Abstract
The Achilles tendon is frequently injured. Data to support specific treatment strategies for complete and partial tears is inconclusive. Regardless of treatment, patients risk re-rupture and typically have long-term functional deficits. We previously showed that pulsed electromagnetic field (PEMF) therapy improved tendon-to-bone healing in a rat rotator cuff model. This study investigated the effects of PEMF on rat ankle function and Achilles tendon properties after (i) complete Achilles tendon tear and repair with immobilization, (ii) partial Achilles tendon tear without repair and with immobilization, and (iii) partial Achilles tendon tear without repair and without immobilization. We hypothesized that PEMF would improve tendon properties, increase collagen organization, and improve joint function, regardless of injury type. After surgical injury, animals were assigned to a treatment group: (a) no treatment control, (b) one hour of PEMF per day, or (c) three hours of PEMF per day. Animals were euthanized at 1, 3, and 6 weeks post-injury. Joint mechanics and gait analysis were assessed over time, and fatigue testing and histology were performed at each time point. Results indicate no clear differences in Achilles healing with PEMF treatment. Some decreases in tendon mechanical properties and ankle function suggest PEMF may be detrimental after complete tear. Some early improvements were seen with PEMF after partial tear with immobilization; however, immobilization was found to be a confounding factor. This body of work emphasizes the distinct effects of PEMF on tendon-to-bone healing and supports trialing potential treatment strategies pre-clinically across tendons before applying them clinically.
Keywords: animal model, orthopaedics, immobilization, gait analysis
INTRODUCTION
The Achilles tendon, while the strongest and largest tendon in the body, is frequently injured, with injury severity ranging from tendinopathic microtrauma to full-width, full-thickness ruptures.1 Due to its role connecting the soleus and gastrocnemius muscles to the calcaneus, the Achilles tendon is critical for ankle and knee flexion as well as foot stabilization.2 Due to these important roles, Achilles tendon injury can cause significant disability, severely impacting daily activities and quality of life. Conclusive evidence to support a specific treatment paradigm for Achilles tendon tears is lacking.3 For full-thickness tears, controversy exists regarding surgical versus non-surgical repair, as well as regarding weight-bearing versus non-weight-bearing rehabilitation strategies and rehabilitation timing post-injury.4,5 Regardless of treatment strategy, patients risk re-rupture and typically have long-term deficits in function, with the rate of return to pre-injury level of activity reported to be as low as 16% after complete rupture.4,5 Treatment of partial-width Achilles tears is typically initially conservative and includes immobilization and rest, though this can be followed by surgical repair if conservative treatment fails.6 However, recent studies suggest that earlier active loading may be beneficial to healing, initiating collagen synthesis and organization.7
To improve Achilles tendon healing outcomes, various non-invasive therapeutics have been investigated, including ultrasound and laser therapy.8,9 One study suggests that pulsed electromagnetic field (PEMF) therapy improved tendon tensile strength after Achilles tendon rupture and repair in a rat model.10 Further, we have previously shown that pulsed electromagnetic field (PEMF) therapy improves tendon-to-bone healing in a rat rotator cuff model over a range of electromagnetic pulse frequencies and treatment durations.11 This treatment strategy is utilized as a non-invasive, adjunctive therapeutic applied after standard-of-care surgical repair. Specifically, PEMF improved tendon modulus and stiffness and increased collagen protein expression and organization, regardless of tested frequency and duration of application.11 However, the effects of a PEMF signal (PhysioStim®, Orthofix Inc., Lewisville, TX; FDA approved for nonunion bone fracture) on ankle joint function and tendon fatigue properties after Achilles tendon injury have not yet been evaluated. We have adapted, utilized, and characterized the effects of three rat models of Achilles tendon injury: complete, full-thickness tear with surgical repair and post-operative immobilization; partial-width, full-thickness tear without repair and with post-operative immobilization; and partial-width, full-thickness tear without repair and without post-operative immobilization.12 These models reflect two different injury severities and two different rehabilitation strategies for partial Achilles tears and have distinct effects on tendon healing, which we have previously characterized.12 The objective of this study was to utilize these animal models to determine the effects of PEMF on Achilles tendon properties and ankle joint function after injury. We hypothesized that both 1 and 3 hours of daily PEMF exposure would improve tendon fatigue properties and in vivo joint function as well as increase collagen fiber organization, regardless of injury severity or immobilization.
METHODS
Study Design & Surgical Procedures
These studies were approved by the University of Pennsylvania Institutional Animal Care and Use Committee (#806038). Adult male Sprague-Dawley rats (400–450 g, approximately 4–5 months; n=432) were used; this age corresponds with slow to no skeletal growth, most closely correlated with a young adult human age range. The animals were housed in a conventional facility with 12-hour light-dark cycles and were fed standard rat chow ad libitum. Animals were divided into 3 injury groups: the first group (n=144; complete tear + immobilization, CT+IM) underwent unilateral full-thickness, blunt transection and modified Kessler repair of the right Achilles tendon followed by one week of limb immobilization.13 Immobilization was performed with the foot in full plantarflexion (160° tibiopedal angle). The second group (n=144; partial tear + immobilization, PT+IM) underwent unilateral full-thickness, partial-width transection of the right Achilles tendon (1.5 mm biopsy punch in central tendon which removes ~60% of tendon width) without repair followed by one week of limb immobilization.12 The third group (n=144; partial tear only, PT) underwent unilateral full-thickness, partial-width transection of the right Achilles tendon without repair and were allowed normal cage activity immediately post-injury. All injuries included resection of the central portion of the plantaris tendon to prevent internal splinting. All animals received a standard three day regimen of buprenorphine. Within each injury group, animals were further randomized into three treatment groups (n=48/treatment group/injury type). The first treatment group did not receive PEMF therapy (non-PEMF). The other treatment groups received either 1 hour of PEMF therapy (1h PEMF) or 3 hours of PEMF therapy (3h PEMF) every day, starting the day after surgery and continuing until the time of euthanasia. Animals were sacrificed at post-operative week 1, 3, or 6 (n=16/time point/treatment group/injury type). Time points were selected to represent timing of early, intermediate, and late rehabilitation, as both temporal improvements and structural changes occur throughout this time period after a complete tear and repair.14
PEMF Exposure
With the exclusion of non-PEMF and uninjured controls, animals received daily PEMF exposure until the time of sacrifice using the commercial PEMF signal PhysioStim® (Orthofix Inc., Lewisville, TX). The maximum amplitude and fundamental frequency of the PhysioStim PEMF signal are 1.19 mT and 3.85 kHz, respectively. The PhysioStim signal was chosen partly because it has received FDA approval for treatment of long-bone fracture nonunions.15,16 Three hours is the standard protocol for long-bone fracture nonunion applications15, and tendon-to-bone healing was improved in the rat rotator cuff model with both 1 and 3 hours of Physio-Stim.11 As detailed in a previous study17, 24 hours after surgery, the animals were placed on custom-built PEMF racks (Orthofix) with an electromagnetic coil for each module, sized to hold a standard rat cage, which was exposed with a uniform electromagnetic field across the volume of the cage.
In vivo Functional Evaluation
Animals in 6 week groups (n=16/treatment group/injury type) underwent longitudinal in vivo ambulatory and passive ankle joint mechanics assessments.13 Quantitative Ambulatory Assessment: Patterns of hindlimb ambulation were quantified using an instrumented walkway, consisting of two load/torque cells and a ventral view camera to provide non-invasive measures of limb function as described.19,20 Each animal was acclimated to the walkway prior to collecting formal measurements. Data were analyzed to calculate ground reaction forces (lateral, braking, propulsion, vertical) and spatiotemporal parameters (step width, stride length, velocity) of the injured limb. Data was collected pre-op for all groups, at 3 and 6 weeks after injury for CT+IM and PT+IM groups, and at 2, 4, and 6 weeks after injury for PT group. Animals were weighed at the time of each assessment, and ground reaction forces were normalized as percent animal body-weight at that time. Data were then normalized to average pre-op values for each group, which represented the uninjured control value for each metric. Ankle Range of Motion and Stiffness: Passive ankle joint range of motion (ROM) and stiffness through plantarflexion and dorsiflexion were quantified using a custom torque cell and orientation device18 on anesthetized animals with LabView software (National Instrument; Austin, TX). A total of two trials, consisting of three plantarflexion/dorsiflexion cycles each, were measured on each testing day. Functional ankle joint properties (toe and linear ankle stiffness; ankle ROM) were evaluated in a blinded fashion using customized MATLAB software.13,18 Data were collected at 2, 3, and 6 weeks after injury for CT+IM and PT+IM groups, and at 2, 4, and 6 weeks after injury for PT group. Pre-operative data was not collected due to an uninjured joint range of motion that was beyond the range of our device.
Mechanical Testing & μCT
Animals assigned to mechanical testing (n=10/time point/treatment group/injury type) were frozen at −20°C at the time of euthanasia and then thawed for dissection. The Achilles tendon-foot complex was removed en bloc and the tendon was fine-dissected under a stereomicroscope to remove any remaining muscle, non-tendinous connective tissue, and any residual plantaris tendon prior to tendon cross-sectional area measurement using a custom laser device.21 Mechanical testing was performed as described13,22 using load-controlled fatigue testing on an Electropuls E3000 testing frame (Instron; Norwood, MA). Specimens were loaded such that the foot and Achilles tendon were perpendicular to each other in order to approximate the physiological loading position, and a consistent gauge length was maintained for all specimens which contained the injury site regardless of injury type. Specimens remained submerged in a 37°C 1x phosphate-buffered saline bath throughout testing. Testing included preloading (0.1N), preconditioning (0.5–1.5% strain at 0.25 Hz for 30 cycles), stress relaxation (6% strain), dynamic frequency sweeps (0.125% strain amplitude, at 0.1, 1, 5, and 10Hz for 10 cycles each), a ramp at 2% strain/s to 35N (used to calculate quasistatic parameters), and fatigue cycling (5–35N at 2 Hz; which corresponds to 10–70% of ultimate failure load) until failure. During loading, force and displacement data were acquired using the WaveMatrix (Instron; Norwood, MA) data acquisition software and analyzed using custom MATLAB code.13,14,22,23 Images were acquired during testing to track strain optically. Fatigue parameters were calculated at 5, 50, and 95% of fatigue life (defined by corresponding percentage of cycles to failures) to describe the three phases of fatigue behavior.23 If tendons could not reliably withstand the initial ramp to begin fatigue testing, fatigue parameters were not calculated. Therefore, fatigue parameters are only reported at 6 weeks for CT+IM and PT+IM models, and at 3 and 6 weeks for the PT model. Post-test, tendons were scanned using μCT (Scanco Medical μCT 35; Wayne, PA) at 55 kVp with a 21μm resolution to assess for presence of heterotopic ossification (HO) at the repair site.
Histology
Achilles tendons were harvested at the time of euthanasia (n=6/time point/treatment group/injury type), processed with paraffin, sagitally sectioned through the middle of the injury at 7 μm, stained with hematoxylin & eosin, and analyzed as described13,14,17,24 including semi-quantitative, blinded grading for cell density and cell nuclear shape as a proxy for overall cell shape, and quantitative polarized light microscopy for collagen alignment within the injury region (n=2–3 ROIs at 200x magnification) per sample which were individually assessed and then averaged).25 Collagen alignment was not able to be measured at one week post-injury for the complete tear model due to lack of organized collagen in granulation tissue.
Statistics
Statistical comparisons were made only within each injury type, comparing each of the PEMF treated groups (1 hour PEMF, 3 hour PEMF) to the non-PEMF control group at each time point, based on our hypothesis. Statistical tests were run using GraphPad Prism 5 (GraphPad Software; San Diego, CA). Comparisons for functional assessments were assessed for each injury type longitudinally with two-way ANOVAs. Mechanics, collagen fiber organization, and μCT metrics were assessed using one way ANOVAs. ANOVAs resulting in significant relationships (α ≤ 0.05) were further analyzed with post-hoc two-tailed Student’s t-tests with Bonferroni corrections for our planned comparisons (p<0.025). Semi-quantitative histological comparisons were made using non-parametric Kruskal-Wallis tests at each time point.
RESULTS
Complete Tear Model With Immobilization
After complete Achilles tear and repair, PEMF treatment altered animal gait patterns. This includes increased operative limb loading rate (Fig 1A) and decreased stance time (total time foot is in contact with floor per gait cycle, Fig 1B) at 3 and 6 weeks post-injury. These changes corresponded with increased walking speed in the 3 hour PEMF group at both time points and in the 1 hour PEMF group at 6 weeks (Fig 1C,D). Operative limb stride length was decreased in the 1 hour PEMF group at 3 weeks and increased in the 3 hour PEMF group at 6 weeks (Fig 1E). No changes were seen in stride length for the contralateral limb compared to controls (Fig 1F). Passive ankle joint motion testing demonstrated that total joint range of motion was increased for 1 hour PEMF at all post-injury time points compared to control (Fig 2A). This group also had increased joint stiffness in plantarflexion at 2 and 3 weeks post-injury compared to control (Fig 2B), but decreased stiffness in dorsiflexion at 2 weeks (Fig 2C).
Figure 1. Complete tear with immobilization functional properties: Gait analysis.
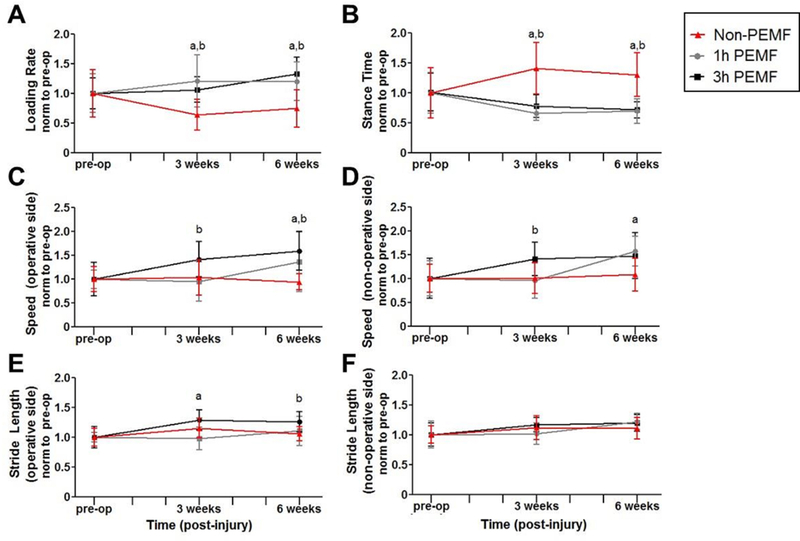
PEMF treatment after complete Achilles tear and subsequent immobilization (A) increased operative limb loading rate and (B) decreased stance time. These changes corresponded with increased walking speed of both the (C) operative limb and (D) contralateral limb in the 3 hour PEMF group at both time points and in the 1 hour PEMF group at 6 weeks. PEMF also altered (E) operative limb stride length; no changes were seen in (F) stride length for the contralateral limb compared to controls. All data is normalized to pre-injury values. Data is presented as mean±SD at each time point. (a) denotes significant difference between non-PEMF and 1 hr PEMF (p<0.025); (b) denotes significant difference between non-PEMF and 3 hrs PEMF (p<0.025).
Figure 2. Complete tear with immobilization functional properties: Passive joint motion.
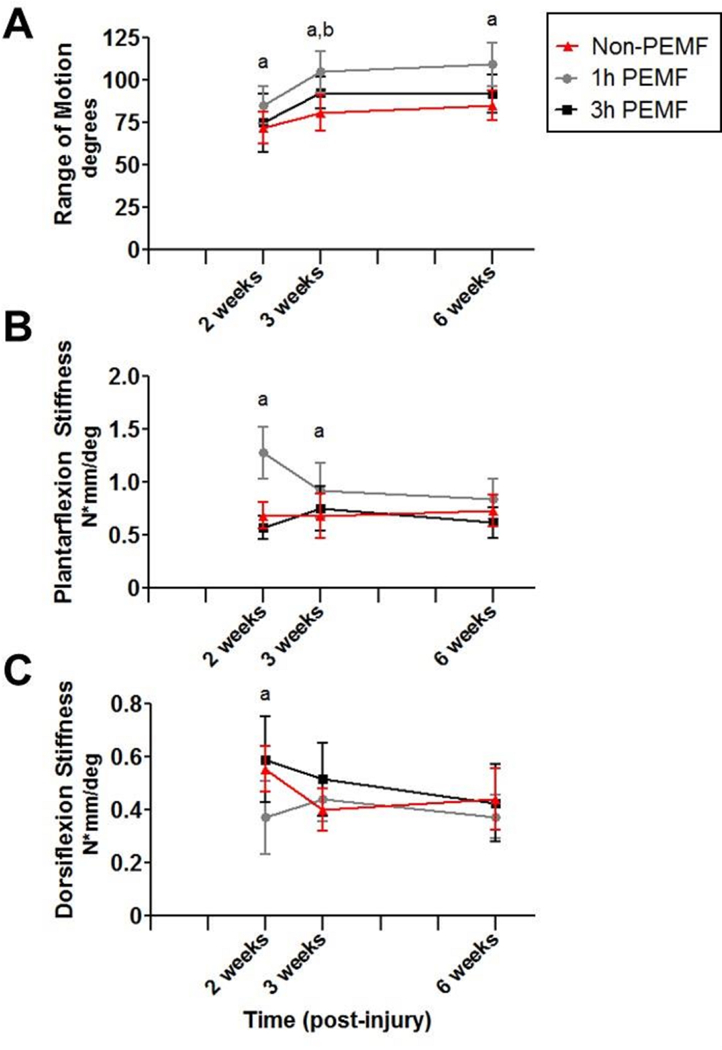
One hour of PEMF treatment (A) increased total joint range of motion at all post-injury time points, (B) increased joint stiffness in plantarflexion at 2 and 3 weeks post-injury compared to controls, and (C) decreased stiffness in dorsiflexion at 2 weeks. Data is presented as mean±SD at each time point. (a) denotes significant difference between non-PEMF and 1 hr PEMF (p<0.025); (b) denotes significant difference between non-PEMF and 3 hrs PEMF (p<0.025).
Tendon mechanical properties were also altered with PEMF treatment after complete tear and repair with immobilization. At 1 week, cross sectional area was larger for the 3 hour PEMF group (Fig 3A). Stiffness was decreased at 1 and 3 weeks for 1 hour PEMF, and at 3 weeks for 3 hour PEMF (Fig 3B). Tissue modulus was decreased at 1 week for both treatment groups compared to controls (Fig 3C). Six weeks post-injury, tanδ, a measure of force dissipation, was improved (decreased) for the 3 hour PEMF group across tested frequencies (Fig 3D). However, dynamic modulus was decreased for both PEMF groups at 3 weeks for a range of tested frequencies (Fig 3E). Fatigue testing of tendons 6 weeks post-injury showed no differences in cycles to failure, initial peak strain, secant stiffness, tangent stiffness, or fatigue modulus (data not shown). However, hysteresis was increased for the 1 hour PEMF group across all phases of fatigue and for the 3 hour PEMF group at 50% fatigue life (Fig 3F). Due to tendon fragility at 1 and 3 weeks post-injury, many samples failed prior to load-controlled cyclic fatigue testing; at 1 week, loading data demonstrated that tendons in the 1 hour PEMF group failed at significantly lower loads than controls (Fig 3G). Note that at 1 week post-op, only quasistatic parameters were measured. At 3 weeks, only quasistatic and viscoelastic parameters were reported due to the majority of samples failing during the initial ramp to 35N (first cycle of fatigue testing).
Figure 3. Complete tear with immobilization mechanical properties.
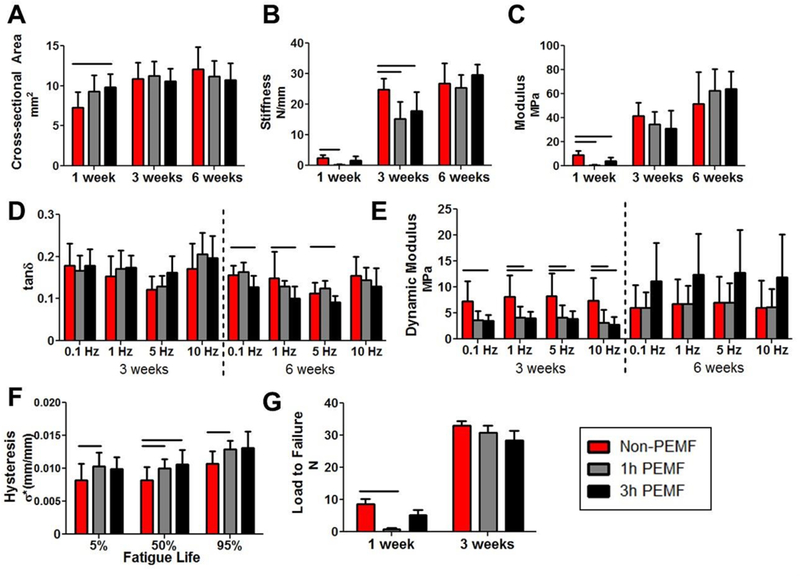
Tendon mechanical properties were altered with PEMF treatment. (A) Cross sectional area was larger for the 3 hour PEMF group at 1 week. (B) Stiffness was decreased at 1 and 3 weeks for 1 hour PEMF, and at 3 weeks for 3 hour PEMF. (C) Tissue modulus was decreased at 1 week for both treatment groups compared to controls. (D) Tanδ was decreased for the 3 hour PEMF group across tested frequencies, but (E) dynamic modulus was decreased for both PEMF groups at 3 weeks. (F) Hysteresis was increased for the 1 hour PEMF group across all phases of fatigue and for the 3 hour PEMF group at 50% fatigue life. (G) At 1 week, tendons in the 1 hour PEMF group failed at significantly lower loads than controls. Note that at 1 week post-op, only quasistatic parameters were measured. At 3 weeks, only quasistatic and viscoelastic parameters were reported due to the majority of samples failing prior to cyclic fatigue loading. Data is presented as mean+SD. Solid bars indicate significant differences (p<0.025).
Representative images of the injury site 1 week post-injury for each group are shown in Figure 4A. At this time point, semi-quantitative grading indicated increases in cellularity for the 1 hour PEMF group (Fig 4B), and a more rounded cell shape in both PEMF groups compared to controls (Fig 4C). No differences were seen in collagen alignment (Fig 4D). μCT imaging showed no differences between groups with regards to volume or mineral density of heterotopic ossification at any time point (Fig 4E,F).
Figure 4. Complete tear with immobilization histological and μCT properties.
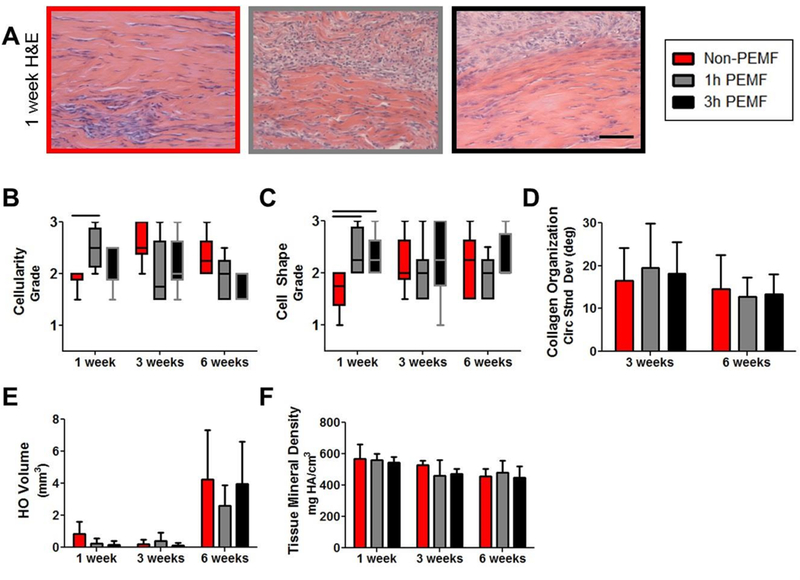
(A) Representative images of the injury site 1 week post-injury. (B) Cellularity was increased for the 1 hour PEMF group at 1 week post-injury, and (C) cells were more rounded in both PEMF groups compared to controls. (D) No differences were seen in collagen alignment. μCT imaging showed no differences between groups with regards to (E) volume or (F) mineral density of heterotopic ossification at any time point. Data in B and C presented as median ± IQR with minimum and maximum whiskers. Data in D-F presented as mean + SD. Scale bar in A: 100 um. Solid bars indicate significant differences (p<0.025).
Partial Tear Model With Immobilization
Effects of PEMF on gait metrics after a partial Achilles tear followed by one week of immobilization were few. Similar to the complete tear model, the 1 hour of PEMF group showed increased loading rate and decreased stance time 3 weeks post-injury (Fig 5A,B); while speed of the operative limb was not significantly altered (Fig 5C), changes in loading rate and stance time did correspond with increased speed of the non-operative limb (Fig 5D). There were no differences in stride length between treatment and control groups (Fig 5E,F). After 2 weeks, the 3 hours of PEMF group demonstrated decreased joint range of motion compared to controls, and at 6 weeks, this metric was decreased for both PEMF groups (Fig 6A). However, there were no significant differences in joint stiffness in either plantarflexion or dorsiflexion (Fig 6B,C).
Figure 5. Partial tear with immobilization functional properties: Gait analysis.
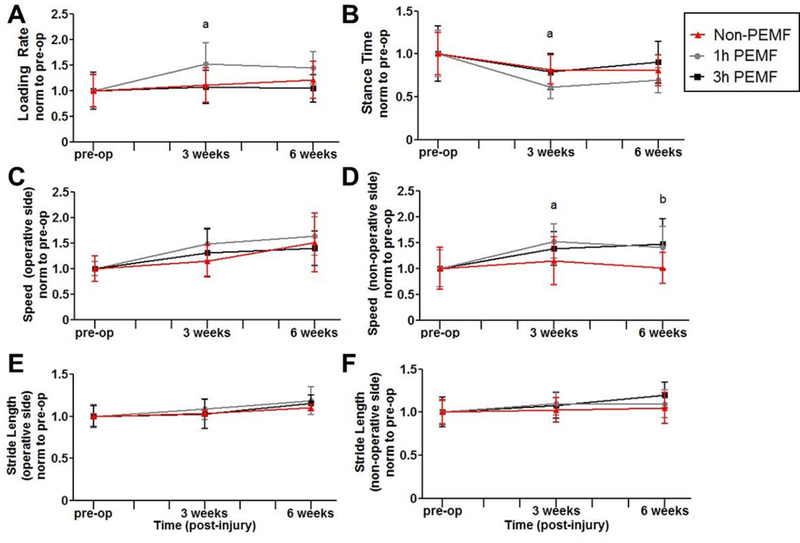
The 1 hour of PEMF group showed (A) increased loading rate and (B) decreased stance time 3 weeks post-injury. (C) Speed of the operative limb was not significantly altered, but (D) non-operative limb speed was increased for the 1 hour PEMF group at 3 weeks and for the 3 hours of PEMF group at 6 weeks. There were no differences in (E) stride length on the operative side or (F) the non-operative side between treatment and control groups. All data is normalized to pre-injury values. Data is presented as mean±SD at each time point. (a) denotes significant difference between non-PEMF and 1 hr Physio-Stim PEMF (p<0.025); (b) denotes significant difference between non-PEMF and 3 hrs Physio-Stim PEMF (p<0.025).
Figure 6. Partial tear with immobilization functional properties: Passive joint motion.
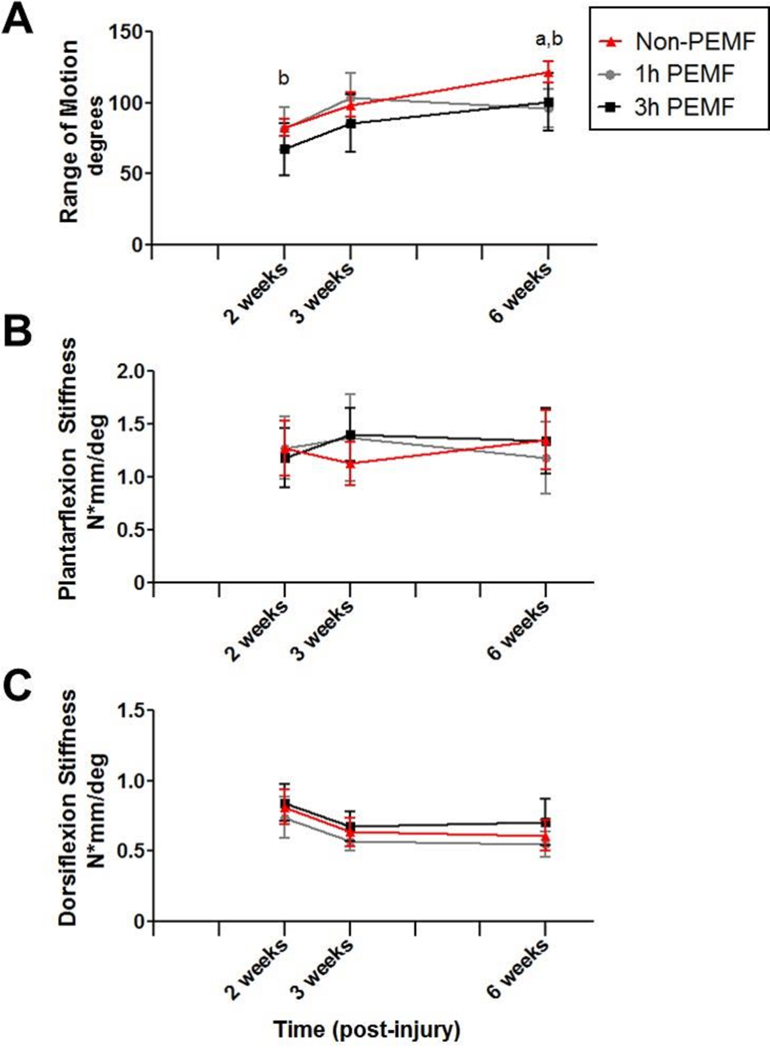
The 3 hours of PEMF group demonstrated (A) decreased joint range of motion at 2 weeks compared to controls, and at 6 weeks, this metric was decreased for both PEMF groups. However, there were no significant differences in (B) joint stiffness in either plantarflexion or (C) dorsiflexion. Data is presented as mean±SD at each time point. (a) denotes significant difference between non-PEMF and 1 hr Physio-Stim PEMF (p<0.025); (b) denotes significant difference between non-PEMF and 3 hrs Physio-Stim PEMF (p<0.025).
There were no differences in tendon cross sectional area with treatment (Fig 7A). At 1 week post-injury, tendons in the 3 hours of PEMF group exhibited increased stiffness (Fig 7B) and modulus (Fig 7C) compared to the non-PEMF tendons. At 3 weeks post-injury, 1 hour of PEMF group also demonstrated increased modulus (Fig 7C). There were no differences in viscoelastic properties tested with frequency sweeps; likewise, fatigue testing of tendons showed no differences in initial peak strain, hysteresis, or fatigue modulus (data not shown). Cycles to failure increased for 3 hours of PEMF tendons at 1 week and 1 hour of PEMF tendons at 3 weeks (Fig 7D). Secant stiffness (not shown) and tangent stiffness (Fig 7E) were also increased at 1 week for the 3 hours of PEMF group throughout fatigue cycling.
Figure 7. Partial tear with immobilization mechanical properties.
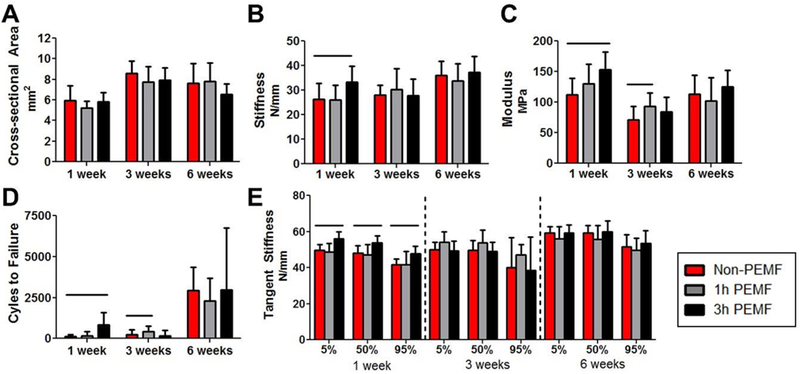
(A) PEMF treatment did not alter cross-sectional area. (B) Stiffness was increased for the 3 hours of PEMF group at 1 week. (C) PEMF increased modulus for the 3 hour group at 1 week and the 1 hour group at 3 weeks, and the same pattern of improvements was seen for (D) cycles to failure. (E) Tangent stiffness was also increased at 1 week for the 3 hours of PEMF group throughout fatigue cycling. Data is presented as mean+SD. Solid bars indicate significant differences (p<0.025).
Representative images of the injury site 3 weeks post-injury for each group are shown in Figure 8A. At this time point, semi-quantitative grading indicated decreased cellularity for the 1 hour PEMF group (Fig 8B), but there were no changes in cell shape (Fig 8C) or collagen alignment (Fig 8D). μCT imaging showed no differences between groups in measured properties of heterotopic ossification at any time point (Fig 8E,F).
Figure 8. Partial tear with immobilization histological and μCT properties.
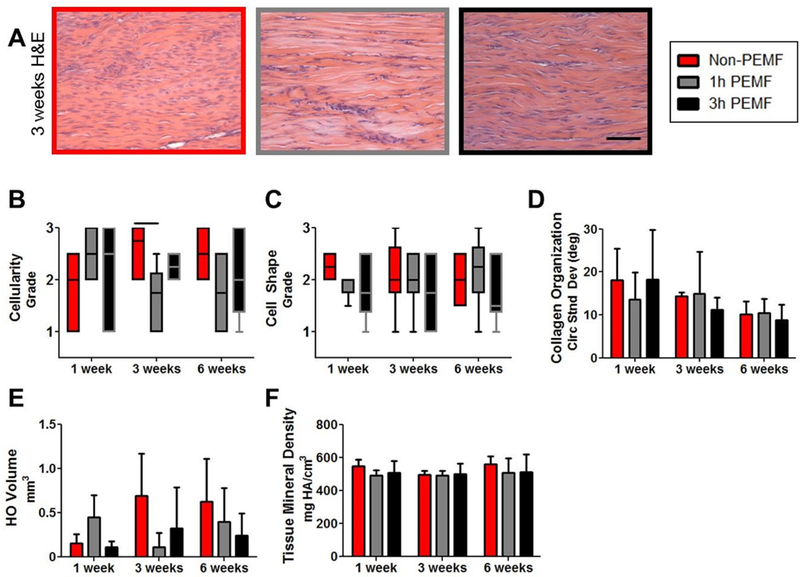
(A) Representative images of the injury site 3 weeks post-injury. (B) Cellularity decreased with 1 hour of PEMF at 3 weeks post-injury compared to controls. There were no changes in (C) cell shape or (D) collagen alignment. μCT imaging showed no differences between groups in (E) heterotopic ossification volume or (F) mineral density. Data in B and C presented as median ± IQR with minimum and maximum whiskers. Data in D-F presented as mean + SD. Scale bar in A: 100 um. Solid bars indicate significant differences (p<0.025).
Partial Tear Model Without Immobilization
There were limited effects of PEMF after a partial Achilles tear without post-injury immobilization. No differences were seen in loading rate or stance time during gait analysis (Fig 9A,B). Animals receiving three hours of PEMF moved their operative limb more quickly than control animals at 2 and 4 weeks post-injury (Fig 9C) but this was not seen on the non-operative side (Fig 9D). There were no differences in stride length (Fig 9E,F). Similarly, no differences were identified in any passive ankle joint metric (Fig 10) or mechanical property (Fig 11).
Figure 9. Partial tear without immobilization functional properties: Gait analysis.
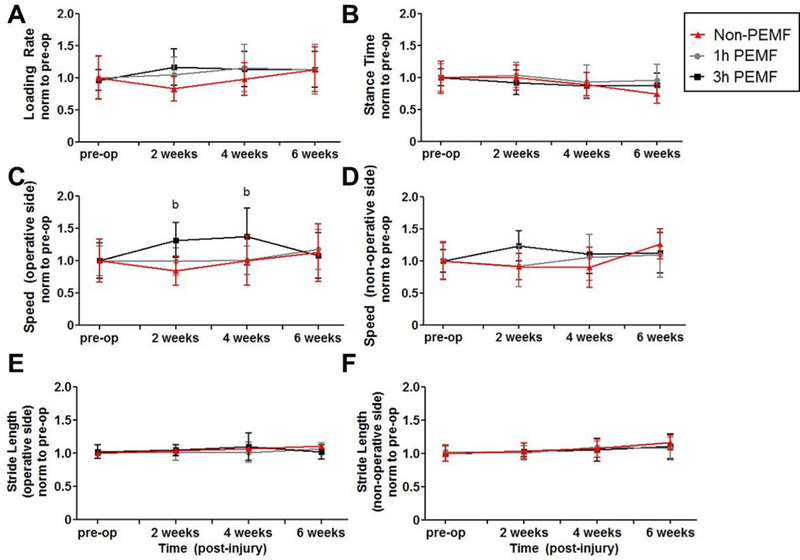
No differences were seen in (A) loading rate or (B) stance time during gait analysis. Animals receiving three hours of PEMF (C) walked more quickly on the operative side than control animals at 2 and 4 weeks post-injury, though no differences were seen (D) on the non-operative side or in (E) operative side stride length or (F) contralateral side stride length. All data is normalized to pre-injury values. Data is presented as mean±SD at each time point. (a) denotes significant difference between non-PEMF and 1 hr Physio-Stim PEMF (p<0.025); (b) denotes significant difference between non-PEMF and 3 hrs Physio-Stim PEMF (p<0.025).
Figure 10. Partial tear without immobilization functional properties: Passive joint motion.
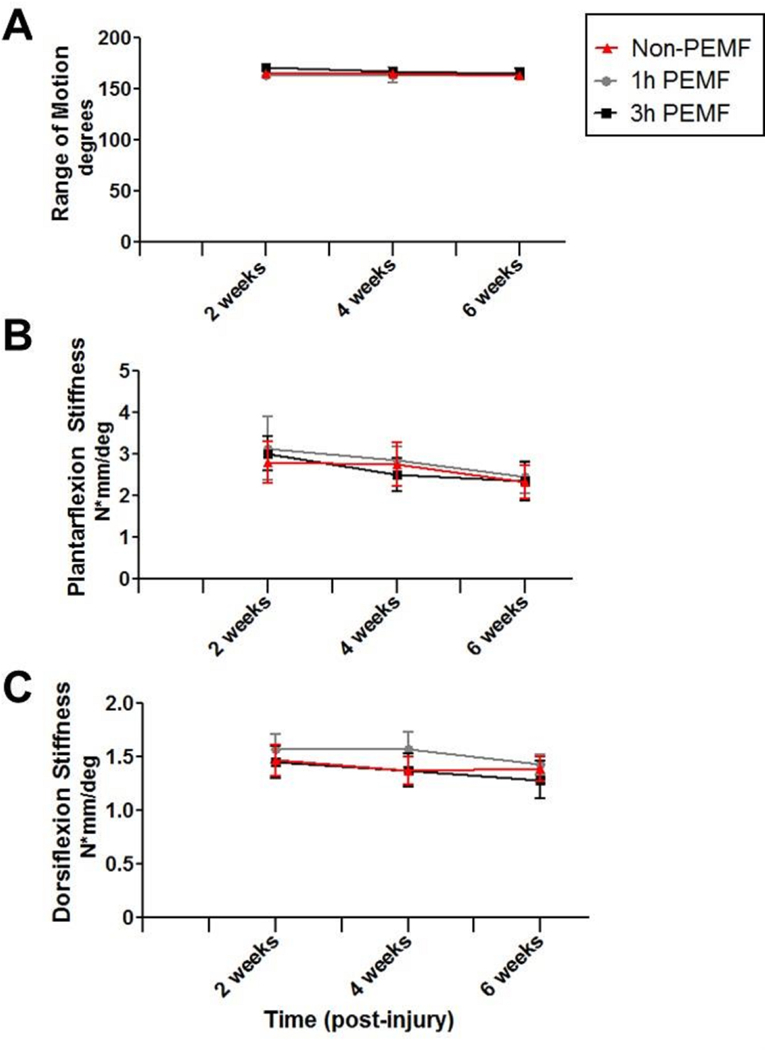
PEMF did not affect (A) range of motion, (B) plantarflexion stiffness, or (C) dorsiflexion stiffness. Data is presented as mean±SD at each time point.
Figure 11. Partial tear without immobilization mechanical properties.
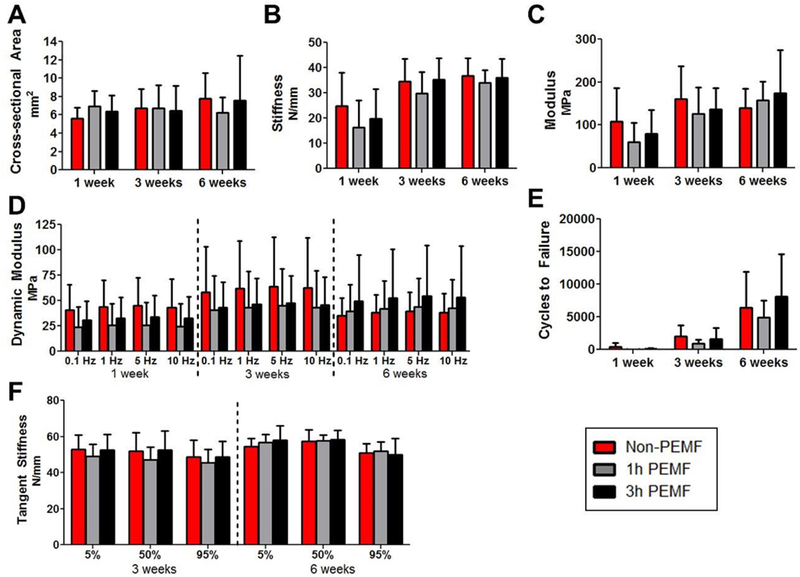
PEMF did not affect (A) tendon cross-sectional area, (B) stiffness, (C) modulus, (D) dynamic modulus, (E) cycles to failure, or (F) tangent stiffness. Data is presented as mean+SD.
Representative images of the injury site 3 weeks post-injury for each group are shown in Figure 12A. At this time point, semi-quantitative grading indicated decreased cellularity for the 3 hour PEMF group (Fig 12B), but no changes in cell shape (Fig 12C). Collagen alignment was also improved (decreased) for the 3 hour PEMF group at 6 weeks post-injury (Fig 12D). μCT imaging showed no differences between groups in heterotopic ossification volume or mineral density (Fig 12E,F).
Figure 12. Partial tear without immobilization histological and μCT properties.
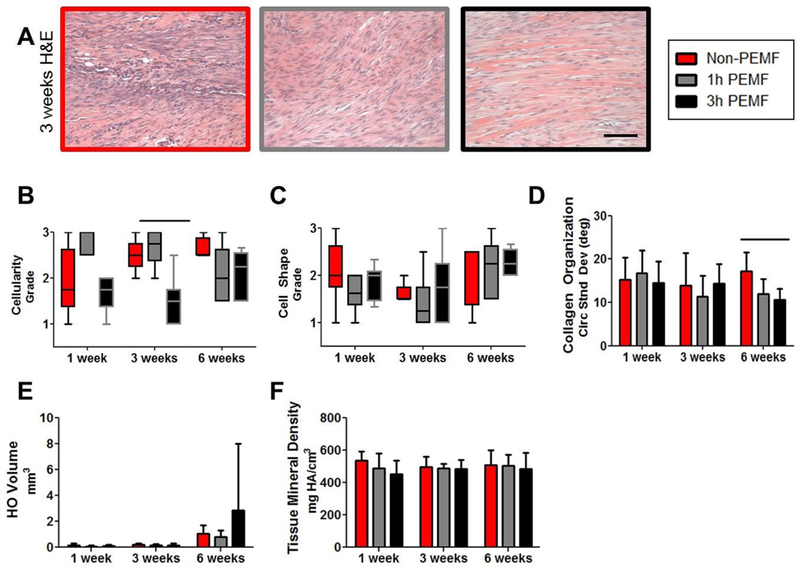
(A) Representative images of the injury site 3 weeks post-injury. (B) Cellularity was decreased at 3 weeks for the 3 hour PEMF group, but there were (C) no changes in cell shape. (D) Collagen alignment was also improved (decreased) for the 3 hour PEMF group at 6 weeks post-injury. μCT imaging showed no differences between groups in (E) heterotopic ossification volume (F) or mineral density. Data in B and C presented as median ± IQR with minimum and maximum whiskers. Data in D-F presented as mean + SD. Scale bar in A: 100 um. Solid bars indicate significant differences (p<0.025).
DISCUSSION
Although our recent pre-clinical studies support the use of non-invasive PEMF therapy to improve early tendon-to-bone rotator cuff repair11,17, the potential effect of PEMF on a soft-tissue tendon injury has not previously been investigated. We used three separate rat models of Achilles injury and rehabilitation in this series of studies to evaluate the effects of PEMF on ankle joint function, tendon mechanics, tendon histology, and formation of heterotopic ossification temporally after Achilles injury.
Overall, there were no clear, distinct effects of PEMF therapy on Achilles tendon healing. In the complete tear model with immobilization, although many parameters did not indicate differences between treatment groups, a few results suggest that PEMF had a detrimental effect on rat Achilles tendon healing for both treatment durations tested in this study. Tendon stiffness and modulus were significantly decreased and tendons failed at lower loads at early time points with PEMF treatment. Viscoelastic and fatigue properties at later time points were also decreased in PEMF groups compared to controls. While increased cell infiltration is a normal response to injury and is typically considered beneficial for long-term healing26, the increased cell density in PEMF-treated tendons compared to non-PEMF controls at early post-injury time points may be impacting tissue mechanical integrity. Increased joint range of motion in the 1 hour PEMF group may be related, at least in part, to a less stiff Achilles tendon with increased hysteresis seen in the same group.27 Additionally, changes in loading rate and stance time of the injured limb indicate that PEMF-treated animals are altering ambulation patterns, which may be due to change in function or pain.19 Together, these results indicate that PEMF may be detrimental to the healing of complete Achilles tears after repair.
Fewer differences were noted in the model utilizing partial tear with post-injury immobilization. 1 hour PEMF-treated animals had altered gait patterns at 3 weeks post-injury, and all PEMF-treated joints exhibited decreased range of motion 6 weeks post-injury. While it is difficult to correlate these findings with specific mechanical properties, 3 hour PEMF tendons demonstrated improved mechanics at 1 week post-injury and 1 hour PEMF tendons demonstrated improved mechanics at 3 weeks post-injury. Cell density was decreased for the 1 hour PEMF group at 3 weeks, suggesting that there is more load-bearing matrix to support improvements in mechanical properties. Ultimately, these changes suggest that PEMF treatment may aide in early tendon healing following partial width, full thickness Achilles injury. However, immobilization for the first week is a confounding factor for tendon healing in this model. From 1 to 3 weeks, tendon cross-sectional area increased, without an increase in structural properties. Therefore, material properties actually decreased between 1 and 3 weeks post-injury, coinciding with the removal of the cast, which occurred after 1 week; animals in the 1 week group were euthanized with their casts on, so no loading occurred in these groups. The unexpected decreases over time in material properties suggest that other properties may be impacted by immobilization in this model12, and make interpretation of the effects of a therapeutic variable difficult.
These results led to our hypothesis that, in the absence of immobilization, PEMF treatment would result in improved healing in the partial tear model compared to control tendons. However, in this model, no differences were observed in joint function or mechanical testing outcome measures generally associated with tendon function and healing. Ultimately, it appears that PEMF treatment does not improve tendon healing in this partial width, full thickness injury model without immobilization. Although there were some improvements in histological properties, these changes did not correlate to improved joint or tendon functional properties. Overall, it is possible that this specific injury model is too conservative to measure potential therapeutic effects in the context of rapid baseline healing in these otherwise healthy Sprague-Dawley rats.12
Contrary to the consistent improvements seen in rotator cuff healing11,17, PEMF therapy does not have a clear effect on Achilles tendon healing. The healing environments in these two injury settings are notably different. First, the supraspinatus tendon forms a portion of a synovial joint capsule, below bony anatomy. Rotator cuff tears do not undergo spontaneous healing.28 Achilles tendons are extrasynovial and are located in a more superficial location, allowing for fibrous tissue formation in response to injury.28 Additionally, supraspinatus tears occur most often at the tendon-to-bone insertion, a complex region of tissues with a gradient of compositional and mechanical properties that is not restored after surgical repair.29 Distinct from this, Achilles tendons primarily tear in the tendon midsubstance, instead requiring only tendon tissue regeneration.1 These differences highlight the importance in assessing tendon-healing therapeutic approaches in multiple models of tendon injury at different sites, as effects will likely differ based on the specific mechanisms targeted by a therapeutic.30
While not different between control and treatment groups for any model at any time point, the observed bone formation in these Achilles tendon injury models increased over time.12 It should be noted that heterotopic bone formation has been observed clinically as well.31 Although PEMF is known to have positive effects on osteoblast stimulation32 and bone formation rates33, this is the first study to measure properties of Achilles heterotopic ossification formation in the context of PEMF treatment. Based on our data, PEMF does not appear to play a role in the initiation or maturation of post-injury heterotopic ossification.
This study was not without limitations. First, our partial tear model does require open surgery to create the injury, therefore not mimicking the clinical condition. Additionally, the study lacks biological data to help explain the potential detrimental effects of PEMF on mechanical properties after complete tear. Further, FDA approved PEMF treatments are applied locally with anatomically specific devices. In this study, PEMF was delivered systemically. Future studies could assess the effects of localized PEMF exposure.
Future studies will also evaluate specific biological mechanisms involved in tendon-to-bone rotator cuff healing that are altered by PEMF treatment. Additional studies could evaluate effects of PEMF therapy for tendinitis or tendinopathy, or on other intrasynovial soft tissue-bone interface applications such as ACL reconstruction.
In conclusion, use of non-invasive PEMF therapy to accelerate tendon healing after Achilles tendon injury did not prove advantageous in the models studied.
ACKNOWLEDGEMENTS
This study was funded by Orthofix, Inc. and the Penn Center for Musculoskeletal Disorders (P30 AR069619). JH was supported by a Ruth L. Kirschstein National Research Service Award (5T32AR053461) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Orthofix Inc. employs co-authors Erik Waldorff, Nianli Zhang, and James Ryaby. They each own stock in Orthofix Inc. We thank Stephanie Weiss, Dr. Daniel Gittings, Dr. Adnan Cheema, and Dr. Mengcun Chen for help with surgeries.
Grant Support: This study was supported by NIH (P30 AR069619) and Orthofix, Inc. JH was supported by a Ruth L. Kirschstein National Research Service Award (5T32AR053461) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Study approved by: University of Pennsylvania IACUC (#806038)
REFERENCES
- 1.Egger AC, Berkowitz MJ. 2017. Achilles tendon injuries. Curr Rev Musculoskelet Med. 10:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doral MN, Alam M, Bozkurt M, et al. 2010. Functional anatomy of the Achilles tendon. Knee Surg Sports Traumatol Arthrosc. 18:638–43. [DOI] [PubMed] [Google Scholar]
- 3.Ochen Y, Beks RB, Mark vH, et al. 2019. Operative treatment versus nonoperative treatment of Achilles tendon ruptures: systematic review and meta-analysis. BMJ 7:k5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barfod KW, Bencke J, Lauridsen HB, et al. 2014. Nonoperative dynamic treatment of acute achilles tendon rupture: the influence of early weight-bearing on clinical outcome: a blinded, randomized controlled trial. J Bone Joint Surg Am. 96:1497–503. [DOI] [PubMed] [Google Scholar]
- 5.Olsson N, Nilsson-Helander K, Karlsson J, et al. 2011. Major functional deficits persist 2 years after acute Achilles tendon rupture. Knee Surg Sports Traumatol Arthrosc. 19:1385–93. [DOI] [PubMed] [Google Scholar]
- 6.Allenmark C. 1992. Partial Achilles tendon tears. Clin Sports Med. 11: 759–69. [PubMed] [Google Scholar]
- 7.Valkering KP, Aufwerber S, Ranuccio F, et al. 2017. Functional weight-bearing mobilization after Achilles tendon rupture enhances early healing response: a single-blinded randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 25:1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng CO, Ng GY, See EK, Leung MC. 2003Therapeutic ultrasound improves strength of Achilles tendon repair in rats. Ultrasound Med Biol. 29:1501–6. [DOI] [PubMed] [Google Scholar]
- 9.Joensen J, Gjerdet NR, Hummelsund S, Iversen V, Lopes-Martins RA, Bjordal JM. 2012. An experimental study of low-level laser therapy in rat Achilles tendon injury. Lasers Med Sci. 27:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strauch B, Patel MK, Rosen DJ, et al. 2006. Pulsed magnetic field therapy increases tensile strength in a rat Achilles’ tendon repair model. J Hand Surg Am. 31:1131–5. [DOI] [PubMed] [Google Scholar]
- 11.Huegel J, Choi DS, Nuss CA, et al. 2018. Effects of pulsed electromagnetic field therapy at different frequencies and durations on rotator cuff tendon-to-bone healing in a rat model. J Shoulder Elbow Surg. 27:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huegel J, Boorman-Padgett JF, Nuss CA, et al. 2019. Quantitative comparison of three rat models of Achilles tendon injury: a multidisciplinary approach. J Biomech. 88:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman BR, Gordon JA, Bhatt PR, et al. 2016. Nonsurgical Treatment and Early Return to Activity Leads to Improved Achilles Tendon Fatigue Mechanics and Functional Outcomes During early Healing in an Animal Model. J Orthop Res. 34:2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman BR, Salka NS, Morris TR, et al. 2017. Temporal Healing of Achilles Tendons After Injury in Rodents Depends on Surgical Treatment and Activity. J Am Acad Orthop Surg. 25:635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garland DE, Moses B, Salyer W. 1991. Long-term follow-up of fracture nonunions treated with PEMFs. Contemp Orthop. 22:295–302. [PubMed] [Google Scholar]
- 16.US Food and Drug Administration. Premarket Approval (PMA) P850007. Silver Spring (MD): US Department of Health & Human Services; 1986. <https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P850007>, accessed February 19, 2019.
- 17.Tucker JJ, Cirone JM, Morris TR, et al. 2017. Pulsed electromagnetic field therapy improves tendon-to-bone healing in a rat rotator cuff repair model. J Orthop Res. 35:902–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peltz CD, Dourte LM, Kuntz AF, et al. 2009The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 91:2421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caro AC, Tucker JJ, Yannascoli SM, et al. 2014. Efficacy of various analgesics on shoulder function and rotator cuff tendon-to-bone healing in a rat (Rattus norvegicus) model. J Am Assoc Lab Anim Sci. 53:185–92. [PMC free article] [PubMed] [Google Scholar]
- 20.Sarver JJ, Dishowitz MI, Kim SY, Soslowsky LJ. 2010. Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model. J Biomech. 43:778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peltz CD, Perry SM, Getz CL, Soslowsky LJ. 2009. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J Orthop Res. 27:416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillin CD, Fryhofer GW, Freedman BR, et al. 2019. Effects of Immobilization Angle on Tendon Healing after Achilles Rupture in a Rat Model. J Orthop Res. 37:562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman BR, Sarver JJ, Buckley MR, et al. 2013. Biomechanical and structural response of healing Achilles tendon to fatigue loading following acute injury. J Biomech. 47:2028–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. 2003. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 21: 413–9. [DOI] [PubMed] [Google Scholar]
- 25.Gimbel JA, Van Kleunen JP, Mehta S, et al. 2004. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 37:739–49. [DOI] [PubMed] [Google Scholar]
- 26.Kajikawa Y, Morihara T, Watanabe N, et al. 2007. GFP chimeric models exhibited a biphasic pattern of mesenchymal cell invasion in tendon healing. J Cell Physiol. 210:684–91. [DOI] [PubMed] [Google Scholar]
- 27.Jung HJ, Fisher MB, Woo SL. 2009. Role of biomechanics in the understanding of normal, injured, and healing ligaments and tendons. Sports Med Arthrosc Rehabil Ther Technol. 1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomopoulos S, Parks WC, Rifkin DB, Derwin KA. 2015. Mechanisms of tendon injury and repair. J Orthop Res 33:832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. 1998. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg 7:599–605. [DOI] [PubMed] [Google Scholar]
- 30.Paredes JJ, Andarawis-Puri N. 2016. Therapeutics for tendon regeneration: a multidisciplinary review of tendon research for improved healing. Ann N Y Acad Sci 1383:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards PJ, Braid JC, Carmont MR, Maffulli N. 2008. Achilles tendon ossification: pathology, imaging and aetiology. Disabil Rehabil. 30:1651–65. [DOI] [PubMed] [Google Scholar]
- 32.Sollazzo V, Palmieri A, Pezzetti F, Massari L, Carinci F. 2010. Effects of pulsed electromagnetic fields on human osteoblastlike cells (MG-63): a pilot study. Clin Orthop Relat Res 468:2260–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chalidis B, Sachinis N, Assiotis A, Maccauro G. 2011. Stimulation of bone formation and fracture healing with pulsed electromagnetic fields: biologic responses and clinical implications. Int J Immunopathol Pharmacol 24(1 Suppl 2):17–20. [DOI] [PubMed] [Google Scholar]


