Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is an adverse effect caused by several classes of widely used anticancer therapeutics. CIPN frequently leads to dose reduction or discontinuation of chemotherapy regimens, and CIPN symptoms can persist long after completion of chemotherapy and severely diminish the quality of life of patients. Differences in the clinical presentation of CIPN by widely diverse classifications of anticancer agents have spawned multiple mechanistic hypotheses that seek to explain the pathogenesis of CIPN. Despite its clinical relevance, common occurrence and extensive investigation, the pathophysiology of CIPN remains unclear. Furthermore, there is no unequivocal gold standard for the prevention and treatment of CIPN. Herein we review in vivo and in vitro models of CIPN with a focus on histopathological changes and morphological features aimed at understanding the pathophysiology of CIPN, and identify gaps requiring deeper exploration. An elucidation of the underlying mechanisms of CIPN is imperative to identify potential targets and approaches for prevention and treatment.
Keywords: Chemotherapy-induced peripheral neuropathy (CIPN), dorsal root ganglion (DRG) neuron, neurotoxicity, axonal degeneration, dying back neuropathy, Wallerian degeneration-like
Introduction
Peripheral neuropathy is a common neurological disorder affecting peripheral nerve fibers including autonomic, motor or sensory fibers 1. Underlying etiologies of peripheral neuropathy are widely diverse and include genetic, infectious, metabolic and toxic causes (Table 1). Peripheral neuropathy can be classified as a spectrum of diseases that are heterogenous and complex 1. The peripheral autonomic, motor and sensory neurons, their afferent and efferent axons, myelinating and non-myelinating Schwann cells, and connective tissue components (blood and lymphatic vessels, endoneurium, epineurium and perineurium) can all be differentially involved; however, an axonal lesion accompanied by Wallerian degeneration-like is a common feature that characterizes peripheral neuropathy, regardless of the underlying cause 1–3.
Table 1.
Examples of agents and conditions associated with peripheral neuropathy
| Classification | Agent/Condition | Proposed Target/ Mechanism | References |
|---|---|---|---|
| Antineoplastic agents | See Table 2 | ||
| Antibiotics | Aminoglycosides: • Amilkacin • Gentamicin • Kanamycin • Neomicin • Streptomycin • Tobramycin Metroniadazole |
Activation of NMDA receptors and lysosomal abnormality Mechanism of metroniadazole-induced peripheral neuropathy not fully understood |
112 113 |
| Anticonvulsants | Phenytoin | “Dying-back” axonal degeneration | 114 |
| Autoimmune disease | Churg-Strauss syndrome (vasculitis) Crohn’s disease Lupus Rheumatoid arthritis Scleroderma Sjögren’s syndrome (vasculitis) |
Heterogenous number of mechanisms | 115 |
| Cardiovascular medications | Aminodarone Perhexiline Statins |
Segmental demyelination with axonal degeneration | 114 |
| Genetic | Charcot-Marie-Tooth disease | Axonal degeneration | 116,117 |
| Heavy metals | Arsenic Gold Lead Mercury Thallium |
Primary axonal damage | 118 |
| Infectious disease | Lyme disease Shingles |
Unclear | 119 |
| Medical condition | Diabetes | Nerve fiber loss, although pathogenesis is poorly understood | 120 |
| Organic solvents | Aliphatic, aromatic, cyclic, halogenated
hydrocarbons Alcohols Esters Ethers Glycols Ketones |
Cross linking of neurofilaments; focal axonal swelling; slow axonal transport; segmental demyelination | 121 |
| Pesticides | Organophosphates Carbamates Organochlorine |
Axonal degeneration | 122 |
| Prescription medication to treat alcoholism | Disulfiram | Unclear, though may involve carbon disulfide | 123 |
| Psychotropic drugs | Methaqualone | Axonal degeneration | 114 |
| Substance abuse condition | Alcoholism | Distal axonopathy; “dying-back” neuropathy; etiology is controversial; may be related to malnutrition | 124 |
Chemotherapy-induced peripheral neuropathy (CIPN) is an adverse effect caused by a spectrum of classes of widely used anticancer therapeutics including platinum-based agents, microtubule disruptors (taxanes and vinca alkaloids), proteasome and angiogenesis inhibitors (Table 2) 4–13. Peripheral nervous system damage has also been reported with targeted cancer therapies,; however, most reports are anecdotal and combination therapies with CIPN drugs are frequently used with target-specific therapeutics 14. CIPN is often dose dependent and progressive during and after treatment 4. It frequently leads to dose reduction or discontinuation of chemotherapy regimens containing CIPN-causing agents 10,15,16. Further, CIPN symptoms can persist long after completion of chemotherapy (“coasting”) and severely diminish the quality of life of patients 5,17. As the number of long-term cancer survivors increases, the long-term effects of CIPN are becoming even more important and mechanistic explanations are needed to find effective treatments.
Table 2.
Commonly used anticancer agents associated with chemotherapy-induced peripheral neuropathy (CIPN) 4–11,13
| Class | Agents | Proposed Mechanism | Clinical Symptoms | Main Target of CIPN Toxicity |
|---|---|---|---|---|
| Taxanes | Paclitaxel Docetaxel Ixabepilone |
Microtubule disruption | Symmetrical painful paresthesias or numbness in a stocking-glove distribution, sensory loss, motor symptoms at high dose | Dorsal root ganglion; axons; distal nerve terminals |
| Platinum-based | Cisplatin Carboplatin Oxaliplatin |
DNA adducts | Symmetrical painful paresthesias or numbness in a stocking-glove distribution; Early: cold allodynia and hyperalgesia; Later: loss of motor function; Long-term chronic sensory neuropathy | Dorsal root ganglion |
| Alkylating agents | Cyclophosphamide Hexamethylmelamine Ifosphamide Procarbazine |
Covalently bind to DNA | Spectrum ranging from numbness and paresthesias to pain | Dorsal root ganglion |
| Vinca alkaloids | Vincristine Vinblastine Vinorelbine Vindesine |
Dysfunction of mitochondria and endoplasmic reticulum; microtubule disruption | Symmetrical sensorimotor painful neuropathy: tingling paresthesias, proprioceptive loss, areflexia, and ataxia; Constipation; Muscle weakness; Gait dysfunction | Dorsal root ganglion; distal nerve terminals |
| Proteosome inhibitors | Bortezomib Carfilzomib Ixazomib |
Binds proteasome complex; mitochondrial disturbance; microtubule disruption | Sensory painful neuropathy | Dorsal root ganglion and peripheral nerves |
| Immunomodulatory | Thalidomide Lenalidomide Pomalidomide |
Antiangiogenesis | Symmetrical distal paresthesias, dysesthesias; Sensory painful neuropathy; Muscle cramps | Dorsal root ganglion; distal nerve terminals |
Peripheral neurotoxicity mechanisms of anticancer drugs are not fully understood, but may result from interactions with DNA, mitochondria, ion channels, glutamate neurotransmission, and/or kinases, involving various targets in the peripheral nervous system such as dorsal root ganglia (DRG), sensory neurons, Schwann cells, and/or satellite glial cells 18–21. While the underlying mechanisms for CIPN are not known, current data identify a “dying back” axon degeneration of distal nerve endings as the major pathology in this disorder 22. Mechanistic understanding of axonal degeneration will provide insights into pathways and molecular dynamics responsible for CIPN. The pathogenesis of CIPN and pathways shared with injury-induced Wallerian degeneration-like have been reviewed 22. Development of effective mechanism-based therapies will need to rely on appropriate use of models that replicate essential features of peripheral neuropathy and use appropriate endpoint measurements that are relevant to the pathogenesis of the disease 23. This review aims to focus on in vivo and in vitro model systems used to explore the underlying pathophysiology and mechanisms that lead to CIPN.
Clinical assessment of CIPN
Differences in the clinical presentation of CIPN have spawned multiple mechanistic hypotheses to explain the pathogenesis of CIPN. Clinical symptoms of peripheral neuropathy are just as diverse as the causes and depend on the type of the nerve fiber affected: involvement of autonomic nerve fibers leads to autonomic dysfunction, which may manifest as orthostatic hypotension, cardiac dysthymia, abnormalities of sweating, incontinence, erectile dysfunction, or gastrointestinal symptoms, such as persistent nausea, vomiting, constipation, or diarrhea; motor nerve fiber involvement can lead to muscle weakness and wasting; sensory fiber involvement can manifest as ataxia, poor balance, and paresthesias, which can be extremely painful 20,23.
Clinically, CIPN is manifested predominately with the loss of sensory function 5,10,24 and presents in a “glove-and-stocking” distribution (Figure 1) 11,12. Diagnosis of peripheral neuropathy is often made based on patient history of symptoms, functional and electrophysiological features to corroborate involvement of specific nerve fiber types, and characteristic neuropathologic features derived from histopathologic methods 1. However, sural or other whole nerve biopsies are rarely indicated in the evaluation of CIPN in patients 9. Nerve conduction velocity testing is routinely used to assess CIPN in patients and animal models 4,16,23,25–28. The reduction in amplitude of sensory nerve action potentials and the ‘dying back’ degeneration of nerve terminals of sensory neurons residing in dorsal root ganglion are both hallmarks of the neurophysiological and histopathological basis of CIPN 5.
Figure 1.

Depiction of the typical “glove-and-stocking” distribution of chemotherapy-induced peripheral neuropathy (CIPN) symptoms with putative targets for CIPN toxicity in the peripheral nervous system depicted from the dorsal root ganglion to axon and axonal components (myelin, microtubules, mitochondria, ion channels, and vascular network) and the distal nerve terminals. (Reprinted from Park, S.B., Goldstein, D., Krishnan, A.V., Lin, C.S.-Y., Friedlander, M.L., Cassidy, J., Koltzenburg, M. and Kiernan, M.C. (2013). Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA: A Cancer Journal for Clinicians, 63, 419–437. Reproduced with permission from John Wiley and Sons.)
Histopathologic evaluation of nerve biopsies is clearly the definitive tool for diagnosing and detecting pathologic changes in peripheral nerves. Since CIPN shows more pronounced distal involvement, the distally located, conveniently accessible and almost purely sensory sural nerve is the optimal choice for nerve biopsy 1; however nerve biopsies are rarely indicated in CIPN patients 9. The role of skin biopsy in CIPN patients is an emerging, minimally invasive tool for assessment of epidermal unmyelinated sensory fibers and small myelinated fibers of the dermis 1,29. A consistent pathologic finding in CIPN patients is intraepidermal nerve fiber (IENF) loss in hands and feet 15. The IENF consists of unmyelinated axons from small-diameter sensory neurons 30. Using immunohistochemistry and image analysis tools, IENF density can be measured to assess small fiber neuropathy in CIPN patients as well as in animal and in vitro models of CIPN 15,31,32. A “dying back” axon degeneration is the hallmark of CIPN in patients and rodent models that is assessed by histopathological examination of peripheral nerve biopsies or skin biopsy, and measurement of IENF density 25.
Sensitive, noninvasive functional techniques to specifically assess small fiber neuropathy include nerve excitability threshold tracking in CIPN patients 33 and Doppler flowmetry to determine axon reflex flare area in patients with peripheral neuropathy of several different etiologies 34. A limitation of these techniques is that structural loss of innervation does not necessarily correlate with the presence of painful neuropathy symptoms in patients 35.
Pathophysiology of CIPN
Over the past two decades, a compelling body of evidence points to four main trends that have emerged from preclinical research on CIPN: neurotoxic anticancer drugs affect the peripheral sensory nerve by (1) directly targeting the mitochondria and producing oxidative stress, (2) functionally impairing ion channels, (3) triggering immunological mechanisms through activation of satellite glial cells, and/or (4) disruption of microtubules 8,13. It is important to note that these various neurotoxic events are not necessarily related to the anticancer mechanisms of action for these agents and may account for the lack of effective treatment. It is hypothesized that a polytherapy which targets multiple mechanisms will most likely be the means to achieve neuroprotection 8.
Different chemotherapies affect distinct components of the peripheral nervous system, from the level of the sensory cell bodies in the dorsal root ganglion (DRG) to the distal axon (Figure 1) 11. DRGs are a prominent target as they are less protected by the blood-nerve barrier and more vulnerable to neurotoxic damage, 36 potentially explaining the predominance of sensory involvement in patients with CIPN. Platinum compounds form DNA adducts that accumulate in DRGs 37,38 and lead to cell death in sensory neurons 39,40. Vinca alkaloids, taxanes and thalidomide have also been associated with DRG damage 41–44.
Disruption of microtubules is another common mechanism of neurotoxicity 6. Microtubules are central to axonal transport of proteins from the cell body into and down the length of the axon 8. Taxanes bind to β-tubulin components of microtubule assemblies, producing over polymerization and interference with normal microtubule dynamics, which has been linked to disruption of axonal transport 45,46. Taxanes have also been shown to induce increased microtubule bundling in axons, which leads to alterations in peripheral nerve mechanical properties when treated in vitro or in vivo 47,48. Vinca alkaloids bind tubulin and inhibit microtubule dynamics, leading to interference with the mitotic spindle 44. Bortezomib, a proteasome inhibitor, also affects tubulin polymerization independent of its anticancer mechanism 49. Bortezomib CIPN may be caused by an indirect increase in stabilization of microtubules due to increased expression of microtubule-associated proteins 50.
Bortezomib is also thought to cause peripheral neuropathy through mitochondrial toxicity and endoplasmic reticulum stress in Schwann cells, leading to pathological adaptive responses including demyelination and macrophage recruitment 51. Finally, accumulation of ubiquitin-conjugated proteins leading to inhibition of transcription, transport and cytoplasmic translation of mRNAs is yet another potential mechanism of bortezomib 41.
Damage to the mitochondria and impairment of mitochondrial function may also play a vital role in the onset and development of CIPN 52. Paclitaxel administration has been reported to cause prominent abnormalities in axonal mitochondria 53, whereas bortezomib has also been reported to affect endoplasmic reticulum and mitochondrial integrity, particularly in Schwann cells 51,54. In addition to energy deficiency, chemotherapies may damage the peripheral vasculature as exemplified by thalidomide, which reduces peripheral nerve blood supply via antiangiogenic effects that lead to axonal degeneration 55.
The role of neuroinflammation in CIPN pathology is becoming increasingly evident 56. Penetration of the blood-nerve barrier and accumulation of chemotherapy agents in the DRG may cause neuroinflammation through activation of immune-like cells, such as satellite glial cells 57, followed by secretion of mediators that enhance neuronal excitability and generate pain hypersensitivity 58.
Additional targets of neurotoxicity include direct axonal toxicity at the distal terminals, which may induce neurotoxicity and Wallerian degeneration-like following treatment with paclitaxel 59, vincristine 60, and thalidomide 61. Oxaliplatin may directly alter axonal voltage-gated sodium (Na+) ion channel function, inducing an acute neurotoxicity manifested by peripheral nerve hyperexcitability 62–64. In some patients, bortezomib may induce primary myelin sheath degeneration 54. However, despite potential diverse mechanisms underlying the development of CIPN, common degenerative pathways may be triggered when the normal processes and energy delivery mechanisms of the peripheral nervous system become disrupted.
Although it is well known that anticancer drugs may act on various subcellular targets of peripheral sensory nerves, and that mechanisms of CIPN may be shared by several chemotherapeutic agents independent of their antitumor properties, there remains no consensus on the molecular mechanisms culminating in CIPN. The development of in vivo and in vitro models has provided valuable tools for studying the pathogenesis of CIPN and intervention strategies 15,16,27,65,66.
Animal models
The first animal model of CIPN was described in 1992 using cisplatin in rats 67. Since then, animal models have been used to reproduce neurotoxic effects of nearly all of the conventional chemotherapeutic agents and investigate the pathophysiology of CIPN using clinically relevant doses (Table 3) 10,15,16,25,27,68,69, with only the exception of thalidomide 23. The outcome measure most commonly reported is evoked limb withdrawal to mechanical monofilaments, and the greatest increase in pain-related behavior is associated with vincristine 70. Other outcomes measured include assessment of locomotor function, memory, reward and attention 70. In animal studies modeling CIPN, mice and rats show similar clinical symptoms and histopathologic features with no significant heterogeneity between them (Table 3) 70. Notably, histomorphologic changes observed in animal models of CIPN are consistent with those revealed in CIPN patients 16.
Table 3.
Comparative pathologic features of select chemotherapeutic agents that cause CIPN in humans and rodent models
| Agent | Class | Clinical Onset of Sensory Symptoms | Animal Model | Treatment (mg/kg/day) | Total Dose (mg/kg) | Total Human Equivalent Dose (mg/kg)a | Highest Dose Recommended in Humansb | Symptoms in Animals | Pathologic Findings in Peripheral Nerves in Rodent Models | Pathologic Findings in Peripheral Nerves in Patients with CIPN |
|---|---|---|---|---|---|---|---|---|---|---|
| Bortezomib | Proteasome inhibitor | Chronic | Mouse Rat |
0.4 – 0.8 0.15 – 0.2 |
4.8 – 6.4 2.4 – 4.8 |
0.39 – 0.52 0.39 – 0.77 |
1.3 mg/m2 (0.035 mg/kg)a | Mechanical allodynia, mechanical hyperalgesia, cold allodynia | IENFc loss; axonal degeneration | IENF loss |
| Cisplatin | DNA adducts | Chronic | Mouse Rat |
2.3 – 6.0 0.5 – 3.0 |
23.0 – 40.0 1.5 – 18.0 |
1.9 – 3.3 0.24 – 2.9 |
80 mg/m2 (2.16 mg/kg)a | Mechanical allodynia, cold allodynia | IENF loss; axonal degeneration | IENF loss |
| Oxaliplatin | DNA adducts | Acute and chronic | Mouse Rat |
0.04 – 0.0 2.0 – 6.0 |
3.0 – 30.0 2.0 – 16.0 |
0.24 – 2.4 0.32 – 2.6 |
110 mg/m2 (2.97 mg/kg)a | Mechanical allodynia; mechanical hyperalgesia; cold allodynia, thermal allodynia, heat allodynia; heat hyperalgesia | IENF loss; axonal degeneration | IENF loss |
| Paclitaxel | Microtubule destabilization | Acute and chronic | Mouse Rat |
4.0 – 18.0 1.0 – 32.0 |
4.0 – 38.0 8.0 – 80.0 |
0.33 – 3.1 1.3 – 12.9 |
175 mg/m2 (4.73 mg/kg)a | Mechanical allodynia and hyperalgesia, cold allodynia and hyperalgesia, heat hyperalgesia | IENF loss; axonal degeneration | IENF loss |
| Vincristine | Microtubule disruption | Chronic | Mouse Rat |
0.1 – 1.7 0.05 – 0.1 |
0.1 – 34.0 0.5 – 1.4 |
0.01 – 2.8 0.08 – 0.23 |
1 mg/m2 (0.027 mg/kg)a | Mechanical allodynia and hyperalgesia, cold allodynia, heat hyperalgesia | IENF loss; axonal degeneration | IENF loss |
Animal models of CIPN generally model the acute phase of CIPN 70, whereas chronic (“coasting”) CIPN is frequently reported clinically 71–73. The most frequent behaviors reported in animal models of CIPN are estimates of gain in sensory function with hypersensitivity in paw withdrawal evoked by mechanical stimuli being the assay most often employed 70. The most commonly reported pain-related behavior in animals is also reflex withdrawal responses 74. This starkly contrasts with chronic clinical CIPN, where the predominant clinical sensory phenotype in CIPN patients is sensory loss 75. Thus, there is a dichotomous sensory profile reported from animal studies and what is observed in chronic CIPN patients, potentially compromising the clinical relevance of these models for chronic CIPN; however, they may have more relevance to acute CIPN 70.
Very little information has been reported regarding CIPN in animals after chronic drug treatment. Electrophysiology and histopathology were examined after chronic chemotherapy treatment in mice given cisplatin, paclitaxel, epothilone-B or bortezomib for four weeks 76. All drugs caused a significant reduction in sensory/motor nerve conduction velocities. Functional toxicity was confirmed by histopathological examination at both the light and electron microscopic level as axonal degeneration 76.
Höke and Ray provide a thorough review of animal models used to study CIPN 27. Many of these animal studies demonstrate similar neuropathological changes seen in human CIPN, and exhibit typical electrophysiological abnormalities expected in peripheral neuropathy, such as reduced nerve action potentials, and in some cases reduced conduction velocities 23. However, there are unsettling differences in outcomes measured, including pathological, electrophysiological, and behavioral abnormalities observed in different laboratories. The source of such discrepancies may include genetic background of animals used, mode of administration (intravenous vs intraperitoneal), dose and duration of drug administration, and extent and detail of outcome measurements used in the evaluation of peripheral neuropathy 23.
Three tubulin-targeting anticancer drugs known to cause CIPN were compared at the maximum tolerated dose (MTD) for two weeks in mice 77. Paclitaxel and ixabepilone produced significant reductions in caudal nerve conduction velocity, caudal amplitude and digital nerve amplitude, whereas eribulin mesylate produced no significant deleterious effects on the same parameters 77. All three agents showed axonal degeneration in DRGs and sciatic nerves with ixabepilone causing the most severe lesions, followed by paclitaxel and eribulin mesylate inducing mild changes (Figure 2). Ixabepilone, at its MTD (3 mg/kg), caused severe and frequent morphologic alterations, both in neuronal and in glial compartments of DRGs. Neurons exhibited dark cytoplasmic inclusions, often localized to the perinuclear area. Clear vacuolations and cytoplasmic swelling were evident in satellite glial cells, which were indicative of severely injured and degenerating cells. Additionally, adverse effects were evident at lower doses of ixabepilone. Cytoplasmic dark inclusions and degenerating neurons, as well as vacuoles of satellite glial cells, were evident in the 0.75 × MTD and MTD ixabepilone groups. Moreover, rare episodes of clear cytoplasmic vacuolation of neurons were also observed.
Figure 2.
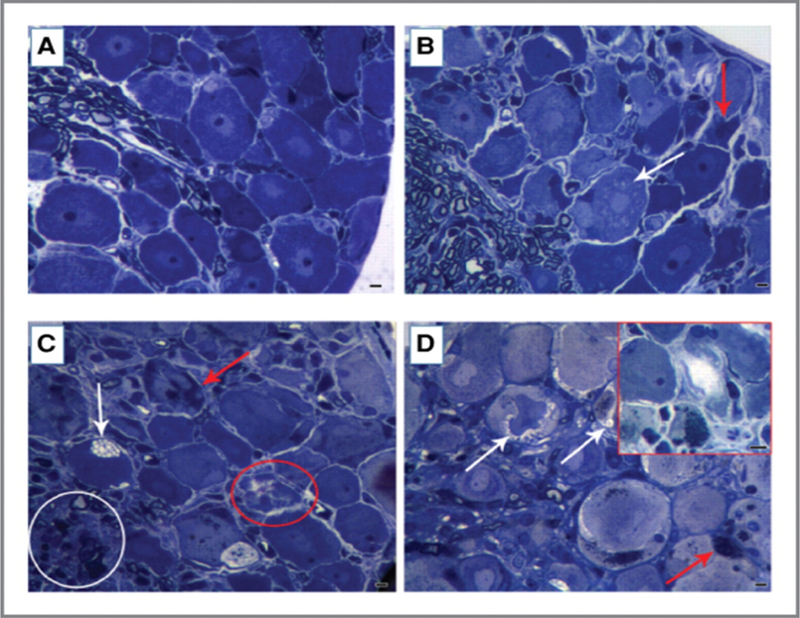
Effect of maximal tolerated dose (MTD) of eribulin mesylate, paclitaxel, and ixabepilone on dorsal root ganglion (DRG) morphology. DRG morphology at the light microscopic level showed changes after each chemotherapy (at its MTD). Ixabepilone (D) caused most severe and frequent morphologic changes both in neuronal and glial compartments. Severely injured, degenerating neurons are outlined (red). Dark cytoplasmic inclusions were evident and were often localized in the perinuclear area. Vacuolations (white arrows) and swelling phenomena (red arrowheads) were evident in the cytoplasm of satellite cells (D). DRGs from paclitaxel-treated mice (C) also displayed degenerating nerve cells (outlined by red circle) with dark cytoplasm (red arrows) and clear vacuolations in cytoplasm of satellite cells (white arrow). Alterations in the proximal axons of DRG were also observed (white circle). DRGs from eribulin mesylate-treated mice (B) showed mild pathologic changes evidenced by some cytoplasmic vacuolation (white arrow) and degenerating nerve cells (red arrow), as compared to vehicle-treated mice (A). Scale bar, 20 μm. (Reprinted from Wozniak, K.M., Nomoto, K., Lapidus, R.G., Wu, Y., Carozzi, V., Cavaletti, G., Hayakawa, K., Hosokawa, S., Towle, M.J., Littlefield, B.A. and Slusher, B.S. (2011). Comparison of neuropathy-inducing effects of eribulin mesylate, paclitaxel, and ixabepilone in mice. Cancer Res, 71, 3952–3962. Reproduced with permission from American Association for Cancer Research.)
A dose-dependent effect of paclitaxel on sciatic nerve morphology was observed at the MTD (30 mg/kg) 77. Severe pathologic changes consistent with axonal degeneration affected both large- and small-diameter fibers (Figure 2). Severity of axonopathy was milder that that seen with ixabepilone, but still clearly evident in groups treated with 0.75 × MTD (22.5 mg/kg) or 0.5 × MTD (15 mg/kg) paclitaxel. Paclitaxel at 0.5 × MTD (15 mg/kg; also caused formation of dark inclusions in the cytoplasm. In addition, DRGs from the 0.75 × MTD (22.5 mg/kg) group had clear vacuolations in the cytoplasm of neurons as well as in satellite glial cells. A proportion of neurons in the DRGs were degenerating and their cytoplasm appeared much darker than normal neurons at the MTD of paclitaxel (30 mg/kg). Dose-dependent axonal degeneration was present in proximal axons at 0.5 × MTD and greater.
Treatment with eribulin mesylate showed a dose-dependent effect on sciatic nerve morphology at 0.5 × MTD (0.875 mg/kg) to the MTD (1.75 mg/kg) 77. At these doses, eribulin mesylate induced mild to moderate pathologic changes consistent with axonal degeneration that affected both large- and small- diameter fibers (Figure 2). Clear cytoplasmic vacuolation of DRG neurons were evident between 0.75 × MTD to the MTD of eribulin mesylate doses, whereas dark inclusions were only rarely observed at the MTD.
Bortezomib-induced sensory neuropathy in rats revealed axonal degeneration in sciatic nerves after eight weeks of treatment (0.15 or 0.20 mg/kg three times/week) and at the end of a four-week follow-up period (0.20 mg/kg only) 78. Complete recovery of morphologic changes was observed in rats treated with 0.15 mg/kg, whereas only partial recovery was observed in the 0.20 mg/kg treated group. Sensory neuropathy in mice treated with 0.40 mg/kg bortezomib three times/week for four weeks showed the same axonal degeneration in sciatic nerves as in rats and a significant reduction in nerve fiber density 26.
The anticancer effects of tubulin-targeting agents generally attributed to their ability to bind microtubules, interfere with mitotic spindle formation, and ultimately block mitosis, resulting in cell death 79. However, somatic neurons do not divide; therefore, neurotoxic effects of tubulin-targeting agents appear to be independent of their anticancer activity. An understanding of the mechanisms behind the neurotoxic effects of tubulin-targeting and other CIPN-causing agents is far from complete and further studies of the peripheral neuropathy of tubulin-targeting chemotherapies are warranted.
In vitro models
Primary DRG explants or dissociated cell cultures obtained from rodents have been used as in vitro models to recapitulate the pathophysiological feature of a predominantly sensory neuropathy and axonal degeneration in DRG neurons and to elucidate underlying mechanisms of CIPN 15,22. Image analysis of neurite outgrowth is the endpoint most commonly employed to quantify morphological alterations caused by CIPN-inducing agents 15,22,31,66,80–84. Using adult DRG neurons isolated from 3- to 6-month-old male Wistar rats, Malgrange et al. observed significant inhibition of neurite outgrowth by cisplatin, vinblastine, taxol and vincristine at a clinically relevant concentration not altering the overt cell viability 85. Time- and dose-dependent inhibition of neurite outgrowth was also observed in DRG explants exposed to cisplatin and/or taxol 86,87. Toxic effects of vincristine and taxol that preferentially target neurites, especially distal axons, and contribute to the commonly featured pathological pattern of “dying back” in clinical CIPN were reproduced by a compartmentalized microfluidic culture platform that enables culture of DRG neuronal cell bodies (somas) and their axons (neurites) in separate chambers, hence, allowing local treatment of drugs to identify the primary site of action 60,88.
Measurement of electrical activity from compartmentalized somas and neurites with a multielectrode assay (MEA) substrate demonstrated a progressive loss of electrophysiological function in neurites but not somas in cultured neurons from 15-day-old rat embryos exposed to vincristine 89, substantiating the finding of reduced conduction velocity that has been observed frequently in clinical and animal models of CIPN. Ultrastructural analysis of taxol treated DRG organotypic cultures or explants revealed an unusual aggregation of microtubules in neurons, and the presence of microtubule-endoplasmic reticulum arrays and necrotic features in the neuronal somas and neurites 87,90, whereas apoptotic cell death was noted in rat DRG neurons and PC12 cells exposed to cisplatin 91,92. Mitochondrial impairment caused by taxol and cisplatin in DRG neurons were evident with loss of mitochondrial membrane potential, induction of mitochondrial DNA damage, cristae lysis and autophagic vacuoles 92–94, and abnormalities in mitochondrial bioenergetics, glycolysis or mRNA transport in axons in DRG neurons isolated from rodents that developed taxol-induced painful neuropathy 95,96.
Exposure of rat DRG explants or dissociated neurons to bortezomib at clinically relevant doses caused time- and dose-dependent inhibition of neurite outgrowth without overt cytotoxicity, but was accompanied by somatic aggregation of β-tubulin and disruption in axonal trafficking of mitochondria 97. In cultured rat DRG neurons, augmentation of the stimulated release of neuropeptide calcitonin gene-related peptide (CGRP) was observed with taxol treatment, which may play a role in taxol-altered neuronal sensitivity 98. Inhibition of neurite outgrowth by cisplatin and bortezomib varied in DRG neurons of different rodent strains, indicating genetic background may contribute to variability in patient susceptibility to CIPN 99.
An increasing body of evidence indicates that supporting, non-neuronal cells in DRG-derived cultures are also involved in the pathogenesis of CIPN 11,100. Our laboratory established a multiparametric morphology-centered rat DRG culture model allowing assessment of toxic effects of CIPN drugs on both sensory neuronal and non-neuronal (mainly Schwann cells) cell populations simultaneously 31. In this model, CIPN-inducing agents bortezomib, cisplatin, eribulin, taxol and vincristine induced a dose-dependent loss of neurite process area without overt cytotoxic effects on cell bodies, recapitulating the feature of ‘dying back’ axonopathy observed in clinical or animal models of CIPN 31. Compound-specific effects, such as neurite fragmentation by cisplatin or bortezomib and enlarged neuronal cell bodies by paclitaxel, were also observed, supporting the usefulness of this multicellular culture model to identify risk and examine mechanisms of CIPN 31.
Recent advances in stem cell technology to differentiate hESCs or hiPSCs into DRG-like, peripheral sensory neurons have provided novel human-based, clinically-relevant, in vitro models to study peripheral sensory neuronal development and injury 80,82–84,101–104.
The application of hiPSC-derived DRG-like sensory neurons to assess CIPN-inducing agents has been evaluated by several laboratories using commercially available cells; results support the use of hiPSC DRG-like cells for toxicity testing of compounds with CIPN liability 66,82,84.
Prevention, restoration and other intervention strategies
It is beyond the scope of this paper to discuss the plethora of agents and strategies that have been tested to prevent or treat CIPN in patients and animal models of CIPN. The reader is referred to several review articles that cover the topic 24,105–108. Suffice it to say that to date, there is no unequivocal gold standard for the prevention and/or treatment of CIPN 24,108.
Unmet needs
There is a need for reliable, standardized and validated clinical assessment endpoints or diagnostic tools to better identify the presence and severity of CIPN 68,109. The clinical utility of such tools is critical when employed as endpoints in clinical trials designed to measure outcomes of prevention, mitigation or treatment strategies for CIPN 109.
Factors to explore that likely contribute to the risk of CIPN include cumulative dose, therapy duration, synergistic neuropathy caused by previously given chemotherapies and concomitant chemotherapies, and the role of pre-existent neuropathy 6,110. Further complicating the epidemiology of CIPN is the existence of immediate, short-term “acute pain syndrome” with some chemotherapeutics, which may be severe enough to lead to reduced dosing or termination of life-saving treatment, whereas other chemotherapeutics induce pain after several treatment cycles 10,110. A better understanding of the epidemiology of CIPN, combined with understanding of the anticancer mechanism of the chemotherapeutic, should help to clarify the mechanisms underlying CIPN and possibly define intervention strategies 69.
Although somatosensory symptoms of CIPN can generally be characterized as “neuropathic pain,” given that the symptoms share similarities with other painful peripheral neuropathies, such as diabetic neuropathy, with symmetrical tingling and “burning pain” that starts distally in the limbs and with a stocking- and-glove distribution 21, there are differential symptomologies evoked by different classes of chemotherapeutic agents. It is likely that each class or individual drug will have unique neurotoxicity mechanisms associated with them. Although a single therapy to treat CIPN is desirable, it is more realistic that several different therapeutics are required. Clearly, there is a need to understand the mechanisms of CIPN to develop effective therapeutic strategies 69,110,111.
Animal models of CIPN generally model the acute phase of CIPN 70, whereas chronic (“coasting”) CIPN is frequently reported clinically 71–73. The mismatch between timing of clinical and nonclinical findings illustrate the need for closer scrutiny of animal models of CIPN to bridge the gap between nonclinical and clinical studies 69.
Typically, most animal models of CIPN involve administration of a chemotherapeutic agent in the absence of a tumor burden 106. Although tumor-bearing rodent models of CIPN are more clinically relevant, the practical and ethical issues are not to be underestimated 106. In situations where chemotherapy is administered after surgical removal of the tumor, modeling CIPN by chemotherapy administration alone is a valid approach 106. The use of intermittent dosing schedules to generate CIPN models and consideration of the same dosing schedules across different laboratories would further understanding of causal mechanisms of CIPN and enhance reproducibility 106.
Conclusions
Despite the myriad etiologies and symptoms, and difficulty in diagnosing CIPN, histopathology is the same, but is seldom used to characterize peripheral neuropathy. Axonal degeneration is the common histomorphologic change observed during CIPN and is the consistent pathological process in most drug-induced neuropathies. Although experimental studies have contributed to the understanding of the pathogenesis of certain drug-induced peripheral neuropathies, the basic mechanisms remain poorly understood. It is through mechanistic insights that new avenues for development of therapies to prevent or treat CIPN will be unveiled.
Acknowledgments
Funding: This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported [in part] by the Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute.
Footnotes
Competing interests: The authors declare no real, perceived or potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Katona I, Weis J. Diseases of the peripheral nerves. Handbook Clin Neurol 2018;145:453–474. [DOI] [PubMed] [Google Scholar]
- 2.Carroll SL, Worley SH. Wallerian Degeneration☆. In: Reference Module in Neuroscience and Biobehavioral Psychology Elsevier; 2017. [Google Scholar]
- 3.Kaufmann W, Bolon B, Bradley A, et al. Proliferative and nonproliferative lesions of the rat and mouse central and peripheral nervous systems. Toxicol Pathol 2012;40(4 Suppl):87S–157S. [DOI] [PubMed] [Google Scholar]
- 4.Addington J, Freimer M. Chemotherapy-induced peripheral neuropathy: an update on the current understanding. F1000Research 2016;5:F1000 Faculty Rev-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer Management and Research 2014;6:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brzeziński K Chemotherapy-induced polyneuropathy. Part I. Pathophysiology. Contemporary oncology (Poznan, Poland) 2012;16(1):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cioroiu C, Weimer LH. Update on Chemotherapy-Induced Peripheral Neuropathy. Curr Neurol Neurosci Rep 2017;17(6):47. [DOI] [PubMed] [Google Scholar]
- 8.Ferrier J, Pereira V, Busserolles J, Authier N, Balayssac D. Emerging trends in understanding chemotherapy-induced peripheral neuropathy. Current pain and headache reports 2013;17(10):364–372. [DOI] [PubMed] [Google Scholar]
- 9.Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro-oncology 2012;14 Suppl 4(Suppl 4):iv45–iv54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treatment Reviews 2014;40(7):872–882. [DOI] [PubMed] [Google Scholar]
- 11.Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA: A Cancer Journal for Clinicians 2013;63(6):419–437. [DOI] [PubMed] [Google Scholar]
- 12.Staff NP, Grisold A, Grisold W, Windebank AJ. Chemotherapy-induced peripheral neuropathy: A current review. Annals of neurology 2017;81(6):772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starobova H, Vetter I. Pathophysiology of Chemotherapy-Induced Peripheral Neuropathy. Frontiers in Molecular Neuroscience 2017;10(174). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavaletti G Chemotherapy-induced peripheral neurotoxicity (CIPN): what we need and what we know. Journal of the peripheral nervous system : JPNS 2014;19(2):66–76. [DOI] [PubMed] [Google Scholar]
- 15.Fehrenbacher JC. Chemotherapy-induced peripheral neuropathy. Prog Mol Biol Transl Sci 2015;131:471–508. [DOI] [PubMed] [Google Scholar]
- 16.Han Y, Smith MT. Pathobiology of cancer chemotherapy-induced peripheral neuropathy (CIPN). Front Pharmacol 2013;4:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. Journal of the Peripheral Nervous System 2008;13(1):27–46. [DOI] [PubMed] [Google Scholar]
- 18.Balayssac D, Ferrier J, Descoeur J, et al. Chemotherapy-induced peripheral neuropathies: from clinical relevance to preclinical evidence. Expert Opin Drug Saf 2011;10(3):407–417. [DOI] [PubMed] [Google Scholar]
- 19.Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neuroscience Letters 2015;596:90–107. [DOI] [PubMed] [Google Scholar]
- 20.Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, Management, and Evaluation of Chemotherapy-Induced Peripheral Neuropathy. Seminars in Oncology 2006;33(1):15–49. [DOI] [PubMed] [Google Scholar]
- 21.Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. PAIN® 2014;155(12):2461–2470. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda Y, Li Y, Segal RA. A Mechanistic Understanding of Axon Degeneration in Chemotherapy-Induced Peripheral Neuropathy. Frontiers in neuroscience 2017;11:481–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Höke A Animal Models of Peripheral Neuropathies. Neurotherapeutics 2012;9(2):262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014;32(18):1941–1967. [DOI] [PubMed] [Google Scholar]
- 25.Authier N, Balayssac D, Marchand F, et al. Animal models of chemotherapy-evoked painful peripheral neuropathies. Neurotherapeutics 2009;6(4):620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehmerle W, Huehnchen P, Peruzzaro S, Balkaya M, Endres M. Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib-induced neuropathy in C57Bl/6 mice. Scientific Reports 2014;4:6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Höke A, Ray M. Rodent Models of Chemotherapy-Induced Peripheral Neuropathy. ILAR Journal 2014;54(3):273–281. [DOI] [PubMed] [Google Scholar]
- 28.Majithia N, Loprinzi CL, Smith TJ. New practical approaches to chemotherapy-induced neuropathic pain: prevention, assessment, and treatment. Oncology 2016;30(11):1020–1029. [PubMed] [Google Scholar]
- 29.Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010;17(7):903–912, e944–909. [DOI] [PubMed] [Google Scholar]
- 30.Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. Journal of the peripheral nervous system : JPNS 2010;15(3):202–207. [DOI] [PubMed] [Google Scholar]
- 31.Guo L, Hamre J 3rd, Eldridge S, et al. Multiparametric Image Analysis of Rat Dorsal Root Ganglion Cultures to Evaluate Peripheral Neuropathy-Inducing Chemotherapeutics. Toxicol Sci 2017;156(1):275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lauria G, Morbin M, Lombardi R, et al. Axonal swellings predict the degeneration of epidermal nerve fibers in painful neuropathies. Neurology 2003;61(5):631–636. [DOI] [PubMed] [Google Scholar]
- 33.Park SB, Lin CS, Kiernan MC. Nerve excitability assessment in chemotherapy-induced neurotoxicity. Journal of visualized experiments : JoVE 2012(62). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namer B, Pfeffer S, Handwerker HO, Schmelz M, Bickel A. Axon reflex flare and quantitative sudomotor axon reflex contribute in the diagnosis of small fiber neuropathy. Muscle Nerve 2013;47(3):357–363. [DOI] [PubMed] [Google Scholar]
- 35.Kalliomaki M, Kieseritzky JV, Schmidt R, et al. Structural and functional differences between neuropathy with and without pain? Experimental neurology 2011;231(2):199–206. [DOI] [PubMed] [Google Scholar]
- 36.Allen DT, Kiernan JA. Permeation of proteins from the blood into peripheral nerves and ganglia. Neuroscience 1994;59(3):755–764. [DOI] [PubMed] [Google Scholar]
- 37.Gregg RW, Molepo JM, Monpetit VJ, et al. Cisplatin neurotoxicity: the relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. Journal of Clinical Oncology 1992;10(5):795–803. [DOI] [PubMed] [Google Scholar]
- 38.Krarup-Hansen Rietz, Krarup Heydorn, Rørth Schmalbruch. Histology and platinum content of sensory ganglia and sural nerves in patients treated with cisplatin and carboplatin: an autopsy study. Neuropathology and Applied Neurobiology 1999;25(1):28–39. [DOI] [PubMed] [Google Scholar]
- 39.Dzagnidze A, Katsarava Z, Makhalova J, et al. Repair Capacity for Platinum-DNA Adducts Determines the Severity of Cisplatin-Induced Peripheral Neuropathy. The Journal of Neuroscience 2007;27(35):9451–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. NeuroToxicology 2006;27(6):992–1002. [DOI] [PubMed] [Google Scholar]
- 41.Casafont I, Berciano MT, Lafarga M. Bortezomib Induces the Formation of Nuclear poly(A) RNA Granules Enriched in Sam68 and PABPN1 in Sensory Ganglia Neurons. Neurotoxicity Research 2009;17(2):167. [DOI] [PubMed] [Google Scholar]
- 42.Giannini F, Volpi N, Rossi S, Passero S, Fimiani M, Cerase A. Thalidomide-induced neuropathy: A ganglionopathy? Neurology 2003;60(5):877–878. [DOI] [PubMed] [Google Scholar]
- 43.Peters CM, Jimenez-Andrade JM, Kuskowski MA, Ghilardi JR, Mantyh PW. An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Research 2007;1168:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Topp KS, Tanner KD, Levine JD. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. Journal of Comparative Neurology 2000;424(4):563–576. [PubMed] [Google Scholar]
- 45.LaPointe NE, Morfini G, Brady ST, Feinstein SC, Wilson L, Jordan MA. Effects of eribulin, vincristine, paclitaxel and ixabepilone on fast axonal transport and kinesin-1 driven microtubule gliding: Implications for chemotherapy-induced peripheral neuropathy. NeuroToxicology 2013;37:231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Theiss C, Meller K. Taxol impairs anterograde axonal transport of microinjected horseradish peroxidase in dorsal root ganglia neurons in vitro. Cell and Tissue Research 2000;299(2):213–224. [DOI] [PubMed] [Google Scholar]
- 47.Bober BG, Shah SB. Paclitaxel alters sensory nerve biomechanical properties. J Biomech 2015;48(13):3559–3567. [DOI] [PubMed] [Google Scholar]
- 48.Liu CN, Berryman E, Zakur D, et al. A novel endpoint for the assessment of chemotherapy-induced peripheral neuropathy in rodents: biomechanical properties of peripheral nerve. J Appl Toxicol 2018;38(2):193–200. [DOI] [PubMed] [Google Scholar]
- 49.Arastu-Kapur S, Anderl JL, Kraus M, et al. Nonproteasomal Targets of the Proteasome Inhibitors Bortezomib and Carfilzomib: a Link to Clinical Adverse Events. Clinical Cancer Research 2011;17(9):2734–2743. [DOI] [PubMed] [Google Scholar]
- 50.Poruchynsky MS, Sackett DL, Robey RW, Ward Y, Annunziata C, Fojo T. Proteasome inhibitors increase tubulin polymerization and stabilization in tissue culture cells: A possible mechanism contributing to peripheral neuropathy and cellular toxicity following proteasome inhibition. Cell Cycle 2008;7(7):940–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shin YK, Jang SY, Lee HK, et al. Pathological adaptive responses of Schwann cells to endoplasmic reticulum stress in bortezomib-induced peripheral neuropathy. Glia 2010;58(16):1961–1976. [DOI] [PubMed] [Google Scholar]
- 52.Canta A, Pozzi E, Carozzi VA. Mitochondrial Dysfunction in Chemotherapy-Induced Peripheral Neuropathy (CIPN). Toxics 2015;3(2):198–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain 2006;122(3):245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavaletti G, Gilardini A, Canta A, et al. Bortezomib-induced peripheral neurotoxicity: A neurophysiological and pathological study in the rat. Experimental neurology 2007;204(1):317–325. [DOI] [PubMed] [Google Scholar]
- 55.Kirchmair R, Tietz AB, Panagiotou E, et al. Therapeutic Angiogenesis Inhibits or Rescues Chemotherapy-induced Peripheral Neuropathy: Taxol- and Thalidomide-induced Injury of Vasa Nervorum is Ameliorated by VEGF. Molecular Therapy 2007;15(1):69–75. [DOI] [PubMed] [Google Scholar]
- 56.Makker PGS, Duffy SS, Lees JG, et al. Characterisation of Immune and Neuroinflammatory Changes Associated with Chemotherapy-Induced Peripheral Neuropathy. PloS one 2017;12(1):e0170814–e0170814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warwick RA, Hanani M. The contribution of satellite glial cells to chemotherapy-induced neuropathic pain. European Journal of Pain 2013;17(4):571–580. [DOI] [PubMed] [Google Scholar]
- 58.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nature Reviews Neuroscience 2009;10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Melli G, Jack C, Lambrinos GL, Ringkamp M, Höke A. Erythropoietin protects sensory axons against paclitaxel-induced distal degeneration. Neurobiology of Disease 2006;24(3):525–530. [DOI] [PubMed] [Google Scholar]
- 60.Silva A, Wang Q, Wang M, Ravula SK, Glass JD. Evidence for direct axonal toxicity in vincristine neuropathy. Journal of the Peripheral Nervous System 2006;11(3):211–216. [DOI] [PubMed] [Google Scholar]
- 61.Isoardo G, Bergui M, Durelli L, et al. Thalidomide neuropathy: clinical, electrophysiological and neuroradiological features. Acta Neurologica Scandinavica 2004;109(3):188–193. [DOI] [PubMed] [Google Scholar]
- 62.Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltage-gated Na+ channel kinetics on rat sensory neurons. European Journal of Pharmacology 2000;406(1):25–32. [DOI] [PubMed] [Google Scholar]
- 63.Sittl R, Lampert A, Huth T, et al. Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype Na(V)1.6-resurgent and persistent current. Proceedings of the National Academy of Sciences of the United States of America 2012;109(17):6704–6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webster RG, Brain KL, Wilson RH, Grem JL, Vincent A. Oxaliplatin induces hyperexcitability at motor and autonomic neuromuscular junctions through effects on voltage-gated sodium channels. British journal of pharmacology 2005;146(7):1027–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marmiroli P, Nicolini G, Miloso M, Scuteri A, Cavaletti G. The fundamental role of morphology in experimental neurotoxicology: the example of chemotherapy-induced peripheral neurotoxicity. Ital J Anat Embryol 2012;117(2):75–97. [PubMed] [Google Scholar]
- 66.Snyder C, Yu L, Ngo T, et al. In vitro assessment of chemotherapy-induced neuronal toxicity. Toxicology in Vitro 2018;50:109–123. [DOI] [PubMed] [Google Scholar]
- 67.Cavaletti G, Tredici G, Marmiroli P, Petruccioli MG, Barajon I, Fabbrica D. Morphometric study of the sensory neuron and peripheral nerve changes induced by chronic cisplatin (DDP) administration in rats. Acta Neuropathol 1992;84(4):364–371. [DOI] [PubMed] [Google Scholar]
- 68.Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit Rev Oncol Hematol 2012;82(1):51–77. [DOI] [PubMed] [Google Scholar]
- 69.Hama A, Takamatsu H. Chemotherapy-Induced Peripheral Neuropathic Pain and Rodent Models. CNS Neurol Disord Drug Targets 2016;15(1):7–19. [DOI] [PubMed] [Google Scholar]
- 70.Currie GL, Angel-Scott H, Colvin L, et al. Animal models of chemotherapy-induced peripheral neuropathy: a machine-assisted systematic review and meta-analysis A comprehensive summary of the field to inform robust experimental design. bioRxiv 2018:293480. [DOI] [PMC free article] [PubMed]
- 71.Hershman DL, Weimer LH, Wang A, et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat 2011;125(3):767–774. [DOI] [PubMed] [Google Scholar]
- 72.Kidwell KM, Yothers G, Ganz PA, et al. Long-term neurotoxicity effects of oxaliplatin added to fluorouracil and leucovorin as adjuvant therapy for colon cancer: results from National Surgical Adjuvant Breast and Bowel Project trials C-07 and LTS-01. Cancer 2012;118(22):5614–5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC. Long-term neuropathy after oxaliplatin treatment: challenging the dictum of reversibility. Oncologist 2011;16(5):708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mogil JS, Crager SE. What should we be measuring in behavioral studies of chronic pain in animals? Pain 2004;112(1–2):12–15. [DOI] [PubMed] [Google Scholar]
- 75.Ventzel L, Madsen CS, Karlsson P, et al. Chronic Pain and Neuropathy Following Adjuvant Chemotherapy. Pain Medicine 2017;19(9):1813–1824. [DOI] [PubMed] [Google Scholar]
- 76.Carozzi VA, Canta A, Oggioni N, et al. Neurophysiological and neuropathological characterization of new murine models of chemotherapy-induced chronic peripheral neuropathies. Experimental neurology 2010;226(2):301–309. [DOI] [PubMed] [Google Scholar]
- 77.Wozniak KM, Nomoto K, Lapidus RG, et al. Comparison of neuropathy-inducing effects of eribulin mesylate, paclitaxel, and ixabepilone in mice. Cancer Res 2011;71(11):3952–3962. [DOI] [PubMed] [Google Scholar]
- 78.Meregalli C, Canta A, Carozzi VA, et al. Bortezomib-induced painful neuropathy in rats: A behavioral, neurophysiological and pathological study in rats. European Journal of Pain 2010;14(4):343–350. [DOI] [PubMed] [Google Scholar]
- 79.Zhou J, Giannakakou P. Targeting Microtubules for Cancer Chemotherapy Anti-Cancer Agents Vol 52005. [DOI] [PubMed] [Google Scholar]
- 80.Hoelting L, Klima S, Karreman C, et al. Stem Cell-Derived Immature Human Dorsal Root Ganglia Neurons to Identify Peripheral Neurotoxicants. Stem Cells Transl Med 2016;5(4):476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Livni L, Lees JG, Barkl-Luke ME, Goldstein D, Moalem-Taylor G. Dorsal root ganglion explants derived from chemotherapy-treated mice have reduced neurite outgrowth in culture. Neuroscience Letters 2019;694:14–19. [DOI] [PubMed] [Google Scholar]
- 82.Rana P, Luerman G, Hess D, Rubitski E, Adkins K, Somps C. Utilization of iPSC-derived human neurons for high-throughput drug-induced peripheral neuropathy screening. Toxicol In Vitro 2017;45(Pt 1):111–118. [DOI] [PubMed] [Google Scholar]
- 83.Sherman SP, Bang AG. High-throughput screen for compounds that modulate neurite growth of human induced pluripotent stem cell-derived neurons. Disease Models & Mechanisms 2018;11(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wing C, Komatsu M, Delaney SM, Krause M, Wheeler HE, Dolan ME. Application of stem cell derived neuronal cells to evaluate neurotoxic chemotherapy. Stem Cell Res 2017;22:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malgrange B, Delree P, Rigo JM, Baron H, Moonen G. Image analysis of neuritic regeneration by adult rat dorsal root ganglion neurons in culture: quantification of the neurotoxicity of anticancer agents and of its prevention by nerve growth factor or basic fibroblast growth factor but not brain-derived neurotrophic factor or neurotrophin-3. J Neurosci Methods 1994;53(1):111–122. [DOI] [PubMed] [Google Scholar]
- 86.Hol EM, Mandys V, Sodaar P, Gispen WH, Bar PR. Protection by an ACTH4–9 analogue against the toxic effects of cisplatin and taxol on sensory neurons and glial cells in vitro. Journal of neuroscience research 1994;39(2):178–185. [DOI] [PubMed] [Google Scholar]
- 87.Scuteri A, Nicolini G, Miloso M, et al. Paclitaxel toxicity in post-mitotic dorsal root ganglion (DRG) cells. Anticancer Research 2006;26(2 A):1065–1070. [PubMed] [Google Scholar]
- 88.Yang IH, Siddique R, Hosmane S, Thakor N, Hoke A. Compartmentalized microfluidic culture platform to study mechanism of paclitaxel-induced axonal degeneration. Experimental neurology 2009;218(1):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ravula SK, Wang MS, Asress SA, Glass JD, Bruno Frazier A. A compartmented neuronal culture system in microdevice format. J Neurosci Methods 2007;159(1):78–85. [DOI] [PubMed] [Google Scholar]
- 90.Masurovsky EB, Peterson ER, Crain SM, Horwitz SB. Microtubule arrays in taxol-treated mouse dorsal root ganglion-spinal cord cultures. Brain Res 1981;217(2):392–398. [DOI] [PubMed] [Google Scholar]
- 91.Gill JS, Windebank AJ. Suramin induced ceramide accumulation leads to apoptotic cell death in dorsal root ganglion neurons. Cell Death Differ 1998;5(10):876–883. [DOI] [PubMed] [Google Scholar]
- 92.Podratz JL, Knight AM, Ta LE, et al. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis 2011;41(3):661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCormick B, Lowes DA, Colvin L, Torsney C, Galley HF. MitoVitE, a mitochondria-targeted antioxidant, limits paclitaxel-induced oxidative stress and mitochondrial damage in vitro, and paclitaxel-induced mechanical hypersensitivity in a rat pain model. British Journal of Anaesthesia 2016;117(5):659–666. [DOI] [PubMed] [Google Scholar]
- 94.Melli G, Taiana M, Camozzi F, et al. Alpha-lipoic acid prevents mitochondrial damage and neurotoxicity in experimental chemotherapy neuropathy. Experimental neurology 2008;214(2):276–284. [DOI] [PubMed] [Google Scholar]
- 95.Bobylev I, Joshi AR, Barham M, et al. Paclitaxel inhibits mRNA transport in axons. Neurobiol Dis 2015;82:321–331. [DOI] [PubMed] [Google Scholar]
- 96.Duggett NA, Griffiths LA, Flatters SJL. Paclitaxel-induced painful neuropathy is associated with changes in mitochondrial bioenergetics, glycolysis, and an energy deficit in dorsal root ganglia neurons. Pain 2017;158(8):1499–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Staff NP, Podratz JL, Grassner L, et al. Bortezomib alters microtubule polymerization and axonal transport in rat dorsal root ganglion neurons. Neurotoxicology 2013;39:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pittman SK, Gracias NG, Vasko MR, Fehrenbacher JC. Paclitaxel alters the evoked release of calcitonin gene-related peptide from rat sensory neurons in culture. Experimental neurology 2014;253:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Podratz JL, Kulkarni A, Pleticha J, et al. Neurotoxicity to DRG neurons varies between rodent strains treated with cisplatin and bortezomib. J Neurol Sci 2016;362:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Imai S, Koyanagi M, Azimi Z, et al. Taxanes and platinum derivatives impair Schwann cells via distinct mechanisms. Sci Rep 2017;7(1):5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jones I, Yelhekar TD, Wiberg R, et al. Development and validation of an in vitro model system to study peripheral sensory neuron development and injury. Sci Rep 2018;8(1):15961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ryan KR, Sirenko O, Parham F, et al. Neurite outgrowth in human induced pluripotent stem cell-derived neurons as a high-throughput screen for developmental neurotoxicity or neurotoxicity. Neurotoxicology 2016;53:271–281. [DOI] [PubMed] [Google Scholar]
- 103.Wheeler HE, Wing C, Delaney SM, Komatsu M, Dolan ME. Modeling chemotherapeutic neurotoxicity with human induced pluripotent stem cell-derived neuronal cells. PLoS One 2015;10(2):e0118020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wilson MS, Graham JR, Ball AJ. Multiparametric High Content Analysis for assessment of neurotoxicity in differentiated neuronal cell lines and human embryonic stem cell-derived neurons. Neurotoxicology 2014;42:33–48. [DOI] [PubMed] [Google Scholar]
- 105.Bakogeorgos M, Georgoulias V. Risk-reduction and treatment of chemotherapy-induced peripheral neuropathy. Expert Review of Anticancer Therapy 2017;17(11):1045–1060. [DOI] [PubMed] [Google Scholar]
- 106.Flatters SJL, Dougherty PM, Colvin LA. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): a narrative review. British Journal of Anaesthesia 2017;119(4):737–749. [DOI] [PubMed] [Google Scholar]
- 107.Lang-Yue H, Wen-Li M, Gen-Cheng W, Yan-Qing W, Qi-Liang M-Y. Prevention and Treatment for Chemotherapy-Induced Peripheral Neuropathy: Therapies Based on CIPN Mechanisms. Current Neuropharmacology 2019;17(2):184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Poupon L, Kerckhove N, Vein J, et al. Minimizing chemotherapy-induced peripheral neuropathy: preclinical and clinical development of new perspectives. Expert Opin Drug Saf 2015;14(8):1269–1282. [DOI] [PubMed] [Google Scholar]
- 109.Cleeland CS, Farrar JT, Hausheer FH. Assessment of Cancer-Related Neuropathy and Neuropathic Pain. The Oncologist 2010;15(suppl 2):13–18. [DOI] [PubMed] [Google Scholar]
- 110.Wolf S, Barton D, Kottschade L, Grothey A, Loprinzi C. Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. Eur J Cancer 2008;44(11):1507–1515. [DOI] [PubMed] [Google Scholar]
- 111.Dorsey SG, Kleckner IR, Barton D, et al. NCI Clinical Trials Planning Meeting for prevention and treatment of chemotherapy-induced peripheral neuropathy. J Natl Cancer Inst 2019. [DOI] [PMC free article] [PubMed]
- 112.Grill MF, Maganti RK. Neurotoxic effects associated with antibiotic use: management considerations. British journal of clinical pharmacology 2011;72(3):381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goolsby TA, Jakeman B, Gaynes RP. Clinical relevance of metronidazole and peripheral neuropathy: a systematic review of the literature. International Journal of Antimicrobial Agents 2018;51(3):319–325. [DOI] [PubMed] [Google Scholar]
- 114.Argov Z, Mastaglia FL. Drug-induced peripheral neuropathies. British medical journal 1979;1(6164):663–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cojocaru IM, Cojocaru M, Silosi I, Vrabie CD. Peripheral nervous system manifestations in systemic autoimmune diseases. Maedica 2014;9(3):289–294. [PMC free article] [PubMed] [Google Scholar]
- 116.Krajewski KM, Lewis RA, Fuerst DR, et al. Neurological dysfunction and axonal degeneration in Charcot–Marie–Tooth disease type 1A. Brain 2000;123(7):1516–1527. [DOI] [PubMed] [Google Scholar]
- 117.Martini R, Klein D, Groh J. Similarities between Inherited Demyelinating Neuropathies and Wallerian Degeneration: An Old Repair Program May Cause Myelin and Axon Perturbation under Nonlesion Conditions. The American Journal of Pathology 2013;183(3):655–660. [DOI] [PubMed] [Google Scholar]
- 118.Brandner S Chapter 30. Toxic neuropathies. In: Vallat J-M, Weiss J, eds. Peripheral Nerve Disorders: Pathology and Genetics Wiley-Blackwell; 2014:320. [Google Scholar]
- 119.Brizzi KT, Lyons JL. Peripheral nervous system manifestations of infectious diseases. The Neurohospitalist 2014;4(4):230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Juster-Switlyk K, Smith AG. Updates in diabetic peripheral neuropathy. F1000Research 2016;5:F1000 Faculty Rev-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dick FD. Solvent neurotoxicity. Occup Environ Med 2006;63(3):221–226, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Keifer MC, Firestone J. Neurotoxicity of pesticides. J Agromedicine 2007;12(1):17–25. [DOI] [PubMed] [Google Scholar]
- 123.Filosto M, Tentorio M, Broglio L, et al. Disulfiram neuropathy: Two cases of distal axonopathy. Clinical Toxicology 2008;46(4):314–316. [DOI] [PubMed] [Google Scholar]
- 124.Mellion M, Gilchrist JM, de la Monte S. Alcohol-related peripheral neuropathy: nutritional, toxic, or both? Muscle & nerve 2011;43(3):309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Food and Drug Administration CfDEaRC. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers https://www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf%23search=%27guidekines+for+industry+sfe+starting%27. Published 2005. Accessed.
- 126.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep 1966;50(4):219–244. [PubMed] [Google Scholar]
- 127.Liston DR, Davis M. Clinically Relevant Concentrations of Anticancer Drugs: A Guide for Nonclinical Studies. Clin Cancer Res 2017;23(14):3489–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]


