Abstract
Background
Platelet-rich plasma (PRP) has been used in different non-transfusion indications due to its role in tissue regeneration and healing. The aim of this overview of systematic reviews (umbrella review) is to provide a summary of the existing research syntheses related to PRP use for sports-related muscle, tendon and ligament injuries.
Materials and methods
Literature searches were performed in MEDLINE, Embase, and Cochrane Library to identify systematic reviews focusing on PRP use for sports-related muscle, tendon and ligament injuries. The methodological quality of included studies was assessed using the checklist for systematic reviews and research syntheses developed by the Joanna Briggs Institute and the GRADE assessment.
Results
Twenty-two studies met the inclusion criteria. Five studies evaluated PRP use for acute muscle injury, and 17 evaluated PRP use for tendon and ligament injury. Studies were heterogeneous in terms of the dose and number of PRP injections, and the control groups. Three of the 5 reviews evaluating acute muscle injury concluded that PRP had no effect on the outcomes considered. One review shows superior efficacy of rehabilitation exercise compared to PRP. One review shows that PRP may result in an earlier return to sport for acute grade I-II injury. Eight out of the 17 reviews evaluating PRP for tendon and ligament injuries show a statistically significant (p<0.05) difference in pain and/or function outcome measures favouring PRP compared to controls, although most of the observed differences were small. Adverse events data and quality of life outcomes were rarely analysed or reported in the included studies and were considered clinically insignificant.
Discussion
In most of the included reviews, the available evidence was judged to be of low/very low quality due to risk of bias, inconsistency and imprecision, thus making the level of certainty of these findings low and not adequate to support the general use of PRP in this setting.
Keywords: platelet-rich plasma, sports medicine, acute muscle injury, tendon injury, ligament injury
Introduction
Transfusion medicine has evolved rapidly in recent years, mostly thanks to the development of Patient Blood Management (PBM), a revolutionary multimodality and multidisciplinary approach adopted to limit the use and the need for allogeneic blood transfusion in all at-risk patients with the aim of improving their clinical outcomes1–19. Another significant advance that has emerged in the last two decades regards the development of blood components for non-transfusion use, in particular, platelet-rich plasma (PRP)-based technologies20. The term PRP is used to describe an autologous blood product generated from a two-phase centrifugation process of a patient’s whole blood to yield a concentration of platelets in a small volume of plasma20. Besides platelets, PRP contains some inflammatory cells (i.e., monocytes and polymorphonuclear neutrophils) and large amounts of proteins, including platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), epithelial growth factor (EGF) and adhesion molecules (i.e., fibrin, fibronectin and vitronectin). Such growth factors and cells have been shown to promote cell recruitment, proliferation and angiogenesis, which may be implicated in tissue regeneration and healing21–24. Such tissue regeneration properties that emerged from animal studies have been extensively studied in humans in a wide range of clinical situations in areas such as orthopaedics, dermatology and dentistry25,26. An interesting field, which has received increasing attention in recent years, is that of PRP use in musculoskeletal soft tissue injuries and tendinopathies. These are very common, especially in adults who take part in sport activities, and they represent a significant burden to society in terms of health care resources and personal disability27. The continuously growing number of pilot studies over recent years on the use of PRP in sports medicine has prompted a number of systematic reviews aimed at evaluating the safety and efficacy of this procedure to treat sports-related injuries28–35. Overviews of existing systematic reviews, also called umbrella reviews, are a relatively new approach to synthesising evidence from systematic reviews and meta-analyses36. The aim of this umbrella review is to provide a summary of the existing research syntheses related to PRP use for sports-related muscle, tendon and ligament injuries.
Material and methods
For the purposes of an umbrella review, the term “studies” refers exclusively to syntheses of research evidence including systematic reviews and meta-analyses.
Review question/objective
The objective of this umbrella review is to evaluate the efficacy of PRP for the treatment of acute lesions of the musculoskeletal system, ligaments and tendinopathies related to sports injuries.
Inclusion and exclusion criteria
We considered for inclusion systematic reviews that included randomised controlled trials (RCTs) or quasi-randomised studies in humans in which the PRP was administered to treat common sports-related injuries such as acute muscle injury, ligament injury and tendinopathies. Studies including RCTs and other study designs (e.g., cohort studies, case series) were also considered, but the qualitative/quantitative synthesis was limited to RCTs only. Studies including PRP use in surgery (repair or reconstruction) and osteoarthritis were excluded. In order to be included, studies had to evaluate RCTs in which the intervention was described as PRP; studies evaluating PRP and other types of interventions (e.g., autologous blood injection, non-steroidal anti-inflammatory drugs, rehabilitation exercises) were also considered, but the quantitative synthesis was limited to the PRP subset analysis.
Participants
Acute muscle injuries and musculoskeletal soft tissue injuries are very common, particularly in adults who take part in sports activities, including professional and recreational athletes. However, many of these conditions have a bimodal distribution and occur in both athletes and sedentary subjects28,35. They are more common in middle age, and with the increase in sports activity in older age groups, they are becoming more frequent.
For this umbrella review we considered studies that included populations with differing levels of physical activity, including studies on the sporting population (professional and/or recreational athletes) and studies that did not explicitly mention involving a sporting population.
Details of patients’ demographic, including sex, age, and level of activity, where available, were extracted.
Outcomes
We included functional outcomes (assessed by subjective assessment questionnaires such as Disabilities of the Arm, Shoulder and Hand questionnaire (DASH), Victorian Institute of Sports Assessment - Achilles questionnaire (VISA-A), and American Orthopedic Foot and Ankle Society (AOFAS) foot questionnaire) and pain outcomes assessed by subjective scales such as visual analogue scales (VAS).
We also included local and systemic adverse effects of PRP administration and controls (including infection at the injection site), recovery time (return to sports, and return to day-to-day or work activities), patient satisfaction and quality of life measures.
We categorised the outcome measurements as short-term (up to 12 weeks follow up), medium-term (between 12 weeks and one year follow up), and long-term (more than one year follow up).
Search strategy
A literature search was performed in mid-October 2019 in Medline (through PubMed), Embase, Scopus and Cochrane library. Searches were performed by one author without language restrictions. A combination of the following text words was used to maximise search specificity and sensitivity: PRP/ OR platelet- rich plasma AND muscle injury AND tendinopathy/ OR tendinitis/OR tendinosis/ OR epicondylitis/OR patellar tendon/OR Achilles tendon AND randomised clinical trial/OR clinical trial AND meta-analysis/ OR systematic review. In addition to the electronic search, we checked the reference lists of the most relevant items (original studies and reviews) in order to identify potentially eligible studies not captured by the initial literature search.
Study selection and data extraction
All titles were screened by two independent assessors (MC and MF). Eligibility assessment was based on the title or abstract and on the full text if required. Full texts of possibly eligible articles were obtained and assessed independently by two reviewers (MC and MF). Both reviewers compared the articles identified. Studies were selected independently by two reviewers (MF and MC), with disagreements resolved through discussion and on the basis of the opinion of a third reviewer (CM).
The two assessors also independently extracted quantitative and qualitative data from each selected study, grouped by the type of clinical indication (acute muscle injury and tendinopathy). Findings are presented in tabular format with supporting text. Quantitative tabulation of results include: first author name and year of publication, the clinical condition under evaluation, principal characteristics of the study population, number of RCTs included in the systematic review, findings related to the PRP and comparator regimens used, the outcomes assessed, a quantitative synthesis (when available) of the estimates of interest (e.g., mean difference with the 95% confidence intervals (CI) in pain and function outcomes), the conclusions drawn taking into account the findings, and the methodological assessment of the review.
Assessment of methodological quality
We used the Joanna Bring Institute critical appraisal checklist for systematic reviews, a tool that evaluates both quantitative and qualitative reviews that is based on principles common across accepted quality assessment tools36. There are 11 questions (Q1–Q11) to guide the appraisal of systematic reviews or meta-analyses with the following checklist. (See the Appendix for details of each question).
Q1. Is the review question clearly and explicitly stated?
Q2. Were the inclusion criteria appropriate for the review question?
Q3. Was the search strategy appropriate?
Q4. Were the sources of studies adequate?
Q5. Were the criteria for appraising studies appropriate?
Q6. Was critical appraisal conducted by two or more reviewers independently?
Q7. Were there methods to minimise errors in data extraction?
Q8. Were the methods used to combine studies appropriate?
Q9. Was the likelihood of publication bias assessed?
Q10. Were recommendations for policy and/or practice supported by the reported data?
Q11. Were the specific directives for new research appropriate?
Each question was to be answered as “yes”, “no”, or “unclear” or not applicable (NA).
The tool (available at: https://ro.uow.edu.au/cgi/viewcontent.cgi?article=4367&context=smhpapers) was used by the two independent reviewers conducting the critical appraisal of each research synthesis selected (MC and MF), with disagreements resolved through discussion.
Appraisal of the quality of evidence
The quality of evidence was appraised following the GRADE approach (Grades of Recommendation, Assessment, Development, and Evaluation). Whenever available, the grading of the quality of evidence reported in the included reviews was considered to determine the quality of evidence. In a situation in which the grading of evidence was not reported by the authors of the study, the GRADE approach was applied in its five domains (risk of bias, indirectness, imprecision, inconsistency and publication bias) based on the information available from the study.
Results
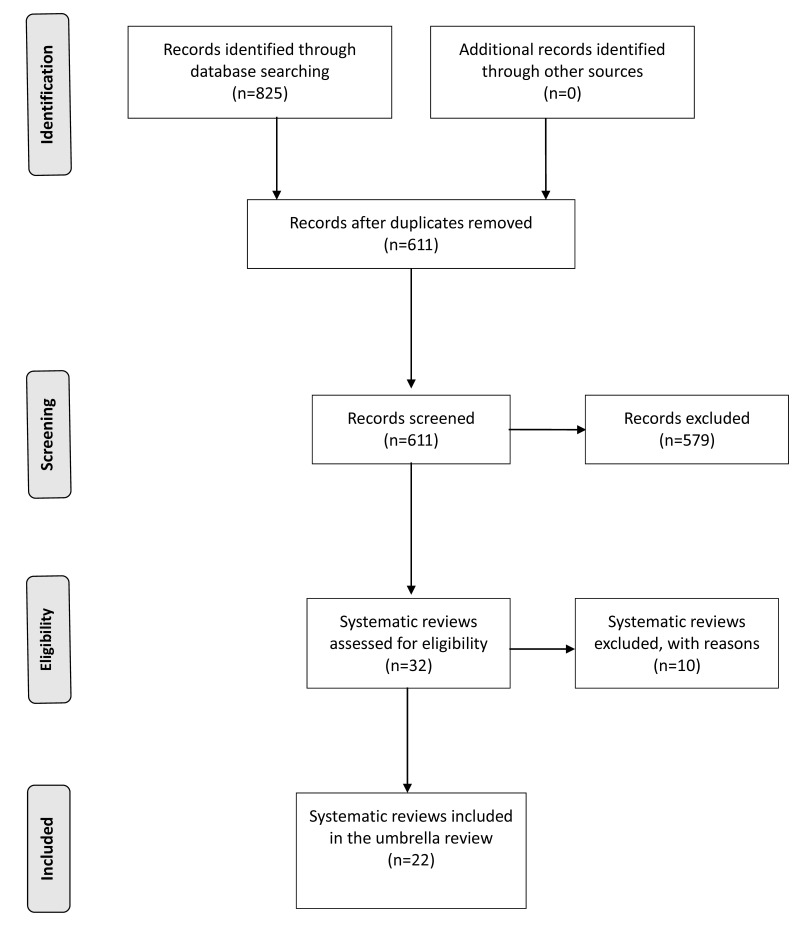
The electronic search retrieved 825 references. At the first stage of screening titles and abstracts, 32 references were selected (Figure 1). After the full texts were scrutinised against the inclusion and exclusion criteria, 22 studies were included in the umbrella review28–35,37–50 and 10 studies were excluded23,51–59. Reasons for exclusion were: duplicate paper51, systematic reviews of PRP use for osteoarthritis (3)52,53,55, PRP use for arthroscopic rotator cuff repair56, an overview of systematic reviews evaluating several injection therapy for lateral epicondylitis57, a network meta-analysis evaluating several injection therapies for lateral epicondylitis58, a systematic review of basic science literature59.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram.
Description of studies
Of the 22 studies included in the overview, 5 were systematic reviews on the use of PRP for acute muscle injury28,29,32–34, and 17 systematic reviews on the use of PRP for tendon and ligament injuries30,31,35,37–50. The 22 studies included were based on 176 overlapping primary RCTs. The main characteristics of the studies included are summarised in Table I.
Table I.
Main characteristics of included studies.
| 1st author, yearref. | Clinical condition | Population | No. RCTs | PRP | Comparators | Outcome | Quantitative synthesis | notes |
|---|---|---|---|---|---|---|---|---|
| ACUTE MUSCLE INJURY | ||||||||
| Manduca 201833 | Acute hamstring injury | College athletes | 3 (2 DB, 1 SB) | PRP + rehabilitation | Rehabilitation alone | Recovery time | Not assessed | PRP injections cannot be recommended as having value for hamstring injuries, compared to rehabilitation alone |
| Moraes 201328 | People with musculoskeletal soft tissue injuries. 8 clinical conditions: rotator cuff tears (arthroscopic repair) (6 trials); shoulder impingement syndrome surgery (1 trial); elbow epicondylitis (3 trials); anterior cruciate ligament (ACL) reconstruction (4 trials), ACL reconstruction (donor graft site application) (2 trials), patellar tendinopathy (1 trial), Achilles tendinopathy (1 trial) and acute Achilles rupture surgical repair (1 trial) |
Participant characteristics differed among study populations. Populations in studies concerning mainly sports injuries (lateral epicondylitis, ACL reconstruction, patellar tendinopathy, Achilles tendinopathy and Achilles ruptures) included mainly young and active adults, whereas studies concerning degenerative conditions (chronic impingement syndrome and rotator cuff tears) mainly included an older population. Most of the studies did not specify whether the participants had a previous history of sports activity |
19 trials (17 randomised and two quasi-randomised; 1,088 participants). Eleven trials reported that participants and follow-up assessors were blinded or partially blinded to the procedure |
PRP. The trials used different ways of preparing and applying PRP | Placebo, autologous whole blood, dry needling or no platelet-rich therapy | Primary outcomes: functional status (e.g., DASH, VISA-A, AOFAS), pain (VAS) and adverse effects. Secondary outcomes: recovery time: return to sports, or work activities. Non-return to previous activities: sports, work or decrease in the level of activity. Quality of life; recurrence of the condition; need for a secondary treatment procedure (for example, surgery); participant satisfaction |
5 studies: SMD of function in short term (≤3 months) =0.24 (−00.7, 0.56) 6 studies: SMD of function at medium term (6 months) =0.06 (−0.39, 0.51) 10 studies: SMD of function at long term (1 year)=0.25, (−0.07 to 0.57) 5 studies: MD of pain in short term =−0.95, (−1.41 to −0.48). 4 studies: incidence of adverse effects, RR=1.31, (0.48 to 3.59) |
The evidence for all primary outcomes was judged as being of very low quality |
| Pas 201529 | Acute hamstring injury | Participants from different sports were used in seven studies; two studies used a specific sport population; one study did not explicitly mention using a sporting population. | 10 RCTs (526 participants) | Only 3 studies used PRP injections | PRP was compared to standardised physiotherapy programme with placebo injections, platelet-poor plasma injections or no injection. The other studies mostly evaluated rehabilitation exercises. | Time to RTP; re-injury; adverse events; change in pain; Hamstring outcome score; patient satisfaction | HR for return to play =1.03(0.87, 1.22); 2 studies: RR for reinjury =0.88 (0.45. 1.71) | Meta-analysis showed superior efficacy for rehabilitation exercises. PRP injection had no effect on acute hamstring injury |
| Grassi 201834 | Acute muscle injuries | Patients were predominantly professional male athletes | Six RCTs, involving 374 patients. All studies reported that outcome assessors were blinded, but only two studies were considered to be double-blind | PRP injections | Conventional physical therapy (n=3), physical therapy and hematoma aspiration (n=1) or physical therapy and isotonic saline injection (n=1). One study had three arms, comparing PRP, platelet-poor plasma (PPP), and no injections. |
Time to return to sport, re-injuries, complications, pain, muscle strength, range of motion /flexibility, muscle function, and imaging | MD for RTS=−7.17 (−12.26, −2.08); 4 studies: RD for reinjury= −0.03 (−0.10, 0.05); 6 studies: RD for complications =0.01 (−0.05, 0.06) | Any benefit in terms of clinical outcomes, return to sport, and recurrence using PRP injections for the treatment of acute muscle injuries is not supported by the available literature. The evidence should be considered to be of low or very low quality |
| Sheth 201832 | Acute muscle injuries (acute grade I or II muscle strains). Subgroup analysis was performed to examine the efficacy of PRP in hamstring muscle strains alone |
Athletes | Five RCTs (268 patients). Two studies34,35 described blinding participants, investigators, and outcome assessors with the use of a placebo (normal saline or platelet poor plasma) | PRP injection + standardised rehabilitation protocol | Standardised rehabilitation protocol (in 2 studies + saline injection or PPR) | Primary outcome: time to return to play. Secondary outcome: rate of reinjury at ≥6 months of follow-up. | MD for RTS=−5.57 (−9.57, −1.58); 3 studies: OR for risk of reinjury=0.76 (0.34, 1.69). Subgroup analysis of studies evaluating grade I or II hamstring muscle strains alone revealed no significant difference in the time to return to sport (3 studies, 159 patients); MD, 3.92 (95% CI, 9.73 to 1.89; p 0.19). Subgroup analysis of studies of higher methodologic quality showed no significant difference in time to return to sport between PRP and control therapy (4 studies; 234 patients); MD, 3.28 (95% CI, 6.61 to 0.05); p 0.05). There was also no difference in return to sport between PRP and control therapy among the pooled studies that included only acute grade II muscle strains (2 studies, 99 patients); MD, 8.21 (95% CI, 19.42 to 3.00; p 0.15) |
The use of PRP may result in an earlier return to sport among patients with acute grade I or II muscle strains without significantly increasing the risk of reinjury at 6 months of follow-up. However, no difference in time to return to sport was revealed when specifically evaluating those with a grade I or II hamstring muscle strain, and in subgroup analysis including only studies of high methodological quality. Overall, our results should be interpreted with caution because of the heterogeneous nature of the studies, PRP preparation, muscle groups studied, and outcome measures used. |
| TENDINOPATHY | ||||||||
| Chen 201837 | Tendon and ligament injuries. Six different pathology subgroups were identified: rotator cuff injury, lateral epicondylitis, patellar tendinopathy, Achilles tendinopathy, anterior cruciate ligament injury, and hamstring tendinopathy | No detailed information of the population included were provided. The mean patient age ranged from 23 to 66 years. There was clinical heterogeneity in the review population, which included both young and active adults with sports injuries (as in lateral epicondylitis (8 studies, 390 participants), ACL reconstruction, patellar tendinopathy, Achilles tendinopathy and Achilles ruptures) and older population with degenerative conditions (e.g., rotator cuff tears (8 studies, 469 participants) | 37 RCTs | “Platelet-rich plasma injection (PRP) and platelet-rich fibrin matrix (PRFM) were considered for inclusion” but not further information is provided | Eleven different controls were used, with over half of studies using surgical repair without additional treatment as a control Saline injection, dry needling, autologous whole blood injection, and corticosteroid injection were deemed appropriate controls | 21 studies reported VAS (at baseline, short term [up to 6.5 months follow-up], and long term [1 year or more follow-up]) | 17 studies: MD of VAS at short term=−0.72 (−1.1, −0.34); 14 studies: MD of VAS at long term=−0.84 (−1.23, −0.44). | “PRP may reduce the pain associated with lateral epicondylitis and rotator cuff pathology”. However, there was substantial heterogeneity in the included studies, and funnel plots appeared to be asymmetric, suggesting publication bias. There were other potential bias caused by small-study effects, reporting bias, and lack of blinding, but the authors did not perform subgroup analyses according to bias assessment |
| Zhang 201838 | Chronic Achilles tendinopathy | The studies considered included in this meta-analysis drew patients from the general population with few competitive elite athletes, and there were fewer women than men in the study groups. Mean age in included studies ranged 40–50 yrs |
4 RCTs (170 participants). All the included studies had low risk of bias. No evidence of publication bias was detected |
PRP injection with eccentric training. The technique used in all PRP groups of included studies was described as single or multiple injections under ultrasonographic guidance, intratendinous and peritendinous, with or without local anesthetic. After the injection, all patients received a standardized rehabilitation and eccentric program | Saline injection and eccentric training | VISA-A score. Secondary outcomes were tendon thickness, color Doppler activity, and other functional measures (such as pain and return to sports activity) | MD of VISA-A =5.3 (−0.7 to 11.3). MD of tendon US thickness change =0.2 mm (−0.6 to 1 mm). MD of tendon US thickness change =0.2 mm (−0.6 to 1 mm). MD of tendon color Doppler activity=0.1 mm (−0.7 to 0.4) | PRP injection with eccentric training did not improve VISA-A scores, reduce tendon thickness, or reduce color Doppler activity in patients with chronic Achilles tendinopathy compared with saline injection. Two studies compared the number of patients returning to their desired sport between the PRP and saline groups; with the numbers available, they found no differences in the likelihood that a patient would return to sport after treatment with PRP compared with saline |
| Gholami 201630 | Lateral epicondylitis (4 studies), Achillean tendinopathy (3 studies, patellar tendon (2 studies), rotator cuff (2 studies) plantar fasciitis (1 study), knee (1 study), talus (1 study) | Professional athletes treated with PRP for sports-related injuries or orthopedic problems | 18 RCTs included in the qualitative synthesis, and 12 RCTs in the meta-analysis | “Every form of PRP (e.g., injection and gel) was included, and there was not any limitation in preparing the process of PRP” | “there was no limitation in comparators”, which included saline, steroids, eccentric loading program, dry needling, Autologous whole blood, Focused extracorporeal shock wave therapy (ESWT) | VAS, VISA, DASH. Return to sports was addressed in some studies, but quantitative analysis was not possible | 6 studies: MD of VAS at 3 months= −0.21 (−2.29, 1.87). | The analysis of the results of pain scores and physical activity/function did not show any superiority for PRP as opposed to the other options. Of 18 RCTs, 11 did not show any clinical benefit for PRP, and the rest was not strong enough in terms of population and effect size to support the efficacy of PRP in pain reduction (p=0.663) or function improvement (p=0.820) |
| Taylor 201131 | Patellar and elbow tendinosis, Achilles tendon injuries, rotator cuff repair, and anterior cruciate ligament (ACL) reconstruction | Heterogeneous population. Authors state that articles that did not pertain to sports medicine (eg, dental ligament cells) were excluded, bur sports activity was rarely reported in the included trials | Of the 13 studies included, only 4 were RCTs (1 quasi-randomised); the others were prospective cohort studies (3 studies), and case reports or case-control studies (6 reports) | The articles reported nonhomogeneous methods for PRP preparation (different volume blood drawn, non homogeneous platelet separation system, different activating agent) and application (injection, PRP gel, PRP scaffold, PRP fibrin membrane) | Controls included eccentric exercise program, steroids and saline injection | Various outcomes, including AOFAS, DASH, EQ-VAS, IKDC, magnetic resonance imaging; power Doppler ultrasonography, ROM, range of motion; SF 36, Short Form, VISA-A | NA | Studies, population included, methods for PRP preparation and application, levels of the evidence were too heterogeneous to draw firm conclusions. The authors state “..despite some benefits demonstrated to date, it must be acknowledged that the uses of PRP in soft tissue applications are still weakly supported...” Establishment of platelet therapy as a reliable, efficacious, and safe therapy in managing the pathology of tendons and ligaments will require the completion of high-quality clinical trials with long-term follow-up” |
| De Vos 201035 | Chronic tendinopathy included wrist extensors, flexors, plantar fasciopathy and patellar tendinopathy | Heterogeneous population | Of the 11 included studies, only 3 evaluated PRP (2 controlled trials, and one case series) | Eight studies used autologous blood injections. Only3 studies used PRP injections, one of which used an additional local anesthetic and 2 did not report whether local anesthesia was used | Exercise or anesthetic injection | 10 studies reported VAS. In 4 studies, the elbow function was quantified using the Nirschl score. One study used the Victorian Institute of Sports Assessment-Patella (VISA-P). The 2 other studies on patellar tendinopathy used the Tegner score to quantify activity level. One study on plantar fasciopathy used AOFAS | In the 3 included PRP trials, no direct comparison was done between PRP treated and control groups | If PRP injections were to be considered separately, three low-quality studies were included, and so there is limited evidence that these injections improve pain and/or function in chronic tendinopathy |
| De Vos 201439 | Chronic lateral epicondylar tendinopathy | Atlethes, but no further information provided | 6 RCTs | Although all studies examined PRP, the exact method and composition varied between studies | Control groups varied between studies (autologous whole blood, steroids, anesthetics, saline) | VAS, DASH, Liverpool elbow score, Patient Rated Tennis Elbow Evaluation (PRTEE) questionnaire. Outcome success was determined differently in each study | NA due to heterogeneity of the outcome measures and methodological Quality. Three high-quality studies and 2 low-quality studies showed no significant benefit of PRP when compared with a control group. One high-quality study showed a beneficial effect of a PRP injection when compared with corticosteroid injection | There is strong evidence that PRP injections are not efficacious in the management of chronic lateral elbow tendinopathy |
| TENDINOPATHY | ||||||||
| Di Matteo 201441 | Patellar and Achilles tendinopathy | Heterogeneous population. The mean age of patients in the RCT on Achilles tendon was notably higher than the common sport-active population | Twenty-two studies were included and analysed. Two studies on patellar tendinopathy were RCTs, whereas just one RCT was published on Achilles tendon | 1 or 2 PRP injection | Dry needling, External shock waves, saline injection | VISA-A, VISA-P, VAS | NA. Considering patellar tendinopathy, 2 RCTs documented superior results for PRP, that was at least capable of accelerating healing times. In the case of Achilles tendon, despite the encouraging findings reported by case series, the only RCT available showed no significant clinical difference between PRP and saline solution |
“The main finding of this review was the paucity of high-level literature regarding the application of PRP in the management of patellar and Achilles tendinopathy. However, the clinical data currently available, although not univocal, suggest considering PRP as a therapeutic option for recalcitrant patellar and Achilles tendinopathies.” |
| Everhart 201742 | Patellar tendinopathy | In the only RCT with PRP, 46 consecutive athletes with jumper’s knee were selected | Of the 15 included studies, only 2 evaluated PRP (1 RCT and one cohort study) | 1 or 2 PRP injection | Extracorporeal shock wave therapy in the PRP RCT | VISA-P | NA | The authors conclude that initial treatment of patella tendinopathy can consist of eccentric squat-based therapy, shockwave, or PRP as monotherapy or an adjunct to accelerate recovery. Corticosteroid therapy should not be used in the treatment of patellar tendinopathy |
| Liu 201943 | Achilles tendinopathy | No information provided. Mean age ranged from 43 to 51 yrs in PRP and controls | 5 RCTs included in quantitative meta-analysis | PRP was administered in 1 study 4 times at 2 wks intervals, and in 4 studies one time | Saline (4 studies), eccentric loading (1 study) | VISA-A, VAS, tendon tickness | VISA-A: SMD at 6 weeks (4 studies)=0.46 (0.15, 0.77); SMD at 12 weeks (5 studies)=0.20 (−0.36, 0.76); SMD at 24 weeks (5 studies)=0.77 (−0.10, 1.65); SMD at 1 year (2 studies)=0.83 (−0.76, 2.42). VAS ( 2 studies): SMD at 6 weeks=1.35 (−1.04, 3.74); SMD at 12 weeks=1.10 (0.53, 1.68); SMD at 24 weeks=1.48 (−1.59, 4.55). Tendon thickness: 2 studies: SMD=1.51 (0.39, 2.63) |
The efficacy of PRP at short/medium term does not significantly differ from that of the placebo |
| Franceschi 201444 | chronic plantar fasciopathy | Ninety-three patients were male and 163 were female. The mean age of the patients involved in all the studies was 45.43 years | All the 8 included studies were prospective: 3 RCTs, 1 non-randomized controlled trial, 3 cohort studies and 1 case series | Each study used a different device to prepare PRP. All but two studies treated patients with only one injection of PRP. One study used two injections, with a 2-week interval, and one study injected each foot three times. | In the 4 controlled trial: steroids injection (3 studies); dextrose prolotherapy (1 study) | VAS, AOFAS, Foot Functional Index (FFI), FHSQ (Foot Health Status Questionnaire). Plantar fascia bands thickness was evaluated by ultrasound in one article |
1 RCT vs DP: FFI was improved for both groups, from 151.5±37.9 at the baseline to 81.6±55.3 for the PRP group and from 132.5±31.1 to 97.7±52.5 for the DP group. PRP vs steroids (2 RCTs): in one trial a statistically significant difference was recorded for VAS and FHSQ between the PRP and control groups at 6 weeks (2.6±2 vs 6.5±2.6, p=0.001 and 25.1±12.4 vs 49.0±19.1 p=0.001); in another RCT a clinically significant difference of AOFAS in favour of PRP (p=0.001, 95% confidence interval) at 3-, 6-, 12- and 24-month follow-up evaluations was observed | There was no statistically significant difference between PRP and DP at any follow-up. Conversely, in two RCTs PRP had a significantly greater efficacy than steroids at short-term follow-up and after a longer period (24 months). Patients treated with steroids significantly improved at 3 months, but subsequently worsened up to values similar to baseline. Although the current evidence suggests that PRP may be of benefit as an injection therapy to treat. plantar fasciopathy, a greater number of studies are needed to draw better conclusions on the use of PRP in PF. To date, the total amount of patients treated with this therapy is still too limited to properly assess both effectiveness and safety |
| Fizpatrick, 201645 | Various tendinophaties: achillean (3 trials), patellar tendinitis (jumper’s knee, 2 trials); rotator cuff (2 trial); tennis elbow (lateral epicondylitis, 11 trial). Trials including surgery, tendon tears, and muscle or ligament injuries were excluded. |
Heterogeneous conditions and population | 18 RCTs, of which 11 with LR-PRP, 2 with LP-PRP | 9 studies used a single injection, and 4 used 2 injections of LR-PRP. | Injection: corticosteroid, 6 studies; saline, 4 studies. Local anesthetic, 2 studies; dry needling, 4 studies. Non-injections: eccentric training, 1 study; Shock wave treatment, 2 studies |
SPDI; VAS; VISA-A, VISA-P; WORC; DASH | In the network meta-analysis, data were pooled as the change in pain from baseline to each time point. No direct comparison was done between treated and control group | The outcome of PRP is different depending on the method of preparation of PRP and the injection technique. There were 4 different types of PRP preparations and techniques studied. Highly cellular LR-PRP shows strongly positive outcomes in treating tendinopathy when assessed in the network meta-analysis; it was not possible to perform subgroup analyses according to the type of tendinopathy due to the low number of trials |
| TENDINOPATHY | ||||||||
| Dupley, 201748 | Patellar tendinosis | In 1 trial defined as atlethic participants aged 18–50 yrs; the other trial included subjects >18 yrs, but no other information provided | 2 RCTs. | 1 PRP injection (1 trial) or 2 PRP injection 2 weeks apart (1 trial) | Extracorporeal shockwave therapy (1 trial) and dry needling of the tendon (1 trial) | VISA-P at short (2–3 months) and long term (≥6 months). Data were extracted in a standardised manner | Mean difference VISA-P at 2 or 3 months: 11.9; 95% CI, −2.7 to 26.4; VISA-P at ≥6 months 12.7; 95% CI, 4.1 to 21.3; (p=0.004) | No significant difference in “mean VISA-P scores between PRP injection and control at early assessment. However, PRP was statistically better than control with regards to VISA-P scores at longer follow-up. Conclusions: There is a paucity of RCTs evaluating the role of PRP in patellar tendinosis.” Moreover, an assessment of the quality of evidence was not performed |
| Yang 201749 | Plantar fascitis | Age varied from 30 to 55 (mean) across studies; higher prevalence of women than men | 9 RCTs | 1 PRP injection (from 2.5 to 8 ml) | steroid injections. Various steroids were used ± lidocaine | VAS, FADI, AOFAS, and RMS | VAS at 4 wks: WMD=0.56, 95% CI: 1.10 to 2.23, P=.51.VAS at 12 weeks: WMD= −0.49 (95% CIs, −1.42/0.44; p=0.30). VAS at 24 weeks: WMD= −0.95, 95% CI: −1.80 to −0.11, p=0.03. FADI after 12 weeks (2 trials): WMD=14.08, 95% CI:11.57 to 39.73, p=0.28). AOFAS after 12 weeks (3 trials): WMD=0.94, 95% CI: 5.99 to 7.86, p=0.79,). RMS after 6 months (2 trials): RR=1.75, 95% CI: 0.27–11.38, p=0.56). |
Significant differences in the VAS were not observed between the 2 groups after 4 or 12 weeks of treatment However, PRP exhibited better efficacy than the steroid treatment after 24 weeks. No significant differences in the FADI, AOFAS, and RMS were observed. In all the comparisons, there was evidence of substantial heterogeneity (I2>75%) |
| Andriolo, 201950 | Patellar tendinopathy | In one trial defined as atlethic participants aged 18–50 yrs; one trial included subjects >18 yrs, and the remaining male subjects aged (means) 29.5–31.1 | Of the 70 trials included in this review, only 3 were RCTs evaluating PRP vs controls | 1 PRP injection (1 trial) or 2 PRP injection 2 weeks apart (1 trial); 1 trial compared single PRP vs multiple PRP injections | Extracorporeal shockwave therapy (1 trial) and dry needling of the tendon (1 trial) | VISA-P at short (2–3 months) and long term (≥6 months) | Data were pooled as the change in VISA-P score from baseline to each time point (short term < 6 months, long term (>= 6 months). No direct comparison was done between PRP treated and control group The authors provide forest plots with MD of VISA-P for PRP and controls pooling together case series studies, cohort and controlled studies | In one study, the application of 1 or 2 infiltrations of PRP did not reveal any difference. As for reference 48 PRP was statistically better than control with regards to VISA-P scores only at longer follow-up. No firm conclusion can be drawn for the paucity of data and low-level of evidence. Eccentric exercises may seem the strategy of choice in the short-term, for the treatment of patellar tendinopathy |
PRP: platelet rich plasma; LR-PRP: leukocyte-rich PRP; LP-PRP: leukocyte-poor PRP; MD: mean difference; WMD: weighted mean difference; US: ultrasonographic. CI, confidence interval. ACL: anterior cruciate ligamentVAS: pain, visual analogue score. VISA-A: Victorian Institute of Sports Assessment-Achilles; VISA-P: Victorian Institute of Sport Assessment–Patella; SPDI: Shoulder Pain and Disability Index; WORC: Western Ontario Rotator Cuff Index. DASH, Disabilities of the Arm, Shoulder and Hand; MAYO, Modified Mayo performance index; FADI, Foot and Ankle Disability Index); AOFAS: American Orthopedic Foot and Ankle Society scale; RMS: Roles and Maudsley Score.
Methodological quality
Methodological quality was determined by the Joanna Bring critical appraisal checklist for Systematic Reviews and Research Syntheses (see Appendix)36. Table II shows the overall results of the critical appraisal. Critical appraisal of the methodological quality of included reviews varied among studies, but 50% of studies met ≥90% of the criteria, and >80% of studies met at least 75% of the criteria.
Table II.
Joanna Bring Institute (JBI) critical appraisal checklist for Systematic Reviews and Research Syntheses35.
| Study: 1st author, year reference | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Manduca, 201833 | Y | Y | Y | Y | Y | U | U | N | N | Y | Y |
| Moraes, 201328 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Pas, 201529 | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Grassi, 201834 | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | Y |
| Sheth, 201832 | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Chen, 201837 | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y |
| Zhang, 201838 | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | Y |
| Gholami, 201630 | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | Y |
| Taylor, 201131 | Y | U | U | Y | N | U | U | N | N | Y | Y |
| De Vos 201035 | Y | Y | Y | Y | Y | Y | Y | N | N | Y | Y |
| De Vos, 201439 | Y | Y | Y | Y | N | N | U | Y | N | Y | Y |
| Andia, 201440 | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Di Matteo, 201441 | Y | Y | Y | N | N | N | U | N | N | Y | Y |
| Everhart, 201742 | Y | Y | Y | Y | N | U | U | Y | Y | Y | Y |
| Liu, 201943 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Franceschi, 201444 | Y | Y | Y | Y | Y | Y | Y | N | N | Y | Y |
| Fizpatrick, 201645 | Y | Y | Y | Y | Y | Y | Y | N | N | Y | Y |
| Xu, 201946 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Tsikopoulos47 | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Dupley, 201748 | Y | Y | U | Y | N | U | U | Y | N | N | Y |
| Yang 2017,49 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Andriolo, 201950 | Y | Y | Y | N | Y | Y | Y | N | N | Y | Y |
Q1. Is the review question clearly and explicitly stated?
Q2. Were the inclusion criteria appropriate for the review question?
Q3. Was the search strategy appropriate?
Q4. Were the sources of studies adequate?
Q5. Were the criteria for appraising studies appropriate?
Q6. Was critical appraisal conducted by two or more reviewers independently?
Q7. Were there methods to minimise errors in data extraction?
Q8. Were the methods used to combine studies appropriate?
Q9. Was the likelihood of publication bias assessed?
Q10. Were recommendations for policy and/or practice supported by the reported data?
Q11. Were the specific directives for new research appropriate?
Y: yes; N: no; U: unclear.
The most common unmet item was related to the assessment of publication bias (Q9) that was assessed in 10 out of 22 studies (45%) (Table II). Sixteen studies (73%) used tools or scales to assess the methodological quality of included trials (Q5), while 6 studies did not (Table II). Six studies used the Cochrane risk of bias assessment tool60. Other studies used the Physiotherapy Evidence Database (PEDro) scale (n=2 studies), the Coleman methodology score (n=2), the CASP (Critical Appraisal Skills Program) checklist for RCTs (n=1), the Clear-NPT (n=1), and the Down and Black scale (n=1)61–65.
We judged 15 studies (68%) to be adequate in terms of the methods used to combine studies; five studies did not perform a formal assessment of statistical heterogeneity31,33,35,41,44, and three studies42,45,50 did not perform a direct comparison between PRP and control groups. Not all the studies were clear as to the methods used to minimise errors in data extraction (Q6: 77%, and Q7: 54%); the search strategy (Q3) was judged appropriate in 91% of studies. Other items (Q1, Q2, Q4, Q10, Q11) were fulfilled in all or nearly all the studies.
Summary of evidence
Acute muscle injury
In 4 studies, participants were predominantly athletes with acute hamstring injury29,32–34. A Cochrane review evaluated acute or chronic musculoskeletal soft tissue injuries, including arthroscopic rotator cuff repair, shoulder impingement surgery, and different tendinopathies. In this study, participants were predominantly young, active adults, but studies concerning degenerative conditions (e.g., chronic impingement syndrome, rotator cuff tears) included an older population28. There was heterogeneity in terms of PRP preparation and administration protocols, and type of controls. All the studies but one33 reported a quantitative synthesis. The outcomes evaluated included recovery time, return to sports activity, and pain and function scores. One study showed superior activity of rehabilitation exercise compared to PRP injections29. One study showed that PRP may result in an earlier return to sport for acute grade I-II injury, but the difference was no longer significant when the analysis was limited to high-quality studies32. Two studies did not show any benefit of PRP use compared to controls28,34. There was consistency among studies in rating the available evidence as being of low quality and insufficient to support the use of PRP for acute muscle injury.
Tendon and ligament injuries
Seventeen studies evaluating PRP for tendon and ligament injuries were identified. Eight studies30,31,35,37,40,41,45,47 evaluated PRP for the treatment of different types of tendinopathies, including Achilles tendinopathy, lateral epicondylitis, patellar tendinopathy, plantar fasciitis. Two studies38,43 evaluated achillean tendinopathy, 2 studies39,46 lateral epicondylitis, 3 studies42,48,50 patellar tendinopathy, and 2 studies44,49 plantar fasciitis. There was heterogeneity in the included population across studies since individuals with differing levels of physical activity, including the sporting (competitive and recreational) and non-sporting populations, were considered. Different PRP preparation and administration protocols were used, and different control groups (including steroids injection, dry needling, whole blood, saline injection, eccentric loading programme, extracorporeal shock wave therapy) were compared to PRP.
Five studies did not perform a quantitative synthesis of data because of the heterogeneity and low methodological quality of the included trials, and/or low number of available trials/patients31,35,39,41,42. Three studies reported changes in pain and function scores from baseline to each time point (short-, medium-, long-term) in treated and controls, but no direct comparison was made between PRP and comparators42,45,50. In two studies there were no differences in summary outcome measures between PRP and controls30,38.
Eight out of the 17 reviews evaluating PRP for sports-related tendon and ligament injuries show a nominally statistically significant (p<0.05) difference in pain and/or function outcome measures (e.g., VAS, VISA-A, VISA-P, AOFAS) favouring PRP compared to controls37,40,43,44,46–49. These between-group differences were limited to subset analyses of different periods of observation (and not to the whole period of observation) and to subset analyses defined according to the control groups, clinical condition and outcome measures (Table I). Most of the observed differences were small and, even if statistically significant, are unlikely to be of clinical significance. Six studies37,40,44,46,47,49 found a statistically significant decrease in VAS at some points of the short-term evaluation (1–6 months), but in most of these studies the differences were small, ranging from 2 to 9 mm. Two studies37,40 provided long-term (≥12 months) VAS data, showing benefits of PRP treatment over controls; the difference in WMD (−0.84; 95%CI: −1.23/−0.44; and −1.56 (−2.29/−0.83), although statistically significant, can be regarded as clinically marginal.
There was also little evidence from 4 studies43,46–48 of benefit of PRP compared to controls on other outcome measures such as VISA-A, VISA-P, MAYO Clinic Performance Index, DASH, at short- and medium-term follow up but, again, this was of marginal clinical significance despite the statistical significance of the differences (Table II).
As for studies on acute muscle injury, there was consistency among studies in rating the available evidence of low quality (due to heterogeneity, imprecision, and risk of biases), which was, therefore, considered insufficient to support the general use of PRP for tendon and ligament injuries.
Adverse events
In the 22 studies included, no participant was reported to have developed any serious events in the follow-up period in either the PRP or the control groups. Seventeen studies did not mention adverse events at all30–35,38,39,41–45,47–50, while one study stated that adverse events were extracted from primary studies, but did not provide any further information47. Two studies reported only a single statement on the absence of adverse events29,37. One study stated that no complication or adverse events were reported in relation to PRP injections apart from injection-related pain (local pain and discomfort after PRP injection). Only two studies describe monitoring processes for identifying and recording complications28,46. One of these study describes that 4 trials reported adverse events, while another 7 trials reported the absence of adverse events; there was no difference between treatment groups in the numbers of participants with adverse effects (7/241 vs 5/245; RR 1.31, 95%CI: 0.48–3.59; I2=0%; 486 participants). The other study states that a total of 4 RCTs reported the outcome of post-injection adverse events; only one case of superficial infection occurred (RD=0.012; 95%CI: −0.059, 0.035), and no severe adverse event was found.
Quality of life/Patient satisfaction
Only 2 of the 22 studies included describe monitoring processes for identifying and recording quality of life data28,30. In one of these studies, no difference between groups was found for quality of life assessed using the SF-12 (MD −1.60, 95% CI: −5.66 to 2.46). The second study states that quality of life and patient satisfaction were assessed in 3 studies. The results indicated that PRP was not superior to the other comparators in terms of quality of life outcomes. Moreover, there was also inconsistency in the methods used to assess patient satisfaction, and there was no significant difference between groups in this outcome measure.
Appraisal of the quality of evidence
The GRADE assessment was made in five studies28,34,43,46,47. In 2 studies, the quality of the evidence was graded as very low28,34, in 1 study as low46, and in 243,47 as moderate/low depending on the outcome of interest; these judgements were made consistently across the 5 reviews. In the remaining 17 studies, we tried to apply the GRADE approach in its 5 domains (risk of bias, indirectness, imprecision, inconsistency, and publication bias). Although many studies did not report information on all the domains of interest (e.g., publication bias and risk of bias assessment), it was possible to make a judgment of low and/or very low quality of evidence for all these studies. The most common reasons for downgrading were inconsistency (11 studies), imprecision (16 studies), and indirectness (11 studies).
Discussion
Platelet-rich plasma has been used in different non-transfusion indications due to its role in tissue regeneration and healing, including orthopaedics and traumatology, dermatology, ocular surface diseases, dentistry, and other settings. The increase in its use in the field of sports medicine prompted us to undertake an umbrella review on the use of PRP for the treatment of soft tissue injuries, including Achilles tendinopathy, lateral epicondylitis, patellar tendinopathy, plantar fasciitis, rotator cuff tears, and muscle injuries. In this review, which included 22 systematic reviews based on 176 overlapping primary studies, we found low/very low certainty of evidence associated to the use of PRP for sports-related muscle, tendon and ligaments injuries.
There was consistency among the 5 studies evaluating PRP for acute muscle injury in rating the available evidence to be of low quality and insufficient to support its use for this indication. For tendon and ligament injuries, there was little evidence from some studies of benefit of PRP compared to controls on VAS at some points of the period of evaluation (short-, medium- and long-term), but in most of these studies the differences were small, ranging from 2 to 9 mm, and unlikely to be clinically important. The minimum clinically significant difference in VAS pain scores is taken to be 8 mm for average pain and 19 mm for first step pain66,67. Differences of less than this amount, even if statistically significant, can be regarded as clinically marginal.
There was also little evidence from some studies of benefit of PRP compared to controls on other outcomes measures such as VISA-A, VISA-P, MAYO Clinic Performance Index, DASH, at short- and medium-term follow up, but of marginal clinical significance.
In the majority of the included studies, adverse events were not included among the predefined outcomes, and the reporting was incomplete and inadequate. Injecting PRP involves using an individual’s own platelets, and the possibility of systemic adverse reactions to the injections is unlikely; however, it is possible that patients may have pain, bleeding and local infection at the injection site. Most of the included studies did not mention adverse events at all, or reported a single statement of the absence of adverse event. Thus, the risk of reporting bias and imprecision (reflecting the inadequate numbers of participants to detect rare events) from the available evidence should be taken into account.
As for adverse events, quality of life outcomes were rarely reported. Only 2 of the 22 studies describe quality of life data. No differences were found between the PRP and the control groups.
With the rising cost of health care, more attention is being focused on evidence-based medicine to determine the best treatment opportunities for different disease conditions. If the certainty of the evidence is low or very low, we should be concerned about using this evidence alone to inform our clinical decision making. Traditionally, tendinopathy and muscle injuries have been treated with oral and injectable anti-inflammatory (NSAIDs and steroids) medications, physical therapy, eccentric training programmes, extracorporeal shock wave therapy, and other approaches68,69. On the basis of the findings of this umbrella review, considering the low or very low quality of the available evidence, PRP should not be recommended in persons with sport-related injuries.
Conclusions
Implications for clinical practice
In the treatment of acute muscle injuries, PRP does not seem to be superior to usual care. These findings are based on low/very low quality evidence. In the treatment of tendon and ligament injuries, there is little evidence to favour PRP compared to controls. Most of the observed differences were small and, even if statistically significant, are unlikely to be of clinical significance. Moreover, the level of certainty of the evidence was low/very low. Overall, there is currently insufficient evidence to support the use of PRT for treating these injuries.
Implications for research
According to the GRADE recommendations, the level of available evidence reflects a high uncertainty in results. Indeed, future research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. The findings of this review, and the identified limitations of most of the RCTs, should guide the design of future RCTs in this setting. An important preliminary to further PRT clinical research would be the development of a standardised methodology for PRP preparation and schedule of administration. This may need some additional input from basic scientific research.
Short-term (less than three months), medium-term (3–12 months), and long-term assessment (one year or longer) of pain and functional outcome data should be collected, with blind assessment of subjective measurement scale outcomes.
Moreover, a subgroup analysis of the probable effects according to the clinical condition and to the comparator should be implemented. Studies should also include adverse effects, patient satisfaction, and quality of life measures among the predefined outcomes.
Appendix.
Joanna Bring Institute (JBI) critical appraisal checklist36
-
Is the review question clearly and explicitly stated?
The review question is an essential step in the systematic review process. A well articulated question defines the scope of the review and aids in the development of the search strategy to locate the relevant evidence. An explicitly stated question, formulated around its PICO (Population, Intervention, Comparator, Outcome) elements aids both the review team in the conduct of the review and the reader in determining if the review has achieved its objectives. Ideally, the review question should be articulated in a published protocol; however, this will not always be the case with many reviews that are located.
-
Were the inclusion criteria appropriate for the review question?
The inclusion criteria should be identifiable from and match the review question.
The necessary elements of the PICO should be explicit and clearly defined. The inclusion criteria should be detailed and the included reviews should clearly be eligible when matched against the stated inclusion criteria. Appraisers of meta-analyses will find that inclusion criteria may include criteria around the ability to conduct statistical analyses which would not be the norm for a systematic review. The types of included studies should be relevant to the review question, for example, an umbrella review aiming to summarise a range of effective non-pharmacological interventions for aggressive behaviours amongst elderly patients with dementia will limit itself to including systematic reviews and meta-analyses that synthesise quantitative studies assessing the various interventions; qualitative or economic reviews would not be included.
-
Was the search strategy appropriate?
A systematic review should provide evidence of the search strategy that has been used to locate the evidence. This may be found in the methods section of the review report in some cases, or as an appendix that may be provided as supplementary information to the review publication. A systematic review should present a clear search strategy that addresses each of the identifiable PICO components of the review question. Some reviews may also provide a description of the approach to searching and how the terms that were ultimately used were derived, though due to limits on word counts in journals this may be more the norm in online only publications. There should be evidence of logical and relevant keywords and terms, and also evidence that Subject Headings and Indexing terms have been used in the conduct of the search. Limits on the search should also be considered and their potential impact; for example, if a date limit was used, was this appropriate and/or justified? If only English language studies were included, will such a language bias have an impact on the review? The response to these considerations will depend, in part, on the review question.
-
Were the sources of studies adequate?
A systematic review should attempt to identify “all” the available evidence and as such there should be evidence of a comprehensive search strategy. Multiple electronic databases should be searched including major bibliographic citation databases such as MEDLINE and CINAHL. Ideally, other databases that are relevant to the review question should also be searched, for example, a systematic review with a question about a physical therapy intervention should also look to search the PEDro database, whilst a review focussing on an educational intervention should also search the ERIC. Reviews of effectiveness should aim to search trial registries. A comprehensive search is the ideal way to minimise publication bias. As a result, a well conducted systematic review should also attempt to search for grey literature, or “unpublished” studies; this may involve searching websites relevant to the review question, or thesis repositories.
-
Were the criteria for appraising studies appropriate?
The systematic review should present a clear statement that critical appraisal was conducted and provide the details of the items that were used to assess the included studies. This may be presented in the methods of the review, as an appendix of supplementary information, or as a reference to a source that can be located. The tools or instruments used should be appropriate for the review question asked and the type of research conducted. For example, a systematic review of effectiveness should present a tool or instrument that addresses aspects of validity for experimental studies and randomised controlled trials such as randomisation and blinding – if the review includes observational research to answer the same question a different tool would be more appropriate. Similarly, a review assessing diagnostic test accuracy may refer to the recognised QUADAS tool.
-
Was critical appraisal conducted by two or more reviewers independently?
Critical appraisal or some similar assessment of the quality of the literature included in a systematic review is essential. A key characteristic to minimise bias or systematic error in the conduct of a systematic review is to have the critical appraisal of the included studies completed independently and in duplicate by members of the review team. The systematic review should present a clear statement that critical appraisal was conducted by at least two reviewers working independently from each other and conferring where necessary to reach a decision regarding study quality and eligibility on the basis of quality.
-
Were there methods to minimise errors in data extraction?
Efforts made by review authors during data extraction can also minimise bias or systematic errors in the conduct of a systematic review. Strategies to minimise bias may include conducting all data extraction in duplicate and independently, using specific tools or instruments to guide data extraction, and some evidence of piloting or training around their use.
-
Were the methods used to combine studies appropriate?
A synthesis of the evidence is a key feature of a systematic review. The synthesis that is presented should be appropriate for the review question and the stated type of systematic review and the evidence it refers to. If a meta-analysis has been conducted, this needs to be reviewed carefully. Was it appropriate to combine the studies? Have the reviewers assessed heterogeneity statistically and provided some explanation for heterogeneity that may be present? Often, where heterogeneous studies are included in the systematic review, narrative synthesis will be an appropriate method for presenting the results of multiple studies. If a qualitative review, are the methods that have been used to synthesise findings congruent with the stated methodology of the review? Is there adequate descriptive and explanatory information to support the final synthesised findings that have been constructed from the findings sourced from the original research?
-
Was the likelihood of publication bias assessed?
As mentioned, a comprehensive search strategy is the best means by which a review author may alleviate the impact of publication bias on the results of the review. Reviews may also present statistical tests such as Egger’s test or funnel plots to also assess the potential presence of publication bias and its potential impact on the results of the review.
-
Were recommendations for policy and/or practice supported by the reported data?
Whilst the first nine questions specifically look to identify potential bias in the conduct of a systematic review, the final questions are more indictors of review quality rather than validity. Ideally, a review should present recommendations for policy and practice. Where these recommendations are made, there should be a clear link to the results of the review. Is there evidence that the strength of the findings and the quality of the research been considered in the formulation of review recommendations?
-
Were the specific directives for new research appropriate?
The systematic review process is recognised for its ability to identify where gaps in the research, or knowledge base, around a particular topic exist. Most systematic review authors will provide some indication, often in the discussion section of the report, of where future research direction should lie. Where evidence is scarce or sample sizes that support overall estimates of effect are small and effect estimates are imprecise, repeating similar research to those identified by the review may be called for and appropriate. In other instances, the case for new research questions to investigate the topic may be warranted.
Footnotes
Disclosure of conflicts of interest
Giancarlo M. Liumbruno is the Editor-in-Chief of Blood Transfusion. Given this, this manuscript was subjected to an additional external review. The other Authors declare no conflicts of interest.
References
- 1.Franchini M, Muñoz M. Towards the implementation of patient blood management across Europe. Blood Transfus. 2017;15:292–3. doi: 10.2450/2017.0078-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerra R, Velati C, Liumbruno GM, Grazzini G. Patient blood management in Italy. Blood Transfus. 2016;14:1–2. doi: 10.2450/2015.0171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaglio S, Prisco D, Biancofiore G, et al. Recommendations for the implementation of a patient blood management programme. Application to elective major orthopaedic surgery in adults. Blood Transfus. 2016;14:23–65. doi: 10.2450/2015.0172-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaglio S, Gentili S, Marano G, et al. The Italian regulatory guidelines for the implementation of patient blood management. Blood Transfus. 2017;15:325–8. doi: 10.2450/2017.0060-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muñoz M, Franchini M, Liumbruno GM. The post-operative management of anaemia: more efforts are needed. Blood Transfus. 2018;16:324–5. doi: 10.2450/2018.0036-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velati C, Romanò L, Piccinini V, et al. Prevalence, incidence and residual risk of transfusion-transmitted hepatitis C virus and human immunodeficiency virus after the implementation of nucleic acid testing in Italy: a 7-year (2009–2015) survey. Blood Transfus. 2018;16:422–32. doi: 10.2450/2018.0069-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riva L, Petrini C. Blood safety policy: should cautionary policies be adopted with caution? Blood Transfus. 2018;16:405–7. doi: 10.2450/2018.0135-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Der Linde R, Favaloro EJ. Tranexamic acid to prevent post-partum haemorrhage. Blood Transfus. 2018;16:321–3. doi: 10.2450/2018.0067-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchini M, Mengoli C, Cruciani M, et al. Safety and efficacy of tranexamic acid for prevention of obstetric haemorrhage: an updated systematic review and meta-analysis. Blood Transfus. 2018;16:329–37. doi: 10.2450/2018.0026-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franchini M, Liumbruno GM. The key role of tranexamic acid in Patient Blood Management programmes. Blood Transfus. 2018;16:471–2. doi: 10.2450/2018.0177-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchini M, Mengoli C, Marietta M, et al. Safety of intravenous tranexamic acid in patients undergoing major orthopaedic surgery: a meta-analysis of randomised controlled trials. Blood Transfus. 2018;16:36–43. doi: 10.2450//2017.0219-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hourlier H, Fennema P. Tranexamic acid use and risk of thrombosis in regular users of antithrombotics undergoing primary total knee arthroplasty: a prospective cohort study. Blood Transfus. 2018;16:44–52. doi: 10.2450/2016.0160-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Jimeno N, Munoz M, Mateo J, et al. Efficacy of topical tranexamic acid within a blood saving program for primary total hip arthroplasty: a pragmatic, open-label randomised study. Blood Transfus. 2018;16:490–7. doi: 10.2450/2018.0133-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laso-Morales MJ, Gomez-Ramirez S, Pallisera-Lloveras A, Pontes C. Intravenous iron administration for postoperative anemia management after colorectal cancer surgery in clinical practice: a single centre, retrospective study. Blood Transfus. 2018;16:338–42. doi: 10.2450/2018.0004-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basora M, Pereira A, Coca M, Tió M, et al. Cost-effectiveness analysis of ferric carboxymaltose in pre-operative haemoglobin optimisation in patients undergoing primary knee arthroplasty. Blood Transfus. 2018;16:438–42. doi: 10.2450/2018.0031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franchini M, Marano G, Veropalumbo E, et al. Patient Blood Management: a revolutionary approach to transfusion medicine. Blood Transfus. 2019;17:191–5. doi: 10.2450/2019.0109-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franchini M, Liumbruno GM. Implementation of a patient blood management programme in obstetrics: let’s do it! Blood Transfus. 2019;17:87–8. doi: 10.2450/2019.0269-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kleinerüschkamp A, Meybohm P, Straub N, et al. A model-based cost-effectiveness analysis of Patient Blood Management. Blood Transfus. 2019;17:16–26. doi: 10.2450/2018.0213-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muñoz M, Stensballe J, Ducloy-Bouthors AS, et al. Patient Blood Management in obstetrics: prevention and treatment of postpartum haemorrhage. A NATA consensus statement. Blood Transfus. 2019;17:112–36. doi: 10.2450/2019.0245-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piccin A, Di Pierro AM, Canzian L, et al. Platelet gel: a new therapeutic tool with great potential. Blood Transfus. 2017;15:333–40. doi: 10.2450/2016.0038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–96. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Martinez CE, Smith PC, Palma Alvarado VA. The influence of platelet-derived products on angiogenesis and tissue repair: a concise update. Fron Physiol. 2015;6:290. doi: 10.3389/fphys.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pachito DV, Latorraca COC, Riera R. Efficacy of platelet-rich plasma for non-transfusion use: Overview of systematic reviews. Int J Clin Pract. 2019:e13402. doi: 10.1111/ijcp.13402. [DOI] [PubMed] [Google Scholar]
- 24.Foster TE, Puskas BL, Mandelbaum BR, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–72. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 25.Franchini M, Cruciani M, Mengoli C, et al. Efficacy of platelet-rich plasma as conservative treatment in orthopaedics: a systematic review and meta-analysis. Blood Transfus. 2018;16:502–13. doi: 10.2450/2018.0111-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franchini M, Cruciani M, Mengoli C, et al. The use of platelet-rich plasma in oral surgery: a systematic review and meta-analysis. Blood Transfus. 2019;17:357–67. doi: 10.2450/2019.0177-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beitzel K, Allen D, Apostolakos J, et al. US definitions, current use, and FDA stance on use of platelet-rich plasma in sports medicine. J Knee Surg. 2015;28:29–34. doi: 10.1055/s-0034-1390030. [DOI] [PubMed] [Google Scholar]
- 28.Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, et al. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2014;4:CD010071. doi: 10.1002/14651858.CD010071.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pas HI, Reurink G, Tol JL, et al. Efficacy of rehabilitation (lengthening) exercises, platelet-rich plasma injections, and other conservative interventions in acute hamstring injuries: an updated systematic review and meta-analysis. Br J Sports Med. 2015;49:1197–205. doi: 10.1136/bjsports-2015-094879. [DOI] [PubMed] [Google Scholar]
- 30.Gholami M, Ravaghi H, Salehi M, et al. A systematic review and meta-analysis of the application of platelet rich plasma in sports medicine. Electron Physician. 2016;8:2325–32. doi: 10.19082/2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21:344–52. doi: 10.1097/JSM.0b013e31821d0f65. [DOI] [PubMed] [Google Scholar]
- 32.Sheth U, Dwyer T, Smith I, et al. Does platelet-rich plasma lead to earlier return to sport when compared with conservative treatment in acute muscle injuries? A Systematic Review and Meta-analysis. Arthroscopy. 2018;34:281–8. doi: 10.1016/j.arthro.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 33.Manduca ML, Straub SJ. Effectiveness of PRP injection in reducing recovery time of acute hamstring injury: a critically appraised topic. J Sport Rehabil. 2018;27:480–4. doi: 10.1123/jsr.2016-0066. [DOI] [PubMed] [Google Scholar]
- 34.Grassi A, Napoli F, Romandini I, et al. Is platelet-rich plasma (PRP) effective in the treatment of acute muscle injuries? A systematic review and meta-analysis. Sports Med. 2018;48:971–89. doi: 10.1007/s40279-018-0860-1. [DOI] [PubMed] [Google Scholar]
- 35.de Vos RJ, van Veldhoven PL, Moen MH, et al. Autologous growth factor injections in chronic tendinopathy: a systematic review. Br Med Bull. 2010;95:63–77. doi: 10.1093/bmb/ldq006. [DOI] [PubMed] [Google Scholar]
- 36.Aromataris E, Fernandez R, Godfrey CM, et al. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13:132–4. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Jones IA, Park C, Vangsness CT., Jr The efficacy of platelet-rich plasma on tendon and ligament healing: a systematic review and meta-analysis with bias assessment. Am J Sports Med. 2018;46:2020–32. doi: 10.1177/0363546517743746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang YJ, Xu SZ, Gu PC, et al. Is platelet-rich plasma injection effective for chronic achilles tendinopathy? A meta-analysis. Clin Orthop Relat Res. 2018;476:1633–41. doi: 10.1007/s11999.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Vos RJ, Windt J, Weir A. Strong evidence against platelet-rich plasma injections for chronic lateral epicondylar tendinopathy: a systematic review. Br J Sports Med. 2014;48:952–6. doi: 10.1136/bjsports-2013-093281. [DOI] [PubMed] [Google Scholar]
- 40.Andia I, Latorre PM, Gomez MC, et al. Platelet-rich plasma in the conservative treatment of painful tendinopathy: a systematic review and meta-analysis of controlled studies. Br Med Bull. 2014;110:99–115. doi: 10.1093/bmb/ldu007. [DOI] [PubMed] [Google Scholar]
- 41.Di Matteo B, Filardo G, Kon E, Marcacci M. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy--a systematic review. Musculoskelet Surg. 2015;99:1–9. doi: 10.1007/s12306-014-0340-1. [DOI] [PubMed] [Google Scholar]
- 42.Everhart JS, Cole D, Sojka JH, et al. Treatment options for patellar tendinopathy: a systematic review. Arthroscopy. 2017;33:861–72. doi: 10.1016/j.arthro.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Liu CJ, Yu KL, Bai JB, et al. Platelet-rich plasma injection for the treatment of chronic Achilles tendinopathy: A meta-analysis. Medicine (Baltimore) 2019;98:e15278. doi: 10.1097/MD.0000000000015278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franceschi F, Papalia R, Franceschetti E, et al. Platelet-rich plasma injections for chronic plantar fasciopathy: a systematic review. Br Med Bull. 2014;112:83–95. doi: 10.1093/bmb/ldu025. [DOI] [PubMed] [Google Scholar]
- 45.Fitzpatrick J, Bulsara M, Zheng MH. The Effectiveness of platelet-rich plasma in the treatment of tendinopathy: a meta-analysis of randomized controlled clinical trials. Am J Sports Med. 2016;45:226–33. doi: 10.1177/0363546516643716. [DOI] [PubMed] [Google Scholar]
- 46.Xu Q, Chen J, Cheng L. Xu Comparison of platelet rich plasma and corticosteroids in the management of lateral epicondylitis: A meta-analysis of randomized controlled trials. Int J Surg. 2019;67:37–46. doi: 10.1016/j.ijsu.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Tsikopoulos K, Tsikopoulos A, Natsis K. Autologous whole blood or corticosteroid injections for the treatment of epicondylopathy and plantar fasciopathy? A systematic review and meta-analysis of randomized controlled trials. Phys Ther Sport. 2016;22:114–22. doi: 10.1016/j.ptsp.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Dupley L, Charalambous CP. Platelet-rich plasma injections as a treatment for refractory patellar tendinosis: a meta-analysis of randomised trials. Knee Surg Relat Res. 2017;29:165–71. doi: 10.5792/ksrr.16.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang WY, Han YH, Cao XW, et al. Platelet-rich plasma as a treatment for plantar fasciitis: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 2017;96:e8475. doi: 10.1097/MD.0000000000008475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andriolo L, Altamura SA, Reale D, et al. Nonsurgical treatments of patellar tendinopathy: multiple injections of platelet-rich plasma are a suitable option: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1001–18. doi: 10.1177/0363546518759674. [DOI] [PubMed] [Google Scholar]
- 51.Reurink G, Goudswaard GJ, Tol JL, et al. Therapeutic interventions for acute hamstring injuries: a systematic review. Br J Sports Med. 2012;46:103–9. doi: 10.1136/bjsports-2011-090447. [DOI] [PubMed] [Google Scholar]
- 52.Laudy AB, Bakker EW, Rekers M, Moen MH. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657–72. doi: 10.1136/bjsports-2014-094036. [DOI] [PubMed] [Google Scholar]
- 53.Dold AP, Zywiel MG, Taylor DW, et al. Platelet-rich plasma in the management of articular cartilage pathology: a systematic review. Clin J Sport Med. 2014;24:31–43. doi: 10.1097/01.jsm.0000432855.85143.e5. [DOI] [PubMed] [Google Scholar]
- 54.Hurley ET, Lim Fat D, Moran CJ, Mullett H. The efficacy of platelet-rich plasma and platelet-rich fibrin in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Am J Sports Med. 2019;47:753–61. doi: 10.1177/0363546517751397. [DOI] [PubMed] [Google Scholar]
- 55.Campbell KA, Saltzman BM, Mascarenhas R, et al. Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:2213–21. doi: 10.1016/j.arthro.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 56.Saltzman BM, Jain A, Campbell KA, et al. Does the use of platelet-rich plasma at the time of surgery improve clinical outcomes in arthroscopic rotator cuff repair when compared with control cohorts? A systematic review of meta-analyses. Arthroscopy. 2016;32:906–18. doi: 10.1016/j.arthro.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Houck DA, Kraeutler MJ, Thornton LB, et al. Treatment of lateral epicondylitis with autologous blood, platelet-rich plasma, or corticosteroid injections: a systematic review of overlapping meta-analyses. Orthop J Sports Med. 2019;7 doi: 10.1177/2325967119831052. 2325967119831052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krogh TP, Bartels EM, Ellingsen T, et al. Comparative effectiveness of injection therapies in lateral epicondylitis: a systematic review and network meta-analysis of randomized controlled trials. Am J Sports Med. 41:1435–46. doi: 10.1177/0363546512458237. 201. [DOI] [PubMed] [Google Scholar]
- 59.Kunze KN, Hannon CP, Fialkoff JD, et al. Platelet-rich plasma for muscle injuries: A systematic review of the basic science literature. World J Orthop. 2019;10:278–91. doi: 10.5312/wjo.v10.i7.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55:129–33. doi: 10.1016/s0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 62.Tallon C, Coleman BD, Khan KM, et al. Outcome of surgery for chronic Achilles tendinopathy. A critical review. Am J Sports Med. 2001;29:315–20. doi: 10.1177/03635465010290031101. [DOI] [PubMed] [Google Scholar]
- 63.Critical Appraisal Skill Programme - CASP [internet] CASP Checklist: 11 questions to help you make sense of a randomised controlled trial. [Accessed on 22/10/2019]. Available at: https://casp-uk.net/wp-content/uploads/2018/01/CASP-Randomised-Controlled-Trial-Checklist-2018.pdf.
- 64.Boutron I, Moher D, Tugwell P, et al. A checklist to evaluate a report of a nonpharmacological trial (CLEAR NPT) was developed using consensus. J Clin Epidemiol. 2005;58:1233–40. doi: 10.1016/j.jclinepi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 65.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelly AM. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Acad Emerg Med. 1998;5:1086–90. doi: 10.1111/j.1553-2712.1998.tb02667.x. [DOI] [PubMed] [Google Scholar]
- 67.Landorf KB, Radford JA, Hudson S. Minimal important difference (MID) of two commonly used outcome measures for foot problems. J Foot Ankle Res. 2010;3:7. doi: 10.1186/1757-1146-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andres BM, Murrell GAC. Treatment of Tendinopathy: What Works, What Does Not, and What is on the Horizon. Clin Orthop Relat Res. 2008;466:1539–54. doi: 10.1007/s11999-008-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baoge L, Van Den Steen E, Rimbaut S, et al. Treatment of skeletal muscle injury: a review. ISRN Orthop. 2012;2012 doi: 10.5402/2012/689012. 689012. [DOI] [PMC free article] [PubMed] [Google Scholar]