Abstract
Background:
There has been growing interest in limited resection and nonsurgical treatment for small lung cancers. Our objective was to examine the pattern and rate of occult N1 nodal metastasis in patients with peripheral, small (≤2 cm), clinically node-negative non-small cell lung cancer (NSCLC).
Methods:
Patients with peripheral <2 cm NSCLC with no evidence of locally advanced or metastatic disease (clinical T1a-bN0M0, 8th edition) who were deemed eligible for either lobectomy or sublobar resection were identified from preregistration eligibility screening logs for the Alliance/CALGB 140503 trial at our institution. All patients undergoing anatomic resection with mediastinal and hilar lymphadenectomy were examined for pathologic outcomes.
Results:
In total, 58 patients treated between November 2014 and January 2017 who met the inclusion criteria were included: 51 underwent lobectomy; 7 underwent segmentectomy. Mean tumor diameter on computed tomography was 1.5 cm, and mean positron emission tomography maximal standardized uptake value was 3.9; the mean consolidation-to-tumor ratio was 0.77. Eight of 58 patients (14%) had occult nodal metastases in N1 stations, and the majority of these nodes were found in interlobar or peribronchial stations (11 or 12). An additional 2 patients (3%) had occult positive N2 nodes. Overall, the false-negative rate for clinical staging was 16%.
Conclusions:
Occult nodal disease was frequently identified in peripheral N1 stations (11–13) in patients with small (≤2 cm) clinical N0 NSCLC. Hilar lymphadenectomy is essential for accurate staging in the management of patients with small clinical N0 NSCLC.
Small (≤2 cm) peripheral non-small cell lung cancers (NSCLCs) without evidence of nodal metastasis are generally perceived to have a favorable prognosis [1]. Although lobectomy has been considered the standard treatment, there has been increasing interest in pursuing limited resections for these presumably low-risk tumors, and two large multicenter randomized trials (JCOG 0802 and Alliance/CALGB 140503) comparing lobectomy with limited resection have now completed accrual [2, 3].
Although several studies have examined the rates of upstaging in early-stage patients, data are scant on upstaging specifically among this subset of patients with smaller (≤2 cm) peripheral tumors, who are increasingly considered for limited resection. In the CALGB 140503 trial, nonanatomic wedge resections were permitted for these lesions [3]. At the same time, nonsurgical modalities such as stereotactic body radiotherapy (SBRT) are increasingly being offered to patients with these small lesions, which may address the primary tumor but not the lymph nodes [4]. Previous studies of early-stage disease, both prospective and retrospective, have varied in methodology and most often included patients with larger tumors up to 3 cm in diameter, reflecting prior classifications of what constitutes stage IA NSCLC [5–15]. It is crucial to understand the location, in addition to the incidence, of occult nodal metastases if we are to diverge from the traditional gold standard of lobectomy with complete lymphadenectomy [16].
In this study, we examined a prospectively identified cohort of patients with peripheral, small (≤2 cm), clinically node-negative NSCLC who were deemed amenable to limited resection and who underwent anatomic resection with hilar and mediastinal lymph node dissection, to report on the locations and rates of occult nodal metastasis in this population.
MATERIAL AND METHODS
Patients
Patients with peripheral, small (≤2 cm on computed tomography [CT]) NSCLC with no evidence of locally advanced or metastatic disease (clinical T1a-bN0M0, 8th edition AJCC Cancer Staging Manual) were prospectively identified at our institution on the basis of preregistration eligibility screening for the Alliance/CALGB 140503 trial. Preregistration criteria included age ≥18 years, peripheral lung nodule ≤2 cm on CT scan presumed to be lung cancer, Eastern Cooperative Oncology Group performance status of 0–2, no history of malignancy within 3 years, no prior chemotherapy or radiation therapy for this malignancy, and no evidence of locally advanced or metastatic disease. All patients had a tumor location that was deemed amenable, by a surgeon, for either sublobar or lobar resection. Consecutively screened patients meeting the above eligibility criteria who underwent anatomic resection were followed for pathologic outcomes, regardless of whether they were ultimately enrolled in the trial. To ensure assessment of the more peripheral interlobar, peribronchial, and segmental lymph nodes (N1 stations 11, 12, or 13), all patients undergoing anatomic resection who underwent hilar and mediastinal lymph node dissection were included [17]. Patients found to have any diagnosis other than NSCLC on final pathologic analysis were excluded. The institutional review board at our center approved this study.
Radiographic Assessment
Tumor diameter was recorded from the CT reports that were screened for initial trial eligibility. Maximal standardized uptake value (SUVmax) was recorded from the positron emission tomography (PET)/CT report when available (N = 54; PET/CT was not a requirement for CALGB 14053 study eligibility). Consolidation/tumor ratio (CTR) was assessed post hoc from the preoperative CT for each patient by an attending thoracic radiologist (A.P.).
Pathologic Assessment
Hematoxylin and eosin–stained tumor slides of resected specimens were reviewed and reported as part of general care delivery. In addition to NSCLC classifications (e.g., adenocarcinoma, squamous cell carcinoma), the histologic subtypes of adenocarcinoma, including lepidic, acinar, papillary, micropapillary, and solid, were recorded in 5% increments when available. Tumors with mixed histologic subtypes were classified on the basis of the largest component (predominant subtype). A histologic subtype was considered to be present if it composed at least 5% of the tumor [18].
Lymph node status was assessed through review of operative and surgical pathology reports. Nodal stations sampled were quantified and reported. The method of nodal disease identification was also noted. For example, in some cases of upstaging, occult metastases were identified in surgically dissected nodes during the operation and labeled and submitted by the surgeon. In other instances, the pathologist identified additional nodes—typically peribronchial nodes—during the inspection and processing of the resection specimen in the pathology lab. The total number of lymph nodes examined in the resection specimen during processing was recorded (lymph nodes retrieved from the resected specimen).
Statistical Analysis
Patient and tumor characteristics were analyzed using descriptive statistics. Univariable associations between occult lymph node metastasis and clinicopathologic variables were assessed using Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
RESULTS
Patient and Clinicopathologic Features
In total, 96 patients treated between November 2014 and January 2017 initially met the preregistration eligibility criteria, and 58 patients ultimately underwent anatomic resection for histologically confirmed NSCLC (Table 1). Of these 58 patients, 51 underwent lobectomy, and 7 underwent segmentectomy. Operative and final pathologic features are summarized in Table 2.
Table 1.
Demographic and clinical characteristics
| Characteristic | Patients (N=58) |
|---|---|
| Age, years, median (IQR) | 69 (64−74) |
| Female | 39 (67.2) |
| ECOG performance status | |
| 0 | 46 (79.3) |
| 1 | 10 (17.2) |
| 2 | 1 (1.7) |
| Unknown | 1 (1.7) |
| Charlson comorbidity indexa | |
| Median (IQR) | 0 (0−2) |
| Never smoker | 10 (17.2) |
| Pack-years | |
| Median (IQR) | 35.0 (13.5−51.0) |
| FEV1, % | |
| Median (IQR) | 95.5 (82−108) |
| DLCO, % | |
| Median (IQR) | 82 (67−98) |
| CT tumor diameter, cm, median (IQR) | 1.4 (1.2−1.8) |
| Consolidation-to-tumor ratio, median (IQR) | 1.0 (0.6−1.0) |
| SUVmax, median (IQR) | 3.0 (1.7−5.5) |
Unless otherwise note, data are no. (%). CT, computed tomography; DLco, diffusing capacity of the lung for carbon monoxide; ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in 1 second; IQR, interquartile range; SD, standard deviation; SUVmax, maximal standardized uptake value.
Charlson comorbidity index reported without age assessment.
Table 2.
Operative and pathologic characteristics
| Characteristic | Patients (N=58) |
|---|---|
| Surgical approach | |
| VATS | 51 (88.0) |
| Thoracotomy | 7 (12.1) |
| Lobectomy | 51 (88.0) |
| RUL | 11 (21.6) |
| RML | 6 (11.8) |
| RLL | 13 (25.5) |
| LLL | 9 (17.6) |
| LUL | 12 (23.5) |
| Segmentectomy | 7 (12.0) |
| RLL | 4 (57.1) |
| LLL | 2 (28.6) |
| LUL | 2 (28.6) |
| Histologic subtype (n=58) | |
| Adenocarcinoma | 48 (82.8) |
| Squamous cell/other | 10 (17.2) |
| Micropapillary and/or solid histologic component | |
| Present (≥5%) | 28 (48.2) |
| Absent (<5%) | 17 (29.3) |
| N/A | 13 (22.4) |
Data are no. (%). LLL, left lower lobe; LUL, left upper lobe; NA, not applicable; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; VATS, video-assisted thoracoscopic surgery.
Occult Nodal Distribution
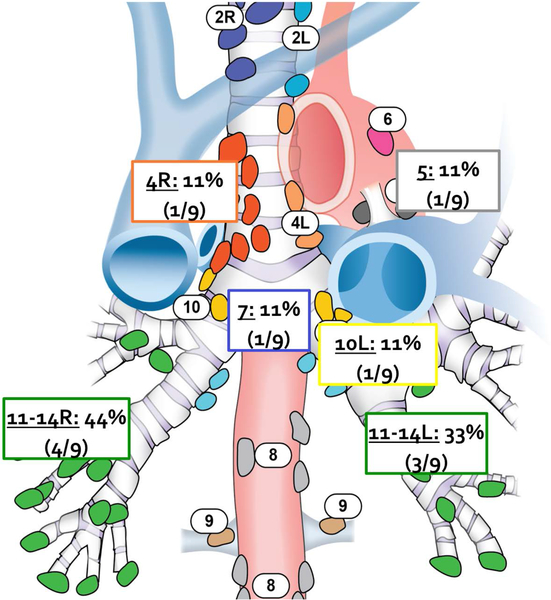
Occult N1 nodal metastases were present in 8 of 58 patients (14%), and almost all of these occult metastases occurred in peripheral stations, including interlobar, lobar, and segmental peribronchial nodes (7/8 patients; 88%) (Figure 1 and Table 3). In over half of these cases (5/8, 63%), the occult N1 disease was found in nodes identified during processing of the specimen by the pathologist. Occult N2 metastasis was present in 2 of 58 patients (3%), 1 of whom also had N1 disease. Overall, 7 patients were upstaged to N1 (12%), and 2 patients were upstaged to N2 (3%), for an overall false-negative rate for clinical staging of 15.5% (SE = 5.5%−25.0%).
Figure 1.
Location of positive occult lymph nodes among upstaged patients (n=9).
Table 3.
Nodal staging and assessment
| Characteristic | Patients (n=58) |
|---|---|
| Pathologic nodal staging | |
| pN0 | 49 (84.5) |
| pN+ | 9 (15.5) |
| pN1 | 7 (12.1) |
| pN2* | 2 (3.4) |
| Number of nodal stations assessed, median (IQR) | 5.0 (4.0–6.0) |
| Location of positive occult lymph nodes among upstaged patients (n=9) | |
| N1 Interlobar to Segmental (Station 11–13) | 7/9 (77.8) |
| Hilar (Station 10) | 1/9 (11.1) |
| N2 Subcarinal (Station 7) | 1/9 (11.1) |
| Aortopulmonary (Station 5) | 1/9 (11.1) |
| Paratracheal (Station 4) | 1/9 (11.1) |
Unless otherwise note, data are no. (%). IQR, interquartile range; LN, lymph node; pN, pathologic node.
One patient had occult N1 and N2 nodes
Preoperative and Radiographic Features Associated with Occult Nodal Disease
Fisher’s exact test was used to test univariate associations between occult lymph node metastasis and clinicopathologic variables. No differences in patient characteristics or demographic characteristics were observed between upstaged patients and non-upstaged patients (Table 4). PET SUVmax and CT tumor diameter did not differ between upstaged patients and non-upstaged patients (P>0.05). There was a trend toward higher CTR values in patients with occult nodal disease, compared with node-negative patients (0.93 vs. 0.74; P=0.09).
Table 4.
Clinical and pathologic features stratified by nodal disease
| Characteristic | pN0 (N=49) | pN+ (N=9) | P |
|---|---|---|---|
| Age, years, mean (SD)a | 67.5 (9.9) | 71.1 (5.8) | 0.38 |
| Sex | |||
| Male | 16 (32.7) | 3 (33.3) | 1.00 |
| Female | 33 (67.3) | 6 (66.7) | |
| ECOG performance status | |||
| 0 | 40 (81.6) | 6 (66.7) | 0.35 |
| 1 or 2 | 8 (16.3) | 3 (33.3) | |
| Charlson comorbidity indexa, b | |||
| Median (IQR) | 0 (0–2) | 1 (0–3) | 0.46 |
| Smoking status | 1.00 | ||
| CS/FS | 40 (81.6) | 8 (88.9) | |
| NS | 9 (18.4) | 1 (11.1) | |
| Pack-yearsa | |||
| Median (IQR) | 35 (15–50) | 35 (11.25–55) | 0.63 |
| SUVmaxa | |||
| Median (IQR) | 2.8 (1.7–5.6) | 4.6 (2.6–5.4) | 0.16 |
| CT tumor diameter, cma | |||
| Median (IQR) | 1.4 (1.2–1.7) | 1.8 (1.3–1.9) | 0.13 |
| Consolidation-to-tumor ratioa | |||
| Median (IQR) | 0.89 (0.53–1.0) | 0.93 (1.0–1.0) | 0.09 |
| Histologic subtype | |||
| Adenocarcinoma | 42 (85.7) | 6 (66.7) | 0.1770 |
| Squamous cell/other | 7 (14.3) | 3 (33.3) | |
| Micropapillary and/or solid histologic component | |||
| Present (≥5%) | 20 (54.1) | 8 (100.0) | 0.0171 |
| Absent (<5%) | 17 (45.9) | 0 (0.0) | |
CS, current smoker; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; FS, former smoker; IQR, interquartile range; NS, never smoker; SD, standard deviation; SUVmax, maximal standardized uptake value.
The Wilcoxon rank-sum test was used. For all other variables, Fisher’s exact test was used.
Charlson comorbidity index reported without age assessment.
Clinicopathologic features associated with occult nodal disease
The clinicopathologic features associated with occult nodal disease are presented in Tables 4 and 5. The rate of upstaging between adenocarcinoma and squamous/other was not significantly different. Micropapillary and/or solid component was present (≥5%) in all 8 upstaged cases of adenocarcinoma (P=0.017).
Table 5.
Patient and disease characteristics in patients with occult nodal disease
| Sex | Smoking Status | Diameter cm | SUVmax | CTR | Lobe | Procedure | Histology | Micropapillary and/or solid component present | Positive Nodal Station |
|---|---|---|---|---|---|---|---|---|---|
| F | CS/FS | 1.7 | 8.3 | 1.0 | RLL | Lobectomy | Squamous | NA | 11 R |
| M | NS | 1.8 | 2.6 | 1.0 | RLL | Lobectomy | Adenocarcinoma | Yes | 7, 4 R |
| F | CS/FS | 2.0 | 9.5 | 0.72 | RLL | Segmentectomy | Pleomorphic | Yes | 13 R |
| M | CS/FS | 1.2 | 5.3 | 1.0 | LUL | Lobectomy | Pleomorphic | Yes | 11 L |
| M | CS/FS | 1.8 | 5.4 | 1.0 | LUL | Lobectomy | Adenocarcinoma | Yes | 12 L |
| F | CS/FS | 1.3 | 1.6 | 1.0 | LLL | Lobectomy | Adenocarcinoma | Yes | 10 L |
| F | CS/FS | 2.0 | 4.6 | 1.0 | RLL | Lobectomy | Adenocarcinoma | Yes | 11 R,12 R |
| M | CS/FS | 1.9 | 4.1 | 0.62 | LUL | Lobectomy | Adenocarcinoma | Yes | 5 L,12 L |
| F | CS/FS | 0.9 | 2.5 | 1.0 | RLL | Lobectomy | Adenocarcinoma | Yes | 12 R |
CS, current smoker; CTR, consolidation-to-tumor ratio; F, female; FS, former smoker; L, left; LLL, left lower lobe; LUL, left upper lobe; M, male; NS, never smoker; R, right; RLL, right lower lobe; SUVmax, maximal standardized uptake value.
COMMENT
In this prospectively identified patient cohort with peripheral, small (≤2 cm), clinical N0 NSCLC equally amenable for either limited resection or lobectomy, occult nodal metastases in N1 stations were common. Although these patients were deemed sufficiently low-risk to offer them limited resections, the overall rate of nodal upstaging remained high, approaching nearly 16%. The majority of occult N1 nodes were found in peripheral interlobar, lobar, or segmental stations that were identified during hilar lymph node dissection and by thorough examination of the peribronchial nodes during processing of the specimen by the pathologist.
The majority of these peripheral occult nodes would likely not have been identified in the absence of a hilar nodal dissection and anatomic resection, and these patients would have risked being understaged and undertreated with a nonanatomic wedge resection or SBRT. Although the mediastinal N2 and station 10 nodes can be easily sampled during a nonanatomic wedge resection, the interlobar, lobar, and segmental N1 nodes (stations 11–13) cannot without a hilar dissection.
The use of wedge resection and SBRT could result in the undertreatment of patients who harbor occult N1 nodes, from both a local control standpoint with respect to the removal of involved nodes and a radiotherapy standpoint with respect to the localization of nodal disease that should have been factored into a treatment field. These unrecognized occult nodes would also represent a missed opportunity in those settings to offer adjuvant chemotherapy to the patient, a recommendation for which we have level 1 evidence [19]. Indeed, the fact that most of the patients randomized to the limited resection arm in the CALGB trial underwent wedge resection (59%) suggests that many surgeons believe wedge resection is acceptable under these circumstances and underscores these concerns [20].
Although previous studies have examined the issue of upstaging in stage I NSCLC, few papers have specifically examined N1 upstaging or focused on the cohort of patients with smaller T1 (<2 cm) tumors—the cohort being studied in the CALGB and JCOG randomized trials. Furthermore, previous retrospective studies were unable to retroactively determine whether a given lesion was equally amenable to limited resection and lobectomy, an important aspect that was confirmed a priori by a surgeon for the patients in the present study.
Previous studies have reported somewhat variable findings but have generally identified similar, if not lower, overall rates of upstaging as in the present study [5, 8, 9, 21]. In a meta-analysis of 10 studies (n=691 patients), Detterbeck reported a false-negative rate of 10% for CT staging of peripheral stage I disease, but this analysis included tumors up to 3 cm in size [22]. Khullar analyzed 13,606 cases of preoperative clinical T1A N0 NSCLC (≤2 cm) from the Surveillance, Epidemiology, and End Results database and reported overall rates of nodal upstaging of 2.1% for wedge resection, 3.6% for segmentectomy, and 7.3% for lobectomy [17]. Altorki et al. examined 347 patients (lobectomy=294, sublobar=53) with peripheral stage I disease—again, up to 3 cm in size—and found N2 upstaging in 7% (2/29) of sublobar patients and 4% (9/231) of lobectomy patients [23]. The varying criteria of these investigations (≤3 cm vs. ≤2 cm) and the inclusion of patients who underwent sublobar resection and/or were inadequately evaluated for nodal disease (Nx) underscore the limitations of the present literature. That we found substantial rates of occult nodal metastasis, if not higher rates, in our cohort of smaller tumors is noteworthy.
Other studies, however, have reported different findings. In a study of 292 patients, Wang et al. arrived at the exact opposite conclusion in their analysis, stating that “the rate of metastasis to the lymph nodes is very low in clinical stage IA peripheral lung cancer patients” [24]. Unlike the studies mentioned above, which drew from a North American population, the study from Wang et al. focused on an Asian population, which could present important regional differences with a higher proportion of ground glass lesions and consequently the rate of nodal metastasis. Indeed, our rate of N1 nodal metastasis was twice the rate they reported.
Our study found a comparable false-negative rate to that reported by Ghaly et al., and our study complements their study by providing added information about the specific location of the occult metastases [21]. The authors found a false-negative rate for PET/CT of 9.6% (43/449), with a false-negative rate of 4.5% (20/449) for pN1 metastasis and 5.1% (23/449) for pN2 metastasis. Ghaly et al. found that high SUVmax on PET was associated with nodal metastasis in this patient cohort. Although SUVmax was also higher in patients with nodal metastasis in our analysis, our study was likely underpowered to be able to detect a statistical significance.
The yield of N1 nodes is contingent on the thoroughness of both the hilar dissection by the surgeon and the pathologic examination of the resection specimen. Work by Ramirez and Osarogiagbon underlines the degree of incomplete assessment of N1 nodes during routine pathologic examination of resected lung cancers [25]. In a study of patients deemed pathologically N0 after initial pathologic review, a subsequent and more thorough examination of the discarded surgical specimen yielded unexpected lymph node metastasis in a surprising 12% of these presumed pN0 cases [25]. These data and subsequent work suggest that positive nodes may be overlooked in a clinically significant proportion of patients, with resultant consequences on long-term survival [25–27].
Troublingly, several studies have also reported that a considerable proportion of patients with early-stage disease are simply not adequately evaluated for nodal disease (so-called Nx patients), likely underrepresenting the true incidence of N1 disease [17, 25, 28–31]. In their analysis of data from the National Cancer Database, Khullar et al. found that no lymph node sampling was performed in 51% of wedge resections, 21% of segmentectomies, and even 3.5% of lobectomies [17]. Specifically, they report that patients undergoing wedge resection had a 55% higher incidence of having three or fewer lymph nodes examined. Together, these data suggest that nodal status may have been underassessed historically and that the incidence of occult N1 disease may be higher than previously reported.
Examination of the histologic subtype of the tumors in this study suggested a possible association between micropapillary and solid histologic subtype and occult nodal disease. This would be consistent with previous findings that micropapillary pattern histologic subtype is an independent predictor of occult mediastinal (N2) lymph node metastasis [32]. Future subanalysis of histologic subtypes across patients in the recently completed CALGB 140503 and JCOG 0802 trials may yield more information about predictors of occult metastasis and their anatomic patterns in these patients with presumed early-stage disease.
The number of patients in this study is a potential limitation, preventing our findings from being conclusive. It may be that the true rate of occult nodal metastasis in our cohort is in fact no different from what others have found, given differences in sample size. However, what is not in dispute is that these occult nodes occurred in these smaller, <2 cm tumors and that they were located in areas that would not be routinely found in the course of a wedge resection (even with a mediastinal nodal dissection) or with SBRT. As with all retrospective analyses, these findings are meant to be hypothesis-generating. In our opinion, the ramifications of this, with respect to decisions about treatment selection for a peripheral <2 cm tumor, would have significant clinical impact and deserves further study and validation
Although this was a retrospective cohort study, unlike previous retrospective reports, patients in this study were prospectively identified through preregistration eligibility screening and had been assessed and deemed eligible by a surgeon for either lobectomy or limited resection before undergoing surgery. Our study population is thus less prone to the potential bias that can arise from assembling a study population retrospectively, as accurately determining clinical stage retroactively is often difficult.
This study also provides a greater focus on the prevalence of occult N1 disease, and importantly, the location of these occult nodal metastases, but this should be substantiated in larger studies. Although the relatively small numbers of patients limit the generalizability of our interpretations, the results and potential implications of this exploratory analysis warrant further exploration in larger data sets to confirm the locations and incidence of occult nodal metastasis, as well as to identify potential radiographic or histologic predictors of nodal metastasis. More-focused examination of radiographic features, such as CTR or PET-SUV, in future analyses of larger data sets, such as the CALGB or JCOG study populations, may identify preoperative predictors of occult nodal metastasis that may ultimately help guide treatment decisions.
In summary, we found that occult N1 nodal metastases were common in patients with clinical N0 peripheral, small (≤2 cm) NSCLC, and these were found in more peripheral interlobar, lobar, and segmental stations. Treating these tumors without performing a hilar nodal dissection may miss occult nodes and risk understaging the patient. As many of these occult nodes were discovered through examination of the resection specimen, careful attention should be paid to its processing, with a thorough inspection of the specimen conducted by the pathologist.
References
- 1.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest 2017;151(1):193–203. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura K, Saji H, Nakajima R et al. A phase iii randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (jcog0802/wjog4607l). Jpn J Clin Oncol 2010;40(3):271–274. [DOI] [PubMed] [Google Scholar]
- 3.Fox N, Bauer T. Calgb 140503: A randomized phase iii trial of lobectomy versus sublobar resection for small (< 2cm) peripheral non-small cell lung cancer. Oncology Issues 2008;23(6):20–21. [Google Scholar]
- 4.Bryant AK, Mundt RC, Sandhu AP et al. Stereotactic body radiation therapy versus surgery for early lung cancer among us veterans. Ann Thorac Surg 2018;105(2):425–431. [DOI] [PubMed] [Google Scholar]
- 5.Meyers BF, Haddad F, Siegel BA et al. Cost-effectiveness of routine mediastinoscopy in computed tomography- and positron emission tomography-screened patients with stage i lung cancer. J Thorac Cardiovasc Surg 2006;131(4):822–829; discussion 822–829. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Welch K, Wang L, Kong FM. Negative predictive value of positron emission tomography and computed tomography for stage t1–2n0 non-small-cell lung cancer: A meta-analysis. Clin Lung Cancer 2012;13(2):81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Caro A, Boada M, Cabanas M et al. False-negative rate after positron emission tomography/computer tomography scan for mediastinal staging in ci stage non-small-cell lung cancer. Eur J Cardiothorac Surg 2012;42(1):93–100; discussion 100. [DOI] [PubMed] [Google Scholar]
- 8.Kent M, Landreneau R, Mandrekar S et al. Segmentectomy versus wedge resection for non-small cell lung cancer in high-risk operable patients. Ann Thorac Surg 2013;96(5):1747–1754; discussion 1754–1745. [DOI] [PubMed] [Google Scholar]
- 9.Pozo-Rodriguez F, Martin de Nicolas JL, Sanchez-Nistal MA et al. Accuracy of helical computed tomography and [18f] fluorodeoxyglucose positron emission tomography for identifying lymph node mediastinal metastases in potentially resectable non-small-cell lung cancer. J Clin Oncol 2005;23(33):8348–8356. [DOI] [PubMed] [Google Scholar]
- 10.Boffa DJ, Kosinski AS, Paul S, Mitchell JD, Onaitis M. Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann Thorac Surg 2012;94(2):347–353; discussion 353. [DOI] [PubMed] [Google Scholar]
- 11.Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for n0 lung cancer. Ann Thorac Surg 2013;96(4):1171–1177. [DOI] [PubMed] [Google Scholar]
- 12.Martin JT, Durbin EB, Chen L et al. Nodal upstaging during lung cancer resection is associated with surgical approach. Ann Thorac Surg 2016;101(1):238–244; discussion 244–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bott MJ, Patel AP, Crabtree TD et al. Pathologic upstaging in patients undergoing resection for stage i non-small cell lung cancer: Are there modifiable predictors? Ann Thorac Surg 2015;100(6):2048–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veeramachaneni NK, Battafarano RJ, Meyers BF, Zoole JB, Patterson GA. Risk factors for occult nodal metastasis in clinical t1n0 lung cancer: A negative impact on survival. Eur J Cardiothorac Surg 2008;33(3):466–469. [DOI] [PubMed] [Google Scholar]
- 15.Licht PB, Jorgensen OD, Ladegaard L, Jakobsen E. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage i lung cancer. Ann Thorac Surg 2013;96(3):943–949; discussion 949–950. [DOI] [PubMed] [Google Scholar]
- 16.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for t1 n0 non-small cell lung cancer. Lung cancer study group. Ann Thorac Surg 1995;60(3):615–622; discussion 622–613. [DOI] [PubMed] [Google Scholar]
- 17.Khullar OV, Liu Y, Gillespie T et al. Survival after sublobar resection versus lobectomy for clinical stage ia lung cancer: An analysis from the national cancer data base. J Thorac Oncol 2015;10(11):1625–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Travis WD, Brambilla E, Noguchi M et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6(2):244–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pignon JP, Tribodet H, Scagliotti GV et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the lace collaborative group. J Clin Oncol 2008;26(21):3552–3559. [DOI] [PubMed] [Google Scholar]
- 20.Altorki NK, Wang X, Wigle D, et al. Perioperative mortality and morbidity after sublobar versus lobar resection for early-stage non-small-cell lung cancer: post-hoc analysis of an international, randomised, phase 3 trial (CALGB/Alliance 140503). Lancet Respir Med 2018;6(12):915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghaly G, Rahouma M, Kamel MK et al. Clinical predictors of nodal metastases in peripherally clinical t1a n0 non-small cell lung cancer. Ann Thorac Surg 2017;104(4):1153–1158. [DOI] [PubMed] [Google Scholar]
- 22.Detterbeck FC. Diagnosis and treatment of lung cancer: An evidence-based guide for the practicing clinician. WB Saunders Company; 2001. [Google Scholar]
- 23.Altorki NK, Yip R, Hanaoka T et al. Sublobar resection is equivalent to lobectomy for clinical stage 1a lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147(2):754–762; Discussion 762–754. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Jiang W, Zhan C, et al. Lymph node metastasis in clinical stage IA peripheral lung cancer. Lung Cancer 2015;90(1):41–6 [DOI] [PubMed] [Google Scholar]
- 25.Ramirez RA, Wang CG, Miller LE et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol 2012;30(23):2823–2828. [DOI] [PubMed] [Google Scholar]
- 26.Osarogiagbon RU, Hilsenbeck HL, Sales EW et al. Improving the pathologic evaluation of lung cancer resection specimens. Transl Lung Cancer Res 2015;4(4):432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smeltzer MP, Faris NR, Ray MA, Osarogiagbon RU. Association of pathologic nodal staging quality with survival among patients with non-small cell lung cancer after resection with curative intent. JAMA Oncol 2018;4(1):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang M, Cao H, Guo X et al. The number of resected lymph nodes (nlns) combined with tumor size as a prognostic factor in patients with pathologic n0 and nx non-small cell lung cancer. PLoS One 2013;8(9):e73220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.David EA, Cooke DT, Chen Y, Nijar K, Canter RJ, Cress RD. Does lymph node count influence survival in surgically resected non-small cell lung cancer? Ann Thorac Surg 2017;103(1):226–235. [DOI] [PubMed] [Google Scholar]
- 30.Qu X, Wang K, Zhang T et al. Long-term outcomes of stage i nsclc (</=3 cm) patients following segmentectomy are equivalent to lobectomy under analogous extent of lymph node removal: A psm based analysis. J Thorac Dis 2017;9(11):4561–4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu F, Qi L, Yue D, Wang C. The effect of the extent of lymph node dissection for stage ia non-small-cell lung cancer on patient disease-free survival. Clin Lung Cancer 2013;14(2):181–187. [DOI] [PubMed] [Google Scholar]
- 32.Nitadori J, Bograd AJ, Kadota K et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013;105(16):1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]