Abstract
In tendon, type-I collagen assembles together into fibrils, fibers, and fascicles that exhibit a wavy or crimped pattern that uncrimps with applied tensile loading. This structural property has been observed across multiple tendons throughout aging and may play an important role in tendon viscoelasticity, response to fatigue loading, tendon healing, and development. Previous work has shown that crimp is permanently altered with the application of fatigue loading. This opens the possibility of evaluating tendon crimp as a clinical surrogate of tissue damage. The purpose of this study was to determine how fatigue loading in tendon affects crimp and mechanical properties throughout aging and between tendon types. Mouse patellar tendons (PT) and flexor digitorum longus (FDL) tendons were fatigue loaded while an integrated plane polariscope simultaneously assessed crimp properties at P150 and P570 days of age to model mature and aged tendon phenotypes (N=10–11/group). Tendon type, fatigue loading, and aging were found to differentially affect tendon mechanical and crimp properties. FDL tendons had higher modulus and hysteresis, whereas the PT showed more laxity and toe region strain throughout aging. Crimp frequency was consistently higher in FDL compared to PT throughout fatigue loading, whereas the crimp amplitude was cycle dependent. This differential response based on tendon type and age further suggests that the FDL and the PT respond differently to fatigue loading and that this response is age-dependent. Together, our findings suggest that the mechanical and structural effects of fatigue loading are specific to tendon type and age in mice.
Introduction
Tendinopathy affects many tendon types, with MRI-defined patellar tendinopathy in one study having a prevalence of 28.3%.1 Although the underlying pathophysiology of tendinopathy is not completely defined, the various hierarchical structures of tendon may be prone to damage from the repetitive application of load, and as such contribute in this degenerative process.2–4 The ultrastructural characteristic waviness of collagen, termed crimp, is one of the emergent properties of tendon that may be important in the development of fatigue-induced sub-rupture damage.5 This cumulative damage may be a contributing factor in tissue failure.5
Previous work has shown that crimp is permanently altered with the application of fatigue loading, which opens the possibility of evaluating tendon crimp as a clinical surrogate of tissue damage. Historically, crimp was studied using histological tools such as snap freezing following various applied strains through mechanical testing.6; 7 As tendons are tensioned, the crimp waveform straightens (termed uncrimping). The strain-dependent uncrimping corresponds to the toe region of a tendon’s stress-strain curve.6; 8 Recent work has taken advantage of techniques in which the whole tissue can be analyzed using polarized light imaging integrated into the mechanical testing apparatus.5 In this and our previous work2 we advance the use of this non-destructive, ex-vivo imaging method for analyzing fascicle-level crimp during fatigue testing, which has been shown to be a sensitive metric to assess a tendon’s response to cyclic loading.3; 9–12
Mechanical and crimp properties of tendons exhibit known variations based on tendon type and tendon region.5; 13 Similarly, some of the favorable biochemical and biomechanical properties of tendons are known to decrease with age such as collagen content and modulus.14; 15 The utility of crimp assessment as a potential diagnostic tool for tendinopathy or overuse requires an understanding of how crimp is affected by specific tissue perturbations (e.g., tendon type or age).
While the relationship between aging and tendon structure-function relationships remains an active area of tendon research, there is also evidence that not all tendons age similarly.16–18 In this work, we extend the understanding of the interplay between fatigue-induced and age-related changes in crimp in the murine patellar tendon (PT) and flexor digitorum longus (FDL) tendon. Clinically, while patellar tendinopathy represents the highest incidence of running-related musculoskeletal injuries,19 the FDL is an oft-harvested tendon in the surgical correction of flatfoot deformity.20 Additionally, the structural properties of energy storing tendons, such as the patellar tendon, have been shown to respond differently to fatigue loading.21 Consequently, as emerging work points to the differences in structure, function and biochemistry of tendons based on physio-anatomical type,22–24 we elected to compare the PT, an energy-storing tendon, with the FDL, a positional tendon of the foot.25
The purpose of this study was to evaluate the effect of tendon type and aging on the structural (crimp) and mechanical response of tendon to fatigue loading. We hypothesized that the PT and FDL tendons would show greater mechanical evidence of laxity with advanced age and with increasing fatigue cycling. We also hypothesized that the crimp properties of aged tendons would react differently to fatigue loading when compared with young adult tendons.
Methods
Tissue Preparation
Murine patellar and flexor digitorum longus tendons at 150 and 570 days of age were used, as representative young adult and aged adult tendons (IACUC approved) (Figure 1a).26 Animals were housed (n=5 mice/cage separated by age) in a conventional facility with 12 h light/dark cycles and were fed standard chow and provided water ad libitum. While we did not monitor gait in this mouse model, we have previously identified deficits in hind limb propulsion force, speed, stride length, and step width in our other rodent model, which may relate to potential changes in tissue properties.27 Following euthanasia by carbon dioxide inhalation, mice were weighed (P150: 24.7+/− 1.6 g, P570: 38.8+/− 7.6 g). Patellar tendons (N=10–11/age) from female C57BL/6 mice were prepared using the following protocol: Animals were thawed and the surrounding musculature and soft tissue was removed from the patella-patellar tendon-tibia unit. Each specimen was fine dissected and its cross sectional area was measured in an unloaded state using a custom, high-resolution laser-based device (2µm resolution, <2.7% error 28) coupled with a LVDT stage to control specimen position (5µm resolution (x-axis) and 38µm resolution (y-axis)), whereby equally spaced passes (0.5mm) in the transverse plane covered each tendon’s length.28 The laser system has excellent repeatability for the mouse patellar tendon, with a coefficient of variation of 4.08%.28 The patella-patellar tendon-tibia unit was potted in polymethylmethacrylate, the pot and the patella were gripped with custom fixture and the sample was ready for mechanical testing. FDL tendons (N=10–11/age) from female C57BL/6 mice at 150 and 570 days of age were prepared per the following protocol: The distal FDL, proximal to its split into its terminal tendinous slips in the plantar aspect of the foot was isolated and traced proximally through the tarsal tunnel, where it was released. The tendon was then gently avulsed from its proximal myotendinous origin and each distal slip was transected near its phalangeal insertion. Each specimen was fine dissected and its cross sectional area was measured using the aforementioned custom laser based device.28 FDL specimens were mounted in custom sandpaper grips proximally and distally and were then ready for mechanical testing in a manner analogous to the patellar tendon.
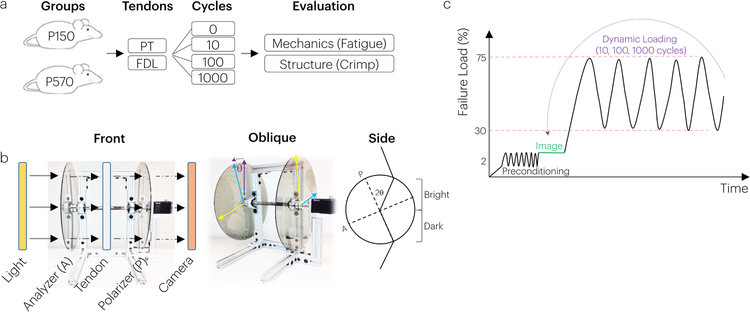
Figure 1. Study Design.
(a) Adult (P150) and aged (P570) mice had their PT and FDL tendons harvested and prepared for fatigue mechanical testing. During loading, mechanical fatigue properties were computed and crimp properties were assessed using a (b) crossed polarizer. (c) The mechanical testing protocol consisted of pre-conditioning and imaging crimp in the toe region followed by cyclic loading and re-imaging.
Mechanical Testing and Image Capture
For mechanical testing, mounted tendons were placed in a 1x PBS bath positioned between two parallel polarized sheets offset at 90 degrees. Image capture of tendon crimp for both PT and FDL was achieved using a set-up in which light would exit a backlight then go through a linear polarizer, the tendon, another linear polarizer and, finally, enter the camera (Basler GigE aca2040gm; resolution: resolution: 3µm, 2048×2048 pixels; Exton, PA) and lens (AF Micro-Nikkor 200mm F/4D IF-ED, TC-201 2X) (Figure 1b). The linear polarizers were oriented at 90 degrees to each other such that the crimp band pattern was visually maximized for each specimen. Tendons were first preloaded (0.05N) and preconditioned between 0.05 and 0.1N. Although this is a low level of preconditioning, even lower levels have been shown to affect collagen fiber reorganization in the mouse supraspinatus tendon.29; 30 Tendons were then imaged at 0.1N, 0.5N and 2.0N, and fatigue tested at 1Hz between 2 and 4N corresponding to 30–75% of their respective failure load and stress of P150 tendons using an Instron Microtester (Instron 5848, Natick MA) as detailed previously (Figure 1c).5 After 10, 100, and 1000 fatigue loading cycles, tendons were returned to imaging loads and re-imaged. Imaging loads were selected to represent the toe, transition, and linear regions of both tendons.5 For each specimen, the tangent modulus (calculated as the slope between the maximum and minimum stress and strain for each loading cycle), hysteresis (calculated as the area enclosed by the stress-strain curve for a given cycle) and laxity (calculated as the ratio of displacement to starting gauge length and assessed at the minimum cyclic force throughout fatigue testing) were computed during loading.5
Image Processing
All images were analyzed using a custom MATLAB program (Version: R2012a, Natick, MA). For every tendon, the midsubstance was evaluated. All images were then processed using a Gaussian low-pass filter; a Fast Fourier Transform (FFT) was then applied and integrated to obtain the cumulative spectral power (CSP). Crimp frequency and crimp amplitude were evaluated at the median CSP. To account for differences in light intensity across samples, samples were evaluated for the change in crimp amplitude (Acrimp,i – Acrimp,0).
Statistical Evaluation
Data normality was assessed and confirmed with Shapiro Wilk tests (SPSS). The effect of cycle number and tendon type was evaluated using two-way repeated measures ANOVAs. The effect of aging and tendon type was evaluated using two-way ANOVAs. Significant factors were evaluated with post-hoc paired or Student’s T-tests with Bonferroni corrections. Significance was set at p<0.05.
Results
Tendon Type and Fatigue Loading Affect Tendon Mechanical and Crimp Properties
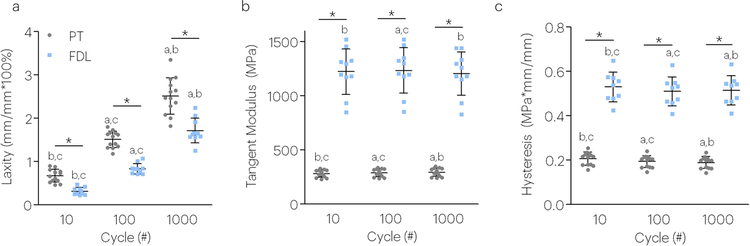
All tendon samples displayed the classic primary and secondary phases of fatigue loading, but did not enter the tertiary phase.31–33 Both tendon type and cycle number significantly affected tendon mechanical fatigue properties. Specifically, tendon laxity increased in response to fatigue loading and both the tangent modulus and hysteresis decreased in both tendon types (Figure 2). Additionally, PT’s had increased laxity throughout fatigue loading compared to FDL tendons (Figure 2a). The decreased laxity of the FDL may be due to its increased tangent modulus and increased hysteresis (Figure 2b,c).
Figure 2. Tendon fatigue properties depend on cycle number and tendon type (P150 tendons).
Both cycle number and tendon type was a significant factor in tendon (a) laxity, (b) tangent modulus, and (c) hysteresis. Data shown as mean and standard deviation. Lines with (*) indicate significant differences between tendons and letters indicate significant differences by cycle number (a-10, b-100, c-1000).
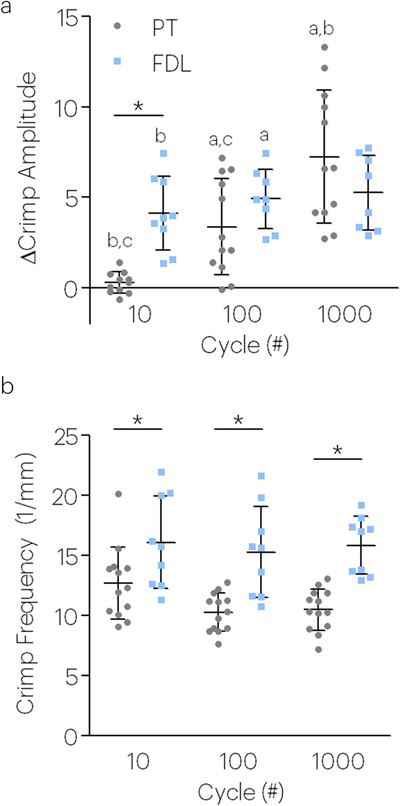
In agreement with the increased laxity observed after fatigue loading in both tendon types, the crimp amplitude also increased after fatigue loading (Figure 3a). Following a similar pattern with the tangent modulus and hysteresis, the crimp frequency was lower in PT than FDL tendons (Figure 3b).
Figure 3. Tendon crimp properties depend on fatigue loading cycle number and tendon type (P150 tendons).
Both cycle number and tendon type were a significant factor in the (a) change in crimp amplitude and (b) crimp frequency. Data for crimp properties was taken at 0.1N. Data shown as mean and standard deviation. Lines with (*) indicate significant differences between tendons and letters indicate significant differences by cycle number (a-10, b-100, c-1000).
Age and Tendon Type Affect Tendon Mechanical and Crimp Properties
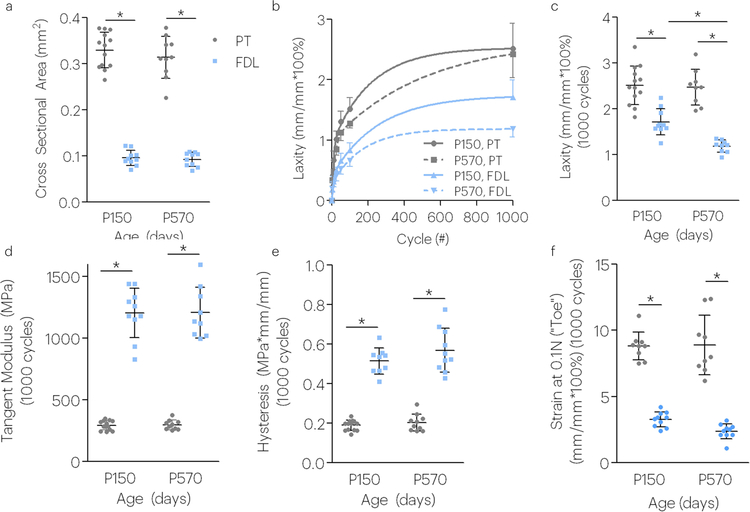
The cross-sectional area was larger in the PT compared to the FDL regardless of age (Figure 4a). After 1000 fatigue loading cycles, tendon laxity was greater in the PT compared to the FDL at both P150 and P570 ages. P150 FDL tendon laxity, however, was greater than that for P570 FDL (Figure 4b,c). The tangent modulus and hysteresis was increased in FDL tendons; however, no effect of aging was detected after 1000 cycles (Figure 4d,e). To evaluate whether changes in the linear region of the stress-strain curve also occurred in the toe region, we evaluated tissue strain at low stresses of these tissues during image capture between fatigue loading cycles (termed low (0.1N). In agreement with the laxity results, the strains assessed at low stress depended on tendon type and age. Aged FDL tendons experienced less strain in response to applied stress, suggesting increased stiffening of the toe region of these groups unlike patellar tendons (Figure 4f).
Figure 4. Aging affects laxity in positional but not energy storing tendons during fatigue loading (P150 and P570 tendons).
Low stress creep response depends on tendon type and aging. Aging affects low stress creep response in positional tendons. Tendon type was a significant factor on the (a) cross sectional area, (b,c) laxity, (d) tangent modulus, (e) hysteresis, and (f) toe region strain. Aging significantly affected the (b,c) tendon laxity. All properties in figures were evaluated after 1000 fatigue loading cycles. Data shown as mean and standard deviation. Lines with (*) indicate significant differences. N=7–12/group.
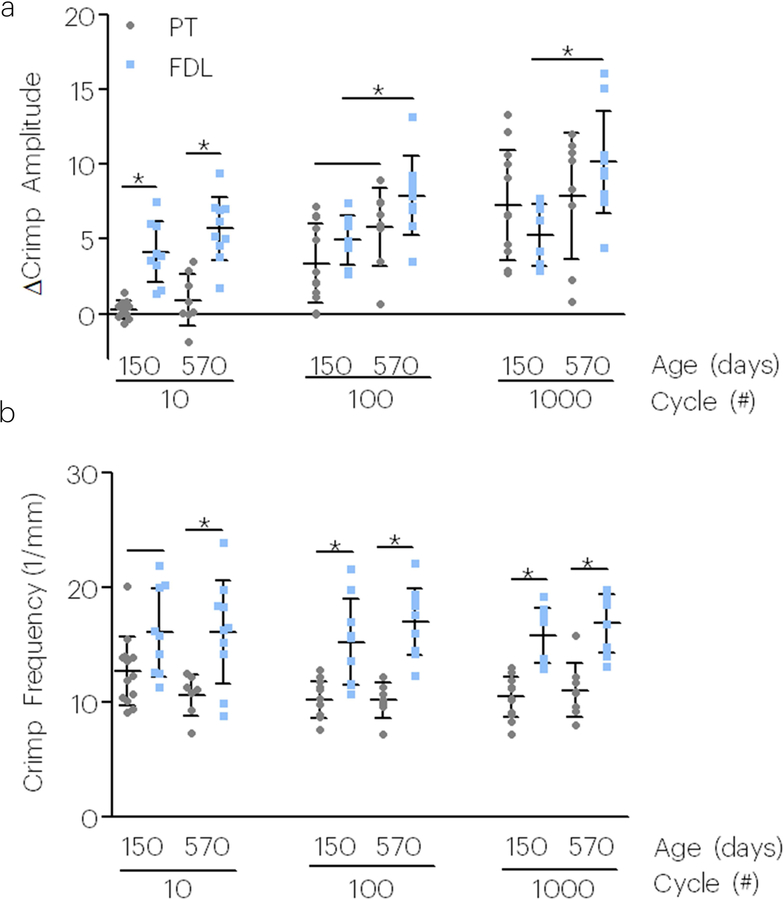
The change in crimp amplitude was greater in young adult and aged FDL tendons compared to PT; however, this was only observed in early fatigue loading. The change in crimp amplitude was not statistically different between PT and FDL tendons at 100 or 1000 fatigue cycles of loading (Figure 5a). However, aged FDL tendons showed an inferior response to cyclic loading, showing increased changes in crimp amplitude in the toe region. The FDL also exhibited increased crimp frequency compared to the PT regardless of tendon age (Figure 5b).
Figure 5. Aging and tendon type affect tendon crimp properties during fatigue loading (P150 and P570 tendons).
Tendon type affected the (a) change in crimp amplitude and (b) crimp frequency, however, tendon age only affected the change in (a) crimp amplitude. Data shown as mean and standard deviation. Lines with (*) indicate significant differences and single lines indicate trends.
Discussion
In clinical orthopaedics, it is well documented that tendons and ligaments degenerate with aging.34; 35 As our tendons are subject to mechanical loads that may be several times body weight, it is important that these tissues withstand repeated loading as we age. However, the capacity of tendons to withstand fatigue loading during aging has remained poorly understood. This study investigated the effects of fatigue loading, tendon type, and aging on tendon mechanical and crimp properties. Tendon crimp is well documented in many tendon types and is commonly studied in humans,36 mice,5 and rabbits.37 This work investigated how tendon mechanics and crimp respond to fatigue loading in tendons of different type and age.
We chose to study the PT and FDL tendons for several reasons. We chose the PT for this and previous studies due to the high prevalence of patellar tendinopathy,38 its ease of harvest, and its ability to allow light transmission for polarized light imaging. More broadly, studying murine tendons enables use in transgenic animals.39; 40 The FDL is a well-studied tendon of the foot and an oft-harvested tendon in clinical orthopaedics.20 Moreover, flexor tendon injuries in the hand are often associated with poor clinical outcomes and the murine FDL is a commonly used model of flexor tendon injury and repair.41–43 Using these two tendons allows for comparison across both tendon structure and physiologic function types (e.g., energy storing versus positional).21
We identified several changes in tissue mechanics and structure between the PT and FDL tendons, including tendon cross sectional area, modulus, hysteresis, laxity, crimp frequency, and crimp amplitude, which agree with similar differences in the superficial digital extensor tendon (SDFT) and common digital extensor tendon (CDET) in the horse.21 We found the positional mouse FDL, for instance, like the positional equine CDET had a higher modulus and hysteresis compared to the energy storing murine PT, and equine SDFT. It is important to acknowledge, however, that the SDFT and CDET are opposing tendons in equine hindfoot function, the PT and FDL are not opposing tendons. Our study also showed that the FDL experienced lower strains at low applied stresses compared to the PT, and showed a significantly increased crimp frequency than PT during fatigue testing. We speculate that the increased crimp frequency in the FDL may be a result of lower strain in the toe region during image capture. This finding is corroborated by equine work which showed increased crimp angles in positional tendons compared to energy storing tendons, which we also observed, but only early in fatigue loading.44 Taken together, this further supports the notion that crimp morphology is unique to tendon anatomical location and age, and similarities may exist across species.
Tendons are known to become more prone to injury and rupture during aging, yet, the biomechanical underpinning of this clinical presentation remains poorly understood.17; 45; 46 The ability of tendon to maintain homeostasis following fatigue loading may be affected by restoration of native multiscale strain transfer mechanisms.3 The differential mechanical and structural response between tendon type and age to fatigue loading may therefore have important implications for multiscale strain transfer and ECM stress transmission. Previously, we demonstrated that fatigue loading increased the crimp amplitude across the tendon width and length, and these structural alterations were shown to be both region and load dependent.5 Later work discovered that fatigue loading negatively altered tendon macroscale mechanical and structural properties.3 At the microscale, fatigue loading abrogated collagen and nuclear reorganization with applied strain, leading to reduced nuclear strain transfer and deficits in ECM stress transmission.3 Recent work investigating aging in craniofacial tendons in zebrafish showed that the overall decline in the tendon modulus of aged tendons is attributable primarily to the loss of non-linearity in the stress-strain curve of aged tendons compared to their young counterparts.47 Indeed, this physiologic non-linearity (toe region) further highlights the role of tendon crimping during aging. Interestingly, while the crimp frequency of PT and FDL was not affected by age or fatigue loading, after 100 cycles of loading, aged FDL tendons exhibited a higher change in crimp amplitude. This could indicate that as flexor tendons age, the ability of the collagenous component of the tendon to return to its pre-fatigue state becomes progressively impaired. Although our results show that there is no significant difference in cross sectional area, modulus, hysteresis, or toe region strain in the mouse PT or FDL with aging, aged FDL tendons had significantly lower laxity and a higher change in crimp amplitude than young tendons. Additionally, the modulus of FDL tendons in aged mice was not different from young specimens, in agreement with recent data,48 however fatigue analysis revealed changes in tissue laxity and crimp amplitude with aging.
Tendon uncrimping plays important roles throughout the body during aging and across species. For instance, both rat tail tendon and equine SDFT tendon crimp frequency and angle decrease with aging,7; 49–51 In this study, aged FDL tendons had increased changes in crimp amplitude across fatigue life compared to adult tendons, suggesting that similar structure-function mechanisms in response to fatigue loading may also occur in smaller animal models. This exciting finding may provide both key insight into broader pathways for tendinopathy across species and become a tool for focused genetic evaluation of these mechanisms using genetic knockout models.
While the use of a non-destructive tool to study tendon crimp properties brings new possibilities, this study is not without limitations. As polarized-light analysis is becoming more widely used to study the structure and function of tendon,12; 52; 53 the interpretation of the outcome parameters such as crimp amplitude may be limited since the values measured are a surrogate for crimp angle. Our previous study has examined specific strains at which crimp disappears during loading.52 Future work will determine the specific strains at which uncrimping occurs following fatigue loading. Another limitation is that this study tests only the acellular component of tendon. Resident cells that comprise tendons are not at play in the tissues we studied, but have been suggested to drive formation of crimp in tendon.54; 55 Future work using the optical measurement of crimp will focus on additional tendon types and regions, genetic knockouts, response to injury48 and eventually in vivo optical measurement of crimp with all the attendant benefits and complexities of a biologically active tissue responding to physiologic load.
Acknowledgements
This study was funded by an NIH/NIAMS supported Penn Center for Musculoskeletal Disorders (NIH, P30 AR050950), a National Science Foundation Graduate Fellowship, and the NIH/NIA (F32 AG057135–02).
Grant Support: This study was funded by an NIH/NIAMS supported Penn Center for Musculoskeletal Disorders (NIH, P30 AR050950), a National Science Foundation Graduate Fellowship, and the NIH/NIA (F32 AG057135–02).
References
- 1.Fairley J, Toppi J, Cicuttini FM, et al. 2014. Association between obesity and magnetic resonance imaging defined patellar tendinopathy in community-based adults: a cross-sectional study. BMC Musculoskelet Disord 15:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thorpe CT, Riley GP, Birch HL, et al. 2014. Effect of fatigue loading on structure and functional behaviour of fascicles from energy-storing tendons. Acta Biomater 10:3217–3224. [DOI] [PubMed] [Google Scholar]
- 3.Freedman BR, Rodriguez AB, Leiphart RJ, et al. 2018. Dynamic Loading and Tendon Healing Affect Multiscale Tendon Properties and ECM Stress Transmission. Sci Rep 8:10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman BR, Mooney DJ. 2019. Biomaterials to Mimic and Heal Connective Tissues. Adv Mater 31:e1806695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman BR, Zuskov A, Sarver JJ, et al. 2015. Evaluating changes in tendon crimp with fatigue loading as an ex vivo structural assessment of tendon damage. J Orthop Res 33:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller KS, Connizzo BK, Feeney E, et al. 2012. Characterizing local collagen fiber re-alignment and crimp behavior throughout mechanical testing in a mature mouse supraspinatus tendon model. J Biomech 45:2061–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gathercole LJ, Keller A. 1991. Crimp morphology in the fibre-forming collagens. Matrix 11:214–234. [DOI] [PubMed] [Google Scholar]
- 8.Franchi M, Fini M, Quaranta M, et al. 2007. Crimp morphology in relaxed and stretched rat Achilles tendon. J Anat 210:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman BR, Fryhofer GW, Salka NS, et al. 2017. Mechanical, histological, and functional properties remain inferior in conservatively treated Achilles tendons in rodents: Long term evaluation. J Biomech 56:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman BR, Gordon JA, Bhatt PR, et al. 2016. Nonsurgical treatment and early return to activity leads to improved Achilles tendon fatigue mechanics and functional outcomes during early healing in an animal model. J Orthop Res 34:2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman BR, Salka NS, Morris TR, et al. 2017. Temporal Healing of Achilles Tendons After Injury in Rodents Depends on Surgical Treatment and Activity. J Am Acad Orthop Surg 25:635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman BR, Sarver JJ, Buckley MR, et al. 2014. Biomechanical and structural response of healing Achilles tendon to fatigue loading following acute injury. J Biomech 47:2028–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hast MW, Zuskov A, Soslowsky LJ. 2014. The role of animal models in tendon research. Bone Joint Res 3:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svensson RB, Heinemeier KM, Couppe C, et al. 2016. Effect of aging and exercise on the tendon. J Appl Physiol (1985) 121:1237–1246. [DOI] [PubMed] [Google Scholar]
- 15.Haut RC, Lancaster RL, DeCamp CE. 1992. Mechanical properties of the canine patellar tendon: some correlations with age and the content of collagen. J Biomech 25:163–173. [DOI] [PubMed] [Google Scholar]
- 16.Agabalyan NA, Evans DJ, Stanley RL. 2013. Investigating tendon mineralisation in the avian hindlimb: a model for tendon ageing, injury and disease. J Anat 223:262–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dressler MR, Butler DL, Wenstrup R, et al. 2002. A potential mechanism for age-related declines in patellar tendon biomechanics. J Orthop Res 20:1315–1322. [DOI] [PubMed] [Google Scholar]
- 18.Legerlotz K, Dorn J, Richter J, et al. 2014. Age-dependent regulation of tendon crimp structure, cell length and gap width with strain. Acta Biomater 10:4447–4455. [DOI] [PubMed] [Google Scholar]
- 19.Lopes AD, Hespanhol Junior LC, Yeung SS, et al. 2012. What are the main running-related musculoskeletal injuries? A Systematic Review. Sports Med 42:891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Backus JD, McCormick JJ. 2014. Tendon transfers in the treatment of the adult flatfoot. Foot Ankle Clin 19:29–48. [DOI] [PubMed] [Google Scholar]
- 21.Thorpe CT, Riley GP, Birch HL, et al. 2014. Fascicles from energy-storing tendons show an age-specific response to cyclic fatigue loading. J R Soc Interface 11:20131058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorpe CT, Peffers MJ, Simpson D, et al. 2016. Anatomical heterogeneity of tendon: Fascicular and interfascicular tendon compartments have distinct proteomic composition. Sci Rep 6:20455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorpe CT, Karunaseelan KJ, Ng Chieng Hin J, et al. 2016. Distribution of proteins within different compartments of tendon varies according to tendon type. J Anat 229:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birch HL. 2007. Tendon matrix composition and turnover in relation to functional requirements. Int J Exp Pathol 88:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Screen HR, Toorani S, Shelton JC. 2013. Microstructural stress relaxation mechanics in functionally different tendons. Med Eng Phys 35:96–102. [DOI] [PubMed] [Google Scholar]
- 26.Dunkman AA, Buckley MR, Mienaltowski MJ, et al. 2013. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol 32:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pardes AM, Beach ZM, Raja H, et al. 2017. Aging leads to inferior Achilles tendon mechanics and altered ankle function in rodents. J Biomech 60:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Favata M 2006. Scarless healing in the fetus: Implications and strategies for postnatal tendon repair Dissertation, University of Pennsylvania Philadelphia. [Google Scholar]
- 29.Miller KS, Connizzo BK, Feeney E, et al. 2012. Examining differences in local collagen fiber crimp frequency throughout mechanical testing in a developmental mouse supraspinatus tendon model. J Biomech Eng 134:041004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller KS, Edelstein L, Connizzo BK, et al. 2012. Effect of preconditioning and stress relaxation on local collagen fiber re-alignment: inhomogeneous properties of rat supraspinatus tendon. J Biomech Eng 134:031007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freedman BR, Zuskov A, Sarver JJ, et al. 2014. Evaluating Changes in Tendon Crimp with Fatigue Loading as an ex vivo Structural Assessment of Tendon Damage. Journal of Orthopaedic Research [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fung DT, Wang VM, Laudier DM, et al. 2009. Subrupture tendon fatigue damage. J Orthop Res 27:264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wren TA, Lindsey DP, Beaupre GS, et al. 2003. Effects of creep and cyclic loading on the mechanical properties and failure of human Achilles tendons. Ann Biomed Eng 31:710–717. [DOI] [PubMed] [Google Scholar]
- 34.Teunis T, Lubberts B, Reilly BT, et al. 2014. A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age. J Shoulder Elbow Surg 23:1913–1921. [DOI] [PubMed] [Google Scholar]
- 35.Milgrom C, Schaffler M, Gilbert S, et al. 1995. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J Bone Joint Surg Br 77:296–298. [PubMed] [Google Scholar]
- 36.Weiss M, Unterhauser FN, Weiler A. 2012. Crimp frequency is strongly correlated to myofibroblast density in the human anterior cruciate ligament and its autologous tendon grafts. Knee Surg Sports Traumatol Arthrosc 20:889–895. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto E, Iwanaga W, Miyazaki H, et al. 2002. Effects of static stress on the mechanical properties of cultured collagen fascicles from the rabbit patellar tendon. J Biomech Eng 124:85–93. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz A, Watson JN, Hutchinson MR. 2015. Patellar Tendinopathy. Sports Health 7:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon JA, Freedman BR, Zuskov A, et al. 2015. Achilles tendons from decorin- and biglycan-null mouse models have inferior mechanical and structural properties predicted by an image-based empirical damage model. J Biomech 48:2110–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connizzo BK, Freedman BR, Fried JH, et al. 2015. Regulatory role of collagen V in establishing mechanical properties of tendons and ligaments is tissue dependent. J Orthop Res 33:882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ackerman JE, Best KT, O’Keefe RJ, et al. 2017. Deletion of EP4 in S100a4-lineage cells reduces scar tissue formation during early but not later stages of tendon healing. Sci Rep 7:8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beason DP, Kuntz AF, Hsu JE, et al. 2012. Development and evaluation of multiple tendon injury models in the mouse. J Biomech 45:1550–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee DJ, Southgate RD, Farhat YM, et al. 2015. Parathyroid hormone 1–34 enhances extracellular matrix deposition and organization during flexor tendon repair. J Orthop Res 33:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spiesz EM, Thorpe CT, Thurner PJ, et al. 2018. Structure and collagen crimp patterns of functionally distinct equine tendons, revealed by quantitative polarised light microscopy (qPLM). Acta Biomater 70:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dudhia J, Scott CM, Draper ER, et al. 2007. Aging enhances a mechanically-induced reduction in tendon strength by an active process involving matrix metalloproteinase activity. Aging Cell 6:547–556. [DOI] [PubMed] [Google Scholar]
- 46.Baumann CW, Kwak D, Liu HM, et al. 2016. Age-induced oxidative stress: how does it influence skeletal muscle quantity and quality? J Appl Physiol (1985) 121:1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah RR, Nerurkar NL, Wang CC, et al. 2015. Tensile properties of craniofacial tendons in the mature and aged zebrafish. J Orthop Res 33:867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackerman JE, Bah I, Jonason JH, et al. 2017. Aging does not alter tendon mechanical properties during homeostasis, but does impair flexor tendon healing. J Orthop Res 35:2716–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diamant J, Keller A, Baer E, et al. 1972. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc Lond B Biol Sci 180:293–315. [DOI] [PubMed] [Google Scholar]
- 50.Patterson-Kane JC, Firth EC, Goodship AE, et al. 1997. Age-related differences in collagen crimp patterns in the superficial digital flexor tendon core region of untrained horses. Aust Vet J 75:39–44. [DOI] [PubMed] [Google Scholar]
- 51.Smith RK, Birch H, Patterson-Kane J, et al. 1999. Should equine athletes commence training during skeletal development?: changes in tendon matrix associated with development, ageing, function and exercise. Equine Vet J Suppl:201–209. [DOI] [PubMed] [Google Scholar]
- 52.Buckley MR, Sarver JJ, Freedman BR, et al. 2013. The dynamics of collagen uncrimping and lateral contraction in tendon and the effect of ionic concentration. J Biomech 46:2242–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freedman B, Rodriguez A, Hillin C, et al. 2017. Tendon healing affects multiscale mechanical, structural, and compositional response of tendon to quasi-static tensile loading [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schiele NR, von Flotow F, Tochka ZL, et al. 2015. Actin cytoskeleton contributes to the elastic modulus of embryonic tendon during early development. J Orthop Res 33:874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Freedman BR, Bade ND, Riggin CN, et al. 2015. The (dys)functional extracellular matrix. Biochim Biophys Acta 1853:3153–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]