Abstract
Background:
The optimal dose of S-adenosyl methionine (SAMe) for major depressive disorder (MDD) remains unclear. The objective of this analysis was to address whether a dose increase provided further improvement in cases of insufficient response using data from an existing randomized clinical trial.
Methods:
Sixty-five patients with MDD who failed to respond to SAMe 1,600 mg/day, escitalopram 10 mg/day, or placebo for 6 weeks were treated with doubled doses of the allocated treatments for the following 6 weeks. Changes in 17-item Hamilton Depression Rating Scale, Inventory of Depressive Symptomatology-Self Rated, and Systematic Assessment for Treatment Emergent Events-Specific Inquiry were compared between the lower and higher dose treatments in each treatment group and among the higher dose treatments of SAMe, escitalopram, and placebo.
Results:
Various depression severity scores decreased significantly for all three treatment arms during the higher dose treatment. No within-group and between-group differences were found in any of the efficacy measures when comparing the doses and treatments. There was a significant difference in reported abdominal discomfort among patients receiving the higher dose of SAMe (31.3%), compared to escitalopram (8.7%) and placebo (3.8%) (χ2=7.32, p=0.026).
Limitations:
The sample size was relatively small. The study duration for dose increase was relatively short.
Conclusions:
Patients with MDD failing to respond to 1,600 mg/day of SAMe may improve after increasing the dose to 3,200 mg/day, but we cannot rule out the contribution of a placebo effect and time-related improvement. The risk of abdominal discomfort may be increased with higher doses of SAMe.
Keywords: dose escalation, escitalopram, major depressive disorder, S-adenosyl methionine, SAMe
Introduction
Depression is a major mental illness that is associated with significant morbidity (Baldessarini et al., 2017) and causes significant global burden and disability (Park and Zarate, 2019). While antidepressants play a crucial role in the treatment of depression, adverse effects are frequent and can be a common reason for early discontinuation from treatment (Crawford et al., 2014). Furthermore, the overall effectiveness of antidepressants as a whole has hardly changed for decades (Cipriani et al., 2018). Thus, the tolerability issue and limited novelty of current pharmacotherapy for depression represent a significant need for exploring new and safer treatments with good tolerability (Chang and Fava, 2010).
S-adenosyl methionine (SAMe) is an endogenous, intracellular amino acid metabolite and enzyme co-substrate involved in multiple crucial biochemical pathways, including the one-carbon cycle (Sarris et al., 2016; Sharma et al., 2017). SAMe may improve depressed mood via enhanced methylation of catecholamines and increased serotonin turnover, reuptake inhibition of norepinephrine, enhanced dopaminergic activity, decreased prolactin secretion, and increased phosphatidylcholine conversion (Papakostas, 2009). SAMe has also been reported to increase the genetic expression of brain-derived neurotrophic factor (BDNF) (Li et al., 2016). SAMe has generally shown promise as monotherapy or augmentation therapy to an antidepressant for major depressive disorder (MDD) or treatment-resistant depression in open-label studies and double-blind randomized clinical trials (RCTs) (De Berardis et al., 2013; Hardy et al., 2003; Sharma et al., 2017). However, while doses ranging from 200–3,200 mg/day of SAMe through different routes of administration have been used in clinical trials, SAMe’s optimal oral dose for depression is still unknown. More notably, there is little evidence regarding the best starting dose or whether sequential dose increases bring further clinical benefit.
In our recent 12-week, 3-arm, double-blind RCT, the efficacies of SAMe monotherapy, escitalopram monotherapy, and placebo were compared in 189 patients with MDD (Mischoulon et al., 2014). Unlike past studies, initial doses of active drugs (1,600 mg/day for SAMe and 10 mg/day for escitalopram) could be doubled (i.e. to 3,200 mg/day of SAMe and 20 mg/day of escitalopram) in subjects who did not respond to initial doses by week 6 of this study, the former representing the highest dose of SAMe ever used in a clinical trial. While all three treatments produced a significant improvement in depression by the end of the 12-week study period, no significant differences were observed between the three treatments, so the study was considered failed.
To develop effective and safe SAMe-based treatment strategies in depression, it is important to characterize the dose-response relationship of this treatment. While clinically effective doses tend to be on average 1,600 mg/day (Alpert et al., 2004; Papakostas et al., 2010), recent clinical trials by Sarris and colleagues showed no advantage for SAMe 800 mg/day over placebo (Sarris et al., 2018). To better characterize the comparative efficacy of two different dose regimens of SAMe, we compared 6-week clinical outcomes among SAMe, escitalopram, and placebo during the dose increase phase in patients with MDD who did not respond to starting doses, based on the previously published report. We also examined symptom changes in the same subjects during the 6-week starting dose period and following the 6-week increased dose period. We thus addressed whether the increase in dose of SAMe from 1,600 to 3,200 mg/day would provide further improvement in itself, and also compared to escitalopram. We hypothesized that the dose increase would result in significantly greater symptom improvement during the second phase compared to the first phase for SAMe and escitalopram, and that the difference between the two active treatments would be nonsignificant.
Methods
Study Design
In the parent study, patients were enrolled at the Massachusetts General Hospital in Boston and at Butler Hospital in Providence, Rhode Island from April 2005 to December 2009 and the detailed protocol was published with the primary paper in 2014 (Mischoulon et al., 2014). The Institutional Review Boards at the participating sites approved the study. Patients were recruited through clinician referral and advertisements in local newspapers, radio, and television. After complete description of the study, written informed consent was obtained from all participating subjects. A total of 189 outpatients of both sexes with MDD were randomly assigned to SAMe 1,600 mg/day, escitalopram 10 mg/day, or placebo during the first 6 weeks (i.e. lower dose treatment). A dose increase was allowed for nonresponders (i.e. patients who did not show ⩾50% reduction in the 17-item Hamilton Depression Rating Scale (HDRS-17) (Hamilton, 1960) score) at week 6; in this case, SAMe was increased to 3,200 mg/day and escitalopram to 20 mg/day for weeks 7–12 (i.e. higher dose treatment). On the other hand, responders at week 6 stayed on the same dose of each treatment during weeks 7–12. The data of non-responders at week 6 who received the increased dose of SAMe, escitalopram, or equivalent placebo during weeks 7–12 and responders at week 6 were separately analyzed in this report.
Randomization numbers were consecutively assigned by a biostatistician and stratified by site. A double-dummy design was used, due to differences in appearance between SAMe and escitalopram tablets. Each patient was provided two bottles, with one bottle containing either SAMe or SAMe-placebo and the other containing either escitalopram or escitalopram-placebo. SAMe tosylate and matching placebo were supplied by Pharmavite LLC (Mission Hills, California). Escitalopram and matching placebo were purchased from Forest Pharmaceuticals (New York, New York).
Study Population
Inclusion and exclusion criteria of the parent study were reported elsewhere (Mischoulon et al., 2014). Briefly, inclusion criteria were (1) outpatients aged between 18 and 80 years (2) who had a diagnosis of MDD according to the Structured Clinical Interview for DSM-IV Axis I disorders-Patient Edition (SCID-I/P) (First et al., 1995) (3) a score ⩾25 on the Inventory of Depressive Symptomatology-Clinician Rated (IDS-C) (Rush et al., 1996) at the screen and baseline visits. Patients were excluded if they met any of the following criteria: pregnancy or women of child-bearing potential who were not using a medically accepted means of contraception; serious suicidality or homicidality; unstable medical illness; organic mental disorders; substance use disorders active within the preceding 6 months, which were assessed by the SCID-I/P and through urine toxicology screens at the screening visit; schizophrenia and other psychotic disorders or psychotic features; bipolar disorder; acute bereavement; severe borderline or antisocial personality disorder; current primary diagnoses of panic disorder or obsessive-compulsive disorder; seizure disorder; concurrent use of other psychotropic drugs; hypothyroidism; prior ⩾6-week courses of either SAMe ⩾1,200 mg/day or escitalopram ⩾10 mg/day during the current depressive episode; intolerance to SAMe or escitalopram; having taken an investigational psychotropic drug within the last year; failure to respond to 2 or more antidepressant trials at adequate doses and duration (⩾6 weeks) during the current depressive episode; any depression-focused ongoing psychotherapy; history of bleeding diatheses, low platelet counts, gastrointestinal bleeding, or use of medications that alter bleeding risk; and a Clinical Global Impressions-Improvement scale (CGI-I) (Guy, 1976) score of “much” or “very much improved” between the screen and baseline visits and/or an IDS-C score <25 at either the screen or the baseline visit. Intellectual disability (intellectual development disorder) was assessed for at the screening visit, through the clinical interview. Patients were asked about developmental disorders and those who would be deemed unable to participate were excluded from the study. We also excluded patients receiving any depression-focused ongoing psychotherapy (family or marital counseling were allowed).
Assessment Measures
The following assessments were performed at screening, baseline, weeks 1, 2, 4, 6, 8, 10, and 12: HDRS-17, Inventory of Depressive Symptomatology-Self Rated (IDS-SR), Clinical Global Impressions Severity (CGI-S) and Improvement (CGI-I) scales, Systematic Assessment for Treatment Emergent Events-Specific Inquiry (SAFTEE-SI) (Rabkin et al., 1992).
Statistical Analysis
The HDRS-17, IDS-SR, CGI-S and CGI-I scores were compared between weeks 6 and 12 in non-responders to each treatment at week 6 by the paired t test or the Wilcoxon signed-rank test on intention-to-treat (ITT) basis using last observation carried forward (LOCF), which is a common way to handle missing values. Effect sizes for the dose increase of the three treatments were measured using Cohen’s d statistic (Cohen J, 1988). To examine the effectiveness of a dose increase for each drug in non-responders, the changes in the HDRS-17, IDS-SR, and CGI-S scores were compared between the lower and higher dose treatments in each treatment group by the paired t test or the Wilcoxon signed-rank test using LOCF. To compare the effectiveness of higher dose treatments of each drug for nonresponders, the changes in the HDRS-17, IDS-SR, CGI-S scores, and the final scores of the CGI-I during the higher dose treatment phase (weeks 6–12) were compared among the SAMe, escitalopram, and placebo groups by the one-way analysis of variance (ANOVA) or the Kruskal-Wallis test using LOCF. Response rates (i.e. proportion of patients who showed a ⩾50% reduction in the HDRS-17 total score compared to week 6) and remission rates (i.e. proportion of patients who showed a total score <7 on the HDRS-17) at week 12 were similarly compared by the Pearson’s chi-squared test. Changes in the HDRS-17 scores from week 6 to week 12 were also compared among the three treatment groups, using the mixed-effects model for repeated measures (MMRM) that contained treatment group, week, and group-by-week interaction as factors with autoregressive AR(1) correlation matrix among time points. Those analyses were conducted for responders at week 6 who stayed on the same dose of each treatment during the second 6-week phase.
Side effects reported on the SAFTEE-SI scale were classified by severity into 0 (none), 1 (mild), 2 (moderate), 3 (severe). Treatment-emergent was defined as any SAFTEE-SI side effect for which severity increased by 2 or 3 levels (e.g. from 2 to 4 or from 1 to 4) from week 6 to week 12. The rates of these side effects reported at any time during the higher dose treatment in non-responders were compared among the three treatment groups by Pearson’s chi-squared test. The outcome of principal interest was the change in the HDRS-17 score. A two-tailed P value of <0.05 was considered statistically significant for all tests. The Bonferroni correction was used to control the familywise error rate when group pairs were compared head to head after multiple comparisons. All statistical analyses are conducted using the Statistical Package for Social Science (SPSS) version 24.0 for Windows (IBM Corporation, Armonk, NY).
Results
Subject Characteristics
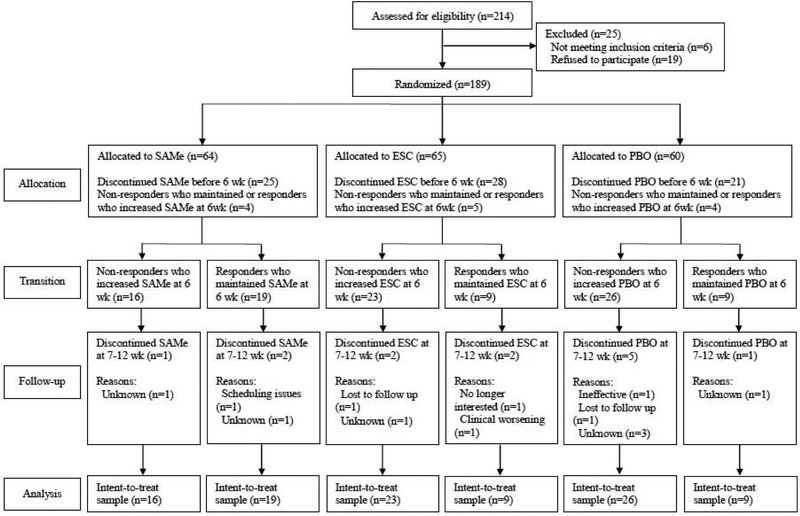
Of the189 participants randomized in the parent study, 65 non-responders increased the dose of their allocated drug at week 6 and continued the treatment up to week 12 (Figure 1). On the other hand, 37 responders at week 6 stayed on the same dose and continued the treatment. Table 1 summarizes sociodemographic and clinical characteristics of the study sample.
Figure 1. Patient Flow.
Abbreviations: ESC = escitalopram; PBO = placebo; SAMe = S-adenosyl-L-methionine; wk = weeks
Table 1.
Baseline Demographic and Clinical Characteristics
| Variable | Non-responders (n=65) | Responders (n=37) |
|---|---|---|
| Study site, n (%) | ||
| Massachusetts General Hospital | 42 (64.6%) | 25 (67.6%) |
| Butler Hospital | 23 (35.4%) | 12 (32.4%) |
| Age, mean (SD) [range], years | 46.0 (14.3) [18–78] | 45.7 (15.1) [21–76] |
| Female gender, n (%) | 29 (44.6%) | 20 (54.1%) |
| Race/ethnicity, n (%) | ||
| White | 46 (70.8%) | 28 (75.7%) |
| African American | 12 (18.5%) | 3 (8.1%) |
| Asian | 2 (3.1%) | 0 (0%) |
| Hispanic/Latino | 2 (3.1%) | 4 (10.8%) |
| No response | 3 (4.6%) | 2 (5.4%) |
| Education, n (%) | ||
| Did not graduate high school | 6 (9.2%) | 4 (10.8%) |
| Graduated high school | 10 (15.4%) | 5 (13.5%) |
| Some college | 18 (27.7%) | 5 (13.5%) |
| Graduated 2-year college | 6 (9.2%) | 2 (5.4%) |
| Graduated 4-year college | 11 (16.9%) | 9 (24.3%) |
| Some graduate school | 1 (1.5%) | 4 (10.8%) |
| Graduated graduate school | 10 (15.4%) | 7 (18.9%) |
| No response | 3 (4.6%) | 0 (0%) |
| Current marital status, n (%) | ||
| Never married | 28 (43.1%) | 15 (40.5%) |
| Married/cohabitating | 14 (21.5%) | 11 (29.7%) |
| Separated/divorced | 16 (24.6%) | 7 (18.9%) |
| Widowed | 4 (6.2%) | 3 (8.1%) |
| No response | 3 (4.6%) | 1 (2.7%) |
| Employment status, n (%) | ||
| Employed full-time | 17 (26.2%) | 9 (24.3%) |
| Employed part-time | 9 (13.8%) | 6 (16.2%) |
| Student | 1 (1.5%) | 1 (2.7%) |
| Retired | 6 (9.2%) | 6 (16.2%) |
| Unemployed | 16 (24.6%) | 11 (29.7%) |
| Volunteer | 1 (1.5%) | 1 (2.7%) |
| Disabled | 8 (12.3%) | 2 (5.4%) |
| Homemaker | 1 (1.5%) | 0 (0%) |
| Other/no response | 6 (9.2%) | 1 (2.7%) |
| IDS-SR score at lower dose start (baseline), mean (SD) [range] | 38.6 (11.3) [12–66] | 34.8 (9.9) [20–57] |
| IDS-SR score at higher dose start (week 6), mean (SD) [range] | 31.8 (10.8) [4–56] | 15.5 (10.5) [0–45] |
| HDRS-17 score at lower dose start (baseline), mean (SD) [range] | 19.6 (3.9) [11–28] | 18.2 (5.5) [12–32] |
| HDRS-17 score at higher dose start (week 6), mean (SD) [range] | 16.8 (4.2) [8–26] | 5.6 (2.6) [0–12] |
Abbreviations: HDRS-17 = 17-item Hamilton Depression Rating Scale, IDS-SR = Inventory of Depressive Symptomatology Self Report, SD = standard deviation
Within-group differences in non-responders
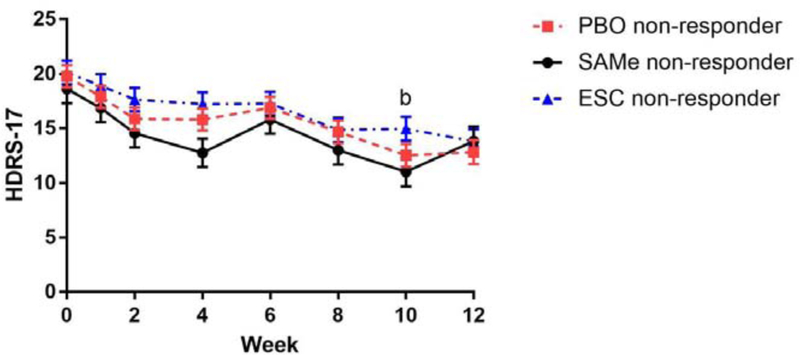
While there was only a trend to significance in the decrease in the mean HDRS-17 score in the SAMe group during the higher dose phase (t(15)=1.82, p=0.089, Cohen’s d=0.51), the mean IDS-SR score (t(15)=2.89, p=0.011), CGI-S score (z=−2.52, p=0.012), and CGI-I score (z=−2.14, p=0.032) decreased significantly in the SAMe group during this period. The mean HDRS-17 score (t(22)=3.06, p=0.006, Cohen’s d=0.65) and CGI-S score (z=−2.04, p=0.041) decreased significantly in the escitalopram group during the higher dose phase. Finally, the mean HDRS-17 score (t(25)=2.88, p=0.008, Cohen’s d=0.69), IDS-SR score (t(25)=2.07, p=0.049), CGI-S score (z=−2.29, p=0.022), and CGI-I score (z=−2.08, p=0.038) in the placebo group also significantly decreased during the higher dose phase (Table 2). In the mixed-effects model for changes in the HDRS-17 score in non-responders, there were significant differences between weeks 6 and 10 in the placebo group (mean [standard error (SE)], 16.9 [1.0] vs 12.5 [1.0], p=0.008) and a trend to significance in the SAMe group (15.8 [1.3] vs 11.0 [1.4], p=0.054) (Figure 2a). On the other hand, no statistically significant differences were found across the four time points during the higher dose phase in the escitalopram group.
Table 2.
Outcome Measures in Non-responders at Week 6
| Characteristics | SAMe (n=16) | ESC (n=23) | PBO (n=26) | |||
|---|---|---|---|---|---|---|
| Score | Score change | Score | Score change | Score | Score change | |
| HDRS-17 score, mean (SD) | ||||||
| Lower dose phase start | 18.6 (4.2) | n.a. | 20.1 (3.6) | n.a. | 19.8 (3.8) | n.a. |
| Lower dose phase end/higher dose phase start | 15.8 (4.3) | −2.8 (4.4) | 17.3 (4.7) | −2.8 (5.0) | 16.9 (3.6) | −2.9 (4.4) |
| Higher dose phase end | 13.4 (5.4) | −2.4 (5.2) | 14.0 (5.3) | −3.2 (5.0)a | 13.3 (6.3) | −3.5 (6.1)a |
| IDS-SR score, mean (SD) | ||||||
| Lower dose phase start | 35.2 (8.8) | n.a. | 38.9 (12.2) | n.a. | 40.5 (11.4) | n.a. |
| Lower dose phase end/higher dose phase start | 27.7 (8.6) | −7.5 (10.4) | 33.3 (11.8) | −5.6 (8.6) | 32.9 (10.5) | −7.6 (8.1) |
| Higher dose phase end | 22.6 (8.8) | −5.1 (6.9)a | 30.1 (14.5) | −3.2 (8.6) | 27.3 (14.7) | −5.7 (13.7)a |
| CGI-S score, mean (SD) | ||||||
| Lower dose phase start | 4.4 (0.7) | n.a. | 4.5 (0.7) | n.a. | 4.4 (0.7) | n.a. |
| Lower dose phase end/higher dose phase start | 3.8 (0.8) | −0.7 (0.8) | 3.8 (0.8) | −0.7 (1.0) | 3.8 (0.8) | −0.6 (0.8) |
| Higher dose phase end | 3.0 (1.1) | −0.8 (0.9)a | 3.3 (1.2) | −0.6 (1.2)a | 3.2 (1.3)a | −0.7 (1.3)a |
| CGI-I score, mean (SD) | ||||||
| Lower dose phase end | 3.4 (0.9) | 3.3 (0.8) | 3.3 (0.7) | |||
| Higher dose phase end | 2.8 (1.0)a | 3.0 (1.1) | 2.8 (1.1)a | |||
| Response rates during higher dose phase, % (n) | 18.8% (3) | 17.4% (4) | 26.9% (7) | |||
| Remission rates at higher dose phase end, % (n) | 12.5% (2) | 8.7% (2) | 19.2% (5) | |||
There were significant changes during the higher dose phase.
bThere were no significant differences in any of the score changes between the higher and lower dose phases in any treatment groups. There were no significant differences in any of the outcome measures during the higher dose phase among the three treatment groups.
Abbreviations: CGI-I = Clinical Global Impressions-Improvement scale, CGI-S = Clinical Global Impressions-Severity of Illness scale, ESC = escitalopram, HDRS-17 = 17-item Hamilton Depression Rating Scale, IDS-SR = Inventory of Depressive Symptomatology Self Report, n.a. = not available, PBO = placebo, SAMe = S-adenosyl-L-methionine.
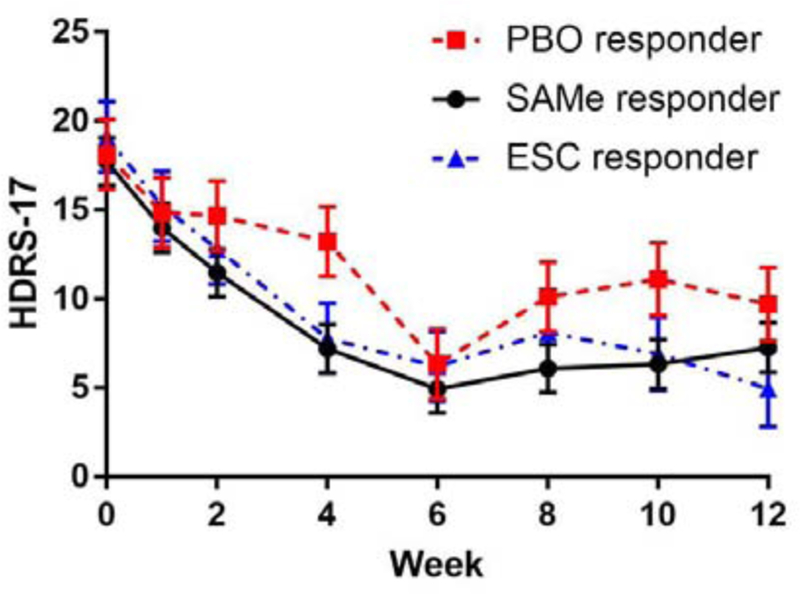
Figure 2. Time Course of the HDRS-17 Scores during the Higher Dose Treatment.
(2a) Non-responder at week 6
(2b) Responder at week 6
aClosed circles, triangles, and squares represent mean values. Bars represent standard errors (SE).
bSignificant differences were found from week 6 in the placebo group (mean [SE], 16.9 [1.0] vs 12.5 [1.0], p=0.008) and the SAMe group as a trend level (15.8 [1.3] vs 11.0 [1.4], p=0.054).
Abbreviations: ESC = escitalopram; HDRS-17 = 17-item Hamilton Depression Rating Scale, PBO = placebo; SAMe = S-adenosyl-L-methionine
No statistically significant differences were found in the changes in any of the outcome measures between the lower and higher dose treatments in any treatment groups (Table 2), indicating that clinical benefit during the higher dose treatment was similar to that in lower dose treatment for non-responders receiving SAMe, escitalopram, and placebo.
Between-group differences in non-responders
No statistically significant differences were found in any of the efficacy outcome measures during the higher dose treatment phase among non-responders receiving SAMe, escitalopram, or placebo, indicating no significant differences in effectiveness among higher doses of SAMe, escitalopram, and placebo (Table 2). In the mixed-effects model for changes in the HDRS-17 score in non-responders, there were no significant differences among the three treatment groups (F(2, 91.72)=2.45, p=0.09) nor a significant interaction between time and treatment groups (F(14, 344.82)=0.96, p=0.49), which indicate time courses of the HDRS-17 score in non-responders are similar among the three treatment groups (Figure 2a).
Within-group and between-group differences in responders
There were no significant changes in any of the outcome measures during the second 6-week phase in any treatment groups (Table 3). No statistically significant differences were found in any of the efficacy outcome measures during the second 6-week phase among responders receiving SAMe, escitalopram, or placebo. In the mixed-effects model for changes in the HDRS-17 score, no statistically significant differences were found across the four time points during the second 6-week phase in any treatment groups (Figure 2b). There were no significant differences among the three treatment groups (F(2, 43.41)=1.71, p=0.19) nor a significant interaction between time and treatment groups (F(14, 189.68)=1.05, p=0.41), which indicate time courses of the HDRS-17 score in responders are similar among the three treatment groups.
Table 3.
Outcome Measures in Responders at Week 6
| Characteristics | SAMe (n=19) | ESC (n=9) | PBO (n=9) | |||
|---|---|---|---|---|---|---|
| Score | Score change | Score | Score change | Score | Score change | |
| HDRS-17 score, mean (SD) | ||||||
| First 6-week phase start | 17.7 (5.4) | n.a. | 19.1 (6.3) | n.a. | 18.1 (4.4) | n.a. |
| First 6-week phase end/second 6-week phase start | 4.9 (2.5) | −12.8 (5.2) | 6.2 (2.8) | −12.9 (5.2) | 6.3 (2.3) | −11.8 (3.6) |
| Second 6-week phase end | 7.0 (6.0) | 2.0 (4.9) | 5.8 (7.1) | −0.4 (5.3) | 10.7 (7.7) | 4.3 (7.6) |
| IDS-SR score, mean (SD) | ||||||
| First 6-week phase start | 34.0 (10.1) | n.a. | 37.0 (10.2) | n.a. | 34.1 (8.8) | n.a. |
| First 6-week phase end/second 6-week phase start | 13.3 (10.3) | −20.8 (12.0) | 14.7 (9.2) | −22.3 (9.3) | 20.8 (10.4) | −13.3 (4.6) |
| Second 6-week phase end | 12.6 (10.8) | −0.7 (5.5) | 15.8 (14.2) | 1.1 (7.1) | 22.3 (13.4) | 1.4 (13.0) |
| CGI-S score, mean (SD) | ||||||
| First 6-week phase start | 4.2 (0.8) | n.a. | 4.2 (1.0) | n.a. | 4.3 (0.5) | n.a. |
| First 6-week phase end/second 6-week phase start | 1.7 (1.0) | −2.5 (1.2) | 2.0 (0.8) | −2.2 (0.8) | 2.1 (1.0) | −2.2 (0.9) |
| Second 6-week phase end | 1.9 (1.0) | 0.3 (0.9) | 1.6 (1.3) | −0.4 (0.7) | 2.6 (1.6) | 0.4 (1.7) |
| CGI-I score, mean (SD) | ||||||
| First 6-week phase end | 1.8 (0.9) | 1.8 (0.6) | 1.8 (0.6) | |||
| Second 6-week phase end | 1.8 (1.0) | 1.3 (0.9) | 2.1 (1.4) | |||
aThere were no significant changes in any of the outcome measures during the second 6-week phase in any treatment groups. There were no significant differences in any of the outcome measures during the second 6-week phase among the three treatment groups.
Abbreviations: CGI-I = Clinical Global Impressions-Improvement scale, CGI-S = Clinical Global Impressions-Severity of Illness scale, ESC = escitalopram, HDRS-17 = 17-item Hamilton Depression Rating Scale, IDS-SR = Inventory of Depressive Symptomatology Self Report, n.a. = not available, PBO = placebo, SAMe = S-adenosyl-L-methionine.
Adverse Events Observed with Higher Doses of SAMe or Escitalopram, and Placebo
The most frequently reported adverse events in the higher dose SAMe group were stomach or abdominal discomfort (31.3%) and fluid retention or swelling (25.0%). In multiple comparisons, we found significant difference in reporting of stomach or abdominal discomfort among subjects receiving higher doses of SAMe (31.3%), escitalopram (8.7%), and placebo (3.8%) (χ2(2)=7.32, p=0.026). While stomach or abdominal discomfort were reported most frequently in the higher dose SAMe group, no significant disadvantage was found when group pairs were compared head to head.
Discussion
To our knowledge, this is the first study to examine the effectiveness of a dose increase of SAMe for patients with MDD who failed to respond to an initial dose. While there were no significant differences in effectiveness among SAMe 3,200 mg/day, escitalopram 20 mg/day, and placebo, increased doses of SAMe and of escitalopram provided improvement for nonresponders in various rating scales, which might be a time-related rather than dose-related improvement. Effect sizes recorded for changes in the HDRS-17 scale in the dose increase period were in the medium range at 0.51 for SAMe and 0.65 for escitalopram. Notably, placebo also produced a continued improvement in the dose increase phase, with significant outcomes in all four outcome measures, and an effect size of 0.69 per the HDRS-17 scale. This robust placebo effect may have been due to a relative enrichment in factors that have been shown to be associated with an increased response to placebo (Trivedi et al., 2018). Likewise, there were no significant differences in subsequent effective outcome among responders to SAMe 1,600 mg/day, escitalopram 10 mg/day, and placebo. Maintaining initial dose would therefore be reasonable choice for responders to SAMe and escitalopram in clinical settings, particularly with a view toward minimizing risk of new or worseningside effects.
The findings are complicated by the fact that there was a trend-level improvement in the SAMe group by week 10 in the mixed-effects model for repeated measures, and this was apparently lost by week 12. This was similar to the pattern seen in the parent study, where SAMe significantly separated from placebo at weeks 8 and 10, followed by the loss of significance by week 12. This effect may have to do with psychological worsening on the part of the patients as they face termination in the study and uncertainty about what may await them regarding their condition and clinical care (Mischoulon et al., 2014).
Furthermore, it should be noted that participants in this study may have been more depressed than what is considered optimal severity for SAMe treatment. According to reviews of published articles so far, general clinical recommendations for SAMe monotherapy suggest targeting patients with mildly symptomatic depression who do not necessarily require a prompt effective antidepressant treatment (De Berardis et al., 2016). Non-response to previous treatment or moderate severity of illness (which is reflective of our study sample, with each arm having mean baseline HDRS-17 scores in the range of 18–19, indicating moderate depression) may not represent a good indication for SAMe treatment, though in previous studies, subjects improved with addition of SAMe despite moderate severity of illness and earlier non or partial response to SSRIs and SNRIs (Alpert et al., 2004; Papakostas et al., 2010).
The effectiveness of SAMe may vary according to subjects’ pharmacokinetic variations. Cerebrospinal fluid (CSF) SAMe levels, which are low in patients with severe depression, have been reported to rise to normal level by intravenous SAMe 200 mg daily for 14 days (Bottiglieri et al., 1990). However, there are no data assessing the relationship between oral SAMe administration and CSF SAMe levels. SAMe may show efficacy only in cases of low baseline CSF levels, which were not assessed in this study. Additionally, while different nutraceuticals used for treating depression have a range of frequently used doses in clinical trials, these may not necessarily indicate the most effective doses (Mischoulon and Rapaport, 2018). In fact, dose-response relationships for depression remain unclear for many nutraceuticals. Only in the meta-analysis of omega-3 polyunsaturated fatty acid, the effective dose and combination of eicosapentaenoic acid and docosahexaenoic acid have been examined (Iovieno et al., 2011). More drug monitoring and dose-response research are necessary to investigate the contribution of a specific doses of nutraceuticals for depression.
In the present study, stomach or abdominal discomfort was reported most frequently with higher dose SAMe compared to the other higher dose treatments, which is consistent with our observations in the parent study (Mischoulon et al., 2014). While gastrointestinal symptoms as well as sweating, vertigo dizziness, irritability, insomnia, tachycardia, restlessness, and anxiety have been often reported in past studies, SAMe treatment has generally been considered safe and well tolerated (De Berardis et al., 2016). This discrepancy probably occurs because previous studies are limited to doses of 1,600 mg/day or less of SAMe, and this study represents the first trial testing 3,200 mg/day of SAMe. While this preliminary finding must be reproduced in larger studies, caution should likely be undertaken by clinicians who are considering increasing the dose of SAMe in partial and non-responders to lower doses. On the other hand, it bears mention that no patient dropped out due to adverse events during the higher dose treatment, suggesting that tolerability was good overall.
This study has several limitations. First, the sample in the higher dose period in the present analysis was one-third as large of that from the parent study, which limits power to detect differences between treatments. Second, the duration of six weeks is relatively short to optimally compare the effectiveness and tolerance of the three higher dosing strategies, since antidepressants may require up to 8–12 weeks to produce a full effect. The treatment duration was selected based on what is known about response to antidepressants, usually occurring within 4–6 weeks (Nierenberg et al., 1995; Posternak et al., 2011), and also taking into account the burden on patients that a very long double blind trial of > 3 months represents, which can reduce adherence and completion rates. This may explain in part why response and remission rates during the higher dose SAMe and escitalopram treatments were only about half the level of those obtained with the step 2 treatments in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial (Rush et al., 2006). Third, since only patients who failed to respond to a certain starting dose of the allocated treatments were included, any extrapolation of these findings to other depressed populations or other doses must be made with caution. For example, these findings may not be extended to more severely depressed or treatment resistant populations than the one we studied, or to patients who start treatment on higher doses than ours.
In conclusion, we found that increasing the dose of SAMe to 3,200 mg/day and escitalopram to 20 mg/day produced additional but modest improvement over a 6-week treatment period in patients with MDD who did not respond to SAMe 1,600 mg/day or escitalopram 10 mg/day. Unfortunately, the high placebo effects prevent us from determining whether changes associated with doubling SAMe and escitalopram are purely time effects rather than dose effects. Of some concern, SAMe 3,200 mg/day resulted in the most frequently reported abdominal discomfort among the investigated higher dose treatments, but this did not appear to result in any early terminations from the study, suggesting that the benefit may outweigh the discomfort. We still need to better characterize the overall efficacy of SAMe compared to standard antidepressants. Clinicians whose patients are using SAMe for depression should carefully consider the pros and cons of dose increases beyond 1,600 mg/day in cases of non-response at this dose or when deciding whether to pursue further optimization in patients who respond well to 1,600 mg/day. Further research is needed in larger samples and for longer study periods to develop more effective dosing strategies.
Highlights.
A dose increase of S-adenosyl methionine (SAMe) in depression was examined.
Symptoms in non-responders improved after a dose increase from 1,600 to 3,200 mg/d.
No significant difference in efficacy was found between each SAMe dose.
No superiority was found among SAMe 3,200 mg/d, escitalopram 20 mg/d, and placebo.
Abdominal discomfort was reported most frequently with SAMe 3,200 mg/d treatment.
Role of Funding:
This study was supported by the NIH and the National Center for Complementary and Alternative Medicine (NCCAM), R01 grant R01AT001638. SAMe tosylate and matching placebo were supplied by Pharmavite LLC, California. Escitalopram and matching placebo were purchased from Forest Pharmaceuticals (New York, NY).
Footnotes
Previous presentation:
None
Declarations of interest:
Dr. Sakurai has received manuscript or speaker’s honoraria from Dainippon Sumitomo, Eli Lilly, Meiji-Seika Pharma, Otsuka Pharmaceutical, Tanabe Mitsubishi Pharma, and Yoshitomi Yakuhin within the past three years. Dr. Sakurai also receives grants from the Japanese Society of Clinical Neuropsychopharmacology and the Uehara Memorial Foundation. Dr. Carpenter has no relevant financial interests. Dr. Tyrka has no relevant financial interests. Dr. Price has received grant/research support from the National Institutes of Health. Dr. Price has received travel funds from Springer. Dr. Price has served as a consultant for Wiley, Springer, U Texas (Austin), Baylor Univ, Cleveland Clinic, Fordham Univ. Dr. Price has received financial support from Wiley. Dr. Papakostas has served as a consultant for Abbott Laboratories, Acadia Pharmaceuticals, Inc*, Alkermes, Inc, AstraZeneca PLC, Avanir Pharmaceuticals, Axsome Therapeutics*, Boston Pharmaceuticals, Inc., Brainsway Ltd, Bristol-Myers Squibb Company, Cala Health, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., Genentech, Inc*, Genomind, Inc*, GlaxoSmithKline, Evotec AG, H. Lundbeck A/S, Inflabloc Pharmaceuticals, Janssen Global Services LLC*, Jazz Pharmaceuticals, Johnson & Johnson Companies*, Methylation Sciences Inc, Mylan Inc*, Novartis Pharma AG, One Carbon Therapeutics, Inc*, Osmotica Pharmaceutical Corp.*, Otsuka Pharmaceuticals, PAMLAB LLC, Pfizer Inc., Pierre Fabre Laboratories, Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Sage Therapeutics, Shire Pharmaceuticals, Sunovion Pharmaceuticals, Taisho Pharmaceutical Co, Ltd, Takeda Pharmaceutical Company LTD, Theracos, Inc., and Wyeth, Inc. Dr. Papakostas has received honoraria (for lectures or consultancy) from Abbott Laboratories, Acadia Pharmaceuticals Inc, Alkermes Inc, Asopharma America Cntral Y Caribe, Astra Zeneca PLC, Avanir Pharmaceuticals, Bristol-Myers Squibb Company, Brainsway Ltd, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., Evotec AG, Forest Pharmaceuticals, GlaxoSmithKline, Inflabloc Pharmaceuticals, Grunbiotics Pty LTD, Jazz Pharmaceuticals, H. Lundbeck A/S, Medichem Pharmaceuticals, Inc, Meiji Seika Pharma Co. Ltd, Novartis Pharma AG, Otsuka Pharmaceuticals, PAMLAB LLC, Pfizer, Pharma Trade SAS, Pierre Fabre Laboratories, Ridge Diagnostics, Shire Pharmaceuticals, Sunovion Pharmaceuticals, Takeda Pharmaceutical Company LTD, Theracos, Inc., Titan Pharmaceuticals, and Wyeth Inc. Dr. Papakostas has received research support (paid to hospital) from AstraZeneca PLC, Bristol-Myers Squibb Company, Forest Pharmaceuticals, the National Institute of Mental Health, Neuralstem, Inc, PAMLAB LLC, Pfizer Inc., Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Sunovion Pharmaceuticals, Tal Medical, and Theracos, Inc. Dr. Papakostas has served (not currently) on the speaker’s bureau for BristolMyersSquibb Co and Pfizer, Inc. (* Asterisk denotes activity undertaken on behalf of Massachusetts General Hospital.) Dr. Dording has no relevant financial interests. Dr. Yeung has no relevant financial interests. Dr. Cusin has received speaking and consulting fees from Janssen, Takeda, Boehringer, Alkermes. –Equity: None. –Royalty/patent: PCT/US15/56192; 070919.00032 Acyclic cucurbit[N]uril type molecular containers to treat intoxication and substance abuse. Dr. Ludington has no relevant financial interests. Mr. Bernard has no relevant financial interests. Dr. Fava reports 3-year disclosures as below: All disclosures can be view on line at: http://mghcme.org/faculty/faculty-detail/maurizio_fava; Research Support: Abbott Laboratories; Acadia Pharmaceuticals; Alkermes, Inc.; American Cyanamid;Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; AXSOME Therapeutics; Biohaven; BioResearch; BrainCells Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Cerecor; Clarus Funds; Clintara, LLC; Covance; Covidien; Eli Lilly and Company;EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; FORUM Pharmaceuticals; Ganeden Biotech, Inc.; GlaxoSmithKline; Harvard Clinical Research Institute; Hoffman-LaRoche; Icon Clinical Research; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck Inc.; Marinus Pharmaceuticals; MedAvante; Methylation Sciences Inc; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM);National Coordinating Center for Integrated Medicine (NiiCM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Neuralstem, Inc.; NeuroRx; Novartis AG; Organon Pharmaceuticals; Otsuka Pharmaceutical Development, Inc.; PamLab, LLC.; Pfizer Inc.; Pharmacia-Upjohn; Pharmaceutical Research Associates., Inc.; Pharmavite® LLC; PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Sanofi-Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute (SMRI); Synthelabo; Taisho Pharmaceuticals; Takeda Pharmaceuticals; Tal Medical; VistaGen; Wyeth-Ayerst Laboratories; Advisory Board/ Consultant: Abbott Laboratories; Acadia; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Avanir Pharmaceuticals; AXSOME Therapeutics; Bayer AG; Best Practice Project Management, Inc.; Biogen; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; Boehringer Ingelheim; Boston Pharmaceuticals; BrainCells Inc; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Cerecor; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co. Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; Forum Pharmaceuticals; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; Indivior; i3 Innovus/Ingenis; Intracellular; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; Marinus Pharmaceuticals; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Navitor Pharmaceuticals, Inc.; Nestle Health Sciences; Neuralstem, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG; Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Osmotica; Otsuka Pharmaceuticals; Pamlab, LLC.; Pfizer Inc.; PharmaStar; Pharmavite® LLC.; PharmoRx Therapeutics; Polaris Partners; Praxis Precision Medicines; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; PPD; Purdue Pharma; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Relmada Therapeutics, Inc.; Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; Sanofi-Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering-Plough Corporation; Shenox Pharmaceuticals; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Taisho Pharmaceuticals; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex; Teva Pharmaceuticals; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Usona Institute,Inc.; Vanda Pharmaceuticals, Inc.; Versant Venture Management, LLC; VistaGen; Speaking/Publishing: Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource,Corp.; Wyeth-Ayerst Laboratories.; Stock/Other Financial Options: Equity Holdings: Compellis; PsyBrain, Inc.; Royalty/patent, other income: Patents for Sequential Parallel Comparison Design (SPCD), licensed by MGH to Pharmaceutical Product Development, LLC (PPD) (US_7840419, US_7647235, US_7983936, US_8145504, US_8145505); and patent application for a combination of Ketamine plus Scopolamine in Major Depressive Disorder (MDD), licensed by MGH to Biohaven. Patents for pharmacogenomics of Depression Treatment with Folate (US_9546401, US_9540691).; Copyright for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), Symptoms of Depression Questionnaire (SDQ), and SAFER; Lippincott, Williams & Wilkins; Wolkers Kluwer; World Scientific Publishing Co. Pte.Ltd. Dr. Mischoulon has received research support from Nordic Naturals. He has provided unpaid consulting for Pharmavite LLC and Gnosis USA, Inc. He has received honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy, Blackmores, Harvard Blog, and PeerPoint Medical Education Institute, LLC. He has received royalties from Lippincott Williams & Wilkins for published book “Natural Medications for Psychiatric Disorders: Considering the Alternatives.”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alpert JE, Papakostas G, Mischoulon D, Worthington JJ, Petersen T, Mahal Y, Burns A, Bottiglieri T, Nierenberg AA, Fava M, 2004. S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J. Clin. Psychopharmacol 24, 661–664. https://www.ncbi.nlm.nih.gov/pubmed/15538131 [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Forte A, Selle V, Sim K, Tondo L, Undurraga J, Vázquez GH, 2017. Morbidity in Depressive Disorders. Psychother. Psychosom 86, 65–72. 10.1159/000448661 [DOI] [PubMed] [Google Scholar]
- Bottiglieri T, Godfrey P, Flynn T, Carney MW, Toone BK, Reynolds EH, 1990. Cerebrospinal fluid S-adenosylmethionine in depression and dementia: effects of treatment with parenteral and oral S-adenosylmethionine. J. Neurol. Neurosurg. Psychiatry 53, 1096–1098. 10.1136/jnnp.53.12.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T, Fava M, 2010. The future of psychopharmacology of depression. J. Clin. Psychiatry 71, 971–975. 10.4088/JCP.10m06223blu [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, Egger M, Takeshima N, Hayasaka Y, Imai H, Shinohara K, Tajika A, Ioannidis JPA, Geddes JR, 2018. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet Lond. Engl 391, 1357–1366. 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical power analysis for the behavioral sciences (2nd ed.). Erlbaum, Hillsdale, NJ: 10.4324/9780203771587 [DOI] [Google Scholar]
- Crawford AA, Lewis S, Nutt D, Peters TJ, Cowen P, O’Donovan MC, Wiles N, Lewis G, 2014. Adverse effects from antidepressant treatment: randomised controlled trial of 601 depressed individuals. Psychopharmacology (Berl.) 231, 2921–2931. 10.1007/s00213-014-3467-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Berardis D, Marini S, Serroni N, Rapini G, Iasevoli F, Valchera A, Signorelli M, Aguglia E, Perna G, Salone A, Di Iorio G, Martinotti G, Di Giannantonio M, 2013. S-Adenosyl-L-Methionine augmentation in patients with stage II treatment-resistant major depressive disorder: an open label, fixed dose, single-blind study. ScientificWorldJournal 2013, 204649 10.1155/2013/204649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Berardis D, Orsolini L, Serroni N, Girinelli G, Iasevoli F, Tomasetti C, de Bartolomeis A, Mazza M, Valchera A, Fornaro M, Perna G, Piersanti M, Di Nicola M, Cavuto M, Martinotti G, Di Giannantonio M, 2016. A comprehensive review on the efficacy of S-Adenosyl-L-methionine in Major Depressive Disorder. CNS Neurol. Disord. Drug Targets 15, 35–44. https://www.ncbi.nlm.nih.gov/pubmed/26295824 [DOI] [PubMed] [Google Scholar]
- First BM, Spitzer RL, Gibbon M, Williams JBW, 1995. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID I/P) Biometrics Research Department, New York State Psychiatric Institute, NY. [Google Scholar]
- Guy W (ed), 1976. ECDEU Assessment Manual for Psychopharmacology, revised DHEW Pub. No. (ADM) 76–338. National Institute of Mental Health, Rockville, MD. [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy ML, Coulter I, Morton SC, Favreau J, Venuturupalli S, Chiappelli F, Rossi F, Orshansky G, Jungvig LK, Roth EA, Suttorp MJ, Shekelle P, 2003. S-adenosyl-L-methionine for treatment of depression, osteoarthritis, and liver disease. Evid. Rep. Technol. Assess (Summ.) 1–3. https://www.ncbi.nlm.nih.gov/pubmed/12899148 [PMC free article] [PubMed] [Google Scholar]
- Iovieno N, Dalton ED, Fava M, Mischoulon D, 2011. Second-tier natural antidepressants: review and critique. J. Affect. Disord 130, 343–357. 10.1016/j.jad.2010.06.010 [DOI] [PubMed] [Google Scholar]
- Li Q, Cui J, Fang C, Zhang X, Li L, 2016. S-adenosylmethionine Administration Attenuates Low Brain-Derived Neurotrophic Factor Expression Induced by Chronic Cerebrovascular Hypoperfusion or Beta Amyloid Treatment. Neurosci. Bull 32, 153–161. 10.1007/s12264-016-0023-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischoulon D, Price LH, Carpenter LL, Tyrka AR, Papakostas GI, Baer L, Dording CM, Clain AJ, Durham K, Walker R, Ludington E, Fava M, 2014. A double-blind, randomized, placebo-controlled clinical trial of S-adenosyl-L-methionine (SAMe) versus escitalopram in major depressive disorder. J. Clin. Psychiatry 75, 370–376. 10.4088/JCP.13m08591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischoulon D, Rapaport MH, 2019. Current Role of Herbal and Natural Preparations. Handb. Exp. Pharmacol 250, 225–252. 10.1007/164_2018_152 [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, McLean NE, Alpert JE, Worthington JJ, Rosenbaum JF, Fava M, 1995. Early nonresponse to fluoxetine as a predictor of poor 8-week outcome. Am. J. Psychiatry 152, 1500–1503. 10.1176/ajp.152.10.1500 [DOI] [PubMed] [Google Scholar]
- Papakostas GI, 2009. Evidence for S-adenosyl-L-methionine (SAM-e) for the treatment of major depressive disorder. J. Clin. Psychiatry 70 Suppl 5, 18–22. 10.4088/JCP.8157su1c.04 [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M, 2010. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am. J. Psychiatry 167, 942–948. 10.1176/appi.ajp.2009.09081198 [DOI] [PubMed] [Google Scholar]
- Park LT, Zarate CA, 2019. Depression in the Primary Care Setting. N. Engl. J. Med 380, 559–568. 10.1056/NEJMcp1712493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posternak MA, Baer L, Nierenberg AA, Fava M, 2011. Response rates to fluoxetine in subjects who initially show no improvement. J. Clin. Psychiatry 72, 949–954. 10.4088/JCP.10m06098 [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Markowitz JS, Ocepek-Welikson K, Wager SS, 1992. General versus systematic inquiry about emergent clinical events with SAFTEE: implications for clinical research. J. Clin. Psychopharmacol 12, 3–10. https://www.ncbi.nlm.nih.gov/pubmed/1552037 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH, 1996. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol. Med 26, 477–486. https://www.ncbi.nlm.nih.gov/pubmed/8733206 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M, 2006. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry 163, 1905–1917. 10.1176/ajp.2006.163.11.1905 [DOI] [PubMed] [Google Scholar]
- Sarris J, Byrne GJ, Bousman C, Stough C, Murphy J, MacDonald P, Adams L, Nazareth S, Oliver G, Cribb L, Savage K, Menon R, Chamoli S, Berk M, Ng C, Mischoulon D, 2018. Adjunctive S-adenosylmethionine (SAMe) in treating non-remittent major depressive disorder: An 8-week double-blind, randomized, controlled trial,. Eur. Neuropsychopharmacol 28, 1126–1136. 10.1016/j.euroneuro.2018.07.098 [DOI] [PubMed] [Google Scholar]
- Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, Ng CH, 2016. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am. J. Psychiatry 173, 575–587. 10.1176/appi.ajp.2016.15091228 [DOI] [PubMed] [Google Scholar]
- Sharma A, Gerbarg P, Bottiglieri T, Massoumi L, Carpenter LL, Lavretsky H, Muskin PR, Brown RP, Mischoulon D, as Work Group of the American Psychiatric Association Council on Research, 2017. S-Adenosylmethionine (SAMe) for Neuropsychiatric Disorders: A Clinician-Oriented Review of Research. J. Clin. Psychiatry 78, e656–e667. 10.4088/JCP.16r11113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, South C, Jha MK, Rush AJ, Cao J, Kurian B, Phillips M, Pizzagalli DA, Trombello JM, Oquendo MA, Cooper C, Dillon DG, Webb C, Grannemann BD, Bruder G, McGrath PJ, Parsey R, Weissman M, Fava M, 2018. A Novel Strategy to Identify Placebo Responders: Prediction Index of Clinical and Biological Markers in the EMBARC Trial. Psychother. Psychosom. 87, 285–295. 10.1159/000491093 [DOI] [PMC free article] [PubMed] [Google Scholar]