Abstract
Household dust is a reservoir of various consumer product chemicals. Thus, characterizing comprehensive chemical profiles of house dust may help improve our understanding of residential chemical exposure. We have previously developed a method for detecting a broad spectrum of chemicals in dust by applying a combination of target, suspect screening, and non-target methods with mass spectrometry preceded by liquid chromatography and gas chromatography. Building upon a previous study that detected 271 compounds in 38 dust samples, we presented concentrations of 144 compounds that were confirmed and quantified by standards in the same set of samples. Ten compounds were measured with median concentrations greater than 10,000 ng/g of dust: cis-hexadec-6-enoic acid, squalene, cholesterol, vitamin E, bis(2-ethylhexyl) phthalate, dioctyl terephthalate, linoleic acid, tricaprylin, tris(1-chloroisopropyl) phosphate, and oxybenzone. We also reviewed in vitro toxicity screening data to identify compounds that were not previously detected in indoor dust but have potential for adverse health effects. Among 119 newly detected compounds, 13 had endocrine disrupting potential and 7 had neurotoxic potential. Toxicity screening data were not available for eight biocides, which may adversely affect health. Our results strive to provide more comprehensive chemical profiles of house dust and identified information gaps for future health studies.
Keywords: concentration, dust, in vitro bioactivity assays, non-target, suspect screening, target
1. Introduction
Thousands of chemicals are currently used in consumer products. Many of the consumer product chemicals have not been studied for exposure potential in the indoor environment where people in developed countries spend most of their time.1 The most studied chemical classes in the indoor environment include pesticides, flame retardants, plasticizers, polycyclic aromatic hydrocarbons (PAHs), and per- and polyfluoroalkyl substances (PFAS).2, 3 Because of potential health concerns over exposures to some of these chemical classes, a large number of alternative chemicals are being introduced into consumer products every year, following legislative activities or advocacy campaigns.4–6 However, exposure and toxicity information needed to evaluate potential human health effects are limited for the alternative chemicals and other chemicals that were not previously measured in indoor environmental media.7
Chemical concentrations in indoor environmental media including air, airborne particles, and settled floor dust have been used to characterize residential exposure to indoor contaminants.2, 8–10 Many consumer product chemicals of current and emerging health concerns are semivolatile organic compounds (SVOCs).2 When released from their original sources, SVOCs are redistributed over time and primarily partitioned to dust and other indoor surfaces.3, 11, 12 When dust concentrations are known but other media concentrations are not measured, partitioning models among dust, gas-phase, and airborne particles can be used to characterize residential chemical exposure.2, 10 Thus, there have been growing efforts in detecting and quantifying SVOCs in house dust.3 However, a complete picture of the chemical fingerprint of dust (i.e., identity and quantity of all chemicals present) is missing, because most previous studies analyzed known chemical classes via a targeted analytical method.2, 13–17 Therefore, development of advanced environmental monitoring methods has emerged as a prominent topic in indoor environmental research in order to detect both known chemical classes and those that were previously not targeted for detection in dust.
Advances in high-resolution mass spectrometry make it possible not only to detect known compounds for which reference standards are available (targets), but also to detect expected compounds using existing databases, libraries, or software matching algorithms (suspects) and even to identify previously unknown compounds (non-targets) through careful examination of high-resolution mass spectra.18, 19 To date, four studies have applied suspect screening and non-target methods to dust samples. Hilton et al.20 first applied a non-target method to one household dust sample obtained from the National Institute of Standards and Technology (NIST) using two-dimensional gas chromatography time-of-flight mass spectrometry (GC×GC-TOF/MS). Ouyang et al.21 carried out a non-target analysis for one household dust sample collected in Sweden using two-dimensional liquid chromatography (LC×LC)-TOF/MS. Rager et al.22 applied suspect screening and non-target methods to 50 household dust samples collected in the U.S. from 2005 to 2006 using LC-TOF/MS. A comparative study of a non-target analysis was conducted in a composite house dust sample as part of a collaborative effort using LC-MS and GC-MS.23 The methods used in the four studies are useful for identifying previously unknown and even unexpected chemicals in dust, but none of them presented concentrations that were confirmed and quantified by standards.
As part of an effort to evaluate a large number of environmental chemicals for potential health effects efficiently, the U.S. Environmental Protection Agency (EPA)’s Toxicity Forecaster (ToxCast) program utilizes hundreds of in vitro high-throughput screening (HTS) assays to support prediction of in vivo toxicities.24 In a parallel effort to screen a larger of number of chemicals based on exposure, several high-throughput (HT) methods including exposure models were developed to characterize and quantify exposures.10, 25–28 As indoor dust is a reservoir for SVOCs released indoors and can provide reasonable surrogates for characterizing exposures, we have previously developed a method for detecting a broad spectrum of chemicals in dust by applying a combination of target, suspect screening, and non-target methods using both LC-quadrupole time-of-flight (QTOF)/MS and GC-QTOF/MS.18 Building upon this previous publication,18 this current study presents chemical concentrations that were quantified in the same set of dust samples for target, suspect and selected nontarget compounds. In addition, we investigated whether the compounds detected in our samples had either endocrine-disrupting or neurotoxic potential, and discussed possible applications of our findings to future health studies.
2. Materials and Methods
2.1. Overview and scope of this study
The aim of this study is to inform key data gaps for assessing potential health effects for consumer product chemicals by integrating our measured dust concentrations with existing exposure and toxicity potential data. Our previously published study comprehensively characterized compounds found in house dust samples, detecting diverse and numerous consumer product chemicals. The present study extends those findings by quantifying concentrations, assessing household level variability, and considering potential exposure and toxicity of the compounds detected. Five steps were taken toward achieving the overall aim. First, we classified detected chemicals by their chemical class (e.g., phthalate) and their common use category (e.g., plasticizer). Second, we compiled information on chemical analysis techniques used to detect the compounds, including analytical instrument (LC or GC) and method (target, suspect screening, or non-target), the limit of detection (LOD), and information on whether identities were confirmed and concentrations were quantified by standards. Third, we summarized results from the chemical analysis, including the number of samples in which a compound was detected, information on whether a compound was newly detected in our house dust, and summary statistics of measured concentrations. Fourth, we added information on whether each detected compound has endocrine disrupting or neurotoxic potential based on in vitro HTS assays. Fifth, we indicated whether the compounds detected in our dust have been biomonitored in the U.S. National Health and Nutrition Examination Survey (NHANES).29
Note that we did not compare our measured concentrations to those reported in other peer-reviewed studies but focused on summarizing our concentrations by chemical class or use category. Other studies have already ascertained that differences in concentrations among studies may result from different sampling methods as well as geographic and temporal variation in chemical use.2, 30 In addition, we did not describe sample collection, dust extraction and analytical methods in detail in the current study because the details are available in the previous study.18 The whole analytical method and workflow were completely validated, results of quality assurance (QA) and quality control (QC) for each of the analytical approaches were provided, and strengths and weaknesses of the various approaches and analytical instruments were discussed previously.18 Thus, we briefly described sampling and analytical methods to the extent necessary for others to quickly extract key information regarding environmental monitoring, such as sample size, sampling method, period and location, and type of analytical instruments. Results of in vitro HTS assays presented in this study do not necessarily represent in vivo toxicities. Factors influencing toxicity such as pharmacokinetics and metabolism, early-life susceptibility, and genetic variability are not addressed by ToxCast.24, 31 Thus, toxicity potentials presented in this study need to be interpreted with caution. Other limitations of using in vitro HTS assays for predicting in vivo response are discussed elsewhere.31
2.2. Sampling and analytical methods
We recruited 38 families in Northern California from May 2015 to August 2016. From each household, we collected one dust sample from an approximate 2 m2 area in the main living room using a high-volume small surface sampler (HVS3), following a standard protocol.32 Dust samples were sieved and 100 mg aliquots were sonication-extracted with hexane/acetone (3:1 v/v) and acetone (100%). The extracts were then analyzed by both LC-QTOF/MS and GC-QTOF/MS with methods that were able to analyze compounds from various compound classes with widely differing chemical properties (e.g., molecular size, logP). In addition to the classical target analysis using reference standards and isotope-labelled internal standards, additional suspect screening and non-target analysis were performed. In order to unambiguously confirm the identity of suspected and non-targeted compounds, additional reference standards were purchased if they were available. Details of quantification methods are available elsewhere.18
2.3. Selection of target compounds
For the targeted method, we selected 76 chemicals for GC analysis and 56 chemicals for LC analysis (see Supporting Information [SI], Table S1). The targeted compounds included personal care products (PCPs; antimicrobial compounds, fragrance ingredients, parabens, ultraviolet [UV] filters), markers of human inputs (skin oils, metabolites), flame retardants (polybrominated diphenyl ethers [PBDEs], organophosphate flame retardants [OP-FRs], and other FRs), pesticides (insecticides, fungicides, herbicides), and a variety of other compounds widely detected in homes (phenols, phthalates, other plasticizers, PAHs, PFAS, and surfactants). The selection criteria included one (or multiple) indicator compounds from substance classes identified in our previous study7 or compounds present in products listed in the U.S. EPA’s Consumer Product Chemical Profiles database (CPCPdb).33
2.3. Chemical use categorization
Chemicals identified in the current study were classified into the most common and primary use category to understand the distribution of measured compounds by use category. For most compounds, we relied on “product” or “use” categorization available in databases such as CPCPdb and the U.S. National Library of Medicine’s Household Product Database (https://householdproducts.nlm.nih.gov/index.htm) to find the most common and primary use category of the compounds associated with at least one consumer product. For compounds with multiple uses, we also relied on web searches to find common uses. Multiple-use compounds were assigned to a primary use category and their secondary or tertiary use categories were further discussed in Results and Discussion. Thus, use categorization may be imprecise. For compounds that are consumed via dietary sources and also formulated in cosmetic products (e.g., linoleic acid, palmitic acid, cholesterol, fatty acids),34 it is likely that emissions from cooking are their dominant source to residential floor dust. Therefore, we preferentially assigned their primary use category to food sources. We further discussed this in Results and Discussion.
2.4. Data sources of endocrine-disrupting potential or neurotoxic potential
Many chemicals present in consumer products exhibit endocrine-disrupting potential35 or neurotoxic potential.36 To determine whether the compounds detected in our samples have either endocrine-disrupting or neurotoxic potential, we used in vitro HTS assays, most of which are included in the U.S. EPA’s ToxCast program. For endocrine-disrupting potential, we evaluated four main processes, including androgen, estrogen, thyroid, and steroidogenic. For androgen, we utilized androgen receptor (AR) pathway activity integrated from 11 AR-related in vitro HTS ToxCast assays and considered compounds with area under the curve (AUC) of ≥ 0.1 to be active in at least one AR pathway assay (active, inactive).37 For estrogen, we utilized estrogen receptor (ER) interaction scores integrated from 13 ER-related in vitro ToxCast assays and considered compounds with an AUC score of ≥ 0.1 to be active in at least one ER pathway assay (active, inactive).38 For thyroid, we utilized the results from the in vitro Amplex UltraRed thyroperoxidase or thyroid peroxidase (AUR-TPO) assay39 and a thyroid-specific in vitro HTS ToxCast assay.40 Because decreased TPO activity reduces thyroid hormone synthesis, compounds that elicited a ≥ 20% reduction in maximal TPO activity were considered to inhibit TPO (active, inactive).39 We also identified compounds that exhibited thyroid receptor activity measured by the in vitro ToxCast assay (active, inactive).40 For steroidogenesis, we utilized results from a method that considered 10 steroid hormones, including progestogens, glucocorticoids, androgens, and estrogens using an in vitro HTS assay with H295R human adrenocortical carcinoma cells.41 Among 2,060 evaluated compounds, we considered compounds that altered at least 4 steroid hormones at the maximum tolerated concentration to be active (or inactive), the same criteria used in Karmaus et al.41 For a neurotoxic indicator, we utilized microelectrode array hits as a measure of neural network activity in vitro (yes, no).42 A summary of toxicological endpoint data is provided in Table S2.
2.5. Statistical analysis
Statistical analyses were performed using Microsoft Excel 2018. For concentrations between the limit of quantification (LOQ) and the LOD, we assigned a value of the LOQ divided by 2. For concentrations below the LOD, we assigned a value of the LOD divided by the square root of 2.43 For compounds detected in more than 50% of the samples, we summarized measured median concentrations by five levels (<500, 500–1,000, 1,000–5,000, 5,000–10,000, >10,000 ng/g of dust) to investigate which compound classes were measured and present at high concentrations. We also computed coefficients of variation (CV) to examine the variability of concentrations in dust across homes.
3. Results
3.1. Measured dust concentrations
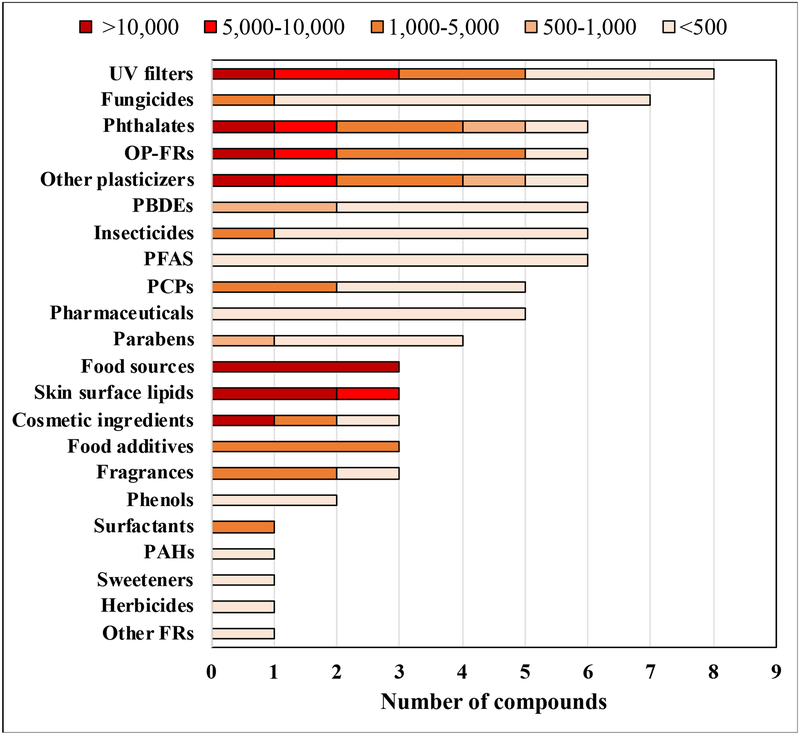
A total of 276 compounds were detected in our dust samples in which 5 additional compounds were later detected after a previous study was published.18 For 14 compounds, identification was not possible and only molecular formula (e.g., C4H7FO) could be assigned. Table S2 summarizes information of analytical methods, results from the analysis, exposure and toxicity potential for 262 detected compounds that could be identified with structure and formula. Additional summary for all 262 compounds detected in our dust is provided in the Supporting Information (see Data S1 for overall description and Table S3 for summary by chemical class and analytical instruments/methods). Overall, a large number of UV filters, phthalates, and OP-FRs were detected in our dust samples and median concentrations for some of them were above 10,000 ng/g of dust (Figure 1). Ten compounds were measured with median concentrations greater than 10,000 ng/g of dust: cis-hexadec-6-enoic acid, squalene, cholesterol, vitamin E, linoleic acid, tricaprylin, bis(2-ethylhexyl) phthalate [DEHP], dioctyl terephthalate [DOTP], tris(1-chloroisopropyl) phosphate [TCIPP], and one UV filter (oxybenzone). Cis-hexadec-6-enoic acid, squalene, cholesterol and vitamin E comprise or are found in skin surface lipids.44 Linoleic acid, cholesterol, and tricaprylin are widely used in cosmetics and personal care products. However, it is likely that emissions from cooking may significantly contribute to the measured dust levels of linoleic acid, cholesterol, and vitamin E.34 Consumer products (e.g., electronics, plastic products, shower curtains), building materials (e.g., vinyl flooring), and furniture (e.g., couches) are well-known emission sources of DEHP, DOTP, or TCIPP in the indoor environment. High concentrations of other chemical classes (e.g., skin oils, cosmetic ingredients, UV filters) detected in the current study highlight that humans and their activities, and possibly pets, play a role as sources of SVOCs in the indoor environment. Fungicides, PBDEs, PFAS, and pharmaceuticals were also abundant in our samples, but most were measured at concentrations below 500 ng/g of dust.
Figure 1.
Summary of median concentrations (ng/g of dust) for 87 compounds (target + suspect + non-target) detected in more than 50% of samples.
In the present study, 119 compounds were identified and/or quantified for the first time in household dust (Figure S1). Some of these compounds were previously measured in U.S. wastewater samples via target analysis but had not been measured in indoor dust. The majority of these compounds was detected via LC non-target (45%) and LC suspect (31%) approaches (see inset of Figure S1). These newly measured compounds mainly comprised surfactants (n = 25), pharmaceuticals (n = 19), compounds with unknown use information (n = 17), and human metabolites (n = 12), because of the polarity of these compounds. We also identified 6 phenols (some are also used as biocides) and 11 biocides (4 insecticides, 7 fungicides) in dust for the first time mostly via GC target and/or LC target analyses.
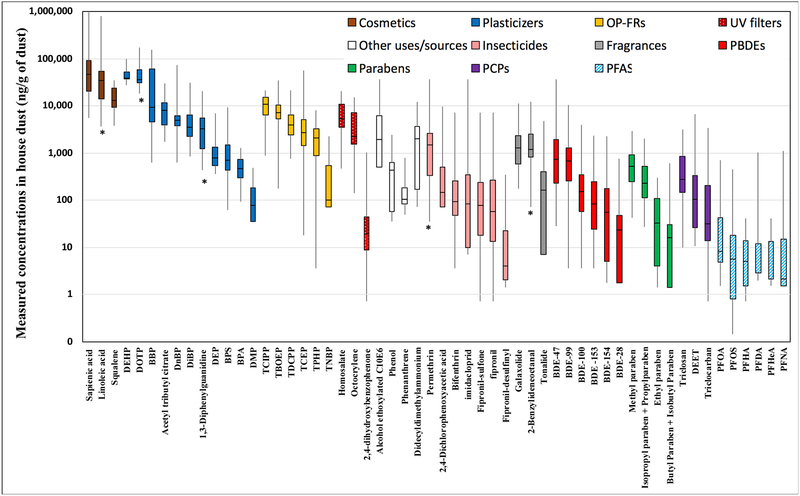
Overall, dust concentrations varied by almost three orders of magnitude across household samples and by almost four orders of magnitude across compounds (Figure 2). PFAS were measured at the lowest concentrations and had relatively large variability in concentrations. DEHP was shown to have the smallest variability (coefficient of variation, CV = 0.35) across the samples. Except for tri-n-butyl phosphate (TNBP), OP-FRs were measured at higher concentrations than PBDEs, and bisphenol S (BPS) was measured at higher concentrations than bisphenol A (BPA). This is consistent with recent changes in consumer use due to changes in product formulation and regulations affecting PBDEs and BPA. Variability metrics for all compounds including CVs are available in Table S2.
Figure 2.
Distributions of dust concentrations (ng/g of dust) for 56 target compounds detected in more than 50% of samples.
Two skin oils (Sapienic acid, squalene) and linoleic acid (used in cosmetics and emitted during cooking) were grouped into ‘cosmetics’ in this figure. Compounds with asterisk (*) indicate the first measurement in household dust. This figure excludes two phenols (tetrachlorophenols, cresol) that were detected in most samples (above LOD) but that were below LOQ. See Table 1 for identification of abbreviations.
Below, we summarized our measured dust concentrations along with other exposure and toxicity potential information by four categories: (1) chemical classes other than biocides that have received considerable public attention in indoor dust (e.g., phthalates, PBDEs, OP-FRs, PFAS), (2) biocides (e.g., insecticides, fungicides), (3) compounds in PCPs (e.g., fragrance ingredients, UV filters), and (4) chemical classes whose dust concentrations are of less concern for environmental exposure calculations (e.g., food additives, skin oils).
3.2. Chemical classes of current and emerging concerns in indoor dust
3.2.1. Phthalates and other plasticizers
Among 7 target phthalates, benzyl butyl phthalate (BBP), DEHP, di-isobutyl phthalate (DiBP), and di-n-butyl phthalate (DnBP) were detected in all of our samples. Median concentrations of these four phthalates were above 3,000 ng/g of dust (Table 1). Diethyl phthalate (DEP) and dimethyl phthalate (DMP) were detected in 79% and 71% of the samples, respectively, at relatively low concentrations (medians were below 1,000 ng/g of dust). Di-n-octyl phthalate (DOP), a target compound of the current study, was not detected in our dust, whereas it was detected in 100% of other California house dust samples collected in 2006.2
Table 1.
Summary of chemical classes that were detected in our dust samples (n = 38) and have been receiving considerable public attention
| Chemical class | Compound Name | Abbreviation | Instrument1 | Method2 | LOD (ng/g of dust) |
Standard3 | # detections | New detection4 | Median conc. (ng/g of dust) |
95th percentile (ng/g of dust) | Endocrine5 | Neurotoxic6 | NHANES7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phthalates | Benzyl butyl phthalate | BBP | G | T | 50 | 1 | 38 | 9181 | 134764 | 1 | 1 | 1 | |
| Bis(2-ethylhexyl) phthalate | DEHP | G | T | 50 | 1 | 38 | 39124 | 77532 | 0 | 0 | 1 | ||
| Di-isobutyl phthalate | DiBP | G | T | 100 | 1 | 38 | 3465 | 25917 | 0 | 1 | 1 | ||
| Di-n-butyl phthalate | DnBP | G | T | 100 | 1 | 38 | 4974 | 15983 | 1 | 1 | 1 | ||
| Diethyl phthalate | DEP | G | T | 500 | 1 | 30 | 966 | 4184 | 0 | 0 | 1 | ||
| Dimethyl phthalate | DMP | G | T | 50 | 1 | 27 | 103 | 388 | 0 | 0 | 1 | ||
| Other plasticizers | Bis(2-ethylhexyl) adipate | DEHA | G | T | 5000 | 1 | 15 | 17522 | 0 | 0 | |||
| Acetyl tributyl citrate | ATBC | G | T | 50 | 1 | 38 | 7969 | 27543 | 0 | 0 | |||
| Dioctyl terephthalate | DOTP | G | T | 50 | 1 | 38 | 1 | 35678 | 115606 | 0 | 0 | ||
| 1,3-Diphenylguanidine | L | T | 30 | 1 | 38 | 1 | 3218 | 9659 | 1 | 1 | |||
| Toluene-2-sulfonamide | L | S8 | <100 | 1 | 38 | 1 | 1922 | 6313 | |||||
| Diethylene glycol dibenzoate | G | N9 | 34 | 1 | 0 | 0 | |||||||
| Bisphenols | Bisphenol A | BPA | L | T | 50 | 1 | 38 | 461 | 1043 | 1 | 1 | 1 | |
| Bisphenol S | BPS | L | T | 55 | 1 | 38 | 691 | 5225 | 1 | 1 | |||
| Bisphenol A bis (2,3-dihydroxypropyl) ether | BADGE.2H2O | L | T | 1000 | 1 | 16 | 11237 | ||||||
| Bisphenol A (3-chloro-2-hydroxypropyl) (2,3-dihydroxypropyl) ether | BADGE-HCl-H2O | L | T | 125 | 1 | 7 | 2205 | ||||||
| Bisphenol AF | BPAF | L | S | 1 | 1 | 3 | 1 | 1 | |||||
| PBDEs | 2,4,4’-Tribromodiphenyl ether | BDE-28 | G | T | 2.5 | 1 | 26 | 38 | 363 | 1 | |||
| 2,2’,4,4’-Tetrabromodiphenyl ether | BDE-47 | G | T | 1.0 | 1 | 38 | 720 | 6878 | 1 | ||||
| 2,2’,4,4’,5-Pentabromodiphenyl ether | BDE-99 | G | T | 5.0 | 1 | 36 | 721 | 5090 | 1 | ||||
| 2,2’,4,4’,5,5’-Hexabromodiphenyl ether | BDE −153 | G | T | 5.0 | 1 | 31 | 111 | 1033 | 1 | ||||
| 2,2’,4,4’,5,6’-Hexabromodiphenyl ether | BDE-154 | G | T | 2.5 | 1 | 31 | 88 | 872 | 1 | ||||
| 2,2’,4,4’,6-Pentabromodiphenyl ether | BDE-100 | G | T | 5.0 | 1 | 35 | 165 | 1394 | 1 | ||||
| 2,2’,3,4,4’,5’,6-Heptabromodiphenyl ether | BDE-183 | G | T | 50 | 1 | 2 | 215 | 1 | |||||
| OP-FRs | Tri-n-butyl phosphate | TNBP | G | T | 100 | 1 | 19 | 100 | 2024 | 0 | 1 | 1 | |
| Triphenyl phosphate | TPHP | G | T | 5.0 | 1 | 37 | 2105 | 7120 | 1 | 1 | 1 | ||
| Tris(1-chloroisopropyl) phosphate | TCIPP | G | T | 50 | 1 | 38 | 10666 | 20407 | 0 | 1 | |||
| Tris(4-butyl-phenyl) phosphate | TBPP | G | T | 25 | 1 | 14 | 217 | ||||||
| Tris(2-butoxyethyl) phosphate | TBOEP | G | T | 250 | 1 | 37 | 7445 | 14159 | 0 | ||||
| Tris(1,3-dichloro-2-propyl) phosphate | TDCPP | G | T | 50 | 1 | 38 | 3955 | 15338 | 1 | 1 | 1 | ||
| Tris(2-chloroethyl) phosphate | TCEP | G | T | 25 | 1 | 37 | 2810 | 20946 | 0 | 0 | 1 | ||
| Triethyl phosphate | TEP | L | S8 | <100 | 1 | 8 | 154 | 0 | |||||
| Tricresyl phosphate | TCP | L | S | 25 | 1 | 8 | 8529 | 1 | 1 | ||||
| Octyl diphenyl phosphate | L | S | 3 | 1 | |||||||||
| Other FRs | Bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate | BEH-TEBP | G | T | 1000 | 1 | 3 | 13761 | 0 | ||||
| 2,4-Dibromophenol | G | T | 20 | 1 | 2 | 50 | |||||||
| 1,2-bis(2,4,6-tribromophenoxy) ethane | BTBPE | G | T | 100 | 1 | 1 | |||||||
| 3,3’,5,5’-Tetrabromobisphenol A | TBBPA | L | S8 | <100 | 1 | 36 | 18 | 91 | 1 | 1 | |||
| Melamine | L | S8 | <100 | 1 | 17 | 3162 | |||||||
| PFAS | N-ethyl perfluorooctane sulfonamide ethanol | MeFOSE | L | T | 8.0 | 1 | 6 | 90 | 1 | ||||
| Perfluorobutane sulfonate | PFBS | L | T | 2.0 | 1 | 9 | 23 | 1 | |||||
| Perfluorohexane sulfonate | PFHxS | L | T | 1.0 | 1 | 16 | 21 | 1 | |||||
| Perfluorooctane sulfonate | PFOS | L | T | 0.2 | 1 | 36 | 6 | 221 | 1 | 1 | 1 | ||
| Perfluorodecanoic acid | PFDA | L | T | 4.0 | 1 | 21 | 11 | 97 | 1 | 0 | 1 | ||
| Perfluoroheptanoic acid | PFHpA | L | T | 3.0 | 1 | 23 | 9 | 39 | 0 | 0 | 1 | ||
| Perfluorohexanoic acid | PFHxA | L | T | 1.0 | 1 | 35 | 6 | 30 | 0 | 0 | 1 | ||
| Perfluorononanoic acid | PFNA | L | T | 3.0 | 1 | 29 | 8 | 217 | 1 | 0 | 1 | ||
| Perfluorooctanoic acid | PFOA | L | T | 3.0 | 1 | 34 | 10 | 138 | 0 | 0 | 1 | ||
| Perfluoropentanoic acid | PFPeA | L | T | 3.0 | 1 | 12 | 10 | 1 | |||||
| Perfluoroundecanoic acid | PFUnDA | L | S8 | <100 | 1 | 5 | 125 | 0 | 0 | 1 | |||
| 6:2 polyfluoroalkyl phosphate diester | 6:2diPAP | L | N9 | 1 | 38 | ||||||||
| 6:2diPAP/8:2diPAP | L | N9 | 1 | 38 | |||||||||
| Fluorotelomer sulfonic acid | FTSA 6:2 | L | N9 | 1 | 38 | ||||||||
| 1h-Perfluoroheptane | L | N | 37 | 1 | |||||||||
| Phenols | 2-Chlorophenol | G | T | 50 | 1 | 1 | 1 | 1 | 0 | ||||
| 2,6-Dichlorophenol | G | T | 100 | 1 | 1 | 1 | |||||||
| 3,4,5-Trichlorophenol | G | T | 500 | 1 | 1 | 1 | |||||||
| Trichlorophenols (2,3,4-; 2,3,5-; 2,3,6-; 2,4,5-; 2,4,6-)10 | G | T | 100 | 1 | 1 | 1 | |||||||
| Phenol | G | T | 50 | 1 | 28 | 580 | 1669 | 1 | 0 | ||||
| Tetrachlorophenol | G | T | 250 | 1 | 19 | 1 | 250 | ||||||
| Cresol (o-,m-,p)10, 11 | G | T | 100 | 1 | 34 | 1 | 250 | 250 | |||||
| 2,4-Dinitrophenol | L | S9 | 50000 | 1 | 7 | 0 | 1 | ||||||
| PAHs | Anthracene | G | T | 5.0 | 1 | 6 | 24 | ||||||
| Benzo(a)anthracene | G | T | 5.0 | 1 | 10 | 89 | 1 | 1 | 1 | ||||
| Benzo(g,h,i)perylene | G | T | 50 | 1 | 17 | 289 | |||||||
| Chrysene | G | T | 5.0 | 1 | 18 | 130 | 1 | ||||||
| Phenanthrene | G | T | 10 | 1 | 38 | 106 | 367 | 1 | 0 | 1 | |||
| Dibenzo(a,h)anthracene + Indeno(1,2,3-cd)pyrene12 | G | T | 50 | 1 | 5 | 260 | 0 | ||||||
| Fluorene | G | T | 10 | 1 | 1 | 0 | 0 | 1 |
Type of analytical instruments used to detect compounds (G = gas chromatography, L = liquid chromatography).
Type of analytical methods used to detect compounds (T = target analysis, S = suspect screening, N = non-target analysis).
Compounds confirmed by standards (1 = Yes, blank = No).
Compounds newly detected in our dust (1 = Yes, blank = No).
Compounds having endocrine-disrupting potential from in vitro high-throughput screening assays (1 = active, 0 = inactive, blank = not tested or data not available).
Compounds having neurotoxic potential from in vitro high-throughput screening assays (1 = active, 0 = inactive, blank = not tested or data not available).
Compounds biomonitored in NHANES (1 = Yes, blank = No).
Detected by suspect screening or non-target methods first and quantified with standards later, thus concentrations are semi-quantitative.
Detected by suspect screening or non-target approach and quantified with standards later, but concentrations were not quantified.
Detected with multiple isomers.
Detected in most samples (above LOD) but were below the limit of quantification (LOQ).
Structures of these compounds were too close to discriminate one from the other.
Abbreviations: LOD, limit of detection; NHANES, National Health and Nutrition Examination Survey; PBDEs, polybrominated diphenyl ethers; OP-FRs, organophosphate flame retardants; PAHs, polycyclic aromatic hydrocarbons; PFAS, per- and polyfluoroalkyl substances
We detected 6 non-phthalate plasticizers and two were newly detected in our dust (dioctyl terephthalate (DOTP), 1,3-diphenylguanidine, toluene-2-sulfonamide, and diethylene glycol dibenzoate). Note that 1,3-diphenylguanidine is primarily used in various solid items including rubber footwear and automobile tires. The four compounds were widely detected in our samples (≥ 34 out of 38 samples). The latter two were detected via suspect screening and non-target methods, respectively. The median concentration of DOTP, a direct replacement for DEHP, was as high as DEHP. Among the four newly detected compounds, 1,3-diphenylguanidine has both endocrine and neurotoxic potential, but has not been biomonitored in NHANES.
3.2.2. Bisphenols and bisphenol analogues (hereafter referred as ‘bisphenols’)
BPA and BPS were detected in all of our samples, and two bisphenol analogues that can serve as replacements for BPA were measured in our dust samples via a target method, including bisphenol A bis (2,3-dihydroxypropyl) ether [BADGE.2H2O] and bisphenol A (3-chloro-2-hydroxypropyl) (2,3-dihydroxypropyl) ether [BADGE-HCl-H2O]. Bisphenol AF (BPAF) was also detected in our samples via a suspect screening method. We confirmed that BPAF has both endocrine and neurotoxic potential, but has not been biomonitored in NHANES. BADGE.2H2O and BADGE-HCl-H2O were not tested for endocrine-disrupting and neurotoxic potential using in vitro HTS assays and have not been biomonitored in NHANES.
3.2.3. PBDEs, OP-FRs, and other FRs
Seven PBDEs were detected and quantified in our samples via a target method and they have been biomonitored in NHANES. Although in vitro toxicity screening data were not available for all PBDEs, other adverse health effects of PBDEs have been summarized elsewhere.45 BDE-209 has been widely detected in other California house dust.46, 47 However, we could not have detected BDE-209 in our samples because our GC method was not designed to measure compounds with such low volatility.
Ten OP-FRs, including 7 target compounds, were detected in our dust samples. Five OP-FRs were ubiquitous (>97%) in our samples. Overall, median concentrations of OP-FRs were higher than those of PBDEs by one order of magnitude. Even though tris(2-butoxyethyl) phosphate (TBOEP) was ubiquitous (>97%) in our samples and measured at high concentrations (median was 7,445 ng/g of dust), it has not been biomonitored in NHANES. Octyl diphenyl phosphate was newly detected in our dust via suspect screening. Four OP-FRs have either endocrine-disrupting or neurotoxic potential, but in vitro toxicity screening data were not available for tris(4-butyl-phenyl) phosphate (TBPP) and octyl diphenyl phosphate.
In addition to PBDEs and OP-FRs, we detected five compounds that are used as flame retardants. Three compounds were detected in a few samples (<4) via a target method. Melamine and 3,3’,5,5’-Tetrabromobisphenol A (TBBPA) were detected in 17 and 36 samples, respectively, via suspect screening. TBBPA has both endocrine-disrupting potential and neurotoxic potential, but has not been biomonitored in NHANES. Because melamine was detected in almost half of our samples and has been detected in urine specimens of children who consumed milk products,48 toxicity testing and biomonitoring are recommended for this compound.
3.2.4. PFAS
A total of 15 PFAS were detected in our dust, including 10 target compounds. We newly detected 3-(perfluorooctyl)propyl iodide in our dust (37 out of 38 samples) via a non-target method. Compared to other chemical classes, PFAS median concentrations were relatively low (<12 ng/g of dust). Eleven PFAS have been biomonitored in NHANES and seven of them were tested for endocrine-disrupting and neurotoxic potential using in vitro HTS assays.
3.2.5. Phenols
Among 15 targeted phenols, only 7 phenols were detected in our samples where trichlorophenols and cresols were detected with multiple isomers. Four phenols were newly detected in our dust via a target method, but in only one sample. We additionally detected 2,4-dinitrophenol via suspect screening. Overall, due to the low detection frequency, median concentrations were computed only for phenol and cresols (580 and 250 ng/g of dust, respectively). None of the 8 detected phenols was biomonitored in NHANES and only three phenols were tested for toxicity using in vitro HTS assays. Cresols were detected with multiple isomers (o-, m-, p-) in 34 out of 38 samples and thus are recommended to be included in future biomonitoring and in vitro toxicity screening testing.
3.2.6. PAHs
Among 12 targeted PAHs, 8 PAHs were detected in our samples. Because structures of dibenzo(a,h)anthracene and indeno(1,2,3-cd)pyrene were too close to discriminate one from the other, results were reported together in Table 1. Except for phenanthrene, 7 other PAHs were detected in fewer than 50% of our samples. Four PAHs were or have been biomonitored in NHANES. In vitro toxicity screening testing data for endocrine-disrupting potential or neurotoxic potential were not available for three PAHs, but other toxic endpoints are available from in vitro HTS assays.49
3.3. Biocides
3.3.1. Insecticides
We detected 22 insecticides mostly via a target method (Table 2). Four insecticides were newly detected in our samples, but in fewer than 25% of our samples. Permethrin was measured at the highest median concentration (1,922 ng/g of dust), but the 95th percentile concentration of other four insecticides (imidacloprid, etofenprox, cypermethrin, tetrachlorvinphos) was higher than that of permethrin. In addition to common insecticides used to control pests indoors, other potential indoor sources of fipronil, imidacloprid, pyriproxifen and permethrin may be associated with their use as topical flea control agents for dogs and cats. About 53% of the participating homes had at least one indoor cat or dog. Fipronil products have been shown to persist on pets for over 28 days50 and this is likely true for other active ingredients based on the relatively low frequency of application required for these products. These active ingredients will accumulate in dust as pets shed treated fur and skin cells. The less frequent detection of some of the insecticides in this study, coupled with their relatively high coefficients of variation (see Table S2), likely reflects the fact that pet ownership and indoor insecticide applications are not as ubiquitous as other indoor product uses. Compared to toxicity testing data (n = 16), biomonitoring data are limited (n = 9). For fipronil-sulfone, fipronil, fipronil-desulfinyl, and fipronil-sulfide that were detected in around or greater than 50% of the samples, both toxicity testing and biomonitoring are recommended.
Table 2.
Summary of biocides that were detected in our dust samples (n = 38)
| Chemical class | Compound Name | Instrument1 | Method2 | LOD (ng/g of dust) |
Standard3 | # detections | New detection4 | Median conc. (ng/g of dust) |
95th percentile (ng/g of dust) | Endocrine5 | Neurotoxic6 | NHANES7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insecticides | Fipronil-sulfone | L | T | 1.0 | 1 | 37 | 77 | 3004 | ||||
| Fipronil | L | T | 1.0 | 1 | 37 | 62 | 5718 | 1 | 1 | |||
| Permethrin | G | T | 50 | 1 | 34 | 1922 | 8151 | 1 | 0 | 1 | ||
| Imidacloprid | L | T | 10 | 1 | 32 | 123 | 22455 | 0 | 0 | 1 | ||
| Bifenthrin | G | T | 5.0 | 1 | 31 | 140 | 3336 | 1 | 0 | |||
| Fipronil-desulfinyl | L | T | 2.0 | 1 | 28 | 11 | 248 | |||||
| Fipronil-sulfide | L | T | 1.0 | 1 | 17 | 136 | ||||||
| Novaluron | L | T | 1.0 | 1 | 9 | 1 | 1826 | 1 | 0 | |||
| Chlorpyrifos | G | T | 5.0 | 1 | 8 | 365 | 1 | 0 | 1 | |||
| Methoxyfenozide | L | T | 2.0 | 1 | 7 | 1 | 204 | 1 | 0 | |||
| Propoxur | L | T | 6.0 | 1 | 6 | 60 | 0 | 0 | 1 | |||
| Chlorantraniprole | L | T | 3.0 | 1 | 4 | 1 | 69 | |||||
| Pyriproxyfen | L | T | 4.0 | 1 | 4 | 791 | 0 | 1 | ||||
| Deltamethrin | G | T | 500 | 1 | 4 | 6658 | 0 | 1 | ||||
| Etofenprox | G | T | 10 | 1 | 4 | 38059 | 1 | 0 | ||||
| Cyhalothrin | G | T | 50 | 1 | 2 | 7449 | 0 | 1 | ||||
| Cypermethrin | G | T | 500 | 1 | 2 | 24222 | 1 | |||||
| 1,3-benzothiazole | L | T | 600 | 1 | 2 | 1 | 2714 | 0 | ||||
| Esfenvalerate | G | T | 250 | 1 | 1 | 0 | 1 | |||||
| Tetrachlorvinphos | L | S8 | <100 | 1 | 6 | 43222 | 1 | 1 | ||||
| Cyfluthrin | G | N9 | 1 | 1 | 1 | |||||||
| Piperonyl butoxide | G | N | 26 | 1 | 0 | |||||||
| Fungicides | Boscalid | L | T | 3.0 | 1 | 10 | 262 | 0 | 1 | |||
| Azoxystrobin | L | T | 5.0 | 1 | 11 | 1 | 77 | 1 | 1 | |||
| Difenoconazole | L | T | 9.0 | 1 | 2 | 1 | 18 | 1 | 1 | |||
| Didecyldimethylammonium chloride | L | T | 100 | 1 | 28 | 1 | 2859 | 10579 | 1 | 1 | ||
| Pentachlorophenol | G | T | 1000 | 1 | 6 | 11511 | 1 | 0 | 1 | |||
| Carbendazim | L | S8 | <100 | 1 | 11 | 2568 | 1 | 0 | ||||
| Imazalil | L | S8 | <100 | 1 | 29 | 18 | 210 | 1 | 1 | |||
| Octhilinone | L | S8 | <100 | 1 | 22 | 5 | 44 | 1 | 1 | |||
| Propiconazole | L | S8 | <100 | 1 | 31 | 34 | 194 | 1 | 1 | |||
| Thiabendazole | L | S8 | <100 | 1 | 37 | 54 | 713 | 0 | 0 | |||
| Fludioxonil | L | S8 | <100 | 1 | 37 | 1 | 12 | 61 | 1 | 1 | ||
| Physcion | L | S8 | <100 | 1 | 38 | 177 | 866 | |||||
| 4-hydroxychlorothalonil | L | N9 | 1 | 38 | 1 | |||||||
| Pyraclostrobin | L | S9 | 1 | 7 | 0 | 1 | ||||||
| Dichlorophen | L | S | 250 | 1 | 1 | 1 | 1 | |||||
| 3-Iodo-2-propynyl-N-butyLarbamate | L | S | 50 | 1 | 16 | 1 | 385 | 1 | 1 | |||
| Herbicides | 2,4-Dichlorophenoxyacetic acid | L | T | 100 | 1 | 20 | 500 | 5057 | 0 | 0 | 1 | |
| Propanil | L | T | 2.0 | 1 | 13 | 512 | 1 | 1 | ||||
| Diuron | L | T | 15 | 1 | 11 | 1116 | 1 | 0 | ||||
| Pendimethalin | L | T | 150 | 1 | 5 | 591 | 1 | 1 | ||||
| Simazine | L | T | 3.0 | 1 | 1 | 0 | 0 |
3.3.2. Fungicides
A total of 15 fungicides were detected, 11 of them via suspect screening or non-target methods. Seven fungicides were newly detected in our dust. Eight fungicides mostly detected via suspect screening or non-target methods had a detection frequency above 50%, indicating widespread use of fungicides in the indoor environment. Except for didecyldimethylammonium chloride (DDAC; median concentration = 2,859 ng/g of dust), measured median concentrations of fungicides were low compared to insecticides. In addition to a fungicidal use, DDAC is used as an antibacterial agent and has wide indoor applications where it is used on walls, floors, tables, toilets and fixtures.51 Of the 15 detected fungicides, 13 fungicides were previously tested for either endocrine-disrupting potential or neurotoxic potential. Except for pentachlorophenol, none of the 14 detected fungicides has been biomonitored in NHANES. Among the compounds that were newly detected and have both endocrine-disrupting and neurotoxic potential, two fungicides (DDAC, fludioxonil) were commonly detected (>70%) in our samples; two other fungicides (azoxystrobin, difenoconazole) were detected infrequently (29% and 5%, respectively). Thus, fungicides with a high detection frequency are recommended to be included in future biomonitoring studies.
3.3.3. Herbicides
Five herbicides were detected in our dust via a target method. A detection frequency was below 50% for 4 out of 5 herbicides. Given the detection frequency, they might be attributable to applications in agricultural fields or gardens. Except for 2,4-dichlorophenoxyacetic acid, none of them was biomonitored in NHANES. Propanil and pendimethalin showed both endocrine-disrupting potential and neurotoxic potential, and diuron showed endocrine-disrupting potential from the in vitro testing. Thus, they might need to be included in future biomonitoring studies.
3.4. Compounds in personal care products
We detected parabens, fragrance ingredients, UV filters, cosmetic ingredients, and those with other personal care uses in our dust (Table 3). Overall, they were widely detected, and median concentrations were on the order of 1,000 to 10,000 ng/g of dust for 11 compounds. For users of products containing these compounds, direct dermal uptake is likely to be a primary exposure route. However, for non-users, such as young children who spend most of their time on the floors and have high dust ingestion rates, dust may be an important exposure medium for these compounds.52 Eight compounds in PCPs were newly detected in our dust and four compounds are cosmetic ingredients. Among newly detected compounds, toxicity testing and biomonitoring are recommended for dexpanthenol because it was detected in all samples with a median of 1,311 ng/g of dust. There are only 8 compounds that have been biomonitored in NHANES and that were tested for endocrine-disrupting and/or neurotoxic potential. However, we observed that there are many PCP compounds that may require biomonitoring and toxicity testing based on the detection frequency and high median concentrations.
Table 3.
Summary of compound classes that were detected in our dust samples (n = 38) and mainly used in dermal applications
| Chemical class | Compound Name | Instrument1 | Method2 | LOD (ng/g of dust) |
Standard3 | # detections | New detection4 | Median conc. (ng/g of dust) |
95th percentile (ng/g of dust) | Endocrine5 | Neurotoxic6 | NHANES7 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parabens | Ethyl paraben | L | T | 2.0 | 1 | 35 | 48 | 241 | 1 | 1 | 1 | |
| Methyl paraben | L | T | 60 | 1 | 37 | 524 | 1559 | 0 | 0 | 1 | ||
| Butyl Paraben + Isobutyl Paraben9 | L | T | 2.0 | 1 | 26 | 23 | 235 | 1 | 1 | 1 | ||
| Isopropyl paraben + Propyl Paraben9 | L | T | 2.0 | 1 | 38 | 226 | 1457 | 1 | 1 | 1 | ||
| Frag-rance | 2-Benzylideneoctanal | G | T | 100 | 1 | 37 | 1 | 1194 | 3950 | 0 | 0 | |
| Galaxolide | G | T | 5.0 | 1 | 38 | 1294 | 5521 | |||||
| Tonalide | G | T | 10 | 1 | 27 | 219 | 818 | 0 | ||||
| UV filters | Homosalate | G | T | 25 | 1 | 38 | 5233 | 17398 | 0 | |||
| Octocrylene | G | T | 25 | 1 | 38 | 2245 | 13656 | |||||
| 2,4-dihydroxybenzophenone | L | T | 1.0 | 1 | 35 | 22 | 148 | 1 | ||||
| Benzophenone-4 | L | S8 | <100 | 1 | 30 | 37 | 230 | |||||
| Octyl methoxycinnamate | L | S8 | <100 | 1 | 37 | 1450 | 8037 | |||||
| 2-Ethylhexyl salicylate | G | N8 | n.d. | 1 | 38 | 7057 | 26531 | |||||
| Benzophenone | G | N8 | n.d. | 1 | 35 | 441 | 1326 | 0 | 0 | |||
| Benzophenone-3 | G | N8 | n.d. | 1 | 38 | 19170 | 64114 | 0 | 1 | |||
| Other personal care | Triclocarban | L | T | 1.0 | 1 | 37 | 33 | 1144 | 1 | 1 | 1 | |
| Triclosan | L | T | 5.0 | 1 | 38 | 275 | 1391 | 1 | 1 | 1 | ||
| N,N-Diethyl-meta-toluamide (DEET) | L | T | 15 | 1 | 30 | 154 | 5536 | 1 | 0 | 1 | ||
| Dexpanthenol | L | S8 | <100 | 1 | 38 | 1 | 1311 | 13243 | ||||
| Benzyl Benzoate | G | N8 | n.d. | 1 | 38 | 1 | 1280 | 10628 | 0 | |||
| Salnacedin | L | N | 38 | 1 | ||||||||
| Coumarin | G | N | 33 | 0 | 0 | |||||||
| Ethyl N-acetyl-N-butyl-β-alaninate | L | S | 4 | |||||||||
| Cosmetics | Tricaprylin | G | N8 | n.d. | 1 | 33 | 27882 | 75596 | ||||
| Isopropyl myristate | G | N8 | n.d. | 1 | 38 | 1 | 1836 | 8485 | 0 | |||
| Lilial | G | N8 | n.d. | 1 | 38 | 1 | 319 | 3557 | 0 | |||
| Cholesteryl benzoate | G | N | 38 | 1 | ||||||||
| Isopropyl palmitate | G | N | 38 | |||||||||
| 12-Hydroxystearic acid | L | N | 38 | 1 | ||||||||
| Benzyl salicylate | G | N | 38 | 0 |
For 1 through 8, refer to footnote of Table 1.
Structures of these compounds were too close to discriminate one from the other.
Abbreviations: n.d., not determined;
3.5. Chemical classes whose dust concentrations are of less concern for environmental exposure calculations
In our samples, we detected 10 food additives, 5 sweeteners,11 food sources, 29 pharmaceuticals, 3 skin oils, 14 human metabolites, 31 surfactants, and 17 compounds whose use is not known (Table S2). Most of them were detected via suspect screening or non-target methods. These compound classes were not of interest in identifying or measuring dust concentrations in previous indoor environmental monitoring studies. Thus, their presence was rarely reported in the literature and 87 out of 119 newly detected compounds fell in this category. Among the compounds classified in this category, only 18 compounds were tested for endocrine-disrupting and/or neurotoxic potential. We found that sorbic acid (food additive), ketoconazole (pharmaceutical), nicotine (pharmaceutical), linoleic acid (food sources), linolenic acid (food sources), and genistein (food sources) have endocrine-disrupting and/or neurotoxic potential. Exposure to genistein occurs primarily through foods made with soybeans and soy protein.53 Biomonitoring is recommended for linoleic acid because it was detected in all samples at a high median concentration (34,308 ng/g of dust) and has endocrine-disrupting potential.
4. Discussion
Results from this study provided more comprehensive chemical profiles of house dust. We detected a total of 276 compounds in our dust samples and quantified concentrations of 144 compounds using standards. In addition to the compounds that were previously measured in indoor dust, we tried to identify overlooked compounds that were not previously measured in dust but were shown to have potential for adverse health effects from the HTS toxicity testing. We were also able to expand the list of compounds present in indoor dust by applying both LC-MS and GC-MS with three analytical approaches. For example, 75% of the newly measured chemicals were observed via LC non-target or LC suspect approaches (see Figure S1). The newly measured compounds in our study mainly comprised surfactants, pharmaceuticals, and human metabolites. Because of the polarity of these compounds, we were able to detect a large number of compounds via LC-MS. Another reason we could extend the list of compounds present in indoor dust is that our samples were recently collected (2015–2016). Compared to Rager et al.22 who investigated non-targeted compounds using LC-MS in U.S household dust samples collected from 2005 to 2006, we were able to newly detect four replacement plasticizers (e.g., acetyl tributyl citrate, DOTP) in our samples, reflecting currently used products.
Our study showed that indoor dust contains chemicals from various consumer product uses and also supported the idea that dust can serve as a marker of use. For example, most of the food additives and sweeteners detected in our dust are used in processed foods or drinks. Thus, the presence of food additives or sweeteners in dust indicates that they exist outside their intended use, which is to be consumed via direct food intake. Cholesterol (found in skin and emitted during cooking) and skin oils were ubiquitously measured in Danish homes and daycare centers.44 In addition to these compounds, we observed cosmetic ingredients and vitamin E with median concentrations greater than 10,000 ng/g. In a separate study in which we analyzed skin wipe samples,54 11 compounds (triethyl citrate, butylated hydroxytoluene, cholesta-3,5-diene, vitamin E, cholesterol, tridecanoic acid, arachidonic acid, palmidrol, palmitic acid, pentadecanoic acid, linolenic acid) were detected, and they were also detected in our dust samples. This indicates that human activities, including cooking, cosmetic use, skin sloughing, dropping food residue or debris unintentionally on floors, could be sources of these compounds. Moreover, because we analyzed recently collected dust samples, we observed that relatively new chemicals (e.g., OP-FRs, BPS) were measured at higher concentrations than those for controversial or banned chemicals in consumer products (e.g., PBDEs, BPA). This reflects the dynamic nature of consumer product formulations, especially given heightened consumer awareness and concerns about the safety of product ingredients.
Compiling existing exposure and toxicity potential data of our detected compounds allowed us to inform key data gaps for assessing potential health effects for previously overlooked chemicals. For example, we found that in vitro HTS toxicity data were not available for some of the detected plasticizers, bisphenols, and biocides, which may adversely affect human health. Of most interest are one plasticizer (toluene-2-sulfonamide), two bisphenols (BADGE.2H2O, BADGE-HCl-H2O), and eight biocides and biocide transformation products (fipronil-desulfinyl, fipronil-sulfide, fipronil-sulfone, chlorantraniprole, cypermethrin, cyfluthrin, 4-hydroxychlorothalonil, physcion). Because most of these compounds were ubiquitous in our samples and may have toxicity potential, they are recommended to be included in future in vitro toxicity screening.
In conclusion, following the identification of a broad spectrum of chemicals from a previous study,18 this study integrated their measured dust concentrations with existing exposure and toxicity information to inform key data gaps for assessing potential health effects for consumer product chemicals. We found that 13 newly detected compounds may potentially disrupt endocrine systems and/or be neurotoxic based on in vitro bioactivity assays. These results expand our knowledge of chemicals present in indoor residential environments where vulnerable populations, especially young children, spend most of their time on the floors.56 Consequently, we expect that our findings may trigger further environmental health research regarding previously overlooked compounds. Many of the pharmaceuticals and PCPs newly detected in our dust have been extensively studied in various aquatic environments, including drinking water, wastewater, surface water, and groundwater55 because they may pose a threat to the ecosystem and/or human health. Given that people spend most of their time indoors,56 more studies are needed to examine the presence of these compounds in residential dust and to investigate potential health effects associated with indoor non-dietary exposure routes. Additional studies are also recommended to confirm the presence of compounds that were less frequently detected in the current study and not yet confirmed by standards.
Supplementary Material
Practical Implications:
Our dust samples contain chemicals from various consumer product uses, including cleaning and personal care products, furniture, plastics, and pesticides. This supports the idea that dust can serve as a marker of use. We expect that this comprehensive investigation of chemicals present in dust will form the basis for future work to develop new hypotheses of adverse health effects due to exposures to previously overlooked compounds.
Acknowledgments
Research reported in this publication was supported by the U.S. Environmental Protection Agency (EPA-G2013-STAR-K1) and the UC Davis Superfund Research Center, National Institutes of Health, NIEHS award (P42-ES004699). All authors declare they have no actual or potential competing financial interests.
Footnotes
Conflict of interest
The authors declare that they have no competing interest.
Appendix A. Supporting Information
Supplementary data associated with this article can be found in the online version at.
References
- 1.Egeghy PP; Vallero DA; Hubal EAC, Exposure-based prioritization of chemicals for risk assessment. Environ Sci Policy 2011, 14, (8), 950–964. [Google Scholar]
- 2.Dodson RE; Camann DE; Morello-Frosch R; Brody JG; Rudel RA, Semivolatile Organic Compounds in Homes: Strategies for Efficient and Systematic Exposure Measurement Based on Empirical and Theoretical Factors. Environmental Science & Technology 2015, 49, (1), 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitro SD; Dodson RE; Singla V; Adarnkiewicz G; Elmi AF; Tilly MK; Zota AR, Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-analysis of US Studies. Environmental Science & Technology 2016, 50, (19), 10661–10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zota AR; Calafat AM; Woodruff TJ, Temporal Trends in Phthalate Exposures: Findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ Health Persp 2014, 122, (3), 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calafat AM; Valentin-Blasini L; Ye X, Trends in Exposure to Chemicals in Personal Care and Consumer Products. Curr Environ Health Rep 2015, 2, (4), 348–55. [DOI] [PubMed] [Google Scholar]
- 6.Cordner A; Mulcahy M; Brown P, Chemical regulation on fire: rapid policy advances on flame retardants. Environ Sci Technol 2013, 47, (13), 7067–76. [DOI] [PubMed] [Google Scholar]
- 7.Shin HM; Ernstoff A; Arnot JA; Wetmore BA; Csiszar SA; Fantke P; Zhang XM; McKone TE; Jolliet O; Bennett DH, Risk-Based High-Throughput Chemical Screening and Prioritization using Exposure Models and in Vitro Bioactivity Assays. Environmental Science & Technology 2015, 49, (11), 6760–6771. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y; Hubal EAC; Little JC, Predicting Residential Exposure to Phthalate Plasticizer Emitted from Vinyl Flooring: Sensitivity, Uncertainty, and Implications for Biomonitoring. Environ Health Persp 2010, 118, (2), 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beko G; Weschler CJ; Langer S; Callesen M; Toftum J; Clausen G, Children’s Phthalate Intakes and Resultant Cumulative Exposures Estimated from Urine Compared with Estimates from Dust Ingestion, Inhalation and Dermal Absorption in Their Homes and Daycare Centers. Plos One 2013, 8, (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Little JC; Weschler CJ; Nazaroff WW; Liu Z; Hubal EAC, Rapid methods to estimate potential exposure to semivolatile organic compounds in the indoor environment. Environmental Science & Technology 2012, 46, (20), 11171–11178. [DOI] [PubMed] [Google Scholar]
- 11.Shin HM; McKone TE; Tulve NS; Clifton MS; Bennett DH, Indoor Residence Times of Semivolatile Organic Compounds: Model Estimation and Field Evaluation. Environmental Science & Technology 2013, 47, (2), 859–867. [DOI] [PubMed] [Google Scholar]
- 12.Weschler CJ; Nazaroff WW, SVOC partitioning between the gas phase and settled dust indoors. Atmos Environ 2010, 44, (30), 3609–3620. [Google Scholar]
- 13.Ao JJ; Yuan T; Ma YN; Gao L; Ni N; Li D, Identification, characteristics and human exposure assessments of triclosan, bisphenol-A, and four commonly used organic UV filters in indoor dust collected from Shanghai, China. Chemosphere 2017, 184, 575–583. [DOI] [PubMed] [Google Scholar]
- 14.Hwang HM; Park EK; Young TM; Hammock BD, Occurrence of endocrine-disrupting chemicals in indoor dust. Sci Total Environ 2008, 404, (1), 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lankova D; Svarcova A; Kalachova K; Lacina O; Pulkrabova J; Hajslova J, Multi-analyte method for the analysis of various organohalogen compounds in house dust. Analytica Chimica Acta 2015, 854, 61–69. [DOI] [PubMed] [Google Scholar]
- 16.Dodson RE; Perovich LJ; Covaci A; Van den Eede N; Ionas AC; Dirtu AC; Brody JG; Rudel RA, After the PBDE Phase-Out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environmental Science & Technology 2012, 46, (24), 13056–13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudel RA; Camann DE; Spengler JD; Korn LR; Brody JG, Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environmental Science & Technology 2003, 37, (20), 4543–4553. [DOI] [PubMed] [Google Scholar]
- 18.Moschet C; Anumol T; Lew BM; Bennett DH; Young TM, Household dust as a repository of chemical accumulation: New insights from a comprehensive high-resolution mass spectrometry study. Environmental Science & Technology 2018, 52, 2878–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krauss M; Singer H; Hollender J, LC-high resolution MS in environmental analysis: from target screening to the identification of unknowns. Anal Bioanal Chem 2010, 397, (3), 943–951. [DOI] [PubMed] [Google Scholar]
- 20.Hilton DC; Jones RS; Sjodin A, A method for rapid, non-targeted screening for environmental contaminants in household dust. Journal of Chromatography A 2010, 1217, (44), 6851–6856. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang XY; Weiss JM; de Boer J; Lamoree MH; Leonards PEG, Non-target analysis of household dust and laundry dryer lint using comprehensive two-dimensional liquid chromatography coupled with time-of-flight mass spectrometry. Chemosphere 2017, 166, 431–437. [DOI] [PubMed] [Google Scholar]
- 22.Rager JE; Strynar MJ; Liang S; McMahen RL; Richard AM; Grulke CM; Wambaugh JF; Isaacs KK; Judson R; Williams AJ; Sobus JR, Linking high resolution mass spectrometry data with exposure and toxicity forecasts to advance high-throughput environmental monitoring. Environment International 2016, 88, 269–280. [DOI] [PubMed] [Google Scholar]
- 23.Rostkowski P; Haglund P; Aalizadeh R; Alygizakis N; Thomaidis N; Arandes JB; Nizzetto PB; Booij P; Budzinski H; Brunswick P; Covaci A; Gallampois C; Grosse S; Hindle R; Ipolyi I; Jobst K; Kaserzon SL; Leonards P; Lestremau F; Letzel T; Magner J; Matsukami H; Moschet C; Oswald P; Plassmann M; Slobodnik J; Yang C, The strength in numbers: comprehensive characterization of house dust using complementary mass spectrometric techniques. Anal Bioanal Chem 2019, 411, (10), 1957–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Judson RS; Houck KA; Kavlock RJ; Knudsen TB; Martin MT; Mortensen HM; Reif DM; Rotroff DM; Shah I; Richard AM; Dix DJ, In vitro screening of environmental chemicals for targeted testing prioritization: the ToxCast project. Environ Health Perspect 2010, 118, (4), 485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isaacs KK; Glen WG; Egeghy P; Goldsmith MR; Smith L; Vallero D; Brooks R; Grulke CM; Ozkaynak H, SHEDS-HT: An Integrated Probabilistic Exposure Model for Prioritizing Exposures to Chemicals with Near-Field and Dietary Sources. Environmental Science & Technology 2014, 48, (21), 12750–12759. [DOI] [PubMed] [Google Scholar]
- 26.Shin HM; McKone TE; Bennett DH, Intake Fraction for the Indoor Environment: A Tool for Prioritizing Indoor Chemical Sources. Environmental Science & Technology 2012, 46, (18), 10063–10072. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X; Arnot JA; Wania F, Model for screening-level assessment of near-field human exposure to neutral organic chemicals released indoors. Environmental Science & Technology 2014, 48, (20), 12312–12319. [DOI] [PubMed] [Google Scholar]
- 28.Wenger Y; Li D; Jolliet O, Indoor intake fraction considering surface sorption of air organic compounds for life cycle assessment. International Journal of Life Cycle Assessment 2012, 17, (7), 919–931. [Google Scholar]
- 29.Centers for Disease Control and Prevetion (CDC), Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA, 2019; pp 1–235. [Google Scholar]
- 30.Blanchard O; Glorennec P; Mercier F; Bonvallot N; Chevrier C; Ramalho O; Mandin C; Le Bot B, Semivolatile Organic Compounds in Indoor Air and Settled Dust in 30 French Dwellings. Environmental Science & Technology 2014, 48, (7), 3959–3969. [DOI] [PubMed] [Google Scholar]
- 31.Pham N; Iyer S; Hackett E; Lock BH; Sandy M; Zeise L; Solomon G; Marty M, Using ToxCast to Explore Chemical Activities and Hazard Traits: A Case Study With Ortho-Phthalates. Toxicol Sci 2016, 151, (2), 286–301. [DOI] [PubMed] [Google Scholar]
- 32.Roberts JW; Budd WT; Ruby MG; Bond AE; Lewis RG; Wiener RW; Camann DE, Development and field testing of a high volume sampler for pesticides and toxics in dust. J Expo Anal Environ Epidemiol 1991, 1, (2), 143–55. [PubMed] [Google Scholar]
- 33.Goldsmith MR; Grulke CM; Brooks RD; Transue TR; Tan YM; Frame A; Egeghy PP; Edwards R; Chang DT; Tornero-Velez R; Isaacs K; Wang A; Johnson J; Holm K; Reich M; Mitchell J; Vallero DA; Phillips L; Phillips M; Wambaugh JF; Judson RS; Buckley TJ; Dary CC, Development of a consumer product ingredient database for chemical exposure screening and prioritization. Food and Chemical Toxicology 2014, 65, 269–279. [DOI] [PubMed] [Google Scholar]
- 34.He LY; Hu M; Huang XF; Yu BD; Zhang YH; Liu DQ, Measurement of emissions of fine particulate organic matter from Chinese cooking. Atmos Environ 2004, 38, (38), 6557–6564. [Google Scholar]
- 35.Dodson RE; Nishioka M; Standley LJ; Perovich LJ; Brody JG; Rudel RA, Endocrine Disruptors and Asthma-Associated Chemicals in Consumer Products. Environ Health Persp 2012, 120, (7), 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith MN; Grice J; Cullen A; Faustman EM, A Toxicological Framework for the Prioritization of Children’s Safe Product Act Data. International Journal of Environmental Research and Public Health 2016, 13, (4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleinstreuer NC; Ceger P; Watt ED; Martin M; Houck K; Browne P; Thomas RS; Casey WM; Dix DJ; Allen D; Sakamuru S; Xia M; Huang R; Judson R, Development and Validation of a Computational Model for Androgen Receptor Activity. Chemical Research in Toxicology 2017, 30, (4), 946–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rotroff DM; Martin MT; Dix DJ; Filer DL; Houck KA; Knudsen TB; Sipes NS; Reif DM; Xia MH; Huang RL; Judson RS, Predictive Endocrine Testing in the 21st Century Using in Vitro Assays of Estrogen Receptor Signaling Responses. Environmental Science & Technology 2014, 48, (15), 8706–8716. [DOI] [PubMed] [Google Scholar]
- 39.Friedman KP; Watt ED; Hornung MW; Hedge JM; Judson RS; Crofton KM; Houck KA; Simmons SO, Tiered High-Throughput Screening Approach to Identify Thyroperoxidase Inhibitors Within the ToxCast Phase I and II Chemical Libraries. Toxicol Sci 2016, 151, (1), 160–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rotroff DM; Dix DJ; Houck KA; Knudsen TB; Martin MT; McLaurin KW; Reif DM; Crofton KM; Singh AV; Xia MH; Huang RL; Judson RS, Using in Vitro High Throughput Screening Assays to Identify Potential Endocrine-Disrupting Chemicals. Environ Health Persp 2013, 121, (1), 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karmaus AL; Toole CM; Filer DL; Lewis KC; Martin MT, High-Throughput Screening of Chemical Effects on Steroidogenesis Using H295R Human Adrenocortical Carcinoma Cells. Toxicol Sci 2016, 150, (2), 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strickland JD; Martin MT; Richard AM; Houck KA; Shafer TJ, Screening the ToxCast phase II libraries for alterations in network function using cortical neurons grown on multi-well microelectrode array (mwMEA) plates. Archives of Toxicology 2018, 92, (1), 487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hornung RW; Reed LD, Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene 1990, 5, (1), 46–51. [Google Scholar]
- 44.Weschler CJ; Langer S; Fischer A; Beko G; Toftum J; Clausen G, Squalene and Cholesterol in Dust from Danish Homes and Daycare Centers. Environmental Science & Technology 2011, 45, (9), 3872–3879. [DOI] [PubMed] [Google Scholar]
- 45.ASTDR Toxicological profile for polybromiated diphenyl ethers (PBDEs) Agency for Toxic Substances and Disease Registry: Atlanta, Georgia, 2017. [PubMed] [Google Scholar]
- 46.Guo WH; Park JS; Wang YZ; Gardner S; Baek C; Petreas M; Hooper K, High polybrominated diphenyl ether levels in California house cats: House dust a primary source? Environ Toxicol Chem 2012, 31, (2), 301–306. [DOI] [PubMed] [Google Scholar]
- 47.Shen B; Whitehead TP; McNeel S; Brown FR; Dhaliwal J; Das R; Israel L; Park JS; Petreas M, High Levels of Polybrominated Diphenyl Ethers in Vacuum Cleaner Dust from California Fire Stations. Environmental Science & Technology 2015, 49, (8), 4988–4994. [DOI] [PubMed] [Google Scholar]
- 48.Gabriels G; Lambert M; Smith P; Wiesner L; Hiss D, Melamine contamination in nutritional supplements - Is it an alarm bell for the general consumer, athletes, and ‘Weekend Warriors’? Nutr J 2015, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams AJ; Grulke CM; Edwards J; McEachran AD; Mansouri K; Baker NC; Patlewicz G; Shah I; Wambaugh JF; Judson RS; Richard AM, The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J Cheminformatics 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teerlink J; Hernandez J; Budd R, Fipronil washoff to municipal wastewater from dogs treated with spot-on products. Sci Total Environ 2017, 599, 960–966. [DOI] [PubMed] [Google Scholar]
- 51.National Center for Biotechnology Information. PubChem Database. Accessed: July 29, 2019 https://pubchem.ncbi.nlm.nih.gov/compound/Didecyldimethylammonium-chloride#section=Overview [Google Scholar]
- 52.U.S. Environmental Protection Agency (U.S. EPA). Exposure Factors Handbook; U.S. Environmental Protection Agency: Washington, DC, 2011. [Google Scholar]
- 53.Barlow J; Johnson JAP; Scofield L Early Life Exposure to the Phytoestrogen Genistein and Breast Cancer Risk in Later Years: Fact Sheet – Phytoestrogen Genistein; Breast Cancer & The Environment Research Centers; 2007. [Google Scholar]
- 54.Alfonso-Garrido J; Bennett DH; Parthasarathy S; Moschet C; Young TM; McKone TE, Exposure Assessment For Air-To-Skin Uptake of Semivolatile Organic Compounds (SVOCs) Indoors. Environmental Science & Technology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ebele AJ; Abou-Elwafa Abdallah M; Harrad S, Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerging Contaminants 2017, 3, (1), 1–16. [Google Scholar]
- 56.Klepeis NE; Nelson WC; Ott WR; Robinson JP; Tsang AM; Switzer P; Behar JV; Hern SC; Engelmann WH, The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. Journal of Exposure Analysis and Environmental Epidemiology 2001, 11, (3), 231–252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.