Abstract
Background:
We report our intermediate-term results following Norwood procedure, including use of an interstage inpatient management strategy for high-risk patients, and sought to create a predictive model for probability of discharge.
Methods:
A single-site retrospective review was conducted for all patients undergoing Norwood from 2006–2016 (n=177). We compared those discharged home with those who either remained hospitalized until Glenn or died prior to Norwood discharge. Multivariable logistic regression was used to develop a predictive model for discharge.
Results:
During the study period, 120(68%) patients were discharged home, 45(25%) remained hospitalized and 12(7%) died before Glenn (median age: 71 days). Interstage survival for those discharged after Norwood was 100%. Longitudinal survival for the cohort was 86%, 81% and 77% at 1, 5, and 10 years. Ten year survival was significantly greater for the discharged group compared to the interstage inpatients (86% vs. 56%, p<0.0001). A reduced predictive model of discharge included lower gestational age (OR=0.95), lower median income for zip code (OR=0.4), lower birth weight-for-age z-score (OR=0.56), longer cardiopulmonary bypass time (OR=0.45), and BT shunt (OR=0.32).
Conclusions:
Survival up to 10 years after Norwood procedure is good using a strategy of inpatient care for a subset of high-risk patients to mitigate home interstage mortality. A probabilistic model used after Norwood was able to predict interstage discharge with good accuracy, but will require external validation to ensure generalizability. Further work is also needed to determine optimal palliative pathways for the high-risk patients due to the notable attrition beyond successful bidirectional Glenn.
The Norwood procedure (NP) is the most common initial palliation for hypoplastic left heart syndrome (HLHS) and similar single ventricle variants with hypoplasia of the aorta. There have been notable improvements in post-operative survival following NP(1,2) and in survival during the first interstage period, the time period between NP discharge and bidirectional Glenn (BDG)(3). Improvements are associated with refinements in surgical techniques, improved post-operative care, comprehensive monitoring at home and a decrease in the time at risk(4–6). Some patients are deemed to be at exceptionally high risk for interstage mortality despite home monitoring and are managed as inpatients until BDG(5,7).
Since 2006, the Children’s Hospital of Wisconsin has employed a strategy of inpatient interstage care for those patients deemed highest risk. As little data exists on the intermediate or long term survival after the NP in the recent era, we present intermediate term outcomes for the cohort of patients undergoing NP since that time. Additionally, we created a predictive model that can be used shortly after NP to assess likelihood of interstage discharge.
Patients and Methods
Patients
After Children’s Hospital of Wisconsin Institutional Review Board approval and waiver of consent, we performed a retrospective chart review for all patients undergoing a NP (no hybrid palliations were done) between 1/1/2006 and 12/31/2015. We excluded 2 patients who received interstage care and subsequent palliation at a referring institution. For statistical analysis, all deaths prior to NP discharge were presumed to have high risk characteristics and were included in the inpatient interstage group. Those undergoing heart transplant or biventricular repair after NP remained in the cohort to assess longer term survival. Patients discharged home after NP were compared to those remaining as inpatients during the interstage period.
Variable Definitions
Pre-surgical high-risk status was assigned to patients who had moderate or greater ventricular dysfunction, moderate or greater atrioventricular valve insufficiency, a highly restrictive (mean gradient > 8 mmHg) or intact atrial septum, or obstructed anomalous pulmonary venous return based on review of pre-operative echo reports. Maternal zip code at the time of delivery was used to obtain the median annual income and percent below the poverty line for that zip code based on 2010 United States census bureau data(8). The vasoactive inotrope score (VIS)(9) was calculated at 24 hours and 7 days after return to the cardiac intensive care unit following the NP.
General practices
Delayed sternal closure after NP was routine. Shunt type was determined by randomization as part of the Single Ventricle Reconstruction trial for part of the time period (2006–2008)(10), or at the discretion of the surgeon. Intraoperative support and postoperative management was standardized as previously described(11). For each patient, the decision for inpatient interstage management was made based on clinical judgment, without defined criteria, by the interstage team which included cardiac intensivists familiar with each patient’s perioperative course and physiologic challenges. Those discharged home had comprehensive monitoring as previously published(4,5).
Statistical analysis
Patient and clinical data were described using medians with interquartile ranges or frequencies with percent of total unless otherwise specified. Differences according to discharge status were tested by the Wilcoxon rank-sum test and Fisher’s exact test. Kaplan-Meier survival curves were created with the log rank test used to evaluate differences in survival between these groups. A competing risks analysis was also performed with heart transplant or Fontan completion as the outcomes modeled as competing risks for death. A predictive model for the probability of discharge during the interstage period was developed using logistic regression as implemented in the rms package(12) (version 5.1.1) in R (version 3.4.0). Restricted cubic splines (three knots) for continuous predictors and two way interactions were retained where the Wald Chi-Square p-value was < 0.3. Penalized and reduced models were developed using penalized regression and by manual selection of predictors associated with discharge status at p<0.05 (two-sided) in the full model. The optimum AIC based on a grid search was selected as the penalty. Optimism corrected estimates of model performance were obtained using bootstrap resampling (n=1000). Missing data were multiply imputed (n=10 datasets) using the Hmisc:: aregImpute(13) function with predictive mean matching. All variables included in the full model were used to inform the imputations. Predictive models were fit to the multiply imputed datasets as appropriate. Odds ratios (OR) and 95% confidence intervals for continuous predictors are presented using a contrast of the 75th versus the 25th percentile of the observed data to facilitate interpretation. The interquartile range OR was chosen as it provides a consistent cut-point for comparisons within meaningful ranges of the observed data. A nomogram was created from the reduced model using the rms::nomogram function.
Results
Cohort characteristics
The cohort included 177 patients over10 years. Demographic and diagnostic data are shown in Table 1. Most patients were prenatally diagnosed (n=141, 79.7%) with 21 (11.9%) born at <37 weeks gestation. Median birth weight was 3105 grams (range: 1240–4430) with a median birth weight-for-age z score of −0.37 (range: −5.2 to2.4). Median age at NP was 7 days (range: 1–128) with 86 (48.6%) having NP with a modified Blalock-Taussig (BT) shunt and 91 (51.4%) with a right ventricle to pulmonary artery (RV-PA) shunt.
Table 1: Cohort demographics and pre-Norwood management characteristics.
AA aortic atresia, AS aortic stenosis, AVSD atrioventricular septal defect, DILV double inlet left ventricle, DORV double outlet right ventricle, HLHS hypoplastic left heart syndrome, MA mitral atresia, MS mitral stenosis, NP Norwood procedure.
| Cohort characteristics | |
|---|---|
| N (%) | |
| MALE GENDER | 99 (55.9%) |
| RACE/ETHNICITY | |
| CAUCASIAN/WHITE | 128 (72.3%) |
| AFRICAN AMERICAN/BLACK | 19 (10.7%) |
| HISPANIC | 19 (10.7%) |
| OTHER | 11 (6.3%) |
| DIAGNOSIS | |
| HLHS (MA/AA) | 55 (31.1%) |
| HLHS (MS/AA) | 24 (13.6%) |
| HLHS (MS/AS) | 40 (22.6%) |
| DORV | 17 (9.6%) |
| DILV | 15 (8.5%) |
| TRICUSPID ATRESIA | 10 (5.7%) |
| AVSD | 11 (6.2%) |
| OTHER | 5 (2.8%) |
| MECHANICAL VENTILATION PRE-OPERATIVELY | 61 (34.5%) |
| PRE-OPERATIVE INOTROPES | 44 (25%) |
| INTERVENTION PRIOR TO NP | 20 (11.3%) |
Outcomes prior to BDG
From birth to time of BDG, 33 patients (18.6%) required ECMO, predominantly for low cardiac output, and 23 patients (n=13%) required cardiopulmonary resuscitation (CPR). Re-intubation after planned extubation following NP was observed in 40 (23.3%) patients and re-intervention on residual anatomic lesions, either by catheterization or surgery, occurred in 57 (32.2%) prior to BDG. These interventions were on the shunt (76.8%), the aortic arch (7.1%), pulmonary arteries (3.6%) or on multiple areas (7.1%). The median age at intervention was 16 days (range 1–188 days).
Predictors of discharge prior to the BDG procedure
Home monitoring during the interstage period occurred for 120 (68%) patients. Forty-five (25%) patients were managed as inpatients until BDG. Twelve (7%) patients died prior to discharge or BDG and were included in the inpatient group for comparisons. Differences between home monitored and inpatient groups are shown in Table 2. Comparisons between patients that died prior to discharge and those that remained inpatient until BDG are presented in Table 3 with details of the patients that died in Table 4.
Table 2: Univariate comparison of demographic and clinical characteristics by groups based on discharge status. Data presented as median (IQR) or n (%) with P-values generated by Wilcoxon test for continuous variables and Fisher’s exact test for categorical variables.
AA aortic atresia, AS aortic stenosis, AVSD atrioventricular septal defect, BDG bidirectional Glenn, BT Blalock-Taussig, DILV double inlet left ventricle, DORV double outlet right ventricle, HLHS hypoplastic left heart syndrome, MA mitral atresia, MS mitral stenosis, NP Norwood procedure, RV-PA right ventricle to pulmonary artery.
| Univariate inpatient to discharge group comparison | |||
|---|---|---|---|
| INPATIENT (N=57) |
DISCHARGED (N=120) |
P-VALUE | |
| MATERNAL AGE (YEARS) | 29 (25–32) | 28 (23–33) | 0.89 |
| GESTATION (WEEKS) | 38.4 (37.1–39) | 38.8 (38–39.3) | 0.05 |
| MEDIAN ANNUAL INCOME FOR ZIP CODE | $42,598 (36819–60036) | $53,950 (44724–69293) | <0.01 |
| % BELOW POVERTY LINE FOR ZIP CODE | 17.2 (9.7–24.5) | 9.7 (7.9) | <0.01 |
| BIRTH WEIGHT-FOR-AGE Z-SCORE | −0.5 (−1.4–0.3) | −0.3 (1.3) | 0.10 |
| PRE-SURGICAL HIGH RISK | 17 (29.8) | 15 (12.5) | 0.01 |
| INOTROPES PRIOR TO NP | 0.35 | ||
| NO | 40 (70.2) | 92 (76.7) | |
| YES | 17 (29.8) | 27 (22.5) | |
| MISSING | 0 (0.0) | 1 (0.8) | |
| MECHANICAL VENTILATION PRIOR TO NP | 27 (47.4) | 34 (28.3) | 0.02 |
| CARDIAC DIAGNOSIS | 0.11 | ||
| HLHS (MA/AA) | 18 (31.6) | 37 (30.8) | |
| HLHS (MS/AA) | 5 (8.8) | 19 (15.8) | |
| HLHS (MS/AS) | 13 (22.8) | 27 (22.5) | |
| DORV | 10 (17.5) | 7 (5.8) | |
| DILV | 2 (3.5) | 13 (10.8) | |
| TRICUSPID ATRESIA | 2 (3.5) | 8 (6.7) | |
| ASVD | 4 (7.0) | 7 (5.8) | |
| OTHER | 3 (5.3) | 2 (1.7) | |
| GENETIC SYNDROME PRESENT | 9 (15.8) | 10 (8.3) | 0.19 |
| CPR FOLLOWING NP | 15 (26.3) | 8 (6.7) | <0.01 |
| ECMO PRIOR TO BDG | 26 (45.6) | 7 (5.8) | <0.01 |
| CATHETER OR SURGICAL REINTERVENTION | 28 (49.1) | 29 (24.2) | <0.01 |
| TYPE OF SHUNT AT NP | |||
| BT SHUNT | 35 (61.4) | 51 (42.5) | 0.02 |
| RV-PA SHUNT | 22 (38.6) | 69 (57.5) | |
| NP CARDIOPULMONARY BYPASS TIME | 190 (160–272) | 166 (145–200) | <0.01 |
| DAYS ON VENTILATOR AFTER NP | 17 (9–33) | 7 (5–11) | <0.01 |
| CHANGE IN INOTROPE SCORE FROM 24 HOURS TO 7 DAYS | −9 (−13–0) | −10 (−15–−7) | 0.01 |
| DURATION OF OPEN CHEST AFTER NP | 9 (4–17) | 3 (2–6) | <0.01 |
| AGE AT BDG (DAYS) | 107 (92–142) | 120 (103–139) | 0.17 |
Table 3: Univariate comparison of pre-operative and operative factors between those who died prior to discharge and those remaining inpatient until bidirectional Glenn. Data presented as median (IQR) or n (%).
AA aortic atresia, AS aortic stenosis, AVSD atrioventricular septal defect, BDG bidirectional Glenn, BT Blalock-Taussig, DILV double inlet left ventricle, DORV double outlet right ventricle, HLHS hypoplastic left heart syndrome, MA mitral atresia, MS mitral stenosis, NP Norwood procedure, RV-PA right ventricle to pulmonary artery.
| Univariate death to inpatient group comparison | |||
|---|---|---|---|
| INPATIENT (N=45) |
DEATH (N=12) | P- VALUE | |
| MATERNAL AGE (YEARS) | 29 (25–32) | 30 (25–34) | 0.78 |
| GESTATION (WEEKS) | 38.8 (37.7–39) | 37.1 (35–38.2) | <0.01 |
| MEDIAN ANNUAL INCOME FOR ZIP CODE | $42,599 (39825–58408) | $45433 (33005–61321) | 0.71 |
| % BELOW POVERTY LINE FOR ZIP CODE | 17.3 (10.3–24.3) | 16.5 (9–29.9) | 0.71 |
| BIRTH WEIGHT-FOR-AGE Z-SCORE | −0.3 (−1–0.4) | −1.5 (−2.7–−0.3) | 0.03 |
| PRE-SURGICAL HIGH RISK | 11 (24.4) | 6 (50) | 0.15 |
| INOTROPES PRIOR TO NP | 12 (26.7) | 5 (41.7) | 0.48 |
| MECHANICAL VENTILATION PRIOR TO | 18 (40) | 9 (75) | 0.05 |
| NP | |||
| CARDIAC DIAGNOSIS | 0.48 | ||
| HLHS (MA/AA) | 16 (35.6) | 2 (16.7) | |
| HLHS (MS/AA) | 4 (8.9) | 1 (8.3) | |
| HLHS (MS/AS) | 11 (24.4) | 2 (16.7) | |
| DORV | 6 (13.3) | 4 (33.3) | |
| DILV | 2 (4.4) | 0 (0) | |
| TRICUSPID ATRESIA | 1 (2.2) | 1 (8.3) | |
| ASVD | 3 (6.7) | 1 (8.3) | |
| OTHER | 2 (4.4) | 1 (8.3) | |
| GENETIC SYNDROME PRESENT | 8 (17.8) | 1 (8.3) | 0.67 |
| CPR FOLLOWING NP | 11 (4.4) | 4 (33.3) | 0.71 |
| ECMO PRIOR TO BDG | 15 (33.3) | 11 (91.7) | <0.01 |
| CATHETER OR SURGICAL REINTERVENTION | 20 (44.4) | 8 (66.7) | 0.21 |
| TYPE OF SHUNT AT NP | 0.04 | ||
| BT SHUNT | 31 (68.9) | 4 (33.3) | |
| RV-PA SHUNT | 14 (31.1) | 8 (67.7) | |
| NP CARDIOPULMONARY BYPASS TIME | 183 (156–246) | 255 (202–288) | 0.06 |
Table 4:
Details of pre-Norwood risk factors for patients that died prior to Glenn
| Age at death (days) | Risk factors pre-Norwood |
|---|---|
| 259 | Prematurity (30 weeks), 1.2 kg, post-natal diagnosis, tracheoesophageal fistula, total anomalous pulmonary venous return (unobstructed) |
| 132 | Left coronary from the pulmonary artery |
| 29 | Post-natal diagnosis; restrictive atrial septum |
| 9 | Twin gestation, prematurity (36 weeks), 1.6 kg, restrictive atrial septum, circumflex coronary from right pulmonary artery |
| 13 | Obstructed partial anomalous pulmonary venous return, circumflex coronary from main pulmonary artery, right diaphragm eventration |
| 80 | Restrictive atrial septum |
| 62 | Premature (32 weeks), 2.1 kg |
| 44 | Restrictive atrial septum |
| 87 | None |
| 84 | Prematurity (35 weeks), 2.3 kg |
| 94 | ≥Moderate tricuspid regurgitation |
| 32 | Prematurity (35 weeks), 2.2 kg |
All those discharged survived to BDG. There were 86 unplanned readmissions in 63 (53%) patients with a median total hospitalized days during the interstage period of 8 days (range: 1–89). The median time at home prior to BDG was 60.5 days (range: 3–296). Median age at BDG was 120 days (range: 51–390) for discharged patients versus 107 (range: 41–214) for those remaining hospitalized who survived to BDG (p=0.17). Operative mortality after BDG for the entire cohort was 3.0%, with all deaths observed in inpatient interstage patients (Figure 1). As a cost comparison, the current cost of inpatient admission alone is $7056 per week ($1008 per day) versus outpatient costs of $552 per week, comprised of a weekly clinic visit ($440), home scale ($42 per week) and home pulse oximeter ($70 per week) rental fees.
Figure 1:
Flow diagram of patient outcomes following Norwood Procedure.
Predictive models were developed to evaluate the likelihood of discharge prior to BDG (Table 5). The Brier score (B) and concordance statistic (c) were examined as measures of model performance. Values were B=0.17 and c=0.78 and Bc=0.18 and cc=0.76 for the reduced model. The calibration intercept and slope for the agreement between observed outcomes and predicted probabilities from the reduced model were 0.07 and 0.88, respectively. The nomogram for likelihood of discharge can be seen in Figure 2. Application of the model would be expected in the 2 weeks following NP.
Table 5: Results of reduced predictive model with odds ratio for discharge home obtained by logistic regression. Missing data was multiply imputed (n=10 datasets). The reference and contrast columns provide the reference group and contrast used in the calculation of the odds ratio and 95% confidence intervals. Odds ratios for continuous variables reflect the exponentiation of the log-odds when contrasting the observed values at the 75th versus the 25th percentile for each predictor (interquartile odds ratios).
BT Blalock-Taussig, NP Norwood procedure, RV-PA right ventricle to pulmonary artery.
| Multivariable predictors of discharge | |||
|---|---|---|---|
| Ref. | Contrast | OR (95% CI) | |
| Gestation (weeks) | 39.1 | 37.8 | 0.95 (0.66; 1.37) |
| Median annual income for zip code | $65,764 | $41,426 | 0.4 (0.23; 0.7) |
| Birth weight-for-age z-score | 0.49 | −0.87 | 0.56 (0.33; 0.96) |
| Cardiopulmonary bypass time at NP (min) | 148 | 209 | 0.45 (0.30; 0.66) |
| Type of shunt at Norwood | RV-PA shunt | BT shunt | 0.32 (0.15; 0.69) |
Figure 2:
Nomogram for likelihood of discharge from Children’s Hospital of Wisconsin following Norwood procedure. To use this nomogram each of the 5 factors is plotted to generate points from line 1. These points are added together and plotted on the ‘total points’ line which corresponds with a probability of discharge on the final line.
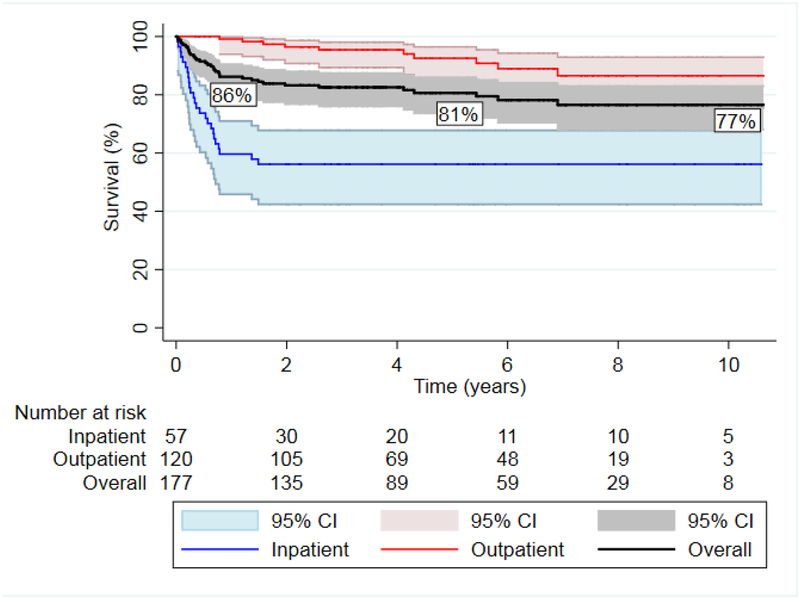
Intermediate survival
Overall survival was 86% at 1 year, 81% at 5 years and 77% at 10 years. Survival to 1 year of age, contingent on survival to BDG, was 75.6% (95% CI 60.2–85.7) in the inpatient interstage group and 99.1% (95% CI 93.9–99.9) in the home monitored group (p<0.001).
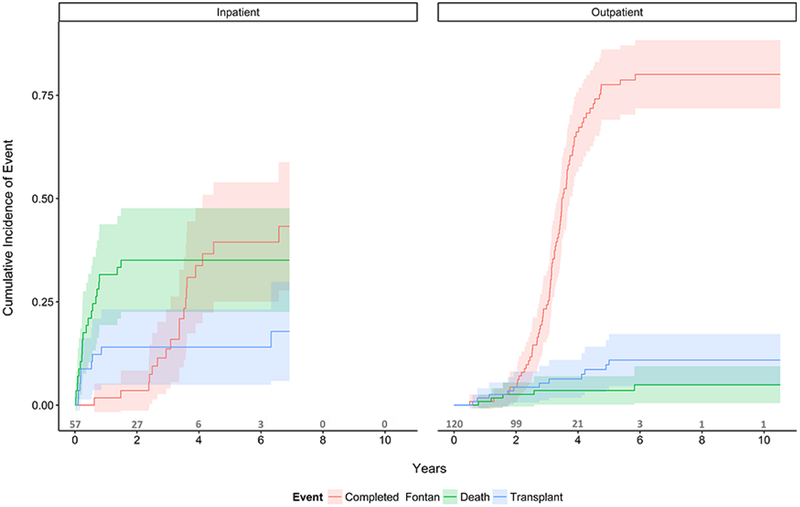
Kaplan-Meier survival curves for the overall cohort as well as the inpatient and home interstage groups can be seen in Figure 3. There was a significant difference in long-term survival between groups (56% vs. 86% at 10 years, p<0.0001). Competing risk analysis results can be seen in figure 4 for the inpatient and discharged groups.
Figure 3:
Kaplan-Meier survival curve after Norwood palliation for all patients and separated by those remaining inpatient from Norwood to Glenn and those discharged home. Overall survival was 86% at 1 year, 81% at 5 years and 77% at 10 years after Norwood procedure with a significant difference between the inpatient and home interstage groups (logrank p < 0.001).
Figure 4:
Competing risks analysis with outcomes of heart transplantation, death and Fontan completion as the outcomes between those managed as inpatient and those managed as outpatients during the interstage period.
Comment
In our aggregate patient population, outcomes after the NP are very good, with a 77% survival at 10 years. Currently, there is a paucity of longer term reporting of outcomes for this population, as most studies have focused on early/hospital mortality, interstage mortality or long term outcomes of those surviving to Fontan. Recently, Newburger et al. published the 6 year outcomes of the Single Ventricle Reconstruction (SVR) trial with 190 deaths from the origin cohort of 549 patients, resulting in a survival of 65.4% assuming survival of the 22 patients undergoing heart transplant(14). Siffel et al. reported improvement over time but in their most recent era (1999–2005) 10 year survival was 43%(15). Our observed higher survival at 10 year is likely a function of several factors including increased vigilance through home monitoring, having a dedicated single ventricle care team, and selective use of inpatient management to BDG for the highest risk patients.
This study presents a novel strategy of selective inpatient interstage management to minimize mortality between the Norwood and BDG procedures in addition to a comprehensive interstage home monitoring program. Recent studies report interstage mortality rates of 5–12%(3,7,16) suggesting that interstage home monitoring alone has not eliminated interstage mortality. Our results indicate that we were able to correctly identify those at highest risk for mortality and submit them to inpatient interstage management, thus leading to 100% interstage survival to BDG in those discharged home after NP. From these data, we were able to identify a predictive model comprised of relatively few factors that may be used to prospectively assess the likelihood of discharge home. Though our nomogram will require external validation, we are hopeful it can be used early in the post-operative period to assess the likelihood of individual patient discharge. This will be useful in risk assessment and guiding care team and parental expectations.
The variables included in this predictive model have previously been identified as risk factors for poor outcomes in this patient population. Lower gestational age has repeatedly been shown to be a risk factor for both hospital and interstage mortality after NP and the hybrid alternative(7,17,18). This is likely related to an increased risk of comorbid conditions as well as the association of prematurity with birth weight which has also been demonstrated to be a risk factor for mortality(18,19). The difficulties of the NP in low birth weight infants is well documented(20,21) and likely relates to technical challenges of the surgery as well as shunt to patient size mismatch.
Median income for maternal zip code was similar to the findings from the SVR trial, where census block poverty level was a risk factor for interstage mortality(7) and lower socioeconomic status had a linear correlation with 1 year transplant-free survival(22). This measure is likely representative of generalized health disparities rather than just poverty as socioeconomic status has been closely linked to health care literacy(23). These disparities have been recognized in the wider congenital heart disease population with higher mortality rates and more health care utilization after congenital heart surgery in children from lower income neighborhoods, even when adjusting for race, payer, and care center(24). Bypass time is likely both a marker and a cause of high risk status. Those with more complex anatomic subtypes or additional lesion have longer bypass times, but the impact of increased bypass time is also well known(25,26). Finally, shunt selection has been greatly debated even after the SVR trial in which there was significantly lower survival at 14 months of age in the BT shunt group with the greatest difference in mortality between groups occurring during the first interstage period(10). Despite early differences in mortality between shunt groups, similar survival was noted at 3 and 6 years of age(14,27). Our data also shows similar early survival with BT shunts compared to RV-PA conduits(28). These results might in fact be due to inpatient interstage management of high risk or seemingly physiologically more vulnerable patients, more commonly identified as those with BT shunts in our series.
While a full cost analysis is certainly beyond the scope of this manuscript, we did evaluate the average cost of admission and compare that to the cost of weekly interstage clinic visits and home monitoring equipment. While this cost difference is substantial at $6500 per week it is unknown what the impact on prevention of presentation in extremis and quality-adjusted life-years saved really is with an inpatient interstage. Further work is required to fully evaluate the cost of an inpatient interstage but given the cost difference, inpatient management should be reserved for those at highest risk only.
The finding of continued increased risk for mortality in the inpatient interstage group beyond BDG is also interesting. While the increased operative mortality at BDG should be anticipated as these represent higher risk patients, the continued divergence of survival and vast difference in survival at 1 year for those surviving to BDG is concerning. Until this point, intensive monitoring of single ventricle patients ended at the time of BDG. Our data suggest that there is a vulnerable group that may benefit from more intensive outpatient surveillance in a specialized clinic, and/or potentially benefit from earlier evaluation for cardiac transplantation. Further investigation is needed to better understand the intermediate mortality during the second interstage period.
This study is limited by being a single center retrospective study. There also may be relevant factors considered in the decision of whether to discharge patients that were not available or collected for this study. Additionally, our criteria for discharge may not be generalizable outside of our center. Our strategy of including the early deaths without knowledge of what their interstage status would have been increases the risk of not finding a difference between groups that may actually exist, a type II error.
In conclusion, ten year survival after the Norwood procedure is good. A strategy of inpatient interstage monitoring of high risk patients can be used as part of a comprehensive management program to effectively mitigate interstage mortality, but these patients remain higher risk even after successful bidirectional Glenn. Application of the criteria for discharge presented here requires further study to assess generalizability. Potential strategies to reduce mortality after BDG in a vulnerable group that remains high risk despite improving the circulatory status require investigation.
Abbreviations
- NP
Norwood procedure
- HLHS
Hypoplastic left heart syndrome
- BDG
Bidirectional Glenn
- BT
Blalock-Tausssig
- VIS
Vasoactive inotrope score
- RV-PA
Right ventricle to pulmonary artery
- CPR
Cardiopulmonary resuscitation
- SVR
Single Ventricle Reconstruction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dean PN, Hillman DG, McHugh KE, Gutgesell HP. Inpatient costs and charges for surgical treatment of hypoplastic left heart syndrome. Pediatrics 2011;128:e1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Czosek RJ, Anderson JB, Heaton PC, Cassedy A, Schnell B, Cnota JF. Staged palliation of hypoplastic left heart syndrome: trends in mortality, cost, and length of stay using a national database from 2000 through 2009. AmJCardiol 2013;111:1792. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JB, Beekman RH 3rd, Kugler JD et al. Improvement in Interstage Survival in a National Pediatric Cardiology Learning Network. CircCardiovascQualOutcomes 2015;8:428. [DOI] [PubMed] [Google Scholar]
- 4.Ghanayem NS, Hoffman GM, Mussatto KA et al. Home surveillance program prevents interstage mortality after the Norwood procedure. JThoracCardiovascSurg 2003;126:1367. [DOI] [PubMed] [Google Scholar]
- 5.Rudd NA, Frommelt MA, Tweddell JS et al. Improving interstage survival after Norwood operation: outcomes from 10 years of home monitoring. JThoracCardiovascSurg 2014;148:1540. [DOI] [PubMed] [Google Scholar]
- 6.Hill GD, Rudd NA, Ghanayem NS, Hehir DA, Bartz PJ. Center Variability in Timing of Stage 2 Palliation and Association with Interstage Mortality: A Report from the National Pediatric Cardiology Quality Improvement Collaborative. Pediatr Cardiol 2016;37:1516–1524. [DOI] [PubMed] [Google Scholar]
- 7.Ghanayem NS, Allen KR, Tabbutt S et al. Interstage mortality after the Norwood procedure: Results of the multicenter Single Ventricle Reconstruction trial. JThoracCardiovascSurg 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.United States Census Bureau American Fact Finder.
- 9.Davidson J, Tong S, Hancock H, Hauck A, da Cruz E, Kaufman J. Prospective validation of the vasoactive-inotropic score and correlation to short-term outcomes in neonates and infants after cardiothoracic surgery. Intensive Care Med 2012;38:1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohye RG, Sleeper LA, Mahony L et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. NEnglJMed 2010;362:1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman GM, Stuth EA, Jaquiss RD et al. Changes in cerebral and somatic oxygenation during stage 1 palliation of hypoplastic left heart syndrome using continuous regional cerebral perfusion. J Thorac Cardiovasc Surg 2004;127:223–33. [DOI] [PubMed] [Google Scholar]
- 12.Rms:Regression modeling strategies. R package version 5.1–1. [Google Scholar]
- 13.Hmisc:Harrell miscellaneous. R package version 4.0–3. [Google Scholar]
- 14.Newburger JW, Sleeper LA, Gaynor JW et al. Transplant-Free Survival and Interventions at 6 Years in the SVR Trial. Circulation 2018;137:2246–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siffel C, Riehle-Colarusso T, Oster ME, Correa A. Survival of Children With Hypoplastic Left Heart Syndrome. Pediatrics 2015;136:e864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hehir DA, Dominguez TE, Ballweg JA et al. Risk factors for interstage death after stage 1 reconstruction of hypoplastic left heart syndrome and variants. JThoracCardiovascSurg 2008;136:94. [DOI] [PubMed] [Google Scholar]
- 17.Cross RR, Harahsheh AS, McCarter R, Martin GR, for the National Pediatric Cardiology Quality Improvement C. Identified mortality risk factors associated with presentation, initial hospitalisation, and interstage period for the Norwood operation in a multi-centre registry: a report from the National Pediatric Cardiology-Quality Improvement Collaborative. CardiolYoung 2013:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sano S, Huang SC, Kasahara S, Yoshizumi K, Kotani Y, Ishino K. Risk factors for mortality after the Norwood procedure using right ventricle to pulmonary artery shunt. AnnThoracSurg 2009;87:178. [DOI] [PubMed] [Google Scholar]
- 19.Tabbutt S, Ghanayem N, Ravishankar C et al. Risk factors for hospital morbidity and mortality after the Norwood procedure: A report from the Pediatric Heart Network Single Ventricle Reconstruction trial. JThoracCardiovascSurg 2012;144:882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsoufi B, McCracken C, Ehrlich A et al. Single ventricle palliation in low weight patients is associated with worse early and midterm outcomes. AnnThoracSurg 2015;99:668. [DOI] [PubMed] [Google Scholar]
- 21.Pizarro C, Davis DA, Galantowicz ME, Munro H, Gidding SS, Norwood WI. Stage I palliation for hypoplastic left heart syndrome in low birth weight neonates: can we justify it? EurJCardiothoracSurg 2002;21:716. [DOI] [PubMed] [Google Scholar]
- 22.Bucholz EM, Sleeper LA, Newburger JW. Neighborhood Socioeconomic Status and Outcomes Following the Norwood Procedure: An Analysis of the Pediatric Heart Network Single Ventricle Reconstruction Trial Public Data Set. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorensen K, Pelikan JM, Rothlin F et al. Health literacy in Europe: comparative results of the European health literacy survey (HLS-EU). EurJPublic Health 2015;25:1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson BR, Fieldston ES, Newburger JW, Bacha EA, Glied SA. Disparities in Outcomes and Resource Use After Hospitalization for Cardiac Surgery by Neighborhood Income. Pediatrics 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meri S, Aronen M, Leijala M. Complement activation during cardiopulmonary bypass in children. Complement 1988;5:46. [DOI] [PubMed] [Google Scholar]
- 26.Kozik DJ, Tweddell JS. Characterizing the inflammatory response to cardiopulmonary bypass in children. AnnThoracSurg 2006;81:S2347. [DOI] [PubMed] [Google Scholar]
- 27.Newburger JW, Sleeper LA, Frommelt PC et al. Transplantation-free survival and interventions at 3 years in the single ventricle reconstruction trial. Circulation 2014;129:2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frommelt MA. Challenges and controversies in fetal diagnosis and treatment: hypoplastic left heart syndrome. ClinPerinatol 2014;41:787. [DOI] [PubMed] [Google Scholar]