Abstract
The Ehlers-Danlos syndromes (EDS) and associated hypermobility spectrum disorders (HSD) are a heterogenous group of connective tissue disorders associated with significant morbidity. The urogenital aspects of these disorders are understudied and there is little guidance on the prevalence, types, or outcomes of urogenital complications in EDS/HSD. Our objective was to perform a scoping review to characterize and synthesize the literature reporting urogenital and pelvic complications in EDS/HSD patients. We performed a systematic search of three databases (Medline, CINAHL, Embase) to January 2019. English language, full-text articles reporting on urogenital or pelvic complications in EDS/HSD were included. A total of 105 studies were included (62 case reports/series, 43 observational) involving patients with hypermobile (23%), vascular (20%), classical (12%) EDS, and HSD (24%). Some studies looked at multiple subtypes (11%) or did not report subtype (33%). Reported complications included urinary (41%), gynecological (36%), obstetrical (25%), renal (9%), and men’s health problems (7%), with some studies reporting on multiple areas. Urinary and gynecological complications were most prevalent in patients with HSD, while a broad range of complications were reported in EDS. While further research is required, results suggest a higher index of suspicion for urogenital problems is probably warranted in this population.
Keywords: Ehlers-Danlos syndrome, generalized joint hypermobility, joint hypermobility syndrome, urogenital abnormalities, urogenital system
1 |. INTRODUCTION
The Ehlers-Danlos syndromes (EDS) are a heterogenous group of connective tissue disorders predominantly associated with defective collagen production. The heterogeneity of the syndromes is significant, with 19 genes and hundreds of mutations identified.1 Clinically, EDS is characterized by degrees of connective tissue fragility, particularly in the skin, tendons, ligaments, blood vessels, and hollow organs.1–3 EDS is associated with significant morbidity, including life threatening vessel and organ rupture, debilitating joint instability and pain, and prolonged, complicated wound healing.1,2,4 Significant disability and reduced quality of life frequently occur5–7 due to musculoskeletal problems,8 severe chronic pain,9 and persistent fatigue.10 Overall, EDS remains understudied and there is a lack of data on prevalence, presentations, and natural history.11,12
The prevalence of EDS is reported to be approximately 1 in 5000 births,13,14 however increasing awareness has been tied to increasing incidence.14 A pair of surveys15 involving 10,000 patients with 16 rare diseases found those with EDS experienced the longest delay in diagnosis, with more than half having received an initial misdiagnosis, and 70% of those received inappropriate treatment as a consequence. As EDS may be encountered clinically by a variety of providers, there is a need for broad awareness and increased understanding.
The complexity of EDS has led to several revisions in the recognized subtypes, as shown in Table 1, with the most recent in 2017.1,16,17 This classification recognizes 13 distinct types, with the genetic basis of all known except for hypermobile EDS (hEDS). Hypermobility spectrum disorders (HSD) are a related set of diagnoses often studied in conjunction with hEDS due to phenotypic similarity and uncertainty if HSD represent distinct disorders or are part of a spectrum with hEDS.19,20 In 2018, a 14th type of EDS was identified,18 associated with pathogenic variants in the AEBP1 gene.
TABLE 1.
Classification of EDS subtypes over time
| 1988, Berlin | 1998, Villefranchea | 2017, International |
|---|---|---|
| Type I, Gravis | ||
| Classical type | Classical EDS (cEDS) | |
| Type II, Mitis | ||
| Type III | Hypermobility type | Hypermobile EDS (hEDS) Hypermobility spectrum disordersb (HSD) |
| Type IV (A, B, C, D) | vascular type | Vascular EDS (vEDS) |
| Type VI | Kyphoscoliosis type | Kyphoscoliotic EDS (kEDS) |
| Type VII | ||
| A | Arthrochalasia type | Arthrochalasia EDS (aEDS) |
| B | ||
| C | Dermatospraxis type | Dermatospraxis EDS (dEDS) |
| Type VIII | Periodontitis type | Peridontal EDS (pEDS) |
| Type V | X-linked type | |
| Type IX | Occipital horn syndrome | |
| Type X | Fibronectin-deficient type |
No longer part of EDS |
| Type XI | Familial hypermobility syndrome | |
| Progeroid type | Spondylodysplastic EDS (spEDS) Brittle cornea syndrome (BCS) Cardiac-valvular EDS (cvEDS) Classical-like EDS (clEDS) Myopathic EDS (mEDS) Musculocontractural EDS (mcEDS) AEBP1 variant EDSc |
|
Often referred to as Villefranche, 1997, however not published until 1998.
HSD may include diagnoses such as Joint hypermobility (JH or JHM), Joint hypermobility syndrome (JHS), Benign joint hypermobility (bJHS), and generalized joint hypermobility (GJH).
Not part of the 2017 International classification; was published separately18 in 2018.
The urogenital system and pelvic region contain many collagen-rich tissues including the bladder, uterus, and pelvic ligaments, increasing the concern for related complications in those with EDS.21–23 While there has been some recognition of this clinically, particularly in the field of obstetrics, little information is available on the prevalence, types, or outcomes associated with the urogenital complications of EDS more broadly.1,21
We were unable to identify any prior reviews on this topic, or sufficient studies suitable for a meta-analysis. We therefore undertook a scoping review, a methodology employing a broadly defined research area, systematic search, and primarily descriptive results.24–26 Our goal was to broadly map the existing literature reporting urogenital and pelvic complications in people with EDS and summarize reported complications. We then present a discussion that provides context for findings with complication rates in the general population, as well as research and clinical implications.
2 |. MATERIALS AND METHODS
2.1 |. Search strategy
A systematic review of Medline (PubMed), CINAHL Plus (EBSCOhost), and Embase was conducted until January 2019. Medline was searched using with the following terms:
(“Ehlers-Danlos Syndrome” OR “Ehlers Danlos” OR “Joint Instability” OR “Joint Hypermobility Syndrome” OR “Benign Joint Hypermobility Syndrome” OR “Generalized Joint Hypermobility”) AND (“Sexual Health” OR “Reproductive Health” OR “Reproductive Medicine” OR “Urogenital System” OR “Urogenital Abnormalities” OR “Urogenital Surgical Procedures” OR “Male Urogenital Diseases” OR “Female Urogenital Diseases” OR “Pelvis” OR “Pelvic Floor” OR “Pelvic Floor Disorders” OR “Uterine Rupture”).
Equivalent searches were used with each database. All databases included renal complications under urogenital terms. No filters were applied. Gray literature, including conference abstracts, was searched using ClinicalTrials.gov, CENTRAL, Greylit.org, and Opengrey.eu. References and citations of relevant articles were searched by hand. Full details of the search strategy, including the review protocol, can be found in the Appendix S1.
2.2 |. Study selection
Study titles and abstracts were screened for the following inclusion criteria: (a) Study population diagnosed with any type of EDS included in the 2017 classification or equivalent type under older classifications, or any HSD, (b) study reported on urogenital, including renal, or pelvic complications, (c) English language, (d) full text available.
Venous or arterial complications related to the urogenital system were excluded as vascular in nature. Obstetrical complications relating to the fetus, including placental and fetal membrane problems, and miscarriage, were excluded, but maternal urogenital or pelvic complications such as uterine rupture were included. Opinion, basic science, animal, and review articles were excluded.
2.3 |. Data collection and study assessment
The following information was extracted from included studies: (a) Study design (see Appendix S1 for details); (b) Type(s) of urogenital or pelvic complication reported; (c) For obstetric complications, whether they occurred in the ante-, intra-, or post-partum period; (d) EDS subtype(s) or HSD type(s) in the study population; (e) Sample size; (f) Publication date; (g) Country of origin. When available, participant age, gender, and method(s) of diagnosis were recorded.
3 |. RESULTS
3.1 |. Study selection
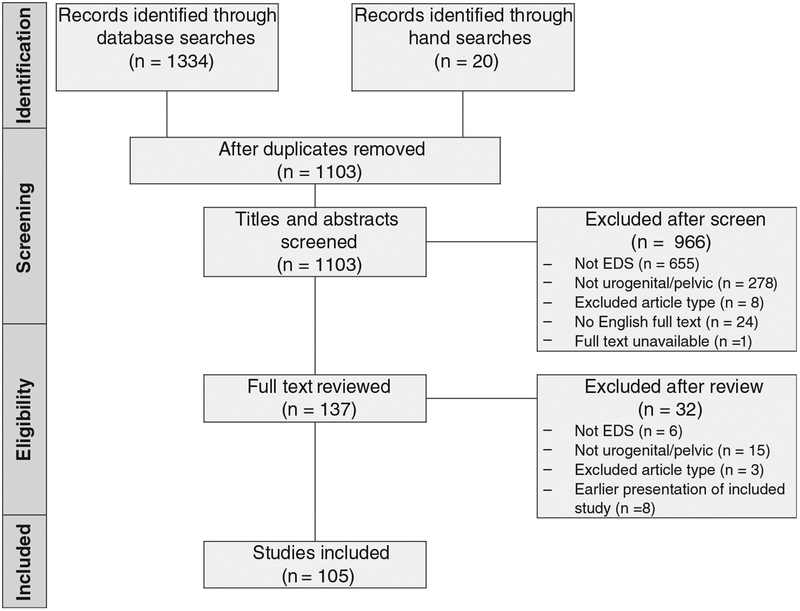
The selection process is summarized in Figure 1. Abstracts for 1103 unique records were screened and 966 were excluded. Of those excluded, 24 were considered potentially relevant but the full texts were unavailable in English. Following screening, 137 records underwent full text review, with the exclusion of an additional 32; thus 105 unique studies were included in our review.
FIGURE 1.
Study selection flowchart
3.2 |. Study characteristics
Table 2 summarizes the design and publication date characteristics of the 105 included studies.27–131 Studies consisted of case reports/series (N = 62, 60%) and observational studies (N = 43, 40%), including cross sectional (N = 29), case-controlled (N = 12), and cohort (N = 2) designs. No clinical trials or treatment outcome studies were found. Publication dates ranged from 1956 to 2018, with 46% published between 2009 and 2018. Publications came from 28 countries, with 52% being from the United States or United Kingdom and 22% from other European and Scandinavia nations. In the observational studies, the mean sample size including controls was 246 (range 11–3475). The total number of patients with EDS or HSD included in all reviewed studies was 5282.
TABLE 2.
Included studies by design and date of publication
| Total | Case report/series | Observational: cross sectional | Observational: case controlled | Observational: cohort | |
|---|---|---|---|---|---|
| 1988 or earlier | 21 (20%) | 19 (90%) | 1 (5%) | 1 (5%) | 0 |
| 1989–1998 | 16 (15%) | 10 (63%) | 5 (31%) | 1 (6%) | 0 |
| 1999–2008 | 20 (19%) | 11 (55%) | 4 (20%) | 5 (25%) | 0 |
| 2009–2019 | 48 (46%) | 22 (46%) | 19 (40%) | 5 (10%) | 2 (4%) |
3.3 |. Population characteristics
The majority (80% of all studies) reported on patients with EDS, including hEDS (23%), vEDS (20%), and cEDS (12%). Of the studies with EDS patients, 43% did not identify the subtype, 43% reported on one subtype, and 11% reported on two or more subtypes. Patients with HSD were included in 24% of reviewed studies. Occurrence of subtypes between observational studies and case reports/series were similar.
The most common primary methods of diagnosis were prior diagnosis from medical records (30%) and use of a standardized criteria, score, or scale (26%). There was significant overlap in the use of the Beighton, Brighton, and Villefranche scales or criteria for diagnosis of hEDS and HSD. Other methods were physical exam without specific criteria (16%), patient self-report (7.5%), genetic testing (7.5%), and skin biopsies (5%). In 8% of studies, diagnostic methodology was unclear or not reported.
The gender distribution of EDS or HSD patients in the studies skewed female, with 63% female only, 20% male only, and 18% both. Gender distribution was similar between study types, and when studies with hEDS or HSD patients were excluded.
There was heterogeneity in the reporting of subject age, including age at time of study participation, age of diagnosis, and age of first complication. Mean age was not reported in 16 studies (13 observational and 3 case reports/series). Of those with available data, the mean patient age was 36 years for observational studies, 25 years for case reports/series. Overall 38 studies included pediatric patients (≤18 years) and nine included geriatric patients (≥65 years).
3.4 |. Urogenital and pelvic complications reported
Complications clustered into five interrelated domains, as shown in Table 3 (a) urinary (including the urinary system, other than the kidneys), (b) renal, (c) gynecological (including the female reproductive tract, genitalia, and sex hormones), (d) obstetric (related to or occurring during the ante-, intra-, or post-partum period), (e) Men’s health (including the male internal or external genitalia, or sex hormones). Some studies reported on multiple domains.
TABLE 3.
Number of studies reporting each complication domain, by disease subtype
| All typesa | cEDS | hEDS | vEDS | Other/multiple EDS types | HSD | EDS and HSD | EDS type NRb | |
|---|---|---|---|---|---|---|---|---|
| Urinary | 43 | 2 | 4 | 1 | 6 | 13 | 2 | 15 |
| Gynecological | 38 | 0 | 8 | 3 | 7 | 10 | 1 | 9 |
| Obstetric | 26 | 3 | 4 | 9 | 2 | 0 | 1 | 7 |
| Renal | 10 | 0 | 1 | 1 | 1 | 1 | 0 | 6 |
| Men’s health | 7 | 0 | 0 | 1 | 2 | 0 | 0 | 4 |
Exceeds total number of studies in review (105) because some studies reported multiple complication domains.
NR, not reported.
3.4.1 |. Urinary
Urinary complications were the most frequently reported overall, and were particularly common in studies of patients with HSD. Specific complications included stress, urge, intercourse, and nocturnal urinary incontinence (UI), vesicoureteral reflux (VUR), multiple and reoccurring bladder diverticula, recurrent urinary tract infections (UTIs), bladder outlet obstruction, bladder pain, dysuria, hematuria, megacystis, urinary retention, and voiding dysfunction.
Several studies found associations between VUR and HSD in pediatric populations. In a sample of 15 children with benign joint hypermobility syndrome (bJHS), a subtype of HSD, Beiraghdar et al36 found 60% had VUR, and van Eerde et al125 identified a significantly higher rate of generalized joint hypermobility (GJH), another subtype of HSD, in VUR patients compared to controls (24.0% vs 6.7%). In a study of 313 children with a history of UTI, Pournasiri et al107 found that GJH was more common in children that also had VUR, and that the prevalence of GJH increased with VUR severity.
Bladder diverticula were reported in 44% of the studies mentioning urinary complications, although the majority (90%) was case reports/series.
Findings on UI were mixed, with 453,81,93,94 of 18 studies finding no association between either EDS or HSD and UI. Among the 14 studies reporting a positive correlation for EDS or HSD and UI, rates between 68% and 84% were reported for adult women.31,45 A case-controlled study by Mastoroude et al91 found the prevalence of UI in women with bJHS to be significantly greater than controls (73% vs 48%). In a prospective longitudinal cohort study of children with hEDS or joint hypermobility syndrome (JHS), Scheper et al112 found UI in 23% overall and 40% among a subset more severely affected by EDS or JHS.
3.4.2 |. Gynecological
The second most frequently reported domain was gynecologic complications. This included multiple types of pelvic organ prolapse (POP) including anterior, posterior, and apical; menstrual complications including irregular menses, intermenstrual bleeding, menorrhagia, and dysmenorrhea; ovarian and uterine abnormalities including premature pubarche, recurrent anovulation, primary ovarian failure, polycystic ovary syndrome, endometriosis, endometrial cysts, endometrial hyperplasia, uterine fibromas, and uterine polyps; pelvic or vulva varicose veins; and pelvic floor dysfunction.
Problems with the genital mucosa were also reported, including vaginal dryness, spontaneous genital skin fissures, genital edema, genital lacerations and bleeding after intercourse, perineal tearing during medical treatment, and recurrent vaginal infections. Berglund and Björck38 found in a sample of 250 women with EDS, 67% reported genital mucosal problems, while Sorokin et al118 reported 25% of women with EDS had vaginal dryness and 8.5% had post-coital bleeding. In two case reports,70,124 such bleeding required emergency medical care.
Pain as a primary gynecological complaint was also reported with vulvodynia, vestibulodynia, dyspareunia, and generalized pelvic pain. In a retrospective cohort study of 386 women with hEDS, Hugon-Rodin et al71 found that over 60% reported dyspareunia. Earlier cross sectional studies43,44,93,118 reported rates in hEDS and other types of EDS of between 30% and 61%.
Women with EDS or HSD are thought to be at higher risk for POP due to the reliance on collagenous tissues for support of these structures. In this review, most (21/23) studies supported this correlation, reporting associations between hypermobility and POP30,33,119 and higher rates or greater severity of POP in those with EDS or HSD as compared to controls.90,101 Two studies reported negative findings. Knoepp et al81 found no associated between bJHS and POP, while McIntosh et al94 found no associated between degree of hypermobility and presence of POP in women with EDS.
3.4.3 |. Obstetric
EDS is known to be associated with the potential for obstetric complications, particularly uterine rupture and post-partum hemorrhage, both of which were found in this review. Seven of the 26 studies reporting obstetric complications included uterine rupture. All patients had vEDS. Two studies reported similar rates, with 2.6% out of 76 pregnancies97 and 2.7% of 183.106
Hemorrhage was reported in 14 studies, and included ante-, intra-, and post-partum hemorrhage. Lind and Wallenburg87 found post-partum hemorrhage in 18.8% of births among EDS patients, vs 7% in a general population group. Several small35,58,88,97 studies reported rates in EDS patients between 10% and 16.7%, with one44 reporting a combined intra and post-partum hemorrhage rate of 19.4%. One retrospective cohort study71 reported a rate of 4.8% from 747 pregnancies, however it was unclear how post-partum hemorrhage was defined and occurrence was self-reported by patients.
Additional obstetric complications included uterine torsion, cervical incompetence, preterm labor, severe perineal tearing, and failure of sutures for episiotomies and C-sections. Separation of the pubic symphysis and coccyx dislocation were also reported.
Several studies reported high rates of complications associated with peripartum joint laxity. Ainsworth and Aulicino29 reported increased laxity during pregnancy in 60% to 79% of cEDS, hEDS, and vEDS patients, while Lind found over 70% of women with various types of EDS reported pelvic pain or instability during pregnancy. Karthikeyan and Venkat-Raman79 found seven out of eight pregnant women with hEDS experienced significant laxity-associated pelvic girdle pain. Two case reports32,66 also highlighted disabling pelvic girdle laxity during pregnancy.
3.4.4 |. Renal
There were two reports each of renal failure and renal insufficiency. Other renal complaints appeared in one study each: anuria, infantile polycystic disease of kidney, kidney stones, medullary sponge kidney, polycystic kidneys, polyuria, renal cysts, renal ptosis, renal hypoplasia, renal tubular acidosis, renomegaly, and tubulointerstitial nephritis.
3.4.5 |. Men’s health
Complications specific to men’s health were limited to case reports/series in patients with EDS, and included four reports of cryptorchidism, two reports of hypogonadism, and one report each of testicular torsion, and tight foreskin requiring surgical correction.
4 |. DISCUSSION
This review found that both male and female patients with EDS and HSD can experience a wide range of urogenital and pelvic complications throughout their lifespan.
Reports of urinary complications were most frequent, with over 40% of studies reporting one or more related problem. While we recognize that comparisons of rates across heterogeneous literature can be problematic, we include reported rates in the general population to provide greater context for our findings. In the general population, VUR is estimated to affect 25% to 40% of children,132 while this review found rates of VUR in EDS and HSD up to 60%. Similarly, this review found higher rates of UI in EDS or HSD (68%-84% in adult women, 23%-40% in children) than has been reported in the general population (25%-45% in adult women,133,134 10% in children133,135). Nearly half of the studies which discussed urinary complications reported patients with bladder diverticulum, although most were case reports. Bladder diverticulum are uncommon in the general population, with one study136 of incidental findings from abdominal CT scans reporting a rate of 0.22% out of over 3000 patients. In general, the literature on urinary complications was suggestive of an increased association with problems such as UI, bladder diverticula, and VUR, but many studies were small and were primarily in HSD. These results warrant further systematic investigations.
This review found significant breadth in the gynecological problems reported. In particular, the rate of dyspareunia (30%-61%) may be greater than in the general population. Dyspareunia in the general population has been reported137 in 7.5% of sexually active women, with some geographic variation138 (3.6% in Australia, 18.6% in Brazil) and higher rates among women post-partum139 (24%). One prior review140 of pain in EDS also noted the occurrence of vulvodynia and dyspareunia. Previous research141 has found that less than a third of women with pelvic pain report it to a medical provider, thus the already high rates found in this review may underrepresent this problem.
Given the tissue fragility associated with EDS there is a plausible mechanism for mucosal complications and the results of this review suggest further research would be beneficial. As compared to the rates in EDS and HSD in this review (25%), rates of vaginal dryness in women among the general population are generally lower, from 5.8% to 19.7%.138 The reported142 general prevalence of post-coital bleeding in women varies widely, but is generally ≤9%, similar to what was found in EDS (8.5%) in this review. The severity of the bleeding reported in some of the EDS patients is notable.
Among the gynecological problems reported were some without a clear physiological, connective tissue explanation, such as ovarian and endometrial problems. Future research should examine the possibility of additional mechanistic pathways not currently recognized which may connect these problems to EDS.
Obstetric risks have long been associated with EDS, and specifically uterine rupture and post-partum hemorrhage. In this review, uterine rupture was only reported in vEDS patients, with a rate of ~3% per pregnancy, as compared to a general population rate of 0.035% out of 110 000 pregnancies.143 Future research should confirm whether uterine rupture is a risk specific to vEDS. The included studies support a risk of hemorrhage for EDS patients, although there was variation in the rates reported (4.8%-18.8%). Rates of post-partum hemorrhage in the general population,144 based on objective measurements of blood loss >500 mL, is reported as 10.6%, with rate based on subjective measurements being 6.09%. Further investigations might seek to identify the extent to which risk in EDS patients differs from the general population, and whether this risk differs between the subtypes of EDS or extends to those with HSD. Aside from rupture and hemorrhage, the reports of disabling increases in joint laxity during pregnancy (60%-87%) were of particular note. Pregnancy has been found to increase both local and generalized joint laxity in healthy subjects,145,146 although specific rates were not available.147 Primary care providers and obstetricians should be aware of this risk for women that have EDS or HSD and are or are planning to become pregnant.
Reports of renal and men’s health complications were infrequent and diffuse. A link with EDS or HSD appears to be less likely.
Due to the genetic and phenotypic heterogeneity of EDS, research that evaluates complications on a subtype specific basis is particularly important for guiding clinical care. In this review, patients with hEDS or HSD most often reported gynecological or urinary complications, and those subtypes combined accounted for greater than 40% of the reports in those domains. The frequency of such complications among those with HSD is notable, as these disorders are commonly thought to involve primarily joint hypermobility and musculoskeletal problems, with few systemic effects. While reports involving HSD were almost exclusively limited to gynecological and urinary domains, complications in patients with hEDS were widely distributed. Similarly, vEDS patients reported a range of urogenital complications, in addition to prominent obstetric risks.
Interestingly, the literature to date has disproportionally reported on female patients, despite EDS being a possibly autosomal disorder. Previous research3,4,148 has found a higher than expected prevalence of hEDS in women relative to men, with hormonal differences considered a possible factor. In this review, we found that a significant gender gap in the literature existed even when studies with hEDS and HSD patients were excluded. Further investigation into gender-specific prevalence and risks may be warranted.
4.1 |. Research and clinical considerations
Nearly half of the research on urogenital and pelvic complications in EDS and HSD has been produced within the past decade, however the literature remains saturated with case reports and observational studies with inherently limited insight on prevalence and risk. This gap, in light of the numerous existing reports of complications, represents an important area for future investigation.
Because of the heterogeneity of EDS and the ongoing efforts to clarify and describe its subtypes, the application of consistent and valid diagnostic methodologies is essential. We suggest future studies utilize current, standardized diagnostic criteria specific to each sub-type, evaluate patients to exclude related connective tissue disorders, and use genetic testing for diagnostic confirmation whenever available. Reporting data particular to subtypes in a mixed population would be informative. Additionally, because of the ongoing efforts to clarify whether hEDS and HSD are related, we suggest future studies include a comprehensive evaluation using the 2017 International criteria to either diagnose or exclude a diagnosis of hEDS. The most common diagnostic methods found in this review are not adequate to differentiate between hEDS and HSD.
Due to the variety of problems reported, patients in this review presented to emergency providers, urologists, nephrologists, gynecologists, obstetricians, and primary care physicians with both acute and chronic complications, highlighting the need for a broad awareness of EDS and HSD. Clinicians seeing patients with these disorders should be aware of the potential for increased risk of urogenital and pelvic complications. While the findings of this review cannot recommend specific screening or management strategies, results suggest an increased level of attention to related symptoms is appropriate. All providers should be alert to reports or complaints by patients of urogenital symptoms, and primary care or relevant specialists should consider proactively asking patient with EDS/HDS about urinary or gynecological symptoms. Specific areas of concern include urinary symptoms, such as recurrent UTIs or incontinence, and gynecological symptoms such as pain or prolapse. Further research is necessary to direct clinical practice, including identifying patients who may benefit from screening or preventative strategies, and assessing treatment outcomes to determine best practices in management.
5 |. LIMITATIONS
The conclusions of this article are limited by the inherent preliminary nature of scoping reviews and the limitations of the included papers. Specific estimates of prevalence and risk cannot be drawn. Included studies did not undergo critical appraisal. Studies in the review included those examining urogenital or pelvic complications as the primary topic, and those reporting such problems as incidental findings. While results were presented by subtype when possible, many included studies did not identify the subtype or did not report results by subtype and so the findings of this review may not accurately represent subtype-specific risk or prevalence. Nearly two dozen potentially relevant non-English language articles were excluded. As the majority was case studies, we do not believe their exclusions significantly altered the findings of this review.
6 |. CONCLUSION
While there has been a significant increase in reporting on urogenital and pelvic complications of EDS within the past decade, the literature remains saturated with case reports and series and observational studies in which statistical insights and generalizability are limited. While specific recommendations cannot be made based on the results of this review, the overall findings do suggest that clinicians should have an elevated index of suspicion for urogenital complications in patients with EDS or HSD. The lack of rigorous studies on complication prevalence or risk represents a significant area of need and important opportunity for future research.
Supplementary Material
ACKNOWLEDGEMENTS
Dr. Yeh was supported by NIH NCCIH K24AT009465. This work was not supported by any other funding.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author (EG), upon reasonable request.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Malfait F, Francomano C, Byers P, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175(1):8–26. 10.1002/ajmg.c.31552. [DOI] [PubMed] [Google Scholar]
- 2.Jobling R, D’Souza R, Baker N, et al. The collagenopathies: review of clinical phenotypes and molecular correlations. Curr Rheumatol Rep. 2014;16(1):394 10.1007/s11926-013-0394-3. [DOI] [PubMed] [Google Scholar]
- 3.Tinkle B, Castori M, Berglund B, et al. Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome type III and Ehlers-Danlos syndrome hypermobility type): clinical description and natural history. Am J Med Genet C Semin Med Genet. 2017;175(1):48–69. 10.1002/ajmg.c.31538. [DOI] [PubMed] [Google Scholar]
- 4.De Paepe A, Malfait F. The Ehlers-Danlos syndrome, a disorder with many faces. Clin Genet. 2012;82(1):1–11. 10.1111/j.1399-0004.2012.01858.x. [DOI] [PubMed] [Google Scholar]
- 5.Bovet C, Carlson M, Taylor M. Quality of life, unmet needs, and iatrogenic injuries in rehabilitation of patients with Ehlers-Danlos syndrome hypermobility type/joint hypermobility syndrome. Am J Med Genet A. 2016;170(8):2044–2051. 10.1002/ajmg.a.37774. [DOI] [PubMed] [Google Scholar]
- 6.Murray B, Yashar BM, Uhlmann WR, Clauw DJ, Petty EM. Ehlers-Danlos syndrome, hypermobility type: a characterization of the patients’ lived experience. Am J Med Genet A. 2013;161(12):2981–2988. 10.1002/ajmg.a.36293. [DOI] [PubMed] [Google Scholar]
- 7.Scheper MC, Juul-Kristensen B, Rombaut L, Rameckers EA, Verbunt J, Engelbert RH. Disability in adolescents and adults diagnosed with hypermobility-related disorders: a meta-analysis. Arch Phys Med Rehabil. 2016;97(12):2174–2187. 10.1016/j.apmr.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Rombaut L, Malfait F, Cools A, De Paepe A, Calders P. Musculoskeletal complaints, physical activity and health-related quality of life among patients with the Ehlers–Danlos syndrome hypermobility type. Disabil Rehabil. 2010;32(16):1339–1345. 10.3109/09638280903514739. [DOI] [PubMed] [Google Scholar]
- 9.Voermans NC, Knoop H, Bleijenberg G, van Engelen BG. Pain in Ehlers-Danlos syndrome is common, severe, and associated with functional impairment. J Pain Symptom Manage. 2010;40(3):370–378. 10.1016/j.jpainsymman.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Voermans NC, Knoop H, van de Kamp N, Hamel BC, Bleijenberg G, van Engelen BG. Fatigue is a frequent and clinically relevant problem in Ehlers-Danlos syndrome. Semin Arthritis Rheum. 2010;40(3):267–274. 10.1016/j.semarthrit.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Castori M Ehlers-Danlos syndrome, hypermobility type: an under-diagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations. ISRN Dermatol. 2012;2012:1–22. 10.5402/2012/751768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulli A, Talarico R, Scirè CA, et al. Ehlers-Danlos syndromes: state of the art on clinical practice guidelines. RMD Open. 2018;4(suppl 1): e000790 10.1136/rmdopen-2018-000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castori M, Morlino S, Celletti C, et al. Re-writing the natural history of pain and related symptoms in the joint hypermobility syndrome/-Ehlers-Danlos syndrome, hypermobility type. Am J Med Genet A. 2013;161(12):2989–3004. 10.1002/ajmg.a.36315. [DOI] [PubMed] [Google Scholar]
- 14.Vanakker O, Callewaert B, Malfait F, Coucke P. The genetics of soft connective tissue disorders. Annu Rev Genomics Hum Genet. 2015;16:229–255. 10.1146/annurev-genom-090314-050039. [DOI] [PubMed] [Google Scholar]
- 15.Kole A, Faurisson F. The Voice of 12,000 Patients. Rare Diseases, Europe: EURORDIS; 2009. [Google Scholar]
- 16.Beighton P, De Paepe A, Danks D, et al. International nosology of heritable disorders of connective tissue, Berlin, 1986. Am J Med Genet. 1988;29(3):581–594. [DOI] [PubMed] [Google Scholar]
- 17.Beighton P, Paepe AD, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Am J Med Genet. 1998;77(1):31–37. . [DOI] [PubMed] [Google Scholar]
- 18.Blackburn PR, Xu Z, Tumelty KE, et al. Bi-allelic alterations in AEBP1 lead to defective collagen assembly and connective tissue structure resulting in a variant of Ehlers-Danlos syndrome. Am J Hum Genet. 2018;102(4):696–705. 10.1016/j.ajhg.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castori M Hakim A, Contemporary approach to joint hypermobility and related disorders. Curr Opin Pediatr. 2017;29(6):640–649. 10.1097/MOP.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 20.Castori M, Tinkle B, Levy H, Grahame R, Malfait F, Hakim A. A framework for the classification of joint hypermobility and related conditions. Am J Med Genet C Semin Med Genet. 2017;175(1):148–157. 10.1002/ajmg.c.31539. [DOI] [PubMed] [Google Scholar]
- 21.Hakim AJ, Sahota A. Joint hypermobility and skin elasticity: the hereditary disorders of connective tissue. Clin Dermatol. 2006;24(6):521–533. 10.1016/j.clindermatol.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Ionescu O-C, Bacalbaa N, B[notdef]anceanu G. The importance of the pathophysiology of the pelvic organ prolapse in understanding the symptoms of the pelvic floor disorders. Ginecoeu. 2017;13(4):151–154. 10.18643/gieu.2017.151. [DOI] [Google Scholar]
- 23.Norton PA. Pelvic floor disorders: the role of fascia and ligaments. Clin Obstet Gynecol. 1993;36(4):926–938. [DOI] [PubMed] [Google Scholar]
- 24.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 25.Colquhoun HL, Levac D, O’Brien KK, et al. Scoping reviews: time for clarity in definition, methods, and reporting. J Clin Epidemiol. 2014;67(12):1291–1294. 10.1016/j.jclinepi.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5(5, 1):69 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams B, Tharma S. Bilateral sacrospinous ligament fixation in a patient with Ehlers-Danlos syndrome. J Obstet Gynaecol J Inst Obstet Gynaecol. 2005;25(2):226–227. 10.1080/01443610500051619. [DOI] [PubMed] [Google Scholar]
- 28.Adib N, Davies K, Grahame R, Woo P, Murray KJ. Joint hypermobility syndrome in childhood. A not so benign multisystem disorder? Rheumatology. 2005;44(6):744–750. 10.1093/rheumatology/keh557. [DOI] [PubMed] [Google Scholar]
- 29.Ainsworth SR, Aulicino PL. A survey of patients with ehlers-danlos syndrome. Clin Orthop. 1993;286:250–256. 10.1097/00003086-199301000-00037. [DOI] [PubMed] [Google Scholar]
- 30.Al-Rawi ZS, Al-Rawi ZT. Joint hypermobility in women with genital prolapse. Lancet Lond Engl. 1982;1(8287):1439–1441. [DOI] [PubMed] [Google Scholar]
- 31.Arunkalaivanan AS, Morrison A, Jha S, Blann A. Prevalence of urinary and faecal incontinence among female members of the hypermobility syndrome association (HMSA). J Obstet Gynaecol J Inst Obstet Gynaecol. 2009;29(2):126–128. 10.1080/01443610802664747. [DOI] [PubMed] [Google Scholar]
- 32.Atalla A, Page I. Ehlers-Danlos syndrome type III in pregnancy. Obstet Gynecol. 1988;71(3 Pt 2):508–509. [PubMed] [Google Scholar]
- 33.Aydeniz A, Dikensoy E, Cebesoy B, Altındağ Ö, Gürsoy S, Balat Ö. The relation between genitourinary prolapse and joint hypermobility in Turkish women. Arch Gynecol Obstet. 2010;281(2):301–304. 10.1007/s00404-009-1103-3. [DOI] [PubMed] [Google Scholar]
- 34.Bade JJ, Ypma AFGVM, van Elk P, Mensink HJA. A pelvic mass: bladder diverticulum with haemorrhage in Ehlers-Danlos patient. Scand J Urol Nephrol. 1994;28(3):319–321. 10.3109/00365599409181289. [DOI] [PubMed] [Google Scholar]
- 35.Beighton P Obstetric aspects of the Ehlers-Danlos syndrome. BJOG. 1969;76(2):97–100. 10.1111/j.1471-0528.1969.tb05801.x. [DOI] [PubMed] [Google Scholar]
- 36.Beiraghdar F, Rostami Z, Panahi Y, Einollahi B, Teimoori M. Vesicourethral reflux in pediatrics with hypermobility syndrome. Nephro-Urol Mon. 2013;5(4):924–927. 10.5812/numonthly.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger J, Lang E, Schaeffer EM. Urological radiographic manifestations of the Ehlers-Danlos syndrome. J Urol. 2007;178(4):1490 10.1016/j.juro.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 38.Berglund B, Björck E. Women with Ehlers-Danlos syndrome experience low oral health–related quality of life. J Orofac Pain. 2012;26:307–314. [PubMed] [Google Scholar]
- 39.Breivik N, Refsum S Jr, Oppedal BR, Vesterhus P. Ehlers-Danlos syndrome and diverticula of the bladder. Z Kinderchir. 1985;40(4):243–246. [DOI] [PubMed] [Google Scholar]
- 40.Burlacu D, Landon C. The prevalence of joint hypermobility syndrome in women undergoing pelvic floor surgery and the risk of repeat surgery: a prospective study. BJOG. 2017;124:46–49. 10.1111/1471-0528.14_14571.28856860 [DOI] [Google Scholar]
- 41.Burrows M, Harrison P. Giant bladder diverticulum in Ehlers-Danlos syndrome type I causing outflow obstruction. Clin Exp Dermatol. 1998;23(3):109–112. 10.1046/j.1365-2230.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 42.Carley ME, Schaffer J. Urinary incontinence and pelvic organ prolapse in women with Marfan or Ehlers-Danlos syndrome. Am J Obstet Gynecol. 2000;182(5):1021–1023. 10.1067/mob.2000.105410. [DOI] [PubMed] [Google Scholar]
- 43.Castori M, Camerota F, Celletti C, et al. Natural history and manifestations of the hypermobility type Ehlers-Danlos syndrome: a pilot study on 21 patients. Am J Med Genet A. 2010;152A(3):556–564. 10.1002/ajmg.a.33231. [DOI] [PubMed] [Google Scholar]
- 44.Castori M, Morlino S, Dordoni C, et al. Gynecologic and obstetric implications of the joint hypermobility syndrome (a.k.a. Ehlers-Danlos syndrome hypermobility type) in 82 Italian patients. Am J Med Genet A. 2012;158A(9):2176–2182. 10.1002/ajmg.a.35506. [DOI] [PubMed] [Google Scholar]
- 45.Chan C, Krahe A, Lee YT, Nicholson LL. Prevalence and frequency of self-perceived systemic features in people with joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type. Clin Rheumatol. 2018;38:503–511. 10.1007/s10067-018-4296-7. [DOI] [PubMed] [Google Scholar]
- 46.Chiu L, Chiu N, Poon M, et al. Radiotherapy for a cervix cancer patient with Ehlers-Danlos syndrome: a case report. 2013;5. [Google Scholar]
- 47.Cuckow PM, Blackhall RJS, Mouriquand PD. Huge bladder diverticula associated with Ehlers-Danlos syndrome. 1994;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das MN, Ghorpade A, Mercy P, Pandey T, Sharma R. Ehlers danlos syndrome in two siblings. Indian J Dermatol Venereol Leprol. 2005;71(3):186 10.4103/0378-6323.16235. [DOI] [PubMed] [Google Scholar]
- 49.Day HJ, Zarafonetis CJ. Coagulation studies in four patients with Ehlers-Danlos syndrome. Am J Med Sci. 1961;242:565–573. [PubMed] [Google Scholar]
- 50.De Cunto CL, Ferraris J, Liberatore D. Hypermobility of the joints in pediatric patients with endstage renal disease. J Rheumatol. 1992;19(6):1003. [PubMed] [Google Scholar]
- 51.de Kort LMO, Verhulst JAPM, Engelbert RHH, Uiterwaal CSPM, de Jong TPVM. Lower urinary tract dysfunction in children with generalized hypermobility of joints. J Urol. 2003;170(5):1971–1974. 10.1097/01.ju.0000091643.35118.d3. [DOI] [PubMed] [Google Scholar]
- 52.De Paepe A, Thaler B, Van Gijsegem M, Van Hoecke D, Matton M. Obstetrical problems in patients with Ehlers-Danlos syndrome type IV; a case report. Eur J Obstet Gynecol Reprod Biol. 1989;33(2):189–193. 10.1016/0028-2243(89)90214-1.. [DOI] [PubMed] [Google Scholar]
- 53.Derpapas A, Cartwright R, Upadhyaya P, Bhide AA, Digesu AG, Khullar V. Lack of association of joint hypermobility with urinary incontinence subtypes and pelvic organ prolapse. BJU Int. 2015;115(4):639–643. 10.1111/bju.12823. [DOI] [PubMed] [Google Scholar]
- 54.Deveaud CM, Kennedy WA, Zderic SA, Howard PS. Biochemical and physiological characterization of the urinary bladder in Ehlers-Danlos syndrome. Adv Exp Med Biol. 1999;462:201–214. discussion 225–233. [DOI] [PubMed] [Google Scholar]
- 55.Dickson MJ, Mahmood S. Urinary stress incontinence in nulliparous teenagers and adolescent women as the presenting feature of underlying medical disorders. BJOG. 2013;120:347–356. 10.1111/1471-0528.12298. [DOI] [Google Scholar]
- 56.Dordoni C, Ritelli M, Venturini M, et al. Recurring and generalized visceroptosis in Ehlers-Danlos syndrome hypermobility type. Am J Med Genet A. 2013;161(5):1143–1147. 10.1002/ajmg.a.35825. [DOI] [PubMed] [Google Scholar]
- 57.Dragomir Kirchner S, Bayle N, Hutin E, Hamonet C, Gracies J-M, Loche C-M. Urodynamic study of lower urinary tract symptoms in Ehlers Danlos syndrome. Ann Phys Rehabil Med. 2013;56:e231–e232. 10.1016/j.rehab.2013.07.595. [DOI] [Google Scholar]
- 58.Drera B, Zoppi N, Ritelli M, et al. Diagnosis of vascular Ehlers-Danlos syndrome in Italy: clinical findings and novel COL3A1 mutations. J Dermatol Sci. 2011;64(3):237–240. 10.1016/j.jdermsci.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Eadie DGA, Wilkins JL. Bladder-neck obstruction and the Ehlers-Danlos syndrome. Br J Urol. 1967;39(3):353–358. 10.1111/j.1464-410X.1967.tb09815.x. [DOI] [PubMed] [Google Scholar]
- 60.Eskandar O, Eckford SD. Pelvic haematoma and severe gluteal bruising associated with Ehler-Danlos syndrome following posterior colpoperineorrhaphy. J Obstet Gynaecol J Inst Obstet Gynaecol. 2009;29(1):68–69. 10.1080/01443610802484443. [DOI] [PubMed] [Google Scholar]
- 61.Fogel S Surgical failures: is it the surgeon or the patient? The all too often missed diagnosis of Ehlers-Danlos syndrome. 2013;79(6):7. [PubMed] [Google Scholar]
- 62.Fowler AL, Bouchier Hayes D, Feher E. Testicular torsion in a patient with Ehlers-Danlos syndrome. BMJ Case Rep. 2018;2018:bcr-2017–222679. 10.1136/bcr-2017-222679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garat JM, Angerri O, Caffaratti J, Moscatiello P, Villavicencio H. Primary congenital bladder diverticula in children. Urology. 2007;70(5):984–988. 10.1016/j.urology.2007.06.1108. [DOI] [PubMed] [Google Scholar]
- 64.Georgy MS, Anwar K, Oates SE, Redford DHA. Perineal delivery in Ehlers-Danlos syndrome. BJOG. 1997;104(4):505–506. 10.1111/j.1471-0528.1997.tb11506.x. [DOI] [PubMed] [Google Scholar]
- 65.Ghosh AK, O’Bryan T. Ehlers-Danlos syndrome with reflux nephropathy. Nephron. 1995;70(2):266–266. 10.1159/000188598. [DOI] [PubMed] [Google Scholar]
- 66.Golfier F, Peyrol S, Attia-Sobol J, Marret H, Raudrant D, Plauchu H. Hypermobility type of Ehlers-Danlos syndrome: influence of pregnancies. Clin Genet. 2002;60(3):240–241. 10.1034/j.1399-0004.2001.600312.x. [DOI] [PubMed] [Google Scholar]
- 67.Handa S, Sethuraman G, Mohan A, Sharma VK. Ehlers-Danlos syndrome with bladder diverticula. Br J Dermatol. 2001;144(5):1084–1085. 10.1046/j.1365-2133.2001.04205.x. [DOI] [PubMed] [Google Scholar]
- 68.Hernández A, Aguirre-Negrete MG, Ramírez-Soltero S, et al. A distinct variant of the Ehlers-Danlos syndrome. Clin Genet. 1979;16(5):335–339. 10.1111/j.1399-0004.1979.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 69.Hernandez AM, Dietrich JE. Gynecologic management of pediatric and adolescent patients with Ehlers-Danlos syndrome. J Pediatr Adolesc Gynecol. 2018;31(2):196 10.1016/j.jpag.2018.02.088. [DOI] [PubMed] [Google Scholar]
- 70.Howard JM, Diaz MCG, Soler ME. Hemorrhagic shock resulting from post-coital vaginal bleeding in an adolescent with Ehlers-Danlos type IV. Pediatr Emerg Care. 2009;25(6):397–398. 10.1097/PEC.0b013e3181a79295. [DOI] [PubMed] [Google Scholar]
- 71.Hugon-Rodin J, Lebègue G, Becourt S, Hamonet C, Gompel A. Gynecologic symptoms and the influence on reproductive life in 386 women with hypermobility type Ehlers-Danlos syndrome: a cohort study. Orphanet J Rare Dis. 2016;11(1):124 10.1186/s13023-016-0511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hurst BS, Lange SS, Kullstam SM, et al. Obstetric and gynecologic challenges in women with Ehlers-Danlos syndrome. Obstet Gynecol. 2014;123(3):506–513. 10.1097/AOG.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 73.Imahori S, Bannerman RM, Graf CJ, Brennan JC. Ehlers-Danlos syndrome with multiple arterial lesions. Am J Med. 1969;47(6):967–977. 10.1016/0002-9343(69)90210-1. [DOI] [PubMed] [Google Scholar]
- 74.Jha S, Arunkalaivanan AS, Situnayake RD. Prevalence of incontinence in women with benign joint hypermobility syndrome. Int Urogynecol J. 2007;18(1):61–64. 10.1007/s00192-006-0096-8. [DOI] [PubMed] [Google Scholar]
- 75.Jorion JL, Michel M. Spontaneous rupture of bladder diverticula in a girl with Ehlers-Danlos syndrome. J Pediatr Surg. 1999;34(3):483–484. [DOI] [PubMed] [Google Scholar]
- 76.Kahn T, Reiser M, Gmeinwieser J, Heuck A. The Ehlers-Danlos syndrome, type IV, with an unusual combination of organ malformations. Cardiovasc Intervent Radiol. 1988;11(5):288–291. 10.1007/BF02577038. [DOI] [PubMed] [Google Scholar]
- 77.Kajbafzadeh A-M, Sharifi-Rad L, Ladi Seyedian SS, Mozafarpour S, Paydary K. Generalized joint hypermobility and voiding dysfunction in children: is there any relationship? Eur J Pediatr. 2014;173(2):197–201. 10.1007/s00431-013-2120-6. [DOI] [PubMed] [Google Scholar]
- 78.Karan A, Isikoglu M, Aksac B, Attar E, Eskiyurt N, Yalcin O. Hypermobility syndrome in 105 women with pure urinary stress incontinence and in 105 controls. Arch Gynecol Obstet. 2004;269(2):89–90. 10.1007/s00404-002-0429-x. [DOI] [PubMed] [Google Scholar]
- 79.Karthikeyan A, Venkat-Raman N. Hypermobile Ehlers-Danlos syndrome and pregnancy. Obstet Med. 2018;11(3):104–109. 10.1177/1753495X18754577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kashizaki F, Hatamochi A, Kamiya K, Yoshizu A, Okamoto H. Vascular-type Ehlers-Danlos syndrome caused by a hitherto unknown genetic mutation: a case report. J Med Case Rep. 2013;7(1):35 10.1186/1752-1947-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knoepp LR, McDermott KC, Muñoz A, Blomquist JL, Handa VL. Joint hypermobility, obstetrical outcomes, and pelvic floor disorders. Int Urogynecol J. 2013;24(5):735–740. 10.1007/s00192-012-1913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Knuuti E, Kauppila S, Kotila V, Risteli J, Nissi R. Genitourinary prolapse and joint hypermobility are associated with altered type I and III collagen metabolism. Arch Gynecol Obstet. 2011;283(5):1081–1085. 10.1007/s00404-010-1518-x. [DOI] [PubMed] [Google Scholar]
- 83.Krapf JM, Goldstein AT. Two case presentations of profound labial edema as a presenting symptom of hypermobility-type Ehlers–Danlos syndrome. J Sex Med. 2013;10(9):2347–2350. 10.1111/jsm.12229. [DOI] [PubMed] [Google Scholar]
- 84.Leduc L, Wasserstrum N. Successful treatment with the Smith-Hodge pessary of cervical incompetence due to defective connective tissue in Ehlers-Danlos syndrome. Am J Perinatol. 1992;9(01):25–27. 10.1055/s-2007-994664. [DOI] [PubMed] [Google Scholar]
- 85.Levine AS, Michael AF. Ehlers-Danlos syndrome with renal tubular acidosis and medullary sponge kidneys. A report of a case and studies of renal acidification in other patients with the Ehlers-Danlos syndrome. J Pediatr. 1967;71(1):107–113. [DOI] [PubMed] [Google Scholar]
- 86.Lewitus Z Ehlers-Danlos syndrome: report of two cases with hypophyseal dysfunction. AMA Arch Dermatol. 1956;73(2):158–161. 10.1001/archderm.1956.01550020058008. [DOI] [PubMed] [Google Scholar]
- 87.Lind J Wallenburg HCS, Pregnancy and the Ehlers–Danlos syndrome: a retrospective study in a Dutch population. 2002;8. [DOI] [PubMed] [Google Scholar]
- 88.Makatsariya A, Radetskaya L, Bitsadze V, Khizroeva J, Khamani N, Makatsariya N. Prenatal care and labor in patients with Mesenchimal dysplasias (Marfan syndrome, Ehlers-Danlos syndrome, hereditary hemorrhagic telangiectasia). J Matern-Fetal Neonatal Med. 2018;1–161. 10.1080/14767058.2018.1493102. [DOI] [PubMed] [Google Scholar]
- 89.Manning J, Korda A, Benness C, Solomon M. The association of obstructive defecation, lower urinary tract dysfunction and the benign joint hypermobility syndrome: a case control study. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(2):128–132. 10.1007/s00192-002-1025-0. [DOI] [PubMed] [Google Scholar]
- 90.Mastoroudes H, Giarenis I, Cardozo L, et al. Prolapse and sexual function in women with benign joint hypermobility syndrome. BJOG. 2013;120(2):187–192. 10.1111/1471-0528.12082. [DOI] [PubMed] [Google Scholar]
- 91.Mastoroudes H, Giarenis I, Cardozo L, et al. Lower urinary tract symptoms in women with benign joint hypermobility syndrome: a case–control study. Int Urogynecol J. 2013;24(9):1553–1558. 10.1007/s00192-013-2065-3. [DOI] [PubMed] [Google Scholar]
- 92.Mauseth R, Lieberman E, Heuser ET. Infantile polycystic disease of the kidneys and Ehlers-Danlos syndrome in an 11-year-old patient. J Pediatr. 1977;90(1):81–83. 10.1016/S0022-3476(77)80770-1. [DOI] [PubMed] [Google Scholar]
- 93.McIntosh LJ, Mallett VT, Frahm JD, Richardson DA, Evans MI. Gynecologic disorders in women with Ehlers-Danlos syndrome. J Soc Gynecol Investig. 1995;2(3):559–564. [DOI] [PubMed] [Google Scholar]
- 94.McIntosh LJ, Stanitski DF, Mallett VT, Frahm JD, Richardson DA, Evans MI. Ehlers-Danlos syndrome: relationship between joint hypermobility, urinary incontinence, and pelvic floor prolapse. Gynecol Obstet Invest. 1996;41(2):135–139. 10.1159/000292060. [DOI] [PubMed] [Google Scholar]
- 95.Moysés-Oliveira M, Mancini TI, Takeno SS, et al. Congenital adrenal hyperplasia, ovarian failure and Ehlers-Danlos syndrome due to a 6p deletion. Sex Dev. 2014;8(4):139–145. 10.1159/000363779. [DOI] [PubMed] [Google Scholar]
- 96.Mukerji S Ehlers-Danlos syndrome with pregnancy. J Indian Med Assoc. 1975;64(6):149–151. [PubMed] [Google Scholar]
- 97.Murray ML, Pepin M, Peterson S, Byers PH. Pregnancy-related deaths and complications in women with vascular Ehlers–Danlos syndrome. Genet Med. 2014;16(12):874–880. 10.1038/gim.2014.53. [DOI] [PubMed] [Google Scholar]
- 98.Nakamura Y, Hada Y, Sada I, Nagayama M. Ehlers-Danlos syndrome and pregnancy: a case of uterine rupture. Asia Oceania J Obstet Gynaecol. 1983;9(3):303–307. [DOI] [PubMed] [Google Scholar]
- 99.Nash K, Oji VC, Mitra S. Uterine torsion, a rare cause of acute abdominal pain in the third trimester of pregnancy: a case report. J Obstet Gynaecol. 2016;36(5):668–669. 10.3109/01443615.2015.1133580. [DOI] [PubMed] [Google Scholar]
- 100.Nethra S, Dickson MJ. Failure of continence surgery due to a hitherto undiagnosed collagen disorder. BJOG. 2013;120:293–312. 10.1111/1471-0528.12296. [DOI] [Google Scholar]
- 101.Norton PA, Baker JE, Sharp HC, Warenski JC. Genitourinary prolapse and joint hypermobility in women. Obstet Gynecol. 1995;85(2):225–228. 10.1016/0029-7844(94)00386-R. [DOI] [PubMed] [Google Scholar]
- 102.O’Brennan E, Eustace K, Scanlon J, Bell H. Fragile fissuring genital skin associated with Ehlers Danlos syndrome. Br J Dermatol. 2018;179:83–84. [Google Scholar]
- 103.Oxenham J Complexities of management of a urostomy in Ehlers-Danlos syndrome: a reflective account. Br J Nurs. 2016;25(5):S14–S19. 10.12968/bjon.2016.25.5.S14. [DOI] [PubMed] [Google Scholar]
- 104.Park MA, Shin SY, Kim YJ, Park MJ, Lee SH. Vascular Ehlers–Danlos syndrome with cryptorchidism, recurrent pneumothorax, and pulmonary capillary hemangiomatosis-like foci: a case report. Medicine (Baltimore). 2017;96(47):e8853 10.1097/MD.0000000000008853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peaceman AM, Cruikshank DP. Ehlers-Danlos syndrome and pregnancy: association of type IV disease with maternal death. Obstet Gynecol. 1987;69(3 Pt 2):428–431. [PubMed] [Google Scholar]
- 106.Pepin M Clinical and genetic features of Ehlers–Danlos syndrome type IV, the vascular type. N Engl J Med. 2000;342(10):673–680. [DOI] [PubMed] [Google Scholar]
- 107.Pournasiri Z, Madani A, Zandi H, Salehpour S, Gorji FA, Ahmadzahe A. Relationship of generalized joint hypermobility with vesicoureteral reflux and urinary tract infection. 2014;8(3):5. [PubMed] [Google Scholar]
- 108.Ryan N, Walkden G, Akbar S. Some wounds are hard to heal: an interesting presentation of Ehlers-Danlos syndrome. J Wound Care. 2012;21(5):223–226. 10.12968/jowc.2012.21.5.223. [DOI] [PubMed] [Google Scholar]
- 109.Rudd N Pregnancy complications in type IV Ehlers-Danlos syndrome. The Lancet. 1983;321(8314–8315):50–53. 10.1016/S0140-6736(83)91577-5. [DOI] [PubMed] [Google Scholar]
- 110.Sardeli C, Axelsen SM, Bek KM. Use of porcine small intestinal sub-mucosa in the surgical treatment of recurrent rectocele in a patient with Ehlers–Danlos syndrome type III. Int Urogynecol J. 2005;16(6):504–505. 10.1007/s00192-004-1265-2. [DOI] [PubMed] [Google Scholar]
- 111.Sato T, Ito H, Miyazaki S, Komine S, Hayashida Y. Megacystis and megacolon in an infant with Ehlers-Danlos syndrome. Acta Paediatr Jpn Overseas Ed. 1993;35(4):358–360. [DOI] [PubMed] [Google Scholar]
- 112.Scheper MC, Nicholson LL, Adams RD, Tofts L, Pacey V. The natural history of children with joint hypermobility syndrome and Ehlers–Danlos hypermobility type: a longitudinal cohort study. Rheumatology. 2017;56(12):2073–2083. 10.1093/rheumatology/kex148. [DOI] [PubMed] [Google Scholar]
- 113.Schippers E, Dittler HJ. Multiple hollow organ dysplasia in ehlersdanlos syndrome. J Pediatr Surg. 1989;24(11):1181–1183. 10.1016/S0022-3468(89)80114-9. [DOI] [PubMed] [Google Scholar]
- 114.Shukla AR, Bellah RA, Canning DA, Carr MC, Snyder HM, Zderic SA. Giant bladder diverticula causing bladder outlet obstruction in children. J Urol. 2004;172(5):1977–1979. 10.1097/01.ju.0000140450.50242.50. [DOI] [PubMed] [Google Scholar]
- 115.Smith CV, Phelan JP. Pregnancy and the Ehlers-Danlos syndrome. A report of two cases. J Reprod Med. 1982;27(12):757–760. [PubMed] [Google Scholar]
- 116.Smith MD, Hussain M, Seth JH, Kazkaz H, Panicker JN. Stress urinary incontinence as the presenting complaint of benign joint hypermobility syndrome. JRSM Short Rep. 2012;3(9):1–3. 10.1258/shorts.2012.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Smith SA, Powell LC, Essin EM. Ehlers-Danlos syndrome and pregnancy: report of a case. Obstet Gynecol. 1968;32(3):331–335. [PubMed] [Google Scholar]
- 118.Sorokin Y, Johnson MP, Rogowski N, Richardson DA, Evans MI. Obstetric and gynecologic dysfunction in the Ehlers-Danlos syndrome. J Reprod Med. 1994;39(4):281–284. [PubMed] [Google Scholar]
- 119.Sorrentino F, Cartwright R, Greco P, et al. The association of pelvic organ prolapse and joint hypermobility: results from the TwinsUK adult twin registry. Int Urogynecol J Pelvic Floor Dysfunct. 2014;25(1):S200–S201. 10.1007/s00192-014-2429-3. [DOI] [Google Scholar]
- 120.Stage KH, Tank ES. Primary congenital bladder diverticula in boys. Urology. 1992;40(6):536–538. 10.1016/0090-4295(92)90410-X. [DOI] [PubMed] [Google Scholar]
- 121.Stoddard FJ, Myers RE. Connective tissue disorders in obstetrics and gynecology. Am J Obstet Gynecol. 1968;102(2):240–243. 10.1016/0002-9378(68)90325-6. [DOI] [PubMed] [Google Scholar]
- 122.Tarrass F, Benjelloun M, Hachim K, et al. Ehlers-Danlos syndrome coexisting with juvenile nephronophtisis. Nephrol Ther. 2006;11(2):117–119. 10.1111/j.1440-1797.2006.00498.x. [DOI] [PubMed] [Google Scholar]
- 123.Toyohara T, Kaneko T, Araki H, Takahashi K, Nakamura T. Giant epi-phrenic diverticulum in a boy with Ehlers-Danlos syndrome. Pediatr Radiol. 1989;19(6–7):437–437. 10.1007/BF02387645. [DOI] [PubMed] [Google Scholar]
- 124.Tucker SC, Yell JA. Dramatic postcoital vulval laceration and bruising in Ehlers-Danlos syndrome. Br J Dermatol. 1999;140(5):974. [DOI] [PubMed] [Google Scholar]
- 125.van Eerde AM, Verhoeven VJM, de Jong TPVM, van de Putte EM, Giltay JC, Engelbert RHH. Is joint hypermobility associated with vesico-ureteral reflux? An assessment of 50 patients. BJU Int. 2012;109(8):1243–1248. 10.1111/j.1464-410X.2011.10469.x. [DOI] [PubMed] [Google Scholar]
- 126.Vo KT, Grooms L, Klima J, Holland-Hall C, O’Brien SH. Menstrual bleeding patterns and prevalence of bleeding disorders in a multi-disciplinary adolescent haematology clinic. Haemophilia. 2013;19(1):71–75. 10.1111/hae.12012. [DOI] [PubMed] [Google Scholar]
- 127.Watson V, Watson R. Complications of classic Ehlers-Danlos syndrome in pregnancy: a rare collagen disorder. BJOG. 2015;122:312–313. 10.1111/14710528.13383.24844913 [DOI] [Google Scholar]
- 128.Wheeler SM, Russo M, Wilson-Murphy M, Shen W. Gynecologic and surgical complications in type IV Ehlers-Danlos syndrome. Obstet Gynecol. 2014;123:431–433. 10.1097/AOG.0000000000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamashita M, Narita M, Ishihara H, Matsuki A, Oyama T. Uterine rupture in a case with Ehlers-Danlos syndrome type IV-anesthetic considerations. Middle East J Anaesthesiol. 1987;9(3):277–281. [PubMed] [Google Scholar]
- 130.Zalis EG, Roberts DC. Ehlers-Danlos syndrome with a hypoplastic kidney, bladder diverticulum, and diaphragmatic hernia. Arch Dermatol. 1967;96(5):540–544. [DOI] [PubMed] [Google Scholar]
- 131.Zarrinkhoo E, Towfigh S. Robotic-assisted abdomino-inguinoplasty to treat groin pain in patient with Ehlers-Danlos syndrome. Hernia. 2016;20(1):S133–S138. 10.1007/s10029-016-1468-8. [DOI] [Google Scholar]
- 132.Tullus K Vesicoureteric reflux in children. The Lancet. 2015;385(9965):371–379. 10.1016/S0140-6736(14)60383-4. [DOI] [PubMed] [Google Scholar]
- 133.Buckley BS, Lapitan MCM. Prevalence of urinary incontinence in men, women, and children—current evidence: findings of the fourth international consultation on incontinence. Urology. 2010;76(2):265–270. 10.1016/j.urology.2009.11.078. [DOI] [PubMed] [Google Scholar]
- 134.Milsom I, Gyhagen M. The prevalence of urinary incontinence. Climacteric. 2018;22:1–6. 10.1080/13697137.2018.1543263. [DOI] [PubMed] [Google Scholar]
- 135.Loening-Baucke V Prevalence rates for constipation and faecal and urinary incontinence. Arch Dis Child. 2007;92(6):486–489. 10.1136/adc.2006.098335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ekeh AP, Walusimbi M, Brigham E, Woods RJ, McCarthy MC. The prevalence of incidental findings on abdominal computed tomography scans of trauma patients. J Emerg Med. 2010;38(4):484–489. 10.1016/j.jemermed.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 137.Mitchell K, Geary R, Graham C, et al. Painful sex (dyspareunia) in women: prevalence and associated factors in a British population probability survey. BJOG. 2017;124(11):1689–1697. 10.1111/1471-0528.14518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leiblum SR, Hayes RD, Wanser RA, Nelson JS. Vaginal dryness: a comparison of prevalence and interventions in 11 countries. J Sex Med. 2009;6(9):2425–2433. 10.1111/j.1743-6109.2009.01369.x. [DOI] [PubMed] [Google Scholar]
- 139.McDonald E, Gartland D, Small R, Brown S. Dyspareunia and childbirth: a prospective cohort study. BJOG. 2015;122(5):672–679. 10.1111/1471-0528.13263. [DOI] [PubMed] [Google Scholar]
- 140.Chopra P, Tinkle B, Hamonet C, et al. Pain management in the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175(1):212–219. 10.1002/ajmg.c.31554. [DOI] [PubMed] [Google Scholar]
- 141.Mann J, Shuster J, Moawad N. Attributes and barriers to care of pelvic pain in university women. J Minim Invasive Gynecol. 2013;20(6):811–818. 10.1016/j.jmig.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shapley M, Jordan J, Croft PR. A systematic review of postcoital bleeding and risk of cervical cancer. Br J Gen Pract. 2006;56(527):453–460. [PMC free article] [PubMed] [Google Scholar]
- 143.Ofir K, Sheiner E, Levy A, Katz M, Mazor M. Uterine rupture: risk factors and pregnancy outcome. Am J Obstet Gynecol. 2003;189(4):1042–1046. 10.1067/S0002-9378(03)01052-4. [DOI] [PubMed] [Google Scholar]
- 144.Oyelese Y, Ananth CV. Postpartum hemorrhage: epidemiology, risk factors, and causes. Clin Obstet Gynecol. 2010;53(1):147–156. 10.1097/GRF.0b013e3181cc406d. [DOI] [PubMed] [Google Scholar]
- 145.Calguneri M, Bird HA, Wright V. Changes in joint laxity occurring during pregnancy. Ann Rheum Dis. 1982;41(2):126–128. 10.1136/ard.41.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schauberger CW, Rooney BL, Goldsmith L, Shenton D, Silva PD, Schaper A. Peripheral joint laxity increases in pregnancy but does not correlate with serum relaxin levels. Am J Obstet Gynecol. 1996;174(2):667–671. 10.1016/S0002-9378(96)70447-7. [DOI] [PubMed] [Google Scholar]
- 147.Albert HB, Godskesen M, Westergaard JG. Incidence of four syndromes of pregnancy-related pelvic joint pain. Spine. 2002;27(24):2831–2834. [DOI] [PubMed] [Google Scholar]
- 148.Castori M, Camerota F, Celletti C, Grammatico P, Padua L. Ehlers-Danlos syndrome hypermobility type and the excess of affected females: possible mechanisms and perspectives. Am J Med Genet A. 2010;152A(9):2406–2408. 10.1002/ajmg.a.33585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.