Abstract
Considerable research in rodents and humans indicates the hippocampus and prefrontal cortex are essential for remembering temporal relationships among stimuli, and accumulating evidence suggests the perirhinal cortex may also be involved. However, experimental parameters differ substantially across studies, which limits our ability to fully understand the fundamental contributions of these structures. In fact, previous studies vary in the type of temporal memory they emphasize (e.g., order, sequence, or separation in time), the stimuli and responses they use (e.g., trial-unique or repeated sequences, and incidental or rewarded behavior), and the degree to which they control for potential confounding factors (e.g., primary and recency effects or order memory deficits secondary to item memory impairments). To help integrate these findings, we developed a new paradigm testing incidental memory for trial-unique series of events, and concurrently assessed order and item memory in animals with damage to the hippocampus, prefrontal cortex, or perirhinal cortex. We found that this new approach led to robust order and item memory, and that hippocampal, prefrontal and perirhinal damage selectively impaired order memory. These findings suggest the hippocampus, prefrontal cortex and perirhinal cortex are part of a broad network of structures essential for incidentally learning the order of events in episodic memory.
INTRODUCTION
The ability to temporally organize personal experiences in memory is a defining aspect of episodic memory. A number of approaches have been developed to investigate the memory for “when” events occur in rodents and humans (e.g., Hannesson et al., 2004a,b; Dere et al., 2005; Babb and Crystal, 2006; Kart-Teke et al., 2006; Barker et al., 2007; Fouquet et al., 2010; Allen et al., 2014) and considerable evidence indicates the hippocampus (HC) plays a central role in this capacity (Eichenbaum, 2013; Davachi & DuBrow, 2015). For instance, in rodents, HC lesions impair temporal order memory, but not item memory (Chiba et al., 1994; Fortin et al., 2002; Kesner et al., 2002; DeVito & Eichenbaum, 2011; Barker & Warburton, 2011; 2013). Further, HC neurons strengthen and replay spatial sequences in the order that they fired during learning, suggesting memory for sequences of spatial locations (Skaggs & McNaughton, 1996; Farooq et al., 2019). HC neurons have also been found to reliably fire at specific moments during gaps between stimuli (“time cells”; Pastalkova et al., 2008; MacDonald et al., 2013) and to differentiate between items presented in the correct or incorrect sequential position (“sequence cells”; Allen et al., 2016). Similarly, in humans, fMRI studies have shown that the HC is significantly activated during encoding or retrieval of different forms of temporal information about one’s experiences (Cabeza et al., 1997; Hayes et al., 2004; Kumaran & Maguire, 2006; Lehn, et al., 2009; Ross et al., 2009; Ekstrom et al., 2011; Tubridy & Davachi, 2011; Hsieh et al., 2014; Davachi & Dubrow, 2015; Reeders et al., 2018).
The prefrontal cortex (PFC) is another structure thought to play a key role in the temporal organization of memories. In rodents, lesions and temporary inactivations of the medial PFC impair temporal order discriminations for objects and spatial locations (Mitchell & Laiacona, 1998; Hannesson, et al., 2004a,b; Barker, et al., 2007; DeVito & Eichenbaum, 2011; Jayachandran et al., 2019). Further, medial PFC neurons exhibit sustained firing in the gap between stimuli, which may help bridge stimulus associations across time (e.g., Cowen & McNaughton, 2007; Gilmartin & McEchron, 2005). There is also ample evidence from human studies implicating PFC in comparable functions (see St. Jacques, et al., 2008; Jenkins & Ranganath, 2010; Preston & Eichenbaum; 2013; Hsieh & Ranganath, 2015; Reeders et al., 2018).
In addition to the HC and PFC, the perirhinal cortex (PER) may also play an important role. Although PER has been most commonly associated with item memory (Murray et al., 2000; Bussey et al., 2002; Murray et al., 2007; Barker & Warburton, 2011; Feinberg et al., 2012), accumulating evidence suggests it may also contribute to order memory. For example, PER is thought to facilitate unitized representations of events that occur across time, combining temporally discontinuous features into a single perceptual object in memory (Allen et al., 2007; Kholodar-Smith et al., 2008a; Kent & Brown, 2012). PER neurons exhibit persistent firing elicited by synaptic stimulation in vitro, and can last for more than a minute after the stimulation stops, suggesting that PER neurons are capable of linking events across temporal gaps (Navaroli et al., 2012). Most recently, it was shown that silencing synaptic activity in medial PFC-PER projections abolishes memory for well-trained sequences of odors (Jayachandran et al., 2019).
However, it is important to note that there is considerable variation in the paradigms used in the above experiments, which makes it difficult to fully understand the specific contributions of the HC, PFC, and PER. First, paradigms vary in the type of temporal memory they emphasize, including memory for the relative order of events (e.g., B occurred before D), for the specific sequence in which they occurred (e.g., A was followed by B, then by C, then D), or for the temporal separation between items (e.g., A occurred ~5 min ago, B ~1 min ago; see Friedman, 1993; Allen & Fortin, 2013). Second, some paradigms involve incidental learning, a key aspect of episodic memory (Zhou et al., 2012), whereas others (primarily in rodents) reward stimulus presentations or order judgments. Third, some paradigms involve trial-unique series of events, a key feature of episodic memory, whereas others involve repeated presentations of the same events. Finally, some paradigms, also typically in rodents, involve short lists of stimuli (2 or 3 items) so order probes have to include the first and/or last sample items. In such cases, temporal memory judgments cannot control for primacy or recency effects, which may result in differences in memory strength between the items, and for the fact that they could solved by remembering only one of the sample items (e.g., the animal could remember only the last item and then avoid it in the probe test).
The objective of the present study is to help integrate previous findings by concurrently assessing the contribution of HC, PFC and PER to both order and item memory using a new paradigm in the rat. First, building on previous spontaneous preference approaches, we developed a task that tests incidental order and item memory for trial-unique series of events. Notably, the task uses a longer series of events (5 odor presentations), which mitigates the influence of primacy and recency effects, reduces the possibility of using memory for only one item in order judgments, and also offers a better parallel with human studies. Second, we performed selective damage to HC, PFC or PER and directly compared the performance of each group on order and item memory for the same series of events. We found that our new approach led to robust order and item memory, and that HC, PFC or PER damage selectively impaired order memory. These findings suggest that a broad network of structures is critical for incidentally learning the order of events in episodic memory.
METHODS
Subjects
Subjects were male Long Evans rats weighing 250-300 g on arrival (n = 52). Rats were individually housed in clear rectangular polycarbonate cages and maintained on a 12hr light-dark cycle (lights off at 8:00 am). All behavioral testing took place during the dark phase (active period) under ambient red lighting conditions. Access to food and water was unrestricted before surgery. Following surgery, rats were mildly food restricted to maintain 85% of their free-feeding body weight with free access to water throughout testing. All surgical and behavioral methods were in compliance with the University of California Irvine Institutional Animal Care and Use Committee.
Surgeries
Excitotoxic lesions were produced using local infusions of NMDA (Sigma, St. Louis, MO). General anesthesia was induced (5%) and maintained by isoflurane (1 – 2.5%) mixed with oxygen (800 ml/min). Rats were then placed into the stereotaxic apparatus (Stoelting Instruments, Wood Dale, IL) and the scalp was anesthetized with Marcaine® (7.5 mg/ml, 0.5 ml, s.c.). The skull was exposed following a midline incision and adjustments were made to ensure bregma, lambda, and sites ± 0.2 mm lateral to the midline were level. During surgery, all rats were administered glycopyrrulate (0.2 mg/ml, 0.5 mg/kg, s.c.) to help prevent respiratory difficulties and 5 ml Ringer’s solution with 5% dextrose (s.c.) for hydration. After removing the bone overlaying the infusion sites (see below), NMDA was infused into the brain using a 33-gauge 10 μl syringe (Hamilton Company, Reno, NV) driven by a motorized infusion pump (World Precision Instruments, Sarasota, FL) mounted onto a stereotaxic manipulator arm. The needle remained at the injection site for at 5 min after drug infusion to allow for diffusion. Dorsoventral (DV) coordinates were measured from the dura mater. Subjects were randomly assigned to one of the five groups: HC lesion, PFC lesion, PER lesion, secondary visual cortex (V2) control lesions, or sham-operated controls.
HC lesions (n = 11).
The section of bone overlaying the seven HC infusion sites was resected bilaterally and remained hydrated in sterile saline during infusions. The bone section was returned following the infusions. Three bilateral dorsal HC sites were targeted as follows: −2.2 A/P, ±1.0M/L, −3.0 D/V; −3.0 A/P, ±1.8/L, −2.8 D/V; −4.0 A/P, ±2.8 M/L, −2.6 D/V. Four bilateral ventral HC sites were targeted as follows: −4.8 A/P, ±4.8 M/L, 6.5 D/V; −4.8 A/P, ±4.5 M/L, −3.3 D/V; −5.7 A/P, ±4.9 M/L, −2.8, D/V; −5.7 A/P, ±5.1 M/L, −5.8 D/V. Each HC site was infused with 200-225 nL of NMDA (85 mM; 50mg/mL) at 200-250 nL/min.
PFC lesions (n = 12).
Small holes were drilled dorsal to the infusion sites targeting the prelimbic cortex of PFC. PFC was infused bilaterally with 250 nL NMDA (85 mM; 50mg/mL) at 200 nL/min (3.2 A/P, ±0.75 M/L, −3.0 D/V from dura) similar to Sharpe and Killcross (2012).
PER lesions (n = 11).
Two holes were drilled bilaterally (~−4 and −7 mm A/P, ~1 mm medial to the temporal ridge) for anchor screws to hold a tissue spreader (Kholodar-Smith et al., 2008a). Temporal muscles were then pulled away to expose the temporal and parietal bones until the zygomatic arch was visible. The tissue spreader was secured between the anchor screws and the inner surface of the temporal muscles. The bone overlaying the temporal cortex (~2 mm x 5 mm) was resected and the fragment was placed in sterile saline. The bone fragment was returned following the infusions. The syringe (non-coring needle; Hamilton Company, Reno, NV) was positioned at a 45° angle from the vertical surface of the temporal cortex, with the needle eye facing ventral and posterior to direct flow of NMDA toward PER. NMDA infusions (85 mM; 50 mg/mL) were made at 7-8 sites (80 nL per infusion; 70 nL/min; equally spaced at ~0.5 mm) spanning the rostrocaudal extent of PER from −2.8 to −7.6 A/P relative to bregma (Burwell, 2001). Seven injections were made when a large blood vessel was present at an intended infusion site. The needle tip was inserted ~1.5 mm into the cortex relative to dura.
Secondary visual cortex (V2) controls (n = 8).
Small holes were drilled dorsal to the V2 infusion sites. V2 sites were infused with 250 nL NMDA (85 mM) at 200 nL/min (−4.5 A/P, ±2.5 M/L; −0.8 D/V from dura).
Sham-operated controls (n = 10).
These subjects underwent the same surgical procedures as their corresponding lesion group (counts: HC, 4; PFC, 4; PER, 2), except no NMDA infusion was made.
Following lesions, incisions were sutured and dressed with a topical antibiotic. Rats were returned to their home cages and monitored until they woke up. One day following surgery, rats were given an analgesic (Flunixin, 50 mg/ml, 2.5 mg/kg, s.c.) and a topical antibiotic was applied to the incision site. Rats were allowed to recover from surgery for approximately two weeks before behavioral testing.
Odor stimuli
Odorants were presented on 1” round wooden beads (Woodworks Ltd., Haltom City, TX), each scented with a household spice (see Feinberg et al., 2012). Beads were scented for 48 hr in a mixture of playground sand and a single spice. For each rat, odors were selected pseudorandomly to counterbalance odorants over serial positions across subjects, and to avoid repeated odors. Odors were selected from the following list: allspice, anise, basil, bay leaves, cardamom, celery, cinnamon, clove, coriander, cumin, dill weed, fennel, ginger, lemon peel, nutmeg, rosemary, sage, marjoram, mint, orange peel, paprika, thyme, and turmeric. Sand was included to dilute odorants and serve as a consistent background odor for all beads. The odor list, as well as odor concentrations in sand, was determined empirically using an independent cohort of naïve rats to help ensure equal levels of innate preference to the individual odors (data not shown). Rats were familiarized with wooden beads prior to testing by placing several unscented beads in their home cages for at least two days prior to behavioral testing (Spinetta et al., 2008; O’Dell et al., 2011; Feinberg et al., 2012). The familiarity with wooden beads ensured that, during testing, animals focused their investigation on the odor added to the experimental beads.
Testing odor and item memory
Naïve rats were briefly handled for 3 - 5 days after initial arrival and throughout behavioral procedures. All behavioral sessions were performed within each individual rat’s home cage. Behavioral testing started after postsurgical recovery and took place during the dark phase (active period) under ambient red lighting conditions. Rats were maintained at 85% of their free-feeding weight during behavioral testing because we found pilot rats would investigate beads longer and more consistently when mildly food restricted (see also Feinberg, et al., 2012). An hour prior to behavioral testing, food hoppers and water bottles were removed to acclimate rats to testing conditions. A series of five odors was presented as an event sequence, with each odor presentation separated by a 20 min interval (see Figure 1A). Each bead was presented at the center of the front-most quadrant of the cage and investigation times (defined as sniffing and whisking within ~1 cm of the bead) were recorded on a laptop computer using ODLog software (www.macropodsoftware.com). Importantly, to ensure equivalent sampling of all odors in the series, the amount of time spent sampling the first odor (available for a total of 30 s) determined how much time each rat was allowed to sample each subsequent odor (e.g., if a rat spent 4 s investigating odor A, we would ensure that odors B through E were each sampled for 4 s). Testing sessions in which a rat did not explore any sample odor to the same level as the first odor (within a 5 min time window) were not included in the analysis. To prevent cross-contamination, each bead was discarded after any presentation during sampling or testing, and the experimenter changed gloves each time a new bead was used.
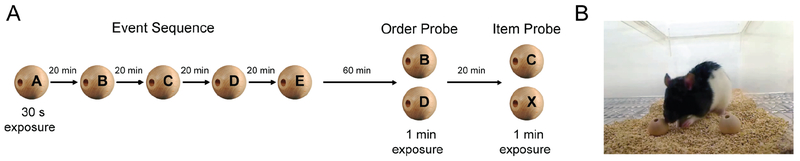
Figure 1. Incidental order and item memory task.
A, Odors (household spices) were presented one at a time on wooden beads in front of the cage and centered. The time spent exploring the first odor (during a 30 s exposure) set the criterion exploration time for the remaining odors in the sequence. After a 60 min retention interval, subjects were given an order probe in which two odors from the sequence were presented (B vs. D) separated by ~6 cm. Preferential exploration on the odor that came earlier (B) indicated memory for order. Twenty minutes later, an item probe was presented in the same way except that it involved a comparison between another odor from the sequence and a novel odor (C vs. X). Preferential exploration toward the novel odor (X) indicated memory for the items presented in the sequence. B, All behavior was performed within each individual rat’s home cage, and active investigation time (sniffing and whisking within 1 cm of bead) was scored for each odor. Each bead was only used once to eliminate the possibility of contamination and/or change in odor strength. Therefore, when a sample odor was presented in the order or item probe, it was on a different bead (which was incubated in the same container for the same period of time).
Memory for the order in which odors were presented, and memory for the odors themselves, was then assessed using an order probe and an item probe (see Figure 1A). The order probe was administered 60 min after the sample list and involved the presentation of two odors from the list (B vs. D). Our pilot work indicated that rats could also perform other order probes above chance levels (e.g. A vs. C, C vs. D), but that performance could vary (similar to findings in Fortin et al., 2002). Thus, a single odor pairing was chosen here to maximize statistical power. Consistent with previous work (e.g., Dere et al., 2005), we expected animals to express memory for the order in which events occurred by preferentially investigating the item that appeared earlier in the series. The item memory probe was administered 20 min after the order probe (~80 min after the sample list) and involved the presentation of two odors: the middle odor from the list and a novel odor (C vs. X). The item probe is an important control to ensure that rats remembered the odors presented on the list, which is expressed as preferential investigation of the novel odor (over the previously encountered odor). Note that for both order and item probes, beads were placed in the same cage quadrant as the sample bead and positioned approximately 3 cm apart (see Figure 1B), with the left/right position counterbalanced across rats. Exploration time for each bead was recorded in ODLog.
Rapidly-presented sequence condition
We also tested the same groups in a more challenging version of the paradigm, in which the sequence of items is more rapidly presented (~45s between items). All procedures, including the retention intervals before the order and item memory probes (60 and 80 min, respectively), were otherwise identical.
Memory strength control condition
We ran a control experiment in a separate cohort of naïve animals to account for the possibility that performance on the order probe simply depends on differences in the memory strength of sampled items. Here, rats were given a series of five odors, with each odor presentation separated by a 20 min interval matching the main task parameters. Subsequently, each rat was presented an odor from the sequence alongside a novel odor (e.g., A vs. V, B vs. W, C vs. X, D vs. Y, E. vs. Z). The interval between the last sample odor and the probe test was 60 min. Each rat received five sessions (in a counterbalanced fashion), in which all comparisons were made (one comparison per session). Only one sequence position was tested per session, per day, with at least one day off between testing sessions. Each session involved a new non-overlapping set of odors.
Data analysis
We computed the total investigation time for each odor and calculated a normalized discrimination index (DI) to quantify preference in the order memory probe (earlier odor vs later odor) and the item memory probe (novel odor vs presented odor):
DI values range from +100 to −100%. Positive values correspond to a preference toward the earlier odor in the order probe, and the novel odor in the item probe. Negative scores correspond to a preference toward the later odor in the order probe, or the previously encountered odor in the item probe. A score of zero indicates no preference for either odor (“chance”). DI scores significantly different from zero are interpreted as evidence of order or item memory, respectively. Each animal was tested three times on each task (using different sets of odors) and the mean score of each rat was used for data analysis.
Statistics were performed using Prism 8 (www.graphpad.com). Group data were analyzed using analysis of variance (ANOVAs) with posthoc tests controlling for the number of comparisons performed (using Holm-Sidak tests or the Bonferroni correction). Group data is expressed as the mean ± standard error of the mean (SEM). Statistical significance was determined using p <. 0.05.
Histology
Rats were administered an overdose of sodium pentobarbital (Euthasol, 390 mg/ml, 150 mg/kg, i.p.) and were transcardially perfused with 100 ml PBS followed by 200 ml of 4% paraformaldehyde (pH 7.4; Sigma-Aldrich, St. Louis, MO). Brains were post-fixed overnight in 4% paraformaldehyde and afterwards placed in a 30% sucrose solution for cryoprotection. Frozen brains were sectioned on a sliding microtome (50 μm; coronal orientation) into four sets of immediately-adjacent sections for a cell body-specific cresyl violet stain and a neuron-specific NeuN stain. Exact methods for each stain are described in detail elsewhere (see Supplementary Materials from Kholodar-Smith et al., 2008a).
Lesion analysis
Using Image J software and Photoshop (version CS6), the extent of neurotoxic damage to the HC, PER, PFC, and V2, as well as lateral entorhinal cortex, infralimbic cortex, and anterior cingulate cortex was estimated on the basis of serial NeuN-stained sections.
RESULTS
Lesion extent
HC lesions.
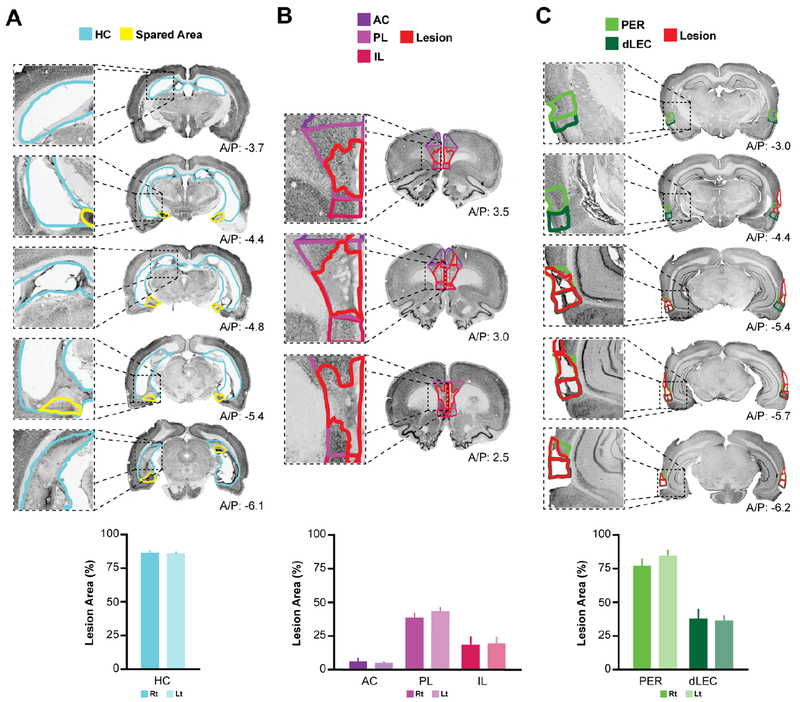
HC lesioned subjects had large and complete lesions to the entire HC while surrounding fibers were spared (Figure 3A). There was a clear lack of HC tissue throughout the rostral-caudal extent of the brains. Two-dimensional lesion area analysis was performed using NeuN-stained sections. Overall, 85.5 ± 2.52% of the hippocampus was lesioned. There was no difference in damage produced in the left hemisphere (85.72 ± 2.77%) compared to the right hemisphere (85.36 ± 2.26%; t10 = 0.17, p = 0.87, paired-samples t-test).
Figure 3. HC, PFC, and PER lesions.
Neurotoxic lesions were made using multiple localized injections of NMDA, which resulted in selective cell loss and atrophy. Slices were stained for NeuN and the percent lesion area was calculated for each region of interest. A, Sample slices from a representative HC lesioned subject (top), and mean lesion area percentage across subjects (bottom; n = 11). B, Sample slices from PFC lesioned subject including all subregions analyzed (AC, PL, and IL), and the mean lesion area percentage across subjects (n = 12). C, Sample slices from a PER lesioned subject and lesion area percentage across subjects (n = 12) in PER (A/P −4.0 to −7.2), and dorsal lateral entorhinal cortex (dLEC).
PFC lesions.
PFC lesioned subjects had large lesions to prelimbic cortex (PL), and to a lesser extent infralimbic cortex (IL; Figure 3B). PL, IL and ACC were included in a quantitative two-dimensional lesion area analysis. PL was the most damaged (40.34 ± 3.25%), followed by IL (18.23 ± 5.85%) and there was very little damage to ACC (5.03 ± 1.60%). The amount of damage to PL is similar to what has been previously found with a similar lesion technique (DeVito & Eichenbaum, 2011), however the extent of damage to extra-PL regions was vastly reduced in this study. Thus, despite minor damage outside the region, any effects of these lesions likely primarily reflect PL function. Using a paired-samples t-test, we found no significant difference in damage in PL to the left hemisphere (37.96 ± 3.55%) compared to the right hemisphere (42.72 ± 3.86%; t11 = −1.40, p = 0.09).
PER lesions.
In PER lesioned subjects, damage was centered in the cortical tissue surrounding the mid-posterior rhinal sulcus (Figure 3C) with 58.32 ± 4.27% of the full extent of PER lesioned (A/P −2.0 to −7.2). The majority of the damage occurred in the posterior PER (A/P −4.0 to −7.2), where the average damage overall was 80.23 ± 4.54%. Using a paired-samples t-test, we found that there was no difference in damage to posterior PER in the left hemisphere (76.34 ± 5.30%) compared to the right hemisphere (84.13 ± 5.08%; t10 = −1.62, p = 0.14). There was also minor damage to the part of lateral entorhinal cortex (LEC) situated immediately ventral to area 35 of PER (36.71 ± 4.21%). The amount of damage is similar to what has been previously found when using this lesion technique (Kholodar-Smith et al., 2008a; Feinberg et al. 2012).
V2 lesions.
V2 lesion rats served as a negative control to demonstrate that damage to cortex overlying HC, in a region not previously associated with sequence memory, does not affect performance in our task. Damage was largely restricted to V2, with 40.38 ± 3.27% damage overall across rats. In four of the rats there was minor damage to CA1 unilaterally, and in two rats there was minor CA1 damage bilaterally. However, this damage did not appear to affect their performance on either order or item probes.
Sham lesions.
HC, PFC, and PER sham (n = 4, 4, and 2, respectively) rats did not show any noticeable evidence of brain damage as assessed with NeuN histological stains. Thus, shams were interpreted as having full and normal neural capabilities during all behavioral experiments, and were combined for subsequent analyses.
Order and item memory
As expected, performance levels were equally high in sham-operated animals and V2-lesioned animals, so we combined them to form the Control group.
Performance on order memory probes.
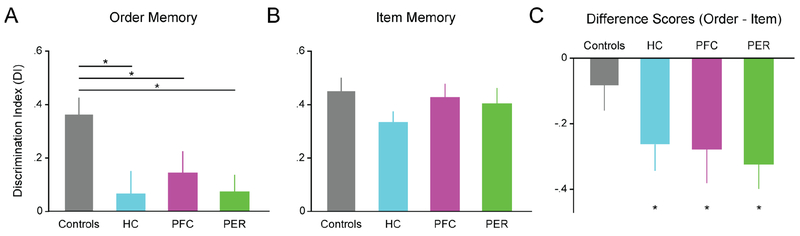
A one-way ANOVA was used to examine differences in Discrimination Index (DI) on the order probe across lesion groups. There was a significant main effect of Group (F3, 48 = 5.084, p=0.0039), and posthoc comparisons showed the control group was significantly different from the HC, PFC and PER groups (Holm-Sidak tests p’s < 0.05). One-sample t-tests showed that the control group was significantly different from chance (DI = 0; t17 = 6.560, p <0.0001), but the lesioned groups were not (HC: t10 = 0.8667, p = 0.4064; PFC: t11 = 1.941, p = 0.0783; PER: t10 = 1.310, p = 0.2196). To limit the number of posthoc tests, pairwise comparisons among HC, PFC, and PER groups were not directly tested; instead, group differences were examined using a Group X Probe interaction (see below). See Figure 2A for a graphical representation of these data.
Figure 2. HC, PFC, or PER lesions impair order memory.
A, Lesions to HC, PFC, or PER significantly impaired order memory compared to controls. B, No significant differences were found following HC, PFC, or PER lesions on item memory. C, Difference scores (order DI minus item DI) were significantly lower than chance (difference score = 0) following HC, PFC, or PER lesions. Controls were not different from chance. Note that each animal was tested three times on each task (using different sets of odors) and the mean score of each rat was used for data analysis. Data shows group means ± SEM.
Performance on item memory probes.
A one-way ANOVA on item memory performance did not show a significant difference across groups (Group effect: F3, 48 = 1.167, p=0.3320), and no group was significantly different from the control group (Holm-Sidak tests p’s > 0.05). Using one-sample t-tests against chance (DI = 0), all groups demonstrated significant preference for the novel odor (odor X) compared to the odor presented in the sequence (odor C; all p’s <0.001). See Figure 2B for a graphical representation of these data.
Direct comparison of order and item probes.
A two-way repeated-measures ANOVA was used to compare group performance across probe types. We found significant main effects of Group (F3, 48 = 5.80, p = 0.002) and Probe (F1, 48 = 32.55, p<0.001). However, the Group X Probe interaction did not reach significance (F3, 48 = 1.96, p=0.133) indicating that the pattern of results did not significantly differ across lesion groups. Post-hoc comparisons revealed that DI scores were significantly lower on the order probes relative to the item probes for the HC, PFC and PER groups (Bonferroni-corrected one-sample t-tests; p’s < 0.017), whereas the control group showed no significant difference. These findings strongly suggest that the deficit observed is selective to order memory and cannot be attributed to a secondary impairment in item memory. These data are displayed in the form of difference scores (DIOrder - DIItem) in Figure 2C.
Control conditions and analyses
Rapid presentation of sample list.
In an effort to dissociate performance across groups, we tested the same animals on a more challenging version of the task in which the sample list was presented more rapidly (~45s between items). We found that all groups showed strong item memory (non-significant Group effect: F3, 41 = 1.48, p=0.24; all groups showing one-sample t-tests above 0, p’s < 0.05). However, none of the groups, including the control group, showed clear order memory under this condition (non-significant Group effect: F3, 41 = 1.09, p = 0.365; mean DI for all groups < 0.2), which makes it difficult to further interpret these results.
Memory strength.
We ran a control experiment in a separate cohort of naïve animals to determine whether the memory strength of the different sample odors was significantly different at the time of the order probe. We found no significant differences in item memory across odor positions (F4, 20 = 0.88, p = 0.49), suggesting that all positions are remembered equally well (i.e., they have the same memory strength). Furthermore, all odor positions were significantly greater than chance exploration times for the novel odor (DI > 0). Thus, it is highly unlikely that memory strength can account for order memory judgments our paradigm.
Odor sampling.
The first odor bead of the sample phase was available to the rat for a total of 30s. Overall, rats actively investigated it for 4.14 ± 1.49 s (mean ± 1 std; averaged over 3 sessions for each subject). This sampling time was compared across sessions and lesion groups using a repeated-measures ANOVA. We found that rats decreased their sampling time over the three sessions (means of 4.82s, 4.67s, and 4.13s respectively; significant main effect of sampling time; F3, 138 = 24.06, p<0.001), but that this effect did not differ across groups (non-significant session x lesion interaction; F9, 138 = 1.18, p=0.31). There was a significant main effect of group (F1,46 = 3.339, p=0.027), though the means were very close (3.85s, 4.39s, 5.00s, and 3.88s for Controls, HC, PFC, and PER, respectively). A post-hoc Holm-Sidak test revealed slightly longer sample times in PFC animals relative to controls (p < 0.037), but no other group differences were observed (p’s > 0.05). This small group difference is unlikely to have confounded our results; although this could have led to slightly higher order and item memory performance in the PFC group. Importantly, that effect is essentially factored out by the difference scores shown in Fig 2C. The key control here is that, for each animal, we equated investigation time within a sequence presentation.
DISCUSSION
Using a new incidental memory paradigm, we assessed the effects of selective damage to the HC, PFC, or PER on order and item memory. We found that each of the three lesioned groups was significantly impaired on order memory relative to controls, and that the deficits were of comparable magnitude. Importantly, we also found that all lesion groups showed normal item memory, indicating that their ability to remember the presented items remained intact (i.e., their deficit was specific to an inability to remember their order). While these structures had previously been shown to be important for different forms of temporal memory, there was considerable variation in task demands across studies and thus a need to assess their contributions within the same experiment. The present study helps integrate these previous findings by demonstrating that the HC, PFC, and PER each play a key role in remembering trial-unique sequences of events, a fundamental feature of episodic memory.
Integrating key features of episodic memory into a single paradigm
Episodic memory involves remembering the series of experiences of our daily life, which are incidentally encoded and retrieved as needed (Tulving, 1972; Allen & Fortin, 2013). Therefore, when modeling episodic memory in animals, it is important to capture the incidental nature of episodic encoding (e.g., Dere et al., 2005; Zhou et al., 2012). To do so, we developed an incidental version of a paradigm we previously used to assess order and item memory, in which animals were explicitly rewarded during item sampling and probe tests (Fortin et al., 2002).
This paradigm goes beyond previous efforts by integrating into a single approach key features of other models of episodic memory (e.g., Chiba et al., 1994; Mitchell & Laiacona, 1998; Fortin et al., 2002; Kesner et al., 2002; Hannesson et al., 2004a,b; Babb and Crystal, 2006; DeVito & Eichenbaum, 2011; Barker & Warburton, 2011; Warburton et al., 2013). First, to focus on incidental encoding and retrieval, odor presentations were not rewarded during the sample list or probe tests. Instead, we took advantage of rodents’ tendency to preferentially explore novel stimuli to assess memory, an approach that has developed and validated by others (see Ennaceur, 2010). More specifically, when presented with two stimuli, we found that control animals preferentially explored the earlier of the two items on the order probe, and the novel odor on the item probe, which we used as an indicator of order and item memory, respectively. We believe preference for the earlier odor is an ethologically-relevant strategy related to optimal foraging behavior (e.g., the rat is more likely to find food or water replenished in an earlier position than a later one because more time has passed; see Allen & Fortin, 2013). This behavior could be supported by associating specific items with their sequential position or representation of the temporal context, or through sequential paired associates (e.g., Allen et al., 2014, Jayachandran et al., 2019; Long & Kahana, 2019). Second, to focus the encoding on the olfactory stimulus, odors were presented on wooden beads that are otherwise identical in all other sensory attributes and each bead was used only once to avoid contamination with the animal or bedding (see Feinberg, et al., 2012; O’Dell, et al., 2012; Spinetta, et al., 2008). In addition, all beads were presented in the same location, and the left/right configurations on the probe tests were counterbalanced across animals and sessions, to make spatial location irrelevant to performance. Third, to control for the possibility that order memory could simply be due to differences in memory strength between the two items, we ensured that the investigation time was equivalent across odors (for each list and animal). Finally, we used a longer sample list than other paradigms (in this case, 5 odor presentations). The use of five items in the sample list allowed us to focus our order and item probes on the middle three items, which were shown to be of comparable memory strength in our control experiment (Dl’s of ~0.6), and avoid order probes involving the first or last sample items (which may be confounded by primacy or recency effects).
One unexpected behavioral finding was that the rapid version of the task (~45 s between items during sampling) failed to show reliable order memory in controls. While our pilot work showed that this rapid version could result in detectable order memory, the control group in the present study was not significantly different from chance. A deeper look at the performance of individual control animals suggests that a subset behaved like our pilot animals while the rest failed to show the effect, which resulted in increased variability. Future use of this task may benefit from systematically varying the interval lengths to help illuminate the relationship between item delays and the reliability/strength of the resultant order memory.
Contributions of HC, PFC and PER to memory for the order of events
In the order probe, we found that controls, a group combining HC, PFC, and PER shams as well as V2 lesions (a negative control), demonstrated a significant preference for the odor that occurred earlier in the sequence (odor B), suggesting that they have intact memory for the order of events. However, rats given a lesion to either HC, PFC, or PER all showed a lack of preference for either odor B or D, and therefore no evidence of order memory. On the item probe, all groups demonstrated significant preference for the novel odor (odor X), suggesting that they had comparable memory for the items presented on the list and, thus, that the order memory deficit was not simply a consequence of a failure to remember the presented odors. This finding of spared item memory following HC, PFC, or PER damage is consistent with previous reports. For instance, it was previously shown that HC or PFC are not necessary for novelty discriminations (Feinberg et al., 2012; Barker, et al., 2007; Fortin, et al., 2002; Mitchell & Laicona, 1998). Furthermore, in a related task, PER lesions did not lead to deficits in odor recognition memory for the type of odorants used here (household odors) at any of the retention intervals tested (5 min to 48 hr), though recognition of social odors was impaired at long retention intervals (Feinberg, et al. 2012).
HC.
Our findings are consistent with previous lesion studies in rodents, which have implicated the HC in order memory using a variety of paradigms (Kesner & Novak, 1982; Chiba, et al., 1994; Mitchell & Laiacona, 1998; Fortin, et al., 2002; Kesner, et al. 2002; DeVito & Eichenbaum, 2011; Barker & Warburton, 2011; 2013) and with neuropsychological and neuroimaging studies in humans (Cabeza et al., 1997; Hayes et al., 2004; Kumaran & Maguire, 2006; Lehn, et al., 2009; Ross et al., 2009; Ekstrom et al., 2011; Tubridy & Davachi, 2011; Hsieh et al., 2014; Davachi & Dubrow, 2015; Reeders et al., 2018; for review Long & Kahana, 2019). Our findings build on these previous studies by showing that the HC plays a key role in the incidental encoding and retrieval of sequences of nonspatial episodes, after controlling for the confounding influence of primacy and recency effects. How the HC performs this function remains to be determined. HC neurons have been shown to provide information about the temporal context in which events occurred (e.g., Manns et al., 2007; MacDonald et al., 2013; Allen et al., 2016) and the HC is thought to use this type of spatiotemporal signal to form episodic memories by binding information about individual events with the spatial and temporal contexts in which they occurred (Allen & Fortin, 2013; Knierim, 2015; Eichenbaum, 2017). Elucidating this process will require recording electrophysiological activity during the incidental encoding and retrieval of sequence of events.
PFC.
PFC has also been implicated in order memory in both spatial and object discrimination tasks in rodents (Barker et al., 2007; Hannesson et al., 2004a,b; DeVito & Eichenbaum, 2011; Mitchell & Laicona, 1998; Fuster, 2001) and humans (Staresina & Davachi, 2009; Jenkins & Ranganath, 2010; Tubridy & Davachi, 2011; Allen & Fortin, 2013). Our data are consistent with this and contributes to the growing body of evidence that PFC is necessary to incidental memory for sequences of nonspatial episodes. Recent findings suggest that PFC may be involved in controlling how sequences are retrieved from HC memory stores dependent on current behavioral demands (Jayachandran et al., 2019; Schmidt et al., 2019). Future studies using transient inactivations in this task may be useful in elucidating the specific role of PFC in the encoding and retrieval of trial-unique event sequences.
PER.
Although silencing medial PFC terminals in PER has been shown to disrupt sequence memory (Jayachandran et al., 2019), this is the first report showing that lesions to PER cause a deficit specific to incidental order memory. This effect is consistent with prior evidence that PER is implicated in bridging temporal memories in trace fear conditioning and unitizing discontinuous stimuli (Kholodar-Smith, et al., 2008a,b; Navaroli, 2012). Additionally, Barker et al., (2007) reported that rats with PER lesions have deficits in order memory, but the selectivity of that effect was unclear as they also found significant deficits in recognition memory. There is also the concern that their study relied on object recognition, which is sensitive to PER lesions (e.g., Murray & Richmond, 2001; Bussey et al., 2005) and that their sequence was only comprised of two-items which can be confounded by primacy and recency effects. The PER effects observed here clarify that the role of PER in memory extends beyond multi-feature object perception by showing the effects of lesions can be specific to memory for order. PER is known to be involved in modulating the flow of information among HC, PFC and entorhinal regions (e.g., Paz et al., 2007), and this modulatory role may be key to the encoding and retrieval of event sequences.
Conclusions
We developed a new incidental order and item memory paradigm that integrates key features from other models of episodic memory, and demonstrated that the HC, PFC and PER are all critical for order memory. While these are important findings, the main shortcoming of the study is that the pattern of results was not significantly different across the three lesion groups and, thus, did not shed light onto the respective contributions of these structures. Our inability to find differences between the lesion groups was primarily due to the experimental design, which included many groups. While this design allowed us to test the role of each structure within the same experiment (a key objective of the study), pairwise comparisons between lesioned groups were impractical due to the need to control for the number of posthoc tests performed. We had hoped the rapidly presented version of this task could help differentiate the roles of these structures across timescales, but unfortunately that alternative version did not result in robust order memory in the control subjects. Another factor that may also have contributed to the lack of differentiation among HC, PFC and PER effects is our use of pretraining lesions, which affected all memory stages (i.e., encoding, consolidation, and retrieval). Future studies using transient inactivations may be more appropriate for revealing differential impairments, by providing an opportunity to target a specific stage. Collectively, these findings suggest that the HC, PFC, and PER are part of a broad network of structures essential for incidentally learning the order of events in episodic memory. Elucidating the specific nature of their respective contributions, as well as their underlying neural mechanisms, will require further investigation.
Acknowledgments:
We would like to thank Clare Quirk and Collin Fuhrman for assistance with behavioral and histological procedures. This research was supported, in part, by the National Science Foundation (awards IOS-1150292 and BCS-1439267 to N.J.F.), NIMH (award MH115697 to N.J.F.), the Whitehall Foundation (award 2010-05-84 to N.J.F.) and the University of California, Irvine.
Footnotes
Conflicts of Interest: The authors declare no competing financial interests.
REFERENCES
- Allen TA, & Fortin N (2013). The evolution of episodic memory. Proceedings of the National Academy of Sciences USA, 110, 10379–10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Furtak SC, & Brown TH (2007). Single-unit responses to 22 kHz ultrasonic vocalizations in rat perirhinal cortex. Behavioural Brain Research, 182(2), 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Morris AM, Mattfeld AT, Stark C, & Fortin NJ (2014). A Sequence of events model of episodic memory shows parallels in rats and humans. Hippocampus 24, 1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TA, Salz DM, McKenzie SA, & Fortin NJ (2016). Nonspatial sequence coding in CA1 neurons. The Journal of Neuroscience, 36(5), 1547–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb SJ, & Crystal JD (2006). Episodic-like memory in the rat. Current Biology, 16(11), 1317–1321. [DOI] [PubMed] [Google Scholar]
- Barker G, Bird F, Alexander V, & Warburton E (2007). Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. The Journal of Neuroscience, 27(11), 2948–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker G, & Warburton E (2011). When is the hippocampus involved in recognition memory? The Journal of Neuroscience, 31(29), 10721–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker G, & Warburton E (2013). Object-in-place associative recognition memory depends on glutamate receptor neurotransmission within two defined hippocampal-cortical circuits: A critical role for AMPA and NMDA receptors in the hippocampus, perirhinal, and prefrontal cortices. Cerebral Cortex, 25(2), 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey T, Saksida L, & Murray E (2002). Perirhinal cortex resolves feature ambiguity in complex visual discriminations. The European journal of neuroscience, 15(2), 365–374. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, & Murray EA (2005). The perceptual-mnemonic/feature conjunction model of perirhinal cortex function. Quarterly Journal of Experimental Psychology B, 58, 269–282. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Mangels J, Nyberg L, Habib R, Houle S, McIntosh AR, & Tulving E (1997). Brain Regions Differentially Involved in Remembering What and When: a PET Study. Neuron, 19, 863–870. [DOI] [PubMed] [Google Scholar]
- Chiba A, Kesner R, & Reynolds A (1994). Memory for spatial location as a function of temporal lag in rats: role of hippocampus and medial prefrontal cortex. Behavioral and Neural Biology, 61(2), 123–131. [DOI] [PubMed] [Google Scholar]
- Cowen SL, & McNaughton BL (2007). Selective delay activity in the medial prefrontal cortex of the rat: contribution of sensorimotor information and contingency. Journal of Neurophysiology, 98(1), 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L & Dubrow S (2015). How the hippocampus preserves order: the role of prediction and context. Trends in cognitive sciences, 19(2), 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Huston JP, & De Souza Silva MA (2005). Integrated memory for objects, places, and temporal order: Evidence for episodic-like memory in mice. Neurobiology of Learning and Memory, 84, 214–221. [DOI] [PubMed] [Google Scholar]
- DeVito L, & Eichenbaum H (2011). Memory for the order of events in specific sequences: Contributions of the hippocampus and medial prefrontal cortex. The Journal of Neuroscience, 31(9), 3169–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Copara MS, Isham EA, Wang W, & Yonelinas AP (2011). Dissociable networks involved in spatial and temporal order source retrieval. NeuroImage, 56, 1803–1813. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H (2013). Memory on time. Trends in Cognitive Sciences, 17(5), 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2017). On the integration of space, time, and memory. Neuron, 95(5), 1007–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A (2010). One-trial object recognition in rats and mice: Methodological and theoretical issues. Behavioural Brain Research, 215(2), 244–254. [DOI] [PubMed] [Google Scholar]
- Farooq U, Sibille J, Liu K, & Dragoi G (2019). Strengthened temporal coordination within pre-existing sequential cell assemblies supports trajectory replay. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg L, Allen T, Ly D, & Fortin N (2012). Recognition memory for social and non-social odors: differential effects of neurotoxic lesions to the hippocampus and perirhinal cortex. Neurobiology of Learning and Memory, 97(1), 7–16. [DOI] [PubMed] [Google Scholar]
- Fouquet C, Tobin C, & Rondi-Reig L (2010). A new approach for modeling episodic memory from rodents to humans: The temporal order memory. Behavioural Brain Research, 215(2), 172–179. [DOI] [PubMed] [Google Scholar]
- Fortin N, Agster K, & Eichenbaum H (2002). Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience, 5(5), 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman WJ (1993) Memory for the time of past events. Psychol Bull 113, 44–66. [Google Scholar]
- Fuster JM (2001). The prefrontal cortex—an update: time is of the essence. Neuron 30(2): 319–333. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, & McEchron MD (2005). Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behavioral Neuroscience, 119(6), 1496–1510. [DOI] [PubMed] [Google Scholar]
- Hannesson D, Howland J, & Phillips A (2004a). Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. The Journal of Neuroscience, 24(19), 4596–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannesson D, Vacca G, Howland J, & Phillips A (2004b). Medial prefrontal cortex is involved in spatial temporal order memory but not spatial recognition memory in tests relying on spontaneous exploration in rats. Behavioural Brain Research, 153(1), 273–285. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Ryan L, Schnyer DM, & Nadel L (2004). An fMRI study of episodic memory: retrieval of object, spatial, and temporal information. Behavioral Neuroscience, 118, 885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LT, Gruber MJ, Jenkins LJ, & Ranganath C (2014). Hippocampal activity patterns carry information about objects in temporal context. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh LT, & Ranganath C (2015). Cortical and subcortical contributions to sequence retrieval: Schematic coding of temporal context in the neocortical recollection network. Neuroimage, 121, 78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayachandran M, Linley S, Schlecht M, Mahler SV, Vertes RP, & Allen TA (2019). Prefrontal pathways provide top down control of memory for sequence of events. Cell Reports, 28, 640–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LJ, & Ranganath C (2010). Prefrontal and medial temporal lobe activity at encoding predicts temporal context memory. The Journal of Neuroscience 30(46): 15558–15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kart-Teke E, De Souza Silva MA, Huston JP, & Dere E (2006). Wistar rats show episodic-like memory for unique experiences. Neurobiology of Learning and Memory, 85, 173–182. [DOI] [PubMed] [Google Scholar]
- Kesner RP, & Novak JM (1982). Serial position curve in rats: Role of the dorsal hippocampus. Science, 218, 173–175. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, & Barua LA (2002). The role of the hippocampus in memory for the temporal order of a sequence of odors. Behavioral Neuroscience, 116(2), 286–290. [DOI] [PubMed] [Google Scholar]
- Kent B, & Brown T (2012). Dual functions of perirhinal cortex in fear conditioning. Hippocampus, 22(10), 2068–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodar-Smith D, Allen T, & Brown T (2008a). Fear conditioning to discontinuous auditory cues requires perirhinal cortical function. Behavioral Neuroscience, 122(5), 1178–1185. [DOI] [PubMed] [Google Scholar]
- Kholodar-Smith D, Boguszewski P, & Brown T (2008b). Auditory trace fear conditioning requires perirhinal cortex. Neurobiology of Learning and Memory, 90(3), 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ (2015). The hippocampus. Current Biology 25(23), R1116–R1121. [DOI] [PubMed] [Google Scholar]
- Kumaran D, & Maguire EA (2006). An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biology, 4(12), e424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn H, Steffenach A, van Strien NM, Veltman DJ, Witter MP, & Håberg AK (2009). A specific role of the human hippocampus in recall of temporal sequences. The Journal of Neuroscience, 29(11), 3475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long NM, & Kahana MJ (2019). Hippocampal contributions to serial-order memory. Hippocampus, 29, 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Carrow S, Place R, & Eichenbaum H (2013). Distinct time cell sequences represent odor memories in immobilized rats. The Journal of Neuroscience, 33(36), 14607–14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Howard MW, & Eichenbaum H (2007). Gradual changes in hippocampal activity support remembering the order of events. Neuron, 56(3), 530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, & Laiacona J (1998). The medial frontal cortex and temporal memory: tests using spontaneous exploratory behaviour in the rat. Behavioural Brain Research 97, 107–113. [DOI] [PubMed] [Google Scholar]
- Murray E, Bussey T, & Saksida L (2007). Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annual Review of Neuroscience, 30, 99–122. [DOI] [PubMed] [Google Scholar]
- Murray E, Bussey T, Hampton R, & Saksida L (2000). The parahippocampal region and object identification. Annals of the New York Academy of Sciences, 911, 166–174. [DOI] [PubMed] [Google Scholar]
- Murray EA, & Richmond BJ (2001). Role of perirhinal cortex in object perception, memory, and associations. Current Opinion in Neurobiology, 11(2), 188–193. [DOI] [PubMed] [Google Scholar]
- Navaroli V, Zhao Y, Boguszewski P, & Brown T (2012). Muscarinic receptor activation enables persistent firing in pyramidal neurons from superficial layers of dorsal perirhinal cortex. Hippocampus, 22(6), 1392–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell S, Feinberg L, & Marshall J (2011). A neurotoxic regimen of methamphetamine impairs novelty recognition as measured by a social odor-based task. Behavioural Brain Research, 216(1), 396–401. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itsov V, Amarasingham A, & Buzsáki G (2008). Internally generated cell assembly sequences in the rat hippocampus. Science, 321, 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (1998). The rat brain in stereotaxic coordinates. New York: Academic Press. [DOI] [PubMed] [Google Scholar]
- Paz R, Bauer EP, & Paré D (2007). Learning-related facilitation of rhinal interaction by medial prefrontal inputs. The Journal of Neuroscience, 27(24), 6542–6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, & Eichenbaum F (2013). Interplay of hippocampus and prefrontal cortex in memory. Current Biology, 23(17), R764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeders PC, Allen TA, & Mattfeld AT (2018). Hippocampus activations reflect temporal contexts while medial prefrontal cortex activations reflect ordinal positions during sequence memory in humans. bioRxiv, 501122. [Google Scholar]
- Ross RS, Brown TI, & Stern CE (2009). The retrieval of learned sequences engages the hippocampus: Evidence from fMRI. Hippocampus, 19(9):790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Duin AA, & Redish AD (2019). Disrupting the medial prefrontal cortex alters hippocampal sequences during deliberative decision making. Journal of Neurophysiology, 121, 1981–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE & McNaughton BL (1996). Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science, 271(5257), 1870–3. [DOI] [PubMed] [Google Scholar]
- Spinetta M, Woodlee M, Feinberg L, Stroud C, Schallert K, Cormack L, & Schallert T (2008). Alcohol-induced retrograde memory impairment in rats: prevention by caffeine. Psychopharmacology, 201(3), 361–371. [DOI] [PubMed] [Google Scholar]
- Staresina BP, & Davachi L (2009). Mind the gap: binding experiences across space and time in the human hippocampus. Neuron, 63(2), 267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Jacques PL, Rubin DC, LaBar K, &Cabeza R. (2008). The short and long of it: Neural correlates of temporal-order memory for autobiographical events. Journal of Cognitive Neuroscience, 20(7), 1327–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubridy S, & Davachi L (2011). Medial temporal lobe contributions to episodic sequence encoding. Cerebral Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E (1972). The organization of memory Organization of Memory, eds Tulving E and Donaldson W New York: Academic, 381–402. [Google Scholar]
- Warburton E, Barker G, & Brown M (2013). Investigations into the involvement of NMDA mechanisms in recognition memory. Neuropharmacology, 74, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Hohmann AG & Crystal JD (2012). Rats answer an unexpected question after incidental encoding. Current Biology, 22(12), 1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]