Abstract
Background
The gut microbiome is being associated increasingly with development of infections besides Clostridium difficile infection. A recent study found an association between butyrate‐producing gut (BPG) bacteria and less frequent development of lower respiratory viral infections in allogeneic hematopoietic stem cell transplant recipients (Haak et al, Blood 131(26): 2978, 2018). In this investigation, we examine the relationship between the abundance of BPG bacteria and the development of viral infections in a cohort of kidney transplant recipients.
Methods
We recruited 168 kidney transplant recipients who provided 510 fecal specimens in the first 3 months after transplantation and profiled the gut microbiota using 16S rRNA gene sequencing of the V4‐V5 hypervariable region. We classified the kidney transplant recipients into higher BPG Bacteria Group and lower BPG Bacteria Group using the same criteria of 1% relative gut abundance of BPG bacteria as the Haak et al study.
Results
Administration of antibiotics against anaerobes was associated with a significant decrease in the relative gut abundance of BPG bacteria. The higher BPG Bacteria Group was associated with less development of respiratory viral infections (Hazard Ratio [HR]: 0.28, P = .01) but not with less development of CMV viremia (HR: 0.38, P = .13) or BK viremia (HR: 1.02, P = .98) at 2 years post transplantation.
Conclusion
Our pilot investigation supports future validation of the relationship between high relative gut abundance of BPG bacteria and decreased risk for development of respiratory viral infections.
Keywords: butyrate, gut microbiome, gut microbiota, respiratory viral infections, viral infections
Abbreviations
- BPG
butyrate‐producing gut
- CMV
cytomegalovirus
- HSCT
hematopoietic stem cell transplant
1. INTRODUCTION
The gut microbiome is now considered to have a role in the development of infectious processes beyond Clostridium difficile infection. With respect to bacterial complications, Taur et al performed a study of 94 allogeneic hematopoietic stem cell transplant (HSCT) recipients and reported that Enterococcus gut domination increased the risk for future development of Enterococcus sepsis by 9‐fold.1 In a different cohort of allogeneic HSCT recipients, Tamburini et al did a strain level analysis on bloodstream isolates and reported that strains of Escherichia coli and Klebsiella pneumoniae that caused septicemia likely originated from the gut.2
The relationship between the gut microbiota and development of viral infections, however, is not well described. Studies in mice have shown a relationship between the gut microbiota and impaired viral clearance. Abt et al investigated antibiotic administration in a mouse model of lymphocytic choriomeningitis virus and found that antibiotic administration led to decreased innate viral immunity response as well as delayed clearance.3 Further studies have revealed that butyrate, a product of certain gut anaerobic bacteria, can have an immunomodulatory role and contributes to overall health in distant sites such as the lung.4
Haak et al investigated the role of butyrate‐producing gut (BPG) bacteria on future development of viral infections. In a cohort of 360 allogeneic HSCT recipients, they reported that having a >1% relative gut abundance of BPG bacteria is associated with 5‐fold less future development of lower respiratory viral infections.5 Based upon this study, we profiled the gut microbiota using 16S rRNA gene sequencing of the V4‐V5 region in 510 fecal specimens from 168 kidney transplant recipients. We report that having a >1% relative abundance of BPG bacteria is associated with less risk for development of respiratory viral infections in kidney transplant recipients, which provides further support for the findings from the Haak et al study.5
2. PATIENTS AND METHODS
2.1. Kidney transplant cohort
From August 2015 to November 2016, 280 kidney transplant recipients were consented for serial collection of fecal specimens, and 168 kidney transplant recipients provided at least one fecal specimen for gut microbial profiling. Among the 168 kidney transplant recipients, 121 subjects provided a fecal specimen at post‐transplant week 2 (between post‐operative day 8 and post‐operative day 24); 162 subjects provided at least one fecal specimen in the first 30 days after transplantation for the pooled individual mean analysis. Demographics and clinical characteristics were collected from chart review. The study was approved by the Weill Cornell Institutional Review Board, and all subjects provided written informed consent.
2.2. Fecal specimen collections
Kidney transplant recipients provided fecal specimens using the Fisherbrand™ commode specimen collection kit (Thermo Fisher Science). Fecal specimens were aliquoted into approximately 200 mg aliquots and subsequently stored at −80°C. The recipients were asked to provide the specimens at post‐transplant week 1, 2, 4, and 12.
2.3. 16S rRNA gene amplification and sequencing
DNA extraction and 16S rRNA gene amplification of the 16S rRNA gene V4‐V5 region (563F and 926R) were performed as described in Lee et al.6 Sequencing of the PCR amplicons was performed on an Illumina MiSeq platform (250 base pair × 250 base pair).
2.4. Bioinformatics and taxonomic classification
Bioinformatics and taxonomic classification were performed as described in Lee et al.6 Briefly, taxonomy was determined using nucleotide BLAST7 with the reference training set, NCBI RefSeq8 and a minimum E‐value threshold of 1 × 10−10.
2.5. Viral infection monitoring and definitions
Respiratory virus infections, CMV viremia, and BK viremia were determined by review of electronic medical records of each subject. A positive respiratory virus infection was defined by a positive test result using the BioFire™ FilmArray™ Respiratory panel (BioFire Diagnostics, LLC). CMV viremia was defined as a positive result above detection level using PCR assay at NewYork‐Presbyterian Hospital–Weill Cornell. CMV PCR testing was performed routinely in all subjects every 3 months in the first 2 years after transplantation. BK viremia was defined as a positive result above detection level using PCR assay at NewYork‐Presbyterian Hospital–Weill Cornell or at QUEST Diagnostics, LLC. BK virus PCR testing was performed routinely in all subjects every month in the first year after transplantation and every 3 months in the second year after transplantation.
2.6. Statistical analyses
The distribution of continuous variables was analyzed using the two‐tailed Wilcoxon rank‐sum test for unpaired values and the two‐tailed Wilcoxon signed‐rank test for paired values; the distribution of categorical variables was analyzed using two‐tailed Fisher's exact test. A Cox Regression Hazard Model was used to estimate whether a relative gut abundance of BPG bacteria was associated with a decreased risk for development of respiratory virus infections, CMV viremia, or BK viremia. Survival curves were constructed and compared using the log‐rank test. All analyses were performed in R 3.3.3 in RStudio 1.1.463.
3. RESULTS
3.1. Top butyrate‐producing gut bacteria in the cohort
Among the 510 fecal specimens from the 168 kidney transplant recipients, the mean ± standard deviation amount of high quality 16S rRNA bacterial gene sequences was 18 179 ± 11 033. The overall demographics and transplant characteristics of these subjects are presented in Table 1. Out of the 61 BPG species, 40 species were detected (Table S1). The sum relative gut abundance of BPG bacteria was calculated for each fecal specimen (defined hereafter as relative gut abundance of BPG bacteria). The mean relative gut abundance of BPG bacteria among all of the 510 fecal specimens was 14.2% with a standard deviation of 13.1%. The top BPG species were: Faecalibacterium prausnitzii (3.7%, mean), Holdemanella biformis (2.7%), Eubacterium dolichum (2.6%), Coprococcus comes (1.2%), Subdoligranulum variabile (0.8%), and Eubacterium rectale (0.7%). A heatmap of the relative gut abundance of these top species among all the fecal specimens is presented in Figure 1.
Table 1.
Characteristics of the kidney transplant cohort
| Characteristic |
Kidney transplant cohort (N=168) Median/Number (%) |
|---|---|
| Age | 54 |
| Female gender | 76 (45%) |
| African American race | 44 (26%) |
| History of Diabetes mellitus | 49 (29%) |
| Prior kidney transplantation | 24 (14%) |
| Liver/kidney dual transplantation | 2 (1%) |
| Kidney/pancreas dual transplantation | 3 (2%) |
| Deceased donor transplantation | 49 (29%) |
| CMV antibody donor Positive/recipient negativea | 31 (19%) |
| Delayed graft function | 28 (17%) |
| Induction therapy | |
| Anti‐thymocyte globulin | 128 (76%) |
| Basiliximab | 39 (23%) |
| None | 1 (1%) |
| Preoperative antibiotic Prophylaxis | |
| Cefazolin | 139 (83%) |
| Vancomycin | 20 (12%) |
| Other | 9 (5%) |
| Pneumocystis jiroveci prophylaxis | |
| Trimethoprim/sulfamethoxazole | 159 (95%) |
| Dapsone | 2 (1%) |
| Atovaquone | 7 (4%) |
| Steroid maintenance protocol | 45 (27%) |
One subject had donor indeterminate CMV antibody status and was unable to be classified so the total number evaluated was 167.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 1.
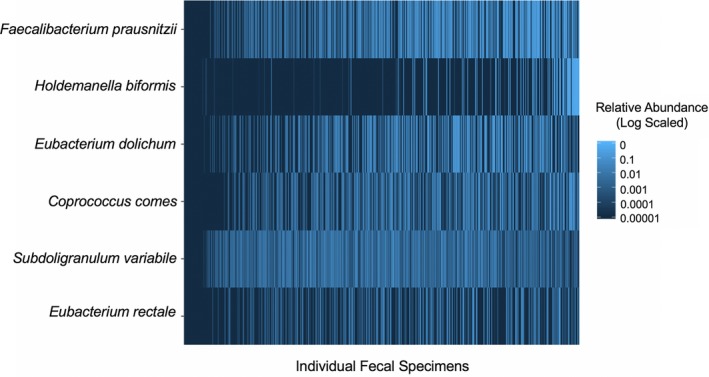
Heatmap of the top abundant butyrate‐producing gut bacteria in the kidney transplant cohort. The 510 fecal specimens from the 168 kidney transplant recipients are represented on the x‐axis and the top 6 butyrate‐producing gut species are on the y‐axis. The relative abundance of each species is represented in blue, log‐scaled. There is marked variability in the top butyrate‐producing species among the specimens
3.2. Antibiotics and butyrate‐producing gut bacteria over time in the cohort
Figure 2A shows the changes in the relative gut abundance of BPG bacteria over the first 3 months after transplantation in the 510 fecal specimens from the 168 kidney transplant recipients. We further evaluated the role of antibiotics (besides Pneumocystis jiroveci prophylaxis and preoperative antibiotic prophylaxis) on the relative gut abundance of BPG bacteria over the first 3 months after transplantation. Among the 168 kidney transplant recipients, 90 subjects received additional antibiotics during the first 3 months after transplantation and provided 279 fecal specimens (Abx Group) and 78 subjects did not receive additional antibiotics and provided 231 fecal specimens (No Abx Group). The relative gut abundance of BPG bacteria was significantly lower in the Abx Group than the No Abx Group (median 12.5% vs median 16.3%, P < .001, Wilcoxon rank‐sum test; Figure 2B).
Figure 2.
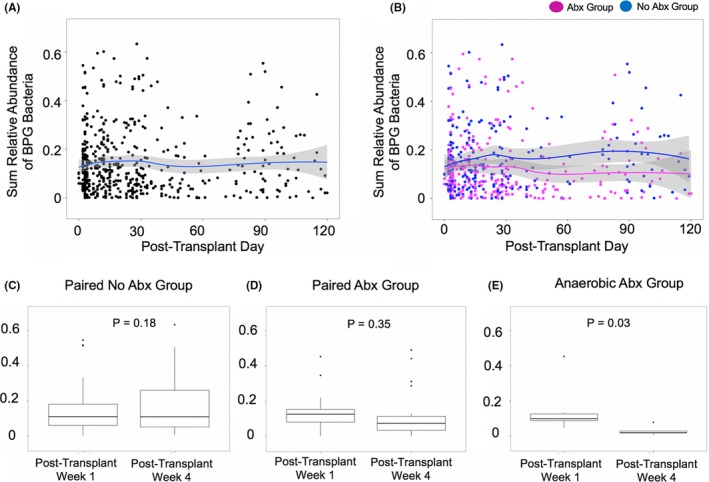
The abundance of butyrate‐producing gut bacteria over time in the kidney transplant cohort. Panel A. Each point represents a single fecal specimen with the day the specimen was produced on the x‐axis and the relative gut abundance of butyrate‐producing gut (BPG) bacteria on the y‐axis. The line represents a locally estimated scatterplot smoothing (LOESS) curve with 95% confidence intervals indicated by the shaded band. Panel B. Each point represents a single fecal specimen with the day the specimen was produced on the x‐axis and the relative gut abundance of butyrate‐producing gut (BPG) bacteria on the y‐axis. A point in blue represents an individual fecal specimen from the 231 specimens from the 78 patients in the No Abx Group and a point in magenta represents an individual fecal specimen from the 279 specimens from the 90 patients in the Abx Group. The line represents a LOESS curve with 95% confidence intervals indicated by the shaded bands. Panel C. Box and whisker plot with the relative gut abundance of BPG bacteria on the y‐axis and the fecal specimen post‐transplant week on the x‐axis for the 54 subjects in the Paired No Abx Group. In each box and whisker plot, the line represents the median, the edges of the box plot 25% and 75%, the whiskers 1.5 times the median, and points represent outliers. The P value was calculated using the Wilcoxon signed‐rank test. Panel D. Box and whisker plot with the relative gut abundance of BPG bacteria on the y‐axis and the fecal specimen post‐transplant week on the x‐axis for the 22 subjects in the Paired Abx Group. In each box and whisker plot, the line represents the median, the edges of the box plot 25% and 75%, the whiskers 1.5 times the median, and points represent outliers. The P value was calculated using the Wilcoxon signed‐rank test. Panel E. Box and whisker plot with the relative gut abundance of BPG bacteria on the y‐axis and the fecal specimen post‐transplant week on the x‐axis for the 6 subjects in the Paired Abx Group who received antibiotics with anaerobic coverage. In each box and whisker plot, the line represents the median, the edges of the box plot 25% and 75%, the whiskers 1.5 times the median, and points represent outliers. The P value was calculated using the Wilcoxon signed‐rank test
In order to understand individual subject's changes in the relative gut abundance of BPG bacteria over time, we evaluated 76 subjects who had paired post‐transplant week 1 and post‐transplant week 4 fecal specimens. Among these 76 subjects, 54 subjects did not receive additional antibiotics (Paired No Abx Group) and 22 subjects received antibiotics between the day the post‐transplant week 1 fecal specimen was collected and the day the post‐transplant week 4 fecal specimen was collected (Paired Abx Group). The list of antibiotics administered in the Paired Abx Group is found in Table S2.
In the Paired No Abx Group, there was no significant change in the relative gut abundance of BPG bacteria from post‐transplant week 1 to post‐transplant week 4 (median 11.0% vs. 10.9%, respectively, P = .18, Wilcoxon signed‐rank test; Figure 2C). In the Paired Abx Group, there was no significant change in the relative gut abundance of BPG bacteria from post‐transplant week 1 to post‐transplant week 4 (median 12.6% vs 7.5%, respectively, P = .35, Wilcoxon signed‐rank test; Figure 2D). We next evaluated the subgroup of subjects in the Paired Abx Group who received anaerobic antibiotic coverage. Anaerobic antibiotic coverage included the following antibiotics: metronidazole, carbapenems, clindamycin, combination of penicillin and beta‐lactamase inhibitor, and oral vancomycin.9, 10 The rationale for evaluating antibiotics with anaerobic coverage was based on the strict anaerobic nature of many of the BPG bacteria (eg, Faecalibacterium prausnitzii and Eubacterium rectale).11 Among the Paired Abx Group, 6 subjects had anaerobic antibiotic coverage and all 6 had a significant decrease in the relative gut abundance of BPG bacteria from post‐transplant week 1 to post‐transplant week 4 (median 9.9% vs 1.9%, respectively, P = .03, Wilcoxon signed‐rank test; Figure 2E).
We also evaluated whether the relative gut abundance of BPG bacteria was associated with gender, age greater than 65 years old, African American race, induction therapy, steroid maintenance therapy, and kidney function at 1 month post transplantation. As antibiotics are associated with decreased abundance of BPG bacteria, we evaluated the paired specimens from the 54 subjects who did not receive antibiotics from post‐transplant week 1 and post‐transplant week 4 (Paired No Abx Group). The relative gut abundance of BPG bacteria was significantly lower in the female group than in the male group (median 11.4% vs 17.7%, P = .03, Wilcoxon rank‐sum test; Figure S1A) and significantly lower in the steroid maintenance group than in the in steroid free group (median 9.4% vs 17.6%, P = .01, Wilcoxon rank‐sum test; Figure S1E). There were no significance associations between the relative gut abundance of BPG bacteria and age greater than 65 years old, African American race, induction therapy, and kidney function at 1 month post transplantation (Figure S1B‐D,F).
3.3. High butyrate‐producing gut bacteria status and development of viral infections
We evaluated whether the relative gut abundance of BPG bacteria was associated with decreased risk for viral infection development in kidney transplant recipients at three intervals: 6 months post transplantation, 1 year post transplantation, and 2 years post transplantation. We evaluated the relative gut abundance of BPG bacteria in fecal specimens collected at post‐transplant week 2 to examine this association. The week 2 timeframe is approximately the engraftment timing in the allogeneic HSCT recipient study.5 Given the intra‐individual variability in gut microbiota, we also evaluated the mean relative gut abundance of BPG bacteria in fecal specimens obtained within the first 30 days after transplantation in each subject to also examine this association. Finally, with respect to the BPG relative gut abundance cutoff between the Higher BPG Group and the Lower BPG Group, we analyzed a 1% relative gut abundance cutoff as utilized in the Haak et al study,5 a bottom quartile cutoff, and a median cutoff.
Among the cohort, 36 kidney graft recipients developed a documented respiratory viral (RV) infection by the BioFire™ FilmArray™ Respiratory Panel in the first 2 years after transplantation. The most common first detected RV infections included: rhinovirus/enterovirus(n = 18), coronavirus(n = 8), and influenza(n = 5). The median time to first RV infection was 152 days with an interquartile range of 83 days to 357 days after transplantation. There were 20 patients who had documented CMV viremia in the first 2 years after transplantation. The median time to first detection of CMV viremia was 247 days with an interquartile range of 184 days and 329 days after transplantation. There were 29 patients who had documented BK viremia in the first 2 years after transplantation. The median time to first detection of BK viremia was 120 days with an interquartile range of 76 days and 164 days after transplantation.
With a cutoff of 1% relative gut abundance of BPG bacteria, there were 121 subjects who provided a post‐transplant week 2 fecal specimen with 10 subjects in the Lower BPG Bacteria Group and 111 subjects in the Higher BPG Bacteria Group. The Higher BPG Bacteria Group had a decreased risk for development of RV infections at 6 months post transplantation (HR[Hazard Ratio]:0.31, 95% CI [Confidence Interval]: 0.09‐1.09, P = .07; Figure 3A), at 1 year post transplantation (HR:0.29, 95% CI: 0.10‐0.87, P = .03; Figure 3B), and at 2 years post transplantation (HR:0.28, 95% CI: 0.11‐0.74, P = .01; Figure 3C) compared with the Lower BPG Bacteria Group. The Higher BPG Bacteria Group had a decreased risk for development of CMV viremia at 1 year post transplantation (HR:0.33, 95% CI: 0.09‐1.17, P = .09; Figure 3E) but not at 2 years post transplantation (HR:0.38, 95% CI: 0.11‐1.31, P = .13; Figure 3F) compared with the Lower BPG Bacteria Group. There were no significant differences in the hazard ratio between the Higher BPG Bacteria Group and the Lower BPG Bacteria Group with respect to future development of BK viremia at 6 months post transplantation (P > .10; Figure 3G), 1 year post transplantation (P > .10; Figure 3H), or at 2 years post transplantation (HR:1.02, 95% CI: 0.21‐4.34, P = .98; Figure 3I).
Figure 3.
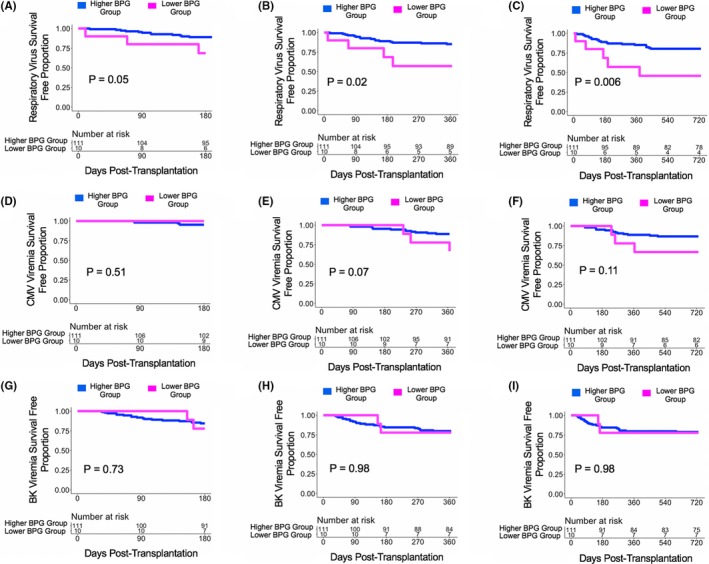
Survival curves based on a 1% cutoff of the relative gut abundance of BPG bacteria. Survival curves show days post transplant on the x‐axis and virus survival free proportion on the y‐axis. The blue line represents the cohort of the Higher BPG Bacteria Group and the magenta line represents the cohort of the Lower BPG Bacteria Group. The number at risk is shown in the table below the survival curve. The P value was calculated using a log‐rank test. Panel A. Survival curve for respiratory virus survival free proportion at 6 mo post transplantation. Panel B. Survival curve for respiratory virus survival free proportion at 1 y post transplantation. Panel C. Survival curve for respiratory virus survival free proportion at 2 y post transplantation. Panel D. Survival curve for CMV viremia survival free proportion at 6 mo post transplantation. Panel E. Survival curve for CMV viremia survival free proportion at 1 y post transplantation. Panel F. Survival curve for CMV viremia survival free proportion at 2 y post transplantation. Panel G. Survival curve for BK viremia survival free proportion at 6 mo post transplantation. Panel H. Survival curve for BK viremia survival free proportion at 1 y post transplantation. Panel I. Survival curve for BK viremia survival free proportion at 2 y post transplantation
With a bottom quartile cutoff for the relative gut abundance of BPG bacteria (5.93%), there were 121 subjects who provided a post‐transplant week 2 fecal specimen with 31 subjects in the Lower BPG Bacteria Group and 90 subjects in the Higher BPG Bacteria Group. The Higher BPG Bacteria Group had a decreased risk for development of RV infections at 6 months post transplantation (HR:0.35, 95% CI: 0.13‐0.97, P = .04; Figure 4A) and at 1 year post transplantation (HR:0.45, 95% CI: 0.18‐1.10, P = .08; Figure 4B) compared with the Lower BPG Bacteria Group. There were no significant differences in the hazard ratio between the Higher BPG Bacteria Group and the Lower BPG Bacteria Group with respect to future development of CMV viremia or BK viremia at 6 months, 1 year, or 2 years post transplantation (P > .10; Figure 4D‐I).
Figure 4.
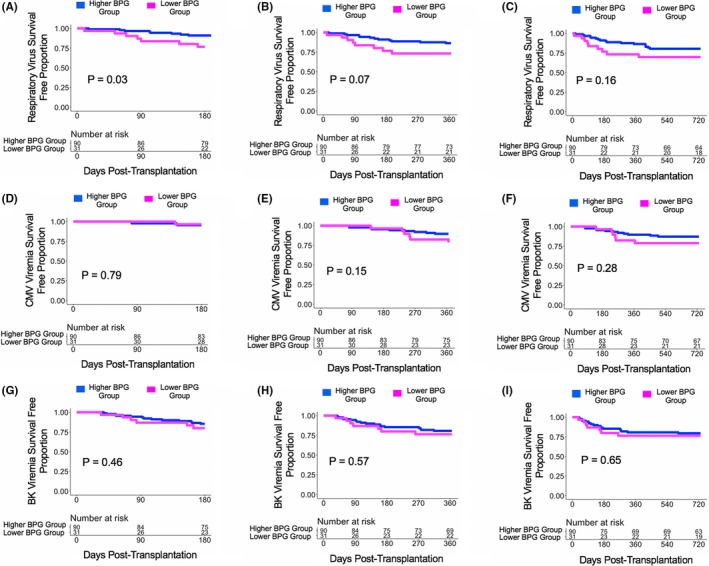
Survival curves based on a bottom quartile cutoff of the relative gut abundance of BPG bacteria. Survival curves show days post transplant on the x‐axis and virus survival free proportion on the y‐axis. The blue line represents the cohort of the Higher BPG Bacteria Group and the magenta line represents the cohort of the Lower BPG Bacteria Group. The number at risk is shown in the table below the survival curve. The P value was calculated using a log‐rank test. Panel A. Survival curve for respiratory virus survival free proportion at 6 mo post transplantation. Panel B. Survival curve for respiratory virus survival free proportion at 1 y post transplantation. Panel C. Survival curve for respiratory virus survival free proportion at 2 y post transplantation. Panel D. Survival curve for CMV viremia survival free proportion at 6 mo post transplantation. Panel E. Survival curve for CMV viremia survival free proportion at 1 y post transplantation. Panel F. Survival curve for CMV viremia survival free proportion at 2 y post transplantation. Panel G. Survival curve for BK viremia survival free proportion at 6 mo post transplantation. Panel H. Survival curve for BK viremia survival free proportion at 1 y post transplantation. Panel I. Survival curve for BK viremia survival free proportion at 2 y post transplantation
With a median cutoff for the relative gut abundance of BPG bacteria (12.96%), there were 121 subjects who provided a post‐transplant week 2 fecal specimen with 61 subjects in the Lower BPG Bacteria Group and 60 subjects in the Higher BPG Bacteria Group. There were no significant differences in the hazard ratio between the Higher BPG Bacteria Group and the Lower BPG Bacteria Group with respect to future development of RV infections, CMV viremia, or BK viremia at 6 months, 1 year, or 2 years post transplantation (P > .10; Figure S2A‐I).
There were 163 subjects who provided at least one fecal specimen in the first month after transplantation. For each subject, the mean relative gut abundance of BPG bacteria among fecal specimens in the first month after transplantation was calculated. With a 1% cutoff of the mean relative abundance of BPG bacteria, there were 7 subjects in the Lower BPG Bacteria Group and 156 subjects in the Higher BPG Bacteria Group. The Higher BPG Bacteria Group had a decreased risk for development of RV infections at 6 months post transplantation (HR:0.10, 95% CI: 0.03‐0.30, P < .001; Figure 5A), at 1 year post transplantation (HR:0.13, 95% CI: 0.04‐0.38, P < .001; Figure 5B), and at 2 years post transplantation (HR:0.16, 95% CI: 0.06‐0.46, P < .001; Figure 5C) compared with the Lower BPG Bacteria Group. The Higher BPG Bacteria Group had a decreased risk for development of CMV viremia at 1 year post transplantation (HR:0.28, 95% CI: 0.06‐1.23, P = .09; Figure 5E) compared with the Lower BPG Bacteria Group. There were no significant differences in the hazard ratio between the Higher BPG Bacteria Group and the Lower BPG Bacteria Group with respect to future development of BK viremia at 6 months, 1 year, or 2 years post transplantation (P > .10; Figure 5G‐I).
Figure 5.
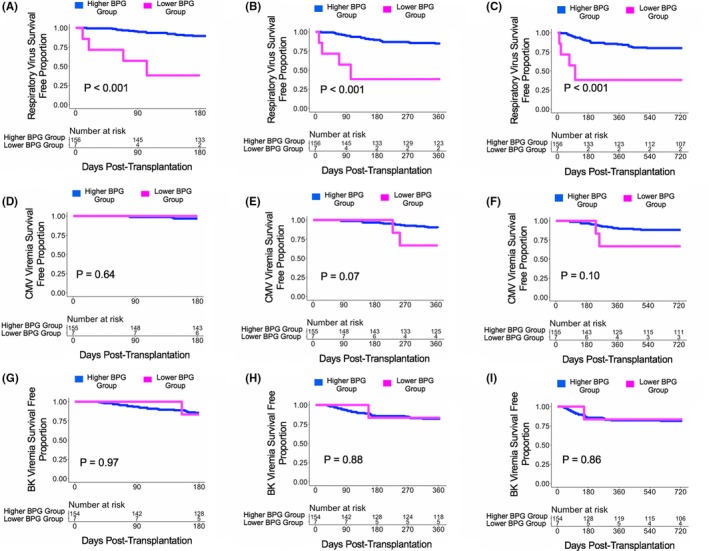
Survival curves based on a 1% cutoff of the mean relative gut abundance of BPG bacteria cutoff. Survival curves show days post transplant on the x‐axis and virus survival free proportion on the y‐axis. The blue line represents the cohort of the Higher BPG Bacteria Group and the magenta line represents the cohort of the Lower BPG Bacteria Group. The number at risk is shown in the table below the survival curve. There was 1 less subject for CMV viremia analysis and 2 less subjects for BK viremia analysis because measurements of the viruses were never performed in these subjects. The P value was calculated using a log‐rank test. Panel A. Survival curve for respiratory virus survival free proportion at 6 mo post transplantation. Panel B. Survival curve for respiratory virus survival free proportion at 1 y post transplantation. Panel C. Survival curve for respiratory virus survival free proportion at 2 y post transplantation. Panel D. Survival curve for CMV viremia survival free proportion at 6 mo post transplantation. Panel E. Survival curve for CMV viremia survival free proportion at 1 y post transplantation. Panel F. Survival curve for CMV viremia survival free proportion at 2 y post transplantation. Panel G. Survival curve for BK viremia survival free proportion at 6 mo post transplantation. Panel H. Survival curve for BK viremia survival free proportion at 1 y post transplantation. Panel I. Survival curve for BK viremia survival free proportion at 2 y post transplantation
With a bottom quartile cutoff of the mean relative gut abundance of BPG bacteria (6.76%), there were 41 subjects in the Lower BPG Bacteria Group and 122 subjects in the Higher BPG Bacteria Group. The Higher BPG Bacteria Group had a decreased risk for development of RV infections at 6 months post transplantation (HR:0.25, 95% CI: 0.10‐0.59, P = .002; Figure 6A), at 1 year post transplantation (HR:0.36, 95% CI: 0.17‐0.78, P = .009; Figure 6B), and at 2 years post transplantation (HR:0.41, 95% CI: 0.21‐0.82, P = .01; Figure 6C) compared with the Lower BPG Bacteria Group. There were no significant differences in the hazard ratio between the Higher BPG Bacteria Group and the Lower BPG Bacteria Group with respect to future development of CMV viremia or BK viremia at 6 months, 1 year, or 2 years post transplantation (P > .10; Figure 6D‐I).
Figure 6.
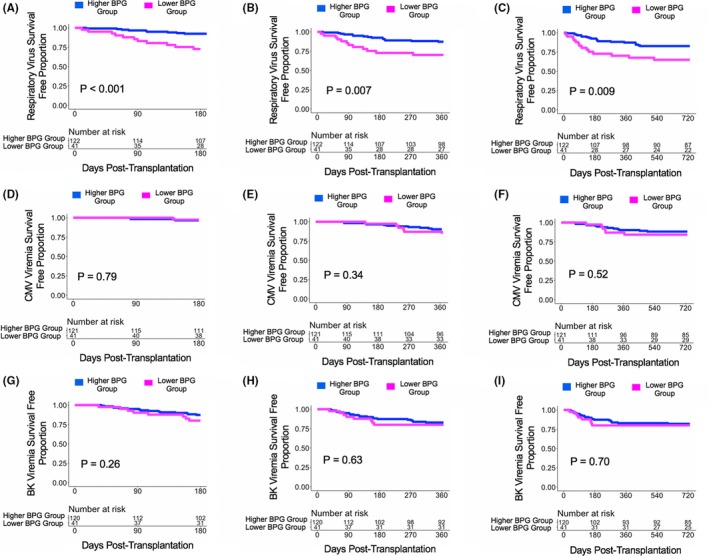
Survival curves based on a bottom quartile cutoff of the mean relative gut abundance of BPG bacteria cutoff. Survival curves show days post transplant on the x‐axis and virus survival free proportion on the y‐axis. The blue line represents the cohort of the Higher BPG Bacteria Group and the magenta line represents the cohort of the Lower BPG Bacteria Group. The number at risk is shown in the table below the survival curve. There was 1 less subject for CMV viremia analysis and 2 less subjects for BK viremia analysis because measurements of the viruses were never performed in these subjects. The P value was calculated using a log‐rank test. Panel A. Survival curve for respiratory virus survival free proportion at 6 mo post transplantation. Panel B. Survival curve for respiratory virus survival free proportion at 1 y post transplantation. Panel C. Survival curve for respiratory virus survival free proportion at 2 y post transplantation. Panel D. Survival curve for CMV viremia survival free proportion at 6 mo post transplantation. Panel E. Survival curve for CMV viremia survival free proportion at 1 y post transplantation. Panel F. Survival curve for CMV viremia survival free proportion at 2 y post transplantation. Panel G. Survival curve for BK viremia survival free proportion at 6 mo post transplantation. Panel H. Survival curve for BK viremia survival free proportion at 1 y post transplantation. Panel I. Survival curve for BK viremia survival free proportion at 2 y post transplantation
With a median quartile cutoff of the mean relative gut abundance of BPG bacteria (10.95%), there were 82 subjects in the Lower BPG Bacteria Group and 81 subjects in the Higher BPG Bacteria Group. The Higher BPG Bacteria Group had a decreased risk for development of RV infections at 6 months post transplantation (HR:0.40, 95% CI 0.15‐1.05, P = .06; Figure S3A) compared with the Lower BPG Bacteria Group. There were no significant differences in the hazard ratio between the Higher BPG Bacteria Group and the Lower BPG Bacteria Group with respect to future development of CMV viremia or BK viremia at 6 months, 1 year, or 2 years post transplantation (P > .10; Figure S3D‐I).
4. DISCUSSION
In this study, we report one of the first description of butyrate‐producing gut bacteria over time in the kidney transplant population. Our data reveal that antibiotics with anaerobic coverage is associated with a decrease in relative gut abundance of BPG bacteria. Our data also support a relationship between high relative gut abundance of BPG bacteria and less risk for development of respiratory viral infections, a finding first observed by Haak et al in the allogeneic HSCT population.5
It is important to note the allogeneic HSCT subjects had a much higher proportion of patients with a less than 1% relative gut abundance of BPG bacteria than the kidney transplant recipients in our study (81% vs. 8%, respectively). We speculate that this difference is likely because of the higher number of antibiotics given to the allogeneic HSCT population. In our study, we found that antibiotics with anaerobic coverage was associated with a decrease in relative gut abundance of BPG bacteria from post‐transplant week 1 to post‐transplant week 4. Kidney transplant recipients usually receive a preoperative antibiotic for surgical prophylaxis and a Pneumocystis jiroveci prophylaxis, but do not routinely receive broad‐spectrum antibiotics with anaerobic coverage so differences in antibiotic regimens may explain the dichotomy in the relative gut abundance of BPG bacteria between the two populations. We also found that gender and steroid maintenance therapy were associated with a lower relative gut abundance of BPG bacteria, which could have also contributed to the dichotomy. With respect to gender, little is known about the differences in the relative gut abundance of BPG between men and women. A recent study by Gao et al noted differences in the gut microbiota in obese individuals stratified by gender12 so it is possible that gender may contribute to the differences in relative gut abundances of BPG bacteria, which may have implications for immune system modulation beyond viral infections.
There are several limitations related to our study. First, there are significant gaps in the timing of the stool specimen evaluation and the timing of viral diagnoses. While our limited data suggest the stability of the relative gut abundance of BPG bacteria in subjects who did not receive antibiotics, the relative gut abundance of BPG bacteria may have changed over the course of time in the setting of antibiotic usage, which we did not account for in our survival curve analyses. In addition, we used the cutoff of 1% relative gut abundance of BPG bacteria, which was utilized in the allogeneic HSCT recipient population.5 Notably, there was a significance difference in the proportion of HSCT recipients and in the proportion of kidney transplant recipients with less than 1% relative gut abundance of BPG bacteria and whether this cutoff is also relevant for the kidney transplant population is unknown. In order to address this concern, we evaluated both a bottom quartile cutoff as well as a median cutoff and we did find an association between high relative gut abundance of BPG bacteria and decreased risk for respiratory viral infections using the bottom quartile cutoff. Another limitation is that respiratory viral panels were performed by clinicians on a for‐cause indication basis unlike BK virus and CMV virus screening which was performed routinely per protocol. Not all subjects with respiratory viral symptoms were screened using the respiratory viral panels, which may have influenced our results. Furthermore, subjects may have asymptomatic long term respiratory viral infections prior to transplantation and whether our detection was the result of a new infection or asymptomatic colonization is not known. We also note that our study evaluated upper respiratory viral infections while the Haak et al study assessed lower respiratory viral infections, so whether our data applies to lower respiratory viral infections in the kidney transplant population is unknown. Given the limited number of outcomes, we did not perform multivariable analyses so it is possible that other factors may have confounded the results.
Despite these limitations, our preliminary study provides one of the first descriptions of BPG bacteria in kidney transplant recipients early after kidney transplantation. We find that antibiotics with anaerobic coverage are associated with a decrease in the relative gut abundance of BPG bacteria and we also report an association between the relative gut abundance of BPG bacteria and the risk for development of respiratory viral infections in kidney transplant recipients. Our results are preliminary in nature and require replication in a larger cohort of kidney transplant recipients. In particular, we did report a trend toward significance of the association between the relative gut abundance of BPG bacteria and CMV viremia, which would need further validation for the CMV viremia finding as well as for CMV disease. Our study examined the relative gut abundance of BPG bacteria, but future studies examining the absolute gut abundance of BPG bacteria and/or fecal butyrate concentrations may provide more insight into the relationship between BPG bacteria and development of viral complications. Nevertheless, if our findings are confirmed, our data support therapeutic options in the form of modulating the gut microbiota for decreasing viral infection rates.
CONFLICT OF INTEREST
The authors of this manuscript have the following competing interests to disclose. EGP has received speaker honoraria from Bristol Myers Squibb, Celgene, Seres Therapeutics, MedImmune, Novartis, and Ferring Pharmaceuticals and is an inventor on patent application # WPO2015179437A1, entitled “Methods and compositions for reducing Clostridium difficile infection” and #WO2017091753A1, entitled “Methods and compositions for reducing vancomycin‐resistant enterococci infection or colonization” and holds patents that receive royalties from Seres Therapeutics, Inc; JRL receives research support from BioFire Diagnostics, LLC. DD, MS, and JRL are inventors on patent application #W02018187521A2 entitled “Methods of detecting cell‐free dna in biological samples.”
AUTHOR CONTRIBUTIONS
MM, LTZ, SA, and EE participated in the processing of specimens and data analysis; JH, CG, ANS, and YT participated in data analysis; TM, ML, and DMD participated in data analysis and writing of the manuscript; JRL, EGP, and MS participated in the study design, data analysis, and writing of the manuscript.
Supporting information
ACKNOWLEDGMENTS
We thank the National Institutes of Allergy and Infectious Diseases for their research support via NIH grant K23 AI 124464 (JRL) and R37 AI 051652 (MS).
Lee JR, Huang J, Magruder M, et al. Butyrate‐producing gut bacteria and viral infections in kidney transplant recipients: A pilot study. Transpl Infect Dis. 2019;21:e13180 10.1111/tid.13180
DATA AVAILABILITY STATEMENT
Sequencing data along with the clinical data will be made available before publication in the database of Genotypes and Phenotypes, accession number phs001879.v1.p1. Local institutional review board approval will be needed to access the data.
REFERENCES
- 1. Taur Y, Xavier JB, Lipuma L, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis. 2012;55(7):905‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tamburini FB, Andermann TM, Tkachenko E, Senchyna F, Banaei N, Bhatt AS. Precision identification of diverse bloodstream pathogens in the gut microbiome. Nat Med. 2018;24(12):1809‐1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Abt MC, Osborne LC, Monticelli LA, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37(1):158‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu H, Wang J, He T, et al. Butyrate: a double‐edged sword for health? Adv Nutr. 2018;9(1):21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haak BW, Littmann ER, Chaubard JL, et al. Impact of gut colonization with butyrate‐producing microbiota on respiratory viral infection following allo‐HCT. Blood. 2018;131(26):2978‐2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee JR, Magruder M, Zhang L, et al. Gut microbiota dysbiosis and diarrhea in kidney transplant recipients. Am J Transplant. 2019;19(2):488‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403‐410. [DOI] [PubMed] [Google Scholar]
- 8. Tatusova T, Ciufo S, Fedorov B, O'Neill K, Tolstoy I. RefSeq microbial genomes database: new representation and annotation strategy. Nucleic Acids Res. 2014;42(D1):D553‐D559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brook I. Treatment of anaerobic infection. Expert Rev Anti Infect Ther. 2007;5(6):991‐1006. [DOI] [PubMed] [Google Scholar]
- 10. Robbins MJ, Marais R, Felmingham D, Ridgway GL, Gruneberg RN. In vitro activity of vancomycin and teicoplanin against anaerobic bacteria. Drugs Exp Clin Res. 1987;13(9):551‐554. [PubMed] [Google Scholar]
- 11. Riviere A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate‐producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gao X, Zhang M, Xue J, et al. Body mass index differences in the gut microbiota are gender specific. Front Microbiol. 2018;9:1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequencing data along with the clinical data will be made available before publication in the database of Genotypes and Phenotypes, accession number phs001879.v1.p1. Local institutional review board approval will be needed to access the data.


