Abstract
Time-based interventions have emerged as promising treatments for disorders associated with impulsivity. These interventions can be implemented to test their efficacy in preventing or treating impulsive choice in animal models of diseases related to impulsivity such as drug abuse. Impulsive choice is typically defined as choosing a smaller-sooner (SS) reward over a larger-later (LL) reward when the LL is relatively more optimal. Previous research has shown that these interventions promote LL choices in males and females, but sex differences have not been assessed. Because sex differences can complicate the application of therapies, it is critical to compare the effects of the intervention in males and females. The intervention group received exposure to 10-s and 30-s interval schedules, and the control rats received no delay to reward. Different impulsive choice tasks were used to assess the intervention efficacy across the two experiments. Following the intervention, reductions in impulsive choice were found in male and female rats, but the degree of improvement was inconsistent across sex and task. Bayesian analyses that combined the results revealed robust evidence of an overall intervention effect with the intervention group showing greater self-control, but there was no evidence for the intervention affecting males and females differently. Taken together, these results suggest that time-based interventions are effective tools to treat impulsivity in both males and females and offer promising translational capability to humans.
Keywords: impulsive choice, intervention, female, Bayesian, rat
1. Introduction
Heightened impulsive choices are associated with multiple maladaptive behaviors such as substance abuse [1–5], gambling [6], and eating a poor diet [7, 8]. Impulsive choice is a stable trait [9] that is associated with diseases such as Attention-Deficit/Hyperactivity Disorder [10–12] and has been suggested to operate as a trans-disease process [13, 14]. To measure impulsive choices, individuals are presented with choices between smaller rewards available sooner (SS) and larger rewards available later (LL) and the impulsive choice is the sub-optimal SS [15, 16]. Given the trans-disease nature of impulsive choice, it is critical to develop effective interventions that promote self-control.
Impulse control disorders such as Attention-Deficit/Hyperactivity Disorder (ADHD) may potentially arise because of errors in time perception [17–19] by overestimating delays [19, 20] or through poor temporal precision [21–23]. Poor timing of delays may lead impulsive individuals to avoid longer delays, known as delay aversion [24–26]. Delay aversion may cultivate further temporal processing deficits because avoiding delays limits experience with longer time periods, which decreases the ability to learn those delays [27]. Along similar lines, individuals with impulsivity may have a low tolerance for waiting for extended periods of time, also known as delay intolerance. The inability or unwillingness to wait may result in more impulsive choices [26, 28]. Although the mechanism(s) underlying impulsive choices remains a debate, time-based interventions have a robust and successful history of increasing self-control.
Time-based interventions have been established in both young and middle-aged male rats using a variety of different interval schedules including differential reinforcement of low rate, fixed-interval (FI), variable-interval (VI), and delay exposure [4, 29–35]. While there is some debate about whether timing [32, 33] or delay tolerance [35, 36] is the primary target of the interventions, the interventions do significantly improve self-control. In addition, the FI and delay exposure interventions lasted for several months [29, 31] and generalized to multiple choice tasks [31], indicating both longevity and generalizability. However, the VI intervention was not long-lasting, suggesting that interventions are most efficacious when they involve exposure to fixed delays [31]. Overall, time-based interventions are a successful treatment for impulsivity in male rats [30, 35, 37, 38]. However, only one study has measured the effects of an FI intervention in a group of females and found reduced impulsive choices and some improvements in timing processes, but there were no comparisons with males [39].
The success of time-based interventions appears to generalize to humans, suggesting that research evaluating any differences in efficacy between sexes is needed. Typically, individuals receive interventions with fading (progressively increasing or decreasing) SS or LL delays within an impulsive choice task. Exposure to delays increases LL choices in children with ADHD [40, 41], children and adults with developmental and/or intellectual disabilities [42–44], children and adults with severe behavior disorders [45] as well as typically-developing children [28]. While some of these studies included female participants, the efficacy of the interventions in each sex was not investigated. Few studies have directly tested time-based interventions in both sexes despite evidence that men and women may differ in impulsivity [46, 47]. For example, women show greater impulsivity with hypothetical rewards compared to men, but men are more impulsive when making real-money choices compared to women [47]. These differences in impulsive choice suggest that time-based interventions may affect males and females differently.
Despite the reported sex differences in impulsive choice, to our knowledge no previous studies have tested sex as a biological variable regarding intervention efficacy. Interventions could potentially operate differently in males and females if their impulsive choices are driven by different factors such as immediacy biases, sensitivities to delay, or magnitude discrimination. The current pair of experiments investigated intervention efficacy in male and female rats. In addition, an ancillary goal of Experiment 2 was to develop high throughput choice and timing tasks to assess intervention efficacy in males and females under conditions of more limited observations, which may be useful for future neuroscientific investigations to assess sex differences. Taken together, these studies offer specific mechanisms to further target when developing effective interventions for males and females to decrease impulsivity associated with animal models of impulse control disorders.
2. Method
2.1. Animals
The subjects used in each experiment were 24 male and 24 female experimentally-naïve Sprague Dawley rats (Charles River, Kingston, NY). The sample sizes were determined based on previous intervention studies from our laboratory in male rats, in which sample sizes of 12 per group were sufficient to reliably detect intervention effects with observed power levels over 80%. The rats arrived at Kansas State University at 21 days of age. They were pair-housed and maintained on 12-hr light:dark schedule (lights off at approximately 7 am) and were tested during the dark phase of the cycle. There was ad libitum access to water in the home cages and experimental chambers. The experiments were approved by the Institutional Animal Care and Use Committee at Kansas State University and all experiments were performed in accordance with relevant guidelines and regulations.
2.2. Procedure
Figure 1 shows the timeline of Experiments 1 and 2.
Figure 1.
Timelines of Experiments 1 and 2. Both cohorts of rats received the same magazine, lever press, and intervention training. Rats in Experiment 1 completed the standard impulsive choice task while rats in Experiment 2 completed shortened and extended versions in a counterbalanced order.
2.2.1. Magazine and lever press training.
Initial training consisted of training to eat from the food cup (1 session) and press the levers (4 sessions). Food cup training involved the delivery of food pellets on a random-time 60-s schedule. During lever training, rats received 20 food pellets on each lever on a series of schedules over the session: fixed ratio 1 → random ratio 3 → random ratio 5.
2.2.2. Time-based intervention.
Rats were randomly assigned to two groups (n = 12 males and 12 females each) – FI or ND (No Delay).
2.2.2.1. Fixed Interval (FI) intervention.
The FI intervention [33] involved separate training on FI 10-s and FI 30-s schedules in a counterbalanced order. A single lever was inserted, and a response initiated the delay. The first response after the delay resulted in one food pellet on the FI 10 and 2 pellets on the FI 30. The inter-trial interval (ITI) lasted 60 s. The FI 10 s was delivered on the SS lever from the choice task and the FI 30 s was delivered on the LL lever from the choice task. The sessions lasted until 100 total reinforcers were earned or 2 hours elapsed. The intervention consisted of 45 total sessions: 15 for the SS lever and 30 for the LL lever to equate the number of trials on each lever.
2.2.2.2. No Delay (ND) control.
The ND control received an identical task to the FI group except that there was no programmed delays to food on either lever [4, 31]. They were exposed to a FR 2 schedule on both levers to match the minimum response requirement on the FI. The ITI for the ND task was 70 or 90 s to match the FI 10 or 30 s (plus the 60-s ITI) so that the rate of reinforcement matched the FI schedule. The 70-s ND was delivered on the SS lever for 1 pellet and the 90-s ND on the LL lever for 2 pellets.
2.2.3. Post-intervention testing.
Following the intervention, the rats in Experiment 1 completed the impulsive choice task. Rats in Experiment 2 completed a peak task and extended and shortened impulsive choice tasks (Figure 1). In all three choice tasks, the rats received a mixture of forced choice and free choice trials as in previous studies [33]. Forced choice trials were identical to the FI intervention trials in all respects except that the SS delay varied across sessions. On free choice trials, the rats were simultaneously presented with the SS and LL levers. A choice response on one lever retracted the opposite lever and initiated the delay. The remainder of the trial was the same as a forced choice trial. The impulsive choice task in Experiment 1 included peak trials, which involved presentations of the SS or LL lever and were the same as forced choice trials except that there was no food delivery and the lever remained inserted for 90 s.
2.2.3.1. Impulsive choice task (Experiment 1).
Rats chose between the SS and LL with manipulations of SS delay across blocks of 10 sessions each: 5→10→20 s. In total this task lasted 30 sessions. Each session was comprised of randomly intermixed trials: 48 free choice, 12 SS forced choice, 12 LL forced choice, 3 SS peak, and 3 LL peak. Analyses of the peak trials embedded in the impulsive choice task in Experiment 1 could not be conducted as there were insufficient observations to yield stable peak functions.
2.2.3.2. Peak procedure (Experiment 2).
Following the intervention period, the rats experienced a peak procedure. It consisted of 2 sessions, one session for SS and one session for LL. Each session comprised 50% peak trials and 50% forced choice in a random order. For a peak trial, the lever was inserted for 90 s and then retracted with no reinforcement. On forced choice trials, the rats received the lever that was previously received with reinforcement from the intervention period. This task was conducted to allow for the analysis of the perceived timing for either SS or LL rewards. However, peak task analyses are not included in the manuscript as the functions were generally very noisy and flat. One day per delay with the 50% peak trials and 50% FI trials did not appear to be sufficient to produce stable functions.
2.2.3.3. Extended impulsive choice task (Experiment 2).
This task was the same as the impulsive choice task except that there was an added 30-s SS delay during a fourth phase and peak trials were replaced by an additional block of forced choice trials that occurred at the start of each session. The task lasted for 40 sessions. The 30-s SS delay was added to directly evaluate preference for the larger magnitude.
2.2.3.4. Shortened impulsive choice task (Experiment 2).
This task was used to determine if the intervention effects on the extended impulsive choice task could be replicated in a higher throughput task with fewer sessions and more delays. Rats received a 5-s SS delay for 4 consecutive sessions after which the SS delay increased each session: 7.5→10→15→20→25→30 s.
2.2.3.5. Bisection task (Experiment 2).
A high throughput bisection task was conducted to assess the rats’ ability to discriminate the delays received in the choice tasks following their experience with the initial impulsive choice task. This task differed from more typical bisection tasks in the following ways: (1) rats received longer durations that matched the delays received in the choice task (most bisection tasks use delays in the 2–10 s range); (2) the test trials were interweaved with training trials from the beginning rather than being included following an acquisition phase; and (3) there were only four sessions of total training and testing. Rats received a houselight cue on each trial that lasted for a short (10 s) or long (30 s) duration after which two levers were inserted. A response on the correct lever (e.g., left for short, right for long, counterbalanced across rats) resulted in food delivery, whereas incorrect responses resulted in a 5-s ITI followed by a repetition of the previous trial. Correction trials continued until a correct response occurred. Rats also received nonreinforced intermixed test trials with signal durations of 10, 15, 20, 25, and 30 s to generate a psychophysical function. There were no correction trials following test trials. Sessions were composed of 4, 40-trial blocks that comprised 10 non-reinforced testing trials (2 for each delay), 15 reinforced short anchor, and 15 reinforced long anchor trials. The task lasted for a total of four 2-hr sessions. Unfortunately, we were not able to conduct analyses on the bisection data because the functions were relatively flat and noisy, indicating that bisection performance appears to require considerably more training to obtain stable functions (even though the rats were already highly familiar with the delays).
3. Results
3.1. Experiment 1
Impulsive choices were analyzed using a repeated measures mixed effects logistic regression [48] using MATLAB 2016A (The Mathworks, Natick, MA). Mixed effects models are the recommended analytical approach in the fields of Psychology and Neuroscience [49] because they increase generalizability to the population and reduce Type I error rates [49, 50]. The mixed effects regression models estimate fixed (group level variables) and random effects (individual differences). The hypothesized effects were tested as fixed effects, and the Akaike Information Criterion (AIC) was used to determine the best random effects structure. When the random effects were highly correlated, a more parsimonious random effects model was selected to avoid overparameterization [51, 52]. Categorical variables (group and sex) were effects coded so that all tests were conducted relative to the grand mean of the data. SS delay was coded as a continuous variable and scaled with two different intercepts: 0-s and 30-s delays [31, 39, 53]. The 0-s delay intercept predicted choices at a 0-s SS delay to assess the intervention effects on bias for immediacy. The 30-s delay intercept predicted (Experiment 1) or analyzed (Experiment 2) choices at a 30-s SS delay where the SS and LL were equal. This provided an assessment of the preference for the larger (2-pellet) reward in the absence of any difference in delay. For all experiments, the fixed effects were tested in a full factorial model with the variables of group (FI or ND), sex, and SS delay with intercept as the random effect. The addition of a random intercept allows the intercept to vary across individual rats. The slope (LL delay) was not included as a random effect because it was significantly correlated with the intercept. A total of 29,494 choices were analyzed over the last five sessions of each SS delay.
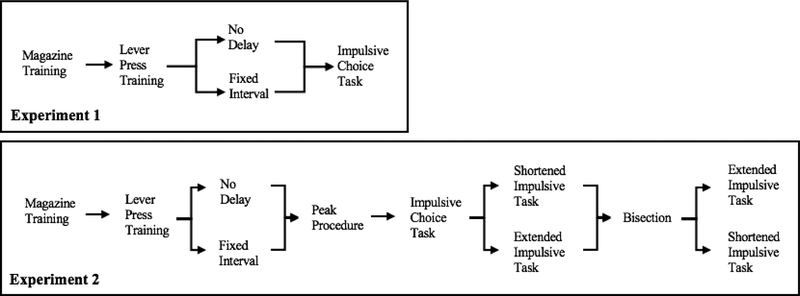
Figure 2 shows mean proportion of LL choices in the impulsive choice task. At the 0-s delay intercept, females made more LL choices than males, t = 3.62, p < .001, b = 0.71 [0.33, 1.10], and the FI group made more LL choices than the ND group, t = 4.04, p < .001, b = 0.80 [0.41, 1.18], but there was no interaction. At the 30-s delay intercept, the FI group made more LL choices than the ND group, t = 2.56, p < .05, b = 0.51 [0.12, 0.90], but there was no sex difference. Thus, the intervention induced a preference for longer delays and larger magnitudes as rats in the intervention group made more LL choices (relative to the ND group) at both intercepts. Interestingly, there was no main effect of Sex on the 30-s delay intercept suggesting that sex differences in impulsive choice behavior may be localized to a reduced preference for immediacy in females (Figure 2).
Figure 2.
Proportion of LL choices in the impulsive choice task. The log odds are included on the secondary axis as a reference for interpreting the b values. The error bars are +/− one standard error of the mean determined from the error estimates in the regression model.
The analysis of sensitivity to delay (slope) revealed that females had a shallower slope than males, t = −5.38, p < .001, b = −0.52 [−0.71, −0.33], indicating that they were less sensitive to delay than male rats. The FI group had a shallower slope than the ND group, t = −2.96, p < .05, b = −0.29 [−0.48, −0.10]. Finally, there was a significant Group × SS Delay × Sex interaction, t = −3.35, p = .001, b = −0.32 [−0.51, −0.13]. Post-hoc analyses revealed that FI females (b = 6.50) had a significantly shallower slope than ND females (b = 7.72), FI males (b = 8.18), and ND males (b = 8.11). Thus, female rats showed significant improvement following the intervention in reducing their sensitivity to delay, whereas males did not show this effect.
3.2. Experiment 2
The last five sessions of each delay were analyzed for the extended impulsive choice task, which included a total of 33,674 choices. The last session of the 5-s delay and all subsequent sessions were analyzed for the shortened impulsive choice task, resulting in a total of 12,726 choices. The effect of the order of delivery of the impulsive choice tasks was tested as a fixed effect in the full factorial model (Group × Sex × SS Delay × Order). In the extended task, the only effect involving order was the 4-way interaction of Group × Sex × SS Delay × Order. There was no overall effect of order or any other interactions. Adding order to the model only made a small improvement in AIC. In the shortened task, there was an overall main effect of order. However, adding order as a variable to the shortened task model made the AIC value worse, indicating a poorer fit of the model to the data. Overall, it seems that although there were some order effects, they did not interact with Sex or Group effects. Therefore, the results included below are from the most parsimonious models that did not include order as a fixed effect.
3.2.1. Extended impulsive choice task.
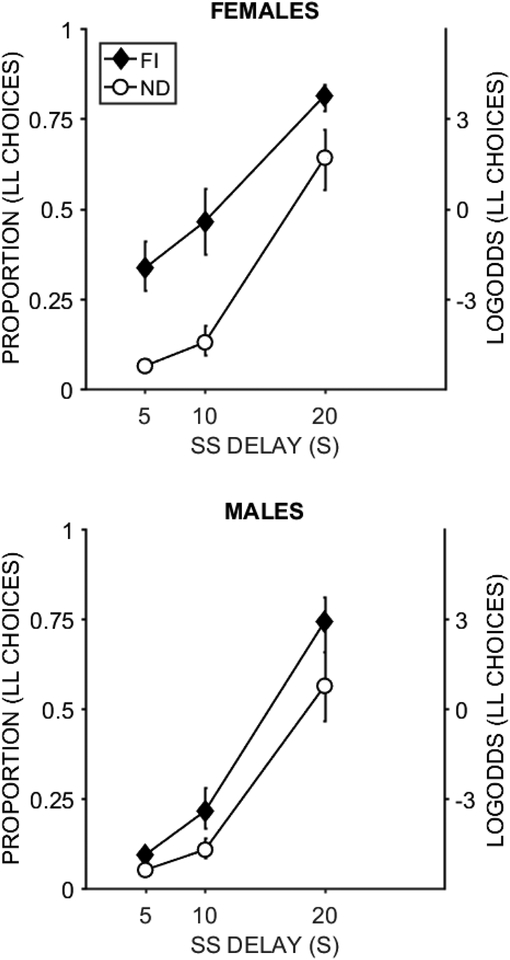
Figure 3 depicts mean proportion of LL choices in the extended impulsive choice task. At the 0-s delay intercept there were main effects of Group, t = 3.11, p = .002, b = 0.47 [0.17, 0.76] and Sex, t = 3.56, p < .001, b = 0.53 [0.24, 0.83], indicating that the FI group and females made more LL choices than their counterparts. There was a significant Group × Sex interaction, t = −2.82, p = .005, b = −0.42 [−0.72, −0.13]. The FI males made significantly more LL choices at the intercept (b = −2.49) compared to the ND males (b = −4.80), t = 5.06, p < .001. However, the FI and ND females did not differ (bFI = −2.80; bND = −2.89), t = 0.21, p = .835. There was no group or sex effect at the 30-s delay intercept.
Figure 3.
Proportion of LL choices in the extended impulsive choice task. The log odds are included on the secondary axis as a reference for interpreting the b values. The error bars are +/− one standard error of the mean determined from the error estimates in the regression model.
Analysis of choice function slopes showed that females were less sensitive to delay than males, t = −12.92, p < .001, b = −0.82 [−0.95, −0.70]. The intervention reduced sensitivity to delay with the FI group having shallower slopes than the ND group, t = −5.98, p < .001, b = −0.38 [−0.50, −0.26]. A significant Group × SS Delay × Sex interaction, t = 3.58, p < .001, b = 0.23 [0.10, 0.35], revealed that the FI males had a significantly shallower slope (b = 6.12) compared to the ND males (b = 7.34), t = 6.05, p < .001. However, the FI and ND females did not differ significantly, although the FI group tended towards shallower slopes (bFI = 4.94; bND = 5.24), t = 1.96, p = .05.
Overall, male rats showed an intervention effect with less bias for immediacy and less delay sensitivity, while the females did not. However, there were overall sex differences where the female rats were less biased for the immediate reward and less sensitive to changes in delay overall (Figure 3). There were no sex or intervention effects on preference for larger magnitudes at the 30-s delay.
3.2.2. Shortened impulsive choice task.
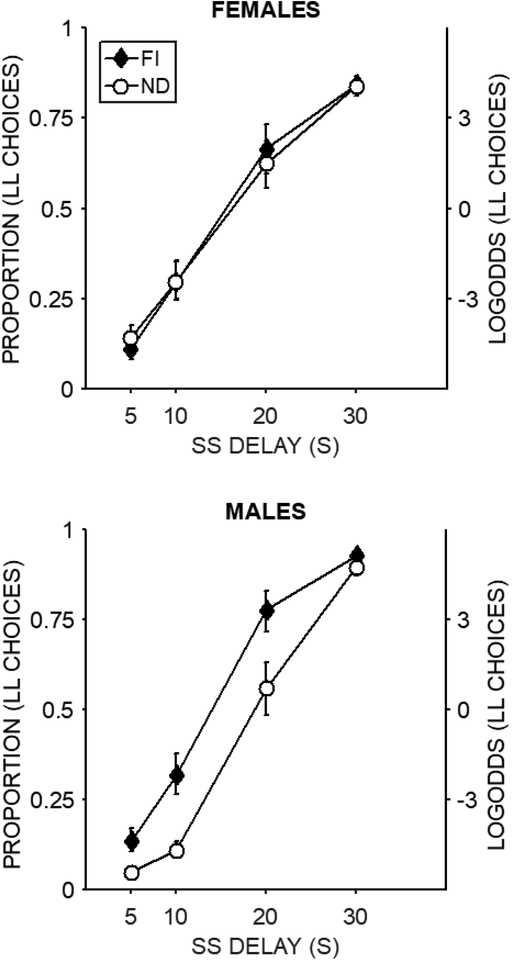
Figure 4 displays mean proportion of LL choices in the shortened impulsive choice task. At the 0-s delay intercept the FI group made more LL choices than the ND group, t = 2.26, p = .02, b = 0.43 [0.06, 0.80], but there was no sex effect. At the 30-s delay intercept, there were no significant main effects or interactions. The slope analysis showed a significant Group × SS Delay effect, t = −2.68, p = .007, b = −0.24 [−0.42, −0.06] that was due to shallower slopes in the FI group. There also was a significant Sex × SS Delay effect, t = −3.77, p < .001, b = −0.33 [−0.51, −0.16] that was due to shallower slopes in the female rats compare to the male rats. There was no Group × Sex × SS Delay interaction.
Figure 4.
Proportion of LL choices in the shortened impulsive choice task. The log odds are included on the secondary axis as a reference for interpreting the b values. The error bars are +/− one standard error of the mean determined from the error estimates in the regression model.
Overall, the FI intervention was effective at reducing bias for immediacy and delay sensitivity regardless of sex (Figure 4). In addition, females were less sensitive to delay than males overall. There were no sex or intervention effects on preference for larger magnitudes at the 30-s delay.
3.2.3. Inter-task correlations.
To assess whether there was cross-task reliability in measuring choice behavior in individual animals, we assess the correlation between the extended and shortened impulsive choice tasks on the common delays that were experienced as well as the overall proportion of LL choices. There were significant inter-task correlations at all delays and overall, and the correlations were stronger at longer delays, r5 = .37, r10 = .43, r20 = .46, r30 = .51, roverall = .54, p ≤ .01.
3.3. Experiments 1 and 2: Bayesian Multi-Level Modeling
There was a nested replication of Experiment 1 in the Experiment 2 extended task which created an opportunity to employ Bayesian modeling to examine the reliability of the parameter estimates produced across the two experiments. This is particularly beneficial given that Experiment 2 only partially replicated Experiment 1 as we can use the Bayesian analysis to determine a better estimate of the true expected effect. Bayesian analysis incorporates prior data to predict current data by including previous data as a prior distribution, which is a set of values that represent what we already know about an effect. For example, an original study suggests that treatment A increases outcome B. In another study examining the same relationship between treatment A and outcome B, the previous information about treatment A, specifically the estimate value and standard error of the estimate from the original study, is specified as the prior distribution for treatment A in the new study. The Bayesian analysis results in a posterior distribution of values that represent the accumulation of information across studies. Unlike null hypothesis testing, we can use results from previous experiments to give the statistical model as much information as possible. Behind the scenes, likelihoods of observing the new data are calculated using Markov chain Monte Carlo sampling. These likelihoods are the posterior probabilities, which are combined with the prior probability specified in the prior distributions. The combination of these probabilities results in a posterior distribution of each parameter estimate that was informed by prior knowledge but not constrained to it. Researchers can use the posterior distributions to understand the relative likelihood that each of the parameter values generated the data. For further information about Bayesian data analysis, see [54] and [55] for explanation and examples.
Parameter estimates and their standard errors from the impulsive choice model in Experiment 1 were specified as the prior distributions for the impulsive choice model in Experiment 2 (for the extended choice task) to indicate the strength of evidence for each parameter value. This analysis examines the likelihood of observing interactions between sex and intervention on impulsive choice behavior. Without a robust sex differences literature to rely on, this analysis begins to compile critical evidence on differences between males and females to develop a clearer picture of any sex differences.
3.3.1. Model specifications.
The 0-s delay intercept model was selected for Bayesian analysis because of the sex differences in this model across experiments. Estimates and standard error values from Experiment 1 were used as priors. For instance, the prior specified for the main effect of SS delay was a normal distribution with a mean of 7.63 and a standard deviation of 0.10. All effects followed this suit except for the Group × Sex interaction. A normal distribution was specified with the model-derived estimate as the mean but a standard deviation of 1. The Group × Sex effect was not significant in the original model, so the standard deviation was specified as 1 to indicate uncertainty in the estimate.
The model formula, scaling, and coding structures entered into the Bayesian analysis were the same as the 0-s delay intercept repeated measures logistic regression in Experiment 2 except that the priors from Experiment 1 were added to the model. Analyses were conducted in the brms package in R [56]. The total number of iterations conducted by the model was 4,500 over 3 chains, 3,000 of which were post-warmup samples. Specific hypothesis tests were conducted to compare evidence for effects to 0 (no effect). The hypothesis tests assessed each effect for evidence that it could be a null effect. This provides an index of how robust and reliable an effect is likely to be with evidence ratios at or near 0 indicating highly robust and reliable effects with strong evidence against the null hypothesis. Group, Sex, Group × Sex, Group × SS Delay, SS Delay × Sex, and Group × SS Delay × Sex were tested to investigate the evidence for each effect. Bayesian results can be illustrated with prior and posterior distributions of estimated effect sizes. The degree of overlap in the prior and posterior distributions indicates the degree of replicability across experiments. In other words, overlap indicates that the previous experiment(s) and current experiment resulted in a highly similar distribution of effect sizes. In many cases, the posterior distribution appears narrower and more pointed compared to the prior distribution. This indicates that the analysis resulted in more instances or higher relative density of the specific values encompassed in the distribution suggesting increased certainty of the estimate effect value. No overlap in distributions suggests the analysis resulted in values unlike those represented by the prior distribution. In addition, posterior distributions centered around zero suggest little to no evidence for the effect (i.e., an estimated effect size near zero). The Bayesian model computes an evidence ratio based on the posterior distributions, which is the overall likelihood that the effect’s estimate value is 0 (i.e., a null result). Larger evidence ratios are more indicative of an overall null result.
3.3.2. Results.
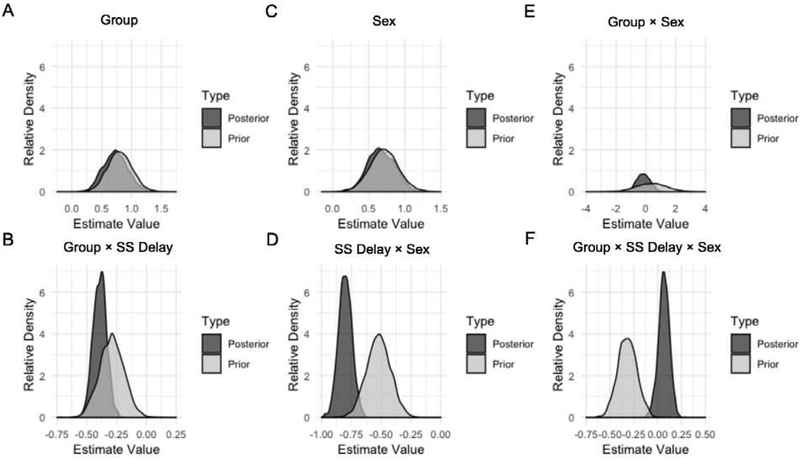
Figure 5 shows prior and posterior distributions of the effect size estimates. The FI intervention (Group effect) resulted in little evidence for the null at the 0-s delay intercept (evidence ratio = 1.33). Figure 5A shows that the prior and posterior distributions were nearly identical, which indicates strong replicability of Experiment 1 results in the Experiment 2 extended task. The hypothesis test on the Group × SS Delay effect resulted in no evidence for the null hypothesis (evidence ratio = 0). Figure 5B depicts the prior and posterior distributions for the Group × SS Delay interaction. The posterior distribution was more pointed than the prior distribution suggesting increased certainty of the estimate value. Posterior distributions for Group and Group × SS Delay effects did not incorporate zero. Thus, the intervention robustly reduced preferences for the immediate reward and sensitivity to delay, consistent with previous studies [31–33, 39].
Figure 5.
Posterior and prior distributions of hypotheses.
Further hypothesis testing was conducted to examine sex differences in impulsive choice and intervention efficacy. The hypothesis test on the Sex main effect resulted in some evidence in favor of a null effect (evidence ratio = 6.25). The SS Delay × Sex effect resulted in almost no evidence in favor of a null effect (evidence ratio = 0.1). The posterior distributions for both effects did not incorporate zero. This indicates that females made more LL choices and showed flatter slopes than males in both experiments (Figures 5C and 5D). The Group × Sex test showed some evidence in favor of a null effect (evidence ratio = 2.22). The Bayesian model found little evidence for the effect, as shown in the posterior distribution centered around zero (Figure 5E). Finally, the Group × SS Delay × Sex effect showed strong evidence in favor of a null effect (evidence ratio = 92.83). In Experiments 1 and 2, the three-way interaction was significant, but this was not replicated in the Bayesian analysis. The three-way interaction was driven by FI females in Experiment 1 and FI males in Experiment 2. This highlights that Bayesian models are not overly sensitive to prior information. The Bayesian model found little evidence for the effect, as shown in the posterior distribution which clearly incorporated zero. The Bayesian model confirms that the three-way interactions in Experiments 1 and 2 were likely due to chance or to the influence of an extraneous variable (Figure 5F).
4. Discussion
The present experiments sought to compare the efficacy of an FI intervention in promoting self-control in male and female rats under different testing conditions. A Bayesian analysis was used to compare the results from the common choice tasks used in Experiments 1 and 2 and provided insight into intervention and sex effects on impulsive choice. In Experiment 1, the intervention was effective at promoting LL choices at the 0-s delay intercept and reducing sensitivity to delay. Similar results were replicated in Experiment 2 with Bayesian analysis confirming a robust intervention effect. This supports previous research suggesting time-based interventions are effective at decreasing impulsive choices by reducing bias for immediacy and sensitivity to delay [29–31, 33, 36].
Along with intervention effects on impulsive choice, the experiments examined sex as a biological variable. In Experiment 1, the intervention reduced impulsive choice behavior in females more so than in males. In the extended task in Experiment 2, the intervention reduced impulsive choice in males but not in females. Bayesian analysis suggested that these inconsistencies are likely chance effects, or to the influence of an unmeasured extraneous variable, with the intervention robustly reducing impulsive choice regardless of sex. This aligns with previous work done in our laboratory with single sex samples [31, 33, 39]. The results are further supported by a strong intervention effect and a lack of a sex effect in the shortened task in Experiment 2. Overall, it seems sex differences in intervention efficacy are inconsistent and unreliable in rats. Further research examining intervention efficacy is needed in male and female humans to better understand treatment outcomes when translating time-based interventions to clinical settings. Previous literature suggests that time-based interventions are successful in both sexes in humans [28, 40, 41, 44, 45], but it is unknown whether the interventions may be effective to varying degrees in males and females.
There do appear to be differences in overall impulsive choices between sexes. Experiment 1 and the extended task in Experiment 2 showed that females were less biased for immediate rewards and were less sensitive to delay and the shortened task in Experiment 2 found they were less sensitive to delay. Bayesian analysis confirmed that the differences in sensitivity to delay between sexes were robust and reliable. This suggests that expression of sex differences may be task-dependent. Interestingly, two previous studies indicated some evidence for greater impulsivity in female rats and mice [57, 58]. Koot, van den Bos, Adriani and Laviola [57] assessed impulsive choice in female and male mice in which the delay to the SS reward was 0 s and the delay to the LL started at 0 s and increased by several seconds each day up to 150 s. There were no overall sex effects on choice behavior. However, there were two sub-populations of mice: some had steep functions and some had flat functions. In the mice with steep functions, females showed greater SS preferences at longer LL delays compared to males. There were no sex differences in the mice with flat functions. This suggests that female mice with high delay sensitivity drove the effects in their study.
van Haaren, van Hest and van de Poll [58] measured choices for 1 versus 3 pellets that were experienced either both delayed (e.g., 6 s) or where the smaller reward was available after .1 or 6 s and the larger reward was associated with a longer delay. Female rats showed similar preferences for larger magnitudes in the absence of differential delays, consistent with the observations in the current Experiment 1. However, when the larger reward was delayed relative to the SS, females tended to make more impulsive choices. This is inconsistent with the present results. Their study involved a small sample size (4–8 per group) and did not include formal statistical analyses, so it is not clear if these results were statistically significant. There were considerable individual differences, particularly in the female rats that may have contributed to the findings.
One key factor that may have contributed to the results is the experience with an immediate SS reward. In Koot et al. the mice only experienced a 0-s SS delay, and in the van Haaran et al. experiment the rats experienced both 0.1-s (effectively an immediate delay) and 6-s SS delays. The females tended to show more impulsive choices in both cases. In the van Haaren et al. study, the rats initially experienced non-differential 6-s delays to both rewards, followed by a 0.1-s SS delay compared to a 6-s LL delay. It is possible that exposure to the immediate delay may have introduced an impulsive choice tendency that persisted when the SS was delayed in later phases. The current studies did not include an immediate reward but instead estimated preferences for immediacy. Differences between experienced versus estimated preferences for immediacy may be a fruitful area for future study.
Relatedly, several of the previous rodent and human intervention studies directly assessed effects on impulsive choices with an immediate SS delay rather than estimating preferences for immediacy. A 0-s delay, which is a few milliseconds in practice, would invoke the sub-second cerebellar motor timing system, whereas delays in the seconds range would rely on the interval timing system which involves the frontal-striatal loops [59–61]. Because interval timing has been implicated as a potentially key factor in impulsive choices [19, 62–64], we opted to focus on parameters that would maximize the reliance on the interval timing system. However, impulsive choices to a 0-s SS do not predict choices when the SS is delayed [65]. With regard to intervention effects, exposure to delayed rewards promotes LL choices, but exposure to immediate delays have little effect on subsequent choices [30]. Thus, 0-s delay paradigms may invoke different processes that could complicate the expression of sex effects on choice behavior. Further research is needed to understand how these task differences can impact behavior in both sexes.
An additional goal of Experiment 2 was to develop high throughput tasks for measuring the effects of the intervention on impulsive choice and timing. The shortened impulsive choice task replicated the intervention effects on the long task (when ignoring sex as a variable) and the two tasks were well correlated in Experiment 2. The shortened task collected measurements at more delays, but with fewer observations per delay. This task could be beneficial in experiments where limited testing may be necessary, such as in testing of impulsive choices in conjunction with pharmacological or neurobiological manipulations. Regarding timing measurements, the high throughput peak and bisection tasks in Experiment 2 yielded noisy flat functions. This is interesting given that the rats had already experienced the delays from these tasks both in the intervention and impulsive choice tasks. The choice behavior indicates that the rats had learned the delays, and previous research with these tasks has also verified that rats time the delays within choice tasks to a good degree of accuracy and precision [33]. The results of Experiment 2 suggest that quality timing assessments require considerably more data than what we were able to obtain here, perhaps due to the inherent noise in the timing system which may create challenges in analyzing timing data with limited observations [66].
In summary, the experiments utilized three tasks to assess the intervention effects on impulsive choice. The intervention was successful at reducing impulsive choice in all tasks, and the Bayesian analysis indicated that the effects were robust and reliable regardless of sex. This is important as it indicates that the intervention can be successfully applied to animal model research in both sexes. These experiments provide a good foundation for further research examining the psychological and physiological mechanisms that the intervention targets along with how such mechanisms interact with the intervention’s potency. Pinning down these mechanisms could provide diagnostic criterion allowing the FI intervention to be tailored to individual treatment needs. Overall, the present experiments provide a strong foundation for time-based behavioral interventions to improve impulsive choice in male and female rats.
Highlights.
Male rats made more impulsive choices overall than female rats
The time-based intervention reduced impulsive choices
Efficacy of the intervention did not differ consistently between males and females
Acknowledgements
This research was supported by NIMH R01 Grant 085739 and by NIGMS Grant 113109 awarded to Kimberly Kirkpatrick and Kansas State University. Subsets of this data were presented at Society for Quantitative Analyses of Behavior 2018 Annual Conference and Kansas Association for Behavior Analysis 2018 Annual Conference.
Footnotes
Competing Interests Statement
Authors have no competing interests to declare.
Conflict of Interest
There are no conflicts of interest/financial disclosures to report for KP, CB, ID, AM, and KK. The manuscript has not been published previously and is not under consideration for publication elsewhere. All authors have contributed significantly to the manuscript and consent to having their names on the manuscript.
References
- [1].Perry JL, Nelson SE, Carroll ME, Impulsive choice as a predictor of acquisition of IV cocaine self- administration and reinstatement of cocaine-seeking behavior in male and female rats, Experimental and clinical psychopharmacology 16(2) (2008) 165–177. [DOI] [PubMed] [Google Scholar]
- [2].Bickel WK, Odum AL, Madden GJ, Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers, Psychopharmacology (Berlin) 146(4) (1999) 447–454. [DOI] [PubMed] [Google Scholar]
- [3].Fuemmeler BF, Kollins SH, McClernon FJ, Attention deficit hyperactivity disorder symptoms predict nicotine dependence and progression to regular smoking from adolescence to young adulthood, Journal of pediatric psychology 32(10) (2007) 1203–1213. [DOI] [PubMed] [Google Scholar]
- [4].Stein JS, Johnson PS, Renda CR, Smits RR, Liston KJ, Shahan TA, Madden GJ, Early and prolonged exposure to reward delay: Effects on impulsive choice and alcohol self-administration in male rats, Experimental and clinical psychopharmacology 21(2) (2013) 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Perry JL, Carroll ME, The role of impulsive behavior in drug abuse, Psychopharmacology 200 (2008) 1–26. [DOI] [PubMed] [Google Scholar]
- [6].Dixon MR, Marley J, Jacobs EA, Delay discounting by pathological gamblers, Journal of applied behavior analysis 36(4) (2003) 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dassen FCM, Houben K, Jansen A, Time orientation and eating behavior: Unhealthy eaters consider immediate consequences, while healthy eaters focus on future health, Appetite 91 (2015) 13–19. [DOI] [PubMed] [Google Scholar]
- [8].Garza KB, Ding M, Owensby JK, Zizza CA, Impulsivity and fast-food consumption: a cross-sectional study among working adults, Journal of the Academy of Nutrition and Dietetics 116(1) (2016) 61–68. [DOI] [PubMed] [Google Scholar]
- [9].Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC, Psychiatric aspects of impulsivity, Am J Psychiatry 158 (2011) 1783–1793. [DOI] [PubMed] [Google Scholar]
- [10].Fox AT, Hand DJ, Reilly MP, Impulsive choice in a rodent model of attention-deficit/hyperactivity disorder, Behavioural Brain Research 187(1) (2008) 146–152. [DOI] [PubMed] [Google Scholar]
- [11].Marco R, Miranda A, Melia A, Muller U, Butler L, Gabriels I, Albrecht B, Uebel H, Banaschewski T, Kuntsi J, Oades R, Steinhausen HC, Faraone SV, Schlotz W, Mulligan A, Andreou P, Christiansen H, Meded S, Asherson P, Gill M, Mulas F, Roeyers H, Rothenberger A, Sonuga-Barke EJS, Delay and reward choice in ADHD: An experimental test of the role of delay aversion, Neuropsychology 23(3) (2009) 367–380. [DOI] [PubMed] [Google Scholar]
- [12].Antrop I, Stock P, Verte S, Wiersema JR, Baeyens D, Roeyers H, ADHD and delay aversion: the influence of non-temporal stimulation on choice for delayed rewards, J Child Psychol Psychiatry 47(11) (2006) 1152–8. [DOI] [PubMed] [Google Scholar]
- [13].Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM, Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: Emerging evidence, Pharmacology & Therapeutics 134(3) (2012) 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bickel WK, Mueller ET, Toward the Study of Trans-Disease Processes: A Novel Approach With Special Reference to the Study of Co-morbidity, Journal of dual diagnosis 5(2) (2009) 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mazur JE, Tradeoffs among delay, rate, and amount of reinforcement, Behavioural processes 49(1) (2000) 1–10. [DOI] [PubMed] [Google Scholar]
- [16].Odum AL, Delay discounting: Trait variable?, Behavioural processes 87(1) (2011) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Litrownik AJ, Franzini L, Geller S, Geller M, Delay of gratification: decisional self-control and experience with delay intervals, American Journal of Mental Deficiency 82(2) (1977) 149–154. [PubMed] [Google Scholar]
- [18].Takahashi T, Loss of self-control in intertemporal choice may be attributable to logarithmic time-perception, Medical Hypotheses 65(4) (2005) 691–693. [DOI] [PubMed] [Google Scholar]
- [19].Wittmann M, Paulus MP, Decision making, impulsivity and time perception, Trends in cognitive sciences 12(1) (2008) 7–12. [DOI] [PubMed] [Google Scholar]
- [20].Wilson VB, Mitchell SH, Musser ED, Schmitt CF, Nigg JT, Delay discounting of reward in ADHD: application in young children, Journal of Child Psychology and Psychiatry 52(3) (2011) 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Noreika V, Falter CM, Rubia K, Timing deficits in attention-deficit/hyperactivity disorder (ADHD): evidence from neurocognitive and neuroimaging studies, Neuropsychologia 51(2) (2013) 235–266. [DOI] [PubMed] [Google Scholar]
- [22].Scott TL, Vonder Haar C, Frontal brain injury chronically impairs timing behavior in rats, Behavioural Brain Research 356 (2019) 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Baldwin RL, Chelonis JJ, Flake RA, Edwards MC, Feild CR, Meaux JB, Paule MG, Effect of methylphenidate on time perception in children with attention-deficit/hyperactivity disorder, Experimental and clinical psychopharmacology 12(1) (2004) 57–64. [DOI] [PubMed] [Google Scholar]
- [24].Winstanley CA, Eagle DM, Robbins TW, Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies, Clinical psychology review 26(4) (2006) 379–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kirkpatrick K, Marshall AT, Smith AP, Mechanisms of individual differences in impulsive and risky choice in rats, Comparative cognition & behavior reviews 10 (2015) 45–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sonuga-Barke EJS, Taylor E, Sembi S, Smith J, Hyperactivity and delay aversion. I: The effect of delay on choice, Journal of Child Psychology and Psychiatry 33(2) (1992) 387–398. [DOI] [PubMed] [Google Scholar]
- [27].Galtress T, Garcia A, Kirkpatrick K, Individual differences in impulsive choice and timing in rats, Journal of the experimental analysis of behavior 98(1) (2012) 65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schweitzer JB, Sulzer-Azaroff B, Self-control: Teaching tolerance for delay in impulsive children, Journal of the experimental analysis of behavior 50(2) (1988) 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Renda CR, Madden GJ, Impulsive choice and pre-exposure to delays: III. Four-month test-retest outcomes in male wistar rats, Behavioural processes 126 (2016) 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Renda CR, Rung JM, Hinnenkamp JE, Lenzini SN, Madden GJ, Impulsive choice and pre-exposure to delays: iv. effects of delay- and immediacy-exposure training relative to maturational changes in impulsivity, Journal of the experimental analysis of behavior 109(3) (2018) 587–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bailey C, Peterson JR, Schnegelsiepen A, Stuebing SL, Kirkpatrick K, Durability and generalizability of time-based intervention effects on impulsive choice in rats, Behavioural processes 152 (2018) 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peterson JR, Kirkpatrick K, The effects of a time-based intervention on experienced middle-aged rats, Behavioural processes 133 (2016) 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Smith AP, Marshall AT, Kirkpatrick K, Mechanisms of impulsive choice: II. Time-based interventions to improve self-control, Behavioural processes 112 (2015) 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Stein JS, Renda CR, Hinnenkamp JE, Madden GJ, Impulsive choice, alcohol consumption, and pre-exposure to delayed rewards: II. Potential mechanisms, Journal of the experimental analysis of behavior 103(1) (2015) 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fox AE, Visser EJ, Nicholson AM, Interventions aimed at changing impulsive choice in rats: Effects of immediate and relatively long delay to reward training, Behavioural processes 158 (2019) 126–136. [DOI] [PubMed] [Google Scholar]
- [36].Rung JM, Buhusi CV, Madden GJ, Reducing impulsive choice: V. The role of timing in delay-exposure training, Behavioural processes 157 (2018) 557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Smith T, Panfil K, Bailey C, Kirkpatrick K, Cognitive and behavioral training interventions to promote self-control, Journal of Experimental Psychology: Animal Learning and Cognition (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rung JM, Peck S, Hinnenkamp JE, Preston E, Madden GJ, Changing Delay Discounting and Impulsive Choice: Implications for Addictions, Prevention, and Human Health, Perspectives on Behavior Science (2019) 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Stuebing SL, Marshall AT, Triplett A, Kirkpatrick K, Females in the forefront: Time-based intervention effects on impulsive choice and interval timing in female rats, Animal Cognition 21(6) (2018) 759–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Binder LM, Dixon MR, Ghezzi PM, A procedure to teach self-control to children with attention deficit hyperactivity disorder, Journal of Applied Behavioral Science 33(2) (2000) 233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Neef NA, Bicard DF, Endo S, Assessment of impulsivity and the development of self-control in students with attention deficit hyperactivity disorder, Journal of applied behavior analysis 34(4) (2001) 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Vessells J, Sy JR, Wilson A, Green L, Effects of delay fading and signals on self-control choices by children, Journal of applied behavior analysis 51(2) (2018) 374–381. [DOI] [PubMed] [Google Scholar]
- [43].Dixon MR, Rehfeldt RA, Randich L, Enhancing tolerance to delayed reinforcers: The role of intervening activities, Journal of applied behavior analysis 36(2) (2003) 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dixon MR, Hayes LJ, Binder LM, Manthey S, Sigman C, Zdanowski DM, Using a self-control training procedure to increase appropriate behavior, Journal of applied behavior analysis 31(2) (1998) 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fisher WW, Thompson RH, Hagopian LP, Bowman LG, Krug A, Facilitating tolerance of delayed reinforcement during functional communication training, Behavior Modification 24(1) (2000) 3–29. [DOI] [PubMed] [Google Scholar]
- [46].Mitchell MR, Potenza MN, Importance of sex differences in impulse control and addictions, Frontiers in psychiatry 6 (2015) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Weafer J, de Wit H, Sex differences in impulsive action and impulsive choice, Addictive behaviors 39(11) (2014) 1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wileyto EP, Audrain-Mcgovern J, Epstein LH, Lerman C, Using logistic regression to estimate delay-discounting functions, Behavior Research Methods, Instruments, & Computers 36(1) (2004) 41–51. [DOI] [PubMed] [Google Scholar]
- [49].Boisgontier MP, Cheval B, The anova to mixed model transition, Neuroscience & Biobehavioral Reviews (2016) 12–14. [DOI] [PubMed] [Google Scholar]
- [50].Moscatelli A, Mezzetti M, Lacquanti F, Modeling psychophysical data at the population-level: the generalized linear mixed model, Journal of Vision 12(11) (2012) 26. [DOI] [PubMed] [Google Scholar]
- [51].Baayen RH, Davidson DJ, Bates DM, Mixed-effects modeling with crossed random effects for subjects and items, Journal of Memory and Language 59 (2008) 390–412. [Google Scholar]
- [52].Bates D, Kliegl R, Vasishth S, Baayen H, Parsimonious mixed models, arXiv preprint arXiv:1506.04967 (2015). [Google Scholar]
- [53].Steele CC, Pirkle JRA, Kirkpatrick K, Diet-induced impulsivity: Effects of a high-fat and a high-sugar diet on impulsive choice in rats, PLOS ONE 12(6) (2017) e0180510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kruschke JK, Liddell TM, Bayesian data analysis for newcomers, Psychonomic bulletin & review 25(1) (2018) 155–177. [DOI] [PubMed] [Google Scholar]
- [55].Young ME, Bayesian data analysis as a tool for behavior analysts, Journal of the experimental analysis of behavior 111(2) (2019) 225–238. [DOI] [PubMed] [Google Scholar]
- [56].Bürkner P, brms: An R package for Bayesian multilevel models using Stan, Journal of Statistical Software 80(1) (2017) 1–28. [Google Scholar]
- [57].Koot S, van den Bos R, Adriani W, Laviola G, Gender differences in delay-discounting under mild food restriction, Behavioural Brain Research 200(1) (2009) 134–143. [DOI] [PubMed] [Google Scholar]
- [58].van Haaren F, van Hest A, van de Poll NE, Self-control in male and female rats, Journal of the experimental analysis of behavior 49 (1988) 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Buhusi CV, Meck WH, What makes us tick? Functional and neural mechanisms of interval timing, Nature Reviews Neuroscience 6 (2005) 755–765. [DOI] [PubMed] [Google Scholar]
- [60].Allman MJ, Teki S, Griffiths TD, Meck WH, Properties of the internal clock: first- and second-order principles of subjective time, Annu Rev Psychol 65 (2014) 743–71. [DOI] [PubMed] [Google Scholar]
- [61].Droit-Volet S, Time perception in children: A neurodevelopmental approach, Neuropsychologia 51(2) (2013) 220–234. [DOI] [PubMed] [Google Scholar]
- [62].Marshall AT, Smith AP, Kirkpatrick K, Mechanisms of impulsive choice: I. Individual differences in interval timing and reward processing, Journal of the experimental analysis of behavior 102(1) (2014) 86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].McClure J, Podos J, Richardson HN, Isolating the delay component of impulsive choice in adolescent rats, Frontiers in integrative neuroscience 8(3) (2014) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Baumann AA, Odum AL, Impulsivity, risk taking, and timing, Behavioural Processes 90 (2012) 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Mitchell SH, Wilson VB, Differences in delay discounting between smokers and nonsmokers remain when both rewards are delayed, Psychopharmacology (Berlin) 219 (2012) 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gibbon J, Scalar expectancy theory and Weber’s law in animal timing, Psychological Review 84 (1977) 279–325. [Google Scholar]