Abstract
This study presents a rare sesarmid cavernicolous crab, Karstama boholano (Ng, 2002), from Taiwan. This genus and species are both new to Taiwan. We describe the diagnostic characteristics of the Taiwanese specimen and provide illustrations of the adult and first zoea, as well as photographs of an adult in its natural habitat. The identity was confirmed by the COI gene sequence and morphological data. In addition, the zoeal morphology and breeding ecology of the genus Karstama Davie and Ng, 2007 are reported for the first time.
Keywords: Cavernicolous Crab, Morphology, Taxonomy, Karstama, Zoea
BACKGROUND
The members of the genus Karstama Davie and Ng, 2007 are known as cavernicolous crabs (Davie and Ng 2007), and are typically found in anchialine cave environments (Ng 2002; Naruse et al. 2005; Davie and Ng 2007; Naruse and Ng 2007; Ng et al. 2008; Fujita and Naruse 2016). This taxon has been reported from Java and Ambon Islands (Indonesia), Solomon Islands, New Britain (Papua New Guinea), Christmas Island, the Philippines, Okinawa Islands (Japan), and Guam (Ng 2002; Naruse et al. 2005; Davie and Ng 2007; Naruse and Ng 2007; Ng et al. 2008; Wowor and Ng 2009; Fujita and Naruse 2016). To date, no study on K. boholano (Ng, 2002) from Taiwan has been published (Ng et al. 2017). In addition, larval morphology is known for 59 species in 21 genera of sesarmid crabs worldwide: Aratus H. Milne Edwards, 1853, Armases Abele, 1992, Bresedium Serène and Soh, 1970, Chiromantes Gistel, 1848, Clistocoeloma Milne-Edwards, 1873, Episesarma De Man, 1895, Geosesarma De Man, 1892, Labuanium Serène and Soh, 1970, Metasesarma H. Milne Edwards, 1853, Metopaulias Rathbun, 1896, Muradium Serène and Soh, 1970, Nanosesarma Tweedie, 1950, Neosarmarium Serène and Soh, 1970, Parasesarma De Man, 1895, Pseudosesarma Serène and Soh, 1970, Sarmatium Dana, 1851, Scandarma Schubart, Liu and Cuesta, 2003, Selatium Serène and Soh, 1970, Sesarma Say, 1817, Sesarmops Serène and Soh, 1970, and Stelgistra Ng and Liu, 1999 (Guerao et al. 2004; Cuesta et al. 2006; Guerao et al. 2011 2012; Rebolledo et al. 2015). However, no larval morphology has been described for members of Karstama.
Since 2014, the first author has found three female Karstama individuals of an unknown species in Taiwan (one each in 2014, 2017 and 2018), two of which were ovigerous; the first zoea was also collected. However, no male has been observed, making it difficult to identify the species by morphology. We used the COI gene to identify these individuals as K. boholano and classified the species as cavernicolous. In addition, the present paper describes the zoeal morphology of this species, making it the first study to provide information on larva and breeding ecology for the genus.
MATERIALS AND METHODS
Three female crabs of an unknown species were observed in total. Two ovigerous crabs were collected from Green Island (eastern Taiwan)—one in 2014 and the other in 2017—and one female crab was collected from Kenting National Park (KTNP, southern Taiwan) in 2018. Both ovigerous crabs released larvae. The first stage zoea larvae of the second ovigerous crab were hatched in plastic containers containing sea water and preserved directly in 70% ethanol for one day. The specimens were deposited in National Taiwan Ocean University (NTOU) in Keelung, Taiwan; examined specimens were deposited for comparative analysis in National Taiwan Museum (TMCD) in Taipei, Taiwan and the Zoological Reference Collection of the Lee Kong Chian Natural History Museum of the National University of Singapore, Singapore (ZRC). Classification followed that of Ng et al. (2008). Carapace length and width were measured in millimeters.
Ten larvae from the first stage were dissected and examined on glass slides under a stereo microscope (Olympus SZX12) using fine entomological needles. Appendages were drawn using a camera lucida installed on a compound microscope (Olympus BX50). Larval descriptions and setal counts were obtained following the method proposed by Clark et al. (1998), and the Sesarmidae larvae were described using the setal terminology from Cuesta et al. (2006).
The following measurements were made: rostro-dorsal length (rdl.), from the tip of the rostral spine to the tip of the dorsal spine; carapace length (cl.), from the postorbital margin to the posteromedian end of the carapace; and carapace width (cw.), the greatest distance across the carapace. Descriptions and figures were arranged according to the standard proposed by Clark et al. (1998). The plumose natatory setae of maxilliped exopods and the terminal part of the furcal arms of the telson were drawn truncated. Parental vouchers and samples of the larvae were deposited into NTOU in Keelung, Taiwan.
DNA extraction and PCR amplification
For the phylogenetic analysis, DNA was extracted from specimens of Sesarmoides kraussi (De Man, 1887) and K. boholano. DNA samples were prepared from the walking leg muscle or eggs. Total genomic DNA was extracted using the PUREENE D-7000A DNA isolation kit (QBIOgene, Carlsbad, CA) according to the manufacturer’s instructions. Total DNA was stored at -20°C in a refrigerator until the next step. The following COI primers were used: LCO-1490; 5’-GGTCAACAAATCATAAAGATATTGG-3’ and HCO-2198; 5’-TAAACTTCAGGGTGACCAAAAA TCA-3’ (Folmer et al. 1994). Each reaction mixture was 25 μL: 1 μL DNA template, 12.5 μL Master Mix buffer (2.5 unit/25 μL), 0.5 μL of each forward and reverse primer (1 μL, 0.4 μM), and 10.5 μL double-distilled water. Thermal cycling for PCR was an initial denaturing temperature of 94°C for 2 min, followed by 40 cycles at 94°C for 35 s, 50°C–55°C for 35 s, and 72°C for 60 s, and a final extension step at 72°C for 10 min. Electrophoresis was performed using 5 μL of each PCR product on a 1% agarose gel to confirm that the PCR worked correctly. The gel was stained with 0.5 μg/mL ethidium bromide and imaged under an ultraviolet transillumination system. All positive PCR amplicons were cut from the gel, purified, and sequenced by Mission Biotech Ltd.
Molecular analyses
Eleven COI sequences were obtained (refer to the GenBank accession numbers in Table 1). Sequences from individual specimens were aligned using the CLUSTAL-W program in BioEdit version 7.2.5 (Hall 2013). After the alignment, 569 bp sequences of COI were obtained. Pairwise divergence distances were analyzed using Kimura 2-parameter (K2P) (Kimura 1980) and MEGA X software (Kumar et al. 2018).
Table 1.
List of species, their catalogue numbers, sampling locality and GenBank accession numbers for gene sequences used in this study
| No. | Species | Catalogue numbers | Sampling locality | GenBank numbers of 16S rDNA |
| 1 | Sesarmoides kruasii | NMNS 0 | Ryukyu Island, Japan | MK432842 |
| 2 | Karstama boholano | NMNS 1 | Green Island, Taiwan | MK432843 |
| 3 | Karstama boholano | ZRC2012.0433-1 | Bohol, Philippines | MK432844 |
| 4 | Karstama boholano | NMNS 2 | Green Island, Taiwan | MK432845 |
| 5 | Karstama boholano | Paratype TMCD | Bohol, Philippines | MK432846 |
| 6 | Karstama boholano | ZRC2012.0433-2 | Bohol, Philippines | MK432847 |
RESULTS
TAXONOMY
Family Sesarmidae
Genus Karstama Davie and Ng, 2007
Karstama boholano (Ng, 2002)
Sesarmoides boholano Ng 2002: 428, figs. 11, 12, 15A, B, 16B, 17b; Naruse et al. 2005: 80.
Karstama boholano Ng et al. 2008: 221; Fujita and Naruse 2016: 23.
Material examined: 1 female (19.3 × 15.6) (DNA voucher, NTOU 1), eastern Green Island, 17 September 2017, C.-Y. Lu; 1 female (20.3 × 16.3) (DNA voucher, NTOU 2), western Green Island, 18 September 2014, J. J. Li; 1 female (19.2 × 15.2) (NTOU 3), Siaobalidao, KTNP, 20 August 2018, J.-J. Li.
Additional material: Karstama boholano (Ng, 2002). — Paratypes: 2 males (14.0 × 12.0, 11.3 by 9.8 mm), 2 females (10.5 by 8.8 mm, 10.4 by 9.2 mm) (largest female as DNA voucher, TMCD), Panglao Island, Bohol, Philippines, coll. H.-C. Liu, 26 Nov. 2001; 1 female (18.1 × 14.9) (DNA voucher, ZRC2012.0433-1), 1 male (17.2 × 14.6) (DNA voucher, ZRC2012.0433-2) Philippines: Bohol, Central Panglao, Panglao Nature Resort grounds, Karst Forest, inside cave, Dec. 2010, P. K. L. Ng and P. Y. C. Ng.
Comparative material: Sesarmoides kraussi (De Man, 1887). — (24.1 × 18.5) (DNA voucher, NTOU 0), Ryukyu Island, Japan, 18 July 2016, J. J. Li.
Diagnosis: Detailed diagnoses of adults were provided by Ng (2002) (specimens from Philippines), Naruse et al. (2005), and Fujita and Naruse (2016) (specimens from Japan). The females from Taiwan generally corresponded to the above description.
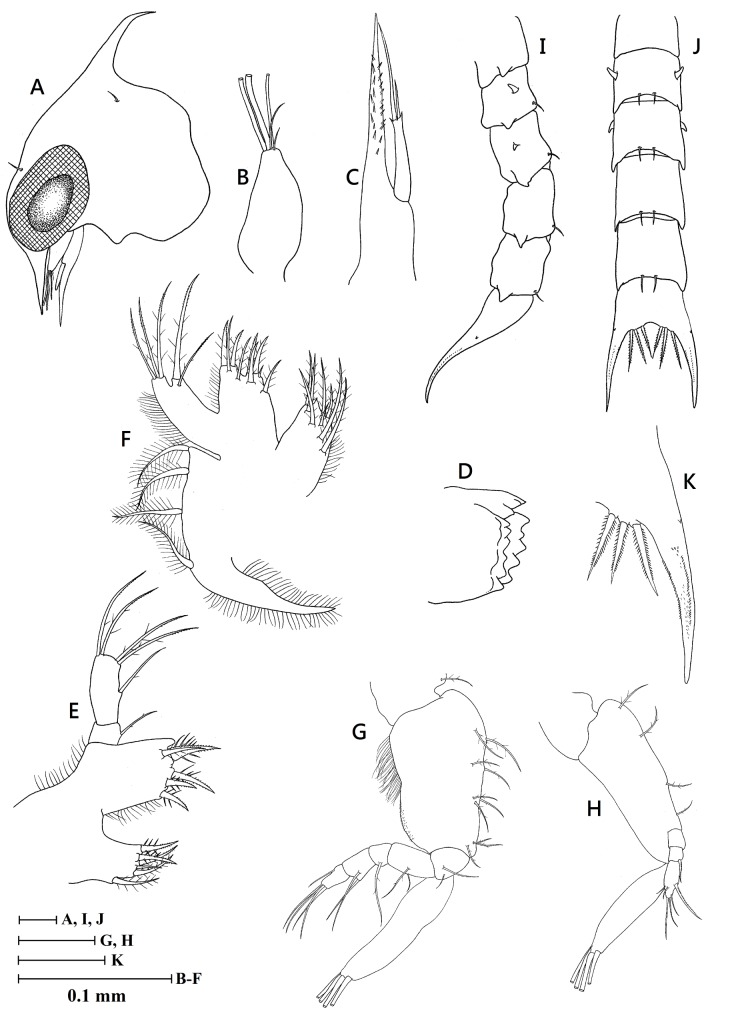
Description of the first zoeal stage: Size: CL, mean 0.35 mm (range 0.30-0.40 mm; n = 10)
Fig. 1.
Zoea I of Karstama boholano (Ng, 2002) from western Green Island, Taitung (NTOU 1), new record genus/species in Taiwan. A, lateral view of carapace; B, antennule; C, antenna; D, mandible; E, maxillule; F, maxilla; G, first maxilliped; H, second maxilliped; I, lateral view of abdomen; J, dorsal view of abdomen; K, telson.
Carapace (Fig. 1A): Lateral spine absent; dorsal spine present, without tubercles, relatively short and curved; rostral spine present, without tubercles, approximately same length as dorsal spine; rostral spine shorter than antennal protopod, without distal spinulation; 1 pair of anterodorsal and posterodorsal setae; ventral margin smooth and without setae; eyes sessile.
Antennule (Fig. 1B): Uniramous, endopod absent; exopod unsegmented with 3 (2 broad and 1 slender) terminal asethetascs and 2 terminal setae.
Antenna (Fig. 1C): Protopod longer than antennule and bearing two rows of small lateral spines; endopod absent; exopod elongated, approximately 1/3 of protopod length and with 1 long, 1 short terminal setae and 3 small terminal spines.
Mandible (Fig. 1D): Endopod palp absent.
Maxillule (Fig. 1E): Epipod seta absent; coxal endite with 6 plumose setae; basial endite with 5 setal processes; endopod 2-segmented, proximal segment with 1 seta, distal segment with 1 subterminal seta and 4 terminal setae; exopod seta absent.
Maxilla (Fig. 1F): Coxal endite bilobed, proximal lobe with 5 setae, distal lobe with 3 setae + 1 rudimentary seta; basial endite bilobed with 5 (1 short simple seta + 4 plumose setae) + 4 plumose setae; endopod with 2 + 3 terminal setae; scaphognathite margin with 4 setae and 1 long stout plumose distal process.
First maxilliped (Fig. 1G): Coxa with 1 seta; basis with 10 setae arranged as 2, 2, 3, 3; endopod 5-segmented with 2, 2, 1, 2, 5 (1 subterminal + 4 terminal) setae, and with a cluster long fine setae on the outer side; exopod unsegmented, distal segment with 4 long terminal plumose natatory setae.
Second maxilliped (Fig. 1H): Coxa without setae; basis with 4 setae arranged as 1, 1, 1, 1; endopod 3-segmented with 0, 1, 6 (3 subterminal + 3 terminal) setae; exopod unsegmented, distal segment with 4 long terminal plumose natatory setae.
Third maxilliped: Absent.
Pereiopods: Absent.
Abdomen (Fig. 1I, J): With 5 somites; somites 2-3 with pair of dorsolateral processes; somites 1-2 with short posterolateral spinous processes, somites 3-5 with subacute posterolateral spinous processes; somites 2-5 with 1 pair of posterodorsal setae; pleopods absent.
Telson (Fig. 1I-K): Telson bifid, curved upward distally; with 1 pair of minute lateral spines; generally spinulated on each furca; posterior margin with 3 pairs of stout spinulate spines.
Distribution: Karstama boholano has been reported from both northern and southern Taiwan; and Panglao Island, Bohol, Philippines (type locality); Ishigaki Island and Tarama-jima Island, Ryukyu Islands; and southwestern Japan (Ng 2002; Naruse et al. 2005; Fujita and Naruse 2016). As such, the discovery of this species in Taiwan was predicted and is finally confirmed here.
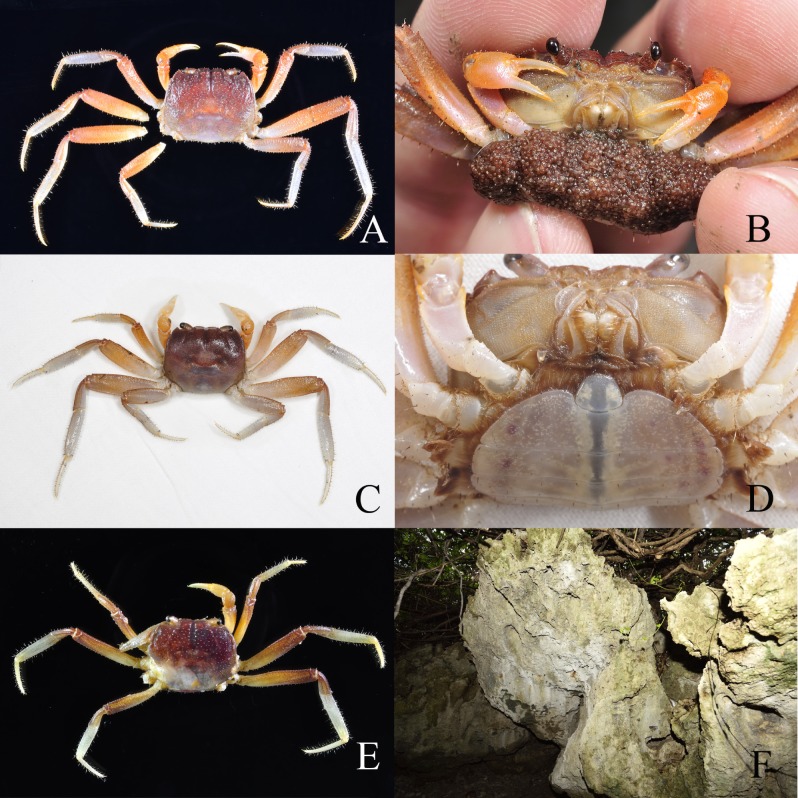
Ecological notes: Two ovigerous female specimens of Karstama boholano were collected together with ovigerous Metasesarma aubryi (A. Milne Edwards, 1869) during their breeding migration. Metasesarma aubryi is a common crab species from Taiwan and adjacent areas, ovigerous females of the species usually release their larvae during the last quarter of the lunar month from July to September (unpublished data). The first female K. boholano (Fig. 2A, B) was found at approximately 03:30 on September 18, 2014, walking under a streetlight. The first author immediately took the crab to the intertidal zone and allowed it to release its free-swimming zoeae into the sea (the zoeae were not collected). The second female (Fig. 2C, D) was found at 20:00 on September 17, 2017. A Green Island resident collected the ovigerous female in a container filled with seawater, and the free-living zoeae were released between 03:00 and 05:00. We believe that the larval release time and rhythm follow a lunar rhythm, peaking in September. A third female (Fig. 2E) was collected from the corrosion cleft on the limestone located in the coastal forest of KTNP, which the literature (Ng 2002; Naruse et al. 2005; Fujita and Naruse 2016) suggests is not a typical cavernicolous environment (Fig. 2F).
Fig. 2.
Karstama boholano (Ng, 2002), associated with the habitat in Taiwan, females. A, B, western Green Island, Taitung (NTOU 1); C, D, eastern Green Island, Taitung (NTOU 2); E, Kenting National Park, Pingtung (NTOU 3); F, natural habitat. A, C, E, dorsal view; B, frontal view, laying eggs before larvae released; D, ventral view.
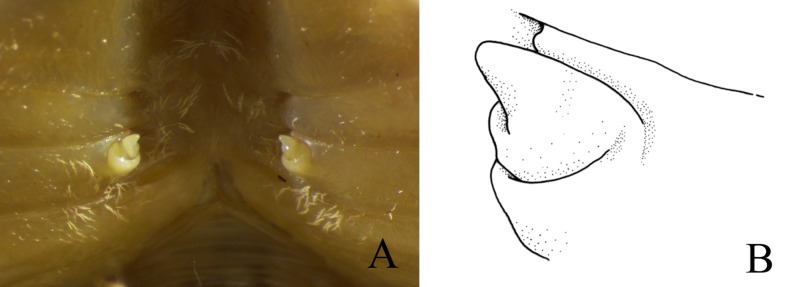
Remarks: Fujita and Naruse (2016: 26, Fig. 3) provide a clear description of female vulvae morphology based on Japanese material, which is a diagnostic character that can be compared to the Taiwanese material. The material in both areas had the same characters: the vulvae’s central operculum protruded in a cone shape and there was no raised sternal vulvar cover (Fig. 3A, B). On the other congener species, the central operculum was mostly rounded (Wowor and Ng 2009). In addition, our data revealed that the female K. boholano from Taiwan has a larger body (average cw. 19.6 mm, n = 3) than specimens from Panglao (average cw. 13.0 mm, n = 6) and Okinawa (average cw. 16.7 mm, n = 3) (measurements of females from Ng 2002; Naruse et al. 2005; Fujita and Naruse 2016).
Molecular analyses: Karstama boholano had belonged to the genus Sesarmoides; therefore, S. kruasii was chosen as the outgroup in this study. The result revealed that all the specimens collected from Green Island or the Philippines emerged from the same branch of K. boholano. Moreover, the intra-specific threshold was around 0 to 0.5% divergence of K2P distances, and the threshold between the two genera was shown to be 41.3% in this study (Table 2).
Fig. 3.
Karstama boholano (Ng, 2002), female valvae, western Green Island, Taitung (NTOU 1). A, general view; B, left gonopore.
Table 2.
The pairwise divergence distances by Kimura 2-parameter in this study
| (1) | (2) | (3) | (4) | (5) | |
| (1) Karstama boholano_NMNS1_Green Island, Taiwan | |||||
| (2) Karstama boholano_ZRC2012.0433-2_Bohol Island, Philippines | 0.5% | ||||
| (3) Karstama boholano_NMNS2_Green Island, Taiwan | 0.5% | 0.0% | |||
| (4) Karstama boholano_ZRC2012.0433-1_Bohol Island, Philippines | 0.0% | 0.5% | 0.5% | ||
| (5) Karstama boholano_Paratype, ASIZ_Bohol Island, Philippines | 0.5% | 0.0% | 0.0% | 0.5% | |
| (6) Sesarmoides kruasii | 41.3% | 41.3% | 41.3% | 41.3% | 41.3% |
DISCUSSION
Fifteen species in the genus Karstarma have been recorded from Indo-West Pacific (Ng et al. 2008; Wowor and Ng 2009; Husana et al. 2010). The present study describes a new record genus/species of the cavernicolous crab K. boholano from Taiwan and establishes the first zoeal morphology of this genus. According to Cuesta et al. (2006), which reported the zoeal morphology of eleven species from Taiwan and adjacent areas, the common characteristics of sesarmid first zoeal are 1) cephalothorax without lateral spines and with a pair of anterodorsal setae; 2) antenna: exopod with terminal small spines and setae of different sizes; exopod with variable lengths, commonly between 1/4 and 2/3 of the protopod length; protopod with well-developed spines distributed in two rows, normally with an unequal number of spines; 3) maxilla endopod bilobed with 2 + 3 setae; 4) first maxilliped: basis with 2 + 2 + 3 + 3 setae; endopod setation 2,2,1,2,5; 5) second maxilliped: basis with 1+1+1+1 setae, endopod setation 0,1,6; 6) abdomen with five somites and dorsolateral processes only found on somites 2–3; and 7) telson with three serrulate setae on the posterior margin throughout development and furcal arms with two dorsal rows of spinules of varying sizes. Based on this study, the first zoeal stage of K. boholano has characteristics in accordance with those of sesarmid larvae, except for the antenna exopod, which had variable lengths and was 1/3 as long as the protopod length.
Based on the information from this study and Cuesta et al. (2006), 12 species of sesarmid larvae have been described from West Pacific in total— Clistocoeloma merguiense De Man, 1888, Karstarma boholano, Labuanium politum (De Man, 1888), L. rotundatum (Hess 1865), L. scandens Ng and Liu, 2003, L. trapezoideum (H. Milne Edwards, 1837), Metasesarma aubryi, M. obesum (Dana, 1851), Pseudosesarma crassimanum (De Man, 1887), Sesarmops impressus (H. Milne Edwards, 1837), S. intermedium (De Haan, 1835), and Stelgistra stormi (De Man, 1895). The first zoeae of these species are difficult to distinguish from the characters and setal appendage patterns, but the zoeae of these species differ in the number of minute lateral spines on the outer margin of their telson furca. The outer margin of the telson furca shows a variable number of minute lateral spines: two in L. politum, M. obesum, and Se. intermedium; one in C. merguiense, K. boholano, L. rotundatum, L. scandens, L. trapezoideum, M. aubryi, and S. impressum; zero in P. crassimanum and St. stormi.
The habitat of Karstama boholano from the Philippines and Okinawa is anchialine caves near the coastline (Ng 2002; Naruse et al. 2005; Fujita and Naruse 2016). However, the anchialine caves in Taiwan are poorly explored (Chung 1994 2008; Chi 1995; Chi and Ho 2017), and to date no cavernicolous crabs have been reported. However, in southern and eastern Taiwan (including offshore islands), the coastal forests contain several small-scale anchialine caves that constitute clefts (Fig. 2F), which are suitable for and may sustain populations of K. boholano. There are anecdotal reports of K. boholano within KTNP that suggest that they may have been present in Taiwan for a long time. The first author, third author, and Hung-Chang Liu observed a long-legged sesarmid crab (initially thought to be Karstama or Sesarmoides) in the coastal forest and limestone rocks of KTNP in the 1990s and again in 2015. However, Sesarmoides inhabits areas near mangroves (Davie and Ng 2007), and, as such environments are not present in KTNP, it can be confidently stated that they were not Sesarmoides. According to the evidence and observations in this present study, we suggest that the long-legged sesarmid crab in Taiwan is K. boholano.
Moreover, the divergence values vary greatly among the genera (Chu et al. 2015), but the previous study showed a threshold of 16 to 17.16% COI divergence of intergenic K2P distance (Kimura 1980). In this study, the pairwise divergence distances in intra-species (Table 2) are small and can be confirmed as the same species as those collected from Taiwan or the Philippines. Moreover, K. boholano had been in the genus Sesarmoides, but was transferred to the genus Karstamae based on morphology (Ng et al. 2008). In this case, the divergence distances between K. boholano and S. kruasii was shown to be 41.3% (Table 2). The value was also higher than previously reported intergenic COI divergences. However, the sequences of these two genera were rare in the database, which limited the molecular analysis in this study. Future studies should sequence the other species DNA fragments in this genus.
Ng et al. (2017) published an updated checklist of brachyuran crabs from Taiwan, which recorded 800 species. Recent publications on Taiwan decapods (Ayoung and Ng 2017; Hsu and Shih 2018; Hsueh 2018; Li et al. 2018; Ng et al. 2018; Wong et al. 2018; Shih et al. 2019) and the present study have added seven new records, bringing the total number of brachyuran crabs recorded in Taiwan to 807 species.
CONCLUSIONS
This study documents a new genus and species record of the cavernicolous crab K. boholano from Taiwan, and describes for the first time the first zoeal morphology for this genus. Comparison of the first zoeal morphology of K. boholano and the larvae of eleven other sesarmid species from Taiwan and adjacent areas revealed that the zoeae of these species differ in the number of minute lateral spines on the outer margin of their telson furca. Evidence and observations presented in this study suggest that K. boholano may have been present in Taiwan for a long time. Karstama and Sesarmoides are sesarmids with long legs, but Sesarmoides inhabits areas near mangroves, but such habitats are not present in Green Island or Kenting National Park. Specimen from this study were collected from coral karsts, and their morphology strongly suggests that they do not belong the genus Sesarmoides. In the COI divergence of intergenic K2P distance, this study’s intra-specific pairwise divergence distances are small and can be confirmed as the same species as those collected from Taiwan or the Philippines.
Acknowledgments
This work was partially supported by a research grant, awarded to the first author, from Kenting National Park, Taiwan and Kaltis International Co., Ltd, Taiwan. We are grateful to Mr. Chin-Yu Lu for his help with the field investigations, Dr. Hsiu-Chin Lin from National Sun Yat-sen University for helping conduct the molecular lab work, and Dr. Peter K. L. Ng from National University of Singapore and Dr. Hung-Chang Liu for kindly providing the Karstama boholano specimens from the Philippines. Furthermore, we would like to thank Mr. Alvin X. R., Ms. Ling, Sing-Ying Chua, and Trevor Padgett for their help enhancing the manuscript, and Noah Last of Third Draft Editing for editing its English language.
Footnotes
Authors’ contributions: Li JJ designed this study, examined the morphological characters, and drafted the manuscript. Shih YJ analyzed the DNA data. Ho PH revised the manuscript. Jiang GC processed the larvae samples and descriptions of the morphology of the first zoea stage, and submitted the manuscript. All authors are in agreement with the content of the manuscript.
Competing interests: Li JJ, Shih YJ, Ho PH, and Jiang GC declare that they have no competing interests.
Availability of data and materials: Parental vouchers and larvae samples have been deposited at the National Taiwan Ocean University (NTOU), Keelung, Taiwan.
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Ahyong ST, Ng PKL. 2017. East Asian cymonomid crabs (Crustacea: Brachyura). Zool Stud 56:24. doi:10.6620/ZS.2017.56-24. [DOI] [PMC free article] [PubMed]
- Chi SC, Ho LD. 2017. Exploring in Shoushan – Landscape. Preparatory Office of Shoushan National Nature Park, Taiwan.
- Chi SC. 1995. Doline in Kenting National Park. Landscape Conservation in Taiwan (2):29–33. (in Chinese)
- Chu KH, Schubart CD, Shih HT, Tsang LM. 2015. Genetic diversity and evolution of Brachyura. In: Castro P, Davie PJF, Guinot D, Schram FR, von Vaupel Klein JC (ed) Treatise on zoology -anatomy, taxonomy, biology – the Crustacea, complementary to the volumes translated from the French of the Traité de Zoologie. Brill, Leiden, 9(C)(I), Decapoda: Brachyura (Part 1), pp. 775– 820.
- Chung KJ. 1994. Landscape and Conservation of Limestone Geomorphology in Southwestern Taiwan. Landscape Conservation in Taiwan (1):9–12. (in Chinese)
- Chung KJ. 2008. Limestone Geomorphology from Taiwan, Taiwan Geography Encyclopedia. Walkers Cultural Enterprises, Ltd, Taiwan.
- Clark PF, Calazans D, Pohle GW. 1998. Accuracy and standardization of brachyuran larval descriptions. Invertebr Reprod Dev 33(2-3):127–144. doi:10.1080/07924259.1998.9652627.
- Cuesta JA, Guerao G, Liu HC, Schubart CD. 2006. Morphology of the first zoeal stages of eleven Sesarmidae (Crustacea, Brachyura, Thoracotremata) from the Indo-West Pacific, with a revision of larval characters of the family. Invertebr Reprod Dev 49(3):151– 173. doi:10.1080/07924259.2006.9652206.
- Davie PJ, Ng PKL. 2007. A new genus for cave-dwelling crabs previously assigned to Sesarmoides (Crustacea: Decapoda: Brachyura: Sesarmidae). Raffles B Zool 16:227–231.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299. [PubMed]
- Fujita Y, Naruse T. 2016. Karstama boholano (Ng 2002) (Decapoda: Brachyura: Sesarmidae) from Tarama-jima Island, Ryukyu Islands, southwestern Japan. Fauna Ryukyuana 28:23–27.
- Guerao G, Anger K, Nettelmann U, Schubart CD. 2004. Complete larval and juvenile development of the mangrove crab Perisesarma fasciatum (Crustacea: Brachyura: Sesarmidae) from Singapore, with a larval comparison of Parasesarma and Perisesarma. J Plankton Res 26:1389–1408.
- Guerao G, Anger K, Simoni R, Cannicci S. 2012. The early life history of Chiromantes ortmanni (Crosnier, 1965) (Decapoda: Brachyura: Sesarmidae): morphology of larval and juvenile stages. Zootaxa 3347:36–62. doi:10.11646/zootaxa.3347.1.2.
- Guerao G, Simoni R, Cannicci S, Anger K. 2011. Morphological description of the megalopa and the first juvenile crab stage of Chiromantes eulimene (Decapoda, Brachyura, Sesarmidae), with a revision of zoeal morphology. Invertebr Reprod Dev 55:100– 109. doi:10.1080/07924259.2011.553416.
- Hall T. 2013. BioEdit, version 7.2.5. Ibis Biosciences, Carlsbad, CA, USA.
- Hsu PY, Shih HT. 2018. A new record crab of Parasesarma lepidum (Decapoda: Brachyura: Sesarmidae) from southern Taiwan. Coll Res 31:85–90.
- Hsueh PW. 2018. A new species of Neorhynchoplax (Crustacea: Decapoda: Brachyura: Hymenosomatidae) from Taiwan. Zootaxa 4461(3):350–358. doi:1646/zootaxa.4461.3.2. [DOI] [PubMed]
- Husana DEM, Naruse T, Kase T. 2010. A new species of the genus Karstarma (Crustacea: Decapoda: Brachyura: Sesarmidae) from anchialine caves in the Philippines. Raffles B Zool 58(1):51–55.
- Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. [DOI] [PubMed]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi:10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed]
- Li JJ, Rahayu DL, Ng PKL. 2018. Identity of the tree-spider crab, Parasesarma leptosoma (Hilgendorf, 1869) (Decapoda: Brachyura: Sesarmidae), with descriptions of seven new species from the western Pacific. Zootaxa 4482:451–490. doi:10.11646/zootaxa.4482.3.2. [DOI] [PubMed]
- Naruse T, Nakai H, Tamura H. 2005. A new record of cavernicolous crab Sesarmoides boholano Ng, 2002 (Brachyura, Sesarmidae) from Ishigaki Island, southern Ryukyu Islands, Japan. Biogeography 7:79–84.
- Naruse T, Ng PKL. 2007. On a new species of cavernicolous crab of the genus Sesarmoides Serène & Soh, 1970 (Crustacea: Decapoda: Brachyura: Sesarmidae) from Sulawesi, Indonesia. Raffles B Zool 55(1):127–130.
- Ng PKL. 2002. New species of cavernicolous crabs of the genus Sesarmoides from the western Pacific, with a key to the genus (Crustacea: Decapoda: Brachyura: Sesarmidae). Raffles B Zool 50(2):419–435.
- Ng PKL, Guinot D, Davie PJ. 2008. Systema Brachyurorum: Part I. An annotated checklist of extant brachyuran crabs of the world. Raffles B Zool 17(1):1–286.
- Ng PKL, Lin CW, Ho PH. 2018. On three species of reef-dwelling pilumnid crabs from Taiwan, with notes on Heteropilumnus De Man, 1895 (Crustacea: Brachyura). Zool Stud 57:12. doi:10.6620/ZS.2018.57-12. [DOI] [PMC free article] [PubMed]
- Ng PKL, Shih HT, Ho PH, Wang CH. 2017. An updated annotated checklist of brachyuran crabs from Taiwan (Crustacea: Decapoda). J Natl Taiwan Mus 70(3-4):1–185. doi:10.6532/JNTM.201712_70(3;4).01.
- Rebolledo AP, Wehrtmann IS, Cuesta JA. 2015. Morphological and morphometric comparison of the first zoeal stage of the mangrove crabs of the genus Aratus H. Milne Edwards, 1853 (Decapoda: Sesarmidae). Zootaxa 3949(2):217–228. doi:10.11646/zootaxa.3949.2.4. [DOI] [PubMed]
- Shih HT, Hsu PY, Shahdadi A, Schubart CD, Li JJ. 2019. The synonymy of the supratidal crab species Parasesarma cognatum Rahayu & Li, 2013 with P. liho Koller, Liu & Schubart, 2010 (Decapoda: Brachyura: Sesarmidae) based on morphological and molecular evidence, with a note on P. paucitorum Rahayu and Ng, 2009. Zool Stud 58:21. doi:10.6620/ZS.2019.58-21. [DOI] [PMC free article] [PubMed]
- Wowor D, Ng PKL. 2009. Two new species of sesarmid crabs (Crustacea: Decapoda: Brachyura) associated with limestone formations in West Papua, Indonesia. Zootaxa 2025(1):21–31. doi:10.5281/zenodo.186160. [DOI] [PubMed]
- Wong KJH, Ng PKL, Jeng MS. 2018. From an old eroded carapace: rediscovery of the majid crab Leptomithrax sinensis Rathbun, 1916 (Crustacea, Brachyura, Majidae) from Taiwan and Japan. Zool Stud 57:49. doi:10.6620/ZS.2018.57-49. [DOI] [PMC free article] [PubMed]