Abstract
Extant Crocodylia are exceptional because they employ almost the full range of quadrupedal footfall patterns (“gaits”) used by mammals; including asymmetrical gaits such as galloping and bounding. Perhaps this capacity evolved in stem Crocodylomorpha, during the Triassic when taxa were smaller, terrestrial, and long-legged. However, confusion about which Crocodylia use asymmetrical gaits and why persists, impeding reconstructions of locomotor evolution. Our experimental gait analysis of locomotor kinematics across 42 individuals from 15 species of Crocodylia obtained 184 data points for a wide velocity range (0.15–4.35 ms−1). Our results suggest either that asymmetrical gaits are ancestral for Crocodylia and lost in the alligator lineage, or that asymmetrical gaits evolved within Crocodylia at the base of the crocodile line. Regardless, we recorded usage of asymmetrical gaits in 7 species of Crocodyloidea (crocodiles); including novel documentation of these behaviours in 5 species (3 critically endangered). Larger Crocodylia use relatively less extreme gait kinematics consistent with steeply decreasing athletic ability with size. We found differences between asymmetrical and symmetrical gaits in Crocodylia: asymmetrical gaits involved greater size-normalized stride frequencies and smaller duty factors (relative ground contact times), consistent with increased mechanical demands. Remarkably, these gaits did not differ in maximal velocities obtained: whether in Alligatoroidea or Crocodyloidea, trotting or bounding achieved similar velocities, revealing that the alligator lineage is capable of hitherto unappreciated extreme locomotor performance despite a lack of asymmetrical gait usage. Hence asymmetrical gaits have benefits other than velocity capacity that explain their prevalence in Crocodyloidea and absence in Alligatoroidea—and their broader evolution.
Subject terms: Evolution, Biomechanics, Herpetology
Introduction
Extant Crocodylia have long been known to use almost all forms of walking and running locomotor modes (e.g., footfall patterns) present in quadrupedal mammals. These gaits include symmetrical (e.g., lateral/diagonal sequence walks; walking and running trots vide1,2) and asymmetrical (e.g., galloping, bounds and half-bounds) footfall patterns. Nevertheless, the diversity, scaling (body size correlations), and underlying mechanisms of this impressive locomotor repertoire are based only on a few studies of select species from the >23 extant members of Crocodylia. Symmetrical walking gaits have been fairly well studied for Crocodylia, mainly in alligatoroids such as Alligator mississippiensis (also Caiman crocodilus) [3–20 and see below]. There have been speculations or anatomical hints of divergent abilities in the “alligator lineage” (Alligatoroidea) vs. “crocodile lineage” (Crocodyloidea)21–24, but empirical evidence of any differences in locomotor abilities remains absent. Charig’s25 anecdote of sustained bipedal running in a crocodile (repeated in more recent studies; e.g.26) is highly specious, never having been reliably documented in that or any subsequent studies.
Bounding (synchronized left-right forelimb and hindlimb motions separated by an aerial phase) and galloping (slightly asynchronous left-right motions; essentially a slower version of bounding) gaits have been described for some Crocodylus species, especially C. johnstoni27,28, C. porosus29 and C. niloticus30,31. It is often misquoted in general media or natural history accounts that C. johnstoni is the only crocodylian known to gallop or bound; some27 wondered if its bounding ability was “unique” but it is clear that this capacity is more broadly distributed within Crocodylia. There is a brief report of putative asymmetrical gaits in juvenile Gavialis5,32, and anecdotes of these gaits in C. palustris, C. novaeguineae and Osteolaemus tetraspis33. It thus is poorly documented what species do use asymmetrical gaits — popular reviews of crocodylian biology often lament this gap in knowledge or recite old misinformation34–36.
In Alligatoroidea, studied velocities are almost exclusively slow, sustained walking <<2 ms−1 3–20. When asymmetrical gaits are used by Crocodylia, they involve the fastest velocities that those species can attain on land, although few accurate measurements exist. Zug29 alleged bounding in C. porosus at up to 18 ms−1, which is faster than most land animals37; this was an error and ~1.8 ms−1 was intended27. Bornhauser and Ziswiler31 obtained similar velocities (0.4 to 2.0 ms−1) for a galloping C. niloticus. Velocities faster than 5 ms−1 have never been recorded for Crocodylia — up to 4.7 ms−1 maximal velocities were measured for C. johnstoni27,28, with similar estimates for C. niloticus30. Considering that bounding tends to be 2–4 times faster than trotting in C. johnstoni27, can species that do not use a gait faster than a relatively rapid, symmetrical trot (i.e. diagonal limbs in phase) only move 2–4 times slower than those that can use asymmetrical gaits?
The kinematics of asymmetrical gaits have been noted to be highly variable within and among Crocodylia, covering almost all possible footfall combinations1,38 and disparate correlations of velocity and kinematic parameters such as stride length or frequency27–29. Some of this variation appears size-related — Zug29 noted that only young C. porosus <2 m total length bound or gallop, and Webb and Gans27 claimed such gaits to occur “only in juveniles” of similar size. This apparent size bias has been speculated to be a biomechanical constraint22,39. Yet the dearth of empirical studies of asymmetrical gaits impairs broader understanding. Indeed, it remains uncertain what enables or constrains asymmetrical gait usage in Crocodylia. Complex interplay between vertebrae, osteoderms, connective tissue, skin and muscle39–42 as well as flexibility and stiffness of the intervertebral joints43,44 may be critical elements. This complexity is augmented by the fact that the axial column switches from its plesiomorphic function of lateral undulation to a derived dorsoventral undulatory motion in bounding and galloping Crocodylia28,31. The amount of lateral undulation was presumably reduced in more erect stem archosaurs and then increased back to near-ancestral levels in later Crocodylomorpha [e.g.15,31].
Despite intense study of locomotion in alligatoroids, at least at slow velocities, no members of this lineage have been clearly shown to truly bound or gallop21. Studies have also noted (sometimes subtle) differences in exercise physiology45, foot form and resulting tracks46, track-making kinematics47, pectoral girdle and humerus shape48, limb musculature and its allometry22 and limb or vertebral proportions23,24 that might relate to differences in locomotor function or even behavior among crocodylian lineages. Small A. mississippiensis “attempted to gallop”11 but a full stride was not achieved, and alligators “could not be induced to gallop”9. Numerous studies have proposed that asymmetrical gaits are ancestral for Crocodylia21,27,39,49, inherited from more terrestrial ancestors in Crocodylomorpha (or even Archosauria). Given that most prior data were based on studies of C. johnstoni and C. porosus, what can a broader sample of locomotor data from Crocodylia tell us about the evolution of asymmetrical gaits? Is there clear evidence for an ancestral capacity for bounding and galloping gaits in Crocodylia and do all lineages, at least at small body sizes, retain this ability?
Here we report our study of crocodylian locomotor dynamics that includes video documentation of gait usage in 15 species from a variety of body sizes within the range expected to move quickly (≤2 m total length; ≤50 kg body mass). First, we reconstruct the evolutionary history of asymmetrical gaits, which have been speculated to only be restricted to a few species. Using basic phylogenetic theory, we test the hypothesis (H1) that routine asymmetrical gait usage is homologous for all Crocodylia (i.e., present in Alligatoroidea and Crocodyloidea). Second, we quantify biomechanical constraints on gait usage in Crocodylia, especially the scaling of asymmetrical gait kinematics. We test the hypothesis (H2) that capacity for asymmetrical gaits declines with increasing body mass (i.e., negatively allometric scaling), for key kinematic parameters such as maximal velocity (absolute and size-normalized), minimal duty factor (relative ground contact time per stride; correlated with peak limb force50); and maximal relative stride frequency. Third, if we do find differences in gait usage (via H1 being falsified) between the lineages of Alligatoroidea and Crocodyloidea, we test the hypotheses that (H3) gait kinematics are different between asymmetrical and symmetrical gaits in the two clades; and (H4) asymmetrical gaits in Crocodyloidea are faster than symmetrical gaits in Alligatoroidea.
Methods
We studied 42 individuals of 15 species of Crocodylia (body mass range 0.5 kg to 43 kg; Supplementary Dataset S1) at the St. Augustine Alligator Farm Zoological Park (Florida), outdoors during the daytime at mean summer temperatures ~30 °C (±2 °C approximately). An additional 14 individual Crocodylia (26 attempted trials; 12 species, including three not in the final dataset: Paleosuchus trigonatus, Caiman jacare and Melanosuchus niger) were studied but did not provide useful data and were excluded from the study; whereas 7 of our 42 individuals were measured twice in separate data collection sessions on different years (and here are treated as the same individual where relevant for statistical analyses). Crocodylians were caught from their enclosures, weighed and measured, and marked with white poster paint or infrared-reflective motion capture markers (1–2 cm diameter) around their joint centres of rotation. The animals were allowed to rest and recover from capture to minimize fatigue. Rest times between capture and between trials varied depending on keeper assessments of the condition of animals, and data collection sessions terminated when animals showed clear signs of fatigue or reluctance to locomote. Due to diverse constraints on available time for staff, experiments and animals, these rest times could not be standardized; nor could periodic cloacal temperature or blood lactate tests be performed.
For data collection, crocodylians were released at one end of a ~5 m long, 1–1.5 m wide runway on level ground, with its left central side facing a lateral view camera(s) and a second camera for dorsal view footage suspended above the central region. Animals were encouraged to move across the runway by simple release, auditory/visual cues, gentle prodding, and/or placement of refugia (bushes/water) at the end of the runway. The techniques used varied based on keepers’ advice customized to the individual animal and situation. The central runway flooring was either a force platform with top plate (force data not presented here), solid wooden board, or woodchips, depending on accessible space during the three years of data collection sessions (see below). Crocodylians were not harmed for the purposes of this study. The experimental protocol was reviewed and approved by the Royal Veterinary College’s Ethics and Welfare Committee; approval number URN 2012 1187 R. All experiments were performed in accordance with relevant guidelines and regulations.
The above methods were consistently used, but as we collected data during three different years (2002, 2004, 2005), the hardware and software used to collect and analyse the kinematic data varied. Video footage of trials was recorded in 2002 at 200 Hz (720 × 480 pixels), in 2004 at 60 Hz (720 × 480 pixels), and in 2005 at 50 Hz (720 × 576 pixels). All video data were then digitally captured (Ulead Visual Studio 9.0; Ulead Systems, Taipei, Taiwan) as video files trimmed down to individual trials for initial analysis in Virtual Dub software (http://www.virtualdub.org/), in which foot touchdown/liftoff timings (from video fields) were recorded for each visible limb for all complete strides (cycle of footfalls). We classified footfall patterns using the limb phases1,2,28,38 as a fraction of a stride between foot touchdown events; with the left hindlimb as the “0” reference. These limb phases corresponded to categories of footfall patterns (“gaits”) coded as trot (1), lateral sequence (2), diagonal sequence (3), rotary gallop (4), transverse gallop (5), half-bound (6), and bound (7); codes 1–3 were symmetrical gaits and 4–7 asymmetrical. We also calculated stance phase duration (time from touchdown to liftoff) and swing phase duration (time from liftoff to touchdown) for each limb, stride duration (mean stance + swing phase durations for all limbs), stride frequency (SF; inverse of stride duration; as Hz), and duty factor (DF; stance phase duration as a fraction of stride duration; as mean of all limbs) as kinematic parameters used in our statistical analyses.
In Matlab software (The MathWorks, Inc., Natick, MA), we digitized hip and shoulder markers from videos to calculate mean forward velocities (u) across a stride, using objects of known scale in the field of view to calibrate from pixels to meters of distance. In the 2005 data collection session, we also had four motion capture cameras (MCU 500; Qualisys AB, Göteborg, Sweden) arranged around the runway area; instead of a lateral view camera. These cameras were used to record (at 240 Hz) the 3D positions of the infrared markers around the body and limb joints of subjects, replacing the 50 Hz videos for velocity but not footfall analysis in that dataset. Only trials that were deemed to involve relatively straight-ahead, steady-state locomotion were used for kinematic analysis. Yet as crocodylians did not normally move quickly in a true steady state (e.g., >10% velocity change within a stride), we used a rough subjective criterion for “steady”, erring on the side of maximal inclusivity to favor natural – and near-maximal, where feasible – locomotor patterns (inevitably including variation that would introduce noise into our dataset). All valid occurrences of different gaits within that final dataset were recorded for characterizing which species used each locomotor mode.
To facilitate comparisons between Crocodylia of different sizes moving with comparable relative kinematics (i.e. more dynamically similar51,52), we normalized kinematic parameters. Relative stride frequency (RSF) was computed using g = 9.81 ms−2 and h = extended hindlimb length to tip of third digit (in m):
| 1 |
Dimensionless velocity (); or the square root of the Froude number; was:
| 2 |
Statistical analyses were conducted using SPSS Statistics software version 25 (IBM Corp., Armonk, NY). Phylogenetic statistics were not conducted as we had already split our sample into the lineages Crocodyloidea and Alligatoroidea and judged our sample inappropriate for available methods in this context. Linear models (LM) or linear mixed effects (LME) models (accounting for repeated measures from the same individuals using random effects) were used to assess hypotheses H2, H3 and H4. Residual variances were allowed to vary depending on the camera recording frequency (Hz) in both LM and LME models, because the higher frame rate and thus temporal precision of the 2002 (and 2004) datasets vs. 2005 might introduce non-systematic biases. Normality of the residuals was assessed visually and data were log-transformed where necessary. We analyzed three datasets: (1) all data for all velocities and gaits pooled (n = 42 individuals; 8 Alligatoroidea); (2) all “running” data obtained, where running was identified based on DF < 0.50 and an asymmetrical gait (n = 12 individuals, 36 strides); and (3) the single fastest stride (for values ≥0.90) per individual (n = 22 Crocodyloidea; 5 Alligatoroidea), as follows.
H1 was tested qualitatively based on footfall patterns; the absence of asymmetrical gaits in Alligatoroidea (or Crocodyloidea) would falsify it. We tested H2 using datasets 2 and 3 (comparing patterns for our fastest vs. any running trials), with log body mass as the predictor and log u, log , DF, and RSF as the outcome variables. LME was used for analysis of all running trials (dataset 2), and LM was used for the analysis of each single fastest stride (dataset 3).
For testing H3, we used dataset 1 (all data), processed using LME as above; but comparing symmetrical gaits in Alligatoroidea with symmetrical and asymmetrical gaits in Crocodyloidea (i.e., three groups) to test for differences in adjusted means for log u, log , DF, and RSF between the three groups.
We tested H4 only with dataset 3 (fastest trial per individual with ≥ 0.90), using LM, where only symmetrical gaits of Alligatoroidea were compared with asymmetrical gaits of Crocodyloidea.
All data including the videos of trials analyzed, and statistical code and analysis outputs, are available on Figshare [http://figshare.com/articles/Video_data_Crocodylian_locomotor_kinematics/11322035]. Additional kinematic changes with speed are described, using nonlinear regression analyses, in the Supplementary text S1, Supplementary Figs. S1–S3, and Supplementary Tables S1–S3.
Results
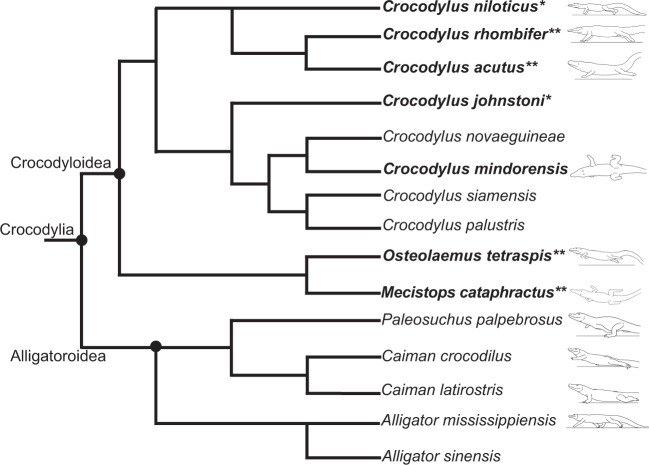
We obtained 184 useable trials from slow to near-maximal velocities (0.15–4.4 ms−1). 42 trials involved asymmetrical gaits, from 17 of our 42 individual subjects (Supplementary Dataset S2). Supplementary Movies S1–S10 illustrate the diverse range of rapid symmetrical and asymmetrical gait performance that we recorded across Crocodylia. We found that Crocodyloidea and Alligatoroidea used different gaits — the former adopted a wide range of asymmetrical gaits (normally a bound at their fastest velocity28), whereas the latter only employed symmetrical gaits (and normally a trot at their fastest velocities). No Alligatoroidea ever galloped or bounded; thus our Hypothesis 1 (H1) was not supported (Fig. 1).
Figure 1.
Distribution of asymmetrical gaits within Crocodylia. Examples of asymmetrical and symmetrical gaits (single frames from videos) from our analysis, mapped onto a phylogeny of Crocodylia (composite from67–71); for testing our H1. Taxa in bold font are known to use asymmetrical gaits. *Indicates taxa with recorded asymmetrical gaits in prior studies and this one; **Indicates taxa with new discoveries of asymmetrical gaits in this study. Line drawings on the right side (by Scott Hartman) are outlines from screen captures from experimental videos of the fastest strides of representative individuals from dataset 3, taken from visible hindfoot-off timings and emphasizing symmetrical gaits for Alligatoroidea vs. bounding asymmetrical gaits for Crocodyloidea. Overhead views of Crocodylus mindorensis and Mecistops cataphractus were reversed so that all are facing left. Not to scale.
We recorded the first documentation of asymmetrical gaits in the critically endangered Philippine crocodile Crocodylus mindorensis — all four of the juveniles studied were remarkably adept at the full range of crocodylian gaits (e.g., Supplementary Movie S6). Likewise, we obtained data for asymmetrical gaits (1 subject, 2 trials; rotary gallop at 2.36 ms−1 and bounding at 2.98 ms−1) in the critically endangered African slender-snouted crocodile Mecistops cataphractus. Additionally, we recorded the only published dataset for asymmetrical gait usage in C. rhombifer (Supplementary Movie S8), C. acutus and (in quantitative detail; cf. Cott, 1961; Bornhauser and Ziswiler, 1983) C. niloticus (Supplementary Movie S7); plus one instance of a very rapid symmetrical gait (3.1 ms−1 trotting) in C. siamensis. Previous evidence for terrestrial locomotor behavior in these taxa was anecdotal at best. We also confirmed purported bounding and galloping ability in Osteolaemus tetraspis (Whitaker and Andrews, 1988), providing the only quantitative data on asymmetrical gaits in this unusual dwarf crocodile, such as bounding at up to 2.98 ms−1 (Supplementary Movie S9). Our data for the more well-studied C. johnstoni contributed new quantitative information on gaits of adult individuals, augmenting a detailed study of small juveniles28, although our adults did not reach the most rapid velocities of those animals or the field study subjects27.
Our study’s measurements of high speed locomotion in alligatoroids are novel. For example, the fastest published, reliably recorded velocities are 0.62 ms−1 for fast trotting in juvenile A. mississippiensis, with duty factors (DF) ~0.7010,14. In contrast, the maximal velocities and minimal DF we obtained were >3 ms−1 and <0.50 DF for alligatoroids, with our fastest young A. mississippiensis trotting at 2.0 ms−1 and 0.50 DF (Supplementary Movie S1).
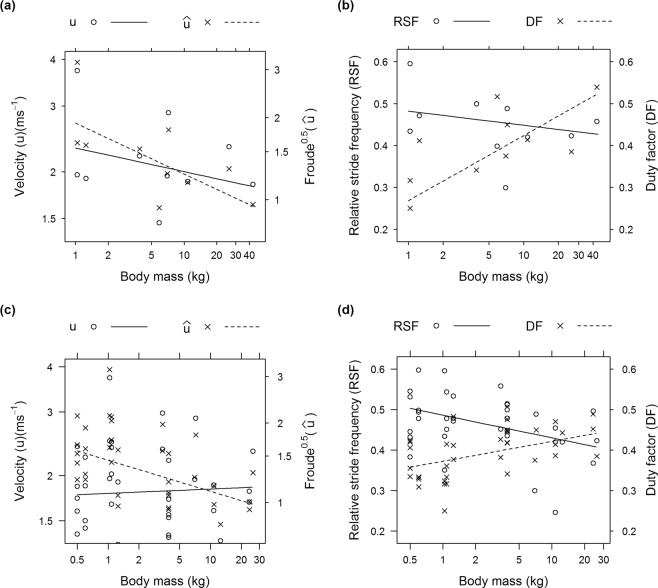
Body mass did not have a significant correlation with maximal velocity (u; in ms−1) although our subjects’ size range, despite spanning two orders of magnitude (0.5–43.2 kg), was restricted to smaller, presumably faster individuals (~2 m total length or less) under 50 kg body mass, far from the >500 kg mass that some of our study species can reach (Fig. 2). However, we found body mass effects on size-normalized kinematics across our subjects that indicated a decline in dimensionless locomotor performance — at the maximal velocities measured (dataset 3), heavier Crocodylia had slower relative velocities () and greater DF (p = 0.026, 0.013). Thus smaller Crocodylia were more athletic, with greater capacity for asymmetrical gaits as per our H2. As dataset 3 was a limited sample size (n = 10 individual Crocodyloidea using asymmetrical gaits at their maximal u), we checked if these results were still upheld with our dataset for all Crocodyloidea using fast asymmetrical gaits (dataset 2; n = 12 individuals, 36 trials), which produced congruent correlations; additionally emphasizing that RSF decreased weakly with body mass (p = 0.014; Table 1). These findings are robust support for our Hypothesis 2 (H2) that the capacity for asymmetrical gaits declines with increasing body mass (i.e., negatively allometric scaling) within Crocodylia; particularly Crocodyloidea.
Figure 2.
Bivariate plots from analyses using linear mixed effects models (a,b) and linear models (c,d), depicting the relationships of kinematic y-variables with body mass, based on only Crocodyloidea and asymmetrical gait data. (a,b): dataset 3 (fastest running stride per individual); (c,d): dataset 2; all “running” strides (DF < 0.50), accounting for repeated measures per individual. See Table 1 for adjusted means and standard errors of the coefficients.
Table 1.
Results from tests of H2 and H3, from a linear mixed effects model analysis (see Methods), focusing on four kinematic parameters: log velocity (u), log Froude0.5 (), duty factor (DF) and relative stride frequency (RSF).
| Test + data | log(u) | log() | DF | RSF |
|---|---|---|---|---|
| H2: dataset 3 | −0.06 ± 0.03 | −0.19 ± 0.03 | 0.07 ± 0.01 | −0.01 ± 0.02 |
| maximal u | (p = 0.180) | (p = 0.026) | (p = 0.013) | (p = 0.417) |
| H2: dataset 2 | 0.01 ± 0.05 | −0.12 ± 0.04 | 0.02 ± 0.01 | −0.02 ± 0.01 |
| running (DF < 0.50) | (p = 0.806) | (p = 0.044) | (p = 0.029) | (p = 0.014) |
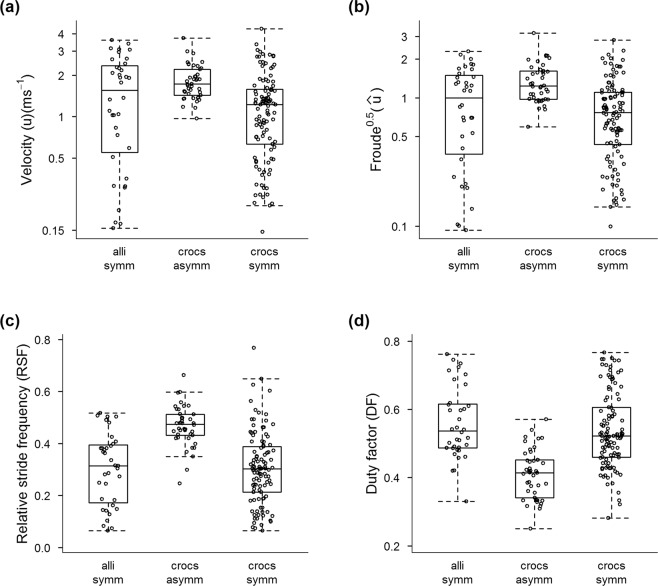
| H3: Alli-symm | 0.05 ± 0.21ab | −0.41 ± 0.22ab | 0.57 ± 0.03a | 0.30 ± 0.03a |
| H3: Crocs-symm | −0.05 ± 0.11a | −0.48 ± 0.12a | 0.56 ± 0.02a | 0.30 ± 0.02a |
| H3: Crocs-asymm | 0.46 ± 0.14b | 0.04 ± 0.14b | 0.46 ± 0.02b | 0.41 ± 0.02b |
| H3: Overall p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Residual variances were allowed to vary depending on the recording frequency (Hz). Bold font emphasizes where p < 0.05. For H2, relationships with log(body mass) are based only on Crocodyloidea and asymmetrical gait data. Adjusted regression coefficients (i.e. slope of the regression line) ± standard errors are shown. Results from dataset 3 are compared with those from dataset 2, in which the model accounted for repeated measures from the same subjects. For H3, we present a comparison of three groups (“alli-symm” = Alligatoroidea symmetrical gaits; “crocs-symm” = Crocodyloidea symmetrical gaits; “crocs-asymm” = Crocodyloidea asymmetrical gaits), using dataset 1 (all valid strides), focusing on adjusted means ± standard errors. Individual number was used as a random effect in the analysis. There was no statistical difference between groups sharing the same letters (superscript a or b).
Considering that our H1 was falsified, we inspected what kinematic parameters differentiated Crocodyloidea and Alligatoroidea for all velocities and gaits (dataset 1; symmetrical and asymmetrical gaits). Our analysis (Fig. 3) supported Hypothesis 3, that symmetrical vs. asymmetrical gait kinematics differ between the two major clades of Crocodylia (p < 0.0001; Table 1). Velocities in ms−1 were only different within Crocodyloidea: asymmetrical gaits tended to be faster than symmetrical gaits; but across all trials and subjects Crocodyloidea was no faster than Alligatoroidea. This same pattern was reproduced when we inspected . Yet asymmetrical gaits had greater RSF and smaller DF than symmetrical gaits (e.g., trotting), both within Crocodyloidea and for Crocodyloidea vs. Alligatoroidea; a trend that was also observable within individuals (Supplementary Dataset S2). Symmetrical gaits, in contrast, did not differ between the two clades of Crocodylia. Other aspects of basic locomotor kinematics were indistinguishable between the two major lineages of Crocodylia, although there was high variability (Fig. 3; Supplementary Figs. S1–S3).
Figure 3.
Box-and-whisker plots comparing four kinematic parameters (a–d) for three categories of locomotor data from Crocodylia (“alli symm” = Alligatoroidea symmetrical gaits; “crocs asymm” = Crocodyloidea asymmetrical gaits; “crocs symm” = Crocodyloidea symmetrical gaits), based on dataset 1 (all gait data). See Table 1 for adjusted means and standard errors from the linear mixed effects modeling analyses.
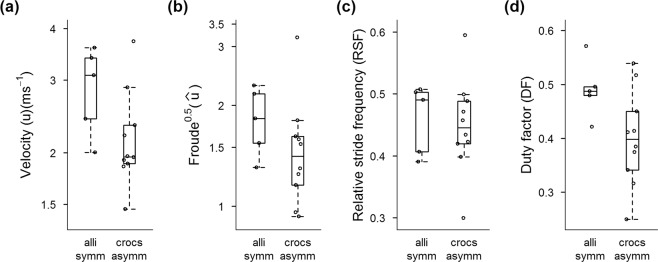
Our Hypothesis 4 posited that asymmetrical gaits are faster than symmetrical gaits (from dataset 3). The maximal velocities (u) and in each individual studied for Alligatoroidea vs. Crocodyloidea (Fig. 4) showed unexpected differences: in particular, Crocodyloidea was ~30–50% slower than Alligatoroidea (p = 0.030, 0.031; Table 2). However, given the small sample sizes and high variation we are wary of accepting that inference, leaving our H4 open to interpretation but certainly disfavouring slower absolute or relative velocities for Alligatoroidea. Notably, we obtained a maximal u of 3.7 and 4.4 ms−1 for our fastest individuals from these two clades (Supplementary Dataset S2); respectively Caiman crocodilus, trotting (Supplementary Movie S3); and Crocodylus acutus, using a diagonal sequence running gait (Supplementary Movie S2). We also did not find any prominent differences between DF or RSF for Alligatoroidea vs. Crocodyloidea, although DF was weakly smaller (by 0.07 or ~14% vs. Alligatoroidea) in the latter clade (p = 0.075).
Figure 4.
Box-and-whisker plots comparing four kinematic parameters (a–d) for two clades of Crocodylia (“alli symm” = Alligatoroidea symmetrical gaits; “crocs asymm” = Crocodyloidea asymmetrical gaits) based on dataset 3 (fastest running stride per individual). See Table 2 for adjusted means and standard errors from the linear modeling analyses.
Table 2.
Results from tests of H4, from a linear mixed effects model analysis (see Methods), focusing on four kinematic parameters: log velocity (u), log Froude0.5 (), duty factor (DF) and relative stride frequency (RSF) from dataset 3 (fastest running stride per individual); comparing the adjusted means ± standard errors for two groups (“alli-symm” = Alligatoroidea symmetrical gaits; “crocs-asymm” = Crocodyloidea asymmetrical gaits); with p values (bold font for < 0.05). Residual variances were allowed to vary depending on the recording frequency (Hz).
| Data | log(u) | log() | DF | RSF |
|---|---|---|---|---|
| Alli-symm | 1.02 ± 0.10 | 0.60 ± 0.10 | 0.49 ± 0.03 | 0.47 ± 0.03 |
| Crocs-asymm | 0.72 ± 0.07 | 0.28 ± 0.08 | 0.42 ± 0.02 | 0.45 ± 0.02 |
| H4: Overall p-value | 0.030 | 0.031 | 0.075 | 0.437 |
Furthermore, 25 individuals of 13 species (87 trials in our broader dataset 2; including symmetrical gait data) achieved relatively rapid running gaits with aerial phases (mean DF < 0.5), with 5 and 4 of these individuals and species (~25% of our sample) being from Alligatoroidea. We often found that, within individual crocodiles using both symmetrical and asymmetrical running gaits, both gait categories could reach similar velocities (e.g., 17 individuals used asymmetrical gaits but it was their fastest gait in only 10 of them; Supplementary Dataset S2). For example, one 0.5 kg C. mindorensis used a diagonal sequence symmetrical gait at 2.44 ms−1 and a transverse gallop at 2.41 ms−1; its two fastest trials; in addition to a lateral sequence symmetrical gait at 1.99 ms−1 and a half-bound at 1.87 ms−1. Crocodyloidea that bounded, nonetheless, usually used it as their fastest gait.
Discussion
First, we have shown that only members of Crocodyloidea in our sample used asymmetrical gaits, falsifying our H1. Second, our H2 was supported, that capacity for asymmetrical gait usage declines with increasing body mass (from 0.5 to 43 kg; two orders of magnitude in scaling) in crocodiles. Third, we demonstrated that there are no clear differences between the symmetrical gait kinematics of Alligatoroidea and Crocodyloidea, but that asymmetrical gaits in crocodiles involve greater relative stride frequencies and smaller duty factors than symmetrical gaits in either lineage (supporting H3). Finally, our H4 was falsified — Alligatoroidea and Crocodyloidea can reach similar speeds even though only the latter uses asymmetrical gaits to achieve them. We now explore the implications of each hypothesis test.
Our data establish that small representatives of Crocodyloidea, whether immature members of large-bodied species or adult members of dwarf species (e.g., Osteolaemus), can bound and gallop, and this capacity seems to be ancestral at least for this lineage. Most studies have concluded that asymmetrical gaits were ancestral for Crocodylia, based on the functional morphology of fossil Crocodylomorpha [39–41,49 but see44]. We concur that the morphofunctional evidence renders this the most plausible hypothesis. If that scenario is correct, then ancestral Alligatoroidea lost this ability (or do not express it). Conversely, an alternative hypothesis that the common ancestor of Crocodylia lacked the capacity for asymmetrical gaits and Crocodyloidea uniquely evolved it still deserves examination; a possibility that is almost never acknowledged. The reported presence of asymmetrical gaits in young Gavialis5,32 is important because if that taxon lies outside of Crocodyloidea + Alligatoroidea (Fig. 1) then this would bolster the inference that asymmetrical gaits are ancestral for Crocodylia. The locomotion of its purported sister taxon in Crocodyloidea, Tomistoma, remains unstudied to our knowledge. Indeed, asymmetrical gaits in Gavialis are not documented with concrete photo or video imagery and hence deserve empirical examination.
Why alligatoroids do not bound or gallop remains uncertain. Some candidate explanations such as muscle leverage or exercise physiology are not known to be appreciably different for these clades but have not been deeply investigated. Habitats frequented by these lineages of Crocodylia differ, with some Alligatoroidea ranging into more temperate climes. Here, we studied all of our Crocodylia at about the same ambient “field” temperature, which they had been acclimatized to in captivity, so this variable was removed in our experimental design but body temperature was not explicitly controlled.
We have also made discoveries and confirmed anecdotes about previously obscure behaviors of Crocodylia: featuring the first documented evidence of asymmetrical gaits in Crocodylus mindorensis, C. rhombifer, C. acutus, Mecistops cataphractus and Osteolaemus tetraspis (Fig. 1; Supplementary Movies S6, S8, S9). These records are important given the critically endangered status of the first three taxa. Anecdotes of asymmetrical gait usage in C. palustris and C. novaeguineae33 are now plausible in light of the prevalence of these gaits in other Crocodyloidea. We were unable to obtain more than symmetrical gaits from these two taxa; respectively only 2 and 3 valid trials each. Together, our new observations of asymmetrical gaits and our broader dataset on locomotor kinematics spanning the clade Crocodylia considerably expand our knowledge of their behaviours and natural history. Importantly, this combined evidence strongly refutes the popular notion that only a few crocodiles (mainly C. johnstoni; also C. porosus) use asymmetrical gaits.
Webb and Gans27 noted that smaller Crocodylus johnstoni used greater stride frequencies than larger individuals, but these freshwater crocodiles were capable of increasing maximal velocities across a snout-vent length range of ~20–85 cm, consistent with positive allometry of absolute athletic performance (albeit within less than one order of body size range). As DF in our analysis would have a strong inverse correlation with ground reaction forces incurred by the limbs50, our observation of increased DF in larger Crocodyloidea (across two orders of magnitude of body mass range) is consistent with data from muscle architecture, demonstrating negative allometry of the relative capacity to generate muscular forces in proportion to body weight22. This pattern of reduced relative athletic capacity is strongly reinforced by the negative allometry of and RSF—larger crocodiles moved more slowly relative to their size using fewer strides per unit time. At some body mass beyond the range of our subjects’, true running gaits with DF < 0.50 should become impossible.
Interestingly, symmetrical gait kinematics did not differ between Crocodyloidea and Alligatoroidea in our sample, but asymmetrical gaits had smaller DF and greater RSF; hence involving more extreme kinematics at a given speed. This result (from H3, focusing on dataset 1) was somewhat reflected at maximal speeds (H4; dataset 3; Table 2 for DF) although differences were marginal. Overall, our data for Crocodyloidea correspond well with the few detailed records for bounding and galloping kinematics. In particular, for Crocodylus johnstoni, Renous et al.28 obtained minimal DF ~0.2 and maximal >1.0, with SF > 2.5 Hz, and their individuals used asymmetrical gaits between 0.4–4 ms−1, only bounding past 2.0 ms−1, and generally maintaining forelimb greater than hindlimb DF values; matching general kinematic patterns in this study (e.g., Supplementary Text S1; Supplementary Figs. S1–S3; Supplementary Tables S1–S3).
In addition to their importance for basic understanding of maximal performance in Crocodylia, these findings have implications for bone safety factors (ratios of failure stress to peak in vivo stress). Past experimental assessment of bone safety factors10 has made important contributions towards understanding crocodylian functional design but may have underestimated safety factors at maximal performance and, thus, deserves some re-evaluation. Perhaps the limb bones of Crocodylia are not as “overbuilt” as previously inferred10 because those calculations were based on locomotor speeds and gaits that impose lower stresses on the bones, thereby reflecting local maximum bone stresses of that given speed/gait rather than the absolute maximum (‘peak’) stresses for a species/individual. The extreme minimal DF values presented here, close to 0.40 (vs. 0.70 in prior studies), imply that peak limb forces (and thus tissue stresses) should be ~1.75 times greater than prior measurements10, which would lead to proportionately reduced safety factors (e.g., from mean values respectively for the femur and tibia of 6.7 and 2.7 to 3.8 and 1.5). However, Blob and Biewener10 also calculated “worst case” values of 3.2 and 1.3, which our data suggest reducing to 1.8 and 0.74 (with the caveat that these are purely theoretical values). The revised estimates suggested here, while making the assumption that limb angular kinematics (and thus moments) do not change with speed or gait, nonetheless fall near the ~2–4 range of safety factors estimated for birds and mammals53.
Thus more investigation of how peak “field” (non-laboratory) performance might impact conclusions drawn from safety factor analysis, or other characterizations of locomotion, is needed for Crocodylia. The grossly similar morphology of limb bones across Crocodylia23 and the prevalence of asymmetrical gaits in Crocodyloidea, involving some faster speeds and smaller duty factors (hence even greater loads50), are further cause for caution. Prior studies of Alligatoroidea recorded maximal speeds of 0.62 ms−1 or less and DF ≥ 0.663–20. These are undeniably valuable data on high walks/slow running trots at close to preferred or moderate speeds, but this study shows even the fastest prior data are far from maximal speeds or minimal duty factors.
We acknowledge challenging limitations to this study. It was difficult to motivate many individuals, hence 14 from our initial sample were excluded, and the majority of these (nine total) were from Alligatoroidea. Indeed, we noticed that members of the alligator lineage seemed (qualitatively) more likely to sit and hiss, struggle, or fight rather than run away from stimuli or release from captivity, compared with Crocodyloidea. We speculate that this is a behavioral tendency that may partly underlie the divergent locomotor abilities within Crocodylia. Even so, our main sample was disproportionately represented by Crocodyloidea (34 vs. 8 Alligatoroidea individuals), which partly was by design. Intensive prior studies of the alligator lineage have failed to identify any asymmetrical gaits in this clade, so we focused on collecting data from under-sampled species (e.g., not Alligator mississippiensis) and from individuals expected by keepers to be highly active—these constraints limited our sample of Alligatoroidea. Regardless, we found hitherto unreported extreme locomotor performance in this clade, albeit only using symmetrical gaits. Our conclusion that Alligatoroidea does not employ asymmetrical gaits could be reinvestigated with a broader sample, but our study and existing literature on Crocodyloidea and Alligatoroidea strongly point toward a divergence in their gait usage.
Performance of individual Crocodylia varied tremendously, and surely was influenced by complex factors including not only motivation but also fatigue, body temperature, personnel, equipment, environment (e.g. substrate stiffness) and more. Our statistical models took into account the main factors we could identify as likely confounding agents, and our experimental design attempted to maximally control these factors within the constraints of the setting, staff, and animals. As an additional check, we re-ran the LME analyses for H2 and H4 (repeated measures) including the effects of stride and trial numbers within an individual as covariates in the models, to test if later strides or trials within our datasets had slower speeds. We found no such effect – indeed, where any effect was found it was a very slight increase of speed in later trials. Hence we conclude that animals did not suffer clear fatigue across trials.
The surprising result that maximal velocities did not differ between Alligatoroidea and Crocodyloidea (H4; Fig. 4, Table 2), or were sometimes even faster in Alligatoroidea, reveals that the key benefit of asymmetrical gaits in Crocodylia is not maximal speed capacity. Thus a vexing mystery is why Crocodyloidea bound or gallop, given that they tend to choose to do so at faster speeds, and why this capacity originated, if more deeply embedded within Crocodylomorpha. Allen et al.22 inferred that longer limb muscle fascicles correlate with asymmetrical gaits in Crocodylia (also see54). Webb and Gans27 postulated that asymmetrical gaits in Crocodylus johnstoni are useful for crossing rough terrain. A study of mouse gaits suggested that bounding might have benefits for stability against perturbations55, consistent with the latter notion and paralleling suggestions of stability/maneuverability tradeoffs in bounding and galloping C. johnstoni28. Reilly et al.56 also proposed benefits for velocity and energetics in bounding toads; aspects of metabolism related to fatigue or endurance remain unexplored candidate explanations for asymmetrical gaits in Crocodylia.
Asymmetrical galloping and bounding gaits are recognized to have evolved multiple times in sarcopterygian vertebrates, including lungfish57, toads56, turtles54, and mammals [e.g.1,58] — and at least one spider and one insect use analogous mechanisms59,60. Thus these locomotor mechanisms are far from being restricted to cursorial mammals as some analyses imply [e.g.61–63]. Notably, as in prior studies27–31, all of our Crocodyloidea using asymmetrical gaits had a single extended period (e.g., Fig. 1) during their aerial phase (“suspension”), not a single gathered (“collected”) suspension or alternating gathered/extended suspensions as typify various mammals61,62. The reasons for this singular axial undulatory pattern remain obscure. The aforementioned cases of convergent evolution provide potential to understand the truly fundamental principles of these gait mechanisms vs. which patterns (e.g., axial undulatory motions) are divergent. Such understanding could test if stability or other benefits, such as circumventing breathing constraints64, broadly underlie the evolution of asymmetrical gaits, and could be useful in crocodylian-inspired robotic design [e.g.65,66].
Supplementary information
Acknowledgements
We thank David Drysdale, owner of the St. Augustine Alligator Farm Zoological Park, for allowing us access to his remarkable crocodylian collection and for making staff members available. We appreciate the help of undergraduate students Rebecca Parkes and Georgina Disney, and Karin Jespers and Andrew Greenhalgh for expert technical assistance; as well as other staff of the St. Augustine Alligator Farm Zoological Park, and Structure and Motion Laboratory. We thank Gregory Erickson, Rodger Kram and Max Donelan for their assistance in early stages of this work, and Sandy Kawano for comments on part of this manuscript. Scott Hartman is thanked for drawing parts of Fig. 1. J.R.H. was supported by funding from a bioinformatics fellowship from the National Science Foundation, an internal grant from The Royal Veterinary College, and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant Agreement #695517).
Author contributions
Conceived the study: J.R.H. Designed the methods: J.R.H. Conducted experimental data collection: J.R.H., D.K., K.A.V., J.B., D.F. Analyzed experimental data: J.R.H., D.F., K.H. Designed, conducted and interpreted statistical analyses: Y.-M.C., J.R.H. Wrote main paper: J.R.H. All authors contributed to editing the paper and approved the final draft.
Competing interests
J.B. is employed by the St. Augustine Alligator Farm Zoological Park.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-55768-6.
References
- 1.Hildebrand M. The quadrupedal gaits of vertebrates. BioSci. 1989;39:766–775. doi: 10.2307/1311182. [DOI] [Google Scholar]
- 2.Lee DV, Harris SL. Linking gait dynamics to mechanical cost of legged locomotion. Front. Robotics AI. 2018;5:111. doi: 10.3389/frobt.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huene F von. Beobachtungen über die Bewegungsart der Extremitäten bei Krokodilien. Biologisch. Centralbl. 1913;33:468–472. [Google Scholar]
- 4.Schaeffer B. The morphological and functional evolution of the tarsus in amphibians and reptiles. Bull. Am. Mus. Nat. Hist. 1941;78:395–472. [Google Scholar]
- 5.Bustard HR, Singh LAK. Studies on the Indian Gharial Gavialis gangeticus (Gmelin) (Reptilia, Crocodilia)- change in terrestrial locomotory pattern with age. J. Bombay. Nat. Hist. Soc. 1977;74:534–536. [Google Scholar]
- 6.Brinkman D. The hind limb step cycle of Caiman sclerops and the mechanics of the crocodile tarsus and metatarsus. Can. J. Zool. 1980;58:2187–2200. doi: 10.1139/z80-301. [DOI] [Google Scholar]
- 7.Padian K, Olsen PE. The fossil trackway Pteraichnus: not pterosaurian, but crocodilian. J. Paleontol. 1984;58:178–184. [Google Scholar]
- 8.Gatesy SM. Hind limb movements of the American alligator (Alligator mississippiensis) and postural grades. J. Zool. 1991;224:577–588. doi: 10.1111/j.1469-7998.1991.tb03786.x. [DOI] [Google Scholar]
- 9.Gatesy SM. An electromyographic analysis of hindlimb function in Alligator during terrestrial locomotion. J. Morphol. 1997;234:197–212. doi: 10.1002/(SICI)1097-4687(199711)234:2<197::AID-JMOR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Blob RW, Biewener AA. In vivo locomotor strain in the hindlimb bones of Alligator mississippiensis and Iguana iguana: implications for the evolution of limb bone safety factor and non-sprawling limb posture. J. Exp. Biol. 2001;202:1023–1046. doi: 10.1242/jeb.202.9.1023. [DOI] [PubMed] [Google Scholar]
- 11.Reilly SM, Elias JA. Locomotion in Alligator mississippiensis: kinematic effects of speed and posture and their relevance to the sprawling-to-erect paradigm. J. Exp. Biol. 1998;201:2559–2574. doi: 10.1242/jeb.201.18.2559. [DOI] [PubMed] [Google Scholar]
- 12.Reilly SM, Blob RW. Motor control of locomotor hindlimb posture in the American alligator (Alligator mississippiensis) J. Exp. Biol. 2003;206:4327–4340. doi: 10.1242/jeb.00688. [DOI] [PubMed] [Google Scholar]
- 13.Willey JS, Biknevicius AR, Reilly SM, Earls KD. The tale of the tail: limb function and locomotor mechanics in Alligator mississippiensis. J. Exp. Biol. 2004;207:553–563. doi: 10.1242/jeb.00774. [DOI] [PubMed] [Google Scholar]
- 14.Reilly SM, Willey JS, Biknevicius AR, Blob RW. Hindlimb function in the alligator: integrating movements, motor patterns, ground reaction forces and bone strain of terrestrial locomotion. J. Exp. Biol. 2005;208:993–1009. doi: 10.1242/jeb.01473. [DOI] [PubMed] [Google Scholar]
- 15.Carpenter K. Role of lateral body bending in crocodylian track making. Ichnos. 2009;16:202–207. doi: 10.1080/10420940802686137. [DOI] [Google Scholar]
- 16.Baier DB, Gatesy SM. Three-dimensional skeletal kinematics of the shoulder girdle and forelimb in walking. Alligator. J. Anat. 2013;223:462–473. doi: 10.1111/joa.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farlow JO, et al. Trackways of the American crocodile (Crocodylus acutus) in northwestern Costa Rica: implications for crocodylian ichnology. Ichnos. 2018;25:30–65. doi: 10.1080/10420940.2017.1350856. [DOI] [Google Scholar]
- 18.Pashchenko DI. A new interpretation of the crocodile forelimb morphological features as adaptation to parasagittal quadrupedal locomotion on the ground. Doklady Biol. Sci. 2018;483:235–238. doi: 10.1134/S0012496618060054. [DOI] [PubMed] [Google Scholar]
- 19.Baier DB, Garrity BM, Moritz S, Carney RM. Alligator mississippiensis sternal and shoulder girdle mobility increase stride length during high walks. J. Exp. Biol. 2019;221:jeb186791. doi: 10.1242/jeb.186791. [DOI] [PubMed] [Google Scholar]
- 20.Nyakatura JA, et al. Reverse-engineering the locomotion of a stem amniote. Nature. 2019;565:351–355. doi: 10.1038/s41586-018-0851-2. [DOI] [PubMed] [Google Scholar]
- 21.Gauthier JA, et al. The bipedal stem crocodilian Poposaurus gracilis: inferring function in fossils and innovation in archosaur locomotion. Bull. Peabody. Mus. Nat. Hist. 2011;52:107–126. doi: 10.3374/014.052.0102. [DOI] [Google Scholar]
- 22.Allen V, et al. Comparative architectural properties of limb muscles in Crocodylidae and Alligatoridae and their relevance to divergent use of asymmetrical gaits in extant Crocodylia. J. Anat. 2014;225:569–582. doi: 10.1111/joa.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iijima M, Kubo T, Kobayashi Y. Comparative limb proportions reveal differential locomotor morphofunctions of alligatoroids and crocodyloids. Roy. Soc. Open Sci. 2018;5:171774. doi: 10.1098/rsos.171774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iijima M, Kubo T. Comparative morphology of presacral vertebrae in extant crocodylians: taxonomic, functional and ecological implications. Zool. J. Linn. Soc. 2019;186:1006–1025. [Google Scholar]
- 25.Charig, A. J. The evolution of the archosaur pelvis and hindlimb: an explanation in functional terms. [Joysey, K. A. & Kemp, T. S. (eds.)] Studies in Vertebrate Evolution, 121–171 (Oliver & Boyd, 1972).
- 26.Padian K, Li C, Pchelnikova J. The trackmaker of Apatopus (Late Triassic, North America): implications for the evolution of archosaur stance and gait. Palaeontol. 2010;53:175–189. doi: 10.1111/j.1475-4983.2009.00924.x. [DOI] [Google Scholar]
- 27.Webb GJ, Gans C. Galloping in Crocodylus johnstoni- a reflection of terrestrial activity? Rec. Austral. Mus. 1982;34:607–618. doi: 10.3853/j.0067-1975.34.1982.244. [DOI] [Google Scholar]
- 28.Renous S, Gasc JP, Bels VL, Wicker R. Asymmetrical gaits of juvenile Crocodylus johnstoni, galloping Australian crocodiles. J. Zool. 2002;256:311–325. doi: 10.1017/S0952836902000353. [DOI] [Google Scholar]
- 29.Zug GR. Crocodilian galloping: an unique gait for reptiles. Copeia. 1974;1974:550–552. doi: 10.2307/1442557. [DOI] [Google Scholar]
- 30.Cott HB. Scientific results of an inquiry into the ecology and economic status of the Nile crocodile (Crocodilus niloticus) in Uganda and Northern Rhodesia. Trans. Zool. Soc. Lond. 1961;29:211–356. doi: 10.1111/j.1096-3642.1961.tb00220.x. [DOI] [Google Scholar]
- 31.Bornhauser C, Ziswiler V. Lokomotionsstudie und Funktionsanalyse der epaxonischen Muskulatur beim Nilkrokodil Crocodilus niloticus (Reptilia, Crocodylia) Rev. Suisse. Zool. 1983;90:789–798. doi: 10.5962/bhl.part.117743. [DOI] [Google Scholar]
- 32.Singh LAK, Bustard HR. Locomotory behaviour during basking and spoor formation in the gharial (Gavialis gangeticus) Brit. J. Herpetol. 1977;5:673–676. [Google Scholar]
- 33.Whitaker R, Andrews H. Notes on crocodilian locomotion. J. Bombay Nat. Hist. Soc. 1988;85:621–622. [Google Scholar]
- 34.Steel, R. Crocodiles. (Christopher Helm, 1989).
- 35.Richardson, K. C., Webb, G. J. W. & Manolis, S. C. Crocodiles: Inside Out. A Guide to the Crocodilians and their Functional Morphology. (Surrey Beatty & Sons, 2002).
- 36.Grigg, G. & Kirshner, D. Biology and Evolution of Crocodylians. (Csiro Publishing, 2015).
- 37.Garland T. The relation between maximal running speed and body mass in terrestrial mammals. J. Zool. 1983;199:157–170. doi: 10.1111/j.1469-7998.1983.tb02087.x. [DOI] [Google Scholar]
- 38.Biknevicius AR, Reilly SM. Correlation of symmetrical gaits and whole body mechanics: debunking myths in locomotor biodynamics. J. Exp. Zool. A: Comp. Exp. Biol. 2006;305:923–934. doi: 10.1002/jez.a.332. [DOI] [PubMed] [Google Scholar]
- 39.Salisbury S. W. & Frey, E. A biomechanical transformation model for the evolution of semi-spheroidal articulations between adjoining vertebral bodies in crocodilians. [Grigg, G. C., Seebacher, F. & Franklink, C. E. (eds.)] Crocodilian Biology and Evolution. 121–148. (Surrey Beatty & Sons, 2001).
- 40.Frey E. Das Tragsystem der Krododiele: eine biomechanische und phylogenetische Analyse. Stuttg. Beitr. Naturk. Ser. A. 1988;426:1–60. [Google Scholar]
- 41.Rossmann T. Studien an känozoischen krokodilen: 5. Biomechanische untersuchung am postkranialen skelett des paläogenen krokodils Pristichampsus rollinatii (Eusuchia: Pristichampsidae) Neues Jahrb. Geol. Paläontol.-Abh. 2000;217:289–330. doi: 10.1127/njgpa/217/2000/289. [DOI] [Google Scholar]
- 42.Schwarz-Wings D, Frey E, Martin T. Reconstruction of the bracing system of the trunk and tail in hyposaurine dyrosaurids (Crocodylomorpha; Mesoeucrocodylia) J. Vert. Paleontol. 2009;29:453–472. doi: 10.1671/039.029.0228. [DOI] [Google Scholar]
- 43.Molnar JL, Pierce SE, Hutchinson JR. An experimental and morphometric test of the relationship between vertebral morphology and joint stiffness in Nile crocodiles (Crocodylus niloticus) J. Exp. Biol. 2014;217:758–768. doi: 10.1242/jeb.089904. [DOI] [PubMed] [Google Scholar]
- 44.Molnar JL, et al. Morphological and functional changes in the vertebral column with increasing aquatic adaptation in crocodylomorphs. Roy. Soc. Open Sci. 2015;2:150439. doi: 10.1098/rsos.150439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Owerkowicz T, Baudinette RV. Exercise training enhances aerobic capacity in juvenile estuarine crocodiles (Crocodylus porosus) Comp. Biochem. Physiol. A: Mol. Integr. Physiol. 2008;150:211–216. doi: 10.1016/j.cbpa.2008.04.594. [DOI] [PubMed] [Google Scholar]
- 46.Milàn J, Hedegaard R. Interspecific variation in tracks and trackways from extant crocodylians. Crocodyle Tracks and Traces. New Mexico Mus. Nat. Hist. Sci. Bull. 2010;51:15–29. [Google Scholar]
- 47.Kubo T. Variation in modern crocodylian limb kinematics and its effect on trackways. Crocodyle Tracks and Traces. New Mexico Mus. Nat. Hist. Sci. Bull. 2010;51:51–53. [Google Scholar]
- 48.Chamero B, Buscalioni ÁD, Marugán-Lobón J. Pectoral girdle and forelimb variation in extant Crocodylia: the coracoid–humerus pair as an evolutionary module. Biol. J. Linn. Soc. 2013;108:600–618. doi: 10.1111/j.1095-8312.2012.02037.x. [DOI] [Google Scholar]
- 49.Parrish JM. The origin of crocodilian locomotion. Paleobiol. 1987;13:396–414. doi: 10.1017/S0094837300009003. [DOI] [Google Scholar]
- 50.Witte TH, Knill K, Wilson AM. Determination of peak vertical ground reaction force from duty factor in the horse (Equus caballus) J. Exp. Biol. 2004;207:3639–3648. doi: 10.1242/jeb.01182. [DOI] [PubMed] [Google Scholar]
- 51.Alexander RM, Jayes AS. A dynamic similarity hypothesis for the gaits of quadrupedal mammals. J. Zool. 1983;201:135–152. doi: 10.1111/j.1469-7998.1983.tb04266.x. [DOI] [Google Scholar]
- 52.Hof AL. Scaling gait data to body size. Gait & Posture. 1996;4:222–223. doi: 10.1016/0966-6362(95)01057-2. [DOI] [Google Scholar]
- 53.Biewener AA. Bone strength in small mammals and bipedal birds: do safety factors change with body size? J. Exp. Biol. 1982;98:289–301. doi: 10.1242/jeb.98.1.289. [DOI] [PubMed] [Google Scholar]
- 54.Mayerl CJ, Blob RW. A novel, bounding gait in swimming turtles: implications for aquatic locomotor diversity. J. Exp. Biol. 2017;220:3611–3615. doi: 10.1242/jeb.164103. [DOI] [PubMed] [Google Scholar]
- 55.Vahedipour A, et al. Uncovering the structure of the mouse gait controller: Mice respond to substrate perturbations with adaptations in gait on a continuum between trot and bound. J. Biomech. 2018;78:77–86. doi: 10.1016/j.jbiomech.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 56.Reilly SM, et al. Conquering the world in leaps and bounds: hopping locomotion in toads is actually bounding. Funct. Ecol. 2015;29:1308–1316. doi: 10.1111/1365-2435.12414. [DOI] [Google Scholar]
- 57.King HM, Shubin NH, Coates MI, Hale ME. Behavioral evidence for the evolution of walking and bounding before terrestriality in sarcopterygian fishes. Proc. Natl. Acad. Sci. 2011;108:21146–21151. doi: 10.1073/pnas.1118669109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Riskin DK, Hermanson JW. Biomechanics: independent evolution of running in vampire bats. Nature. 2005;434:292. doi: 10.1038/434292a. [DOI] [PubMed] [Google Scholar]
- 59.Gorb SN, Barth FG. Locomotor behavior during prey-capture of a fishing spider, Dolomedes plantarius (Araneae: Araneidae): galloping and stopping. J. Arachnol. 1994;22:89–93. [Google Scholar]
- 60.Smolka J, Byrne MJ, Scholtz CH, Dacke M. A new galloping gait in an insect. Curr. Biol. 2013;23:R913–R915. doi: 10.1016/j.cub.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 61.Bertram JE, Gutmann A. Motions of the running horse and cheetah revisited: fundamental mechanics of the transverse and rotary gallop. J. Roy. Soc. Interface. 2008;6:549–559. doi: 10.1098/rsif.2008.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biancardi CM, Minetti AE. Biomechanical determinants of transverse and rotary gallop in cursorial mammals. J. Exp. Biol. 2012;215:4144–4156. doi: 10.1242/jeb.073031. [DOI] [PubMed] [Google Scholar]
- 63.Tanase M, Ambe Y, Aoi S, Matsuno F. A galloping quadruped model using left–right asymmetry in touchdown angles. J. Biomech. 2015;48:3383–3389. doi: 10.1016/j.jbiomech.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 64.Carrier DR. The evolution of locomotor stamina in tetrapods: circumventing a mechanical constraint. Paleobiol. 1987;13:326–341. doi: 10.1017/S0094837300008903. [DOI] [Google Scholar]
- 65.Shriyam S, Mishra A, Nayak D, Thakur A. Design, fabrication and gait planning of alligator-inspired robot. Int. J. Curr. Engin. Tech. Spec. Iss. 2014;2:567–575. doi: 10.14741/ijcet/spl.2.2014.108. [DOI] [Google Scholar]
- 66.Pouya S, Khodabakhsh M, Spröwitz A, Ijspeert A. Spinal joint compliance and actuation in a simulated bounding quadruped robot. Auton. Rob. 2017;41:437–452. doi: 10.1007/s10514-015-9540-2. [DOI] [Google Scholar]
- 67.Brochu CA. Morphology, fossils, divergence timing, and the phylogenetic relationships of Gavialis. Syst. Biol. 1997;46:479–522. doi: 10.1093/sysbio/46.3.479. [DOI] [PubMed] [Google Scholar]
- 68.Gatesy J, Amato G, Norell M, DeSalle R, Hayashi C. Combined support for wholesale taxic atavism in gavialine crocodylians. Syst. Biol. 2003;52:403–422. doi: 10.1080/10635150390197037. [DOI] [PubMed] [Google Scholar]
- 69.Gold MEL, Brochu CA, Norell MA. An expanded combined evidence approach to the Gavialis problem using geometric morphometric data from crocodylian braincases and Eustachian systems. PloS one. 2014;9:e105793. doi: 10.1371/journal.pone.0105793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee MS, Yates AM. Tip-dating and homoplasy: reconciling the shallow molecular divergences of modern gharials with their long fossil record. Proc. Roy. Soc. B. 2018;285:20181071. doi: 10.1098/rspb.2018.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iijima, M. & Kobayashi, Y. Mosaic nature in the skeleton of East Asian crocodylians fills the morphological gap between “Tomistominae” and Gavialinae. Cladistics, 10.1111/cla.12372 (2019). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.