Abstract
Children’s consumer products represent an important exposure source for many toxicants. Chemicals of high concern, as designated by the Washington State Child Safe Product Act include phthalates, Bisphenol A (BPA) and parabens, among others. As regulation and reporting requirements increase, so has demand for safer alternatives. This project examines how predictive toxicology and exposure comparison tools can fill gaps in alternatives assessments for hazardous chemicals found in children’s products. Phthalates, parabens, BPA and their alternatives were assessed for endocrine disruption and reproductive toxicity using authoritative lists and US Environmental Protection Agency’s (EPA) predictive toxicology and exposure comparison tools. Resources included the European Chemical Agency’s Endocrine Disruptor Substances of Concern database, Global Harmonization System and Classification of Labeling Chemicals, Quantitative Structural Activity Relationships from the Toxicity Estimation Software Tool, the Toxicological Prioritization Index (ToxPi) score calculated from the ToxCast Database, and No Observable Adverse Effects Levels (NOAELs)/Highest No Effects Levels (HNEL) from animal studies found in the CompTox Chemistry Dashboard. Exposure was assessed using ExpoCast predictions. Though alternatives were rarely included in authoritative lists, predictive toxicology tools suggested that BPA alternatives may not be safer but paraben and phthalate alternatives may be safer. All four paraben and no bisphenol or phthalate alternatives were listed on EPA’s Safer Chemical Ingredients List. Overall, we found that predictive toxicology tools help fill gaps for alternatives assessments when existing classifications are incomplete.
Keywords: Children’s Health, Consumer Products, Alternatives Assessment, Endocrine Disruption
Introduction
One of the principles of green chemistry is to design safer chemicals considering potential health and ecological hazards from creation through disposal1. Safer chemical substitutions are often identified by alternatives assessments. Guidance or review documents on alternatives assessments have been released by the National Academy of Sciences (NAS)2, Interstate Chemical Clearinghouse3, the U.S. Environmental Protection Agency (EPA)4 and the Organisation for Economic Co-operation and Development5. Like many guidance frameworks, the NAS begins with the identification of hazardous chemicals and then screens for whether alternatives are available. If alternatives are available, they are screened for chemical and physical properties, human health and ecological hazards. Most human health assessments follow the hazard identification step in the traditional risk assessment framework6. The NAS suggests the inclusion of in vitro data to fill data gaps when human and animal data are not available. This call was reiterated in Tickner et al. 2018, with a push to advance the use of predictive toxicology tools for filling data gaps in alternatives assessments7.
In addition to exposure comparison, consideration of function, analysis of economic feasibility and lifecycle assessment, alternatives analyses require the full hazard assessment of at least two chemicals, increasing the likelihood of data gaps, especially for newer chemicals. Lack of data is not an indication of safety and approaches to bridge data gaps are highly important for alternatives assessments. Regrettable substitutions, such as the replacement of methylene chloride with n-hexane in brake fluid can be counter-productive to the overall goals of green chemistry and alternatives assessments.
This paper focuses on three chemical groups of high concern to children with members among the 85 chemicals designated by the Washington State Children’s Safe Product Act (CSPA): Bisphenol A (BPA), Phthalates and Parabens. BPA is used as a coating for plastic consumer products, such as water bottles, food storage containers and electronics. While BPA has not been banned in the United States, the Food and Drug Administration (FDA) has restricted BPA concentrations in baby bottles, sippy cups and some food packaging due to its endocrine disrupting capability and reproductive and developmental toxicity8. Low dose exposures can be particularly damaging during sensitive periods of development9. Despite the lack of more extensive regulation from the Consumer Product Safety Commission and the FDA, consumer concern has driven the development of BPA-free products. Many of these products contain bisphenol-S (BPS) as a replacement compound. While some assessments have found BPS to be safe in children’s products10, others find that BPS has similar toxicological attributes as BPA11,12.
Phthalates are plasticizers found in a wide array of consumer products. Concerns over exposures to phthalates in consumer products have led to regulations at the state and federal level in the United States. The US Consumer Product Safety Improvement Act (CPSIA) of 2008 limits the use of some hazardous chemicals, including six phthalates, lead and cadmium in children’s products13. Three phthalates; DEHP, DBP, and BBP concentrations are restricted to no more than 1000 ppm per individual phthalate in children’s toys and products designed to care for children under age three. DINP, DIOP and DnOP are restricted in concentrations greater than 1000 ppm per individual phthalate in children’s toys that can be placed in a child’s mouth and in products designed for care of children under age three. These and other state regulations, such as CSPA, passed in 2008 in Washington State, which limits the total concentration of phthalates in any child’s product to 1000 ppm14, are pushing industry to identify alternatives for phthalates. While the majority of regulations are focused on children’s products, some companies have voluntarily removed phthalates from their products. Apple removed phthalates from cords and headphones in 2013 and Lowe’s Home Improvement and Home Depot quit selling vinyl flooring that contains phthalates in 201515. While some manufacturers are working to completely remove phthalates, others have begun using less toxic phthalates. For example, manufacturers are using DINP instead of DEHP because DINP has a lower toxicity and a higher molecular weight, making it less likely to migrate out of plastics15. Phthalate alternatives are less publicized than the alternatives to BPA however, the Lowell Center for Sustainable Production at the University of Massachusetts developed a technical briefing to identify these compounds16.
While there have been no limits on parabens in children’s consumer products, there is a consumer-led initiative for paraben free products. A recent guide for pediatricians regarding endocrine disrupting chemicals suggested avoiding personal care products that contained parabens because in vitro studies have found estrogenic and anti-androgenic potential17. Parabens are incorporated into consumer products as preservatives. Thus, it is important to determine which preservatives are replacing parabens and whether these compounds are less toxic.
This case study of BPA, parabens and phthalates will help to determine whether predictive tools (in vitro and in silico models) can fill data gaps in exposure and toxicity assessment of alternative and conventional chemicals found in children’s consumer products. We hypothesize that predictive exposure and toxicology tools will allow us to better characterize the hazard and potential exposures of alternative chemicals. As regulations of chemicals in children’s consumer products are becoming more common, it is important to identify sources of data for determining whether the alternative chemicals are in fact safer.
Methods:
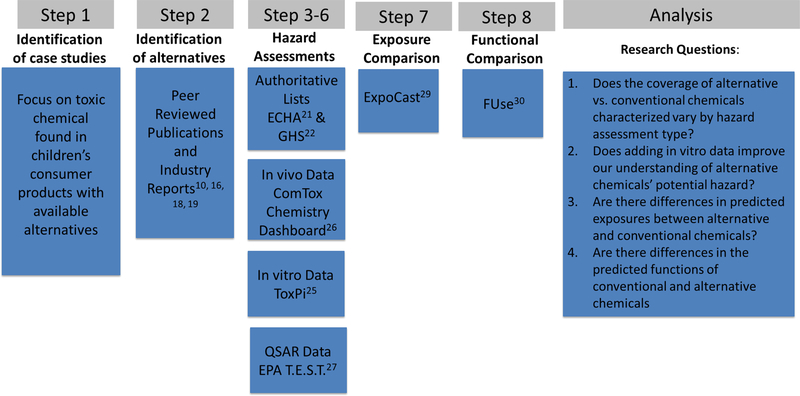
Three case studies were investigated: BPA, phthalates, parabens. For each of these case studies, the relevant substitution chemicals were identified and hazard was assessed for endocrine disruption and reproductive and developmental toxicity. Hazard was characterized using authoritative lists and predictive toxicology tools. Exposure comparisons were made using an in silico predictive exposure model. Figure 1 shows the integration of the various hazard and exposure assessments and data levels.
Figure 1:
The steps used to identify case studies, alternatives and integrate hazard assessments from multiple data levels.
Step 1 and 2: Identification of case studies and alternatives:
Case studies were identified based on the following criteria: 1. these groups (among others) are reported under CSPA in Washington State 2. there are children’s products marketed as being free of these chemicals and 3. there is evidence of toxicity. We conducted a review of reports and peer reviewed literature to identify publications containing alternatives to the case study chemical groups18,19,10,16. We used the search terminology: “chemical group” “alternatives” and “consumer products” in PubMed and Google Scholar to identify articles. We reviewed papers looking specifically for alternative chemicals already in use, not proposed or potential alternatives. This case study did not attempt to identify all potential alternatives to conventional chemicals. Instead, we focused on a narrow group of alternatives as a “proof of principle” for the use of in vitro and in silico tools for filling data gaps. Conventional and alternative compounds are provided in Table S1. The alternatives were compared with the EPA’s Safer Choice Program’s Safer Chemical Ingredients List (SCIL)20. This list is designed to help manufacturers choose safer chemicals for use in their products and ingredients. Chemicals on the list are grouped by function and classified according to criteria for human health, environmental toxicity and environmental fate. The classification is based on experimental and modeled data.
Step 3: Hazard Characterization- Authoritative lists
Authoritative lists are screening tools developed to aid regulatory agencies in chemical prioritization. As such, they are not exhaustive or comprehensive, yet they frequently serve as a starting place for toxicity assessment. Endocrine disruption and reproductive toxicity was based on the European Chemicals Agency (ECHA) Endocrine Disruptor Substances of Concern database21. This list is designed to prioritize chemicals for further assessment. Substances are categorized from 1–3. Category 1 includes known endocrine disruptors while categories 2 and 3 include suspected endocrine disruptors. We also included chemicals listed as reproductive toxicants in the Global Harmonization System (GHS)22. While there are differences in endocrine and reproductive toxicity mechanisms, the in vitro assays considered in this analysis have been used for both endocrine and reproductive toxicity assessment23. The GHS aims to internationally harmonize the classification and labeling of chemicals. Reproductive toxicity is categorized into three groups: 1A (known reproductive toxicity based on human data), 1B (presumed reproductive toxicity based on experimental animal data) and 2 (suspected reproductive toxicity based on human or animal evidence possibly with other information).
For data analysis purposes, if a chemical was included in either of these sources, it was considered to be present on an authoritative list. The categorizations from both of these lists are shown in Table S1.
Step 4: Hazard Characterization- in vitro
ToxCast is a high throughput toxicity screening program that uses 821 assay endpoints assessed using in vitro assays from human and animal cell lines as well as cell free assays. ToxPi is a prioritization support software tool24 that uses in vitro assay data from ToxCast, physicochemical properties, and toxicity pathways to identify potential endocrine disrupting chemicals by considering their responses across a variety of endocrine disrupting assays, including estrogen, androgen, thyroid, glucocorticoid and PPAR disruption25. Data from ToxCast is combined with the logarithm of the water octanol partition coefficient and the bioconcentration factor to develop a score25. ToxPi scores are normalized for cytotoxicity. Scores fall between zero and one with higher scores indicating higher endocrine disruption potential. ToxPi scores for chemicals and alternatives were accessed from those published in Filer et al. 201425 and reflect the prioritization based on 1858 chemicals. A full list of ToxPi Scores is shown in Table S1.
Step 5: Hazard Characterization- in vivo
The EPA’s CompTox Chemistry Dashboard (https://comptox.epa.gov/dashboard)26 was used to access animal data from ECHA and EPA sources including: COSmetics to Optimise Safety (COSMOS http://www.cosmostox.eu/what/databases/), ToxRefDb, Tox21, High Production Volume Information System (HPVIS), Integrated Risk Information System (IRIS), Hazard Evaluation Support System Integrated Platform (HESS) and others. The No Observable Adverse Effects Level (NOAEL) or Highest No Effects Level (HNEL) was used to assess the hazard from in vivo studies. The NOAEL or HNEL from the CompTox Chemistry Dashboard was used to compare the relative hazard between conventional and alternative chemicals. To be protective, the lowest NOAEL from a rodent study with oral exposure was used. Oral exposure was selected because it was the most common and relevant exposure route assessed, and as such it allowed for the most consistent comparison across chemicals. For some chemicals, only the Lowest Observable Adverse Effects Level (LOAEL) or Lowest Effects Level (LEL) was available. In this case, it was converted to a NOAEL by dividing by an uncertainty factor of 10. The studies used followed a repeat exposure framework, with some studies including prenatal or multigenerational dosing structures.
Step 6: Hazard Characterization- Quantitative Structural Activity Relationships
Characterization of unknown chemicals frequently begins with looking at the molecular structure and comparing its features with known toxicants. The EPA Toxicity Estimation Software Tool (T.E.S.T.) can characterize the toxicity of unknown compounds using Quantitative Structural Activity Relationships (QSAR)27. We used T.E.S.T. version 4.2 to predict the developmental toxicity of conventional and alternatives chemicals. T.E.S.T. uses 797 2-dimmensional molecular structure descriptors, such as the number of benzene rings to predict toxicity. We set T.E.S.T to run using the consensus model, which combines results from four models (singular, hierarchical, FDA and nearest neighbor) and has been shown to generate the best results for screening with higher sensitivity than specificity. The formulas for these models is available in the T.E.S.T. manual27. In this case, developmental toxicity was defined as a binary variable that includes any effect interfering with normal development before or after birth. The binary toxicity valued were developed by the CESAR Project28. The model is based on experimental data from the Teratogen Information System and the Food and Drug Administration and consists of 285 chemicals28.
Step 7: Comparative Exposure Prediction- in silico
Exposure comparison is an important step in alternatives assessment2. It can be challenging to find exposure data for both conventional and alternative chemicals. Prediction of exposure is important for chemicals in children’s consumer products as children have a high frequency of hand to mouth activity. The ExpoCast initiative by the EPA aims to create tools for screening the thousands of chemicals that are relevant for human exposure. Chemical use information and fate and transport models are used to estimate exposure. Chemical release information for ExpoCast is derived from production volumes found in the EPA Chemical Data Reporting Rule (previously called the Inventory Update Reporting Rule). From this information ExpoCast generates an estimated exposure for 1936 ToxCast chemicals with sufficient information. Exposure predictions in mg/kg/day from Wambaugh et al. 2014 for children age 6–1129 were used for this analysis and are shown in Table S1.
Step 8: Function Prediction- in silico
Because alternatives assessments aim to determine whether there is a safer alternative, it requires the assessor to consider functionality2. Phillips et al. 2017 developed quantitative structural use relationship (QSUR) models and the Functional Use (FUse) Database to classify the function of chemicals found in consumer products30. QSUR models can help relate physiochemical properties with how chemicals are used in consumer products. In this analysis we compared the functions of conventional and alternative chemicals. EPA’s FUse Database provides functional use predictions for approximately 6400 chemicals. The Fuse Database was accessed through the CompTox Chemistry Dashboard. The Harmonized Functional Use category with the highest predicted probability for use for each chemical was assessed to compare functional use between conventional and alternative chemicals.
Data Analysis:
ToxPi scores, NOAELs and ExpoCast exposure predictions were compared using the average of each chemical group and the standard error. QSAR data was binary and the percent of chemicals within each group that are predicted developmental toxicants was calculated using only chemicals found in the EPA T.E.S.T. database. Because this is a case study and some chemical groups only contained 1 chemical, it was not appropriate to do statistical analyses. In order to compare functional use, the average of the highest predicted probability for harmonized functional use category for each chemical group (alternative vs. conventional) was calculated. We used a ratio approach to determine how hypothetical changes in efficacy, and therefore exposure, might impact public health. For the example of parabens, we calculated the ratio of the ToxPi scores for conventional/alternative chemicals. If this ratio is over one, then the alternatives are less toxic. However, to achieve the same function as the conventional chemical, an alternative might be added at higher concentrations. We chose to only do this analysis for parabens, as the ToxPi Scores for alternative and conventional bisphenols and phthalates shared the same standard error range.
Results
Assessment of Coverage of Conventional and Alternative Chemicals:
Overall, conventional chemicals were more likely included in the list of endocrine disruptors published by ECHA and GHS than alternative chemicals for all three chemical groups (phthalates, parabens and bisphenols). Data from in vivo animal studies improved coverage for alternative chemicals for bisphenols and phthalates, but not for parabens. In vitro data improved coverage for all three chemical groups. Except for paraben alternatives, QSAR data had the greatest coverage of all data types. Table 1 shows the number of conventional chemicals and alternative chemicals found in authoritative lists, in vivo, in vitro and QSAR databases for each of the three chemical groups.
Table 1:
Number chemicals and percentage within each chemical group included in authoritative lists, animal in vivo databases, in vitro databases and QSAR tools for endocrine disruptions or reproductive and developmental toxicity.
| Conventional Chemicals Coverage | ||||
|---|---|---|---|---|
| Chemical Group | Lists (ECHA & GHS) % (n) | in vivo % (n) | In vitro % (n) | QSAR % (n) |
| Bisphenols (n=1) BPA | 100% (1) | 100% (1) | 100% (1) | 100% (1) |
| Parabens (n=4) Methyl, ethyl, propyl and butyl parabens | 50% (2) | 75% (3) | 100% (4) | 100% (4) |
| Phthalates (n=9) DnOP, DIDP, DINP, DnHP, DEHP, DEP, DBP, BBP | 89% (8) | 78% (7) | 67% (6) | 100(9) |
| Alternative Chemicals Coverage | ||||
| Bisphenols (n=2) BPS, BPF | 0 (0) | 100% (2) | 100% (2) | 100% (2) |
| Parabens (n=4) | 25% (1) | 100% (4) | 75% (3) | 50% (4) |
| Benzoic acid, Potassium sorbate, Sodium benzoate, Sorbic acid | ||||
| Phthalates (n=17) Aceyl tributyl citrate, Di-isononyl-cyclohexane-1, 2 dicarboxylate, dioctyl terephthalate, epoxidized soybean oil, alkylsubphonic phenyl ester, tri-2-ethylhexyl trimellitate, acetylated monoglycerides of fully hydrogenated caster oil, 4-benzenedicarboxylate, di-butyl adipate, butylated hydroxytolulene, hyper branched poly, di (2 ethylhexyl) phosphate, tri (2ethylhexyl) phosphate, o-tolulene sulfonamide, 2,2,4 trimethyl 1,3 pentanediol diisobutyrate, diocytl sebate, dibutyl sebate, di (2- ethyl hexyl adipate) |
18% (3) | 59% (10) | 82% (14) | 88% (15) |
The in vivo studies selected for this paper are described in Tables S1 and S2. While conventional chemical studies frequently focused on specific endpoints, such as endocrine disruption or reproductive and developmental toxicity, alternative chemicals were more likely to have non-specific endpoints. NOAELs and study references are shown in Table S1.
Prioritization Comparison
Authoritative Lists Hazard Characterization:
No alternative chemicals were classified as endocrine disruptors ECHA but three were classified as reproductive toxicants by GHS (Table 1, detailed classifications in Table S1). Benzoic Acid (paraben alternative) is classified as a suspected reproductive toxicant by GHS. Ethyl and butyl parabens are both considered endocrine disruptors by ECHA. Di-butyl adipate and butylated hydroxytolulene (phthalate alternatives) are both considered suspected reproductive toxicants by GHS. Eight out of nine of the phthalates considered in this paper were considered known or suspected reproductive toxicants by GHS as well as known or suspected endocrine disruptors by ECHA.
In vitro Hazard Characterization:
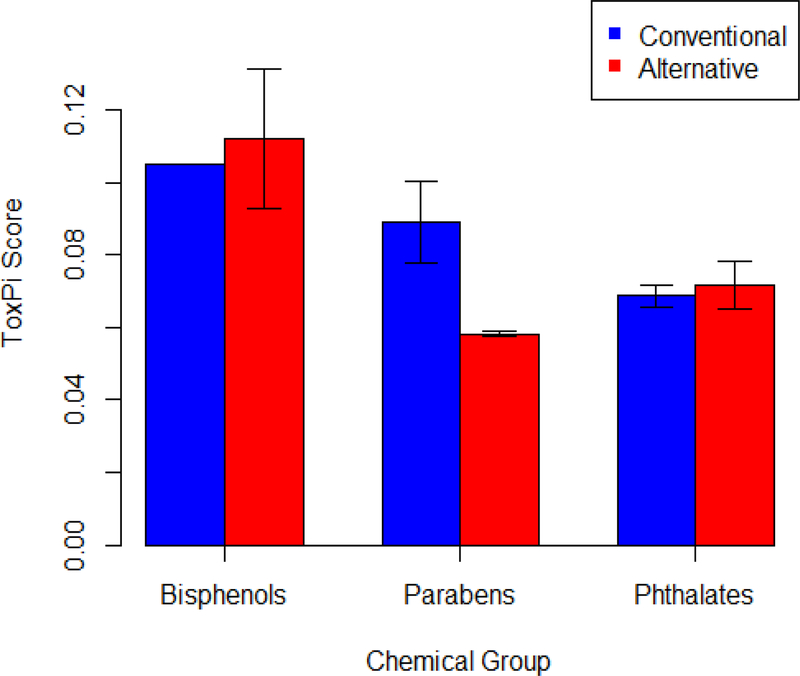
Figure 2 shows the average ToxPi score for conventional and alternative chemicals within each chemical group. For phthalates and bisphenols, the average ToxPi score for conventional chemicals was within the standard error range of the alternatives’ ToxPi score. For parabens, average conventional ToxPi score was above the standard error range of the alternatives’ ToxPi scores.
Figure 2:
Comparison of the average ToxPi score for conventional and alternative chemicals. Error bars represent standard error. Chemical groups without error bars had an N of 1.
In vivo Hazard Characterization:
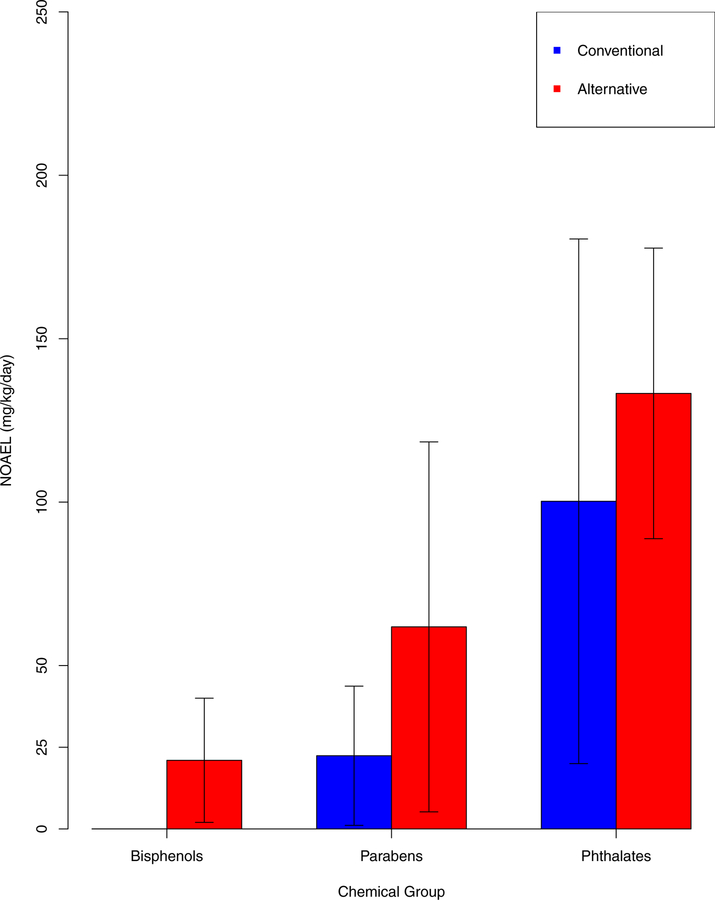
Animal studies from the CompTox Chemistry Dashboard were used to identify the NOAEL for each chemical (shown in Tables S1 and S2). Of the databases accessed through the CompTox Chemistry Dashboard the majority of the studies came from HPVIS, ECHA and COSMOS. The standard error ranges for NOAELs for phthalates and parabens and their alternative chemicals overlapped, suggesting little difference in toxicity (Figure 3). BPA’s NOAEL was lower than the standard error range of its alternatives’ NOAELs. However, the NOAEL for BPA was based on developmental neurotoxicity and similarly sensitive endpoints were not available for BPF and BPS. The NOAEL range for paraben and their alternatives were similar. The types of studies used to derive NOAELs are described in Table S2.
Figure 3:
Average NOAELs for conventional and alternative chemicals. Error Bars represent standard error. Chemical groups without error bars had an N of 1.
QSAR Hazard Characterization:
Conventional chemicals were more likely to be predicted as developmental toxicants. All conventional and alternative bisphenols were predicted as developmental toxicants. Seventy-five percent of conventional parabens and 50% of alternative parabens were predicted developmental toxicants. Conventional and alternative phthalates were predicted to be developmental toxicants at similar rates, 56% for conventional and 47% for alternative.
In silico exposure comparison:
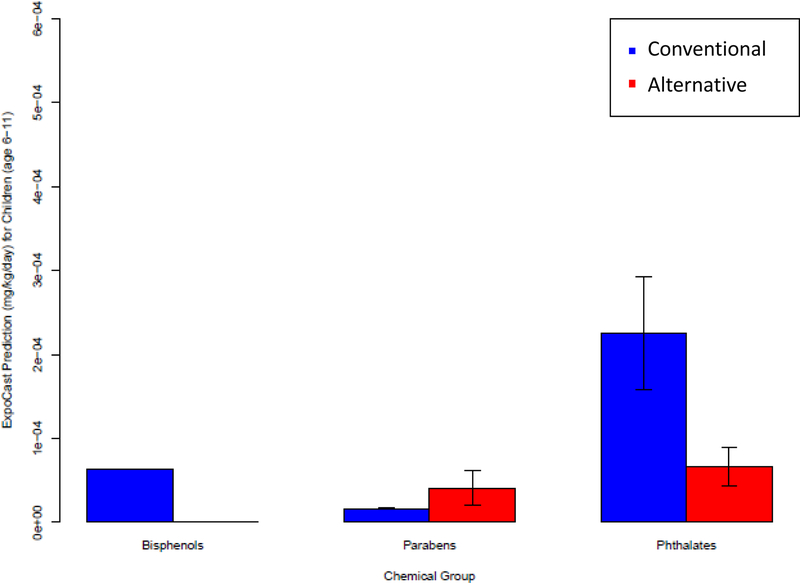
The median exposure predictions for children age six to eleven are shown in Figure 4 and listed in Table S1. The median was used in the comparison, however for most chemicals there is a wide predicted range. For example, BPA has a median exposure prediction of 0.629E-05 mg/kg/day while the 95th percentile is predicted to be 4.98 × 10−3mg/kg/day for children age 6–11. For bisphenols and phthalates, conventional chemicals had higher predicted exposure than alternative chemicals for children age 6–11 (Figure 4). Parabens alternatives had predicted exposure within the standard error range of conventional parabens, suggesting similar exposures (Figure 4).
Figure 4:
The average ExpoCast prediction for alternative and conventional chemicals for bisphenols, parabens and phthalates. Error Bars represent standard error. Chemical groups without error bars had an N of 1.
Functional Prediction:
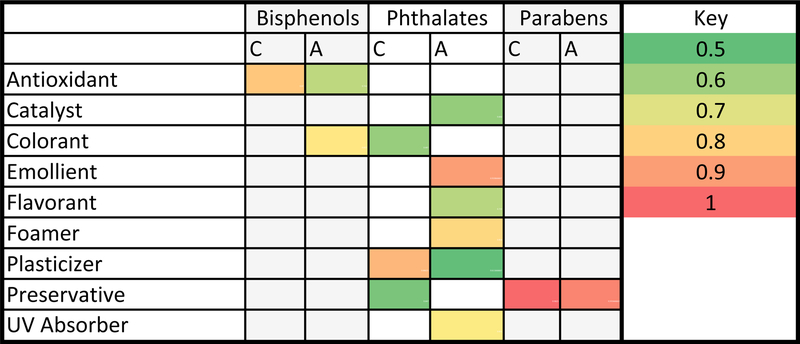
The harmonized functional use categories with the highest probabilities for alternative and conventional chemicals are shown in Figure 5. There was more diversity in the functional category with the highest predicted probability among alternative chemicals for bisphenols and phthalates. Parabens and their alternatives were both most likely to be found in preservatives.
Figure 5:
Harmonized Functional Use Categories from the Fuse Database accessed from the CompTox Chemistry Dashboard for Alternative (A) and Conventional (C) Chemicals. Colors represent the average of the highest predicted probabilities for each chemical group by functional use category. As is shown in the key, red indicates a higher probable function and green indicates a lower probable function.
Selecting Safer Alternatives:
The alternative chemicals identified for this case study were compared with SCIL. None of the phthalate or BPA alternatives were listed on the SCIL, but all four of the paraben alternatives were on SCIL. All the alternatives included in SCIL were classified as a “green circle” meaning the “chemical has been verified to be of low concern based on experimental and modeled data.” This allows us to put the toxicity results shown in Figures 2 and 3 in the context of recommended alternatives. For example, BPS and BPF, the primary BPA alternatives, are not listed as safer chemicals on SCIL and have been identified as potential regrettable substitutions. This is supported by multiple reports in the peer reviewed literature of BPS and BPF having similar toxicity to BPA11,12. The replacement of BPA with BPS and BPF is considered a regrettable substitution. BPF and BPS were not listed on any of the authoritative lists consulted. While the NOAEL for BPA was lower than the standard error ranges for NOAELs of BPA alternatives, the ToxPi scores suggested little difference in toxicity. The NOAEL for BPA is based on developmental neurotoxicity, a highly sensitive endpoint. Developmental neurotoxicity NOAELs were not available for BPF and BPS. The discrepancy in sensitivity of endpoints might account for the observed different in NOAELs between BPA and its alternatives. All four of the alternative paraben chemicals were listed on SCIL. While these chemicals were not included in authoritative lists, the average NOAEL was higher and the average ToxPi score was lower than for parabens, indicating safer options. The results from this analysis are congruous with SCIL.
When considering safer alternatives, it is important to determine whether a higher concentration of the alternative chemical may be needed to achieve the same efficacy. A simple approach to estimate how an increase in the concentration of alternative chemicals, relative to conventional chemicals might affect public health impact is to consider the ratio of the conventional and alternative toxicities. In this case study, we used the ToxPi scores for parabens and their alternatives. Because the ToxPi scores for conventional and alternative phthalates and bisphenols had overlapping standard error ranges, the ratio would be about one and is not informative. The ratio of conventional to alternative paraben ToxPi scores was 1.54 (Table 2). Even if the alternative is half as effective and the concentration has to be increased, it may still lead to a positive public health impact.
Table 2:
Integrating exposure potential and toxicity considerations: the ratio of the average conventional/alternative chemical ToxPi Scores.
| Compound | ToxPi Score | Group Average +/− Standard Deviation | Ratio of Conventional/Alternative Averages | |
|---|---|---|---|---|
| Conventional | Methyl Paraben | 0.061 | 0.089+/−0.023 | 1 |
| Ethyl Paraben | 0.080 | |||
| Propyl Paraben | 0.106 | |||
| Butyl Paraben | 0.110 | |||
| Alternative | Benzoic Acid | 0.057 | 0.058+/− 0.0012 | 1.54 |
| Sodium Benzoate | 0.060 | |||
| Sorbic Acid | 0.057 |
Discussion:
This work shows that in vitro, QSAR and in silico data sources can provide information about hazard and exposure for chemicals in children’s consumer products when authoritative lists are unavailable.
These data sources are particularly useful for conducting alternatives assessments as some alternatives may be newer chemicals with less information available. The need for data sources to complete toxicological and exposure comparisons in alternatives assessment was noted in the 2014 National Academy Report “A Framework to Guide Selection of Chemical Alternatives”2. This report suggested the inclusion of in vitro data to fill gaps where animal and human data were missing. This concept was elaborated on in a recent analysis of the role of predictive toxicology in alternatives assessment31. Malloy et al. 2017 reported that there are “significant gaps in toxicity information for the vast majority of chemicals and exposure pathways” and that this problem is exacerbated in alternatives analysis when often multiple chemicals need to be compared31. In addition, Malloy et al. 2017 made four recommendations for integrating and improving alternatives assessment using predictive toxicology. The recommendations included using case studies as examples, using predictive toxicology tools as screening methods, drawing on existing resources to integrate predictive toxicology in alternatives assessments and supporting interdisciplinary collaborations31. In this work, we use children’s consumer products as a case study for integrating predictive toxicology tools, such as in vitro and in silico data, in alternatives assessments. Jacobs et al. 2016 conducted an extensive review of existing alternatives assessment frameworks32 and found that exposure assessment was a critical area with missing information. By using existing data and publicly available resources for both exposure and toxicity33, we were able to show that predictive toxicology tools not only add to the data available for alternatives assessments, but also fill in key gaps when no animal or human studies are available.
This case study suggests that some alternative chemicals may still be hazardous to children’s health.
While few alternatives were found on authoritative lists, there was overlap in the toxicities of conventional and alternative chemicals using in vivo data, ToxPi and QSAR tools. The ToxPi Scores and QSAR predictions for alternative chemicals were similar to conventional chemicals for bisphenols. Phthalates and their alternatives had similar toxicities in vivo, ToxPi Scores and QSAR predictions. Parabens, had ToxPi scores, but similar in vivo toxicity. This suggests that these alternative chemicals may have similar endocrine disruption activities to the chemicals they are replacing, despite alternative chemicals being included in authoritative lists. Other studies have suggested that bisphenol A alternatives may not be safer12. The results of this case study rely on the identification of chemical alternatives as well as information about the associated exposure and toxicity. One limitation to this case study was the availability of alternatives in use. As more states and governing bodies begin to require toxic chemical reporting, it may be easier to identify alternatives. Having limited access to alternatives in use may impact the results of this case study. However, the additional toxicity, exposure and functional assessment information provides a proof of principle for the utility of predictive toxicology tools for alternatives assessment.
Case studies with alternatives listed on EPA’s Safer Chemical Ingredient List, had lower ToxPi Scores and higher NOAELs.
All four paraben alternatives were on the SCIL. The ToxPi scores and NOAELs reflect this with paraben alternatives showing lower ToxPi scores and higher NOAELs, relative to parabens. BPA and Phthalate alternatives were not found on the SCIL and did not show this trend in vitro. For parabens, the average ToxPi score was 0.89, compared with 0.58 for alternatives. We used the ratio of these two numbers to begin to account for the impact of changes in concentration or exposure between conventional and alternative chemicals. For example, if alternative chemicals are 65% less toxic, then we might tolerate an increase in concentration and exposure potential, while still protecting public health.
The need for data to support alternatives assessments, particularly for children’s consumer products will increase as more states are developing regulations and reporting frameworks.
Oregon, Maine, Minnesota and California are all in various stages of implementing laws similar to Washington, increasing industry’s interest in tools that support safer alternatives assessment, identification and evaluation of both hazard and exposure. One of the challenges to using alternatives assessments in decision-making is dealing with uncertainty in terms of missing data34. Resources are needed to not only address data gaps but also manage the uncertainty associated with incorporating new data streams. As demonstrated in this paper, predictive toxicology and comparative exposure tools can help fill data gaps. In vitro and in silico data, however, have different limitations and tools are needed to understand how to appropriately address these factors. Many State and federal agencies use GreenScreen® for chemical hazard assessment35. GreenScreen® is a chemical hazard assessment approach that provides users with guidance on integrating multiple data sources. While in vitro databases are included in the GreenScreen® lists of recommended resources, there is little guidance on how to interpret and include in vitro data. The methods used in this paper, provide an example of how in vitro data can be can help characterize uncertainty and be useful for managing data gaps in chemical hazard characterization for alternatives assessments.
This manuscript is the first case study to examine the utility of predictive toxicology and exposure comparison tools for alternatives assessments for chemicals in children’s products. While the applications and conclusions provide a useful platform for identifying future directions and data needs, there are some challenges. For example, information on new chemicals in children’s consumer products is hard to find. Additionally, it would be helpful to know how tightly the chemicals are bound to the children’s product. While most of the conventional chemicals, especially phthalates, have well-characterized metabolism pathways, information on alternative chemical metabolism in children would add to our assessments, as children can have differences in enzyme expression34. Because this study focused on endocrine disruption, chemicals with other mechanisms of toxicity might appear less toxic. It is important to assess all types of toxicity when considering an alternative because it would be regrettable to replace an endocrine disruptor with a carcinogen, for example. One of the benefits of ToxCast is availability of assay data for other endpoints, such as reproductive35 and developmental toxicity23 and neurotoxicity35. In vitro databases, such as ToxCast can help understand the range of molecular initiating events that are often not studied in a single in vivo study. Other factors that are important for prioritizing alternatives in children’s consumer products are listed in Table 3.
Table 3:
Information needed to assess toxicity and exposure of alternative chemicals in children’s consumer products
| Alternative Chemical Properties | Exposure- Product Properties | Toxicity Properties |
|---|---|---|
| What alternatives are currently being used? What is the toxicity of the alternatives (in vitro and in vivo)? What are the biokinetic properties of the alternatives? What are the physiochemical properties of the alternatives (QSAR)? Does this alternative have another use/multiple uses? How effective is the alternative? Can it be effective at a similar concentration range or is more needed? |
How tightly is the chemical bound to the product? Is there a difference in the bond between alternative and conventional chemicals? How is the product used and misused? How might product use and misuse change exposure potential? What is the intended age range for this product? What happens to the product after its use (fate and transport)? Does the chemical staybound to the product? Is the chemical stable? What is the concentration of the chemical in the product? What part of the product is the chemical found in? Is it accessible during normal use and misuse? |
What is the timing and dosing structure of in vitro toxicity assessments and how does it related to in vivo development? Are the acute and chronic effects different? How might effects be different during development? What is the specificity of the in vivo toxicity assessments conducted? Is the in vivo toxicity assessment focused on a specific and sensitive endpoint? What was the dosing structure of the in vivo studies? How specific are the in vitro molecular targets? Is the chemical cytotoxic across a wide range of cells or is the response specific? Do the alternative and conventional chemicals affect the safe biological endpoints? If the endpoints are different, is one less serious? Are there differences in the ADME? |
Definitions: Quantitative Structural Activity Relationships (QSAR), Absorption, Distribution, Metabolism and Excretion (ADME)
Conclusions:
This work highlights three case studies for examining the use of in vitro and in silico data for screening existing and alternative chemicals in children’s consumer products based on relative hazard and exposure potential. As more United States and other governments are passing legislation to require manufactures to phase out conventional chemicals of toxicological concern, it will be increasingly important to understand the safety of alternative chemicals.
In vitro and QSAR databases increase coverage of toxicological data for alternative chemicals (Table 1) that may soon be replacing toxic chemicals in children’s products relative to authoritative lists and reports.
In vitro data suggests that paraben alternatives may be safer options (Figures 2 and 3). All paraben alternatives were included in EPA’s Safer Chemical Ingredients List, while no BPA or phthalate alternatives were included. Phthalates, BPA and their alternatives had similar toxicities in vitro.
Exposure to conventional chemicals is currently predicted to be higher than alternatives for phthalates and Bisphenol A and similar for parabens (Figure 4).
While the predicted functions of phthalate and BPA alternatives were broader than the conventional chemicals, parabens and their alternatives had similar predicted functions. This suggests that paraben alternatives, in addition to being less hazardous, may also be more practical substitutes.
Supplementary Material
Acknowledgements:
This project is supported by the Environmental Protection Agency (FP-91779601–0, RD 83573801, RD 83451401) and the National Institute of Environmental Health Sciences (5P01ES009601). We would like to thank Kathie Dionisio for her guidance and Katherine Phillips for technical review of this manuscript. The views expressed in this paper are those of the authors and do not necessarily reflect the views of the U.S. EPA.
Footnotes
Conflict of Interest: The authors declare no conflict of interest
References
- 1.Anastas PT, Warner JC. Green Chemistry: Theory and Practice. Oxford University Press; 1998; New York. [Google Scholar]
- 2.The National Academy of Sciences Press. A Framework to Guide Selection of Chemical Alternatives. https://www.nap.edu/catalog/18872/a-framework-to-guide-selection-of-chemical-alternatives 2014. [PubMed]
- 3.Interstate Chemical Clearninghouse. IC2 Alternatives Assessment Guide http://www.theic2.org/alternatives_assessment_guide 2014.
- 4.United States Environmental Protection Agency. What are the Key Steps to Conducting an Alternatives Assessment. https://www.epa.gov/saferchoice/design-environment-alternatives-assessments.
- 5.Office of Economic Cooperation and Development. Current landscape of alternatives assessment practice: a meta-review. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2013)24&docLanguage=En 2014.
- 6.Faustman and Omenn. Risk Assessment In Klaassen CD, Casarett and Doull’s toxicology: the basic science of poisons. 9th Edition 2019, McGraw-Hill, New York [Google Scholar]
- 7.Tickner J, Jacobs M, Malloy T, Buck T, Stone A, Blake A et al. Advancing alternatives assessment for safer chemical substitution: A research and practice agenda. Integrated environmental assessment and management 2018. doi: 10.1002/ieam.4094. [DOI] [PubMed] [Google Scholar]
- 8.United States Food and Drug Administration, US. Food Additive Regulations Amended to No Longer Provide for the Use of BPA-Based Materials in Baby Bottles, Sippy Cups, and Infant Formula Packaging. http://www.fda.gov/newsevents/publichealthfocus/ucm064437.htm#regulations 2012. [Google Scholar]
- 9.Vandenberg LN, Prins GS. Clarity in the face of confusion: new studies tip the scales on bisphenol A (BPA). Andrology 2016; 4: 561–564. doi: 10.1111/andr.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lioy PJ, Hauser R, Gennings C, Koch HM, Mirkes PE, Schwetz BA et al. Assessment of phthalates/phthalate alternatives in children’s toys and childcare articles: Review of the report including conclusions and recommendation of the Chronic Hazard Advisory Panel of the Consumer Product Safety Commission. J Expo Sci Environ Epidemiol 2015; 25: 343–353. [DOI] [PubMed] [Google Scholar]
- 11.Kinch CD, Ibhazehiebo K, Jeong J-H, Habibi HR, Kurrasch DM. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proceedings of the National Academy of Sciences 2015; 112: 1475–1480. doi: 10.1073/pnas.1417731112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rochester JR, Bolden AL. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environmental health perspectives 2015; 123: 643–650. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.United States Comsumer Product Safety Comission., Us Consumer Product Safety Improvement Act of 2008 https://www.cpsc.gov/s3fs-public/pdfs/blk_pdf_cpsia.pdf 2008. [Google Scholar]
- 14.Washington State Department of Ecology. Children’s Safe Product Act. http://www.ecy.wa.gov/programs/hwtr/RTT/cspa/ 2016. [Google Scholar]
- 15.AH T Plasticizer Makers Want a Piece of the Phthalate Pie. Chemical and Engineering News 2015; 93: 16–18. doi:https://cen.acs.org/articles/93/i25/Plasticizer-Makers-Want-Piece-Phthalates.html. [Google Scholar]
- 16.Lowell Center for Sustainable Production. Technical Briefing: Phthalates and Their Alternatives: Health and Environmental Concerns. http://ec.europa.eu/environment/aarhus/pdf/35/Annex_11_report_from_Lowell_Center.pdf 2011. [Google Scholar]
- 17.Wong KH, Durrani TS. Exposures to Endocrine Disrupting Chemicals in Consumer Products-A Guide for Pediatricians. Current problems in pediatric and adolescent health care 2017; 47: 107–118. doi: 10.1016/j.cppeds.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Benvenuti N 3 Reasons Why Paraben Alternatives Might Be Worse Than Parabens. FutureDerm 2014; https://www.futurederm.com/3-reasons-why-paraben-alternatives-might-be-worse-than-parabens/. [Google Scholar]
- 19.Klaus W New Alternative to Paraben-Based Preservative Blends. Schulke and Myr GmbH, Nordestedt Germany 2018; http://www.schulkemicrosites.de/media-cosmetic-preservation/Literature/New-Alternatives-to-Paraben-Based-Preservative-Blends-C-T-2005.pdf. [Google Scholar]
- 20.United States Environmental Protection Agency. 2018. https://www.epa.gov/saferchoice/safer-ingredients#scil Safer Chemical Ingredents List; Accessed 10/8/2018.
- 21.European Chemicals Agency. Endocrine Disruptions: Substances of Concern. http://ec.europa.eu/environment/chemicals/endocrine/strategy/substances_en.htm#priority_list. [Google Scholar]
- 22.Global Harmonization System. http://www.safe.nite.go.jp/english/ghs/ghs_index.html. Accessed 10/5/2018 Updated 2018.
- 23.Pham N, Iyer S, Hackett E, Lock BH, Sandy M, Zeise L et al. Using ToxCast to Explore Chemical Activities and Hazard Traits: A Case Study With Ortho-Phthalates. Toxicological sciences: an official journal of the Society of Toxicology 2016; 151: 286–301. doi: 10.1093/toxsci/kfw049. [DOI] [PubMed] [Google Scholar]
- 24.Reif David M, Martin Matthew T, Tan Shirlee W, Houck Keith A, Judson Richard S, Richard Ann M et al. Endocrine Profiling and Prioritization of Environmental Chemicals Using ToxCast Data. Environmental health perspectives 2010; 118: 1714–1720. doi: 10.1289/ehp.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filer D, Patisaul HB, Schug T, Reif D,Thayer K Test driving ToxCast: endocrine profiling for 1858 chemicals included in phase II. Current Opinion in Pharmacology 2014; 19: 145–152. doi: 10.1016/j.coph.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC et al. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. J Cheminform 2017; 9: 017–0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.United States Environmental Protection Agency. Toxicity Estimation Software Tool Version 4.2 User Manual. https://www.epa.gov/sites/production/files/2016-05/documents/600r16058.pdf 2016.
- 28.Cassano A, Manganaro A, Martin T, Young D, Piclin N, Pintore M et al. CAESAR models for developmental toxicity. Chemistry Central Journal 2010; 4: S4. doi: 10.1186/1752-153x-4-s1-s4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wambaugh JF, Wang A, Dionisio KL, Frame A, Egeghy P, Judson R et al. High Throughput Heuristics for Prioritizing Human Exposure to Environmental Chemicals. Environmental Science & Technology 2014; 48: 12760–12767. doi: 10.1021/es503583j. [DOI] [PubMed] [Google Scholar]
- 30.Phillips KA, Wambaugh JF, Grulke CM, Dionisio KL, Isaacs KK. High-throughput screening of chemicals as functional substitutes using structure-based classification models. Green Chemistry 2017; 19: 1063–1074. doi: 10.1039/C6GC02744J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malloy T, Zaunbrecher V, Beryt E, Judson R, Tice R, Allard P et al. Advancing alternatives analysis: The role of predictive toxicology in selecting safer chemical products and processes. Integrated environmental assessment and management 2017; 13: 915–925. doi: 10.1002/ieam.1923. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs MM, Malloy TF, Tickner JA, Edwards S Alternatives Assessment Frameworks: Research Needs for the Informed Substitution of Hazardous Chemicals. Environmental health perspectives 2016; 124: 265–280. doi: 10.1289/ehp.1409581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC et al. The CompTox Chemistry Dashboard: a community data resource for environmental chemistry. Journal of Cheminformatics 2017; 9: 61. doi: 10.1186/s13321-017-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malloy TF, Zaunbrecher VM, Batteate CM, Blake A, Carroll WF Jr., Corbett CJ et al. Advancing Alternative Analysis: Integration of Decision Science. Environmental health perspectives 2017; 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wehage K, Chenhansa P, Schoenung JM. An open framework for automated chemical hazard assessment based on GreenScreen for Safer Chemicals: A proof of concept. Integrated environmental assessment and management 2017; 13: 167–176. doi: 10.1002/ieam.1763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.