Abstract
The pattern of ketamine-induced locomotor activity varies substantially across ontogeny and according to sex. Although ketamine is classified as an NMDA channel blocker, it appears to stimulate the locomotor activity of both male and female rats via a monoaminergic mechanism. To more precisely determine the neural mechanisms underlying ketamine’s actions, male and female preweanling and adolescent rats were pretreated with vehicle, the dopamine (DA) synthesis inhibitor ∝-methyl-dl-p-tyrosine (AMPT), or the serotonin (5-HT) synthesis inhibitor 4-chloro-dl-phenylalanine methyl ester hydrochloride (PCPA). After completion of the pretreatment regimen, the locomotor activating effects of saline, ketamine, D-amphetamine, and cocaine were assessed during a 2 h test session. In addition, the ability of AMPT and PCPA to reduce dorsal striatal DA and 5-HT content was measured in male and female preweanling, adolescent, and adult rats. Results showed that AMPT and PCPA reduced, but did not fully attenuate, the ketamine-induced locomotor activity of preweanling rats and female adolescent rats. Ketamine (20 and 40 mg/kg) caused a minimal amount of locomotor activity in male adolescent rats, and this effect was not significantly modified by AMPT or PCPA pretreatment. When compared to ketamine, D-amphetamine and cocaine produced different patterns of locomotor activity across ontogeny; moreover, AMPT and PCPA pretreatment affected psychostimulant- and ketamine-induced locomotion differently. When these results are considered together, it appears that both dopaminergic and serotonergic mechanisms mediate the ketamine-induced locomotor activity of preweanling and female adolescent rats. The dichotomous actions of ketamine relative to the psychostimulants in vehicle-, AMPT-, and PCPA-treated rats, suggests that ketamine modulates DA and 5-HT neurotransmission through an indirect mechanism.
Keywords: Ketamine, cocaine, D-amphetamine, ∝-methyl-dl-p-tyrosine (AMPT), 4-chloro-dl-phenylalanine methyl ester hydrochloride (PCPA), locomotor activity
1. Introduction
Evidence suggests that ketamine use is increasing world-wide. In the United States and other countries, ketamine is often used recreationally as a “rave” or “club” drug [1–3], while in some Southeast Asian nations ketamine is one of the most frequently used illicit drugs [3–5]. In terms of licit uses, ketamine was approved by the Food and Drug Administration nearly five decades ago (1970) for use as a dissociative anesthetic [6–8]. Renewed interest in the therapeutic properties of ketamine was generated when it was reported that a single subanesthetic infusion quickly reduces the symptomology associated with treatment-resistant depression [9,10]. Interestingly, the robustness of this therapeutic effect may be positively correlated with ketamine’s acute dissociative actions [11,12; but see 13].
In rats, ketamine, which is an NMDA receptor open channel blocker [14,15], causes sedation, analgesia, and anesthesia at high doses [16–18]. At subanesthetic doses, ketamine causes prolonged periods of hyperactivity in both rats and mice [16,19,20]. One of the most interesting aspects of this ketamine effect is that it varies greatly according to age and sex [21–27]. More specifically, ketamine causes approximately equal amounts of hyperactivity in female rats across ontogeny (i.e., from the preweanling period, through adolescence, and into adulthood); whereas, male rats show a progressive decline in ketamine-induced locomotor activity with increasing age [25,26]. By adulthood, ketamine causes a much stronger locomotor response in female rats than male rats [21,25,26]. The cause of these age- and sex-dependent behavioral effects has not been fully established; however, pharmacokinetic factors may play a critical role [26,28]. Specifically, ketamine’s locomotor activating effects are mirrored by age- and sex-dependent differences in peak ketamine levels and drug availability in brain [26]. Even if pharmacokinetic factors account for these age- and sex-dependent differences, the neuronal mechanisms underlying ketamine’s locomotor activating effects are still uncertain.
Since dopamine (DA) and serotonin (5-HT) neurotransmission is associated with motor movement [29–31], many researchers have attempted to determine whether ketamine and other NMDA open channel blockers [e.g., MK-801 and phencyclidine (PCP)] either directly or indirectly modulate monoamine system functioning. Indeed, antagonists at D1 and D2 receptors, as well as 5-HT2A receptors, significantly reduce the hyperactivity caused by ketamine and MK-801 [16,32–42]. Using a different approach (i.e., a monoamine depletion strategy), Crawford et al. [43] showed that a dose of reserpine (5 mg/kg) sufficient to reduce dorsal striatal DA and 5-HT levels by 87–96% caused a significant decline in the ketamine-induced locomotor activity of male and female preweanling, adolescent, and adult rats. Similar results were reported when reserpine-treated adult male rats and mice were injected with MK-801 or PCP [33,34,44–46]. Although these results are informative, reserpine’s lack of specificity makes it difficult to parse out the relative importance of the various monoamine neurotransmitters.
The purpose of the present study was to significantly extend the latter results by pretreating male and female preweanling and adolescent rats with DA [∝-methyl-dl-p-tyrosine (AMPT)] or 5-HT [4-chloro-dl-phenylalanine methyl ester hydrochloride (PCPA)] synthesis inhibitors. After receiving their pretreatment regimens, rats were injected with ketamine (20 or 40 mg/kg), D-amphetamine, or cocaine and locomotor activity was assessed for 120 min. Although the effects of DA and 5-HT synthesis inhibitors have not been assessed in ketamine-treated male and female preweanling and adolescent rats, AMPT has alternately been reported to reduce or leave intact the ketamine- and MK-801-induced hyperactivity of male adult mice [34,35]. The reason for this inconsistency is unknown. Moreover, there is equivocal evidence suggesting that PCPA may reduce the locomotor activating effects of MK-801 in male adult rats and mice [38]. As in our companion paper [43], male and female rats were also tested with doses of D-amphetamine (2 mg/kg) and cocaine (15 mg/kg) known to produce robust locomotor activity in rats of these ages [47–49]. These groups were included for comparison purposes, because there is evidence that ketamine may function like a psychostimulant at the presynaptic terminal [16,50,51; but see 52].
2. Materials and Methods
2.1. Subjects
Subjects were 276 preweanling, 396 adolescent, and 36 adult male and female Sprague-Dawley rats. Within these ontogenetic periods, rats were tested at PD 18–PD 21, PD 38–PD 41, and PD 78–PD 81, respectively, which is consistent with established norms [53–57]. Adult rats were purchased from Charles River (Hollister, CA). Preweanling and adolescent rats were born and raised at California State University, San Bernardino (CSUSB). Litters were culled to 10 pups on PD 3 and weaned at PD 21. Rats were group housed in large polycarbonate maternity cages (30.5 × 43 × 19 cm) on ventilated racks. Food and water were freely available. The colony room was maintained at 22–23 °C and kept under a 12:12 light-dark cycle. Testing was conducted during the light phase of the cycle. Subjects were cared for according to the “Guide for the Care and Use of Laboratory Animals” [58] under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
2.2. Apparatus
Locomotor activity was assessed in commercially available activity monitoring chambers (Coulbourn Instruments, Whitehall, PA) that had acrylic walls, a plastic floor, and an open top. Because rats tested on PD 21 and PD 41 differ substantially in body size, preweanling rats were tested in smaller chambers (26 × 26 × 41 cm) than adolescent rats (41 × 41 × 41 cm) [59,60]. Each chamber was equipped with an X–Y photobeam array, with 16 photocells and detectors, that was used to measure distance traveled. Photobeam resolution for the large chambers was 1.27 cm and for the small chambers was 0.76 cm. The position of each rat was assessed every 100 ms (i.e., the sampling interval), thus allowing the Truscan software (Version 1.006, Coulbourn Instruments) to determine distance traveled.
2.3. Drugs
AMPT (∝-methyl-dl-p-tyrosine, product no. M3281), PCPA (4-chloro-dl-phenylalanine methyl ester hydrochloride, product no. C3635), (±)-ketamine hydrochloride (product no. K1068), cocaine hydrochloride (product no. C5776), and D-amphetamine hemisulfate salt (product no. A5880) were dissolved in saline and injected intraperitoneally (ip) at a volume of 2.5 ml/kg (preweanling rats) or 1 ml/kg (adolescent and adult rats). With the exception of ketamine (Spectrum Chemicals, New Brunswick, NJ), all compounds were purchased from Sigma-Aldrich (St. Louis, MO).
2.4. Experimental designs and procedures
2.4.1. Experiment 1a: Effects of AMPT on the ketamine-induced locomotor activity of male and female preweanling rats
On PD 20, rats were injected with saline and habituated to activity chambers for 30 min. On PD 21, rats were injected with vehicle or AMPT (200 mg/kg) 4 h and 2 h before behavioral testing. This two-injection protocol was used in order to maximize DA synthesis inhibition [35,61]; in our hands, AMPT (2 × 200 mg/kg) produces large reductions in the dorsal striatal DA levels of young rats [62,63]. After each vehicle/AMPT injection, rats were returned to their home cage. At the time of behavioral testing (i.e., 2 h after the final AMPT injection), rats (n = 10 per group) were injected with saline or ketamine (5, 10, 20, or 40 mg/kg, ip). A broad dose range of ketamine was used because male and female rats are differentially responsive to this drug [21,22,25,26,43,64]. Immediately after saline/ketamine injections, rats were placed in the testing chamber and locomotor activity, which was operationally defined as distance traveled (cm), was measured continuously across 12 ten-minute time blocks (2 h). Time blocks of this duration are sufficient to show time-dependent changes in the psychopharmacological effects of ketamine [25,26,43] and psychostimulant drugs [47–49].
2.4.2. Experiment 1b: Effects of AMPT on the D-amphetamine- and cocaine-induced locomotor activity of male and female preweanling rats
Habituation, drug pretreatments, and behavioral testing were conducted in the same manner as described in Experiment 1a, except that male and female preweanling rats (n = 10 per group) were injected (ip) with saline, 2 mg/kg D-amphetamine, or 15 mg/kg cocaine before locomotor activity assessment.
2.4.3. Experiment 2: Effects of PCPA on the ketamine-, D-amphetamine-, and cocaine-induced locomotor activity of male and female preweanling rats
Male and female preweanling rats were injected with vehicle or PCPA (200 mg/kg) on PD 18, PD 19, and PD 20 (a three-day administration protocol is standard for PCPA) [38,65–68]. One hour prior to the final PCPA treatment, rats were injected with saline and habituated to the testing chamber for 30 min. On PD 21 (24 h after the final injection of vehicle or PCPA), male and female adolescent rats (n = 8 per group) were injected with saline, ketamine (20 or 40 mg/kg, ip), D-amphetamine (2 mg/kg, ip), or cocaine (15 mg/kg, ip) and distance traveled was measured for 120 min.
2.4.4. Experiment 3: Effects of AMPT on the ketamine-, D-amphetamine-, and cocaine-induced locomotor activity of male and female adolescent rats
On PD 40, male and female rats were injected with saline and habituated to activity chambers for 30 min. On PD 41, vehicle and AMPT (2 × 200 mg/kg) were injected as described in Experiment 1a. Immediately prior to behavioral testing, male and female adolescent rats (n = 10 per group) were injected with saline, ketamine (20 or 40 mg/kg, ip), D-amphetamine (2 mg/kg, ip), or cocaine (15 mg/kg, ip). Distance traveled was measured across 12 ten-minute time blocks.
2.4.5. Experiment 4: Effects of PCPA on the ketamine-, D-amphetamine-, and cocaine-induced locomotor activity of male and female adolescent rats
Male and female adolescent rats were pretreated with vehicle or PCPA (3 × 200 mg/kg) and tested with saline, ketamine (20 or 40 mg/kg, ip), D-amphetamine (2 mg/kg, ip), or cocaine (15 mg/kg, ip) as described in Experiment 3. For adolescent rats (n = 8 per group), PCPA pretreatment was administered on PD 38–PD 40, while the test day drugs were given on PD 41.
2.4.6. Experiment 5: Effects of AMPT and PCPA on monoamine content in the dorsal striatum of male and female preweanling, adolescent, and adult rats
Male and female preweanling and adolescent rats were pretreated with vehicle, AMPT (2 × 200 mg/kg), or PCPA (3 × 200 mg/kg) as described in previous experiments. Adult rats were included for comparison purposes, with AMPT (2 × 200 mg/kg) being injected on PD 81 and PCPA (3 × 200 mg/kg) injections occurring on PD 78–80. Vehicle was injected on either PD 81 or PD 78–80. Male and female preweanling, adolescent, and adult rats (n = 6 per group) were decapitated 2 h after the final AMPT injection or 24 h after the final PCPA injection. Dorsal striatal sections were dissected bilaterally on an ice-cold dissection plate and stored at −80 °C. DA and 5-HT content was assayed using high performance liquid chromatography (HPLC) with electrochemical detection as described previously [43].
2.5. Data analysis
For both preweanling and adolescent rats, litter effects were controlled by assigning no more than one subject per litter per group [69]. Behavioral data were analyzed using repeated measures analyses of variance (ANOVAs). When the assumption of sphericity was violated, as determined by Mauchly’s test, the Huynh-Feldt epsilon statistic was used to adjust degrees of freedom [70]. Corrected degrees of freedom were rounded to the nearest whole number and are indicated by a superscripted “a” in the parenthetical statistical reports. Neurochemical data (i.e., DA and 5-HT content) were analyzed using ANOVAs and graphically presented as percent of same age vehicle controls. Tukey tests were used for making post hoc comparisons. When analyzing statistically significant higher order interactions, the mean square error terms (i.e., MSerror) used for the Tukey calculations were based on separate one- or two-way ANOVAs at each time block. Main effects, interactions, and post hoc tests were considered significant at p < 0.05. The AMPT experiments were conducted simultaneously with reserpine experiments [43], so the two sets of experiments share some of the same rats.
3. Results
3.1. Experiment 1a: Effects of AMPT on the ketamine-induced locomotor activity of male and female preweanling rats
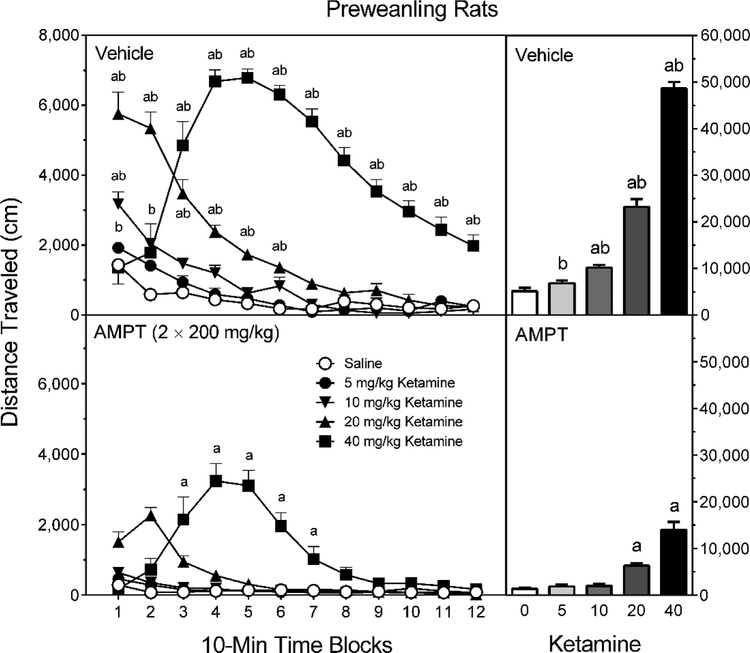
The locomotor activity of preweanling rats was significantly enhanced by the three larger doses of ketamine (10, 20, or 40 mg/kg) (Figure 1, right graphs) [Treatment main effect, F (4, 90) = 295.86, p < 0.001; and Tukey tests, p < 0.05], and significantly reduced by AMPT (2 × 200 mg/kg) [Pretreatment main effect, F (1, 90) = 504.13, p < 0.001]. Vehicle-pretreated rats injected with ketamine (5–40 mg/kg) evidenced more locomotor activity than AMPT-pretreated rats injected with the same dose of ketamine [Pretreatment × Treatment interaction, F (4, 90) = 87.74, p < 0.001; and Tukey tests, p < 0.05]. Even so, 20 and 40 mg/kg ketamine increased locomotor responding in AMPT-pretreated rats [Tukey tests, p < 0.05]. As is often reported, distance traveled scores of preweanling rats did not differ according to sex [Sex main effect, F (1, 80) = 0.003, p = 0.96].
Fig. 1.
Mean distance traveled scores (±SEM) of male and female preweanling rats pretreated with vehicle (upper graphs) or 2 × 200 mg/kg AMPT (lower graphs) and then tested after saline or ketamine (5–40 mg/kg) treatment on PD 21. The right graphs represent total distance traveled collapsed across the testing session. a = Significantly different from saline-treated rats from the same pretreatment condition; b = Significantly different from AMPT-pretreated rats given the same dose of ketamine.
Among vehicle-pretreated rats (Figure 1, upper left graph), 10 mg/kg ketamine increased locomotor activity relative to saline controls on only time block 1; 20 mg/kg ketamine increased locomotor activity on time blocks 1–6; and 40 mg/kg ketamine significantly enhanced locomotor activity on time blocks 3–12 [aPretreatment × Treatment × Time Block interaction, F (12, 281) = 8.09, p < 0.001; and Tukey tests, p < 0.05]. Among AMPT-pretreated rats (Figure 1, lower left graph), only the higher dose of ketamine (40 mg/kg) caused a significant increase in locomotion, which was apparent on time blocks 3–7 [Tukey tests, p < 0.05]. Comparisons between vehicle-and AMPT-pretreated rats showed that the DA synthesis inhibitor significantly reduced the locomotor activity of ketamine-treated rats at various points across the testing session (5 mg/kg ketamine, time block 1; 10 mg/kg ketamine, time blocks 1 and 2; 20 mg/kg ketamine, time blocks 1–6; and 40 mg/kg ketamine, time blocks 3–12) [aPretreatment × Treatment × Time Block interaction, and Tukey tests, p < 0.05].
3.2. Experiment 1b: Effects of AMPT on the D-amphetamine- and cocaine-induced locomotor activity of male and female preweanling rats
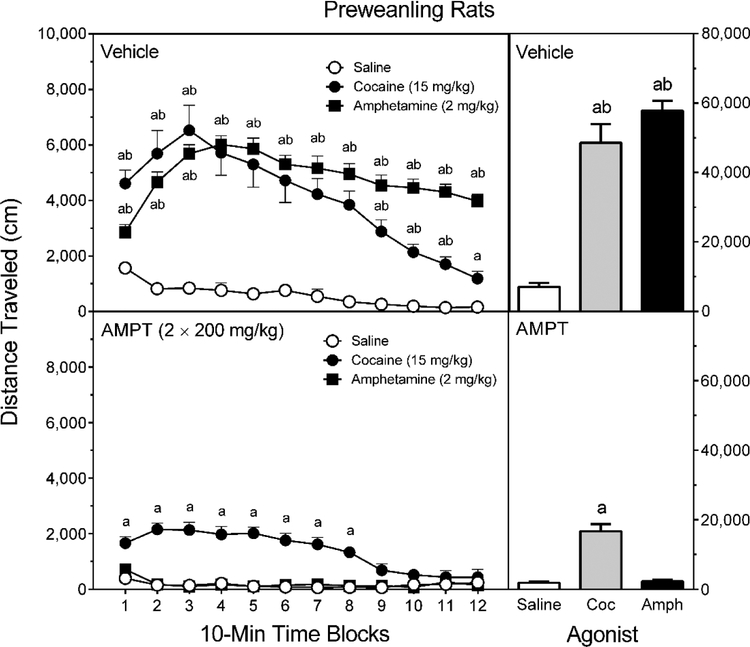
Both psychostimulants caused a large increase in locomotor activity (Figure 2, right graphs) [Treatment main effect, F (3, 54) = 67.35, p < 0.001; and Tukey tests, p < 0.05], but this effect was almost completely prevented by AMPT [Pretreatment main effect, F (1, 54) = 198.32, p < 0.001]. Specifically, AMPT fully attenuated the distance traveled scores of D-amphetamine-treated rats [Pretreatment × Treatment interaction, F (2, 54) = 43.98, p < 0.01; and Tukey tests, p < 0.05]. AMPT also caused a significant reduction in cocaine-induced locomotor activity but, in this case, AMPT-pretreated rats tested with cocaine exhibited more locomotor activity than the AMPT-saline controls [Tukey tests, p < 0.05].
Fig. 2.
Mean distance traveled scores (±SEM) of male and female preweanling rats pretreated with vehicle (upper graphs) or 2 × 200 mg/kg AMPT (lower graphs) and then tested after saline, cocaine (15 mg/kg), or D-amphetamine (2 mg/kg) treatment on PD 21. The right graphs represent total distance traveled collapsed across the testing session. a = Significantly different from saline-treated rats from the same pretreatment condition; b = Significantly different from AMPT-pretreated rats given the same agonist.
Among vehicle-pretreated rats (Figure 2, upper left graph), D-amphetamine and cocaine increased the locomotor activity of preweanling rats on all time blocks (with the exception of time block 12) [aPretreatment × Treatment × Time Block interaction, F (8, 212) = 4.69, p < 0.001; and Tukey tests, p < 0.05]. Among AMPT-pretreated rats, D-amphetamine did not enhance distance traveled scores on any time block, while cocaine increased locomotor activity on time blocks 1–8 [Tukey tests, p < 0.05]. Even so, rats in the AMPT-Cocaine group exhibited significantly less locomotor activity than rats in the Vehicle-Cocaine group on time blocks 1–12 [Tukey tests, p < 0.05]. No sex differences were apparent at any time block [aSex × Pretreatment × Treatment × Time Block interaction, F (9, 212) = 1.11, p = 0.35].
3.3. Experiment 2: Effects of PCPA on the ketamine-, D-amphetamine-, and cocaine-induced locomotor activity of male and female preweanling rats
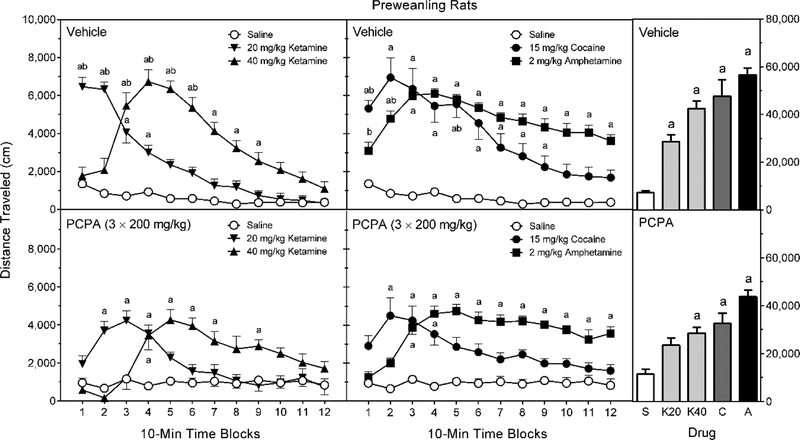
Overall, D-amphetamine produced more locomotor activity than all other compounds (Figure 3, right graphs), while 20 mg/kg ketamine stimulated less locomotion than 40 mg/kg ketamine or 15 mg/kg cocaine [Treatment main effect, F (4, 70) = 40.44, p < 0.001; and Tukey tests, p < 0.05]. Even so, 20 mg/kg ketamine produced significantly greater distance traveled scores than saline [Tukey tests, p < 0.05]. PCPA decreased the distance traveled scores of preweanling rats [Pretreatment main effect, F (1, 70) = 15.89, p < 0.001]; however, post hoc analysis of the marginally significant Pretreatment × Treatment interaction [F (4, 70) = 2.78, p = 0.033] indicated that PCPA did not significantly reduce the locomotor activity of saline-, ketamine-, cocaine-, or D-amphetamine-treated preweanling rats [Tukey tests, p > 0.05].
Fig. 3.
Mean distance traveled scores (±SEM) of preweanling rats pretreated with vehicle (upper graphs) or 3 × 200 mg/kg PCPA (lower graphs) and then tested after saline, ketamine (20 or 40 mg/kg), cocaine (15 mg/kg), or D-amphetamine (2 mg/kg) treatment on PD 21. The right graphs represent total distance traveled collapsed across the testing session. a = Significantly different from saline-treated rats from the same pretreatment condition; b = Significantly different from reserpine-pretreated rats given the same treatment drug.
Statistical analyses involving the time variable provide a more clear picture, as 20 mg/kg ketamine increased the distance traveled scores of vehicle-pretreated rats on time blocks 1–3 (Figure 3, left graphs), while 40 mg/kg ketamine increased distance traveled on time blocks 3–9 [aPretreatment × Treatment × Time Block interaction, F (20, 348) = 3.21, p < 0.001; and Tukey tests, p < 0.05]. PCPA significantly decreased ketamine-induced locomotor activity towards the start of the testing session (20 mg/kg ketamine, time blocks 1 and 2; 40 mg/kg ketamine, time blocks 3–6) [Tukey tests, p < 0.05]. Nonetheless, PCPA-pretreated rats injected with ketamine had greater distance traveled scores than PCPA-pretreated saline controls on various time blocks across the testing session (20 mg/kg ketamine, time blocks 1–3; 40 mg/kg ketamine, time blocks 4–7 and 10) [Tukey tests, p < 0.05].
Among vehicle-pretreated rats, D-amphetamine significantly enhanced distance traveled scores on time blocks 2–12 (Figure 3, middle graphs), while cocaine increased distance traveled on time blocks 1–9 [aPretreatment × Treatment × Time Block interaction, F (20, 348) = 3.21, p < 0.001, p < 0.001; and Tukey tests, p < 0.05]. PCPA significantly depressed D-amphetamine-induced locomotor activity on only time blocks 1 and 2, while cocaine-induced locomotion was reduced on time blocks 1 and 5 [Tukey tests, p < 0.05]. Among PCPA-pretreated rats, D-amphetamine was able to increase locomotor activity on time blocks 3–12 (relative to the PCPA-Saline controls), while cocaine significantly increased distance traveled scores on time blocks 2–4 [Tukey tests, p < 0.05]. Once again, no sex differences were apparent on any time block [aSex × Pretreatment × Treatment × Time Block interaction, F (22, 324) = 0.57, p = 0.94].
3.4. Experiment 3: Effects of AMPT on the ketamine-, D-amphetamine-, and cocaine-induced locomotor activity of male and female adolescent rats
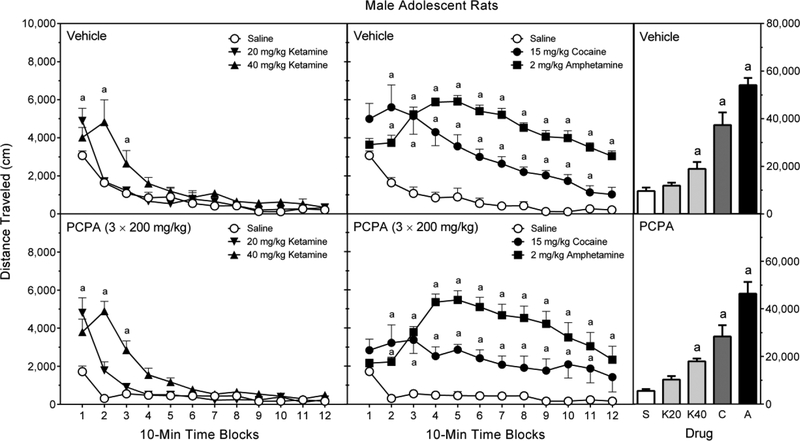
3.4.1. Male adolescent rats
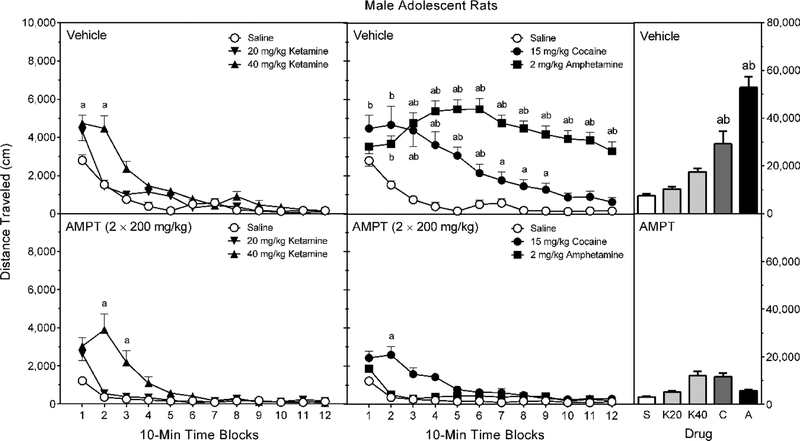
D-Amphetamine, cocaine, and the higher dose of ketamine (40 mg/kg) increased the locomotor activity of male adolescent rats (Figure 4, right graphs) [Treatment main effect, F (4, 90) = 32.15, p < 0.001; and Tukey tests, p < 0.05]; whereas, AMPT caused an overall reduction in locomotion [Pretreatment main effect, F (1, 90) = 108.23, p < 0.001]. Among vehicle-pretreated rats, post hoc analysis of the Pretreatment × Treatment interaction indicated that D-amphetamine and cocaine significantly enhanced distance traveled scores, while 20 and 40 mg/kg ketamine caused only nonsignificant increases in locomotor activity [Pretreatment × Treatment interaction, F (4, 90) = 28.29, p < 0.001; and Tukey tests, p < 0.05]. AMPT reduced the cocaine- and D-amphetamine-induced locomotor activity of male adolescent rats to basal levels [Tukey tests, p < 0.05].
Fig. 4.
Mean distance traveled scores (±SEM) of male adolescent rats pretreated with vehicle (upper graphs) or 2 × 200 mg/kg AMPT (lower graphs) and then tested after saline, ketamine (20 or 40 mg/kg), cocaine (15 mg/kg), or D-amphetamine (2 mg/kg) treatment on PD 41. The right graphs represent total distance traveled collapsed across the testing session. a = Significantly different from saline-treated rats from the same pretreatment condition; b = Significantly different from reserpine-pretreated rats given the same treatment drug.
Analysis of the time factor showed that only 40 mg/kg ketamine, but not 20 mg/kg ketamine, briefly stimulated locomotor activity at the start of the testing session (i.e., time blocks 1 and 2) (Figure 4, left graphs) [aPretreatment × Treatment × Time Block interaction, F (17, 375) = 3.90, p < 0.001; and Tukey tests, p < 0.05]. AMPT did not attenuate the ketamine-induced locomotor activity of male adolescent rats. On the other hand, D-amphetamine increased the distance traveled scores of vehicle-pretreated male rats on time blocks 3–12, while cocaine produced elevated amounts of locomotor activity on time blocks 2–9 (Figure 4, middle graphs) [aPretreatment × Treatment × Time Block interaction, and Tukey tests, p < 0.05]. AMPT significantly reduced psychostimulant-induced locomotor activity on time blocks 1–6 (cocaine) and time blocks 2–12 (D-amphetamine) [Tukey tests, p < 0.05].
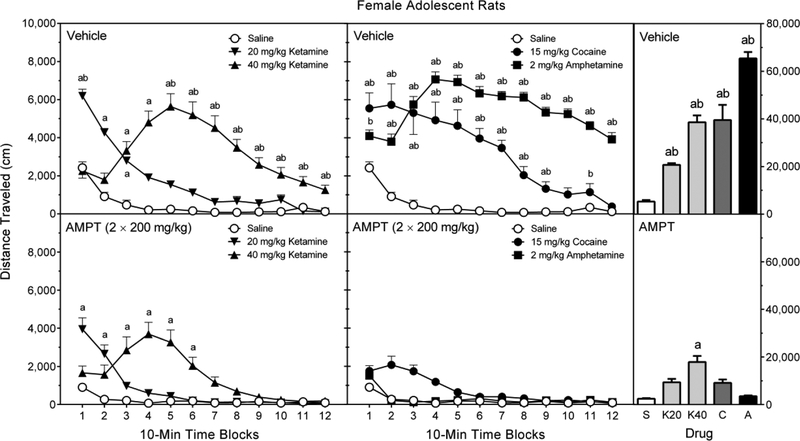
3.4.2. Female adolescent rats
All test compounds significantly increased locomotor activity (Figure 5, right graphs), with D-amphetamine (2 mg/kg) producing the greatest increase in distance traveled scores, 40 mg/kg ketamine and cocaine (15 mg/kg) stimulating an intermediate amount of locomotor activity, and 20 mg/kg ketamine causing a smaller, but still significant, increase in locomotion [Treatment main effect, F (4, 90) = 41.97, p < 0.001; and Tukey tests, p < 0.05]. AMPT significantly reduced the locomotor activity of female adolescent rats [Pretreatment main effect, F (1, 90) = 236.44, p < 0.001], although rats in the AMPT–Ketamine (40 mg/kg) group exhibited greater distance traveled scores than rats in the AMPT–Saline group [Pretreatment × Treatment interaction, F (4, 90) = 38.14, p < 0.001; and Tukey tests, p < 0.05].
Fig. 5.
Mean distance traveled scores (±SEM) of female adolescent rats pretreated with vehicle (upper graphs) or 2 × 200 mg/kg AMPT (lower graphs) and then tested after saline, ketamine (20 or 40 mg/kg), cocaine (15 mg/kg), or D-amphetamine (2 mg/kg) treatment on PD 41. The right graphs represent total distance traveled collapsed across the testing session. a = Significantly different from saline-treated rats from the same pretreatment condition; b = Significantly different from reserpine-pretreated rats given the same treatment drug.
Among vehicle-pretreated rats (Figure 5, upper left graph), 20 mg/kg ketamine increased the locomotor activity of female adolescent rats on time blocks 1–3, while the higher dose of ketamine (40 mg/kg) enhanced locomotor activity on time blocks 3–12 [aPretreatment × Treatment × Time Block interaction, F (16, 359) = 6.66, p < 0.001; and Tukey tests, p < 0.05]. In comparison, 2 mg/kg D-amphetamine increased distance traveled scores on time blocks 2–12 (Figure 5, upper middle graph), whereas cocaine-induced locomotor activity was apparent on time blocks 1–9 [Tukey tests, p < 0.05].
AMPT only decreased the locomotor activating effects of 20 mg/kg ketamine at the very start of the testing session (time block 1), while the actions of 40 mg/kg ketamine were reduced on time blocks 5–12 (Figure 5, lower left graph) [aPretreatment × Treatment × Time Block interaction; and Tukey tests, p < 0.05]. Even so, rats in the AMPT–Ketamine groups exhibited greater distance traveled scores than control rats on time blocks 1 and 2 (20 mg/kg ketamine) and time blocks 3–6 (40 mg/kg ketamine), thus indicating that AMPT only partially attenuated ketamine-induced locomotor activity. In contrast, AMPT fully attenuated the D-amphetamine- and cocaine-induced locomotor activity of female adolescent rats (Figure 5, lower left graph) [Tukey tests, p < 0.05].
3.5. Experiment 4: Effects of PCPA on the ketamine-, D-amphetamine-, and cocaine-induced locomotor activity of male and female adolescent rats
3.5.1. Male adolescent rats
In male adolescent rats, 40 mg/kg ketamine, cocaine, and D-amphetamine caused a step-wise increase in distance traveled scores (Figure 6, right graphs), with D-amphetamine producing the most locomotor activity [Treatment main effect, F (4, 70) = 61.68, p < 0.001; and Tukey tests, p < 0.05]. The lower dose of ketamine (20 mg/kg) did not significantly increase distance traveled. Overall, PCPA caused a slight decrease in locomotor activity [Pretreatment main effect, F (1, 70) = 5.49, p < 0.05], but this effect did not vary according to pretreatment condition [Pretreatment × Treatment interaction, F (4, 70) = 0.64, p = 0.64].
Fig. 6.
Mean distance traveled scores (±SEM) of male adolescent rats pretreated with vehicle (upper graphs) or 3 × 200 mg/kg PCPA (lower graphs) and then tested after saline, ketamine (20 or 40 mg/kg), cocaine (15 mg/kg), or D-amphetamine (2 mg/kg) treatment on PD 41. The right graphs represent total distance traveled collapsed across the testing session. a = Significantly different from saline-treated rats from the same pretreatment condition; b = Significantly different from reserpine-pretreated rats given the same treatment drug.
The three-way interaction was not significant [aPretreatment × Treatment × Time Block interaction, F (14, 254) = 1.06, p = 0.39], suggesting that PCPA had minimal effects on the ketamine-, D-amphetamine-, and cocaine-induced locomotor activity of male adolescent rats. Instead, the significant Treatment × Time Block interaction [F (14, 254) = 14.82, p < 0.001; and Tukey tests, p < 0.05] indicated that ketamine increased the distance traveled scores of male adolescent rats on only time block 1 (20 mg/kg ketamine) and time blocks 2 and 3 (40 mg/kg ketamine) (Figure 6, left graphs). In contrast, D-amphetamine increased distance traveled on time blocks 2–12 (Figure 6, middle graphs), while cocaine stimulated locomotor activity on time blocks 2–11 [Tukey tests, p < 0.05].
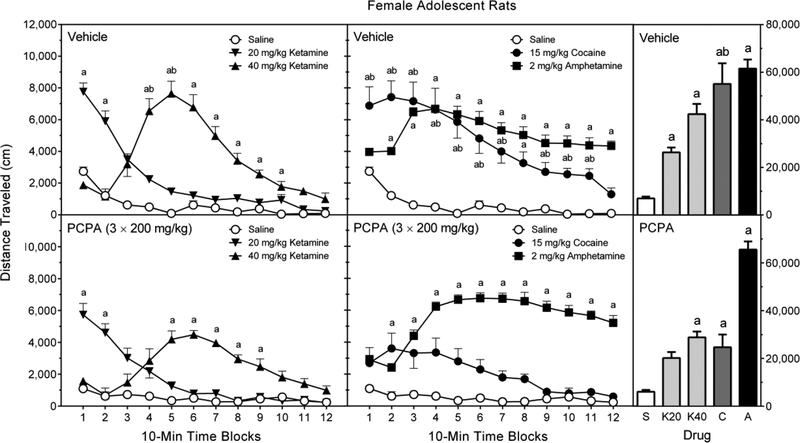
3.5.2. Female adolescent rats
All test compounds increased the distance traveled scores of female adolescent rats (Figure 7, right graphs), with D-amphetamine producing the greatest increase in locomotor activity and 20 mg/kg ketamine causing the smallest increase [Treatment main effect, F (4, 70) = 54.23, p < 0.001; and Tukey tests, p < 0.05]. PCPA caused a significant reduction in distance traveled scores [Pretreatment main effect, F (1, 70) = 13.43, p < 0.001], but this PCPA-induced decline was only evident in cocaine-treated rats [Pretreatment × Treatment interaction, F (4, 70) = 5.48, p < 0.001; and Tukey tests, p < 0.05].
Fig. 7.
Mean distance traveled scores (±SEM) of female adolescent rats pretreated with vehicle (upper graphs) or 3 × 200 mg/kg PCPA (lower graphs) and then tested after saline, ketamine (20 or 40 mg/kg), cocaine (15 mg/kg), or D-amphetamine (2 mg/kg) treatment on PD 41. The right graphs represent total distance traveled collapsed across the testing session. a = Significantly different from saline-treated rats from the same pretreatment condition; b = Significantly different from reserpine-pretreated rats given the same treatment drug.
The lower dose of ketamine (20 mg/kg) enhanced the distance traveled scores of vehicle pretreated rats on time blocks 1 and 2 (Figure 7, left graphs); whereas, 40 mg/kg ketamine significantly increased locomotion on time blocks 4–10 [aPretreatment × Treatment × Time Block interaction, F (17, 298) = 2.75, p < 0.001; and Tukey tests, p < 0.05]. PCPA did not affect the distance traveled scores of female adolescent rats treated with 20 mg/kg ketamine, but the 5-HT synthesis inhibitor did blunt the locomotor activity of rats treated with 40 mg/kg ketamine on time blocks 4 and 5 [Tukey tests, p < 0.05]. In vehicle-pretreated rats, D-amphetamine increased locomotor activity on time blocks 2–12 (Figure 7, middle graphs) [Tukey tests, p < 0.05], which was an effect not modified by PCPA. Cocaine enhanced the locomotor activity of vehicle-pretreated female adolescent rats on time blocks 2–11, with PCPA significantly reducing cocaine-induced distance traveled scores on time blocks 4–7 and 9–11 [aPretreatment × Treatment × Time Block interaction; and Tukey tests, p < 0.05].
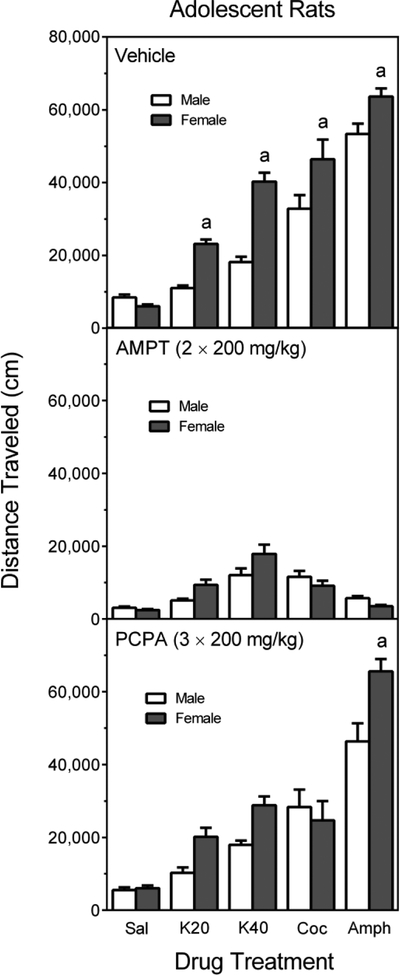
3.6. Cross-sex comparisons involving vehicle-, AMPT-, and PCPA-pretreated adolescent rats
Overall, female adolescent rats had greater distance traveled scores than male rats [Sex main effect, F (1, 330) = 39.85, p < 0.001]. Among vehicle-pretreated adolescent rats (Figure 8, upper graph), females injected with cocaine (15 mg/kg) or either dose of ketamine (20 or 40 mg/kg) exhibited more locomotor activity than male rats [Sex × Pretreatment × Treatment interaction, F (8, 330) = 2.66, p < 0.01; and Tukey tests, p < 0.05]. Although Tukey tests indicated that D-amphetamine-treated male and female rats did not differ, a separate t-test comparing the two groups was significant [Sex effect, t (34) = 2.86, p = 0.007]. No drug-induced sex effects were apparent when rats were pretreated with AMPT. Among PCPA-pretreated adolescent rats (Figure 8, lower graph), the only significant sex difference was between male and female D-amphetamine-treated rats, with females showing more locomotion than male rats [Tukey tests, p < 0.05].
Fig. 8.
Total distance traveled (mean, ±SEM) of male and female adolescent rats pretreated with vehicle (upper graph), AMPT (middle graph), or PCPA (lower graph) and then tested after saline, ketamine (20 or 40 mg/kg), cocaine (15 mg/kg), or D-amphetamine (2 mg/kg) treatment on PD 41. a = Significantly different from male rats given the same drug treatment.
3.7. Effects of AMPT and PCPA on the body weights of male and female preweanling and adolescent rats
Adolescent rats weighed substantially more than preweanling rats (Table 1) [Age main effect, F (1, 232) = 3515.01, p < 0.001]. Overall, male rats weighed more than female rats [Sex main effect, F (1, 232) = 142.59, p < 0.001], but this sex-dependent difference was only statistically significant in adolescent rats [Age × Sex interaction, F (1, 232) = 84.43, p < 0.001; and Tukey tests, p < 0.05]. At both ages, PCPA significantly reduced the body weights of male and female rats [Pretreatment main effect, F (1, 232) = 44.03, p < 0.001].
Table 1.
Mean (SEM) body weights (g) of vehicle- and PCPA-pretreated male and female preweanling (n = 20 per group) and adolescent (n = 40 per group) rats on the test day
| Pretreatment | Age | |||
|---|---|---|---|---|
| Preweanling | Adolescent | |||
| Male | Female | Male | Female | |
| Vehicle | 64.95 (2.3) | 60.15 (2.0) | 207.85 (3.2)a | 158.25 (2.6) |
| PCPA (3 × 200 mg/kg) | 53.85 (1.9)b | 47.60 (1.6)b | 185.85 (2.6)ab | 150.63 (2.2)b |
Significantly different from adolescent female rats.
Significantly different from vehicle-treated rats.
3.8. Experiment 5: Effects of AMPT and PCPA on monoamine content in the dorsal striatum of male and female preweanling, adolescent, and adult rats
3.8.1. Basal DA and 5-HT content
Among vehicle-pretreated rats, dorsal striatal DA values (pg/mg wet weight tissue) of preweanling (M = 4,715, SEM = 87), adolescent (M = 6,659, SEM = 200), and adult (M = 9,763, SEM = 359) rats increased in a step-wise manner according to age [Age main effect, F (2, 33) = 109.96, p < 0.001; and Tukey tests, p < 0.05]. Dorsal striatal 5-HT values of vehicle-pretreated preweanling rats (M = 217.2, SEM = 15.1) were significantly lower than vehicle-pretreated adult rats (M = 312.3, SEM = 22.9), with adolescent rats (M = 292.2, SEM = 26.7) being intermediate between the other two age groups and significantly different from neither [Age main effect, F (2, 33) = 5.15, p < 0.05; and Tukey tests, p < 0.05].
3.8.2. DA and 5-HT content expressed as percent of vehicle-pretreated controls
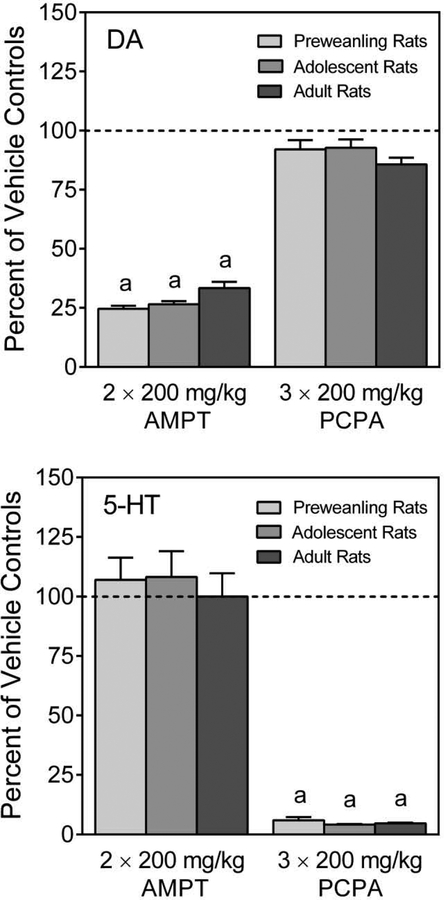
At all ages, AMPT significantly reduced dorsal striatal DA levels relative to vehicle controls (Figure 9, upper graph) [Age main effect, F (2, 99) = 569.10, p < 0.001; and Tukey tests, p < 0.05], whereas PCPA did not affect DA content. Conversely, AMPT did not alter 5-HT levels (Figure 9, lower graph), while PCPA caused a significant reduction in the dorsal striatal 5-HT levels of preweanling, adolescent, and adult rats [Age main effect, F (2, 99) = 175.98, p < 0.001; and Tukey tests, p < 0.05]. The effects of AMPT and PCPA on DA and 5-HT content did not vary according to sex at any age [DA: Sex × Age × Pretreatment interaction, F (2, 60) = 0.94, p = 0.40; 5-HT: Sex × Age × Pretreatment interaction, F (2, 60) = 0.03, p = 0.98].
Fig. 9.
Mean dorsal striatal DA (upper graph) and 5-HT (lower graph) content (±SEM) of male and female preweanling (PD 21), adolescent (PD 41), and adult (PD 81) rats pretreated with vehicle, AMPT (2 × 200 mg/kg) or PCPA (3 × 200 mg/kg). Data are expressed as percent of same age vehicle controls. a = Significantly different from same-age vehicle-pretreated rats.
4. Discussion
Ketamine produced a familiar pattern of age- and sex-dependent behavioral effects [25,26], as the NMDA open channel blocker robustly increased the locomotor activity of preweanling rats and female adolescent rats, while causing only a minor enhancement in the locomotor activity of male adolescent rats. At the highest dose tested, ketamine (40 mg/kg) initially caused a muted locomotor response in preweanling rats and adolescent female rats that lasted for approximately 30 min, which was followed by robust locomotor activity that eventually tapered away (i.e., the pattern of locomotor activity looked like a bell-shaped curve). In contrast, lower doses of ketamine (e.g., 20 mg/kg) produced peak locomotion in the first 5-min time block. This pattern of dose-dependent effects, which is not observed in adolescent male rats, is due to the acute anesthetic and motor debilitating effects of 40 mg/kg ketamine [25,26]. In contrast, few sex- and age-dependent differences were apparent when vehicle-pretreated rats were tested with psychostimulants. At both ages, D-amphetamine (2 mg/kg) tended to produce a more pronounced locomotor response than cocaine (15 mg/kg), but no overarching statements can be made about this effect since dose-response data were not collected. The greater point is that ketamine produced a unique pattern of locomotor activity that varied substantially according to age and sex, while D-amphetamine and cocaine produced only minor age- and sex-dependent differences at the doses tested.
4.1. Effects of DA depletion on ketamine-, D-amphetamine-, and cocaine-induced locomotor activity
AMPT partially attenuated ketamine’s locomotor activating effects in both preweanling rats and female adolescent rats, while having no discernable impact on the ketamine-induced locomotor activity of male adolescent rats. These age and sex effects were not the result of age-dependent differences in the effectiveness of the pretreatment regimen, because AMPT caused a similarly large decline in the dorsal striatal DA levels of preweanling and adolescent rats. AMPT did not significantly reduce dorsal striatal norepinephrine levels (data not shown), indicating that AMPT’s locomotor effects were mediated by DA. Consistent with this interpretation, α- and β-adrenergic antagonists do not modulate ketamine- and MK-801-induced locomotor activity [16,33]. Age- and sex-dependent effects were not apparent when D-amphetamine and cocaine were administered to AMPT-pretreated rats. Specifically, selective DA depletion fully attenuated the D-amphetamine-induced locomotor activity of male and female rats at both ages; whereas, AMPT caused a significant, but less than complete, attenuation of cocaine-induced locomotor activity in preweanling and adolescent rats.
Past research is generally consistent with these results, as AMPT leaves the MK-801-induced locomotor activity of male adult mice unaffected [34], while attenuating the D-amphetamine-induced locomotor activity of male adult rats [61,71]. The only divergent findings involved AMPT and ketamine, as Uchihashi and colleagues [35] reported that AMPT (2 × 100 mg/kg or 2 × 300 mg/kg) reduced the ketamine-induced locomotor activity of male adult mice (we found that AMPT did not affect ketamine-treated male adolescent rats). The reason for this discrepancy is unclear, but it may involve species or age differences since their male adult mice exhibited a far more pronounced locomotor response to ketamine than did the male adolescent rats tested in the present study. Nonetheless, the two most prominent features of these data are: (a) AMPT only partially attenuated the ketamine-induced locomotor activity of preweanling and female adolescent rats, while not affecting the ketamine-induced locomotor activity of adult rats; and (b) ketamine produced a different pattern of behavioral effects in AMPT-pretreated rats than did the psychostimulant compounds.
4.2. Effects of 5-HT depletion on ketamine-, D-amphetamine-, and cocaine-induced locomotor activity
PCPA had a suppressing action on the ketamine-induced locomotor activity of preweanling rats and female adolescent rats. This effect is best seen in Figures 3 and 7 (left graphs), as vehicle- and PCPA-pretreated rats evidenced the same pattern of ketamine-induced locomotor activity across the testing session, but the magnitude of ketamine’s effects were reduced by PCPA. Although ketamine stimulated little locomotor activity in male adolescent rats, the effect was not altered by PCPA pretreatment. Consistent with the latter findings, PCPA did not significantly decrease the MK-801-induced locomotor activity of male adult rats and mice, although a minor suppression was noted [38]. PCPA also did not alter the D-amphetamine-induced locomotor activity of male and female adolescent and preweanling rats (except for two time blocks). This result is generally consistent with past findings [72,73], although PCPA has occasionally been reported to potentiate the D-amphetamine-induced locomotor activity of male adult rats and mice [74,75].
Lastly, PCPA produced a modest and nonsignificant reduction in the cocaine-induced locomotor activity of both preweanling rats and male adolescent rats [see also 76]. Female adolescent rats showed a statistically significant decline, as PCPA caused over a 50% reduction in cocaine-induced locomotor activity (Vehicle–Cocaine, M = 55,066 cm; PCPA–Cocaine, M = 24,708 cm). The reason why cocaine-treated female adolescent rats are particularly susceptible to the effects of PCPA is unclear, but estrogens are known to influence 5-HT system functioning [77–79]. Regardless, these results show that ketamine, D-amphetamine, and cocaine cause very different patterns of sex- and age-dependent locomotor effects in rats depleted of 5-HT.
4.3. Possible neuronal mechanisms mediating ketamine- and psychostimulant-induced locomotor activity
In the companion article to this paper, Crawford et al. [43] reported that 5 mg/kg reserpine fully attenuated the ketamine-induced locomotor activity of preweanling, adolescent, and adult rats, while leaving D-amphetamine-induced locomotor activity largely unaffected. In the present study, AMPT fully attenuated D-amphetamine-induced locomotor activity at all ages, while only partially attenuating the locomotor activity caused by 40 mg/kg ketamine. In terms of D-amphetamine, the different pattern of AMPT- and reserpine-induced effects may be a consequence of the different pools of DA affected by the pretreatment compounds. Specifically, AMPT inhibits tyrosine hydroxylase activity, thereby reducing newly synthesized DA [80], while reserpine depletes DA located in vesicular storage pools [81]. Because D-amphetamine primarily [82], but not exclusively [83,84], releases newly synthesized DA from cytosolic pools, its actions are more sensitive to AMPT than reserpine [61]. Consistent with this model, AMPT fully attenuated D-amphetamine-induced locomotor activity in the present study.
There are at least two possible reasons why reserpine and AMPT differentially affected ketamine-induced locomotor activity. First, ketamine’s locomotor activating effects may preferentially rely on DA located in vesicular storage pools; thus, explaining why reserpine more strongly impacted ketamine-induced locomotor activity than did AMPT. Second, the partial attenuation of ketamine-induced locomotor activity by AMPT may indicate that multiple neurotransmitter systems, both of which are sensitive to reserpine, co-mediate ketamine’s locomotor activating effects. A candidate neurotransmitter is 5-HT because: (a) selective stimulation of certain 5-HT receptor subtypes increases locomotor activity in adult rats and mice [85–87; for a review, see 29]; (b) reserpine significantly reduces both 5-HT and DA levels, and fully attenuates ketamine-induced locomotor activity [43]; and (c) AMPT and PCPA, when given alone, significantly reduce only DA or 5-HT levels (Figure 9), and only partially attenuate ketamine-induced locomotor activity. Consistent with the co-mediation explanation, Carlsson and colleagues concluded, after a decade’s long series of studies, that the MK-801-induced locomotor activity of male adult rats and mice is mediated by both dopaminergic and serotonergic mechanisms [36,37,88]. Even so, they found that 5-HT involvement was approximately neutral (i.e., the excitatory influences mediated by 5-HT2A receptors were “almost completely counterbalanced” by inhibitory influences mediated by other 5-HT receptor subtypes [38]. In the present study, we also found that 5-HT depletion had a neutral-like effect on the ketamine-induced locomotor activity of male adolescent rats. Conversely, 5-HT depletion did not have a “neutral effect” on preweanling and adolescent female rats, as PCPA partially suppressed the ketamine-induced locomotor activity of these groups. Thus, it is possible that both DA and 5-HT systems mediate the ketamine-induced locomotor activity of male and female rats, but the balance of serotonergic excitatory and inhibitory influences may change across ontogeny.
4.4. Nature of the interaction between ketamine and the DA/5-HT systems
Although ketamine has long been recognized as an NMDA channel blocker [89,90], ketamine’s locomotor activating effects were initially believed to be the result of direct actions at the DA presynaptic terminal (i.e., enhancing DA release or blocking reuptake much like D-amphetamine or cocaine) [16,50,51]. More recently, there is accumulating evidence that ketamine indirectly modulates DA system functioning [52,91]. The ventral tegmental area (VTA) and substantia nigra are rich in NMDA receptors [92,93], and stimulation of these receptors alters the firing rate of DA projection neurons [91,94–97]. Although fewer studies have examined the relationship between ketamine and 5-HT neurotransmission, NMDA receptors modulate the firing rate of 5-HT neurons in the raphe [98], and these 5-HT neurons are capable of mediating motor movement via projections to the VTA and structures of the basal ganglia [99]. In sum, results from the present study are consistent with the hypothesis that ketamine indirectly modulates locomotor activity through dopaminergic and serotonergic mechanisms. Not only do DA and 5-HT synthesis inhibitors partially attenuate ketamine-induced locomotor activity, but ketamine does not produce the same pattern of locomotor effects as D-amphetamine or cocaine (i.e., ketamine does not act like a psychostimulant at the presynaptic terminal).
4.5. Sex effects in preweanling and adolescent rats
Multiple examples of sex differences were evident in the present study, with the following being the most notable: (1) ketamine produced substantially more locomotor activity in female adolescent rats than male adolescent rats; (2) among adolescents, the locomotor inhibiting effects of AMPT and PCPA were significantly greater in ketamine-treated females than males; (3) both psychostimulants caused greater locomotor activity in female adolescent rats than males; and (4) preweanling rats did not exhibit any sex differences and, at least in terms of ketamine, responded in the same manner as female adolescent rats. The ability of ketamine to produce more locomotor activity in female adolescent rats than males is almost certainly due to pharmacokinetic factors. Nabeshima was the first to show that (a) adult female rats metabolize PCP (an NMDA receptor open channel blocker) at a slower rate than males, and (b) this effect was due to estrogens inhibiting cytochrome P450 liver enzymes [100,101]. In terms of ketamine, it has recently been reported that administering a single bolus injection to adolescent and adult female rats causes greater drug availability in brain, higher peak drug levels, and slower drug metabolism than when the same dose of ketamine is administered to male rats [26,28]. Sex-dependent differences in monoamine systems may explain why AMPT and PCPA were more effective at reducing the ketamine-induced locomotor activity of female adolescent rats; however, it is also possible that a floor effect obscured the actions of AMPT and PCPA in male rats (i.e., basal levels of ketamine-induced locomotor activity were very low).
In terms of psychostimulants, adult female rats typically exhibit more D-amphetamine- and cocaine-induced locomotor activity than male rats [102–104], and the same effect was evident in the present study. It is well-established that estrogens are responsible for the differential responsiveness of male and female rats to psychostimulant drugs [105–107]; however, instead of affecting cocaine pharmacokinetics [108,109], estrogens may enhance DA release characteristics in brain [102,110]. Unlike adolescent and adult rats, preweanling rats have not yet reached puberty and, as a consequence, have low levels of circulating gonadal hormones [53,111]. For this reason, it is not surprising that the locomotor activating effects of indirect DA agonists [104,112,113] and NMDA receptor open channel blockers [25,114–116] do not differ according to sex. Even so, it is interesting that both preweanling rats (low levels of estrogen) and female adolescent rats (periodic high levels of estrogen) exhibit ketamine-induced hyperresponsiveness, whereas male adolescent and adult rats (high levels of testosterone) show reduced effects. This pattern of results suggests that testosterone may moderate ketamine-induced locomotor activity. Indeed, Nabeshima et al. found that testosterone altered PCP pharmacokinetics [100] and van den Buuse et al. recently reported that gonadectomy depressed the MK-801-induced locomotor activity of adult male mice, but not adult female mice [117].
4.6. Limitations
Although monoamine-depleting agents and synthesis inhibitors are very commonly used in neurochemistry, electrophysiology, and behavioral research, an often ignored limitation is that these compounds cause weight loss, or a reduction in normal weight gain, in rats of various ages. PCPA is especially prone to causing weight loss because it is often administered over a three- or four-day span and at doses of 200–500 mg/kg [38,66,68,75,118]. Although we used a moderate dose of PCPA (3 × 200 mg/kg), the 5-HT synthesis inhibitor caused a relative decline in the body weights of male and female preweanling and adolescent rats. PCPA did not alter the locomotor activity of D-amphetamine-treated male and female rats, suggesting that weight loss alone was not sufficient to significantly affect locomotion. Nonetheless, it cannot be ruled out that PCPA-induced weight loss might have affected distance traveled scores at different ages or after different drug treatments. A second limiting feature is the lack of adult comparison groups. Because of a desire to reduce subject numbers we decided to rely on results from previous behavioral studies using adult rats and mice. Although this research strategy is often employed [22,25,119,120], interpretation might have been enhanced if adult groups were included.
4.7. Conclusions
Both AMPT and PCPA partially attenuated the ketamine-induced locomotor activity of preweanling and female adolescent rats, while leaving the ketamine-induced locomotor activity of male adolescent rats largely unaffected. These results are generally consistent with the findings of Carlsson and colleagues [36–38,88], who reported that the locomotor activating effects of a noncompetitive NMDA receptor antagonist (MK-801) were mediated by dopaminergic and serotonergic neurotransmission. Ketamine caused a different pattern of age- and sex-dependent locomotor effects than D-amphetamine or cocaine, thus indicating that ketamine was not functioning like an indirect DA agonist. When considered together, these results suggest that ketamine may indirectly activate locomotor activity through multiple monoaminergic mechanisms.
Highlights.
DA and 5-HT synthesis inhibitors only partially attenuate the ketamine-induced locomotor activity of preweanling and adolescent rats
This pattern of results suggests that DA and 5-HT systems may jointly mediate ketamine’s locomotor activating effects
In AMPT- and PCPA-pretreated rats, cocaine and amphetamine produce different patterns of behavioral effects than ketamine, thereby indicating that ketamine stimulates locomotion via a different mechanism than prototypical psychostimulant compounds
Acknowledgements
This work was supported by the National Institute of General Medical Sciences [grant number GM083883] and the National Institute of Drug Abuse [grant number DA033877].
References
- [1].Jansen KLR, A review of the nonmedical use of ketamine: use, users and consequences, J. Psychoactive Drugs 32 (2000) 419–433. [DOI] [PubMed] [Google Scholar]
- [2].Dillon P, Copeland J, Jansen K, Patterns of use and harms associated with non-medical ketamine use, Drug Alcohol Depend 69 (2003) 23–28. [DOI] [PubMed] [Google Scholar]
- [3].Kalsi SS, Wood DM, Dargan PI, The epidemiology and patterns of acute and chronic toxicity associated with recreational ketamine use, Emerg. Health Threats J 4 (2011) 7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Morgan CJ, Curran HV, Ketamine use: a review, Addiction 107 (2012) 27–38. [DOI] [PubMed] [Google Scholar]
- [5].Tan S, Lam WP, Wai MS, Yu WH, Yew DT, Chronic ketamine administration modulates midbrain dopamine system in mice, PLoS One 7 (2012) e43947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kohrs R, Durieux ME, Ketamine: teaching an old drug new tricks, Anesth. Analg 87 (1998) 1186–1193. [DOI] [PubMed] [Google Scholar]
- [7].Bergman SA, Ketamine: review of its pharmacology and its use in pediatric anesthesia, Anesth. Prog 46 (1999) 10–20. [PMC free article] [PubMed] [Google Scholar]
- [8].Domino EF, Taming the ketamine tiger. 1965, Anesthesiology 113 (2010) 678–684. [DOI] [PubMed] [Google Scholar]
- [9].Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression, Arch. Gen. Psychiatry 63 (2006) 856–864. [DOI] [PubMed] [Google Scholar]
- [10].aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ, Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression, Biol. Psychiatry 67 (2010) 139–145. [DOI] [PubMed] [Google Scholar]
- [11].Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T, Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression, Neuroendocrinol. Lett 34 (2013) 287–293. [PubMed] [Google Scholar]
- [12].Luckenbaugh DA, Niciu MJ, Ionescu DF, Nolan NM, Richards EM, Brutsche NE, Guevara S, Zarate CA, Do the dissociative side effects of ketamine mediate its antidepressant effects? J. Affect. Disord 159 (2014) 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Valentine GW, Mason GF, Gomez R, Fasula M, Watzl J, Pittman B, Krystal JH, Sanacora G, The antidepressant effect of ketamine is not associated with changes in occipital amino acid neurotransmitter content as measured by [1H]-MRS, Psychiatry Res 191 (2011) 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fernandes A, Wojcik T, Baireddy P, Pieschl R, Newton A, Tian Y, Hong Y, Bristow L, Li YW, Inhibition of in vivo [3H]MK-801 binding by NMDA receptor open channel blockers and GluN2B antagonists in rats and mice, Eur. J. Pharmacol 766 (2015) 1–8. [DOI] [PubMed] [Google Scholar]
- [15].Glasgow NG, Wilcox MR, Johnson JW, Effects of Mg2+ on recovery of NMDA receptors from inhibition by memantine and ketamine reveal properties of a second site, Neuropharmacology 137 (2018) 344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Irifune M, Shimizu T, Nomoto M, Ketamine-induced hyperlocomotion associated with alteration of presynaptic components of dopamine neurons in the nucleus accumbens of mice, Pharmacol. Biochem. Behav 40 (1991) 399–407. [DOI] [PubMed] [Google Scholar]
- [17].Kawamata T, Omote K, Sonoda H, Kawamata M, Namiki A, Analgesic mechanisms of ketamine in the presence and absence of peripheral inflammation, Anesthesiology 93 (2000) 520–528. [DOI] [PubMed] [Google Scholar]
- [18].Rodrigues SF, de Oliveira MA, Martins JO, Sannomiya P, de Cássia Tostes R, Nigro D, Carvalho MH, Fortes ZB, Differential effects of chloral hydrate- and ketamine/xylazine-induced anesthesia by the s.c. route, Life Sci 79 (2006) 1630–1637. [DOI] [PubMed] [Google Scholar]
- [19].Usun Y, Eybrard S, Meyer F, Louilot A, Ketamine increases striatal dopamine release and hyperlocomotion in adult rats after postnatal functional blockade of the prefrontal cortex, Behav. Brain Res 256 (2013) 229–237. [DOI] [PubMed] [Google Scholar]
- [20].Yamamoto T, Nakayama T, Yamaguchi J, Matsuzawa M, Mishina M, Ikeda K, Yamamoto H, Role of the NMDA receptor GluN2D subunit in the expression of ketamine-induced behavioral sensitization and region-specific activation of neuronal nitric oxide synthase, Neurosci. Lett 610 (2016) 48–53. [DOI] [PubMed] [Google Scholar]
- [21].Wilson C, Cone K, Kercher M, Hibbitts J, Fischer J, Van Lake A, Sumner J, Naloxone increases ketamine-induced hyperactivity in the open field in female rats, Pharmacol. Biochem. Behav 81 (2005) 530–534. [DOI] [PubMed] [Google Scholar]
- [22].Wilson C, Kercher M, Quinn B, Murphy A, Fiegel C, McLaurin A, Effects of age and sex on ketamine-induced hyperactivity in rats, Physiol. Behav 91 (2007) 202–207. [DOI] [PubMed] [Google Scholar]
- [23].Wiley JL, Evans RL, Grainger DB, Nicholson KL, Locomotor activity changes in female adolescent and adult rats during repeated treatment with a cannabinoid or club drug, Pharmacol. Rep 63 (2011) 1085–1092. [DOI] [PubMed] [Google Scholar]
- [24].Rocha A, Hart N, Trujillo KA, Differences between adolescents and adults in the acute effects of PCP and ketamine and in sensitization following intermittent administration, Pharmacol. Biochem. Behav 157 (2017) 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].McDougall SA, Moran AE, Baum TJ, Apodaca MG, Real V, Effects of ketamine on the unconditioned and conditioned locomotor activity of preadolescent and adolescent rats: impact of age, sex, and drug dose, Psychopharmacology 234 (2017) 2683–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McDougall SA, Park GI, Ramirez GI, Gomez V, Adame BC, Crawford CA, Sex-dependent changes in ketamine-induced locomotor activity and ketamine pharmacokinetics in preweanling, adolescent, and adult rats, Eur. Neuropsychopharmacol 29 (2019) 740–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bates MLS, Trujillo KA, Long-lasting effects of repeated ketamine administration in adult and adolescent rats, Behav. Brain Res 369 (2019) 111928. doi: 10.1016/j.bbr.2019.111928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Saland SK, Kabbaj M, Sex differences in the pharmacokinetics of low-dose ketamine in plasma and brain of male and female rats, J. Pharmacol. Exp. Ther 367 (2018) 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Geyer MA, Serotonergic functions in arousal and motor activity. Behav. Brain Res 73 (1996) 31–35. [DOI] [PubMed] [Google Scholar]
- [30].Hauber W, Involvement of basal ganglia transmitter systems in movement initiation, Prog. Neurobiol 56 (1998) 507–540. [DOI] [PubMed] [Google Scholar]
- [31].Tzschentke TM, Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog. Neurobiol 63 (2001) 241–320. [DOI] [PubMed] [Google Scholar]
- [32].Yamaguchi K, Nabeshima T, Kameyama T, Role of dopaminergic and GABAergic mechanisms in discrete brain areas in phencyclidine-induced locomotor stimulation and turning behavior, J. Pharmacobiodyn 9 (1986) 975–986. [DOI] [PubMed] [Google Scholar]
- [33].Maj J, Rogóz Z, Skuza G, Locomotor hyperactivity induced by MK-801 in rats. Pol. J. Pharmacol. Pharm 43 (1991) 449–458. [PubMed] [Google Scholar]
- [34].Kuribara H, Asami T, Ida I, Tadokoro S, Characteristics of the ambulation-increasing effect of the noncompetitive NMDA antagonist MK-801 in mice: assessment by the coadministration with central-acting drugs, Jpn. J. Pharmacol 58 (1992) 11–18. [DOI] [PubMed] [Google Scholar]
- [35].Uchihashi Y, Kuribara H, Tadokoro S, Assessment of the ambulation-increasing effect of ketamine by coadministration with central-acting drugs in mice, Jpn. J. Pharmacol 60 (1992) 25–31. [DOI] [PubMed] [Google Scholar]
- [36].Martin P, Svensson A, Carlsson A, Carlsson ML, On the roles of dopamine D-1 vs. D-2 receptors for the hyperactivity response elicited by MK-801, J. Neural Transm. Gen. Sect 95 (1994) 113–121. [DOI] [PubMed] [Google Scholar]
- [37].Martin P, Waters N, Waters S, Carlsson A, Carlsson ML, MK-801-induced hyperlocomotion: differential effects of M100907, SDZ PSD 958 and raclopride, Eur. J. Pharmacol 335 (1997) 107–116. [DOI] [PubMed] [Google Scholar]
- [38].Martin P, Waters N, Schmidt CJ, Carlsson A, Carlsson ML, Rodent data and general hypothesis: antipsychotic action exerted through 5-HT2A receptor antagonism is dependent on increased serotonergic tone, J. Neural Transm 105 (1998) 365–396. [DOI] [PubMed] [Google Scholar]
- [39].Lapin IP, Rogawski MA, Effects of D1 and D2 dopamine receptor antagonists and catecholamine depleting agents on the locomotor stimulation induced by dizocilpine in mice, Behav. Brain Res 70 (1995) 145–151. [DOI] [PubMed] [Google Scholar]
- [40].Yamamoto M, Mizuki Y, Suetsugi M, Ozawa Y, Ooyama M, Suzuki M, Effects of dopamine antagonists on changes in spontaneous EEG and locomotor activity in ketamine-treated rats, Pharmacol. Biochem. Behav 57 (1997) 361–365. [DOI] [PubMed] [Google Scholar]
- [41].Carlsson ML, Martin P, Nilsson M, Sorensen SM, Carlsson A, Waters S, Waters N, The 5-HT2A receptor antagonist M100907 is more effective in counteracting NMDA antagonist- than dopamine agonist-induced hyperactivity in mice, J. Neural Transm 106 (1999) 123–129. [DOI] [PubMed] [Google Scholar]
- [42].Matulewicz P, Kasicki S, Hunt MJ, The effect of dopamine receptor blockade in the rodent nucleus accumbens on local field potential oscillations and motor activity in response to ketamine, Brain Res 1366 (2010) 226–232. [DOI] [PubMed] [Google Scholar]
- [43].Crawford CA, Moran AE, Baum TA, Apodaca MG, Montejano NR, Park GI, Gomez V, McDougall SA, Effects of monoamine depletion on the ketamine-induced locomotor activity of preweanling, adolescent, and adult rats: sex and age differences, Behav. Brain Res in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fessler RG, Sturgeon RD, Meltzer HY, Effects of phencyclidine and methylphenidate on d-amphetamine-induced behaviors in reserpine pretreated rats, Pharmacol. Biochem. Behav 13 (1980) 835–842. [DOI] [PubMed] [Google Scholar]
- [45].Starr MS, Starr BS, Comparison of the effects of NMDA and AMPA antagonists on the locomotor activity induced by selective D1 and D2 dopamine agonists in reserpine-treated mice. Psychopharmacology 114 (1994) 469–476. [DOI] [PubMed] [Google Scholar]
- [46].Narayanan S, Willins D, Dalia A, Wallace L, Uretsky N, Role of dopaminergic mechanisms in the stimulatory effects of MK-801 injected into the ventral tegmental area and the nucleus accumbens, Pharmacol. Biochem. Behav 54 (1996) 565–573. [DOI] [PubMed] [Google Scholar]
- [47].McDougall SA, Kozanian OO, Greenfield VY, Horn LR, Gutierrez A, Mohd-Yusof A, Castellanos KA, One-trial behavioral sensitization in preweanling rats: differential effects of cocaine, methamphetamine, methylphenidate, and D-amphetamine, Psychopharmacology 217 (2011) 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].McDougall SA, Nuqui CM, Quiroz AT, Martinez CM, Early ontogeny of D-amphetamine-induced one-trial behavioral sensitization, Pharmacol. Biochem. Behav 104 (2013) 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McDougall SA, Apodaca MG, Mohd-Yusof A, Mendez AD, Katz CG, Teran A, Garcia-Carachure I, Quiroz AT, Crawford CA, Ontogeny of cocaine-induced behaviors and cocaine pharmacokinetics in male and female neonatal, preweanling, and adult rats, Psychopharmacology 235 (2018) 1967–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nishimura M, Sato K, Okada T, Yoshiya I, Schloss P, Shimada S, Tohyama M, Ketamine inhibits monoamine transporters expressed in human embryonic kidney 293 cells, Anesthesiology 88 (1998) 768–774. [DOI] [PubMed] [Google Scholar]
- [51].Hancock PJ, Stamford JA, Stereospecific effects of ketamine on dopamine efflux and uptake in the rat nucleus accumbens, Br. J. Anaesth 82 (1999) 603–608. [DOI] [PubMed] [Google Scholar]
- [52].Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, Cheer JF, Frost DO, Huang XP, Gould TD, Effects of ketamine and ketamine metabolites on evoked striatal dopamine release, dopamine receptors, and monoamine transporters, J. Pharmacol. Exp. Ther 359 (2016) 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Spear LP, The adolescent brain and age-related behavioral manifestations, Neurosci. Biobehav. Rev 24 (2000) 417–463. [DOI] [PubMed] [Google Scholar]
- [54].Smith RF, Animal models of periadolescent substance abuse, Neurotoxicol. Teratol 25 (2003) 291–301. [DOI] [PubMed] [Google Scholar]
- [55].Caster JM, Walker QD, Kuhn CM, Enhanced behavioral response to repeated-dose cocaine in adolescent rats, Psychopharmacology 183 (2005) 218–225. [DOI] [PubMed] [Google Scholar]
- [56].Badanich KA, Adler KJ, Kirstein CL, Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi, Eur. J. Pharmacol 550 (2006) 95–106. [DOI] [PubMed] [Google Scholar]
- [57].Pautassi RM, Nizhnikov ME, Spear NE, Assessing appetitive, aversive, and negative ethanol-mediated reinforcement through an immature rat model, Neurosci. Biobehav. Rev 33 (2009) 953–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].National Research Council, Guide for the Care and Use of Laboratory Animals, eighth ed., National Academies Press, Washington, 2010. [Google Scholar]
- [59].Campbell BA, Lytle LD, Fibiger HC, Ontogeny of adrenergic arousal and cholinergic inhibitory mechanisms in the rat, Science 166 (1969) 635–637. [DOI] [PubMed] [Google Scholar]
- [60].Shalaby IA, Spear LP, Psychopharmacological effects of low and high doses of apomorphine during ontogeny, Eur. J. Pharmacol 67 (1980) 451–459. [DOI] [PubMed] [Google Scholar]
- [61].Finn IB, Iuvone PM, Holtzman SG, Depletion of catecholamines in the brain of rats differentially affects stimulation of locomotor activity by caffeine, D-amphetamine, and methylphenidate, Neuropharmacology 29 (1990) 625–631. [DOI] [PubMed] [Google Scholar]
- [62].McDougall SA, Garmsen GM, Meier TL, Crawford CA, Kappa opioid mediated locomotor activity in the preweanling rat: role of pre- and postsynaptic dopamine receptors, Psychopharmacology 133 (1997) 62–68. [DOI] [PubMed] [Google Scholar]
- [63].McDougall SA, Hernandez RM, Reichel CM, Farley CM, The partial D2-like dopamine receptor agonist terguride acts as a functional antagonist in states of high and low dopaminergic tone: evidence from preweanling rats, Psychopharmacology 178 (2005) 431–439. [DOI] [PubMed] [Google Scholar]
- [64].Carrier N, Kabbaj M, Sex differences in the antidepressant-like effects of ketamine, Neuropharmacology 70 (2013) 27–34. [DOI] [PubMed] [Google Scholar]
- [65].Bertrand F, Lehmann O, Galani R, Lazarus C, Jeltsch H, Cassel JC, Effects of MDL 73005 on water-maze performances and locomotor activity in scopolamine-treated rats, Pharmacol. Biochem. Behav 68 (2001) 647–660. [DOI] [PubMed] [Google Scholar]
- [66].Szewczyk B, Poleszak E, Wlaź P, Wróbel A, Blicharska E, Cichy A, Dybała M, Siwek A, Pomierny-Chamioło L, Piotrowska A, Brański P, Pilc A, Nowak G, The involvement of serotonergic system in the antidepressant effect of zinc in the forced swim test, Prog. Neuropsychopharmacol. Biol. Psychiatry 33 (2009) 323–329. [DOI] [PubMed] [Google Scholar]
- [67].Limón-Morales O, Soria-Fregozo C, Arteaga-Silva M, Vázquez-Palacios G, Bonilla-Jaime H, Altered expression of 5-HT1A receptors in adult rats induced by neonatal treatment with clomipramine, Physiol. Behav 124 (2014) 37–44. [DOI] [PubMed] [Google Scholar]
- [68].Aristieta A, Morera-Herreras T, Ruiz-Ortega JA, Miguelez C, Vidaurrazaga I, Arrue A, Zumarraga M, Ugedo L, Modulation of the subthalamic nucleus activity by serotonergic agents and fluoxetine administration, Psychopharmacology 231 (2014) 1913–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Holson RR, Pearce B, Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species, Neurotoxicol. Teratol 14 (1992) 221–228. [DOI] [PubMed] [Google Scholar]
- [70].Huynh H, Feldt LS, Estimation of the Box correction for degrees of freedom from sample data in randomized block and split-plot designs, J. Educ. Stat 1 (1976) 69–82. [Google Scholar]
- [71].Di Lullo SL, Martin-Iverson MT, Presynaptic dopaminergic neurotransmission mediates amphetamine-induced unconditioned but not amphetamine-conditioned locomotion and defecation in the rat, Brain Res 568 (1991) 45–54. [DOI] [PubMed] [Google Scholar]
- [72].Breese GR, Cooper BR, Mueller RA, Evidence for involvement of 5-hydroxytryptamine in the actions of amphetamine, Br. J. Pharmacol 52 (1974) 307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Green AR, Kelly PH, Evidence concerning the involvement of 5-hydroxytryptamine in the locomotor activity produced by amphetamine or tranylcypromine plus L-DOPA, Br. J. Pharmacol 57 (1976) 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Segal DS, Differential effects of para-chlorophenylalanine on amphetamine-induced locomotion and stereotypy, Brain Res 116 (1976) 267–276. [DOI] [PubMed] [Google Scholar]
- [75].Avale ME, Nemirovsky SI, Raisman-Vozari R, Rubinstein M, Elevated serotonin is involved in hyperactivity but not in the paradoxical effect of amphetamine in mice neonatally lesioned with 6-hydroxydopamine, J. Neurosci. Res 78 (2004) 289–296. [DOI] [PubMed] [Google Scholar]
- [76].Svingos AL, Hitzemann R, 5-HT3 receptor antagonists block cocaine-induced locomotion via a PCPA-sensitive mechanism, Pharmacol. Biochem. Behav 43 (1992) 871–879. [DOI] [PubMed] [Google Scholar]
- [77].Österlund MK, Hurd YL, Acute 17β-estradiol treatment down-regulates serotonin 5HT1A receptor mRNA expression in the limbic system of female rats, Mol. Brain Res 55 (1998) 169–172. [DOI] [PubMed] [Google Scholar]
- [78].Benmansour S, Weaver RS, Barton AK, Adeniji OS, Frazer A, Comparison of the effects of estradiol and progesterone on serotonergic function, Biol. Psychiatry 71 (2012) 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Creech RD, Li Q, Carrasco GA, Van de Kar LD, Muma NA, Estradiol induces partial desensitization of serotonin 1A receptor signaling in the paraventricular nucleus of the hypothalamus and alters expression and interaction of RGSZ1 and Gαz, Neuropharmacology 62 (2012) 2040–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Watanabe S, Fusa K, Takada K, Aono Y, Saigusa T, Koshikawa N, Cools AR, Effects of alpha-methyl-p-tyrosine on extracellular dopamine levels in the nucleus accumbens and the dorsal striatum of freely moving rats, J. Oral Sci 47 (2005) 185–190. [DOI] [PubMed] [Google Scholar]
- [81].Callaway CW, Kuczenski R, Segal DS, Reserpine enhances amphetamine stereotypies without increasing amphetamine-induced changes in striatal dialysate dopamine, Brain Res 505 (1989) 83–90. [DOI] [PubMed] [Google Scholar]
- [82].Kuczenski R, Biochemical actions of amphetamine and other stimulants, in Creese I (Ed.), Stimulants: Neurochemical, Behavioral and Clinical Perspectives, Raven Press, New York, 1983, pp. 31–61. [Google Scholar]
- [83].Parker EM, Cubeddu LX, Effects of d-amphetamine and dopamine synthesis inhibitors on dopamine and acetylcholine neurotransmission in the striatum. II. Release in the presence of vesicular transmitter stores, J. Pharmacol. Exp. Ther 237 (1986) 193–203. [PubMed] [Google Scholar]
- [84].Watanabe S, Aono Y, Fusa K, Takada K, Saigusa T, Koshikawa N, Cools AR, Contribution of vesicular and cytosolic dopamine to the increased striatal dopamine efflux elicited by intrastriatal injection of dexamphetamine, Neuroscience 136 (2005) 251–257. [DOI] [PubMed] [Google Scholar]
- [85].Cheetham SC, Heal DJ, Evidence that RU 24969-induced locomotor activity in C57/Bl/6 mice is specifically mediated by the 5-HT1B receptor, Br. J. Pharmacol 110 (1993) 1621–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Takahashi H, Takada Y, Urano T, Takada A, 5-HT4 receptors in the hippocampus modulate rat locomotor activity, Hippocampus 12 (2002) 304–310. [DOI] [PubMed] [Google Scholar]
- [87].Fletcher PJ, Sinyard J, Higgins GA, The effects of the 5-HT2C receptor antagonist SB242084 on locomotor activity induced by selective, or mixed, indirect serotonergic and dopaminergic agonists, Psychopharmacology 187 (2006) 515–525. [DOI] [PubMed] [Google Scholar]
- [88].Svensson A, Carlsson A, Carlsson ML, Differential locomotor interactions between dopamine D1/D2 receptor agonists and the NMDA antagonist dizocilpine in monoamine-depleted mice, J. Neural Transm 90 (1992) 199–217. [DOI] [PubMed] [Google Scholar]
- [89].Anis NA, Berry SC, Burton NR, Lodge D, The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methylaspartate, Br. J. Pharmacol 79 (1983) 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Harrison NL, Simmonds MA, Quantitative studies on some antagonists of N-methyl-D-aspartate in slices of rat cerebral cortex, Br. J. Pharmacol 84 (1985) 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, Carter G, Johnson B, Rasmussen K, Rorick-Kehn LM, The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits, J. Pharmacol. Exp. Ther 358 (2016) 71–82. [DOI] [PubMed] [Google Scholar]
- [92].Albin RL, Makowiec RL, Hollingsworth ZR, Dure LS 4th, Penney JB, Young AB, Excitatory amino acid binding sites in the basal ganglia of the rat: a quantitative autoradiographic study, Neuroscience, 46 (1992) 35–48. [DOI] [PubMed] [Google Scholar]
- [93].Standaert DG, Testa CM, Young AB, Penney JB Jr., Organization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J. Comp. Neurol 343 (1994) 1–16. [DOI] [PubMed] [Google Scholar]
- [94].French ED, Ceci A, Non-competitive N-methyl-D-aspartate antagonists are potent activators of ventral tegmental A10 dopamine neurons, Neurosci. Lett 119 (1990) 159–162. [DOI] [PubMed] [Google Scholar]
- [95].Overton P, Clark D, Iontophoretically administered drugs acting at the N-methyl-D-aspartate receptor modulate burst firing in A9 dopamine neurons in the rat, Synapse 10 (1992) 131–140. [DOI] [PubMed] [Google Scholar]
- [96].Chergui K, Charléty PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G, Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo, Eur. J. Neurosci 5 (1993) 137–144. [DOI] [PubMed] [Google Scholar]
- [97].Belujon P, Grace AA, Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity, Biol. Psychiatry 76 (2014) 927–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gartside SE, Cole AJ, Williams AP, McQuade R, Judge SJ, AMPA and NMDA receptor regulation of firing activity in 5-HT neurons of the dorsal and median raphe nuclei, Eur. J. Neurosci 25 (2007) 3001–3008. [DOI] [PubMed] [Google Scholar]
- [99].Mylecharane EJ, Ventral tegmental area 5-HT receptors: mesolimbic dopamine release and behavioural studies, Behav. Brain Res 73 (1996) 1–5. [DOI] [PubMed] [Google Scholar]
- [100].Nabeshima T, Yamaguchi K, Furukawa H, Kameyama T, Role of sex hormones in sex-dependent differences in phencyclidine-induced stereotyped behaviors in rats, Eur. J. Pharmacol 105 (1984) 197–206. [DOI] [PubMed] [Google Scholar]
- [101].Nabeshima T, Yamaguchi K, Yamada K, Hiramatsu M, Kuwabara Y, Furukawa H, Kameyama T, Sex-dependent differences in the pharmacological actions and pharmacokinetics of phencyclidine in rats, Eur. J. Pharmacol 98 (1984) 217–227. [DOI] [PubMed] [Google Scholar]
- [102].Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V, Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels, Neuropharmacology 46 (2004) 672–687. [DOI] [PubMed] [Google Scholar]
- [103].Milesi-Hallé A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM, Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats, Pharmacol. Biochem. Behav 86 (2007) 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].McDougall SA, Eaton SE, Mohd-Yusof A, Crawford CA, Age-dependent changes in cocaine sensitivity across early ontogeny in male and female rats: possible role of dorsal striatal D2High receptors, Psychopharmacology 232 (2015) 2287–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Quiñones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ, Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats, Behav. Brain Res 101 (1999) 15–20. [DOI] [PubMed] [Google Scholar]
- [106].Sell SL, Scalzitti JM, Thomas ML, Cunningham KA, Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats, J. Pharmacol. Exp. Ther 293 (2000) 879–886. [PubMed] [Google Scholar]
- [107].Becker JB, Molenda H, Hummer DL, Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse, Ann. N.Y. Acad. Sci 937 (2001) 172–187. [DOI] [PubMed] [Google Scholar]
- [108].van Haaren F, Garcea M, Anderson KG, Tebbett IR, Cocaine and benzoylecgonine in serum microsamples of intact and gonadectomized male and female Wistar rats. Pharmacol. Biochem. Behav 58 (1997) 421–424. [DOI] [PubMed] [Google Scholar]
- [109].Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM, Effects of sex and gonadectomy on cocaine metabolism in the rat, J. Pharmacol. Exp. Ther 290 (1999) 1316–1323. [PubMed] [Google Scholar]
- [110].Becker JB, Gender differences in dopaminergic function in striatum and nucleus accumbens, Pharmacol. Biochem. Behav 64 (1999) 803–812. [DOI] [PubMed] [Google Scholar]
- [111].Ojeda SR, Urbanski HF, Puberty in the rat, in Knobil R, Neill JD (Eds.), The Physiology of Reproduction (vol. 2), second ed., Raven Press, New York, 1994, pp. 363–409. [Google Scholar]
- [112].Snyder KJ, Katovic NM, Spear LP, Longevity of the expression of behavioral sensitization to cocaine in preweanling rats, Pharmacol. Biochem. Behav 60 (1998) 909–914. [DOI] [PubMed] [Google Scholar]
- [113].Kozanian OO, Gutierrez A, Mohd-Yusof A, McDougall SA, Ontogeny of methamphetamine- and cocaine-induced one-trial behavioral sensitization in preweanling and adolescent rats, Behav. Pharmacol 23 (2012) 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Duke MA, O’Neal J, McDougall SA, Ontogeny of dopamine agonist-induced sensitization: role of NMDA receptors, Psychopharmacology, 129 (1997) 153–160. [DOI] [PubMed] [Google Scholar]
- [115].Frantz K, Van Hartesveldt C, The locomotor effects of MK801 in the nucleus accumbens of developing and adult rats, Eur. J. Pharmacol 368 (1999) 125–135. [DOI] [PubMed] [Google Scholar]
- [116].Frantz K, Van Hartesveldt C, Locomotion elicited by MK801 in developing and adult rats: temporal, environmental, and gender effects, Eur. J. Pharmacol 369 (1999) 145–157. [DOI] [PubMed] [Google Scholar]
- [117].van den Buuse M, Low JK, Kwek P, Martin S, Gogos A, Selective enhancement of NMDA receptor-mediated locomotor hyperactivity by male sex hormones in mice, Psychopharmacology 234 (2017) 2727–2735. [DOI] [PubMed] [Google Scholar]
- [118].Wallace A, Pehrson AL, Sánchez C, Morilak DA, Vortioxetine restores reversal learning impaired by 5-HT depletion or chronic intermittent cold stress in rats, Int. J. Neuropsychopharmacol 17 (2014) 1695–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Moody CA, Spear LP, Ontogenetic differences in the psychopharmacological responses to separate and combined stimulation of D1 and D2 dopamine receptors during the neonatal to weanling age period, Psychopharmacology 106 (1992) 161–168. [DOI] [PubMed] [Google Scholar]
- [120].Varlinskaya EI, Truxell EM, Spear LP, Sex differences in sensitivity to the social consequences of acute ethanol and social drinking during adolescence, Behav. Brain Res 282 (2015) 6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]