Abstract
Historically, individuals with androgen insensitivity syndrome (AIS) were managed with removal of gonadal tissue at various ages to avert the risk of gonadal malignancy. Recently, clinical practice changed, with gonadectomy being postponed until late adolescence. Adolescents and adults with complete AIS have questioned this approach. Additionally, testicular germ cell tumors are increasingly believed to be quite rare with rates as low as 0% in molecularly confirmed individuals with AIS. Gonadectomy deprives patients of the benefits of their endogenous hormones and potential fertility. Furthermore, human rights organizations advocate for deferring irreversible surgery in conditions known as differences of sex development, which includes AIS, to allow patient autonomy in decision-making. Recent literature supports an approach that uses risk stratification to manage gonads in AIS. Herein we review what is known about malignancy risk in the different subtypes of AIS and propose a management protocol for gonad retention.
Keywords: Androgen insensitivity syndrome, Gonads, Gonadectomy, Malignancy risk
Introduction
Historically, individuals with androgen insensitivity syndrome (AIS) were managed with removal of gonadal tissue at various ages to avert the risk of gonadal malignancy. Recently, clinical practice has changed, with gonadectomy being postponed until late adolescence.1 Adolescents and adults with complete AIS have questioned this approach.2 Additionally, testicular germ cell tumors are increasingly believed to be quite rare with rates as low as 0% in molecularly confirmed individuals with AIS.3 Gonadectomy deprives patients of the benefits of their endogenous hormones and potential fertility. Furthermore, human rights organizations advocate for deferring irreversible surgery in conditions known as differences of sex development, which includes AIS, to allow patient autonomy in decision-making. Recent literature supports an approach that uses risk stratification to manage gonads in AIS.4 Herein we review what is known about malignancy risk in the different subtypes of AIS and propose a management protocol for gonad retention.
AIS is an X-linked, recessive genetic condition, caused by an androgen receptor (AR) gene mutation,5,6 that results in resistance to androgens in XY individuals. The condition was first introduced in 1953 and termed “Morris syndrome” or “testicular feminization syndrome.”7 Stigmatizing terminology was replaced with AIS after the discovery that affected individuals were androgen-resistant rather than deficient.8
AIS is divided into subtypes that include complete AIS (CAIS), partial AIS (PAIS), and mild AIS. Overall, the world-wide incidence of CAIS is estimated to range from 1 in 20,000 to 1 in 99,000.9,10 The reported incidence of PAIS is 1 in 130,000; however, PAIS is often a clinical diagnosis and the true incidence might differ in individuals who have undergone AR sequencing.11 PAIS might include multiple genetic diagnoses and a spectrum of multiple anatomical differences; thus, AR sequencing is necessary for a true diagnosis of AIS. The incidence and prevalence for mild AIS, which might remain undetected unless it presents with infertility, is unknown.
AIS has been historically managed by eventual removal of the testes because of a perceived risk of malignancy. However, there is mounting evidence to suggest that the risk of malignancy is overstated.3
Risk for Malignancy in AIS
The risk for germ cell cancer in gonads of patients with AIS varies across the subtypes. It is believed that the number of germ cell tumors is increased in individuals with AIS because of the Y chromosome and presence of the testis specific protein Y-linked 1 (TSPY) gene.12,13 The malignancy risks for AIS have varied in the literature.14–16 A recent review stressed the importance of only including cases in which AR sequencing confirmed the AIS diagnosis.17 The cited published literature is shown in Table 1.
Table 1.
Included Studies
| Reference | Clinical Diagnosis(es) | n | Age at Gonadectomy | AR Sequencing Performed | Germ Cells Present | Precursor Lesions | Malignant Tumors |
|---|---|---|---|---|---|---|---|
| Morris7 | Testicular feminization | 80 | 12-51 Years | * | * | * | 6/80: Seminoma, alveolar carcinoma, malignant teratoma, pelvic sarcoma, and malignant arrhenoblastoma |
| Morris and Mahesh26,† | Testicular feminization | 181 | Postpubertal | * | * | * | 11/50: Seminoma |
| Dewhurst et al27 | Androgen insensitivity (AIS) | 82 | Before puberty to older than 30 years | * | * | * | 0/82 |
| Dewhurst et al15 | AIS, PAIS | 66 (44 CAIS; 22 PAIS) | Younger than 15 to older than 30 years | * | * | * | 0/44 CAIS; 1/12 PAIS: seminoma |
| Manuel et al19 | Testicular feminization | 23 | * | * | * | * | 0/23 |
| Muller21 | CAIS, IAIS | 12 (4 CAIS; 8 IAIS) | 2 Months to 19 years | * | 50% | 3/12 | Carcinoma in situ |
| Hurt et al22 | AIS | 1 | 14 Years | * | * | * | Seminoma |
| Rutgers and Scully23 | CAIS, IAIS | 43 (40 CAIS; 3 IAIS) | 16-83 Years CAIS‡; 14-47 years PAIS | * | 28% CAIS; 0% PAIS | * | 3/40 CAIS: seminomas and sex cord tumor; 0/3 PAIS |
| Ahmed et al28 | CAIS, PAIS | 278 (173 PAIS; 105 CAIS) | 14 Years (0.1-18)§ | Yes‖ | * | * | 0/278 |
| Hannema et al24 | CAIS | 44 | 5.5 Years (1-13)¶ | Yes | 95% | 2/44 | Carcinoma in situ |
| Purves et al25 | CAIS | 16 | 18.2 Years (2-21) | * | * | * | 0/16 |
| Kravarusic et al14 | CAIS, PAIS | 11 (6 CAIS; 5 PAIS) | 3-8 Years‡ | * | * | * | 0/6 CAIS; 2/5 PAIS: bilateral seminoma, gonadoblastoma |
| Liu et al16 | Gonadal dysgenesis, CAIS, PAIS | 102 (30 CAIS; 18 PAIS) | 20.7 Years (16-34)‡ | * | * | 0/48 | 9/30 CAIS: gonadoblastoma; 3/18 PAIS: gonadoblastoma |
| Kaprova-Pleskacova et al18 | CAIS | 19 (36 Gonads) | 3 Months to 18.5 years‡ | Yes | 84% | 6/13 | Precursor lesion types not reported; 1/13: intratubular germ cell neoplasia |
| Cools et al3 | CAIS, PAIS | 52 (42 CAIS; 10 PAIS) | 17.5 Years (14-54)¶ | Yes | 64% CAIS 88% PAIS | 7/52 | Precursor lesions types not reported; 0/52 in situ tumors |
AIS, androgen insensitivity syndrome; IAIS, incomplete androgen insensitivity syndrome; AR, androgen receptor; CAIS, complete androgen insensitivity syndrome; PAIS, partial androgen insensitivity syndrome.
Not reported or unknown.
Literature review of cases.
At clinical presentation.
Sixty-six percent of CAIS cases had gonadectomy before puberty, 29% postpubertal, 5% unknown age; 39% of PAIS cases had gonadectomy before puberty, 9% postpubertal, and 52% unknown age.
In a subset of cases; 51 CAIS, 114 PAIS.
Median.
Although deferral of gonadectomy until adolescence is becoming routine, ultimate removal was usually suggested because the risk of malignancy is believed to increase with age.2 However, reports of invasive testicular germ cell tumor (TGCT) in AIS adults are rare.3 Pre-germ cell neoplasia in situ (pre-GCNIS) is the precursor of TGCT. A risk stratification model has been proposed on the basis of single nucleotide polymorphisms and variations of the KIT ligand (also known as KITLG gene), which is a marker known to be related to TGCT development in typical XY male individuals.3 In a study in 2014, the presence of KITLG was evaluated in 37 gonads from individuals with AR-confirmed CAIS. Seven gonads tested positive for KITLG. Six of those were considered precursor lesions; one was described as having intratubular germ cell neoplasia.18 KITLG was part of the criteria used to classify the lesions as precursor lesions. One study of testicular samples from postpubertal individuals with CAIS and PAIS, ages 14-54 years, showed no TGCT, despite a 10%-15% prevalence of the earliest neoplastic lesion pre-GCNIS.3 Although those with pre-GCNIS were predicted to have greater genetic susceptibility to development of TGCT on the basis of the presence of risk alleles such as KITLG, the absence of TGCT suggests that a small percentage of these lesions will progress to invasive TGCT; however, the incidence of TGCT and the natural history of these precursor lesions are unknown.3
The age at which malignancy can develop is not well understood in this population. In 1975, it was reported that the risk for malignancy in CAIS was 3.6% at the age of 25 years and reached 33% at the age of 50 years.19 In a review published in 1987, it was estimated that the risk for malignancy was 2%-5% in CAIS patients who were older than 25 years of age.20 Muller reported a 38% risk of malignancy in individuals with “incomplete” AIS ranging from 2 months of age to 15.8 years.21 There have been three studies on tumors in female adolescents: one 17-year-old and two 14-year-old girls.7,22,23 Of note, none of these reports discuss AR gene testing to confirm an AIS diagnosis. More recently, two articles reported no malignancies in the biopsies of gonads from individuals with CAIS.24,25
Historical Management of Malignancy Risk for Patients with AIS
Patients with AIS have been described in the medical literature since 1953. Morris reported on a large cohort of 80 patients; 155 gonads were included in the analysis but only 58 were evaluated histologically.7 Of those, six gonads were malignant (Table 1).7 A follow-up paper by Morris in 1963 reported an additional 99 cases of gonadectomy for testicular feminization.26 The indication for surgery in most of these cases was the presence of a pelvic or gonadal mass. Morris concluded, “The incidence of neoplasia appears sufficient to continue to advocate removal of the gonads with substitution therapy after secondary sex characteristics have developed.”26
In 1971, Dewhurst discussed a cohort of 41 patients with CAIS who underwent bilateral gonadectomy, stratified into groups based on pubertal development.27There were no malignancies in any of the pathologic specimens.27In a second report, Dewhurst et al stratified risk on the basis of complete or partial androgen insensitivity, and in the CAIS group, no malignancies were reported.15Among those with PAIS, one malignant tumor was noted in a 55-year-old individual. A later study on the postoperative pathology of gonad specimens in patients with CAIS and PAIS described a frequency of malignant change in 10% of the cohort with only 7.5% having germ cell neoplasia.23More recently, a series of 105 patients with CAIS showed that no patients who underwent gonadectomy had malignancies.28
A review of the literature performed by Cools et al revealed that in patients with CAIS, the prevalence of malignancy among those who underwent prophylactic gonadectomy was low at 0.8%.12 As recently as 2015, gonadectomy was being recommended for female patients who were 46, XY,29 although for CAIS individuals, gonadectomy is recommended to be deferred until adolescence to allow for spontaneous puberty.
One of the limitations of the historic representation of malignancy risk is that the diagnosis and characteristics of AIS have changed over time. AR sequencing was not available until 1988 when the AR gene was cloned in humans.8 As such, the patient populations were often combined into AIS in the literature. In addition, many of the studies diagnosed AIS clinically and likely some of the women had gonadal dysgenesis.2 This higher tumor rate in gonadal dysgenesis likely confounded these results. Moreover, because no specific markers are available to distinguish germ cells with delayed maturation from those who underwent malignant transformation, older studies likely overdiagnosed TGCT and gonadoblastoma, resulting in overtreatment using gonadectomy, especially in young patients.13 Most of these reports lack the AR testing necessary to confirm a diagnosis of AIS. Only four of the reports in the literature discuss AR sequencing in the patient population and only one reported malignant changes. A third limitation is the age at which malignant changes occur if at all. The natural history of TGCT and precursor lesions is not well understood. Whether an individual is a carrier of risk alleles such as KITLG might also play a role in malignant tumor development.
Current Management Recommendations
Current medical and surgical therapies for patients with AIS match historical recommendations with many institutions removing the gonads followed by administration of long-term hormone replacement therapy. There are multiple reasons to change the current therapy protocols.
Hormonal Considerations
The implications of long-term hormone replacement therapy are complex, and currently no studies exist to assess whether pharmacologic hormone replacement is equivalent to endogenous androgens. Women with CAIS self-reported that the optimal timing for gonadectomy was in adolescent or adult years,30 possibly because of the problems associated with hormone replacement therapy or the ability to participate in the decision-making process.
Women with CAIS have reported generally feeling “unwell” after gonadectomy, despite receiving estrogen, and some patients request testosterone replacement. It might be that these women have very high levels of estrogen due to aromatization of testosterone, and thus, the traditional estrogen replacement provided to post-menopausal women is not enough to control vasomotor and other symptoms. Attention should be paid to what is considered normal levels of estrogen replacement for adolescent or adult patients who undergo gonadectomy. Historically, patients were treated with a goal of replacement as if they were postmenopausal. Depending on age and symptoms, different doses and levels might be indicated for different patients and care should be customized. In addition, although these women have AR abnormalities, which preclude androgen function, testosterone might still be associated with effects that are not well understood.31
Elective oophorectomy in perimenopausal women who undergo hysterectomy was advocated as a way to prevent epithelial ovarian cancer, associated with a 1.4% lifetime risk and an overall 5-year mortality rate of 47% in the general population.32,33 Recent studies of women with postsurgical hypogonadism point to a possible negative effect of this practice on cardiovascular health and all-cause mortality.34,35 Gonadectomy might have a similar effect on the cardiovascular health of women with CAIS, although this warrants further investigation and has not been studied in women with CAIS.
Low bone mineral density reported in CAIS has been attributed to a combination of decreased circulating estrogen and skeletal resistance to androgen action.36,37 Testosterone levels are increased relative to XY men, and estrogen levels are decreased relative to XX women.38 In individuals with CAIS, who underwent gonadectomy at the mean age of 14.8 years (range, 13-16.5 years) and underwent a dual-energy X-ray-absorptiometry scan at a mean of 33.8 years (range, 31.4-36.3 years), bone mineral density appeared to remain stable after gonadectomy, even when there was poor compliance with hormone replacement therapy.38 These patients reported a mixture of hormone replacement therapy: 52% reported using oral estradiol/ethinylestradiol, 28% reported using transdermal estradiol, 6% reported using testosterone alone, and 3% selective estrogen receptor modulator; 12% reported no treatment.
There are psychosocial consequences of gonadectomy and hormone replacement therapy as well. A correlation between having had a gonadectomy and lifetime suicidal thoughts was found in a study of how childhood and adolescent treatment experiences influence adult well-being by Schweizer et al.39 These authors suggested correlating the consequences of gonadectomy and subsequent estrogen replacement therapy with endocrine mood effects in other groups, such as estradiol and mood variability in XX adolescents, increased risk of hormone-modulated depression in menopausal women, and antidepressive effects of cross-sex testosterone therapy in XX transmen.39
Fertility Considerations
In 2016, Finlayson et al reported germ cells in 68% of gonad samples from children with a variety of differences of sex development and proposed that consideration be given to preservation of fertility potential using techniques for fertility preservation (FP) in pediatric cancer patients.40 In this article, the presence of gonadal germ cells were found in 6/6 CAIS patients but the number inversely correlated with age, indicating that delaying gonadectomy in CAIS might actually decrease fertility potential. If FP is a realistic possibility, there could be conflicts in the optimal timing of gonadectomy on the basis of considerations of preservation of endogenous hormone function and fertility potential.2 Ethical issues to be considered in counseling families and patients about potential FP include autonomy in reproductive decisions, the emotional effect of such discussion in patients with gender-discordant gonads, cost, and “false hope,” which is “a type of psychological risk that occurs when patients are misled about the possibility of success for a particular treatment.”41 Additionally, some case reports have shown that individuals with AIS assigned male can have a biological child.42,43 As such, physicians should consider fertility potential when recommending gonadectomy.
Ethical Considerations
Efforts in health care reform have encouraged medical providers to shift from a physician-only treatment plan to one in which the patient and the physician make a shared decision about treatment.44 Moreover, numerous governmental and human rights organizations have affirmed children’s rights to bodily autonomy.45–51 In 2016, the American Medical Association Board of Trustees issued a report recommending care that “respects the rights of the patient to participate in decisions and, except when life-threatening circumstances require emergency intervention, defers medical or surgical intervention until the child is able to participate in decision-making.”51
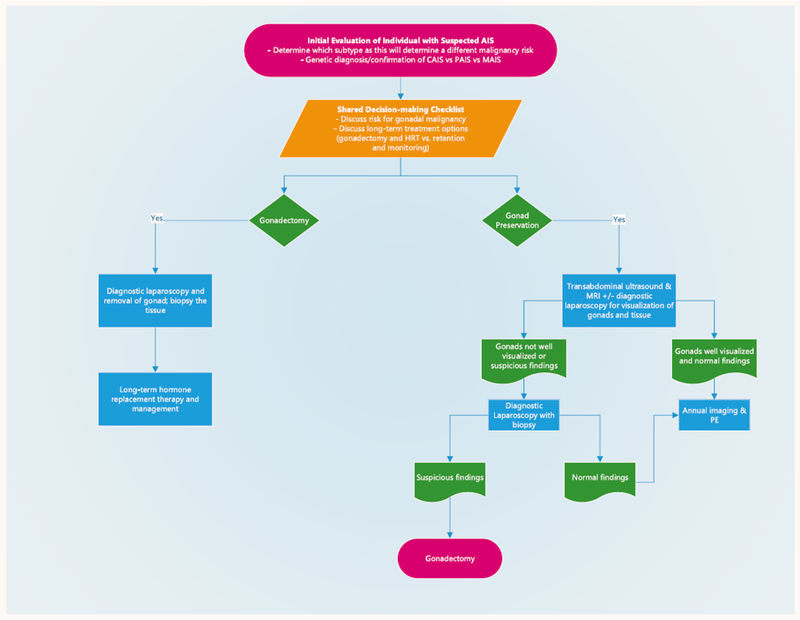
Patel et al first described a clinical algorithm for gonad evaluation and preservation in the CAIS population.4. We expanded this to include all subtypes of AIS and further refined the algorithm for clinical use including a shared decision-making (SDM) process (Fig. 1).
Fig. 1.
Gonad preservation treatment algorithm recommended for clinical process upon initial diagnosis and subsequent clinical follow-up of individuals diagnosed with androgen insensitivity syndrome (AIS). CAIS, complete androgen insensitivity syndrome; HRT, hormone replacement therapy; MAIS, mild androgen insensitivity syndrome; MRI, magnetic resonance imaging; PAIS, partial androgen insensitivity syndrome; PE, physical exam.
Clinical Management for Watchful Waiting
SDM
To determine the best course of action concerning retention or removal of the gonads, a SDM process should be initiated when a patient is first diagnosed with AIS. AIS diagnosis confirmation on the basis of clinical presentation and AR sequencing is necessary before treatment decision-making to understand fully the malignancy risks as well as fertility potential of the individual. The SDM process involves counseling sessions, occurring over the course of several visits, with up to date reviews all pertinent issues. Included is a discussion of the risks and benefits of gonad retention vs removal. An SDM tool can be used to guide discussions to address the surveillance schedule, repercussions of missing annual follow-up, cancer risks, and FP. Finally, the long-term implications of hormone replacement therapy as well as all options for medical therapy post gonadectomy should be discussed.
Management Protocol
For patients who decide to proceed with gonad retention, we recommend a baseline transabdominal ultrasound (TAUS) examination and magnetic resonance imaging (MRI) to locate the position of the patient’s gonads and visualize their features. An exam with the patient under anesthesia and diagnostic laparoscopy can be performed so the treating physician will have baseline knowledge of the appearance of the gonads and be able to compare intraoperative findings with imaging. Diagnostic laparoscopy is not necessary in every case.
In patients whose gonads appear normal without features suggestive of disease, continued monitoring with annual imaging should be pursued. Tumor serum markers in germ cell tumors have not shown to be fully effective as a guidance tool or provide benefit in determining risk for malignancy.3,12 Therefore, to reduce the burden on the patient, we do not recommend tumor markers as part of our clinical management protocol.
Imaging Studies
Ultrasound has been the most used modality in screening patients for malignancy, particularly in patients at risk for other gonadal tumors such as ovarian cancer. Ultrasound has been also used in detecting nonpalpable intraabdominal gonads in cryptorchidism; although sensitivity and specificity are greatly decreased, particularly compared with laparoscopy.52 For PAIS patients with inguinal gonads, a pelvic ultrasound and annual examination is recommended if the patient chooses gonad retention. For CAIS patients who have intra-abdominal gonads, annual TAUS is recommended.
We recommend a contrast-enhanced MRI scan of the abdomen and pelvis as a baseline step in surveillance imaging and especially in those in whom the gonads are not well visualized on TAUS. Although MRI performs superiorly to ultrasound in the detection of nonpalpable gonads in patients with cryptorchidism, no studies have compared ultrasound with MRI specifically in detection of gonads in patients with AIS.52 A recent study compared MRI findings with the histologic findings of gonads after prophylactic gonadectomy.53 MRI did not detect premalignant lesions, which are microscopic. The study did conclude that MRI correlated well with histology of macroscopic findings such as paratesticular cysts and Sertoli cell adenomas, and could depict the gonads sufficiently. We also recommend MRI every five years until TAUS is more reliable between centers. The performance of an MRI scan might require intravenous access, anesthesia, and/or incur an out of pocket cost to the patient and family and these factors have to be considered in each case.
Diagnostic Laparoscopy and Exam under Anesthesia
Currently, it is our practice to consider a baseline diagnostic laparoscopy and exam with the patient under anesthesia to visualize and characterize the internal structures including the gonads, as well as evaluate vaginal depth. In some patients, this might not be desired or necessary. This might be helpful in instances in which the imaging is equivocal. In the event that the gonads are indicative of malignancy in their appearance at the time of laparoscopy, biopsy of the affected gonad(s) might be considered. It should be noted that the formation of cysts is a part of the natural history of AIS gonads and clinicians should consider this as more patients opt for monitoring.
Gonadal Biopsy
To date, there have been no discussions in the literature regarding the utility of either percutaneous or surgical biopsy of the gonads in patients with AIS. However, biopsy has played a role in the management of other gonadal malignancies, such as testicular and ovarian cancer.54–58
Annual Follow-up and Surveillance
In patients who have undergone baseline imaging and/or diagnostic laparoscopy and are found to have normal-appearing gonads, annual monitoring consisting of imaging studies and physical examination should be continued. If imaging findings might be indicative of disease, such as presence of mass, development of cysts, calcifications, unexplained abdominal lymphadenopathy, or asymmetric change in size are encountered on ultrasound, then MRI should be performed to further delineate the characteristics. If MRI is consistent with ultrasound findings, diagnostic laparoscopy might not be warranted. If there are abnormal imaging findings, laparoscopy for direct visualization with possible biopsy might be considered. At any point, if the gonads appear abnormal intraoperatively, biopsy should be considered and/or gonadectomy of the affected gonad(s) should be performed.
Conclusion
Gonadectomy has been the historic recommendation for patients with AIS. However, the literature shows weak evidence that prophylactic gonadectomy in patients with CAIS decreases the risk of malignancy. Current estimates in this population show that the rate of tumor development is relatively low. We propose the current management of prophylactic gonadectomy in patients with CAIS should be adjusted. Because malignancy risk is much higher in PAIS, strict clinical surveillance or discussion of possible gonadectomy might be beneficial to this group.
It remains of utmost importance to discuss with patients the inherent risks of gonad retention and that this management approach is relatively new. Annual monitoring for CAIS and PAIS is our current recommendation for safe clinical management. Ultimately, patient autonomy and shared-decision making will play a large and vital role in the care and management of individuals with AIS.
Acknowledgments
This work was supported, in part, by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R01HD093450.
Footnotes
Erica M. Weidler is a paid board member and Executive Director of Accord Alliance. The remaining authors indicate no conflicts of interest.
References
- 1.Hughes IA, Morel Y, McElreavey K, et al. : Biological assessment of abnormal genitalia. J Pediatr Urol 2012; 8:592. [DOI] [PubMed] [Google Scholar]
- 2.Deans R, Creighton SM, Liao LM, et al. : Timing of gonadectomy in adult women with complete androgen insensitivity syndrome (CAIS): patient preferences and clinical evidence. Clin Endocrinol 2012; 76:894. [DOI] [PubMed] [Google Scholar]
- 3.Cools M, Wolffenbuttel KP, Hersmus R, et al. : Malignant testicular germ cell tumors in postpubertal individuals with androgen insensitivity: prevalence, pathology and relevance of single nucleotide polymorphism-based susceptibility profiling. Hum Reprod 2017; 32:2561. [DOI] [PubMed] [Google Scholar]
- 4.Patel V, Casey RK, Gomez-Lobo V: Timing of gonadectomy in patients with complete androgen insensitivity syndrome-current recommendations and future directions. J Pediatr Adolesc Gynecol 2016; 29:320. [DOI] [PubMed] [Google Scholar]
- 5.Hughes IA, Deeb A: Androgen resistance. Best Pract Res Clin Endocrinol Metab 2006; 20:577. [DOI] [PubMed] [Google Scholar]
- 6.Oakes MB, Eyvazzadeh AD, Quint E, et al. : Complete androgen insensitivity syndrome-a review. J Pediatr Adolesc Gynecol 2008; 21:305. [DOI] [PubMed] [Google Scholar]
- 7.Morris JM:The syndrome of testicular feminization in male pseudohermaphrodites. Am J Obstet Gynecol 1953; 65:1192. [DOI] [PubMed] [Google Scholar]
- 8.Quigley CA, De Bellis A, Marschke KB, et al. : Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev 1995; 16:271. [DOI] [PubMed] [Google Scholar]
- 9.Bangsboll S, Qvist I, Lebech PE, et al. : Testicular feminization syndrome and associated gonadal tumors in Denmark. Acta Obstet Gynecol Scand 1992; 71:63. [DOI] [PubMed] [Google Scholar]
- 10.Boehmer AL, Brinkmann O, Bruggenwirth H, et al. : Genotype versus phenotype in families with androgen insensitivity syndrome. J Clin Endocrinol Metab 2001; 86:4151. [DOI] [PubMed] [Google Scholar]
- 11.Mazen I, El-Ruby M, Kamal R, et al. : Screening of genital anomalies in newborns and infants in two egyptian governorates. Horm Res Paediatr 2010; 73:438. [DOI] [PubMed] [Google Scholar]
- 12.Cools M, Drop SL, Wolffenbuttel KP, et al. : Germ cell tumors in the intersex gonad: old paths, new directions, moving frontiers. Endocr Rev 2006; 27:468. [DOI] [PubMed] [Google Scholar]
- 13.Looijenga LH, Hersmus R, Oosterhuis JW, et al. : Tumor risk in disorders of sex development (DSD). Best Pract Res Clin Endocrinol Metab 2007; 21:480. [DOI] [PubMed] [Google Scholar]
- 14.Kravarusic D, Seguier-Lipszyc E, Feigin E, et al. : Androgen insensitivity syndrome: risk of malignancy and timing of surgery in a paediatric and adolescent population. Afr J Paediatr Surg 2011; 8:194. [DOI] [PubMed] [Google Scholar]
- 15.Dewhurst CJ, Ferreira HP, Gillett PG: Gonadal malignancy in XY females. J Obstet Gynaecol Br Commonw 1971; 78:1077. [DOI] [PubMed] [Google Scholar]
- 16.Liu AX, Shi HY, Cai ZJ, et al. : Increased risk of gonadal malignancy and prophylactic gonadectomy: a study of 102 phenotypic female patients with Y chromosome or Y-derived sequences. Hum Reprod 2014; 29:1413. [DOI] [PubMed] [Google Scholar]
- 17.Cools M, Looijenga L: Update on the pathophysiology and risk factors for the development of malignant testicular germ cell tumors in complete androgen insensitivity syndrome. Sex Dev 2017; 11:175. [DOI] [PubMed] [Google Scholar]
- 18.Kaprova-Pleskacova J, Stoop H, Bruggenwirth H, et al. : Complete androgen insensitivity syndrome: factors influencing gonadal histology including germ cell pathology. Mod Pathol 2014; 27:721. [DOI] [PubMed] [Google Scholar]
- 19.Manuel M, Katayama PK, Jones HW Jr: The age of occurrence of gonadal tumors in intersex patients with a Y chromosome. Am J Obstet Gynecol 1976; 124:293. [DOI] [PubMed] [Google Scholar]
- 20.Verp MS, Simpson JL: Abnormal sexual differentiation and neoplasia. Cancer Genet Cytogenet 1987; 25:191. [DOI] [PubMed] [Google Scholar]
- 21.Muller J: Morphometry and histology of gonads from twelve children and adolescents with the androgen insensitivity (testicular feminization) syndrome. J Clin Endocrinol Metab 1984; 59:785. [DOI] [PubMed] [Google Scholar]
- 22.Hurt WG, Bodurtha JN, McCall JB, et al. : Seminoma in pubertal patient with androgen insensitivity syndrome. Am J Obstet Gynecol 1989; 161:530. [DOI] [PubMed] [Google Scholar]
- 23.Rutgers JL, Scully RE: The androgen insensitivity syndrome (testicular feminization): a clinicopathologic study of 43 cases. Int J Gynecol Pathol 1991; 10:126. [DOI] [PubMed] [Google Scholar]
- 24.Hannema SE, Scott IS, Rajpert-De Meyts E, et al. : Testicular development in the complete androgen insensitivity syndrome. J Pathol 2006; 208:518. [DOI] [PubMed] [Google Scholar]
- 25.Purves JT, Miles-Thomas J, Migeon C, et al. : Complete androgen insensitivity: the role of the surgeon. J Urol 2008; 180(4 suppl):1716. [DOI] [PubMed] [Google Scholar]
- 26.Morris JM, Mahesh VB: Further observations on the syndrome, “testicular feminization”. Am J Obstet Gynecol 1963; 87:731. [PubMed] [Google Scholar]
- 27.Dewhurst CJ: The XY female. Am J Obstet Gynecol 1971; 109:675. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed SF, Cheng A, Dovey L, et al. : Phenotypic features, androgen receptor binding, and mutational analysis in 278 clinical cases reported as androgen insensitivity syndrome. J Clin Endocrinol Metab 2000; 85:658. [DOI] [PubMed] [Google Scholar]
- 29.van der Zwan YG, Biermann K, Wolffenbuttel KP, et al. : Gonadal maldevelopment as risk factor for germ cell cancer: towards a clinical decision model. Eur Urol 2015; 67:692. [DOI] [PubMed] [Google Scholar]
- 30.Wisniewski AB, Migeon CJ, Meyer-Bahlburg HF, et al. : Complete androgen insensitivity syndrome: long-term medical, surgical, and psychosexual outcome. J Clin Endocrinol Metab 2000; 85:2664. [DOI] [PubMed] [Google Scholar]
- 31.Birnbaum W, Bertelloni S: Sex hormone replacement in disorders of sex development. Endocr Dev 2014; 27:149. [DOI] [PubMed] [Google Scholar]
- 32.Pearce CL, Stram DO, Ness RB, et al. : Population distribution of lifetime risk of ovarian cancer in the United States. Cancer Epidemiol Biomarkers Prev 2015; 24:671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Cancer Institute: Surveillance, Epidemiology, and End Results Program: Cancer stat facts: ovarian cancer. Available: https://seer.cancer.gov/statfacts/html/ovary.html. Accessed January 15, 2019.
- 34.Parker WH, Broder MS, Chang E, et al. : Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses’ health study. Obstet Gynecol 2009; 113:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker WH: Bilateral oophorectomy versus ovarian conservation: effects on long-term women’s health. J Minim Invasive Gynecol 2010; 17:161. [DOI] [PubMed] [Google Scholar]
- 36.Han TS, Goswami D, Trikudanathan S, et al. : Comparison of bone mineral density and body proportions between women with complete androgen insensitivity syndrome and women with gonadal dysgenesis. Eur J Endocrinol 2008; 159:179. [DOI] [PubMed] [Google Scholar]
- 37.Soule SG, Conway G, Prelevic GM, et al. : Osteopenia as a feature of the androgen insensitivity syndrome. Clin Endocrinol 1995; 43:671. [DOI] [PubMed] [Google Scholar]
- 38.King TFJ, Wat WZM, Creighton SM, et al. : Bone mineral density in complete androgen insensitivity syndrome and the timing of gonadectomy. Clin Endocrinol 2017; 87:136. [DOI] [PubMed] [Google Scholar]
- 39.Schweizer K, Brunner F, Gedrose B, et al. : Coping with diverse sex development: treatment experiences and psychosocial support during childhood and adolescence and adult well-being. J Pediatr Psychol 2017; 42:504. [DOI] [PubMed] [Google Scholar]
- 40.Finlayson C, Johnson EK, Chen D, et al. : Proceedings of the Working Group Session on Fertility Preservation for Individuals with Gender and Sex Diversity. Transgend Health 2016; 1:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campo-Engelstein L, Chen D, Baratz AB, et al. : The ethics of fertility preservation for pediatric patients with differences (disorders) of sex development. J Endocr Soc 2017; 1:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tordjman KM, Yaron M, Berkovitz A, et al. : Fertility after high-dose testosterone and intracytoplasmic sperm injection in a patient with androgen insensitivity syndrome with a previously unreported androgen receptor mutation. Andrologia 2014; 46:703. [DOI] [PubMed] [Google Scholar]
- 43.Massin N, Bry H, Vija L, et al. : Healthy birth after testicular extraction of sperm and ICSI from an azoospermic man with mild androgen insensitivity syndrome caused by an androgen receptor partial loss-of-function mutation. Clin Endocrinol 2012; 77:593. [DOI] [PubMed] [Google Scholar]
- 44.O’Malley AS, Carrier ER, Docteur E, et al. : Policy Options to Encourage Patient-Physician Shared Decision Making, No. 5. Washington, DC, National Institute for Health Care Reform, 2011 [Google Scholar]
- 45.Sexual Health: Human Rights and the Law. Geneva, Switzerland, WQ200, 2015 [Google Scholar]
- 46.UNFE Intersex Fact Sheet. In: Equal UNF. Geneva, Switzerland, United Nations Human Rights Office, Office of the High Commissioner, 2015, pp 1–2 [Google Scholar]
- 47.Legal Lambda. Providing ethical and compassionate health care to intersex patients: Intersex-affirming hospital policies. Available from: https://www.lambdalegal.org/publications/intersex-affirming; 2018.
- 48.Strangio C: Stop performing nonconsensual, medically unnecessary surgeries on young intersex children. New York, NY, ACLU, LGBT & HIV Project, 2017 [Google Scholar]
- 49.“I want to be like nature made me”: medically unnecessary surgeries on intersex children in the US. New York, NY, Human Rights Watch, 2017 [Google Scholar]
- 50.In recognition of intersex awareness day [press release]. Washington, DC, US Department of State, 2017 [Google Scholar]
- 51.Harris P: Supporting autonomy for patients with differences of sex development (DSD) (Resolution 3-A-16). Chicago, IL, American Medical Association, 2016 [Google Scholar]
- 52.Tasian GE, Copp HL, Baskin LS: Diagnostic imaging in cryptorchidism: utility, indications, and effectiveness. J Pediatr Surg 2011; 46:2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nakhal RS, Hall-Craggs M, Freeman A, et al. : Evaluation of retained testes in adolescent girls and women with complete androgen insensitivity syndrome. Radiology 2013; 268:153. [DOI] [PubMed] [Google Scholar]
- 54.Verma K, Ram TR, Kapila K: Value of fine needle aspiration cytology in the diagnosis of testicular neoplasms. Acta Cytol 1989; 33:631. [PubMed] [Google Scholar]
- 55.Heikkila R, Heilo A, Stenwig AE, et al. : Testicular ultrasonography and 18G biopty biopsy for clinically undetected cancer or carcinoma in situ in patients with germ cell tumours. Br J Urol 1993; 71:214. [DOI] [PubMed] [Google Scholar]
- 56.Capelouto CC, Clark PE, Ransil BJ, et al. : A review of scrotal violation in testicular cancer: is adjuvant local therapy necessary? J Urol 1995; 153:981. [PubMed] [Google Scholar]
- 57.Griffin N, Grant LA, Freeman SJ, et al. : Image-guided biopsy in patients with suspected ovarian carcinoma: a safe and effective technique? Eur Radiol 2009; 19:230. [DOI] [PubMed] [Google Scholar]
- 58.Shaida N, Berman LH: Percutaneous testicular biopsy for indeterminate testicular lesions. Br J Radiol 2012; 85(spec no 1):S54. [DOI] [PMC free article] [PubMed] [Google Scholar]