizophrenia. Am J Psychiatry. 137:191–197. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC. 2010. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. Am J Psychiatry. 167:818–827. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Pi HJ, Zhang Y, Otmakhova NA. 2010. A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry. 68:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fan G, Xu K, Wang F. 2011. Changes in cerebellar functional connectivity and anatomical connectivity in schizophrenia: a combined resting-state functional MRI and diffusion tensor imaging study. J Magn Reson Imaging. 34:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. 2011. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 32:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay AV, Iversen LL, Rossor M, Spokes E, Bird E, Arregui A, Creese I, Synder SH. 1982. Increased brain dopamine and dopamine receptors in schizophrenia. Arch Gen Psychiatry. 39:991–997. [DOI] [PubMed] [Google Scholar]
- Meda SA, Wang Z, Ivleva EI, Poudyal G, Keshavan MS, Tamminga CA, Sweeney JA, Clementz BA, Schretlen DJ, Calhoun VD, et al. 2015. Frequency-specific neural signatures of spontaneous low-frequency resting state fluctuations in psychosis: evidence from Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Consortium. Schizophr Bull. 41:1336–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Mancini-Marie A. 2016. Sex/gender differences in the brain and cognition in schizophrenia. Neurosci Biobehav Rev. 67:57–78. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. 2005. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 62:379–386. [DOI] [PubMed] [Google Scholar]
- Murray JD, Anticevic A, Gancsos M, Ichinose M, Corlett PR, Krystal JH, Wang XJ. 2014. a. Linking microcircuit dysfunction to cognitive impairment: effects of disinhibition associated with schizophrenia in a cortical working memory model. Cereb Cortex. 24:859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JD, Bernacchia A, Freedman DJ, Romo R, Wallis JD, Cai X, Padoa-Schioppa C, Pasternak T, Seo H, Lee D, et al. 2014. b. A hierarchy of intrinsic timescales across primate cortex. Nat Neurosci. 17:1661–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanetti L, Cerliani L, Gazzola V, Renken R, Keysers C. 2009. Group analyses of connectivity-based cortical parcellation using repeated k-means clustering. Neuroimage. 47:1666–1677. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage. 76:439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay IS, MacDonald AW 3rd. 2018. The ups and downs of thalamocortical connectivity in schizophrenia. Biol Psychiatry. 83:473–474. [DOI] [PubMed] [Google Scholar]
- Ramsay IS, Nienow TM, MacDonald AW 3rd. 2017. Increases in intrinsic thalamocortical connectivity and overall cognition following cognitive remediation in chronic schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging. 2:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey NF, Tallent K, van Gelderen P, Frank JA, Moonen CT, Weinberger DR. 1996. Reproducibility of human 3D fMRI brain maps acquired during a motor task. Hum Brain Mapp. 4:113–121. [DOI] [PubMed] [Google Scholar]
- Ray JP, Price JL. 1993. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 337:1–31. [DOI] [PubMed] [Google Scholar]
- Repovs G, Barch DM. 2012. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Front Hum Neurosci. 6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G, Csernansky JG, Barch DM. 2011. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 15:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch N, Spoletini I, Wilke M, Bria P, Di Paola M, Di Iulio F, Martinotti G, Caltagirone C, Spalletta G. 2007. Prefrontal-thalamic-cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr Res. 93:79–89. [DOI] [PubMed] [Google Scholar]
- Sarpal DK, Argyelan M, Robinson DG, Szeszko PR, Karlsgodt KH, John M, Weissman N, Gallego JA, Kane JM, Lencz T, et al. 2016. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am J Psychiatry. 173:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarpal DK, Robinson DG, Lencz T, Argyelan M, Ikuta T, Karlsgodt KH, Gallego JA, Kane JM, Szeszko PR, Malhotra AK. 2015. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 72:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, Gennatas ED, Elliott MA, Smith A, Hakonarson H, et al. 2015. Linked sex differences in cognition and functional connectivity in youth. Cereb Cortex. 25:2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack HG, Nieuwenhuis M, van Haren NE, Abramovic L, Scheewe TW, Brouwer RM, Hulshoff Pol HE, Kahn RS. 2014. Can structural MRI aid in clinical classification? A machine learning study in two independent samples of patients with schizophrenia, bipolar disorder and healthy subjects. Neuroimage. 84:299–306. [DOI] [PubMed] [Google Scholar]
- Scholtens LH, Schmidt R, de Reus MA, van den Heuvel MP. 2014. Linking macroscale graph analytical organization to microscale neuroarchitectonics in the macaque connectome. J Neurosci. 34:12192–12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. 2002. Atypical antipsychotics: mechanism of action. Can J Psychiatry. 47:27–38. [PubMed] [Google Scholar]
- Seeman P, Chau-Wong M, Tedesco J, Wong K. 1975. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proc Natl Acad Sci USA. 72:4376–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Lee T. 1975. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 188:1217–1219. [DOI] [PubMed] [Google Scholar]
- Sheffield JM, Kandala S, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, Clementz BA, Lerman-Sinkoff DB, Hill SK, Barch DM. 2017. Transdiagnostic associations between functional brain network integrity and cognition. JAMA Psychiatry. 74:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn AK, Baker JT, Lewandowski KE, Ongur D, Cohen BM. 2015. Aberrant cerebellar connectivity in motor and association networks in schizophrenia. Front Hum Neurosci. 9:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 44:83–98. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ. 2006. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 59:929–939. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. 2009. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 35:509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA. 2013. Clinical phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 170:1263–1274. [DOI] [PubMed] [Google Scholar]
- Tu PC, Hsieh JC, Li CT, Bai YM, Su TP. 2012. Cortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: a resting fMRI study. Neuroimage. 59:238–47. [DOI] [PubMed] [Google Scholar]
- van der Gaag M, Hoffman T, Remijsen M, Hijman R, de Haan L, van Meijel B, van Harten PN, Valmaggia L, de Hert M, Cuijpers A, et al. 2006. The five-factor model of the Positive and Negative Syndrome Scale II: a ten-fold cross-validation of a revised model. Schizophr Res. 85:280–287. [DOI] [PubMed] [Google Scholar]
- Wang L, Zou F, Shao Y, Ye E, Jin X, Tan S, Hu D, Yang Z. 2014. Disruptive changes of cerebellar functional connectivity with the default mode network in schizophrenia. Schizophr Res. 160:67–72. [DOI] [PubMed] [Google Scholar]
- Welsh RC, Chen AC, Taylor SF. 2010. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 36:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Douaud G, Nichols TE, Smith SM. 2016. Faster permutation inference in brain imaging. Neuroimage. 141:502–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. 2014. Permutation inference for the general linear model. Neuroimage. 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. 2012. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 169:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GJ, Murray JD, Wang XJ, Glahn DC, Pearlson GD, Repovs G, Krystal JH, Anticevic A. 2016. a. Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc Natl Acad Sci USA. 113:E219–E228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GJ, Murray JD, Wang X-J, Glahn DC, Pearlson GD, Repovs G, Krystal JH, Anticevic A. 2016. b. Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc Natl Acad Sci USA. 113:E219–E228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, et al. 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.