Abstract
Generation of olfactory bulb (OB) interneurons requires neural stem/progenitor cell specification, proliferation, differentiation, and young interneuron migration and maturation. Here, we show that the homeobox transcription factors Dlx1/2 are central and essential components in the transcriptional code for generating OB interneurons. In Dlx1/2 constitutive null mutants, the differentiation of GSX2+ and ASCL1+ neural stem/progenitor cells in the dorsal lateral ganglionic eminence is blocked, resulting in a failure of OB interneuron generation. In Dlx1/2 conditional mutants (hGFAP-Cre; Dlx1/2F/− mice), GSX2+ and ASCL1+ neural stem/progenitor cells in the postnatal subventricular zone also fail to differentiate into OB interneurons. In contrast, overexpression of Dlx1&2 in embryonic mouse cortex led to ectopic production of OB-like interneurons that expressed Gad1, Sp8, Sp9, Arx, Pbx3, Etv1, Tshz1, and Prokr2. Pax6 mutants generate cortical ectopia with OB-like interneurons, but do not do so in compound Pax6; Dlx1/2 mutants. We propose that DLX1/2 promote OB interneuron development mainly through activating the expression of Sp8/9, which further promote Tshz1 and Prokr2 expression. Based on this study, in combination with earlier ones, we propose a transcriptional network for the process of OB interneuron development.
Keywords: Ascl1, Pax6; Dlx1; Dlx2; Gsx2; interneuron; olfactory bulb; Sp8; Sp9
Introduction
GABAergic interneurons in the olfactory bulb (OB) are not born within the OB; they are generated by progenitors located in different germinal zones of telencephalon, including the ganglionic eminences, septum, pallium; this process continues postnatally in the subventricular zone (SVZ) and the rostral migratory stream (RMS) (Doetsch et al. 1999; Stenman et al. 2003; Kohwi et al. 2007; Long et al. 2007; Merkle et al. 2007; Ventura and Goldman 2007; Young et al. 2007; Xu et al. 2008; Fuentealba et al. 2015). The lateral ganglionic eminence (LGE) contains 2 distinct compartments: the dorsal LGE (dLGE) generates the interneurons for the OB (Yun et al. 2001; Stenman et al. 2003; Waclaw et al. 2006; Li et al. 2018) and the intercalated cells for the amygdala (Carney et al. 2009; Waclaw et al. 2010; Cocas et al. 2011; Kuerbitz et al. 2018), and the ventral LGE (vLGE) generates striatal medium spiny neurons (MSNs) (Deacon et al. 1994; Olsson et al. 1995, 1998; Anderson et al. 1997; Stenman et al. 2003; Zhang et al. 2016; Xu et al. 2018). The dLGE and vLGE may be further divided into pLGE1/2 and pLGE3/4 (Flames et al. 2007; Xu et al. 2018).
Multiple transcription factors play important roles in OB interneuron development. Gsx2, which is highly expressed in the ventricular zone (VZ) neural stem/progenitor cells of the dLGE, plays a critical role in OB interneuron development (Corbin et al. 2000; Toresson et al. 2000; Toresson and Campbell 2001; Yun et al. 2001, 2003; Wang et al. 2009, 2013, 2013). Ascl1, which is expressed by LGE progenitors, nonautonomously promotes the maintenance of adjacent neural progenitors through activating Notch signaling and lateral inhibition, and autonomously promotes neurogenesis (Casarosa et al. 1999; Long et al. 2009; Castro et al. 2011). Dlx1/2, which are expressed by neural progenitors and migrating OB neuroblasts (immature OB interneurons) (Porteus et al. 1994; Doetsch et al. 2002), promote neuronal differentiation and migration. Dlx1/2−/− null mutant mice lack virtually all GABAergic interneurons in the OB (Porteus et al. 1994; Bulfone et al. 1995; Anderson et al. 1997; Long et al. 2007), whereas Dlx1−/−, Dlx2−/−, or Dlx5−/− mutants have less severe OB phenotypes (Qiu et al. 1995; Long et al. 2003, 2007). The zinc finger transcription factors Sp8/9, which are expressed by dividing, nondividing neuroblasts and mature OB interneurons (Waclaw et al. 2006; Liu et al. 2009; Li et al. 2011), are essential for the neuronal migration and differentiation of OB interneurons through promoting expression of prokineticin receptor 2 (Prokr2), a G protein-coupled receptor, and teashirt zinc finger family member 1 (Tshz1) (Li et al. 2018). The zinc finger transcription factor Tshz1 is mainly expressed by neuroblasts and mature interneurons, and is required for the differentiation and radial migration of neuroblasts within the OB (Ragancokova et al. 2014). Prokineticin 2 (Prok2) and Prokr2 signaling is required for the neuronal migration and differentiation from the SVZ through the RMS to their final layers in the OB (Ng et al. 2005; Matsumoto et al. 2006; Prosser et al. 2007). Loss of Prok2-Prokr2 signaling results in loss of most OB interneurons. This signaling is involved in human Kallmann syndrome, which is characterized by congenital hypogonadotropic hypogonadism (due to gonadotropin-releasing hormone [GnRH] deficiency) and anosmia/hyposmia (due to OB interneuron defects) (Ng et al. 2005; Dode et al. 2006; Matsumoto et al. 2006; Pitteloud et al. 2007; Prosser et al. 2007; Sarfati et al. 2010; Martin et al. 2011). In Sp8/9 double conditional knockout mice, neuroblasts in the dLGE and postnatal SVZ, RMS, and OB fail to express Prokr2 and Tshz1; accordingly, few neuroblasts are able to reach the OB, and those that do fail to migrate tangentially and radially and then undergo apoptotic cell death, resulting in loss of nearly all mature OB interneurons (Li et al. 2018).
Although Dlx1/2−/− mice lack virtually all OB interneurons, there are many gaps in our knowledge of how Dlx1/2 regulate OB interneuron development. Here, by analyzing Dlx1/2−/− double mutant, Pax6Sey/Sey single mutant and Dlx1/2−/−; Pax6Sey/Sey triple mutant mice, we provide new evidence that, without Dlx1/2 function, virtually all neural progenitors in the dLGE fail to differentiate into OB interneurons. Importantly, we show that Dlx1/2 function is required postnatally for OB interneuron development using hGFAP-Cre in the Dlx1/2F/− conditional mutants, as GSX2+ and ASCL1+ neural stem/progenitor cells in the postnatal SVZ fail to differentiate into immature OB interneurons. When Dlx1/2 genes were overexpressed in the E14.5 mouse cortex by in utero electroporation (IUE), neocortical progenitors started to generate immature OB interneurons instead of cortical pyramidal projection neurons. These OB interneurons express Gad1 (Gad67), Sp8, Sp9, Arx, Pbx3, Etv1 (Er81), Tshz1, and Prokr2. We propose that DLX1/2 promote OB interneuron development mainly through activating the expression of Sp8/9, which further promote Tshz1 and Prokr2 expression. This study, in combination with earlier studies, presents evidence for a transcriptional regulatory network that controls the multistep process of OB interneuron development (Fig. 10N).
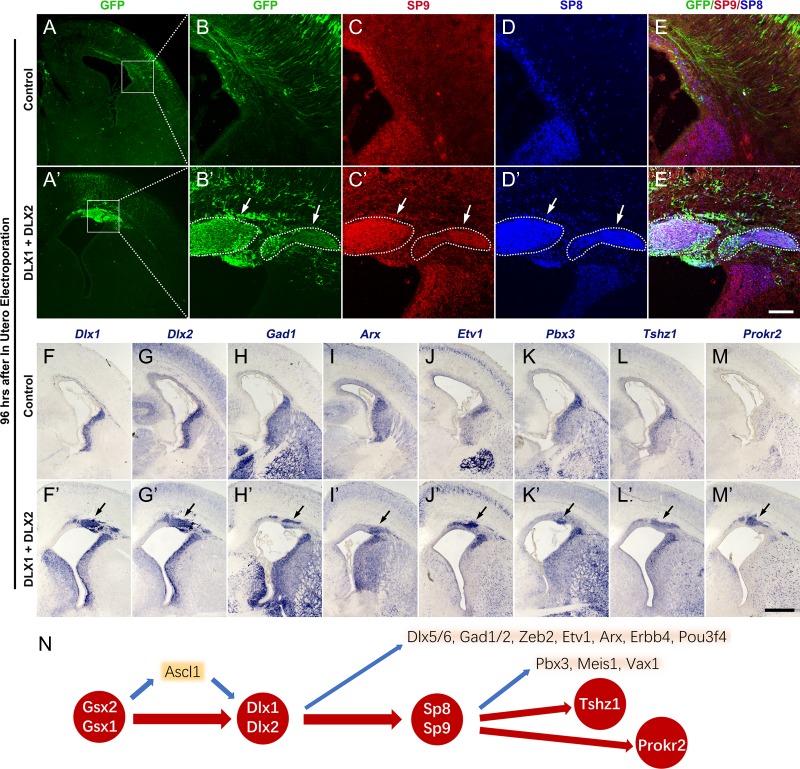
Figure 10.
Overexpression of Dlx1&2 in the dorsal pallium (cortex) induced the generation of immature OB interneurons. (A–E′) Overexpression of Dlx1&2 in the neocortex by IUE of plasmids (pCAGIG-Dlx1-Ires-GFP + pCAGIG-Dlx2-Ires-GFP) at E14.5 induced the ectopic expression of SP8&9 (arrows) in the cortical SVZ at E18.5, whereas overexpression of GFP alone (pCAGIG-Ires-GFP) did not. (F–M′) In situ RNA hybridization showed that ectopic expression of Dlx1&2 induced Gad1, Arx, Etv1, Pbx3, Tshz1, and Prokr2 (arrows). (N) Based on Dlx1/2 loss of function and gain of function analysis, we propose a transcriptional regulatory network that underlies OB interneuron development. Note that loss of Gsx2/1, Dlx1/2, Sp8/9, or Prokr2 function results in loss of all or most OB interneurons (see discussion). Scale bars: 500 μm in M′ for A, A′, F–M′; 100 μm in E′ for B–E′.
Materials and Methods
Mice
Dlx1/2 +/− (Qiu et al. 1997), Pax6Sey/+ (small eye mice carrying the Seyneu mutant allele of Pax6) (Hill et al. 1991), Ascl1-GFP knockin knockout (Kim et al. 2007; Leung et al. 2007), Sp9LacZ/+ (Zhang et al. 2016), Sp9 floxed (Zhang et al. 2016), Sp8 floxed (Bell et al. 2003), Dlx1/2 floxed (Silbereis et al. 2014), hGFAP-Cre (Zhuo et al. 2001), and Dlx5/6-Cre-Ires-EGFP (Stenman et al. 2003) mice were previously described. These mice were maintained in a mixed genetic background of C57BL/6 J and CD1. The day of vaginal plug detection was regarded as embryonic day 0.5, and the day of birth was considered as postnatal day 0. All animal experiments described in this study were approved in accordance with institutional guidelines at Fudan University Shanghai Medical College.
Tissue Preparation
E17–E18 embryos and postnatal mice were perfused intracardially with 4% PFA in 1× phosphate buffered saline (PBS, pH 7.4); embryonic brains (earlier than E17) were immersion fixed in 4% PFA. Postnatal brains were fixed overnight in 4% PFA, cryoprotected in 30% sucrose for at least 24 h, frozen in the embedding medium and cryosectioned.
In Utero Electroporation
In utero electroporation (IUE) of wild type CD1 embryos was performed at E14.5 as described (Saito and Nakatsuji 2001). In brief, plasmids pCAGIG-Ires-GFP (control) or (pCAGIG-Dlx1-Ires-GFP and pCAGIG-Dlx2-Ires-GFP, 1.0 μg/μL, 1:1) or (pCAGIG-Dlx1-Ires-GFP, pCAGIG-Dlx2-Ires-GFP and pCAGIG-Cre, 1.0 μg/μL, 1:1:2) were mixed with 0.05% Fast Green (Sigma), and injected through the uterine wall into the lateral ventricle using a beveled pulled glass micropipette. Five electrical pulses were applied at 35 V (50 ms duration) across the uterine wall at 950 ms intervals using 7-mm platinum electrodes (Tweezertrode 45-0488, BTX, Harvard Apparatus) connected to an electroporator (ECM830, BTX). Embryos were then analyzed at different time points.
Immunohistochemistry
Immunohistochemistry was performed on 6, 12, and 20 μm cryostat sections on slides. For SP9, DLX2, and BCL11b immunohistochemistry, sections were boiled in 10 mM sodium citrate briefly for antigen retrieval. The following primary antibodies were used: goat anti-SP8 (1:3 000, Santa Cruz, sc-104 661), rabbit anti-SP9 (1:500) (Zhang et al. 2016), chicken anti-GFP (1:3 000, Aves Labs, GFP-1020), goat anti-DCX (1:1 000, Santa Cruz, sc-8066), rabbit anti-DCX (1:1 000, Abcam, ab18723), mouse anti-NeuN (1:500, Millipore, MAB-377), rabbit anti-ASCL1 (1:2 000, Cosmo Bio, SK-T01-003), rabbit anti-GSX2 (1:2 000, Millipore, ABN162), rabbit anti-OLIG2 (1:500, Millipore, AB9610), rabbit anti-Ki67 (1:500, Vector Labs, VP-K451), rabbit anti-PAX6 (1:2 000, MBL, PD022), goat anti-EBF (1:200, Santa Cruz, sc-15 888), rabbit anti-EBF1 (1:5 000, Millipore, AB10523), goat anti-SOX2 (1:500, Santa Cruz, sc-17 320), rabbit anti-ISL1 (1:500, Abcam, ab20670), rabbit anti-GFAP (1:500, Dako, Z0334), rabbit anti-EOMES (TBR2, 1:500, Abcam, ab23345), rat anti-BCL11b (1:1 000, Abcam, ab18465), and guinea pig anti DLX2 (1:2000) (Kuwajima et al. 2006; Hansen et al. 2013).
Secondary antibodies against the appropriate species were incubated for 60 min at room temperature (all from Jackson, 1:400). Fluorescently stained sections were then washed, counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma, 200 ng/mL) for 2–5 min and coverslipped with Gel/Mount (Biomeda, Foster City, CA). Omission of primary antibodies eliminated the staining signals.
In Situ RNA Hybridization
All in situ RNA hybridization experiments were performed using digoxigenin riboprobes on 20 μm cryostat sections. Riboprobes were described in previous studies (Zhang et al. 2016; Li et al. 2018; Liu et al. 2018; Xu et al. 2018) or made from cDNAs amplified by PCR using the following primers:
1. Erbb4 Fwd: GCACCGATATTTGCCCCAA
Erbb4 Rev: CAGTCATGACTAGTGGGACCGTTAC
2. Etv1 Fwd: TTCATGGCCTCCCACTGAAAATC
Etv1 Rev: CCTTCGTTGTAGGGGTGAGGGTT
3. Dlx5 Fwd: CAGCTTTCAGCTGGCCGCTT
Dlx5 Rev: CAAGGCACCATTGATAGTGTCCACA
4. Bcl11b Fwd: GTAAAGATGAGCCTTCCAGCTACAT
Bcl11b Rev: TTAGCTCCTCTCAGCCTGCTC
5. Sox1 Fwd: CCCTGTGAAATCGAAACGTGCT
Sox1 Rev: TCCAATGTAGGTTGAGCTCTGGTCT
6. Pou3f4 Fwd: ATGGCCACAGCTGCCTCGAA
Pou3f4 Rev: GCTCACCATGGTCTGGAGGCTC
7. Pax6 Fwd: TGCAGACCCATG
Pax6 Rev: CGGTCTGCCCGTTCAACATCCTTAG
Microscopy
Bright field images (in situ hybridization results) and some fluorescent images were imaged with Olympus BX 51 microscope using a ×4 or ×10 objective. Other fluorescent images were taken with Olympus FV1000 confocal microscope system using ×10, ×20, ×40, or ×60 objectives. Z-stack confocal images were reconstructed using the FV10-ASW software. All images were merged, cropped and optimized in Photoshop CS5 without distorting the original information.
RNA Sequencing Analysis
RNA sequencing (RNA-Seq) analysis was performed as previously described (Xu et al. 2018). The LGE (including VZ, SVZ, and MZ-mantle zone/striatum) from E16.5 Dlx1/2−/− null mutant mice and littermate wild type controls were dissected (n = 3 mice, each group). The neocortex (including VZ, SVZ, CP-cortical plate, and MZ-marginal zone) from E16.5 Pax6Sey/Sey mice and littermate wild type controls were dissected (n = 2 mice, each group). In brief, total RNA was extracted using RNeasy Mini Kit (QIAGEN) according to the manufacturer’s protocol, quantified using NanoDrop ND-2000, and checked for RNA integrity by an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). RNA-Seq libraries were prepared according to the Illumina TruSeq protocol. Levels of gene expression were reported in FPKM (fragments per kilobase of transcript per million mapped reads) (Trapnell et al. 2012; Xu et al. 2018). A gene was considered to be expressed if it had an FPKM >1. For a gene to be called as differentially expressed, it required a P-value <0.05. Data from this experiment have been deposited in the GEO database (GSE121215).
Quantification
DLX2 expression and its colocalization with SP8/9 in the E16.5 dLGE SVZ1 and SVZ2, and adult SVZ, RMS, and OB core (34 292 μm2 area from the lateral SVZ and RMS; 29 000 μm2 from the OB core) were quantified in 2–3 randomly chosen 6-μm sections for E16.5 mice and 12-μm sections for P21 mice, and 3 mice were used for each group.
GSX2+, ASCL1+, ISL1+, SP8+, EBF1+, EBF3+, SP9+, BCL11b+, SP9+/BCL11b+ cells in the dLGE at E16.5 were quantified in 3 randomly chosen 12-μm sections for each group of mice (n = 3).
DLX2, GSX2, KI67, ASCL1, SP8, SP9, and OLIG2 expressions in the SVZ were quantified in 3 randomly chosen 12-μm sections for each group of mice (P50 wild type mice and hGFAP-Cre, Dlx1/2F/− mice were used, n = 3). We counted the positive cells and cell numbers colabeled with DCX within a 200 000 μm2 area in the SVZ per section.
Statistics
Statistical significance was assessed using unpaired Student’s t test. All quantification results were presented as the mean ± SEM. P < 0.05 were considered significant.
Results
Coexpression of DLX2, SP8, and SP9 in Immature OB Interneurons
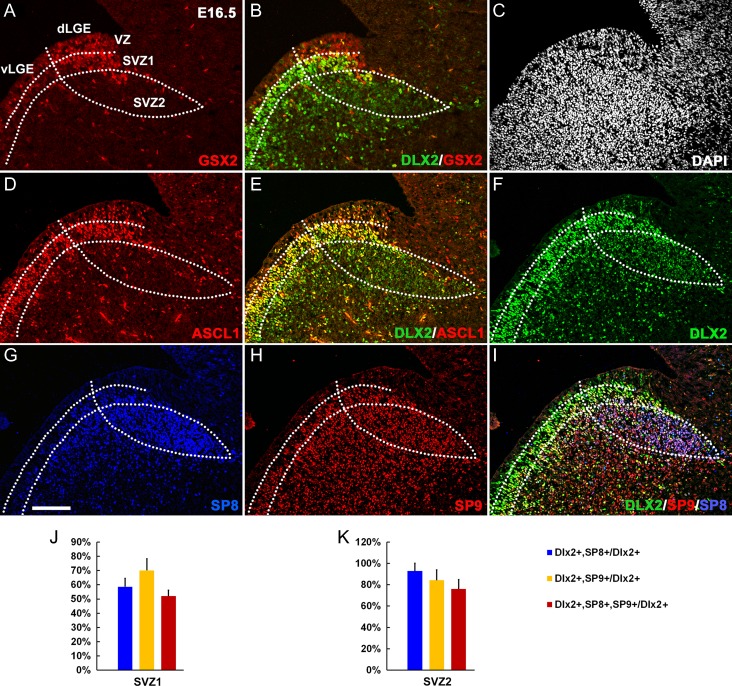
GSX2, DLX2, ASCL1, SP8, and SP9 are essential for OB interneuron development. We compared GSX2, DLX2, ASCL1, SP8, and SP9 protein expression in the dLGE and postnatal SVZ, RMS, and OB using triple immunofluorescence analysis and confocal microscopy. The germinal zone of dLGE consists the VZ, SVZ1 (adjacent to the VZ) and SVZ2 (Fig. 1A) (Petryniak et al. 2007; Wang et al. 2013; Xu et al. 2018). Strong GSX2 expression in the dLGE and weak GSX2 expression in the vLGE was detected at E12.5 (Toresson et al. 2000; Wang et al. 2009), but became more evident at E14.5 (Supplementary Fig. S1A–C). In the dLGE at E16.5, most progenitors in the VZ and SVZ1 expressed strong GSX2, whereas the vLGE expressed relatively weak GSX2 (Fig. 1A). A few ASCL1+ and DLX2+ cells were scattered in the VZ (Fig. 1D–F), but most dLGE SVZ1 progenitors strongly coexpressed ASCL1, DLX2, and GSX2 (Fig. 1B,E) (Eisenstat et al. 1999). In the dLGE SVZ2, only a few GSX2+ and ASCL1+ cells were detected (Fig. 1A,D). However, DLX2 expression was maintained in nearly all SVZ2 cells. DLX2 expression was even higher in SVZ1 (Fig. 1E,F). SP8 and SP9 were robustly expressed in the dLGE SVZ1&2 (Fig. 1G–I); in SVZ2 ~80% of DLX2+ cells coexpressed SP8 and SP9 (Fig. 1I,K). Thus DLX2 expression begins before SP8 and SP9 in the dLGE VZ/SVZ1 and most DLX2+ cells in the SVZ2 coexpressed SP8/9, and vice versa.
Figure 1.
SP8, SP9, and DLX2 are coexpressed in the E16.5 dLGE SVZ. (A, B) GSX2 was strongly expressed in the dLGE VZ and SVZ1; only a few GSX2+ cells were in the SVZ2, whereas DLX2 was mainly expressed in the SVZ1&2. Note weak expression of GSX2 in the vLGE. (C) The section was stained with DAPI. (D, E) Most ASCL1+ cells expressed DLX2. (F–I) DLX2 was strongly expressed in the SVZ1 and relatively weakly expressed in the SVZ2 and DLX2 expression began before SP8/9 expression. Most cells in the SVZ2 expressed DLX2/SP8/SP9. (J, K) Quantification of DLX2+ cells that expressed SP8, SP9, or SP8/9 in the dLGE SVZ1 and SVZ2 at E16.5. Scale bar: 100 μm in G for (A–I).
Expression of DLX2, SP8, and SP9 in the postnatal SVZ, RMS, and OB core showed similar results (Supplementary Fig. S2A–K). In the SVZ and RMS at P21, intermediate progenitors (type C cells) expressed strong DLX2+ but not SP8 or SP9 (Supplementary Fig. S2A–H) (Doetsch et al. 2002; Waclaw et al. 2006; Wei et al. 2011; Li et al. 2018). However, most neuroblasts (type A cells) in the SVZ, RMS, and OB core coexpressed DLX2 and SP8/9 (Supplementary Fig. S2D, H, K, L). Because primary neural stem cells first generate intermediate progenitor cells, which in turn generate neuroblasts that tangentially migrate from SVZ to the OB, based on the DLX2/SP8/SP9 expression patterns, we hypothesized that Dlx2 (and Dlx1) promotes Sp8/9 expression during the process of OB interneuron development, from embryonic to postnatal stages.
Ascl1 Does not Regulate the Expression of Gad1, Sp8/9, Tshz1, and Prokr2 in the dLGE
Proneural transcription factor ASCL1 promotes the maintenance of neural progenitors through activating Notch signaling and lateral inhibition (Casarosa et al. 1999; Long et al. 2009; Castro et al. 2011; Wang et al. 2013). Here, we re-examined Ascl1 function in the dLGE and confirmed that Gad1 expression were increased in the LGE VZ/SVZ in Ascl1GFP/GFP knockin knockouts (null mutants) (Supplementary Fig. S3A–C′). However, expression of Sp8, Sp9, Tshz1, and Prokr2 in the mutant dLGE was comparable with controls (Supplementary Fig. S3D–O′), suggesting that Ascl1 is not required for expression of these key regulators of OB interneuron development. However, consistent with our previous study (Zhang et al. 2016), Sp9 expression was reduced in the vLGE of Ascl1 mutants (Supplementary Fig. S3H–I′), where it regulates striatal MSN development.
Neural Progenitors Fail to Differentiate Into Immature OB Interneurons in the dLGE of Dlx1/2−/− Mice
We previously demonstrated that Dlx1/2−/− mice lack virtually all OB interneurons (Anderson et al. 1997; Bulfone et al. 1998; Long et al. 2007). Currently, there are many more known markers for the OB interneuron lineage; thus we used this more definitive gene set to re-examine the generation of OB interneurons in the dLGE of Dlx1/2−/− mutants. We first examined the LGE at E16.5. Dlx1/2−/− mutants accumulated GSX2+ and ASCL1+ cells in the SVZ of both dLGE and vLGE (Fig. 2A–B′,D), consistent with the known block in neural differentiation (Long et al. 2007, 2009). SP8 expression was largely lost in the dLGE, whereas SP8+ immature (or undifferentiated) MSNs remained in the vLGE SVZ (Fig. 2E,E′,H) (Xu et al. 2018). This suggests that the expression of SP8 in the dLGE, but not in the vLGE, is critically dependent on DLX1/2. On the other hand, SP9 expression was only partially reduced (Fig. 2I–I′,L).
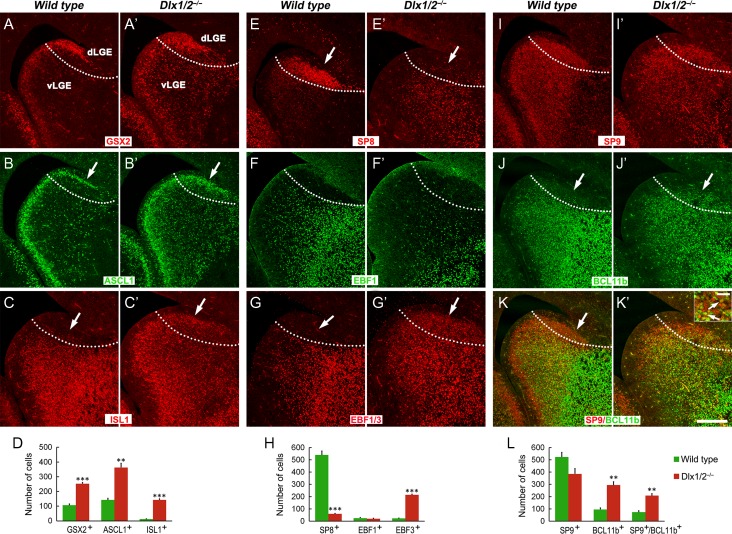
Figure 2.
Neural progenitors accumulate in the dLGE of Dlx1/2−/− mice at E16.5. (A–B′) Compared with controls, there were more GSX2+ and ASCL1+ progenitors in the d/vLGE of Dlx1/2−/− mice (arrows). (C, C′) ISL1 expression was increased in the mutant dLGE (arrows). (E, E′) SP8 was lost in the mutant dLGE (arrows). (F–G′) EBF1 was not expressed in the control and mutant dLGE, but the pan EBF antibody immunostaining showed that EBF3 was expressed in the mutant dLGE (arrows). (I–K′) SP9 expression was reduced in the dLGE; most SP9+ cells coexpressed the immature MSN marker BCL11b (arrows in the inset of K′). (D, H, L) Quantification of cell numbers in the dLGE. Scale bars: 200 μm in K′ for A–K′; 20 μm in the inset of K′.
Since SP9 is expressed in both MSNs and in OB interneurons, the SP9+ cells in the dLGE suggested that the dLGE in the Dlx1/2−/− mice might generate MSNs. Thus, we examined expression of the genes that are associated with MSN development. Most of these SP9+ cells in the mutant dLGE expressed strong BCL11b (Fig. 2I–K′,L), a marker of immature and mature MSNs (Arlotta et al. 2008), whereas BCL11b was not expressed in the dLGE of control mice (Fig. 2J). Furthermore, the expression of ISL1, a Drd1 MSN marker (Ehrman et al. 2013; Lu et al. 2014), was also observed in the mutant dLGE (Fig. 2C,C′,D). Notably, the dLGE in neither the control, nor the mutant, expressed EBF1, another marker of immature Drd1+ MSNs (Fig. 2F,F′) (Lobo et al. 2006). However, the mutant dLGE did ectopically express EBF3 (Fig. 2G,G′,H), a transcription factor that was not expressed in the normal dLGE.
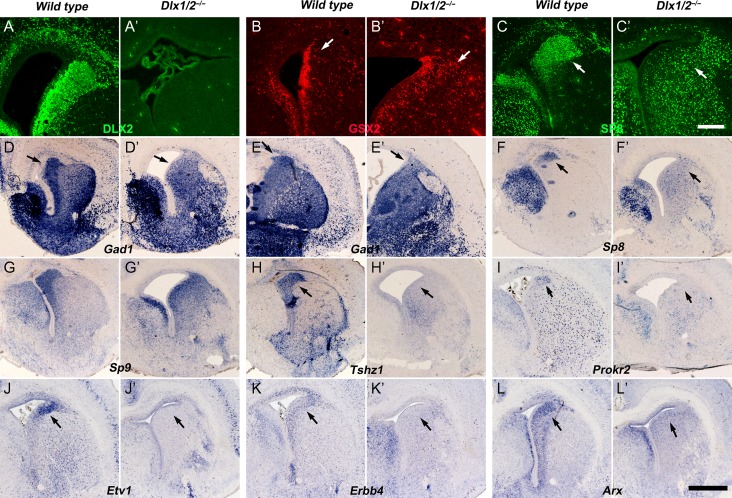
Dlx1/2 −/− mice die within a few hours after birth (Qiu et al. 1997), therefore we analyzed molecular features in the VZ/SVZ of dLGE at E18.5, especially for OB interneuron lineage markers. The Dlx1/2−/− mutants had more GSX2+ cells in the LGE SVZ (Fig. 3A-B′). Once again, SP8 expression was greatly reduced in the mutant dLGE SVZ, whereas many SP8+ immature MSN-like cells were observed throughout the LGE SVZ (Fig. 3C,C′,F,F′). Sp9 expression was still observed in the mutant dLGE SVZ (Fig. 3G,G′). OB immature interneuron markers Gad1, Tshz1, Prokr2, Etv1, Erbb4, and Arx were either lost or severely reduced in the dLGE (Fig. 3D–E′,H–L′). Thus, the Sp9+ cells in the dLGE SVZ are not immature OB interneurons.
Figure 3.
Immature OB interneurons are not generated in the dLGE of Dlx1/2−/− Mice at E18.5. (A–A′) No DLX2 protein was detected in the DLX1/2 mutant telencephalon. (B–B′) More GSX2+ cells were in the mutant dLGE SVZ (arrows). (C–C′, F–F′) The expression of Sp8 mRNA and SP8 protein was greatly reduced in the mutant dLGE (arrows). (D–E′) In situ hybridization showed Gad1 expression was reduced in the dLGE SVZ of mutants compared with controls (arrows). (G–G′) Sp9 expression was maintained in the mutant dLGE. (H–L′) Expression of OB interneuron lineage markers, Tshz1, Prokr2, Etv1, Erbb4, and Arx, were either lost or severely reduced in the mutant dLGE (arrows). Scale bars: 200 μm in C′ for A–C′; 600 μm in L′ for D–L′.
Previously we have reported that SP8 and SP9 coordinately promote the differentiation of OB interneurons by activating Tshz1 and Prokr2 expression (Li et al. 2018). The loss or reduced expressions of Sp8, Sp9, Tshz1, and Prokr2 in the dLGE of Dlx1/2−/− mice (Fig. 3C–I′), and the persistent expression of Dlx1/2/5 in the dLGE of Sp8/9 single or double conditional mutants (Supplementary Fig. 4A-F”’) suggested that DLX1/2 act upstream of Sp8/Sp9 to promote the differentiation of OB interneurons. Thus, in the absence of Dlx1/2, the dLGE fail to produce OB immature interneurons. In contrast, some of dLGE progenitors began to generate immature striatal MSN-like cells that express ISL1, SP9, and BCL11b (Fig. 2C′,I–K′).
RNA-Seq Analysis Reveals Key Molecular Defects in the LGE of Dlx1/2−/− Mice
To further characterize the molecular changes in the LGE of Dlx1/2−/− mice, we performed RNA-Seq analysis. Gene expression profiles from the E16.5 LGE (including VZ, SVZ, MZ-striatum) of Dlx1/2−/− mice and littermate wild type controls were analyzed (n = 3 biological replicas each group, GEO accession number: GSE121215). The levels of gene expression were reported in FPKM (Trapnell et al. 2012). For example, the expression levels (FPKM) of Dlx1 and Dlx2 genes in E16.5 wild type LGE were 80.2 and 139.4, respectively, whereas they were 11.6 and 5.5 in the null mutants, respectively (P-value = 4.29 E-100 and 2.02E-232, respectively) (Supplementary Table S1). The expression levels of Dlx5/6 genes, direct downstream targets of Dlx/1/2, were almost undetectable (Dlx5: 49.0 vs. 1.4; Dlx6: 22.3 vs. 0.6; wild types vs. Dlx1/2 mutants) (Supplementary Table S1).
Our RNA-Seq analysis revealed about 3300 dysregulated genes in the mutants (1890 with reduced RNA expression and 1410 with increased RNA expression), suggesting that Dlx1/2 have profound effects on the LGE development. To further analyze the impact of Dlx1/2 deletion on different cell types of OB interneuron lineage in the dLGE, such as neural stem/progenitors and immature OB interneurons, we explored expression levels for genes that are enriched in these populations (Supplementary Table S1). Compared with controls, we found that mRNA levels of LGE progenitor-enriched genes in Dlx1/2−/− mice were significantly increased, such as Gsx1/2, Ascl1, Egfr, Lhx2, Dll1/3, Hes5/6, Nr2e1, Nr2f1, Notch1, Gli2/3, Sox9, and Nes (Supplementary Table S1). However, mRNA levels of immature OB interneuron-enriched genes were all significantly reduced or undetectable, such as Dlx1/2/5/6, Arx, Etv1, Erbb4, Sp8, Neto1, Meis1/2, Gad1/2, Vax1, Tshz1, and Errbb4 (Supplementary Table S1). In general, this is consistent with our RNA in situ hybridization results and previous transcriptomic analyses using microarrays (Long et al. 2009). Thus, our results confirm that lack of OB interneuron genesis in the dLGE of Dlx1/2−/− mice.
Pax6 Sey/Sey Mice Have a Large OB Interneuron Ectopia in the Neocortex
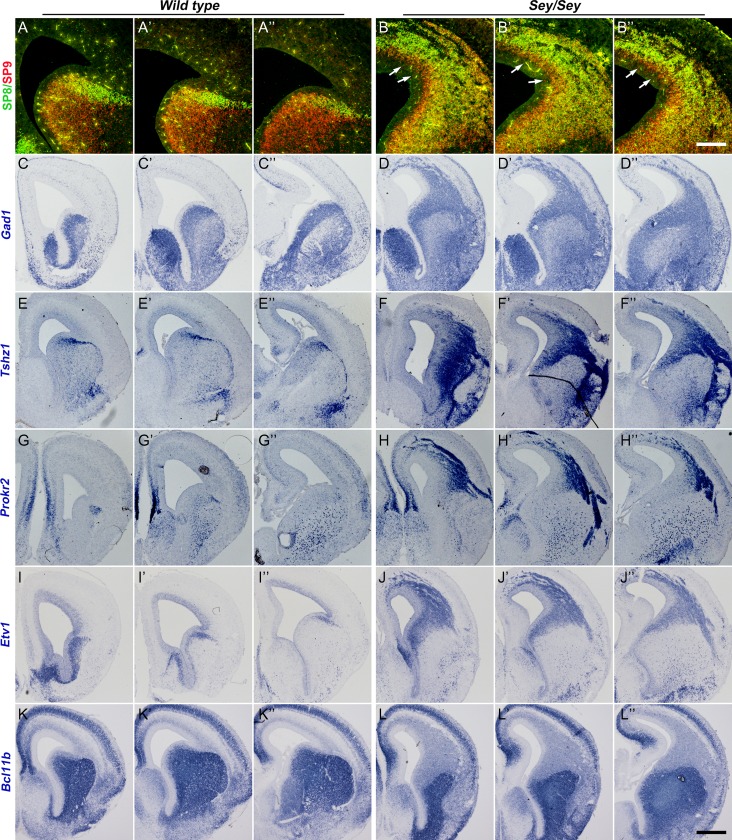
Pax6 Sey/Sey mice lack functional PAX6 protein, lack an OB and die at birth (Hill et al. 1991). However, the generation of OB interneurons in the embryos of Pax6Sey/Sey mice has not been fully investigated. At E16.5, in situ RNA hybridization and immunohistochemistry staining revealed that both dLGE and neocortex generated a large numbers of immature OB interneurons as they expressed the following markers of OB interneurons: Gad1, SP9, SP8, Tshz1, Prokr2, Etv1 (Fig. 4A–J″). Perhaps because Pax6Sey/Sey mice do not form OBs, these interneurons lost their target destination, and thus formed ectopic collections in the dLGE and neocortex (Fig. 4A–J″) (Kroll and ’Leary 2005). To assess whether vLGE fate was also generated in the dLGE and cortex, we assessed expression of Bcl11b+, a marker of striatal MSNs. Strong Bcl11b+ expression was not present in the ectopias (Fig. 4K–L″), suggesting that neocortical progenitors in Pax6Sey/Sey mice acquired the dLGE fate, but not the vLGE fate.
Figure 4.
Pax6 Sey/Sey mice have a large ectopia containing OB interneurons in the cortex and dLGE at E16.5. (A–B″) SP8 and SP9 were ectopically expressed in the cortex of Pax6Sey/Sey mice. Note SP9 expression appears in more immature cells (i.e., are closer to the ventricle) than SP8 in the cortical SVZ (arrows). (C–J″) Increased Gad1, Tshz1, Prokr2, and Etv1 expression was seen in the mutant dLGE and cortex. (K–L″) Strong Bcl11b expression was not observed in the region of the ectopia mutant cortex. Scale bars: 200 μm in (B″) for (A–B″); 400 μm in (L″) for (C–L″).
RNA-Seq Analysis Provided Further Molecular Evidence for Ectopic Generation of OB Interneurons in the Cortex of Pax6Sey/Sey Mice
Gene expression profiles from the E16.5 cortex (including VZ, SVZ, CP, and MZ) of Pax6Sey/Sey mice and littermate wild type controls were analyzed using RNA-Seq (n = 2 biological replicas each group, GEO accession number: GSE121215). The expression levels (FPKM) of Pax6 gene in E16.5 wild type cortex was 27.9, whereas in E16.5 Pax6Sey/Sey cortex was 110.5 (P = 3.41E-13) (Supplementary Table S2), indicating that loss of functional PAX6 protein greatly increased Pax6 transcripts. This is consistent with previous observations (Stoykova et al. 1996). On the other hand, the expression levels of Neurog2, the direct downstream target of Pax6 (Scardigli et al. 2003; Sansom et al. 2009), in wild type cortex was 32.6, whereas in Pax6Sey/Sey cortex was reduced to 1.8 (P = 3.75E-8). Accordingly, we observed reduced expression of Eomes (Tbr2), a gene expressed highly in intermediate progenitors of cortical pyramidal projection neurons (Bulfone et al. 1995; Englund et al. 2005; Elsen et al. 2018), and Tbr1, a gene expressed in postmitotic cortical pyramidal neurons (Hevner et al. 2001; McKenna et al. 2011; Fazel Darbandi et al. 2018) (Wild types vs. Mutants; Eomes: 53.7 vs. 11.2; P = 1.76E-07; Tbr1: 114.3 vs. 62.4; P = 1.62E-04) (Supplementary Table S2).
Compared with controls, RNA levels of OB interneuron lineage-enriched genes (from genes that highly expressed in progenitors to genes that highly expressed in immature OB interneurons) in the Pax6Sey/Sey cortex were significantly increased, levels ranging from a 1.5- to 18-fold change (Supplementary Table S2). These include Gsx2, Ascl1, Dlx1/2/5/6, Ccnd2, Cdca7, Mki67, Nr2e1, Nr2f1, Pcna, Pax6, Sox1/2, and Etv1, Gad1/2, Arx, Sp8/9, Tshz1, Pbx3, Pou3f4, Prokr2, Errbb4, and others. In contrast, the expression levels of maturing/mature OB interneuron-enriched genes Th (tyrosine hydroxylase), Pvalb, Calb1 (calbindin 1) and Calb2 (calbindin 2, calretinin, CR) were very low in the Pax6Sey/Sey cortex (Supplementary Table S2). Likewise, the Pax6Sey/Sey cortex had low cortical expression of MSNs-enriched genes, such as Drd1, Chrm4, Ebf1, Isl1, Pdyn, Tac1, and Drd2, Adora2a, Penk, Ptprm, Gpr6 (Supplementary Table S2). Thus, we conclude that cortical progenitors in Pax6Sey/Sey mice acquired the dLGE fate and produced cells with properties of immature OB interneurons.
Dlx1/2 are Required for the Differentiation of Ectopic Immature OB Interneurons in Pax6Sey/Sey Mice
Our results demonstrated that the Dlx1/2−/− dLGE fails to produce OB immature interneurons. However, it is quite difficult to further analyze the functions of Dlx1/2 in OB interneuron development, as the dLGE domain is much smaller than the vLGE domain. In addition, the dLGE and vLGE share many common molecular features and both exhibit blocking neuronal differentiation phenotypes in Dlx1/2−/− mice (Anderson et al. 1997; Long et al. 2007, 2009). However, the enlarged dLGE and dLGE-like neocortex of Pax6Sey/Sey mice provide an excellent model to analyze Dlx1/2 functions in OB interneuron development.
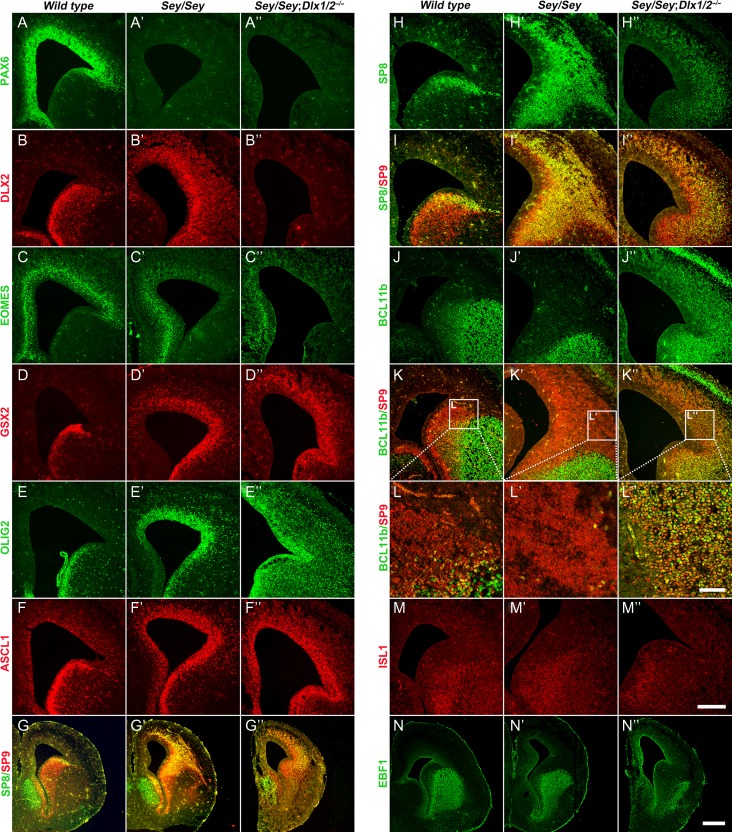
The mouse Pax6 and Dlx1/2 genes are both located in chromosome 2 and separated by ~12 cm. We obtained Pax6Sey/+; Dlx1/2+/− mice through meiotic recombination. We then generated Pax6Sey/Sey; Dlx1/2−/− and studied their cortex and dLGE. We found that the Pax6Sey/Sey; Dlx1/2−/− mutants lacked PAX6, EOMES, and DLX2 proteins, and had increased GSX2, ASCL1 and OLIG2 expression (Fig. 5A–F″). Sox1+ progenitors were also increased (Fig. 6L–L″). Some SP8+/SP9+ cells were observed in the Pax6Sey/Sey; Dlx1/2−/− cortex, but these cells coexpressed BCL11b, a striatal MSN marker, providing evidence that they were not OB interneurons (Fig. 5G–L″). However, we did not observe the expression of ISL1 and EBF1 in the cortex of Pax6Sey/Sey; Dlx1/2−/− triple mutant mice (Fig. 5M–N″), suggesting that MSNs were not generated either.
Figure 5.
More subpallial neural progenitors are in the cortex of Pax6Sey/Sey; Dlx1/2−/− Mice at E16.5. (A–B″) Immunostaining of PAX6 and DLX2 confirmed the genotypes of mice used. (C–C″) EOMES expression was greatly reduced in both Pax6Sey/Seyand Pax6Sey/Sey; Dlx1/2−/− mice. (D–F″) GSX2+, OLIG2+, and ASCL1+ progenitors ectopically accumulated in the neocortex of Pax6Sey/Sey mice; their expression increased further in Pax6Sey/Sey; Dlx1/2−/− mice. (G–I″) The ectopic SP8/9 cortical expression in the Pax6Sey/Sey mice was reduced in the Pax6Sey/Sey; Dlx1/2−/− cortex; furthermore, the SP8/9+ cells showed a scattered pattern. (J–L″) Most SP9/8+ cells coexpressed BCL11b in the cortex of Pax6Sey/Sey; Dlx1/2−/− mice. (M–N″) ISL1 and EBF1 expression was not observed in the neocortex of these mutants. Scale bars: 70 μm in L″ for L–L″; 250 μm in M″ for A–F″, H–K″, M–M″; 500 μm in N″ for G–G″, N–N″.
Figure 6.
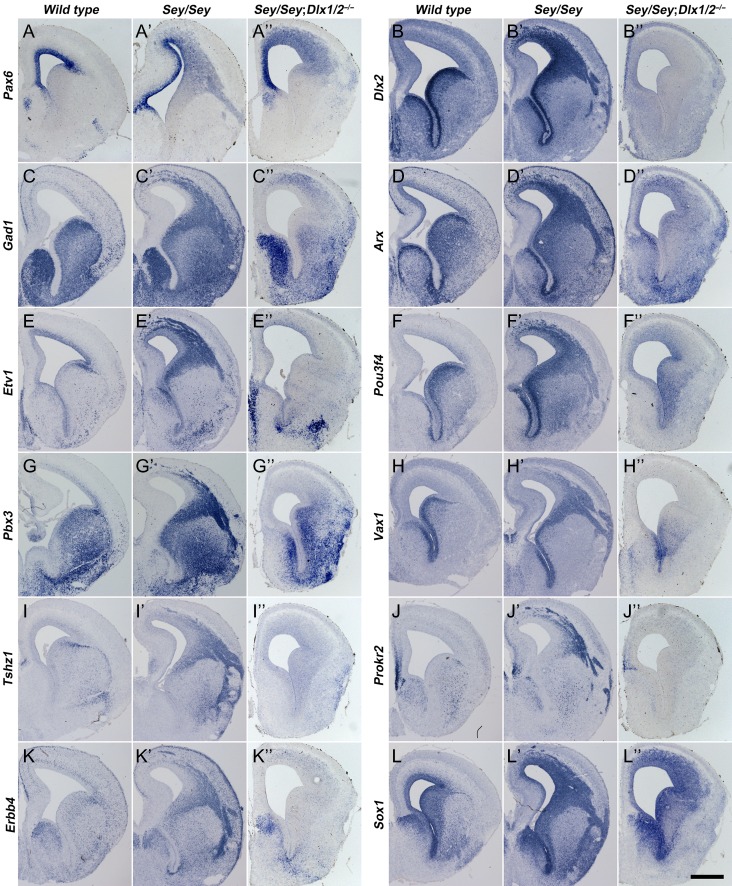
Immature OB interneurons are not generated in the cortex of Pax6Sey/Sey; Dlx1/2−/− mice at E16.5. (A–L″) In situ RNA hybridization indicated that immature OB interneuron markers (Gad1, Etv1, Pbx3, Tshz1, Erbb4, Arx, pou3f4, Vax1, Prokr2) were not detected in Pax6Sey/Sey; Dlx1/2−/− mice compared with Pax6Sey/Sey mice. Note that Pax6 transcripts and Sox1 expression were increased in the cortex of these mutants. Scale bar: 600 μm in L″ for A–L″.
Pax6 Sey/Sey Dlx1/2 −/− mutants lacked the ectopic cortical expression of Gad1, Arx, Etv1, Pou3f4, Pbx3, Vax1, Tshz1, Prokr2, and Erbb4 that were observed in the cortex of Pax6Sey/Sey mice (Fig. 6A–K″). This provides strong evidence for a block of interneuron generation. Thus, the analysis of Pax6Sey/Sey; Dlx1/2−/− triple mutant mice further demonstrated that Dlx1/2 are absolutely required for making nearly all OB interneurons.
Postnatal SVZ Progenitors Fail to Generate OB Interneurons in hGFAP-Cre; Dlx1/2F/− Conditional Knockout Mice
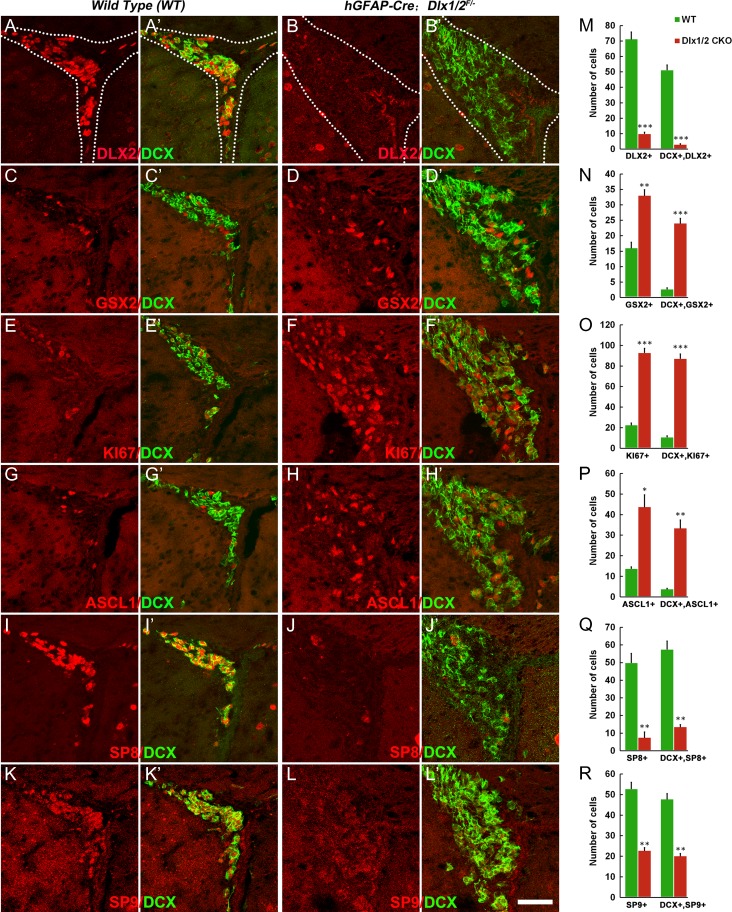
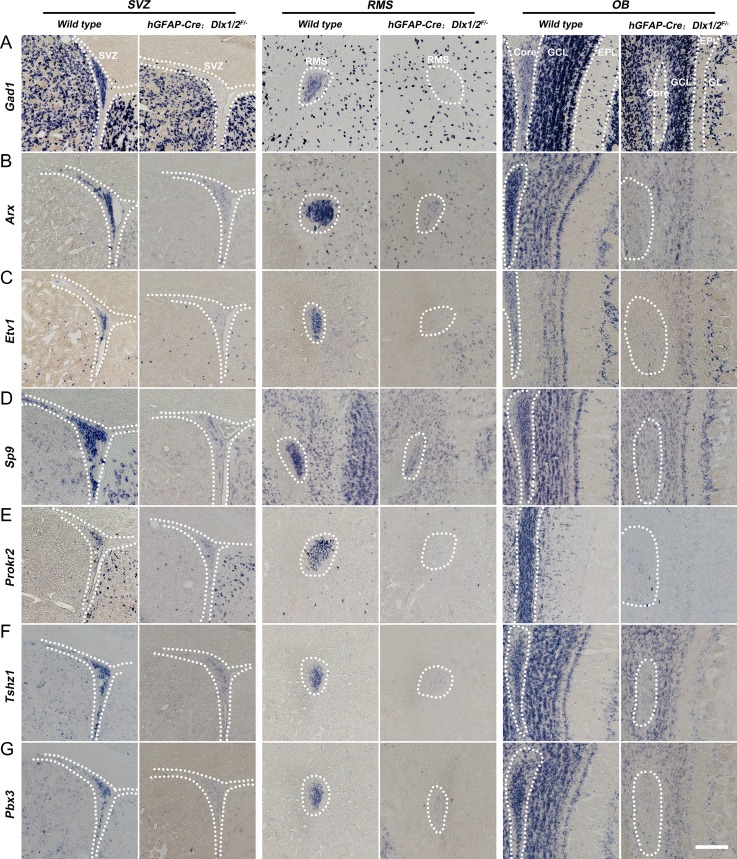
Next, we assessed Dlx1/2 function in the postnatal generation of OB interneurons. To this end we use a Dlx1/2 conditional allele (Dlx1/2F/F mice) (Silbereis et al. 2014; Pla et al. 2018). Dlx1/2+/− mice were crossed with hGFAP-Cre mice (Zhuo et al. 2001), and male hGFAP-Cre; Dlx1/2+/− mice were crossed female Dlx1/2F/F mice to generate hGFAP-Cre; Dlx1/2F/− conditional knockouts (Dlx1/2-CKO). hGFAP-Cre starts to be expressed in the E13.5 cortical VZ and E18.5 LGE VZ (Zhuo et al. 2001; Malatesta et al. 2003), and can delete floxed alleles from ~95% postnatal SVZ stem cells (Lim et al. 2009). Indeed, DLX2 expression was almost undetectable in the SVZ of Dlx1/2-CKO mice at P50 (Fig. 7A–B′,M). Furthermore, SP8/9 SVZ expression was greatly reduced (Fig. 7I–L′,Q,R). In contrast, there were increased numbers of SVZ cells expressing progenitor markers: GSX2+, Ki67+, ASCL1+, GSX2+/DCX+, Ki67+/DCX+, and ASCL1+/DCX+ (Fig. 7C–H′,N–P). Similar expression patterns of these genes were also observed in the RMS and the core of the OB at P50 (Supplementary Fig. S5A–L′). Notably, while wild type controls had very few GSX2+, Ki67+, ASCL1+ cells in the OB core, these cells were abundant in the OB core of Dlx1/2-CKO mice at P50 (Supplementary Fig. S5D′,F′,H′). Consistent with a block in neuronal differentiation in the SVZ, there was a large increase of GFAP+ (based on strong GFAP expression) and OLIG2+ cells (7.23 ± 1.18 vs. 23.23 ± 0.27 per section; wild type vs. Dlx1/2-CKO, P = 0.0004) (Supplementary Fig. S6A–F′). Finally, the expression of OB interneuron-marker genes, Gad1, Arx, Etv1, Sp9, Prokr2, Tshz1, and Pbx3, in the adult SVZ, RMS, and OB core, were either severely reduced or undetectable (Fig. 8A–G). Thus, Dlx1/2 function remains essential in the adult SVZ and RMS for the differentiation of OB neural progenitors.
Figure 7.
Neuronal differentiation of stem/progenitors in the adult SVZ (P50) of hGFAP-Cre; Dlx1/2F/− conditional knockout (Dlx1/2-CKO) mice was blocked. (A–L′) DLX2+, SP8+, SP9+, DLX2+/DCX+, SP8+/DCX+, and SP9+/DCX+ cells were greatly reduced whereas GSX2+, Ki67+, ASCL1+, GSX2+/DCX+, Ki67+/DCX+, and ASCL1+/DCX+ cells were significantly increased in the SVZ of Dlx1/2-CKO mice compared with wild type control mice at P50. (M–R) Quantification of these experiments. Scale bar: 50 μm in L′ for A–L′.
Figure 8.
Loss of immature OB interneurons in the adult (P50) Dlx1/2-CKO SVZ, RMS and the core of the OB. (A–G) In situ RNA hybridization showed that expression of Gad1, Arx, Etv1, Sp9, Prokr2, Tshz1, and Pbx3 in the adult SVZ, RMS, and OB core of Dlx1/2-CKO mice were either severely reduced or undetectable. Scale bar: 100 μm in G for A–G.
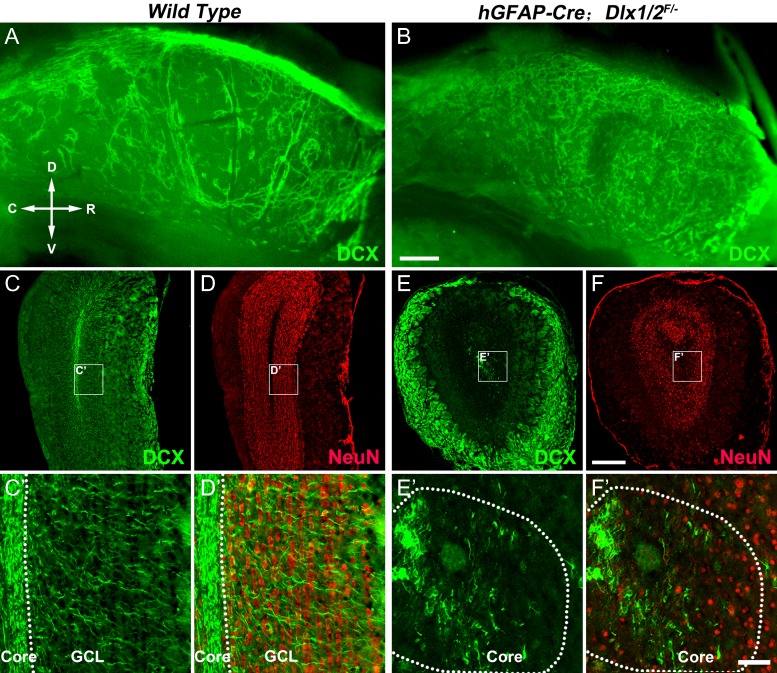
We then examined DCX+ cell migration in the lateral wall of the lateral ventricle in adult mice (P50) using wholemount immunostaining (Doetsch and Alvarez-Buylla 1996; Mirzadeh et al. 2010). DCX+ cells in the normal adult mouse SVZ are dividing and nondividing neuroblasts (Ponti et al. 2013), but DCX+ cells in the SVZ of adult Dlx1/2-CKO mice are mainly GSX2+, Ki67+, ASCL1+ progenitors (Fig. 7C–H′). Control mice displayed DCX+ cells (neuroblasts) arranged in orderly chains (Fig. 9A), whereas in Dlx1/2-CKO mice, DCX+ cells (progenitors) did not form migration chains, appearing disorganized (Fig. 9B), indicative of a defect in tangential migration (n = 7 mice per group). The sizes of OBs were reduced in adult Dlx1/2-CKO mice and the number of NeuN+ cells in the OB was significantly decreased compared with wild types (Fig. 9C–F′). While laminar structures were present in the Dlx1/2-CKO mouse OB, these could correspond the projection neurons and outer nerve layer (Fig. 9C–F′) (Bulfone et al. 1998). A severe reduction of DCX+ cells was seen in the mutant OB, and their lack of clear radial orientation suggested that their radial migration may be abnormal (Fig. 9C′–F′).
Figure 9.
Tangential and radial migration defects of DCX+ cells in the adult SVZ and OB of Dlx1/2-CKO mice. (A, B) Wholemount DCX immunostaining of the lateral wall of the lateral ventricle showed DCX+ cells in the SVZ of Dlx1/2-CKO mice (P50) did not form migration chains. (C–F) OB coronal sections stained for NeuN and DCX. Note that Dlx1/2-CKO mouse OB was smaller than wild type OB. (C′–F′) High magnification images of boxed areas in (C–F) showing the defect of the radial migration of DCX+ cells. R, rostral; C, caudal; D, dorsal; V, ventral. Scale bars: 300 μm in B for A–B′; 350 μm in F for C–F; 50 μm in F′ for C′–F′.
Overexpression of Dlx1, Dlx2, or Dlx1&2 in the Neocortex Promoted the Generation of Immature OB Interneurons From Cortical Progenitors
Previous studies have shown that ectopic expression of Dlx genes in the developing neocortex is sufficient to induce the ectopic expression of GAD1 (Stuhmer et al. 2002). Thus, we investigated whether overexpression of Dlx1&2 genes in the neocortex was sufficient for the cortical progenitors to generate OB interneurons. We ectopically expressed Dlx1&2 by IUE of pCAGIG-Dlx1-Ires-GFP plasmids and pCAGIG-Dlx2-Ires-GFP plasmids together or control pCAGIG-GFP plasmids into the cortical VZ of E14.5 wild type CD1 mice, and examined the brains at different time points after IUE.
Twelve hours after Dlx1&2 IUE, strong DLX1 (data not shown) and DLX2 protein expression was observed in GFP+ cortical progenitors (Supplementary Fig. S7A–A″); these GFP+ cells did not express SP8 or SP9 protein (Supplementary Fig. S7B–C″). Twenty-four hours after Dlx1&2 IUE, SP8 was strongly expressed in most GFP+ cells whereas SP9 expression was not turned on yet (Supplementary Fig. S7D–F″). Forty-eight hours later, a subset of GFP+ cells started to express SP9 (Supplementary Fig. S8A–C″). Sixty hours after IUE, coexpression of SP8 and SP9 was observed in most GFP+ cells in the cortical SVZ (Supplementary Fig. S8D–F″). Furthermore, Dlx1&2 IUE induced cortical SVZ expression of Gad1, Arx, Etv1, Pbx3, Tshz1 and Prokr2, markers of immature OB interneurons (Supplementary Fig. S8G–L). Ninety-six hours after Dlx1&2 IUE (at E18.5), DLX1/2+/GFP+ cells appeared to form clusters; these cells expressed Gad1, SP8/9, Arx, Etv1, Pbx3, Tshz1, and Prokr2 (Fig. 10A–M′, Supplementary Fig. S9A–L). Notably, we observed that SP8 was expressed in nearly all DLX1&2+ cells, including those cells that already migrated into the different layers of the cortex (Supplementary Fig. 9M–O′), providing further evidence that DLX1&2 can induce SP8 expression. OB interneuron markers were not expressed in the cortical SVZ IUE of the negative control pCAGIG-GFP plasmid (Fig. 10A–E,F–M).
Finally, we asked whether IUE of Dlx1 or Dlx2 alone in the cortex could induce OB interneuron generation in the cortical SVZ. Consistent with IUE of Dlx1&2, 96 h after Dlx1 or Dlx2 IUE alone, DLX1+/GFP+ cells or DLX2+/GFP+ cells expressed the OB interneuron markers Dlx1, Dlx2, Gad1, SP8/9, Etv1, Tshz1, and Prokr2 (Supplementary Fig. S10A–T). Thus, overexpression of either Dlx1, Dlx2, or Dlx1&2 in cortical neural progenitors induces the genetic program that promotes the generation of immature OB interneurons in the cortical SVZ.
Discussion
We propose that Dlx1/2 promote OB interneuron development largely through activating the expression of OB interneuron genes, especially Sp8/9, which further activate Tshz1 and Prokr2 expression. This study, in combination with earlier studies, presents evidence for a transcriptional regulatory network that controls the multistep process of OB interneuron development (Fig. 10N).
A Model of the Transcriptional Regulation Network That Regulates OB Interneuron Development
Loss of function and gain of function analysis reveal that Dlx1/2 are necessary and sufficient for making all OB interneurons. Base on this study and earlier studies, we present a common model of the transcriptional network that regulates OB interneuron development: Gsx2/1-Dlx2/1-Sp8/9-Tshz1-Prokr2 (Fig. 10N).
The fundamental feature of the dLGE is expression of strong GSX2, and GSX2 is at the top of the hierarchy in the dLGE (Wang et al. 2009, 2013). Many OB interneurons were still generated in the Gsx2 mutant mice (Corbin et al. 2000; Toresson and Campbell 2001; Yun et al. 2001). However, Gsx1 expression is upregulated in the dLGE in Gsx2 mutants; Gsx1 partially rescues the phenotypes of Gsx2 mutants (Toresson and Campbell 2001; Yun et al. 2003). Loss of Gsx1/2 function results in loss of Ascl1 and Dlx genes’ expression in the dLGE, and none of interneurons is generated in the OB (Toresson and Campbell 2001; Yun et al. 2003). Ectopic expression of Gsx2 in the embryonic cortex induces Dlx and Ascl1 expression, which further induces the generation of SP8+ immature OB interneurons (Waclaw et al. 2009). Therefore, Gsx2 promotes OB interneuron development through activating Dlx1/2 and Ascl1 (Fig. 10N).
Ascl1 maintains progenitor state (Casarosa et al. 1999; Yun et al. 2002; Long et al. 2009; Castro et al. 2011; Wang et al. 2013), and partially regulates Dlx1/2 expression through the I12b intergenic enhancer (Poitras et al. 2007). GSX2, ASCL1, and DLX2 are coexpressed in the VZ/SVZ1 of the dLGE (Fig. 1A–F). Although GSX2 and ASCL1 expression are downregulated in SVZ2 of the dLGE, DLX1/2 continue to be expressed there prenatally, and in the postnatal SVZ, RMS, and OB. There is evidence that DLX2 can directly bind 2 of its enhancers (I12b or URE2) and thus may be able to promote its own expression (Poitras et al. 2007; Potter et al. 2009). Consistent with this, in the present study, we also show that overexpression of either Dlx1 or Dlx2 alone in the embryonic cortex could induce the expression of Dlx1/2 in the cortical SVZ, providing another strong evidence that DLX1/2 can promote their own expression.
Following Dlx1/2 function, we propose that the next step of OB interneuron development is controlled by Sp8/9. Sp8/9 mutants lack Tshz1 and Prokr2 expression and have severely reduced Pbx3, Vax1, and Meis1 expression (Li et al. 2018); however, Dlx1/2/5, Etv1, Arx, and Erbb4 expression are maintained in the dLGE (Supplementary Fig. S4A–F″′). There is evidence that Dlx1/2 directly promote Sp8/9, Gad1/2, Etv1, Arx, Erbb4, and Zeb2 expression (Long et al. 2007, 2009; Colasante et al. 2008; McKinsey et al. 2013; Le et al. 2017; Pla et al. 2018) (data not shown) (Fig. 10N).
The Functions of Dlx1/2 Genes in the OB Interneuron Development
Dlx1/2 constitutive null mutants fail to induce Dlx5/6 expression in most of the forebrain (Anderson et al. 1997; Long et al. 2009) (Supplementary Table S1). Conditional deletion of Dlx1/2 (probably in SVZ1 or SVZ2), about 24 h after their expression is initiated, does not eliminate Dlx5/6 expression (Pla et al. 2018). In these mutants the generation and migration of OB and cortical interneurons is largely intact (Pla et al. 2018) (Rubenstein J.L., unpublished data). Thus, the lack of expression of all 4 Dlx genes leads to the virtually complete absence of OB interneurons in the Dlx1/2 mutants. Dlx1/2 promote OB interneuron genesis through positively regulating a subset of transcription factors (Fig. 10N), but may also through repressing Gsx2/1, Ascl1, and Olig2 expression as these transcription factors promote the progenitor and/or glia cell state (Petryniak et al. 2007; Long et al. 2009; Chapman et al. 2013, 2018; Wang et al. 2013).
Dlx Genes and Other Transcription Factors are Required for the Specification of OB Interneuron Subtypes
Regional identity, and the identity of neural stem cells, is determined in the VZ. The LGE may consist of multiple progenitor domains along the dorsoventral axis (Flames et al. 2007; Xu et al. 2018). The dLGE, generates OB interneurons, may consist of pLGE1 and pLGE2, whereas the vLGE, generates largely striatal MSNs and some OB interneurons, may consist of pLGE3 and pLGE4 (Zhang et al. 2016; Xu et al. 2018). pLGE1 is implicated in the generation of Dlx2+Pax6+ TH+ OB interneurons (Long et al., 2007), whereas pLGE2 may generate the majority of OB interneurons. Furthermore, distinct OB interneuron subtypes are produced by the dLGE, vLGE, cortex, and septum (Kohwi et al. 2007; Merkle et al. 2007, 2014; Ventura and Goldman 2007; Young et al. 2007; Kriegstein and Alvarez-Buylla 2009; Lopez-Juarez et al. 2013; Fuentealba et al. 2015; Hoch et al. 2015; Qin et al. 2017). Gsx2/1-Dlx2/1-Sp8/9-Tshz1-Prokr2 transcriptional network operates in each of these domains, where several of these transcription factors are implicated in specification of OB interneuron subtypes. Sp8 is required for the generation of Calb2+ and Pvalb+ OB interneurons (Waclaw et al. 2006; Li et al. 2011). Sp9 is required for the generation of a small population of Pvalb+ OB interneurons (Li et al. 2018). Pax6, Dlx2, and Etv1 are required for the generation of the TH+ subpopulation (Qiu et al. 1995; Dellovade et al. 1998; Hack et al. 2005; Kohwi et al. 2005; Cave et al. 2010). The Calb1+ subpopulation requires Tshz1 (Ragancokova et al. 2014). It is likely that the specification of OB interneuron subtypes results from combinatorial transcription factor codes; these remain to be elucidated (Allen et al., 2007; Alvarez-Buylla et al. 2008; Bartolini et al. 2013; Zhou et al. 2015; Fujiwara and Cave 2016).
Supplementary Material
Notes
The authors thank Dr Kazuaki Yoshikawa for providing the DLX2 antibody. The GEO accession number for the RNA-Seq data reported in this article is GSE121215. Conflict of Interest: J.L.R. is cofounder, stockholder, and currently serve on the scientific board of Neurona, a company studying the potential therapeutic use of interneuron transplantation.
Authors’ Contributions
T.G. and G.L. performed experiments and analysis. H.D., Y.W., S.W., Z.L., G.T., Z.S., X.S., Z.Z., Z.X., and Y.Y. helped conduct experiments and analyze the data. B.C. and J.L.R. helped guide the project and discussed some of results. Z.Y. designed the experiments and analyzed the results. Z.Y. and J.L.R. wrote the article.
Funding
Research grants to Z.Y. from National Key Research and Development Program of China (2018YFA0108000), National Natural Science Foundation of China (NSFC 31820103006, 31630032, 31425011, and 31429002), research grant to Y.Y. (NSFC 31700889), research grants to B.C. from the National Institute of Mental Health (R01MH094589) and from the National Institute of Neurological Disorders and Stroke (R01NS089777), and to J.L. Rubenstein from the National Institute of Mental Health (R37MH049428).
References
- Allen ZJ 2nd, Waclaw RR, Colbert MC, Campbell K. 2007. Molecular identity of olfactory bulb interneurons: transcriptional codes of periglomerular neuron subtypes. J Mol Histol. 38:517–525. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. 2008. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb Symp Quant Biol. 73:357–365. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. 1997. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 19:27–37. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Jabaudon D, Yoshida Y, Macklis JD. 2008. Ctip2 controls the differentiation of medium spiny neurons and the establishment of the cellular architecture of the striatum. J Neurosci. 28:622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini G, Ciceri G, Marin O. 2013. Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. Neuron. 79:849–864. [DOI] [PubMed] [Google Scholar]
- Bell SM, Schreiner CM, Waclaw RR, Campbell K, Potter SS, Scott WJ. 2003. Sp8 is crucial for limb outgrowth and neuropore closure. Proc Natl Acad Sci USA. 100:12195–12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfone A, Smiga SM, Shimamura K, Peterson A, Puelles L, Rubenstein JL. 1995. T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron. 15:63–78. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Wang F, Hevner R, Anderson S, Cutforth T, Chen S, Meneses J, Pedersen R, Axel R, Rubenstein JL. 1998. An olfactory sensory map develops in the absence of normal projection neurons or GABAergic interneurons. Neuron. 21:1273–1282. [DOI] [PubMed] [Google Scholar]
- Carney RS, Cocas LA, Hirata T, Mansfield K, Corbin JG. 2009. Differential regulation of telencephalic pallial-subpallial boundary patterning by Pax6 and Gsh2. Cereb Cortex. 19:745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarosa S, Fode C, Guillemot F. 1999. Mash1 regulates neurogenesis in the ventral telencephalon. Development. 126:525–534. [DOI] [PubMed] [Google Scholar]
- Castro DS, Martynoga B, Parras C, Ramesh V, Pacary E, Johnston C, Drechsel D, Lebel-Potter M, Garcia LG, Hunt C, et al. 2011. A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome-wide characterization of its targets. Genes Dev. 25:930–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cave JW, Akiba Y, Banerjee K, Bhosle S, Berlin R, Baker H. 2010. Differential regulation of dopaminergic gene expression by Er81. J Neurosci. 30:4717–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H, Riesenberg A, Ehrman LA, Kohli V, Nardini D, Nakafuku M, Campbell K, Waclaw RR. 2018. Gsx transcription factors control neuronal versus glial specification in ventricular zone progenitors of the mouse lateral ganglionic eminence. Dev Biol. 442:115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H, Waclaw RR, Pei Z, Nakafuku M, Campbell K. 2013. The homeobox gene Gsx2 controls the timing of oligodendroglial fate specification in mouse lateral ganglionic eminence progenitors. Development. 140:2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocas LA, Georgala PA, Mangin JM, Clegg JM, Kessaris N, Haydar TF, Gallo V, Price DJ, Corbin JG. 2011. Pax6 is required at the telencephalic pallial-subpallial boundary for the generation of neuronal diversity in the postnatal limbic system. J Neurosci. 31:5313–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasante G, Collombat P, Raimondi V, Bonanomi D, Ferrai C, Maira M, Yoshikawa K, Mansouri A, Valtorta F, Rubenstein JL, et al. 2008. Arx is a direct target of Dlx2 and thereby contributes to the tangential migration of GABAergic interneurons. J Neurosci. 28:10674–10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G. 2000. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development. 127:5007–5020. [DOI] [PubMed] [Google Scholar]
- Deacon TW, Pakzaban P, Isacson O. 1994. The lateral ganglionic eminence is the origin of cells committed to striatal phenotypes: neural transplantation and developmental evidence. Brain Res. 668:211–219. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Pfaff DW, Schwanzel-Fukuda M. 1998. Olfactory bulb development is altered in small-eye (Sey) mice. J Comp Neurol. 402:402–418. [PubMed] [Google Scholar]
- Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, et al. 2006. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet. 2:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Alvarez-Buylla A. 1996. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci USA. 93:14895–14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. 1999. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 97:703–716. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. 2002. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 36:1021–1034. [DOI] [PubMed] [Google Scholar]
- Ehrman LA, Mu X, Waclaw RR, Yoshida Y, Vorhees CV, Klein WH, Campbell K. 2013. The LIM homeobox gene Isl1 is required for the correct development of the striatonigral pathway in the mouse. Proc Natl Acad Sci USA. 110:E4026–E4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstat DD, Liu JK, Mione M, Zhong W, Yu G, Anderson SA, Ghattas I, Puelles L, Rubenstein JL. 1999. DLX-1, DLX-2, and DLX-5 expression define distinct stages of basal forebrain differentiation. J Comp Neurol. 414:217–237. [DOI] [PubMed] [Google Scholar]
- Elsen GE, Bedogni F, Hodge RD, Bammler TK, MacDonald JW, Lindtner S, Rubenstein JLR, Hevner RF. 2018. The epigenetic factor landscape of developing neocortex is regulated by transcription factors Pax6--> Tbr2--> Tbr1. Front Neurosci. 12:571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. 2005. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 25:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel Darbandi S, Robinson Schwartz SE, Qi Q, Catta-Preta R, Pai EL, Mandell JD, Everitt A, Rubin A, Krasnoff RA, Katzman S, et al. 2018. Neonatal Tbr1 dosage controls cortical layer 6 connectivity. Neuron. 100:831–845 e837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marin O. 2007. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 27:9682–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba LC, Rompani SB, Parraguez JI, Obernier K, Romero R, Cepko CL, Alvarez-Buylla A. 2015. Embryonic origin of postnatal neural stem. Cells. Cell. 161:1644–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara N, Cave JW. 2016. Partial conservation between mice and humans in olfactory bulb interneuron transcription factor codes. Front Neurosci. 10:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack MA, Saghatelyan A, de Chevigny A, Pfeifer A, Ashery-Padan R, Lledo PM, Gotz M. 2005. Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci. 8:865–872. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Flandin P, Yoshikawa K, Rubenstein JL, Alvarez-Buylla A, Kriegstein AR. 2013. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat Neurosci. 16:1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL. 2001. Tbr1 regulates differentiation of the preplate and layer 6. Neuron. 29:353–366. [DOI] [PubMed] [Google Scholar]
- Hill RE, Favor J, Hogan BL, Ton CC, Saunders GF, Hanson IM, Prosser J, Jordan T, Hastie ND, van Heyningen V. 1991. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 354:522–525. [DOI] [PubMed] [Google Scholar]
- Hoch RV, Clarke JA, Rubenstein JL. 2015. Fgf signaling controls the telencephalic distribution of Fgf-expressing progenitors generated in the rostral patterning center. Neural Dev. 10:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Leung CT, Reed RR, Johnson JE. 2007. In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci. 27:12764–12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Osumi N, Rubenstein JL, Alvarez-Buylla A. 2005. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 25:6997–7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Petryniak MA, Long JE, Ekker M, Obata K, Yanagawa Y, Rubenstein JL, Alvarez-Buylla A. 2007. A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J Neurosci. 27:6878–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. 2009. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 32:149–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll TT, O’Leary DD. 2005. Ventralized dorsal telencephalic progenitors in Pax6 mutant mice generate GABA interneurons of a lateral ganglionic eminence fate. Proc Natl Acad Sci USA. 102:7374–7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerbitz J, Arnett M, Ehrman S, Williams MT, Vorhees CV, Fisher SE, Garratt AN, Muglia LJ, Waclaw RR, Campbell K. 2018. Loss of intercalated cells (ITCs) in the mouse amygdala of Tshz1 mutants correlates with fear, depression, and social interaction phenotypes. J Neurosci. 38:1160–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima T, Nishimura I, Yoshikawa K. 2006. Necdin promotes GABAergic neuron differentiation in cooperation with Dlx homeodomain proteins. J Neurosci. 26:5383–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TN, Zhou QP, Cobos I, Zhang S, Zagozewski J, Japoni S, Vriend J, Parkinson T, Du G, Rubenstein JL, et al. 2017. GABAergic interneuron differentiation in the basal forebrain is mediated through direct regulation of glutamic acid decarboxylase isoforms by Dlx homeobox transcription factors. J Neurosci. 37:8816–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR. 2007. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 10:720–726. [DOI] [PubMed] [Google Scholar]
- Li X, Sun C, Lin C, Ma T, Madhavan MC, Campbell K, Yang Z. 2011. The transcription factor Sp8 is required for the production of parvalbumin-expressing interneurons in the olfactory bulb. J Neurosci. 31:8450–8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang C, Zhang Z, Wen Y, An L, Liang Q, Xu Z, Wei S, Li W, Guo T, et al. 2018. Transcription factors Sp8 and Sp9 coordinately regulate olfactory bulb interneuron development. Cereb Cortex. 28:3278–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Huang YC, Swigut T, Mirick AL, Garcia-Verdugo JM, Wysocka J, Ernst P, Alvarez-Buylla A. 2009. Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature. 458:529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, You Y, Li X, Ma T, Nie Y, Wei B, Li T, Lin H, Yang Z. 2009. Brain injury does not alter the intrinsic differentiation potential of adult neuroblasts. J Neurosci. 29:5075–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhang Z, Lindtner S, Li Z, Xu Z, Wei S, Liang Q, Wen Y, Tao G, You Y et al. 2018. Sp9 regulates medial ganglionic eminence-derived cortical interneuron development. Cereb Cortex, doi: 10.1093/cercor/bhy133. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. 2006. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 9:443–452. [DOI] [PubMed] [Google Scholar]
- Long JE, Garel S, Alvarez-Dolado M, Yoshikawa K, Osumi N, Alvarez-Buylla A, Rubenstein JL. 2007. Dlx-dependent and -independent regulation of olfactory bulb interneuron differentiation. J Neurosci. 27:3230–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JE, Garel S, Depew MJ, Tobet S, Rubenstein JL. 2003. DLX5 regulates development of peripheral and central components of the olfactory system. J Neurosci. 23:568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JE, Swan C, Liang WS, Cobos I, Potter GB, Rubenstein JL. 2009. Dlx1&2 and Mash1 transcription factors control striatal patterning and differentiation through parallel and overlapping pathways. J Comp Neurol. 512:556–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Juarez A, Howard J, Ullom K, Howard L, Grande A, Pardo A, Waclaw R, Sun YY, Yang D, Kuan CY, et al. 2013. Gsx2 controls region-specific activation of neural stem cells and injury-induced neurogenesis in the adult subventricular zone. Genes Dev. 27:1272–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KM, Evans SM, Hirano S, Liu FC. 2014. Dual role for Islet-1 in promoting striatonigral and repressing striatopallidal genetic programs to specify striatonigral cell identity. Proc Natl Acad Sci USA. 111:E168–E177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M. 2003. Neuronal or glial progeny: regional differences in radial glia fate. Neuron 37:751–764. [DOI] [PubMed] [Google Scholar]
- Martin C, Balasubramanian R, Dwyer AA, Au MG, Sidis Y, Kaiser UB, Seminara SB, Pitteloud N, Zhou QY, Crowley WF Jr.. 2011. The role of the prokineticin 2 pathway in human reproduction: evidence from the study of human and murine gene mutations. Endocr Rev. 32:225–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, et al. 2006. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci USA. 103:4140–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna WL, Betancourt J, Larkin KA, Abrams B, Guo C, Rubenstein JL, Chen B. 2011. Tbr1 and Fezf2 regulate alternate corticofugal neuronal identities during neocortical development. J Neurosci. 31:549–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey GL, Lindtner S, Trzcinski B, Visel A, Pennacchio LA, Huylebroeck D, Higashi Y, Rubenstein JL. 2013. Dlx1&2-dependent expression of Zfhx1b (Sip1, Zeb2) regulates the fate switch between cortical and striatal interneurons. Neuron. 77:83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Fuentealba LC, Sanders TA, Magno L, Kessaris N, Alvarez-Buylla A. 2014. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat Neurosci. 17:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. 2007. Mosaic organization of neural stem cells in the adult brain. Science. 317:381–384. [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z, Doetsch F, Sawamoto K, Wichterle H, Alvarez-Buylla A. 2010. The subventricular zone en-face: wholemount staining and ependymal flow. J Vis Exp, doi: 10.3791/1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. 2005. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 308:1923–1927. [DOI] [PubMed] [Google Scholar]
- Olsson M, Bjorklund A, Campbell K. 1998. Early specification of striatal projection neurons and interneuronal subtypes in the lateral and medial ganglionic eminence. Neuroscience. 84:867–876. [DOI] [PubMed] [Google Scholar]
- Olsson M, Campbell K, Wictorin K, Bjorklund A. 1995. Projection neurons in fetal striatal transplants are predominantly derived from the lateral ganglionic eminence. Neuroscience. 69:1169–1182. [DOI] [PubMed] [Google Scholar]
- Petryniak MA, Potter GB, Rowitch DH, Rubenstein JL. 2007. Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron. 55:417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, et al. 2007. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA. 104:17447–17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla R, Stanco A, Howard MA, Rubin AN, Vogt D, Mortimer N, Cobos I, Potter GB, Lindtner S, Price JD, et al. 2018. Dlx1 and Dlx2 promote interneuron GABA synthesis, synaptogenesis, and dendritogenesis. Cereb Cortex. 28:3797–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poitras L, Ghanem N, Hatch G, Ekker M. 2007. The proneural determinant MASH1 regulates forebrain Dlx1/2 expression through the I12b intergenic enhancer. Development. 134:1755–1765. [DOI] [PubMed] [Google Scholar]
- Ponti G, Obernier K, Guinto C, Jose L, Bonfanti L, Alvarez-Buylla A. 2013. Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc Natl Acad Sci USA. 110:E1045–E1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteus MH, Bulfone A, Liu JK, Puelles L, Lo LC, Rubenstein JL. 1994. DLX-2, MASH-1, and MAP-2 expression and bromodeoxyuridine incorporation define molecularly distinct cell populations in the embryonic mouse forebrain. J Neurosci. 14:6370–6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GB, Petryniak MA, Shevchenko E, McKinsey GL, Ekker M, Rubenstein JL. 2009. Generation of Cre-transgenic mice using Dlx1/Dlx2 enhancers and their characterization in GABAergic interneurons. Mol Cell Neurosci. 40:167–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser HM, Bradley A, Caldwell MA. 2007. Olfactory bulb hypoplasia in Prokr2 null mice stems from defective neuronal progenitor migration and differentiation. Eur J Neurosci. 26:3339–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Ware SM, Waclaw RR, Campbell K. 2017. Septal contributions to olfactory bulb interneuron diversity in the embryonic mouse telencephalon: role of the homeobox gene Gsx2. Neural Dev. 12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M, Bulfone A, Ghattas I, Meneses JJ, Christensen L, Sharpe PT, Presley R, Pedersen RA, Rubenstein JL. 1997. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: mutations of Dlx-1, Dlx-2, and Dlx-1 and −2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev Biol. 185:165–184. [DOI] [PubMed] [Google Scholar]
- Qiu M, Bulfone A, Martinez S, Meneses JJ, Shimamura K, Pedersen RA, Rubenstein JL. 1995. Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev. 9:2523–2538. [DOI] [PubMed] [Google Scholar]
- Ragancokova D, Rocca E, Oonk AM, Schulz H, Rohde E, Bednarsch J, Feenstra I, Pennings RJ, Wende H, Garratt AN. 2014. TSHZ1-dependent gene regulation is essential for olfactory bulb development and olfaction. J Clin Invest. 124:1214–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Nakatsuji N. 2001. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev Biol. 240:237–246. [DOI] [PubMed] [Google Scholar]
- Sansom SN, Griffiths DS, Faedo A, Kleinjan DJ, Ruan Y, Smith J, van Heyningen V, Rubenstein JL, Livesey FJ. 2009. The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genet. 5:e1000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfati J, Dode C, Young J. 2010. Kallmann syndrome caused by mutations in the PROK2 and PROKR2 genes: pathophysiology and genotype-phenotype correlations. Front Horm Res. 39:121–132. [DOI] [PubMed] [Google Scholar]
- Scardigli R, Baumer N, Gruss P, Guillemot F, Le Roux I. 2003. Direct and concentration-dependent regulation of the proneural gene Neurogenin2 by Pax6. Development. 130:3269–3281. [DOI] [PubMed] [Google Scholar]
- Silbereis JC, Nobuta H, Tsai HH, Heine VM, McKinsey GL, Meijer DH, Howard MA, Petryniak MA, Potter GB, Alberta JA, et al. 2014. Olig1 function is required to repress dlx1/2 and interneuron production in Mammalian brain. Neuron. 81:574–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman J, Toresson H, Campbell K. 2003. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 23:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoykova A, Fritsch R, Walther C, Gruss P. 1996. Forebrain patterning defects in small eye mutant mice. Development. 122:3453–3465. [DOI] [PubMed] [Google Scholar]
- Stuhmer T, Anderson SA, Ekker M, Rubenstein JL. 2002. Ectopic expression of the Dlx genes induces glutamic acid decarboxylase and Dlx expression. Development. 129:245–252. [DOI] [PubMed] [Google Scholar]
- Toresson H, Campbell K. 2001. A role for Gsh1 in the developing striatum and olfactory bulb of Gsh2 mutant mice. Development. 128:4769–4780. [DOI] [PubMed] [Google Scholar]
- Toresson H, Potter SS, Campbell K. 2000. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 127:4361–4371. [DOI] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura RE, Goldman JE. 2007. Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J Neurosci. 27:4297–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Allen ZJ 2nd, Bell SM, Erdelyi F, Szabo G, Potter SS, Campbell K. 2006. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 49:503–516. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Ehrman LA, Pierani A, Campbell K. 2010. Developmental origin of the neuronal subtypes that comprise the amygdalar fear circuit in the mouse. J Neurosci. 30:6944–6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Wang B, Pei Z, Ehrman LA, Campbell K. 2009. Distinct temporal requirements for the homeobox gene Gsx2 in specifying striatal and olfactory bulb neuronal fates. Neuron. 63:451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Long JE, Flandin P, Pla R, Waclaw RR, Campbell K, Rubenstein JL. 2013. Loss of Gsx1 and Gsx2 function rescues distinct phenotypes in Dlx1/2 mutants. J Comp Neurol. 521:1561–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Waclaw RR, Allen ZJ 2nd, Guillemot F, Campbell K. 2009. Ascl1 is a required downstream effector of Gsx gene function in the embryonic mouse telencephalon. Neural Dev. 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Nie Y, Li X, Wang C, Ma T, Huang Z, Tian M, Sun C, Cai Y, You Y, et al. 2011. Emx1-expressing neural stem cells in the subventricular zone give rise to new interneurons in the ischemic injured striatum. Eur J Neurosci. 33:819–830. [DOI] [PubMed] [Google Scholar]
- Xu Z, Liang Q, Song X, Zhang Z, Lindtner S, Li Z, Wen Y, Liu G, Guo T, Qi D, et al. 2018. SP8 and SP9 coordinately promote D2-type medium spiny neuron production by activating Six3 expression. Development. 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. 2008. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 506:16–29. [DOI] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. 2007. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 27:8286–8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun K, Fischman S, Johnson J, Hrabe de Angelis M, Weinmaster G, Rubenstein JL. 2002. Modulation of the notch signaling by Mash1 and Dlx1/2 regulates sequential specification and differentiation of progenitor cell types in the subcortical telencephalon. Development. 129:5029–5040. [DOI] [PubMed] [Google Scholar]
- Yun K, Garel S, Fischman S, Rubenstein JL. 2003. Patterning of the lateral ganglionic eminence by the Gsh1 and Gsh2 homeobox genes regulates striatal and olfactory bulb histogenesis and the growth of axons through the basal ganglia. J Comp Neurol. 461:151–165. [DOI] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JL. 2001. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 128:193–205. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhang Y, Wang C, Xu Z, Liang Q, An L, Li J, Liu Z, You Y, He M, et al. 2016. The zinc finger transcription factor Sp9 is required for the development of striatopallidal projection neurons. Cell Rep. 16:1431–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Liu F, Tian M, Xu Z, Liang Q, Wang C, Li J, Liu Z, Tang K, He M, et al. 2015. Transcription factors COUP-TFI and COUP-TFII are required for the production of granule cells in the mouse olfactory bulb. Development. 142:1593–1605. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. 2001. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 31:85–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.