Abstract
The treatment for patients with stage IVc nasopharyngeal carcinoma (NPC) at diagnosis was still controversial. In this study, we tried to build a prognostic score model and optimize the treatment for the patients. The prognostic model was based on the primary cohort involving 289 patients from 2002 to 2011 and the validation involving another 156 patients from 2012 to 2015.The prognostic model was built based on the hazard ratios of significant prognostic factors for overall survival (OS). By multivariate analysis, factors associated with poor OS were Karnofsky performance score ≤70, liver metastases, multiple-organ metastases, ≥2 metastatic lesions, lactate dehydrogenase >245 IU/I and poor response to chemotherapy (all P < 0.01). Based on these prognostic factors, patients were divided into the low-risk (0–2 points), intermediate-risk (3–6 points) and high-risk (≥7 points) groups. Five-year OS rates for the low-, intermediate- and high-risk groups were 49.3%, 9.7% and 0.0%, respectively (P < 0.01). Furthermore, loco-regional radiotherapy was associated with significantly better OS in low- and intermediate-risk patients, but not in high-risk patients. These results demonstrated that the prognostic score model based on six negative factors can effectively predict OS in patients with stage IVc NPC at diagnosis. Loco-regional radiotherapy may be beneficial for low- and intermediate-risk patients, but not for high-risk patients.
Subject terms: Oncology, Radiotherapy
Introduction
The epithelial malignancy nasopharyngeal carcinoma (NPC) is disproportionally common in Southern China1. Due to its proximity to an abundant lymphatic network, poorly differentiated and undifferentiated NPC have a higher propensity to metastasize than other squamous head and neck cancers2,3. The presence of distant metastasis is the most significant negative prognostic factor in NPC; median overall survival (OS) for patients with distant metastasis at diagnosis ranges from only 9 to 20 months4–7.
Patients with distant metastasis at diagnosis can have a different treatment response and prognosis compared to patients who develop distant metastasis after treatment8–12. However, the American Joint Committee on Cancer (AJCC) staging system classifies patients with distant metastasis as a single group, which does not enable stratification of prognosis13,14. Therefore, there is significant variation in the survival outcomes of patients who receive similar therapy. Moreover, the optimal treatment for patients with distant metastasis at diagnosis remains controversial. Systemic chemotherapy has been shown to be a critical component of comprehensive treatment and has a high objective response rate. However, the duration of disease control is limited when chemotherapy ceases15,16. The value of radiotherapy to the primary tumor is still uncertain, and the balance between survival benefits and radiation-related complications needs to be considered17,18. Therefore, it is necessary to create a risk-stratification system that enables the survival outcomes of patients with stage IV NPC at initial diagnosis to be predicted; this would have value from both a therapeutic and research point of view.
Materials and Methods
Patients
The inclusion criteria were: (1) patients with pathologically confirmed WHO type II or III NPC; (2) diagnosed with distant metastasis based on clinical symptoms, imaging finding and follow-up data; (3) who received at least one anti-cancer treatment including chemotherapy and/or radiotherapy. A total of 334 eligible cases were referred to Sun Yat-Sen University Cancer Center (278 cases) and Hui Zhou Municipal Central Hospital (56 cases) between January 2002 and June 2011; 45 patients were excluded due to missing clinical data (19 cases) or no treatment (26 cases). The remaining 289 patients were included in the primary cohort. One-hundred and fifty-six consecutive patients from 2012 to 2015 were included in the validation cohort.
Pre-treatment evaluation included a complete patient history, physical examination, complete blood count, renal and liver function tests, lactate hydrogenase (LDH), magnetic resonance imaging (MRI) of the nasopharynx and neck, chest radiographs or computed tomography (CT), abdominal sonography or CT, and single photon emission computed tomography whole-body bone scan. This protocol was in compliance with ethical standards and was approved by the institutional ethics committee of Sun Yat-sen University Cancer Center. Written informed consent was obtained from all participants. All methods were performed in accordance with the relevant guidelines and regulations.
Treatment
The majorityof patients were treated with cisplatinum-based doublets: PF regimen (cisplatin + 5-fluorouracil), TP regimen (cisplatin + paclitaxel) and the TPF regimen (all three drugs). Due to poor medical condition or patient choice, some received mono-therapy.
Primary tumor radiotherapy was recommended for patients with distant metastasis in limited sites, stable metastatic disease after systemic chemotherapy, or serious symptoms caused by the primary tumor. Radiotherapy techniques ranged from conventional two-dimensional radiotherapy to three-dimensional conformal or intensity-modulated radiotherapy. Median dose prescribed to the primary tumor was 70.0 Gy. Local therapy for metastases included the palliative radiotherapy, radiofrequency ablation and surgery.
Treatment evaluations were performed after every two courses of chemotherapy, and then every 3 months after treatment. Tumor response was evaluated using the Response Evaluation Criteria in Solid Tumors (RECIST). Radiation toxicities were assessed and scored according to the radiation morbidity scoring criteria of the Radiation Therapy Oncology Group.
Statistical analysis
OS was defined as the duration from date of diagnosis to death or censorship at last follow-up, calculated using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate analysis were performed using the Cox proportion hazards model. Factors considered included patient factors (Karnofsky performance score, gender, age), basic laboratory characteristics (LDH, ALP, hemoglobin), and tumor features (T category, N category, features of metastases). A two-tailed P-value < 0.05 was considered significant.
The regression coefficients (β) of the independent prognostic factor were derived from the Cox regression equation (HR = eβ), then converted into integers to provide a score index for the prognostic score model.
Results
Patient characteristics and survival
The characteristics of 289 patients in the primary cohort and 156 patients in the validation cohort are summarized in Table 1.Among the patients in the primary cohort,median age was 52-years-old (range, 15–75 years). The incidence of bone metastasis, liver metastasis and lung metastasis was 67.4% (195/289), 33.2% (96/289), and 15.9% (46/289), respectively. The rates of objective response (complete and partial responses), stabilization, and progressive disease were 56.1% (162/289), 32.2% (93/289), and 11.8% (34/289), respectively.
Table 1.
Characteristics of the patients in the primary cohort and validation cohort.
| Number of patients(%) | ||
|---|---|---|
| Characteristic | Primary cohort (n = 289) | Validation cohort (n = 156) |
| Gender | ||
| Male | 249 (86.1) | 129 (82.7) |
| Female | 40 (13.9) | 27 (17.3) |
| Age (years) | ||
| Median | 52 | 49 |
| Range | 15-73 | 24–77 |
| Karnofsky performance score (KPS) | ||
| >70 | 240 (83.0) | 127 (81.4) |
| ≤70 | 49 (17.0) | 29 (18.6) |
| T category | ||
| T1-2 | 100 (34.6) | 20 (12.8) |
| T3-4 | 189 (65.4) | 136 (87.2) |
| N category | ||
| N0-1 | 81 (28.0) | 22 (14.1) |
| N2-3 | 208 (72.0) | 134 (85.9) |
| Sites of metastasis | ||
| Bone | 195 (67.4) | 100 (76.3) |
| Liver | 96 (33.2) | 40 (25.6) |
| Lung | 56 (17.6) | 34 (21.8) |
| Others | 20 (6.9) | 18 (11.5) |
| Multi-organ metastases (≥ 2) | ||
| Yes | 71 (24.6) | 50 (32.1) |
| No | 218 (75.4) | 106 (67.9) |
| Numbers of metastatic lesions | ||
| 1 | 56 (19.3) | 31 (19.9) |
| 2-5 | 105 (36.3) | 42 (26. 9) |
| ≥ 6 | 128 (44.4) | 83 (53.2) |
| Hemoglobin, Hb (g/L) | ||
| <120 | 39 (13.4) | 17 (10.9) |
| ≥120 | 250 (86.6) | 139 (89.1) |
| Alkaline phosphatase, ALP (IU/L) | ||
| ≤110 | 224 (77.6) | 126 (81.8) |
| >110 | 65 (22.4) | 30(19.2) |
| Lactate dehydrogenase, LDH (IU/L) | ||
| ≤245 | 187 (64.7) | 95 (60.9) |
| >245 | 102 (35.3) | 61 (39.1) |
| Chemotherapy cycles | ||
| 1-3 | 86 (29.8) | 39 (25.0) |
| ≥4 | 203 (70.2) | 117 (75.0) |
| Chemotherapy response | ||
| CR+PR | 162 (56.1) | 88 (56.4) |
| SD | 93 (32.2) | 36 (23.1) |
| PD | 34 (11.8) | 32 (20.5) |
Acute treatment-related toxicities were recorded. Grade III–IV leucopenia occurred in 47.4% (93/289) of patients during chemotherapy. Three patients died of treatment-related toxicities: one due to a severe infection caused by grade IV leucopenia, one due to gastric hemorrhage and another due to hepatic failure. Grade II-III mucositis occurred in 44.0% (80/183) of patients who received radiotherapy to the primary tumor.
Median survival time was 24 months (range, 2–144 months) and 25months (range, 4–86 months) for the primary cohort and validation cohort, and the 5-year overall survival rates were 23.0% and 24.3%,respectively.
Univariate and multivariate analyses in primary cohort
The factors analyzed in univariate analysis are listed in Table 2. The negative prognostic factors for OS were a Karnofsky performance score (KPS) ≤ 70 (HR = 6.78, P < 0.01), N2-3 (HR = 1.30, P = 0.01), liver metastases (HR = 2.89, P < 0.01), multiple-organ metastases (HR = 2.76, P < 0.01), number of lesions (2–5: HR = 1.64, P = 0.04; ≥6: HR = 6.40, P < 0.01), lactate dehydrogenase (LDH) > 245 IU/I (HR = 3.11, P < 0.01), alkaline phosphatase (ALP) > 110 IU/I (HR = 2.15, P < 0.01) and poor response to chemotherapy (SD: HR = 1.87, P = 0.03; PD: HR = 7.12, P < 0.01).
Table 2.
Univariate analysis of variables correlated with overall survival in primary cohort.
| Factor | 5-year OS (%) | HR (95% CI) | P-value |
|---|---|---|---|
| Gender, male/female | 21.2/40.8 | 1.57 (0.94–2.64) | 0.08 |
| Age (years), ≤50/>50 | 22.5/25.8 | 1.43 (0.73–1.68) | 0.76 |
| KPS, >70/≤70 | 28.6/0.0 | 6.78 (4.17–9.25) | <0.01a |
| T category, T1–2/T3–4 | 24.9/22.3 | 1.13 (0.64–1.24) | 0.41 |
| N category, N0–1/N2–3 | 32.8/20.5 | 1.30 (1.09–1.56) | 0.01 |
| Metastasis sites | |||
| Bone, no/yes | 21.5/25.2 | 0.87 (0.61–2.04) | 0.84 |
| Liver, no/yes | 32.4/8.5 | 2.89 (2.03–3.81) | <0.01a |
| Lung, no/yes | 23.4/31.4 | 1.22 (0.82–3.81) | 0.21 |
| Others, no/yes | 24.4/21.1 | 1.24 (0.70–2.20) | 0.12 |
| Multiple-organ metastases, no/yes | 28.7/4.3 | 2.76 (1.98–3.84) | <0.01a |
| Number of lesions | |||
| 1 | 50.5 | Baseline | — |
| 2–5 | 35.7 | 1.64 (1.01–2.64) | 0.04 |
| ≥6 | 3.5 | 6.40 (4.05–10.1) | <0.01 |
| Hemoglobin, ≥120 vs. < 120 g/L | 25.3/15.4 | 1.15 (0.78–1.70) | 0.48 |
| Serum LDH, ≤245 vs. >245IU/L | 35.3/3.3 | 3.11 (2.24–4.20) | <0.01a |
| Serum ALP, ≤110 vs. >110IU/L | 29.3/7.7 | 2.15 (1.59–2.93) | <0.01a |
| Chemotherapy response | |||
| CR + PR | 31.3 | Baseline | — |
| SD | 19.3 | 1.87 (1.25–2.32) | 0.03a |
| PD | 0.0 | 7.12 (4.68–10.8) | <0.01a |
HR, hazard ratio; CI, confidence interval; a statistically significant.
Cox multivariate analysis identified a KPS ≤ 70 (HR = 6.78, P = 0.003), liver metastases (HR = 2.89, P < 0.01), multiple-organ metastases (HR = 2.76, P < 0.01), number of lesions (2–5: HR = 1.64, P = 0.04; ≥6: HR = 6.40, P < 0.01), LDH > 245 IU/I (HR = 1.69, P < 0.01) and poor response to chemotherapy (SD: HR = 1.87, P = 0.03; PD: HR = 7.12, P < 0.01) were negative independent prognostic factors for OS. N2–3 and ALP > 110 IU/I were not significantly associated with OS in Cox multivariate analysis (Table 3).
Table 3.
Multivariate analysis of variables correlated with overall survival in the primary cohort.
| Factor | HR (95% CI) | P-value |
|---|---|---|
| KPS, >70/≤70 | 3.48 (1.74–6.44) | <0.01 |
| Liver metastases, no/yes | 1.63 (1.32–2.53) | <0.01 |
| Multiple-organ metastases, no/yes | 1.52 (1.08–2.17) | <0.01 |
| Number of lesions | ||
| 1 | Baseline | — |
| 2 to 5 | 2.31 (1.42–3.33) | 0.03 |
| ≥6 | 3.33 (2.34–5.95) | <0.01 |
| Serum LDH, ≤245 vs. >245 IU/L | 1.69 (1.22–2.66) | <0.01 |
| Chemotherapy response | ||
| CR + PR | Baseline | — |
| SD | 2.25 (1.42–3.09) | <0.01 |
| PD | 3.73 (2.31–6.98) | <0.01 |
Establishment and validation of the prognostic model
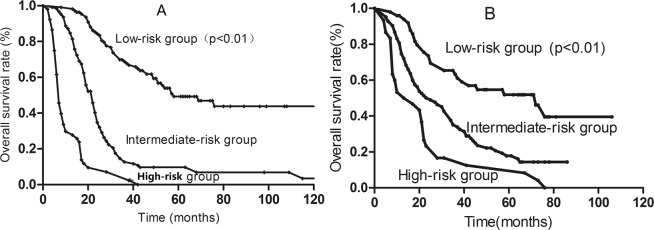
The prognostic-score model was built based on the six negative independent prognostic variables. A score of 1, 2 or 3 was assigned for each factor according to the HR (n) value (Table 4). The maximum possible score for each patient was 12. The prognostic score for each patient was calculated by summing the individual scores for each factor. Patients were then assigned to three risk stratification groups: low-risk group (total score, 0–2; 38.5% of patients [111/289]); intermediate-risk group (total score, 3–6; 43.5% of patients [126/289]); and high-risk group (total score, 7–12; 18.0% of patients [52/289]). The median survival time of the low-, intermediate- and high-risk groups were 48.0, 22.0 and 7.0 months, respectively. The respective 5-year OS rates were 49.3%, 9.7% and 0.0%, respectively (P < 0.001; Fig. 1A) in the primary cohort. In validation cohort, the survival for three groups was also significantly different and the 5-year OS rates were 51.9%, 14.9% and 0.0%, respectively (P < 0.01; Fig. 1B).
Table 4.
Prognostic index score based on patients in the primary cohort.
| Characteristic | Score | n (HR = en) |
|---|---|---|
| Multiple-organ metastases | 1 | 0.42 |
| Liver metastases | 1 | 0.48 |
| Serum LDH > 245 IU/L | 1 | 0.52 |
| Number of lesions | ||
| 1 | Baseline | — |
| 2 to 5 | 2 | 0.83 |
| ≥6 | 3 | 1.20 |
| KPS ≤ 70 | 3 | 1.24 |
| Chemotherapy response | ||
| CR + PR | Baseline | — |
| SD | 2 | 0.81 |
| PD | 3 | 1.30 |
Figure 1.
Kaplan-Meier survival curves for patients in the low-, intermediate- and high-risk groups in the primary cohort (A) and validation cohort (B).
Response to treatment modalities in the three risk stratification groups
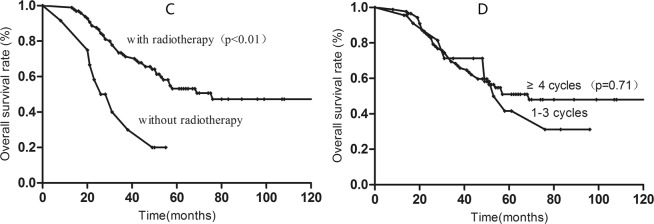
In the low-risk group, 5-year OS for patients treated with radiotherapy was 53.7%; no patients in this group survived more than five years without radiotherapy. However, patients who received 1–3 cycles and ≥4 cycles of chemotherapy achieved similar 5-year OS (41.7% vs. 51.0%; P = 0.71) (shown in Fig. 2).
Figure 2.
Kaplan-Meier survival curves for low-risk patients with radiotherapy (C) and chemotherapy cycles (D).
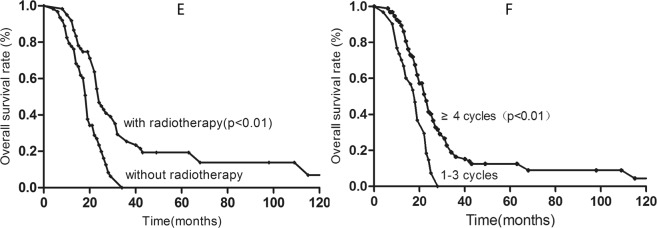
In the intermediate-risk group, patients who received ≥4 cycles of chemotherapy achieved better OS than those who received 1–3 cycles (12.0% vs. 0%; P < 0.01). Furthermore, radiotherapy to the primary tumor was associated with better OS than no radiotherapy in the intermediate-risk group (19.4% vs. 0.0%; P < 0.01) (shown in Fig. 3).
Figure 3.
Kaplan-Meier survival curves for intermediate-risk patients with radiotherapy (E) and chemotherapy cycles (F).
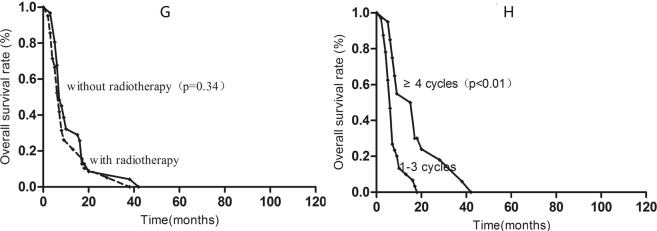
In the high-risk group, chemotherapy was an independent factor associated with OS: patients who received ≥4 cycles of chemotherapy had better 1-year OS than patients who received 1–3 cycles (13.4% vs. 50.0%; P < 0.01). However, radiotherapy to the primary tumor provided no benefit in terms of 1-year OS in the high-risk group (32.0% vs. 26.5%; P = 0.32) (shown in Fig. 4).
Figure 4.
Kaplan-Meier survival curves for high-risk patients with radiotherapy (G) and chemotherapy cycles (H).
Discussion
Traditionally, NPC with distant metastasis has a poor OS rate and is considered incurable. Yeh et al. reported the median OS duration of 125 patients with distant metastasis at diagnosis was only 9.7 months; 2-year OS was 14%4. However, the introduction of comprehensive treatment has greatly improved OS. Lin et al. retrospectively analyzed 105 patients treated with chemoradiotherapy: median OS duration was 25 months and estimated 5-year OS was 17%5. Furthermore, patients with distant metastasis at diagnosis have heterogenous treatment outcomes17,18. In a study of five patients who received comprehensive treatment, two had no evidence of disease at last follow-up (29 and 91 months) while the other three patients suffered disease progression within 12 months of the start of treatment17. Similarly, patients with different prognostic factors had variable survival outcomes in this study. The 5-year OS rates for patients in the low-, intermediate- and high-risk groups generated by the prognostic model were 49.3%, 9.7%, and 0.0%, respectively.
KPS is a significant prognostic factor in other tumor types, including lung cancer and esophageal cancer19,20. Poor performance status may be related to old age, severe comorbidity or more advanced disease. Jin et al. confirmed that poor performance status was associated with poorer survival in 718 patients with NPC with distant metastasis (hazard ratio of up to 2.1)11. Similarly, poor performance status was an important negative prognostic factor in this cohort of patients, with a hazard ratio of 3.48. Moreover, the 5-year OS rate for patients with a KPS >70 was 28.6%, while no patients with poor performance status survived for more than five years.
The sites and extension of distant metastases have long been considered to be prognostic factors in patients with NPC11,18,21,22. Compared to bone metastases and lung metastases, liver metastases are associated with poorer 5-year OS (32.4% vs. 8.5%). Ong et al. proposed a prognostic scoring system for OS after retrospectively reviewing 220 patients with distant metastasis, though only 21% of these patients had metastases at initial diagnosis. Liver metastasis was negatively associated with OS (hazard ratio, 1.54) in their prognostic model11. Consistent with these previous studies, we found patients with multiple-organ metastases or more than one lesion had poorer OS than those with single-site or single metastases. The 5-year OS rates for patients with 1, 2–5, and ≥6 lesions were 50.5%, 35.7%, and 3.5% respectively. These observations could be explained by the theory of oligometastases, a status extending from disease that remains localized to wide-spread dissemination that can be potentially cured23–25.
Our prognostic model based on the six independent negative factors may help to predict survival outcomes and identify patients who may benefit from comprehensive treatment. Systemic chemotherapy remains the recommended first-line treatment for NPC and is the most commonly used strategy in patients with distant metastasis at diagnosis. The value of loco-regional radiotherapy to the primary tumor remains a major controversy. However, increasing evidence indicates loco-regional radiotherapy provides a survival benefit by reducing primary tumor bulk and the severity of symptoms caused by the tumor4–7,17,18. Hu et al. retrospectively 41 patients treated with systemic chemotherapy followed by loco-regional definitive intensity-modulated radiation therapy as a first-line treatment; the 5-year OS rate was 22.5% and five patients were alive without evidence of disease after 52 to >101 months18. Partly consistent with those results, we found loco-regional radiotherapy could significantly prolong the survival of patients in the low- and intermediate-risk groups, but not the high-risk group. Furthermore, the survival benefits of systemic chemotherapy were variable. Patients in the intermediate- and high-risk groups who received at least four cycles of chemotherapy achieved significantly better OS than those who received less than four cycles, while the number of cycles of chemotherapy had no significant effect on OS in low-risk patients.
There were several weaknesses in the study. First, this prognostic model was based on retrospective analysis with inevitable selection bias in the treatment modalities. Second, for the lack of serum EBV DNA data, we did not investigate it in the study which has been demonstrated as an independent prognostic factor in disseminated NPC25. Finally, the data used to build the model was from endemic area which was need to be validated in the non-endemic area.
In conclusion, our prognostic score model based on six negative independent prognostic factors can effectively predict the survival of patients with NPC who have distant metastasis at initial diagnosis. Furthermore, loco-regional radiotherapy may be necessary for low-and intermediate-risk patients, but not for high-risk patients. However, due to the selection from the retrospective analysis, more prospective studies are needed to validate the prognostic score model.
Author contributions
Conception and design: Fei Han, Li Bai. Provision of study materials or patients: Yun-ming Tian, Wei-zeng Huang, Yu-hong Lan, Chong Zhao, Li Bai, Fei Han. Collection and assembly of data: All authors. Data analysis and interpretation: Yun-ming Tian, Li Bai, Fei Han. Manuscript writing: Li Bai, Fei Han. Final approval of manuscript: All authors. Accountable for all aspects of the work: All authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yun-ming Tian and Wei-zeng Huang.
Contributor Information
Yun-ming Tian, Email: tianyunmingzlyy@126.com.
Li Bai, Email: bailihuizhou@126.com.
Fei Han, Email: hanfeisysucc@126.com.
References
- 1.Yu MC, Yuan JM. Epidemiology of nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:421–429. doi: 10.1016/S1044579X02000858. [DOI] [PubMed] [Google Scholar]
- 2.Razak AR, et al. Nasopharyngeal carcinoma: the next challenges. Eur J Cancer. 2010;46:1967–1978. doi: 10.1016/j.ejca.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Lee AW, et al. The battle against nasopharyngeal cancer. Radiother Oncol. 2012;104:272–278. doi: 10.1016/j.radonc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Yeh SA, et al. Treatment outcomes of patients with AJCC stage IVC nasopharyngeal carcinoma: benefits of primary radiotherapy. Jpn J Clin Oncol. 2006;36:132–136. doi: 10.1093/jjco/hyi245. [DOI] [PubMed] [Google Scholar]
- 5.Lin S, et al. Combined high-dose radiation therapy and systemic chemotherapy improves survival in patients with newly diagnosed metastatic nasopharyngeal cancer. Am J Clin Oncol. 2012;35:474–479. doi: 10.1097/COC.0b013e31821a9452. [DOI] [PubMed] [Google Scholar]
- 6.Chen MY, et al. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer. 2013;32:604–613. doi: 10.5732/cjc.013.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CT, Cao KJ, Li Y, Xie GF. Prognosis analysis of nasopharyngeal carcinoma patients with distant metastasis. Ai Zheng. 2007;26:212–215. [PubMed] [Google Scholar]
- 8.Teo PM, et al. Prognosticators determining survival subsequent to distant metastasis from nasopharyngeal carcinoma. Cancer. 1996;77:2423–2431. doi: 10.1002/(SICI)1097-0142(19960615)77:12<2423::AID-CNCR2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 9.Khanfir A, et al. Prognostic factors in metastatic nasopharyngeal carcinoma. Cancer Radiotherapy. 2007;11:461–464. doi: 10.1016/j.canrad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Fandi Abderrahim, Bachouchi Mounir, Azli Nacer, Taamma Abdelkrim, Boussen Hammouda, Wibault Pierre, Eschwege François, Armand Jean-Pierre, Simon Jonathan, Cvitkovic Esteban. Long-Term Disease-Free Survivors in Metastatic Undifferentiated Carcinoma of Nasopharyngeal Type. Journal of Clinical Oncology. 2000;18(6):1324–1330. doi: 10.1200/JCO.2000.18.6.1324. [DOI] [PubMed] [Google Scholar]
- 11.Ong YK. Design of a prognostic index score for metastatic nasopharyngeal carcinoma. Eur J Cancer. 2003;39:1535–1541. doi: 10.1016/S0959-8049(03)00310-1. [DOI] [PubMed] [Google Scholar]
- 12.Jin Y, et al. To build a prognostic score model containing indispensible tumour markers for metastatic nasopharyngeal carcinoma in an epidemic area. Eur J Cancer. 2012;48:882–888. doi: 10.1016/j.ejca.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Edge, S. B. et al. AJCC cancer staging manual. 7th ed. Philadelphia: Lippincott-Raven; (2009).
- 14.Pan CC, et al. Challenges in the modification of the M1 stage of the TNM staging system for nasopharyngeal carcinoma: A study of 1027 cases and review of the literature. Exp Ther Med. 2012;4:334–338. doi: 10.3892/etm.2012.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Au E, Ang PT. A phase II trial of 5-fluorouracil and cisplatinum in recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol. 1994;5:87–89. doi: 10.1093/oxfordjournals.annonc.a058703. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, et al. Triplet combination with paclitaxel, cisplatin and 5-FU is effective in metastatic and/or recurrent nasopharyngeal carcinoma. Cancer Chemother Pharmacol. 2013;71:371–378. doi: 10.1007/s00280-012-2020-x. [DOI] [PubMed] [Google Scholar]
- 17.Setton J, Wolden S, Caria N, Lee N. Definitive treatment of metastatic nasopharyngeal carcinoma: Report of 5 cases with review of literature. Head Neck. 2012;34:753–757. doi: 10.1002/hed.21608. [DOI] [PubMed] [Google Scholar]
- 18.Hu SX, et al. Systemic chemotherapy followed by locoregional definitive intensity-modulated radiation therapy yields prolonged survival in nasopharyngeal carcinoma patients with distant metastasis at initial diagnosis. Med Oncol. 2015;32:224. doi: 10.1007/s12032-015-0663-2. [DOI] [PubMed] [Google Scholar]
- 19.Paesmans M, et al. Prognostic factors for survival in advanced non-small-cell lung cancer: univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1,052 patients. The European Lung Cancer Working Party. J Clin Oncol. 1995;13:1221–1230. doi: 10.1200/JCO.1995.13.5.1221. [DOI] [PubMed] [Google Scholar]
- 20.Polee MB, et al. Prognostic factors for survival in patients with advanced oesophageal cancer treated with cisplatin-based combination chemotherapy. Br J Cancer. 2003;89:2045–2050. doi: 10.1038/sj.bjc.6601364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui EP, et al. Lung metastasis alone in nasopharyngeal carcinoma: a relatively favorable prognostic group. A study by the Hong Kong nasopharyngeal carcinoma study group. Cancer. 2004;101:300–306. doi: 10.1002/cncr.20358. [DOI] [PubMed] [Google Scholar]
- 22.Tian YM, et al. Prognostic factors in nasopharyngeal carcinoma with synchronous liver metastasis: a retrospective study for the management of treatment. Radiat Oncol. 2013;8:272. doi: 10.1186/1748-717X-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh D, et al. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol Biol Phys. 2004;58:3–10. doi: 10.1016/S0360-3016(03)01442-1. [DOI] [PubMed] [Google Scholar]
- 24.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 25.An X, et al. Plasma Epstein-Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer. 2011;117:3750–3757. doi: 10.1002/cncr.25932. [DOI] [PubMed] [Google Scholar]