Abstract
A new species of freshwater crab of the genus Qianguimon Huang, 2018, Q. rongxianense sp. nov., is described from Rong County, Yulin City, Guangxi Zhuang Autonomous region, southern China. This new species resembles its congeners and some species in the genus Yarepotamon Dai & Türkay, 1997, but can be distinguished from these by its combination of the carapace, third maxilliped, male gonopod, female vulvae characters and size. Molecular data derived from the mitochondrial 16S rDNA supports the establishment of the new species, but does not provide further evidence as to its generic placement.
Keywords: Qianguimon, Freshwater crab, Guangxi, New species, 16S rDNA
BACKGROUND
China has the richest freshwater crab fauna and shrimp in the world (Cai and Ng 2018; Cumberlidge et al. 2011), and the full extent of this biodiversity is yet to be discovered (Yeo et al. 2007). Since 2010, numerous new potamid genera and species from Guangdong and Guangxi have been published (Do et al. 2016; Huang et al. 2014 2016 2017 2018; Naruse et al. 2010 2013; Zou et al. 2018). Upon checking specimens deposited in the Department of Parasitology of the Medical College of Nanchang University, we discovered an undescribed species of potamid crab that was collected from Rongxian County, Yulin City, Guangxi. This new species has a boot-shaped male first gonopod terminal segment that is very similar to those of Qianguimon species, whereas other characters closely resemble species from Yarepotamon. In an attempt to collect additional fresh specimens and record its ecological data, the authors conducted a collection trip to the locality in November 2018. Unfortunately, the new species could not be collected; instead, Eurusamon guangdongense Huang, 2018 and an undescribed species of Nanhaipotamon Bott, 1968, which will be described in a separate study, were found in the listed locality. Nonetheless, the original series of specimens are in good condition and of sufficient numbers for morphological examination. Phylogenetic analysis of the mitochondrial 16S rDNA failed to provide evidence for the generic placement of this new species; however, there is good support for it belonging a clade within the China-East Asia Islands Clade (Shih et al. 2009), which contains genera from southern China.
MATERIALS AND METHODS
Specimens were collected by Ye-Song Cheng from Shan Xin Dui (22.5049°N, 110.7282°E), Sixian Village, Licun Town, Rong County, Guangxi Zhuang Autonomous Region, southern China; preserved in 95% ethanol and deposited in the Department of Parasitology of the Medical College of Nanchang University, Jiangxi, China (NCUMCP), and the Sun Yat-sen Museum of Biology, Sun Yat-sen University, Guangzhou, China (SYSBM). Measurements, in millimeters, are of the carapace width and length, respectively. The following abbreviations are used: CW – carapace width; G1 – male first gonopod; G2 – male second gonopod. The terminology used primarily follows that of Dai (1999) and Davie et al. (2015).
Approximately 50 mg of muscle tissue was extracted from the ambulatory legs or chelipeds of each sequenced crab with the aid of the DP1902 Tissue Kit (BioTeKe Inc. Beijing). A region of ~550 basepairs (bp) of the 16S rDNA gene was amplified using polymerase chain reaction (PCR) with the primers 1471 (5’-CCTGTTTANCAAAAACAT-3’) and 1472 (5’-AGATAGAAACCAACCTGG-3’) (Crandall and Fitzpatrick 1996; Shih et al. 2004). Parameters for the PCR were as follows: initial denaturation for 50 s at 94°C, annealing for 40 s at 52°C, and extension for 1 min at 72°C (33 cycles) and a subsequent extension for 10 min at 72°C. The PCR products were then sequenced using the ABI 3730 automatic sequencer.
Mitochondrial 16S rRNA gene is a relatively conservative marker with enough variable heterotopic sites suitable for species identification and has been widely used for studying crabs (Bai et al. 2018; Jia et al. 2018). A total of 61 species from 41 genera were used to construct phylogenetic tree. Sequences were aligned using MAFFT vers.7.215 (Katoh and Standley 2013) based on the G-INS-I method. Loop regions were deleted as in Shih et al. (2009). The optimum model for sequence evolution of the 16S dataset, HKY+G+I, was determined by Modelgenerator vers.851 (Katoh and Standley 2013), and selected by Bayesian information criterion (BIC). The BI tree was constructed with MrBayes vers.3.2.6 (Ronquist et al. 2012) with four chains for 2,000,000 generations, with trees sampled every 1,000 generations. Using Tracer vers.1.6 (Rambaut and Drummond 2013) to see ESS values (all greater than 200), the first 25% discarded as burn-in. MEGA vers.X.0 (Kumar et al. 2018) was used to construct the maximum parsimony (MP) tree. The heuristic search used tree bisection-reconnection (TBR) branch-swapping with 100 random addition sequence replicates. Topological robustness was assessed by 2000 bootstrap reiterations.
RESULTS
TAXONOMY
Family Potamidae Ortmann, 1896
Subfamily Potamiscinae Ortmann, 1896
Genus Qianguimon Huang, 2018
Qianguimon rongxianense sp. nov.
(Figs. 1–4)
urn:lsid:zoobank.org:act:ED6D2D89-7DE3-457B-8043-CB3925CE19E3
Type material: Holotype: ♂ (15.2 × 12.8 mm) (NCU MCP 118401), Shan Xin Dui (22.5049°N, 110.7282°E), Sixian Village, Licun Town, Rong County, Yulin City, Guangxi Zhuang Autonomous Region, small stream, coll. Ye-Song Cheng, August 23, 2007. Paratypes: 1♀ (allotype) (20.4 × 16.0 mm) (NCU MCP 118403), same data as holotype; 1♂ (14.9 × 12.6 mm) (NCU MCP 118402).
Additional material examined: 4♂♂ (14.3 × 12.2 mm, 13.3 × 11.7 mm, 15.9 × 13.5 mm, 15.8 × 13.6 mm) (SYSBM, NCU MCP 118405, 118406, 118410), same data as holotype; and 3♀♀ (16.6 × 14.1 mm, 20.1 × 17.1 mm, 17.7 × 14.4 mm) (NCU MCP 118407, 118408, 118409), same data as holotype.
Etymology: This species is named after the type locality, Rong County, Yulin City, Guangxi Zhuang Autonomous Region, southern China.
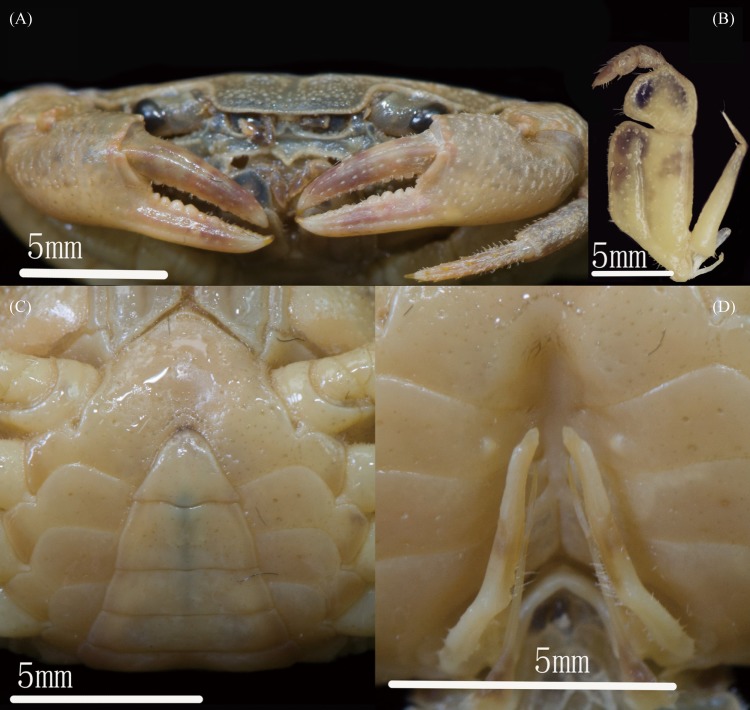
Description: Carapace subquadrate, about 1.2 times as wide as long (n = 10); regions indistinct, dorsal surface slightly convex, pitted, anterolateral region weakly rugose (Figs. 1A, B, 3A). Front slightly deflexed, margin slightly ridged in dorsal view (Figs. 1A, B, 3A). Epigastric cristae prominent, separated by narrow gap (Figs. 1A, B, 3A). Postorbital cristae sharp, laterally expanded, not fused with epigastric cristae or epibranchial teeth (Figs. 1A, B, 3A). Branchial regions not inflated (Figs. 1A, B, 3A). Cervical groove shallow, inconspicuous, H shaped groove between gastric and cardiac regions depressed (Figs. 1A, B, 3A). Mesogastric region slightly convex (Figs. 1A, B, 3A). External orbital angle bluntly triangular, almost confluent with anterolateral margin. Epibranchial tooth small, granular, indistinct (Figs. 1, 3A). Anterolateral margin cristate, lined with approximately 16–20 granules; bent inward posteriorly (Figs. 1A, B, 3A). Posterolateral surface with low, oblique striae, converging towards posterior carapace margin (Figs. 1A, B, 3A). Orbits large; supraorbital and infraorbital margins cristate, lined with numerous granules (Fig. 1C). Sub-orbital and upper parts of pterygostomial regions covered with granules; sub-hepatic regions lined with oblique striae (Fig. 1C). Posterior margin of epistome narrow, concave; median lobe sharply triangular, lateral margins straight (Fig. 1C).
Fig. 1.
Qianguimon rongxianense sp. nov. Holotype male (15.2 × 12.8 mm) (NCU MCP 118401). (A) overall habitus; (B) dorsal view of the carapace; (C) frontal view of the cephalothorax.
Maxilliped 3 exopod reaching proximal 1/4 of merus length, with short flagellum (Fig. 2B). Merus trapezoidal, about 1.1 times as broad as long, with median depression (Fig. 2B). Ischium trapezoidal, about 1.5 times as long as broad, with distinct median sulcus (Fig. 2B).
Fig. 2.
Qianguimon rongxianense sp. nov. Holotype male (15.2 × 12.8 mm) (NCU MCP 118401). (A) outer view of chelipeds; (B) left third maxilliped; (C) ventral view showing anterior thoracic sternum and pleon; (D) ventral view showing sterno-pleonal cavity with G1 in situ.
Chelipeds (pereiopod 1) unequal (Figs. 1A, 2A, 3A). Carpus surface wrinkled with sharp spine at inner-distal angle with spinule at base (Figs. 1A, 3A). Palm of larger chela about 1.3–1.4 times as long as high in males (n = 6), 1.5–1.6 times in females (n = 4); dactylus 0.7 times as long as palm in males (n = 6), 0.7–0.8 times in females (n = 4) (Figs. 1A, 2A, 3A). Inner margin of fingers with granular teeth, with small gap when fingers closed (Fig. 2A).
Ambulatory legs (pereiopods 2–5) slender, with setae; pereiopod 3 merus 0.5 times as long as the carapace in males (n = 6), 0.4 times in females (n = 4) (Figs. 1A, 3A). Pereiopods 5 propodus 1.5–1.6 times as long as broad in males (n = 6), 1.6 times in females (n = 4), about as long as dactylus (Figs. 1A, 3A).
Fig. 3.
Qianguimon rongxianense sp. nov. Paratype female (20.4 × 16.0 mm) (NCU MCP 118403). (A) overall habitus; (B) thoracic sternum showing vulvae.
Male thoracic sterum generally smooth, pitted; sternites 1–4 broad, about 1.6 times as wide as length; sternites 1, 2 forming triangular structure, with obvious suture; suture between sternites 2, 3 inconspicuous; sternites 3, 4 fused without obvious boundary (Fig. 2C). Male sterno-pleonal cavity relatively deep, barely reaching anteriorly to level of midlength of cheliped coxae base; median longitudinal groove between sternites 7, 8 deep and medium (Fig. 2D). Male pleonal locking tubercle positioned at mid-length of sternite 5 (Fig. 2D). Female vulvae ovate, medium-sized, reaching proximal three-quarters width of sternite 6, lateral margin with wide rim (Fig. 3B).
Male pleon triangular (Fig. 2C); somites 3–6 progressively narrower longitudinally, lateral margins slightly concave; somite 6 about 1.3–1.4 times as wide as long in males (n = 6); telson about 2.1 times as wide as long with rounded apex in males (n = 6) (Fig. 2C). Female pleon broadly ovate.
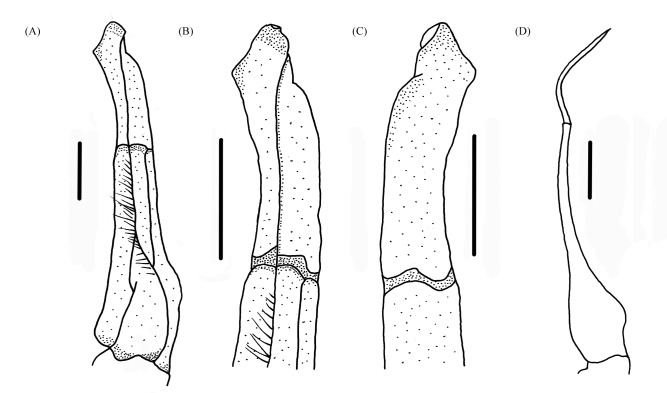
G1 slender, tip of terminal segment exceeding pleonal locking tubercle, almost reaching suture between thoracic sternites 4/5 (Fig. 2D); subterminal segment about 1.6 times length of terminal segment, groove for G2 starts at mid-point; terminal segment boot-shaped, with large triangular sub-distal projection, outer-distal margin swollen (Fig. 4A–C). G2 subterminal segment about 1.8 times length of distal segment, with subovate base and flagelliform distal segment (Fig. 4D).
Fig. 4.
Gonopods. A–D Qianguimon rongxianense sp. nov. Holotype male (15.2 × 12.8 mm) (NCU MCP 118401); (A) ventral view of the left G1; (B) ventral view of the distal part of left G1; (C) dorsal view of the distal part of the left G1; (D) left G2. Scale bars: A–D = 1.0 mm.
Remarks: The new species has a boot-shaped G1 terminal segment typical of that of congeners, but markedly distinct in some characters. Its small size (CW < 20 mm) [(versus < 30 mm in Q. aflagellum (Dai, Song, Li & Liang, 1980) and < 40 mm in Q. elongatum Huang, 2018 and Q. splendidum Huang, 2018 (cf. Table. 1)], confluent external orbital angle and anterolateral region (Figs. 1, 3A) [versus separated by narrow gap in all other Qianguimon (cf. Table. 1)], short third maxilliped exopod flagellum (Fig. 2B) [versus very short to absent in all other Qianguimon (cf. Table. 1)], and swollen outer-distal margin of the G1 terminal segment [versus not swollen in all other Qianguimon (cf. Table. 1)] immediately set it apart from the other species. Qianguimon rongxianense sp. nov. also resembles species from Yarepotamon Dai & Türkay, 1997 and Eurusamon Huang, 2018, but its boot-shaped G1 (Fig. 4A–C) and medium-sized, wide-rimmed vulvae (Fig. 3B) separate it from these species. These comparisons are listed in table 1.
Distribution: The new species is known only from type locality presently, Rong County, Yulin City, Guangxi Zhuang Autonomous Region.
Table 1.
Morphological differences among Qianguimon rongxianense sp. nov., Yarepotamonbreviflagellum Dai & Türkay, 1997, Qianguimon aflagellum Huang, 2018, Qianguimon elongatum Huang, 2018, Qianguimon splendidum Huang, 2018 and Eurusamon guangdongense Huang, 2018
| Species/Character | Q. rongxianense sp. nov. | Q. aflagellum | Q. elongatum |
| External orbital angle and anterolateral region | Almost confluent (Fig. 1A, 1B) | Separated by narrow gap (cf. Huang, 2018: fig. 8A, B) | Separated by narrow gap (cf. Huang, 2018: fig. 9A, B) |
| Flagellum of exopod of third maxilliped | Short (Fig. 2A) | Very short to absent (cf. Dai & Türkay, 1997: fig. 5 (1); unpublished data) | Absent (cf. Huang, 2018: fig. 10D) |
| G1 in situ | Reaching beyond pleonal locking tubercle, not reaching sternites 4/5 suture (Fig. 2D) | Reaching beyond pleonal locking tubercle, reaching to sternites 4/5 suture (cf. Huang, 2018: fig. 8D) | Reaching well beyond pleonal locking tubercle, well exceeding sternites 4/5 suture (cf. Huang, 2018: fig. 9D) |
| G1 | Generally slender, terminal segment boot-shaped, with large triangular sub-distal projection, outer-distal margin swollen (Fig. 4A–C) | Generally slender, terminal segment boot-shaped, with large triangular sub-distal projection (cf. Huang, 2018: fig. 15E) | Very slender, terminal segment boot-shaped, with blunt sub-distal projection (cf. Huang, 2018: fig. 15F) |
| Female vulvae | Medium-sized, reaching proximal three-quarters width of sternite 6, lateral margin with wide rim (Fig. 3B) | Medium-sized, reaching proximal three-quarters width of sternite 6, lateral margin with wide rim (cf. Huang, 2018: fig. 16C) | Medium-sized, reaching proximal three-quarters width of sternite 6, lateral margin with wide rim (cf. Huang, 2018: fig. 16D) |
| Carapace size | Small (CW up to 20 mm) | Medium (CW up to 30 mm) (cf. Huang, 2018) | Medium (CW up to 40 mm) (cf. Huang, 2018) |
| Species/Character | Q. splendidum | E. guangdongense | Y. breviflagellum |
| External orbital angle and anterolateral region | Separated by narrow gap (cf. Huang, 2018: fig. 11A, B) | Almost confluent (cf. Huang, 2018: fig. 13A, B) | Almost confluent (cf. Huang, 2018: fig. 2A, B) |
| Flagellum of exopod of third maxilliped | Absent (cf. Huang, 2018: fig. 12D) | Long (cf. Dai & Türkay, 1997: fig. 8 (1)) | Short (cf. Dai & Türkay, 1997: fig. 6 (1)) |
| G1 in situ | Reaching well beyond pleonal locking tubercle, exceeding sternites 4/5 suture (cf. Huang, 2018: fig. 11D) | Reaching well beyond pleonal locking tubercle, exceeding sternites 4/5 suture (cf. Huang, 2018: fig. 13D) | Reaching beyond pleonal locking tubercle, reaching to sternites 4/5 suture (cf. Huang, 2018: fig. 2D) |
| G1 | Very slender, terminal segment boot-shaped, with large triangular sub-distal projection (cf. Huang, 2018: fig. 15G) | Very slender, terminal segment sinuous (cf. Huang, 2018: fig. 15H) | Generally slender, terminal segment sinuous, with or without sub-distal projection (cf. Huang, 2018: fig. 15A) |
| Female vulvae | Medium-sized, reaching proximal three-quarters width of sternite 6, lateral margin with wide rim (cf. Huang, 2018: fig. 16E) | Relatively small, reaching proximal three-fifths width of sternite 6, lateral margin with narrow rim (cf. Huang, 2018: fig. 16F) | Medium-sized, reaching proximal three-quarters width of sternite 6, lateral margin with narrow rim (cf. Dai & Türkay, 1997: fig. 6 (1)) |
| Carapace size | Medium (CW up to 40 mm) (cf. Huang, 2018) | Large (CW up to 60 mm) (cf. Huang, 2018) | Small (CW up to 30 mm) (cf. Huang, 2018) |
Phylogenetic relationships
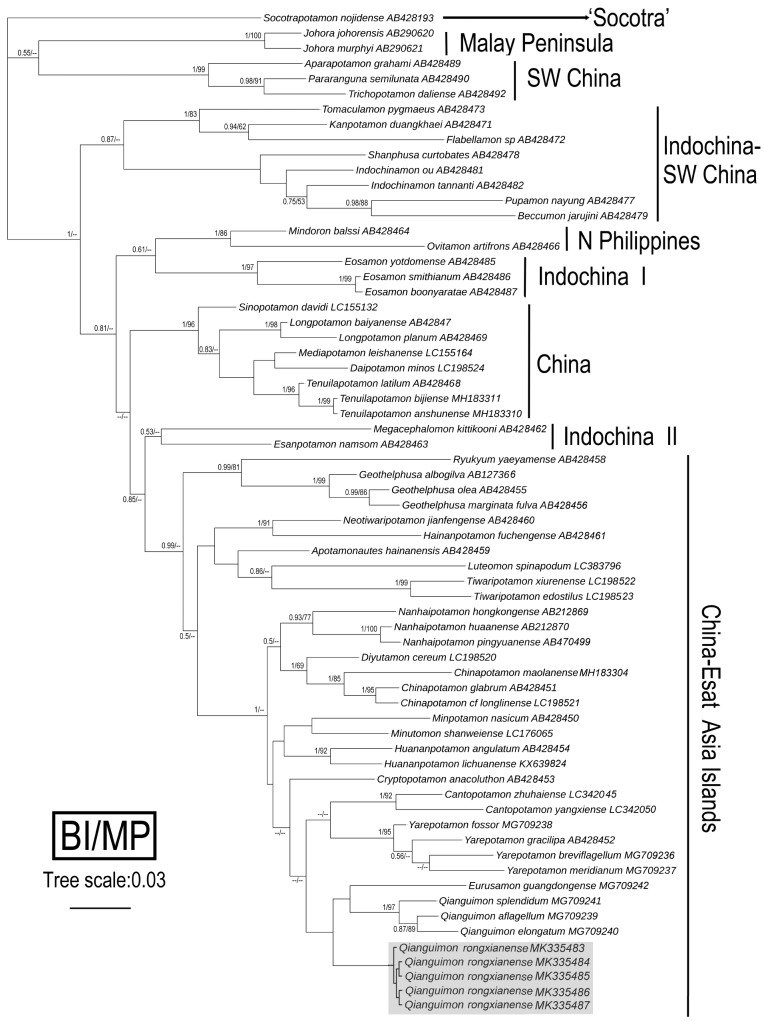
The 16S rDNA sequences of 61 species from 41 genera of potamid crabs, including the new species, which have been published in GenBank, were used for alignment analysis with a total length of 461 bp. The localities and access numbers of these species are listed in figure 5. Overall, the phylogenetic trees constructed by the different methods showed a generally similar topological structure with some well supported terminal nodes, but most basal nodes are very weakly supported (Fig. 5). The results shows that the new species is clustered with Qianguimon and Eurusamon (weak support) in a clade (high support from BI analysis) within the China-East Asia Islands Clade (Shih et al. 2009) that contains other genera from southern China such as Cantopotamon Huang, Ahyong & Shih, 2017, Chinapotamon Dai & Naiyanetr, 1994, Cryptopotamon Ng, 1992, Diyutamon Huang, Shih & Ng, 2017, Huananpotamon Dai & Ng, 1994, Minpotamon Dai & Türkay, 1997, Minutomon Huang, Mao & Huang, 2014, Nanhaipotamon Bott, 1968 and Yarepotamon Dai & Türkay, 1997.
Fig. 5.
Bayesian inference (BI) tree of 16S rDNA of new species (shown in gray) and part of the subfamily Potamiscinae (Shih et al. 2009). The number at the nodes represents Bayesian inference (BI) posterior probabilities and Maximum parsimony (MP) bootstrap values. Only values > 50% are displayed.
DISCUSSION
In a revision of the Chinese freshwater crabs previously placed in the genus Isolapotamon Bott, 1968 placed Malayopotamon gracillipa Dai, Song, Li & Liang, 1980, Isolapotamon aflagellum Dai, Song, Li & Liang, 1980 within Yarepotamon Dai & Türkay, 1997, and described Y. breviflagellum Dai & Türkay, 1997, and Y. guangdongense Dai & Türkay, 1997 (Dai and Türkay 1997). In a revision of Yarepotamon by Huang (2018), the genus was found to be a collection of species from three different genera: Yarepotamon s. str., Eurusamon and Qianguimon, according to morphology and molecular data derived from the mitochondrial 16S rDNA. According to Huang’s (2018) definition, Qianguimon is mainly characterized by the following: medium sized (carapace width up to 40 mm); third maxilliped exopod with short or no flagellum; and G1 generally slender, terminal segment boot-shaped with sub-distal projection. In freshwater crabs, large morphological changes, especially in characters that are under selection pressure from the environment, can happen with rapid adaptation to different habitats (Klaus et al. 2013). Though the new species is unique in smaller size and relatively longer third maxilliped exopod flagellum, which are characters often associated with a peripheral aquatic habitat (Huang et al. 2014), the most taxonomically important character of the G1 agrees well with other Qianguimon species. According to our phylogenetic analyses, however, the generic placement of the new species is inconclusive. Mitochondrial introgression has been reported in mangrove crabs (Cannicci et al. 2017) and is likely also present in freshwater crabs. It is possible that during the cladogenesis of the new species, mitochondrial introgression occurred between it and another unknown taxon, resulting in the loss of phylogenetic information in the mitochondrial genome. The addition of nuclear markers in the analysis is needed to test this hypothesis, though this is unfortunately beyond the limitations of the current study. Therefore, the current placement of this new species within Qianguimon is based on a morphological analysis, while the molecular data only suggests a close relationship between the new species and species from Qianguimon and Eurusamon. With the addition of this new species, the freshwater crab diversity in Guangxi has reached 40 species in 15 genera, next only to Yunnan and Taiwan (Shih and Ng 2011).
CONCLUSIONS
This study describes a new species of freshwater crabs. In the process of morphological comparison with similar populations, we identified that the crab specimen collected from Rongxian County, Yulin City, Guangxi Zhuang Autonomous Region, China is an undescribed species of Qianguimon, and the phylogenetic study based on the mitochondrial 16S rDNA gene also indicates that it is a new species.
Acknowledgments
This work and the new species name have been registered with ZooBank under urn:lsid:zoobank.org:pub:CC5ABF9A-3171-49DF-B0C9-C7410086C37A. The present study is supported by the National Sharing Service Platform for Parasite Resources (TDRC-22), the National Natural Science Foundation of China (No. 31560179, 31460156), the Natural Science Foundation of Jiangxi Province (No. 20171BAB205108), Nanchang University College Students’ Innovation and Entrepreneurship Training Program (No. 2018388). We thank Ye-Song Cheng for collecting the specimens of the new species. Special thanks expressed to Ying-Yi Cui, Ning Gao, Ya-Nan Zhang and Meng-Jun Zhao for their help in the molecular experiments, and to Xin-Nan Jia for his help and advice on constructing the phylogenetic tree. We would also thank Professor Xian-Min Zhou and Zong-Heng Nie for their guidance in the morphological classification of freshwater crabs.
Footnotes
Authors’ contributions: SBW initiated the manuscript; JXZ guided the writing, image processing and molecular analysis; CH edited the content and language of the manuscript. All authors contributed to drafting and revising the manuscript. All authors read and approved the final manuscript.
Competing interests: SBW, CH and JXZ declare that they have no conflict of interest.
Availability of data and materials: Sequences generated in this study have been deposited in the GenBank database (accession numbers in Fig. 5).
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Bai J, Xu SX, Nie ZH, Wang Y, Zhu CC, Wang Y, Min WP, Cai YX, Zou JX, Zhou XM. 2018. The complete mitochondrial genome of Huananpotamon lichuanense (Decapoda: Brachyura) with phylogenetic implications for freshwater crabs. Gene 646:217– 226. doi:10.1016/j.gene.2018.01.015. [DOI] [PubMed]
- Cai Y, Ng PKL. 2018. Freshwater shrimps from karst caves of southern China, with descriptions of seven new species and the identity of Typhlocaridina linyunensis Li and Luo, 2001 (Crustacea: Decapoda: Caridea). Zool Stud 57:27. doi:10.6620/ZS.2018.57-27. [DOI] [PMC free article] [PubMed]
- Cannicci S, Schubart CD, Innocenti G, Dahdouh-Guebas F, Shahdadi A, Fratini S. 2017. A new species of the genus Parasesarma De Man, 1895 from East African mangroves and evidence for mitochondrial introgression in sesarmid crabs. Zool Anz 269:89– 99. doi:10.1016/j.jcz.2017.08.002.
- Crandall KA, Fitzpatrick JF. 1996. Crayfish molecular systematics: Using a combination of procedures to estimate phylogeny. Syst Biol 45:1–26.
- Cumberlidge N, Ng PKL, Yeo DCJ, Naruse T, Meyer KS, Esser LJ. 2011. Diversity, endemism and conservation of the freshwater crabs of China (Brachyura: Potamidae and Gecarcinucidae). Integr Zool 6:45–55. doi:10.1111/j.1749-4877.2010.00228.x. [DOI] [PubMed]
- Dai AY. 1999. Fauna Sinica: Arthropoda Crustacea Malacostraca Decapoda Parathelphusidae Potamidae. Science Press, Beijing.
- Dai AY, Türkay M. 1997. Revision of the Chinese freshwater crabs previously placed in the genus Isolapotamon Bott, 1968 (Crustacea: Decapoda: Brachyura: Potamidae). Raffles B Zool 45:237–264.
- Davie PJ, Guinot D, Ng PKL. 2015. Systematics and classification of Brachyura. In: Treatise on zoology — anatomy, taxonomy, biology. The Crustacea, Vol. 9C–I, pp. 1049–1130. (P. Castro, P.J.F. Davie, D. Guinot, F.R. Schram & J.C. Von Vaupel Klein, eds.). Brill, Leiden. doi:10.1163/9789004190832_021.
- Do VT, Shih HT, Huang C. 2016. A new species of freshwater crab of the genus Tiwaripotamon Bott, 1970 (Crustacea, Brachyura, Potamidae) from northern Vietnam and southern China. Raffles B Zool 64:213–219.
- Huang C. 2018. Revision of Yarepotamon Dai & Türkay, 1997 (Brachyura: Potamidae), freshwater crabs endemic to southern China, with descriptions of two new genera and four new species. J Crustacean Biol 38:173–189. doi:10.1093/jcbiol/rux120.
- Huang C, Ahyong ST, Shih HT. 2017. Cantopotamon, a new genus of freshwater crabs from Guangdong, China, with descriptions of four new species (Crustacea: Decapoda: Brachyura: Potamidae). Zool Stud 56:41. doi:10.6620/ZS.2017.56-41. [DOI] [PMC free article] [PubMed]
- Huang C, Mao SY, Huang JR. 2014. Two new potamid crabs, Yuexipotamon arcophallus new genus, new species and Minutomon shanweiense new genus, new species, (Crustacea: Decapoda: Brachyura: Potamidae) from southern China. Zootaxa 3764:455–466. doi:10.11646/zootaxa.3764.4.5. [DOI] [PubMed]
- Huang C, Shih HT, Ahyong ST. 2018. Two new genera and two new species of narrow-range freshwater crabs from Guangdong, China (Decapoda: Brachyura: Potamidae). J Crustacean Biol 38:614–624. doi:10.1093/jcbiol/ruy050.
- Huang C, Shih HT, Mao SY. 2016. Yuebeipotamon calciatile, a new genus and new species of freshwater crab from southern China (Crustacea, Decapoda, Brachyura, Potamidae). Zookeys 615:61– 72. doi:10.3897/zookeys.615.9964. [DOI] [PMC free article] [PubMed]
- Jia XN, Xu SX, Bai J, Wang YF, Nie ZH, Zhu CC, Wang Y, Cai YX, Zou JX, Zhou XM. 2018. The complete mitochondrial genome of Somanniathelphusa boyangensis and phylogenetic analysis of genus Somanniathelphusa (Crustacea: Decapoda: Parathelphusidae). PLoS ONE 13:e0192601. doi:10.1371/journal.pone.0192601. [DOI] [PMC free article] [PubMed]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol Biol Evol 30:772–780. doi:10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed]
- Klaus S, Mendoza JC, Liew JH, Plath M, Meier R, Yeo DC. 2013. Rapid evolution of troglomorphic characters suggests selection rather than neutral mutation as a driver of eye reduction in cave crabs. Biol Letters 9:20121098. doi:10.1098/rsbl.2012.1098. [DOI] [PMC free article] [PubMed]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi:10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed]
- Naruse T, Zhu CC, Zhou XM. 2010. Two new species of freshwater crabs of the genus Sinolapotamon Tai & Sung, 1975 (Decapoda, Brachyura, Potamidae) from Guangxi Zhuang Autonomous Region, China. Crustaceana 83:245–256. doi:10.1163/00112160 9X12603430877199.
- Naruse T, Zhu CC, Zhou XM. 2013. Two new species of freshwater crabs of the genus Heterochelamon Türkay & Dai, 1997 (Crustacea: Decapoda: Brachyura: Potamidae) from Guangxi Zhuang Autonomous Region, southern China. Zootaxa 3647:567. doi:10.11646/zootaxa.3647.4.6. [DOI] [PubMed]
- Rambaut A, Drummond AJ. 2013. Tracer v1.6. http://beast.bio.ed.ac. uk/Tracer. Accessed 22 March 2018.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539– 542. doi:10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed]
- Shih HT, Ng PKL. 2011. Diversity and biogeography of freshwater crabs (Crustacea: Brachyura: Potamidae, Gecarcinucidae) from East Asia. Syst Biodivers 9:1–16. doi:10.1080/14772000.2011.5 54457.
- Shih HT, Ng PKL, Chang HW. 2004. Systematics of the genus Geothelphusa (Crustacea, Decapoda, Brachyura, Potamidae) from southern Taiwan: A molecular appraisal. Zool Stud 43:561– 570.
- Shih HT, Yeo DCJ, Ng PKL. 2009. The collision of the indian plate with asia: Molecular evidence for its impact on the phylogeny of freshwater crabs (Brachyura: Potamidae). J Biogeogr 36:703– 719. doi:10.1111/j.1365-2699.2008.02024.x.
- Yeo DCJ, Ng PKL, Cumberlidge N, Magalhães C, Daniels SR, Campos MR. 2007. Global diversity of crabs (Crustacea: Decapoda: Brachyura) in freshwater. Hydrobiologia 595:275– 286. doi:10.1007/s10750-007-9023-3.
- Zou JX, Bai J, Zhou XM. 2018. A new species of karst-dwelling freshwater crab of the genus Chinapotamon Dai & Naiyanetr, 1994 (Crustacea: Decapoda: Brachyura: Potamidae), from Guizhou, southwest China. Peerj 6:17. doi:10.7717/peerj.5947. [DOI] [PMC free article] [PubMed]