Abstract
Background
In Cameroon, the prevention of hepatitis B virus (HBV) transmission by blood transfusion is still only based on hepatitis B surface antigen (HBsAg) screening. However, occult HBV infection (OBI) characterised by the absence of detectable HBsAg and low level of viral DNA remains a potential threat for blood safety. The prevalence of OBI was investigated in blood donors from Yaoundé to provide evidence-based recommendations to improve HBV blood safety.
Material and methods
Blood donations from August 1st, 2016 to March 31st, 2017 were routinely screened for HBV, human immunodeficiency virus (HIV), and hepatitis C virus (HCV) infections (Murex HBsAg Version 3, Murex HIV Ag/Ab Combination, and Murex HCV Ag/Ab Combination [DiaSorin]). Additional HBV investigations were performed, including hepatitis B core antibody ([HBc] Monolisa Anti-HBc PLUS; BIO-RAD) and HBV DNA tested in minipools of two samples using the quantitative Cobas Taqman HBV assay (Roche; LoQ: 6 IU/mL) and HBV DNA genotyping by sequencing.
Results
Of 1,162 donations analysed, 91 (7.8%) were reactive for HBsAg. All of them were also anti-HBc positive. Among the 1,071 HBsAg negative samples, 522 (48.7%) were reactive for anti-HBc. Six (0.56% of all donations) samples fulfilled the consensus definition of OBI and showed low HBV DNA loads (all <6 IU/mL). Following nested polymerase chain reaction amplifications, HBV DNA sequences were obtained for 4 of these samples (1 nearly whole genome [3123 nt], 2 Pre-S/S regions [1,356 nt], and 1 S region [445 nt]). Phylogenetic analysis identified genotype E in all samples.
Discussion
Around 1 in 100 Cameroonian blood donors screened who resulted HBsAg negative and anti-HBc positive carried occult HBV infection. HBsAg alone for screening prospective donors is not sufficient to eliminate the risk of HBV transfusion transmission in Cameroon, and because anti-HBc screening does not seem to be feasible without compromising blood supply, implementation of HBV nucleic acid testing could be considered when possible.
Keywords: occult hepatitis B infection, blood donors, Yaoundé-Cameroon
Introduction
Hepatitis B virus (HBV) is an enveloped DNA virus belonging to the Hepadnaviridae family1. The virus is responsible for the fact that chronic hepatitis B represents a major global health problem with more than 240 million chronically infected persons worldwide, particularly in low- and middle-income countries (LMICs)2. HBV infection remains the most common viral infection transmitted by blood transfusion3. Over the past decades, the risk of HBV transfusion transmission has been steadily reduced through the recruitment of volunteer donors, the selection of donors based on behavioural-risk assessment, the development of increasingly more sensitive hepatitis B antigen (HBsAg) assays, and, in some countries, the use of hepatitis B core antibody (anti-HBc) screening and HBV nucleic acid testing (NAT)1.
Occult HBV infection (OBI) is defined by detectable low level of HBV DNA (<200 IU/mL) in liver or serum with undetectable HBsAg and with/without anti-HBc or anti-HBs, excluding the pre-seroconversion window period (WP). The molecular basis of OBI is the persistence of covalently closed circular DNA (cccDNA) in the cell4. OBI has been reported among healthy asymptomatic blood donors, patients with chronic liver disease, and patients with hepatocellular carcinoma5. The prevalence of OBI tends to be higher in regions with high HBV endemicity6.
In Cameroon, as in most developing countries, screening for HBV among blood donors and patients relies only on serological detection of HBsAg7. In the absence of anti-HBc testing, blood transfusion carries the risk of transmitting HBV infection from donors with OBI8,9. Besides the cost, that is not always affordable by LMICs, HBV-DNA detection by NAT has been proven to be a reliable preventive measure against HBV transmission from donors with OBI4. In Cameroon, the only data on OBI have been reported in human immunodeficiency virus (HIV) positive patients showing prevalence rates between 5.9 and 6.9%10,11. Therefore, in order to provide evidence-based recommendations to improve HBV blood safety, a study was carried out to establish OBI prevalence in blood donors from Yaoundé, Cameroon.
Materials and methods
Samples
A total of 1,162 blood donors were included consecutively in the study at the Blood Bank of the Yaoundé University Teaching Hospital (YUTH), Cameroon. Two 1 mL aliquots of serum were obtained from each donor sample. These were stored at −20°C, and later transported under appropriate conditions to the National Reference Centre (NRC) for Infectious Risks in Blood Transfusion of the National Institute of Blood Transfusion in Paris, France, where further HBV investigations were performed. Prior to recruitment at the Blood Bank of the YUTH, we received research authorisation from the General Manager of the YUTH and ethical clearance from the Regional Ethical Committee in Yaoundé. Each participant was informed about the study through an information leaflet and was invited to sign a consent form. Furthermore, to collect information on demographics, each blood donor was asked to fill in a questionnaire as per routine practice. Before the samples were transported for serological and molecular biology testing in France, the research authorisation of the French Ministry of Health was obtained by the National Reference Center for Infectious Risks in Blood Transfusion, Paris, France.
Blood donation testing
Donations were screened routinely for HBV, HIV and hepatitis C virus (HCV) infections in the blood bank of YUTH using the Murex® HBsAg Version 3, Murex® HIV Ag/Ab Combination, and Murex® HCV Ag/Ab Combination (DiaSorin SpA, Saluggia, Italy), respectively. Additional testing performed at the National Institute of Blood Transfusion in Paris, France, included anti-HBc (Monolisa™ Anti-HBc PLUS; Biorad, Marnes-la-Coquette, France) and HBV DNA in minipools of two samples (MP-2) using the quantitative Cobas® Taqman® HBV assay (Roche Diagnostics; Meylan, France; 95% LOQ of 6 IU/mL). When positive, minipools were resolved by testing each individual sample in the pool with the same NAT assay.
Hepatitis B virus DNA analysis
The nearly whole genome (3,123 nt), and the Pre-S/S (1,356 nt) and S (445 nt) regions of HBV were amplified by nested PCR as previously described12. Amplified products were purified and directly sequenced using the Sanger method (GATC Eurofins Biotech, Constance, Germany). Multiple S sequence alignment and phylogenetic analysis were performed as previously described13. The Viral Epidemiology Signature pattern Analysis programme14 was used to compare the deduced amino acid sequences obtained in this study with 66 and 70 corresponding sequences obtained from genotype E-infected HbsAg-positive blood donors collected in France and Guinea, respectively15,16.
Statistical analysis
The Statistical Package of Social Sciences (IBM SPSS® Statistics, Armonk, NY, USA) version 21 was used in statistical analysis. Cross tabulation and χ2 test were used to detect the significant differences between HBV parameters (HBsAg, anti-hepatitis B virus core antibody [HBcAb], HBV DNA) and the donor characteristics (gender, age groups and donor type). p<0.05 was considered statistically significant.
Results
Blood donors included in the study were 1,002 males (86.2%) and 160 females (13.7%) with a mean age of 29.24 years (standard deviation [SD]=8.21). The distribution of voluntary and family donors was 328 (28.2%) and 834 (71.8%), respectively.
Of these 1,162 donors, 91 (7.8%) tested HbsAg positive. All HbsAg positive samples and 522 (48.7%) out of 1,071 HbsAg negative samples were reactive for HBcAb (Figure 1). Repeat testing to confirm HBcAb reactivity was not possible due to limitations of sample volume. There was no relationship between the presence of HBsAg and demographic characteristics (age, gender and donor type). In contrast, HBcAb reactivity was significantly associated with age (p=0.0001) (Table I).
Figure 1.
Flow chart of serological and molecular investigations of hepatitis B in blood donations collected at the University Teaching Hospital Blood Service, Yaoundé, Cameroon.
Table I.
Demographic and viral characteristics of 1,162 Cameroonian blood donors at the Yaoundé University Teaching Hospital in 2017.
| Characteristics | HBsAg | HBcAb | HBV DNA | |||
|---|---|---|---|---|---|---|
| Tested | Positive | Tested | Positive | Tested | Positive | |
| N donors | 1,162 | 91 (7.8%) | 1,071a | 522 (48.7%) | 522b | 6 (1.1%) |
|
| ||||||
| Gender | ||||||
| Male | 1,002 (86.2%) | 81 (8.1%) | 921 (86.0%) | 455 (49.4%) | 455 (87.2%) | 5 (1.1%) |
| Female | 160 (13.8%) | 10 (6.3%) | 150 (14%) | 67 (44.7%) | 67 (12.8%) | 1 (1.5%) |
| p-value | NS | NS | NAa | |||
|
| ||||||
| Donor type | ||||||
| Benevolent | 328 (28.2%) | 25 (7.6%) | 303 (28.3%) | 133 (43.9%) | 133 (25.5%) | 0 (−) |
| Family | 834 (71.8%) | 66 (7.9%) | 768 (71.7%) | 389 (50.7%) | 389 (74.5%) | 6 (1.5%) |
| p-value | NS | NS | NA | |||
|
| ||||||
| Age groups (years) | ||||||
| 18–20 | 98 (8.4%) | 9 (9.2%) | 89 (8.3%) | 34 (38.2%) | 34 (6.5%) | 0 (−) |
| 21–30 | 662 (57.0%) | 51 (7.7%) | 611 (57.1%) | 275 (45.0%) | 275 (52.7%) | 1 (0.4%) |
| 31–40 | 274 (23.6%) | 21 (7.7%) | 253 (23.6%) | 144 (56.9%) | 144 (27.6%) | 5 (3.5%) |
| 41–50 | 98 (8.4%) | 10 (10.2%) | 88 (8.2%) | 48 (55.0%) | 48 (9.2%) | 0 (−) |
| 51–60 | 28 (2.4%) | 0 (−) | 28 (2.6%) | 21 (75.0%) | 21 (4.0%) | 0 (−) |
| 61–65 | 2 (0.2%) | 0 (−) | 2 (0.2%) | 0 (−) | 0 (−) | 0 (−) |
| p-value | NS | 0.0001 | NA | |||
HBsAg: hepatitis B antigen; HBcAb: anti-hepatitis B virus core antibody; HBV DNA: hepatitis B virus deoxyriboinucleic acid; NS: not significant; NA: not applicable.
Only HBsAg negative samples were tested;
Only HBcAb positive samples were tested.
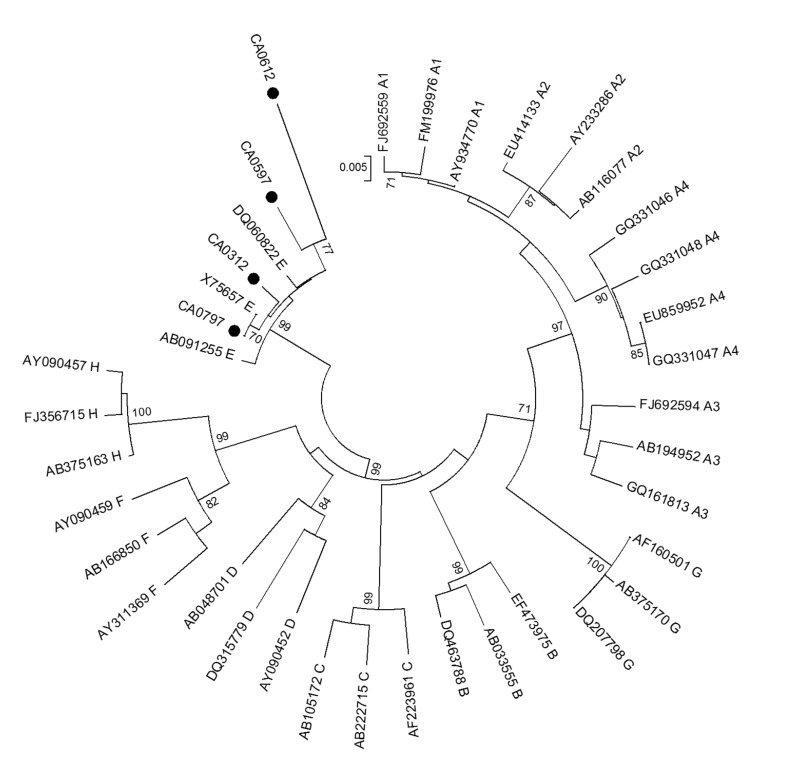
Hepatitis B virus DNA was tested in the 522 HBsAg−/HBcAb+ donors by using MP-2 NAT and 6 out of 255 (2.3%) mini-pools were found reactive. Pool resolution identified 6 HBV DNA reactive samples (one reactive in each initially reactive pool) with individual HBV DNA load <6 IU/mL that were classified as OBI. Five were males, aged 33–40 years old, and one was a 30-year old female. Mean age of OBI carriers (35 years) was higher than those of HBsAg positive (28.5 years) and non-infected donors (28 years) (p=0.7) but not significantly different compared to HBcAb positive only donors (30.6 years). All were family donors and none were infected with HCV or HIV. Nested PCR products and the corresponding sequences were obtained for four OBI samples (1 whole genome, 2 Pre-S/S, and 1 S) definitively confirming OBI carriage. Phylogenetic analysis identified genotype E in all OBI samples (Figure 2). OBI sequences were compared to 66 and 70 sequences obtained from HBV genotype E-infected HBsAg-positive blood donors collected in France and Guinea, respectively. No OBI-specific mutation was observed within the S (4 strains) or Core (1 strain). In the Pol region, sample CA0312 showed two unusual amino acid substitutions (V518A and A731G) within the catalytic area of the RT/polymerase.
Figure 2.
Phylogenetic tree of Cameroonian occult hepatitis B virus (HBV) infection (OBI) strains based on partial S sequences (445 nt).
Phylogenetic analysis was performed with the neighbour-joining algorithm based on Kimura two-parameter distance estimation method. Only bootstrap values ≥75% are shown (1,000 replicates). Cameroonian sequences are identified by a black dot and HBV reference sequences of genotypes/subgenotypes A1–4, B, C, D, E, F, G, and H are identified by their GenBank accession numbers.
Discussion
Overall, 52.7% (613 of 1,162) of blood donors in Yaoundé, Cameroon, had serological markers of HBV active or past infection. This study reports a very high prevalence of HBsAg carriers (approximately 8%), which confirms that hepatitis B infection is still a major problem in the region. But this prevalence was lower than the overall prevalence of 10.5% (95% CI: 8.7–12.4) recently reported in a meta-analysis of 12 studies conducted in Cameroonian blood donors between 2001 and 20167. Difference may be related to the medical selection procedure of blood donors at the YUTH Blood Bank and the evolution of the methodologies used over the 15-year period and/or to regional variations in HBV seroprevalence7.
In the present study, the prevalence of occult HBV carriage was 1.1% (6 of 522) in HbcAb positive donors suggesting an estimated 0.52% (6 of 1,162) prevalence in the total blood donor population of Yaoundé. After elimination of 91 donors who tested HBsAg positive, the potential infectivity of OBI+ donations can be estimated at 0.56% (6 of 1,071). These frequencies are significantly lower than data collected in West and East Africa that showed OBI prevalence of 4.6–17% in selected cohorts of HBsAg-negative donors and 1.5–1.7% in the general blood donor populations17–19. Nevertheless, OBI prevalence also remained lower than that recently reported in HIV co-infected Cameroonian patients (5.9–6.9%)10,11. The heterogeneity of data between studies may be explained by differences in the methods and the strategy used. In the present study, HBV DNA was tested in minipools of two samples in a selected subset of the donor population. The dilution introduced by pooling may affect the efficiency of DNA testing, as suggested by the extremely low viral DNA loads (<6 IU/mL) observed in all OBI samples. Strong suppression of viral replication and gene expression may have resulted from the host immune responses. But it could also be due to viral characteristics (e.g., genotype E)20 and/or to assay performance21.
In the present study, HBV DNA was not investigated in HBcAb-negative donors and a possible underestimation of the number of HBV DNA carriers cannot be ruled out. Cameroonian OBI donors were predominantly males with a mean age of 35 years, and were infected with HBV genotype E (HBVE). Genotype E is the most prevalent genotype in western Africa throughout the crescent from Mauritania to Namibia22. In Cameroon, HBV genotype A was also prevalent in particular populations including Pygmies, Bantus, and HIV-infected individuals22,23. However, the age distribution in Cameroonian OBI donors was similar to that observed in South African OBI donors infected with genotype A1 (30–40 years)21. Occurrence of OBI at a younger age does not seem to be related to any particular African HBV genotype but rather to the specificity of the natural history of HBV infection in sub-Saharan Africa that includes mainly horizontal but also vertical transmission in early childhood24.
The analysis of the limited number of HBV sequences available showed a very limited genetic variability in the OBI strains, similar to that usually observed in non-OBI genotype E strains12,13.
In particular, the S protein was similar to comparison sequences from genotype E-infected HBsAg-positive chronic carrier blood donors. The host immune pressure did not seem to play a major role in the occurrence of OBI in these HBVE-infected donors. However, this analysis is limited by the absence of anti-HBs antibody testing. Many different mechanisms are potentially associated with OBI25. It is tempting to hypothesize that the presence of the unusual V518A and A731G amino acid substitutions within the catalytic area of the RT/polymerase may affect the efficiency of the viral replication, leading to an OBI phenotype. However, further functional analyses are needed to explore this.
Nucleic acid testing for HBV DNA reduces the risk of HBV transfusion-transmission by detecting OBI as well as acute WP infections21. According to the present data, the implementation of HBV NAT at the Yaoundé University Teaching Hospital Blood Bank may intercept at least 52 HBV-infected donations per 10,000 donations. However, the implementation of viral nucleic acid testing is still challenging for low-income countries like Cameroon due to its considerable cost, the limited logistical resources available, and the few adequately trained staff. HBcAb testing might be a potentially affordable alternative for low-income countries, despite the fact it cannot detect WP infections26. In addition, the yield of HBcAb in negative HBsAg Cameroonian donors was 48.7%. Despite the lack of confirmatory testing in the present study, this was similar to the 35.1% and 48.4% values reported previously in Ghanaian donors under 20 years of age and older than 40 years, respectively27. In contrast, lower HBcAb reactivity rates have been observed in Sudanese (33%) and Egyptian (8%) donors19,28,29. Therefore, the introduction of HBcAb screening in the Yaoundé Blood Bank would cause a significant shortage in the blood supply.
In summary, HBsAg screening alone is not sufficient to eliminate the risk of HBV transfusion-transmission in Cameroon. Anti-HBc screening does not seem to be feasible without compromising blood supply and implementation of HBV nucleic acid testing might be considered. However, further studies are needed for a more precise evaluation of the prevalence of HBV DNA in the total blood donor population, the cost-effectiveness, and the specific feasibility issues before considering NAT implementation in Cameroon.
Conclusions
In summary, this study provides clear evidence of OBI infection in blood donors at the Yaoundé University Teaching Hospital, Cameroon. HBV was confirmed highly endemic in Cameroon. More than 50% of blood donors were anti-HBc positive. Around 1 in 100 Cameroonian blood donors screened who tested HBsAg negative and anti-HBc positive carried occult HBV infection. HBsAg alone for screening prospective donors is not sufficient to eliminate the risk of HBV transfusion-transmission in Cameroon, and because anti-HBc screening does not seem to be feasible without compromising blood supply, implementation of HBV nucleic acid testing could be considered when possible. Introducing HBV NAT would certainly reduce transfusion transmitted cases, but the overall impact in limiting HBV epidemic in Cameroon is expected to be negligible in the absence of effective vaccination and treatment campaigns.
Footnotes
Funding and resources
NHLBI training grant K24-HL075036 and NIH Fogarty grant D43-TW010345 to ELM. The National Blood Foundation (USA), Research Grant 2014 to CTT. National Institute of Blood Transfusion (INTS), Department of Blood Borne Agents, National Reference Center for Infectious Risks in Blood Transfusion, Paris, France.
Authorship contributions
DF and DC are co-first authors. DF, CTT, DM, SL, DC and ELM conceived and designed the study, analysed and interpreted the data, wrote the manuscript and approved the final version. HIK and FEC designed the study, interpreted the data, reviewed the manuscript and approved the final version. CTT, SL and ELM provided study material. DF, CD and DC carried out laboratory testing. DF, DC and SL collected, assembled, analysed and interpreted the data.
The Authors declare no conflicts of interest.
References
- 1.Candotti D, Laperche S. Hepatitis B virus blood screening: need for reappraisal of blood safety measures? Front Med. 2018;5:1–10. doi: 10.3389/fmed.2018.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva: WHO guidelines approved by the Guidelines Review Committee; 2015. [PubMed] [Google Scholar]
- 3.Niederhauser C. Reducing the risk of hepatitis B virus transfusion-transmitted infection. J Blood Med. 2011;2:91–102. doi: 10.2147/JBM.S12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raimondo G, Caccamo G, Filomia R, Pollicino T. Occult HBV infection. Semin Immunopathol. 2013;35:39–52. doi: 10.1007/s00281-012-0327-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makvandi M. Update on occult hepatitis B virus infection. World J Gastroenterol. 2016;22:8720–34. doi: 10.3748/wjg.v22.i39.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minuk GY, Sun D, Uhanova J, et al. Occult hepatitis B virus infection in a North American community-based population. J Hepatol. 2005;42:480–5. doi: 10.1016/j.jhep.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 7.Bigna JJ, Amougou MA, Asangbeh SL, et al. Seroprevalence of hepatitis B virus infection in Cameroon: a systematic review and meta-analysis. BMJ Open. 2017;7:e015748. doi: 10.1136/bmjopen-2016-015748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spreafico M, Berzuini A, Foglieni B, et al. Poor efficacy of nucleic acid testing in identifying occult HBV infection and consequences for safety of blood supply in Italy. J Hepatol. 2015;63:1068–76. doi: 10.1016/j.jhep.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Candotti D, Assennato SM, Laperche S, et al. Multiple HBV transfusion transmissions from undetected occult infections: revising the minimal infectious dose. Gut. 2019;68:313–21. doi: 10.1136/gutjnl-2018-316490. [DOI] [PubMed] [Google Scholar]
- 10.Gachara G, Magoro T, Mavhandu L, et al. Characterization of occult hepatitis B virus infection among HIV positive patients in Cameroon. AIDS Res Ther. 2017;14:11. doi: 10.1186/s12981-017-0136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salpini R, Fokam J, Ceccarelli L, et al. High burden of HBV-Infection and atypical HBV strains among HIV-infected Cameroonians. Curr HIV Res. 2016;14:165–71. doi: 10.2174/1570162x13666150930114742. [DOI] [PubMed] [Google Scholar]
- 12.Zahn A, Li C, Danso K, et al. Molecular characterization of occult hepatitis B virus in genotype E-infected subjects. J Gen Virol. 2008;89:409–18. doi: 10.1099/vir.0.83347-0. [DOI] [PubMed] [Google Scholar]
- 13.Candotti D, Diarra B, Bisseye C, et al. Molecular characterization of hepatitis B virus in blood donors from Burkina Faso: prevalence of quasi-subgenotype A3, genotype E, and mixed infections. J Med Virol. 2016;88:2145–56. doi: 10.1002/jmv.24589. [DOI] [PubMed] [Google Scholar]
- 14.Los Alamos National Laboratory [Internet] HIV sequence database: Viral Epidemiology Signature Pattern Analysis - VESPA. [Accessed on: 30/08/2019]. Available at: https://www.hiv.lanl.gov/content/sequence/VESPA/vespa.html.
- 15.Servant-Delmas A, Mercier M, El Ghouzzi M-H, et al. National survey of hepatitis B virus (HBV) polymorphism in asymptomatic HBV blood donors from 1999 to 2007 in France. Transfusion. 2010;50:2607–18. doi: 10.1111/j.1537-2995.2010.02725.x. [DOI] [PubMed] [Google Scholar]
- 16.Garmiri P, Loua A, Haba N, et al. Deletions and recombinations in the core region of hepatitis B virus genotype E strains from asymptomatic blood donors in Guinea, west Africa. J Gen Virol. 2009;90:2442–51. doi: 10.1099/vir.0.012013-0. [DOI] [PubMed] [Google Scholar]
- 17.Diarra B, Yonli AT, Sorgho PA, et al. Occult hepatitis B virus infection and associated genotypes among Hbsag-negative subjects in Burkina Faso. Mediterr J Hematol Infect Dis. 2018;10:e2018007. doi: 10.4084/MJHID.2018.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oluyinka OO, Tong HV, Tien SB, Fagbami AH. Occult hepatitis B virus infection in Nigerian blood donors and hepatitis B virus. PLoS One. 2015;10:e0131912. doi: 10.1371/journal.pone.0131912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahgoub S, Candotti D, El Ekiaby M, Allain JP. Hepatitis B virus (HBV) infection and recombination between HBV genotypes D and E in asymptomatic blood donors from Khartoum, Sudan. J Clin Microbiol. 2011;49:298–306. doi: 10.1128/JCM.00867-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zahn A, Li C, Danso K, et al. Molecular characterization of occult hepatitis B virus in genotype E-infected subjects. J Gen Virol. 2008;89:409–18. doi: 10.1099/vir.0.83347-0. [DOI] [PubMed] [Google Scholar]
- 21.Allain JP, Candotti D. Diagnostic algorithm for HBV safe transfusion. Blood Transfus. 2009;7:174–82. doi: 10.2450/2008.0062-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olinger CM, Venard V, Njayou M, et al. Phylogenetic analysis of the precore/core gene of hepatitis B virus genotypes E and A in West Africa: new subtypes, mixed infections and recombinations. J Gen Virol. 2006;87:1163–73. doi: 10.1099/vir.0.81614-0. [DOI] [PubMed] [Google Scholar]
- 23.Forbi JC, Ben-Ayed Y, Xia G, et al. Disparate distribution of hepatitis B virus genotypes in four sub-Saharan African countries. J Clin Virol. 2013;58:59–66. doi: 10.1016/j.jcv.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botha JF, Dusheiko GM, Ritchie MJJ, et al. Hepatitis B virus carrier state in black children in ovamboland: role of perinatal and horizontal infection. Lancet. 1984;1:1210–2. doi: 10.1016/s0140-6736(84)91694-5. [DOI] [PubMed] [Google Scholar]
- 25.Raimondo G, Locarnini S, Pollicino T, et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71:397–408. doi: 10.1016/j.jhep.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Allain J-P, Opare-Sem O. Screening and diagnosis of HBV in low-income and middle-income countries. Nat Rev Gastroenterol Hepatol. 2016;13:643–53. doi: 10.1038/nrgastro.2016.138. [DOI] [PubMed] [Google Scholar]
- 27.Allain J-P, Candotti D, Soldan K, et al. The risk of hepatitis B virus infection by transfusion in Kumasi, Ghana. Blood. 2003;101:2419–25. doi: 10.1182/blood-2002-04-1084. [DOI] [PubMed] [Google Scholar]
- 28.Antar W, El-Shokry MH, Abd El Hamid WA, Helmy MF. Significance of detecting anti-HBc among Egyptian male blood donors negative for HBsAg. Transfus Med. 2010;20:409–13. doi: 10.1111/j.1365-3148.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- 29.Said ZN, Sayed MH, El Salama, et al. Occult hepatitis B virus infection among Egyptian blood donors. World J Hepatol. 2013;5:64–73. doi: 10.4254/wjh.v5.i2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]