Abstract
Childhood socioeconomic status (SES) is associated with numerous aspects of cognitive development and disparities in academic achievement. The specific environmental factors that contribute to these disparities remain poorly understood. We used observational methods to characterize three aspects of the early environment that may contribute to SES-related differences in cognitive development: violence exposure, cognitive stimulation, and quality of the physical environment. We evaluated the associations of these environmental characteristics with associative memory, cued attention, and memory-guided attention in a sample of 101 children aged 60–75 months. We further investigated whether these specific cognitive abilities mediated the association between SES and academic achievement 18 months later. Violence exposure was specifically associated with poor associative memory, but not cued attention or memory-guided attention. Cognitive stimulation and higher quality physical environment were positively associated with cued attention accuracy, but not after adjusting for all other environmental variables. The quality of the physical environment was associated with memory-guided attention accuracy. Of the cognitive abilities examined, only memory-guided attention contributed to SES-related differences in academic achievement. These findings suggest specificity in how particular aspects of early environmental experience scaffold different types of attention and memory subserved by distinct neural circuits and shed light on a novel cognitive-developmental mechanism underlying SES-related disparities in academic achievement.
Keywords: Cognitive stimulation, Violence exposure, Physical environment, Attention, Memory, Socioeconomic status
1. Introduction
Childhood socioeconomic status (SES) is associated with differences in cognitive development, such that children raised in low-SES environments often perform more poorly on standard cognitive tasks than their higher-SES counterparts, particularly in the domains of language, memory, attention, and executive function (Finn et al., 2016; Noble et al., 2007, 2005; Rosen et al., 2018a; Sheridan et al., 2013; Sirin, 2005). These differences are thought to contribute to the well-documented disparities in academic achievement between children from low- compared to high-SES backgrounds.
SES is a complex exposure that reflects differences in many aspects of the early environment that may contribute to cognitive development and academic performance (Evans, 2004; Evans and English, 2002). Experience-dependent plasticity is elevated early in life, allowing children’s brains adapt to the environment in which they are raised (Ellis et al., 2017; Greenough et al., 1987). Understanding of how specific environmental inputs influence development of neural systems that support different cognitive functions remains remarkably limited. Numerous environmental mechanisms have been proposed to explain the association between low-SES and cognitive outcomes, including low cognitive stimulation, exposure to violence and toxins, reduced environmental predictability and complexity, differences in the physical environment, and exposure to chronic stress (Evans, 2006; Hackman et al., 2010; Johnson et al., 2016; Rosen et al., 2019a). However, few empirical studies have examined how these environmental factors might influence cognitive development and whether specific aspects of experience might play a disproportionate role in shaping some cognitive abilities more than others. In the present study, we focused on three specific environmental factors: violence exposure, cognitive stimulation, and the quality of the physical environment. We focus on these factors because previous studies suggest that they vary with SES and have been linked to cognitive outcomes (Evans and English, 2002; Evans, 2004). We investigated whether these three environmental factors were associated with three domains of cognitive function that rely on distinct neural circuits: associative memory, cued attention, and memory-guided attention. These exposures may have differential associations with our three cognitive outcomes based on the likely neural mechanisms impacted by each aspect of experience as we review below. We further evaluated whether differences in these domains of cognitive function contribute to SES-related differences in academic achievement over time by conducting the study at two time points, 18 months apart.
1.1. Associative memory
Children from low-SES households have poorer performance on some types of memory than children from higher-SES households (Farah et al., 2006; Herrmann and Guadagno, 1997; Noble et al., 2007; Sheridan et al., 2013). This association has been reported for paired associate learning, face memory, recognition memory, and verbal memory (Piccolo et al., 2016a,b; Herrmann and Guadagno, 1997; Markant and Amso, 2016; Noble et al., 2007). Moreover, some studies have reported an association between SES and hippocampal volume (Dufford et al., 2018), a brain region that plays a major role in associative learning and memory development (Eichenbaum et al., 2007; Ghetti and Bunge, 2012). However, the specific environmental mechanisms that explain the association of SES and memory in children remain unclear.
Several potential environmental mechanisms have been proposed to explain these SES-related differences in memory. These include differences in parental nurturance (Farah et al., 2008), exposure to chronic stress (Johnson et al., 2016), and exposure to violence (Hackman et al., 2010). Toxic effects of chronic stress and elevated glucocorticoid exposure early in life on hippocampal neurons have been observed in animal studies (Brunson et al., 2001; Ivy et al., 2010; Lupien et al., 2009). In humans, exposure to violence is associated with smaller hippocampal volume (Hanson et al., 2015; McLaughlin et al., 2016; Teicher et al., 2012). Indeed, some work indicates that even after controlling for SES, exposure to violence in childhood is associated with worse memory performance, reduced hippocampal volume, and atypical hippocampal function during memory tasks (Gustafsson et al., 2008; Lambert et al., 2017). Here, we investigate the hypothesis that exposure to violence will be specifically associated with associative memory performance in early childhood, but not other aspects of attention and memory that do not rely on the hippocampus.
1.2. Attention
SES-related differences in attention and working memory are well-established (Clearfield and Jedd, 2013; Finn et al., 2016; Hackman and Farah, 2009; Kishiyama et al., 2009; Mezzacappa, 2004; Sheridan et al., 2017; Stevens et al., 2009) such that children from low-SES households have lower performance than their higher-SES peers. This association is present for multiple aspects of attention—including alerting, orienting, and filtering (Mezzacappa, 2004)—and both verbal and spatial working memory (Rosen et al., 2019a,b; Sheridan et al., 2017) and is observable from infancy through adolescence (Clearfield and Jedd, 2013; Farah et al., 2006; Lipina et al., 2005). SES is also associated with the structure and function of the frontoparietal network that supports attention and working memory (Finn et al., 2016; Mackey et al., 2015; Noble et al., 2015; Rosen et al., 2018a).
Cognitive stimulation has been proposed as a mechanism explaining SES-related differences in cognitive function including attention and working memory (Rosen et al., 2019a,b). Indeed this specific aspect of early experience has been associated with attention (Razza et al., 2010; Rosen et al., 2019a,b) beginning in infancy (Belsky et al., 1980; Findji et al., 1993; Lawson et al., 1992). Although few studies have examined relations between cognitive stimulation and neural development, a recent study demonstrated that low levels of cognitive stimulation mediated the association between low SES and thinner cortex in the frontoparietal network that supports attention (Rosen et al., 2018a). In the present study, we investigate the hypothesis that cognitive stimulation will be specifically associated with attention in children, but not with associative memory.
1.3. Memory-guided attention
Memory-guided attention reflects the reliance on prior experience, via either explicit or implicit memory, to efficiently direct visual attention in familiar environments (Hutchinson and Turk-Browne, 2012; Rosen et al., 2018a; Summerfield et al., 2006). The ability to use memory to guide attention in familiar contexts is present in children as young as five (Dixon et al., 2010). This function may be particularly important for development of adaptive functioning and academic outcomes because it allows children to use past experience to more effectively guide attention rather than having to rely solely on external cues. To date, the association between SES and memory-guided attention performance has not been explored. Embedded within memory-guided attention are two functions: memory retrieval and deployment of spatial attention. Because these two functions are associated with SES, we hypothesized that SES would demonstrate similar associations with memory-guided attention performance. Indeed, lower SES is associated with thinner cortex and lower surface area in brain regions that support memory-guided attention in children and adolescents (Mackey et al., 2015; Noble et al., 2015; Piccolo et al., 2016a,b). However, as we have outlined below, SES-related differences in performance on these three tasks may be driven by different environmental mechanisms.
The environmental factors that influence the integration of mnemonic and attentional processes during development are unknown. Given that both memory and attention contribute to memory-guided attention, both violence exposure and cognitive stimulation could influence this capacity in children. However, while the hippocampus supports associative memory (Eichenbaum et al., 2007; Squire, 2004) and the frontoparietal network supports attention (Corbetta and Shulman, 2002), a third set of brain regions located in the posterior cognitive control network—including the posterior precuneus, posterior callosal sulcus, and lateral intraparietal sulcus—is preferentially recruited for memory-guided attention in adults (Rosen et al., 2016, 2018b). Therefore it is possible that different environmental factors may impact the development of memory-guided attention. One possibility is that the quality of the physical environment would be related to the integration of memory and attention. Children reared in an environment with lower levels of perceptual complexity or that is overly cluttered and less structured may be less able to consistently rely on previous experience to direct visual attention to important information in the environment.
1.4. Present study
Here, we investigated how distinct aspects of the early environment known to vary as a function of SES were associated with associative memory, cued attention, and memory-guided attention in early childhood. We evaluated these questions in a sample of five to six-year olds using gold-standard observational measures of the home environment. We hypothesized that performance on all three tasks would be associated with SES, but that distinct SES-related effects would be explained by distinct environmental factors. We expected that: (i) violence exposure would be specifically associated with associative memory performance, (ii) cognitive stimulation would be associated with cued attention, and (iii) the quality of the physical environment would be associated with memory-guided attention. We then tested whether SES has an indirect effect on these three cognitive outcomes through distinct environmental factors. Understanding how specific aspects of the early environment are associated with distinct memory and attention processes, that are subserved by different neural systems, has the potential to provide avenues for design of targeted interventions aimed to mitigate SES-related differences in cognitive function. Moreover, we investigated whether these different aspects of cognition contributed to SES-related differences in academic achievement over an 18-month follow up. This investigation of specific cognitive functions as mediators of SES-related differences in academic achievement may highlight an important role for specific cognitive functions in academic outcomes that have not been previously considered (e.g. memory-guided attention).
2. Methods
2.1. Participants
A sample of 101 youths aged 60–75 months (Mean Age 5.55 ± 0.37, 51 females) and their parents participated in the study between February 2016 and September 2017. Families were recruited from the Seattle area via fliers posted at preschools, daycares, clinics, and from the general community. Children were free of developmental disorders and families spoke English as a primary language in the home. To ensure SES-related diversity, our recruitment efforts focused on neighborhoods with wide variability in SES composition. The race and ethnicity of the families was similar to the demographics of the greater Seattle area (67.3.% White, 14.8.% Black, 2.9.% American Indian / Alaska Native, 12.8.% Asian, 0.9.% Native Hawaiian / Pacific Islander, 0.9.% Other; 8.9.% Hispanic or Latino). The Institutional Review Board at the University of Washington approved all procedures. Participants were compensated and written informed consent was obtained from legal guardians. Youths provided verbal assent. Two female participants were excluded from all analyses due to having scores of verbal intelligence as assessed by the Peabody Picture Vocabulary Test (Dunn and Dunn, 2007) two standard deviations below the mean, which was an exclusion criteria for participation. An additional female participant did not complete the memory-guided attention task and was not included in those analyses of that task.
This age range was chosen for a number of reasons. First, children in this age range tend to spend a large proportion of their time in the home and it is likely to be a period of time where different aspects of the home environment may have a greater impact on cognitive development than later periods of development when less time is spent in the home. As children develop and spend more time in other contexts, the relative importance of those environments increases relative to the home environment (Crosnoe et al., 2010). Second, memory-guided attention has been studied and shown to be intact in children as young as five years (e.g. Dixon et al., 2010) but has not been studied in younger children.
2.2. Socioeconomic status
SES was assessed using two measures: the income-to-needs ratio and maximum years parental education. The income-to-needs ratio captures the amount of annual income that a family earns relative to the federal poverty line for a family of that size. Parents reported annual income in 10 bins, and the median of the income bins was used except for the lowest and highest bins, which were assigned $5000 and $200,000 respectively. Income-to-needs ratio was calculated by dividing the total household income by the 2016 U.S. census-defined poverty line for a family of that size, with a value less than one indicating income below the poverty line. Median income-to-needs was 4.49 with 8 % of participants (income to needs less than 1) living in poverty and 23 % of participants living at less than twice the poverty line. Income-to-needs is based on the federal poverty line and does not account for regional variation in cost of living. In the area where data were collected, a 2017 study found that a family of four requires an income of approximately $75,000 per year in order afford basic needs (i.e. food, housing, transportation, health care, and child care; (Pearce, 2017). According to this standard, nearly half of our sample is below or near the self-sufficiency standard for the Seattle area. Income-to-needs values were log-transformed for all analyses, which is common in developmental studies (Noble et al., 2015; Rosen et al., 2018a,b) as SES associations with cognitive development are strongest at the lower end of the SES distribution. We use the term SES to refer to the income-to-needs ratio.
We additionally used caregiver education as another measure of SES, coded as total years of education obtained by the caregiver with the greatest educational attainment (10–22 years). Results using this measure were largely consistent with results using income-to-needs and are presented in the Supplemental Materials.
2.3. Environmental measures
Two experimenters visited the family home to assess the home environment using the Home Observation of the Environment (HOME), Early Childhood version (Bradley et al., 2001). The HOME is composed of both observations by the experimenter and interview questions directed at the parent and a point is given for every item coded as present. The observation component includes information about what the interviewer sees in the home (e.g. books, toys, clutter), observations about the parent (e.g. language use), and observations about parent-child interactions (e.g. whether the parent is affectionate towards the child). The interview portion contains questions about items the child might have (e.g. puzzles), questions about parent behaviors (e.g. parent encourages child to learn numbers) and questions about parent-child interactions (e.g. parent holds child for 10−15 min over the course of the day).
We extracted two sub-scales from the HOME items for further analysis: cognitive stimulation and the physical environment. Several of the original subscales in the HOME assessment (Language Stimulation, Academic Stimulation, Variety, and Learning Materials) include items reflecting cognitive stimulation. Moreover, some of these subscales include items that reflect other aspects of the home environment that reflect constructs other than cognitive stimulation (e.g. parent’s voice conveys positive feelings about child, which reflects warmth and not stimulation). As such, we performed a confirmatory factor analysis of the HOME items based on a conceptual model of the types of experiences underlying cognitive stimulation—including environmental complexity, enriching experiences, interactions with caregivers, and linguistic experience (Rosen et al., 2019a,b). Cognitive stimulation was made up of 20 items that assessed learning materials and complex stimuli for the child in the home (e.g. the number of books in the home, access to toys that teach numbers), the variety of experiences (e.g. being taken to a museum in the last year, being taken on a trip at least 50 miles away within the last year), language in the home (e.g. whether parent uses complex sentence structure or grammar) and caregiver involvement in the child’s learning (e.g. child is encouraged to learn to read a few words, child is encouraged to learn colors). The Physical Environment scale was comprised of six items that assessed the perceptual environment (e.g. house is not perceptually monotonous), cleanliness (e.g. house is reasonably clean and minimally cluttered), and safety of the environment (e.g. building appears safe and free of hazards). Confirmatory factor analysis indicated that our model of the constructs represented in the HOME items fit the data well (RMSEA < 0.001, 95 % C.I.: 0.0, 0.031; Tucker Lewis Index = 1.0; Comparative Fit Index: 1.0). See Supplemental Materials for information on the specific items were included in the cognitive stimulation and physical environment subscales. Within this sample, the cognitive stimulation scale demonstrated good within measure reliability (Chronbach’s α = 0.75) and the physical environment demonstrated acceptable reliability (Chronbach’s α = 0.64).
To assess exposure to violence, parents completed the Violence Exposure Scale for Children-Revised (VEX-R, (Fox and Leavitt, 1995)) in a format adapted for parent rather than child report. This assessment measures the frequency that a child has experienced different types of witnessing violence (e.g., seeing someone be hit really hard; witnessing someone be stabbed or shot) and directly experiencing violence (e.g., being beaten up, being pushed or shoved). A total score reflecting the frequency of experiencing violence was created by summing the items for a maximum score of 22. Within this sample, this scale demonstrated good reliability (Chronbach’s α = 0.77).
Procedure. The first visit (T1) was a home visit during which children completed the tablet tasks and parents completed the HOME interview and the VEX-R. Seventy-six participants (75.2.% of the baseline sample) performed the Woodcock-Johnson tests of academic achievement during a longitudinal follow-up (T2) which was completed an average of 18 months after the T1 assessment (M = 17.45 months, SD = 4.03).
At T1, before beginning any of the tasks, the experimenter first familiarized the participant with using the tablet. The experimenter placed the tablet in front of the child and ensured that the child could see the tablet well with no glare obscuring their view. During the orientation to the tablet, the child viewed a blank grey screen to practice touching. When the child touched the screen, a grey circle would appear in the location that they touched. The experimenter explained that this meant that the tablet “knew” where they were touching. The experimenter had the child practice touching several times and made sure they were not touching too gently, holding their finger down, or dragging their finger. This practice continued until the child was comfortable using the tablet as intended.
3. Behavioral tasks
3.1. Paired associate learning task
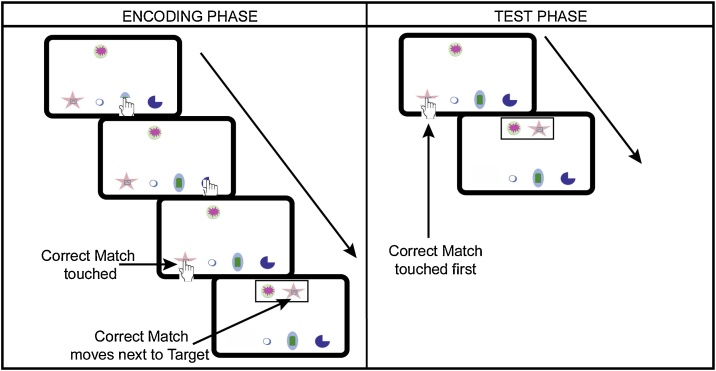
To assess associative memory performance, participants performed a paired associate learning (PAL) task (Hamoudi and Sheridan, 2015); Fig. 1). This task included 12 shapes randomly combined into 6 pairs. The task consisted of two phases, a learning phase and a test phase. Before beginning, the participant was shown the task structure on laminated pages in a binder and watched a brief movie demonstration of the task on the tablet. During the learning phase, participants were presented with a shape (target shape) at the top of the tablet screen. They were instructed to “find the shape’s friend” from a choice of four shapes at the bottom of the screen. They were also instructed that at first they might not know who the “friend” is, but if they saw the shape before, they should try to use their memory to “find the friend.” The participant was instructed to touch the shapes at the bottom of the screen to find the “friend.” If they touched a shape that was not the target shape’s pair, nothing occurred. When the participant touched the target shape’s pair, it moved up the screen and stopped next to the target, a black rectangle appeared around the two shapes, and the two shapes moved side-to-side as if they were doing a dance together. The learning phase was presented over 24 trials and each pair was presented four times, with each shape appearing as the target (at the top of the screen) twice and as a potential the pair (at the bottom of the screen among the other lures) twice.
Fig. 1.
Associative memory task. To assess associative memory, participants performed a paired associate learning task. During the encoding phase, they were presented with a target shape at the top of the screen and four possible items at the bottom of the screen. They were instructed to touch the shapes at the bottom of the screen to find the target’s “friend.” When the correct pair was found, it moved to the top of the screen next to the target, a box appeared around the two shapes and the two shapes moved back and forth as if they were doing a dance, and the trial ended. During the test phase, participants performed the same task, but were instructed to use their memory and try to find the target shape’s “friend.” Accuracy was assessed using the proportion of trials on which they identified the correct pair on the first touch of the trial during the test phase.
The test phase occurred approximately 20 min later. During this phase, the participants were presented with the same instructions and same trial structure as the learning phase. They were reminded again that they should use their memory to find the correct shape. Scores were taken from the test phase as the proportion of trials on which they identified the correct match on the first touch of the trial.
3.2. Attention tasks
The two attention tasks were based on a set of cued and memory-guided attention tasks developed for adults that were adapted for use in children (Rosen et al., 2018b; Fig. 2). A set of eight objects that would be familiar to children in this age range (e.g., truck, kite, flower) were used as targets. These images were split into two lists (List A and List B). The lists were counterbalanced across participants such that half of the subjects received List A as targets for memory-guided attention and List B as targets for cued attention and the other half the reverse. The order of these two tasks was also counterbalanced across participants. Twelve familiar object images (e.g., apple, pillow, cup) served as distractors for both tasks and never appeared as targets for any participants.
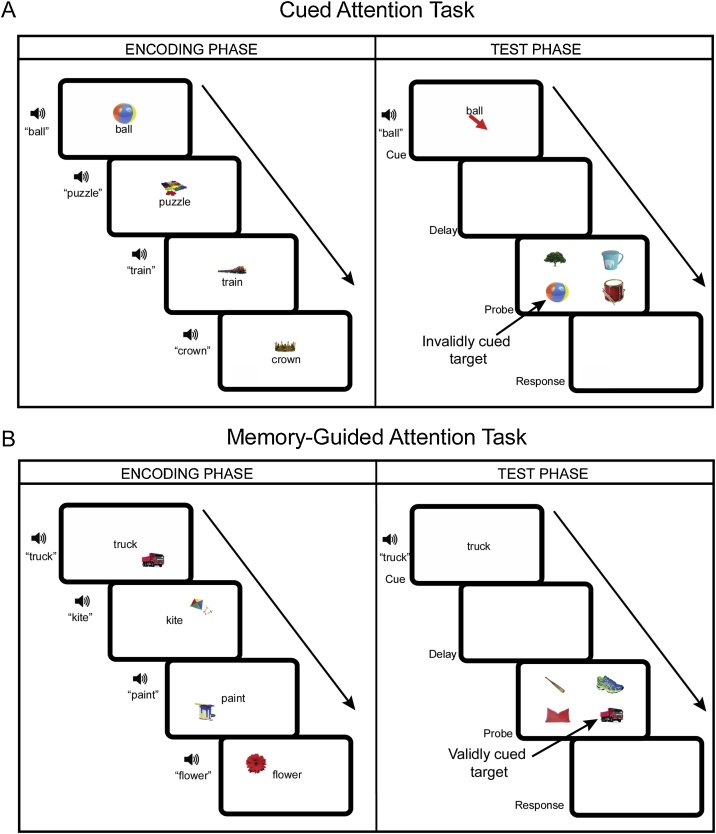
Fig. 2.
Cued attention and memory-guided attention tasks. During encoding of the cued attention task (A), participants simply viewed all of the objects that would be used as targets in the test phase. During the test phase for the cued attention task, a word and an arrow appeared along with an audio cue of the word. Participants were told to direct their attention to the quadrant where the arrow was pointing. After a brief delay, four pictures appeared, one in each quadrant. Participants were instructed to touch the location where the picture appeared. The example pictured above represents an invalidly cued target such that the ball is not presented in the cued location. During the encoding phase of the memory-guided attention task (B), participants learned object-location pairings. During the test phase, a word appeared on the screen along with an audio cue of that word. Participants were instructed to pay attention to the location where the picture belongs. After a brief delay, four pictures appeared, one in each quadrant and participants touched the location where the target appeared. The example pictured above, represents a validly cued target such that the truck appears in the location learned by the participant. Across both tasks, the target appeared in the cued location on 50 % of the trials (valid), and in one of the other locations on the other 50 % of trials (invalid trials). Mean reaction time on validly and invalidly cued trials as well as accuracy across all trials were used to assess performance on both tasks. See text for more details.
3.2.1. Cued attention task
The cued-attention task consisted of two phases: an encoding phase and a test phase (Fig. 2A). The encoding phase was designed to ensure that children had equal exposure to stimuli used in the cued attention and memory-guided attention tasks. The test phase was designed to probe children’s ability to use an external cue (an arrow) to guide spatial attention in a Posner-style cueing task (Posner et al., 1980). During the encoding phase, participants were presented with an image of an object at the center of the screen (e.g. a truck), the written word that corresponded to that object, and an audio cue corresponding to that object (e.g. “truck”). The audio and text were both presented to mitigate any differences in reading ability. Participants were instructed to look at the pictures and try to remember them. Each picture was presented for 1500 ms and a total of eight times for a total of 32 encoding trials and 12 s.
Approximately 20 min later, participants were presented with the test phase. During this task, participants viewed a word on the screen accompanied by an arrow pointing to one of the four quadrants (Cue: 2000 ms). They were instructed to pay attention to where the arrow was pointing and look for the picture that matched the word at that spot. A blank screen then appeared (Delay: 1000 ms). Four object images then appeared on the screen, one in each quadrant (Probe: 500 ms). Participants were instructed to touch the spot on the screen where the target picture appeared; responses were recorded during both the Probe period and a Response period (1000 ms), providing participants had a total of 1500 ms to respond from the onset of the target. The target appeared in the correct location (i.e., where the arrow was pointing) on 50 % of trials, and in one of the other three locations on the other 50 % of trials with equal likelihood of appearing at each of the other locations on a given trial. Each target was presented 8 times in a pseudorandom order for a total of 32 trials. Mean reaction time on validly cued trials (i.e., target appeared in the cued location, which reflects the speed with which the participant is able to use the cue to identify that the target appeared at the cued location) and invalidly cued trials (i.e., target appeared in a different location, which reflects the speed with which the participant disengages from the cued location and reengages at the location where the target appears) was assessed. We also measured accuracy, which reflects overall ability to use the cue to guide attention, across all trials.
3.2.2. Memory-guided attention task
In the memory-guided attention task children first completed an encoding phase in which they learned to bind an object with a spatial location; during a test phase, they were cued with the object word and asked to use their memory to direct their attention to the location the picture should appear (Fig. 2B). The encoding phase consisted of two phases. During the first encoding phase, participants were presented with an object image (e.g. flower) located in one of the four quadrants accompanied by the word that matched that object and the word said aloud by the tablet (e.g. “flower”). Participants were instructed to “look at the screen and try to remember where the picture goes.” The word and the image were presented simultaneously for 2000 ms. Each image was presented four times for a total of 16 trials with each picture only ever appearing in one of the four quadrants. On the second round of encoding, participants viewed and heard the word cue (2000 ms) without the image followed by a blank screen for 1000 ms. Participants were instructed to try to touch the screen where the picture should go (i.e., the spatial location they learned in the first encoding phase) before the picture appeared. Then the picture appeared in the correct location for 1000 ms. This additional encoding was designed to aid in the binding of the picture with the spatial location. During this phase, each object was presented four times for a total of 16 trials. Across the two encoding phases, participants saw each object for total of 12 s, the same amount of time each object was seen during encoding for the cued attention task.
The test phase, which occurred approximately 20 min after encoding, was structured similarly as the test phase for the cued attention task. During this task, participants viewed a word on the screen (Cue: 2000 ms). Unlike the cued attention task, participants did not see an arrow on the screen but instead were instructed to use their memory to direct their attention to the location on the screen where the picture should appear. A blank screen then appeared (Delay: 1000 ms). Four object images then appeared on the screen, one in each quadrant (Probe: 500 ms). Participants were instructed to touch the spot on the screen where the target picture appeared during the probe period; responses were taken during both the Probe period and the Response period (1000 ms), providing participants had a total of 1500 ms to respond from the onset of the target. The target appeared in the correct location on 50 % of trials, and in one of the other three locations on the other 50 % of trials with equal likelihood of appearing at each of the other location on a given trial. Each target was presented eight times in a pseudorandom order for a total of 32 trials. We assessed mean reaction time on validly cued trials, which reflects the speed with which the participant is able to use their memory to identify that the target appeared at the remembered location, and invalidly cued trials, which reflects the speed with which the participant disengages from the remembered location and reengages at the location where the target appears. We also assessed accuracy, which reflects overall ability to use the memory to guide attention across all trials
3.3. Woodcock-johnson IV tests of achievement
During the T2 follow-up, three subsets of the Woodcock-Johnson IV Tests of Achievement (WJ IV) were used as assessments of academic achievement (Schrank et al., 2015): Letter-Word Identification, Spelling, and Calculation. Each test presented the participants with items of increasing difficulty. In the Letter-Word Identification test, participants were asked to identify letters and read lists of words. In the Spelling test, participants were instructed to spell words that were read aloud and used in a sentence by the experimenter. The Calculation test required children to complete a series of arithmetic problems. The Letter-Word Identification, Spelling, and Calculation subsets were all discontinued when the participants answered incorrectly on six consecutive items. Standard scores normed by age were calculated for each subset as measures of the child’s achievement in that academic domain and the Academic Skills Cluster was calculated based on these scores.
3.4. Statistical analyses
We had two overarching goals in this study. The first was to identify the environmental factors that contribute to SES-related differences in cognitive performance on each of the three tasks: associative memory, cued attention, and memory-guided attention. First, we used linear regression to examine the association of SES with cognitive performance. Specifically, we estimated a series of separate multivariate models examining income-to-needs as a predictor of associative memory, cued attention, and memory-guided attention. Next, we examined the associations between income-to-needs and the three measures of the environment (violence exposure, cognitive stimulation, and quality of the physical environment). Then, we examined the associations of the environmental measures with performance on each of the cognitive tasks at T1. Next, given that the environmental factors are correlated with one another, we performed sensitivity analyses to determine which of the environmental variables predicted performance on each task when controlling for the other environmental factors. Finally, we tested whether SES had an indirect effect on cognitive outcomes through the environment. We used a standard test of statistical mediation that estimates the significance of indirect effects using a bootstrapping approach that provides confidence intervals for the indirect effects (Hayes, 2013). Confidence intervals that do not include 0 are considered evidence for statistically significant indirect effects. We tested indirect effects for environmental factors significantly associated with both SES and the cognitive outcomes, while controlling for other aspects of the environment. All regression and mediation analyses included age and sex as covariates.
There was a high correlation between the physical environment and cognitive stimulation (see Table 2 for bivariate correlations). This makes it difficult to disentangle these factors due to multicollinearity. To address the issue of multicollinearity, we calculated the variance inflation factor (VIF), which measures the inflation of the variance of the parameter estimate when another variable that is highly correlated with the predictor is also present in the model. The standard states that a VIF above 10 is considered to have high multicollinearity (Kutner et al., 2005) and that a VIF below 4 is acceptable with low enough risk of inflated coefficients(Sheather, 2009). We calculated VIFs for models including the physical environment, cognitive stimulation, and violence exposure as predictors (with age and sex as covariates) for associative memory, memory-guided attention, and cued attention performance. In all instances, the VIF for cognitive stimulation and the physical environment were below 4 (range: 3.35–3.61), reducing the concern for inflated coefficients in the present study.
Table 2.
Bivariate correlations of all study variables.
| Age T1 | Age T2 | Sex | Log ItNR | Edu. | Cog. | Phys. | Viol. | PAL | Cued Attn | MGA | Cued RT Valid | Cued RT Invalid | MGA RT Valid | MGA RT Invalid | AA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age T1 | ||||||||||||||||
| Age T2 | .702** | |||||||||||||||
| Sex | .007 | -.001 | ||||||||||||||
| Log ItNR | .078 | <.001 | -.016 | |||||||||||||
| Edu | -.014 | .073 | -.093 | .493** | ||||||||||||
| Cog. | .008 | .033 | .032 | .552** | .545** | |||||||||||
| Phys. | .088 | .047 | -.051 | .620** | .483** | .833** | ||||||||||
| Viol. | .063 | -.031 | .010 | -.345** | -.359** | -.230* | -.312** | |||||||||
| PAL | .102 | .198 | .245* | .045 | .067 | .122 | .078 | -.204* | ||||||||
| Cued Attn | .146 | -.032 | -.014 | .235* | .360** | .209* | .255* | -.167 | .012 | |||||||
| MGA | .260** | .006 | .039 | .217* | .218* | .117 | .244* | -.179 | .016 | .599** | ||||||
| Cued RT valid | -.360** | -.200 | .045 | .039 | .003 | .018 | -.061 | -.006 | -.009 | -.260* | -.279* | |||||
| Cued RT Invalid | -.161 | -.109 | .039 | .134 | .037 | .190 | .214* | -.059 | .001 | .045 | .056 | .306** | ||||
| MGA RT valid | -.285** | -.247* | -.005 | -.016 | -.041 | -.043 | -.026 | .024 | -.098 | -.181 | -.186 | .519** | .465** | |||
| MGA RT invalid | -.113 | -.233* | -.088 | -.052 | -.076 | -.060 | -.080 | .112 | -.095 | -.143 | -.094 | .267** | .469** | .515** | ||
| AA | -.022 | -.167 | -.126 | .279 | .267* | .159 | .265* | -.264 | -.032 | .255* | .300** | -.107 | .128 | .025 | .164 |
NOTE: * indicates p < .05, ** indicates p < .01. Log ItNR = Log transformed income-to-needs ratio, Edu = max parental education in years, Cog = cognitive stimulation, Phys = physical environment, viol = violence exposure, PAL = accuracy on the associative memory task, Cued Attn = accuracy on the cued attention task, MGA = accuracy on the memory-guided attention task, Cued RT Valid = reaction time for validly cued target in the Cued attention task, Cued RT Invalid = reaction time for invalidly cued target in the Cued attention task, MGA RT Valid = reaction time for validly cued target in the memory-guided attention task, MGA RT Invalid = reaction time for invalidly cued target in the memory-guided attention task, AA = Academic Achievement as assessed by the Woodcock-Johnson Academic Skills Cluster.
The second goal was to determine whether performance on these cognitive tests serve as a mechanism linking SES with academic achievement. We tested whether income-to-needs was associated with academic achievement and whether each of our three cognitive tests was associated with academic achievement using linear regression. Analysis of academic achievement controlled for age at T2. After testing each of these paths, we used a standard test of statistical mediation (Hayes, 2013). We tested indirect effects for performance on cognitive tasks significantly associated with both SES and academic performance.
4. Results
4.1. Descriptive statistics
Means and standard deviations for all study variables are presented in Table 1, and bivariate correlations between all study variables are presented in Table 2.
Table 1.
Descriptive Statistics.
| Measure | Minimum | Maximum | Mean | Standard Deviation |
|---|---|---|---|---|
| Age T1 (years) | 5.00 | 6.24 | 5.55 | .37 |
| Age T2 (years) | 6.13 | 8.11 | 7.00 | .46 |
| ItNR | .08 | 10.5 | 4.73 | 2.86 |
| Log ItNR | −2.54 | 2.35 | 1.26 | .95 |
| Edu (years) | 10 | 22 | 16.65 | 2.85 |
| Cognitive Stimulation (total score) | 5 | 20 | 15.69 | 3.07 |
| Physical Environment (total score) | 0 | 6 | 4.9 | 1.34 |
| Violence Exposure | 0 | 20 | 3.00 | 3.90 |
| Associative Memory Accuracy | .08 | .83 | .38 | .17 |
| Cued Attention Accuracy | .28 | 1 | .80 | .12 |
| Memory-Guided Attention Accuracy | .38 | 1 | .80 | .13 |
| Cued RT valid (ms) | 680 | 1420 | 926 | 121 |
| Cued RT Invalid (ms) | 770 | 1500 | 1182 | 141 |
| MGA RT valid (ms) | 690 | 1500 | 968 | 152 |
| MGA RT invalid (ms) | 850 | 1660 | 1142 | 129 |
| Academic Achievement | 710 | 141 | 100.42 | 13.39 |
Note: T1 = Time 1, T2 = Time 2, ItNR = Income-to-Needs Ratio, Edu = Education, RT = response time, MGA = memory-guided attention.
4.2. SES and cognitive performance
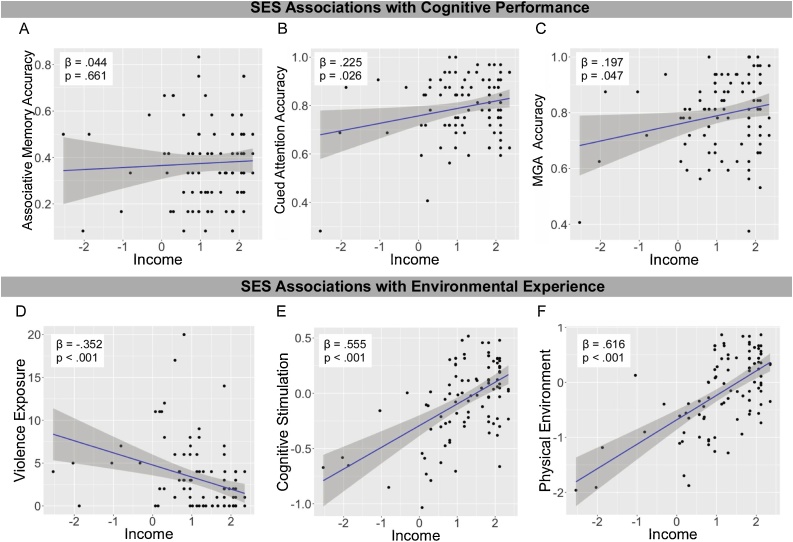
First we assessed the associations between SES and performance on the three cognitive tasks. SES was not associated with associative memory accuracy (β = .044, p = .661 [95 % CI: −.124 to .211]). There was was a positive association between SES and accuracy on both cued attention (β = .225, p = .026 [95 % CI: .057–.393]) and memory-guided attention (β = .197, p = .047 [95 % CI: .029–.365]; Fig. 3A–C). SES was not associated with reaction time for valid or invalid targets on the attention task or the memory-guided attention task (ps > .14).
Fig. 3.
Associations of SES with cognitive function and environmental experience. MGA refers to memory-guided attention.
4.3. SES and environmental measures
SES was strongly associated with all three environmental measures such that income-to-needs was negatively associated with violence exposure (β = −.352, p < .001 [95 % CI: −.184 to -.519]) and positively associated with both cognitive stimulation (β = .555, p < .001 [95 % CI: .387–.722]) and the quality of the physical environment (β = .616, p < .001 [95 % CI: .448–.784]; Fig. 3D–F).
4.4. Environmental measures and cognitive performance
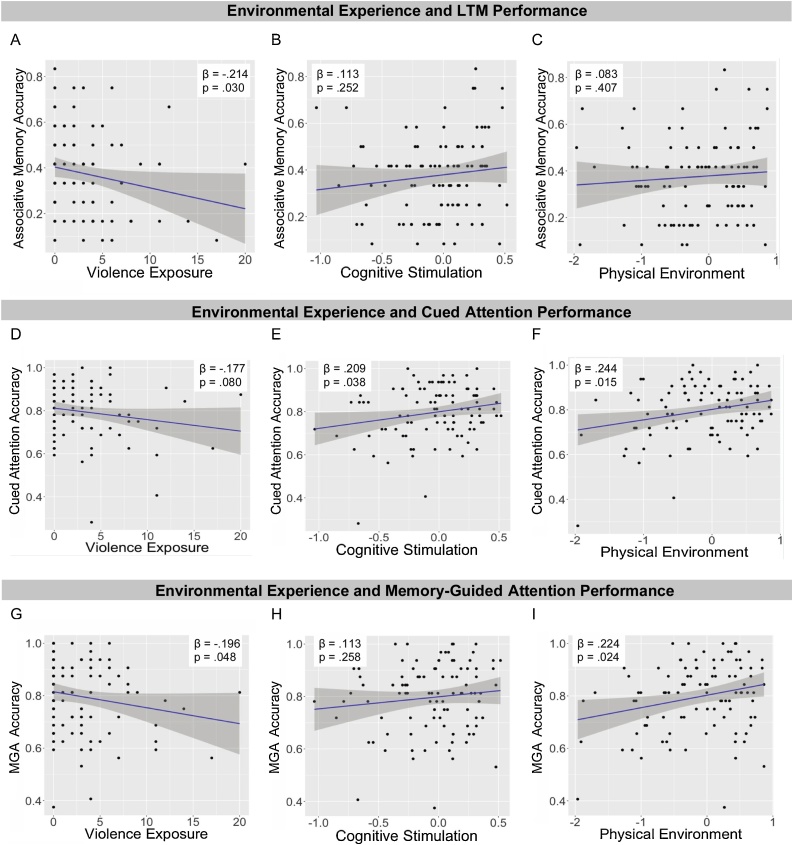
Next, we tested the associations between the three environmental measures and performance on the three cognitive tasks. Violence exposure was associated with reduced PAL accuracy (β = −.214, p = .030 [95 % CI: −.382 to −.046]). PAL was not associated with either cognitive stimulation or the quality of the physical environment (β = .113, p = .252 [95 % CI: -0.055 to .281]; β =.083, p = .407 [95 % CI: -.085 to .251], respectively; Fig. 4A–C).
Fig. 4.
Associations of environmental experience with cognitive performance. MGA refers to memory-guided attention.
In contrast, both cognitive stimulation (β = .209, p = .038 [95 % CI: .041–.377]) and the quality of the physical environment (β = .244, p = .015 [95 % CI: .076–.412]), were associated with cued attention accuracy. Violence exposure was unrelated to attention accuracy (β = −.177, p = .080 [95 % CI: −.345 to −.001]; Fig. 4D–F). None of the environmental measures were associated with RT on validly cued trials in the cued attention task (ps > .75). For invalidly cued targets, the physical environment (β = .232, p = .021 [95 % CI: .064–.400]) and cognitive stimulation (β = .191, p = .058 [95 % CI: .023.359]) were each associated with slower RT; there was no association with violence exposure (β = −.049, p = .628 [95 % CI: −.217−.119]).
Finally, both the quality of the physical environment (β = .224, p = .024 [95 % CI: .057–.392]) and violence exposure (β = −.196, p = .048 [95 % CI: −.364 to −.028]) were associated with memory-guided attention accuracy, but cognitive stimulation was not (β = .113, p = .258 [95 % CI: −.055 to .281]; Fig. 4G–I). None of the measures of the environment were associated with RT for the memory-guided attention task for valid or invalid targets (ps > .65).
4.5. Sensitivity analyses
We next investigated whether each environmental measure was associated with accuracy on each cognitive task while controlling for the other environmental measures. All results are presented in Table 3. Violence exposure continued to be negatively associated with associative memory, even after controlling for cognitive stimulation and the physical environment, which were not significantly associated with PAL accuracy. None of the environmental measures are significantly associated with accuracy on the cued attention task in the fully-adjusted model. The quality of the physical environment was positively associated with memory-guided attention accuracy even after controlling for violence exposure and cognitive stimulation, which were not associated with memory-guided attention accuracy.
Table 3.
Sensitivity Analyses. Regression analyses including violence exposure, cognitive stimulation, and physical environment in the same model. Significant associations are marked in bold.
| Violence Exposure | Cognitive Stimulation | Physical Environment | |
|---|---|---|---|
| PAL | β = .216, p = .039 | β = .092, p = .328 | β = .047, p = .467 |
| Cued Attention | β = -.112, p = .288 | β = .030, p = .869 | β = .183, p = .332 |
| Memory-Guided Attention | β = -.130, p = .203 | β = -.230, p = .194 | β = .377, p = .041 |
4.6. Mediation analysis
We performed tests of formal mediation for environmental factors associated with a cognitive outcome, controlling for other environmental factors. Because the sensitivity analysis revealed that the physical environment was associated with memory-guided attention accuracy over and above the effect of violence and cognitive stimulation, we tested a formal mediation model and found evidence in support of an indirect effect of SES on memory-guided attention through the quality of the physical environment (95 % CI: .000–.0248). We did not examine a mediating role of the environment on cued attention because none of the environmental factors predicted performance over and above the other environmental factors. Additionally, because there was no direct effect of SES on associative memory, the indirect effect through violence exposure was not examined.
4.7. SES and academic achievement
SES was positively associated with academic achievement such that higher income-to-needs predicted higher performance on the Woodcock-Johnson Academic Skills Cluster (β = 0.287, p = .011 [95 % CI: .119–.455]).
4.8. Cognitive performance and academic achievement
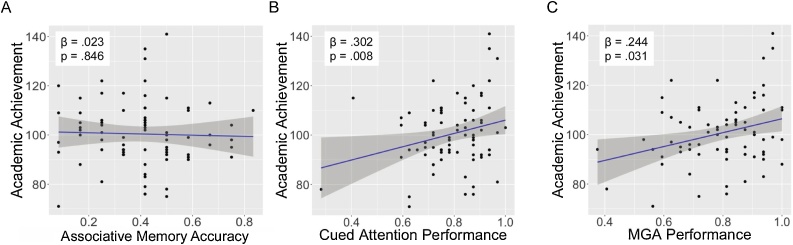
PAL accuracy was not associated with academic achievement (β = .023, p = .846 [95 % CI: −.145 to .191]). In contrast, higher accuracy on both the cued attention task and the memory-guided attention task were associated with higher academic achievement (β =.302, p = .008 [95 % CI: .134–.470]; β =.244, p = .031 [95 % CI: .076–.412], respectively; Fig. 5).
Fig. 5.
Associations of cognitive performance with academic achievement. MGA refers to memory-guided attention.
4.9. Mediation analyses
Because both cued attention and memory-guided attention accuracy were related to academic achievement, we tested a formal mediation model to determine whether there is an indirect effect of SES on academic achievement over the 18-month follow up through these cognitive functions. Memory-guided attention mediated the association between income and academic achievement (95 % CI: .018–2.87); accuracy on the cued attention task did not (95 % CI: −.21 to 3.14).
5. Discussion
We investigated how specific aspects of the early environment were related to three domains of cognitive performance supported by distinct neural circuits, including associative memory, cued attention, and memory-guided attention. Our findings revealed distinct environmental associations with different domains of cognitive function. Violence exposure was specifically associated with associative memory accuracy and the quality of the physical environment was specifically associated with memory-guided attention accuracy. Furthermore, we found that SES had an indirect effect on memory-guided attention through the physical environment. Although cognitive stimulation and the quality of the physical environment were each associated with cued attention accuracy, these associations were no longer significant after adjusting for all forms of environmental experience, indicating that multiple environmental factors may drive cued attention accuracy. Furthermore, we investigated how these three domains of cognitive function were associated with academic achievement over an 18-month follow-up. While better memory-guided attention and cued attention accuracy were each associated with higher academic achievement, only memory-guided attention mediated SES-related differences in academic achievement.
While previous studies have established that children as young as five years can use prior experience to direct attention (Dixon et al., 2010; Nussenbaum et al., 2019), existing studies have not investigated the environmental factors that may influence memory-guided attention. Here, we demonstrate that higher SES is associated with better memory-guided attention performance. Importantly, the quality of the physical environment was the strongest environmental predictor of memory-guided attention performance, even after accounting for other aspects of the environment including violence exposure and cognitive stimulation.
There are a several reasons that a higher quality physical environment might be associated with better memory-guided attention. If an environment has low levels of consistency and structure in a cluttered and overcrowded home, children may not often encounter situations in which they can reliably use their previous experience to guide attention. Similarly, if an environment has low levels of visual complexity, children may not require the development of the ability to effectively integrate mnemonic-based and stimulus-based information that is required for memory-guided attention. In adults, three posterior nodes of the cognitive control network — including the posterior precuneus, lateral intraparietal sulcus and posterior callosal sulcus — are recruited for memory-guided attention (Rosen et al., 2016, 2018b). Children reared in severely deprived environments with very low levels of perceptual stimulation have reduced cortical thickness in these regions, and thinning in these regions mediated the association between time in the deprived environment and inattentiveness (McLaughlin et al., 2014). It is possible that low environmental complexity leads to increased synaptic pruning of regions that support memory-guided attention and that this accelerated pruning has downstream effects on attention (McLaughlin et al., 2017). Future studies will need to investigate the associations between the physical environment in more normative environments, such as those in the present study, with structure and function of the posterior nodes of the cognitive control network that support memory-guided attention.
We additionally found that memory-guided attention partially explains SES-related differences in academic achievement over an 18-month delay. Memory-guided attention is likely important for adaptive academic and behavioral functioning in school. When a child arrives at a new school they must rely heavily on external cues in order to know where to direct their attention (e.g. where to put their backpack, where the teacher stands, where other children are sitting, what needs to be on the desk), which can be overwhelming and impair the child’s ability to focus on one task. As the school year progresses, children begin to learn about the environment and can thus use this previous experience to guide attention to the most relevant information for a given task. When using memory-guided attention, children can more effectively utilize their limited attentional capacity to focus on a particular task because they no longer need to use these resources to process external cues to guide their attention. The present findings that the physical environment was important for memory-guided attention and that memory-guided attention was in turn associated with academic achievement are broadly consistent with a recent study demonstrating that the quality of the physical environment was positively associated with cortical thickness and that greater cortical thickness was associated with higher levels of academic achievement (Uy et al., 2019). Together with our results, these findings highlight the physical environment and its association with memory-guided attention as important environmental and cognitive mechanisms explaining SES-related differences in achievement.
Although chronic stress and trauma exposure have well-established associations with the structure of the hippocampus in animal and human studies (Ivy et al., 2010; Lambert et al., 2017; Lupien et al., 2009), remarkably little research has investigated how these environmental factors influence cognitive functions subserved by the hippocampus in young children, including associative memory. Our findings suggest that violence exposure is negatively associated with associative memory in early childhood, even after accounting for variation in cognitive stimulation and the quality of the physical environment. These findings are in line with prior work documenting poor associative learning among older children, adolescents, and adults exposed to violence (Hanson et al., 2017; Lambert et al., 2019) and extend these findings by demonstrating these associations are present in early childhood, in children far younger than previously examined to assess the impact of violence exposure on memory. Associative learning of two previously unassociated stimuli depends on the hippocampus and other medial temporal lobe structures including the parahippocampal cortex (Eichenbaum and Bunsey, 1995; Yoon et al., 2012). Animal studies demonstrate that chronic stress and high levels of associated glucocorticoids have deleterious effects on hippocampal neurons (Brunson et al., 2001; Ivy et al., 2010; Lupien et al., 2009). It is possible that a high levels of violence exposure in children negatively influences associative memory via a similar mechanism. These findings point to a strikingly early emergence of differences in associative memory function after violence exposure. Future studies should investigate whether these differences are mediated by disruptions in hippocampal and medial temporal lobe structure and function in young children who have experienced violence.
Consistent with previous studies showing positive associations between SES and attention and working memory, SES was positively associated with cued attention performance (Clearfield and Jedd, 2013; Finn et al., 2016; Kishiyama et al., 2009; Mezzacappa, 2004; Sheridan et al., 2017; Stevens et al., 2009). Higher levels of cognitive stimulation and a higher quality physical environment were each associated with better cued attention performance, although when all three environmental measures were included in the model, no single factor remained significantly associated with cued attention performance. These results suggest that multiple environmental factors may contribute to the development of this attentional process. These findings are broadly consistent with other work demonstrating that cognitive stimulation is positively associated with attention in children. A study that directly assessed how different aspects of the home environment were associated with focused attention demonstrated that while cognitive stimulation was associated with focused attention among children living in poverty, the quality of the physical environment was associated with focused attention among children living near poverty (Razza et al., 2010). Additionally, work in deprived early environments as seen in institutional rearing which include both severely low levels of cognitive and perceptual stimulation has found lasting effects on working memory and attention even among children who were placed in high quality foster care before the age of 24 months (Merz et al., 2016; Slopen et al., 2012). Finally, both greater cognitive stimulation and higher quality of the physical environment are associated with greater cortical thickness in the middle frontal gyrus and superior parietal lobule, brain regions that support top-down attention (Rosen et al., 2018a; Uy et al., 2019). Together with our findings, these studies suggest that low levels of cognitive stimulation and poor quality of the physical environment may influence the development of the frontoparietal network, which may in turn contribute to SES-related differences in attentional function.
Somewhat counterintuitively, we also found that higher cognitive stimulation and quality of the physical environment were associated with slower reaction time for invalidly cued targets. This could indicate that these children were more focused on the cued location of the target, and slower to disengage and reorient attention when the target appeared in an unexpected location (Posner and Cohen, 1984). Future studies should further probe whether children reared with low cognitive stimulation and poorer quality physical environments demonstrate less of a cost of selective attention (i.e. missing an unexpected stimulus because of hyper focused attention in a particular location) and perhaps are better able to attend more diffusely to the environment (Plebanek and Sloutsky, 2017), which may indeed be adaptive in certain environmental contexts.
The present study has several limitations that should be acknowledged. First, because the measures of the environment and cognitive function were collected concurrently, we are limited in our ability to establish a directional link between these environmental factors and development of associative memory, cued attention, and memory-guided attention. Additionally, while we established that SES had an indirect effect on memory-guided attention through the physical environment, mediation analyses performed when all study variables are collected at the same time point should be interpreted with caution (Maxwell and Cole, 2007). Although the children in the present study were relatively young, it will be important to determine the precise developmental window in which these environmental factors have the greatest impact on these cognitive outcomes. Future studies should take a longitudinal approach beginning in infancy to investigating how distinct aspects of the environment impact different types of cognitive development, given that relevant nonverbal tasks are beginning to emerge in the literature (e.g.,Weiss et al., 2018). Second, cognitive stimulation and the physical environment were highly correlated in our sample. This introduces challenges in separating the unique associations of these aspects of the environment with cognitive outcomes. Therefore, replication of these findings in additional samples is an important goal for future research. Third, the sample size of the present study is relatively small to disentangle different aspects of the early home environment and therefore, these results should be interpreted as preliminary. Relatedly, while the sample in the present study ranged widely in SES levels from well below the poverty line to more than ten times the poverty line, the sample was on average, relatively high SES based on the income-to-needs ratio. Importantly, nearly half of our sample was at or below the self-sufficiency standard for the metropolitan area in which the data were collected. It will be important for future studies to replicate the present findings using a larger sample that is representative of the U.S. income distribution. Fourth, while the same stimuli of everyday objects were used in the memory-guided attention and cued attention tasks and were counterbalanced across participants, a distinct set of stimuli made up of abstract shapes was used in the associative memory task as part of an established tablet task used in young children (Hamoudi and Sheridan, 2015). It is possible that these differences contributed to the distinct associations seen. However, given that other studies have found similar associations between violence exposure and memory for real world objects (Lambert et al., 2019), it seems unlikely that these stimuli differences are driving our effects. Still, future work should structure three tasks (associative memory, cued attention, and memory-guided attention) using the same stimulus sets to rule out this possibility. Finally, given that we are unable to randomly assign individuals to different SES levels, we are limited in our ability to make causal inferences about how SES contributes to cognitive development. However, we see the dissociable associations with different environmental experience and cognitive outcomes that are subserved by different neural systems as a strength of the present study. Evaluating whether this specificity replicates in randomized trials focused on providing income supplements to families with young children is an important goal for future research.
6. Conclusions and future directions
The present study demonstrates that specific aspects of early environmental experience contribute to the development of different types of cognitive function which are subserved by distinct neural mechanisms. Violence exposure is negatively associated with associative memory, which is dependent on the hippocampus. In contrast, a higher quality physical environment was associated with memory-guided attention, which is supported by the posterior cognitive control network. Finally, multiple aspects of the environment were associated with cued attention, which is supported by the frontoparietal network. The findings from the present study highlight the importance of careful quantification of the home environment to understand the particular mechanisms linking SES and cognitive development. Interventions intended to mitigate SES-related differences in cognitive function should be informed by studies like this one that investigate what specific aspect of the environment might be driving differences.
This is the first study to highlight memory-guided attention as a potential cognitive mechanism linking SES and academic achievement. Understanding the environmental pathways through which differences in SES ultimately shape cognitive development is important for identifying malleable targets for interventions to reduce SES-related disparities in cognitive outcomes. Given this, our findings suggest that incorporating techniques aimed at improving memory-guided attention in such interventions could be potentially promising. One avenue through which this could be accomplished is by improving the quality of the physical environment, for example through interventions aimed at helping low-SES families access affordable high-quality housing. Future studies focused on how the early environment impacts the neural systems that support memory-guided attention may provide important insights into neural mechanisms contributing to the income-achievement gap. This may, in turn, point to research, practices, and societal changes that can help ameliorate this gap.
Funding
This work was supported by the National Institute of Child Health and Human Development [F32 HD089514 to MR], National Institute of Mental Health [R01-MH103291 and R01-MH106482 to KM], the Brain and Behavior Foundation NARSAD Early Investigator Award, an Early Career Research Fellowship from the Jacobs Foundation, and the IMHRO Rising Star Award to KM, and the Institute for Learning & Brain Science Ready Mind Project.
Declaration of Competing Interest
None.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100731.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Belsky J., Goode M.K., Most R.K. Maternal stimulation and infant exploratory competence: cross-sectional, correlational, and experimental analyses. Child Dev. 1980;51(4):1168–1178. [PubMed] [Google Scholar]
- Bradley R.H., Corwyn R.F., McAdoo H.P., Garcia Coll C. The home environments of children in the United States part I: variations by age, ethnicity, and poverty status. Child Dev. 2001;72(6):1844–1867. doi: 10.1111/1467-8624.t01-1-00382. [DOI] [PubMed] [Google Scholar]
- Brunson K.L., Eghbal-Ahmadi M., Bender R., Chen Y., Baram T.Z. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc. Natl. Acad. Sci. 2001;98(15):8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clearfield M.W., Jedd K.E. The effects of socio-economic status on infant attention. Infant Child Dev. 2013;22:53–67. [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crosnoe R., Leventhal T., Wirth R.J., Pierce K.M., Planta R.C., NICHD Early Child Care Research Network Family socioeconomic status and consistent environmental stimulation in early childhood. Child Dev. 2010;81(3):972–987. doi: 10.1111/j.1467-8624.2010.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rosa Piccolo L., Arteche A.X., Fonseca R.P., Grassi-Oliveira R., Salles J.F. Influence of family socioeconomic status on IQ, language, memory and executive functions of Brazilian children. Psicologia: Reflexao e Critica. 2016;29(1) [Google Scholar]
- Dixon M.L., Zelazo P.D., De Rosa E. Evidence for intact memory-guided attention in school-aged children. Dev. Sci. 2010;13(1):161–169. doi: 10.1111/j.1467-7687.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- Dufford A.J., Bianco H., Kim P. Socioeconomic disadvantage, brain morphometry, and attentional bias to threat in middle childhood. Cogn. Affect. Behav. Neurosci. 2018;19(2):309–326. doi: 10.3758/s13415-018-00670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L.M., Dunn D.M. Vol. 30. 2007. (Peabody Picture Vocabulary Test 4. Summary). [Google Scholar]
- Eichenbaum H., Yonelinas A.P., Ranganath C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 2007;30(1):123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum Howard, Bunsey M. On the binding of associations in memory: clues from studies on the role of the hippocampal region in paired-associate learning. Curr. Dir. Psychol. Sci. 1995;4(1):19–23. [Google Scholar]
- Ellis B.J., Bianchi J.M., Griskevicius V., Frankenhuis W.E. Beyond risk and protective factors: an adaptation-based approach to resilience. Perspect. Psychol. Sci. 2017 doi: 10.1177/1745691617693054. [DOI] [PubMed] [Google Scholar]
- Evans G.W. The environment of childhood poverty. Am. Psychol. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Evans G.W. Child development and the physical environment. Annu. Rev. Psychol. 2006;57(1):423–451. doi: 10.1146/annurev.psych.57.102904.190057. [DOI] [PubMed] [Google Scholar]
- Evans G.W., English K. The environment of poverty: multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Dev. 2002;73(4):1238–1248. doi: 10.1111/1467-8624.00469. [DOI] [PubMed] [Google Scholar]
- Farah M.J., Betancourt L., Shera D.M., Savage J.H., Giannetta J.M., Brodsky N.L. Environmental stimulation, parental nurturance and cognitive development in humans. Dev. Sci. 2008;11(5):793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Farah M.J., Shera D.M., Savage J.H., Betancourt L., Giannetta J.M., Brodsky N.L. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110(1):166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Findji F., Pêcheux M.G., Ruel J. Dyadic activities and attention in the infant: a developmental study. Eur. J. Psychol. Educ. 1993;8:23. [Google Scholar]
- Finn A.S., Minas J.E., Leonard J.A., Mackey A.P., Salvatore J., Goetz C. Functional brain organization of working memory in adolescents varies in relation to family income and academic achievement. Dev. Sci. 2016:1–15. doi: 10.1111/desc.12450. [DOI] [PubMed] [Google Scholar]
- Fox N.A., Leavitt L.A. 1995. The Violence Exposure Scale for Children-Revised (VEX-R) [Google Scholar]
- Ghetti S., Bunge S.A. Neural changes underlying the development of episodic memory during middle childhood. Dev. Cogn. Neurosci. 2012;2(4):381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough W.T., Black J.E., Wallace C.S. Experience and brain development. Child Dev. 1987;58(3):539–559. [PubMed] [Google Scholar]
- Gustafsson H.C., Coffman J.L., Langley H.A., Cox M.J. Intimate partner violence and children’s memory. J. Fam. Psychol. 2008;15(10):1203–1214. doi: 10.1037/a0034592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D.A., Farah M.J., Meaney M.J. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010 doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman D., Farah M. Socioeconomic status and the developing brain. Trends Cogn. Sci. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamoudi A., Sheridan M. 2015. Unpacking the Black Box of Cognitive Ability a Novel Tool for Assessment in a Population Based Survey. [Google Scholar]
- Hanson J.L., Nacewicz B.M., Sutterer M.J., Cayo A.A., Schaefer S.M., Rudolph K.D. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol. Psychiatry. 2015;77(4):314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., van den Bos W., Roeber B.J., Rudolph K.D., Davidson R.J., Pollak S.D. Early adversity and learning: implications for typical and atypical behavioral development. J. Child Psychol. Psychiatry. 2017;58(7):770–778. doi: 10.1111/jcpp.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. Guilford; New York, NY: 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis; pp. 3–4. https://doi.org/978-1-60918-230-4. [Google Scholar]
- Herrmann D., Guadagno M.A. Memory performance and socio-economic status. Appl. Cogn. Psychol. 1997;11(2):113–120. [Google Scholar]
- Hutchinson J.B., Turk-Browne N.B. Memory-guided attention: control from multiple memory systems. Trends Cogn. Sci. 2012;16(12):576–579. doi: 10.1016/j.tics.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy A.S., Rex C.S., Chen Y., Dubé C., Maras P.M., Grigoriadis D.E. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci. 2010;30(39):13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.B., Riis J.L., Noble K.G. State of the art review: poverty and the developing brain. Pediatrics. 2016;137(4):1–17. doi: 10.1542/peds.2015-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishiyama M.M., Thomas Boyce W., Jimenez A.M., Perry L.M., Knight R.T. Socioeconomic disparities affect prefrontal function in children. J. Cogn. Neurosci. 2009;21(6):1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- Kutner M.H., Nachtsheim C.J., Neter J., Li W. 2005. Applied Linear Statistical Models. [Google Scholar]
- Lambert H.K., Peverill M., Sambrook K.A., Rosen M.L., Sheridan M.A., McLaughlin K.A. Altered development of hippocampus-dependent associative learning following early-life adversity. Dev. Cogn. Neurosci. 2019 doi: 10.1016/j.dcn.2019.100666. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert H.K., Sheridan M.A., Sambrook K.A., Rosen M.L., Askren M.K., McLaughlin K.A. Hippocampal contribution to context encoding across development is disrupted following early-life adversity. J. Neurosci. 2017;37(7) doi: 10.1523/JNEUROSCI.2618-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson K.R., Parrinello R., Ruff H.A. Maternal behavior and infant attention. Infant Behav. Dev. 1992;15:209–229. [Google Scholar]
- Lipina S.J., Martelli M.I., Vuelta B., Colombo J.A. Performance on the A-not-B task of argentinean infants from unsatisfied and satisfied basic needs homes. Interamerican J. Psychol. 2005;39(1):49–60. [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Mackey A.P., Finn A.S., Leonard J.A., Jacoby-Senghor D.S., West M.R., Gabrieli C.F.O., Gabrieli J.D.E. Neuroanatomical correlates of the income-achievement gap. Psychol. Sci. 2015;26(6):925–933. doi: 10.1177/0956797615572233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant J., Amso D. The development of selective attention orienting is an agent of change in learning and memory efficacy. Infancy. 2016;21(2):154–176. doi: 10.1111/infa.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S.E., Cole D.A. Bias in cross-sectional analyses of longitudinal mediation. Psychol. Methods. 2007;12(1):23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Gold A.L., Duys A., Lambert H.K., Peverill M. Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology. 2016;41(8):1956–1964. doi: 10.1038/npp.2015.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin Katie A., Sheridan M.A., Nelson C.A. Neglect as a violation of species-expectant experience: neurodevelopmental consequences. Biol. Psychiatry. 2017;82(7):462–471. doi: 10.1016/j.biopsych.2017.02.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Winter W., Fox N.A., Zeanah C.H., Nelson C.A. Widespread reductions in cortical thickness following severe early-life deprivation: a neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2014;76(8):629–638. doi: 10.1016/j.biopsych.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz E.C., Harlé K.M., Noble K.G., Mccall R.B. Executive function in previously institutionalized children. Child Dev. Perspect. 2016;10(2):105–110. doi: 10.1111/cdep.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzacappa E. Alerting, orienting, and executive attention: developmental properties and sociodemographic correlates in an epidemiological sample of young, urban children author (s): enrico Mezzacappa published by: wiley on behalf of the society for research in. Child Dev. 2004;75(5):1373–1386. doi: 10.1111/j.1467-8624.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Houston S.M., Brito N.H., Bartsch H., Kan E., Kuperman J.M. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 2015;18(5):773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., McCandliss B.D., Farah M.J. Socioeconomic gradients predict individual differences in neurocognitive abilities. Dev. Sci. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble K.G., Norman M.F., Farah M.J. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev. Sci. 2005;8(1):74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Nussenbaum K., Scerif G., Nobre A.C. Differential effects of salient visual events on memory-guided attention in adults and children. Child Dev. 2019;90(4):1369–1388. doi: 10.1111/cdev.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce . 2017. The Self-Sufficiency Standard for Washington State. [Google Scholar]
- Piccolo L.R., Merz E.C., He X., Sowell E.R., Noble K.G. Age-related differences in cortical thickness vary by socioeconomic status. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0162511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebanek D.J., Sloutsky V.M. Costs of selective attention: when children notice what adults miss. Psychol. Sci. 2017;28(6):723–732. doi: 10.1177/0956797617693005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M., Cohen Y. Attention and Performance X: Control of Language Processes. 1984. Components of visual orienting. [Google Scholar]
- Posner M.I., Snyder C., Davidson B. Attention and the Detection of Signals. J. Exp. Psychol. Gen. 1980;109(2):160–174. [PubMed] [Google Scholar]
- Razza R.A., Martin A., Brooks-Gunn J. Associations among family environment, sustained attention, and school readiness for low-income children. Dev. Psychol. 2010;46(6):1528–1542. doi: 10.1037/a0020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M.L., Amso D., McLaughlin K.A. The role of the ventral visual stream in scaffolding prefrontal cortex development: a novel mechanism linking socioeconomic status and executive function. Dev. Cognit. Neurosci. 2019;39 doi: 10.1016/j.dcn.2019.100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M.L., Hagen M.P., Lurie Lucy, A Miles, Zoe E., Sheridan Margaret A., Meltzoff Andrew N., McLaughlin K.A. Cognitive stimulation as a mechanism linking socioeconomic status with executive function: a longitudinal investigation of the gap in academic achievement. Child Dev. 2019 doi: 10.1111/cdev.13315. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M.L., Stern C.E., Michalka S.W., Devaney K.J., Somers D.C. Cognitive control network contributions to memory-guided visual attention. Cereb. Cortex. 2016;26(5) doi: 10.1093/cercor/bhv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M.L., Sheridan M.A., Sambrook K.A., Meltzoff A.N., McLaughlin K.A. Socioeconomic disparities in academic achievement: a multi-modal investigation of neural mechanisms in children and adolescents. NeuroImage. 2018;173:298–310. doi: 10.1016/j.neuroimage.2018.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M.L., Stern C.E., Devaney K.J., Somers D.C. Cortical and subcortical contributions to long-term memory-guided visuospatial attention. Cereb. Cortex. 2018 doi: 10.1093/cercor/bhx172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank F.A., Mather N., McGrew K.S. Test review: woodcock-johnson IV tests of achievement. J. Psychoeduc. Assess. 2015;33(4):391–398. [Google Scholar]
- Sheather S.J. 2009. A Modern Approach to Regression with R. [Google Scholar]
- Sheridan M.A., How J., Araujo M., Schamberg M.A., Nelson C.A. What are the links between maternal social status, hippocampal function, and HPA axis function in children? Dev. Sci. 2013;16(5):665–675. doi: 10.1111/desc.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan M.A., Peverill M., Finn A.S., Mclaughlin K.A., Psychopathol Author manuscript, D Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning HHS Public Access Author manuscript. Dev. Psychopathol. 2017;29(5):1777–1794. doi: 10.1017/S0954579417001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirin S.R. Socioeconomic status and academic achievement : a meta-analytic review of a meta-analytic review of research. Rev. Educ. Res. 2005;75(3):417–453. [Google Scholar]
- Slopen N., McLaughlin K.A., Fox N.A., Zeanah C.H., Nelson C.A. Alterations in neural processing and psychopathology in children raised in institutions. Arch. Gen. Psychiatry. 2012;69(10):1022–1030. doi: 10.1001/archgenpsychiatry.2012.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L.R. Memory systems of the brain: a brief history and current perspective. Neurobiol. Learn. Mem. 2004;82(3):171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Stevens C., Lauinger B., Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an event-related brain potential study. Dev. Sci. 2009;12(4):634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield J.J., Lepsien J., Gitelman D.R., Mesulam M.M., Nobre A.C. Orienting attention based on long-term memory experience. Neuron. 2006;49(6):905–916. doi: 10.1016/j.neuron.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Anderson C.M., Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. 2012;109(9):E563–572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uy J.P., Goldenberg D., Tashjian S.M., Do K.T., Galván A. Physical home environment is associated with prefrontal cortical thickness in adolescents. Dev. Sci. 2019:e12834. doi: 10.1111/desc.12834. [DOI] [PubMed] [Google Scholar]
- Weiss S.M., Meltzoff A.N., Marshall P.J. Neural measures of anticipatory bodily attention in children: relations with executive function. Dev. Cogn. Neurosci. 2018;34:148–158. doi: 10.1016/j.dcn.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Seo Y., Kim J., Lee I. Hippocamp us is required for paired associate memory with neither delay nor trial uniqueness. Learn. Mem. 2012;19:1–8. doi: 10.1101/lm.024554.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.